Anticancer and Immunomodulatory Effects of a Thiazolyl Benzodiazepine Targeting HSP90 in ER+ Breast Cancer
Abstract
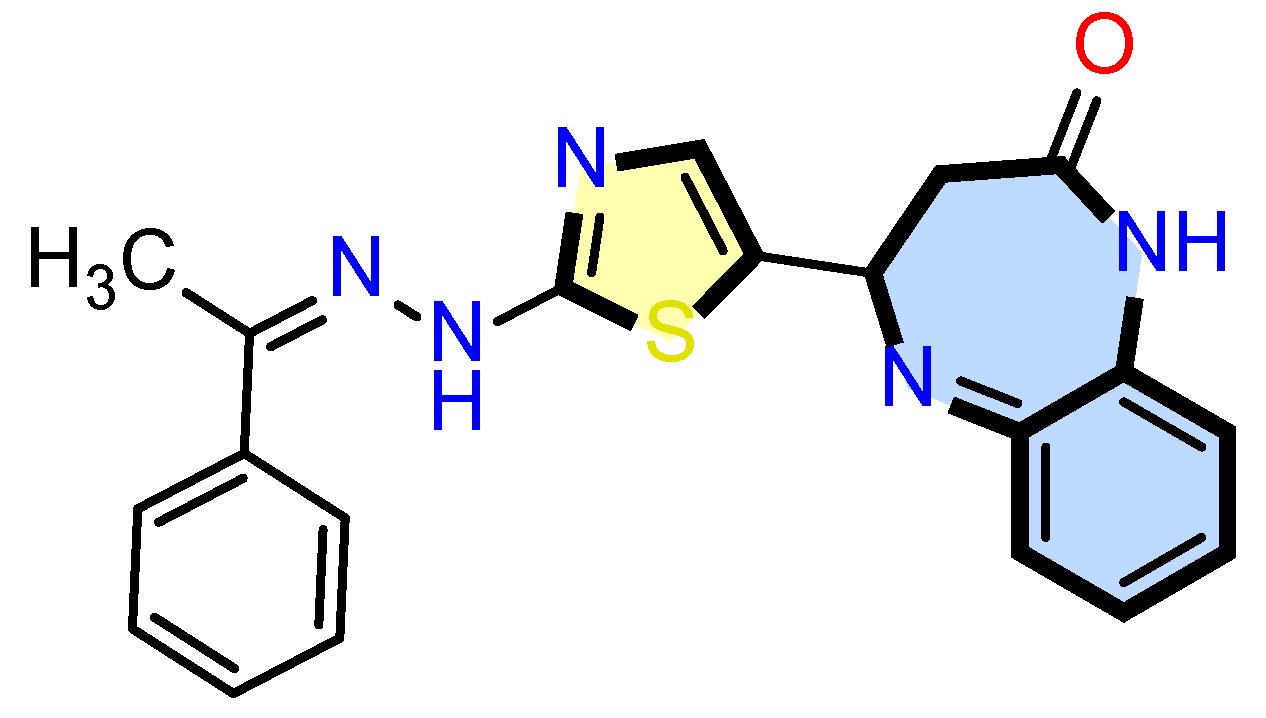
1. Introduction
2. Results
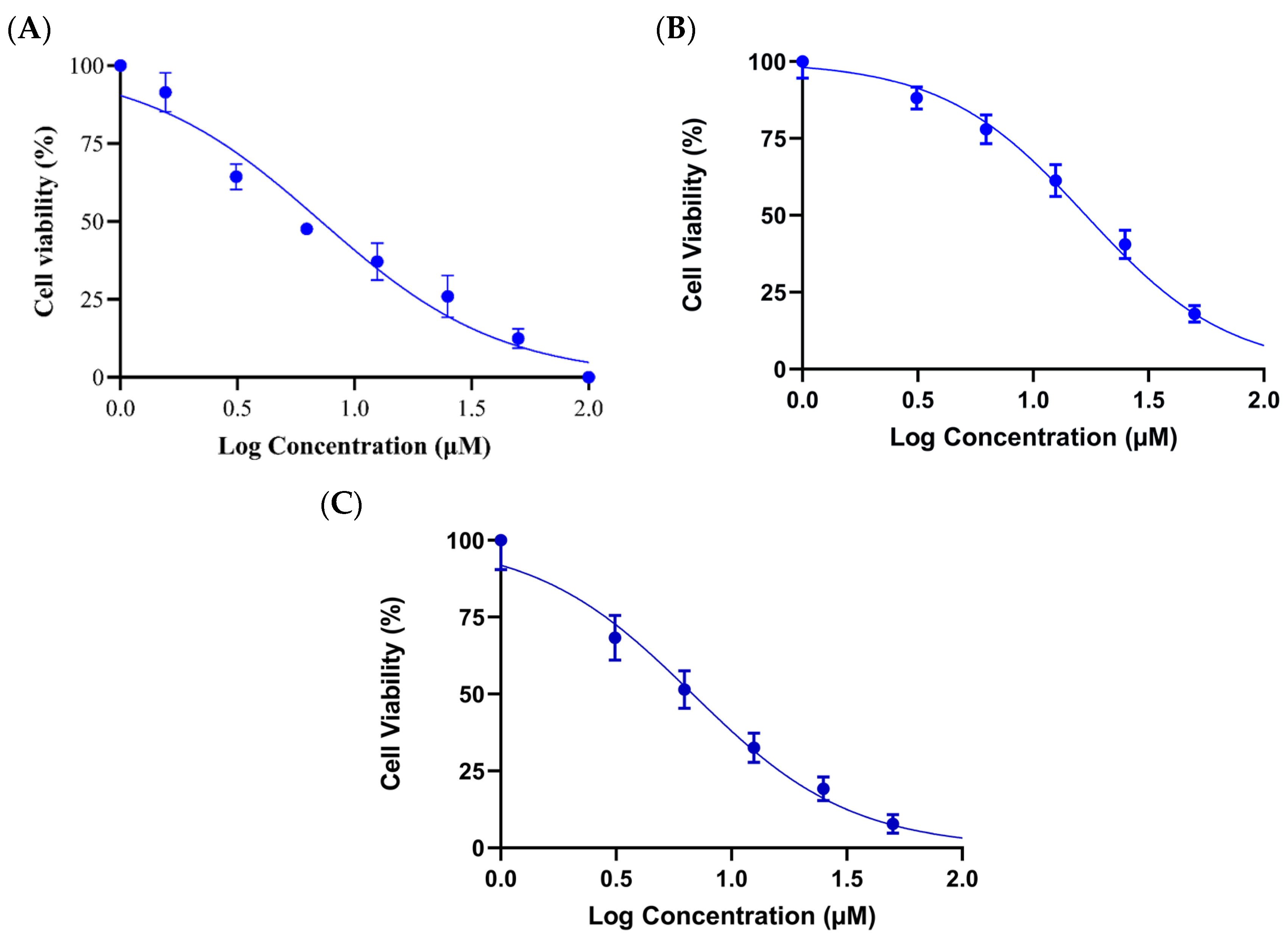
2.1. Cell Cytotoxicity Assay
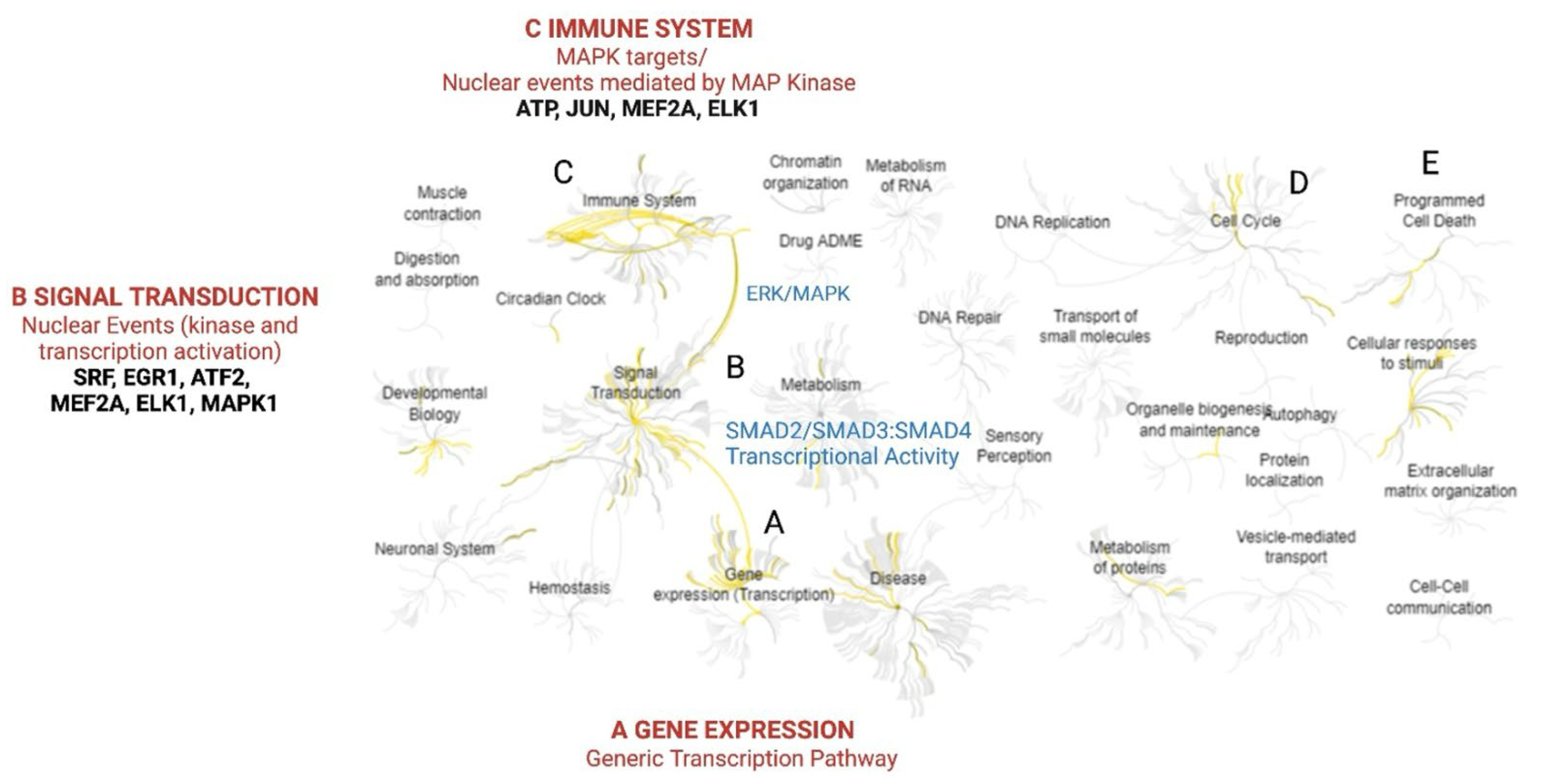
2.2. Array Studies and Gene Enrichment Analysis
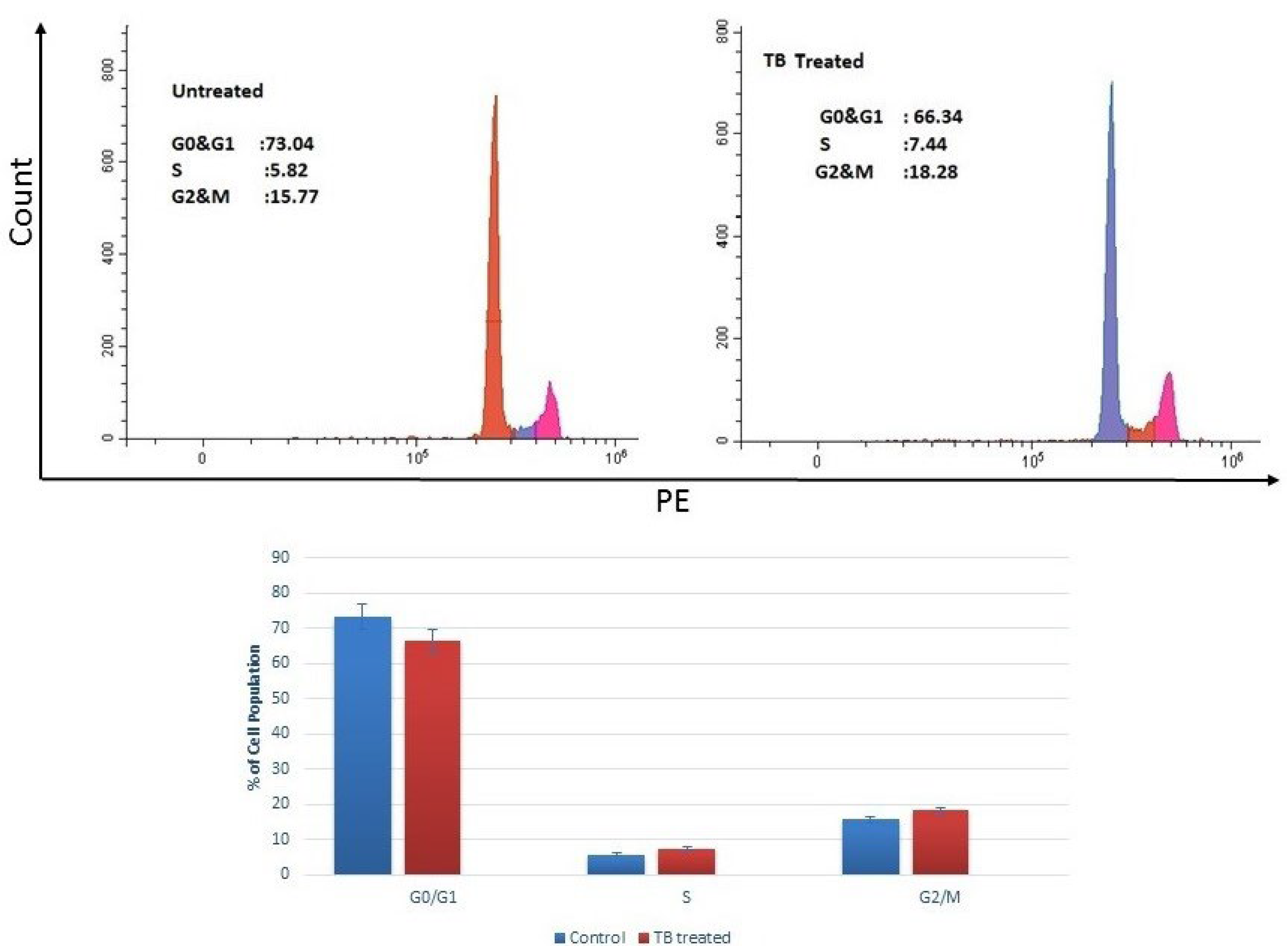
2.3. Cell Cycle Analysis
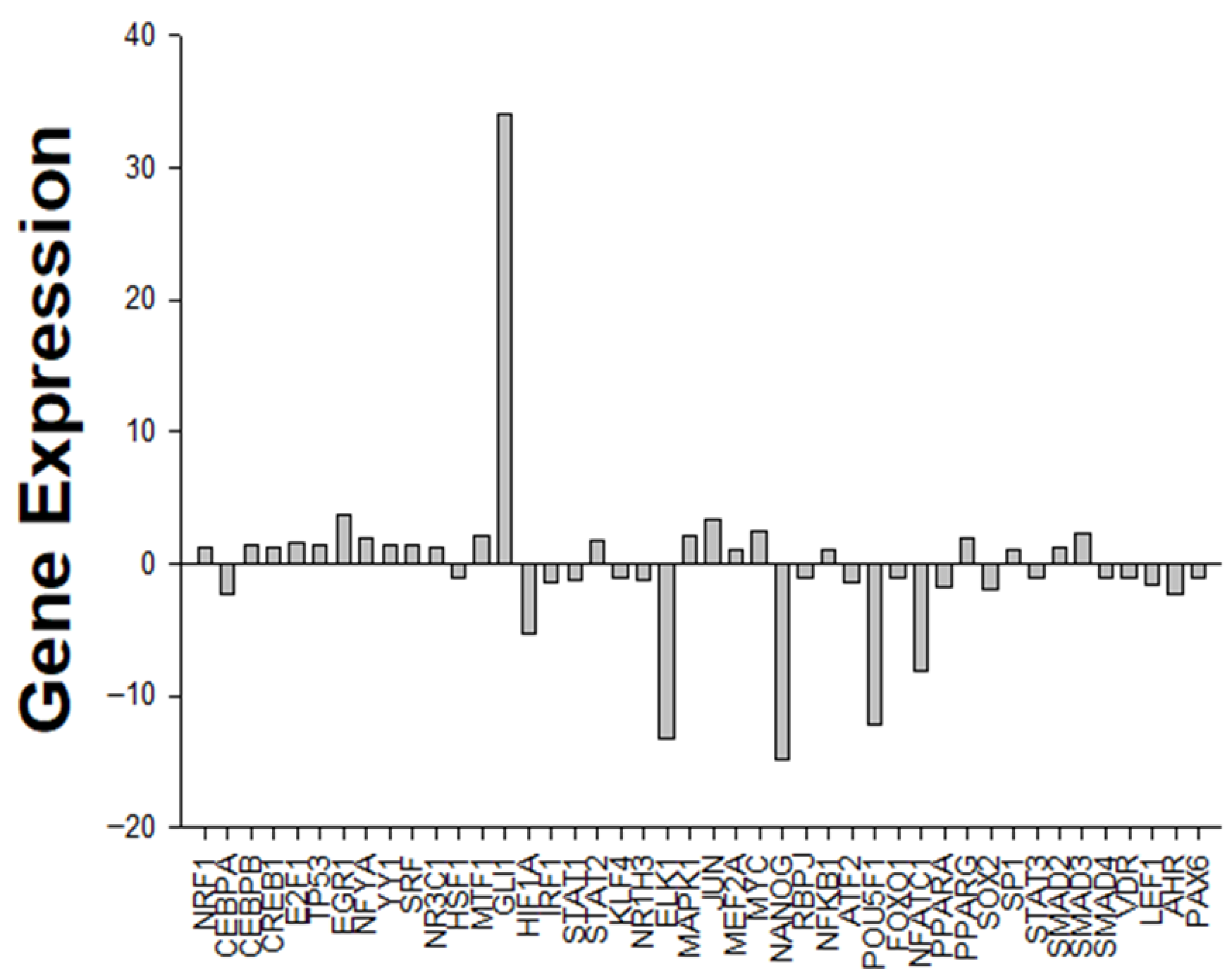
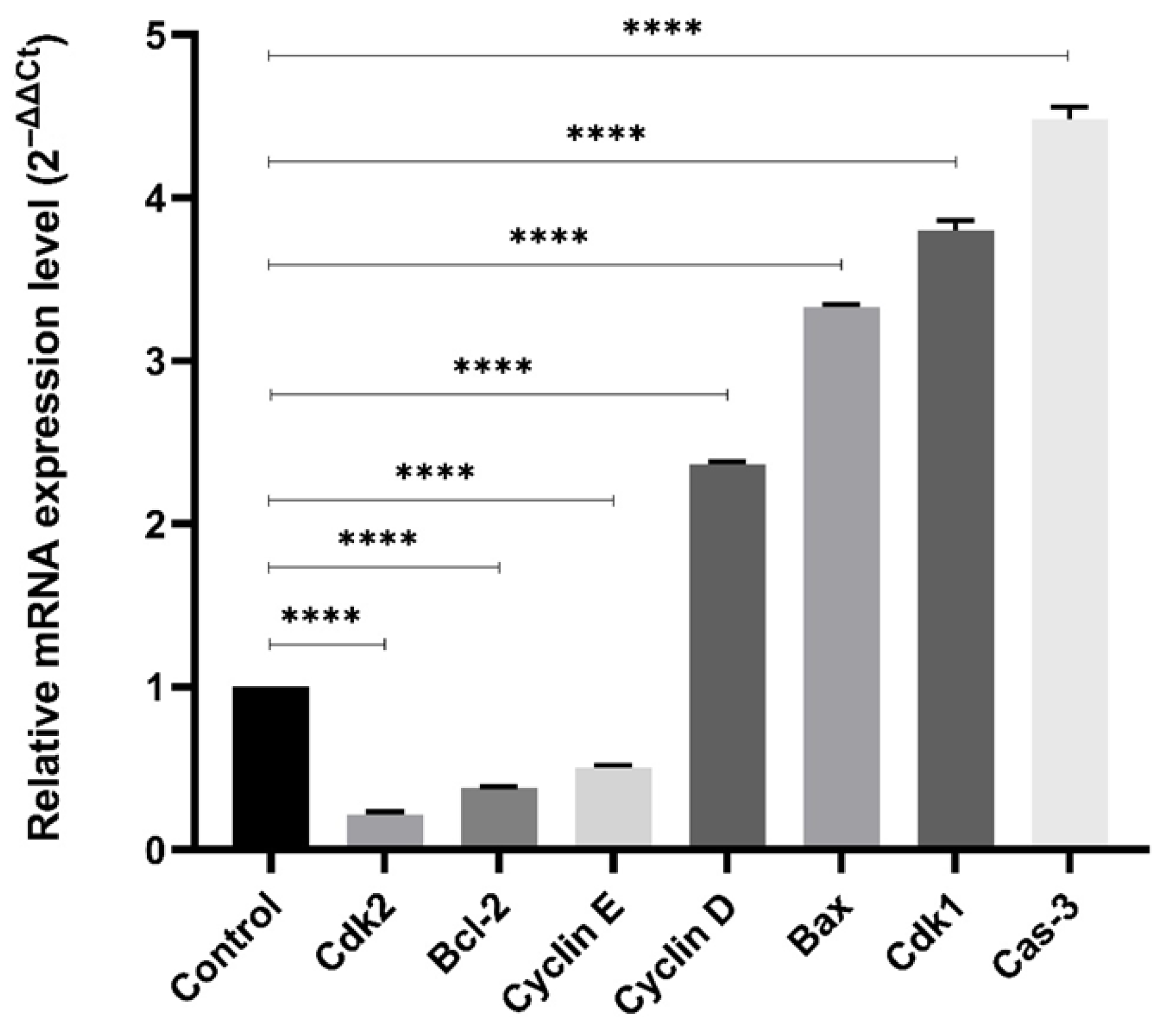
2.4. Gene Expression Profiling
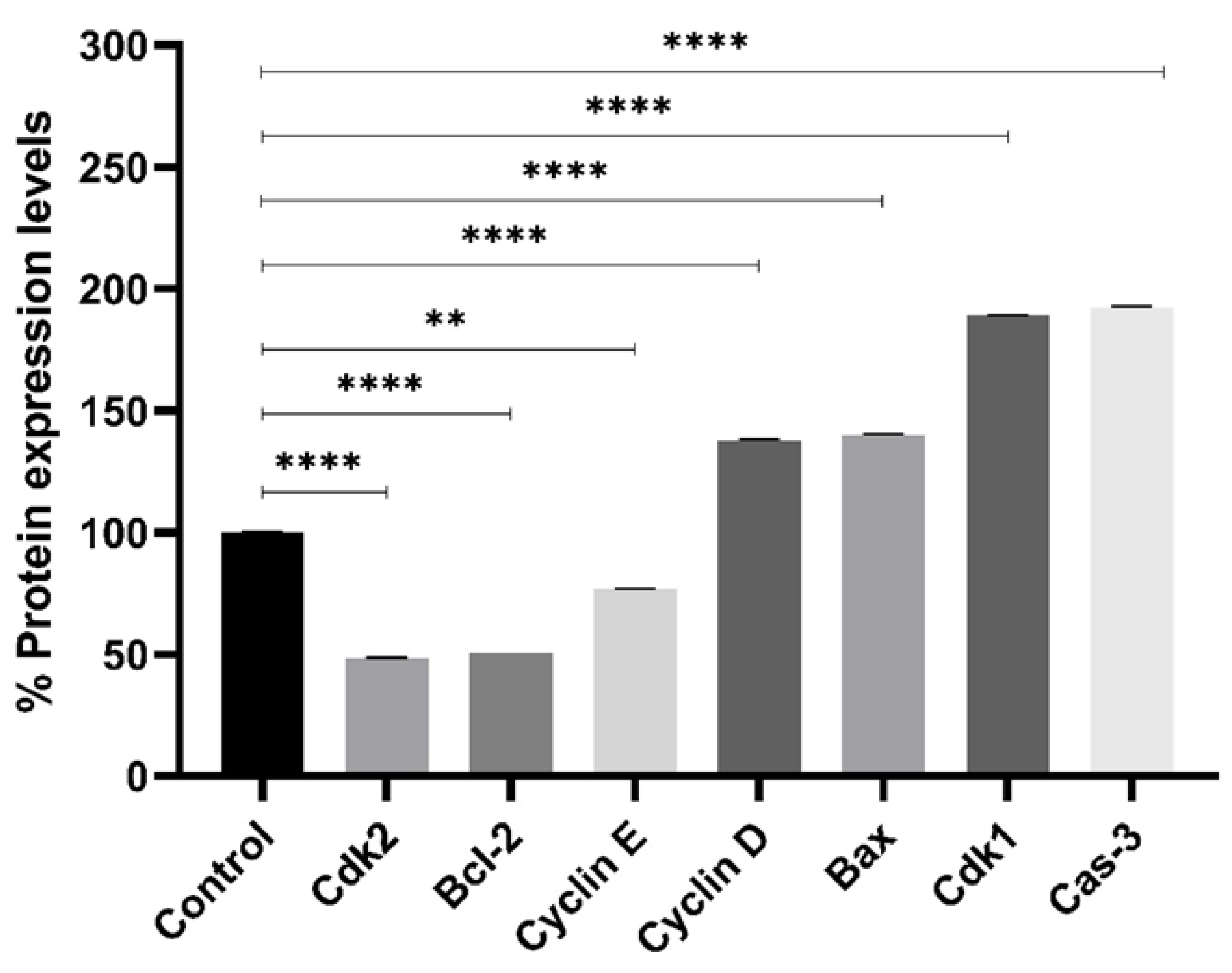
2.5. Protein Expression Profiling
2.6. Binding Assay
3. Discussion
4. Materials and Methods
4.1. Cell Culture, PCR Array Studies and Gene Enrichment Analysis
4.2. Cell Cycle Analysis
4.3. Gene Expression Profiling
4.4. Protein Expression Profiling
4.5. Binding Assay
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HSP | Heat shock protein |
| TB | Thiazolyl benzodiazepine |
| MCF-7 | Human breast epithelial carcinoma |
| ATCC | American Type Culture Collection |
