Preclinical Evaluation of Stable Integrin αvβ3-Specific [198Au]Gold Nanoparticles for Tumor Therapy
Abstract
1. Introduction
2. Results
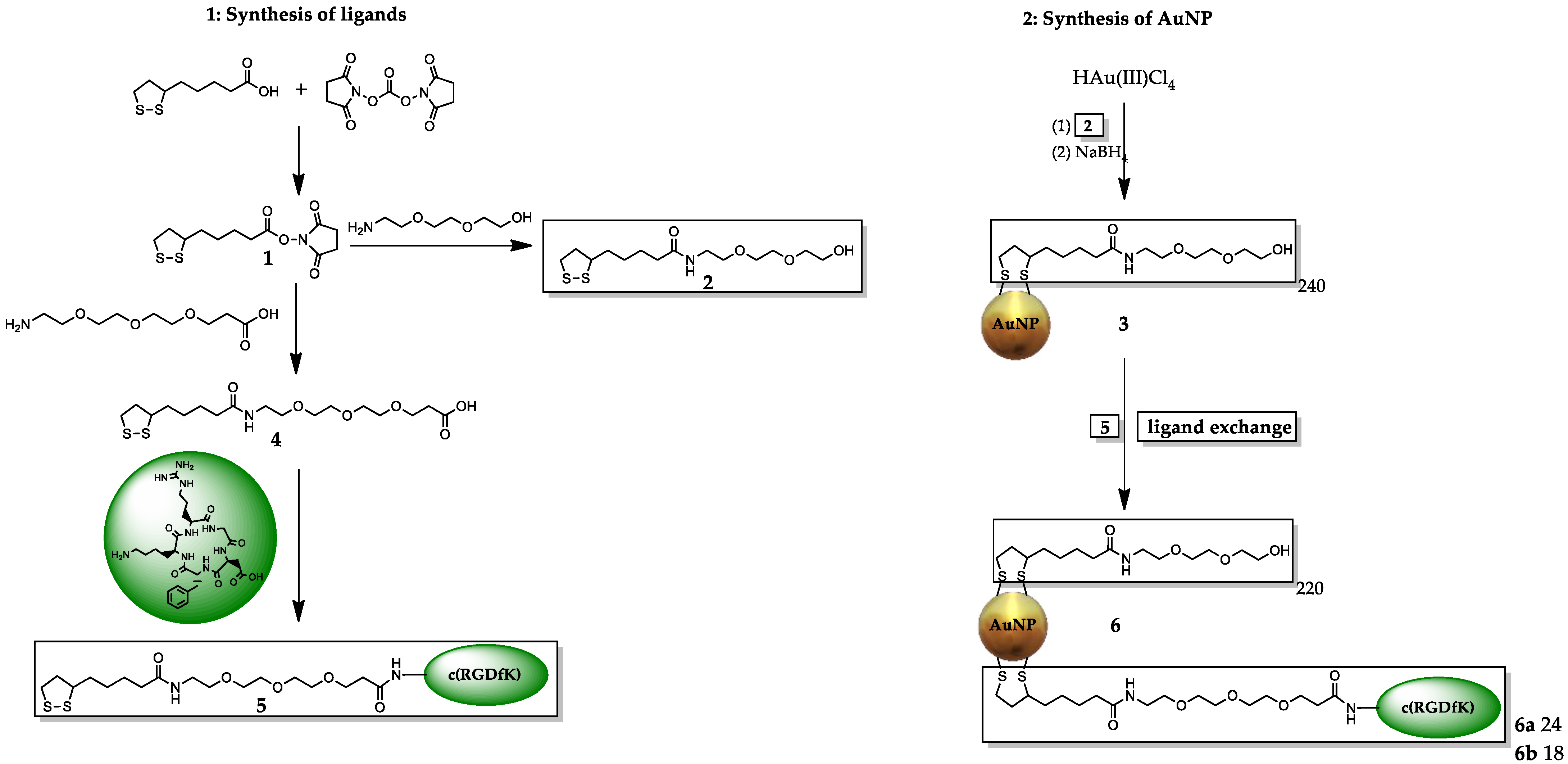
2.1. Synthesis of AuNPs and Functionalization with the Integrin αvβ3-Specific Peptide c(RGDfK)
2.2. Neutron Irradiation of Developed AuNPs 3, 6a and 6b
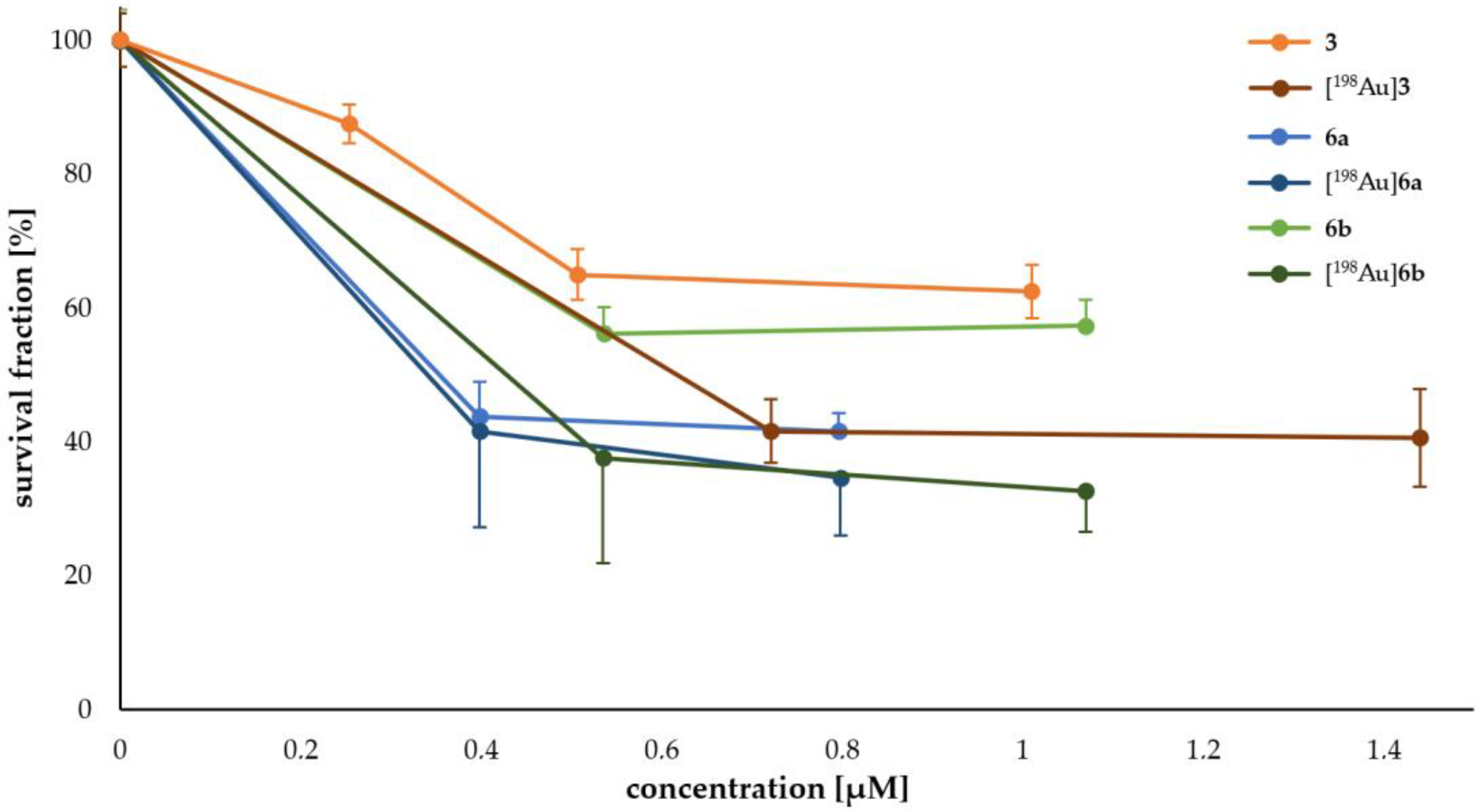
