Recent Advances in Androgen Receptor Pathway Inhibitors for Castration-Sensitive Prostate Cancer
Abstract
1. Introduction
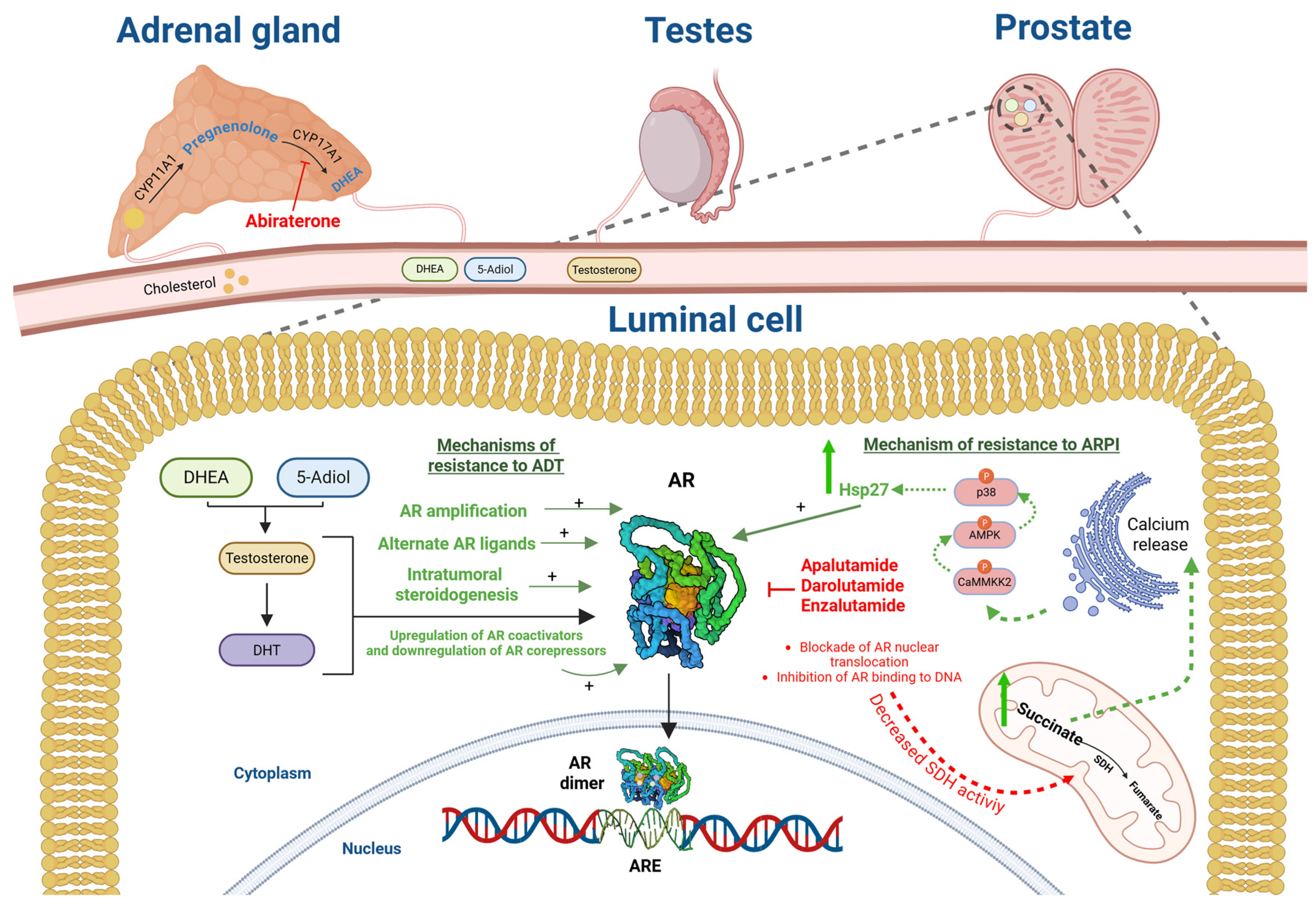
1.1. ARPI Molecular Features and Mechanism of Action
1.2. ARPI in Localized Prostate Cancer: A Radiation Oncologist’s Perspective
1.3. ARPI in Localized Prostate Cancer: A Surgeon Perspective
| Author, Year | Therapy | Design | Arms of Neoadjuvant | No of Patients | Positive Margins | pCR | MRD | pCR + MRD | Survival |
|---|---|---|---|---|---|---|---|---|---|
| NEOADJUVANT TREATMENTS | |||||||||
| Taplin, 2014 [39] | ABI | Phase II RCT, 58 high-risk men | A: 12-w ABI + L vs. B: 24-w ABI + L | A: 28 pts B: 30 pts | 19% vs. 10% (p NR) | 4% vs. 10% (p NR) | 0% vs. 14% (p NR) | 4% vs. 24% (p NR) | - |
| Montgomery, 2017 [40] | ENZA | Phase II RCT, 52 intermediate/high-risk men | A: 6-mo ENZA vs. B: 6-mo ENZA + dutasteride + L | A: 27 pts B: 25 pts | 16% vs. 22% (p NR) | 0% vs. 4% (p NR) | 0% vs. 13% (p NR) | 0% vs. 17% (p NR) | - |
| Efstathiou, 2019 [33] | ABI | Phase II RCT, 65 high-risk men | A: 3-mo L vs. B: 3-mo ABI + L | A: 21 pts B: 44 pts | 14% vs. 5% (p 0.17) | - | - | - | 71% vs. 75% 3-yr RFS (p 0.28) |
| McKay, 2019 [32] | ABI + ENZA | Phase II RCT, 75 intermediate/high-risk men | A: 24-w ENZA + L vs. B: 24-w ABI + ENZA + L | A: 25 pts B: 50 pts | 12% vs. 18% (p NR) | 8% vs. 10% (p NR) | 8% vs. 20% (p NR) | 16% vs. 30% (p 0.2) | - |
| McKay, 2021 [36] | ABI + APA | Phase II RCT, 118 intermediate/high-risk men | A: 6-mo ABI + L vs. B: 6-mo ABI + APA + L | A: 59 pts B: 59 pts | 12% vs. 7% (p NR) | 10% vs. 13% (p NR) | 10% vs. 9% (p NR) | 20% vs. 22% (p 0.4) | - |
| Lee, 2022 (NEAR trial) [34] | APA | Phase II single-arm, 30 intermediate/high-risk men | 12-w APA | 30 pts | 16% | 0% | NR | NR | 86% 2-yr bRFS |
| Devos, 2023 (ARNEO trial) [30] | APA+ Degarelix | Phase II RCT, 89 high-risk men | A: 3-mo Degarelix + APA vs. B: 3-mo Degarelix + placebo | A: 45 pts B: 44 pts | 18% vs. 18% (p NR) | 0% vs. 0% (p NR) | 38% vs. 9% (p 0.002) | 38% vs. 9% (p 0.002) | - |
| Wei, 2023 [29] | APA | Phase II single-arm, 7 advanced PCa | 4-mo APA + ADT | 7 pts | - | 14% | - | - | - |
| Zhuang, 2024 [31] | DARO | Phase II single-arm, 30 high-risk men | 6-mo DARO + ADT | 30 pts | 13% | 7% | 33% | 40% | 90% 1-yr PFS |
| ADJUVANT TREATMENTS | |||||||||
| McKay, 2023 [38] | ABI + APA | Phase II RCT, 82 IR/HR men | A: 12-mo ABI + APA + L vs. B: observation | A: 42 pts B: 40 pts | 14 (17%) | 4-yr: 67% vs. 61% | - | - | - |
| Shore, 2024 (NCT04523207) [37] | APA | Phase II single-arm, 108 HR men | 12-mo APA + ADT | 108 pts | 6 (6%) | 2-yr 100% | - | - | - |
1.4. ARPI in De Novo Metastatic Castration-Sensitive Prostate Cancer
| Author, Year | Therapy | Design | Arms of Treatment | No of Patients | OS | PSAP | rPFS | CRFS |
|---|---|---|---|---|---|---|---|---|
| Fizazi, 2017 (LATITUDE trial) [11] | ABI | Phase III RCT, 1119 high-risk mCSPC men | A: ADT B: ADT + ABI | A: 602 pts B: 597 pts | mOS: 36.5 months vs. 53.3 months | mPSAP 7.4 months vs. 33.2 months | mrPFS: 14.8 months vs. 33 months | __ |
| Armstrong, 2019 (ARCHES trial) [14] | ENZA | Phase III RCT, 1150 de novo or with recurrence mCSPC men | A: ADT B: ADT + ENZA | A: 574 pts B: 576 pts | Immature data HR for death 0.81 p = 0.33 for group B | HR 0.19 p < 0.001 for group B | mrPFS: 19 months vs. NR | Median time to castration resistance: 13 months vs. NR |
