Multitarget-Directed Ligands for Alzheimer’s Disease: Recent Novel MTDLs and Mechanistic Insights
Abstract
1. Introduction
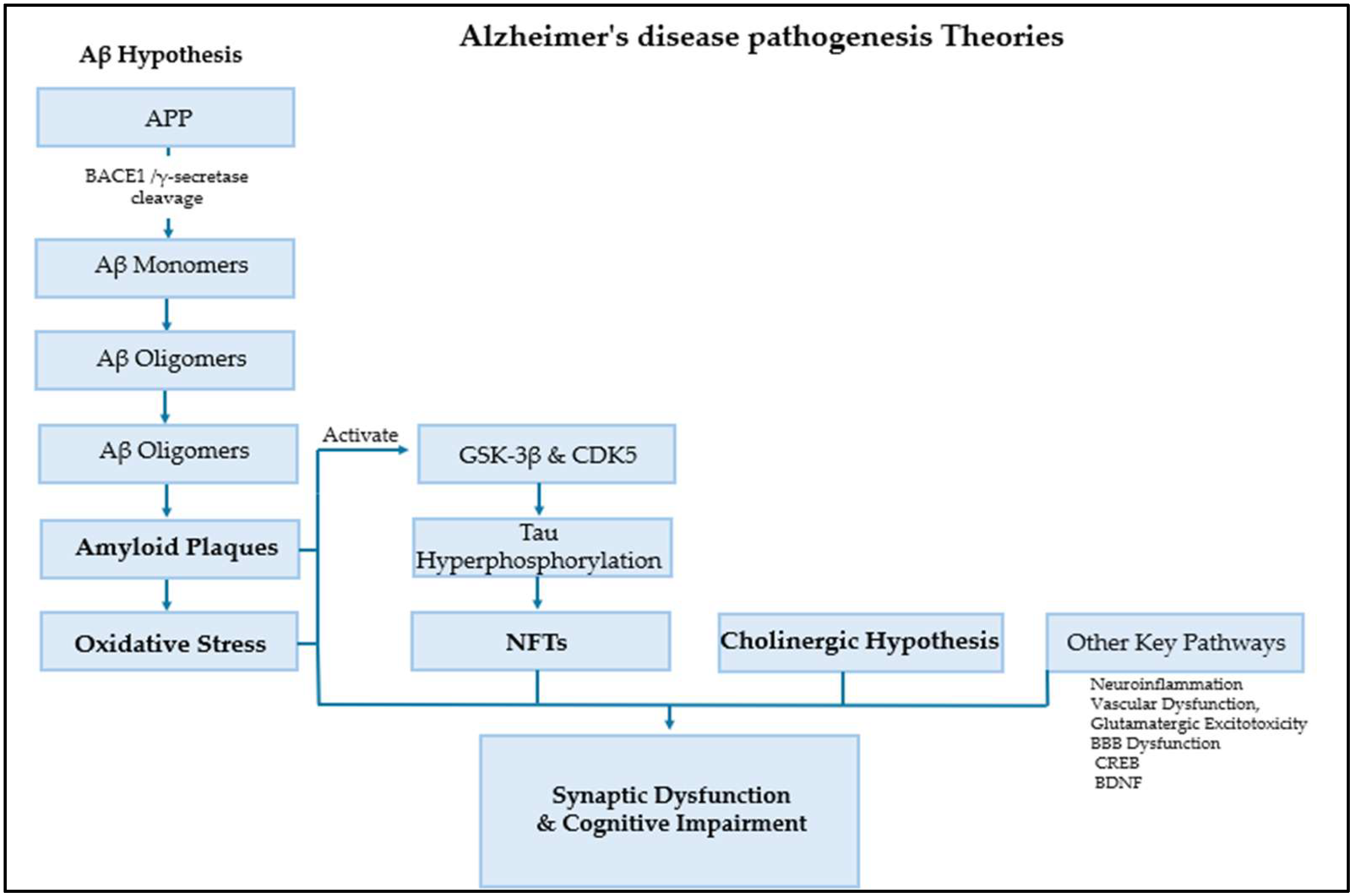
2. Alzheimer’s Disease Pathogenesis
3. Alzheimer’s Disease Treatment
4. Alzheimer’s Disease Multi-Target Treatment
5. Most Recent Novel MTDLs Developed via Pharmacophore Design Strategy
5.1. Linked Pharmacophore Strategies
5.2. Fused Pharmacophore Strategies
5.3. Merged Pharmacophore Strategies
5.4. Natural Product Scaffolds
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MTDLs | Multitarget Directed Ligands |
| AD | Alzheimer’s disease |
| AChE | Acetylcholinesterase |
| BuChE | Butyrylcholinesterase |
| MAO | Monoamine Oxidase |
| NDDs | Neurodegenerative diseases |
| Aβ | Amyloid-Beta |
| BACE1 | β secretase |
| GSK-3β | Glycogen Synthase Kinase 3β |
| CDK5 | Cyclin-Dependent Kinase 5 |
| APP | Amyloid precursor protein |
| NFTs | Neurofibrillary tangles |
| ROS | Reactive oxygen species |
| pTau | Hyperphosphorylated tau |
| NMDAR | N-methyl-D-aspartate receptor |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| IC50 | Inhibitory concentration 50% |
| NQO1 | NQO1 expression (a downstream gene of Nrf2) |
| tBHQ | tert-butylhydroquinone |
| HO1 | HO1 pathway/expression |
| PDE9 | Phosphodiesterase-9 |
| ORAC-FL | Oxygen Radical Absorbance Capacity (test) |
References
- Gadhave, D.G.; Sugandhi, V.V.; Jha, S.K.; Nangare, S.N.; Gupta, G.; Singh, S.K.; Dua, K.; Cho, H.; Hansbro, P.M.; Paudel, K.R. Neurodegenerative disorders: Mechanisms of degeneration and therapeutic approaches with their clinical relevance. Ageing Res. Rev. 2024, 99, 102357. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7, 1161. [Google Scholar] [CrossRef] [PubMed]
- Blaikie, L.; Kay, G.; Lin, P.K.T. Current and emerging therapeutic targets of alzheimer’s disease for the design of multi-target directed ligands. MedChemComm 2019, 10, 2052–2072. [Google Scholar] [CrossRef] [PubMed]
- WHO. A Blueprint for Dementia Research. 2022. Available online: https://www.who.int/publications/i/item/9789240058248 (accessed on 24 December 2024).