| DMEM | Dulbecco′s Modified Eagle′s Medium |
| CCK-8 | Cell Counting Kit-8 |
| HSP90 | Heat Shock Protein 90 |
| ER+ | Estrogen Receptor-Positive |
| IC50 | Half Maximal Inhibitory Concentration |
| MAPK | Mitogen-Activated Protein Kinase |
| MDA-MB-231 | Triple-Negative Human Breast Cancer Cell Line |
| SK-BR-3 | HER2-Positive Human Breast Cancer Cell Line |
References
- Özgür, A.; Tutar, Y. Heat Shock Protein 90 Inhibition in Cancer Drug Discovery. From Chemistry to Futural Clinical Applications. Anticancer Agents Med. Chem. 2016, 16, 280–290. [Google Scholar]
- Gümüş, M.; Özgür, A.; Tutar, L.; Dışlı, A.; Koca, İ.; Tutar, Y. Design, Synthesis, and Evaluation of Heat Shock Protein 90 Inhibitors in Human Breast Cancer and Its Metastasis. Curr. Pharm. Biotechnol. 2016, 17, 1231–1245. [Google Scholar] [CrossRef]
- Li, J.; Van Veldhuizen, J.; Kremer, K.N.; Granner, M.A.; Durrant, D.E.; Roush, W.R.; Zhan, C.; Pei, D.; Zhang, Y.; Cohen, M.S. The Next Generation of Immunotherapy for Cancer: Small Molecules Could Make Big Waves. J. Immunol. 2019, 202, 11–19. [Google Scholar] [CrossRef]