| Davis, 2019 (ENZAMET) [13] | ENZA | Phase III RCT, 1125 mCSPC men | A:ADT B: ADT + ENZA | A: 562 pts B: 563 pts | 72% Vs. 80% 3-yr OS | 67% Vs. 37% 3-yr PSAPFS | __ | __ |
| Chi, 2019 (TITAN trial) [15] | APA | Phase III RCT, 1051 mCSPC men | A: ADT B: ADT + APA | A: 527 pts B: 525 pts | 73.5% vs. 82.4% 2-yr OS | mPSAP12.9 months vs. NR | mrPFS 22.1months vs. NR | __ |
| Smith, 2022 (ARASENS trial) [16] | DARO | Phase III RCT, 1306 mCSPC men | A: ADT + DOCE B: ADT + DOCE + DARO | A: 651 B: 655 | mOS 48.9 months vs. NR | __ | __ | Median time to castration resistance: 19.1 months vs. NR |
| Fizazi, 2022 (PEACE-1 trial) [46] | SOC vs. SOC + ABI | Phase III RCT, 1172 de novo mCSPC men | A: ADT or DOCE B: ADT or DOCE + RT C: ADT or DOCE + ABI D: ADT or DOCE + RT + ABI | Group I A: 296 + B: 293 Group II C: 292 + D: 291 | mOS 4.7 years vs. 5.7 years | __ | mrPFS 2.2 years vs. 4.5 years | mCRPS 1.5 years vs. 3.8 years |
| Davis, 2022 (sub-analysis ENZAMET trial) [48] | ENZA | Phase III RCT, 270 high-volume mCSPC men | A: ADT + DOCE B: ADT + ENZA + DOCE | A: 137 B: 133 | __ | HR OS: 0.79 (0.57–1.10) 5-yr OS | __ | __ |
1.5. ARPI in Metachronous Castration Sensitive Prostate-Cancer
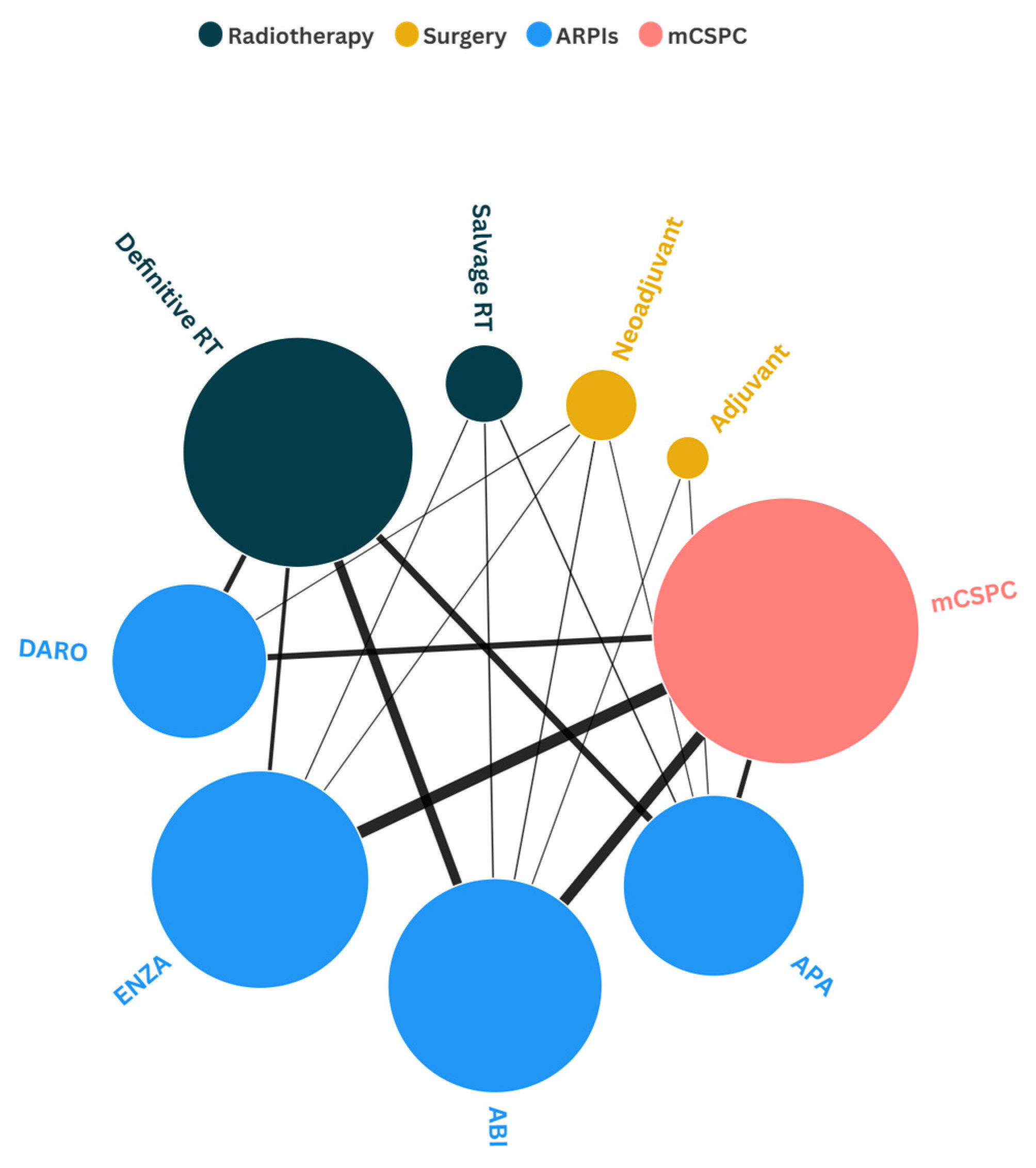
1.6. Network Map Analysis of ARPIs Use Across Different Disease Stages
2. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schafer, E.J.; Laversanne, M.; Sung, H.; Soerjomataram, I.; Briganti, A.; Dahut, W.; Bray, F.; Jemal, A. Recent Patterns and Trends in Global Prostate Cancer Incidence and Mortality: An Update. Eur. Urol. 2025, 87, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Benadada, F.; Saad, F.; Delouya, G.; Taussky, D. Charles Brenton Huggins: A historical review of the Nobel laureate’s pioneering discoveries. Cancer 2024, 130, 1019–1024. [Google Scholar] [CrossRef]
- Mason, M.D.; Parulekar, W.R.; Sydes, M.R.; Brundage, M.; Kirkbride, P.; Gospodarowicz, M.; Cowan, R.; Kostashuk, E.C.; Anderson, J.; Swanson, G.; et al. Final Report of the Intergroup Randomized Study of Combined Androgen-Deprivation Therapy Plus Radiotherapy Versus Androgen-Deprivation Therapy Alone in Locally Advanced Prostate Cancer. J. Clin. Oncol. 2015, 33, 2143–2150. [Google Scholar] [CrossRef]
- Shore, N.D.; Moul, J.W.; Pienta, K.J.; Czernin, J.; King, M.T.; Freedland, S.J. Biochemical recurrence in patients with prostate cancer after primary definitive therapy: Treatment based on risk stratification. Prostate Cancer Prostatic Dis. 2024, 27, 192–201. [Google Scholar] [CrossRef]
- Kato, H.; Goto, Y.; Kojima, S.; Onoda, Y.; Wakai, K.; Hou, K.; Araki, K.; Sakamoto, S.; Ichikawa, T.; Naya, Y. Time to Castration Resistance is Associated With Overall Survival Even After the Achievement of Castration Resistance in Metastatic Prostate Cancer. Prostate 2025, 85, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Ingrosso, G.; Lancia, A.; Bardoscia, L.; Becherini, C.; Bottero, M.; Bertini, N.; Cai, T.; Caini, S.; Caserta, C.; Doccioli, C.; et al. Current diagnostic and therapeutic options in de novo low-volume metastatic hormone-sensitive prostate cancer. Expert Rev. Anticancer. Ther. 2025, 25, 741–754. [Google Scholar] [CrossRef]
