Ozone-Mediated Modulation of Green Tea Extract Enhances Bioactive Compounds and Therapeutic Potential Relevant to Human Health
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenolic and Flavonoid Profile of Green Tea Extract
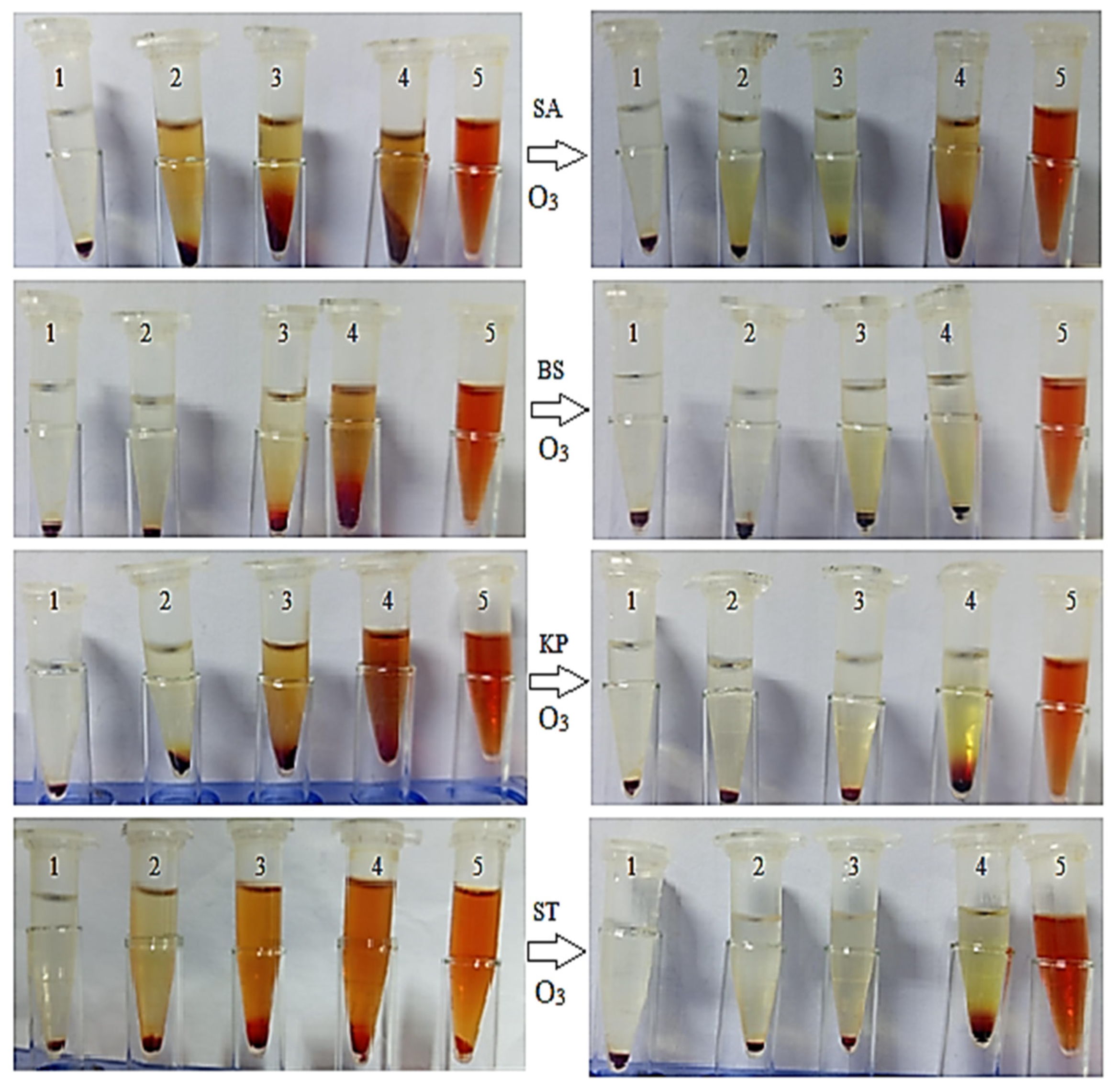
2.2. Antimicrobial Efficacy of Green Tea Extract
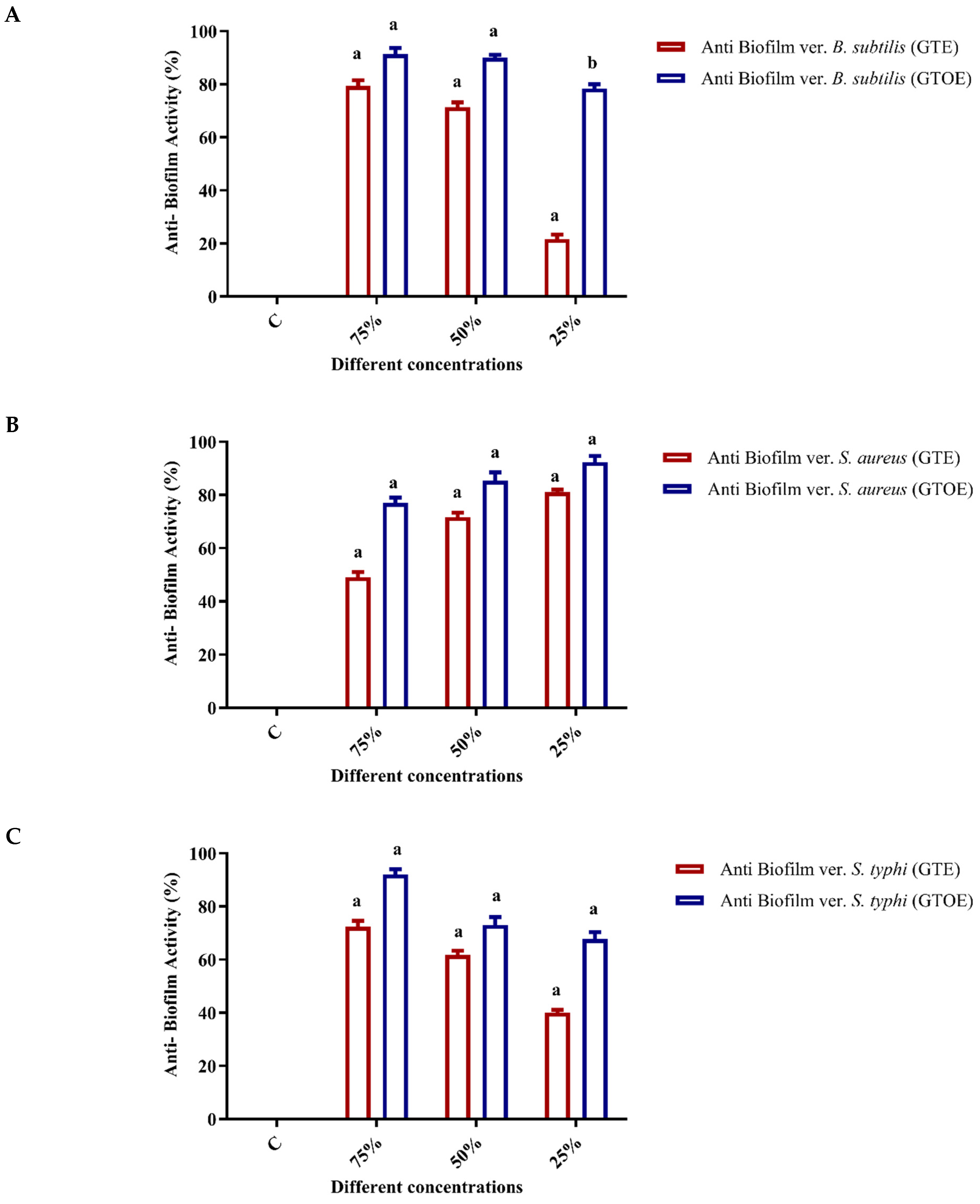
2.3. Anti-Biofilm Activity of Green Tea Extract
2.4. Bactericidal Kinetics of Green Tea Extract
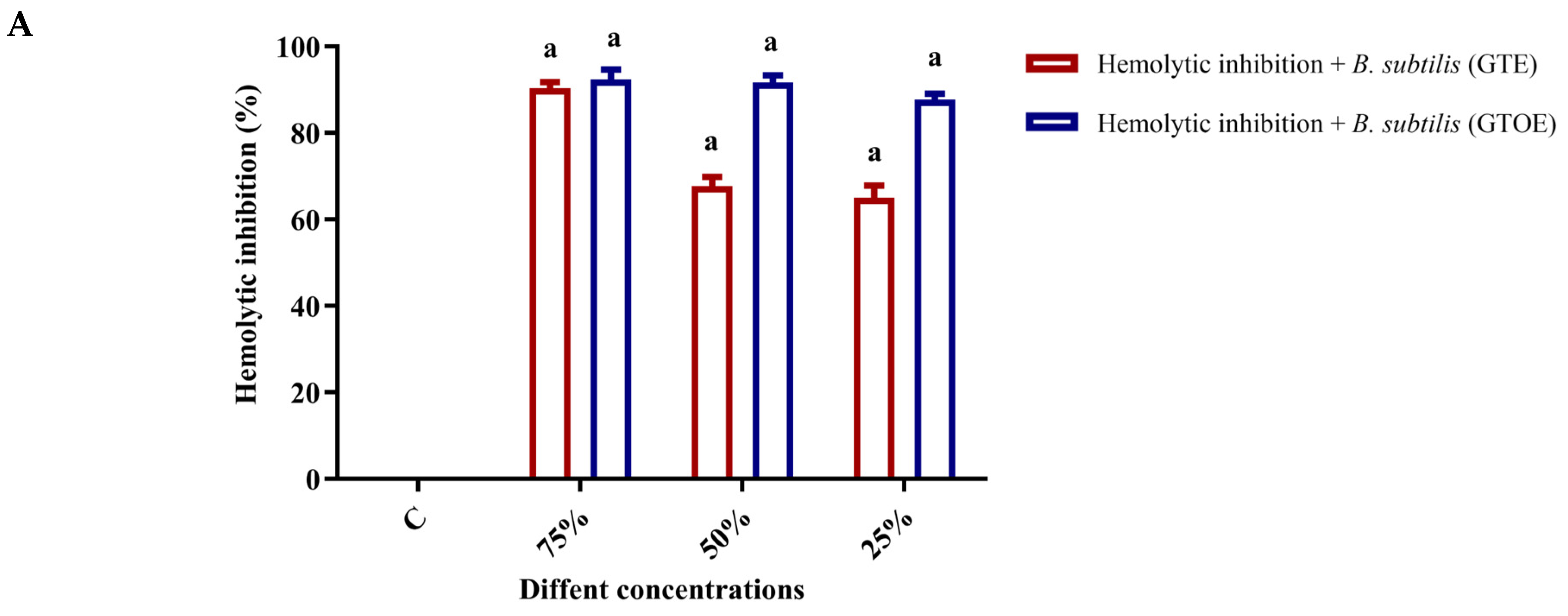
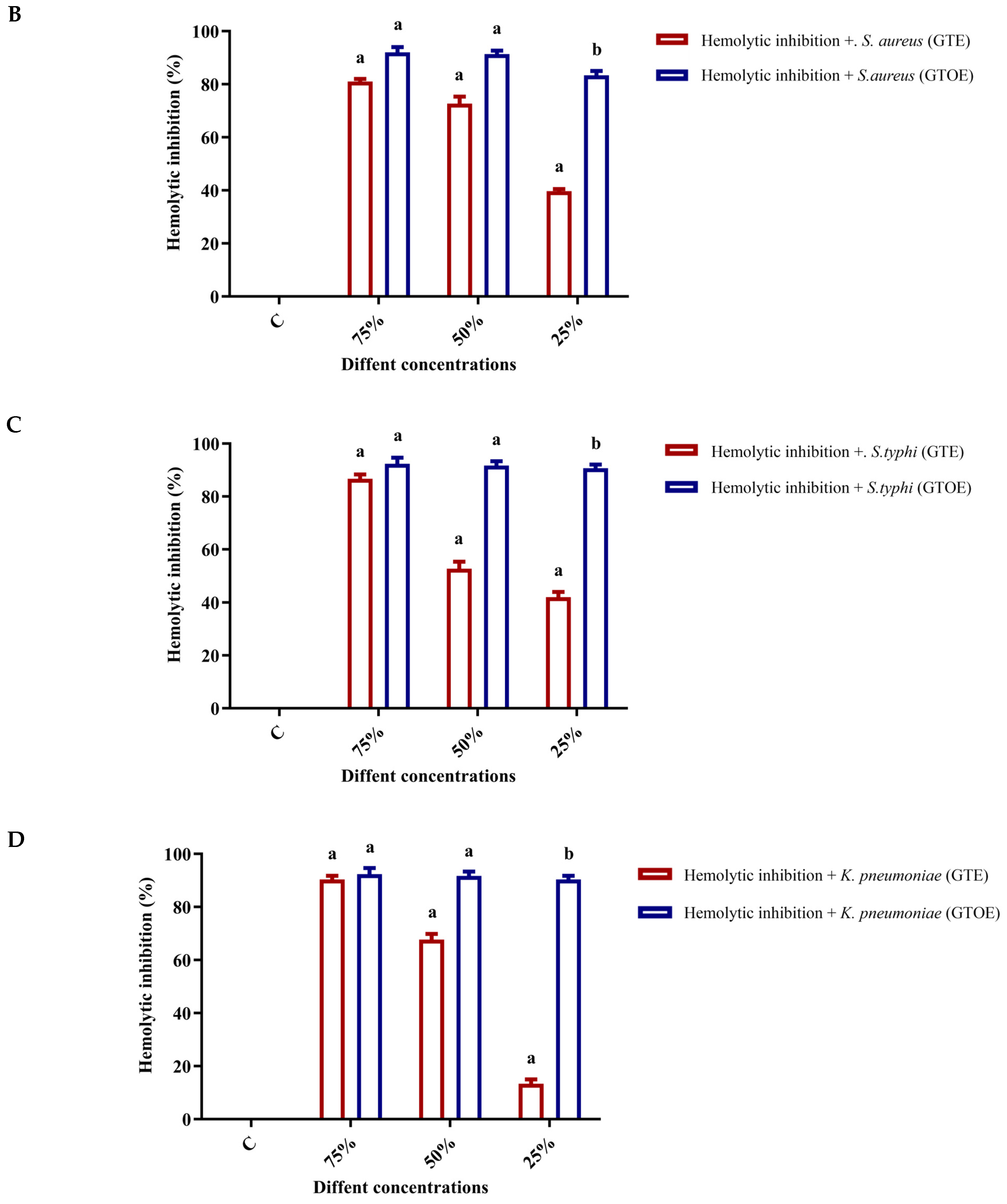
2.5. Anti-Hemolytic Activity of Ozonated Green Tea Extract
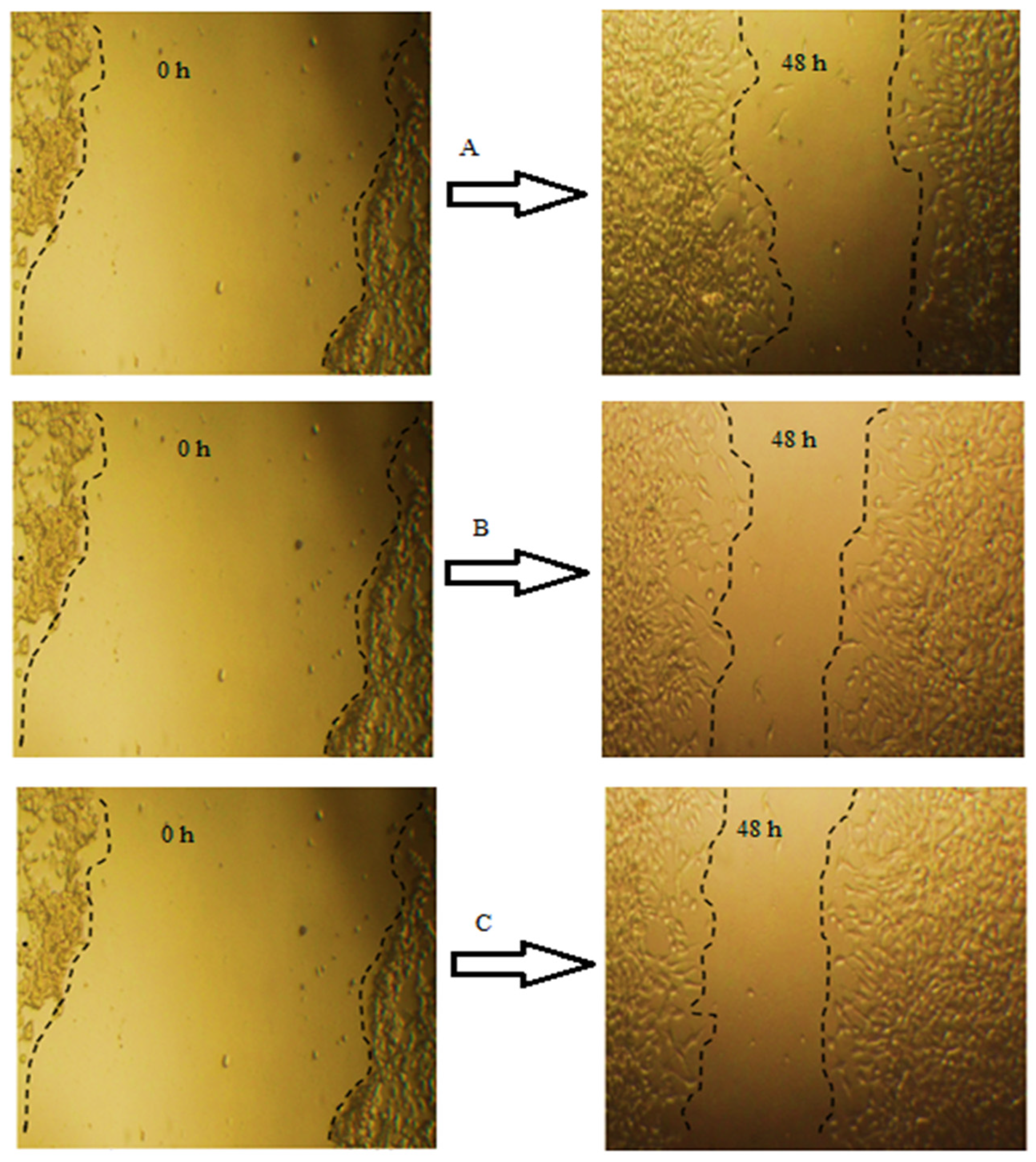
2.6. Wound Healing Properties of GTE and GTOE
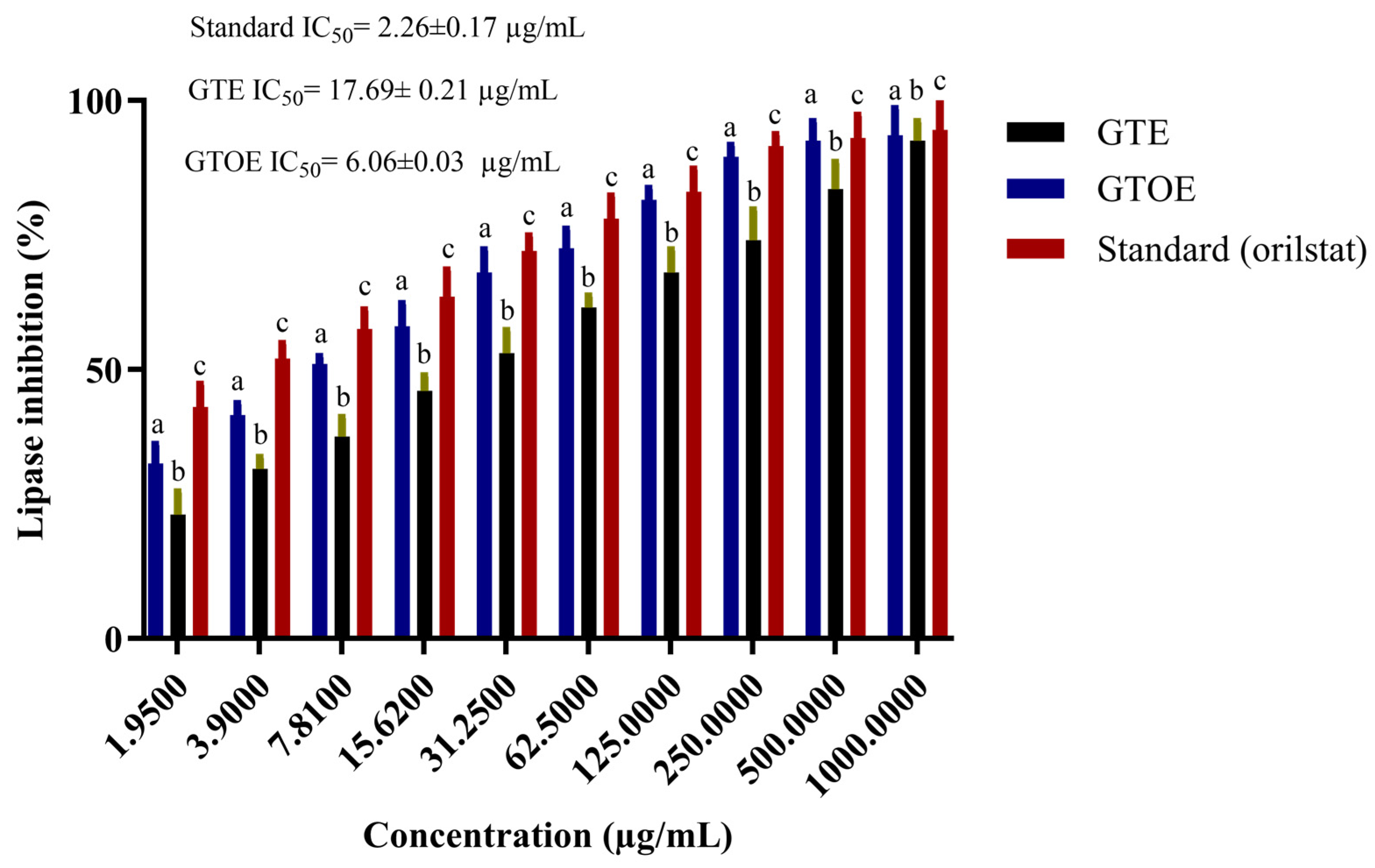
2.7. Potential Anti-Obesity Activities of Green Tea Extract
2.8. Antioxidant Potential of Green Tea Extract
3. Materials and Methods
3.1. Chemicals and Green Tea
3.2. Ozonation of Green Tea Extract
3.3. High-Performance Liquid Chromatography (HPLC) Analysis
3.4. Evaluation of Antimicrobial Activity
3.5. Assessment of Antibiofilm Activity
3.6. Time-Kill Kinetics Assay
3.7. Evaluation of Anti-Hemolytic Activity
3.8. Scratch Assay for Wound Healing Evaluation
3.9. Assessment of Potential Anti-Obesity Activity via Pancreatic Lipase Inhibition
3.10. Determination of Antioxidant Activity Using the DPPH Radical Scavenging Assay
3.11. Statistical Examination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radeva-Ilieva, M.; Stoeva, S.; Hvarchanova, N.; Georgiev, K.D. Green Tea: Current Knowledge and Issues. Foods 2025, 14, 745. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Luo, L.; Zhao, J.; Wang, Y.; Luo, H. Biological potential and mechanisms of Tea’s bioactive compounds: An Updated review. J. Adv. Res. 2024, 65, 345–363. [Google Scholar] [CrossRef]
- Rudolphi-Skórska, E.; Dyba, B.; Kreczmer, B.; Filek, M. Impact of polyphenol-rich green tea extracts on the protection of DOPC monolayer against damage caused by ozone induced lipid oxidation. Acta Biochim. Pol. 2018, 65, 193–197. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Y.; Zhang, X.; Wu, Y.; Zhang, L.; Liu, G.; Fu, J.; Li, X.; Mu, D.; Li, Z. Elevated Ozone Reduces the Quality of Tea Leaves but May Improve the Resistance of Tea Plants. Plants 2024, 13, 1108. [Google Scholar] [CrossRef]
