Design, Synthesis, and Evaluation of Pyrrole-Based Selective MAO-B Inhibitors with Additional AChE Inhibitory and Neuroprotective Properties Identified via Virtual Screening
Abstract
1. Introduction
2. Results and Discussion
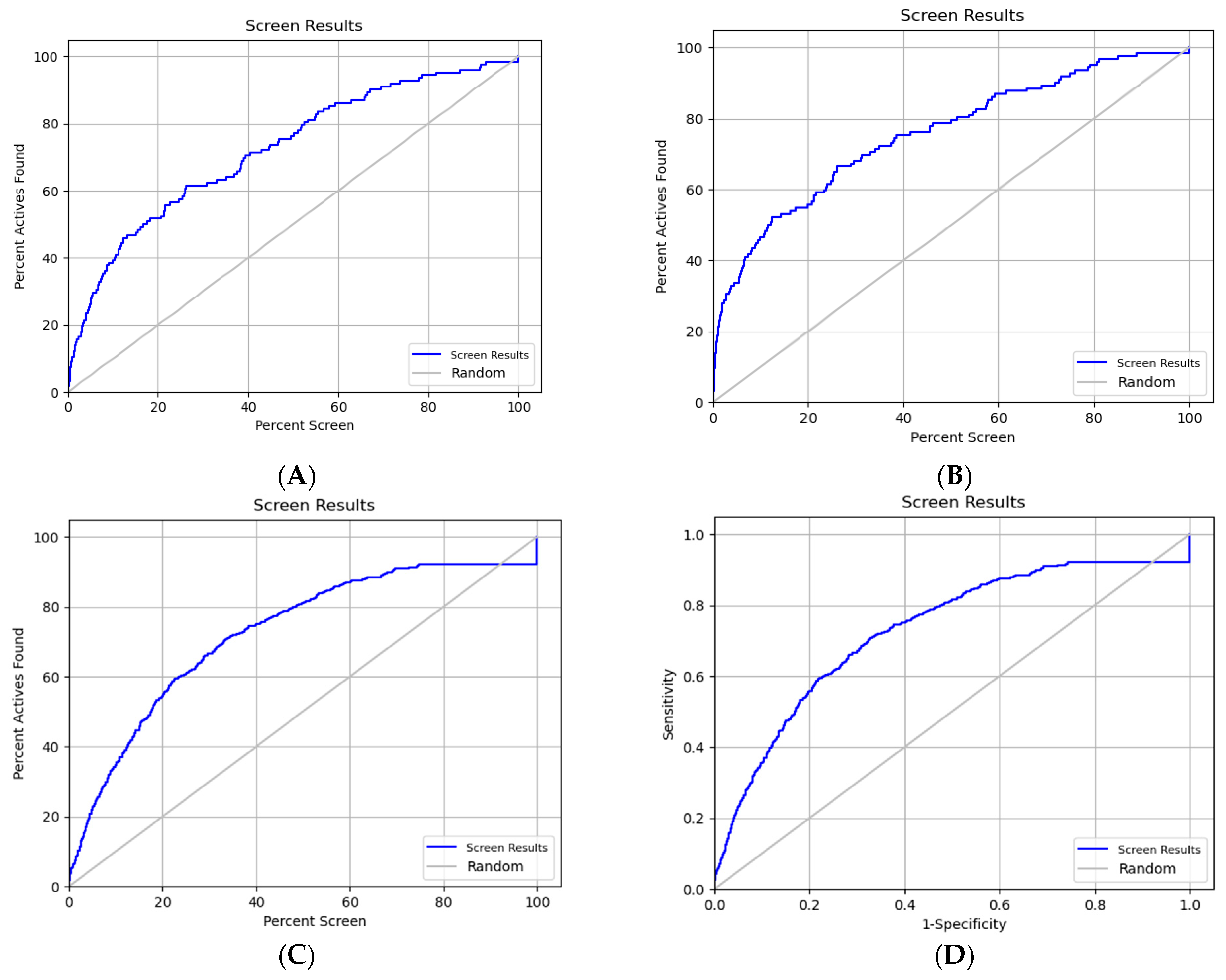
2.1. Validation of the Docking Protocols
2.1.1. Protein Reliability Report
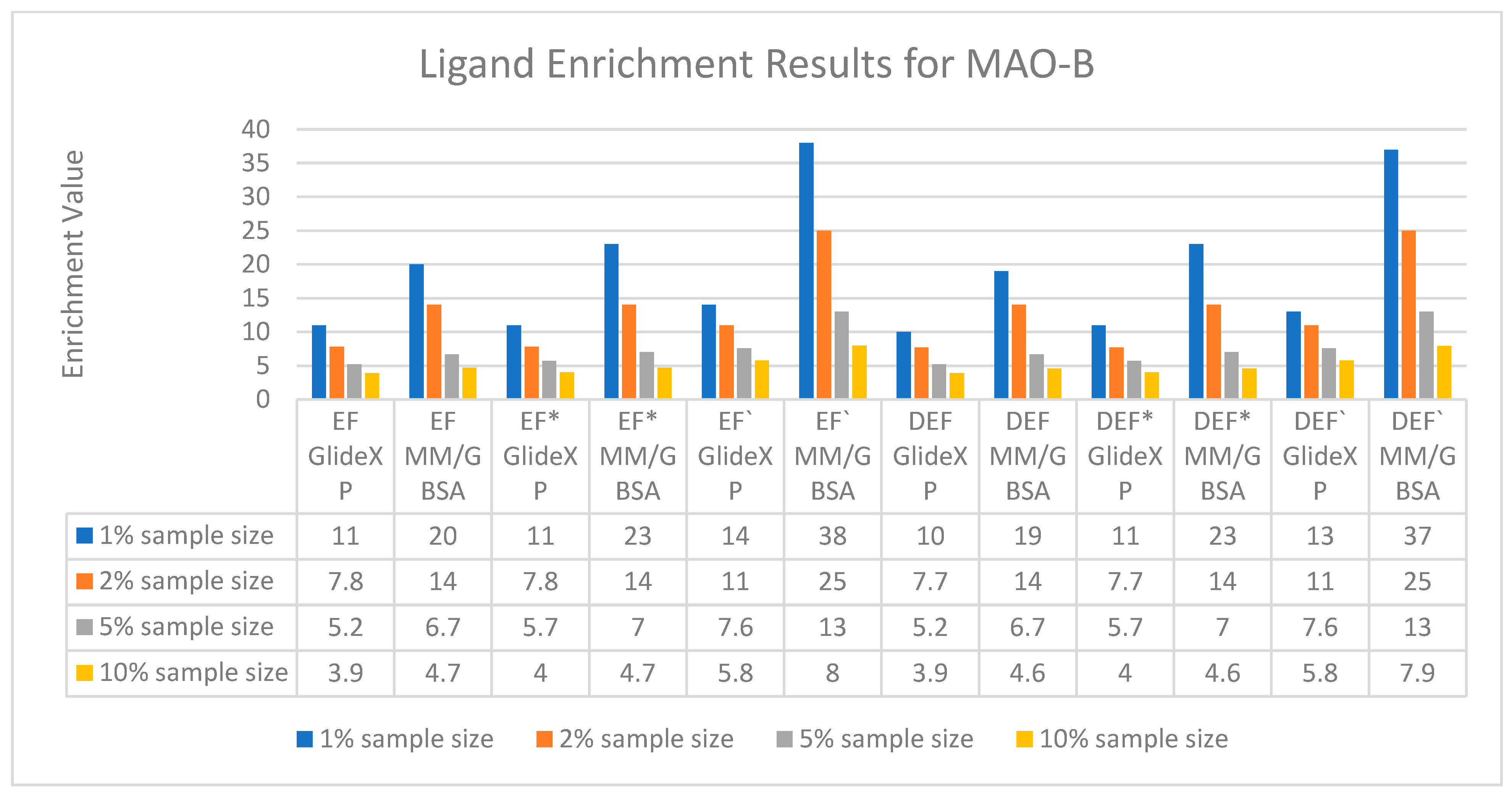
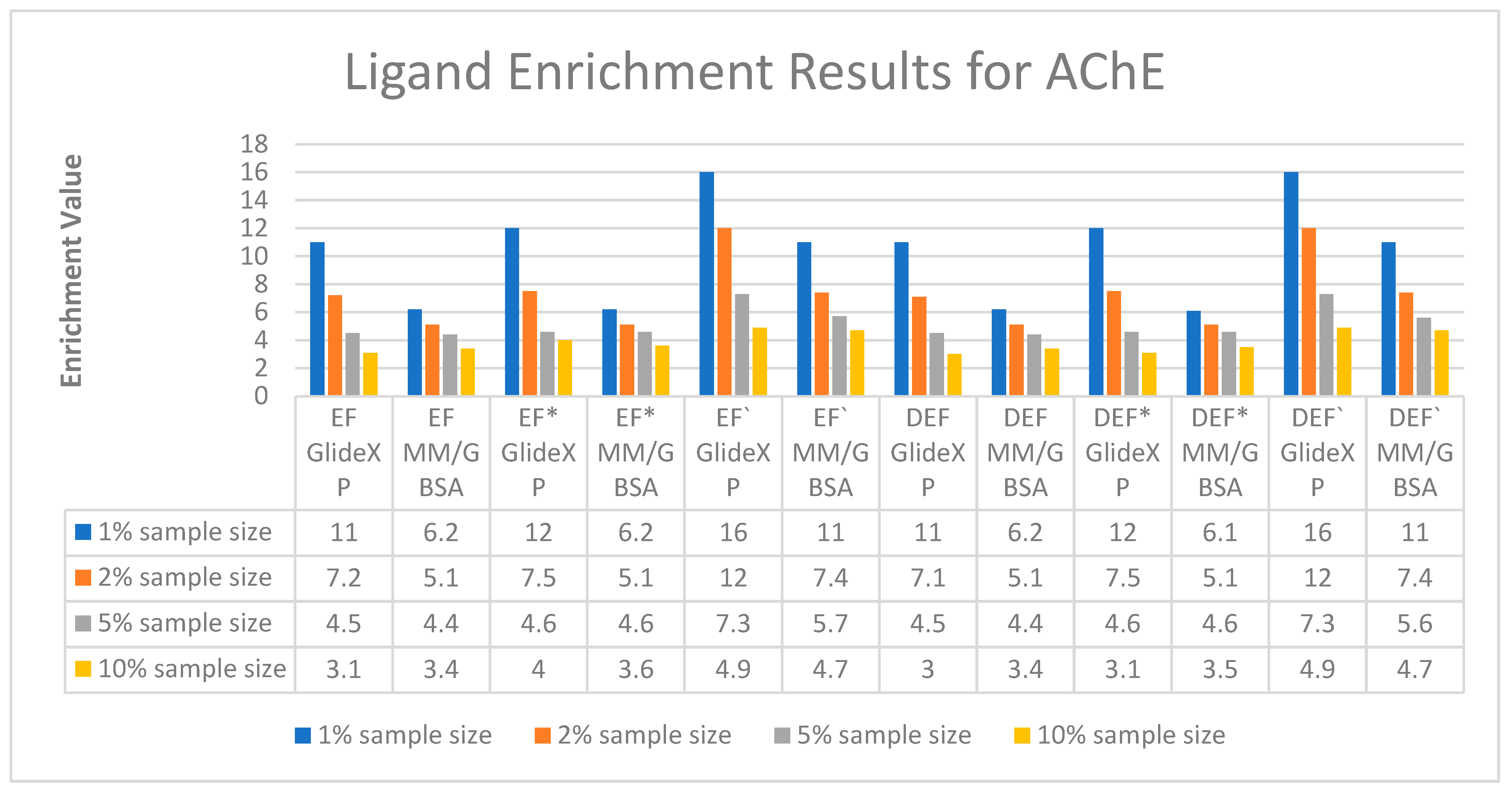
2.1.2. Ligand Enrichments Calculations
2.1.3. Consensus Docking of the Top-Ranked Compounds
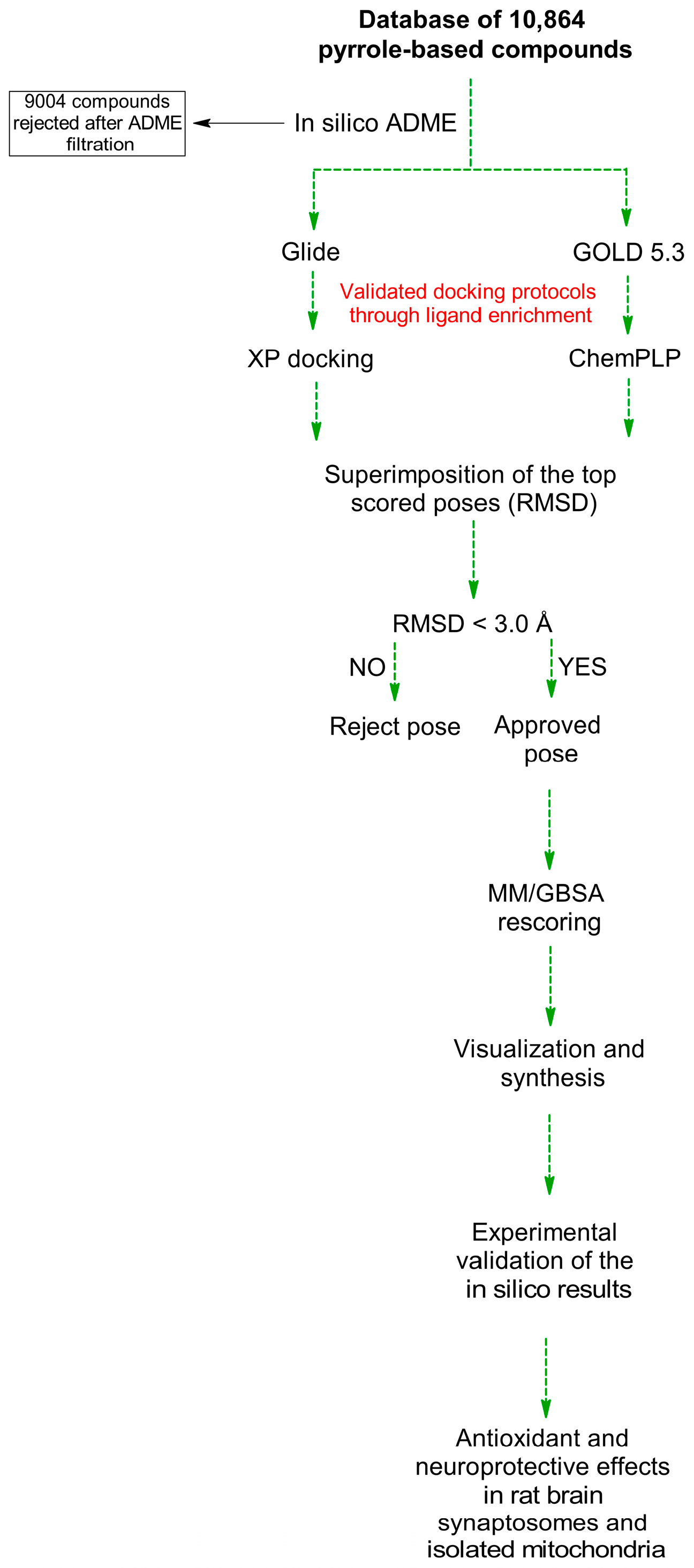
2.2. Virtual Screening of Pyrrole-Based Compounds
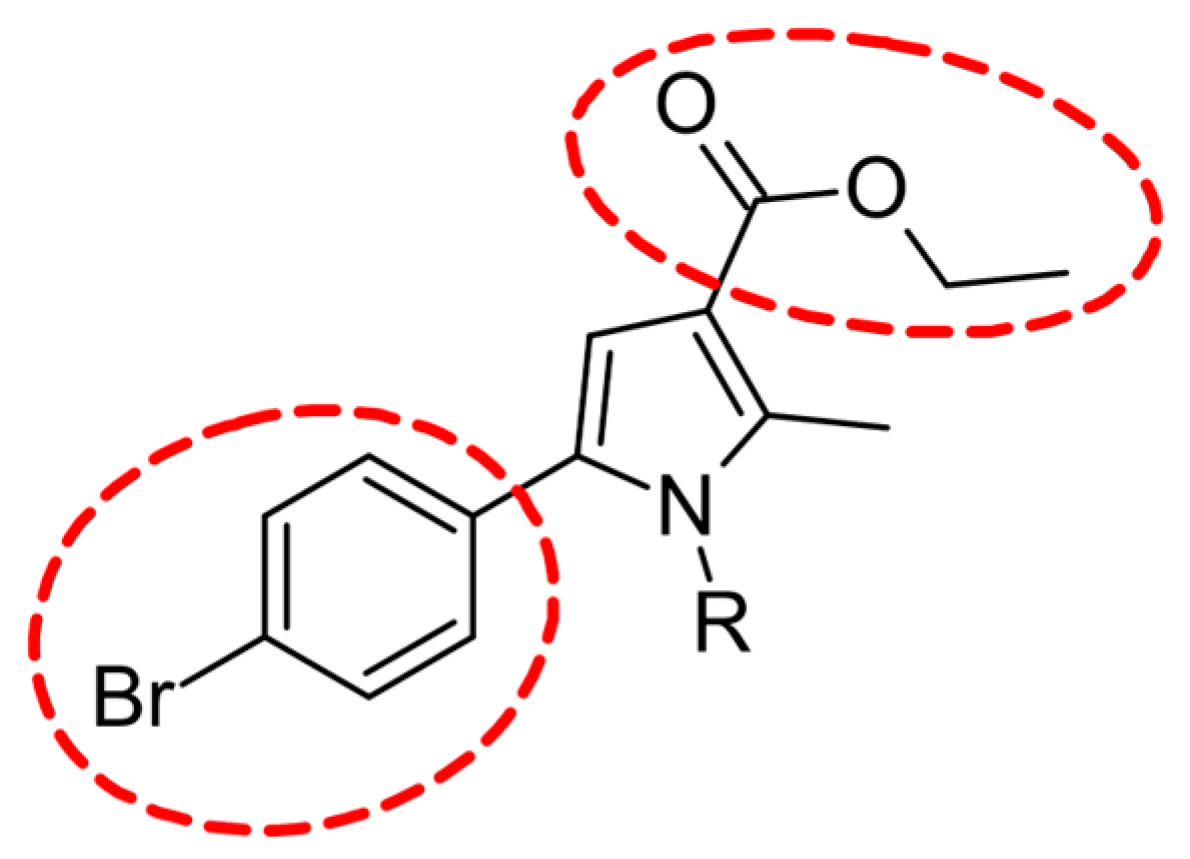
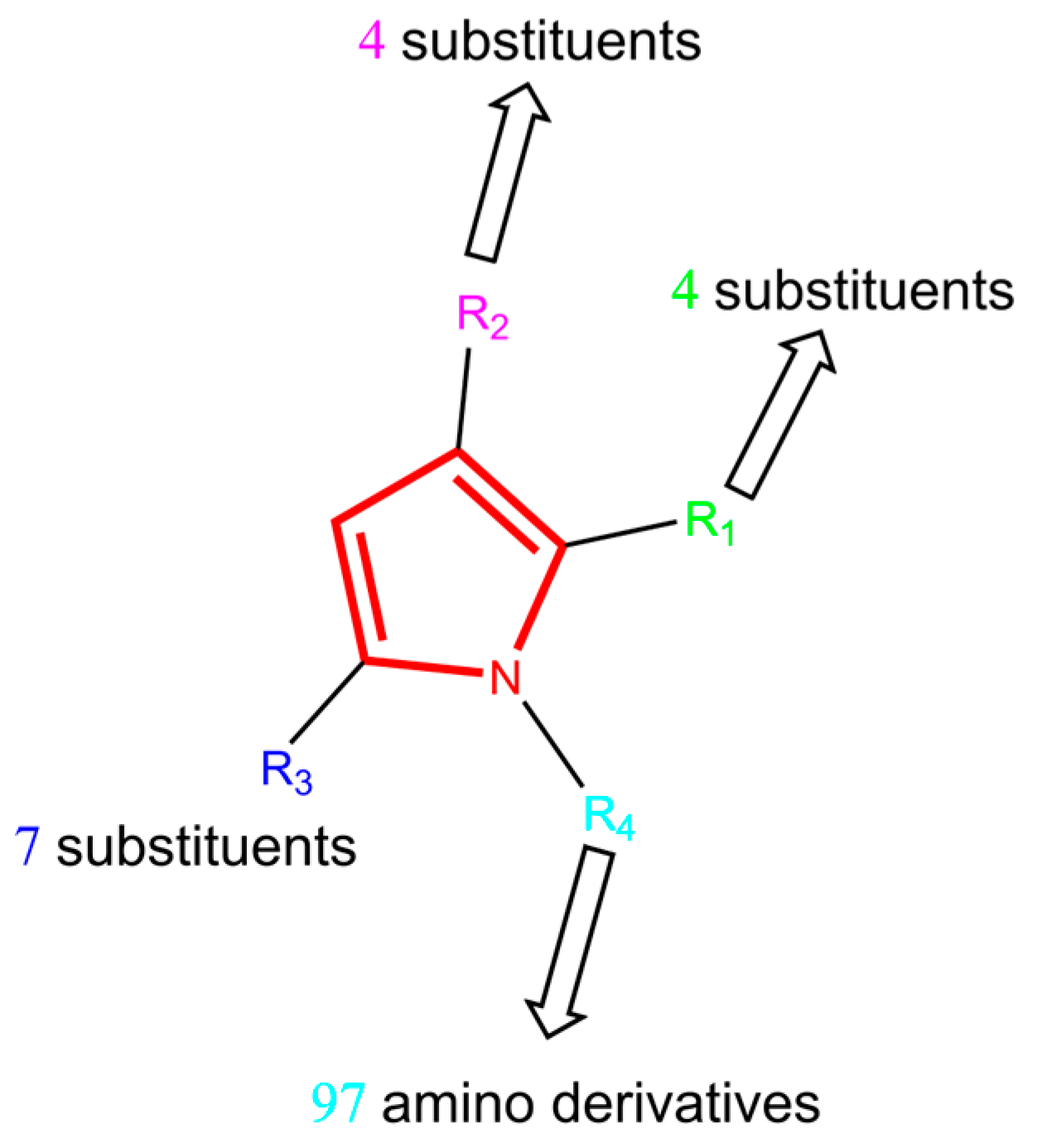
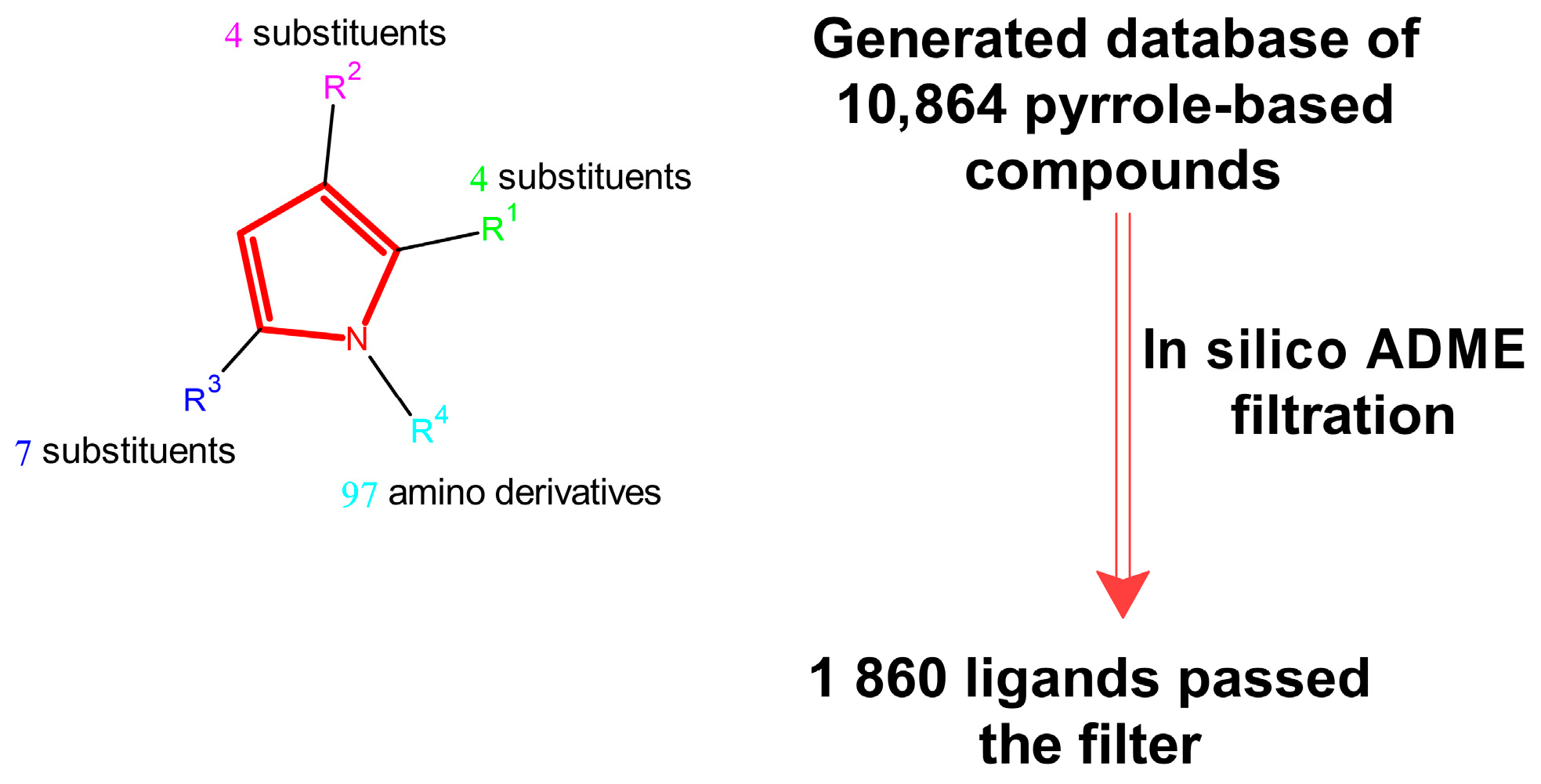
2.2.1. Dataset Selection
2.2.2. Virtual Screening
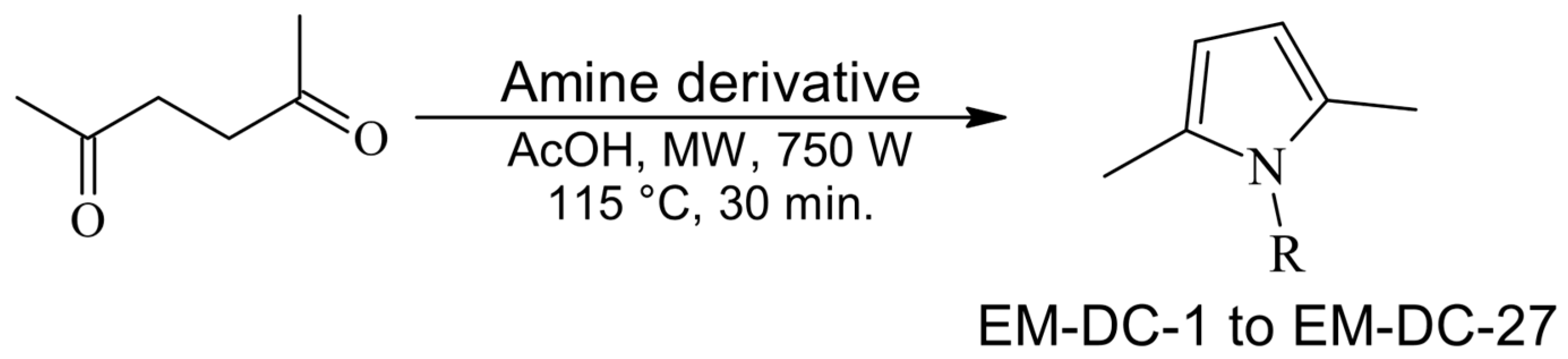
2.3. Synthesis of the Leader Structures
2.4. In Vitro Enzymatic Activity Evaluation of AChE, BChE, MAO-A, and MAO-B
2.5. SAR Analysis
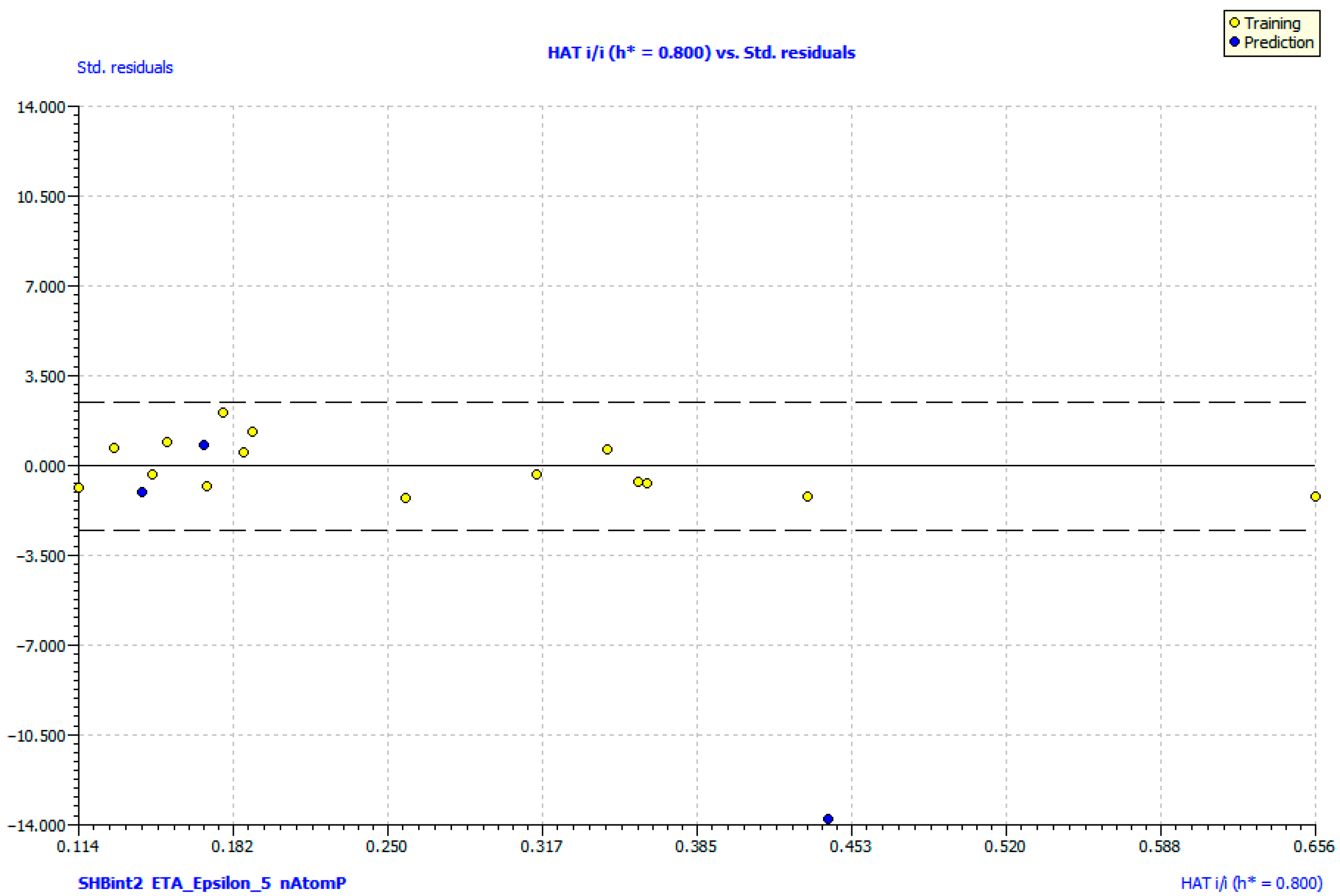
2.6. QSAR Study of BChE Inhibition
- 1.
- SHBint2, a surface-based hydrogen bond descriptor, has a positive coefficient (0.197), suggesting that increased hydrogen bonding enhances inhibitory activity.
- 2.
- ETA_Epsilon_5 has a negative coefficient (−16.205), indicating that higher electronic spatial features decrease activity.
- 3.
- nAtomP, representing the number of atoms in the largest π-system, has a positive coefficient (0.181), showing that extended π-conjugation favors stronger interactions with BChE.
2.7. Molecular Docking in MAO-B and AChE
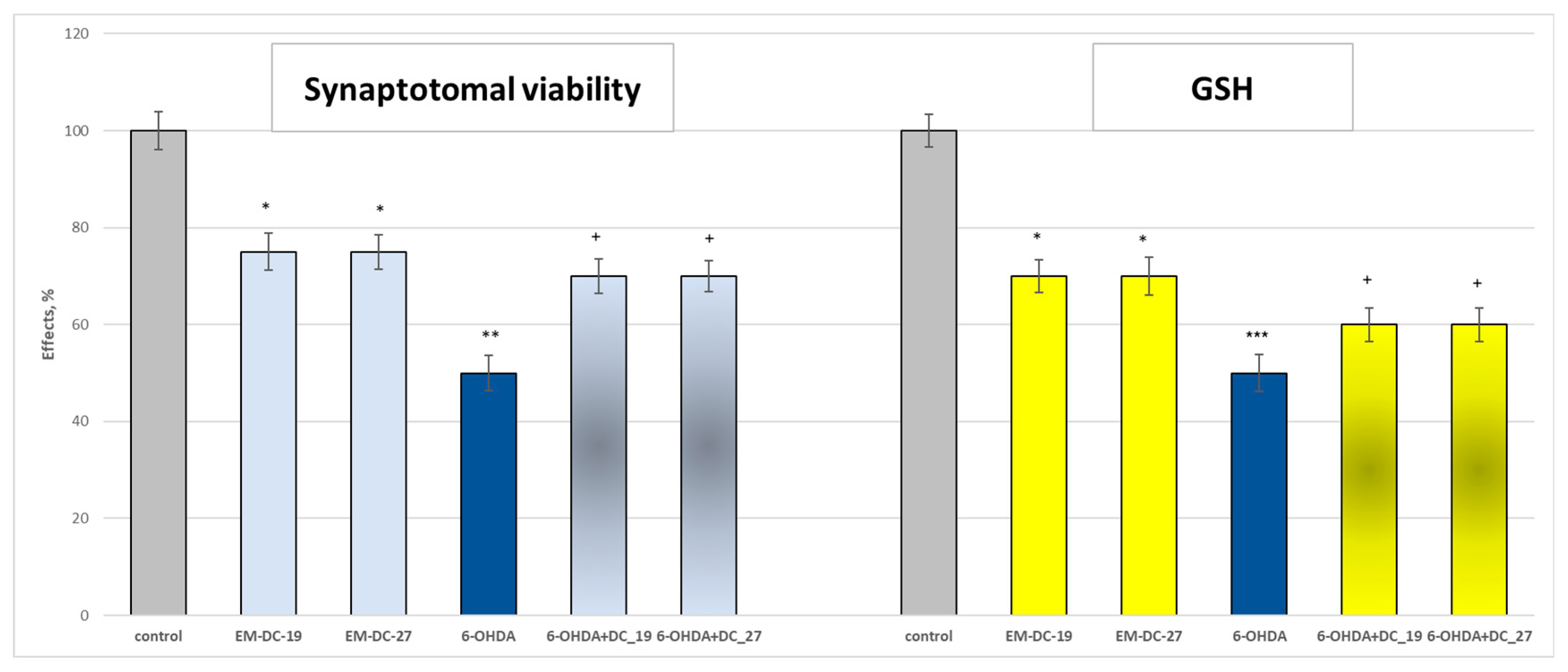
2.8. Effects of the Compounds EM-DC-19 and EM-DC-27 on Isolated Rat Brain Synaptosomes
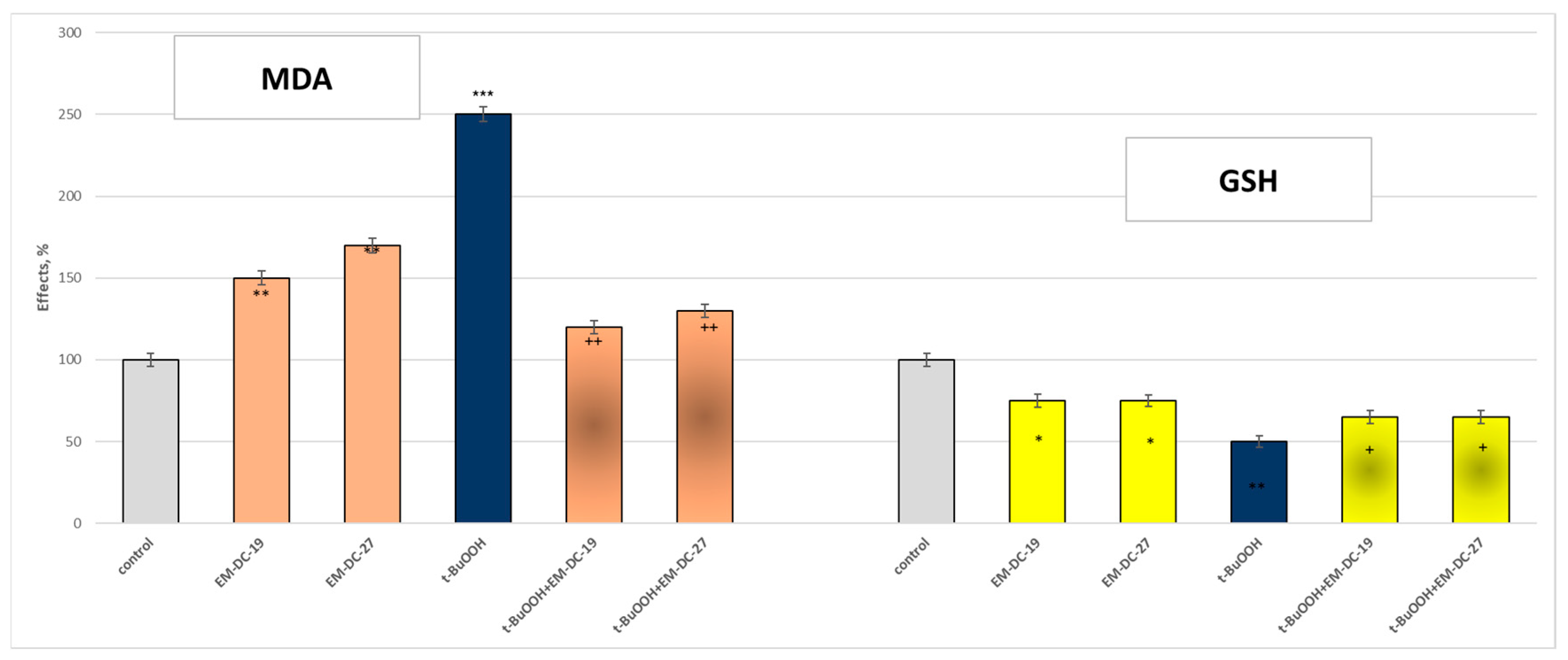
2.9. Effects of the Compounds EM-DC-19 and EM-DC-27 on Isolated Rat Brain Mitochondria
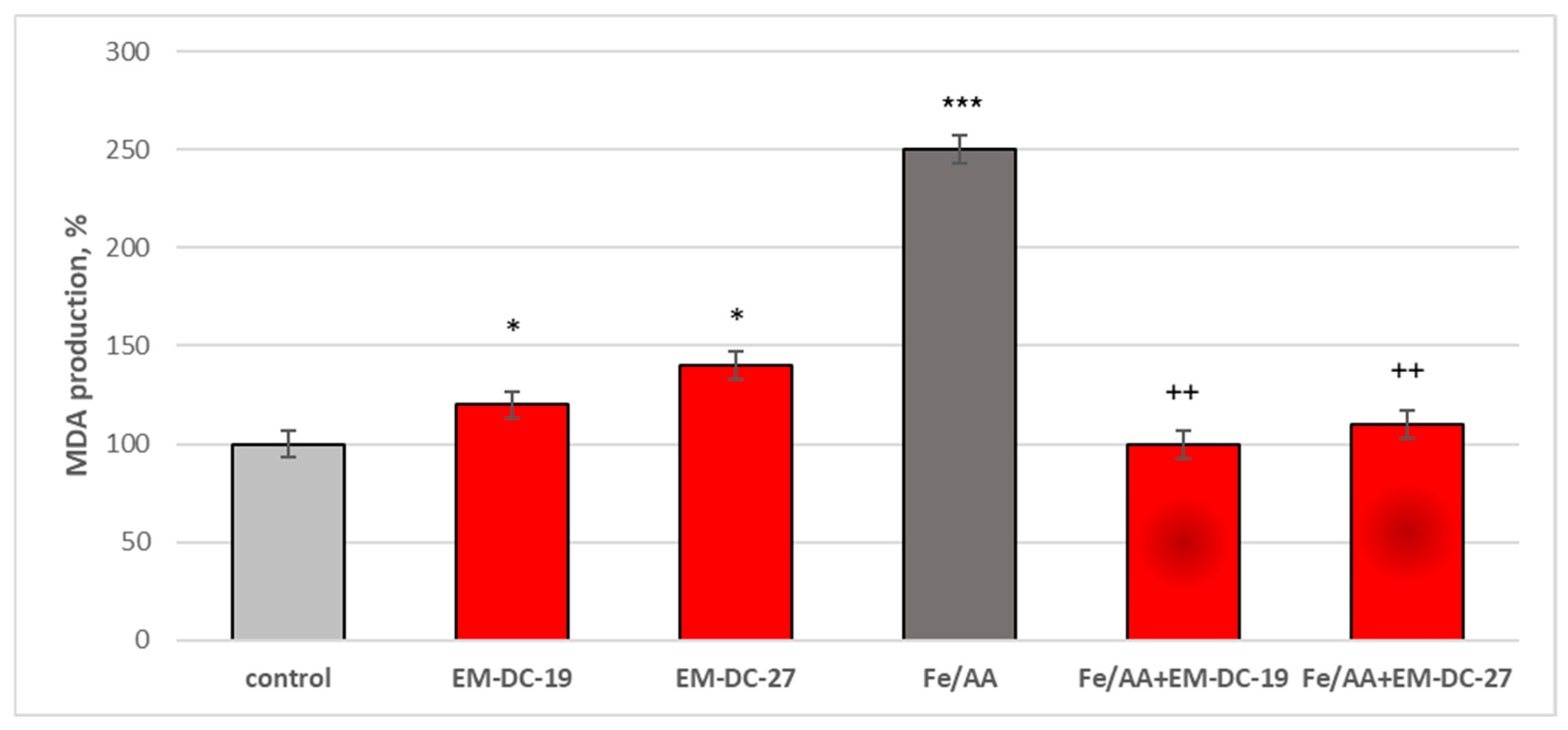
2.10. Effects of the Compounds EM-DC-19 and EM-DC-27 on Isolated Rat Brain Microsomes
2.11. In Silico ADME and Toxicological Profile of the Leader Compounds
3. Materials and Methods
3.1. Virtual Screening
3.2. QSAR Study of BChE Inhibition
3.3. Synthesis
3.4. In Vitro Enzymatic Activity Evaluation of AChE, BChE, MAO-A, and MAO-B
3.5. Animals
3.6. Sub-Cellular in Vitro Studies
3.6.1. Rat Brain Synaptosomes—Isolation and Incubation
3.6.2. Model of 6-OHDA-Induced Neurotoxicity
3.6.3. Synaptosomal Viability
3.6.4. Determination of Reduced Glutathione (GSH)
3.6.5. Rat Brain Microsomes—Preparation
3.6.6. FeSO4/Ascorbic Acid-Induced Lipid Peroxidation (LPO)
3.6.7. Malondialdehyde (MDA) Assay
3.6.8. Rat Brain Mitochondria—Isolation
3.6.9. Tert-Butyl Hydroperoxide (t-BuOOH)-Induced Oxidative Stress
3.6.10. Lipid Peroxidation Assay