- Rifaldi, F.; Tortorella, A.; Sporeni, S.; Lanzetta, I.; Figini, S.; Naspro, R.L.J.; Lancia, A.; Montagna, B.; Secondino, S.; Pedrazzoli, P.; et al. Novel androgen receptor inhibitors in prostate cancer: What do we know so far? Crit. Rev. Oncol. 2025, 215, 104857. [Google Scholar] [CrossRef] [PubMed]
- Ingrosso, G.; Detti, B.; Scartoni, D.; Lancia, A.; Giacomelli, I.; Baki, M.; Carta, G.; Livi, L.; Santoni, R. Current therapeutic options in metastatic castration-resistant prostate cancer. Semin. Oncol. 2018, 45, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Saxena, N.; Beraldi, E.; Fazli, L.; Somasekharan, S.P.; Adomat, H.; Zhang, F.; Molokwu, C.; Gleave, A.; Nappi, L.; Nguyen, K.; et al. Androgen receptor (AR) antagonism triggers acute succinate-mediated adaptive responses to reactivate AR signaling. EMBO Mol. Med. 2021, 13, e13427. [Google Scholar] [CrossRef]
- Akamatsu, S.; Inoue, T.; Ogawa, O.; Gleave, M.E. Clinical and molecular features of treatment-related neuroendocrine prostate cancer. Int. J. Urol. 2018, 25, 345–351. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef]
- James, N.D.; de Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy with Enzalutamide or Placebo in Men with Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2019, 37, 2974–2986. [Google Scholar] [CrossRef]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juárez Soto, Á.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2019, 381, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Kopyltsov, E.; Park, C.H.; Alekseev, B.; Montesa-Pino, Á.; et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2022, 386, 1132–1142. [Google Scholar] [CrossRef]
- Wang, E.C.; Lee, W.R.; Armstrong, A.J. Second generation anti-androgens and androgen deprivation therapy with radiation therapy in the definitive management of high-risk prostate cancer. Prostate Cancer Prostatic Dis. 2023, 26, 30–40. [Google Scholar] [CrossRef]
- Attard, G.; Murphy, L.; Clarke, N.W.; Cross, W.; Jones, R.J.; Parker, C.C.; Gillessen, S.; Cook, A.; Brawley, C.; Amos, C.L.; et al. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: A meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet 2022, 399, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Freedland, S.J.; de Almeida Luz, M.; De Giorgi, U.; Gleave, M.; Gotto, G.T.; Pieczonka, C.M.; Haas, G.P.; Kim, C.S.; Ramirez-Backhaus, M.; Rannikko, A.; et al. Improved outcomes with enzalutamide in biochemically recurrent prostate cancer. N. Engl. J. Med. 2023, 389, 1453–1465. [Google Scholar] [CrossRef]
- Tran, P.T.; Lowe, K.; Tsai, H.L.; Song, D.Y.; Hung, A.Y.; Hearn, J.W.D.; Miller, S.; Proudfoot, J.A.; Deek, M.P.; Phillips, R.; et al. Phase II Randomized Study of Salvage Radiation Therapy Plus Enzalutamide or Placebo for High-Risk Prostate-Specific Antigen Recurrent Prostate Cancer After Radical Prostatectomy: The SALV-ENZA Trial. J. Clin. Oncol. 2023, 41, 1307–1317. [Google Scholar] [CrossRef]
- Nguyen, P.L.; Kollmeier, M.; Rathkopf, D.E.; Hoffman, K.E.; Zurita, A.J.; Spratt, D.E.; Dess, R.T.; Liauw, S.L.; Szmulewitz, R.Z.; Einstein, D.J.; et al. FORMULA-509: A multicenter randomized trial of post-operative salvage radiotherapy (SRT) and 6 months of GnRH agonist with or without abiraterone acetate/prednisone (AAP) and apalutamide (Apa) post-radical prostatectomy (RP). J. Clin. Oncol. 2023, 41 (Suppl. 6), 303. [Google Scholar] [CrossRef]