- Alawlaqi, M.M.; Al-Rajhi, A.M.H.; Abdelghany, T.M.; Ganash, M.; Moawad, H. Evaluation of Biomedical Applications for Linseed Extract: Antimicrobial, Antioxidant, Anti-Diabetic, and Anti-Inflammatory Activities In Vitro. J. Funct. Biomater. 2023, 14, 300. [Google Scholar] [CrossRef]
- Alsolami, A.; Bazaid, A.S.; Alshammari, M.A.; Qanash, H.; Amin, B.H.; Bakri, M.M.; Abdelghany, T.M. Ecofriendly fabrication of natural jojoba nanoemulsion and chitosan/jojoba nanoemulsion with studying the antimicrobial, anti-biofilm, and anti-diabetic activities in vitro. Biomass Convers. Biorefin. 2025, 15, 1283–1294. [Google Scholar] [CrossRef]
- Qanash, H.; Bazaid, A.S.; Aldarhami, A.; Alharbi, B.; Almashjary, M.N.; Hazzazi, M.S.; Felemban, H.R.; Abdelghany, T.M. Phytochemical Characterization and Efficacy of Artemisia judaica Extract Loaded Chitosan Nanoparticles as Inhibitors of Cancer Proliferation and Microbial Growth. Polymers 2023, 15, 391. [Google Scholar] [CrossRef] [PubMed]
- Bazaid, A.S.; Binsaleh, N.K.; Barnawi, H.; Alharbi, B.; Alsolami, A.; Selim, S.; Al Jaouni, S.K.; Saddiq, A.A.; Ganash, M.; Abdelghany, T.M.; et al. Unveiling the in vitro activity of extracted Euphorbia trigona via Supercritical Fluid Extraction against pathogenic yeasts, obesity, cancer, and its wound healing properties. Bioresour. Bioprocess. 2025, 12, 28. [Google Scholar] [CrossRef]
- Suzuki, T.; Pervin, M.; Goto, S.; Isemura, M.; Nakamura, Y. Beneficial Effects of Tea and the Green Tea Catechin Epigallocatechin-3-gallate on Obesity. Molecules 2016, 21, 1305. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, H.; Byun, S. A review on the effect of green tea extract against obesity. Food Sci. Biotechnol. 2025, 34, 1661–1678. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Binsaleh, N.K.; Bazaid, A.S.; Barnawi, H.; Alharbi, B.; Alsolami, A.; Babsail, A.Y.; Selim, S.; Abdelghany, T.M.; Elbaz, R.M.; Qanash, H. Chemical characterization, anticancer, antioxidant and anti-obesity activities with molecular docking studies of Pleurotus ostreatus biomass exposed to moist heat. J. Food Meas. Charact. 2025, 19, 2476–2495. [Google Scholar] [CrossRef]
- Wakamatsu, M.; Yamanouchi, H.; Sahara, H.; Iwanaga, T.; Kuroda, R.; Yamamoto, A.; Minami, Y.; Sekijima, M.; Yamada, K.; Kajiya, K. Catechin and caffeine contents in green tea at different harvest periods and their metabolism in miniature swine. Food Sci. Nutr. 2019, 7, 2769–2778. [Google Scholar] [CrossRef]