- Wimo, A.; Ali, G.-C.; Guerchet, M.; Prince, M.; Prina, M.; Wu, Y.-T. World Alzheimer Report 2015 The Global Impact of Dementia an Analysis of Prevalence, Incidence, Cost and Trends. Available online: www.alz.co.uk/worldreport2015corrections (accessed on 30 December 2024).
- Safiri, S.; Jolfayi, A.G.; Fazlollahi, A.; Morsali, S.; Sarkesh, A.; Sorkhabi, A.D.; Golabi, B.; Aletaha, R.; Asghari, K.M.; Hamidi, S.; et al. Alzheimer’s disease: A comprehensive review of epidemiology, risk factors, symptoms diagnosis, management, caregiving, advanced treatments and associated challenges. Front. Med. 2024, 11, 1474043. [Google Scholar] [CrossRef]
- Armstrong, R.A. Risk factors for Alzheimer’s disease. Folia Neuropathol. 2019, 57, 87–105. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Jack, C.R.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimer’s Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef]
- Shawkatova, I.; Javor, J. Alzheimer’s Disease: Recent Developments in Pathogenesis, Diagnosis, and Therapy. Life 2025, 15, 549. [Google Scholar] [CrossRef]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef]
- Koelsch, G. BACE1 Function and inhibition: Implications of intervention in the amyloid pathway of Alzheimer’s disease pathology. Molecules 2017, 22, 1723. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Tamagno, E.; Guglielmotto, M.; Vasciaveo, V.; Tabaton, M. Oxidative Stress and Beta Amyloid in Alzheimer’s Disease. Which Comes First: The Chicken or the Egg? Antioxidants 2021, 10, 1479. [Google Scholar] [CrossRef] [PubMed]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef]
- Lue, L.F.; Brachova, L.; Civin, W.H.; Rogers, J. Inflammation, A beta deposition, and neurofibrillary tangle formation as correlates of Alzheimer’s disease neurodegeneration. J. Neuropathol. Exp. Neurol. 1996, 55, 1083–1088. [Google Scholar] [CrossRef]
- Soares, C.; Ros, L.U.D.; da Rocha, A.S.; Machado, L.S.; Bellaver, B.; Zimmer, E.R. The glutamatergic system in Alzheimer’s disease: A systematic review with meta-analysis. Alzheimer’s Dement. 2022, 18, e064821. [Google Scholar] [CrossRef]
- Nasb, M.; Tao, W.; Chen, N. Alzheimer’s Disease Puzzle: Delving into Pathogenesis Hypotheses. Aging Dis. 2024, 15, 43–73. [Google Scholar] [CrossRef]
- Sousa, J.A.; Bernardes, C.; Bernardo-Castro, S.; Lino, M.; Albino, I.; Ferreira, L.; Brás, J.; Guerreiro, R.; Tábuas-Pereira, M.; Baldeiras, I.; et al. Reconsidering the role of blood-brain barrier in Alzheimer’s disease: From delivery to target. Front. Aging Neurosci. 2023, 15, 1102809. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, D.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Andronie-Cioara, F.L.; Toma, M.M.; Bungau, S.; Bumbu, A.G. Role of Monoamine Oxidase Activity in Alzheimer’s Disease: An Insight into the Therapeutic Potential of Inhibitors. Molecules 2021, 26, 3724. [Google Scholar] [CrossRef] [PubMed]
- Numakawa, T.; Kajihara, R. Neurotrophins and Other Growth Factors in the Pathogenesis of Alzheimer’s Disease. Life 2023, 13, 647. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J. New approaches to symptomatic treatments for Alzheimer’s disease. Mol. Neurodegener. 2021, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Atri, A. The Alzheimer’s Disease Clinical Spectrum: Diagnosis and Management. Med. Clin. 2019, 103, 263–293. [Google Scholar] [CrossRef]
- Cheong, S.L.; Tiew, J.K.; Fong, Y.H.; Leong, H.W.; Chan, Y.M.; Chan, Z.L.; Kong, E.W.J. Current Pharmacotherapy and Multi-Target Approaches for Alzheimer’s Disease. Pharmaceuticals 2022, 15, 1560. [Google Scholar] [CrossRef]
