Innovative Strategies to Enhance the Bioavailability of Cannabidiol: Nanotechnology and Advanced Delivery Systems
Abstract
1. Introduction
1.1. Cannabidiol (CBD)
1.2. Bioavailability and Bioefficacy
2. Methodology of Literature Review
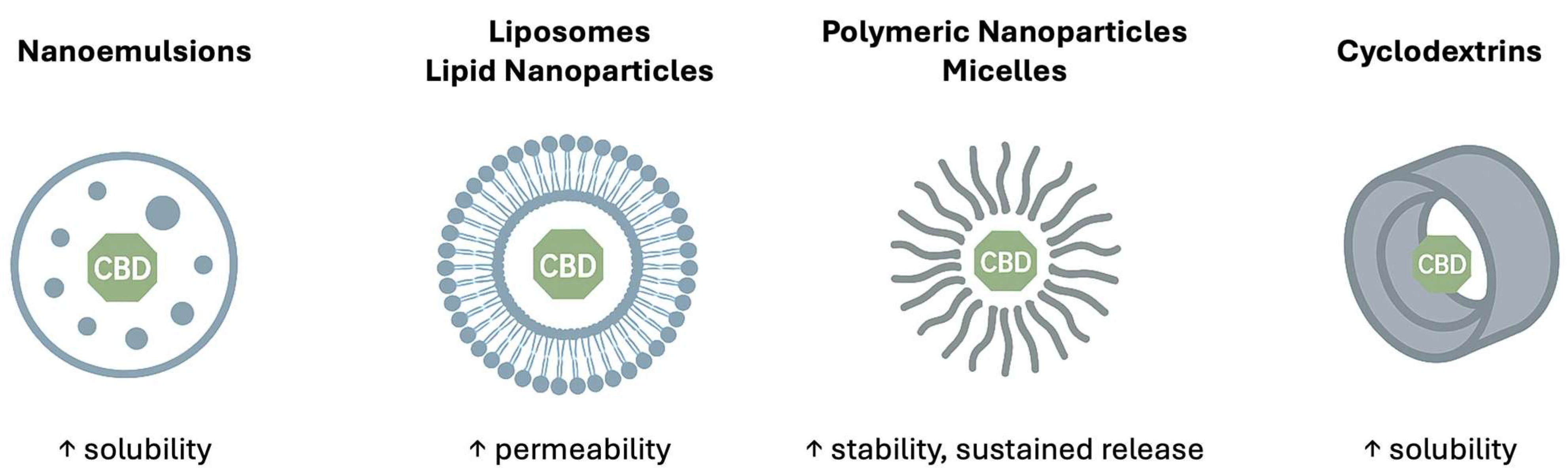
3. Lipid-Based Nanocarriers
3.1. Nanoemulsions
3.2. Nanoliposomes
3.3. Nanosuspension
3.4. Oleosomes
4. Polymeric/Biopolymeric Nanocarriers
4.1. Polymeric Micelles
4.2. Polymeric Nanoparticles
5. Cyclodextrin Inclusion Complex
6. Comparative Synthesis and Ranking
7. Future Perspective
7.1. Manufacturing and Regulatory Challenges
7.2. Safety, Toxicity, and Clinical Translation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CBD | Cannabidiol |
| THC | Δ9-tetrahydrocannabinol |
| GPCR | G-Protein-Coupled Cannabinoid CB1 Receptor |
| GlyRα1/α2/β | Glycine Receptor alpha-1, alpha-3, beta |
| 5-HT1A/2A/3A | 5-hydroxytryptamine Receptor 1A, 2A, 3A |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| ARA1 | Adenosine A1 Receptor |
| FDA | U.S. Food and Drug Administration |
| EMA | European Medicines Agency |
| CBD-NE | Cannabidiol contained Nanoemulsion |
| SNEDDS | Self-Nanoemulsifying Drug Delivery System |
| AUC | Area Under the Curve |
| Cmax | Maximum Serum Concentration |
| PNLs | Pro-Nanolipospheres |
| MLVs | Multilamellar Vesicles |
| MVVs | Multivesicular Vesicles |
| GUVs | Giant Unilamellar Vesicles |
| LUVs | Large Unilamellar Vesicles |
| SUVs | Small Unilamellar Vesicles |
| SPC | Soybean Phosphatidylcholine |
| PEG 400 | Polyethylene Glycol 400 |
| PPD | 20(S)-Protopanaxadiol |
| CP-liposomes | Cannabidiol and 20(S)-Protopanaxadiol contained liposomes |
| GMCP-liposomes | Cannabidiol, 20(S)-Protopanaxadiol and n-Dodecyl β-D-maltoside contained liposomes |
| PBS | Phosphate-Buffered Saline |
| HPMCAS | Hydroxypropylmethylcellulose Acetate Succinate |
| FD | Ester of Fucoidan and Deoxycholic Acid |
| API | Active Pharmaceutical Ingredient |
| PLGA | Poly-lactic-co-glycolic acid |
| NPs | Nanoparticles |
| CAM | Chorioallantoic Membrane |
| WP | Whey Protein |
| β-CD | β-Cyclodextrin |
| DM-β-CD | 2,6-di-O-methyl-β-cyclodextrin |
References
- Maroon, J.; Bost, J. Review of the Neurological Benefits of Phytocannabinoids. Surg. Neurol. Int. 2018, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Morrison, P.D.; Fusar-Poli, P.; Martin-Santos, R.; Borgwardt, S.; Winton-Brown, T.; Nosarti, C.; O’ Carroll, C.M.; Seal, M.; Allen, P.; et al. Opposite Effects of Δ-9-Tetrahydrocannabinol and Cannabidiol on Human Brain Function and Psychopathology. Neuropsychopharmacology 2010, 35, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Sitovs, A.; Logviss, K.; Lauberte, L.; Mohylyuk, V. Oral Delivery of Cannabidiol: Revealing the Formulation and Absorption Challenges. J. Drug Deliv. Sci. Technol. 2024, 92, 105316. [Google Scholar] [CrossRef]
- Bartončíková, M.; Lapčíková, B.; Lapčík, L.; Valenta, T. Hemp-Derived CBD Used in Food and Food Supplements. Molecules 2023, 28, 8047. [Google Scholar] [CrossRef]
