Development of a Drug Delivery System Using a Compound Based on Ethyl Cyanoacrylate and Hancornia speciosa (Gomes) in a Rat Calvaria Model
Abstract
1. Introduction
2. Results
2.1. Micro-Computed Tomography Analysis
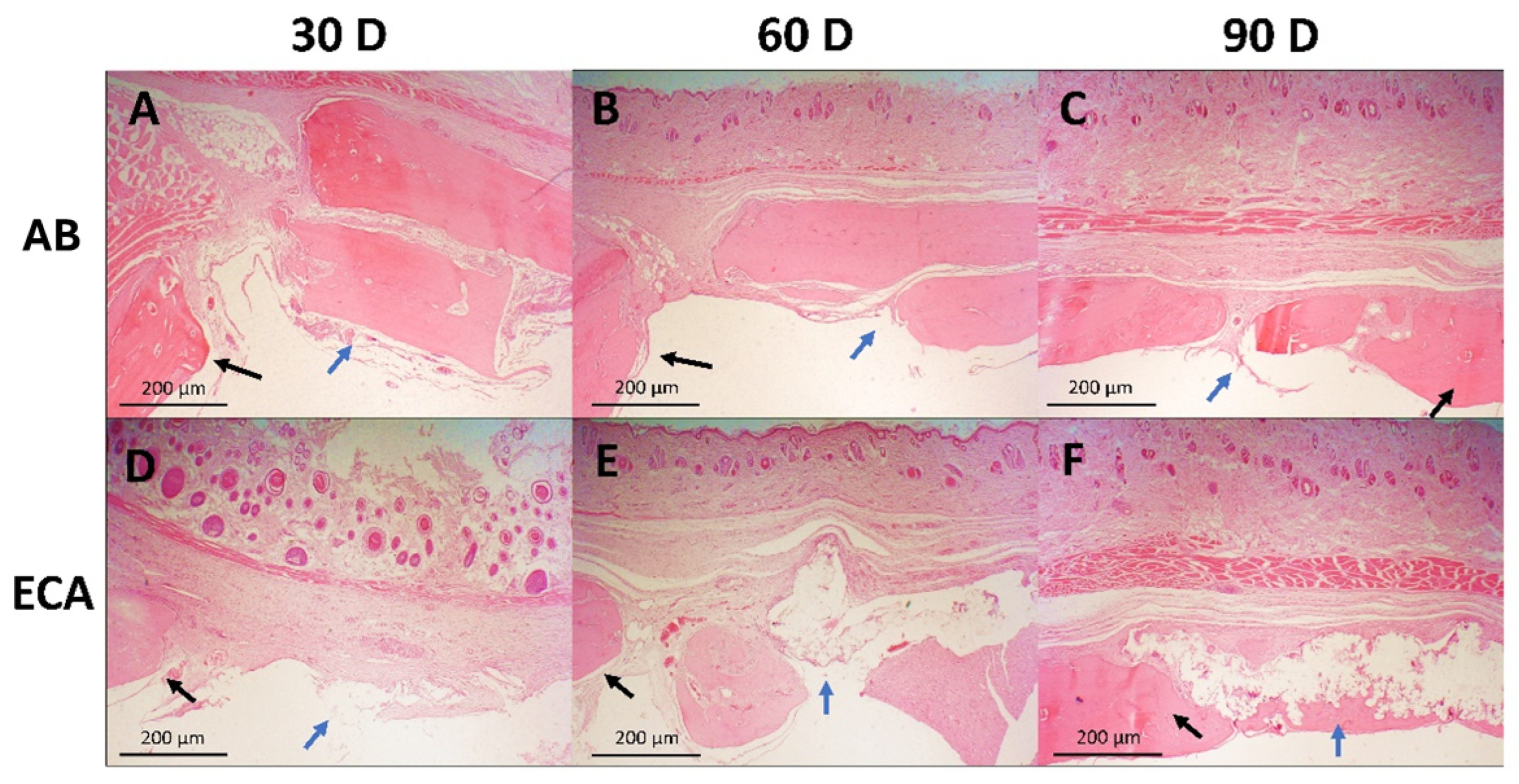
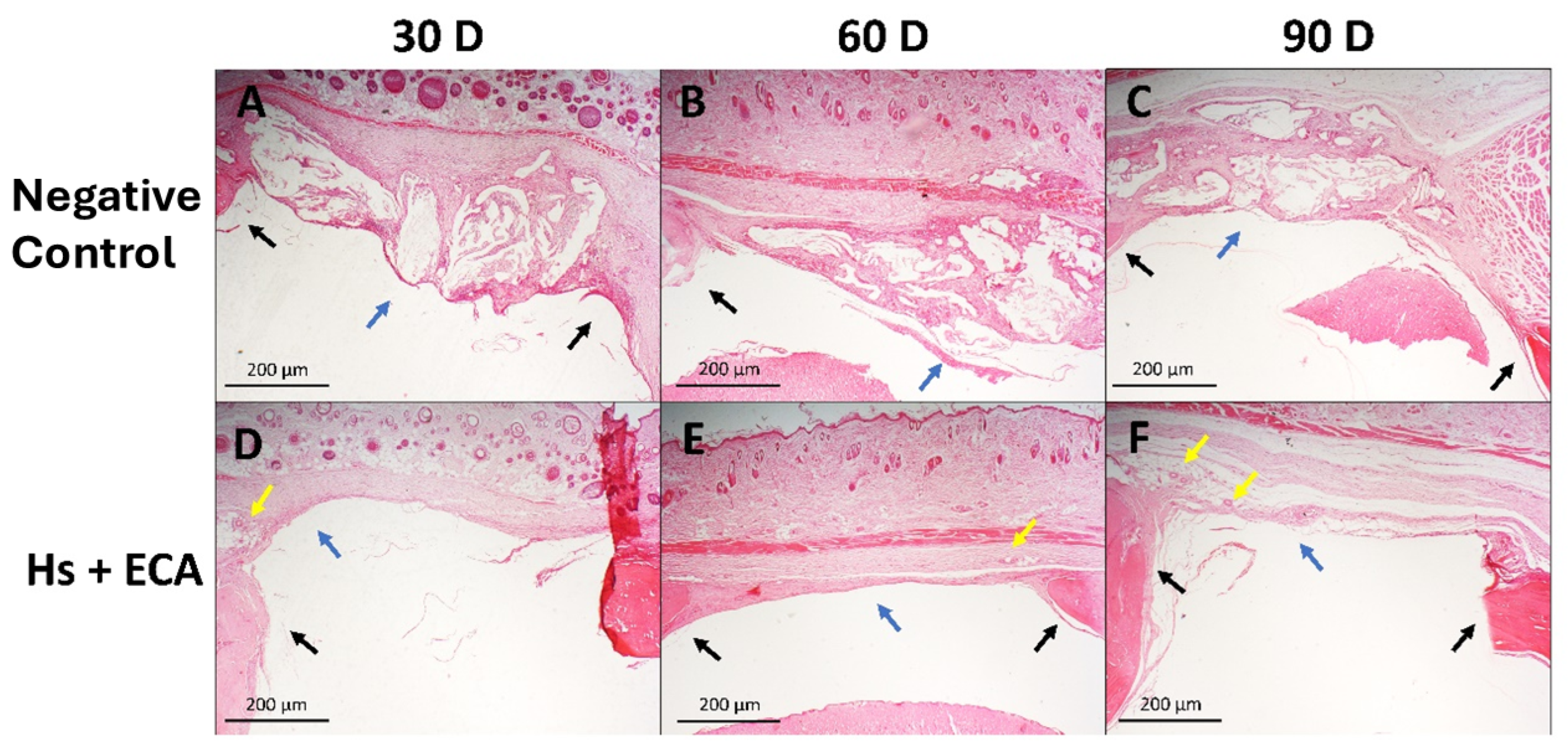
2.2. Qualitative Histological Analysis
3. Discussion
4. Materials and Methods
4.1. Ethical and Legal Considerations
4.2. Sample Size and Animals
4.3. Materials
4.4. Biomaterial Formulation
4.5. Surgical Protocol
4.6. Micro-Computed Tomography Analysis
4.7. Histological Preparation
4.8. Morphometric Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Hs | Hancornia speciosa (Gomes) |
| ECA | Ethyl cyanoacrylate |
| micro-CT | Micro-computed tomography |
| DDS | Drug delivery systems |
| AB | Autogenous bone |
References
- Armiento, A.R.; Hatt, L.P.; Rosenberg, G.S.; Thompson, K.; Stoddart, M.J. Functional biomaterials for bone regeneration: A lesson in complex biology. Adv. Funct. Mater. 2020, 30, 1909874. [Google Scholar] [CrossRef]
- Ogay, V.; Mun, E.A.; Kudaibergen, G.; Baidarbekov, M.; Kassymbek, K.; Zharkinbekov, Z.; Saparov, A. Progress and prospects of polymer-based drug delivery systems for bone tissue regeneration. Polymers 2020, 12, 2881. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Saito, N. Biodegradable polymers as drug delivery systems for bone regeneration. Pharmaceutics 2020, 12, 95. [Google Scholar] [CrossRef]