2.3. Cell Experiments
Determination of Cell Survival
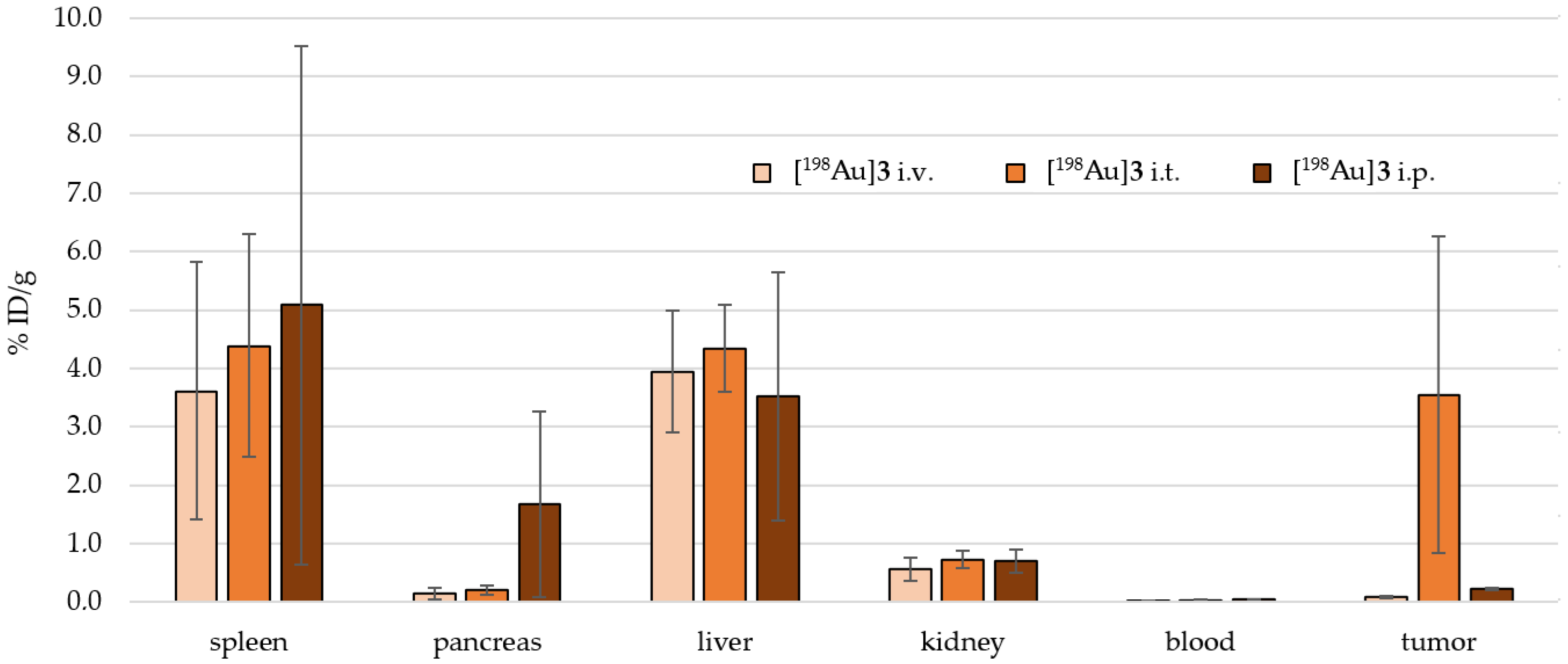
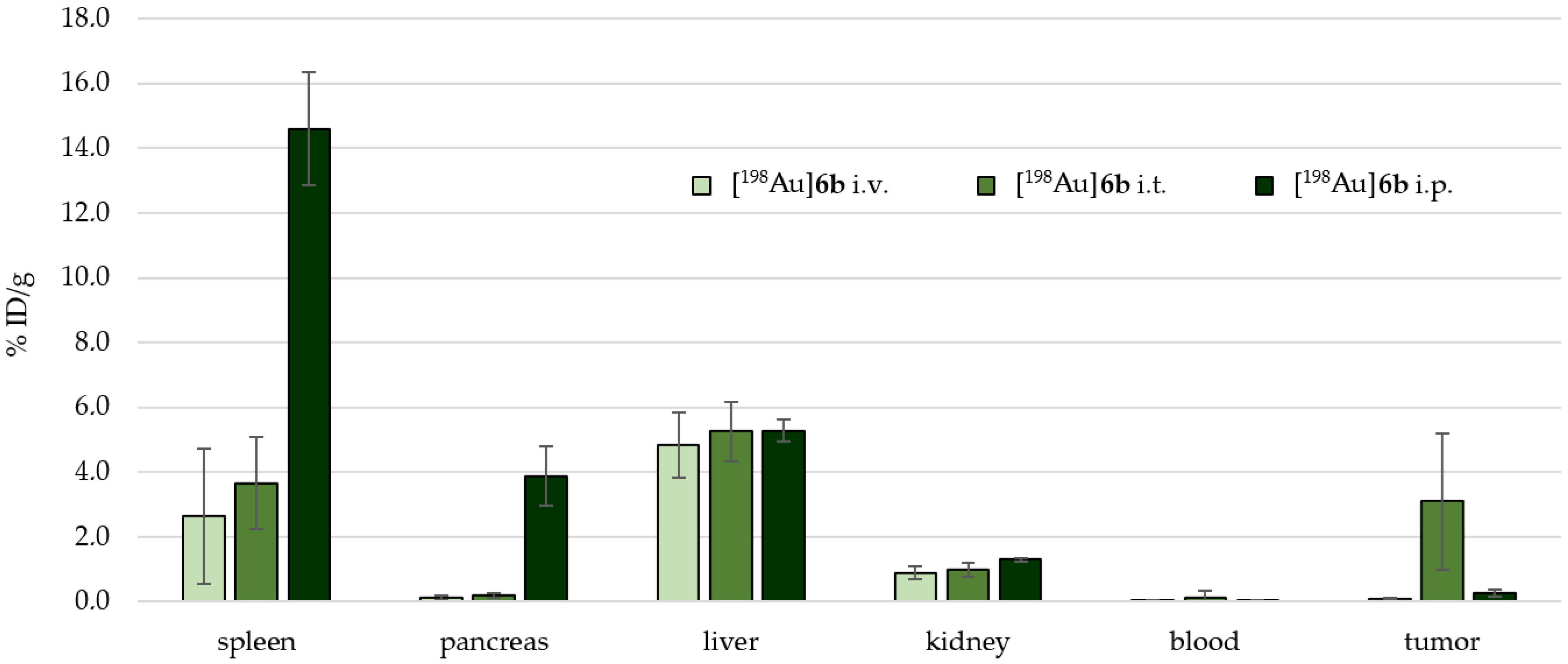
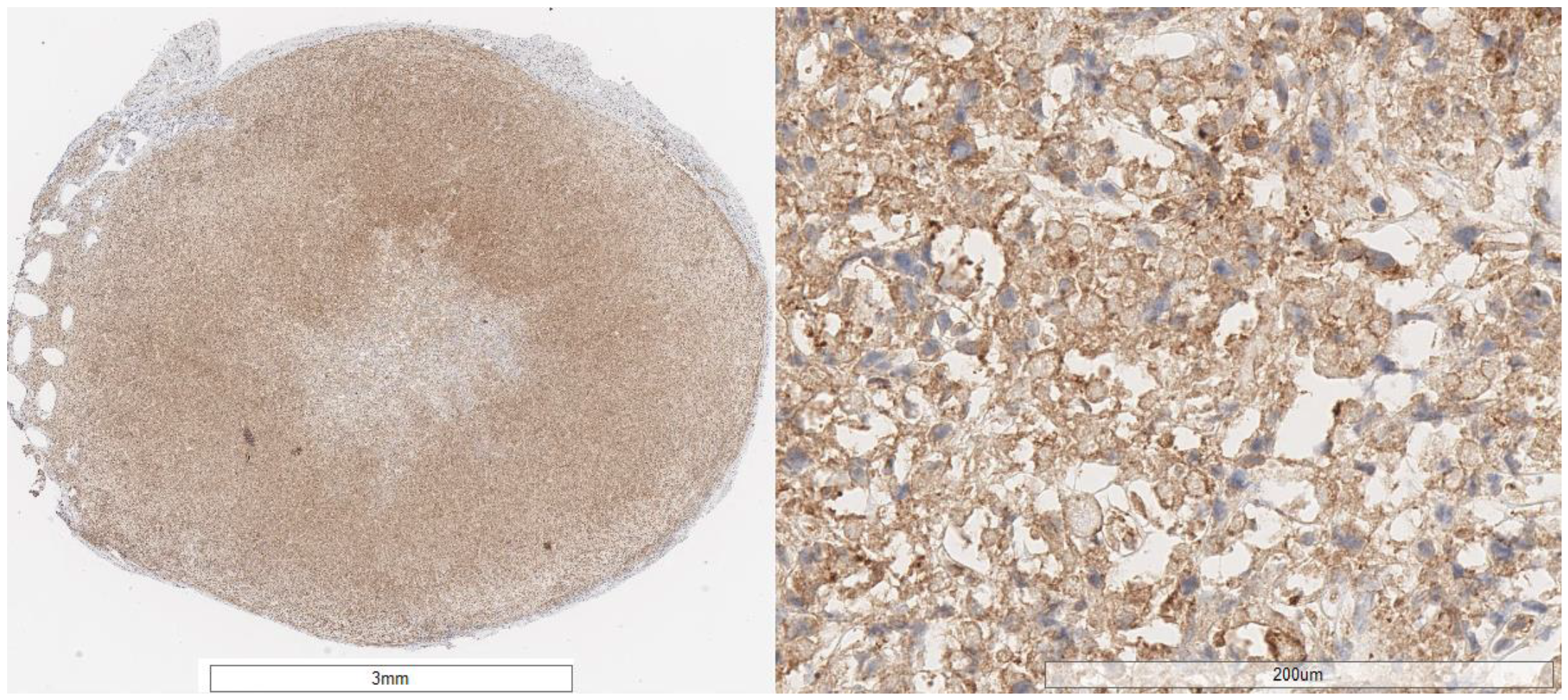
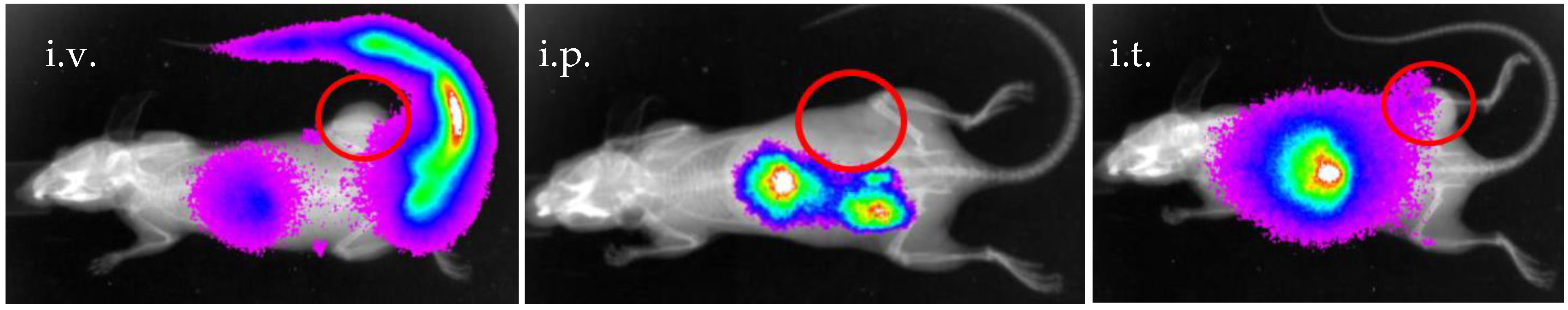
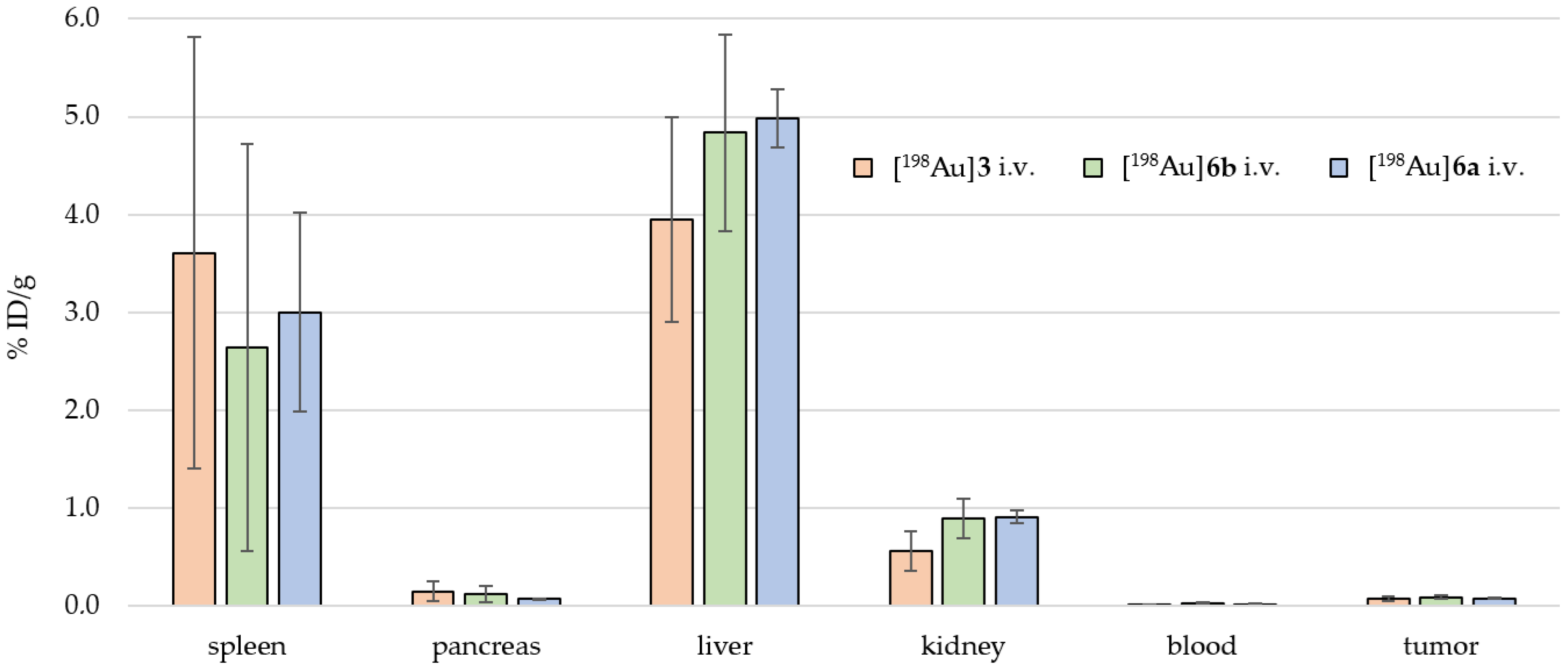
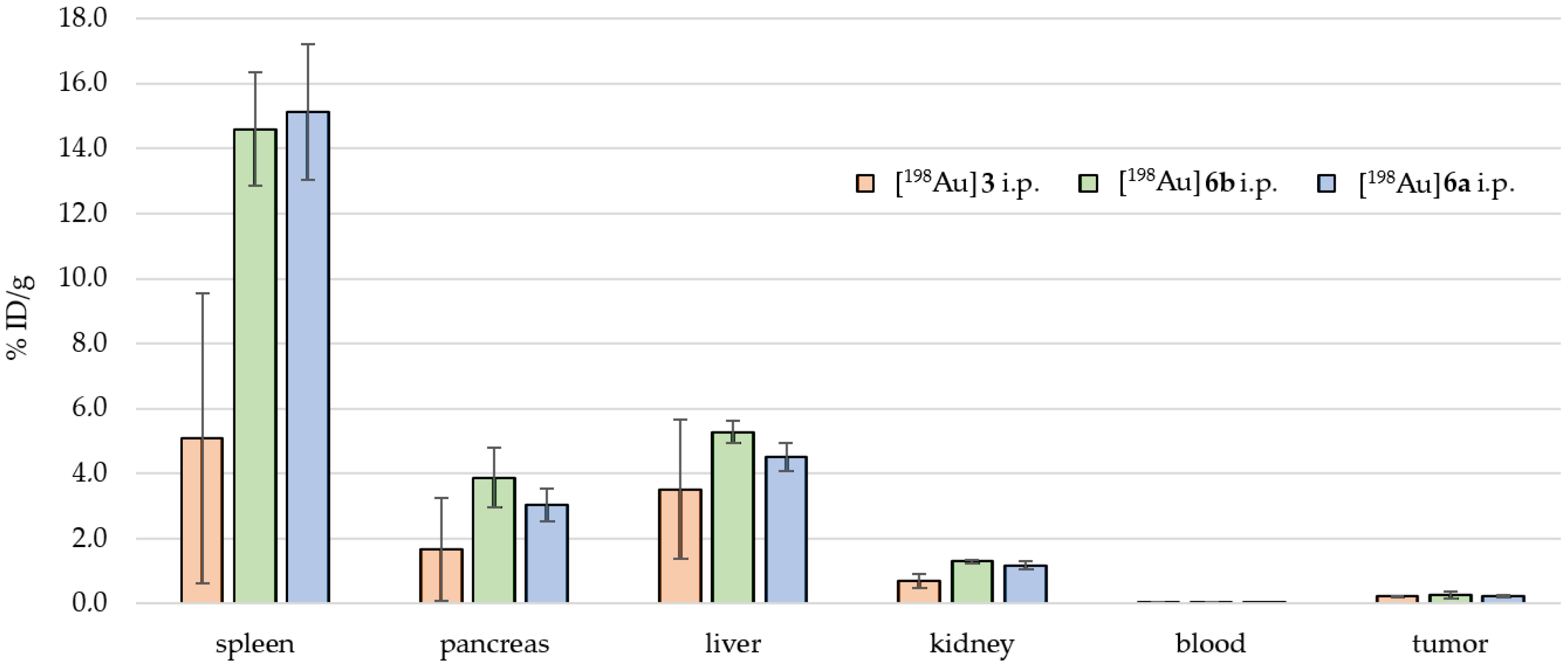
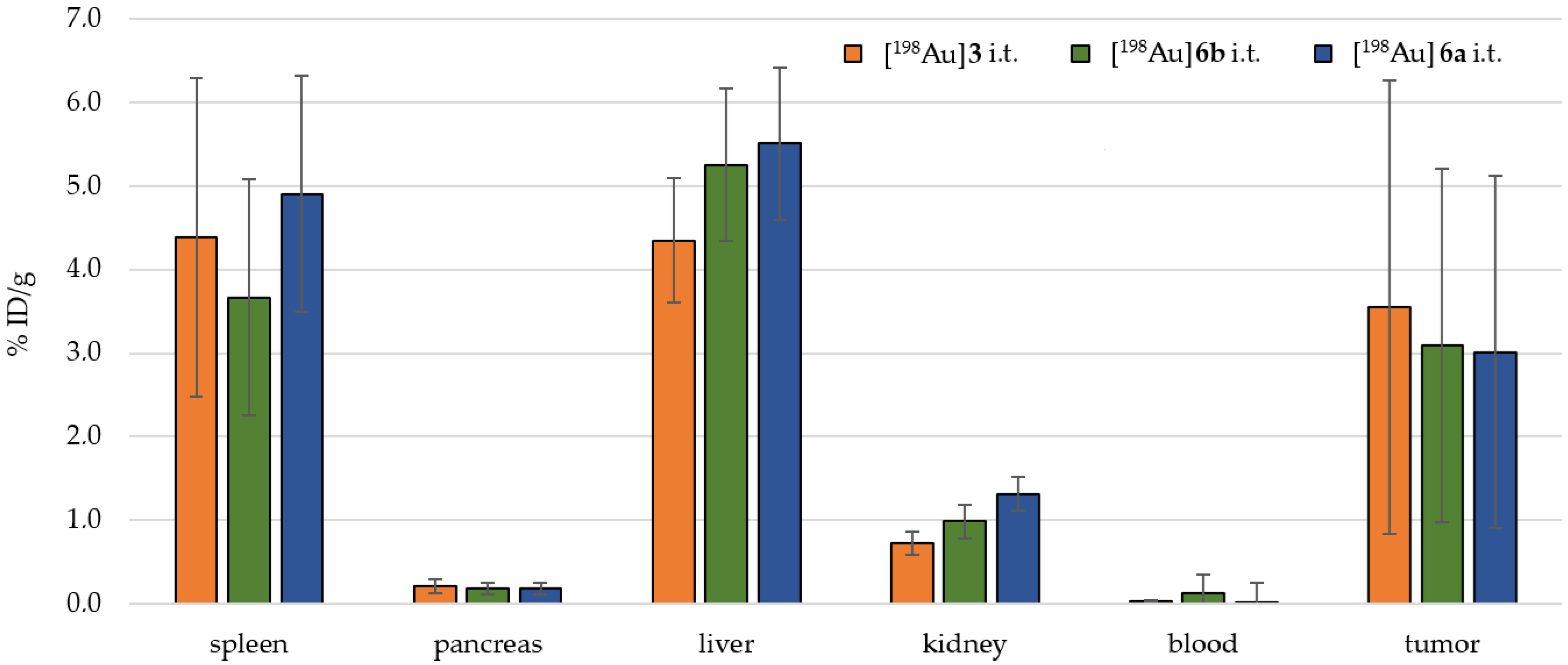