- Bubley, A.; Erofeev, A.; Gorelkin, P.; Beloglazkina, E.; Majouga, A.; Krasnovskaya, O. Tacrine-Based Hybrids: Past, Present, and Future. Int. J. Mol. Sci. 2023, 24, 1717. [Google Scholar] [CrossRef]
- Silva, M.A.; Kiametis, A.S.; Treptow, W. Donepezil Inhibits Acetylcholinesterase via Multiple Binding Modes at Room Temperature. J. Chem. Inf. Model. 2020, 60, 3463–3471. [Google Scholar] [CrossRef]
- Kandiah, N.; Pai, M.-C.; Senanarong, V.; Looi, I.; Ampil, E.; Park, K.W.; Karanam, A.K.; Christopher, S. Rivastigmine: The advantages of dual inhibition of acetylcholinesterase and butyrylcholinesterase and its role in subcortical vascular dementia and Parkinson’s disease dementia. Clin. Interv. Aging 2017, 12, 697–707. [Google Scholar] [CrossRef]
- Olivares, D.; Deshpande, V.K.; Shi, Y.; Lahiri, D.K.; Greig, N.H.; Rogers, J.T.; Huang, X. N-Methyl D-Aspartate (NMDA) Receptor Antagonists and Memantine Treatment for Alzheimer’s Disease, Vascular Dementia and Parkinson’s Disease. Curr. Alzheimer Res. 2012, 9, 746–758. [Google Scholar] [CrossRef]
- Hardy, J. Alzheimer’s Disease: Treatment Challenges for the Future. J. Neurochem. 2025, 169, e70176. [Google Scholar] [CrossRef]
- Papaliagkas, V. Anti-Amyloid Therapies for Alzheimer’s Disease: Progress, Pitfalls, and the Path Ahead. Int. J. Mol. Sci. 2025, 26, 9529. [Google Scholar] [CrossRef]
- Cummings, J.L.; Tong, G.; Ballard, C. Treatment Combinations for Alzheimer’s Disease: Current and Future Pharmacotherapy Options. J. Alzheimer’s Dis. 2019, 67, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G.R.; Lehár, J.; Keith, C.T. Multi-target therapeutics: When the whole is greater than the sum of the parts. Drug Discov. Today 2007, 12, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewski, S.; Gauthier, S.; Wimo, A.; Rosa-Neto, P. Combination Therapy of Anti-Tau and Anti-Amyloid Drugs for Disease Modification in Early-stage Alzheimer’s Disease: Socio-economic Considerations Modeled on Treatments for Tuberculosis, HIV/AIDS and Breast Cancer. J. Prev. Alzheimer’s Dis. 2016, 3, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Proschak, E.; Stark, H.; Merk, D. Polypharmacology by Design: A Medicinal Chemist’s Perspective on Multitargeting Compounds. J. Med. Chem. 2018, 62, 420–444. [Google Scholar] [CrossRef]
- Morphy, R.; Kay, C.; Rankovic, Z. From magic bullets to designed multiple ligands. Drug Discov. Today 2004, 9, 641–651. [Google Scholar] [CrossRef]
- Bansal, Y.; Silakari, O. Multifunctional compounds: Smart molecules for multifactorial diseases. Eur. J. Med. Chem. 2014, 76, 31–42. [Google Scholar] [CrossRef]
- Gontijo, V.S.; Viegas, F.P.D.; Ortiz, C.J.C.; Silva, M.d.F.; Damasio, C.M.; Rosa, M.C.; Campos, T.G.; Couto, D.S.; Dias, K.S.T.; Viegas, C. Molecular Hybridization as a Tool in the Design of Multi-target Directed Drug Candidates for Neurodegenerative Diseases. Curr. Neuropharmacol. 2020, 18, 348–407. [Google Scholar] [CrossRef]
- Talevi, A. Multi-target pharmacology: Possibilities and limitations of the ‘skeleton key approach’ from a medicinal chemist perspective. Front. Pharmacol. 2015, 6, 205. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, X.; He, S.; Jiang, H.; Feng, F.; Liu, W.; Qu, W.; Sun, H. Rational Design of Multitarget-Directed Ligands: Strategies and Emerging Paradigms. J. Med. Chem. 2019, 62, 8881–8914. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Liu, F.; Li, S.; Shi, D. Rational Multitargeted Drug Design Strategy from the Perspective of a Medicinal Chemist. J. Med. Chem. 2021, 64, 10581–10605. [Google Scholar] [CrossRef] [PubMed]