- Grifoni, L.; Vanti, G.; Donato, R.; Sacco, C.; Bilia, A.R. Promising Nanocarriers to Enhance Solubility and Bioavailability of Cannabidiol for a Plethora of Therapeutic Opportunities. Molecules 2022, 27, 6070. [Google Scholar] [CrossRef]
- Żółnowska, I.; Gostyńska-Stawna, A.; Jelińska, A.; Stawny, M. Cannabis Medicine 2.0: Nanotechnology-Based Delivery Systems for Synthetic and Chemically Modified Cannabinoids for Enhanced Therapeutic Performance. Nanomaterials 2025, 15, 1260. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Saharan, V.A.; Banerjee, D.; Ram, V.; Kulhari, H.; Pooja, D.; Singh, A. A Comprehensive Update on Cannabidiol, Its Formulations and Drug Delivery Systems. Phytochem. Rev. 2025, 24, 2723–2757. [Google Scholar] [CrossRef]
- O’Sullivan, S.E.; Jensen, S.S.; Kolli, A.R.; Nikolajsen, G.N.; Bruun, H.Z.; Hoeng, J. Strategies to Improve Cannabidiol Bioavailability and Drug Delivery. Pharmaceuticals 2024, 17, 244. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Masteikova, R.; Lazauskas, R.; Bernatoniene, J. Cannabis Sativa L. Bioactive Compounds and Their Protective Role in Oxidative Stress and Inflammation. Antioxidants 2022, 11, 660. [Google Scholar] [CrossRef]
- Silmore, L.H.; Willmer, A.R.; Capparelli, E.V.; Rosania, G.R. Food Effects on the Formulation, Dosing, and Administration of Cannabidiol (CBD) in Humans: A Systematic Review of Clinical Studies. Pharmacotherapy 2021, 41, 405–420. [Google Scholar] [CrossRef]
- Wechsler, R.T.; Burdette, D.E.; Gidal, B.E.; Hyslop, A.; McGoldrick, P.E.; Thiele, E.A.; Valeriano, J. Consensus Panel Recommendations for the Optimization of EPIDIOLEX® Treatment for Seizures Associated with Lennox-Gastaut Syndrome, Dravet Syndrome, and Tuberous Sclerosis Complex. Epilepsia Open 2024, 9, 1632–1642. [Google Scholar] [CrossRef]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A Narrative Review of Molecular Mechanism and Therapeutic Effect of Cannabidiol (CBD). Basic Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef]
- Iliff, H.A.; Lynch, D.L.; Kotsikorou, E.; Reggio, P.H. Parameterization of Org27569: An Allosteric Modulator of the Cannabinoid CB1 G Protein-Coupled Receptor. J. Comput. Chem. 2011, 32, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Ibeas Bih, C.; Nunn, A.V.W.; Bazelot, M.; Dallas, M.; Whalley, B.J. Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics 2015, 12, 699–730. [Google Scholar] [CrossRef] [PubMed]
- Coomber, B.; O’Donoghue, M.F.; Mason, R. Inhibition of Endocannabinoid Metabolism Attenuates Enhanced Hippocampal Neuronal Activity Induced by Kainic Acid. Synapse 2008, 62, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Laun, A.S.; Shrader, S.H.; Brown, K.J.; Song, Z.-H. GPR3, GPR6, and GPR12 as Novel Molecular Targets: Their Biological Functions and Interaction with Cannabidiol. Acta Pharmacol. Sin. 2019, 40, 300–308. [Google Scholar] [CrossRef]