3.6.11. Measurement of GSH Content
3.7. Statistical Analysis
3.8. In Silico Knowledge-Based Toxicity Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parkinson Study Group. A Controlled Trial of Rasagiline in Early Parkinson Disease. N. Engl. J. Med. 2002, 347, 1614–1622. [Google Scholar] [CrossRef]
- Morales Casado, M.I.; López Ariztegui, N. Use of Safinamide for Treatment of Parkinson Disease: Real-World Data from Spain. Drugs Context 2025, 14, 1–11. [Google Scholar] [CrossRef]
- Boos, J.; Shubbar, A.; Geldenhuys, W.J. Dual monoamine oxidase B and acetylcholine esterase inhibitors for treating movement and cognition deficits in a C. elegans model of Parkinson’s disease. Med. Chem. Res. 2021, 30, 1166–1174. [Google Scholar] [CrossRef]
- Zou, D.; Liu, R.; Lv, Y.; Guo, J.; Zhang, C.; Xie, Y. Latest advances in dual inhibitors of acetylcholinesterase and monoamine oxidase B against Alzheimer’s disease. J. Enzym. Inhib. Med. Chem. 2023, 38, 2270781. [Google Scholar] [CrossRef]
- Walczak-Nowicka, Ł.J.; Herbet, M. Acetylcholinesterase Inhibitors in the Treatment of Neurodegenerative Diseases and the Role of Acetylcholinesterase in their Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9290. [Google Scholar] [CrossRef]
- Lionta, E.; Spyrou, G.; Vassilatis, D.K.; Cournia, Z. Structure-Based Virtual Screening for Drug Discovery: Principles, Applications and Recent Advances. Curr. Top. Med. Chem. 2014, 14(16), 1923–1938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhu, Y.; Zhang, W.; Sun, Y.; Zhang, Z.; Wang, Y.; Tan, Y.; Yu, L.; Chen, S.; Ma, S.; et al. LigDockTailor: Ligand-specific docking tool matching using multidimensional descriptors. Comput. Biol. Chem. 2025, 119, 108554. [Google Scholar] [CrossRef]
- Mateev, E.; Valkova, I.; Angelov, B.; Georgieva, M.; Zlatkov, A. Validation through Redocking, Cross-docking and Ligand Enrichment in Various Well-Resolved MAO-B Receptors. Int. J. Pharm. Sci. Res. 2022, 13, 1099–1107. [Google Scholar] [CrossRef]
- Mateev, E.; Georgieva, M.; Zlatkov, A. Improved Molecular Docking of MAO-B Inhibitors with Glide. Biomolecules 2023, 13, 159. [Google Scholar] [CrossRef]
- Mateev, E.; Georgieva, M.; Zlatkov, A. Benchmarking Docking Protocols for Virtual Screenings of Novel Acetylcholinesterase Inhibitors. Indian J. Pharm. Sci. 2022, 84, 1525–1534. [Google Scholar] [CrossRef]
- Huang, N.; Shoichet, B.K.; Irwin, J.J. Benchmarking sets for molecular docking. J. Med. Chem. 2006, 49, 6789–6801. [Google Scholar] [CrossRef]
- Kashyap, K.; Bhati, G.; Ahmed, S.; Siddiqi, M.I. Deep Convolutional Neural Network-Based Identification and Biological Evaluation of MAO-B Inhibitors. Int. J. Biol. Macromol. 2024, 281, 136438. [Google Scholar] [CrossRef]