- Morphy, R.; Rankovic, Z. Medicinal Chemistry Approaches for Multitarget Drugs. In Burger’s Medicinal Chemistry and Drug Discovery, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 249–274. [Google Scholar] [CrossRef]
- Morphy, R.; Rankovic, Z. Designing multiple ligands—Medicinal chemistry strategies and challenges. Curr. Pharm. Des. 2009, 15, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Morphy, R.; Rankovic, Z. Designed multiple ligands. An emerging drug discovery paradigm. J. Med. Chem. 2005, 48, 6523–6543. [Google Scholar] [CrossRef]
- Hopkins, A.L.; Mason, J.S.; Overington, J.P. Can we rationally design promiscuous drugs? Curr. Opin. Struct. Biol. 2006, 16, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Tavera-Mendoza, L.E.; Quach, T.D.; Dabbas, B.; Hudon, J.; Liao, X.; Palijan, A.; Gleason, J.L.; White, J.H. Incorporation of histone deacetylase inhibition into the structure of a nuclear receptor agonist. Proc. Natl. Acad. Sci. USA 2008, 105, 8250–8255. [Google Scholar] [CrossRef]
- Sterling, J.; Herzig, Y.; Goren, T.; Finkelstein, N.; Lerner, D.; Goldenberg, W.; Miskolczi, I.; Molnar, S.; Rantal, F.; Tamas, T.; et al. Novel dual inhibitors of AChE and MAO derived from hydroxy aminoindan and phenethylamine as potential treatment for Alzheimer’s disease. J. Med. Chem. 2002, 45, 5260–5279. [Google Scholar] [CrossRef]
- Jiang, X.-Y.; Chen, T.-K.; Zhou, J.-T.; He, S.-Y.; Yang, H.-Y.; Chen, Y.; Qu, W.; Feng, F.; Sun, H.-P. Dual GSK-3β/AChE Inhibitors as a New Strategy for Multitargeting Anti-Alzheimer’s Disease Drug Discovery. ACS Med. Chem. Lett. 2018, 9, 171–176. [Google Scholar] [CrossRef]
- Jenwitheesuk, E.; Horst, J.A.; Rivas, K.L.; Van Voorhis, W.C.; Samudrala, R. Novel paradigms for drug discovery: Computational multitarget screening. Trends Pharmacol. Sci. 2008, 29, 62–71. [Google Scholar] [CrossRef]
- Ma, X.H.; Wang, R.; Tan, C.Y.; Jiang, Y.Y.; Lu, T.; Rao, H.B.; Li, X.Y.; Go, M.L.; Low, B.C.; Chen, Y.Z. Virtual Screening of Selective Multitarget Kinase Inhibitors by Combinatorial Support Vector Machines. Mol. Pharm. 2010, 7, 1545–1560. [Google Scholar] [CrossRef]
- Anighoro, A.; Bajorath, J.; Rastelli, G. Polypharmacology: Challenges and Opportunities in Drug Discovery. J. Med. Chem. 2014, 57, 7874–7887. [Google Scholar] [CrossRef]
- Simakov, A.; Chhor, S.; Ismaili, L.; Martin, H. Nrf2 Activation and Antioxidant Properties of Chromone-Containing MTDLs for Alzheimer’s Disease Treatment. Molecules 2025, 30, 2048. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, B.; Zheng, L. Usnic Acid Derivatives as Multi-Target Anti-Alzheimer’s Disease Agents: Design, Synthesis, X-Ray Single Crystal Structure of Zn(II) Complex and Biological Activities. Chem. Biodivers. 2024, 22, e202401548. [Google Scholar] [CrossRef] [PubMed]
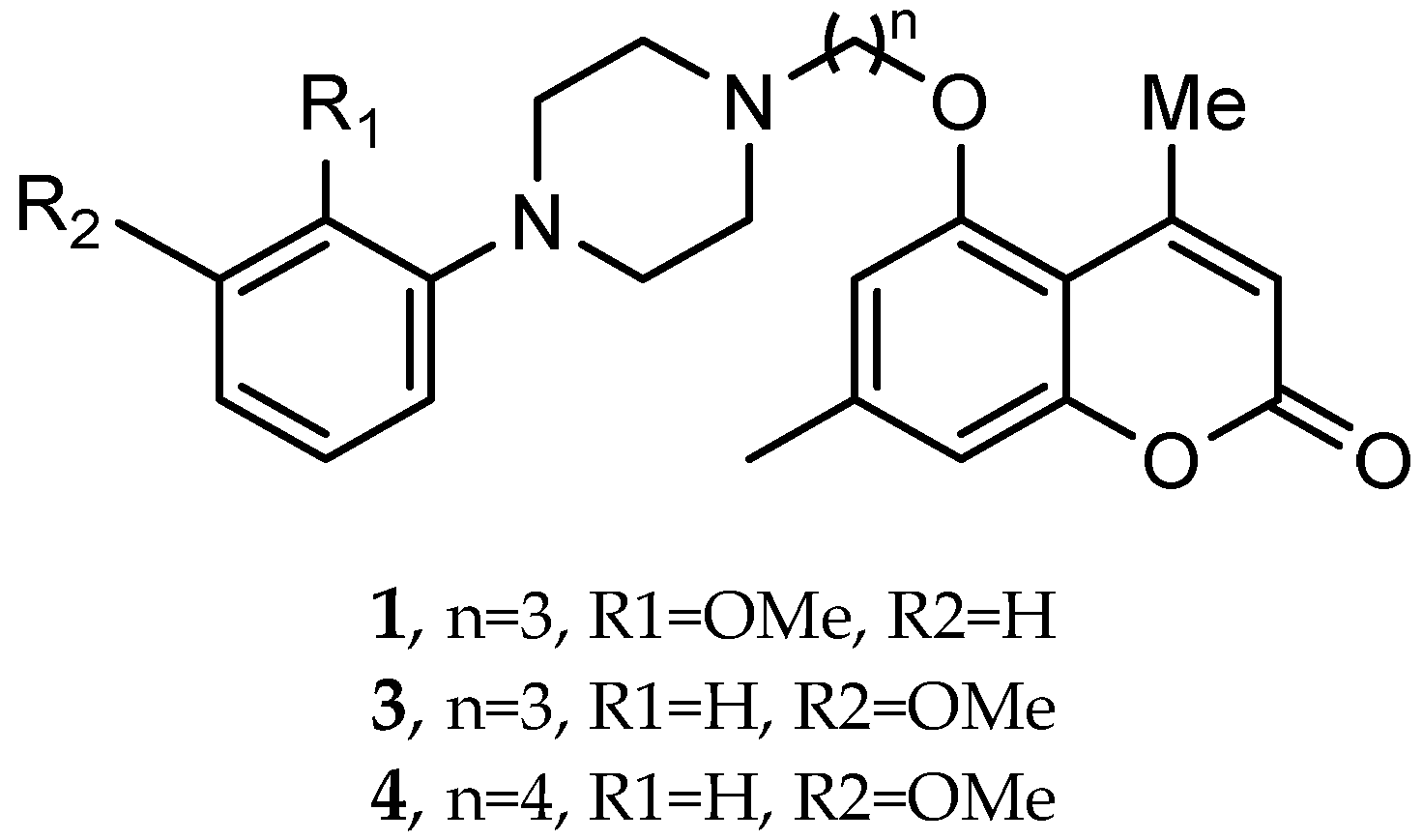
- El-Mageed, M.M.A.; Ezzat, M.A.F.; Moussa, S.A.; Abdel-Aziz, H.A.; Elmasry, G.F. Rational design, synthesis and computational studies of multi-targeted anti-Alzheimer’s agents integrating coumarin scaffold. Bioorganic Chem. 2024, 154, 108024. [Google Scholar] [CrossRef] [PubMed]
- Negi, N.; Ayyannan, S.R.; Tripathi, R.K.P. Multi-targeted benzylpiperidine–isatin hybrids: Design, synthesis, biological and in silico evaluation as monoamine oxidases and acetylcholinesterase inhibitors for neurodegenerative disease therapies. J. Comput. Mol. Des. 2025, 39, 10. [Google Scholar] [CrossRef]
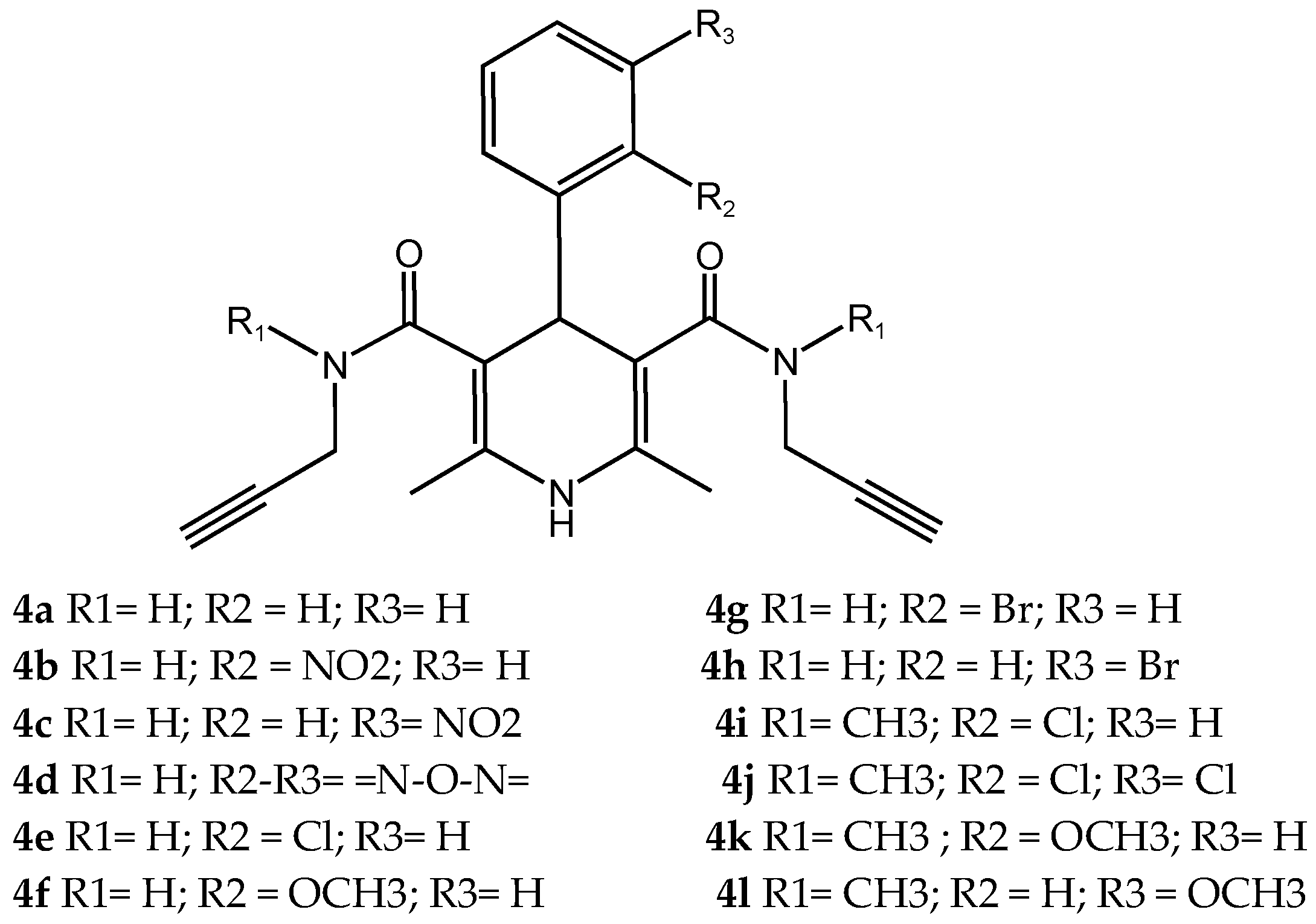
- Shaaban, A.E.; Ali, A.R.; Ayyad, S.N.; Badria, F.A. Unveiling the potential of novel natural product-based MTDLs that integrate cinnamamide scaffold as multifunctional agents for the treatment of Alzheimer’s disease. Bioorganic Chem. 2025, 163, 108666. [Google Scholar] [CrossRef]
