Organoselenium Compounds Derived from Natural Metabolites
Abstract
1. Introduction
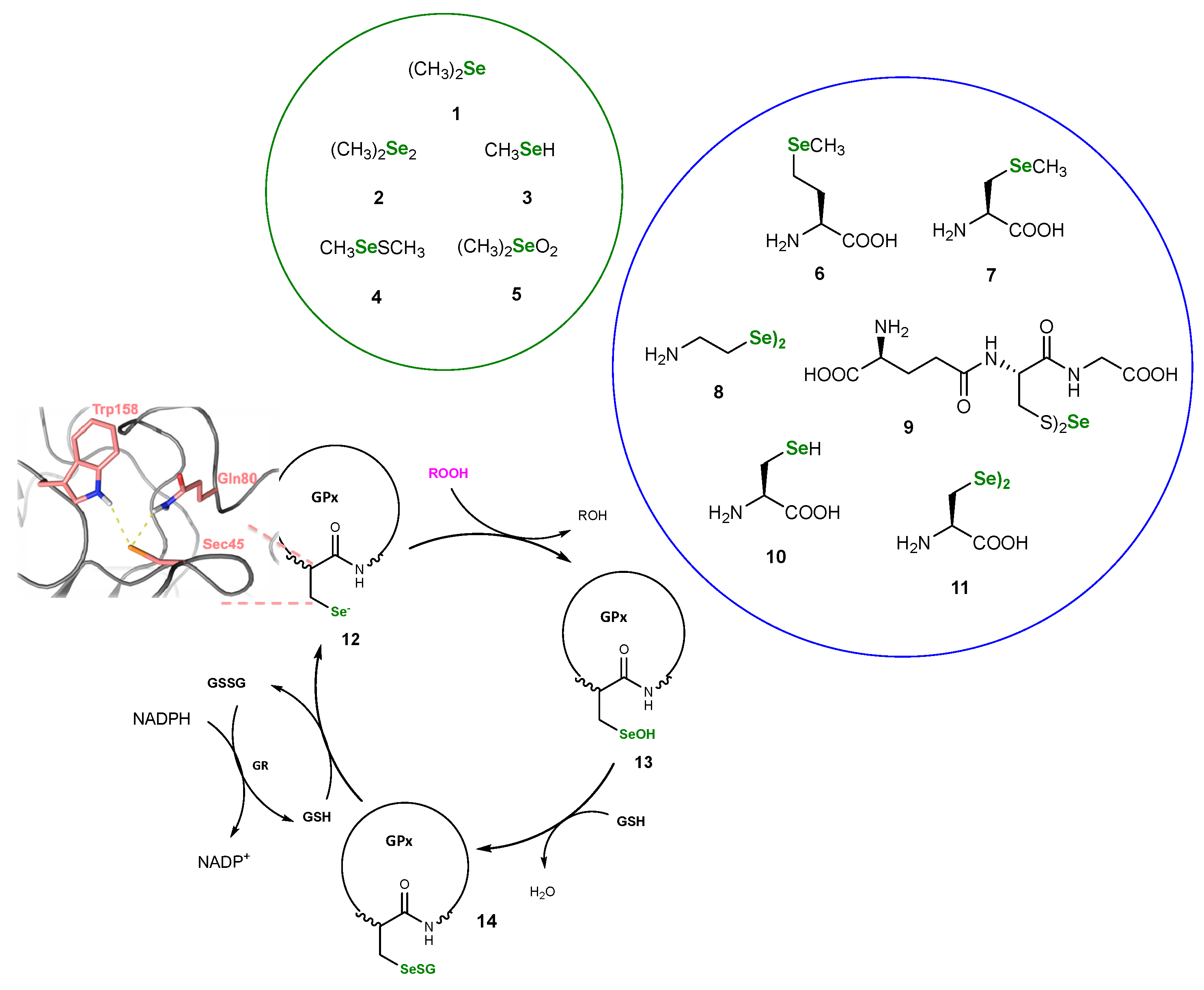
2. Naturally Derived OSeCs as GPx-like Catalysts
2.1. Methods for Assessing GPx-like Antioxidant Activity
2.2. Examples of Organoselenium Compounds Mimicking GPx
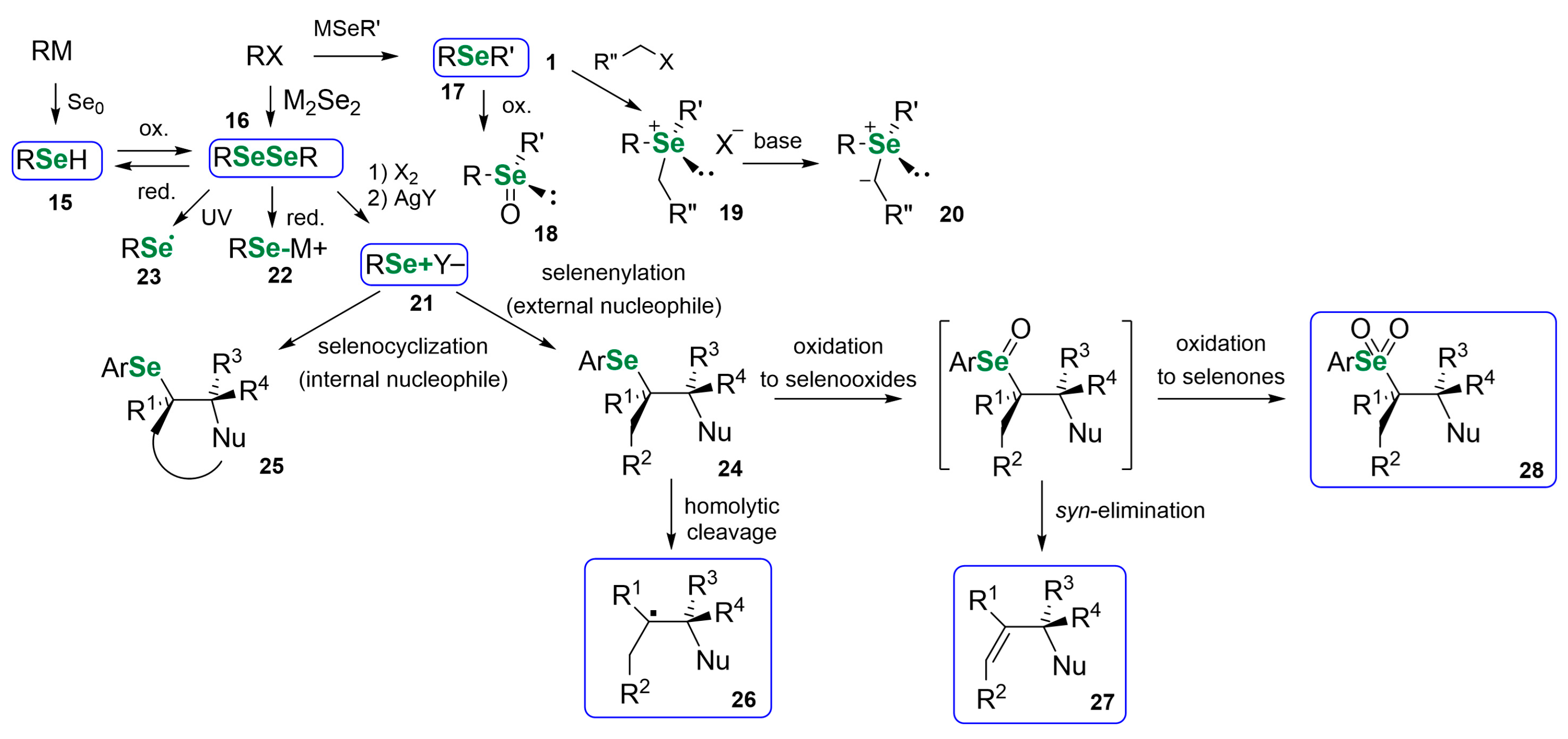
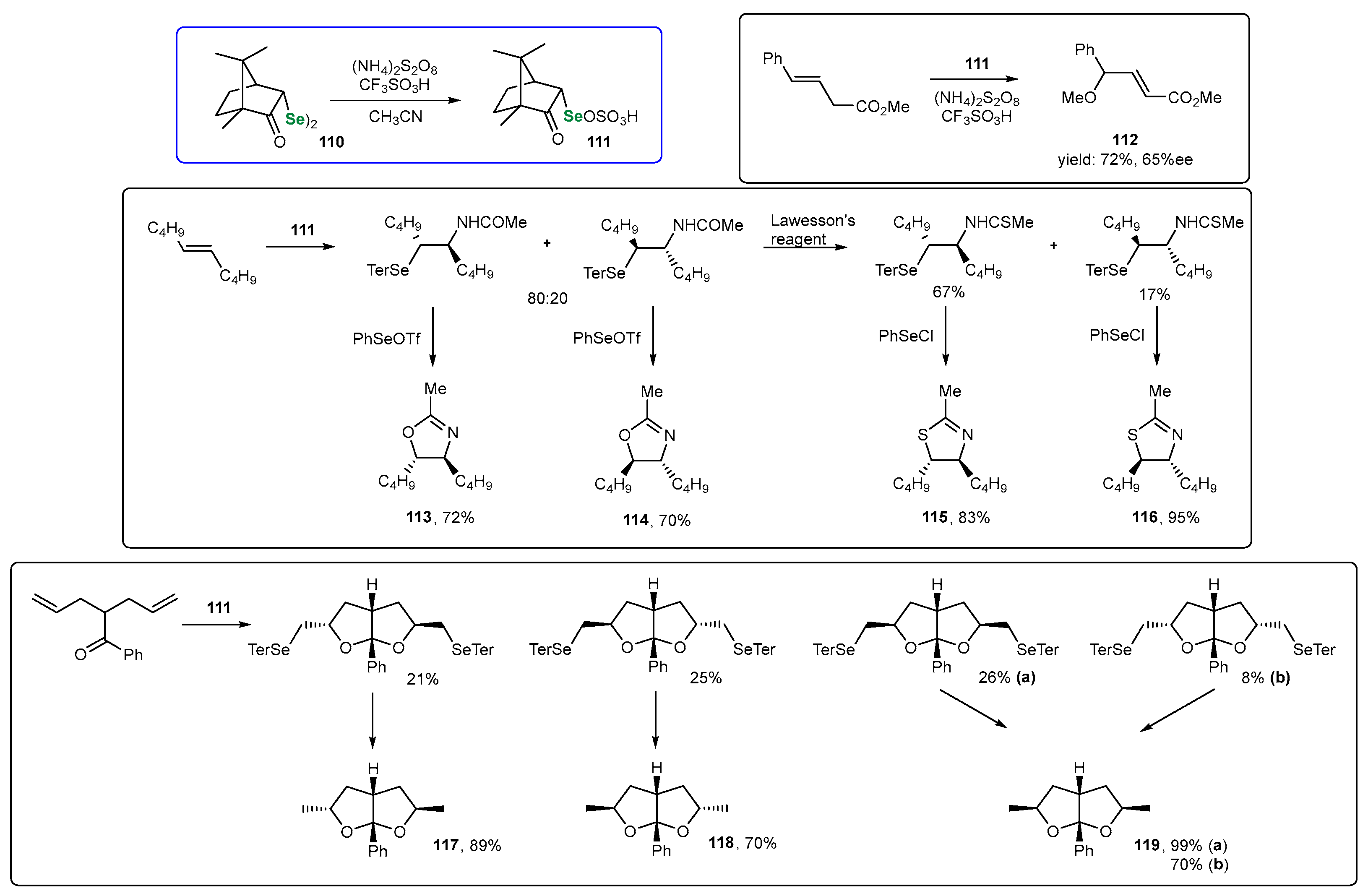
3. Naturally Derived OSeCs as Reagents in Organic Synthesis
3.1. Se Amino Acids and Peptides
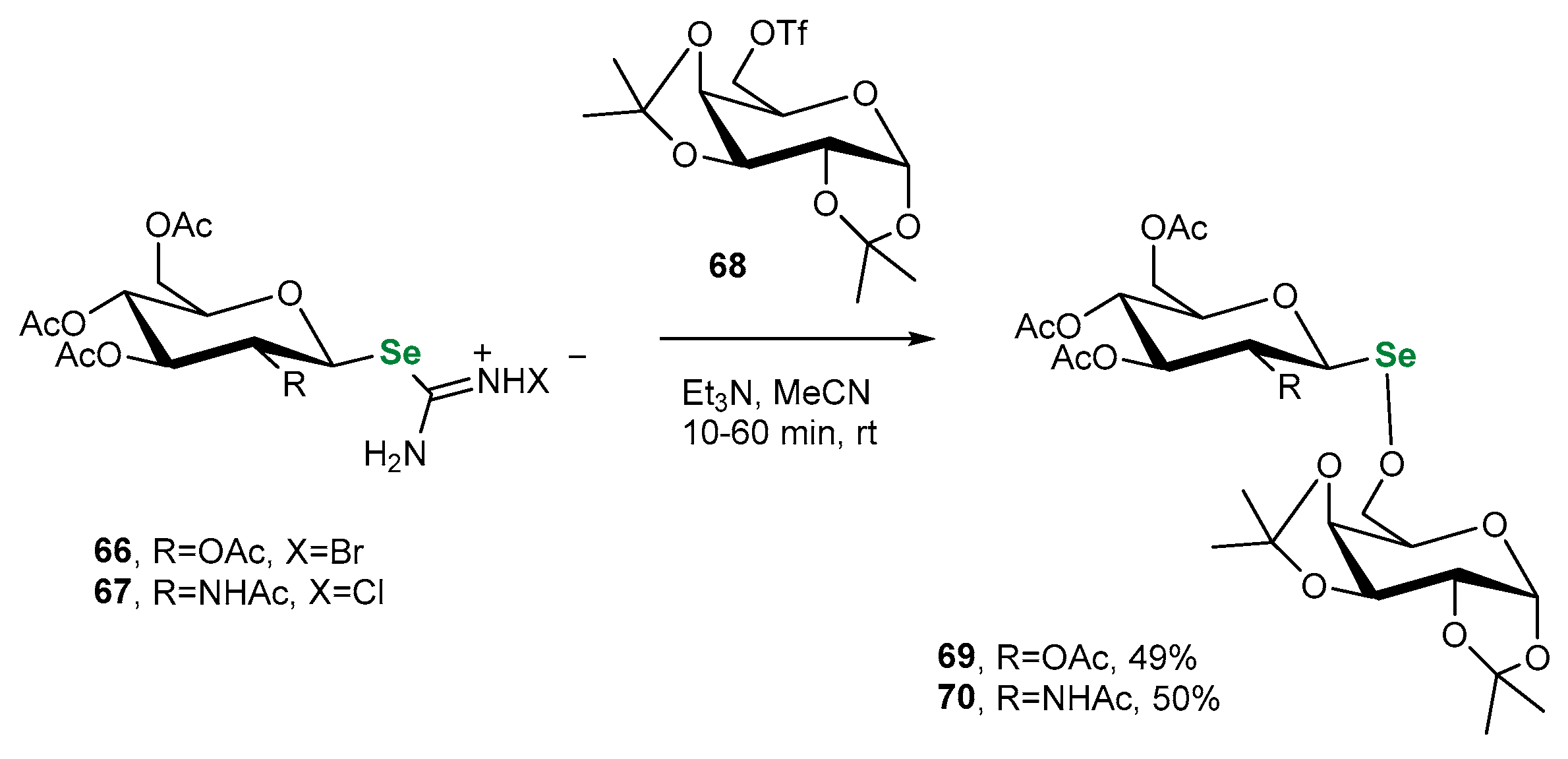
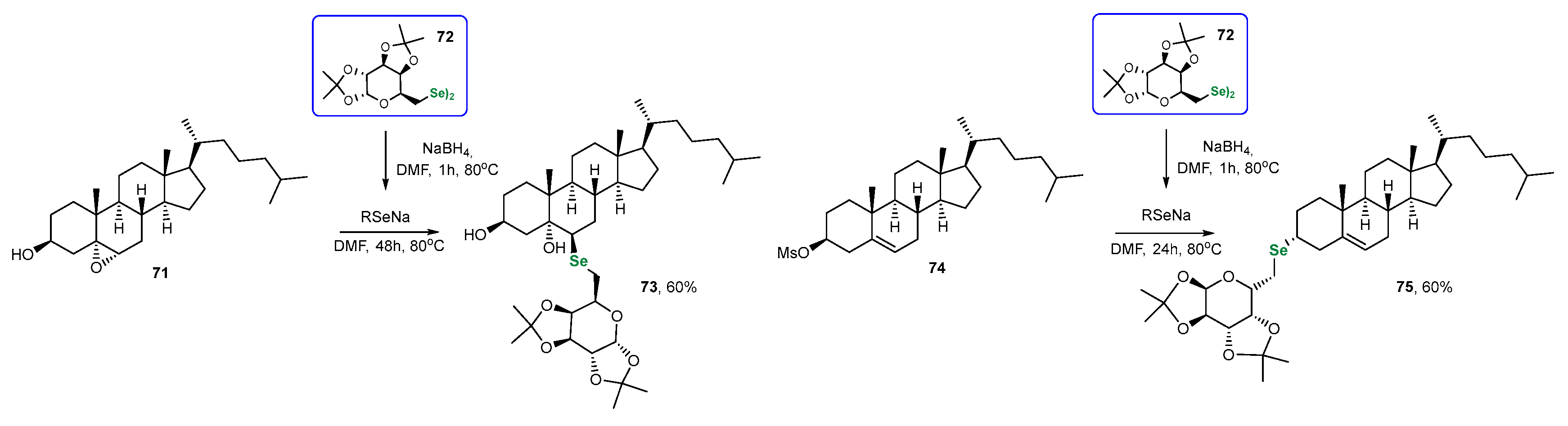
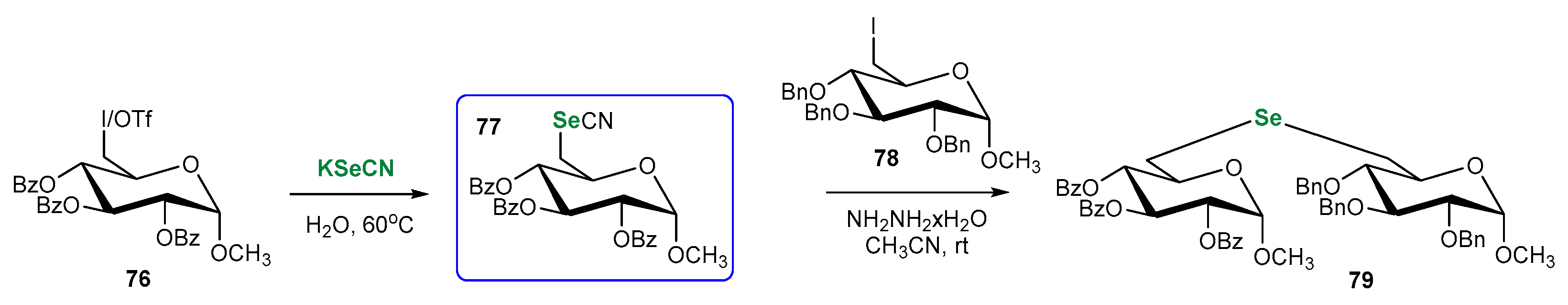
3.2. Se-Sugars
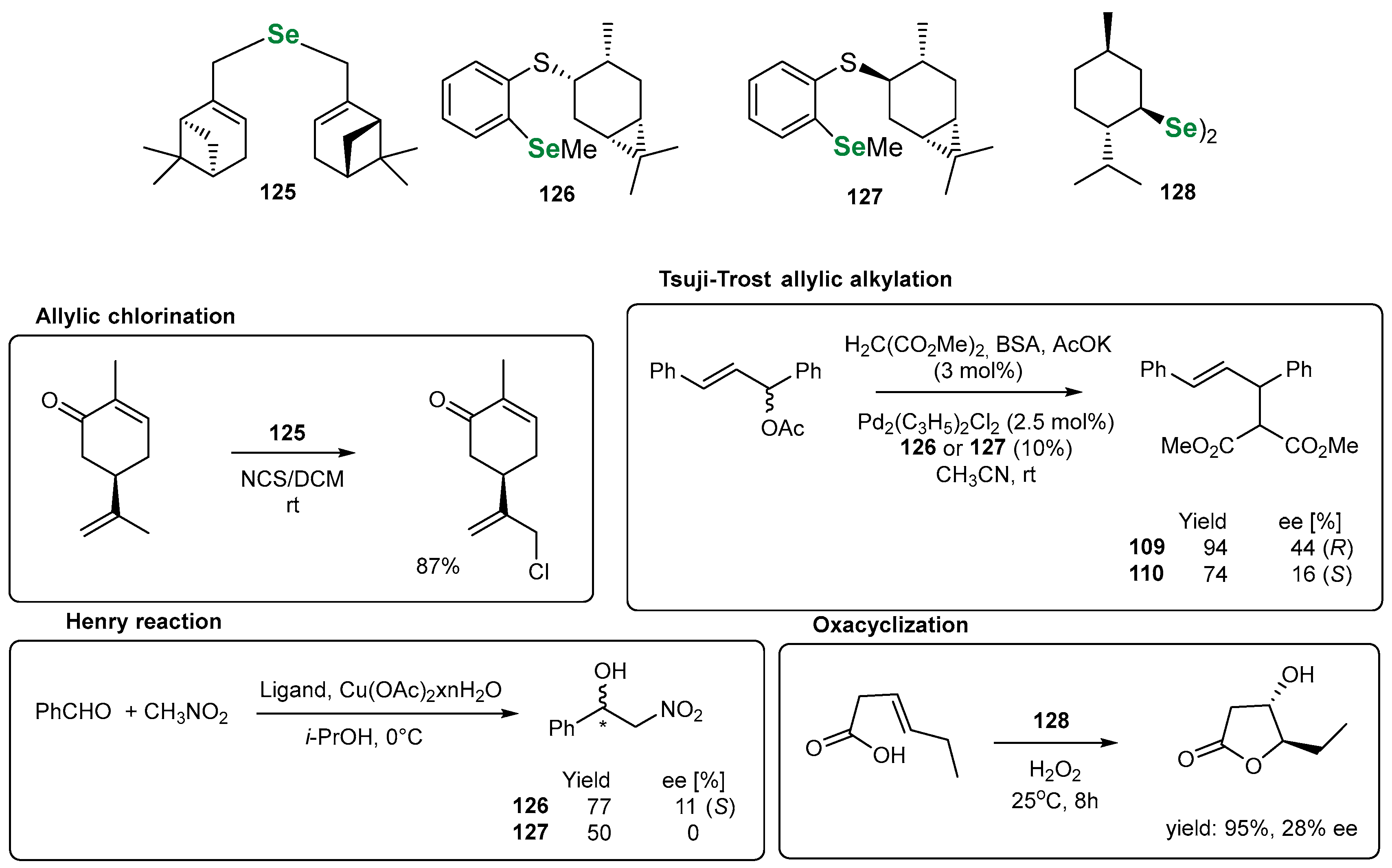
3.3. Organoselenium Reagents Derived from Monoterpenes
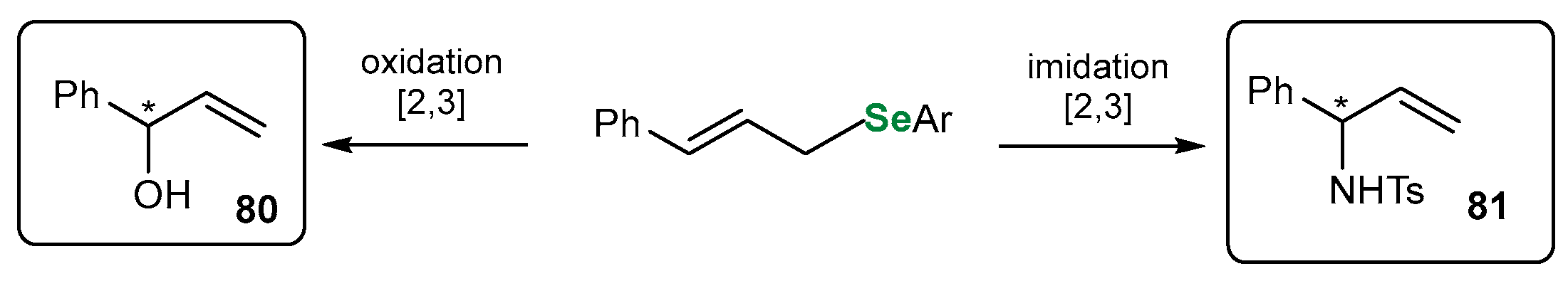
3.3.1. Monoterpene-Based OSeRs in [2,3]-Sigmatropic Rearrangements
3.3.2. Monoterpene-Based OSeCs as Electrophilic Reagents
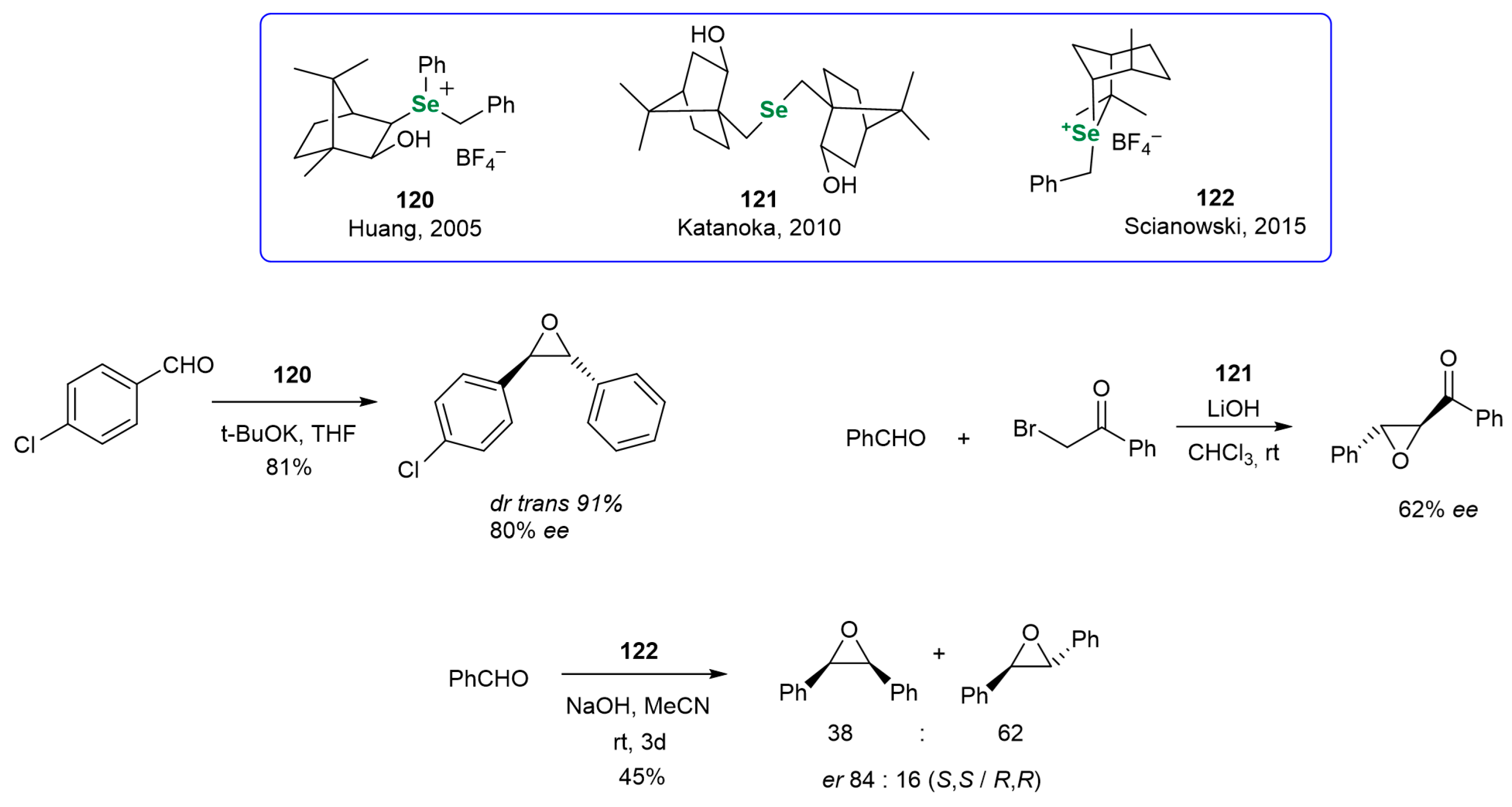
3.3.3. Monoterpene-Based OSeCs in Asymmetric Epoxidation and Cyclopropanation
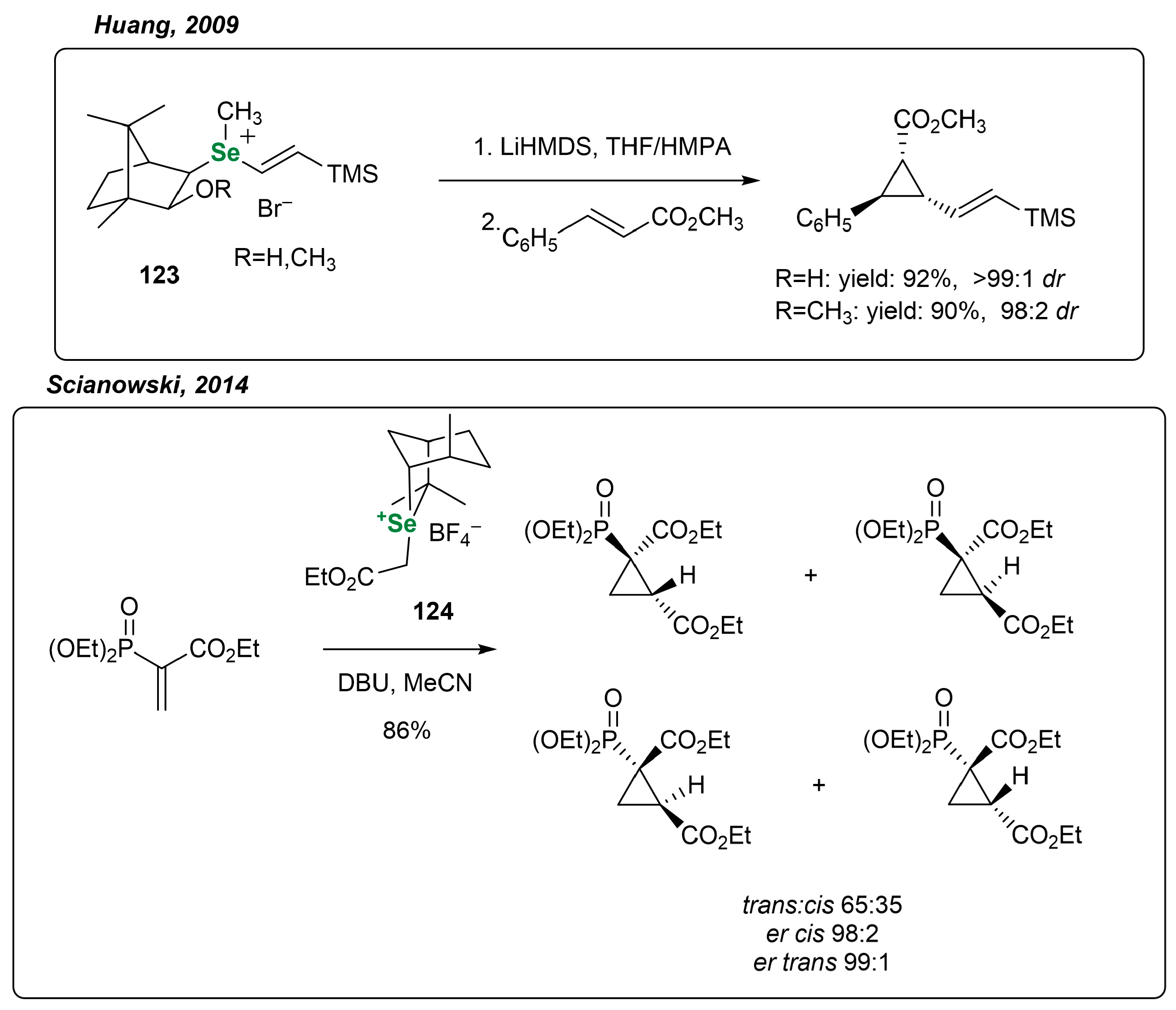
3.3.4. Other Transformations Utilizing Chiral Monoterpene-Based OSeCs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Drew, S.W.; Demain, A.L. Effect of primary metabolites on secondary metabolism. Annu. Rev. Microbiol. 1977, 31, 343–356. [Google Scholar] [CrossRef]
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural products (secondary metabolites). In Biochemistry & Molecular Biology of Plants; Buchanan, B., Gruissem, W., Jones, R., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 2000; pp. 1250–1318. [Google Scholar]
- Li, Q.; Zhang, Y.; Chen, Z.; Pan, X.; Zhang, Z.; Zhu, J.; Zhu, X. Organoselenium chemistry-based polymer synthesis. Org. Chem. Front. 2020, 7, 2815–2841. [Google Scholar] [CrossRef]
- Heredia, A.A.; Bouchet, L.M.; Castro-Godoy, W.D.; Argüello, J.E. Synthesis of organoselenium compounds using electrochemical and photochemical methods as novel approaches in organic chemistry. Tetrahedron 2023, 148, 133667–133682. [Google Scholar] [CrossRef]
- Sonego, J.M.; de Diego, S.I.; Szajnman, S.H.; Gallo-Rodriguez, C.; Rodriguez, J.B. Organoselenium Compounds: Chemistry and Applications in Organic Synthesis. Chem. Eur. J. 2023, 29, e202300030. [Google Scholar] [CrossRef]
- Azeredo, J.B.; Penteado, F.; Nascimento, V.; Sancineto, L.; Braga, A.L.; Lenardao, E.J.; Santi, C. “Green Is the Color”: An Update on Ecofriendly Aspects of Organoselenium Chemistry. Molecules 2022, 27, 1597. [Google Scholar] [CrossRef]
- Cheng, K.; Sun, Y.; Liu, B.; Ming, J.; Wang, L.; Xu, C.; Xiao, Y.; Zhang, C.; Shang, L. Selenium modification of natural products and its research progress. Foods 2023, 12, 3773. [Google Scholar] [CrossRef]
- Weekley, C.M.; Harris, H.H. Which form is that? The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem. Soc. Rev. 2013, 42, 8870–8894. [Google Scholar] [CrossRef]
- Achibat, H.; AlOmari, N.A.; Messina, F.; Sancineto, L.; Khouili, M.; Santi, C. Organoselenium compounds as phytochemicals from the natural kingdom. Nat. Prod. Commun. 2015, 11, 1885–1892. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Z.; Zhang, L.; Peng, J.; Huang, T.; Yang, X.; Jeong, B.R.; Yang, Q. Seleno-amino acids in vegetables: A review of their forms and metabolism. Front. Plant Sci. 2022, 13, 804368–804381. [Google Scholar] [CrossRef] [PubMed]
- Pacuła, A.J.; Mangiavacchi, F.; Sancineto, L.; Lenardao, E.J.; Ścianowski, J.; Santi, C. An update on “Selenium containing compounds from poison to drug candidates: A review on the GPx-like activity”. Curr. Chem. Biol. 2015, 9, 97–112. [Google Scholar] [CrossRef]
- Ge, L.; Liu, P.; Tian, L.; Li, Y.; Chen, L. Se-methylselenocysteine inhibits the progression of non-small cell lung cancer via ROS-mediated NF-κB signalling pathway. Exp. Cell Res. 2024, 440, 114101–114112. [Google Scholar] [CrossRef]
- Dongsoo, K.D.; Ku, B.; Choi, E.-M. Se-methylselenocysteine stimulates migration and antioxidant response in HaCaT keratinocytes: Implications for wound healing. J. Trace Elem. Med. Biol. 2025, 58, 126426–126433. [Google Scholar]
- Dantas de Araujo, A.; Perry, S.R.; Fairlie, D.P. Chemically diverse helix-constrained peptides using selenocysteine crosslinking. Org. Lett. 2018, 20, 1453–1456. [Google Scholar] [CrossRef]
- Yin, Y.; Fei, Q.; Liu, W.; Li, Z.; Suga, H.; Wu, C. Chemical and ribosomal synthesis of topologically controlled bicyclic and tricyclic peptide scaffolds primed by selenoether formation. Angew. Chem. Int. Ed. 2019, 58, 4880–4885. [Google Scholar] [CrossRef]
- Bjornstedt, M.; Kumar, S.; Holmgren, A. Selenodiglutathione is a highly efficient oxidant of reduced thioredoxin and a substrate for mammalian thioredoxin reductase. J. Biol. Chem. 1992, 267, 8030–8034. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Kumakura, F.; Komatsu, I.; Arai, K.; Onuma, Y.; Hojo, H.; Singh, B.G.; Priyadarsini, K.I.; Iwaoka, M. Antioxidative glutathione peroxidase activity of selenoglutathione. Angew. Chem. Int. Ed. 2011, 50, 2125–2128. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, S.; Asano, Y.; Arai, K.; Iwaoka, M. Selenoglutathione diselenide: Unique redox reactions in the GPx-like catalytic cycle and repairing of disulfide bonds in scrambled protein. Biochemistry 2017, 56, 5644–5653. [Google Scholar] [CrossRef] [PubMed]
