Antifungal Mechanism Effect of Artemisinin on Fusarium solani
Abstract
1. Introduction
2. Results
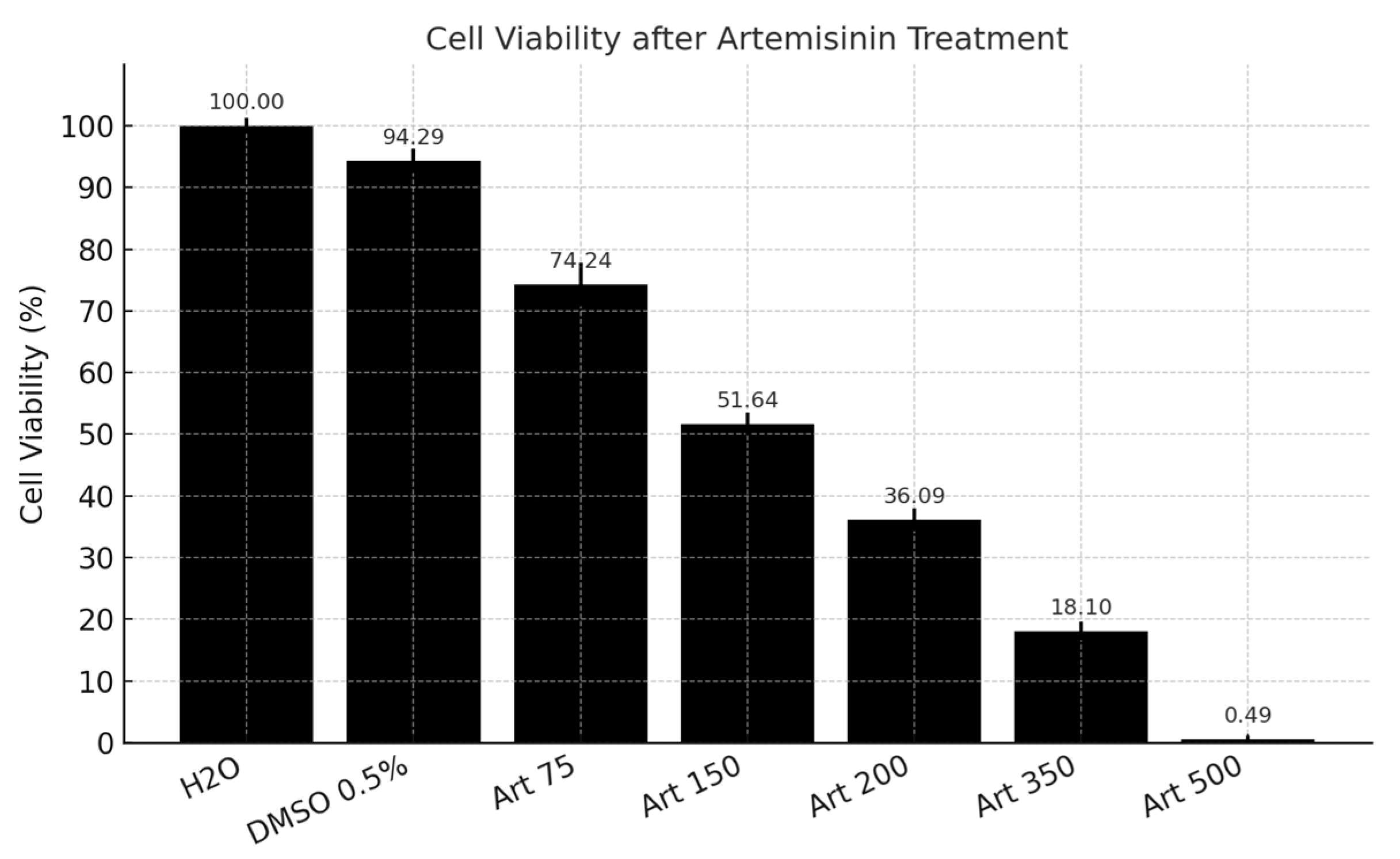
2.1. Cytotoxicity Assay
2.2. In Situ ART-Induced Membrane Permeability
2.3. Oxidative Damage as the Mechanism of ART Fungicidal Activity
3. Discussion
4. Materials and Methods
4.1. Fungal Strain
4.2. Compound Preparation and Fungal Inhibition Assay
4.3. Confocal Scanning Laser Microscopy
4.4. Carbonylation Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ART | Artemisinin |
| Fs | Fusarium solani |
References
- Gomez, L.B.; Ward, T.J.; Badiale-Furlong, E.; Del Ponte, E.M. Species composition, toxigenic potential and pathogenicity of Fusarium graminearum species complex isolates from southern Brazilian rice. Plant Pathol. 2014, 64, 980–987. [Google Scholar] [CrossRef]
- Moreno, G.; Arenas, R. Other fungi causing onychomycosis. Clin. Dermatol. 2010, 28, 160–163. [Google Scholar] [CrossRef]
- Bongomin, F.; Batac, C.; Richardson, M.D.; Denning, D.W. A review of onychomycosis due to Aspergillus species. Mycopathologia 2018, 183, 485–493. [Google Scholar] [CrossRef]
- Szaliński, M.; Zgryźniak, A.; Rubisz, I.; Gajdzis, M.; Kaczmarek, R.; Przeździecka-Dołyk, J. Fusarium keratitis—Review of current treatment possibilities. J. Clin. Med. 2021, 10, 5468. [Google Scholar] [CrossRef]
- Dursun, D.; Ozdek, S.; Mistik, S.; Tamer, C.; Ozturk, F. Advanced Fusarium keratitis progressing to endophthalmitis. Cornea 2003, 22, 300–303. [Google Scholar] [CrossRef]
- Nucci, M.; Anaissie, E. Invasive fusariosis. Clin. Microbiol. Rev. 2023, 36, e0015922. [Google Scholar] [CrossRef] [PubMed]
- Gambhir, N.; Harris, S.D.; Everhart, S.E. Evolutionary significance of fungal hypermutators: Lessons learned from clinical strains and implications for fungal plant pathogens. mSphere 2022, 7, e0008722. [Google Scholar] [CrossRef] [PubMed]
- Meza-Menchaca, T.; Singh, R.K.; Quiroz-Chávez, J.; García-Pérez, L.M.; Rodríguez-Mora, N.; Soto-Luna, M.; Gastélum-Contreras, G.; Vanzzini-Zago, V.; Sharma, L.; Quiroz-Figueroa, F.R. First demonstration of clinical Fusarium strains causing cross-kingdom infections from humans to plants. Microorganisms 2020, 8, 947. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Liu, F.; Liang, J.; Zhao, P.; Tsui, C.K.; Cai, L. Cross-kingdom synthetic microbiota supports tomato suppression of Fusarium wilt disease. Nat. Commun. 2022, 13, 7890. [Google Scholar] [CrossRef]