- Dias, I.; Bon, L.; Banas, A.; Chavarria, D.; Borges, F.; Guerreiro-Oliveira, C.; Cardoso, S.M.; Sanna, D.; Garribba, E.; Chaves, S.; et al. Exploiting the potential of rivastigmine-melatonin derivatives as multitarget metal-modulating drugs for neurodegenerative diseases. J. Inorg. Biochem. 2024, 262, 112734. [Google Scholar] [CrossRef]
- Asghar, S.; Mushtaq, N.; Ahmed, A.; Anwar, L.; Munawar, R.; Akhtar, S. Potential of Tryptamine Derivatives as Multi-Target Directed Ligands for Alzheimer’s Disease: AChE, MAO-B, and COX-2 as Molecular Targets. Molecules 2024, 29, 490. [Google Scholar] [CrossRef]
- Wu, X.; Ze, X.; Qin, S.; Zhang, B.; Li, X.; Gong, Q.; Zhang, H.; Zhu, Z.; Xu, J. Design, Synthesis, and Biological Evaluation of Novel Tetrahydroacridin Hybrids with Sulfur-Inserted Linkers as Potential Multitarget Agents for Alzheimer’s Disease. Molecules 2024, 29, 1782. [Google Scholar] [CrossRef]
- Nagani, A.; Shah, M.; Patel, S.; Patel, H.; Parikh, V.; Patel, A.; Patel, S.; Patel, K.; Parmar, H.; Bhimani, B.; et al. Unveiling piperazine-quinoline hybrids as potential multi-target directed anti-Alzheimer’s agents: Design, synthesis and biological evaluation. Mol. Divers. 2024, 29, 1453–1478. [Google Scholar] [CrossRef]
- Żołek, T.; Purgatorio, R.; Kłopotowski, Ł.; Catto, M.; Ostrowska, K. Coumarin Derivative Hybrids: Novel Dual Inhibitors Targeting Acetylcholinesterase and Monoamine Oxidases for Alzheimer’s Therapy. Int. J. Mol. Sci. 2024, 25, 12803. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, X.-N.; Luo, W.-H.; Huang, Q.-H.; Feng, L.-L.; Wu, Y.; Zhang, C. Discovery of Effective Inhibitors Against Phosphodiesterase 9, a Potential Therapeutic Target of Alzheimer’s Disease with Antioxidant Capacities. Antioxidants 2025, 14, 123. [Google Scholar] [CrossRef]
- Polini, B.; Zallocco, L.; Gado, F.; Ferrisi, R.; Ricardi, C.; Zuccarini, M.; Carnicelli, V.; Manera, C.; Ronci, M.; Lucacchini, A.; et al. A Proteomic Approach Identified TFEB as a Key Player in the Protective Action of Novel CB2R Bitopic Ligand FD22a against the Deleterious Effects Induced by β-Amyloid in Glial Cells. Cells 2024, 13, 875. [Google Scholar] [CrossRef]
- Bajad, N.G.; Jangra, J.; Ta, G.; Kumar, A.; Krishnamurthy, S.; Singh, S.K. Discovery of pyrazoline analogs as multi-targeting cholinesterase, β-secretase and Aβ aggregation inhibitors through lead optimization strategy. Int. J. Biol. Macromol. 2025, 301, 140436. [Google Scholar] [CrossRef]
- Ahsan, M.J.; Ali, A.; Ali, A.; Thiriveedhi, A.; Bakht, M.A.; Yusuf, M.; Salahuddin; Afzal, O.; Altamimi, A.S.A. Pyrazoline Containing Compounds as Therapeutic Targets for Neurodegenerative Disorders. ACS Omega 2022, 7, 38207–38245. [Google Scholar] [CrossRef]
- Kumar, V.; Jangid, K.; Kumar, V.; Kumar, N.; Mishra, J.; Arora, T.; Dwivedi, A.R.; Kumar, P.; Bhatti, J.S.; Parkash, J.; et al. In vitro and in vivo Investigations of 4-Substituted 2-Phenylquinazoline derivatives as multipotent ligands for the treatment of Alzheimer’s disease. Bioorganic Chem. 2025, 155, 108126. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Kumar, M.; Dwivedi, A.R.; Kumar, V. Synthesis, Biological Evaluation and Molecular Modeling Studies of Propargyl-Containing 2,4,6-Trisubstituted Pyrimidine Derivatives as Potential Anti-Parkinson Agents. ChemMedChem 2018, 13, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Pachón-Angona, I.; Bernard, P.J.; Simakov, A.; Maj, M.; Jozwiak, K.; Novotna, A.; Lemke, C.; Gütschow, M.; Martin, H.; Oset-Gasque, M.