- Ścianowski, J.; Rafiński, Z. Electrophilic Selenium Reagents: Addition Reactions to Double Bonds and Selenocyclizations. In Organoselenium Chemistry: Between Synthesis and Biochemistry; Santi, C., Ed.; Bentham: Sharjah, United Arab Emirates, 2014; pp. 8–60. [Google Scholar]
- Drabowicz, J.; Lewkowski, J.; Ścianowski, J. Selenium compounds with valency higher than two. In Organoselenium Chemistry: Synthesis and Reactions; Wirth, T., Ed.; Wiley-VCH: Weinheim, Germany, 2012; pp. 191–256. [Google Scholar]
- Bowman, W.R. Selenium compounds in radical reactions. In Organoselenium Chemistry: Synthesis and Reactions; Wirth, T., Ed.; Wiley-VCH: Weinheim, Germany, 2011; pp. 111–146. [Google Scholar]
- Liu, M.; Zhang, X.; Chu, S.; Ge, Y.; Huang, T.; Liu, Y.; Yu, L. Selenization of cotton products with NaHSe endowing the antibacterial activities. Chem. Lett. 2022, 33, 205–208. [Google Scholar] [CrossRef]
- Singh, F.V.; Wirth, T. Selenium reagents as catalysts. Catal. Sci. Technol. 2019, 9, 1073–1091. [Google Scholar] [CrossRef]
- Stadel, J.T.; Back, T.G. Asymmetric synthesis with organoselenium compounds—The past twelve years. Chem. Eur. J. 2024, 30, e202304074. [Google Scholar] [CrossRef] [PubMed]
- Madabeni, A.; Bortoli, M.; Nogara, P.A.; Ribaudo, G.; Dalla Tiezza, M.; Flohé, L.; Rocha, J.B.T.; Orian, L. 50 years of organoselenium chemistry, biochemistry and reactivity: Mechanistic understanding, successful and controversial stories. Chem. Eur. J. 2024, 30, e202403003. [Google Scholar] [CrossRef] [PubMed]
- Avila, D.S.; Gubert, P.; Palma, A.; Colle, D.; Alves, D.; Nogueira, C.W.; Rocha, J.B.; Soares, F.A. An organotellurium compound with antioxidant activity against excitotoxic agents without neurotoxic effects in brain of rats. Brain Res. Bull. 2008, 76, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Ramoutar, R.R.; Brumaghim, J.L. Antioxidant and anticancer properties and mechanisms of inorganic selenium, oxo-sulfur, and oxo-selenium compounds. Cell Biochem. Biophys. 2010, 58, 1–23. [Google Scholar] [CrossRef]
- Mugesh, G.; du Mont, W.W.; Sies, H. Chemistry of biologically important synthetic organoselenium compounds. Chem. Rev. 2001, 101, 2125–2180. [Google Scholar] [CrossRef]
- Ren, X.; Zou, L.; Lu, J.; Holmgren, A. Selenocysteine in mammalian thioredoxin reductase and application of ebselen as a therapeutic. Free Radic. Biol. Med. 2018, 127, 238–247. [Google Scholar] [CrossRef]
- Herbette, S.; Roeckel-Drevet, P.; Drevet, J. Seleno-independent glutathione peroxidases: More than simple antioxidant scavengers. FEBS 2007, 274, 2163–2180. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.M.; Pickard, K.; Nicol, F.; Beckett, G.J.; Duthie, G.G.; Arthur, J.R. Effects of organic and inorganic selenium supplementation on selenoenzyme activity in blood lymphocytes, granulocytes, platelets and erythrocytes. Clin. Sci. 2000, 98, 593–599. [Google Scholar] [CrossRef]
- Johansson, L.; Gafvelin, G.; Arnér, E.S.J. Selenocysteine in proteins—Properties and biotechnological use. Biochim. Biophys. Acta Gen. Subj. 2005, 1726, 1–13. [Google Scholar] [CrossRef]
- Lee, S.R.; Bar-Noy, S.; Kwon, J.; Levine, R.L.; Stadtman, T.C.; Rhee, S.G. Mammalian thioredoxin reductase: Oxidation of the C-terminal cysteine/selenocysteine active site forms a thioselenide, and replacement of selenium with sulfur markedly reduces catalytic activity. Proc. Natl. Acad. Sci. USA 2000, 97, 2521–2526. [Google Scholar] [CrossRef]
- Gasdaska, J.R.; Harney, J.W.; Gasdaska, P.Y.; Powis, G.; Berry, M.J. Regulation of human thioredoxin reductase expression and activity by 3′-untranslated region selenocysteine insertion sequence and mRNA instability elements. J. Biol. Chem. 1999, 274, 25379–25385. [Google Scholar] [CrossRef]
- Zhao, R.; Masayasu, H.; Holmgren, A. Ebselen: A substrate for human thioredoxin reductase strongly stimulating its hydroperoxide reductase activity and a superfast thioredoxin oxidant. Proc. Natl. Acad. Sci. USA 2002, 99, 8579–8584. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Xu, H. Incorporating selenium into heterocycles and natural products—From chemical properties to pharmacological activities. J. Med. Chem. 2002, 65, 4436–4456. [Google Scholar] [CrossRef]
- Wilson, S.R.; Zucker, P.A.; Huang, R.R.C.; Spector, A. Development of synthetic compounds with glutathione peroxidase activity. J. Am. Chem. Soc. 1989, 111, 5936–5939. [Google Scholar] [CrossRef]
- Shaaban, S.; Ashmawy, A.M.; Negm, A.; Wessjohann, L.A. Synthesis and biochemical studies of novel organic selenides with increased selectivity for hepatocellular carcinoma and breast adenocarcinoma. Eur. J. Med. Chem. 2019, 179, 515–526. [Google Scholar] [CrossRef]