- D’Abadia, P.L.; Bailão, E.F.L.C.; Lino Júnior, R.S.; Oliveira, M.G.; Silva, V.B.; Oliveira, L.A.R.; Conceição, E.C.; Melo-Reis, P.R.; Borges, L.L.; Gonçalves, P.J.; et al. Hancornia speciosa serum fraction latex stimulates the angiogenesis and extracellular matrix remodeling processes. An. Acad. Bras. Ciênc. 2020, 92, e20190107. [Google Scholar] [CrossRef] [PubMed]
- Bonete, J.M.; Silva, G.D.; Guidelli, É.J.; Gonçalves, P.J.; Almeida, L.M.; Baffa, O.; Kinoshita, A. Tissue reaction and anti-biofilm action of new biomaterial composed of latex from Hancornia speciosa Gomes and silver nanoparticles. An. Acad. Bras. Ciênc. 2020, 92, e20191584. [Google Scholar] [CrossRef] [PubMed]
- De Melo, W.M.; Maximiano, W.M.; Antunes, A.A.; Beloti, M.M.; Rosa, A.L.; de Oliveira, P.T. Cytotoxicity testing of methyl and ethyl 2-cyanoacrylate using direct contact assay on osteoblast cell cultures. J. Oral Maxillofac. Surg. 2013, 71, 35–41. [Google Scholar] [CrossRef]
- Andreotti Damante, C.; Cardoso, M.V.; Hage Karam, P.S.B.; Haiter, A.C.; Sant’ana, A.C.P.; Greghi, S.L.A.; Zangrando, M.S.R.; De Rezende, M.L.R.; Oliveira, R.C. Evaluation of regular market ethyl cyanoacrylate cytotoxicity for human gingival fibroblasts and osteoblasts. Surg. Infect. 2020, 21, 29–34. [Google Scholar] [CrossRef]
- Sadowska, J.M.; Ginebra, M.P. Inflammation and biomaterials: Role of the immune response in bone regeneration by inorganic scaffolds. J. Mater. Chem. B 2020, 8, 9477–9497. [Google Scholar] [CrossRef]
- Cooper, G.M.; Mooney, M.P.; Gosain, A.K.; Campbell, P.G.; Losee, J.E.; Huard, J. Testing the critical size in calvarial bone defects: Revisiting the concept of a critical-size defect. Plast. Reconstr. Surg. 2010, 125, 1685–1692. [Google Scholar] [CrossRef]