- Saliba, S.W.; Jauch, H.; Gargouri, B.; Keil, A.; Hurrle, T.; Volz, N.; Mohr, F.; van der Stelt, M.; Bräse, S.; Fiebich, B.L. Anti-Neuroinflammatory Effects of GPR55 Antagonists in LPS-Activated Primary Microglial Cells. J. Neuroinflamm. 2018, 15, 322. [Google Scholar] [CrossRef]
- Ahrens, J.; Demir, R.; Leuwer, M.; de la Roche, J.; Krampfl, K.; Foadi, N.; Karst, M.; Haeseler, G. The Nonpsychotropic Cannabinoid Cannabidiol Modulates and Directly Activates Alpha-1 and Alpha-1-Beta Glycine Receptor Function. Pharmacology 2009, 83, 217–222. [Google Scholar] [CrossRef]
- Xiong, W.; Cui, T.; Cheng, K.; Yang, F.; Chen, S.-R.; Willenbring, D.; Guan, Y.; Pan, H.-L.; Ren, K.; Xu, Y.; et al. Cannabinoids Suppress Inflammatory and Neuropathic Pain by Targeting A3 Glycine Receptors. J. Exp. Med. 2012, 209, 1121–1134. [Google Scholar] [CrossRef]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic Properties of Cannabidiol at 5-HT1a Receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef]
- Theodore, W.H.; Wiggs, E.A.; Martinez, A.R.; Dustin, I.H.; Khan, O.I.; Appel, S.; Reeves-Tyer, P.; Sato, S. Serotonin 1A Receptors, Depression, and Memory in Temporal Lobe Epilepsy. Epilepsia 2012, 53, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.-H.; Galadari, S.; Isaev, D.; Petroianu, G.; Shippenberg, T.S.; Oz, M. The Nonpsychoactive Cannabinoid Cannabidiol Inhibits 5-Hydroxytryptamine3A Receptor-Mediated Currents in Xenopus laevis Oocytes. J. Pharmacol. Exp. Ther. 2010, 333, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Ramer, R.; Heinemann, K.; Merkord, J.; Rohde, H.; Salamon, A.; Linnebacher, M.; Hinz, B. COX-2 and PPAR-γ Confer Cannabidiol-Induced Apoptosis of Human Lung Cancer Cells. Mol. Cancer Ther. 2013, 12, 69–82. [Google Scholar] [CrossRef]
- Quintanilla, R.A.; Utreras, E.; Cabezas-Opazo, F.A. Role of PPARγ in the Differentiation and Function of Neurons. PPAR Res. 2014, 2014, 768594. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-Y.; Singer, P.; Lytle, N.; Wei, C.J.; Lan, J.-Q.; Williams-Karnesky, R.L.; Chen, J.-F.; Yee, B.K.; Boison, D. Adenosine Augmentation Ameliorates Psychotic and Cognitive Endophenotypes of Schizophrenia. J. Clin. Investig. 2012, 122, 2567–2577. [Google Scholar] [CrossRef]
- Gonca, E.; Darıcı, F. The Effect of Cannabidiol on Ischemia/Reperfusion-Induced Ventricular Arrhythmias: The Role of Adenosine A1 Receptors. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 76–83. [Google Scholar] [CrossRef]
- Castillo, A.; Tolón, M.R.; Fernández-Ruiz, J.; Romero, J.; Martinez-Orgado, J. The Neuroprotective Effect of Cannabidiol in an in Vitro Model of Newborn Hypoxic-Ischemic Brain Damage in Mice Is Mediated by CB2 and Adenosine Receptors. Neurobiol. Dis. 2010, 37, 434–440. [Google Scholar] [CrossRef]
- Stella, B.; Baratta, F.; Della Pepa, C.; Arpicco, S.; Gastaldi, D.; Dosio, F. Cannabinoid Formulations and Delivery Systems: Current and Future Options to Treat Pain. Drugs 2021, 81, 1513–1557. [Google Scholar] [CrossRef]
- Perucca, E.; Bialer, M. Critical Aspects Affecting Cannabidiol Oral Bioavailability and Metabolic Elimination, and Related Clinical Implications. CNS Drugs 2020, 34, 795–800. [Google Scholar] [CrossRef]