- Iwaoka, M.; Tomoda, S. A model study on the effect of an amino group on the antioxidant activity of glutathione peroxidase. J. Am. Chem. Soc. 1994, 116, 2557–2561. [Google Scholar] [CrossRef]
- Shaaban, S.; Negm, A.; Ashmawy, A.M.; Ahmed, D.M.; Wessjohann, L.A. Combinatorial synthesis, in silico, molecular and biochemical studies of tetrazole-derived organic selenides with increased selectivity against hepatocellular carcinoma. Eur. J. Med. Chem. 2016, 122, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Kumakura, F.; Mishra, B.; Priyadarsini, K.I.; Iwaoka, M. A water-soluble cyclic selenide with enhanced glutathione peroxidase-like catalytic activities. Eur. J. Org. Chem. 2010, 2010, 440–445. [Google Scholar] [CrossRef]
- Vessman, K.; Ekström, M.; Berglund, M.; Andersson, C.M.; Engman, L. Catalytic antioxidant activity of diaryl tellurides in a two-phase lipid peroxidation model. J. Org. Chem. 1995, 60, 4461–4467. [Google Scholar] [CrossRef]
- Kumar, S.; Johansson, H.; Engman, L.; Valgimigli, L.; Amorati, R.; Fumo, M.G.; Pedulli, G.F. Regenerable chain-breaking 2,3-dihydrobenzo[b]selenophene-5-ol antioxidants. J. Org. Chem. 2007, 72, 2583–2595. [Google Scholar] [CrossRef]
- Chilaya, G.S. Induction of chirality in nematic phases. Rev. Phys. Appl. 1981, 16, 193–208. [Google Scholar] [CrossRef]
- Chilaya, G.S.; Lisetski, L.N. Cholesteric liquid crystals: Physical properties and molecular-statistical theories. Mol. Cryst. Liq. Cryst. 1986, 140, 243–286. [Google Scholar] [CrossRef]
- Chilaya, G.S. Effect of various external factors and pretransitional phenomena on structural transformations in cholesteric liquid crystals. Crystallogr. Rep. 2000, 45, 871–886. [Google Scholar] [CrossRef]
- Frizon, T.E.; Rafique, J.; Saba, S.; Bechtold, I.H.; Gallardo, H.; Braga, A.L. Synthesis of functionalized organoselenium materials: Selenides and diselenides containing cholesterol. Eur. J. Org. Chem. 2015, 2015, 3470–3476. [Google Scholar] [CrossRef]
- Combs, G.F., Jr. The Vitamins: Fundamental Aspects in Nutrition and Health, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Singh, V.P.; Poon, J.F.; Butcher, R.J.; Lu, X.; Mestres, G.; Ott, M.K.; Engman, L. Effect of a bromo substituent on the glutathione peroxidase activity of a pyridoxine-like diselenide. J. Org. Chem. 2015, 80, 7385–7395. [Google Scholar] [CrossRef] [PubMed]
- Suhas, R.; Gowda, D.C. Design and synthesis of tryptophan containing peptides as potential analgesic and anti-inflammatory agents. J. Pept. Sci. 2012, 18, 535–540. [Google Scholar] [CrossRef]
- Suresha, G.P.; Prakasha, K.C.; Shiva Kumara, K.N.; Kapfo, W.; Gowda, D.C. Design and Synthesis of Heterocyclic Conjugated Peptides as Novel Antimicrobial Agents. Int. J. Pept. Res. Ther. 2009, 15, 25–30. [Google Scholar] [CrossRef]
- Shantharam, C.S.; Suyoga Vardhan, D.M.; Suhas, R.; Gowda, D.C. Design and synthesis of amino acids-conjugated heterocycle derived ureas/thioureas as potent inhibitors of protein glycation. Russ. J. Bioorg. Chem. 2014, 40, 443–454. [Google Scholar] [CrossRef]
- Sudati, J.H.; Nogara, P.A.; Saraiva, R.A.; Wagner, C.; Alberto, E.E.; Braga, A.L.; Fachinetto, R.; Piquini, P.C.; Rocha, J.B.T. Diselenoamino acid derivatives as GPx mimics and as substrates of TrxR: In vitro and in silico studies. Org. Biomol. Chem. 2018, 16, 3777–3787. [Google Scholar] [CrossRef]
- Pacuła, A.J.; Kaczor, K.B.; Antosiewicz, J.; Janecka, A.; Długosz, A.; Janecki, T.; Wojtczak, A.; Ścianowski, J. New chiral ebselen analogues with antioxidant and cytotoxic potential. Molecules 2017, 22, 492. [Google Scholar] [CrossRef]
- Pacuła, A.J.; Ścianowski, J. Terpenes as green starting materials for new organoselenium and organotellurium compounds. Curr. Green Chem. 2016, 3, 36–50. [Google Scholar] [CrossRef]
- Obieziurska, M.; Pacuła, A.J.; Długosz-Pokorska, A.; Krzemiński, M.; Janecka, A.; Ścianowski, J. Bioselectivity induced by chirality of new terpenyl organoselenium compounds. Materials 2019, 12, 3579. [Google Scholar] [CrossRef]
- Obieziurska-Fabisiak, M.; Pacuła, A.J.; Capoccia, L.; Drogosz-Stachowicz, J.; Janecka, A.; Santi, C.; Ścianowski, J. Phenylselanyl Group Incorporation for “Glutathione Peroxidase-Like” Activity Modulation. Molecules 2020, 25, 3354. [Google Scholar] [CrossRef] [PubMed]
- Laskowska, A.; Pacuła-Miszewska, A.J.; Obieziurska-Fabisiak, M.; Jastrzębska, A.; Długosz-Pokorska, A.; Gach-Janczak, K.; Ścianowski, J. Synthesis of a new class of β-carbonyl selenides functionalized with ester groups with antioxidant and anticancer properties—Part II. Molecules 2024, 29, 2866. [Google Scholar] [CrossRef]
- Telo, J.P.; Veiros, L.F.; André, V.; Ferreira da Silva, J.; Justino, G.C.; Antunes, A.M.M. Monoterpenoid selenophenes derived from (-)-carvone with GPx-like activity. Org. Biomol. Chem. 2025, 23, 2153–2162. [Google Scholar] [CrossRef] [PubMed]