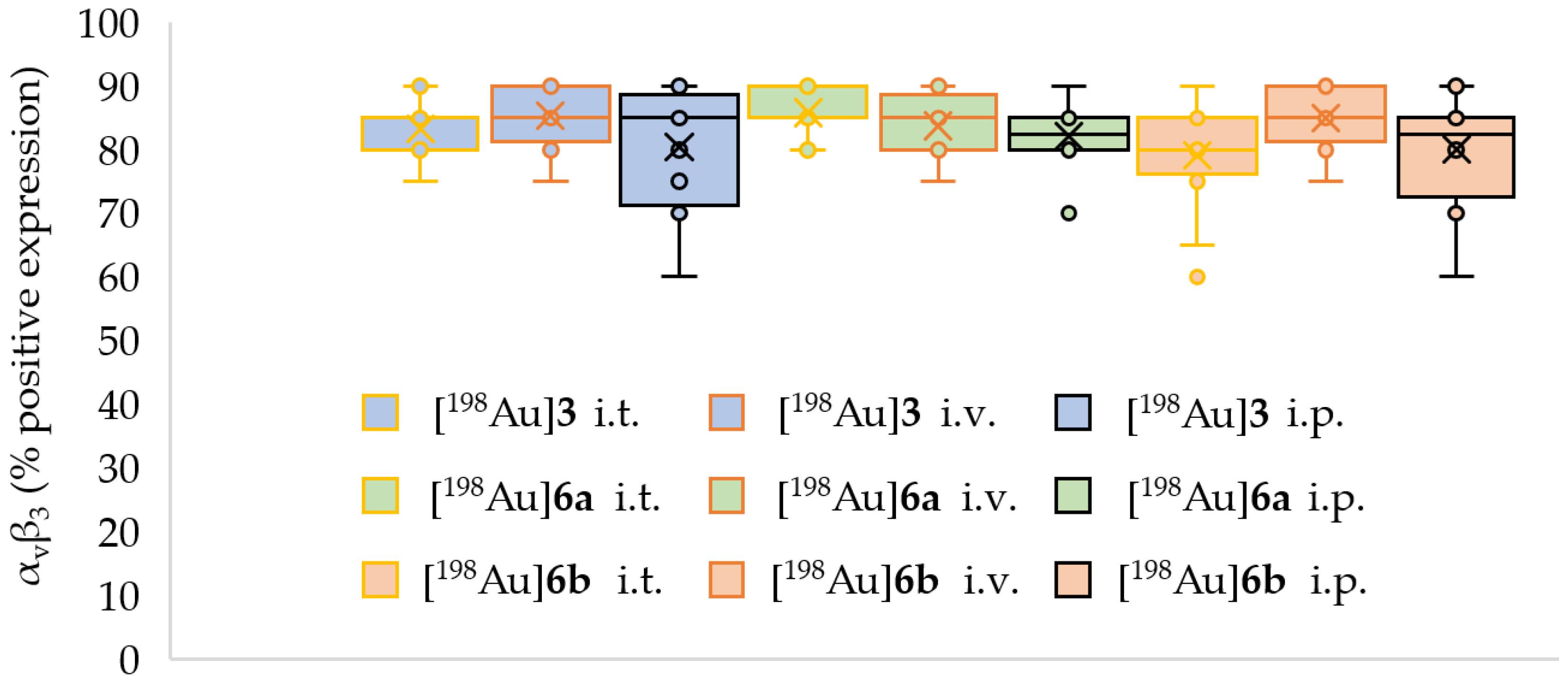
2.4. Preclinical Experiments
3. Discussion
3.1. In Vitro Experiments
3.2. In Vivo Preclinical Experiments
4. Materials and Methods
4.1. General Procedures
4.2. Determination of the Number of Ligands on the Surface of the AuNPs
- The mass loss of the AuNP 3 was ~33.27%, which results in ~240 PEG ligands on the AuNP surface, M ~ 246 kDa.