- Khorasani, E.; Vahidi, B. 3D-Printed Scaffolds for Cranial Bone Regeneration: A Systematic Review of Design, Materials, and Computational Optimization. Biotechnol. Bioeng. 2025, 122, 1982–2008. [Google Scholar] [CrossRef]
- Mohan, S.; Karunanithi, P.; Raman Murali, M.; Anwar Ayob, K.; Megala, J.; Genasan, K.; Kamarul, T.; Balaji Raghavendran, H.R. Potential use of 3D CORAGRAF-loaded PDGF-BB in PLGA microsphere seeded mesenchymal stromal cells in enhancing the repair of calvaria critical-size bone defect in rat model. Mar. Drugs 2022, 20, 561. [Google Scholar] [CrossRef]
- Fernandes, Y.; Mantovani, R.; Reino, D.; Novaes, A., Jr.; Messora, M.; Gustavo Sousa, L.; Palioto, D.; Scombatti de Souza, S. Evaluation of a new porcine bone graft on the repair of surgically created critical bone defects in rat calvaria: Histomorphometric and microtomographic study. J. Funct. Biomater. 2022, 13, 124. [Google Scholar] [CrossRef]
- Carvalho Vasconcelos, R.; Ferreira, C.; de Araújo, E.M.; Motta, F.; Bomio, M.; de Araújo Júnior, R.F.; Paiva, D.F.F.; Pirih, F.Q.; da Silva, J.S.P.; Chan, A.B.; et al. Zirconia/hydroxyapatite (80/20) scaffold repair in critical size calvarial defect increased FGF-2, osteocalcin and OPG immunostaining and IL-10 levels. Am. J. Transl. Res. 2020, 12, 2439–2450. [Google Scholar]
- Egawa, S.; Miura, S.; Yokoyama, H.; Endo, T.; Tamura, K. Growth and differentiation of a long bone in limb development, repair and regeneration. Dev. Growth Differ. 2014, 56, 410–424. [Google Scholar] [CrossRef]