- Alhasan, R.; Nasim, M.J.; Jacob, C.; Gaucher, C. Selenoneine: A unique reactive selenium species from the blood of tuna with implications for human diseases. Curr. Pharmacol. Rep. 2019, 5, 163–173. [Google Scholar] [CrossRef]
- Alhasan, R.; Martins, G.M.; de Castro, P.P.; Saleem, R.S.Z.; Zaiter, A.; Fries-Raeth, I.; Kleinclauss, A.; Perrin-Sarrado, C.; Chaimbault, P.; da Silva Júnior, E.N.; et al. Selenoneine-inspired selenohydantoins with glutathione peroxidase-like activity. Bioorg. Med. Chem. 2023, 94, 117479–117491. [Google Scholar] [CrossRef]
- Carreiro, E.P.; Burke, A.J. Amino acids as chiral building blocks. In Chiral Building Blocks in Asymmetric Synthesis; Wojaczyńska, E., Wojaczyński, J., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 161–196. [Google Scholar]
- Wessjohann, L.A.; Schneider, A. Synthesis of selenocysteine and its derivatives with an emphasis on selenenylsulfide (SeS) formation. Chem. Biodivers. 2008, 5, 375–388. [Google Scholar] [CrossRef]
- Braga, A.L.; Wessjohann, L.A.; Taube, P.S.; Galetto, F.Z.; de Andrade, F.M. Straightforward method for the synthesis of selenocysteine and selenocystine derivatives from L-serine methyl ester. Synthesis 2010, 18, 3131–3137. [Google Scholar] [CrossRef]
- Rooseboom, M.; Vermeulen, N.P.E.; Durgut, F.; Commandeur, J.N.M. Comparative study on the bioactivation mechanisms and cytotoxicity of Te-phenyl-L-tellurocysteine, Se-phenyl-L-selenocysteine, and S-phenyl-L-cysteine. Chem. Res. Toxicol. 2002, 15, 1610–1618. [Google Scholar] [CrossRef]
- Venhorst, J.; Rooseboom, M.; Vermeulen, N.P.E.; Commandeur, J.N.M. Studies on the inhibition of human cytochromes P450 by selenocysteine Se-conjugates. Xenobiotica 2003, 33, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Menge, W.M.P.B.; Commandeur, J.N.M.; Worthington, E.A.; Vermeulen, N.P.E. Synthesis of novel Se-substituted selenocysteine derivatives as potential kidney selective prodrugs of biologically active selenol compounds: Evaluation of kinetics of β-elimination reactions in rat renal cytosol. J. Med. Chem. 1996, 39, 2040–2046. [Google Scholar] [CrossRef]
- Block, E.; Booker, S.J.; Flores-Penalba, S.; George, G.N.; Gundala, S.; Landgraf, B.J.; Liu, J.; Lodge, S.N.; Pushie, M.J.; Rozovsky, S.; et al. Trifluoroselenomethionine: A new unnatural amino acid. ChemBioChem 2016, 17, 1738–1751. [Google Scholar] [CrossRef] [PubMed]
- Poluboyarinov, P.A.; Golubkina, N.A.; Aniskov, A.A.; Moiseeva, I.J.; Glebova, N.N.; Shvets, V.I. The synthesis and biological activity of 3,3′-dimethyl-L-selenocystine, a new selenocystine derivative. Russ. J. Bioorg. Chem. 2019, 45, 241–247. [Google Scholar] [CrossRef]
- Muttenthaler, M.; Alewood, P.F. Selenopeptide chemistry. J. Pept. Sci. 2008, 14, 1223–1239. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Campillo, I.; Blanco-Canosa, J.B. Kinetic and mechanistic studies of native chemical ligation with phenyl α-selenoester peptides. JACS 2024, 4, 4374–4382. [Google Scholar] [CrossRef] [PubMed]
- Guan, I.; Williams, K.; Liu, J.S.T.; Liu, X. Synthetic thiol and selenol derived amino acids for expanding the scope of chemical protein synthesis. Front. Chem. 2022, 9, 826764–826796. [Google Scholar] [CrossRef]
- Raj, M.; Wu, H.; Blosser, S.L.; Vittoria, M.A.; Arora, P.S. Aldehyde capture ligation for synthesis of native peptide bonds. J. Am. Chem. Soc. 2015, 137, 6932–6940. [Google Scholar] [CrossRef]
- Temperini, A.; Piazzolla, F.; Minuti, L.; Curini, M.; Siciliano, C. General, mild, and metal-free synthesis of phenyl selenoesters from anhydrides and their use in peptide synthesis. J. Org. Chem. 2017, 82, 4588–4603. [Google Scholar] [CrossRef]
- Pehlivan, Ö.; Waliczek, M.; Kijewska, M.; Stefanowicz, P. Selenium in peptide chemistry. Molecules 2023, 28, 3198. [Google Scholar] [CrossRef]
- Malins, L.R.; Payne, R.J. Synthesis and utility of β-selenol-phenylalanine for native chemical ligation–deselenization chemistry. Org. Lett. 2012, 14, 3142–3145. [Google Scholar] [CrossRef]
- Townsend, S.D.; Tan, Z.; Dong, S.; Shang, S.; Brailsford, J.A.; Danishefsky, S.J. Advances in proline ligation. J. Am. Chem. Soc. 2012, 134, 3912–3916. [Google Scholar] [CrossRef]
- Lin, Y.A.; Chalker, J.M.; Davis, B.G. Olefin Cross-Metathesis on Proteins: Investigation of Allylic Chalcogen Effects and Guiding Principles in Metathesis Partner Selection. J. Am. Chem. Soc. 2010, 132, 16805–16811. [Google Scholar] [CrossRef] [PubMed]
- Dowman, L.J.; Kulkarni, S.S.; Alegre-Requena, J.V.; Giltrap, A.M.; Norman, A.R.; Sharma, A.; Gallegos, L.C.; Mackay, A.S.; Welegedara, A.P.; Watson, E.E.; et al. Site-selective photocatalytic functionalisation of peptides and proteins at selenocysteine. Nat. Commun. 2022, 13, 6885–6898. [Google Scholar] [CrossRef]
- Boysen, M.M.K. Carbohydrates as synthetic tools in organic chemistry. Chem. Eur. J. 2007, 13, 8648–8659. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Lu, Q.; Xing, D.; Zhang, R. Exploring carbohydrates for therapeutics: A review on future directions. Front. Pharmacol. 2021, 12, 756724–756733. [Google Scholar] [CrossRef]