- Su, X.Z.; Miller, L.H. The discovery of artemisinin and the Nobel Prize in Physiology or Medicine. Sci. China Life Sci. 2015, 58, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Li, W.; Wong, N.K.; Xue, F.; Li, Q.; Zhang, Y.; Xu, J.; Deng, Z.; Zhou, Y. Discovery of artemisinins as microsomal prostaglandin synthase-2 inhibitors for the treatment of colorectal cancer via chemoproteomics. J. Med. Chem. 2024, 67, 2083–2094. [Google Scholar] [CrossRef]
- Meza-Menchaca, T.; Ramos-Ligonio, A.; López-Monteon, A.; Vidal Limón, A.; Kaluzhskiy, L.A.; Shkel, T.V.; Strushkevich, N.V.; Jiménez-García, L.F.; Agredano Moreno, L.T.; Gallegos-García, V.; et al. Insights into ergosterol peroxide’s trypanocidal activity. Biomolecules 2019, 9, 484. [Google Scholar] [CrossRef]
- Espinoza, C.; González, M.C.R.; Mendoza, G.; Creus, A.H.; Trigos, Á.; Fernández, J.J. Exploring photosensitization as an efficient antifungal method. Sci. Rep. 2018, 8, 14489. [Google Scholar] [CrossRef]
- Atta, S.; Perera, C.; Kowalski, R.P.; Jhanji, V. Fungal Keratitis: Clinical Features, Risk Factors, Treatment, and Outcomes. J. Fungi 2022, 8, 962. [Google Scholar] [CrossRef]
- Li, K.M.; Dong, X.; Ma, Y.N.; Wu, Z.H.; Yan, Y.M.; Cheng, Y.X. Antifungal coumarins and lignans from Artemisia annua. Fitoterapia 2019, 134, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.N.; Chen, C.J.; Li, Q.Q.; Xu, F.R.; Cheng, Y.X.; Dong, X. Monitoring antifungal agents of Artemisia annua against Fusarium oxysporum and Fusarium solani, associated with Panax notoginseng root-rot disease. Molecules 2019, 24, 213. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.P.; Guo, Y.T.; Wang, J.W.; Tan, R.X. Nitric oxide potentiates oligosaccharide-induced artemisinin production in Artemisia annua hairy roots. J. Integr. Plant Biol. 2008, 50, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, S.; Saxena, P.; Alam, P.; Wajid, S.; Abdin, M.Z. Modulation of artemisinin biosynthesis by elicitors, inhibitor, and precursor in hairy root cultures of Artemisia annua L. J. Plant Interact. 2014, 9, 811–824. [Google Scholar] [CrossRef]
- Bertea, C.M.; Freije, J.R.; van der Woude, H.; Verstappen, F.W.A.; Perk, L.; Marquez, V.; De Kraker, J.-W.; Posthumus, M.A.; Jansen, B.J.M.; de Groot, A.; et al. Identification of intermediates and enzymes involved in the early steps of artemisinin biosynthesis in Artemisia annua. Planta Med. 2005, 71, 40–47. [Google Scholar] [CrossRef]
- Woerdenbag, H.J.; Bos, R.; Salomons, M.C.; Hendriks, H.; Pras, N.; Malingré, T.M. Volatile constituents of Artemisia annua L. (Asteraceae). Flavour Fragr. J. 1993, 8, 131–137. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, N.; Jian, D.; Jiang, R.; Yang, C.; Lan, X.; Chen, M.; Zhang, F.; Liao, Z. Overexpression of AaPIF3 promotes artemisinin production in Artemisia annua. Ind. Crops Prod. 2019, 138, 111476. [Google Scholar] [CrossRef]