- Allison, J.P. Immune Checkpoint Blockade in Cancer Therapy: The 2015 Lasker-DeBakey Clinical Medical Research Award. JAMA 2015, 314, 1113–1114. [Google Scholar] [CrossRef]
- Leventakos, K.; Mansfield, A.S. Advances in the Treatment of Non-Small Cell Lung Cancer: Focus on Nivolumab, Pembrolizumab, and Atezolizumab. BioDrugs 2016, 30, 397–405. [Google Scholar] [CrossRef]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T Cell Immunotherapy for Human Cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Dai, Y. Small Molecule-Based Immunomodulators for Cancer Therapy. Acta Pharm. Sin. B 2022, 12, 4287–4308. [Google Scholar] [CrossRef] [PubMed]
- Hartley, J.A. The Development of Pyrrolobenzodiazepines as Antitumour Agents. Expert Opin. Investig. Drugs 2011, 20, 733–744. [Google Scholar] [CrossRef]
- Gill, R.K.; Kaushik, S.O.; Bansal, S.; Shah, A.; Bariwal, J. Recent Development in [1,4]Benzodiazepines as Potent Anticancer Agents: A Review. Mini-Rev. Med. Chem. 2014, 14, 229–256. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, L.; Araújo, A.C.; Airoldi, C.; Bini, D. Pyrrolo[2,1-c][1,4]benzodiazepine as a Scaffold for the Design and Synthesis of Anti-Tumour Drugs. Anticancer Agents Med. Chem. 2009, 9, 1–31. [Google Scholar] [CrossRef]
- Wang, J.J.; Shen, Y.K.; Hu, W.P.; Hsieh, M.C.; Lin, F.L.; Hsu, M.K.; Hsu, M.H. Design, Synthesis, and Biological Evaluation of Pyrrolo[2,1-c][1,4]benzodiazepine and Indole Conjugates as Anticancer Agents. J. Med. Chem. 2006, 49, 1442–1449. [Google Scholar] [CrossRef]
- Esteva, F.J.; Sahin, A.A.; Smith, T.L.; Yang, Y.; Pusztai, L.; Nahta, R.; Buchholz, T.A.; Buzdar, A.U.; Hortobagyi, G.N.; Bacus, S.S. Prognostic Significance of Phosphorylated P38 Mitogen-Activated Protein Kinase and HER-2 Expression in Lymph Node-Positive Breast Carcinoma. Cancer 2004, 100, 499–506. [Google Scholar] [CrossRef]
- Bartholomeusz, C.; Gonzalez-Angulo, A.M.; Liu, P.; Hayashi, N.; Lluch, A.; Ferrer-Lozano, J.; Hortobágyi, G.N. High ERK protein expression levels correlate with shorter survival in triple-negative breast cancer patients. Oncologist 2012, 17, 766–774. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weldon, C.B.; Parker, A.P.; Patten, D.; Elliott, S.; Tang, Y.; Frigo, D.E.; Dugan, C.M.; Coakley, E.L.; Butler, N.N.; Clayton, J.L.; et al. Sensitization of Apoptotically-Resistant Breast Carcinoma Cells to TNF and TRAIL by Inhibition of P38 Mitogen-Activated Protein Kinase Signaling. Int. J. Oncol. 2004, 24, 1473–1480. [Google Scholar] [PubMed]
- Shi, Y.Y.; Small, G.W.; Orlowski, R.Z. Proteasome Inhibitors Induce a P38 Mitogen-Activated Protein Kinase (MAPK)-Dependent Anti-Apoptotic Program Involving MAPK Phosphatase-1 and Akt in Models of Breast Cancer. Breast Cancer Res. Treat. 2006, 100, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Chueh, A.C.; Brown, D.V.; Latham, A.M.; Watson, V.J.; Huang, R.Y.; Thomas, M.L.; Barry, S.C.; Kheir, T.B.; Sieber, O.M. Chronic Chromosome Instability Induced by Plk1 Results in Immune Suppression in Breast Cancer. Cell Rep. 2023, 42, 113266. [Google Scholar] [CrossRef]
- Gil-Edo, R.; Espejo, S.; Falomir, E.; Carda, M. Synthesis and Biological Evaluation of Potential Oncoimmunomodulator Agents. Int. J. Mol. Sci. 2023, 24, 2614. [Google Scholar] [CrossRef]
- Oda, E.; Ohki, R.; Murasawa, H.; Nemoto, J.; Shibue, T.; Yamashita, T.; Tokino, T.; Taniguchi, T.; Tanaka, N. Noxa, a BH3-Only Member of the Bcl-2 Family and Candidate Mediator of p53-Induced Apoptosis; PUMA, a Novel Proapoptotic Gene, Is Induced by p53. Science 2000, 288, 1053–1058. [Google Scholar] [CrossRef]
- Neckers, L.; Workman, P. HSP90 molecular chaperone inhibitors: Are we there yet? Clin. Cancer Res. 2012, 18, 64–76. [Google Scholar] [CrossRef]
- Jhaveri, K.; Taldone, T.; Modi, S.; Chiosis, G. Advances in the clinical development of heat shock protein 90 (HSp90) inhibitors in cancers. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2012, 1823, 742–755. [Google Scholar] [CrossRef]
- Tunoğlu, S.; Tutar, L.; Gümüş, M.; Tunoğlu, E.N.Y.; Koca, İ.; Tutar, Y. Hsp Inhibitor Is Affective Against Adenocarcinomic Human Alveolar Basal Epithelial Cells Through Modulating ERK/MAPK Signaling Pathway. Chem. Biodivers. 2024, 21, e202301422. [Google Scholar] [CrossRef] [PubMed]