- Aminoroaya, A.; Nouri Khorasani, S.; Bagheri, R.; Talebi, Z.; Malekkhouyan, R.; Das, O.; Esmaeely Neisiany, R. Facile encapsulation of cyanoacrylate-based bioadhesive by electrospray method and investigation of the process parameters. Sci. Rep. 2024, 14, 5389. [Google Scholar] [CrossRef]
- Tomazi, R.; Figueira, Â.C.; Ferreira, A.M.; Ferreira, D.Q.; de Souza, G.C.; de Souza Pinheiro, W.B.; Pinheiro Neto, J.R.; da Silva, G.A.; de Lima, H.B.; da Silva Hage-Melim, L.I.; et al. Hypoglycemic activity of aqueous extract of latex from Hancornia speciosa Gomes: A study in zebrafish and in silico. Pharmaceuticals 2021, 14, 856. [Google Scholar] [CrossRef]
- dos Santos Neves, J.; Franchin, M.; Rosalen, P.L.; Omar, N.F.; dos Santos, M.A.; Paschoal, J.A.R.; Novaes, P.D. Evaluation of the osteogenic potential of Hancornia speciosa latex in rat calvaria and its phytochemical profile. J. Ethnopharmacol. 2016, 183, 151–158. [Google Scholar] [CrossRef]
- Yun, J.; Nam, I.H.; Kim, M.C.; Park, J.H.; Kang, D.; Cha, H.J. In situ photo-crosslinkable protein bioadhesive for bone graft fixation. J. Dent. Res. 2024, 103, 415–423. [Google Scholar] [CrossRef]
- Gonzalez, E.; Orta, J.; Quero, C.; Niemshik, L.; Galera, R.; Onay, D.; Rojas, O. Ethyl-2-cyanoacrylate fixation of the cranial bone flap after craniotomy. Surg. Neurol. 2000, 53, 288–289. [Google Scholar] [CrossRef]