- Taylor, L.; Gidal, B.; Blakey, G.; Tayo, B.; Morrison, G. A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects. CNS Drugs 2018, 32, 1053–1067. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An Advanced Mode of Drug Delivery System. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Nakano, Y.; Tajima, M.; Sugiyama, E.; Sato, V.H.; Sato, H. Development of a Novel Nano-Emulsion Formulation to Improve Intestinal Absorption of Cannabidiol. Med. Cannabis Cannabinoids 2019, 2, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Zgair, A.; Wong, J.C.; Lee, J.B.; Mistry, J.; Sivak, O.; Wasan, K.M.; Hennig, I.M.; Barrett, D.A.; Constantinescu, C.S.; Fischer, P.M.; et al. Dietary Fats and Pharmaceutical Lipid Excipients Increase Systemic Exposure to Orally Administered Cannabis and Cannabis-Based Medicines. Am. J. Transl. Res. 2016, 8, 3448–3459. [Google Scholar] [PubMed]
- Buya, A.B.; Beloqui, A.; Memvanga, P.B.; Préat, V. Self-Nano-Emulsifying Drug-Delivery Systems: From the Development to the Current Applications and Challenges in Oral Drug Delivery. Pharmaceutics 2020, 12, 1194. [Google Scholar] [CrossRef]
- Snela, A.; Froelich, A.; Jadach, B.; Osmałek, T. Układy samoemulgujące jako nośniki leków do stosowania doustnego. Farm. Współ./Contemp. Pharm. 2018, 11, 164–171. [Google Scholar]
- Wu, F.; Ma, Q.; Tian, G.; Chen, K.; Yang, R.; Shen, J. Formulation and Evaluation of Solid Self-Nanoemulsifying Drug Delivery System of Cannabidiol for Enhanced Solubility and Bioavailability. Pharmaceutics 2025, 17, 340. [Google Scholar] [CrossRef]
- Sandmeier, M.; Wong, E.T.; Nikolajsen, G.N.; Purwanti, A.; Lindner, S.; Bernkop-Schnürch, A.; Xia, W.; Hoeng, J.; Kjær, K.; Bruun, H.Z.; et al. Oral Formulations for Cannabidiol: Improved Absolute Oral Bioavailability of Biodegradable Cannabidiol Self-Emulsifying Drug Delivery Systems. Colloids Surf. B Biointerfaces 2025, 255, 114879. [Google Scholar] [CrossRef]
- Izgelov, D.; Davidson, E.; Barasch, D.; Regev, A.; Domb, A.J.; Hoffman, A. Pharmacokinetic Investigation of Synthetic Cannabidiol Oral Formulations in Healthy Volunteers. Eur. J. Pharm. Biopharm. 2020, 154, 108–115. [Google Scholar] [CrossRef]
- Cherniakov, I.; Izgelov, D.; Barasch, D.; Davidson, E.; Domb, A.J.; Hoffman, A. Piperine-pro-Nanolipospheres as a Novel Oral Delivery System of Cannabinoids: Pharmacokinetic Evaluation in Healthy Volunteers in Comparison to Buccal Spray Administration. J. Control. Release 2017, 266, 1–7. [Google Scholar] [CrossRef]
- Chaves, M.A.; Ferreira, L.S.; Baldino, L.; Pinho, S.C.; Reverchon, E. Current Applications of Liposomes for the Delivery of Vitamins: A Systematic Review. Nanomaterials 2023, 13, 1557. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of Liposomes as Drug Delivery System for Therapeutic Applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Chauhan, N.; Vasava, P.; Khan, S.L.; Siddiqui, F.A.; Islam, F.; Chopra, H.; Emran, T.B. Ethosomes: A Novel Drug Carrier. Ann. Med. Surg. 2022, 82, 104595. [Google Scholar] [CrossRef]