-J.; et al. Design and Synthesis of Multi-Functional Ligands through Hantzsch Reaction: Targeting Ca2+ Channels, Activating Nrf2 and Possessing Cathepsin S Inhibitory, and Antioxidant Properties. Pharmaceutics 2024, 16, 121. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, P.d.S.M.; de Chirico, F.; Loi, M.; Trazzi, S.; Ciani, E.; Rodrigues, D.A.; Alves, M.A.; Lima, L.M.; Milelli, A.; Monti, B.; et al. Design, synthesis and pharmacological evaluation of multitarget GPR40 agonists/HDAC6 inhibitors for Alzheimer’s disease. Eur. J. Med. Chem. 2025, 296, 117868. [Google Scholar] [CrossRef]
- Said, M.F.; Wadie, W.; El-Haleim, E.A.A.; El Shiekh, R.A.; El-Zoheiry, H.H. Probing new 3-hydrazinyl indole phenacetamide derivatives as multitarget anti-Alzheimer: Synthesis, in vivo, in vitro, and in silico studies. Eur. J. Med. Chem. 2025, 295, 117720. [Google Scholar] [CrossRef]
- Vitale, R.M.; Morace, A.M.; D’Errico, A.; Ricciardi, F.; Fusco, A.; Boccella, S.; Guida, F.; Nasso, R.; Rading, S.; Karsak, M.; et al. Identification of Cannabidiolic and Cannabigerolic Acids as MTDL AChE, BuChE, and BACE-1 Inhibitors Against Alzheimer’s Disease by In Silico, In Vitro, and In Vivo Studies. Phytother. Res. 2024, 39, 233–245. [Google Scholar] [CrossRef]
- D’ANiello, E.; Fellous, T.; Iannotti, F.A.; Gentile, A.; Allarà, M.; Balestrieri, F.; Gray, R.; Amodeo, P.; Vitale, R.M.; Di Marzo, V. Identification and characterization of phytocannabinoids as novel dual PPARα/γ agonists by a computational and in vitro experimental approach. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2019, 1863, 586–597. [Google Scholar] [CrossRef]




| Novel MTDLs | Nrf2 Induction Potencies CD (µM) |
|---|---|
| 4a | 1.0 ± 0.1 |
| 4b | 0.4 ± 0.1 |
| 4c | 0.3 ± 0.1 |
| 4d | 0.7 ± 0.1 |
| 4e | 1.0 ± 0.1 |
| 4f | 0.4 ± 0.1 |
| 4g | 0.7 ± 0.2 |
| 4h | 0.5 ± 0.1 |
| tBHQ | 0.6 ± 0.1 |
| Novel MTDLs | (AChE) Inhibition (IC50, μM) | (BuChE) Inhibition (IC50, μM) | OH Scavenging Abilities (IC50, μM) |
|---|---|---|---|
 1 | 0.713 ± 0.005 | 5.934 ± 0.141 | 0.865 ± 0.025 |
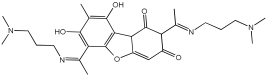 2 | 0.143 ± 0.006 | 0.356 ± 0.008 | 0.572 ± 0.004 |
| Tacrine | 0.0145 ± 0.001 | 0.003 ± 0.000 | |
| Trolox | 0.63 ± 0.010 |
| Novel MTDLs | hAChE Inhibition (IC50 nM ± SD) | hBuChE Inhibition (IC50 nM ± SD) | GSK-3β Inhibition (IC50 nM ± SD) | (Aβ) Aggregation Inhibition IC50 uM | Tau Protein Aggregation Inhibition IC50 uM |
|---|---|---|---|---|---|
 6c | 28.88 ± 3.19 | 131.90 ± 8.17 | 51.42 ± 2.94 | 22.45 ± 1.05 | 31.22 ± 1.90 |
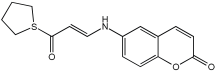 6h | 26.03 ± 3.99 | 90.02 ± 6.71 | 26.91 ± 2.19 | 35.04 ± 1.64 | 56.31 ± 3.43 |
| Donepezil | 31.54 ± 2.16 | 614.50 ± 7.30 | 219.10 ± 5.82 | 75.31 ± 3.53 | 120.1 ± 7.32 |
| Rivastigmine | 107.40 ± 7.49 | − |
| Novel MTDLs | MAO-A IC 50(μM) ± SEM a | MAO-B IC 50(μM) ± SEM a | (AChE) Inhibition IC 50(μM) ± SEM a |
|---|---|---|---|
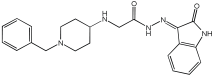 4 | 0.22 ± 0.02 | 0.057 ± 0.001 | 0.27 ± 0.01 |
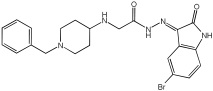 5 | 0.65 ± 0.05 | 39.81 ± 1.04 | 3.25 ± 0.14 |
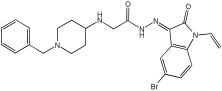 11 | 9.67 ± 0.33 | 0.61 ± 0.03 | 2.16 ± 0.08 |
 12 | 0.12 ± 0.07 | 5.73 ± 0.06 | 2.32 ± 0.17 |
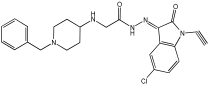 14 | 0.108 ± 0.004 | 0.60 ± 0.003 | 0.034 ± 0.002 |
| Donepezil | 0.084 ± 0.002 |
| Novel MTDLs | AChE IC50 (μM ± SD) | BChE IC50 (μM ± SD) | MAO-A IC50 (μM ± SD) | MAO-B IC50 (μM ± SD) |
|---|---|---|---|---|
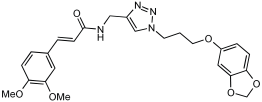 16 | 4.59 ± 0.87 | 13.24 ± 0.56 | 30.78 ± 0.94 | 32.02 ± 0.59 |
 14 | 7.51 ± 0.5 | 21.6 ± 0.85 | 61.44 ± 0.44 | 63.44 ± 0.39 |
 18 | 6.42 ± 0.16 | 35.3 ± 0.38 | 61.2 ± 0.42 | 46.59 ± 0.72 |
 21 | 0.37 ± 0.24 | 13.53 ± 0.52 | 33.68 ± 0.57 | 64.09 ± 1.39 |
| Donepezil 6.01 ± 0.2 | Rivastigmine 5.88 ± 1.86 | Methylene blue 143.6 ± 22.1 | Biperiden HCl 66.4 ± 1.47 |
| Novel MTDLs | Aβ42 Self-Aggr. Inhib. (%) | Aβ42 Cu-Ind Aggr. Inhib. (%) | hMAO-A IC50 (μM) or % Inhibition at 10 μM | hMAO-B IC50 (μM) or % Inhibition at 10 μM |
|---|---|---|---|---|
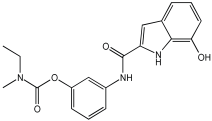 5a3 | 25.0 | 48.3 | 6.66 ± 0.74 | 3.85 ± 0.10 |
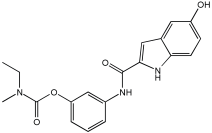 5a2 | 50.3 | 73.5 | 28% | 36% |
| Curcumin | 61.3 | 53.0 | - | - |
| (R)-(-)-Deprenyl | __ | __ | 18 ± 1 | 0.049 ± 0.009 |
| Clorgyline | __ | __ | 0.00200 ± 0.00015 | 2.44 ± 0.49 |
| Novel MTDLs | AChE | (MAO-B) | (COX-2) %Inhibition ± SD |
|---|---|---|---|
| (IC50 ± SD (µM)) | |||
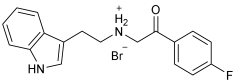 SR10 | 1.00 ± 0.08 | 216.10 ± 0.29 | 72.90 ± 2.42 |
 SR25 | 0.17 ± 0.02 | 85.10 ± 0.26 | 72.43 ± 2.36 |
 SR42 | 0.70 ± 0.21 | 43.21 ± 0.46 | 75.16 ± 2.30 |
| Novel MTDLs | (AChE) Inhibition (IC50, μM) ± SEM | GSK-3β (IC50, μM) ± SEM |
|---|---|---|
| 16a | 2.30 ± 0.14 | 15.10 ± 0.90 |
| 16b | 20.00 ± 1.50 | 38.50 ± 0.70 |
| 16c | 10.30 ± 0.70 | 2.28 ± 0.07 |
| 16d | 1.89 ± 0.21 | 1.69 ± 0.09 |
| 16e | 15.30 ± 0.90 | 0.94 ± 0.02 |
| 16f | 16.50 ± 0.80 | 2.44 ± 0.06 |
| 18a | 0.047 ± 0.002 | 0.93 ± 0.08 |
| 18b | 2.13 ± 0.11 | 0.37 ± 0.02 |
| 18c | 2.24 ± 0.13 | 0.42 ± 0.03 |
| Tacrine | 0.229 ± 0.01 | - |
| Novel MTDLs | hAChE (IC50 ± SD, μM) | eqhBuChE (IC50 ± SD, μM) | DPPH Antioxidant Potential (IC50, μM) |
|---|---|---|---|
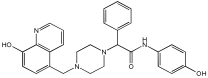 78 | 30.92 ± 0.996 | 12.42 ± 0.219 | 156.06 |
 79 | 214.46 ± 0.657 | 2.28 ± 0.355 | 237.21 |
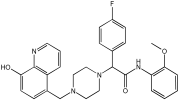 83 | 14.09 ± 0.266 | 1.888 ± 0.300 | 162.16 |
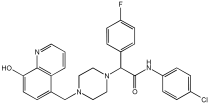 86 | 64.32 ± 0.678 | 2.02 ± 0.270 | 179.69 |
 93 | 76.43 ± 0.145 | 4.953 ± 0.295 | - |
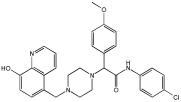 95 | 3.013 ± 0.433 | 3.144 ± 0.189 | 211.99 |
| Donepezil | 0.054 ± 0.002 | - | - |
| Tacrine | - | 0.0047 ± 0.0002 | - |
| Ascorbic acid | - | - | 79.37 |
| Novel MTDLs | (AChE) % Inhibition at 10 µM ± SD | hMAO-A % Inhibition at 10 µM ± SD | hMAO-B % Inhibition at 10 µM ± SD |
|---|---|---|---|
| 1 | 61 ± 1% | 44 ± 4% | 2.18 ± 0.48 |
| 3 | 53 ± 2% | 39 ± 5% | 1.88 ± 0.45 |
| 4 | 56 ± 1% | 38 ± 4% | 3.18 ± 0.63 |
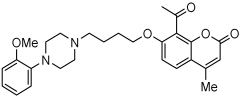 10 | 1.52 ± 0.66 | 52 ± 4% | 24 ± 1% |
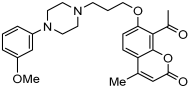 11 | 2.80 ± 0.69 | 6.97 ± 0.76 | 32 ± 1% |
 12 | 4.95 ± 0.48 | 7.65 ± 0.36 | 57 ± 4% |
| Novel MTDLs | PDE9 (IC50, nM) | ORAC µmol of Trolox Equiv/µmol |
|---|---|---|
| 17b | 91 ± 4 | 2 ± 0.27 |
| 17c | 1.8 | 0.32 ± 0.06 |
| 18c | 194 ± 26 | 1.61 ± 0.11 |
| 18d | 214 ± 20 | 1.09 ± 0.02 |
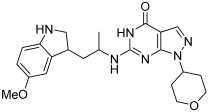 17d | 89 ± 4 | 2.6 ± 0.5 |
| Novel MTDLs | eeAChE IC50 (μM) or % Inhibition | eqBuChE IC50 (μM) or % Inhibition | BACE-1 % Inhibition |
|---|---|---|---|
 48 | 2.89 ± 0.706 | 0.151 ± 0.089 | 36.64 ± 1.343 |
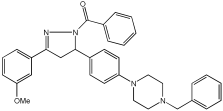 53 | 4.086 ± 0.415 | 0.247 ± 0.072 | 26.19 ± 1.635 |
 54 | 3.393 ± 0.916 | 1.079 ± 0.300 | 41.02 ± 1.290 |
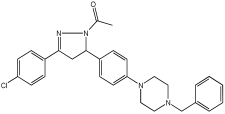 56 | 2.831 ± 0.528 | 0.569 ± 0.031 | 34.64 ± 1.198 |
| Novel MTDLs | eeAChE %Age Inhibition at 10 µM | MAO-B %Age Inhibition at 10 µM | MAO-A %Age Inhibition at 10 µM | eqBuChE %Age Inhibition at 20 µM |
|---|---|---|---|---|
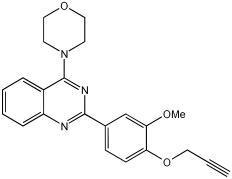 VAV 8 | 96.34% (0.171 ± 0.07) | 98.54% (0.078 ± 0.009) | 43.11% | 7.28% |