- Posadas, E.M.; Gay, H.A.; Rodgers, J.P.; Morgan, T.M.; Xiao, Y.; Yu, J.B.; Michalski, J.M.; Bouchard, M.; Desai, N.B.; Funk, R.; et al. Intensification of ADT with enzalutamide in high-risk patients with biochemical relapse following radical prostatectomy undergoing salvage radiation: Initial results from RTOG 3506 (STEEL). J. Clin. Oncol. 2024, 42 (suppl. 4), 131. [Google Scholar] [CrossRef]
- Schmidt-Hegemann, N.S.; Zamboglou, C.; Mason, M.; Mottet, N.; Hinnen, K.; De Meerleer, G.; Cozzarini, C.; Maingon, P.; Henry, A.; Spahn, M.; et al. ESTRO-ACROP recommendations for evidence-based use of androgen deprivation therapy in combination with external-beam radiotherapy in prostate cancer. Radiother. Oncol. 2023, 183, 109544. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, S.; Turco, F.; Davis, I.D.; Efstathiou, J.A.; Fizazi, K.; James, N.D.; Shore, N.; Small, E.; Smith, M.; Sweeney, C.J.; et al. Management of Patients with Advanced Prostate Cancer. Report from the 2024 Advanced Prostate Cancer Consensus Conference (APCCC). Eur. Urol. 2025, 87, 157–216. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Würnschimmel, C.; Ruvolo, C.C.; Nocera, L.; Tian, Z.; Saad, F.; Briganti, A.; Tilki, D.; Graefen, M.; Kluth, L.A.; et al. Increasing rates of NCCN high and very high-risk prostate cancer versus number of prostate biopsy cores. Prostate 2021, 81, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Akre, O.; Garmo, H.; Adolfsson, J.; Lambe, M.; Bratt, O.; Stattin, P. Mortality among men with locally advanced prostate cancer managed with noncurative intent: A nationwide study in PCBaSe Sweden. Eur. Urol. 2011, 60, 554–563. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Shelley, M.; Harrison, C.; Coles, B.; Wilt, T.J.; Mason, M. Neo-adjuvant and adjuvant hormone therapy for localised and locally advanced prostate cancer. Cochrane Database Syst. Rev. 2006, 2010, CD006019. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, R.; Zhong, D.; Chen, Z.; Chen, G.; Yang, M.; Lin, L.; Li, T.; Ye, L.; Chen, L.; et al. Androgen deprivation therapy plus apalutamide as neoadjuvant therapy prior radical prostatectomy for patients with unresectable prostate cancer. Front. Pharmacol. 2023, 14, 1284899. [Google Scholar] [CrossRef]
- Devos, G.; Tosco, L.; Baldewijns, M.; Gevaert, T.; Goffin, K.; Petit, V.; Mai, C.; Laenen, A.; Raskin, Y.; Van Haute, C.; et al. ARNEO: A Randomized Phase II Trial of Neoadjuvant Degarelix with or Without Apalutamide Prior to Radical Prostatectomy for High-risk Prostate Cancer. Eur. Urol. 2023, 83, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Wang, Y.; Zhang, S.; Qiu, X.; Zhou, F.; Fu, Y.; Wei, X.; Xu, L.; Guo, H. Neoadjuvant darolutamide plus androgen deprivation therapy for high-risk/very high-risk localized prostate cancer: A multicenter, open-labeled, single-arm phase II trial. J. Clin. Oncol. 2024, 42, 321. [Google Scholar] [CrossRef]