- Candela, L.; Formato, M.; Crescente, G.; Piccolella, S.; Pacifico, S. Coumaroyl Flavonol Glycosides and More in Marketed Green Teas: An Intrinsic Value beyond Much-Lauded Catechins. Molecules 2020, 25, 1765. [Google Scholar] [CrossRef]
- Sumpio, B.E.; Cordova, A.C.; Berke-Schlessel, D.W.; Qin, F.; Chen, Q.H. Green tea, the “Asian paradox,” and cardiovascular disease. J. Am. Coll. Surg. 2006, 202, 813–825. [Google Scholar] [CrossRef]
- Alsalamah, S.A.; Alghonaim, M.I.; Abdelghany, T.M.; Almehayawi, M.S.; Selim, S.; Alharbi, M.T. Effect of UV-C radiation on chemical profile and pharmaceutical application in vitro of Aloe vera oil. AMB Express 2025, 15, 83. [Google Scholar] [CrossRef] [PubMed]
- Alsalamah, S.A.; Alghonaim, M.I.; Mohammad, A.M.; Khalel, A.F.; Alqahtani, F.S.; Abdelghany, T.M. Ozone-modified properties of pumpkin seed oil as anti-H. pylori, anticancer, anti-diabetic and anti-obesity agent. Sci. Rep. 2025, 15, 25959. [Google Scholar] [CrossRef]
- Silva, V.; Peirone, C.; Amaral, J.S.; Capita, R.; Alonso-Calleja, C.; Marques-Magallanes, J.A.; Martins, Â.; Carvalho, Á.; Maltez, L.; Pereira, J.E.; et al. High Efficacy of Ozonated Oils on the Removal of Biofilms Produced by Methicillin-Resistant Staphylococcus aureus (MRSA) from Infected Diabetic Foot Ulcers. Molecules 2020, 25, 3601. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.H.; Abdelghany, T.M.; Selim, S.; Almuhayawi, M.S.; Alruhaili, M.H.; Alharbi, M.T.; Al Jaouni, S.K. Phytochemical characterization of peanut oil and its ozonized form to explore biological activities in vitro. AMB Express 2025, 15, 76. [Google Scholar] [CrossRef]
- Khalifa, K.A.; Barghoth, M.; Desouky, S.; Elsayed, H.; Roushdy, M. HIGHLY EFFICIENTLY INACTIVATION OF MICROBIAL PATHOGENS USING ADVANCED OZONE GENERATOR UNIT AS AN ECO- FRIENDLY PROMISING STRATEGY. Al-Azhar J. Pharm. Sci. 2022, 66, 193–207. [Google Scholar] [CrossRef]
- Selim, S.; Alruwaili, Y.; Ejaz, H.; Abdalla, A.E.; Almuhayawi, M.S.; Nagshabandi, M.K.; Tarabulsi, M.K.; Al Jaouni, S.K.; Bazuhair, M.A.; Abdelghany, T.M. Estimation and Action Mechanisms of Cinnamon Bark via Oxidative Enzymes and Ultrastructures as Antimicrobial, Anti-biofilm, Antioxidant, Anti-diabetic, and Anticancer Agents. BioResources 2024, 19, 7019–7041. [Google Scholar] [CrossRef]