- Bartelds, R.; Nematollahi, M.H.; Pols, T.; Stuart, M.C.A.; Pardakhty, A.; Asadikaram, G.; Poolman, B. Niosomes, an Alternative for Liposomal Delivery. PLoS ONE 2018, 13, e0194179. [Google Scholar] [CrossRef]
- Franzè, S.; Angelo, L.; Casiraghi, A.; Minghetti, P.; Cilurzo, F. Design of Liposomal Lidocaine/Cannabidiol Fixed Combinations for Local Neuropathic Pain Treatment. Pharmaceutics 2022, 14, 1915. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhang, K.; Lu, L.; Li, M.; Han, M.; Guo, Y.; Wang, X. Improved Therapeutic Efficacy of CBD with Good Tolerance in the Treatment of Breast Cancer through Nanoencapsulation and in Combination with 20(S)-Protopanaxadiol (PPD). Pharmaceutics 2022, 14, 1533. [Google Scholar] [CrossRef] [PubMed]
- Moqejwa, T.; Marimuthu, T.; Kondiah, P.P.D.; Choonara, Y.E. Development of Stable Nano-Sized Transfersomes as a Rectal Colloid for Enhanced Delivery of Cannabidiol. Pharmaceutics 2022, 14, 703. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Schlich, M.; Pireddu, R.; Corrias, F.; Fadda, A.M.; Sinico, C. Production of Nanosuspensions as a Tool to Improve Drug Bioavailability: Focus on Topical Delivery. Curr. Pharm. Des. 2015, 21, 6089–6103. [Google Scholar] [CrossRef]
- Fu, X.; Xu, S.; Li, Z.; Chen, K.; Fan, H.; Wang, Y.; Xie, Z.; Kou, L.; Zhang, S. Enhanced Intramuscular Bioavailability of Cannabidiol Using Nanocrystals: Formulation, In Vitro Appraisal, and Pharmacokinetics. AAPS PharmSciTech 2022, 23, 85. [Google Scholar] [CrossRef]
- Caggiano, N.J.; Wilson, B.K.; Priestley, R.D.; Prud’homme, R.K. Development of an In Vitro Release Assay for Low-Density Cannabidiol Nanoparticles Prepared by Flash NanoPrecipitation. Mol. Pharm. 2022, 19, 1515–1525. [Google Scholar] [CrossRef]
- Nikiforidis, C.V. Structure and Functions of Oleosomes (Oil Bodies). Adv. Colloid Interface Sci. 2019, 274, 102039. [Google Scholar] [CrossRef]
- Shao, Q.; Liu, X.; Su, T.; Ma, C.; Wang, P. New Insights Into the Role of Seed Oil Body Proteins in Metabolism and Plant Development. Front. Plant Sci. 2019, 10, 1568. [Google Scholar] [CrossRef]
- Ji, L.; Feng, W.; Chen, H.; Chu, Y.; Wong, A.; Zhu, Y.; Sinatra, G.; Bramante, F.; Carrière, F.; Stocks, M.J.; et al. Rapeseed Oleosomes Facilitate Intestinal Lymphatic Delivery and Oral Bioavailability of Cannabidiol. Int. J. Pharm. 2025, 668, 124947. [Google Scholar] [CrossRef]
- Błaszczak-Świątkiewicz, K.; Olszewska, P.; Mikiciuk-Olasik, E. Zastosowanie nanocząsteczek w leczeniu i diagnostyce nowotworów. Nowotwory. J. Oncol. 2013, 63, 320–330. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, X.; Wang, Y.; Li, M.; Yuan, Q.; Zhao, Z. Inflammation-Targeted Cannabidiol-Loaded Nanomicelles for Enhanced Oral Mucositis Treatment. Drug Deliv. 2022, 29, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-T.; Zhang, J. Solubility Enhancement and Antioxidation Maintenance of CBD Encapsulated in the P407-RUB Nano-Micelle System. Curr. Drug Deliv. 