- Liu, F.; Mailhot, O.; Glenn, I.S.; Vigneron, S.F.; Bassim, V.; Xu, X.; Fonseca-Valencia, K.; Smith, M.S.; Radchenko, D.S.; Fraser, J.S.; et al. The Impact of Library Size and Scale of Testing on Virtual Screening. bioRxiv 2024, 21, 1039–1045. [Google Scholar] [CrossRef]
- Méndez, R.; Leplae, R.; Licata, L.; Fernández-Recio, J. Assessment of Blind Predictions of Protein–Protein Interactions: Current Status of Docking Methods. Proteins 2003, 52, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Chilingaryan, G.; Abelyan, N.; Sargsyan, A.; Nazaryan, K.; Serobian, A.; Zakaryan, H. Combination of consensus and ensemble docking strategies for the discovery of human dihydroorotate dehydrogenase inhibitors. Sci. Rep. 2021, 11, 11417. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, R.; Fukunishi, H. Supervised Consensus Scoring for Docking and Virtual Screening. J. Chem. Inf. Model. 2007, 47, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Mateev, E.; Irfan, A.; Mateeva, A.; Kondeva-Burdina, M.; Georgieva, M.; Zlatkov, A. In Silico and In Vitro Screening of Pyrrole-Based Hydrazide-Hydrazones as Novel Acetylcholinesterase Inhibitors. Pharmacia 2024, 71, 1–7. [Google Scholar] [CrossRef]
- Mateev, E.; Karatchobanov, V.; Dedja, M.; Diamantakos, K.; Mateeva, A.; Muhammed, M.T.; Irfan, A.; Kondeva-Burdina, M.; Valkova, I.; Georgieva, M.; et al. Novel Pyrrole Derivatives as Multi-Target Agents for the Treatment of Alzheimer’s Disease: Microwave-Assisted Synthesis, In Silico Studies and Biological Evaluation. Pharmaceuticals 2024, 17, 1171. [Google Scholar] [CrossRef]
- Tzvetkov, N.T.; Stammler, H.-G.; Hristova, S.; Atanasov, A.G.; Antonov, L. (Pyrrolo-pyridin-5-yl)benzamides: BBB Permeable Monoamine Oxidase B Inhibitors with Neuroprotective Effect on Cortical Neurons. Eur. J. Med. Chem. 2019, 162, 793–809. [Google Scholar] [CrossRef]
- Grychowska, K.; Olejarz-Maciej, A.; Blicharz, K.; Pietruś, W.; Karcz, T.; Kurczab, R.; Koczurkiewicz, P.; Doroz-Płonka, A.; Latacz, G.; Keeri, A.R.; et al. Overcoming Undesirable hERG Affinity by Incorporating Fluorine Atoms: A Case of MAO-B Inhibitors Derived from 1 H-Pyrrolo-[3,2-c]quinolines. Eur. J. Med. Chem. 2022, 236, 114329. [Google Scholar] [CrossRef]
- Sharma, A.; Rudrawar, S.; Sharma, A.; Bharate, S.B.; Jadhav, H.R. Design, synthesis, in silico, and in vitro evaluation of pyrrol-2-yl-phenyl allylidene hydrazine carboximidamide derivatives as AChE/BACE 1 dual inhibitors. RSC Adv. 2024, 14, 26703–26722. [Google Scholar] [CrossRef]