- The mass loss of AuNP-PEG-RGDhigh 6a was ~37.1%, and the RGD accounts for ~4% mass loss (~24 RGD ligands per AuNP). Therefore, the molar mass for AuNP-RGDhigh 6a was calculated to be ~262 kDa.
- Furthermore, the AuNP-RGDlow 6b contained ~18 RGD ligands, ~257 kDa.
4.3. Neutron Irradiation Experiments
4.4. Colony Formation Assay
4.5. In Vivo Experiments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
Appendix A.1. Organic Syntheses
- c(RGDfK) [62]
- TA-NHS 1 [63]
- TA-PEG3-OH 2
- TA-PEG4-COOH 4
- TA-PEG4-c(RGDfK) 5
- AuNP-dithio-PEG3-OH 3 [41]
- AuNP-PEG-RGDs by ligand exchange
- AuNP-dithio-PEG-RGDhigh 6a
- AuNP-dithio-PEG-RGDlow 6b
Appendix A.2. Electron Microscopy

| Nuclide | Half-Life | Gamma Energy [keV] Incidence [%] |
|---|---|---|
| 197Au →198Au → 198Hg (stable) | 2.6941 d | 411.80205 (95.6%) |
| 23Na → 24Na → 24Mg (stable) | 14.956 h | 1368.625 (99.9%) 2754.008 (99.9%) |
| 37Cl (24.4% isotopic incidence) → 38Cl → 38Ar (stable) | 37.230 min | 1642.68 (32.9%) 2167.400 (44.0%) |
| 40Ar →41Ar → 41K (stable) | 109.61 min | 1293.64 (99.2%) |
| 55Mn →56Mn → 56Fe (stable) | 2.5789 h | 846.7638 (98.9%) 1810.726 (26.9%) 2113.092 (14.2%) 2523.06 (1.018%) |
| Type of AuNP and dose | Number of Colonys | Standard Deviation (SD) | Survival Fractions [%] | SD [%] | c AuNP [µM] | c Asc [mM] |
|---|---|---|---|---|---|---|
| 101 (control) | 4 | 100.0 | 4.0 | 0 | - | |
| [198Au]6a 5 Gy | 42 | 6 | 41.6 | 14.3 | 0.40 | 1.15 |
| [198Au]6a 10 Gy | 35 | 3 | 34.7 | 8.6 | 0.80 | 2.31 |
| [198Au]6b 5 Gy | 38 | 6 | 37.6 | 15.8 | 0.54 | 1.49 |
| [198Au]6b 10 Gy | 33 | 2 | 32.7 | 6.1 | 1.07 | 2.98 |
| [198Au]3 5 Gy | 42 | 2 | 41.6 | 4.8 | 0.72 | 1.77 |
| [198Au]3 10 Gy | 41 | 3 | 40.6 | 7.3 | 1.44 | 3.55 |
| 89 (control) | 4 | 100.0 | 4.5 | - | - | |
| 6a (c like 5 Gy) | 39 | 2 | 43.8 | 5.1 | 0.40 | 1.15 |
| 6a (c like 10 Gy) | 37 | 1 | 41.6 | 2.7 | 0.80 | 2.31 |
| 6b (c like 5 Gy) | 50 | 2 | 56.2 | 4.0 | 0.54 | 1.49 |
| 6b (c like 10 Gy) | 51 | 2 | 57.3 | 3.9 | 1.07 | 2.98 |
| Mean | ||||||||
| [198Au]3 i.v. [MBq] | 2.37 | 7.54 | 7.29 | 2.14 | 3.45 | 1.72 | 4.09 | |
| Organs | Mouse 01 | Mouse 02 | Mouse 03 | Mouse 04 | Mouse 05 | Mouse 06 | %ID/g n = 6 | ±SD n = 6 |
| intestine small | 0.04 | 0.15 | 0.14 | 0.02 | 0.04 | 0.03 | 0.07 | 0.05 |
| intestine large | 0.08 | 0.20 | 0.19 | 0.03 | 0.09 | 0.04 | 0.10 | 0.07 |
| stomach | 0.07 | 0.24 | 0.17 | 0.04 | 0.08 | 0.05 | 0.11 | 0.07 |
| spleen | 2.71 | 7.06 | 6.07 | 1.58 | 3.08 | 1.16 | 3.61 | 2.21 |
| pancreas | 0.10 | 0.29 | 0.29 | 0.04 | 0.10 | 0.07 | 0.15 | 0.10 |
| liver | 5.22 | 4.04 | 3.54 | 4.14 | 4.81 | 1.95 | 3.95 | 1.05 |
| kidney | 0.46 | 0.83 | 0.84 | 0.42 | 0.48 | 0.33 | 0.56 | 0.20 |
| blood | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.00 |
| heart | 0.14 | 0.66 | 0.57 | 0.11 | 0.19 | 0.12 | 0.30 | 0.23 |
| lung | 0.10 | 0.68 | 0.49 | 0.10 | 0.20 | 0.07 | 0.27 | 0.23 |
| tail | 5.96 | 0.67 | 2.81 | 7.39 | 7.97 | 5.08 | 4.98 | 2.55 |
| brain | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| tumor | 0.06 | 0.11 | 0.11 | 0.06 | 0.06 | 0.05 | 0.07 | 0.02 |
| bone | 0.42 | 0.83 | 0.90 | 0.23 | 0.53 | 0.46 | 0.56 | 0.23 |
| muscle | 0.04 | 0.21 | 0.09 | 0.04 | 0.06 | 0.03 | 0.08 | 0.06 |
| [198Au]3 i.t. [MBq] | 4.16 | 3.95 | 4.87 | 5.73 | 3.68 | 4.03 | 4.40 | |
| Mouse 07 | Mouse 08 | Mouse 09 | Mouse 10 | Mouse 11 | Mouse 12 | %ID/g n = 6 | n = 6 | |
| intestine small | 0.04 | 0.11 | 0.15 | 0.12 | 0.09 | 0.05 | 0.09 | 0.04 |
| intestine large | 0.07 | 0.16 | 0.15 | 0.19 | 0.15 | 0.10 | 0.13 | 0.04 |
| stomach | 0.07 | 0.15 | 0.21 | 0.19 | 0.17 | 0.09 | 0.14 | 0.05 |
| spleen | 1.82 | 3.56 | 7.00 | 6.17 | 5.32 | 2.46 | 4.39 | 1.91 |
| pancreas | 0.08 | 0.25 | 0.26 | 0.30 | 0.24 | 0.11 | 0.21 | 0.08 |
| liver | 3.44 | 4.47 | 4.81 | 4.21 | 5.61 | 3.54 | 4.35 | 0.74 |
| kidney | 0.52 | 0.89 | 0.78 | 0.87 | 0.72 | 0.57 | 0.73 | 0.14 |
| blood | 0.04 | 0.02 | 0.02 | 0.04 | 0.03 | 0.03 | 0.03 | 0.01 |
| heart | 0.15 | 0.45 | 0.52 | 0.59 | 0.42 | 0.22 | 0.39 | 0.16 |
| lung | 0.11 | 0.28 | 0.41 | 0.49 | 0.35 | 0.14 | 0.30 | 0.14 |
| tail | 0.07 | 0.19 | 0.17 | 0.18 | 0.17 | 0.11 | 0.15 | 0.04 |
| brain | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 |
| tumor | 18.42 | 1.44 | 3.50 | 1.34 | 2.70 | 8.73 | 3.54 | 2.71 |
| bone | 0.37 | 0.70 | 0.83 | 1.00 | 0.89 | 0.58 | 0.73 | 0.21 |
| muscle | 0.07 | 0.11 | 0.08 | 0.08 | 0.09 | 0.05 | 0.08 | 0.02 |
| [198Au]3 i.p. [MBq] | 5.13 | 5.20 | 5.07 | 3.68 | 4.77 | |||
| Mouse 13 | Mouse 14 | Mouse 15 | Mouse 16 | Mouse 17 | Mouse 18 | %ID/g n = 6 | n = 6 | |
| intestine small | 0.04 | 0.72 | 0.04 | 0.86 | 0.88 | 0.07 | 0.43 | 0.39 |
| intestine large | 0.06 | 0.32 | 0.06 | 0.91 | 0.61 | 0.08 | 0.34 | 0.32 |
| stomach | 0.11 | 0.76 | 0.05 | 0.56 | 0.44 | 0.12 | 0.34 | 0.26 |
| spleen | 0.75 | 6.56 | 1.05 | 11.01 | 10.36 | 0.79 | 5.09 | 4.45 |
| pancreas | 0.23 | 2.74 | 0.11 | 4.25 | 2.46 | 0.20 | 1.67 | 1.59 |
| liver | 1.36 | 4.46 | 1.51 | 6.90 | 5.18 | 1.71 | 3.52 | 2.12 |
| kidney | 0.43 | 0.80 | 0.47 | 1.00 | 0.86 | 0.62 | 0.70 | 0.21 |
| blood | 0.04 | 0.02 | 0.03 | 0.02 | 0.02 | 0.06 | 0.03 | 0.01 |
| heart | 0.07 | 0.19 | 0.06 | 0.28 | 0.30 | 0.09 | 0.16 | 0.10 |
| lung | 0.06 | 0.10 | 0.06 | 0.54 | 0.21 | 0.08 | 0.17 | 0.17 |
| tail | 0.05 | 0.09 | 0.05 | 0.15 | 0.18 | 0.06 | 0.10 | 0.05 |
| brain | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 |
| tumor | 1.66 | 0.20 | 1.68 | 0.22 | 0.23 | 7.53 | 0.22 | 0.01 |
| bone | 0.16 | 0.53 | 0.15 | 0.66 | 0.84 | 0.23 | 0.43 | 0.26 |
| muscle | 0.03 | 0.05 | 0.03 | 0.06 | 0.07 | 0.07 | 0.05 | 0.02 |
| [198Au]6a i.v. [MBq] | 3.09 | 3.40 | 4.53 | 2.65 | 3.46 | 2.84 | 3.33 | |
| Mouse 19 | Mouse 20 | Mouse 21 | Mouse 22 | Mouse 23 | Mouse 24 | %ID/g n = 6 | n = 6 | |
| intestine small | 0.08 | 0.08 | 0.10 | 0.07 | 0.07 | 0.08 | 0.08 | 0.01 |
| intestine large | 0.08 | 0.06 | 0.07 | 0.05 | 0.05 | 0.06 | 0.06 | 0.01 |
| stomach | 0.07 | 0.08 | 0.10 | 0.06 | 0.10 | 0.08 | 0.08 | 0.01 |
| spleen | 2.52 | 4.10 | 4.30 | 1.58 | 2.08 | 3.43 | 3.00 | 1.02 |
| pancreas | 0.07 | 0.08 | 0.07 | 0.05 | 0.08 | 0.07 | 0.07 | 0.01 |
| liver | 5.32 | 4.95 | 4.62 | 4.63 | 5.04 | 5.36 | 4.99 | 0.29 |
| kidney | 0.90 | 0.89 | 0.99 | 0.79 | 0.93 | 0.97 | 0.91 | 0.06 |
| blood | 0.02 | 0.03 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.00 |
| heart | 0.15 | 0.20 | 0.17 | 0.19 | 0.24 | 0.22 | 0.20 | 0.03 |
| lung | 0.11 | 0.15 | 0.19 | 0.20 | 0.21 | 0.20 | 0.18 | 0.03 |
| tail | 9.64 | 6.55 | 7.75 | 7.19 | 6.96 | 6.88 | 7.50 | 1.03 |
| brain | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| tumor | 0.09 | 0.08 | 0.08 | 0.07 | 0.07 | 0.08 | 0.08 | 0.01 |
| bone | 0.49 | 0.54 | 0.58 | 0.47 | 0.54 | 0.50 | 0.52 | 0.04 |
| muscle | 0.03 | 0.04 | 0.02 | 0.03 | 0.06 | 0.05 | 0.04 | 0.01 |
| [198Au]6a i.t. [MBq] | 4.74 | 4.30 | 4.62 | 4.42 | 4.71 | 4.89 | 4.61 | |
| Mouse 25 | Mouse 26 | Mouse 27 | Mouse 28 | Mouse 29 | Mouse 30 | %ID/g n = 6 | n = 6 | |
| intestine s | 0.08 | 0.25 | 0.18 | 0.19 | 0.19 | 0.26 | 0.19 | 0.06 |
| intestine l | 0.06 | 0.17 | 0.12 | 0.11 | 0.13 | 0.16 | 0.12 | 0.04 |
| stomach | 0.08 | 0.21 | 0.16 | 0.16 | 0.15 | 0.20 | 0.16 | 0.04 |
| spleen | 2.15 | 4.72 | 5.70 | 5.54 | 4.02 | 7.29 | 4.91 | 1.59 |
| pancreas | 0.07 | 0.24 | 0.20 | 0.18 | 0.19 | 0.23 | 0.19 | 0.05 |
| liver | 3.83 | 6.04 | 5.42 | 6.06 | 5.26 | 6.42 | 5.51 | 0.85 |
| kidney | 0.78 | 1.33 | 1.46 | 1.45 | 1.27 | 1.58 | 1.31 | 0.26 |
| blood | 0.03 | 0.02 | 0.02 | 0.02 | 0.01 | 0.03 | 0.02 | 0.01 |
| heart | 0.24 | 0.85 | 0.70 | 0.66 | 0.70 | 0.88 | 0.67 | 0.21 |
| lung | 0.33 | 0.67 | 0.65 | 0.67 | 0.52 | 0.76 | 0.60 | 0.14 |
| tail | 0.05 | 0.13 | 0.09 | 0.10 | 0.14 | 0.13 | 0.11 | 0.03 |
| brain | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 |
| tumor | 23.81 | 2.50 | 2.80 | 4.23 | 3.46 | 2.08 | 3.01 | 0.76 |
| bone | 0.44 | 0.55 | 0.68 | 0.96 | 0.64 | 0.82 | 0.68 | 0.17 |
| muscle | 0.07 | 0.09 | 0.08 | 0.06 | 0.09 | 0.10 | 0.08 | 0.01 |
| [198Au]6a i.p. [MBq] | 5.08 | 5.05 | 5.15 | 5.16 | 5.13 | 5.08 | 5.11 | |
| Mouse 31 | Mouse 32 | Mouse 33 | Mouse 34 | Mouse 35 | Mouse 36 | %ID/g n = 6 | n = 6 | |
| intestine small | 0.57 | 0.80 | 0.65 | 0.69 | 1.40 | 1.10 | 0.87 | 0.29 |
| intestine large | 1.05 | 1.45 | 1.03 | 0.94 | 0.61 | 0.99 | 1.01 | 0.25 |
| stomach | 1.70 | 0.54 | 0.57 | 0.54 | 0.64 | 0.56 | 0.76 | 0.42 |
| spleen | 19.35 | 13.12 | 13.33 | 14.76 | 14.34 | 15.82 | 15.12 | 2.10 |
| pancreas | 2.70 | 2.64 | 3.35 | 2.77 | 4.05 | 2.73 | 3.04 | 0.51 |
| liver | 5.10 | 4.84 | 4.12 | 4.85 | 4.13 | 4.04 | 4.51 | 0.43 |
| kidney | 1.33 | 1.22 | 1.07 | 1.35 | 1.03 | 1.08 | 1.18 | 0.13 |