2024, 21, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I.; Cohen, M.; Delie, F.; Bastida-Ruiz, D.; Yart, L.; Martin-Sabroso, C.; Fernández-Carballido, A. PLGA Nanoparticles for the Intraperitoneal Administration of CBD in the Treatment of Ovarian Cancer: In Vitro and In Ovo Assessment. Pharmaceutics 2020, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.L.; Duan, W.; Lee, B.-J.; Tran, T.T.D. The Use of Zein in the Controlled Release of Poorly Water-Soluble Drugs. Int. J. Pharm. 2019, 566, 557–564. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Sun, Y.; Freeman, K.; Mchenry, M.A.; Wang, C.; Guo, M. Enhanced Stability and Oral Bioavailability of Cannabidiol in Zein and Whey Protein Composite Nanoparticles by a Modified Anti-Solvent Approach. Foods 2022, 11, 376. [Google Scholar] [CrossRef]
- Hiral, M.; Akhilesh, D.; Prabhakara, P.; Kamath, J.V. Enhancement of Solubility by Complexation with Cyclodextrin and Nanocrystallisation. Int. Res. J. Pharm. 2012, 3, 82–87. [Google Scholar]
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.-H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides 2022, 3, 1–31. [Google Scholar] [CrossRef]
- Nelumdeniya, N.R.M.; Ranatunga, R.J.K.U. Complex Forming Behaviour of α, β and γ-Cyclodextrins with Varying Size Probe Particles in Silico. Ceylon J. Sci. 2021, 50, 329. [Google Scholar] [CrossRef]
- Zhou, J.; Jia, J.; He, J.; Li, J.; Cai, J. Cyclodextrin Inclusion Complexes and Their Application in Food Safety Analysis: Recent Developments and Future Prospects. Foods 2022, 11, 3871. [Google Scholar] [CrossRef]
- Rajbanshi, B.; Saha, S.; Das, K.; Barman, B.K.; Sengupta, S.; Bhattacharjee, A.; Roy, M.N. Study to Probe Subsistence of Host-Guest Inclusion Complexes of α and β-Cyclodextrins with Biologically Potent Drugs for Safety Regulatory Dischargement. Sci. Rep. 2018, 8, 13031. [Google Scholar] [CrossRef]
- Li, H.; Chang, S.-L.; Chang, T.-R.; You, Y.; Wang, X.-D.; Wang, L.-W.; Yuan, X.-F.; Tan, M.-H.; Wang, P.-D.; Xu, P.-W.; et al. Inclusion Complexes of Cannabidiol with β-Cyclodextrin and Its Derivative: Physicochemical Properties, Water Solubility, and Antioxidant Activity. J. Mol. Liq. 2021, 334, 116070. [Google Scholar] [CrossRef]
- Musakhanian, J.; Rodier, J.-D.; Dave, M. Oxidative Stability in Lipid Formulations: A Review of the Mechanisms, Drivers, and Inhibitors of Oxidation. AAPS PharmSciTech 2022, 23, 151. [Google Scholar] [CrossRef] [PubMed]
- Cherniakov, I.; Izgelov, D.; Domb, A.J.; Hoffman, A. The Effect of Pro NanoLipospheres (PNL) Formulation Containing Natural Absorption Enhancers on the Oral Bioavailability of Delta-9-Tetrahydrocannabinol (THC) and Cannabidiol (CBD) in a Rat Model. Eur. J. Pharm. Sci. 2017, 109, 21–30. [Google Scholar] [CrossRef]
- Varut, R.M.; Ciolofan, M.S.; Veronica, M.E.; Radivojević, K.; Trasca, D.M.; Popescu, C.; Diaconu, O.; Singer, C.E. Cyclodextrins as Modulators of Gut Microbiota: Pharmaceutical Applications and Impact on Intestinal Health. Pharmaceutics 2025, 17, 752. [Google Scholar] [CrossRef]