- Tang, G.-Y.; Zhao, C.-N.; Xu, X.-Y.; Gan, R.-Y.; Cao, S.-Y.; Liu, Q.; Shang, A.; Mao, Q.-Q.; Li, H.-B. Phytochemical Composition and Antioxidant Capacity of 30 Chinese Teas. Antioxidants 2019, 8, 180. [Google Scholar] [CrossRef]
- Re, L.; Mawsouf, M.N.; Menéndez, S.; León, O.S.; Sánchez, G.M.; Hernández, F. Ozone therapy: Clinical and basic evidence of its therapeutic potential. Arch. Med. Res. 2008, 39, 17–26. [Google Scholar] [CrossRef]
- Li, C.; Wang, S.; Wang, J.; Wu, Z.; Xu, Y.; Wu, Z. Ozone treatment promotes physicochemical properties and antioxidant capacity of fresh-cut red pitaya based on phenolic metabolism. Front. Nutr. 2022, 9, 1016607. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef]
- Kuljarusnont, S.; Iwakami, S.; Iwashina, T.; Tungmunnithum, D. Flavonoids and Other Phenolic Compounds for Physiological Roles, Plant Species Delimitation, and Medical Benefits: A Promising View. Molecules 2024, 29, 5351. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Khan, M.I.; Shin, J.H.; Lee, M.G.; Seo, H.J.; Shin, T.S.; Kim, M.Y. HPLC fractionation and pharmacological assessment of green tea seed saponins for antimicrobial, anti-angiogenic and hemolytic activities. Biotechnol. Bioprocess Eng. 2015, 20, 1035–1043. [Google Scholar] [CrossRef]
- Chang, E.H.; Huang, J.; Lin, Z.; Brown, A.C. Catechin-mediated restructuring of a bacterial toxin inhibits activity. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Brown, A.C. Applications of Catechins in the Treatment of Bacterial Infections. Pathogens 2021, 10, 546. [Google Scholar] [CrossRef]
- Sasagawa, K.; Domon, H.; Sakagami, R.; Hirayama, S.; Maekawa, T.; Isono, T.; Hiyoshi, T.; Tamura, H.; Takizawa, F.; Fukushima, Y.; et al. Matcha Green Tea Exhibits Bactericidal Activity against Streptococcus pneumoniae and Inhibits Functional Pneumolysin. Antibiotics 2021, 10, 1550. [Google Scholar] [CrossRef]
- Siriphap, A.; Kiddee, A.; Duangjai, A.; Yosboonruang, A.; Pook-In, G.; Saokaew, S.; Sutheinkul, O.; Rawangkan, A. Antimicrobial Activity of the Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate (EGCG) against Clinical Isolates of Multidrug-Resistant Vibrio cholerae. Antibiotics 2022, 11, 518. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Fortino, V.; Bocci, V. The dual action of ozone on the skin. Br. J. Dermatol. 2005, 153, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Ugazio, E.; Tullio, V.; Binello, A.; Tagliapietra, S.; Dosio, F. Ozonated Oils as Antimicrobial Systems in Topical Applications. Their Characterization, Current Applications, and Advances in Improved Delivery Techniques. Molecules 2020, 25, 334. [Google Scholar] [CrossRef]
