Is Crocin Effective in Modulating Blood Lipid Levels? An Updated Systematic Review and Meta-Analysis with Dose– and Time–Response Assessments
Abstract
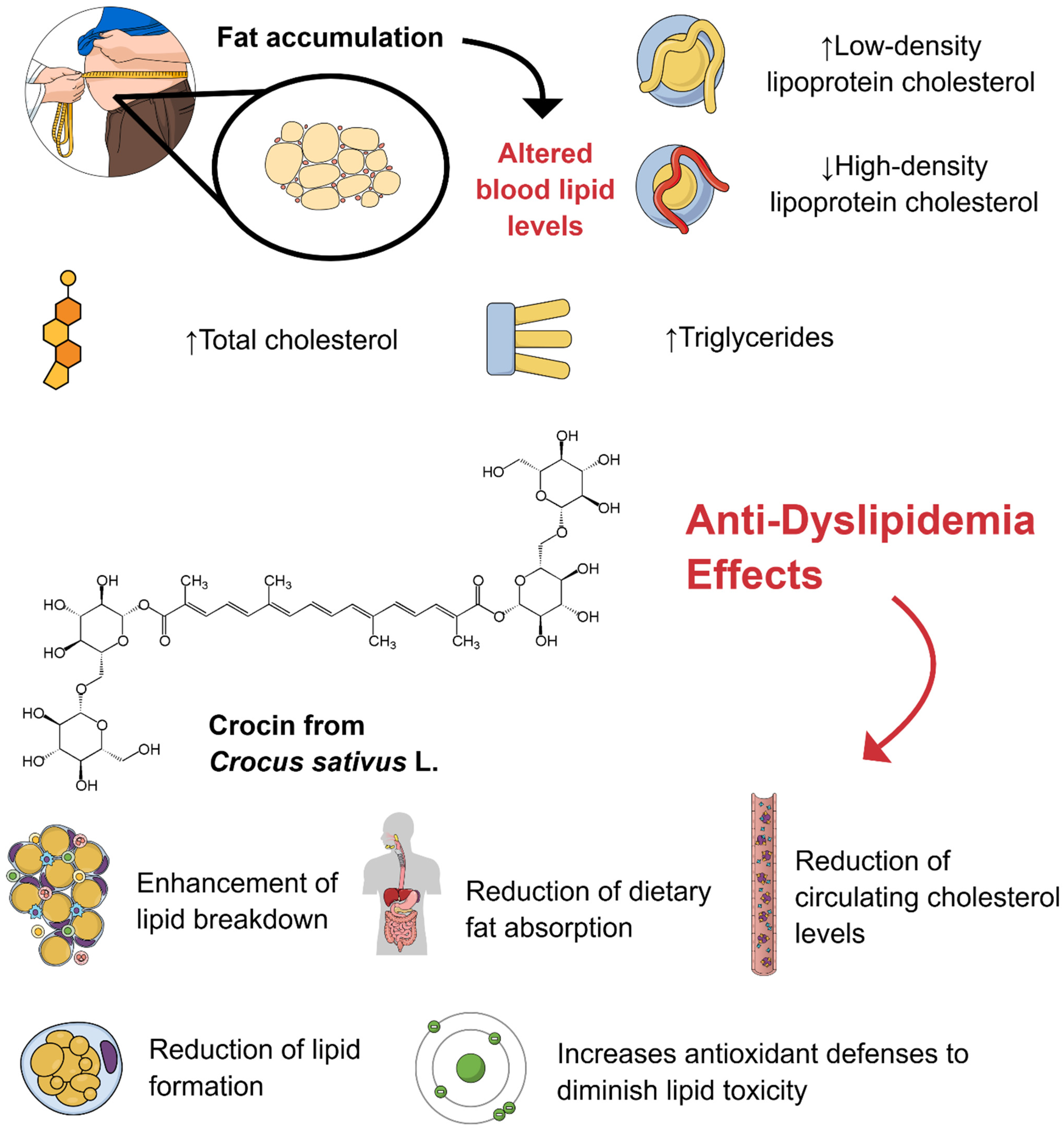
1. Introduction
2. Materials and Methods
2.1. Focused Question
2.2. Language
2.3. Literature Search and Databases
2.4. Study Selection
2.5. Data Extraction
2.6. Search and Selection of Relevant Articles
2.7. Data Items
2.8. Quality Assessment
2.9. Qualitative Analysis
2.10. Synthesis of Results and Summary Measures
3. Results and Discussion
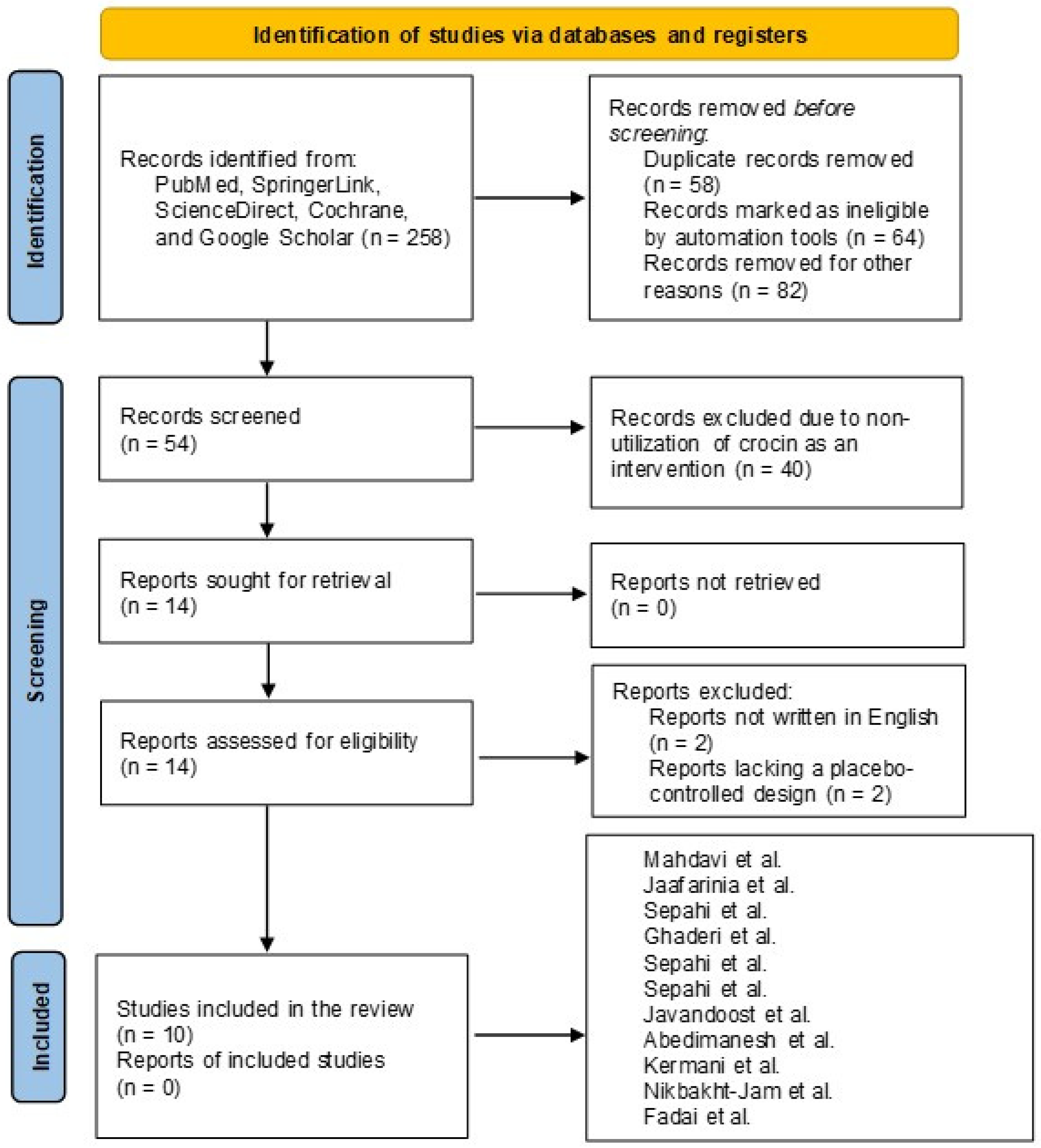
3.1. Literature Search Report
3.2. Overview of the Included Studies and Bias Assessment
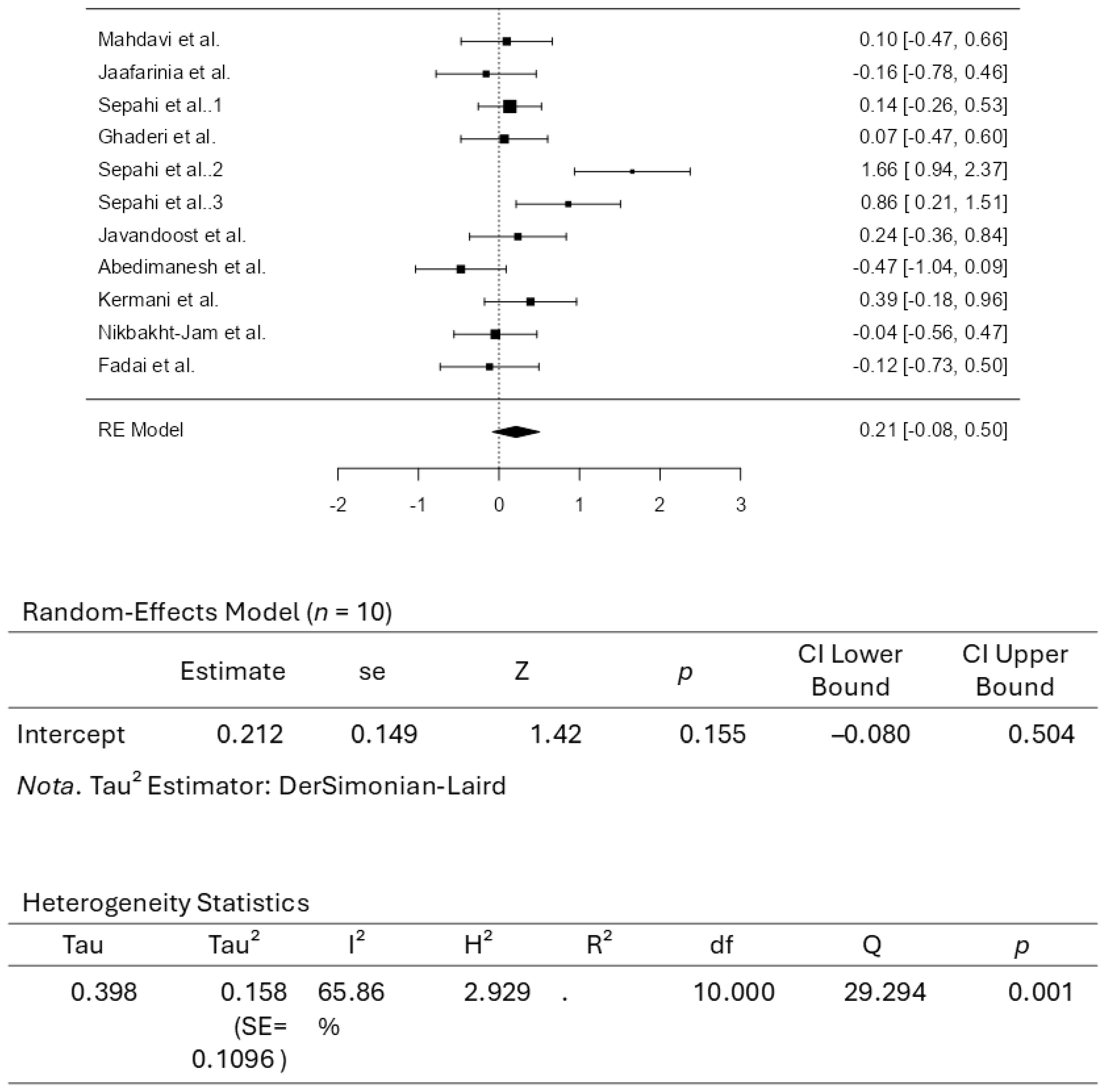
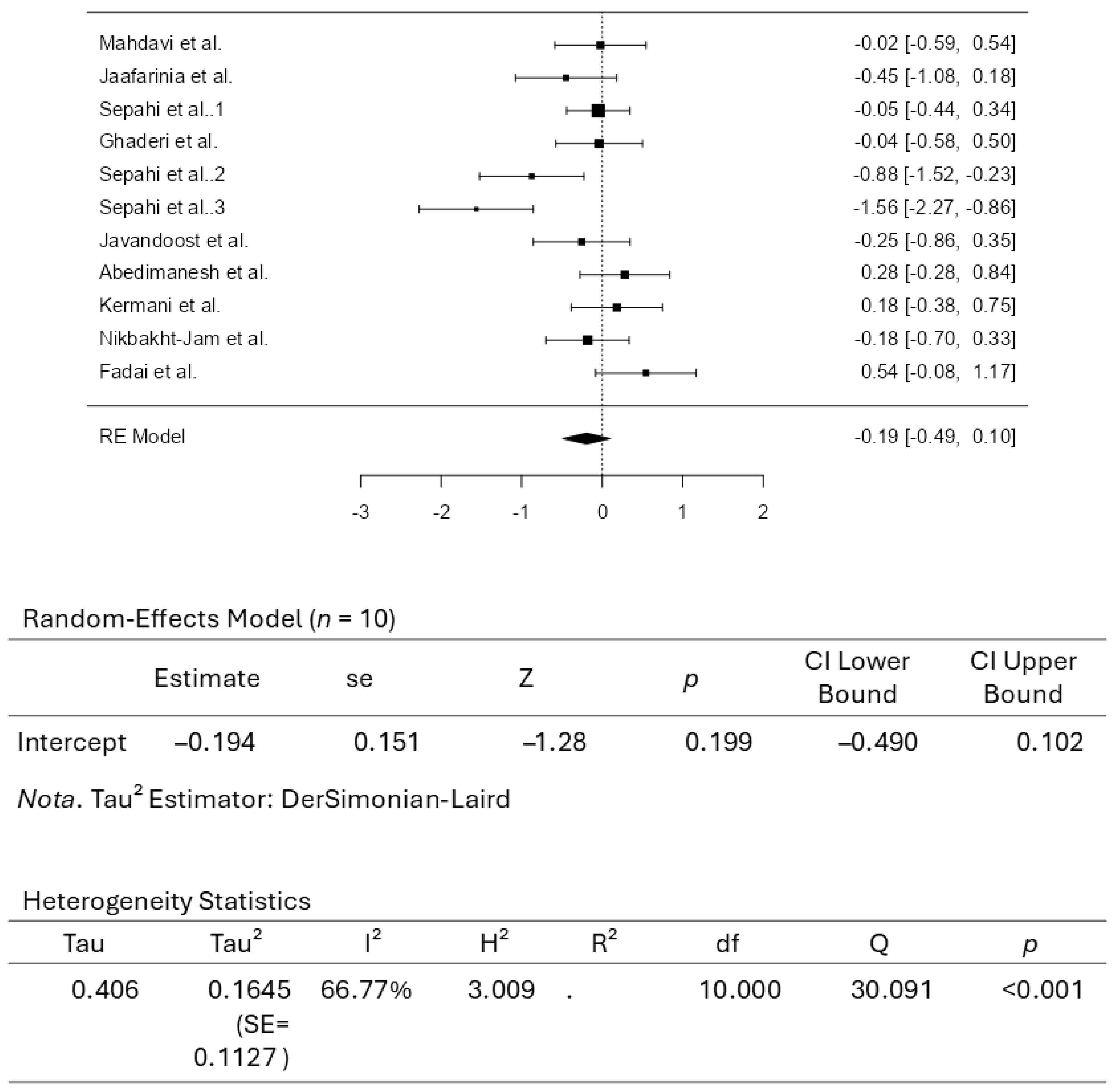
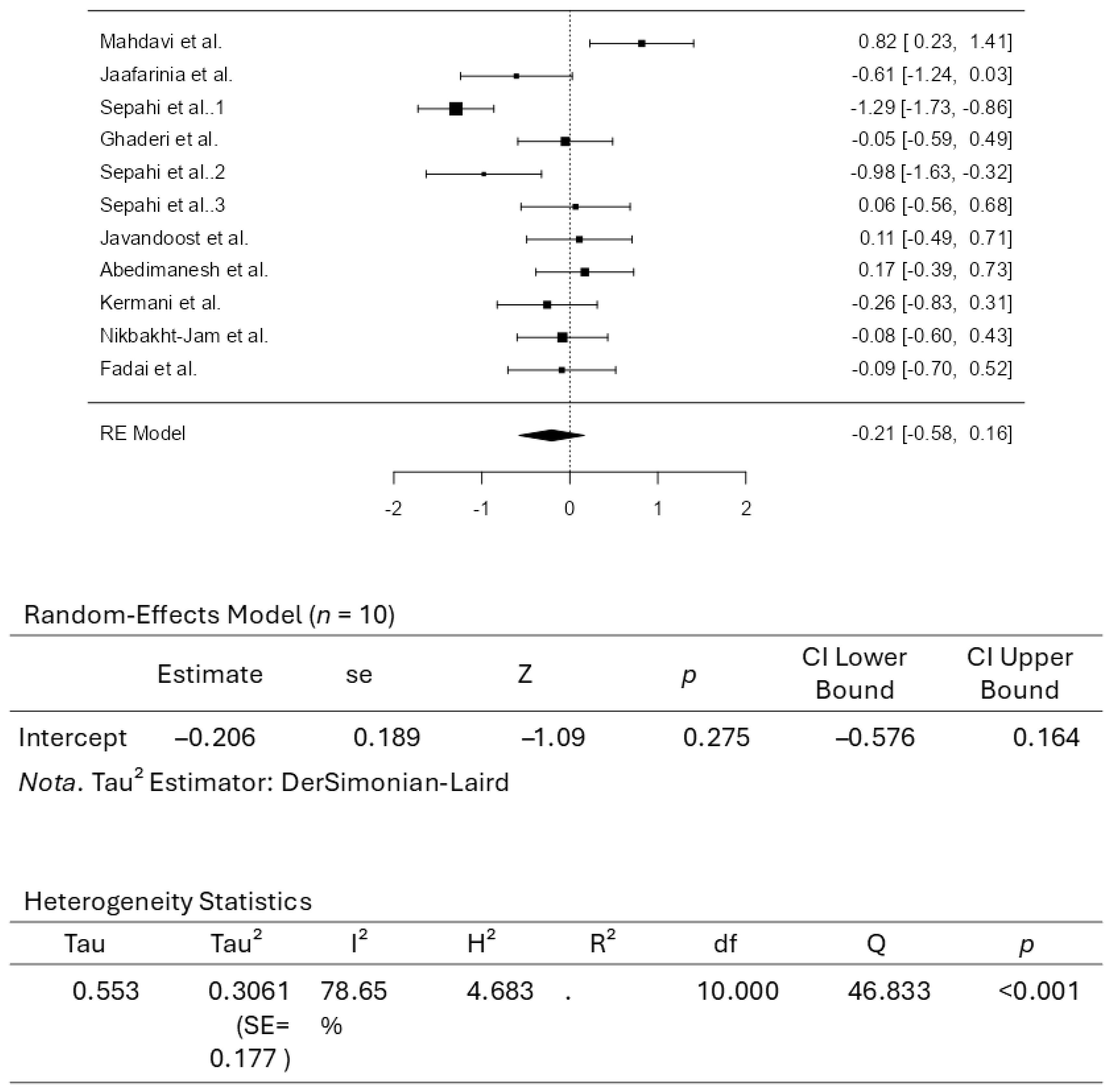
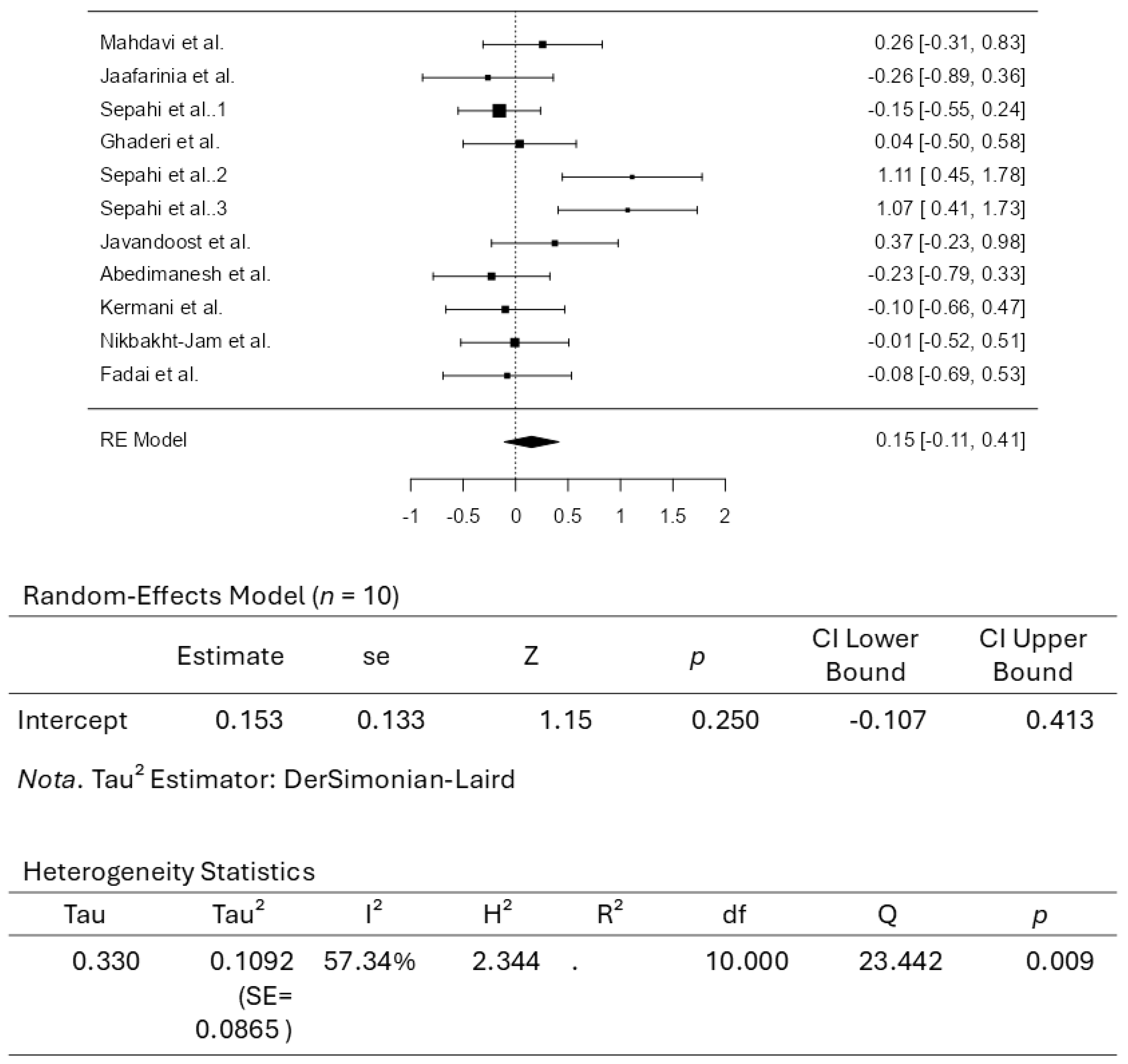
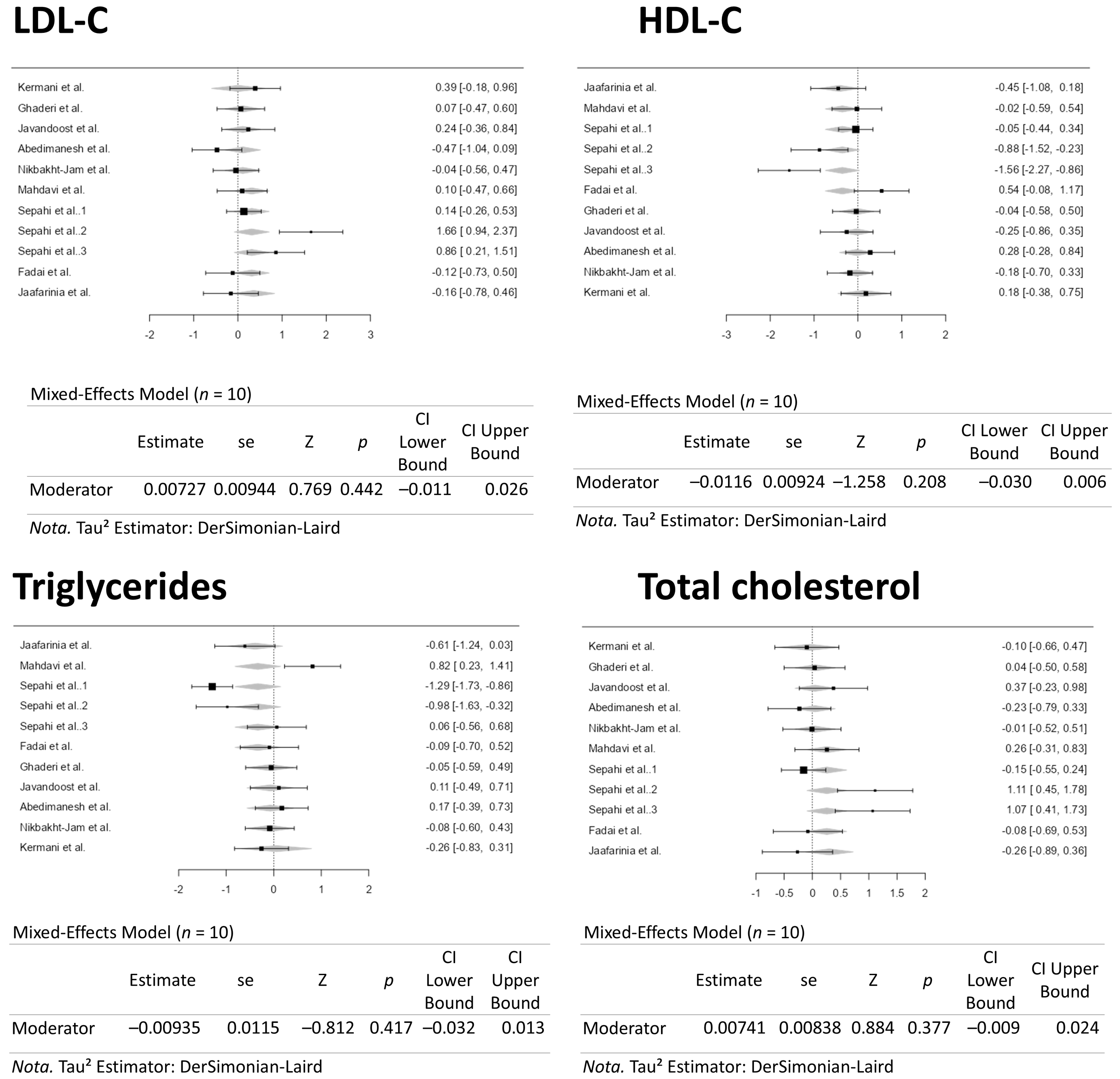
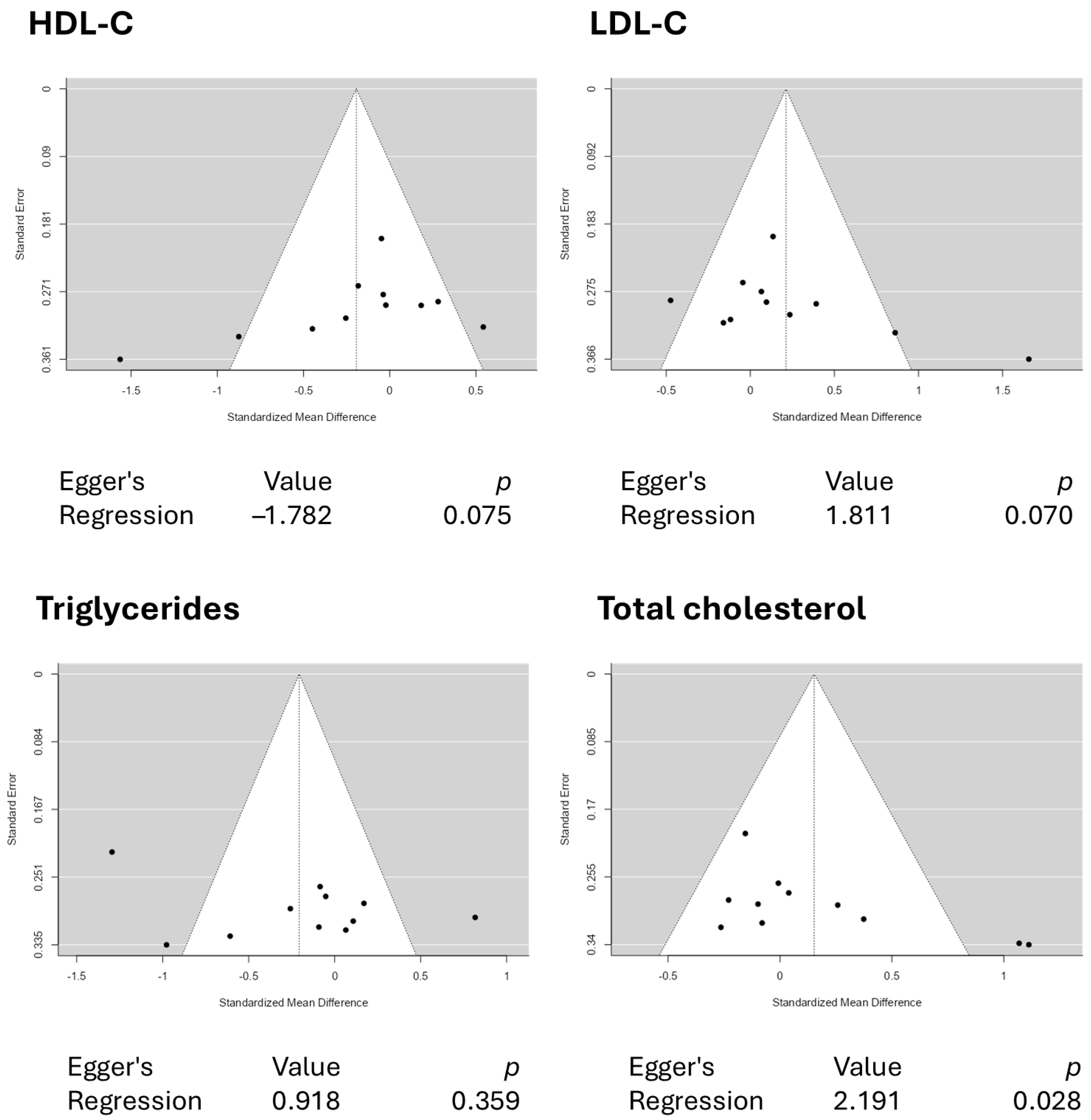
3.3. Results from the Quantitative Assessments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lang, J.M.; Shostak, E.S.; Quinn, W.K.; Chervinskaya, V.D.; Fioraso, E.; Smith, E.; Kotarsky, C.J.; DeBlauw, J.A.; Lloyd, J.L.; Ives, S.J. Dyslipidemia Impacts Cardiometabolic Health and CVD Risk in a Relatively Young Otherwise Healthy Population. J. Clin. Hypertens. 2025, 27, e14972. [Google Scholar] [CrossRef]
- Raja, V.; Aguiar, C.; Alsayed, N.; Chibber, Y.S.; ElBadawi, H.; Ezhov, M.; Hermans, M.P.; Pandey, R.C.; Ray, K.K.; Tokgözoglu, L.; et al. Non-HDL-cholesterol in dyslipidemia: Review of the state-of-the-art literature and outlook. Atherosclerosis 2023, 383, 117312. [Google Scholar] [CrossRef]
- Habib, M.B.; Akbar, N.S.; Batool, G. Investigation of Dyslipidemia and Lipid Profile Ratios Among Patients in Tertiary Care Hospitals. EJIFCC 2025, 36, 124–131. [Google Scholar]
- Huang, J.X.F.; Yousaf, A.; Moon, J.; Ahmed, R.; Uppal, K.; Pemminati, S. Recent Advances in the Management of Dyslipidemia: A Systematic Review. Cureus 2025, 17, e81034. [Google Scholar] [CrossRef]
- Brunham, L.R.; Lonn, E.; Mehta, S.R. Dyslipidemia and the Current State of Cardiovascular Disease: Epidemiology, Risk Factors, and Effect of Lipid Lowering. Can. J. Cardiol. 2024, 40, S4–S12. [Google Scholar] [CrossRef]
- Sikand, G.; Handu, D.; Rozga, M.; de Waal, D.; Wong, N.D. Medical Nutrition Therapy Provided by Dietitians is Effective and Saves Healthcare Costs in the Management of Adults with Dyslipidemia. Curr. Atheroscler. Rep. 2023, 25, 331–342. [Google Scholar] [CrossRef]
- Berisha, H.; Hattab, R.; Comi, L.; Giglione, C.; Migliaccio, S.; Magni, P. Nutrition and Lifestyle Interventions in Managing Dyslipidemia and Cardiometabolic Risk. Nutrients 2025, 17, 776. [Google Scholar] [CrossRef]