- Khan, S.; Iqbal, T.; Khan, M.B.; Hussain, R.; Khan, Y.; Darwish, H.W. Novel Pyrrole Based Triazole Moiety as Therapeutic Hybrid: Synthesis, Characterization and Anti-Alzheimer Potential with Molecular Mechanism of Protein Ligand Profile. BMC Chem. 2024, 18, 223. [Google Scholar] [CrossRef]
- Binda, C.; Wang, J.; Pisani, L.; Caccia, C.; Carotti, A.; Salvati, P.; Edmondson, D.E.; Mattevi, A. Structures of Human Monoamine Oxidase B Complexes with Selective Noncovalent Inhibitors: Safinamide and Coumarin Analogs. J. Med. Chem. 2007, 50, 5848–5852. [Google Scholar] [CrossRef] [PubMed]
- Oprea, T.I. Virtual Screening in Lead Discovery: A Viewpoint. Molecules 2002, 7, 51–62. [Google Scholar] [CrossRef]
- Pacureanu, L.; Bora, A.; Crisan, L. New insights on the activity and selectivity of MAO-B inhibitors through in silico methods. Int. J. Mol. Sci. 2023, 24, 9583. [Google Scholar] [CrossRef]
- Philkhana, S.C.; Badmus, F.O.; Dos Reis, I.C.; Kartika, R. Recent Advancements in Pyrrole Synthesis. Synthesis 2021, 53, 1531–1555. [Google Scholar] [CrossRef]
- Manger, M.; Sobott, F.; Buchholz, S.; Kammerer, B.; Kaufmann, S.H.E. Discovery of Mycobacterium Tuberculosis Protein Tyrosine Phosphatase A (MptpA) Inhibitors Based on Natural Products and a Fragment-Based Approach. ChemBioChem 2005, 6, 1749–1753. [Google Scholar] [CrossRef]
- Marvi, O.; Taherpour Nahzomi, H. Grinding Solvent-Free Paal-Knorr Pyrrole Synthesis on Smectites as Recyclable and Green Catalysts. Bull. Chem. Soc. Ethiop. 2018, 32, 140–150. [Google Scholar] [CrossRef]
- Ahmed, K.; Shaikh, F.; Malik, S.M.; Moku, B.; Shaik, B.; Siddiqui, M.R.H.; Alarifi, A. Convenient Synthesis of Substituted Pyrroles via a Cerium (IV) Ammonium Nitrate (CAN)-Catalyzed Paal-Knorr Reaction. Arab. J. Chem. 2016, 9, 542–549. [Google Scholar] [CrossRef]
- Danks, T.N.; Keller, P.A.; Lockhart, K.J. Microwave Assisted Synthesis of Pyrroles. Tetrahedron Lett. 1999, 40, 3957–3960. [Google Scholar] [CrossRef]
- Akbaşlar, D.; Demirkol, O.; Giray, S. Paal-Knorr Pyrrole Synthesis in Water. Synth. Commun. 2014, 44, 1323–1332. [Google Scholar] [CrossRef]
- Kaur, R.; Rani, V.; Abbot, V.; Kapoor, Y.; Konar, D.; Kumar, K. Recent Synthetic and Medicinal Perspectives of Pyrroles: An Overview. J. Pharm. Chem. Chem. Sci. 2017, 1, 17–32. [Google Scholar]
- Rambabu, D.; Rama Krishna, G.; Basavoju, S.; Reddy, C.M.; Pal, M. Crystal structure and synthesis of 4-(4-hydroxybenzylideneamino)-1-methyl-3-propyl-1H-pyrazole-5-carboxamide. J. Mol. Struct. 2011, 994, 332–334. [Google Scholar] [CrossRef]
- Saletti, M.; Maramai, S.; Reale, A.; Paolino, M.; Brogi, S.; Di Capua, A.; Cappelli, A.; Giorgi, G.; D’Avino, D.; Rossi, A.; et al. Novel analgesic/anti-inflammatory agents: 1,5-Diarylpyrrole nitrooxyethyl sulfides and related compounds as Cyclooxygenase-2 inhibitors containing a nitric oxide donor moiety endowed with vasorelaxant properties. Eur. J. Med. Chem. 2022, 241, 114615. [Google Scholar] [CrossRef]