- Saska, S.; Hochuli-Vieira, E.; Minarelli-Gaspar, A.M.; Gabrielli, M.F.; Capela, M.V.; Gabrielli, M.A. Fixation of autogenous bone grafts with ethyl-cyanoacrylate glue or titanium screws in the calvaria of rabbits. Int. J. Oral Maxillofac. Surg. 2009, 38, 180–186. [Google Scholar] [CrossRef]
- Groppo, M.F.; Caria, P.H.; Freire, A.R.; Figueroba, S.R.; Ribeiro-Neto, W.A.; Bretas, R.E.S.; Prado, F.B.; Haiter-Neto, F.; Aguiar, F.H.; Rossi, A.C. The effect of a hydroxyapatite impregnated PCL membrane in rat subcritical calvarial bone defects. Arch. Oral Biol. 2017, 82, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Felipetti, F.A.; Bereta, R.M.; Piedade, S.M.S.; Novaes, P.D. Oral administrations of Hancornia speciosa Gomes latex do not increase bone neoformation. Rev. Bras. Ortop. 2019, 54, 692–696. [Google Scholar]
- Sánchez-Garcés, M.Á.; Camps-Font, O.; Escoda-Francolí, J.; Muñoz-Guzón, F.; Toledano-Serrabona, J.; Gay-Escoda, C. Short time guided bone regeneration using beta-tricalcium phosphate with and without fibronectin—An experimental study in rats. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e532–e540. [Google Scholar] [CrossRef] [PubMed]
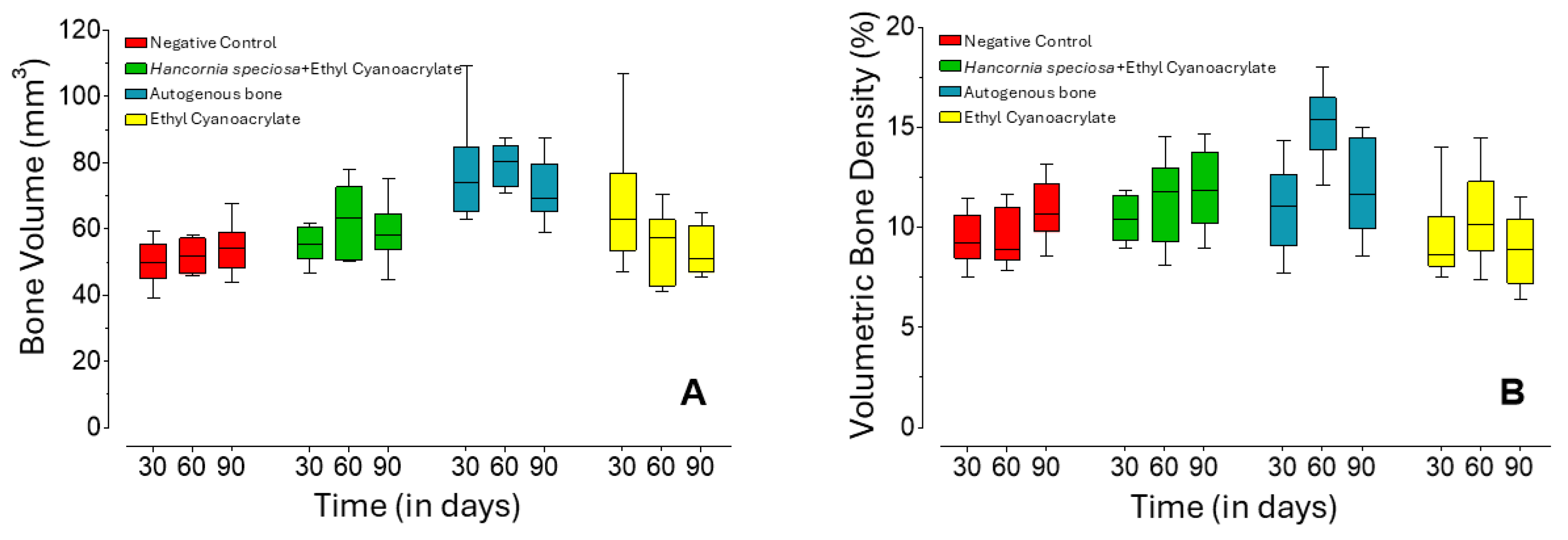
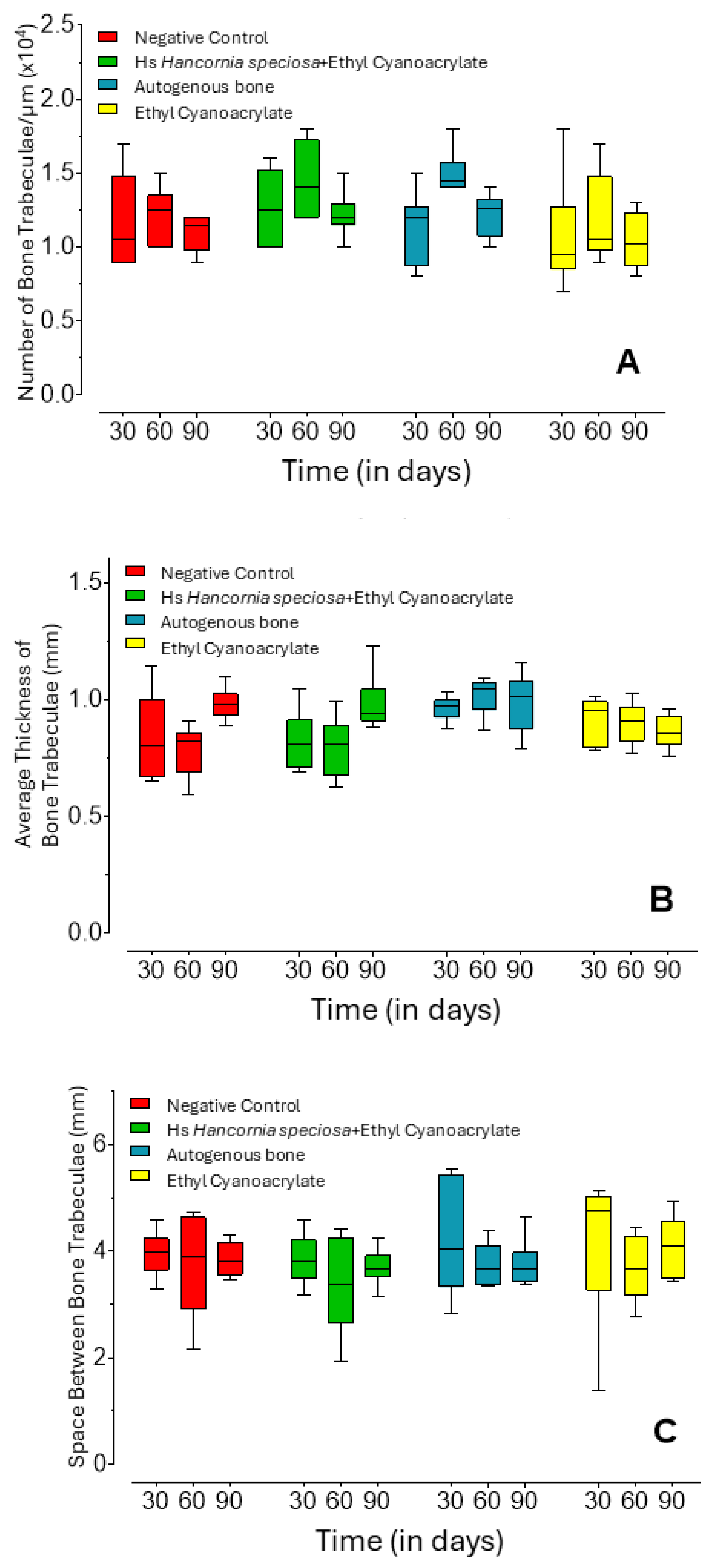
| Group | 30 Days (n = 12) | 60 Days (n = 12) | 90 Days (n = 10) | |
|---|---|---|---|---|
| Bone Volume (mm3) | Hs+ECA | 55.6 b A (53.0–59.3) | 63.2 b A (53.7–69.0) | 57.2 a A (56.7–61.2) |
| Negative Control | 50.0 b A (47.0–53.8) | 51.8 b A (47.2–56.5) | 53,9 b A (49.4–56.4) | |
| AB | 73.9 a A (68.2–76.1) | 80.2 a A (75.1–83.4) | 67.3 a A (67.1–77.2) | |