- Su, W.; Mastova, A.V.; Ul’yanova, M.A.; Kononova, P.A.; Selyutina, O.Y.; Evseenko, V.I.; Meteleva, E.S.; Dushkin, A.V.; Su, W.; Polyakov, N.E. NMR Study of Water-Soluble Carotenoid Crocin: Formation of Mixed Micelles, Interaction with Lipid Membrane and Antioxidant Activity. Int. J. Mol. Sci. 2024, 25, 3194. [Google Scholar] [CrossRef]
- Xu, Z.; Zhu, L.; Wu, J.; Liu, Y.; Du, Z.; Wei, M.; Chen, H.; Qiang, S. Crocin Attenuates Nrf2/GPX4 Mediated Ferroptosis in Obstructive Nephropathy by Selectively Targeting MKK4. Phytother. Res. 2025; early view. [Google Scholar] [CrossRef]
- Karayakali, M.; Altinoz, E.; Elbe, H.; Koca, O.; Onal, M.O.; Bicer, Y.; Demir, M. Crocin treatment exerts anti-inflammatory and anti-oxidative effects in liver tissue damage of pinealectomized diabetic rats. Environ. Sci. Pollut. Res. Int. 2023, 30, 47670–47684. [Google Scholar] [CrossRef]
- Zhao, T.; Lu, H.; Li, M.; Yan, Q.; Gu, J.; Liu, L. Neuroprotective mechanism of crocin via PI3K/Akt/mTOR signaling pathway after cerebral infarction: An in vitro study. Am. J. Transl. Res. 2022, 14, 3164–3171. [Google Scholar]
- Dogan, T.; Yildirim, B.A.; Kapakin, K.A.T.; Kiliclioglu, M.; Alat, O. Effects of Nrf-2/HO-1, NF-κB/Cox-2/TLR-4, and Bax/Bcl-2/caspase-3 pathways in alleviating azithromycin-induced cardiotoxicity in rats: Potential cardioprotective role of crocin. Iran. J. Basic Med. Sci. 2025, 28, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, H.A.; Quinn, G.A.; Nasef, M.M.; Mishra, V.; Aljabali, A.A.A.; El-Tanani, M.; Serrano-Aroca, Á.; Webba Da Silva, M.; McCarron, P.A.; Tambuwala, M.M. Crocin Inhibits Angiogenesis and Metastasis in Colon Cancer via TNF-α/NF-kB/VEGF Pathways. Cells 2022, 11, 1502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, P.; He, Z.; Zhang, L.; He, X.; Liu, F.; Qi, J. Crocin ameliorates atherosclerosis by promoting the reverse cholesterol transport and inhibiting the foam cell formation via regulating PPARγ/LXR-α. Cell Cycle 2022, 21, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Ruan, J.; Wu, R.; Zhao, M.; Zhao, T.; Qi, M.; Yau, S.S.Y.; Yao, G.; Zhang, H.; Hu, Y.; et al. A natural carotenoid crocin exerts antidepressant action by promoting adult hippocampal neurogenesis through Wnt/β-catenin signaling. J. Adv. Res. 2023, 43, 219–231. [Google Scholar] [CrossRef]
- Chen, W.; Su, J.; Liu, Y.; Gao, T.; Ji, X.; Li, H.; Li, H.; Wang, Y.; Zhang, H.; Lv, S. Crocin Ameliorates Diabetic Nephropathy through Regulating Metabolism, CYP4A11/PPARγ, and TGF-β/Smad Pathways in Mice. Curr. Drug Metab. 2023, 24, 709–722. [Google Scholar] [CrossRef]
- Ali, A.; Yu, L.; Kousar, S.; Khalid, W.; Maqbool, Z.; Aziz, A.; Arshad, M.S.; Aadil, R.M.; Trif, M.; Riaz, S.; et al. Crocin: Functional characteristics, extraction, food applications and efficacy against brain related disorders. Front. Nutr. 2022, 9, 1009807. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Maleki, M.; Rashid-Farrokhi, F.; Abdullahi, P.R.; Hemmati, M.A.; Jamialahmadi, T.; Sahebkar, A. Modulating effects of crocin on lipids and lipoproteins: Mechanisms and potential benefits. Heliyon 2024, 10, e28837. [Google Scholar] [CrossRef]
- Demir, M.; Altinoz, E.; Koca, O.; Elbe, H.; Onal, M.O.; Bicer, Y.; Karayakali, M. Antioxidant and anti-inflammatory potential of crocin on the doxorubicin mediated hepatotoxicity in Wistar rats. Tissue Cell 2023, 84, 102182. [Google Scholar] [CrossRef]
- Naserizadeh, S.K.; Taherifard, M.H.; Shekari, M.; Mesrkanlou, H.A.; Asbaghi, O.; Nazarian, B.; Khosroshahi, M.Z.; Heydarpour, F. The effect of crocin supplementation on lipid concentrations and fasting blood glucose: A systematic review and meta-analysis and meta-regression of randomized controlled trials. Complement. Ther. Med. 2020, 52, 102500. [Google Scholar] [CrossRef]
- Bastani, S.; Vahedian, V.; Rashidi, M.; Mir, A.; Mirzaei, S.; Alipourfard, I.; Pouremamali, F.; Nejabati, H.; Kadkhoda, J.; Maroufi, N.F.; et al. An evaluation on potential anti-oxidant and anti-inflammatory effects of Crocin. Biomed. Pharmacother. 2022, 153, 113297. [Google Scholar] [CrossRef]
- Morrison, A.; Polisena, J.; Husereau, D.; Moulton, K.; Clark, M.; Fiander, M.; Mierzwinski-Urban, M.; Clifford, T.; Hutton, B.; Rabb, D. The effect of English-language restriction on systematic review-based meta-analyses: A systematic review of empirical studies. Int. J. Technol. Assess. Health Care 2012, 28, 138–144. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions; Cochrane: London, UK, 2019; pp. 205–228. [Google Scholar]
- Bakbergenuly, I.; Hoaglin, D.C.; Kulinskaya, E. Estimation in meta-analyses of mean difference and standardized mean difference. Stat. Med. 2020, 39, 171–191. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W.; Cheung, M.W. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Spiegelhalter, D.J. A re-evaluation of random-effects meta-analysis. J. R. Stat. Soc. A 2009, 172, 137–159. [Google Scholar] [CrossRef] [PubMed]
- The Jamovi Project. 2025. jamovi (Version 2.6.26). Available online: https://www.jamovi.org (accessed on 29 August 2025).
- Deeks, J.J.; Higgins, J.P.; Altman, D.G.; Cochrane Statistical Methods Group. Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Greenland, S.; Longnecker, M.P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am. J. Epidemiol. 1992, 135, 1301–1309. [Google Scholar] [CrossRef]
- Crippa, A.; Orsini, N. Dose-response meta-analysis of differences in means. BMC Med. Res. Methodol. 2016, 16, 91. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ghaderi, A.; Hazegh, P.; Baseri, M.H.K.; Vahed, N.; Nazemi, S.; Aghajani, A.; Ghoreishi, F.S.; Sadeghi-Gandomani, H.; Kashani, A.T. Oral supplementation with crocin (a constituent of saffron) in subjects with cigarette smoking: A clinical trial. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 5689–5699. [Google Scholar] [CrossRef]
- Jaafarinia, A.; Kafami, B.; Sahebnasagh, A.; Saghafi, F. Evaluation of therapeutic effects of crocin in attenuating the progression of diabetic nephropathy: A preliminary randomized triple-blind placebo-controlled trial. BMC Complement. Med. Ther. 2022, 22, 262. [Google Scholar] [CrossRef]
- Sepahi, S.; Golfakhrabadi, M.; Bonakdaran, S.; Lotfi, H.; Mohajeri, S.A. Effect of crocin on diabetic patients: A placebo-controlled, triple-blinded clinical trial. Clin. Nutr. ESPEN 2022, 50, 255–263. [Google Scholar] [CrossRef]
- Ghaderi, A.; Rasouli-Azad, M.; Vahed, N.; Banafshe, H.R.; Soleimani, A.; Omidi, A.; Ghoreishi, F.S.; Asemi, Z. Clinical and metabolic responses to crocin in patients under methadone maintenance treatment: A randomized clinical trial. Phytother. Res. 2019, 33, 2714–2725. [Google Scholar] [CrossRef] [PubMed]
- Sepahi, S.; Mohajeri, S.A.; Hosseini, S.M.; Khodaverdi, E.; Shoeibi, N.; Namdari, M.; Tabassi, S.A.S. Effects of Crocin on Diabetic Maculopathy: A Placebo-Controlled Randomized Clinical Trial. Am. J. Ophthalmol. 2018, 190, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Kermani, T.; Kazemi, T.; Molki, S.; Ilkhani, K.; Sharifzadeh, G.; Rajabi, O. The Efficacy of Crocin of Saffron (Crocus sativus L.) on the Components of Metabolic Syndrome: A Randomized Controlled Clinical Trial. J. Res. Pharm. Pract. 2017, 6, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Javandoost, A.; Afshari, A.; Nikbakht-Jam, I.; Khademi, M.; Eslami, S.; Nosrati, M.; Foroutan-Tanha, M.; Sahebkar, A.; Tavalaie, S.; Ghayour-Mobarhan, M.; et al. Effect of crocin, a carotenoid from saffron, on plasma cholesteryl ester transfer protein and lipid profile in subjects with metabolic syndrome: A double blind randomized clinical trial. ARYA Atheroscler. 2017, 13, 245–252. [Google Scholar]
- Abedimanesh, N.; Bathaie, S.Z.; Abedimanesh, S.; Motlagh, B.; Separham, A.; Ostadrahimi, A. Saffron and crocin improved appetite, dietary intakes and body composition in patients with coronary artery disease. J. Cardiovasc. Thorac. Res. 2017, 9, 200–208. [Google Scholar] [CrossRef]
- Nikbakht-Jam, I.; Khademi, M.; Nosrati, M.; Eslami, S.; Foroutan-Tanha, M.; Sahebkar, A.; Tavalaie, S.; Ghayour-Mobarhan, M.; Ferns, G.A.A.; Hadizadeh, F.; et al. Effect of crocin extracted from saffron on pro-oxidant–anti-oxidant balance in subjects with metabolic syndrome: A randomized, placebo-controlled clinical trial. Eur. J. Integr. Med. 2016, 8, 307–312. [Google Scholar] [CrossRef]
- Fadai, F.; Mousavi, B.; Ashtari, Z.; Ali Beigi, N.; Farhang, S.; Hashempour, S.; Shahhamzei, N.; Bathaie, S.Z. Saffron aqueous extract prevents metabolic syndrome in patients with schizophrenia on olanzapine treatment: A randomized triple blind placebo controlled study. Pharmacopsychiatry 2014, 47, 156–161. [Google Scholar] [CrossRef]
- Perswani, P.; Ismail, S.M.; Mumtaz, H.; Uddin, N.; Asfand, M.; Khalil, A.B.B.; Ijlal, A.; Khan, S.E.; Usman, M.; Younas, H.; et al. Rethinking HDL-C: An In-Depth Narrative Review of Its Role in Cardiovascular Health. Curr. Probl. Cardiol. 2024, 49, 102152. [Google Scholar] [CrossRef]
- Lee-Rueckert, M.; Escola-Gil, J.C.; Kovanen, P.T. HDL functionality in reverse cholesterol transport—Challenges in translating data emerging from mouse models to human disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 566–583. [Google Scholar] [CrossRef]
- Marques, L.R.; Diniz, T.A.; Antunes, B.M.; Rossi, F.E.; Caperuto, E.C.; Lira, F.S.; Gonçalves, D.C. Reverse Cholesterol Transport: Molecular Mechanisms and the Non-medical Approach to Enhance HDL Cholesterol. Front. Physiol. 2018, 9, 526. [Google Scholar] [CrossRef]
- Mamede, I.; Braga, M.A.P.; Martins, O.C.; Franchini, A.E.O.; Silveira Filho, R.B.; Santos, M.C.F. Association between very high HDL-C levels and mortality: A systematic review and meta-analysis. J. Clin. Lipidol. 2024, 18, e701–e709. [Google Scholar] [CrossRef] [PubMed]
- Madaudo, C.; Bono, G.; Ortello, A.; Astuti, G.; Mingoia, G.; Galassi, A.R.; Sucato, V. Dysfunctional High-Density Lipoprotein Cholesterol and Coronary Artery Disease: A Narrative Review. J. Pers. Med. 2024, 14, 996. [Google Scholar] [CrossRef]
- Cho, K.H. The Current Status of Research on High-Density Lipoproteins (HDL): A Paradigm Shift from HDL Quantity to HDL Quality and HDL Functionality. Int. J. Mol. Sci. 2022, 23, 3967. [Google Scholar] [CrossRef] [PubMed]
- Chehab, O.; Akl, E.; Abdollahi, A.; Zeitoun, R.; Ambale-Venkatesh, B.; Wu, C.; Tracy, R.; Blumenthal, R.S.; Post, W.S.; Lima, J.A.C.; et al. Higher HDL cholesterol levels are associated with increased markers of interstitial myocardial fibrosis in the MultiEthnic Study of Atherosclerosis (MESA). Sci. Rep. 2023, 13, 20115. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Catapano, A.L.; Norata, G.D. Biological Consequences of Dysfunctional HDL. Curr. Med. Chem. 2019, 26, 1644–1664. [Google Scholar] [CrossRef]
- He, Y.; Kothari, V.; Bornfeldt, K.E. High-Density Lipoprotein Function in Cardiovascular Disease and Diabetes Mellitus. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e10–e16. [Google Scholar] [CrossRef]
- Velissaridou, A.; Panoutsopoulou, E.; Prokopiou, V.; Tsoupras, A. Cardio-Protective-Promoting Properties of Functional Foods Inducing HDL-Cholesterol Levels and Functionality. Nutraceuticals 2024, 4, 469–502. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Manjunatha, S.; Damayanthi, D.; Sylaja, P.N.; Gopala, S. Multiple facets of HDLs as modifiable risk factors in stroke—The good, the bad, and the ugly. J. Lipid Res. 2025, 66, 100879. [Google Scholar] [CrossRef] [PubMed]
- Duarte Lau, F.; Giugliano, R.P. Lipoprotein(a) and its Significance in Cardiovascular Disease: A Review. JAMA Cardiol. 2022, 7, 760–769. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Langsted, A. Lipoprotein(a) and cardiovascular disease. Lancet 2024, 404, 1255–1264. [Google Scholar] [CrossRef]
- Boffa, M.B.; Koschinsky, M.L. Lipoprotein(a) and cardiovascular disease. Biochem. J. 2024, 481, 1277–1296. [Google Scholar] [CrossRef] [PubMed]
- Vinci, P.; Di Girolamo, F.G.; Panizon, E.; Tosoni, L.M.; Cerrato, C.; Pellicori, F.; Altamura, N.; Pirulli, A.; Zaccari, M.; Biasinutto, C.; et al. Lipoprotein(a) as a Risk Factor for Cardiovascular Diseases: Pathophysiology and Treatment Perspectives. Int. J. Environ. Res. Public Health 2023, 20, 6721. [Google Scholar] [CrossRef] [PubMed]


| Study | Country | Study Design | Participants | Sex (M/F) | Sample Size | Trial Duration | Age (Years) | BMI (kg/m2) | Intervention | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IG | CG | IG | CG | IG | CG | Type | Dose (mg/d) | Control Group | ||||||
| Mahdavi et al. [35] | Iran | RCT, double-blind | Smokers | 39 M/9 F | 23 | 25 | 3 months | 34.39 ± 10.72 | 36.20 ± 9.23 | NR | NR | Tablets | 30 | Placebo |
| Jaafarinia et al. [36] | Iran | RCT, triple-blind | T2DM | 23 M/17 F | 21 | 19 | 90 days | 63.86 ± 10.62 | 62.68 ± 9.84 | 27.21 ± 3.86 | 27.26 ± 3.34 | Tablets | 15 | Placebo |
| Sepahi et al. [37] | Iran | RCT, triple-blind | T2DM | 47 M/53 F | 50 | 50 | 3 months | 57.58 ± 1.0 | 56.92 ± 1.9 | NR | NR | Tablets | 30 | Placebo |
| Ghaderi et al. [38] | Iran | RCT, double-blind | MMT | NR | 26 | 27 | 8 weeks | 44.5 ± 9.4 | 45.6 ± 9.9 | 24.5 ± 4.4 | 25.2 ± 4.2 | Tablets | 30 | Placebo |
| Sepahi et al. [39] | Iran | RCT, double-blind | Diabetic maculopathy | 29 M/31 F | 40 | 20 | 3 months | 54.31 ± 6.6 (5 mg/d), 56.09 ± 4.3 (15 mg/d) | 57.17 ± 2.9 | NR | NR | Tablets | 5 or 15 | Placebo |
| Kermani et al. [40] | Iran | RCT, double-blind | MetS | 7 M/41 F | 24 | 24 | 6 weeks | 53.8 ± 9.2 | 50.9 ± 8.8 | 29.9 ± 3.9 | 29.8 ± 5.3 | Tablets | 100 | Placebo |
| Javandoost et al. [41] | Iran | RCT, double-blind | MetS | 18 M/26 F | 21 | 22 | 8 weeks | 33.10 (M) and 44.5 (F) | 34.9 (M) and 46.0 (F) | NR | NR | Tablets | 30 | Placebo |
| Abedimanesh et al. [42] | Iran | RCT, double-blind | CAD | 25 M/25 F | 25 | 25 | 8 weeks | 53.36 ± 5.94 | 56.32 ± 5.91 | 27.92 ± 2.57 | 28.05 ± 2.89 | Capsules | 30 | Placebo |
| Nikbakht-Jam et al. [43] | Iran | RCT, double-blind | MetS | 25 M/33 F | 29 | 29 | 8 weeks | 38.97 ± 13.33 | 43.46 ± 12.77 | NR | NR | Tablets | 30 | Placebo |
| Fadai et al. [44] | Iran | RCT, triple-blind | Schizophrenia | 41 M | 20 | 21 | 12 weeks | 48.1 ± 7.7 | 48.1 ± 6.1 | NR | NR | Capsules | 30 | Placebo |
| Study | Question Focus | Appropriate Randomization | Allocation Blinding | Double-Blind | Losses (<20%) | Prognostic or Demographic Characteristics | Outcomes | Intention-to-Treat Analysis | Sample Calculation | Adequate Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| Mahdavi et al. [35] |  |  |  |  |  |  |  |  |  |  |
| Jaafarinia et al. [36] |  |  |  |  |  |  |  |  |  |  |
| Sepahi et al. [37] |  |  |  |  |  |  |  |  |  |  |
| Ghaderi et al. [38] |  |  |  |  |  |  |  |  |  |  |
| Sepahi et al. [39] |  |  |  |  |  |  |  |  |  |  |
| Kermani et al. [40] |  |  |  |  |  |  |  |  |  |  |
| Javandoost et al. [41] |  |  |  |  |  |  |  |  |  |  |
| Abedimanesh et al. [42] |  |  |  |  |  |  |  |  |  |  |
| Nikbakht-Jam et al. [43] |  |  |  |  |  |  |  |  |  |  |
| Fadai et al. [44] |  |  |  |  |  |  |  |  |  |  |
 , Low risk of bias;
, Low risk of bias;  , High risk of bias;
, High risk of bias;  , Unclear risk of bias.
, Unclear risk of bias.| Stratification Category | Observed Variations Across the Included Studies |
|---|---|
| Crocin dosage | Ranged from 5–100 mg daily, with the majority of the studies applying doses from 15–30 mg/d. |
| Intervention duration | Ranged principally from 6 to 12 weeks. |
| Population age | Ranged from adults (~33.10 years) to elderly (63.86 ± 10.62 years) individuals. |
| Health status | The intervention protocols included smokers, diabetic individuals (including those with diabetic complications like diabetic maculopathy), individuals under methadone maintenance treatment, individuals with metabolic syndrome, patients with coronary artery disease, and individuals living with schizophrenia. |
| Intervention protocols | Varied principally regarding frequency of the interventions (once vs. twice daily) and administration timing. |
| Subgroup analysis performed | Conducted mainly regarding crocin dosage or treatment vs. placebo and health statuses of the included participants. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurindo, L.F.; Chagas, E.F.B.; Dogani Rodrigues, V.; Argollo Haber, R.d.; Cristina Castilho Caracio, F.; Capobianco Marangão, M.C.; dos Santos Bueno, M.; Pereira, E.d.S.B.M.; Penteado Detregiachi, C.R.; Valenti, V.E.; et al. Is Crocin Effective in Modulating Blood Lipid Levels? An Updated Systematic Review and Meta-Analysis with Dose– and Time–Response Assessments. Pharmaceuticals 2025, 18, 1735. https://doi.org/10.3390/ph18111735
Laurindo LF, Chagas EFB, Dogani Rodrigues V, Argollo Haber Rd, Cristina Castilho Caracio F, Capobianco Marangão MC, dos Santos Bueno M, Pereira EdSBM, Penteado Detregiachi CR, Valenti VE, et al. Is Crocin Effective in Modulating Blood Lipid Levels? An Updated Systematic Review and Meta-Analysis with Dose– and Time–Response Assessments. Pharmaceuticals. 2025; 18(11):1735. https://doi.org/10.3390/ph18111735
Chicago/Turabian StyleLaurindo, Lucas Fornari, Eduardo Federighi Baisi Chagas, Victória Dogani Rodrigues, Ricardo de Argollo Haber, Flávia Cristina Castilho Caracio, Maria Clara Capobianco Marangão, Manuela dos Santos Bueno, Eliana de Souza Bastos Mazuqueli Pereira, Cláudia Rucco Penteado Detregiachi, Vitor Engrácia Valenti, and et al. 2025. "Is Crocin Effective in Modulating Blood Lipid Levels? An Updated Systematic Review and Meta-Analysis with Dose– and Time–Response Assessments" Pharmaceuticals 18, no. 11: 1735. https://doi.org/10.3390/ph18111735
APA StyleLaurindo, L. F., Chagas, E. F. B., Dogani Rodrigues, V., Argollo Haber, R. d., Cristina Castilho Caracio, F., Capobianco Marangão, M. C., dos Santos Bueno, M., Pereira, E. d. S. B. M., Penteado Detregiachi, C. R., Valenti, V. E., Longui Cabrini, M., & Maria Barbalho, S. (2025). Is Crocin Effective in Modulating Blood Lipid Levels? An Updated Systematic Review and Meta-Analysis with Dose– and Time–Response Assessments. Pharmaceuticals, 18(11), 1735. https://doi.org/10.3390/ph18111735