- Naddeo, S.; Gentile, D.; Margani, F.; Prioglio, G.; Magaletti, F.; Galimberti, M.; Barbera, V. Pyrrole Compounds from the Two-Step One-Pot Conversion of 2,5-Dimethylfuran for Elastomer Composites with Low Dissipation of Energy. Molecules 2024, 29, 861. [Google Scholar] [CrossRef]
- Kappe, C.O. Controlled Microwave Heating in Modern Organic Synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- Lyu, J.; Bender, B.; Gahbauer, S.; Luttens, A.; Webb, C.; Stein, R.; Fink, E.; Balius, T.; Carlsson, J.; Irwin, J.J.; et al. A Practical Guide to Large-Scale Docking. Nat. Protoc. 2021, 16, 5249–5274. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Li, S.; Li, A.J.; Zhao, J.; Sakamuru, S.; Huang, W.; Xia, M.; Huang, R. Identification of potent and selective acetylcholinesterase/butyrylcholinesterase inhibitors by virtual screening. J. Chem. Inf. Model. 2023, 63, 2321–2330. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, H.; Chen, Y.; Sun, H. Recent progress in the identification of selective butyrylcholinesterase inhibitors for Alzheimer’s disease. Eur. J. Med. Chem. 2017, 132, 294–309. [Google Scholar] [CrossRef]
- Budryn, G.; Majak, I.; Grzelczyk, J.; Szwajgier, D.; Rodríguez-Martínez, A.; Pérez-Sánchez, H. Hydroxybenzoic Acids as Acetylcholinesterase Inhibitors: Calorimetric and Docking Simulation Studies. Nutrients 2022, 14, 2476. [Google Scholar] [CrossRef]
- Mathew, B.; Carradori, S.; Guglielmi, P.; Uddin, M.S.; Kim, H. New Aspects of Monoamine Oxidase B Inhibitors: The Key Role of Halogens to Open the Golden Door. Curr. Med. Chem. 2021, 28, 266–283. [Google Scholar] [CrossRef]
- Cavalli, A.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Melchiorre, C. Multi-Target-Directed Ligands to Combat Neurodegenerative Diseases. J. Med. Chem. 2008, 51, 347–372. [Google Scholar] [CrossRef]
- Binda, C.; Li, M.; Hubálek, F.; Restelli, N.; Pollegioni, L.; Edmondson, D.E.; Mattevi, A. Functional Role of the “Aromatic Cage” in Human Monoamine Oxidase B: Structures and Catalytic Properties of Tyr435 Mutant Proteins. Biochemistry 2006, 45, 4775–4784. [Google Scholar] [CrossRef]
- Ariel, N.; Ordentlich, A.; Barak, D.; Bino, T.; Velan, B.; Shafferman, A. The “Aromatic Patch” of Three Proximal Residues in the Human Acetylcholinesterase Active Centre Allows for Versatile Interaction Modes with Inhibitors. Biochem. J. 1998, 335, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Bian, J.-S. Neuroprotective Effects of Hydrogen Sulfide in Parkinson’s Disease Animal Models: Methods and Protocols. Methods Enzymol. 