- McKay, R.R.; Ye, H.; Xie, W.; Lis, R.; Calagua, C.; Zhang, Z.; Trinh, Q.D.; Chang, S.L.; Harshman, L.C.; Ross, A.E.; et al. Evaluation of Intense Androgen Deprivation Before Prostatectomy: A Randomized Phase II Trial of Enzalutamide and Leuprolide with or Without Abiraterone. J. Clin. Oncol. 2019, 37, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Efstathiou, E.; Davis, J.W.; Pisters, L.; Li, W.; Wen, S.; McMullin, R.P.; Gormley, M.; Ricci, D.; Titus, M.; Hoang, A.; et al. Clinical and Biological Characterisation of Localised High-risk Prostate Cancer: Results of a Randomised Preoperative Study of a Luteinising Hormone-releasing Hormone Agonist with or Without Abiraterone Acetate plus Prednisone. Eur. Urol. 2019, 76, 418–424. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, L.S.; Sim, A.Y.L.; Ong, C.W.; Yang, X.; Ng, C.C.Y.; Liu, W.; Rajasegaran, V.; Lim, A.M.S.; Aslim, E.J.; Ngo, N.T.; et al. NEAR trial: A single-arm phase II trial of neoadjuvant apalutamide monotherapy and radical prostatectomy in intermediate- and high-risk prostate cancer. Prostate Cancer Prostatic Dis. 2022, 25, 741–748, Erratum in Prostate Cancer Prostatic Dis. 2022, 25, 803. [Google Scholar] [CrossRef]
- McKay, R.R.; Berchuck, J.; Kwak, L.; Xie, W.; Silver, R.; Bubley, G.J.; Chang, P.K.; Wagner, A.; Zhang, Z.; Kibel, A.S.; et al. Outcomes of Post-Neoadjuvant Intense Hormone Therapy and Surgery for High Risk Localized Prostate Cancer: Results of a Pooled Analysis of Contemporary Clinical Trials. J. Urol. 2021, 205, 1689–1697. [Google Scholar] [CrossRef]
- McKay, R.R.; Xie, W.; Ye, H.; Fennessy, F.M.; Zhang, Z.; Lis, R.; Calagua, C.; Rathkopf, D.; Laudone, V.P.; Bubley, G.J.; et al. Results of a Randomized Phase II Trial of Intense Androgen Deprivation Therapy prior to Radical Prostatectomy in Men with High-Risk Localized Prostate Cancer. J. Urol. 2021, 206, 80–87. [Google Scholar] [CrossRef]
- Shore, N.; Hafron, J.; Saltzstein, D.; Brown, G.; Belkoff, L.; Aggarwal, P.; Phillips, J.; Bhaumik, A.; McGowan, T. Apalutamide for High-Risk Localized Prostate Cancer Following Radical Prostatectomy (Apa-RP). J. Urol. 2024, 212, 682–691. [Google Scholar] [CrossRef]
- McKay, R.R.; Xie, W.; Yang, X.; Acosta, A.; Rathkopf, D.; Laudone, V.P.; Bubley, G.J.; Einstein, D.J.; Chang, P.; Wagner, A.A.; et al. Postradical prostatectomy prostate-specific antigen outcomes after 6 versus 18 months of perioperative androgen-deprivation therapy in men with localized, unfavorable intermediate-risk or high-risk prostate cancer: Results of part 2 of a randomized phase 2 trial. Cancer 2024, 130, 1629–1641. [Google Scholar]
- Taplin, M.E.; Montgomery, B.; Logothetis, C.J.; Bubley, G.J.; Richie, J.P.; Dalkin, B.L.; Sanda, M.G.; Davis, J.W.; Loda, M.; True, L.D.; et al. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: Results of a randomized phase II neoadjuvant study. J. Clin. Oncol. 2014, 32, 3705–3715. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, B.; Tretiakova, M.S.; Joshua, A.M.; Gleave, M.E.; Fleshner, N.; Bubley, G.J.; Mostaghel, E.A.; Chi, K.N.; Lin, D.W.; Sanda, M.; et al. Neoadjuvant Enzalutamide Prior to Prostatectomy. Clin. Cancer Res. 2017, 23, 2169–2176. [Google Scholar] [CrossRef] [PubMed]
- Piombino, C.; Oltrecolli, M.; Tonni, E.; Pirola, M.; Matranga, R.; Baldessari, C.; Pipitone, S.; Dominici, M.; Sabbatini, R.; Vitale, M.G. De Novo Metastatic Prostate Cancer: Are We Moving toward a Personalized Treatment? Cancers 2023, 15, 4945. [Google Scholar] [CrossRef]