- Zahra, M.; Abrahamse, H.; George, B.P. Flavonoids: Antioxidant Powerhouses and Their Role in Nanomedicine. Antioxidants 2024, 13, 922. [Google Scholar] [CrossRef]
- Rafique, S.; Murtaza, M.A.; Hafiz, I.; Ameer, K.; Qayyum, M.M.N.; Yaqub, S.; Mohamed Ahmed, I.A. Investigation of the antimicrobial, antioxidant, hemolytic, and thrombolytic activities of Camellia sinensis, Thymus vulgaris, and Zanthoxylum armatum ethanolic and methanolic extracts. Food Sci. Nutr. 2023, 11, 6303–6311. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.Z.; Xiao, W.J.; Zhou, D.W.; Tan, C.Y.; Tan, Z.L.; Han, X.F.; Zhou, C.S.; Tang, S.X. Effect of tea catechins on regulation of cell proliferation and antioxidant enzyme expression in H2 O2 -induced primary hepatocytes of goat in vitro. J. Anim. Physiol. Anim. Nutr. 2013, 97, 475–484. [Google Scholar] [CrossRef]
- Lan, X.; Liu, Y.; Wang, Y.; Tian, F.; Miao, X.; Wang, H.; Tang, Y. Coaxial electrospun PVA/PCL nanofibers with dual release of tea polyphenols and ε-poly (L-lysine) as antioxidant and antibacterial wound dressing materials. Int. J. Pharm. 2021, 601, 120525. [Google Scholar] [CrossRef]
- Li, L.; Feng, A.c.; Lu, J.; Liu, H.; Xue, W.; Cui, H. Evaluating the therapeutic efficacy of ozone liquid dressing in healing wounds associated with bullous pemphigoid. Sci. Rep. 2025, 15, 7205. [Google Scholar] [CrossRef]
- Fitzpatrick, E.; Holland, O.J.; Vanderlelie, J.J. Ozone therapy for the treatment of chronic wounds: A systematic review. Int. Wound J. 2018, 15, 633–644. [Google Scholar] [CrossRef]
- Li, F.; Qasim, S.; Li, D.; Dou, Q.P. Updated review on green tea polyphenol epigallocatechin-3-gallate as a cancer epigenetic regulator. Semin. Cancer Biol. 2022, 83, 335–352. [Google Scholar] [CrossRef]
- Truong, V.-L.; Jeong, W.-S. Cellular Defensive Mechanisms of Tea Polyphenols: Structure-Activity Relationship. Int. J. Mol. Sci. 2021, 22, 9109. [Google Scholar] [CrossRef]