| blood | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.00 |
| heart | 0.28 | 0.25 | 0.23 | 0.22 | 0.24 | 0.23 | 0.24 | 0.02 |
| lung | 0.43 | 0.32 | 0.40 | 0.32 | 0.27 | 0.58 | 0.39 | 0.10 |
| tail | 0.09 | 0.10 | 0.08 | 0.07 | 0.07 | 0.09 | 0.08 | 0.01 |
| brain | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| tumor | 0.21 | 0.28 | 0.29 | 0.17 | 0.24 | 0.21 | 0.24 | 0.04 |
| bone | 0.62 | 0.64 | 0.50 | 0.21 | 0.46 | 0.40 | 0.47 | 0.15 |
| muscle | 0.05 | 0.05 | 0.04 | 0.05 | 0.03 | 0.04 | 0.04 | 0.01 |
| [198Au]6b i.v. [MBq] | 3.02 | 2.20 | 1.03 | 4.81 | 2.45 | 6.98 | 3.41 | |
| Mouse 37 | Mouse 38 | Mouse 39 | Mouse 40 | Mouse 41 | Mouse 42 | %ID/g n = 6 | n = 6 | |
| intestine small | 0.19 | 0.07 | 0.13 | 0.07 | 0.10 | 0.34 | 0.15 | 0.09 |
| intestine large | 0.09 | 0.06 | 0.08 | 0.05 | 0.10 | 0.20 | 0.10 | 0.05 |
| stomach | 0.13 | 0.08 | 0.09 | 0.07 | 0.06 | 0.19 | 0.10 | 0.05 |
| spleen | 2.18 | 2.05 | 1.44 | 1.58 | 1.37 | 7.24 | 2.64 | 2.08 |
| pancreas | 0.13 | 0.07 | 0.09 | 0.05 | 0.08 | 0.30 | 0.12 | 0.09 |
| liver | 5.73 | 4.62 | 6.45 | 3.64 | 4.83 | 3.75 | 4.84 | 1.01 |
| kidney | 0.99 | 0.71 | 1.00 | 0.75 | 0.68 | 1.23 | 0.89 | 0.20 |
| blood | 0.02 | 0.02 | 0.02 | 0.02 | 0.05 | 0.02 | 0.03 | 0.01 |
| heart | 0.38 | 0.13 | 0.20 | 0.16 | 0.21 | 0.67 | 0.29 | 0.19 |
| lung | 0.31 | 0.14 | 0.18 | 0.13 | 0.21 | 0.35 | 0.22 | 0.08 |
| tail | 6.34 | 10.91 | 12.97 | 7.20 | 8.12 | 1.09 | 7.77 | 3.74 |
| brain | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 |
| tumor | 0.08 | 0.09 | 0.08 | 0.09 | 0.07 | 0.14 | 0.09 | 0.02 |
| bone | 0.61 | 0.54 | 0.87 | 0.30 | 0.47 | 0.91 | 0.62 | 0.21 |
| muscle | 0.05 | 0.02 | 0.05 | 0.12 | 0.02 | 0.11 | 0.06 | 0.04 |
| [198Au]6b i.t. [MBq] | 4.59 | 4.10 | 4.17 | 4.54 | 4.49 | 4.41 | 4.38 | |
| Mouse 43 | Mouse 44 | Mouse 45 | Mouse 46 | Mouse 47 | Mouse 48 | %ID/g n = 6 | n = 6 | |
| intestine small | 0.11 | 0.21 | 0.17 | 0.30 | 0.09 | 0.29 | 0.19 | 0.08 |
| intestine large | 0.08 | 0.16 | 0.13 | 0.18 | 0.08 | 0.17 | 0.13 | 0.04 |
| stomach | 0.08 | 0.17 | 0.15 | 0.21 | 0.08 | 0.25 | 0.16 | 0.06 |
| spleen | 2.00 | 3.94 | 3.62 | 3.90 | 2.24 | 6.30 | 3.67 | 1.41 |
| pancreas | 0.09 | 0.25 | 0.20 | 0.27 | 0.09 | 0.19 | 0.18 | 0.07 |
| liver | 3.92 | 5.06 | 5.71 | 5.75 | 4.40 | 6.68 | 5.25 | 0.91 |
| kidney | 0.68 | 1.01 | 0.98 | 1.20 | 0.77 | 1.25 | 0.98 | 0.21 |
| blood | 0.03 | 0.63 | 0.02 | 0.02 | 0.03 | 0.02 | 0.13 | 0.22 |
| heart | 0.24 | 0.62 | 0.52 | 0.68 | 0.31 | 0.80 | 0.53 | 0.20 |
| lung | 0.16 | 0.55 | 0.41 | 0.48 | 0.25 | 0.80 | 0.44 | 0.21 |
| tail | 0.07 | 0.12 | 0.14 | 0.14 | 0.10 | 0.17 | 0.12 | 0.03 |
| brain | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 | 0.02 | 0.01 | 0.00 |
| tumor | 5.46 | 0.95 | 3.17 | 1.06 | 6.30 | 1.62 | 3.09 | 2.11 |
| bone | 0.40 | 0.61 | 0.94 | 1.07 | 0.43 | 1.51 | 0.83 | 0.39 |
| muscle | 0.07 | 0.08 | 0.07 | 0.13 | 0.05 | 0.10 | 0.08 | 0.02 |
| [198Au]6b i.p. [MBq] | 5.22 | 5.09 | 4.99 | 4.91 | 4.95 | 5.04 | 5.03 | |
| Mouse 49 | Mouse 50 | Mouse 51 | Mouse 52 | Mouse 53 | Mouse 54 | %ID/g n = 6 | n = 6 | |
| intestine small | 1.82 | 2.44 | 1.17 | 0.92 | 1.32 | 1.31 | 1.50 | 0.50 |
| intestine large | 1.74 | 1.31 | 1.82 | 1.76 | 1.47 | 1.08 | 1.53 | 0.27 |
| stomach | 0.66 | 0.84 | 1.02 | 1.63 | 0.68 | 0.74 | 0.93 | 0.34 |
| spleen | 17.77 | 15.20 | 12.58 | 15.36 | 13.35 | 13.30 | 14.59 | 1.75 |
| pancreas | 2.83 | 3.94 | 3.47 | 3.04 | 5.50 | 4.51 | 3.88 | 0.91 |
| liver | 5.46 | 5.32 | 4.68 | 5.46 | 5.76 | 5.00 | 5.28 | 0.35 |
| kidney | 1.32 | 1.28 | 1.20 | 1.34 | 1.33 | 1.35 | 1.30 | 0.05 |
| blood | 0.03 | 0.03 | 0.02 | 0.04 | 0.03 | 0.06 | 0.03 | 0.01 |
| heart | 0.37 | 0.31 | 0.36 | 0.36 | 0.33 | 0.37 | 0.35 | 0.02 |
| lung | 0.33 | 0.38 | 0.50 | 0.24 | 0.41 | 0.56 | 0.40 | 0.11 |
| tail | 0.17 | 0.13 | 0.15 | 0.15 | 0.16 | 0.15 | 0.15 | 0.01 |
| brain | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 |
| tumor | 0.47 | 0.17 | 0.17 | 0.27 | 0.22 | 0.23 | 0.25 | 0.10 |
| bone | 0.64 | 0.66 | 0.88 | 0.67 | 0.77 | 0.82 | 0.74 | 0.09 |
| muscle | 0.05 | 0.06 | 0.11 | 0.08 | 0.05 | 0.05 | 0.07 | 0.02 |
| AuNP | HE Evaluation | CD31 Evaluation | Caspase 3 Evaluation * | αvβ3 Integrin | |
|---|---|---|---|---|---|
| % Positive Expression | Intensity | ||||
| 3 | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma, and a group of atypical cells with cytoplasmic clearing (20–30%); high stromal content, grows in cord-like pattern | 5–10%; scattered elongated vessels | <5% | 75–80% | moderate–strong |
| 3 | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma, in the middle eosinophilic acellular material, and a group of atypical cells with cytoplasmic clearing (15–20%); high stromal content, grows in cord-like pattern | 15–20%; scattered elongated vessels | 25–30% | 85–90% | moderate–strong |
| 3 | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma, in the middle eosinophilic acellular material, and a group of atypical cells with cytoplasmic clearing (80–90%); high stromal content, grows in cord-like pattern | 10–15%; mostly in periphery | 10–15% | 85–90% | moderate–strong |
| 3 | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma, in the middle eosinophilic acellular material, and a group of atypical cells with cytoplasmic clearing (60–70%); high stromal content, grows in cord-like pattern | 5–10%; mostly in periphery | 10–15% | 80–85% | moderate–strong |
| 3 | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma, in the middle eosinophilic acellular material, and a group of atypical cells with cytoplasmic clearing (80–90%); high stromal content, grows in cord-like pattern | 15–20%; scattered elongated vessels | 10–15% | 80–85% | moderate |
| 3 | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma, and a group of atypical cells with cytoplasmic clearing (5%); high stromal content, grows in cord-like pattern | 5–10%; scattered | 5–10% (weak signal) | 80–85% | moderate–strong |
| 3 | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma, and a group of atypical cells with cytoplasmic clearing (5%); high stromal content, grows in cord-like pattern | 15–20%; scattered elongated vessels | 25–30% | 85–90% | moderate–strong |
| 3 | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma the eosinophilic acellular material; and a group of atypical cells with cytoplasmic clearing (10–15%); high stromal content, grows in cord-like pattern | 10–15%; scattered elongated vessels | 5–10% | >90% | moderate–strong |
| 3 | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma and a group of atypical cells with cytoplasmic clearing (5–10%); gland/lumen forming area (5–10%); eosinophilic acellular material; high stromal content, grows in cord-like pattern | 15–20%; scattered elongated vessels | 10–15% | 85–90% | moderate–strong |
| 3 | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma and a group of atypical cells with cytoplasmic clearing (50–60%); eosinophilic acellular material; high stromal content, grows in cord-like pattern | 10–15%; scattered elongated vessels | 15–20% | 75–80% | mild–moderate |
| 3 | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma and a group of atypical cells with cytoplasmic clearing (5%); gland/lumen forming area (10–15%); eosinophilic acellular material; high stromal content, grows in cord-like pattern | 15–20%; scattered elongated vessels | 15–20% | 80–85% | moderate |
| 3 | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma and a group of atypical cells with cytoplasmic clearing (5–10%); the eosinophilic acellular material; high stromal content, grows in cord-like pattern | 10–15%; scattered elongated vessels | 10–15% | 85–90% | moderate–strong |
| 6a | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma and a group of atypical cells with cytoplasmic clearing (5%); gland/lumen forming area (5–10%); the eosinophilic acellular material; high stromal content, grows in cord-like pattern | 15–20%; mostly in periphery | 5–10% | 75–80% | moderate |
| 6a | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma and a group of atypical cells with cytoplasmic clearing (40–50%); the eosinophilic acellular material; high stromal content, grows in cord-like pattern | 5–10%; scattered elongated vessels | 10–15% | 80–85% | moderate |
| 6a | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma a group of atypical cells with cytoplasmic clearing (30–35%); and gland/lumen forming area (35–40%); the eosinophilic acellular material; high stromal content, grows in cord-like pattern | 15–20%; scattered elongated vessels | 10–15% | >90% | strong |