- Buzzoni, C.; Auvinen, A.; Roobol, M.J.; Carlsson, S.; Moss, S.M.; Puliti, D.; de Koning, H.J.; Bangma, C.H.; Denis, L.J.; Kwiatkowski, M.; et al. Metastatic Prostate Cancer Incidence and Prostate-specific Antigen Testing: New Insights from the European Randomized Study of Screening for Prostate Cancer. Eur. Urol. 2015, 68, 885–890. [Google Scholar] [CrossRef]
- Agarwal, N.; Lucas, J.; Aguilar-Bonavides, C.; Thomas, S.; Gormley, M.; Chowdhury, S.; Merseburger, A.S.; Bjartell, A.; Uemura, H.; Özgüroğlu, M.; et al. Genomic aberrations associated with overall survival (OS) in metastatic castration-sensitive prostate cancer (mCSPC) treated with apalutamide (APA) or placebo (PBO) plus androgen deprivation therapy (ADT) in TITAN. J. Clin. Oncol. 2022, 40, 5066. [Google Scholar] [CrossRef]
- Tilki, D.; van den Bergh, R.C.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer. Part II—2024 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2024, 86, 164–182. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. NN. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Foulon, S.; Carles, J.; Roubaud, G.; McDermott, R.; Fléchon, A.; Tombal, B.; Supiot, S.; Berthold, D.; Ronchin, P.; et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitiveprostate cancer (PEACE-1): A multicentre, open-label, randomised, phase 3 study with 2 × 2 factorial design. Lancet 2022, 399, 1695–1707. [Google Scholar] [CrossRef]
- Hussain, M.; Tombal, B.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Shore, N.; Kopyltsov, E.; Kalebasty, A.R.; Bögemann, M.; et al. Darolutamide Plus Androgen-Deprivation Therapy and Docetaxel in Metastatic Hormone-Sensitive Prostate Cancer by Disease Volume and Risk Subgroups in the Phase III ARASENS Trial. J. Clin. Oncol. 2023, 41, 3595–3607. [Google Scholar] [CrossRef] [PubMed]
- Davis, I.D.; Martin, A.J.; Zielinski, R.R.; Thomson, A.; Tan, T.H.; Sandhu, S.; Reaume, M.N.; Pook, D.W.; Parnis, F.; North, S.A.; et al. Updated overall survival outcomes in ENZAMET (ANZUP 1304), an international, cooperative group trial of enzalutamide in metastatic hormone-sensitive prostate cancer (mHSPC). J. Clin. Oncol. 2022, 40 (Suppl. 17), LBA5004. [Google Scholar] [CrossRef]
- Deek, M.P.; Van der Eecken, K.; Phillips, R.; Parikh, N.R.; Isaacsson Velho, P.; Lotan, T.L.; Kishan, A.U.; Maurer, T.; GAP6 Consortium; Boutros, P.C.; et al. The Mutational Landscape of Metastatic Castration-sensitive Prostate Cancer: The Spectrum Theory Revisited. Eur. Urol. 2021, 80, 632–640. [Google Scholar] [CrossRef]
- Stopsack, K.H.; Nandakumar, S.; Wibmer, A.G.; Haywood, S.; Weg, E.S.; Barnett, E.S.; Kim, C.J.; Carbone, E.A.; Vasselman, S.E.; Nguyen, B.; et al. Oncogenic Genomic Alterations, Clinical Phenotypes, and Outcomes in Metastatic Castration-Sensitive Prostate Cancer. Clin. Cancer Res. 2020, 26, 3230–3238. [Google Scholar] [CrossRef]
- Makarov, D.V.; Humphreys, E.B.; Mangold, L.A.; Carducci, M.A.; Partin, A.W.; Eisenberger, M.A.; Walsh, P.C.; Trock, B.J. The natural history of men treated with deferred androgen deprivation therapy in whom metastatic prostate cancer developed following radical prostatectomy. J. Urol. 2008, 179, 156–161; discussion 161–162. [Google Scholar] [CrossRef]