- Kurin, E.; Hajská, M.; Kostovčíková, E.; Dokupilová, K.; Mučaji, P.; Nagy, M.; Novotný, B.; Bittner Fialová, S. Unveiling Synergistic Antioxidant Effects of Green Tea and Peppermint: Role of Polyphenol Interactions and Blend Preparation. Int. J. Mol. Sci. 2025, 26, 6257. [Google Scholar] [CrossRef]
- Capasso, L.; De Masi, L.; Sirignano, C.; Maresca, V.; Basile, A.; Nebbioso, A.; Rigano, D.; Bontempo, P. Epigallocatechin Gallate (EGCG): Pharmacological Properties, Biological Activities and Therapeutic Potential. Molecules 2025, 30, 654. [Google Scholar] [CrossRef]
- Vareltzis, P.; Stergiou, A.; Kalinderi, K.; Chamilaki, M. Antioxidant Potential of Spray- and Freeze-Dried Extract from Oregano Processing Wastes, Using an Optimized Ultrasound-Assisted Method. Foods 2023, 12, 2628. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.H.; Yahya, R.; Abdelghany, T.M.; Fareid, M.A.; Mohamed, A.M.; Amin, B.H.; Masrahi, A.S. Anticancer, Anticoagulant, Antioxidant and Antimicrobial Activities of Thevetia peruviana Latex with Molecular Docking of Antimicrobial and Anticancer Activities. Molecules 2022, 27, 3165. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Hassan, M.M.; El-Naggar, M.A. GC/MS analysis of Juniperus procera extract and its activity with silver nanoparticles against Aspergillus flavus growth and aflatoxins production. Biotechnol. Rep. 2020, 27, e00496. [Google Scholar] [CrossRef] [PubMed]
- Al-Rajhi, A.M.H.; Abdelghany, T.M.; Almuhayawi, M.S.; Alruhaili, M.H.; Saddiq, A.A.; Baghdadi, A.M.; Al Jaouni, S.K.; Albasri, H.M.; Waznah, M.S.; Alraddadi, F.A.; et al. Effect of ozonation on the phytochemicals of black seed oil and its anti-microbial, anti-oxidant, anti-inflammatory, and anti-neoplastic activities in vitro. Sci. Rep. 2024, 14, 30445. [Google Scholar] [CrossRef] [PubMed]
- Al-Rajhi, A.M.H.; Qanash, H.; Almashjary, M.N.; Hazzazi, M.S.; Felemban, H.R.; Abdelghany, T.M. Anti-Helicobacter pylori, Antioxidant, Antidiabetic, and Anti-Alzheimer’s Activities of Laurel Leaf Extract Treated by Moist Heat and Molecular Docking of Its Flavonoid Constituent, Naringenin, against Acetylcholinesterase and Butyrylcholinesterase. Life 2023, 13, 1512. [Google Scholar] [CrossRef] [PubMed]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
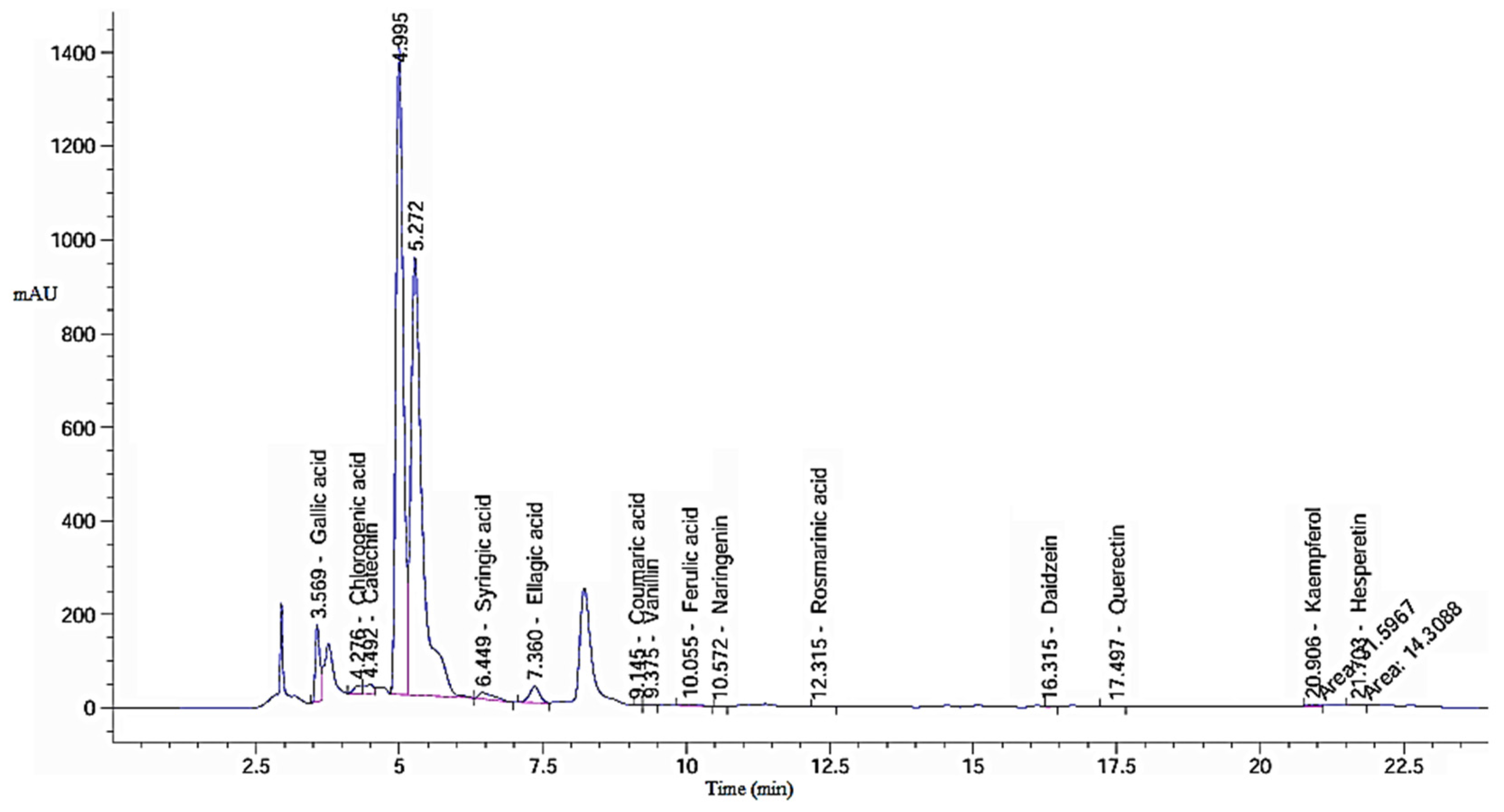
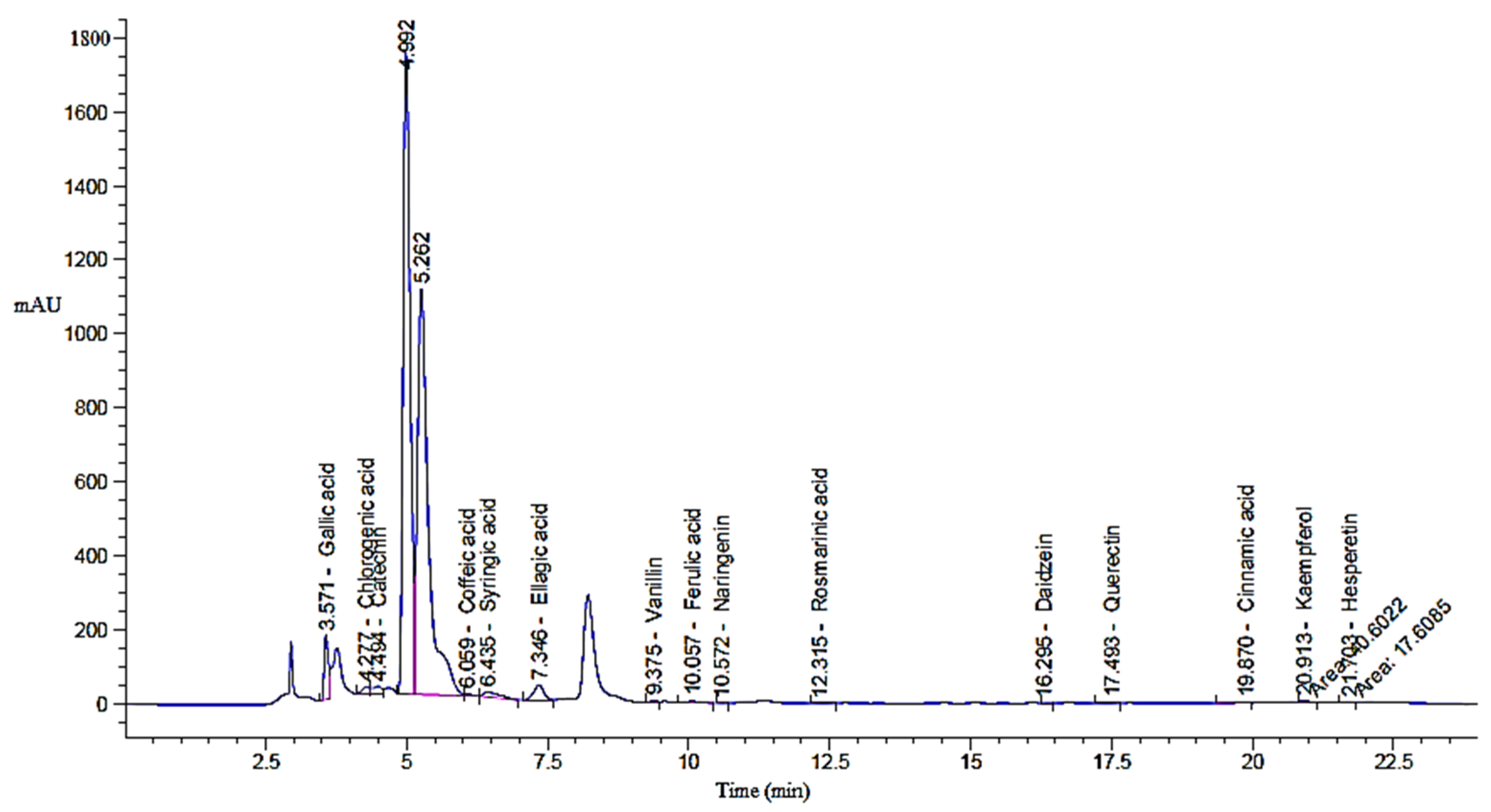


| Detected Compound | GTE | GTOE | ||||