| 6a | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma a group of atypical cells with cytoplasmic clearing (5%); and gland/lumen forming area (20–25%); the eosinophilic acellular material; high stromal content, grows in cord-like pattern | 10–15%; scattered elongated vessels | 10–15% | 80–85% | moderate–strong |
| 6a | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma and a group of atypical cells with cytoplasmic clearing (5%); gland/lumen forming area (60–65%); the eosinophilic acellular material; high stromal content, grows in cord-like pattern | 15–20%; mostly in periphery | 5–10% | 80–85% | moderate–strong |
| 6a | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma and a group of atypical cells with cytoplasmic clearing (60–70%); the eosinophilic acellular material; high stromal content, grows in cord-like pattern | 10–15%; mostly in periphery | 5–10% | 85–90% | moderate–strong |
| 6a | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma and a group of atypical cells with cytoplasmic clearing (5–10%); the eosinophilic acellular material; high stromal content, grows in cord-like pattern | 5–10%; scattered vessels | <5% | 85–90% | strong |
| 6a | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma and a group of atypical cells with cytoplasmic clearing (40–45%); the eosinophilic acellular material; high stromal content, grows in cord-like pattern | 10–15%; scattered vessels | 10–15% | 80–85% | moderate–strong |
| 6a | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma and a group of atypical cells with cytoplasmic clearing (10–15%); gland/lumen forming area (<5%); the eosinophilic acellular material; high stromal content, grows in cord-like pattern | 15–20%; mostly in periphery | 20–25% | 85–90% | moderate–strong |
| 6a | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma and a group of atypical cells with cytoplasmic clearing (60–70%); the eosinophilic acellular material; high stromal content, grows in cord-like pattern | 10–15%; scattered elongated vessels | 20–25% | 80–85% | mild–moderate |
| 6a | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma and a group of atypical cells with cytoplasmic clearing (10–15%); the eosinophilic acellular material; high stromal content, grows in cord-like pattern | 15–20%; mostly in periphery | 20–25% | 85–90% | moderate–strong |
| 6a | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma and a group of atypical cells with cytoplasmic clearing (15–20%); gland/lumen forming area (15–20%); eosinophilic acellular material; high stromal content, grows in cord-like pattern | 10–15%; mostly in periphery | 10–15% (weak) | 85–90% | moderate–strong |
| 6b | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma and a group of atypical cells with cytoplasmic clearing (<5%); gland/lumen forming area (70–75%); eosinophilic acellular material; high stromal content, grows in cord-like pattern | 15–20%; mostly in periphery | 10–15% | 85–90% | moderate–strong |
| 6b | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma and a group of atypical cells with cytoplasmic clearing (<5%); prominent eosinophilic acellular material; high stromal content, grows in cord-like pattern | 10–15%; scattered elongated vessels | 15–20% | 85–90% | moderate-strong |
| 6b | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma, in the middle eosinophilic acellular material, and a group of atypical cells with cytoplasmic clearing (30–40%); high stromal content, grows in cord-like pattern; | 10–15%; mostly in periphery | 5–10% | 80–85% | moderate–strong |
| 6b | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma, in the middle eosinophilic acellular material, and a group of atypical cells with cytoplasmic clearing (60–65%); high stromal content, grows in cord-like pattern | 10–15%; mostly in periphery | 5–10% | 75–80% | moderate |
| 6b | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma, in the middle eosinophilic acellular material, and a group of atypical cells with cytoplasmic clearing (10–15%); high stromal content, grows in cord-like pattern | 20–25%; mostly in periphery | <5% | 85–90% | moderate–strong |
| 6b | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma, in the middle eosinophilic acellular material, and a group of atypical cells with cytoplasmic clearing (80–90%); high stromal content, grows in cord-like pattern | 5–10%; predominantly in periphery | 5–10% | 85–90% | moderate–strong |
| 6b | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma, in the middle eosinophilic acellular material, and a group of atypical cells with cytoplasmic clearing (15–20%); high stromal content, grows in cord-like pattern | 10–15%; scattered vessels | 10–15% | 80–85% | moderate |
| 6b | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma, in the middle eosinophilic acellular material, and a group of atypical cells with cytoplasmic clearing (15–20%); high stromal content, grows in cord-like pattern | 15–20%; scattered elongated vessels | 10–15% | 80–85% | moderate |
| 6b | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma, in the middle eosinophilic acellular material, and a group of atypical cells with cytoplasmic clearing (5–10%); high stromal content, grows in cord-like pattern | 10–15%; scattered vessels | 10–15% | 75–80% | moderate |
| 6b | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma, in the middle eosinophilic acellular material, and a group of atypical cells with cytoplasmic clearing (60–70%); high stromal content, grows in cord-like pattern | 5–10%; predominantly in periphery | 5–10% | 85–90% | moderate–strong |
| 6b | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma, in the middle eosinophilic acellular material, and a group of atypical cells with cytoplasmic clearing (5%); high stromal content, grows in cord-like pattern | 10–15%; scattered vessels | 5–10% | 80–85% | moderate–strong |
| 6b | mass forming, undifferentiated (cells look atypical loss of nuclear polarity, hyperchromatic nuclei), in between stroma, in the middle eosinophilic acellular material, and a group of atypical cells with cytoplasmic clearing (5%); high stromal content, grows in cord-like pattern | 15–20%; scattered vessels | 5–10% | 60–65% | mild–moderate |
References
- Sheppard, C.W.; Goodell, J.P.B.; Hahn, P.F. Colloidal gold containing the radioactive isotope Au198 in the selective internal radiation therapy of diseases of the lymphoid system. J. Lab. Clin. Med. 1947, 12, 1437–1441. [Google Scholar] [PubMed]
- Flocks, R.H.; Kerr, H.D.; Elkins, H.B.; Culp, D. Treatment of carcinoma of the prostate by interstitial radiation with radio-active gold (Au 198): A preliminary report. J. Urol. 1952, 68, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Maeda, H.; Fang, J.; Inutsuka, T.; Kitamoto, Y. Vascular permeability enhancement in solid tumor: Various factors, mechanisms involved and its implications. Int. Immunopharmacol. 2003, 3, 319–328. [Google Scholar] [CrossRef]
- Pretze, M.; Hien, A.; Radle, M.; Schirrmacher, R.; Wängler, C.; Wängler, B. Gastrin-releasing peptide receptor- and prostate-specific membrane antigen-specific ultrasmall gold nanoparticles for characterization and diagnosis of prostate carcinoma via fluorescence imaging. Bioconjug. Chem. 2018, 29, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Pretze, M.; von Kiedrowski, V.; Runge, R.; Freudenberg, R.; Hübner, R.; Davarci, G.; Schirrmacher, R.; Wängler, C.; Wängler, B. αvβ3-Specific gold nanoparticles for fluorescence imaging of tumor angiogenesis. Nanomaterials 2021, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, E.J.; Kim, J.W.; Chung, U.S.; Koh, W.G.; Keum, K.C.; Koom, W.S. Gold nanoparticles enhance anti-tumor effect of radiotherapy to hypoxic tumor. Radiat. Oncol. J. 2016, 34, 230–238. [Google Scholar] [CrossRef]
- Black, K.C.L.; Wang, Y.; Luehmann, H.P.; Cai, X.; Xing, W.; Pang, B.; Zhao, Y.; Cutler, C.S.; Wang, L.V.; Liu, Y.; et al. Radioactive 198Au-Doped Nanostructures with Different Shapes for In Vivo Analyses of Their Biodistribution, Tumor Uptake, and Intratumoral Distribution. ACS Nano 2014, 8, 4385–4394. [Google Scholar] [CrossRef]
- Cui, S.; Yin, D.; Chen, Y.; Di, Y.; Chen, H.; Ma, Y.; Achilefu, S.; Gu, Y. In vivo targeted deep-tissue photodynamic therapy based on near-infrared light triggered upconversion nanoconstruct. ACS Nano 2013, 7, 676–688. [Google Scholar] [CrossRef]
- Aggarwal, R.; Sheikh, A.; Akhtar, M.; Ghazwani, M.; Hani, U.; Sahebkar, A.; Kesharwani, P. Understanding gold nanoparticles and their attributes in ovarian cancer therapy. Mol. Cancer 2025, 24, 88. [Google Scholar] [CrossRef]