- Tripathi, A.; Chen, Y.-H.; Jarrard, D.F.; Hahn, N.M.; Garcia, J.A.; Dreicer, R.; Liu, G.; Hussain, M.H.A.; Shevrin, D.H.; Cooney, M.M.; et al. Eight-year survival rates by baseline prognostic groups in patients with metastatic hormone-sensitive prostate cancer (mHSPC): An analysis from the ECOG-ACRIN 3805 (CHAARTED) trial. J. Clin. Oncol. 2022, 40 (Suppl. 16), 5081. [Google Scholar] [CrossRef]
- Hamid, A.A.; Gray, K.P.; Shaw, G.; MacConaill, L.E.; Evan, C.; Bernard, B.; Loda, M.; Corcoran, N.M.; Van Allen, E.M.; Choudhury, A.D.; et al. Compound Genomic Alterations of TP53, PTEN, and RB1 Tumor Suppressors in Localized and Metastatic Prostate Cancer. Eur. Urol. 2019, 76, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Sutera, P.; Van Der Eecken, K.; Kishan, A.U.; Hamid, A.; Grist, E.; Attard, G.; Lotan, T.; Mendes, A.A.; Paller, C.J.; Carducci, M.A.; et al. Definitions of disease burden across the spectrum of metastatic castration-sensitive prostate cancer: Comparison by disease outcomes and genomics. Prostate Cancer Prostatic Dis. 2022, 25, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Miszczyk, M.; Rajwa, P.; Yanagisawa, T.; Nowicka, Z.; Shim, S.R.; Laukhtina, E.; Kawada, T.; von Deimling, M.; Pradere, B.; Rivas, J.G.; et al. The Efficacy and Safety of Metastasis-directed Therapy in Patients with Prostate Cancer: A Systematic Review and Meta-analysis of Prospective Studies. Eur. Urol. 2024, 85, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Deek, M.P.; Van der Eecken, K.; Sutera, P.; Deek, R.A.; Fonteyne, V.; Mendes, A.A.; Decaestecker, K.; Kiess, A.P.; Lumen, N.; Phillips, R.; et al. Long-Term Outcomes and Genetic Predictors of Response to Metastasis-Directed Therapy Versus Observation in Oligometastatic Prostate Cancer: Analysis of STOMP and ORIOLE Trials. J. Clin. Oncol. 2022, 40, 3377–3382. [Google Scholar] [CrossRef]
- James, N.D.; Tannock, I.; N’Dow, J.; Feng, F.; Gillessen, S.; Ali, S.A.; Trujillo, B.; Al-Lazikani, B.; Attard, G.; Bray, F.; et al. The Lancet Commission on prostate cancer: Planning for the surge in cases. Lancet 2024, 403, 1683–1722, Erratum in Lancet 2024, 403, 1634. [Google Scholar] [CrossRef]
- Litvin, V.; Aprikian, A.G.; Dragomir, A. Cost-Effectiveness Analysis of Contemporary Advanced Prostate Cancer Treatment Sequences. Curr. Oncol. 2025, 32, 240. [Google Scholar] [CrossRef]
- Yoo, M.; Nelson, R.E.; Haaland, B.; Dougherty, M.; Cutshall, Z.A.; Kohli, R.; Beckstead, R.; Kohli, M. Cost-effectiveness analysis of 7 treatments in metastatic hormone-sensitive prostate cancer: A public-payer perspective. JNCI J. Natl. Cancer Inst. 2023, 115, 1374–1382. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ameyaw, D.; Obeng, G.D.; Amuah, R.; Józwiak-Hagymásy, J.; Dóczi, T.; Mezei, D.; Németh, B.; Tordai, A.; Alanya, A.; et al. Systematic Literature Review on Economic Evaluations and Health Economic Models in Metastatic Castration-Sensitive Prostate Cancer. Curr. Oncol. 2025, 32, 412. [Google Scholar] [CrossRef]
- Zhao, J.; Tang, B.; Shen, P.; Zeng, H.; Wei, Q. Empowering PARP inhibition through rational combination: Mechanisms of PARP inhibitors and combinations with a focus on the treatment of metastatic castration-resistant prostate cancer. Crit. Rev. Oncol. 2025, 210, 104698. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Therapy | Design | Arms of Treatment | No of Patients | MFS | OS | FFP | PFS |