- Allan, J.; Belz, S.; Hoeveler, A.; Hugas, M.; Okuda, H.; Patri, A.; Rauscher, H.; Silva, P.; Slikker, W.; Sokull-Kluettgen, B.; et al. Regulatory Landscape of Nanotechnology and Nanoplastics from a Global Perspective. Regul. Toxicol. Pharmacol. 2021, 122, 104885. [Google Scholar] [CrossRef] [PubMed]
| Receptor Type | Action | Effects of Action | Possible Applications | Refs. |
|---|---|---|---|---|
| CB1 | Antagonistic modulator | Anticonvulsant effect | Epilepsy | [15] |
| GPR12 | Inverse agonist | Promotes neurite outgrowth and blocks myelin inhibition in neurons | Neurodegenerative diseases | [16] |
| GPR55 | Antagonist | Anti-inflammatory effect, Analgesic effect | Neurodegenerative diseases, Analgesia/Coanalgesia | [17] |
| GlyRα1 | Allosteric modulator | Antiepileptic effect | Epilepsy | [18] |
| GlyRα1β | Allosteric modulator | Antiepileptic effect | Epilepsy | [18] |
| GlyRα3 | Potentiator | Analgesic effect | Neuropathic pain | [19] |
| 5-HT1A | Agonist | Antiepileptic effect, Anticataleptic effect, Antipsychotic effect | Epilepsy, Neuroleptic malignant syndrome, Anxiety | [20,21] |
| 5-HT2A | Agonist | Antiepileptic effect, Anticataleptic effect | Epilepsy, Neuroleptic malignant syndrome, | [20,21] |
| 5-HT3A | Antagonist | Antiemetic | Vomiting associated with cancer treatment | [22] |
| PPARγ | Agonist | Antitumor effect, Anti-inflammatory effect | Neurodegenerative diseases | [23,24] |
| ARA1 | Agonist | Antiarrhythmic effect, Anti-inflammatory effect, Antipsychotic effect | Ventricular tachycardia, Neurodegenerative diseases, Anxiety | [25,26,27] |
| Cell Line | |||||||
|---|---|---|---|---|---|---|---|
| 4T1 | MCF-1 | A549 | C6 | Hela | HepG2 | ||
| Fold of increased permeability | CP-Liposomes | 36.3 | 8.6 | 2.2 | 1.8 | 4.3 | 3.2 |
| GMCP-Liposomes | 19.4 | 6.5 | 2.2 | 2.2 | 3.5 | 3.5 | |
| Delivery System | Species/Model | Cmax (ng/mL) | AUC0–∞ (ng·h/mL) | Relative Bioavailability | Ref. |
|---|---|---|---|---|---|
| SNNEDSs | Human (healthy volunteers) | 18 ± 9 L-SNNEDS | 66 ± 19 L-SNNEDS | ~7.0-fold ↑ (vs. powder) | [38] |
| Rat | 499.5 ± 144.6 L-SNNEDS 283.7 ± 84.7 S-SNNEDS-SD 146.7 ± 26.0 S-SNEEDS-SCA | 1534.3 ± 438.9 L-SNNEDS 833.8 ± 214.7 S-SNNEDS_SD 642.5 ± 77.4 S-SNEEDS-SCA | ~6–14-fold ↑ (vs. MCT oil) | [36] | |
| Polymeric nanoparticles | Rat | 0.466 ± 0.023 | 2.912 ± 0.310 | ~1.75-fold ↑ (vs. pure CBD) | [59] |
| Oleosomes | Rat | 79 ± 11 | 413 ± 25 | ~1.5-fold ↑ (vs. oil) | [52] |
| Nanocarrier System | Mechanism of Action | Effect on Bioavailability (PK Outcome) |
|---|---|---|
| SNEDDSs/Nanoemulsions | Spontaneous formation of nano-sized emulsions in GI tract → ↑ surface area, partial lymphatic transport | ↑ Solubility, ↑ dissolution rate, ↑ oral absorption, partial bypass of first-pass metabolism |
| Liposomes/Transfersomes | Phospholipid vesicles protect CBD; enhanced membrane fluidity and skin/mucosal penetration | Controlled release, protection from degradation, ↑ permeability, potential for transdermal/oral delivery |