- Choudhury, M.; Brunton, P.; Dias, G.; Schwass, D.; Meledandri, C.; Ratnayake, J.; Pletzer, D.; Tompkins, G. Gold nanoparticles as innovative therapeutics for oral mucositis: A review of current evidence. Drug Deliv. Transl. Res. 2025, 15, 2323–2353. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, K.; Rojas, M. Nanoparticles targeting monocytes and macrophages as diagnostic and therapeutic tools for autoimmune diseases. Heliyon 2023, 9, e19861. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.; Khlebtsov, B.; Khlebtsov, N. Drug delivery using gold nanoparticles. Adv. Drug Deliv. Rev. 2025, 216, 115481. [Google Scholar] [CrossRef] [PubMed]
- Zarschler, K.; Rocks, L.; Licciardello, N.; Boselli, L.; Polo, E.; Garcia, K.P.; De Cola, L.; Stephan, H.; Dawson, K.A. Ultrasmall inorganic nanoparticles: State-of-the-art and perspectives for biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1663–1701. [Google Scholar] [CrossRef]
- Cui, M.; Liu, R.; Deng, Z.; Ge, G.; Liu, Y.; Xie, L. Quantitative study of protein coronas on gold nanoparticles with different surface modifications. Nano Res. 2014, 7, 345–352. [Google Scholar] [CrossRef]
- Dai, Q.; Walkey, C.; Chan, W.C. Polyethylene glycol backfilling mitigates the negative impact of the protein corona on nanoparticle cell targeting. Angew. Chem. Int. Ed. 2014, 53, 5093–5096. [Google Scholar] [CrossRef]
- Shukla, R.; Chanda, N.; Zambre, A.; Upendran, A.; Katti, K.; Kulkarni, R.R.; Nune, S.K.; Casteel, S.W.; Smith, C.J.; Vimal, J.; et al. Laminin receptor specific therapeutic gold nanoparticles (198AuNP-EGCg) show efficacy in treating prostate cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 12426–12431. [Google Scholar] [CrossRef]
- Chanda, N.; Kattumuri, V.; Shukla, R.; Zambre, A.; Katti, K.; Upendran, A.; Kulkarni, R.R.; Kan, P.; Fent, G.M.; Casteel, S.W.; et al. Bombesin functionalized gold nanoparticles show in vitro and in vivo cancer receptor specificity. Proc. Natl. Acad. Sci. USA 2010, 107, 8760–8765. [Google Scholar] [CrossRef]
- Lin, F.-S.; Chen, C.-H.; Tseng, F.-G.; Hwu, Y.; Chen, J.-K.; Lin, S.-Y.; Yang, C.-S. Radiotherapy of the Excretable Radioactive Gold Nanocomposite with Intratumoral Injection. Int. J. Mater. Mech. Manuf. 2013, 1, 265–268. [Google Scholar] [CrossRef]
- Zhu, J.; Chin, J.; Wängler, C.; Wängler, B.; Lennox, R.B.; Schirrmacher, R. Rapid 18F-labeling and loading of PEGylated gold nanoparticles for in vivo applications. Bioconjug. Chem. 2014, 25, 1143–1150. [Google Scholar] [CrossRef]
- Zhao, Y.; Sultan, D.; Detering, L.; Cho, S.; Sun, G.; Pierce, R.; Wooley, K.L.; Liu, Y. Copper-64-alloyed gold nanoparticles for cancer imaging: Improved radiolabel stability and diagnostic accuracy. Angew. Chem. Int. Ed. 2014, 53, 156–159. [Google Scholar] [CrossRef]
- Pretze, M.; van der Meulen, N.P.; Wängler, C.; Schibli, R.; Wängler, B. Targeted 64Cu-labeled gold nanoparticles for dual imaging with positron emission tomography and optical imaging. J. Label. Comp. Radiopharm. 2019, 62, 471–482. [Google Scholar] [CrossRef]
- Jiménez-Mancilla, N.; Ferro-Flores, G.; Santos-Cuevas, C.; Ocampo-García, B.; Luna-Gutiérrez, M.; Azorín-Vega, E.; Isaac-Olivé, K.; Camacho-López, M.; Torres-García, E. Multifunctional targeted therapy system based on 99mTc/177Lu-labeled gold nanoparticles-Tat(49-57)-Lys3-bombesin internalized in nuclei of prostate cancer cells. J. Label. Compd. Radiopharm. 2013, 56, 663–671. [Google Scholar] [CrossRef]
- Eskandari, N.; Yavari, K.; Outokesh, M.; Sadjadi, S.; Ahmadi, S.J. Iodine-131 radiolabeling of poly ethylene glycol-coated gold nanorods for in vivo imaging. J. Label. Compd. Radiopharm. 2013, 56, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Pretze, M.; Hien, A.; Roscher, M.; Richter, K.; Rädle, M.; Wängler, C.; Wängler, B. P422—Efficient modification of GRPR-specific gold nanoparticles for fluorescence imaging of prostate carcinoma. J. Label. Compd. Radiopharm. 2017, 60 (Suppl. 1), S601. [Google Scholar] [CrossRef]
- Hien, A.; Pretze, M.; Braun, F.; Schäfer, E.; Kümmel, T.; Roscher, M.; Schock-Kusch, D.; Waldeck, J.; Müller, B.; Wängler, C.; et al. Non-contact recognition of fluorescently labeled objects in deep tissue via optimized optical arrangement. PLoS ONE 2018, 13, e0208236. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, X. Gold nanoparticles for photoacoustic imaging. Nanomed. Nanotechnol. Biol. Med. 2015, 10, 299–320. [Google Scholar] [CrossRef]
- Häkkinen, H. The gold-sulfur interface at the nanoscale. Nat. Chem. 2012, 4, 443–455. [Google Scholar] [CrossRef]
- Chanda, N.; Shukla, R.; Katti, K.V.; Kannan, R. Gastrin releasing protein receptor specific gold nanorods: Breast and prostate tumor avid nanovectors for molecular imaging. Nano Lett. 2009, 9, 1798–1805. [Google Scholar] [CrossRef]
- Mayo, R.L.; Robinson, F.R.S. Auger and secondary X-ray electrons from gold. Proc. R. Soc. Lond. A 1939, 173, 192–200. [Google Scholar] [CrossRef]
- Zhang, X.D.; Wu, D.; Shen, X.; Chen, J.; Sun, Y.M.; Liu, P.X.; Liang, X.J. Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials 2012, 33, 6408–6419. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309–N315. [Google Scholar] [CrossRef]
- Chanda, N.; Kan, P.; Watkinson, L.D.; Shukla, R.; Zambre, A.; Carmack, T.L.; Engelbrecht, H.; Lever, J.R.; Katti, K.; Fent, G.M.; et al. Radioactive gold nanoparticles in cancer therapy: Therapeutic efficacy studies of GA-198AuNP nanoconstruct in prostate tumor-bearing mice. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 201–209. [Google Scholar] [CrossRef]
- Säterborg, N.E. The distribution of 198Au injected intravenously as a colloid and in solution. Acta Radiol. Ther. Phys. Biol. 1973, 12, 509–528. [Google Scholar] [CrossRef]
- Khan, M.K.; Minc, L.D.; Nigavekar, S.S.; Kariapper, M.S.T.; Nair, B.M.; Schipper, M.; Cook, A.C.; Lesniak, W.G.; Balogh, L.P. Fabrication of {198Au0} radioactive composite nanodevices and their use for nano-brachytherapy. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 57–69. [Google Scholar] [CrossRef]
- Chakravarty, R.; Chakraborty, S.; Guleria, A.; Kumar, C.; Kunwar, A.; Nair, K.V.V.; Sarma, H.D.; Dash, A. Clinical scale synthesis of intrinsically radiolabeled and cyclic RGD peptide functionalized 198Au nanoparticles for targeted cancer therapy. Nucl. Med. Biol. 2019, 72–73, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Aboudzadeh, M.R.; Moassesi, M.E.; Amiri, M.; Shams, H.; Alirezapour, B.; Sadeghi, M.; Sari, M.F.; Keyvani, M. Preparation and characterization of chitosan-capped radioactive gold nanoparticles: Neutron irradiation impact on structural properties. J. Iran. Chem. Soc. 2015, 13, 339–345. [Google Scholar] [CrossRef]
- Quigley, N.G.; Steiger, K.; Hoberück, S.; Czech, N.; Zierke, M.A.; Kossatz, S.; Pretze, M.; Richter, F.; Weichert, W.; Pox, C.; et al. PET/CT imaging of head-and-neck and pancreatic cancer in humans by targeting the “Cancer Integrin” avb6 with Ga-68-Trivehexin. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 1136–1147. [Google Scholar] [CrossRef]
- Rehm, J.; Winzer, R.; Pretze, M.; Müller, J.; Notni, J.; Hempel, S.; Distler, M.; Folprecht, G.; Kotzerke, J. αvβ6-Integrin Targeted PET/CT Imaging in Pancreatic Cancer Patients Using 68Ga-Trivehexin. Front. Nuc. Med. 2024, 4, 1487602. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, F.; Chen, X. Integrin αvβ3-targeted cancer therapy. Drug Dev. Res. 2008, 69, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Turcu, I.; Zarafu, I.; Popa, M.; Chifiriuc, M.C.; Bleotu, C.; Culita, D.; Ghica, C.; Ionita, P. Lipoic acid gold nanoparticles functionalized with organic compounds as bioactive materials. Nanomaterials 2017, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of Thiol-derivatised Gold Nanoparticles in a Two-phase Liquid-Liquid System. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Zhu, J.; Waengler, C.; Lennox, R.B.; Schirrmacher, R. Preparation of water-soluble maleimide-functionalized 3 nm gold nanoparticles: A new bioconjugation template. Langmuir ACS J. Surf. Colloids 2012, 28, 5508–5512. [Google Scholar] [CrossRef]
- Davarci, G.; Wängler, C.; Eberhardt, K.; Geppert, C.; Schirrmacher, R.; Freudenberg, R.; Pretze, M.; Wängler, B. Radiosynthesis of Stable 198Au-Nanoparticles by Neutron Activation of αvβ3-Specific AuNPs for Therapy of Tumor Angiogenesis. Pharmaceuticals 2023, 16, 1670. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, R. Monte-Carlo-Simulation Zur Dosimetrie Bei Der Zellexposition Mit Offenen Radionukliden in Typischen In-Vitro Bestrahlungsgeometrien. Master’s Thesis, Technical University Dresden, Dresden, Germany, 2012. Available online: https://eltab.ub.uni-kl.de/media/103162/ (accessed on 1 September 2025).