| ECA | 62.8 ab A (56.6–66.8) | 57.2 ab A (46.6–59.7) | 48.4 b A (47.2–59.9) | |
| Volumetric Bone Density (%) | Hs+ECA | 10.4 a A (9.6–11.4) | 11.8 b A (10.1–12.4) | 11.8 a A (10.6–13.5) |
| Negative Control | 9.2 a A (8.8–10.1) | 8.9 b A (8.6–10.3) | 10.5 ab A (10.2–11.9) | |
| AB | 11.1 a A (9.8–12.0) | 15.4 a B (14.7–15.9) | 11.4 a AB (10.4–14.3) | |
| ECA | 8.6 a A (8.3–9.2) | 10.2 b A (9.5–11.3) | 8.9 b A (7.5–10.1) | |
| Trabecular Number (1/µm × 10−4) | Hs+ECA | 1.25 a A (1.03–1.48) | 1.40 ab A (1.23–1.65) | 1.20 a A (1.20–1.20) |
| Negative Control | 1.05 a A (0.90–1.35) | 1.25 ab A(1.05–1.30) | 1.20 a A (1.00–1.20) | |
| AB | 1.20 a A (0.98–1.20) | 1.45 a B (1.40–1.50) | 1.30 a AB (1.10–1.30) | |
| ECA | 0.95 a A (0.90–1.08) | 1.05 b A (1.00–1.33) | 1.00 a A (0.90–1.20) | |
| Mean Trabecular Thickness (mm) | Hs+ECA | 0.81 b AB (0.73–0.86) | 0.81 b A (0.72–0.85) | 0.94 a B (0.92–0.95) |
| Negative Control | 0.80 ab A (0.71–0.92) | 0.82 b A (0.74–0.84) | 0.98 a B (0.95–1.00) | |
| AB | 0.97 a A (0.94–0.99) | 1.04 a A (1.00–1.06) | 1.03 a A (0.90–1.05) | |
| ECA | 0.95 ab A (0.83–0.98) | 0.91 ab A (0.85–0.95) | 0.85 a A (0.83–0.92) | |
| Trabecular Separation (mm) | Hs+ECA | 3.80 a A (3.62–4.03) | 3.38 a A (2.98–4.04) | 3.65 a A (3.64–3.81) |
| Negative Control | 3.97 a A (3.80–4.08) | 3.90 a A (3.27–4.50) | 3.75 a A (3.59–4.13) | |
| AB | 4.03 a A (3.55–5.13) | 3.67 a A (3.44–3.95) | 3.59 a A (3.44–3.73) | |