- Kul, P.; Tunçbilek, M.; Ergül, M.; Yenilmez Tunoğlu, E.N.; Tutar, Y. A Novel 6,8,9-Trisubstituted Purine Analogue Drives Breast Cancer Luminal A Subtype MCF-7 to Apoptosis and Senescence Through Hsp70 Inhibition. Anti-Cancer Agents Med. Chem. 2023, 23, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Bronowicka-Adamska, P.; Bentke, A.; Lasota, M.; Wróbel, M. Effect of S-Allyl–L-cysteine on MCF-7 Cell Line 3-Mercaptopyruvate Sulfurtransferase/Sulfane Sulfur System, Viability and Apoptosis. Int. J. Mol. Sci. 2020, 21, 1090. [Google Scholar] [CrossRef] [PubMed]
- Coşkun, K.A.; Koca, İ.; Gümüş, M.; Tutar, Y. Designing Specific HSP70 Substrate Binding Domain Inhibitor for Perturbing Protein Folding Pathways to Inhibit Cancer Mechanism. Anticancer Agents Med. Chem. 2021, 21, 1472–1480. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coskun, K.A.; Tutar, L.; Çifci, K.U.; Al, M.; Koca, I.; Gumus, M.; Gulum, L.; Capkinoglu, E.; Tutar, Y. Anticancer and Immunomodulatory Effects of a Thiazolyl Benzodiazepine Targeting HSP90 in ER+ Breast Cancer. Pharmaceuticals 2025, 18, 1665. https://doi.org/10.3390/ph18111665
Coskun KA, Tutar L, Çifci KU, Al M, Koca I, Gumus M, Gulum L, Capkinoglu E, Tutar Y. Anticancer and Immunomodulatory Effects of a Thiazolyl Benzodiazepine Targeting HSP90 in ER+ Breast Cancer. Pharmaceuticals. 2025; 18(11):1665. https://doi.org/10.3390/ph18111665
Chicago/Turabian StyleCoskun, Kubra Acikalin, Lutfi Tutar, Kezban Uçar Çifci, Mervenur Al, Irfan Koca, Mehmet Gumus, Levent Gulum, Emir Capkinoglu, and Yusuf Tutar. 2025. "Anticancer and Immunomodulatory Effects of a Thiazolyl Benzodiazepine Targeting HSP90 in ER+ Breast Cancer" Pharmaceuticals 18, no. 11: 1665. https://doi.org/10.3390/ph18111665
APA StyleCoskun, K. A., Tutar, L., Çifci, K. U., Al, M., Koca, I., Gumus, M., Gulum, L., Capkinoglu, E., & Tutar, Y. (2025). Anticancer and Immunomodulatory Effects of a Thiazolyl Benzodiazepine Targeting HSP90 in ER+ Breast Cancer. Pharmaceuticals, 18(11), 1665. https://doi.org/10.3390/ph18111665