- Mangiavacchi, F.; Coelho Dias, I.F.; Di Lorenzo, I.; Grzes, P.; Palomba, M.; Rosati, O.; Bagnoli, L.; Marini, F.; Santi, C.; Lenardao, E.J.; et al. Sweet selenium: Synthesis and properties of selenium-containing sugars and derivatives. Pharmaceuticals 2020, 13, 211. [Google Scholar] [CrossRef]
- Mehta, S.; Pinto, B.M. Phenylselenoglycosides as novel, versatile glycosyl donors. Selective activation over thioglycosides. Tetrahedron Lett. 1991, 32, 4435–4438. [Google Scholar] [CrossRef]
- Mehta, S.; Pinto, B.M. Novel glycosidation methodology. The use of phenyl selenoglycosides as glycosyl donors and acceptors in oligosaccharide synthesis. J. Org. Chem. 1993, 58, 3269–3276. [Google Scholar] [CrossRef]
- Spell, M.; Wang, X.; Wahba, A.E.; Conner, E.; Ragains, J. An α-selective, visible light photocatalytic glycosylation of alcohols with selenoglycosides. Carbohydr. Res. 2013, 369, 42–47. [Google Scholar] [CrossRef]
- Khatuntseva, E.A.; Sherman, A.A.; Tsvetkov, Y.E.; Nifantiev, N.E. Phenyl 2-azido-2-deoxy-1-selenogalactosides: A single type of glycosyl donor for the highly stereoselective synthesis of α- and β-2-azido-2-deoxy-d-galactopyranosides. Tetrahedron Lett. 2016, 57, 708–711. [Google Scholar] [CrossRef]
- Kumar, A.A.; Illyes, T.Z.; Kover, K.E.; Szilagyi, L. Convenient syntheses of 1,2-trans selenoglycosides using isoselenuranium salts as glycosylselenenyl transfer reagents. Carbohydr. Res. 2012, 360, 8–18. [Google Scholar] [CrossRef]
- Affeldt, R.; Santos, F.P.; da Silva, R.S.; Rodrigues, O.E.D.; Wessjohann, L.A.; Lüdtke, D.S. Stereoselective glycoconjugation of steroids with selenocarbohydrates. RSC Adv. 2016, 6, 93905–93914. [Google Scholar] [CrossRef]
- Manna, T.; Misra, A.K. On-water synthesis of glycosyl selenocyanate derivatives and their application in the metal free organocatalytic preparation of nonglycosidic selenium linked pseudodisaccharide derivatives. RSC Adv. 2021, 11, 10902–10912. [Google Scholar] [CrossRef] [PubMed]
- Brill, Z.G.; Condakes, M.L.; Ting, C.P.; Maimone, T.J. Navigating the chiral pool in the total synthesis of complex terpene natural products. Chem. Rev. 2017, 117, 11753–11795. [Google Scholar] [CrossRef]
- Ruzicka, L. Isoprene rule and the biogenesis of terpenic compounds. Experientia 1953, 9, 357–367. [Google Scholar] [CrossRef]
- Rubulotta, G.; Quadrelli, E.A. Terpenes: A valuable family of compounds for the production of fine chemicals. Stud. Surf. Sci. Catal. 2019, 238, 215–229. [Google Scholar]
- Sharpless, K.B.; Lauer, R.F. Selenium dioxide oxidation of olefins. Evidence for the intermediacy of allylseleninic acids. J. Am. Chem. Soc. 1972, 94, 7154–7155. [Google Scholar] [CrossRef]
- Sharpless, K.B.; Lauer, R.F. Facile thermal rearrangements of allyl selenides and diselenides. [1,3] and [2,3] shifts. J. Org. Chem. 1972, 37, 3973–3974. [Google Scholar] [CrossRef]
- Sharpless, K.B.; Young, M.W.; Lauer, R. Reactions of selenooxides: Thermal syn-elimination and H218O exchange. Tetrahedron Lett. 1973, 14, 1979–1982. [Google Scholar] [CrossRef]
- Reich, J.; Yelm, K.E. Asymmetric induction in the oxidation of [2.2]paracyclophane-substituted selenides. Application of chirality transfer in the selenoxide [2,3] sigmatropic rearrangement. J. Org. Chem. 1991, 56, 5672–5679. [Google Scholar] [CrossRef]
- Nishibayashi, Y.; Singh, J.D.; Fukuzawa, S.; Uemura, S. Synthesis of [R,S;R,S]- and [S,R;S,R]-Bis[2-[1-(dimethylamino)ethyl]ferrocenyl] diselenides and Their application to asymmetric selenoxide elimination and [2,3]sigmatropic rearrangement. J. Org. Chem. 1995, 60, 4114–4120. [Google Scholar] [CrossRef]
- Fujita, M.; Kanakubo, H.; Ushijima, H.; Oishi, A.; Ikeda, Y.; Taguchi, Y. Asymmetric [2,3] sigmatropic rearrangement of optically active allylic selenides. Synlett 1998, 1998, 987–988. [Google Scholar] [CrossRef]
- Uzarewicz, A.; Ścianowski, J.; Bąkowska-Janiszewska, J. Synthesis and reactions of organic compounds with a nitrogen atom. Part XV. Reactions of (+)-3-chloro-2(10)-pinene and (–)-10-chloro-2-pinene with phenyltelluro- and phenylselenosodium. Pol. J. Chem. 1999, 73, 1791–1796. [Google Scholar]
- Uzarewicz, A.; Ścianowski, J.; Bąkowska-Janiszewska, J. Reaction of (+)-3-carene and (+)-2-carene with t-butyl hypochlorite or N-chlorosuccinimide in the presence of free radical catalysts. Pol. J. Chem. 2000, 74, 777–783. [Google Scholar] [CrossRef]
- Uzarewicz, A.; Ścianowski, J.; Bąkowska-Janiszewska, J. Synthesis and reactions of organic compounds with a nitrogen atom. Part XVI. Reactions of (−)-4-chloro-3(10)-carene and (+)-10-chloro-3-carene with phenyltelluro- and phenylselenosodium. Pol. J. Chem. 2000, 74, 1079–1083. [Google Scholar]