| Polymeric NPs/Micelles | Encapsulation in biodegradable polymer matrix; controlled degradation and drug release | ↑ Stability, sustained release → ↑ AUC, potential for tissue targeting |
| Nanosuspensions | Size reduction to nanocrystals → ↑ surface area for dissolution | Rapid dissolution, ↑ Cmax, possibility of parenteral administration |
| Cyclodextrin complexes | Inclusion of CBD in hydrophobic cavity of cyclodextrin → ↑ aqueous solubility | ↑ Solubility and dissolution, moderate ↑ oral bioavailability |
| Oleosomes | Natural lipid bodies stabilize CBD; promote lymphatic absorption | Protection from degradation, lymphatic transport → partial bypass of first-pass metabolism, ↑ systemic exposure |
| Platform | Clinical Readiness | Safety Data Depth | Scalability/Manufacturability | Key Strengths | Main Limitations |
|---|---|---|---|---|---|
| SNEDDS | High (human PK evidence; closest to translation) | Medium (tolerability events; surfactant load; P-PNL DDI risk) | Medium–High (oil/surfactant systems; solidification adds complexity) | Big oral exposure gains; formulation know-how | First-pass not fully overcome; chronic safety of excipients; DDI with enzyme inhibition |
| Liposomes | Medium–Low (mainly preclinical/transdermal) | Medium (biocompatible lipids but oxidation/irritancy risks) | Low–Medium (stability control and cost) | Controlled release; membrane crossing; skin delivery | Chemical/physical instability; cost and scale-up hurdles; limited clinical data |
| Polymeric NPs | Medium–Low (enhanced effects vs. free CBD, limited clinical) | Medium (novel excipients; biodegradability/chronic-use questions) | Low–Medium (solvents/complex processes) | Stability; tunable release; targeting potential | Regulatory burden for new polymers; manufacturing complexity; burst release |
| Nanosuspensions | Medium–Low (animal PK ↑; parenteral option) | Medium (well-known excipients; needs chronic data) | Medium (unit ops established; CBD-specific know-how evolving) | High drug load; solvent-lean; fast dissolution | Limited clinical data; route/formulation optimization needed |
| Cyclodextrin complexes | Low (early stage; in vitro dissolution focus) | Medium (GRAS history but dose-related concerns) | High (simple processing; excipient supply) | Huge solubility/dissolution boosts | Sparse in vivo efficacy; dose-limit safety considerations |
| Oleosomes | Low (promising animal PK and lymphatic transport) | Medium (natural carriers; standardization pending) | Low–Medium (source variability; standardization challenges) | Natural, stable emulsions; strong lymphatic targeting | Reproducibility/regulatory acceptance; no clinical data yet |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paczkowska-Walendowska, M.; Trzaskoma, P.; Dziopa, A.; Moeini, A.; Soczawa, M.; Krasiński, Z.; Cielecka-Piontek, J. Innovative Strategies to Enhance the Bioavailability of Cannabidiol: Nanotechnology and Advanced Delivery Systems. Pharmaceuticals 2025, 18, 1637. https://doi.org/10.3390/ph18111637
Paczkowska-Walendowska M, