The Role of Sirt3 in Kidney Health and Disease
Abstract
1. Introduction
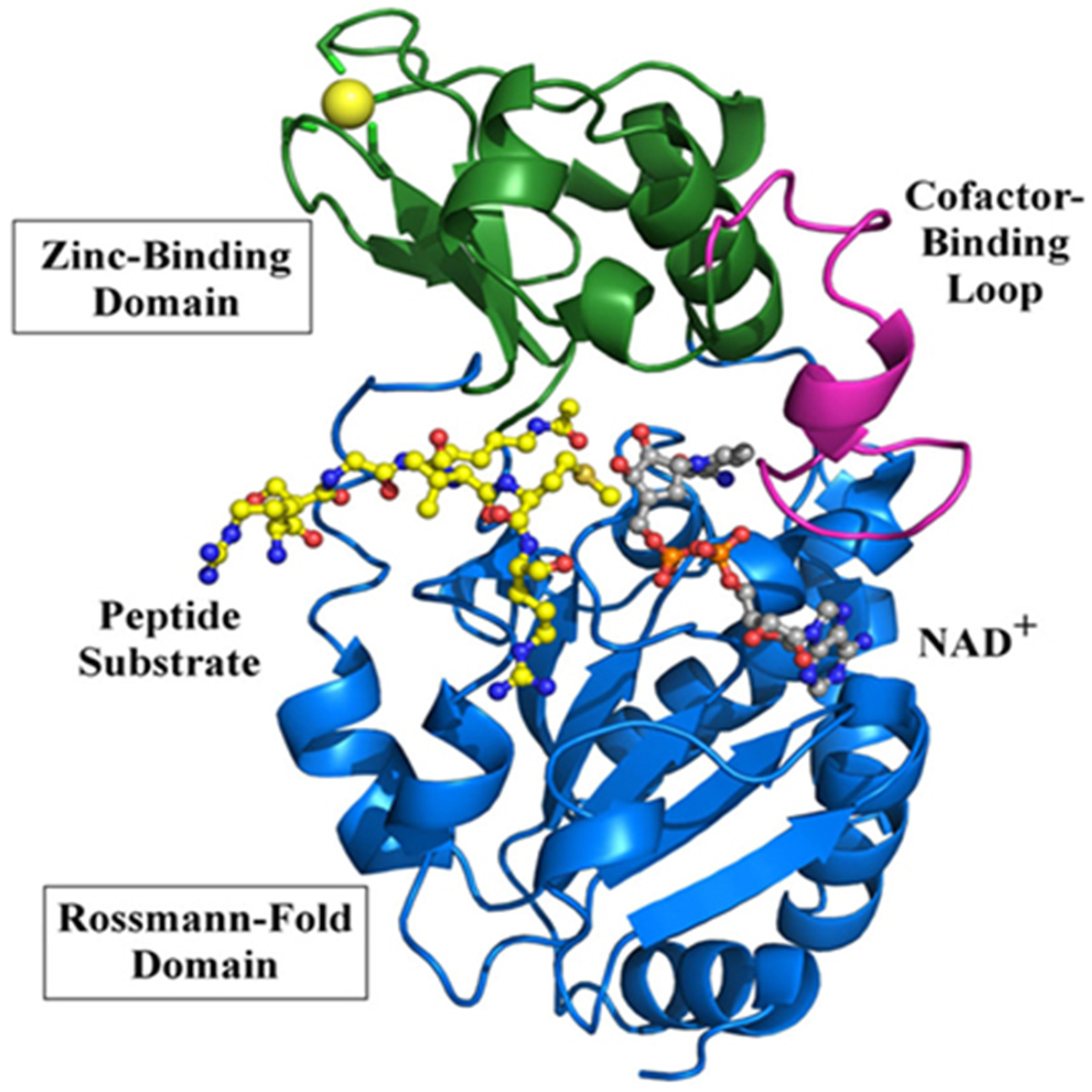
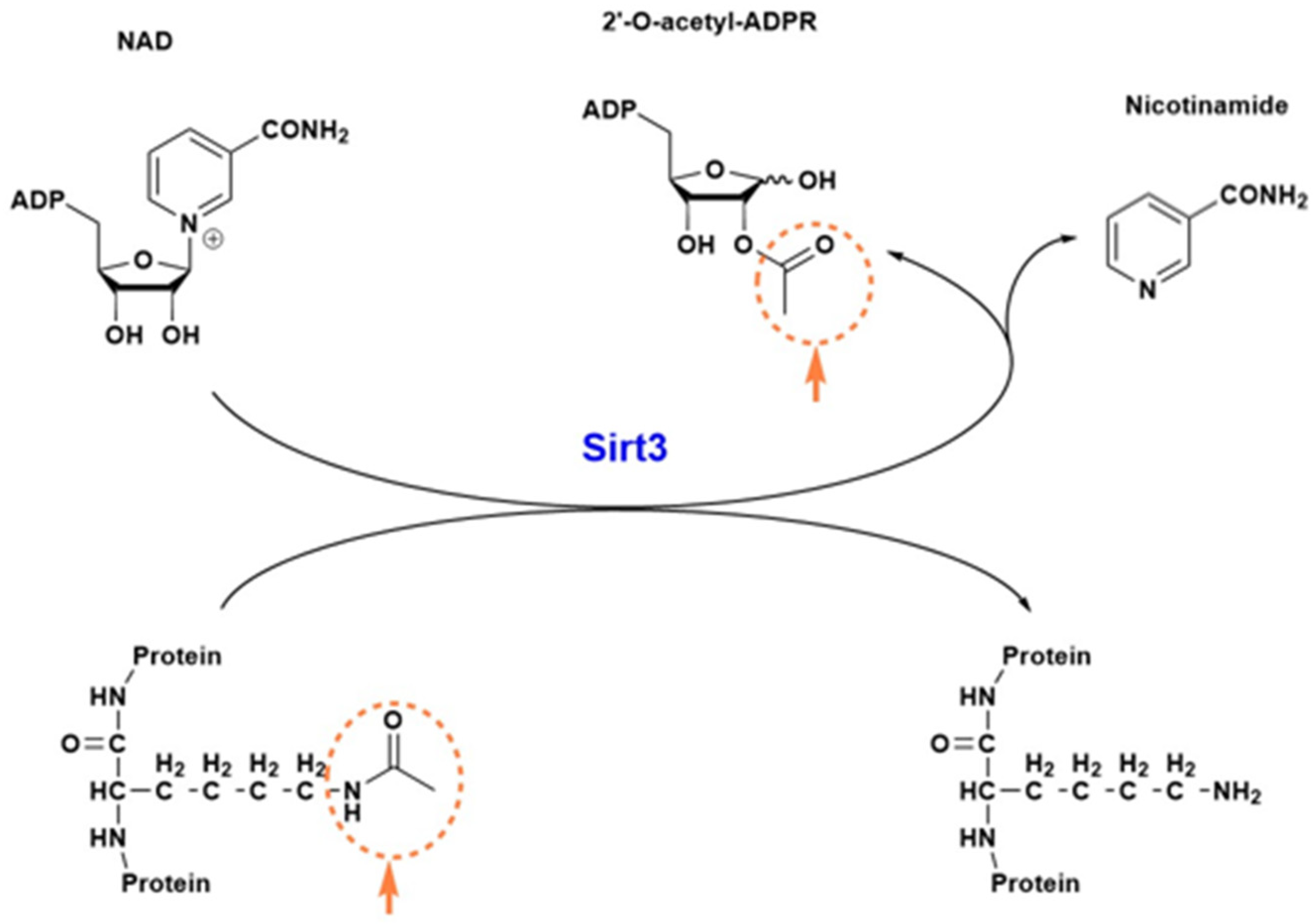
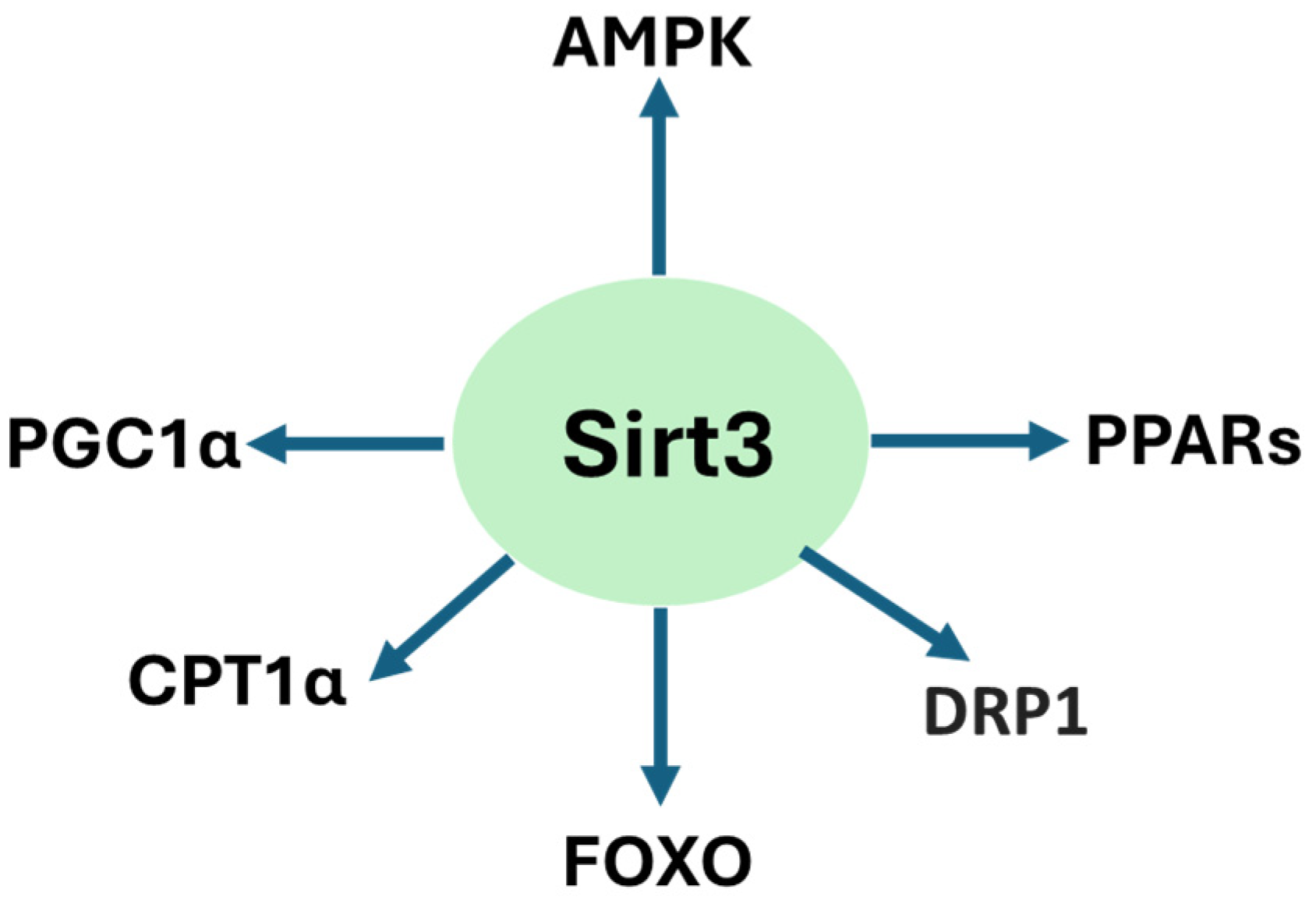
2. Sirt3 Structure and Function
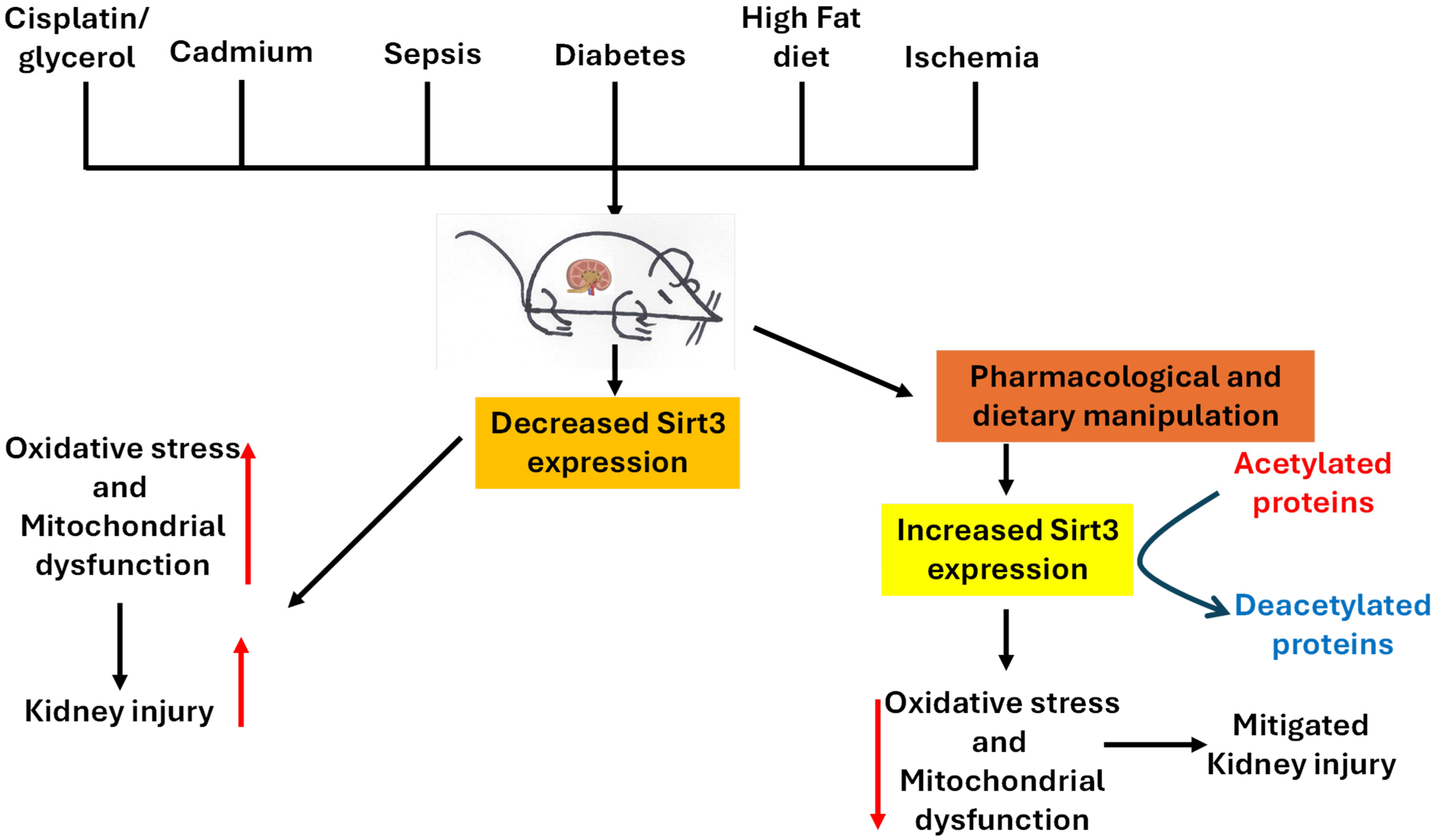
3. Renoprotective Roles of Sirt3
3.1. Sirt3 Protects Against Cisplatin/Glycerol-Induced Acute Kidney Injury
3.2. Sirt3 Protects Against Cadmium-Induced Kidney Injury
3.3. Sirt3 Protects Against Sepsis-Induced AKI
3.4. Sirt3 Deficiency in Diabetic Kidney Disease
3.5. Sirt3 Protects Against High-Fat Diet-Induced Renal Injury
3.6. Sirt3 Protects Against Ischemia-Reperfusion-Induced Kidney Injury
3.7. Dietary Restriction and Pharmacological Activation of Sirt3 in Renal Protection
3.7.1. Dietary Restriction
3.7.2. Pharmacological Activation
| Pharmacological Agents/Natural Products | Kidney Injury Model | Main Mechanisms | References |
|---|---|---|---|
| Apelin | Cisplatin-AKI | Mitochondrial homeostasis | [150] |
| Kaempferol | High glucose-tubular cell damage | Decreased oxidative stress and apoptosis | [151] |
| Calycosin | A variety of models | Anti-oxidative stress | [152] |
| S-nitrosoglutathione | Septic kidney injury | Inhibition of pyroptosis | [153] |
| Sodium thiosulfate | Cisplatin-induced kidney injury | Increased H2S content | [154] |
| β-nicotiamide mononucleotide | Septic AKI | Increased NAD+ content | [147,155] |
| Artemisinin | Diclofenac-induced kidney injury | Mitochondrial homeostasis | [156] |
| Butyrate | High fat-induced glomerulopathy | Decreased oxidative stress | [148] |
| Luseogliflozin (SGLT2 inhibitor) | Ischemia-reperfusion injury | Suppression of ferroptosis | [157] |
| Ligustilide | Ischemia-reperfusion injury | Mitochondrial homeostasis | [158] |
| Tanshinone IIA | Doxorubicin-induced kidney injury | Decreased oxidative stress | [159] |
| Linagliptin | Cisplatin-induced kidney injury | Enhanced mitophagy | [160] |
| Dapagliflozin | Streptozotocin-DKD | Metabolic reprogramming | [117] |
| Dexmedetomidine | IFNα-induced glomerulopathy | Decreased inflammation and oxidative stress | [161] |
| Diosmin | UUO-mouse model | Decreased renal fibrosis | [162] |
| Dihydromyricetin | Gentamicin-induced nephropathy | Anti-oxidative stress | [163] |
| Salvianolic acid B | Diabetic nephropathy | Inhibiting oxidative stress | [120] |
| Dahuang-Gancao decoction | LPS-induced kidney injury | Decreased apoptosis/inflammation | [150] |
| Eprosartan | Ischemic-AKI | Decreased oxidative stress | [164] |
| Shilajit | 5-fluorouracil kidney injury | Decreased oxidative stress | [165] |
| Dexpanthenol | Glycerol-induced kidney injury | Decreased oxidative stress | [166] |
| Nicotinamide riboside | db/db mice | Decreased inflammation | [167] |
| Canagliflozin | High salt renal injury | Decreased oxidative stress | [168] |
| Metrnl | DKD | Mitochondrial homeostasis | [169] |
| Maresin-1 | Cecal ligation and puncture | Anti-inflammation | [170] |
| Notoginsenoside Fc | Acetaminophen-induced nephropathy | Decreased mitochondrial damage | [171] |
| Poricoic acid A | UUO rats | Suppressing renal fibrosis | [172] |
| TUG891/Fatty acid receptor 4 | Ischemic/cisplatin/cecal ligation | Mitigating cell senescence | [173] |
| Magnesium isoglycyrrhizinate | Cisplatin-AKI | Decreased mito-DNA damage | [174] |
| Mitoquinone | Ischemia-reperfusion injury | Decreased oxidative damage | [175] |
| Molineria recurvata | Streptozotocin-DKD | Antioxidation and anti-inflammation | [115] |
| Eplerenone | Ischemia-reperfusion injury | Preserving mitochondrial function | [176] |
| Liquiritigenin | Cisplatin-induced AKI | Improved mitochondrial function | [177] |
| Matrine | Cisplatin-induced AKI | Enhanced mitochondrial function | [178] |
| Fluorofenidone | UUO/ischemic injury | Reduced mitochondrial damage | [179] |
| Honokiol | Cisplatin-AKI | Inhibiting mitochondrial fission | [180] |
| Purple rice husk | DKD | Maintaining redox balance | [181] |
| Melatonin | Contrast-induced AKI | Anti-oxidative stress | [182] |
| Melatonin | Cecal ligation & puncture | Decreased oxidative stress | [183] |
| Melatonin | Ischemic-AKI | Mitochondrial homeostasis | [184] |
| Tenovin-1 | HFD-induced DKD | Antioxidant, anti-inflammation | [111] |
| N-acetylcysteine | Bisphenol A-induced renal injury | Restoring mitochondrial integrity | [185] |
| Mega-3 fatty acids | Rat 5/6 nephrectomy | Nrf2 activation | [186] |
| Apigenin | DKD | Restoring redox balance | [121] |
| Honokiol | DKD | Anti-oxidative stress | [130] |
| Rhein | 5/6 nephrectomy-CKD | Anti-fibrosis & anti-oxidation | [187] |
| Empagliflozin | Streptozotocin-DKD | Inhibiting epithelial-mesenchymal transition | [188] |
| Jian-Pi-Yi-Shen formula | Adenine-induced CKD | Maintaining mitochondrial dynamics | [189] |
| Croton hookeri | Streptozotocin-DKD | Inhibiting oxidative stress | [114] |
| β-lapachone | Cisplatin-AKI | NQO1 activation | [190,191] |
| Spironolactone | 5/6 nephrectomy | Increased p-eNOS production | [192] |
| Renalase | Cisplatin-AKI | Attenuating mitochondrial fission | [193] |
| Stanniocalcin-1 | DKD | Inhibiting BNIP3 | [194] |
| Metformin | Folic acid-AKI, ischemic AKI | Maintaining mitochondrial integrity | [110] |
| Intermedin | 5/6 nephrectomy | Inhibiting oxidative stress | [195] |
| Pyrroloquinoline quinine | High glucose/HK-2 cells | Inhibiting ROS production | [196] |
| Mito-TEMPO | 5/6 nephrectomy | Mitigating renal fibrosis | [197] |
| Fenofibrate | Salt-induced hypertension | Mitochondrial homeostasis | [198] |
| Dioscin | Fructose-induced renal injury | Decreased oxidative stress | [199] |
| Green tea polyphenols | High fat-induced renal damage | Decreased oxidative stress | [112] |
| Lactobacillus rhamnosus GG | Deoxynivalenol-induced kidney injury | Decreased oxidative damage | [200] |
| Annexin A1 | Ischemic-AKI | Increased mitochondrial function | [201] |
| Jinshuiqing | IgA nephropathy | Anti-inflammation | [202] |
| Tert-butylhydroquinone | Contrast-induced nephropathy | Increased antioxidant capacity | [203] |
| Curcumin | Cisplatin-AKI | Mitochondrial homeostasis | [204,205] |
| Silybin | Cisplatin-AKI | Attenuated mitochondrial dysfunction | [44] |
| Resveratrol | Cadmium-induced renal damage | Maintaining redox balance | [78] |
| Resveratrol/exercise | Salt-induced hypertension | Enhanced mitophagy | [206] |
| ACE inhibitor/nicorandil | CKD | Decreased oxidative stress | [207] |
| Coumarin derivative SZC-6 | CKD | Restoring mitochondrial function | [208] |
| Substrate Protein | Disease Model | Reference |
|---|---|---|
| Mitochondrial pyruvate carrier 2 | DKD | [68] |
| Glycogen synthase kinase-3β | UUO | [42] |
| Carnitine palmitoyltransferase 1α | DKD | [209] |
| P53 | Adenine-induced CKD | [47] |
| Krüppel-like factor 15 | DKD | [210] |
| Transcription factor A | LPS-induced sepsis | [211] |
| Mitochondrial SOD2 | Folic acid/ischemia model | [110] |
| Beta-catenin (lysine 49) | UUO | [172] |
| ATP-dependent metalloprotease (YME1L1) | LPS-AKI | [87] |
| Optic atrophy 1 | Cisplatin-AKI | [178] |
| Pyruvate dehydrogenase E1α | UUO | [212] |
| Peroxisome proliferator-activated receptor gamma coactivator-1 alpha | 5/6 Nephrectomy | [186] |
| ATP synthase β | Cisplatin-AKI | [213] |
| Forkhead box protein O3a | Hypertensive renal injury | [214] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diep, T.N.; Liu, H.; Yan, L.J. Beneficial Effects of Butyrate on Kidney Disease. Nutrients 2025, 17, 722. [Google Scholar] [CrossRef]
- Kamt, S.F.; Liu, J.; Yan, L.J. Renal-Protective Roles of Lipoic Acid in Kidney Disease. Nutrients 2023, 15, 1732. [Google Scholar] [CrossRef]