2015, 554, 169–186. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, V.; Burkitt, M.J. Mitochondrial Metabolism of a Hydroperoxide to Free Radicals in Human Endothelial Cells: An Electron Spin Resonance Spin-Trapping Investigation. Biochem. J. 1994, 304, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Gramatica, P.; Chirico, N.; Papa, E.; Cassani, S.; Kovarich, S. QSARINS: A New Software for the Development, Analysis, and Validation of QSAR MLR Models. J. Comput. Chem. 2013, 34, 2121–2132. [Google Scholar] [CrossRef]
- Yap, C.W. PaDEL-descriptor An open source software to calculate molecular descriptors and fingerprints. J. Comput. Chem. 2011, 32, 1466–1474. [Google Scholar] [CrossRef]
- Abchir, O.; Khedraoui, M.; Nour, H.; Yamari, I.; Errougui, A.; Samadi, A.; Chtita, S. Integrative Approach for Designing Novel Triazole Derivatives as α-Glucosidase Inhibitors: QSAR, Molecular Docking, ADMET, and Molecular Dynamics Investigations. Pharmaceuticals 2024, 17, 261. [Google Scholar] [CrossRef]
- Karim, E.M.; Abchir, O.; Nour, H.; Daoui, O.; El Khattabi, S.; Siddique, F.; El Kouali, M.; Talbi, M.; Errougui, A.; Chtita, S. In Silico Design of Novel Piperazine-Based mTORC1 Inhibitors Through DFT, QSAR and ADME Investigations. Biophysica 2024, 4, 517–529. [Google Scholar] [CrossRef]
- Khedraoui, M.; Abchir, O.; Nour, H.; Yamari, I.; Errougui, A.; Samadi, A.; Chtita, S. An In Silico Study Based on QSAR and Molecular Docking and Molecular Dynamics Simulation for the Discovery of Novel Potent Inhibitor against AChE. Pharmaceuticals 2024, 17, 830. [Google Scholar] [CrossRef]
- Bautista-Aguilera, O.M.; Esteban, G.; Bolea, I.; Nikolic, K.; Agbaba, D.; Moraleda, I.; Iriepa, I.; Samadi, A.; Soriano, E.; Unzeta, M.; et al. Design, Synthesis, Pharmacological Evaluation, QSAR Analysis, Molecular Modeling and ADMET of Novel Donepezil–Indolyl Hybrids as Multipotent Cholinesterase/Monoamine Oxidase Inhibitors for the Potential Treatment of Alzheimer’s Disease. Eur. J. Med. Chem. 2014, 75, 82–95. [Google Scholar] [CrossRef]
- Kasabova-Angelova, A.; Kondeva-Burdina, M.; Mitkov, J.; Georgieva, M.; Tzankova, V.; Zlatkov, A. Neuroprotective and MAOB Inhibitory Effects of a Series of Caffeine-8-Thioglycolic Acid Amides. Braz. J. Pharm. Sci. 2020, 56, e18255. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- López, S.; Bastida, J.; Viladomat, F.; Codina, C. Acetylcholinesterase Inhibitory Activity of Some Amaryllidaceae Alkaloids and Narcissus Extracts. Life Sci. 2002, 71, 2521–2529. [Google Scholar] [CrossRef]
- ISO 9001:2008; Quality Management Systems—Requirements. International Organization for Standardization: Geneva, Switzerland, 2008.