|---|---|---|---|---|---|---|---|---|
| DEFINITIVE TREATMENT | ||||||||
| Attard, 2022 (STAMPEDE platform) [18] | RT + ABI | Phase III RCT, 1974 high-risk men | A: RT + ADT alone B: RT + ADT + ABI | A: 988 pts B: 986 pts | 82% vs. 69% vs. 6-yr MFS | 86% vs. 77% vs. 6-yr OS | __ | __ |
| NCT02446444 (ENZARAD trial) ongoing | RT + ENZA | Phase III RCT, 802 non-metastatic high-risk or N1 | A: RT + ENZA B: RT + ADT | 802 pts | __ | ongoing | ongoing | __ |
| NCT04136353 (DASL-HiCaP trial) ongoing | RT + DARO | Phase III RCT, 1100 non metastatic high-risk men | A: RT + ADT + DARO B: RT + ADT | 110 pts | ongoing | ongoing | ongoing | __ |
| NCT02531516 (ATLAS trial) ongoing | RT + APA | Phase III RCT, 1503 non metastatic high-risk men | A: RT + APA + ADT B: RT + ADT | 1503 pts | ongoing | ongoing | ongoing | ongoing |
| SALVAGE TREATMENT | ||||||||
| Freedland, 2023 (EMBARK trial) [19] | ENZA | Phase III RCT, 1068 high-risk men with biochemical recurrence | A: ENZA + ADT B: ADT alone C: ENZA alone | A: 355 pts B: 358 pts C: 355 pts | 87.3% vs. 71.4% vs. 80% 5-yr MFS | 92% vs. 87.2% vs. 89.5% 5-yr OS | __ | __ |
| Tran, 2022 (SALV-ENZA trial) [20] | SRT + ENZA | Phase II RCT, 86 high-risk men with biochemical recurrence | A: SRT + ENZA B: SRT alone | A: 43 pts B:43 pts | __ | __ | 66% vs. 84% 2-yr FFP | __ |
| Nguyen, 2023 (FORMULA-509) [21] | SRT + ABI + APA | RCT, 345 high-risk men with biochemical recurrence | A: SRT + ADT6m + BI B: SRT + ADT6m + ABI + APA | A: 172 B: 173 | A: 66% vs. B: 84% 3-yr MFS | __ | __ | 69% vs. 75% 3-yr PFS |
| Posadas, 2024 (STEEL trial) [22] | SRT + ADT + ENZA | Phase II RCT, 188 high-risk men with biochemical recurrence | A: SRT + ADT B: SRT + ENZA | A: 94 pts B: 94 pts | __ | __ | __ | HR PFS for arm B: 0.72 one side p = 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lancia, A.; Oderda, M.; Camilli, F.; Festa, E.; Bottero, M.; Alì, E.; La Mattina, S.; Bonzano, E.; Saddi, J.; Detti, B.; et al. Recent Advances in Androgen Receptor Pathway Inhibitors for Castration-Sensitive Prostate Cancer. Pharmaceuticals 2025, 18, 1697. https://doi.org/10.3390/ph18111697
Lancia A, Oderda M, Camilli F, Festa E, Bottero M, Alì E, La Mattina S, Bonzano E, Saddi J, Detti B, et al. Recent Advances in Androgen Receptor Pathway Inhibitors for Castration-Sensitive Prostate Cancer. Pharmaceuticals. 2025; 18(11):1697. https://doi.org/10.3390/ph18111697
Chicago/Turabian StyleLancia, Andrea, Marco Oderda, Federico Camilli, Eleonora Festa, Marta Bottero, Emanuele Alì, Salvatore La Mattina, Elisabetta Bonzano, Jessica Saddi, Beatrice Detti, and et al. 2025. "Recent Advances in Androgen Receptor Pathway Inhibitors for Castration-Sensitive Prostate Cancer" Pharmaceuticals 18, no. 11: 1697. https://doi.org/10.3390/ph18111697
APA StyleLancia, A., Oderda, M., Camilli, F., Festa, E., Bottero, M., Alì, E., La Mattina, S., Bonzano, E., Saddi, J., Detti, B., Santos Hernandez, D. A., & Ingrosso, G. (2025). Recent Advances in Androgen Receptor Pathway Inhibitors for Castration-Sensitive Prostate Cancer. Pharmaceuticals, 18(11), 1697. https://doi.org/10.3390/ph18111697