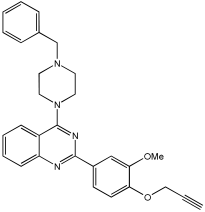 VAV 19 | 95.52% (0.177 ± 0.05) | 71.92% (6.06 ± 0.69) | 47.01% | 7.58% |
| Novel MTDLs5 | Calcium Channel Blockade% at 10 µM | ORAC Trolox Equiv/± SEM | Nrf2 Induction Potencies CD (µM) |
|---|---|---|---|
| 4a | 12 | 0.86 ± 0.5 | n.d. |
| 4b | n.i. | 1.36± 0.16 | 82.8 ± 8.4 |
| 4c | 3 | 1.54 ± 0.15 | n.i. |
| 4d | 10 | 1.23 ± 0.23 | n.i. |
| 4e | n.i. | 1.15± 0.02 | n.i. |
| 4f | 19 | 1.34 ± 0.2 | n.i. |
| 4g | n.i. | 1.43 ± 0.03 | n.i. |
| 4h | 7 | 1.28 ± 0.05 | 55.3 ± 7.4 |
| 4i | 11 | 1.93 ± 0.08 | 69.3 ± 5.2 |
| 4j | 12 | 1.82 ± 0.05 | n.i. |
| 4k | 14 | n.i. | n.i. |
| 4l | 15 | 0.96 ± 0.07 | n.i. |
| nimodipine | 37 | n.d. | n.d. |
| melatonin | n.d. | 2.45 ± 0.09 | n.d. |
| Novel MTDLs | HDAC6 IC50 (nM) | GPR40 EC50 (nM) | Emax GPR40 |
|---|---|---|---|
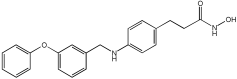 4a | 18% (1 μM) | 9.5 | 93% |
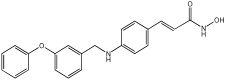 5a | 267 | 5.4 | 88% |
 6a | 1590 | 683 | 55% |
 4b | 31% (1 μM) | ND | −7% |
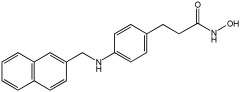 4c | 301 | 30.6 | 109% |
 4d | 160 | 22.9 | 113% |
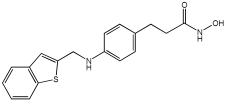 4e | 73 | 22.5 | 95% |
 4h | 551 | 8.9 | 109% |
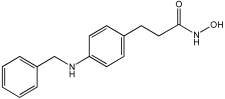 4i | 157 | 766 | 55% |
| GW9508 | – | 7.0 | 100% |
| Novel MTDLs | Edema (mm) ± SEM (% Inhibition) 2 h | (AChE) IC50 ± SD (μM) | (BChE) IC50 ± SD (μM) |
|---|---|---|---|
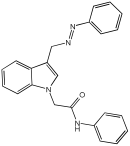 5a | 76.59% | 0.268 ± 0.01 | 2.777 ± 0.45 |
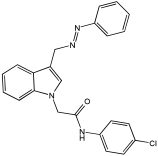 5b | 80.45% | 0.507 ± 0.002 | 2.925 ± 0.10 |
 5c | 73.90% | 0.656 ± 0.001 | 3.131 ± 0.10 |
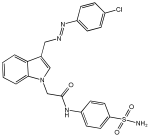 5j | 74.78% | 0.302 ± 0.01 | 2.908 ± 0.15 |
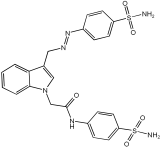 5o | 72.98% | 0.195 ± 0.0001 | 2.321 ± 0.10 |
| Donepezil | 0.304 ± 0.005 | 1.382 ± 0.01 | |
| Indomethacin | 72.26% |
| Novel MTDLs | (AChE) Inhibition (Ki, μM) | (BuChE) Inhibition (Ki, μM) | (BACE1) Inhibition (IC50, μM) | (Aβ) Aggregation Inhibition (%)μM |
|---|---|---|---|---|
 CBD | 7.9 ± 4.3 | 6.7 ± 0.6 | 6.1 ± 0.2 | 57.5 ± 0.8 |
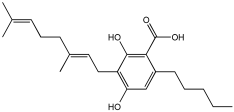 CBGA | 10.5 ± 2.9 | 23.3 ± 12.0 | 1.4 ± 0.1 | 47.7 ± 2.1 |
| Novel MTDLs | Targets Inhibition |
|---|---|
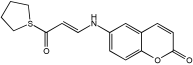 6h MTDLs integrate the Coumarin scaffold | hAChE, hBuChE, GSK-3β, (Aβ) aggregation, and tau protein aggregation [56]. |
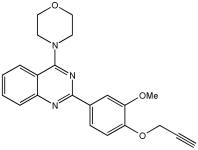 VAV 8 MTDLs integrate the 2-phenylquinazoline scaffold | eeAChE, MAO-B, MAO-A, and eqBuChE [68] |
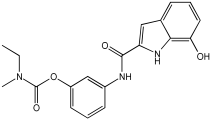 5a3 MTDLs integrate rivastigmine–melatonin scaffolds | Aβ42, Aβ42, hMAO-A, and hMAO-B [59] |
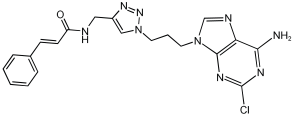 21 Natural product-based MTDLs integrate cinnamamide scaffold | AChE, BChE, MAO-A, and MAO-B [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almaghrabi, M. Multitarget-Directed Ligands for Alzheimer’s Disease: Recent Novel MTDLs and Mechanistic Insights. Pharmaceuticals 2025, 18, 1685. https://doi.org/10.3390/ph18111685
Almaghrabi M. Multitarget-Directed Ligands for Alzheimer’s Disease: Recent Novel MTDLs and Mechanistic Insights. Pharmaceuticals. 2025; 18(11):1685. https://doi.org/10.3390/ph18111685
Chicago/Turabian StyleAlmaghrabi, Mohammed. 2025. "Multitarget-Directed Ligands for Alzheimer’s Disease: Recent Novel MTDLs and Mechanistic Insights" Pharmaceuticals 18, no. 11: 1685. https://doi.org/10.3390/ph18111685
APA StyleAlmaghrabi, M. (2025). Multitarget-Directed Ligands for Alzheimer’s Disease: Recent Novel MTDLs and Mechanistic Insights. Pharmaceuticals, 18(11), 1685. https://doi.org/10.3390/ph18111685