- Shi, J.; Wang, F.; Liu, S. Radiolabeled cyclic RGD peptides as radiotracers for tumor imaging. Biophys. Rep. 2016, 2, 1–20. [Google Scholar] [CrossRef]
- Wängler, C.; Maschauer, S.; Prante, O.; Schäfer, M.; Schirrmacher, R.; Bartenstein, P.; Eisenhut, M.; Wängler, B. Multimerization of cRGD peptides by click chemistry: Synthetic strategies, chemical limitations, and influence on biological properties. Chembiochem 2010, 11, 2168–2181. [Google Scholar] [CrossRef] [PubMed]
- Haubner, R.; Gratias, R.; Diefenbach, B.; Goodman, S.L.; Jonczyk, A.; Kessler, H. Structural and functional aspects of RGD-containing cyclic pentapeptides as highly potent and selective integrin αvβ3 antagonists. J. Am. Chem. Soc. 1996, 118, 7461–7472. [Google Scholar] [CrossRef]
- Hahn, M.B.; Villate, J.M.Z. Combined cell and nanoparticle models for TOPAS to study radiation dose enhancement in cell organelles. Sci. Rep. 2021, 11, 6721. [Google Scholar] [CrossRef]
- Sakr, T.M.; Thipe, V.C.; Katti, K.K.; Watkinson, L.; Carmack, T.; Smith, C.J.; Cutler, C.; Hegde, P.; Hegde, A.; Lugao, A.B.; et al. Immunomodulatory green nanomedicine production, tumor cellular targeting, in vivo biodistributions and preclinical therapeutic efficacy investigations of resveratrol-functionalized gold and theranostic 198gold nanoparticles. J. Mater. Chem. B 2025, 13, 8038–8050. [Google Scholar] [CrossRef]
- Żelechowska-Matysiak, K.; Wawrowicz, K.; Wierzbicki, M.; Budlewski, T.; Bilewicz, A.; Majkowska-Pilip, A. Radiobioconjugate of Kadcyla with Radioactive Gold Nanoparticles for Targeted Therapy of HER2-Overexpressing Cancers. Mol. Pharm. 2025, 22, 4019–4031. [Google Scholar] [CrossRef]
- Phillips, W.T.; Goins, B.; Bao, A.; Vargas, D.; Guttierez, J.E.; Trevino, A.; Miller, J.R.; Henry, J.; Zuniga, R.; Vecil, G.; et al. Rhenium-186 liposomes as convection-enhanced nanoparticle brachytherapy for treatment of glioblastoma. Neuro-Oncology 2012, 14, 416–425. [Google Scholar] [CrossRef]
- Brenner, A.J.; Patel, T.; Bao, A.; Phillips, W.T.; Michalek, J.E.; Youssef, M.; Weinberg, J.S.; Kamiya Matsuoka, C.; Hedrick, M.H.; LaFrance, N.; et al. Convection enhanced delivery of Rhenium [186Re]Obisbemeda (186RNL) in recurrent glioma: A multicenter, single arm, phase 1 clinical trial. Nat. Commun. 2025, 16, 2079. [Google Scholar] [CrossRef]
- Mier, W.; Babich, J.; Haberkorn, U. Is nano too big? Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 4–6. [Google Scholar] [CrossRef]
- Kiessling, F.; Mertens, M.E.; Grimm, J.; Lammers, T. Nanoparticles for imaging: Top or flop? Radiology 2014, 273, 10–28. [Google Scholar] [CrossRef]
- Pompa, P.P.; Vecchio, G.; Galeone, A.; Brunetti, V.; Sabella, S.; Maiorano, G.; Falqui, A.; Bertoni, G.; Cingolani, R. In vivo toxicity assessment of gold nanoparticles in Drosophila melanogaster. Nano Res. 2011, 4, 405–413. [Google Scholar] [CrossRef]
- Vecchio, G.; Galeone, A.; Brunetti, V.; Maiorano, G.; Sabella, S.; Cingolani, R.; Pompa, P.P. Concentration-dependent, size-independent toxicity of citrate capped AuNPs in Drosophila melanogaster. PLoS ONE 2012, 7, e29980. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging macrophages with nanoparticles. Nat. Mat. 2014, 13, 125–138. [Google Scholar] [CrossRef]
- Nankivell, V.; Vidanapathirana, A.K.; Hoogendoorn, A.; Tan, J.T.M.; Verjans, J.; Psaltis, P.J.; Hutchinson, M.R.; Gibson, B.C.; Lu, Y.; Goldys, E.; et al. Targeting macrophages with multifunctional nanoparticles to detect and prevent atherosclerotic cardiovascular disease. Cardiovasc. Res. 2024, 120, 819–838. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.F.; Filipe, H.A.L.; Miguel, S.P.; Ribeiro, M.J.; Coutinho, P. Recent advances in smart gold nanoparticles for photothermal therapy. Nanomed. Nanotechnol. Biol. Med. 2025, 20, 1339–1353. [Google Scholar] [CrossRef]
- Zamora, P.O.; Marek, M.J. Post Labeling Stabilization of Radiolabeled Proteins and Peptides. U.S. Patent 6,261,536 B1, 17 July 2001. [Google Scholar]
- Dai, X.; Su, Z.; Liu, J.O. An improved synthesis of a selective αvβ3-integrin antagonist cyclo(-RGDfK-). Tetrahedron Lett. 2000, 41, 6295–6298. [Google Scholar] [CrossRef]
- Dzwonek, M.; Załubiniak, D.; Piątek, P.; Cichowicz, G.; Męczynska-Wielgosz, S.; Stępkowski, T.; Kruszewski, M.; Więckowska, A.; Bilewicz, R. Towards potent but less toxic nanopharmaceuticals-lipoic acid bioconjugates of ultrasmall gold nanoparticles with an anticancer drug and addressing unit. RSC Adv. 2018, 8, 14947–14957. [Google Scholar] [CrossRef] [PubMed]
| AuNP Sample | Description | Number of Ligands | Molecular Mass [kDa] |
|---|---|---|---|
| 3 | AuNP-dithio-PEG | 240× 2 | 246 |
| 6a | AuNP-dithio-PEG-RGDhigh | 218× 2, 24× 5 | 262 |
| 6b | AuNP-dithio-PEG-RGDlow | 220× 2, 18× 5 | 257 |
| Sample | Amount AuNP | Amount of Gold | Calculated 198Au Activity After Irradiation | Obtained Activity * |
|---|---|---|---|---|
| gold | - | 12.7 mg (100%) | 87 MBq | 60 MBq * |
| 3 | 15.30 mg | 10.10 mg (66%) | 102 MBq | 144.5 MBq ** |
| 3 | 7.11 mg | 4.69 mg (66%) | 50.6 MBq | 65.9 MBq ** |
| 6a | 14.62 mg | 9.21 mg (63%) | 103 MBq | 146.8 MBq ** |
| 6a | 7.07 mg | 4.45 mg (63%) | 50.8 MBq | 65.4 MBq ** |
| 6b | 14.84 mg | 9.65 mg (65%) | 103 MBq | 140.7 MBq ** |
| 6b | 7.00 mg | 4.55 mg (65%) | 50.8 MBq | 69.5 MBq ** |
| Concentration of AuNP 3 | Concentration of Ascorbic Acid | pH (AuNP/AS) | pH (AuNP/AS in Medium) |
|---|---|---|---|
| 0 µM (medium only) | - | - | 7.9 |
| 10.1 µM (stock) | 70.97 mM | 3.5 | - |
| 0.254 µM | 1.77 mM | 4.0 | 7.2 |
| 0.507 µM | 3.55 mM | 3.8 | 7.1 |
| 1.01 µM | 7.10 mM | 3.7 | 6.6 |
| 2.03 µM | 14.19 mM | 3.5 | 5.4 |
| Asc only | 14.19 mM | 3.5 | 5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davarci, G.; Wängler, C.; Eberhardt, K.; Tulessin, M.; Geppert, C.; Schirrmacher, R.; Fricker, G.; Mogler, C.; Pretze, M.; Wängler, B. Preclinical Evaluation of Stable Integrin αvβ3-Specific [198Au]Gold Nanoparticles for Tumor Therapy. Pharmaceuticals 2025, 18, 1670. https://doi.org/10.3390/ph18111670
Davarci G, Wängler C, Eberhardt K, Tulessin M, Geppert C, Schirrmacher R, Fricker G, Mogler C, Pretze M, Wängler B. Preclinical Evaluation of Stable Integrin αvβ3-Specific [198Au]Gold Nanoparticles for Tumor Therapy. Pharmaceuticals. 2025; 18(11):1670. https://doi.org/10.3390/ph18111670
Chicago/Turabian StyleDavarci, Güllü, Carmen Wängler, Klaus Eberhardt, Margaret Tulessin, Christopher Geppert, Ralf Schirrmacher, Gert Fricker, Carolin Mogler, Marc Pretze, and Björn Wängler. 2025. "Preclinical Evaluation of Stable Integrin αvβ3-Specific [198Au]Gold Nanoparticles for Tumor Therapy" Pharmaceuticals 18, no. 11: 1670. https://doi.org/10.3390/ph18111670
APA StyleDavarci, G., Wängler, C., Eberhardt, K., Tulessin, M., Geppert, C., Schirrmacher, R., Fricker, G., Mogler, C., Pretze, M., & Wängler, B. (2025). Preclinical Evaluation of Stable Integrin αvβ3-Specific [198Au]Gold Nanoparticles for Tumor Therapy. Pharmaceuticals, 18(11), 1670. https://doi.org/10.3390/ph18111670