- Uzarewicz, A.; Ścianowski, J.; Bąkowska-Janiszewska, J. Synthesis and reactions of organic compounds with a nitrogen atom. Part XVII. Reactions of acyclic and monocyclic chlorides with phenyltelluro- and phenylselenosodium. Pol. J. Chem. 2001, 74, 649–656. [Google Scholar]
- Ścianowski, J.; Tafelska-Kaczmarek, A. Syntheses and reactions of allyl phenylselenides. A convenient method for the synthesis of allyl methylcarbamates. Pol. J. Chem. 2007, 81, 529–534. [Google Scholar] [CrossRef]
- Ścianowski, J.; Rafalski, J.; Banach, A.; Czaplewska, J.; Komoszyńska, A. Synthesis and reactions of optically active selenols derived from monoterpenes. Tetrahedron Asymmetry 2013, 24, 1089–1096. [Google Scholar] [CrossRef]
- Kurose, N.; Takahashi, T.; Koizumi, T. Asymmetric [2,3]-sigmatropic rearrangement of chiral allylic selenimides. J. Org. Chem. 1996, 61, 2932–2933. [Google Scholar] [CrossRef] [PubMed]
- Kurose, N.; Takahashi, T.; Koizumi, T. Asymmetric [2,3]-sigmatropic rearrangement of chiral selenonium ylides. J. Org. Chem. 1997, 62, 4562–4563. [Google Scholar] [CrossRef]
- Kurose, N.; Takahashi, T.; Koizumi, T. First synthesis of optically pure selenuranes and stereoselective alkaline hydrolysis. Their application to asymmetric [2,3]-sigmatropic rearrangement of allylic selenoxides. Tetrahedron Asymmetry 1997, 8, 12115–12129. [Google Scholar] [CrossRef]
- Wirth, T. Organoselenium chemistry: A tool for asymmetric synthesis. Angew. Chem. Int. Ed. 2000, 39, 3740–3749. [Google Scholar] [CrossRef]
- Back, T.G.; Dyck, B.P.; Parvez, M. Unexpected formation of 1,3-diselenetanes from the reaction of camphor enolate with selenium. J. Chem. Soc. Chem. Commun. 1994, 4, 515–516. [Google Scholar] [CrossRef]
- Back, T.G.; Dyck, B.P.; Parvez, M. 1,3-Diselenetanes and 1,3-dithietanes derived from camphor. Formation, structure, stereochemistry, and oxidation to selenoxide and sulfoxide products. J. Org. Chem. 1995, 60, 703–710. [Google Scholar] [CrossRef]
- Back, T.G.; Dyck, B.P. Asymmetric cyclisation of unsaturated alcohols and carboxylic acids with camphor-based selenium electrophiles. Chem. Commun. 1996, 22, 2567–2568. [Google Scholar] [CrossRef]
- Back, T.G.; Nan, S. Asymmetric methoxyselenenylations with camphor-based selenium electrophiles. J. Chem. Soc. Perkin Trans. 1998, 1, 3123–3124. [Google Scholar] [CrossRef]
- Back, T.G.; Dyck, B.P.; Nan, S. Asymmetric electrophilic methoxyselenenylations and cyclisations with 3-camphorseleno derivatives. Tetrahedron 1999, 55, 3191–3208. [Google Scholar] [CrossRef]
- Back, T.G.; Moussa, Z. New chiral auxiliaries for highly stereoselective asymmetric methoxyselenenylations. Org. Lett. 2000, 2, 3007–3009. [Google Scholar] [CrossRef]
- Back, T.G.; Moussa, Z.; Parvez, M. Asymmetric methoxyselenenylations and cyclisations with 3-camphorseleno electrophiles containing oxime substituents at C-2. Formation of an unusual oxaselenazole from an oxime-substituted selenenyl bromide. J. Org. Chem. 2002, 67, 499–509. [Google Scholar] [CrossRef]
- Rafiński, Z.; Ścianowski, J.; Wojtczak, A. Synthesis and reactions of optically active dialkyl diselenides from pinane group. Lett. Org. Chem. 2009, 6, 321–328. [Google Scholar] [CrossRef]
- Ścianowski, J.; Rafiński, Z.; Szuniewicz, A.; Wojtczak, A. New chiral selenium electrophiles derived from functionalised terpenes. Tetrahedron 2009, 65, 10162–10174. [Google Scholar] [CrossRef]
- Tiecco, M.; Testaferri, L.; Santi, C.; Marini, F.; Bagnoli, L.; Temperini, A. Asymmetric selenomethoxylation of alkenes with camphorselenenyl sulfate. Tetrahedron Lett. 1998, 39, 2809–2812. [Google Scholar] [CrossRef]
- Tiecco, M.; Testaferri, L.; Marini, F.; Santi, C.; Bagnoli, L.; Temperini, A. Asymmetric oxyselenenylation-deselenylation reactions of alkenes induced by camphor diselenide and ammonium persulfate. A convenient one-pot synthesis of enantiomerically enriched allylic alcohols and ethers. Tetrahedron Asymmetry 1999, 10, 747–757. [Google Scholar] [CrossRef]
- Tiecco, M.; Testaferri, L.; Santi, C.; Tomassini, C.; Marini, F.; Bagnoli, L.; Temperini, A. Asymmetric amidoselenenylation of alkenes promoted by camphorselenenyl sulfate: A useful synthetic route to enantiopure oxazolines. Eur. J. Org. Chem. 2000, 2000, 3451–3457. [Google Scholar] [CrossRef]
- Tiecco, M.; Testaferri, L.; Santi, C.; Tomassini, C.; Marini, F.; Bagnoli, L.; Temperini, A. Asymmetric synthesis of thioamido selenides. A simple synthetic route to enantiopure thiazolines. Tetrahedron Asymmetry 2002, 13, 429–435. [Google Scholar] [CrossRef]
- Tiecco, M.; Testaferri, L.; Bagnoli, L.; Scarponi, C.; Purgatorio, V.; Temperini, A.; Marini, F.; Santi, C. Synthesis of enantiomerically pure perhydrofuro[2,3-b]furans. Tetrahedron Asymmetry 2005, 16, 2429–2435. [Google Scholar] [CrossRef]
- Tiecco, M.; Testaferri, L.; Bagnoli, L.; Scarponi, C.; Temperini, A.; Marini, F.; Santi, C. Organoselenium mediated asymmetric cyclisations. Synthesis of enantiomerically pure 1,6-dioxaspiro[4.4]nonanes. Tetrahedron Asymmetry 2006, 17, 2768–2774. [Google Scholar] [CrossRef]