| ECA | 4.75 a A (4.05–4.99) | 3.67 a A (3.38–4.08) | 4.09 a A (3.51–4.42) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paiva, D.F.F.; Mafra, M.A.T.; dos Santos, V.A.B.; Figueroba, S.R.; Dantas, A.C.G.C.; Amorim, K.d.S.; Neto, F.H.; Silva, C.B.d.; Groppo, F.C. Development of a Drug Delivery System Using a Compound Based on Ethyl Cyanoacrylate and Hancornia speciosa (Gomes) in a Rat Calvaria Model. Pharmaceuticals 2025, 18, 1695. https://doi.org/10.3390/ph18111695
Paiva DFF, Mafra MAT, dos Santos VAB, Figueroba SR, Dantas ACGC, Amorim KdS, Neto FH, Silva CBd, Groppo FC. Development of a Drug Delivery System Using a Compound Based on Ethyl Cyanoacrylate and Hancornia speciosa (Gomes) in a Rat Calvaria Model. Pharmaceuticals. 2025; 18(11):1695. https://doi.org/10.3390/ph18111695
Chicago/Turabian StylePaiva, Daniel Felipe Fernandes, Marco Antonio Tridapalli Mafra, Victor Augusto Benedicto dos Santos, Sidney Raimundo Figueroba, Anne Caroline Gercina Carvalho Dantas, Klinger de Souza Amorim, Francisco Haiter Neto, Camila Batista da Silva, and Francisco Carlos Groppo. 2025. "Development of a Drug Delivery System Using a Compound Based on Ethyl Cyanoacrylate and Hancornia speciosa (Gomes) in a Rat Calvaria Model" Pharmaceuticals 18, no. 11: 1695. https://doi.org/10.3390/ph18111695
APA StylePaiva, D. F. F., Mafra, M. A. T., dos Santos, V. A. B., Figueroba, S. R., Dantas, A. C. G. C., Amorim, K. d. S., Neto, F. H., Silva, C. B. d., & Groppo, F. C. (2025). Development of a Drug Delivery System Using a Compound Based on Ethyl Cyanoacrylate and Hancornia speciosa (Gomes) in a Rat Calvaria Model. Pharmaceuticals, 18(11), 1695. https://doi.org/10.3390/ph18111695