- Yuan, J.; Zhao, J.; Qin, Y.; Zhang, Y.; Wang, A.; Ma, R.; Han, M.; Hui, Y.; Guo, S.; Ning, X.; et al. The protective mechanism of SIRT3 and potential therapy in acute kidney injury. QJM Int. J. Med. 2024, 117, 247–255. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Physiology and pathophysiology of potassium homeostasis. Adv. Physiol. Educ. 2016, 40, 480–490. [Google Scholar] [CrossRef]
- Bankir, L.; Bouby, N.; Speth, R.C.; Velho, G.; Crambert, G. Glucagon revisited: Coordinated actions on the liver and kidney. Diabetes Res. Clin. Pract. 2018, 146, 119–129. [Google Scholar] [CrossRef]
- Sharma, R.; Sahoo, B.; Srivastava, A.; Tiwari, S. Reduced insulin signaling and high glucagon in early insulin resistance impaired fast-fed regulation of renal gluconeogenesis via insulin receptor substrate. J. Cell Biochem. 2022, 123, 1327–1339. [Google Scholar] [CrossRef]
- Meyer, C.; Dostou, J.M.; Gerich, J.E. Role of the human kidney in glucose counterregulation. Diabetes 1999, 48, 943–948. [Google Scholar] [CrossRef]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef]
- Liu, H.; Yan, L.-J. The Role of Ketone Bodies in Various Animal Models of Kidney Disease. Endocrines 2023, 4, 236–249. [Google Scholar] [CrossRef]
- Forbes, J.M.; Thorburn, D.R. Mitochondrial dysfunction in diabetic kidney disease. Nat. Rev. Nephrol. 2018, 14, 291–312. [Google Scholar] [CrossRef]
- Mapuskar, K.A.; Vasquez-Martinez, G.; Mayoral-Andrade, G.; Tomanek-Chalkley, A.; Zepeda-Orozco, D.; Allen, B.G. Mitochondrial Oxidative Metabolism: An Emerging Therapeutic Target to Improve CKD Outcomes. Biomedicines 2023, 11, 1573. [Google Scholar] [CrossRef]
- Chang, L.Y.; Chao, Y.L.; Chiu, C.C.; Chen, P.L.; Lin, H.Y. Mitochondrial Signaling, the Mechanisms of AKI-to-CKD Transition and Potential Treatment Targets. Int. J. Mol. Sci. 2024, 25, 1518. [Google Scholar] [CrossRef]
- Cao, M.; Zhao, X.; Xia, F.; Shi, M.; Zhao, D.; Li, L.; Jiang, H. Mitochondrial dysfunction and metabolic reprogramming in acute kidney injury: Mechanisms, therapeutic advances, and clinical challenges. Front Physiol. 2025, 16, 1623500. [Google Scholar] [CrossRef]
- Yan, L.J. Folic acid-induced animal model of kidney disease. Anim. Models Exp. Med. 2021, 4, 329–342. [Google Scholar] [CrossRef]
- Haoxin, L.; Yucheng, W.; Ying, W.; Liang-Jun, Y. Rodent Models of Streptozotocin-Induced Diabetes as Suitable Paradigms for Studying Diabetic Kidney Disease. Free Radic. Antioxid. 2024, 14, 32–33. [Google Scholar]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Emma, F.; Montini, G.; Parikh, S.M.; Salviati, L. Mitochondrial dysfunction in inherited renal disease and acute kidney injury. Nat. Rev. Nephrol. 2016, 12, 267–280. [Google Scholar] [CrossRef]
- Morigi, M.; Perico, L.; Rota, C.; Longaretti, L.; Conti, S.; Rottoli, D.; Novelli, R.; Remuzzi, G.; Benigni, A. Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J. Clin. Investig. 2015, 125, 715–726. [Google Scholar] [CrossRef]
- Takasu, M.; Kishi, S.; Nagasu, H.; Kidokoro, K.; Brooks, C.R.; Kashihara, N. The Role of Mitochondria in Diabetic Kidney Disease and Potential Therapeutic Targets. Kidney Int. Rep. 2025, 10, 328–342. [Google Scholar] [CrossRef]
- Srivastava, S.P.; Li, J.; Kitada, M.; Fujita, H.; Yamada, Y.; Goodwin, J.E.; Kanasaki, K.; Koya, D. SIRT3 deficiency leads to induction of abnormal glycolysis in diabetic kidney with fibrosis. Cell Death Dis. 2018, 9, 997. [Google Scholar] [CrossRef]
- Perico, L.; Remuzzi, G.; Benigni, A. Sirtuins in kidney health and disease. Nat. Rev. Nephrol. 2024, 20, 313–329. [Google Scholar] [CrossRef]
- Wang, X.; Luo, T.; Yang, Y.; Yang, L.; Liu, M.; Zou, Q.; Wang, D.; Yang, C.; Xue, Q.; Liu, S.; et al. TRPA1 protects against contrast-induced renal tubular injury by preserving mitochondrial dynamics via the AMPK/DRP1 pathway. Free Radic. Biol. Med. 2024, 224, 521–539. [Google Scholar] [CrossRef]
- Wang, Z.; Do Carmo, J.M.; Da Silva, A.A.; Fu, Y.; Hall, J.E. Mechanisms of Synergistic Interactions of Diabetes and Hypertension in Chronic Kidney Disease: Role of Mitochondrial Dysfunction and ER Stress. Curr. Hypertens. Rep. 2020, 22, 15. [Google Scholar] [CrossRef]
- Pavlovic, N.; Krizanac, M.; Kumric, M.; Vukojevic, K.; Bozic, J. Mitochondrial Dysfunction: The Silent Catalyst of Kidney Disease Progression. Cells 2025, 14, 794. [Google Scholar] [CrossRef]
- Bosch-Presegue, L.; Vaquero, A. Sirtuins in stress response: Guardians of the genome. Oncogene 2014, 33, 3764–3775. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, B.; Wang, H.; Lv, S.; Chen, H.; Liu, D. Caloric restriction, Sirtuins, and cardiovascular diseases. Chin. Med. J. 2024, 137, 921–935. [Google Scholar] [CrossRef]
- Chong, M.C.; Silva, A.; James, P.F.; Wu, S.S.X.; Howitt, J. Exercise increases the release of NAMPT in extracellular vesicles and alters NAD+ activity in recipient cells. Aging Cell 2022, 21, e13647. [Google Scholar] [CrossRef]
- Veena, K.V.; Siddamalla, S.; Deenadayal, M.; Sisinthy, S.; Bhanoori, M. Histone deacetylase 1, Sirtuin 1, and Sirtuin 3 single-nucleotide polymorphisms and the risk of endometriosis in South Indian women. J. Obstet. Gynaecol. 2022, 42, 3230–3235. [Google Scholar] [CrossRef]
- Mahlknecht, U.; Voelter-Mahlknecht, S. Chromosomal characterization and localization of the NAD+-dependent histone deacetylase gene sirtuin 1 in the mouse. Int. J. Mol. Med. 2009, 23, 245–252. [Google Scholar] [CrossRef]
- Trinh, D.; Al Halabi, L.; Brar, H.; Kametani, M.; Nash, J.E. The role of SIRT3 in homeostasis and cellular health. Front. Cell. Neurosci. 2024, 18, 1434459. [Google Scholar] [CrossRef]
- Yang, J.; Lu, Y.; Zhao, Y.; Wang, X. Mechanisms of SIRT3 regulation of aging and aging-related diseases and advances in drug therapy. Gerontology 2025, 1–22. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Wang, Z. Roles of SIRT3 in aging and aging-related diseases. Int. J. Biol. Sci. 2025, 21, 5135–5163. [Google Scholar] [CrossRef]
- Jiao, X.; Li, Y.; Zhang, T.; Liu, M.; Chi, Y. Role of Sirtuin3 in high glucose-induced apoptosis in renal tubular epithelial cells. Biochem. Biophys. Res. Commun. 2016, 480, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Ni, H.; Kuang, B.; Wang, Z.; Hou, S.; Gu, S.; Gong, N. Sirtuin 3 in renal diseases and aging: From mechanisms to potential therapies. Pharmacol. Res. 2024, 206, 107261. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, T.; Chen, J.; Fan, Z.; Gao, F.; Yu, Z.; Jiang, Y. SIRT3/6: An amazing challenge and opportunity in the fight against fibrosis and aging. Cell. Mol. Life Sci. 2024, 81, 69. [Google Scholar] [CrossRef] [PubMed]
- Juszczak, F.; Arnould, T.; Decleves, A.E. The Role of Mitochondrial Sirtuins (SIRT3, SIRT4 and SIRT5) in Renal Cell Metabolism: Implication for Kidney Diseases. Int. J. Mol. Sci. 2024, 25, 6936. [Google Scholar] [CrossRef]
- Lombard, D.B.; Tishkoff, D.X.; Bao, J. Mitochondrial sirtuins in the regulation of mitochondrial activity and metabolic adaptation. Handb. Exp. Pharmacol. 2011, 206, 163–188. [Google Scholar]
- Chen, Y.; Zhang, J.; Lin, Y.; Lei, Q.; Guan, K.L.; Zhao, S.; Xiong, Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011, 12, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Luo, D.; Huang, S.; Liu, S.; Zhang, B.; Wang, F.; Lu, J.; Chen, J.; Li, S. Impaired Nicotinamide Adenine Dinucleotide Biosynthesis in the Kidney of Chronic Kidney Disease. Front. Physiol. 2021, 12, 723690. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Le, J.W.; Sun, M.; Zhu, J.H. Sirtuin 3 deficiency promotes acute kidney injury induced by sepsis via mitochondrial dysfunction and apoptosis. Iran. J. Basic Med. Sci. 2021, 24, 675–681. [Google Scholar] [PubMed]