|---|---|---|---|---|---|---|
| Area (mAU * s) | Retention Time | Concentration * (µg/g) | Area (mAU * s) | Retention Time | Concentration * (µg/g) | |
| Gallic acid | 871.62 | 3.57 | 3150.92 a | 889.50 | 3.57 | 3229.69 b |
| Chlorogenic acid | 156.63 | 4.28 | 1097.40 a | 155.53 | 4.28 | 1089.72 a |
| Catechin | 226.91 | 4.49 | 2634.09 a | 218.38 | 4.49 | 2535.09 a |
| Caffeic acid | 0.00 | 5.98 | 0.00 a | 48.48 | 6.06 | 145.11 b |
| Syringic acid | 257.72 | 6.45 | 820.94 a | 273.02 | 6.44 | 877.93 a |
| Ellagic acid | 435.65 | 7.36 | 2470.66 a | 489.62 | 7.35 | 2789.48 a |
| Coumaric acid | 0.88 | 9.15 | 1.61 a | 0.00 | 8.82 | 0.00 b |
| Vanillin | 15.27 | 9.38 | 20.85 a | 21.03 | 9.38 | 40.59 b |
| Ferulic acid | 41.59 | 10.06 | 120.25 a | 48.24 | 10.06 | 139.50 a |
| Naringenin | 1.67 | 10.57 | 8.07 a | 1.72 | 10.57 | 8.31 a |
| Rosmarinic acid | 8.76 | 12.32 | 35.90 a | 10.47 | 12.32 | 49.50 a |
| Daidzein | 11.77 | 16.32 | 33.64 a | 11.44 | 16.30 | 32.71 a |
| Quercetin | 17.86 | 17.50 | 112.65 a | 18.53 | 17.49 | 122.96 a |
| Cinnamic acid | 0.00 | 19.77 | 0.00 a | 9.42 | 19.87 | 10.20 b |
| Kaempferol | 31.60 | 20.91 | 161.98 a | 40.60 | 20.91 | 208.15 a |
| Hesperetin | 871.62 | 21.70 | 33.83 a | 17.61 | 21.70 | 41.63 a |
| Investigated Microorganisms | Zone of Inhibition (mm) | MIC (μg/mL) | MBC or MFC (μg/mL) | ||||
|---|---|---|---|---|---|---|---|
| GTE | GTOE | Control | GTE | GTOE | GTE | GTOE | |
| B. subtilis | 13 ± 0.1 | 16 ± 0.3 | 20 ± 0.6 | 31.25 ±0.4 | 15.62 ± 0.2 | 125 ± 0.6 | 31.25 ± 0.1 |
| S. aureus | 19 ± 0.4 | 21 ± 0.7 | 15 ± 0.3 | 62.5 ± 0.6 | 15.62 ± 0.6 | 125 ± 0.6 | 31.25 ± 0.5 |
| K. pneumoniae | 16 ± 0.6 | 20 ± 0.5 | 15 ± 0.5 | 62.5 ± 0.3 | 15.62 ± 0.3 | 125 ± 0.6 | 15.62 ± 0.4 |
| S. typhi | 15 ± 0.8 | 22 ± 0.2 | 16 ± 0.3 | 31.25 ± 0.2 | 15.62 ± 0.2 | 62.5 ± 0.6 | 31.25 ± 0.3 |
| C. albicans | 13 ± 0.3 | 18 ± 0.1 | 21 ± 0.8 | 62.5 ± 0.6 | 31.25 ± 0.2 | 500 ± 0.6 | 125 ± 0.6 |
| A. niger | NA | NA | 30 ± 0.7 | -- | -- | -- | -- |
| Killing Kinetic Time (min) | BS | SA | KP | ST | ||||
|---|---|---|---|---|---|---|---|---|
| GTE | GTOE | GTE | GTOE | GTE | GTOE | GTE | GTOE | |
| 0 | 38 × 105 a ± 1 | 38 × 105 a ± 1 | 24 × 105 a ± 1 | 24 × 105 a ± 1 | 19 × 105 a ± 2 | 19 × 105 a ± 2 | 25 × 105 a ± 1 | 25 × 105 a ± 1 |