- Ferrer, C.; Alio, J.; Rodriguez, A.; Andreu, M.; Colom, F. Endophthalmitis caused by Fusarium proliferatum. J. Clin. Microbiol. 2005, 43, 5372–5375. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Xie, Z.; Huo, H.; Jia, P.; Zhang, A.; Liang, J.; Wang, J. Anti-Fusarium activity of essential oil distilled from artemisinin (Artemisia annua L.) extraction residues. S. Afr. J. Bot. 2023, 158, 180–189. [Google Scholar] [CrossRef]
- Numonov, S.; Sharopov, F.; Salimov, A.; Sukhrobov, P.; Atolikshoeva, S.; Safarzoda, R.; Habasi, M.; Aisa, H. Assessment of artemisinin contents in selected Artemisia species from Tajikistan (Central Asia). Medicines 2019, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Čavar Željković, S.; Maksimović, M.; Vidic, D. Chemical Composition and Antioxidant and Antimicrobial Activity of Essential Oil of Artemisia annua L. from Bosnia. Ind. Crops Prod. 2012, 37, 479–485. [Google Scholar] [CrossRef]
- El-Naggar, E.B.; Azazi, M.; Švajdlenka, E.; Žemlička, M. Artemisinin from Minor to Major Ingredient in Artemisia annua. J. Appl. Pharm. Sci. 2013, 3, 116–123. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza, C.; Quiroz-Figueroa, F.R.; Guzmán-López, O.; Ruiz-May, E.; Gallegos-García, V.; Salinas-Castro, A.; García-Serrano, G.; Meza-Menchaca, T. Antifungal Mechanism Effect of Artemisinin on Fusarium solani. Pharmaceuticals 2025, 18, 1696. https://doi.org/10.3390/ph18111696
Espinoza C, Quiroz-Figueroa FR, Guzmán-López O, Ruiz-May E, Gallegos-García V, Salinas-Castro A, García-Serrano G, Meza-Menchaca T. Antifungal Mechanism Effect of Artemisinin on Fusarium solani. Pharmaceuticals. 2025; 18(11):1696. https://doi.org/10.3390/ph18111696
Chicago/Turabian StyleEspinoza, César, Francisco Roberto Quiroz-Figueroa, Oswaldo Guzmán-López, Eliel Ruiz-May, Verónica Gallegos-García, Alejandro Salinas-Castro, Giovanny García-Serrano, and Thuluz Meza-Menchaca. 2025. "Antifungal Mechanism Effect of Artemisinin on Fusarium solani" Pharmaceuticals 18, no. 11: 1696. https://doi.org/10.3390/ph18111696
APA StyleEspinoza, C., Quiroz-Figueroa, F. R., Guzmán-López, O., Ruiz-May, E., Gallegos-García, V., Salinas-Castro, A., García-Serrano, G., & Meza-Menchaca, T. (2025). Antifungal Mechanism Effect of Artemisinin on Fusarium solani. Pharmaceuticals, 18(11), 1696. https://doi.org/10.3390/ph18111696