- Tiecco, M.; Testaferri, L.; Marini, F.; Sternativo, S.; Santi, C.; Bagnoli, L.; Temperini, A. Asymmetric aldol reactions from titanium enolates of α-seleno ketones and esters. Tetrahedron Asymmetry 2004, 15, 783–791. [Google Scholar] [CrossRef]
- Tiecco, M.; Testaferri, L.; Marini, F.; Sternativo, S.; Santi, C.; Bagnoli, L.; Temperini, A. Conjugated additions of selenium containing enolates to enones—Enantioselective synthesis of δ-oxo-α-seleno esters and their facile transformations. Eur. J. Org. Chem. 2005, 2025, 543–551. [Google Scholar] [CrossRef]
- Li, X.L.; Wang, H.Y.; Huang, Z.Z. New camphor-derived selenonium ylides: Enantioselective synthesis of chiral epoxides. Aust. J. Chem. 2005, 58, 749–752. [Google Scholar] [CrossRef]
- Watanabe, S.; Hasebe, R.; Ouchi, J.; Nagasawa, H.; Kataoka, T. Enantioselective Darzens reaction using organoselenide-lithium hydroxide complex. Tetrahedron Lett. 2010, 51, 5778–5780. [Google Scholar] [CrossRef]
- Banach, A.; Ścianowski, J.; Uzarewicz-Baig, M.; Wojtczak, A. Terpenyl selenides: Synthesis and application in asymmetric epoxidation. Eur. J. Org. Chem. 2015, 16, 3477–3485. [Google Scholar] [CrossRef]
- Wang, H.Y.; Yang, F.; Li, X.L.; Yan, X.M.; Huang, Z.Z. First example of highly stereoselective synthesis of 1,2,3-trisubstituted cyclopropanes via chiral selenonium ylides. Chem. Eur. J. 2009, 15, 3784–3789. [Google Scholar] [CrossRef]
- Midura, W.H.; Ścianowski, J.; Banach, A.; Zając, A. Asymmetric synthesis of cyclopropyl phosphonates using chiral terpenyl sulfonium and selenonium ylides. Tetrahedron Asymmetry 2014, 25, 1488–1493. [Google Scholar] [CrossRef]
- Boualy, B.; El Houssame, S.; Sancineto, L.; Santi, C.; Ait Ali, M.; Stoeckli-Evans, H.; El Firdoussi, L. A mild and efficient method for the synthesis of a new optically active diallyl selenide and its catalytic activity in the allylic chlorination of natural terpenes. New. J. Chem. 2016, 40, 3395–3399. [Google Scholar] [CrossRef]
- Ścianowski, J.; Pacuła, A.J.; Zielińska-Błajet, M.; Wojtczak, A. New diphenyl diselenides o-substituted by an O(S,Se)-caranyl skeleton—Synthesis and application in asymmetric reactions. New J. Chem. 2016, 40, 6697–6705. [Google Scholar] [CrossRef]
- Sancineto, L.; Mangiavacchi, F.; Tidei, C.; Bagnoli, L.; Marini, F.; Gioiello, A.; Scianowski, J.; Santi, C. Selenium-catalysed oxacyclization of alkenoic acids and alkenols. Asian J. Org. Chem. 2017, 6, 988–992. [Google Scholar] [CrossRef]
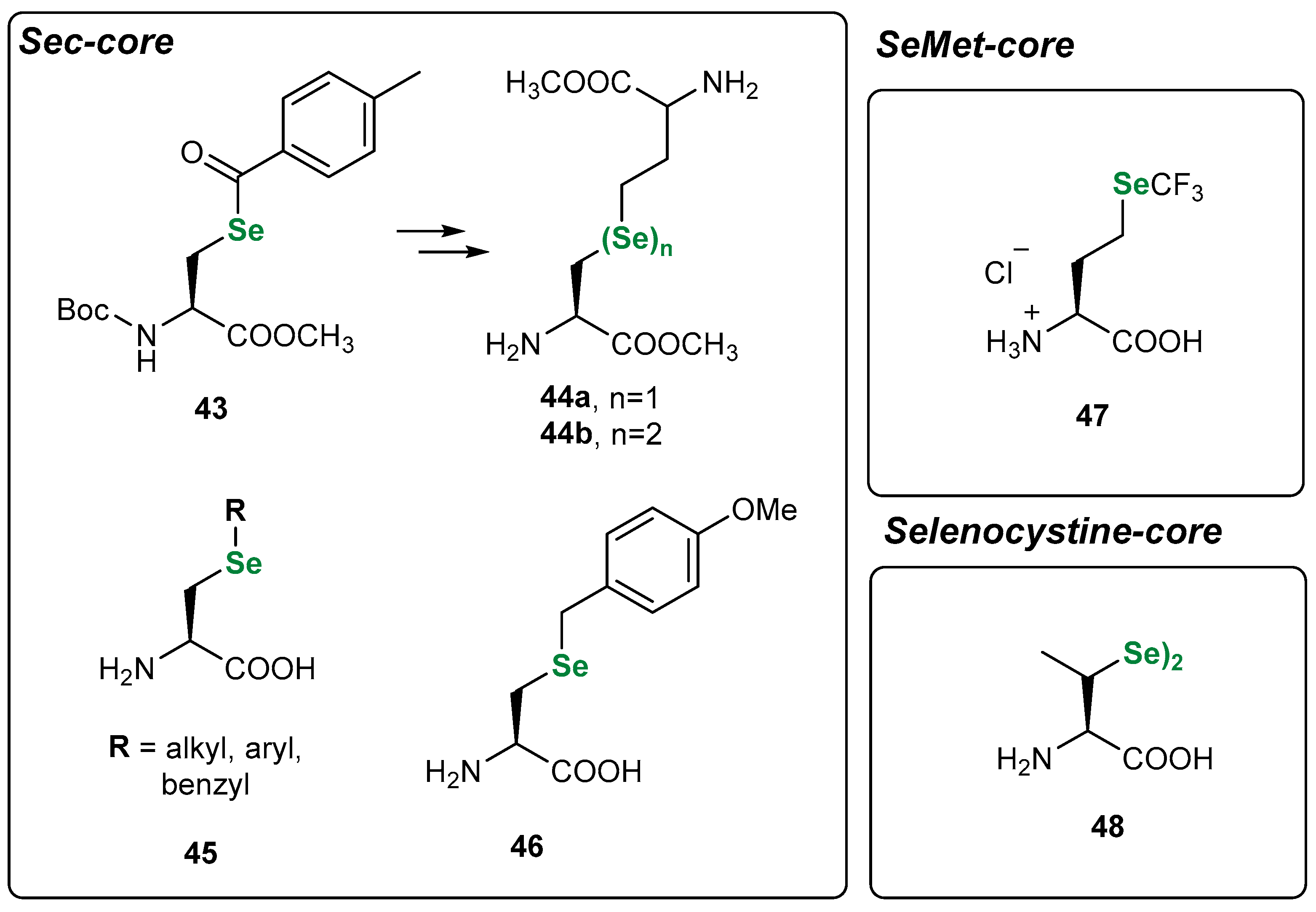
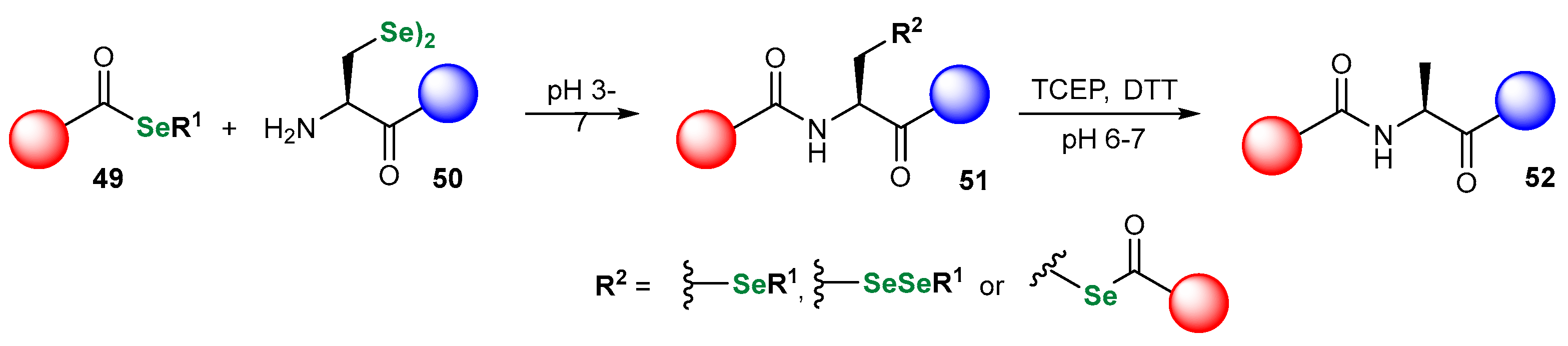
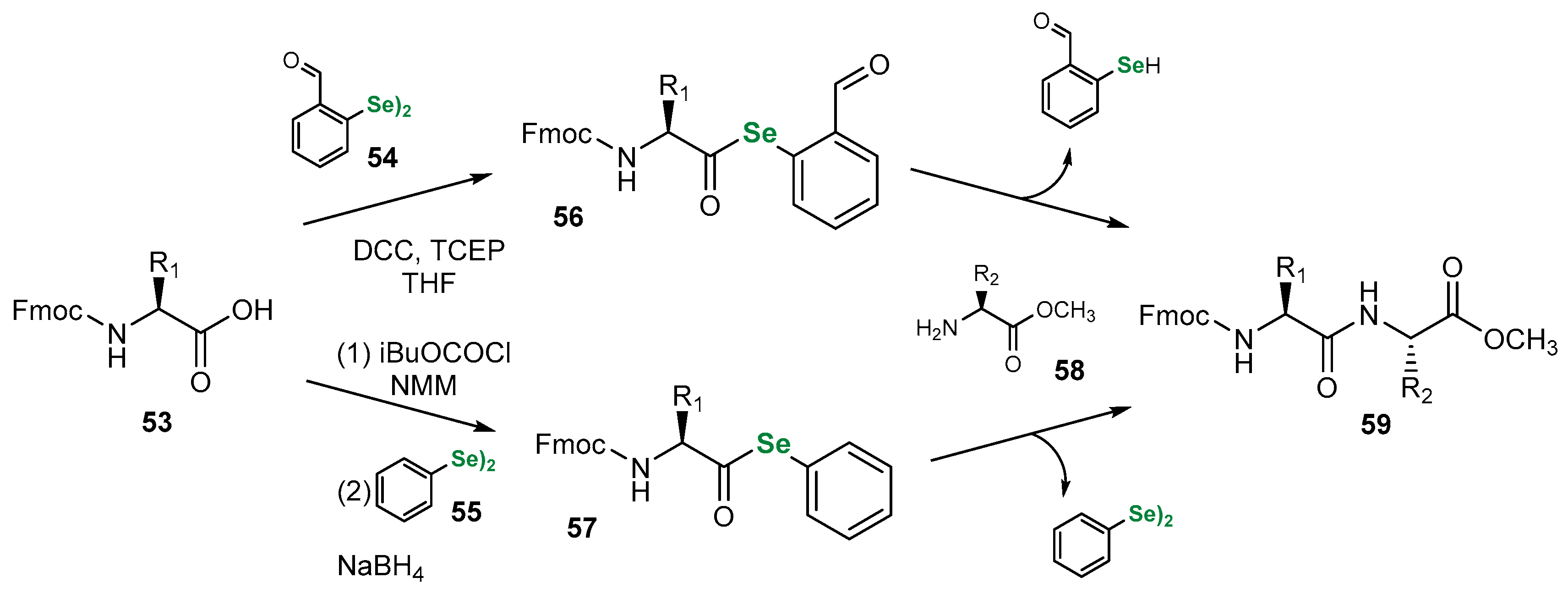
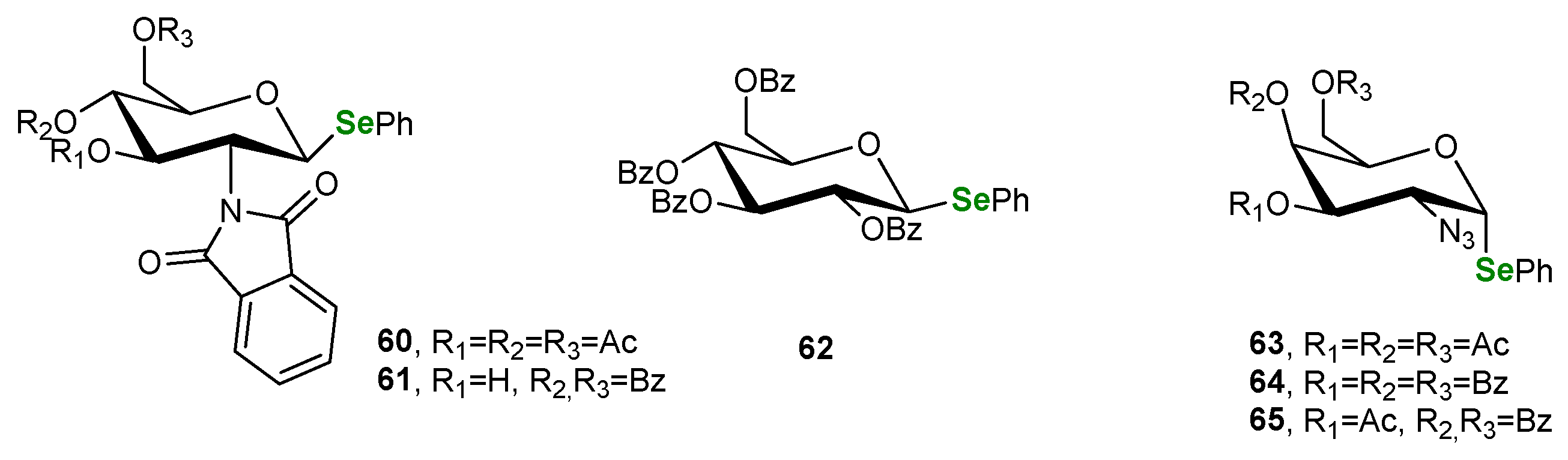
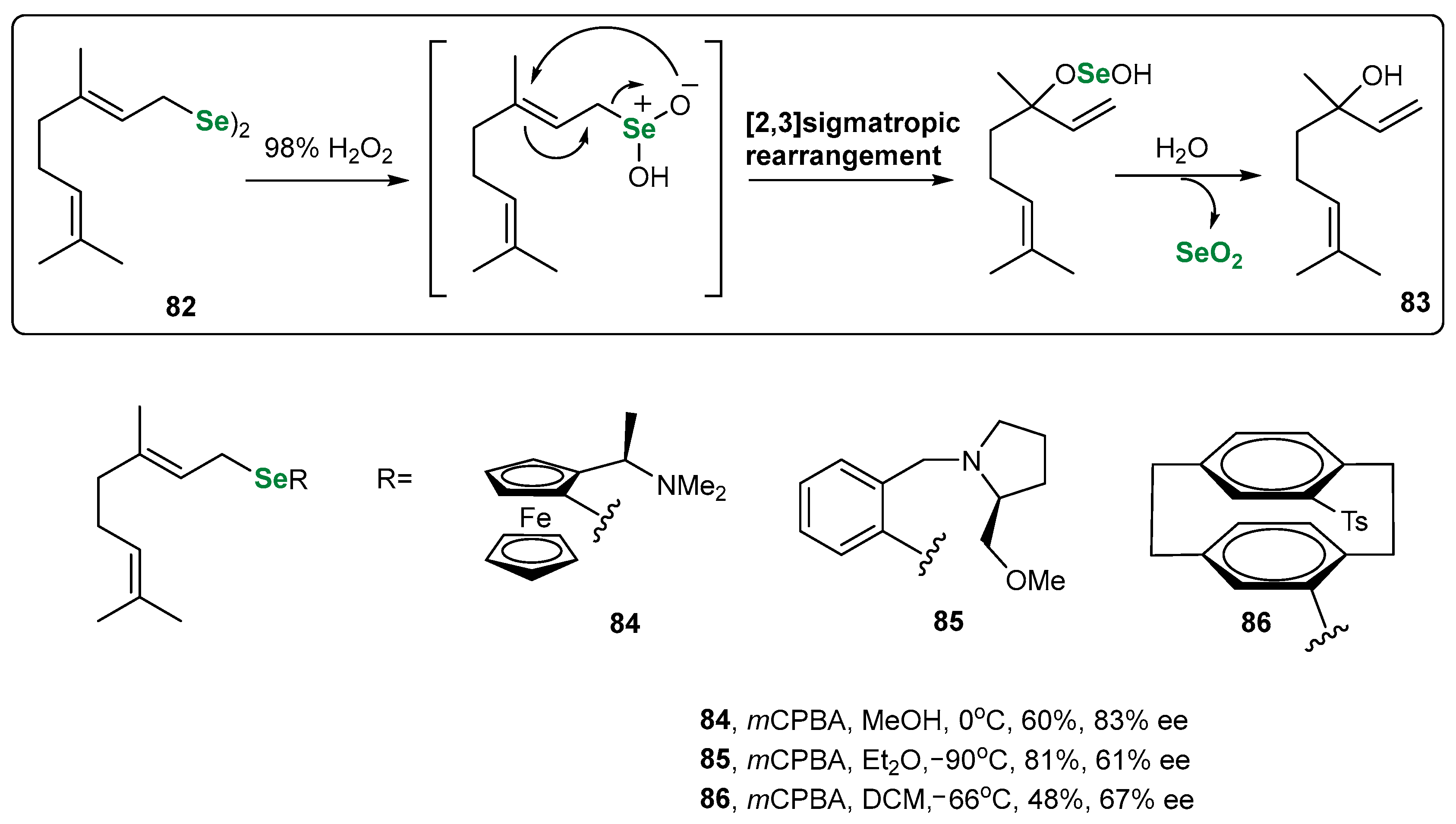
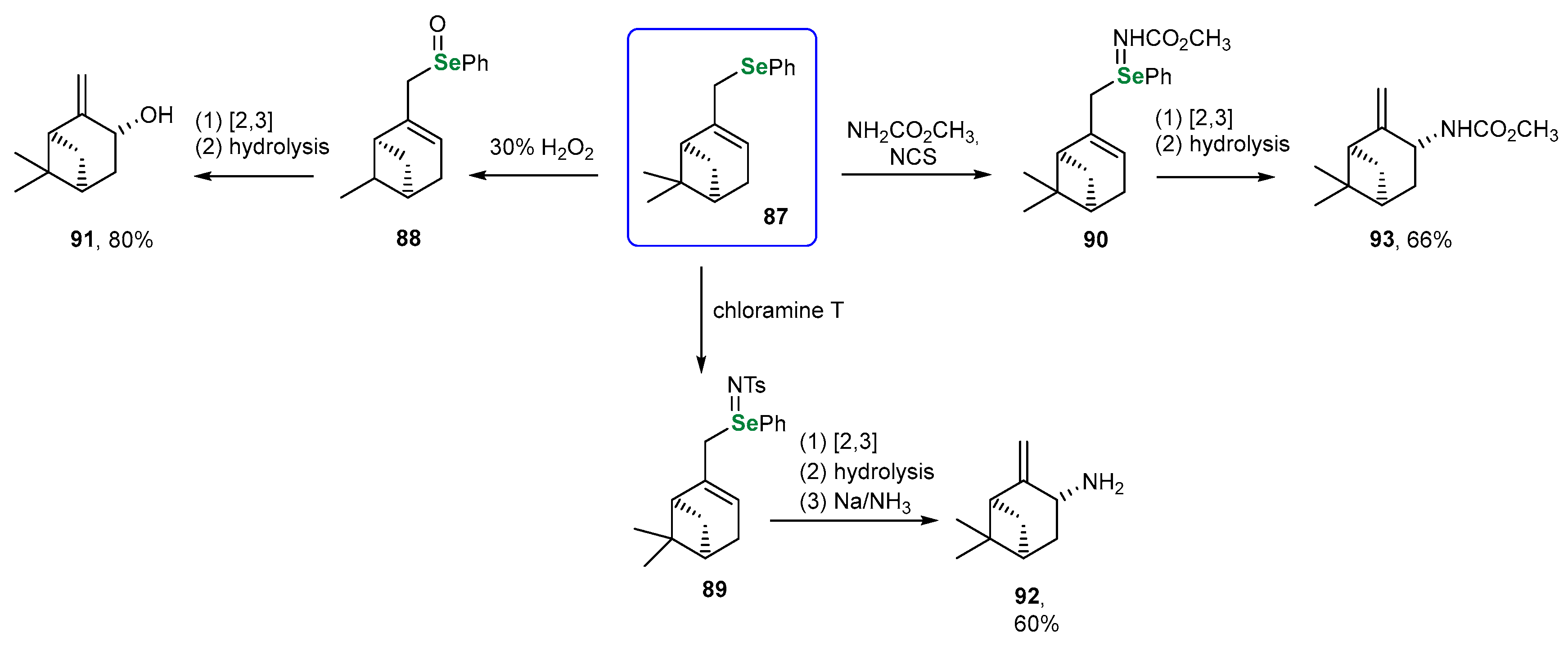
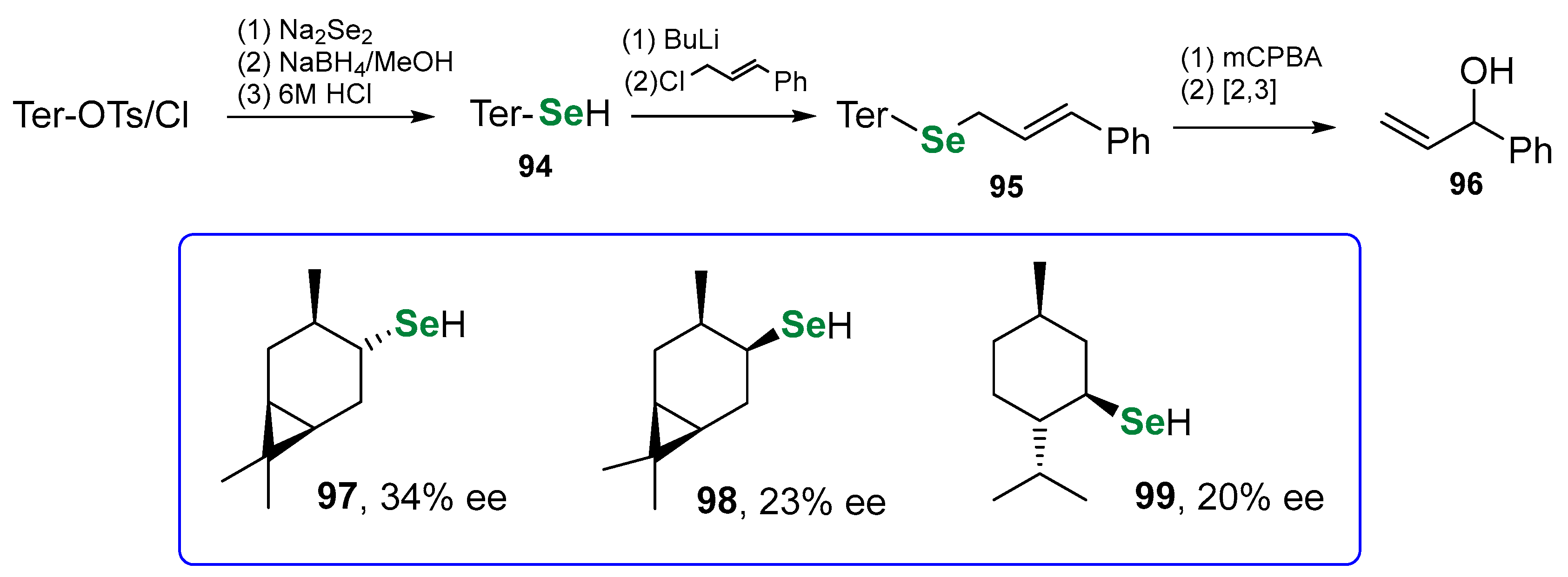
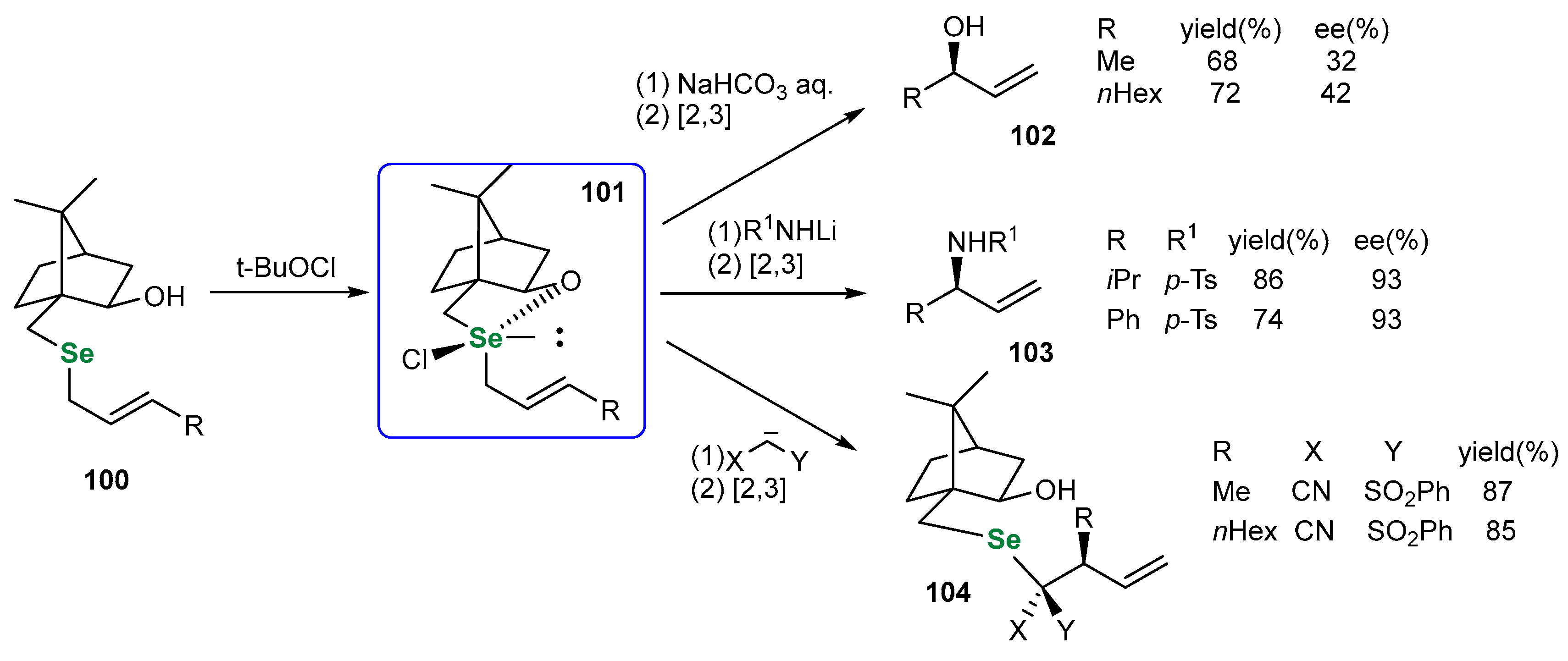
| Methods for Assessing GPx-like Antioxidant Activity | Methods for Assessing Radical-Trapping Antioxidant Activity | |
|---|---|---|
| GSH/GR Coupled Assay (Enzymatic Method) [37] | ABTS Assay [38] | |
| The glutathione reductase (GR)-coupled assay was the pioneering indirect method for assessing GPx-mimic activity, created by Wilson et al. In this process, the GR enzyme utilizes the cofactor NADPH (β-nicotinamide adenine dinucleotide 2′-phosphate) to catalyse the conversion of oxidised glutathione (GSSG), produced during the catalytic reaction, back to its reduced form (GSH). The initial rates of NADPH reduction (νo) are measured using UV spectroscopy at a wavelength of 340 nm. The assay solution is prepared by mixing a potassium phosphate buffer, EDTA, sodium azide, GR, and a suitable amount of the test compound. The subsequent addition of H2O2 starts the reaction. The half and overall equations for the occurring reactions are as follows (Equations (1)–(3)): | In the method introduced by Shaaban et al., the antioxidant activity of organoselenium compounds is evaluated based on their capacity to decolourise ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radicals. The corresponding radical-scavenging activity is determined by measuring the reduction in absorbance at 734 nm. | |
| (1) | ||
| (2) | ||
| (3) | ||
| PhSH assay [39] | DPPH assay [40] | |
| In this method, proposed by Iwaoka and Tomoda, benzenethiol (PhSH) is utilized as a GSH substitute. The reduction of hydrogen peroxide in the presence of PhSH, which leads to the concurrent production of diphenyl disulfide (PhSSPh), is evaluated using various methods: a. spectrophotometric analysis measures the increase in UV absorption at 305 nm resulting from the formation of PhSSPh; b. HPLC analysis determines the quantity of PhSSPh produced by measuring the time needed for 50% of PhSH to convert to PhSSPh (t1/2 values), calculated from the peak areas at different time intervals. The equation for the described reaction is as follows (Equation (4)): | Shaaban et al. presented a straightforward method for evaluating the radical scavenging activities of organoselenium compounds and nutritional products. The antioxidant capacity of a compound is determined by its ability to convert the stable DPPH·radical (which appears purple in methanol) to DPPH (colourless), as indicated by a reduction in absorbance at 517 nm. | |