| 30 | 150 × 103 a ± 3 | 17 × 103 b ± 2 | 172 × 103 a ± 1 | 121 × 103 a ± 1 | 125 × 104 a ± 1 | 110 × 104 a ± 1 | 211 × 104 a ± 2 | 174 × 104 a ± 2 |
| 60 | 113 × 102 a ± 2 | 21 × 102 b ± 1 | 27 × 103 a ± 2 | 142 × 102 b ± 1 | 171 × 103 a ± 2 | 197 × 102 a ± 1 | 19 × 103 a ± 2 | 32 × 102 b ± 1 |
| 120 | 154 × 10 a ± 1 | 14 b ± 1 | 30 × 102 a ± 2 | 230 b ± 1 | 124 × 102 a ± 2 | 180 b ± 1 | 16 × 102 a ± 3 | 194 b |
| 150 | 12 a ± 1 | 0 b | 160 a ± 1 | 47 b ± 1 | 25 × 102 a ± 2 | 36 b | 233 a | 0.0 b |
| 180 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Treated HFB4 | At 24 h | At 48 h | RM µm | Wound Closure % µm2 | Area Difference | ||
|---|---|---|---|---|---|---|---|
| Width | Area | Width | Area | ||||
| Control | 1462.882 a ± 11 | 1,579,505.469 a ± 10 | 719.4707 a ± 16 | 776,768.5 a ± 13 | 15.48774 a ± 2 | 50.82205 a ± 1 | 802,737.0 a ± 14 |
| GTE | 1462.882 a ± 14 | 1,579,505.469 a ± 8 | 632.3957 a ± 14 | 682,776.5 a ± 9 | 17.3018 a ± 1 | 56.77276 a ± 2 | 896,728.9 a ± 13 |
| GTOE | 1462.88 a ± 12 | 1,579,505.469 a ± 7 | 568.6807 a ± 13 | 614,004.7 a ± 14 | 18.62919 a ± 4 | 61.12677 a ± 3 | 965,500.8 a ± 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazaid, A.S.; Alsalamah, S.A.; Qanash, H.; Alghonaim, M.I.; Saeedi, N.H.; Aldarhami, A. Ozone-Mediated Modulation of Green Tea Extract Enhances Bioactive Compounds and Therapeutic Potential Relevant to Human Health. Pharmaceuticals 2025, 18, 1694. https://doi.org/10.3390/ph18111694
Bazaid AS, Alsalamah SA, Qanash H, Alghonaim MI, Saeedi NH, Aldarhami A. Ozone-Mediated Modulation of Green Tea Extract Enhances Bioactive Compounds and Therapeutic Potential Relevant to Human Health. Pharmaceuticals. 2025; 18(11):1694. https://doi.org/10.3390/ph18111694
Chicago/Turabian StyleBazaid, Abdulrahman S., Sulaiman A. Alsalamah, Husam Qanash, Mohammed Ibrahim Alghonaim, Nizar H. Saeedi, and Abdu Aldarhami. 2025. "Ozone-Mediated Modulation of Green Tea Extract Enhances Bioactive Compounds and Therapeutic Potential Relevant to Human Health" Pharmaceuticals 18, no. 11: 1694. https://doi.org/10.3390/ph18111694
APA StyleBazaid, A. S., Alsalamah, S. A., Qanash, H., Alghonaim, M. I., Saeedi, N. H., & Aldarhami, A. (2025). Ozone-Mediated Modulation of Green Tea Extract Enhances Bioactive Compounds and Therapeutic Potential Relevant to Human Health. Pharmaceuticals, 18(11), 1694. https://doi.org/10.3390/ph18111694