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Taupin, P.; Zini, S.; Cesselin, F.; Ben-Ari, Y.; Roisin, M.P. Subcellular fractionation on Percoll gradient of mossy fiber synaptosomes: Morphological and biochemical characterization in control and degranulated rat hippocampus. J. Neurochem. 1994, 62, 1586–1595. [Google Scholar] [CrossRef]
- Mungarro-Menchaca, X.; Ferrera, P.; Morán, J.; Arias, C. Beta-Amyloid Peptide Induces Ultrastructural Changes in Synaptosomes and Potentiates Mitochondrial Dysfunction in the Presence of Ryanodine. J. Neurosci. Res. 2002, 68, 89–96. [Google Scholar] [CrossRef]
- Ravindranath, V.; Anandatheerthavarada, H.K. Preparation of Brain Microsomes with Cytochrome P450 Activity Using Calcium Aggregation Method. Anal. Biochem. 1990, 187, 310–313. [Google Scholar] [CrossRef]
- Mansuy, D.; Sassi, A.; Dansette, P.M.; Plat, M. A New Potent Inhibitor of Lipid Peroxidation In Vitro and In Vivo, the Hepatoprotective Drug Anisyldithiolthione. Biochem. Biophys. Res. Commun. 1986, 135, 1015–1021. [Google Scholar] [CrossRef]
- Deby, C.; Goutier, R. New Perspectives on the Biochemistry of Superoxide Anion and the Efficiency of Superoxide Dismutases. Biochem. Pharmacol. 1990, 39, 399–405. [Google Scholar] [CrossRef]
- Sims, N.R.; Anderson, M.F. Isolation of Mitochondria from Rat Brain Using Percoll Density Gradient Centrifugation. Nat. Protoc. 2008, 3, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; Emgard, M.; Brundin, P.; Burkitt, M.J. trans-Resveratrol Protects Embryonic Mesencephalic Cells from tert-Butyl Hydroperoxide: Electron Paramagnetic Resonance Spin-Trapping Investigation. J. Neurochem. 2001, 75, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Shirani, M.; Alizadeh, S.; Mahdavinia, M.; Dehghani, M.A. The Ameliorative Effect of Quercetin on Bisphenol A-Induced Toxicity in Mitochondria Isolated from Rats. Environ. Sci. Pollut. Res. 2019, 26, 7688–7696. [Google Scholar] [CrossRef] [PubMed]




 |  |
| A | B |
| Compound | MAO-B (PDB: 2V5Z) | RMSD Value (Å) | AChE (PDB: 4EY6) | RMSD Value (Å) | Synthetic Accessibility (SA) * | ||
|---|---|---|---|---|---|---|---|
| MM/GBSA (kcal/mol) | GOLD 5.3 (ChemPLP) | MM/GBSA (kcal/mol) | GOLD 5.3 (ChemPLP) | ||||
| EM-DC-1 | −61.54 | 152.21 | 1.47 | −52.28 | 110.31 | 1.25 | 1.50 |
| EM-DC-2 | −60.32 | 142.63 | 1.54 | −53.43 | 123.26 | 1.22 | 1.55 |
| EM-DC-3 | −60.18 | 150.23 | 2.24 | −50.53 | 112.53 | 1.63 | 1.75 |
| EM-DC-4 | −59.84 | 146.72 | 0.84 | −53.28 | 106.77 | 1.58 | 1.64 |
| EM-DC-5 | −59.79 | 142.48 | 0.33 | −48.45 | 102.73 | 0.71 | 1.90 |
| EM-DC-6 | −59.74 | 147.36 | 2.87 | −54.67 | 107.54 | 1.52 | 2.34 |
| EM-DC-7 | −59.69 | 151.53 | 0.91 | −55.48 | 111.92 | 2.42 | 1.57 |
| EM-DC-8 | −59.62 | 152.48 | 0.58 | −52.81 | 122.68 | 1.52 | 1.72 |
| EM-DC-9 | −59.55 | 145.27 | 2.94 | −54.23 | 105.65 | 1.33 | 1.60 |
| EM-DC-10 | −59.57 | 150.64 | 1.59 | −51.51 | 115.51 | 2.03 | 2.27 |
| EM-DC-11 | −59.51 | 149.63 | 0.46 | −47.28 | 100.57 | 1.62 | 2.15 |
| EM-DC-12 | −59.45 | 144.72 | 1.25 | −53.59 | 107.48 | 2.73 | 1.57 |
| EM-DC-13 | −58.28 | 147.62 | 1.91 | −57.92 | 101.83 | 1.04 | 2.00 |
| EM-DC-14 | −58.24 | 148.26 | 1.07 | −58.54 | 103.54 | 2.43 | 1.49 |
| EM-DC-15 | −58.17 | 150.62 | 1.46 | −48.56 | 112.34 | 1.63 | 2.26 |
| EM-DC-16 | −57.92 | 151.71 | 1.82 | −48.37 | 108.45 | 1.34 | 2.03 |
| EM-DC-17 | −57.84 | 148.64 | 0.87 | −51.91 | 115.34 | 2.38 | 1.83 |
| EM-DC-18 | −57.83 | 149.13 | 2.64 | −56.57 | 108.94 | 1.28 | 1.78 |
| EM-DC-19 | −57.76 | 150.27 | 1.57 | −55.43 | 122.45 | 1.36 | 2.94 |
| EM-DC-20 | −57.72 | 148.60 | 1.44 | −54.41 | 107.94 | 1.72 | 2.42 |
| EM-DC-21 | −52.37 | 116.22 | 1.67 | −43.20 | 103.47 | 2.53 | 2.04 |
| EM-DC-22 | −51.32 | 112.67 | 0.84 | −53.13 | 122.15 | 1.48 | 2.12 |
| EM-DC-23 | −54.02 | 120.31 | 0.67 | −47.27 | 104.37 | 2.23 | 2.56 |
| EM-DC-24 | −53.41 | 112.64 | 1.31 | −48.85 | 121.84 | 1.08 | 1.79 |
| EM-DC-25 | −53.92 | 113.79 | 0.83 | −51.54 | 114.15 | 1.48 | 2.75 |
| EM-DC-26 | −50.28 | 121.37 | 2.74 | −47.16 | 111.28 | 1.57 | 1.76 |
| EM-DC-27 | −54.24 | 138.82 | 1.94 | −43.81 | 101.28 | 2.82 | 1.71 |
| Galanthamine | n/a | n/a | n/a | −62.58 | 121.25 | 1.08 | |
| Donepezil | n/a | n/a | n/a | −83.76 | 154.81 | 0.84 | |
| Selegiline | −55.28 | 144.51 | 0.47 | n/a | n/a | n/a | |
| Compound | Inhibition Activity 200 µM AchE a | AChE IC50 (µM) b | Inhibition Activity 200 µM BChE a | BChE IC50 (µM) b | Inhibition Activity 1 µM MAO-A a | MAO-A IC50 (µM) b | Inhibition Activity 1 µM MAO-B a | MAO-B IC50 (µM) b |
|---|---|---|---|---|---|---|---|---|
| EM-DC-1 | 7.52 ± 2.07 | >200 | 53.93 ± 2.14 | 172.70 ± 7.30 | 55 ± 7.1 | 0.32 ± 0.10 | 99 ± 7.1 | >100 |
| EM-DC-2 | 93.60 ± 4.66 | 0.75 ± 0.06 | 57.25 ± 0.58 | 158.06 ± 11.76 | 75 ± 6.9 | 0.43 ± 0.10 | 75 ± 7.1 | 0.44 ± 0.10 |
| EM-DC-3 | 8.32 ± 2.64 | >200 | 0.04 ± 9.01 | >200 | 70 ± 7.2 | 0.52 ± 0.10 | 75 ± 7.1 | 0.56 ± 0.10 |
| EM-DC-4 | 1.40 ± 0.38 | >200 | 0 | >200 | 60 ± 6.8 | 0.32 ± 0.10 | 98 ± 7.1 | >100 |
| EM-DC-5 | 39.78 ± 2.64 | 280.46 ± 21.27 | 74.02 ± 1.56 | 73.81 ± 5.96 | 98 ± 7.3 | >100 | 99 ± 7.1 | >100 |
| EM-DC-6 | 64.98 ± 1.14 | 128.30 ± 1.87 | 36.26 ± 3.54 | 330.20 ± 59.77 | 97 ± 7.1 | >100 | 98 ± 7.1 | >100 |
| EM-DC-7 | 11.90 ± 2.33 | >200 | 43.86 ± 1.70 | 224.26 ± 8.12 | 80 ± 7.2 | 0.51 ± 0.10 | 75 ± 7.1 | 0.49 ± 0.10 |
| EM-DC-8 | 31.12 ± 2.48 | 455.33 ± 80.71 | 35.42 ± 2.91 | 259.73 ± 13.47 | 99 ± 7.3 | >100 | 99 ± 7.1 | >100 |
| EM-DC-9 | 72.86 ± 3.02 | 76.84 ± 4.68 | 73.70 ± 4.50 | 69.51 ± 7.52 | 98 ± 6.8 | >100 | 98 ± 7.1 | >100 |
| EM-DC-10 | 60.35 ± 1.05 | 147.53 ± 5.91 | 87.17 ± 1.60 | 11.33 ± 0.87 | 99 ± 6.8 | >100 | 99 ± 7.1 | >100 |
| EM-DC-11 | 29.00 ± 2.47 | 306.70 ± 13.71 | 30.56 ± 2.11 | 425.70 ± 54.80 | 97 ± 6.9 | >100 | 97 ± 7.1 | >100 |
| EM-DC-12 | 65.69 ± 3.25 | 102.57 ± 5.85 | 73.25 ± 1.80 | 72.79 ± 2.53 | 70 ± 6.9 | 0.49 ± 0.10 | 70 ± 7.1 | 0.51 ± 0.10 |
| EM-DC-13 | 91.15 ± 1.22 | 3.81 ± 0.26 | 83.81 ± 0.67 | 10.52 ± 0.17 | 80 ± 7.1 | 0.38 ± 0.10 | 80 ± 7.1 | 0.41 ± 0.10 |
| EM-DC-14 | 33.24 ± 2.03 | 362.70 ± 48.63 | 0 | >200 | 75 ± 7.1 | 0.44 ± 0.10 | 75 ± 7.1 | 0.46 ± 0.10 |
| EM-DC-15 | 63.86 ± 1.29 | 130.83 ± 1.85 | 19.06 ± 2.47 | >200 | 70 ± 7.2 | 0.55 ± 0.10 | 70 ± 7.1 | 0.53 ± 0.10 |
| EM-DC-16 | 85.26 ± 2.43 | 11.79 ± 1.10 | 48.75 ± 1.50 | 204.83 ± 9.62 | 80 ± 7.3 | 0.46 ± 0.10 | 80 ± 7.1 | 0.47 ± 0.10 |
| EM-DC-17 | 0 | >200 | 3.85 ± 2.99 | >200 | 99 ± 6.6 | >100 | 99 ± 7.1 | >100 |
| EM-DC-18 | 15.99 ± 1.40 | >200 | 0 | >200 | 98 ± 6.5 | >100 | 98 ± 7.1 | >100 |
| EM-DC-19 | 73.98 ± 0.59 | 76.15 ± 6.12 | 18.03 ± 0.17 | >200 | 99 ± 6.1 | >100 | 50 ± 7.1 | 0.29 ± 0.10 |
| EM-DC-20 | 0 | >200 | 0 | >200 | 75 ± 6.4 | 0.60 ± 0.10 | 75 ± 7.1 | 0.62 ± 0.10 |
| EM-DC-21 | 0 | >200 | 0 | >200 | 99 ± 7.1 | >100 | 99 ± 7.1 | >100 |
| EM-DC-22 | 0 | >200 | 0 | >200 | 99 ± 7.2 | >100 | 99 ± 7.1 | >100 |
| EM-DC-23 | 21.81 ± 2.14 | 480.03 ± 26.56 | 0 | >200 | 98 ± 7.3 | >100 | 98 ± 7.1 | >100 |
| EM-DC-24 | 24.08 ± 4.10 | 348.80 ± 25.17 | 0 | >200 | 80 ± 7.1 | 0.58 ± 0.10 | 80 ± 7.1 | 0.59 ± 0.10 |
| EM-DC-25 | 74.76 ± 1.47 | 87.19 ± 5.70 | 69.76 ± 0.81 | 103.93 ± 2.50 | 60 ± 7.1 | 0.33 ± 0.10 | 98 ± 7.1 | >100 |
| EM-DC-26 | 12.84 ± 3.54 | >200 | 68.25 ± 1.60 | 106.50 ± 0.70 | 97 ± 7.1 | >100 | 97 ± 7.1 | >100 |
| EM-DC-27 | 26.46 ± 2.36 | 375.20 ± 52.99 | 0 | >200 | 99 ± 7.1 | >100 | 50 ± 7.1 | 0.34 ± 0.10 |
| Galanthamine | 93.24 ± 0.52 | 1.31 ± 0.07 | 89.16 ± 0.44 | 26.62 ± 0.79 | - | - | - | - |
| Donepezil | 91.01 ± 0.53 | 0.0632 ± 0.0081 | 92.25 ± 0.22 | 6.88 ± 0.26 | - | - | - | - |
| Selegiline | - | - | - | - | - | - | 45% | 0.32 ± 0.09 |
| Chlorgyline | - | - | - | - | 45 ± 6.6 | 0.33 ± 0.09 | - | - |
| Model Equation: pIC50 = 14.588 + 0.197 SHBint2 − 16.205 ETA_Epsilon_5 + 0.181 nAtomP | ||||
| R | 0.930 | Descriptor | VIF | Ttest |
| R2 | 0.867 | SHBint2 | 1.840 | 5.291 |
| Q2 | 0.735 | ETA_Epsilon_5 | 1.842 | −3.947 |
| R2adj | 0.830 | nAtomP | 1.004 | 6.872 |
| R2 test | 0.600 | |||
| Compound | Mol Weight (a) | QLogP o/w (b) | QPlogBB (c) | PSA (d) | Rule of Five Violations (e) | Toxicological Alerts (f) |
|---|---|---|---|---|---|---|
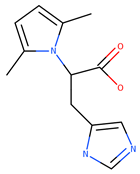 | 233.26 | 2.41 | −0.624 | 68.38 | 0 | 0 |
| EM-DC-19 | ||||||
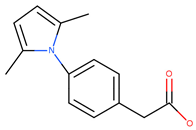 | 229.27 | 3.50 | −0.451 | 51.91 | 0 | 1 |
| EM-DC-27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateev, E.; Chtita, S.; Pavlova, E.; Irfan, A.; Tzankova, D.; Sharma, S.; Georgiev, B.; Mateeva, A.; Momekov, G.; Georgieva, M.; et al. Design, Synthesis, and Evaluation of Pyrrole-Based Selective MAO-B Inhibitors with Additional AChE Inhibitory and Neuroprotective Properties Identified via Virtual Screening. Pharmaceuticals 2025, 18, 1677. https://doi.org/10.3390/ph18111677
Mateev E, Chtita S, Pavlova E, Irfan A, Tzankova D, Sharma S, Georgiev B, Mateeva A, Momekov G, Georgieva M, et al. Design, Synthesis, and Evaluation of Pyrrole-Based Selective MAO-B Inhibitors with Additional AChE Inhibitory and Neuroprotective Properties Identified via Virtual Screening. Pharmaceuticals. 2025; 18(11):1677. https://doi.org/10.3390/ph18111677
Chicago/Turabian StyleMateev, Emilio, Samir Chtita, Ekaterina Pavlova, Ali Irfan, Diana Tzankova, Shubham Sharma, Borislav Georgiev, Alexandrina Mateeva, Georgi Momekov, Maya Georgieva, and et al. 2025. "Design, Synthesis, and Evaluation of Pyrrole-Based Selective MAO-B Inhibitors with Additional AChE Inhibitory and Neuroprotective Properties Identified via Virtual Screening" Pharmaceuticals 18, no. 11: 1677. https://doi.org/10.3390/ph18111677
APA StyleMateev, E., Chtita, S., Pavlova, E., Irfan, A., Tzankova, D., Sharma, S., Georgiev, B., Mateeva, A., Momekov, G., Georgieva, M., Zlatkov, A., & Kondeva-Burdina, M. (2025). Design, Synthesis, and Evaluation of Pyrrole-Based Selective MAO-B Inhibitors with Additional AChE Inhibitory and Neuroprotective Properties Identified via Virtual Screening. Pharmaceuticals, 18(11), 1677. https://doi.org/10.3390/ph18111677