| (4) | ||
| DTTred/DTTox NMR assay [41] | HPLC Lipid Peroxidation assay [42,43] | |
| The GPx-like antioxidant activity of compounds can be assessed using the protocol developed by Iwaoka et al. In this approach, the organoselenium catalyst reduces H2O2 and is subsequently regenerated in the presence of dithiothreitol (DTTred). The kinetics of this reaction are analysed using 1H NMR spectroscopy in either CD3OD or D2O. Signals corresponding to the disulfide (DTTox) produced at specific intervals are recorded. The chemical equation that describes the reaction is as follows (Equation (5)): | To evaluate the antioxidant properties of organochalcogen compounds, Engman et al. routinely employed azo-initiated peroxidation of linoleic acid and its derivatives. A recent study described a refined experimental model for determining inhibition times (Tinh) and rates of peroxidation inhibition (Rinh) within a biphasic system. In this modified approach, the lipid phase contained linoleic acid and the test antioxidant, while the aqueous phase included a water-soluble co-antioxidant, such as N-acetylcysteine (NAC), capable of regenerating the active antioxidant species. The reaction mixture, chlorobenzene containing linoleic acid and the antioxidant, was vigorously stirred at 42 °C with the NAC solution. Peroxidation was initiated by 2,2′-Azobis(2,4-dimethylvaleronitrile) (AMVN), and the formation of conjugated dienes was monitored by HPLC with UV detection at 234 nm. The inhibition rate (Rinh) was subsequently calculated using least-squares analysis of absorbance versus time data. | |
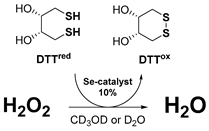 | (5) | |
| The Structure of the Compound | Origin | Method for Assessing GPx-like Antioxidant Activity | Author |
|---|---|---|---|
 | Cholesterol-derived diselenide | PhSH assay | Braga et al. |
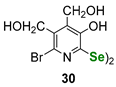 | Pyridoxine (vitamin B6)-derived diselenide | GSH/GR coupled assay | Singh et al. |
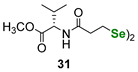 | Amino acid (L-valine)-derived diselenide | PhSH assay | Braga, Rocha et al. |
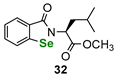 | Amino acid (L-leucine)-derived benzisoselenazolone | DTTred/DTTox NMR assay | Ścianowski et al. |
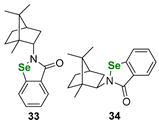 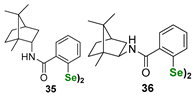 | Terpene (camphane)-derived benzisoselenazolones and diselenides | DTTred/DTTox NMR assay | Ścianowski et al. |
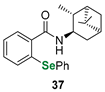 | Terpene (pinene)-derived phenylselenide | DTTred/DTTox NMR assay | Ścianowski et al. |
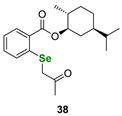 | Terpene (O-menthyl)-derived selenide | DPPH assay | Ścianowski et al. |
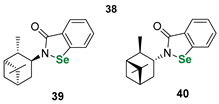 | Terpene (pinene)-derived benzisoselenazolones | DTTred/DTTox NMR assay | Ścianowski et al. |
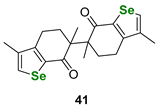 | Terpene carvone-derived selenophenes | DTTred/DTTox NMR assay | João P. Telo et al. |
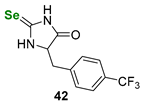 | Selenoneine-derived selenohydantoin | GSH/GR coupled assay | Gaucher, da Silva Júnior et al. |
| Reagent | Substrate | Product | Yield [%], d.r. | Author |
|---|---|---|---|---|
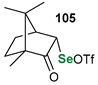 | 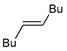 | 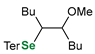 | 88, 94:6 | Back et al. |
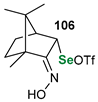 | 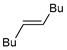 |  | 88, 98:2 | Back et al. |
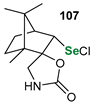 | 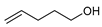 | 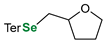 | 87, 84:16 | Back et al. |
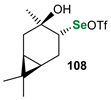 |  | 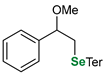 | 54, 90:10 | Ścianowski et al. |
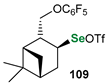 |  |  | 49, 86:14 | Ścianowski et al. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacuła-Miszewska, A.J.; Obieziurska-Fabisiak, M.; Ścianowski, J. Organoselenium Compounds Derived from Natural Metabolites. Pharmaceuticals 2025, 18, 1749. https://doi.org/10.3390/ph18111749
Pacuła-Miszewska AJ, Obieziurska-Fabisiak M, Ścianowski J. Organoselenium Compounds Derived from Natural Metabolites. Pharmaceuticals. 2025; 18(11):1749. https://doi.org/10.3390/ph18111749
Chicago/Turabian StylePacuła-Miszewska, Agata J., Magdalena Obieziurska-Fabisiak, and Jacek Ścianowski. 2025. "Organoselenium Compounds Derived from Natural Metabolites" Pharmaceuticals 18, no. 11: 1749. https://doi.org/10.3390/ph18111749
APA StylePacuła-Miszewska, A. J., Obieziurska-Fabisiak, M., & Ścianowski, J. (2025). Organoselenium Compounds Derived from Natural Metabolites. Pharmaceuticals, 18(11), 1749. https://doi.org/10.3390/ph18111749