- Pezzotta, A.; Perico, L.; Corna, D.; Morigi, M.; Remuzzi, G.; Benigni, A.; Imberti, B. Sirt3 deficiency promotes endothelial dysfunction and aggravates renal injury. PLoS ONE 2023, 18, e0291909. [Google Scholar] [CrossRef]
- Wang, J.; Ren, X.; Lu, H.; Guo, Z.; Li, X.; Tian, Y.; Yin, Y.; Qin, Z.; Yun, K.; Wu, M.; et al. Restoration of SIRT3 Expression in Aged Mice Alleviates UUO-Induced Renal Fibrosis by Reducing GSK-3beta Hyperacetylation. Adv. Sci. 2025. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Zhang, L.; Sui, M.X.; Zhu, Y.H.; Zeng, L. Protective effects of sirtuin 3 in a murine model of sepsis-induced acute kidney injury. Sci. Rep. 2016, 6, 33201. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Zhang, L.; Sui, M.X.; Zhu, Y.H.; Zeng, L. Activation of Sirtuin 3 by Silybin Attenuates Mitochondrial Dysfunction in Cisplatin-induced Acute Kidney Injury. Front. Pharmacol. 2017, 8, 178. [Google Scholar] [CrossRef]
- Locatelli, M.; Macconi, D.; Corna, D.; Cerullo, D.; Rottoli, D.; Remuzzi, G.; Benigni, A.; Zoja, C. Sirtuin 3 Deficiency Aggravates Kidney Disease in Response to High-Fat Diet through Lipotoxicity-Induced Mitochondrial Damage. Int. J. Mol. Sci. 2022, 23, 8345. [Google Scholar] [CrossRef] [PubMed]
- Kobroob, A.; Kumfu, S.; Chattipakorn, N.; Wongmekiat, O. Modulation of Sirtuin 3 by N-Acetylcysteine Preserves Mitochondrial Oxidative Phosphorylation and Restores Bisphenol A-Induced Kidney Damage in High-Fat-Diet-Fed Rats. Curr. Issues Mol. Biol. 2024, 46, 4935–4950. [Google Scholar] [CrossRef]
- Xiong, Z.; Hu, X.; Wang, R.; Li, C.; Cheng, H.; Zhao, W.; Shen, Y.; Wang, L.; Li, W.; Zhu, X.; et al. Jingtian granule alleviates adenine-induced renal fibrosis in mice through SIRT3-Mediated deacetylation of P53. Front. Pharmacol. 2025, 16, 1526414. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, H.; Li, J. A review on SIRT3 and its natural small molecule activators as a potential Preventive and therapeutic target. Eur. J. Pharmacol. 2024, 963, 176155. [Google Scholar] [CrossRef]
- Pillai, V.B.; Sundaresan, N.R.; Jeevanandam, V.; Gupta, M.P. Mitochondrial SIRT3 and heart disease. Cardiovasc. Res. 2010, 88, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hu, Y.; Ding, N.; Wei, J.; Li, C. The role of Sirt1 in kidney disease. Int. Urol. Nephrol. 2025, 57, 147–158. [Google Scholar] [CrossRef]
- Kitada, M.; Kume, S.; Takeda-Watanabe, A.; Kanasaki, K.; Koya, D. Sirtuins and renal diseases: Relationship with aging and diabetic nephropathy. Clin. Sci. 2013, 124, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Perico, L.; Morigi, M.; Benigni, A. Mitochondrial Sirtuin 3 and Renal Diseases. Nephron 2016, 134, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Yu, D.; Zhao, H.; Li, P. Sirtuin 3 in diabetic kidney disease: Mechanisms and pharmacotherapy. Ren. Fail. 2025, 47, 2543927. [Google Scholar] [CrossRef]
- Ye, K.; Zhao, Y.; Huang, W.; Zhu, Y. Sodium butyrate improves renal injury in diabetic nephropathy through AMPK/SIRT1/PGC-1alpha signaling pathway. Sci. Rep. 2024, 14, 17867. [Google Scholar]
- Chu, W.; Sun, X.; Yan, Y. Study on the regulation of renal tubular cell apoptosis by SIRT1/NF-kappaB signaling pathway in septic acute kidney injury. Ren. Fail. 2025, 47, 2499904. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, T.; Ma, F.; Fu, T.; Yang, L.; Mao, H.; Wang, Y.; Peng, L.; Li, P.; Zhan, Y. Roles of Sirt1 and its modulators in diabetic microangiopathy: A review. Int. J. Biol. Macromol. 2024, 264 Pt 2, 130761. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Lee, K.; He, J.C. SIRT1 Is a Potential Drug Target for Treatment of Diabetic Kidney Disease. Front. Endocrinol. 2018, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, R.; Lee, K.; He, J.C. The Role of SIRT1 in Diabetic Kidney Disease. Front. Endocrinol. 2014, 5, 166. [Google Scholar] [CrossRef]
- Guan, Y.; Hao, C.M. SIRT1 and Kidney Function. Kidney Dis. 2016, 1, 258–265. [Google Scholar] [CrossRef]
- Moniot, S.; Weyand, M.; Steegborn, C. Structures, substrates, and regulators of Mammalian sirtuins—Opportunities and challenges for drug development. Front. Pharmacol. 2012, 3, 16. [Google Scholar] [CrossRef]
- Morris, B.J. Seven sirtuins for seven deadly diseases of aging. Free Radic. Biol. Med. 2013, 56, 133–171. [Google Scholar] [CrossRef] [PubMed]
- Yu, D. Sirtuin 3 as a promising target in disease therapy: Model action and drug discovery. Eur. J. Med. Chem. 2025, 297, 117929. [Google Scholar] [CrossRef]
- Osborne, B.; Bentley, N.L.; Montgomery, M.K.; Turner, N. The role of mitochondrial sirtuins in health and disease. Free Radic. Biol. Med. 2016, 100, 164–174. [Google Scholar] [CrossRef]
- Benigni, A.; Perico, L.; Macconi, D. Mitochondrial Dynamics Is Linked to Longevity and Protects from End-Organ Injury: The Emerging Role of Sirtuin 3. Antioxid. Redox Signal. 2016, 25, 185–199. [Google Scholar] [CrossRef]
- Ilari, S.; Giancotti, L.A.; Lauro, F.; Dagostino, C.; Gliozzi, M.; Malafoglia, V.; Sansone, L.; Palma, E.; Tafani, M.; Russo, M.A.; et al. Antioxidant modulation of sirtuin 3 during acute inflammatory pain: The ROS control. Pharmacol. Res. 2020, 157, 104851. [Google Scholar] [CrossRef]
- Guo, Y.; Cheng, X.; Huang, C.; Gao, J.; Shen, W. Frataxin Loss Promotes Angiotensin II-Induced Endothelial-to-Mesenchymal Transition. J. Am. Heart Assoc. 2024, 13, e034316. [Google Scholar] [CrossRef]
- Oppedisano, F.; Nesci, S.; Spagnoletta, A. Mitochondrial sirtuin 3 and role of natural compounds: The effect of post-translational modifications on cellular metabolism. Crit. Rev. Biochem. Mol. Biol. 2024, 59, 199–220. [Google Scholar] [CrossRef]
- Sharma, A.; Mahur, P.; Muthukumaran, J.; Singh, A.K.; Jain, M. Shedding light on structure, function and regulation of human sirtuins: A comprehensive review. 3 Biotech 2023, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Feng, L.; Yan, Y.; Ye, H.; Tang, K.; Guo, X.; Ma, Y. SIRT3 deficiency aggravates mitochondrial metabolic disorder and podocyte injury in DKD via MPC2 acetylation. Cell. Signal. 2025, 135, 112029. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Luo, R.; Xu, Z.; Zhang, C.; Zhang, Z.; Ren, P.; He, X.; Zhang, J.; Liu, Y. Fe-flavonoid nanozyme as dual modulator of oxidative stress and autophagy for acute kidney injury repair. Theranostics 2025, 15, 8658–8674. [Google Scholar] [CrossRef] [PubMed]
- Iskander, A.; Yan, L.J. Cisplatin-Induced Kidney Toxicity: Potential Roles of Major NAD+-Dependent Enzymes and Plant-Derived Natural Products. Biomolecules 2022, 12, 1078. [Google Scholar] [CrossRef]
- Yan, L.J. Analysis of oxidative modification of proteins. Curr. Protoc. Protein Sci. 2009, 14, 14–24. [Google Scholar]
- Ye, S.; Zhang, M.; Tang, S.C.W.; Li, B.; Chen, W. PGC1-alpha in diabetic kidney disease: Unraveling renoprotection and molecular mechanisms. Mol. Biol. Rep. 2024, 51, 304. [Google Scholar] [CrossRef]
- Brooks, C.; Wei, Q.; Cho, S.G.; Dong, Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J. Clin. Investig. 2009, 119, 1275–1285. [Google Scholar] [CrossRef]
- Canto, C.; Auwerx, J. NAD+ as a signaling molecule modulating metabolism. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J.; Allen, D.C. Cadmium-Induced Kidney Injury: Oxidative Damage as a Unifying Mechanism. Biomolecules 2021, 11, 1575. [Google Scholar] [CrossRef]
- Fu, B.; Zhao, J.; Peng, W.; Wu, H.; Zhang, Y. Resveratrol rescues cadmium-induced mitochondrial injury by enhancing transcriptional regulation of PGC-1alpha and SOD2 via the Sirt3/FoxO3a pathway in TCMK-1 cells. Biochem. Biophys. Res. Commun. 2017, 486, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Y.; Liang, N.N.; Zhang, X.Y.; Ren, Y.H.; Wu, W.Z.; Liu, Z.B.; He, Y.Z.; Zhang, Y.H.; Huang, Y.C.; Zhang, T.; et al. Mitochondrial GPX4 acetylation is involved in cadmium-induced renal cell ferroptosis. Redox Biol. 2024, 73, 103179. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, C.; Ge, J.; Lv, M.W.; Talukder, M.; Guo, K.; Li, Y.H.; Li, J.L. Ameliorative effects of resveratrol against cadmium-induced nephrotoxicity via modulating nuclear xenobiotic receptor response and PINK1/Parkin-mediated Mitophagy. Food Funct. 2020, 11, 1856–1868. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Karl, I.E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003, 348, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef]
- Qiu, X.; Brown, K.; Hirschey, M.D.; Verdin, E.; Chen, D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010, 12, 662–667. [Google Scholar] [CrossRef]
- Lv, W.; Xue, L.; Liang, L.; Liu, D.; Li, C.; Liao, J.; Jin, Y. Endotoxin induced acute kidney injury modulates expression of AQP1, P53 and P21 in rat kidney, heart, lung and small intestine. PLoS ONE 2023, 18, e0288507. [Google Scholar] [CrossRef]
- Won, J.P.; Lee, H.G.; Yoon, H.J.; Seo, H.G. Biochanin A-mediated anti-ferroptosis is associated with reduction of septic kidney injury. Life Sci. 2024, 358, 123124. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Gu, J.; Li, T.; Chen, H.; Liu, K.; Liu, M.; Zhang, H.; Xiao, X. Inhibition of aerobic glycolysis alleviates sepsis-induced acute kidney injury by promoting lactate/Sirtuin 3/AMPK-regulated autophagy. Int. J. Mol. Med. 2021, 47, 19. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.; Yang, Y.; Cheng, L.; Yang, X.; Liu, H.; Li, W.; Wan, Y.; Yang, D. Sirt3 mitigates LPS-induced mitochondrial damage in renal tubular epithelial cells by deacetylating YME1L1. Cell Prolif. 2023, 56, e13362. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, Z.; Zhang, H.; Wang, L.; Sun, F.; Li, Q.; Cao, T.; Wang, B.; Ma, H.; You, M.; et al. Sodium-glucose exchanger 2 inhibitor canagliflozin promotes mitochondrial metabolism and alleviates salt-induced cardiac hypertrophy via preserving SIRT3 expression. J. Adv. Res. 2025, 70, 255–269. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, Y.; Song, J.; Wang, S.; Lu, J.; Wei, F.; Li, X. Urinary exosomes exacerbate diabetic kidney disease by promoting NLRP3 inflammasome activation via the microRNA-516b-5p/SIRT3/AMPK pathway. Am. J. Physiol. Endocrinol. Metab. 2025, 328, E911–E923. [Google Scholar] [CrossRef]
- Wu, J.; Luo, X.; Thangthaeng, N.; Sumien, N.; Chen, Z.; Rutledge, M.A.; Jing, S.; Forster, M.J.; Yan, L.J. Pancreatic mitochondrial complex I exhibits aberrant hyperactivity in diabetes. Biochem. Biophys. Rep. 2017, 11, 119–129. [Google Scholar] [CrossRef]
- Wu, J.; Jin, Z.; Yan, L.J. Redox imbalance and mitochondrial abnormalities in the diabetic lung. Redox Biol. 2017, 11, 51–59. [Google Scholar] [CrossRef]
- Roxburgh, S.A.; Kattla, J.J.; Curran, S.P.; O’Meara, Y.M.; Pollock, C.A.; Goldschmeding, R.; Godson, C.; Martin, F.; Brazil, D.P. Allelic depletion of grem1 attenuates diabetic kidney disease. Diabetes 2009, 58, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Hanai, J.; Sugimoto, H.; Mammoto, T.; Charytan, D.; Strutz, F.; Kalluri, R. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 2003, 9, 964–968. [Google Scholar] [CrossRef]
- Kanasaki, K.; Taduri, G.; Koya, D. Diabetic nephropathy: The role of inflammation in fibroblast activation and kidney fibrosis. Front Endocrinol 2013, 4, 7. [Google Scholar] [CrossRef]
- LeBleu, V.S.; Taduri, G.; O’Connell, J.; Teng, Y.; Cooke, V.G.; Woda, C.; Sugimoto, H.; Kalluri, R. Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 2013, 19, 1047–1053. [Google Scholar] [CrossRef]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef]
- Zeisberg, M.; Neilson, E.G. Mechanisms of tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 2010, 21, 1819–1834. [Google Scholar] [CrossRef]
- Grande, M.T.; Lopez-Novoa, J.M. Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat. Rev. Nephrol. 2009, 5, 319–328. [Google Scholar] [CrossRef]
- Grgic, I.; Duffield, J.S.; Humphreys, B.D. The origin of interstitial myofibroblasts in chronic kidney disease. Pediatr. Nephrol. 2012, 27, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.P.; Koya, D.; Kanasaki, K. MicroRNAs in kidney fibrosis and diabetic nephropathy: Roles on EMT and EndMT. BioMed Res. Int. 2013, 2013, 125469. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; Grahovac, G.; Zeisberg, M.; Kalluri, R. Renal fibrosis and glomerulosclerosis in a new mouse model of diabetic nephropathy and its regression by bone morphogenic protein-7 and advanced glycation end product inhibitors. Diabetes 2007, 56, 1825–1833. [Google Scholar] [CrossRef]
- Srivastava, S.P.; Shi, S.; Kanasaki, M.; Nagai, T.; Kitada, M.; He, J.; Nakamura, Y.; Ishigaki, Y.; Kanasaki, K.; Koya, D. Effect of Antifibrotic MicroRNAs Crosstalk on the Action of N-acetyl-seryl-aspartyl-lysyl-proline in Diabetes-related Kidney Fibrosis. Sci. Rep. 2016, 6, 29884. [Google Scholar] [CrossRef]
- Kanasaki, K.; Shi, S.; Kanasaki, M.; He, J.; Nagai, T.; Nakamura, Y.; Ishigaki, Y.; Kitada, M.; Srivastava, S.P.; Koya, D. Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes 2014, 63, 2120–2131. [Google Scholar] [CrossRef] [PubMed]
- Palsson-McDermott, E.M.; Curtis, A.M.; Goel, G.; Lauterbach, M.A.R.; Sheedy, F.J.; Gleeson, L.E.; Van den Bosch, M.W.M.; Quinn, S.R.; Domingo-Fernandez, R.; Johnston, D.G.W.; et al. Pyruvate Kinase M2 Regulates Hif-1alpha Activity and IL-1beta Induction and Is a Critical Determinant of the Warburg Effect in LPS-Activated Macrophages. Cell Metab. 2015, 21, 65–80. [Google Scholar] [CrossRef]
- Hamabe, A.; Konno, M.; Tanuma, N.; Shima, H.; Tsunekuni, K.; Kawamoto, K.; Nishida, N.; Koseki, J.; Mimori, K.; Gotoh, N.; et al. Role of pyruvate kinase M2 in transcriptional regulation leading to epithelial-mesenchymal transition. Proc. Natl. Acad. Sci. USA 2014, 111, 15526–15531. [Google Scholar] [CrossRef]
- Liang, J.; Cao, R.; Zhang, Y.; Xia, Y.; Zheng, Y.; Li, X.; Wang, L.; Yang, Y.; Lu, Z. PKM2 dephosphorylation by Cdc25A promotes the Warburg effect and tumorigenesis. Nat. Commun. 2016, 7, 12431. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.M.; Ahn, S.H.; Choi, P.; Ko, Y.A.; Han, S.H.; Chinga, F.; Park, A.S.; Tao, J.; Sharma, K.; Pullman, J.; et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 2015, 21, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J. Clin. Investig. 2013, 123, 3664–3671. [Google Scholar] [CrossRef]
- Morita, M.; Kanasaki, K. Sodium-glucose cotransporter-2 inhibitors for diabetic kidney disease: Targeting Warburg effects in proximal tubular cells. Diabetes Metab. 2020, 46, 353–361. [Google Scholar] [CrossRef]
- Zhu, M.; He, J.; Xu, Y.; Zuo, Y.; Zhou, W.; Yue, Z.; Shao, X.; Cheng, J.; Wang, T.; Mou, S. AMPK activation coupling SENP1-Sirt3 axis protects against acute kidney injury. Mol. Ther. 2023, 31, 3052–3066. [Google Scholar] [CrossRef]
- Yi, W.; Xie, X.; Du, M.; Bu, Y.; Wu, N.; Yang, H.; Tian, C.; Xu, F.; Xiang, S.; Zhang, P.; et al. Green Tea Polyphenols Ameliorate the Early Renal Damage Induced by a High-Fat Diet via Ketogenesis/SIRT3 Pathway. Oxid. Med. Cell. Longev. 2017, 2017, 9032792. [Google Scholar] [CrossRef]
- Kundu, A.; Gali, S.; Sharma, S.; Park, J.H.; Kyung, S.Y.; Kacew, S.; Kim, I.S.; Lee, K.Y.; Kim, H.S. Tenovin-1 Ameliorates Renal Fibrosis in High-Fat-Diet-Induced Diabetic Nephropathy via Antioxidant and Anti-Inflammatory Pathways. Antioxidants 2022, 11, 1812. [Google Scholar] [CrossRef]
- Wang, L.F.; Li, Q.; Le Zhao, J.; Wen, K.; Zhang, Y.T.; Zhao, Q.H.; Ding, Q.; Li, J.H.; Guan, X.H.; Xiao, Y.F.; et al. CD38 deficiency prevents diabetic nephropathy by inhibiting lipid accumulation and oxidative stress through activation of the SIRT3 pathway. Biochem. Cell Biol. 2025, 103, 1–12. [Google Scholar] [PubMed]
- Wang, X.X.; Wang, D.; Luo, Y.; Myakala, K.; Dobrinskikh, E.; Rosenberg, A.Z.; Levi, J.; Kopp, J.B.; Field, A.; Hill, A.; et al. FXR/TGR5 Dual Agonist Prevents Progression of Nephropathy in Diabetes and Obesity. J. Am. Soc. Nephrol. 2018, 29, 118–137. [Google Scholar] [CrossRef]
- Kundu, A.; Dey, P.; Sarkar, P.; Karmakar, S.; Tae, I.H.; Kim, K.S.; Park, J.H.; Lee, S.H.; Lee, B.M.; Renthlei, L.; et al. Protective effects of Croton hookeri on streptozotocin-induced diabetic nephropathy. Food Chem. Toxicol. 2020, 135, 110873. [Google Scholar] [CrossRef]
- Dey, P.; Kundu, A.; Lee, H.E.; Kar, B.; Vishal, V.; Dash, S.; Kim, I.S.; Bhakta, T.; Kim, H.S. Molineria recurvata Ameliorates Streptozotocin-Induced Diabetic Nephropathy through Antioxidant and Anti-Inflammatory Pathways. Molecules 2022, 27, 4985. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, J.Y.; Wei, Z.T.; Liu, S.K.; Sun, J.L.; Mao, Y.H.; Xu, Y.D.; Yang, Y. Therapeutic effect and mechanism of combination therapy with ursolic acid and insulin on diabetic nephropathy in a type I diabetic rat model. Front. Pharmacol. 2022, 13, 969207. [Google Scholar] [CrossRef]
- Li, H.; Xia, Y.; Zha, H.; Zhang, Y.; Shi, L.; Wang, J.; Huang, H.; Yue, R.; Hu, B.; Zhu, J.; et al. Dapagliflozin attenuates AKI to CKD transition in diabetes by activating SIRT3/PGC1-alpha signaling and alleviating aberrant metabolic reprogramming. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167433. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Song, S.; Liu, S.; Li, Q.; Zou, W.; Ke, J.; Wang, C. (Pro)renin receptor mediates tubular epithelial cell pyroptosis in diabetic kidney disease via DPP4-JNK pathway. J. Transl. Med. 2024, 22, 26. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Zhang, S.; Sun, L.; Qian, M.; Xu, H. Salvianolic Acid B Inhibits Oxidative Stress in Glomerular Mesangial Cells Alleviating Diabetic Nephropathy by Regulating SIRT3/FOXO1 Signaling. Kidney Blood Press. Res. 2023, 48, 738–751. [Google Scholar] [CrossRef]
- Ogura, Y.; Kitada, M.; Xu, J.; Monno, I.; Koya, D. CD38 inhibition by apigenin ameliorates mitochondrial oxidative stress through restoration of the intracellular NAD+/NADH ratio and Sirt3 activity in renal tubular cells in diabetic rats. Aging 2020, 12, 11325–11336. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Richa, S.; Dey, P.; Kim, K.S.; Son, J.Y.; Kim, H.R.; Lee, S.Y.; Lee, B.H.; Lee, K.Y.; Kacew, S.; et al. Protective effect of EX-527 against high-fat diet-induced diabetic nephropathy in Zucker rats. Toxicol. Appl. Pharmacol. 2020, 390, 114899. [Google Scholar] [CrossRef]
- Ogura, Y.; Kitada, M.; Monno, I.; Kanasaki, K.; Watanabe, A.; Koya, D. Renal mitochondrial oxidative stress is enhanced by the reduction of Sirt3 activity, in Zucker diabetic fatty rats. Redox Rep. 2018, 23, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef]
- Singh, A.K.; Kari, J.A. Metabolic syndrome and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2013, 22, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, W.; Jiang, W.; Yu, W.; Lin, Y.; Zhang, T.; Yao, J.; Zhou, L.; Zeng, Y.; Li, H.; et al. Regulation of cellular metabolism by protein lysine acetylation. Science 2010, 327, 1000–1004. [Google Scholar] [CrossRef]
- Hallows, W.C.; Yu, W.; Smith, B.C.; Devries, M.K.; Ellinger, J.J.; Someya, S.; Shortreed, M.R.; Prolla, T.; Markley, J.L.; Smith, L.M.; et al. Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol. Cell 2011, 41, 139–149. [Google Scholar] [CrossRef]
- Locatelli, M.; Zoja, C.; Zanchi, C.; Corna, D.; Villa, S.; Bolognini, S.; Novelli, R.; Perico, L.; Remuzzi, G.; Benigni, A.; et al. Manipulating Sirtuin 3 pathway ameliorates renal damage in experimental diabetes. Sci. Rep. 2020, 10, 8418. [Google Scholar] [CrossRef]
- Fu, Y.; Xiang, Y.; Wei, Q.; Ilatovskaya, D.; Dong, Z. Rodent models of AKI and AKI-CKD transition: An update in 2024. Am. J. Physiol. Ren. Physiol. 2024, 326, F563–F583. [Google Scholar] [CrossRef]
- Smith, T.; Zaidi, A.; Brown, C.V.M.; Pino-Chavez, G.; Bowen, T.; Meran, S.; Fraser, D.; Chavez, R.; Khalid, U. Robust Rat and Mouse Models of Bilateral Renal Ischemia Reperfusion Injury. In Vivo 2024, 38, 1049–1057. [Google Scholar] [CrossRef]
- Yan, Y.; Bai, J.; Zhou, X.; Tang, J.; Jiang, C.; Tolbert, E.; Bayliss, G.; Gong, R.; Zhao, T.C.; Zhuang, S. P2X7 receptor inhibition protects against ischemic acute kidney injury in mice. Am. J. Physiol.-Cell Physiol. 2015, 308, C463–C472. [Google Scholar] [CrossRef]
- Chang-Panesso, M.; Kadyrov, F.F.; Lalli, M.; Wu, H.; Ikeda, S.; Kefaloyianni, E.; Abdelmageed, M.M.; Herrlich, A.; Kobayashi, A.; Humphreys, B.D. FOXM1 drives proximal tubule proliferation during repair from acute ischemic kidney injury. J. Clin. Investig. 2019, 129, 5501–5517. [Google Scholar] [CrossRef]
- Ouyang, J.; Zeng, Z.; Fang, H.; Li, F.; Zhang, X.; Tan, W. SIRT3 Inactivation Promotes Acute Kidney Injury Through Elevated Acetylation of SOD2 and p53. J. Surg. Res. 2019, 233, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yang, X.; Jian, Y.; Liu, J.; Ke, X.; Chen, S.; Yang, D.; Yang, D. SIRT3 deficiency exacerbates early-stage fibrosis after ischaemia-reperfusion-induced AKI. Cell. Signal. 2022, 93, 110284. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, J.; Li, X.; Liu, Z.; Han, Y.; Xu, X.; Li, X.; Tang, Y.; Liu, Y.; Yu, T.; et al. Sirt3 modulate renal ischemia-reperfusion injury through enhancing mitochondrial fusion and activating the ERK-OPA1 signaling pathway. J. Cell. Physiol. 2019, 234, 23495–23506. [Google Scholar] [CrossRef]
- Zhang, R.; Wei, X.; Xu, Y.; Han, C.; Cai, X.; Wu, Y.; Geng, Y.; Liu, C. Low-protein Calorie-restriction Mitigates Diabetic Mice Kidney Injury via the Gut-Kidney Axis. Int. J. Vitam. Nutr. Res. 2025, 95, 37311. [Google Scholar] [CrossRef]
- Taufani, I.P.; Laila, S.R.; Tasminatun, S.; Simaremare, S.R.S.; Mardiana, M.; Situmorang, J.H. Calorie and time-restricted feeding improves liver and kidney histopathology in streptozotocin-induced type 1 diabetic rats. Front. Physiol. 2025, 16, 1629751. [Google Scholar] [CrossRef]
- Sohal, R.S.; Forster, M.J. Caloric restriction and the aging process: A critique. Free Radic. Biol. Med. 2014, 73, 366–382. [Google Scholar] [CrossRef] [PubMed]
- Andrianova, N.V.; Zorova, L.D.; Pevzner, I.B.; Kolosova, N.G.; Plotnikov, E.Y.; Zorov, D.B. Calorie Restriction Provides Kidney Ischemic Tolerance in Senescence-Accelerated OXYS Rats. Int. J. Mol. Sci. 2022, 23, 15224. [Google Scholar] [CrossRef] [PubMed]
- Andrianova, N.V.; Zorova, L.D.; Pevzner, I.B.; Popkov, V.A.; Chernikov, V.P.; Silachev, D.N.; Plotnikov, E.Y.; Zorov, D.B. Resemblance and differences in dietary restriction nephroprotective mechanisms in young and old rats. Aging 2020, 12, 18693–18715. [Google Scholar] [CrossRef]
- Dittenhafer-Reed, K.E.; Richards, A.L.; Fan, J.; Smallegan, M.J.; Fotuhi Siahpirani, A.; Kemmerer, Z.A.; Prolla, T.A.; Roy, S.; Coon, J.J.; Denu, J.M. SIRT3 mediates multi-tissue coupling for metabolic fuel switching. Cell Metab. 2015, 21, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Palacios, O.M.; Carmona, J.J.; Michan, S.; Chen, K.Y.; Manabe, Y.; Ward, J.L., 3rd; Goodyear, L.J.; Tong, Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging 2009, 1, 771–783. [Google Scholar] [CrossRef]
- Zhang, B.; Cui, S.; Bai, X.; Zhuo, L.; Sun, X.; Hong, Q.; Fu, B.; Wang, J.; Chen, X.; Cai, G. SIRT3 overexpression antagonizes high glucose accelerated cellular senescence in human diploid fibroblasts via the SIRT3-FOXO1 signaling pathway. Age 2013, 35, 2237–2253. [Google Scholar] [CrossRef]
- Yang, H.; Zuo, X.Z.; Tian, C.; He, D.L.; Yi, W.J.; Chen, Z.; Zhang, P.W.; Ding, S.B.; Ying, C.J. Green Tea Polyphenols Attenuate High-Fat Diet-Induced Renal Oxidative Stress through SIRT3-Dependent Deacetylation. Biomed. Environ. Sci. 2015, 28, 455–459. [Google Scholar]
- Sun, C.; Xiong, H.; Guo, T. beta-Nicotinamide Mononucleotide Alleviates Sepsis-associated Acute Kidney Injury by Activating NAD+/SIRT3 Signaling. Cell Biochem. Biophys. 2025, 83, 2089–2099. [Google Scholar] [CrossRef]
- Shi, Y.; Xing, L.; Zheng, R.; Luo, X.; Yue, F.; Xiang, X.; Qiu, A.; Xie, J.; Russell, R.; Zhang, D. Butyrate attenuates high-fat diet-induced glomerulopathy through GPR43-Sirt3 pathway. Br. J. Nutr. 2025, 133, 1–10. [Google Scholar] [CrossRef]
- Sung, P.H.; Yue, Y.; Chen, Y.L.; Chiang, J.Y.; Cheng, B.C.; Yang, C.C.; Chai, H.T.; Yip, H.K. Combined dapagliflozin and roxadustat effectively protected heart and kidney against cardiorenal syndrome-induced damage in rodent through activation of cell stress-Nfr2/ARE signalings and stabilizing HIF-1alpha. Biomed. Pharmacother. 2024, 180, 117567. [Google Scholar] [CrossRef]
- Guan, Y.M.; Diao, Z.L.; Huang, H.D.; Zheng, J.F.; Zhang, Q.D.; Wang, L.Y.; Liu, W.H. Bioactive peptide apelin rescues acute kidney injury by protecting the function of renal tubular mitochondria. Amino Acids 2021, 53, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.C.; Li, Y.; Wu, D.; Zhang, X.G.; Hou, F. The protective effect of kaempferol on high glucose-stimulated renal tubular epithelial cells. BMC Nephrol. 2025, 26, 477. [Google Scholar] [CrossRef]
- Dalal, D.; Singh, L.; Singh, A. Calycosin and kidney health: A molecular perspective on its protective mechanisms. Pharmacol. Rep. 2025, 77, 658–669. [Google Scholar] [CrossRef]
- Fan, H.; Sun, M.; Zhu, J.H. S-nitrosoglutathione inhibits pyroptosis of kidney tubular epithelial cells in sepsis via the SIRT3/SOD2/mtROS signaling pathway. Ren. Fail. 2025, 47, 2472987. [Google Scholar] [CrossRef]
- Dugbartey, G.J.; Alornyo, K.K.; Adams, I.; Adjei, S.; Amoah, D.; Obeng-Kyeremeh, R. Chemoprotective Mechanism of Sodium Thiosulfate Against Cisplatin-Induced Nephrotoxicity Is via Renal Hydrogen Sulfide, Arginine/cAMP and NO/cGMP Signaling Pathways. Int. J. Mol. Sci. 2025, 26, 384. [Google Scholar] [CrossRef]
- Cao, T.; Ni, R.; Ding, W.; Ji, X.; Fan, G.C.; Zhang, Z.; Peng, T. Nicotinamide mononucleotide as a therapeutic agent to alleviate multi-organ failure in sepsis. J. Transl. Med. 2023, 21, 883. [Google Scholar] [CrossRef] [PubMed]
- Hellal, D.; El-Khalik, S.R.A.; Arakeep, H.M.; Radwan, D.A.; Abo Safia, H.S.; Farrag, E.A.E. Activation of sirtuin 3 and maintenance of mitochondrial homeostasis by artemisinin protect against diclofenac-induced kidney injury in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 5593–5609. [Google Scholar] [CrossRef]
- Hirashima, Y.; Nakano, T.; Torisu, K.; Aihara, S.; Wakisaka, M.; Kitazono, T. SGLT2 inhibition mitigates transition from acute kidney injury to chronic kidney disease by suppressing ferroptosis. Sci. Rep. 2024, 14, 20386. [Google Scholar] [CrossRef]
- Xia, K.; Jin, Z.; Qiu, Q.; Zhou, Y.; Lu, Y.; Qiu, T.; Zhou, J.; Chen, Z. Ligustilide alleviates oxidative stress during renal ischemia-reperfusion injury through maintaining Sirt3-dependent mitochondrial homeostasis. Phytomedicine 2024, 134, 155975. [Google Scholar] [CrossRef]
- Fan, Y.; Kang, S.; Shao, T.; Xu, L.; Chen, J. Activation of SIRT3 by Tanshinone IIA ameliorates renal fibrosis by suppressing the TGF-beta/TSP-1 pathway and attenuating oxidative stress. Cell. Signal. 2024, 122, 111348. [Google Scholar] [CrossRef] [PubMed]
- Ewees, M.G.E.; Mostafa-Hadeab, G.; Saber, S.; El-Meguid, E.A.A.; Sree, H.T.A.; Abdel Rahman, F.E.S.; Mahmoud, N.I. Linagliptin mitigates cisplatin-induced kidney impairment via mitophagy regulation in rats, with emphasis on SIRT-3/PGC-1alpha, PINK-1 and Parkin-2. Toxicol. Appl. Pharmacol. 2024, 491, 117048. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Li, X.; Wu, J. Sirtuin 3 is required for the dexmedetomidine-mediated alleviation of inflammation and oxidative stress in nephritis. Immun. Inflamm. Dis. 2024, 12, e1135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.M.; Li, X.L.; Zhu, Y.; Shi, R.; Wang, Z.J.; Xiao, J.P.; Wang, D.G. Diosmin ameliorates renal fibrosis through inhibition of inflammation by regulating SIRT3-mediated NF-kappaB p65 nuclear translocation. BMC Complement. Med. Ther. 2024, 24, 29. [Google Scholar] [CrossRef]
- Matouk, A.I.; Awad, E.M.; Mousa, A.A.K.; Abdelhafez, S.M.N.; Fahmy, U.A.; El-Moselhy, M.A.; Abdel-Naim, A.B.; Anter, A. Dihydromyricetin protects against gentamicin-induced nephrotoxicity via upregulation of renal SIRT3 and PAX2. Life Sci. 2024, 336, 122318. [Google Scholar] [CrossRef]
- Lotfi, B.; Bagheri, Y.; Abdollahpour, A.; Ahmadian, E.; Matin, S.; Firouzfar, A.; Zununi Vahed, S.; Khajepour, F. Protective effect of Eprosartan against ischemic acute renal injury: Acting on NF-kappaB, caspase 3, and Sirtuin 1. Int. Immunopharmacol. 2023, 115, 109690. [Google Scholar] [CrossRef]
- Ezer, M.; Ozturkler, M.; Yildiz-Dalginli, K.; Atakisi, E.; Beseren-Havadar, H.; Atakisi, O. Investigation of the molecular and cellular effects of Shilajit on 5-fluorouracil (5-FU)-induced nephrotoxicity in rats. Iran. J. Basic Med. Sci. 2025, 28, 565–574. [Google Scholar]
- Koyuncu, F.; Solmaz, F.A.; Gulle, K.; Ilhan, I.; Tepebasi, M.Y.; Ozden, E.S.; Kirdemir, P. Effect of dexpanthenol on glycerol-induced acute kidney injury by targeting the PGC-1alpha/SIRT3 pathway. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 12299–12306. [Google Scholar]
- Myakala, K.; Wang, X.X.; Shults, N.V.; Krawczyk, E.; Jones, B.A.; Yang, X.; Rosenberg, A.Z.; Ginley, B.; Sarder, P.; Brodsky, L.; et al. NAD metabolism modulates inflammation and mitochondria function in diabetic kidney disease. J. Biol. Chem. 2023, 299, 104975. [Google Scholar] [CrossRef]
- Wang, Z.; Zhai, J.; Zhang, T.; He, L.; Ma, S.; Zuo, Q.; Zhang, G.; Wang, Y.; Guo, Y. Canagliflozin ameliorates epithelial-mesenchymal transition in high-salt diet-induced hypertensive renal injury through restoration of sirtuin 3 expression and the reduction of oxidative stress. Biochem. Biophys. Res. Commun. 2023, 653, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, L.; Jin, B.; Wu, Y.; Xu, L.; Chang, X.; Hu, L.; Wang, G.; Huang, Y.; Song, L.; et al. Metrnl Alleviates Lipid Accumulation by Modulating Mitochondrial Homeostasis in Diabetic Nephropathy. Diabetes 2023, 72, 611–626. [Google Scholar] [CrossRef]
- Sun, M.; Wang, F.; Li, H.; Li, M.; Wang, Y.; Wang, C.; Zhang, Y.; Zhang, D.; Li, J.; Yao, S. Maresin-1 Attenuates Sepsis-Associated Acute Kidney Injury via Suppressing Inflammation, Endoplasmic Reticulum Stress and Pyroptosis by Activating the AMPK/SIRT3 Pathway. J. Inflamm. Res. 2024, 17, 1349–1364. [Google Scholar] [CrossRef]
- Wei, M.; Gao, Y.; Cheng, D.; Zhang, H.; Zhang, W.; Shen, Y.; Huang, Q.; An, X.; Wang, B.; Yu, Z.; et al. Notoginsenoside Fc ameliorates renal tubular injury and mitochondrial damage in acetaminophen-induced acute kidney injury partly by regulating SIRT3/SOD2 pathway. Front. Med. 2022, 9, 1055252. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Q.; Chen, L.; Guo, Y.; Wu, X.Q.; Zhao, T.T.; Zhao, H.L.; Zhang, H.J.; Yan, M.H.; Zhang, G.Q.; Li, P. Poricoic acid A suppresses renal fibroblast activation and interstitial fibrosis in UUO rats via upregulating Sirt3 and promoting beta-catenin K49 deacetylation. Acta Pharmacol. Sin. 2023, 44, 1038–1050. [Google Scholar] [CrossRef]
- Yang, L.; Wang, B.; Guo, F.; Huang, R.; Liang, Y.; Li, L.; Tao, S.; Yin, T.; Fu, P.; Ma, L. FFAR4 improves the senescence of tubular epithelial cells by AMPK/SirT3 signaling in acute kidney injury. Signal Transduct. Target. Ther. 2022, 7, 384. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, H.; Hu, J.; Li, H.; Guo, S.; Chen, B.; Liu, C.; Wang, G.; Zhou, F. Magnesium Isoglycyrrhizinate Reduces the Target-Binding Amount of Cisplatin to Mitochondrial DNA and Renal Injury through SIRT3. Int. J. Mol. Sci. 2022, 23, 13093. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Zhang, Y.; Xiong, Y.; Zhu, Z.; Wang, L.; Liu, X. Mitochondria-Targeted Antioxidant Mitoquinone Maintains Mitochondrial Homeostasis through the Sirt3-Dependent Pathway to Mitigate Oxidative Damage Caused by Renal Ischemia/Reperfusion. Oxid. Med. Cell. Longev. 2022, 2022, 2213503. [Google Scholar] [CrossRef] [PubMed]
- Barati, A.; Rahbar Saadat, Y.; Meybodi, S.M.; Nouraei, S.; Moradi, K.; Kamrani Moghaddam, F.; Malekinejad, Z.; Hosseiniyan Khatibi, S.M.; Zununi Vahed, S.; Bagheri, Y. Eplerenone reduces renal ischaemia/reperfusion injury by modulating Klotho, NF-kappaB and SIRT1/SIRT3/PGC-1alpha signalling pathways. J. Pharm. Pharmacol. 2023, 75, 819–827. [Google Scholar] [CrossRef]
- Zhou, M.; Dai, Y.; Ma, Y.; Yan, Y.; Hua, M.; Gao, Q.; Geng, X.; Zhou, Q. Protective Effects of Liquiritigenin against Cisplatin-Induced Nephrotoxicity via NRF2/SIRT3-Mediated Improvement of Mitochondrial Function. Molecules 2022, 27, 3823. [Google Scholar] [CrossRef]
- Yuan, L.; Yang, J.; Li, Y.; Yuan, L.; Liu, F.; Yuan, Y.; Tang, X. Matrine alleviates cisplatin-induced acute kidney injury by inhibiting mitochondrial dysfunction and inflammation via SIRT3/OPA1 pathway. J. Cell. Mol. Med. 2022, 26, 3702–3715. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Lv, X.; Zhang, Y.; Han, Y.; Li, J.; Zeng, J.; Tang, D.; Meng, J.; Yuan, X.; Peng, Z.; et al. Fluorofenidone Inhibits UUO/IRI-Induced Renal Fibrosis by Reducing Mitochondrial Damage. Oxid. Med. Cell. Longev. 2022, 2022, 2453617. [Google Scholar] [CrossRef]
- Mao, R.W.; He, S.P.; Lan, J.G.; Zhu, W.Z. Honokiol ameliorates cisplatin-induced acute kidney injury via inhibition of mitochondrial fission. Br. J. Pharmacol. 2022, 179, 3886–3904. [Google Scholar] [CrossRef]
- Wongmekiat, O.; Lailerd, N.; Kobroob, A.; Peerapanyasut, W. Protective Effects of Purple Rice Husk against Diabetic Nephropathy by Modulating PGC-1alpha/SIRT3/SOD2 Signaling and Maintaining Mitochondrial Redox Equilibrium in Rats. Biomolecules 2021, 11, 1224. [Google Scholar] [CrossRef]
- Zhang, C.; Suo, M.; Liu, L.; Qi, Y.; Zhang, C.; Xie, L.; Zheng, X.; Ma, C.; Li, J.; Yang, J.; et al. Melatonin Alleviates Contrast-Induced Acute Kidney Injury by Activation of Sirt3. Oxid. Med. Cell. Longev. 2021, 2021, 6668887. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, L.; Wu, J.; An, S.; Fang, H.; Han, Y.; Huang, Q.; Chen, Z.; Zeng, Z. Melatonin Attenuates Sepsis-Induced Small-Intestine Injury by Upregulating SIRT3-Mediated Oxidative-Stress Inhibition, Mitochondrial Protection, and Autophagy Induction. Front. Immunol. 2021, 12, 625627. [Google Scholar] [CrossRef]
- Kobroob, A.; Kongkaew, A.; Wongmekiat, O. Melatonin Reduces Aggravation of Renal Ischemia-Reperfusion Injury in Obese Rats by Maintaining Mitochondrial Homeostasis and Integrity through AMPK/PGC-1alpha/SIRT3/SOD2 Activation. Curr. Issues Mol. Biol. 2023, 45, 8239–8254. [Google Scholar] [CrossRef]
- Kobroob, A.; Peerapanyasut, W.; Kumfu, S.; Chattipakorn, N.; Wongmekiat, O. Effectiveness of N-Acetylcysteine in the Treatment of Renal Deterioration Caused by Long-Term Exposure to Bisphenol A. Biomolecules 2021, 11, 655. [Google Scholar] [CrossRef]
- Son, S.H.; Lee, S.M.; Lee, M.H.; Son, Y.K.; Kim, S.E.; Won, S.A. Omega-3 Fatty Acids Upregulate SIRT1/3, Activate PGC-1alpha via Deacetylation, and Induce Nrf1 Production in 5/6 Nephrectomy Rat Model. Mar. Drugs 2021, 19, 182. [Google Scholar] [CrossRef]
- Wu, X.; Liu, M.; Wei, G.; Guan, Y.; Duan, J.; Xi, M.; Wang, J. Renal protection of rhein against 5/6 nephrectomied-induced chronic kidney disease: Role of SIRT3-FOXO3alpha signalling pathway. J. Pharm. Pharmacol. 2020, 72, 699–708. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Takagi, S.; Nitta, K.; Kitada, M.; Srivastava, S.P.; Takagaki, Y.; Kanasaki, K.; Koya, D. Renal protective effects of empagliflozin via inhibition of EMT and aberrant glycolysis in proximal tubules. JCI Insight 2020, 5, e129034. [Google Scholar] [CrossRef]
- Liu, X.; Liu, S.; Zhang, B.; Luo, D.; Huang, S.; Wang, F.; Zheng, L.; Lu, J.; Chen, J.; Li, S. Jian-Pi-Yi-Shen Formula Alleviates Chronic Kidney Disease in Two Rat Models by Modulating QPRT/NAD+/SIRT3/Mitochondrial Dynamics Pathway. Evid.-Based Complement. Altern. Nat. Med. 2021, 2021, 6625345. [Google Scholar] [CrossRef]
- Liu, H.; Diep, T.N.; Yan, L.-J. Mitigating Oxidative Stress and Inflammation: The Protective Role of β-lapachone in Kidney Disease. In Inflammation: From Chemical Mediators and Pathophysiology to Practice and Treatment; Wang, Y.-X., Ed.; Springer Nature Switzerland: Cham, Switzerland, 2025; pp. 1–18. [Google Scholar]
- Oh, G.S.; Kim, H.J.; Choi, J.H.; Shen, A.; Choe, S.K.; Karna, A.; Lee, S.H.; Jo, H.J.; Yang, S.H.; Kwak, T.H.; et al. Pharmacological activation of NQO1 increases NAD+ levels and attenuates cisplatin-mediated acute kidney injury in mice. Kidney Int. 2014, 85, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Lee, A.S.; Liu, S.H.; Chang, K.C.; Shen, M.Y.; Chang, C.T. Spironolactone ameliorates endothelial dysfunction through inhibition of the AGE/RAGE axis in a chronic renal failure rat model. BMC Nephrol. 2019, 20, 351. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Li, Q.; Yuan, Y.; Zhang, C.; Wu, L.; Liu, X.; Cao, W.; Guo, H.; Duan, S.; Xu, X.; et al. Renalase attenuates mitochondrial fission in cisplatin-induced acute kidney injury via modulating sirtuin-3. Life Sci. 2019, 222, 78–87. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, H.; Xiao, L.; Liu, G.; Sun, L.; He, L. STC-1 ameliorates renal injury in diabetic nephropathy by inhibiting the expression of BNIP3 through the AMPK/SIRT3 pathway. Lab. Investig. 2019, 99, 684–697. [Google Scholar] [CrossRef]
- Liu, S.M.; Zhang, Y.R.; Chen, Y.; Ji, D.R.; Zhao, J.; Fu, S.; Jia, M.Z.; Yu, Y.R.; Tang, C.S.; Huang, W.; et al. Intermedin Alleviates Vascular Calcification in CKD through Sirtuin 3-Mediated Inhibition of Mitochondrial Oxidative Stress. Pharmaceuticals 2022, 15, 1224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Wang, Y.; Zhao, K.; Chi, Y.; Wang, B. Pyrroloquinoline quinine protects HK-2 cells against high glucose-induced oxidative stress and apoptosis through Sirt3 and PI3K/Akt/FoxO3a signaling pathway. Biochem. Biophys. Res. Commun. 2019, 508, 398–404. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Ding, W.; Wang, Y. Mito-TEMPO Alleviates Renal Fibrosis by Reducing Inflammation, Mitochondrial Dysfunction, and Endoplasmic Reticulum Stress. Oxid. Med. Cell. Longev. 2018, 2018, 5828120. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, L.; Pignieri, A.; Fiasche, M.; Giudici, M.; Crestani, M.; Mitro, N.; Abbate, M.; Zoja, C.; Rottoli, D.; Foray, C.; et al. Fenofibrate attenuates cardiac and renal alterations in young salt-loaded spontaneously hypertensive stroke-prone rats through mitochondrial protection. J. Hypertens. 2018, 36, 1129–1146. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Xu, L.; Tao, X.; Yin, L.; Qi, Y.; Xu, Y.; Han, X.; Tang, Z.; Ma, X.; Liu, K.; et al. Protective effects of dioscin against fructose-induced renal damage via adjusting Sirt3-mediated oxidative stress, fibrosis, lipid metabolism and inflammation. Toxicol. Lett. 2018, 284, 37–45. [Google Scholar] [CrossRef]
- Ma, K.; Bai, Y.; Li, J.; Ren, Z.; Li, J.; Zhang, J.; Shan, A. Lactobacillus rhamnosus GG ameliorates deoxynivalenol-induced kidney oxidative damage and mitochondrial injury in weaned piglets. Food Funct. 2022, 13, 3905–3916. [Google Scholar] [CrossRef]
- Suliman, H.; Ma, Q.; Zhang, Z.; Ren, J.; Morris, B.T.; Crowley, S.D.; Ulloa, L.; Privratsky, J.R. Annexin A1 Tripeptide Mimetic Increases Sirtuin-3 and Augments Mitochondrial Function to Limit Ischemic Kidney Injury. Front. Physiol. 2021, 12, 683098. [Google Scholar] [CrossRef]
- Zha, Z.; Song, W.; Wang, R.; Zhang, X.; Liu, X.; Wang, L. Therapeutic Mechanisms of Jinshuiqing in IgA Nephropathy: A Transcriptomic Analysis. Med. Sci. Monit. Basic Res. 2025, 31, e950343. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, X.; Shao, X.; Wang, H.; Liu, X.; Ke, X.; Xiong, C.; Wei, L.; Zou, H. tert-Butylhydroquinone Treatment Alleviates Contrast-Induced Nephropathy in Rats by Activating the Nrf2/Sirt3/SOD2 Signaling Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 4657651. [Google Scholar] [CrossRef]
- Ugur, S.; Ulu, R.; Dogukan, A.; Gurel, A.; Yigit, I.P.; Gozel, N.; Aygen, B.; Ilhan, N. The renoprotective effect of curcumin in cisplatin-induced nephrotoxicity. Ren. Fail. 2015, 37, 332–336. [Google Scholar] [CrossRef]
- Ortega-Dominguez, B.; Aparicio-Trejo, O.E.; Garcia-Arroyo, F.E.; Leon-Contreras, J.C.; Tapia, E.; Molina-Jijon, E.; Hernandez-Pando, R.; Sanchez-Lozada, L.G.; Barrera-Oviedo, D.; Pedraza-Chaverri, J. Curcumin prevents cisplatin-induced renal alterations in mitochondrial bioenergetics and dynamic. Food Chem. Toxicol. 2017, 107 Pt A, 373–385. [Google Scholar] [CrossRef]
- Bal, N.B.; Sadi, G.; Bostanci, A.; Kiremitci, S.; Adanir, I.; Uludag, M.O.; Demirel-Yilmaz, E. Effect of regular exercise and resveratrol on hypertension-induced cellular stress response and senescence in renal and vascular tissues of rats. J. Cardiovasc. Pharmacol. 2025, 10, 1097. [Google Scholar] [CrossRef]
- Shiraishi, T.; Tamura, Y.; Taniguchi, K.; Higaki, M.; Ueda, S.; Shima, T.; Nagura, M.; Nakagawa, T.; Johnson, R.J.; Uchida, S. Combination of ACE inhibitor with nicorandil provides further protection in chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2014, 307, F1313–F1322. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xuan, A.; Zheng, L.; Li, D.; Chen, C.; Liu, H.; Lu, G.; Cheng, Z.; Zou, Y.; Zhi, S.; et al. Novel coumarin derivative SZC-6 as an allosteric activator of SIRT3 alleviates diabetic kidney disease via the SIRT3-Foxo3a signaling axis. Free Radic. Biol. Med. 2025, 240, 29–45. [Google Scholar] [CrossRef]
- Yang, S.; Wang, X.; Zhang, Y.; Ke, Q.; Su, W.; Zhou, Y.; Jiang, L.; Dai, C.; Wen, P. SIRT3 regulates CPT1a acetylation and fatty acid oxidation in renal tubular epithelial cells under diabetic condition. Mol. Biol. Rep. 2025, 52, 603. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Chang, J.; Liu, Y.; Yin, J.; Zeng, X. Apelin/APJ alleviates diabetic nephropathy by improving glomerular endothelial cells dysfunction via SIRT3-KLF15. Mol. Med. Rep. 2025, 31, 122. [Google Scholar] [CrossRef]
- Deng, Z.; He, M.; Hu, H.; Zhang, W.; Zhang, Y.; Ge, Y.; Ma, T.; Wu, J.; Li, L.; Sun, M.; et al. Melatonin attenuates sepsis-induced acute kidney injury by promoting mitophagy through SIRT3-mediated TFAM deacetylation. Autophagy 2024, 20, 151–165. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, P.; Luo, J.; Ding, H.; Cao, H.; He, W.; Zen, K.; Zhou, Y.; Yang, J.; Jiang, L. Sirtuin 3 regulates mitochondrial protein acetylation and metabolism in tubular epithelial cells during renal fibrosis. Cell Death Dis. 2021, 12, 847. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhu, L.; Li, L.; Liu, J.; Chen, Y.; Cheng, J.; Peng, T.; Lu, Y. S-Sulfhydration of SIRT3 by Hydrogen Sulfide Attenuates Mitochondrial Dysfunction in Cisplatin-Induced Acute Kidney Injury. Antioxid. Redox Signal. 2019, 31, 1302–1319. [Google Scholar] [CrossRef]
- Lin, J.R.; Zheng, Y.J.; Zhang, Z.B.; Shen, W.L.; Li, X.D.; Wei, T.; Ruan, C.C.; Chen, X.H.; Zhu, D.L.; Gao, P.J. Suppression of Endothelial-to-Mesenchymal Transition by SIRT (Sirtuin) 3 Alleviated the Development of Hypertensive Renal Injury. Hypertension 2018, 72, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Jiang, S.; Gao, S.; Wang, Y.; Cai, X.; Fang, J.; Yan, T.; Craig Wan, C.; Cai, Y. Sirtuins: Research advances on the therapeutic role in acute kidney injury. Phytomedicine 2022, 101, 154122. [Google Scholar] [CrossRef]
- Lambona, C.; Zwergel, C.; Valente, S.; Mai, A. SIRT3 Activation a Promise in Drug Development? New Insights into SIRT3 Biology and Its Implications on the Drug Discovery Process. J. Med. Chem. 2024, 67, 1662–1689. [Google Scholar] [CrossRef]
- Aventaggiato, M.; Arcangeli, T.; Vernucci, E.; Barreca, F.; Sansone, L.; Pellegrini, L.; Pontemezzo, E.; Valente, S.; Fioravanti, E.; Russo, M.A.; et al. Pharmacological Activation of SIRT3 Modulates the Response of Cancer Cells to Acidic pH. Pharmaceuticals 2024, 17, 810. [Google Scholar] [CrossRef]
- Dai, H.; Sinclair, D.A.; Ellis, J.L.; Steegborn, C. Sirtuin activators and inhibitors: Promises, achievements, and challenges. Pharmacol. Ther. 2018, 188, 140–154. [Google Scholar] [CrossRef]
- Huang, J.; Liang, Y.; Zhou, L. Natural products for kidney disease treatment: Focus on targeting mitochondrial dysfunction. Front. Pharmacol. 2023, 14, 1142001. [Google Scholar] [CrossRef]
- Oka, S.I.; Titus, A.S.; Zablocki, D.; Sadoshima, J. Molecular properties and regulation of NAD+ kinase (NADK). Redox Biol. 2023, 59, 102561. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J. NADH/NAD+ Redox Imbalance and Diabetic Kidney Disease. Biomolecules 2021, 11, 730. [Google Scholar] [CrossRef]
- Rabelink, T.J.; de Vries, A.P.J. CD38—A new target in renal immune disease. Nat. Rev. Nephrol. 2024, 20, 641–642. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, R.; Li, W.; Ren, M.; Thangthaeng, N.; Sumien, N.; Liu, R.; Yang, S.; Simpkins, J.W.; Forster, M.J.; et al. Administration of 5-methoxyindole-2-carboxylic acid that potentially targets mitochondrial dihydrolipoamide dehydrogenase confers cerebral preconditioning against ischemic stroke injury. Free Radic. Biol. Med. 2017, 113, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J.; Wang, Y. Roles of Dihydrolipoamide Dehydrogenase in Health and Disease. Antioxid. Redox Signal. 2023, 39, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Lu, G.; Gao, C.; Wang, Y.; Sun, H. Tissue-specific and nutrient regulation of the branched-chain alpha-keto acid dehydrogenase phosphatase, protein phosphatase 2Cm (PP2Cm). J. Biol. Chem. 2012, 287, 23397–23406. [Google Scholar] [CrossRef]
- Szabo, E.; Nagy, B.; Czajlik, A.; Komlodi, T.; Ozohanics, O.; Tretter, L.; Ambrus, A. Mitochondrial Alpha-Keto Acid Dehydrogenase Complexes: Recent Developments on Structure and Function in Health and Disease. Subcell. Biochem. 2024, 104, 295–381. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzouz, R.S.; Yan, L.-J. The Role of Sirt3 in Kidney Health and Disease. Pharmaceuticals 2025, 18, 1668. https://doi.org/10.3390/ph18111668
Azzouz RS, Yan L-J. The Role of Sirt3 in Kidney Health and Disease. Pharmaceuticals. 2025; 18(11):1668. https://doi.org/10.3390/ph18111668
Chicago/Turabian StyleAzzouz, Ryan S., and Liang-Jun Yan. 2025. "The Role of Sirt3 in Kidney Health and Disease" Pharmaceuticals 18, no. 11: 1668. https://doi.org/10.3390/ph18111668
APA StyleAzzouz, R. S., & Yan, L.-J. (2025). The Role of Sirt3 in Kidney Health and Disease. Pharmaceuticals, 18(11), 1668. https://doi.org/10.3390/ph18111668







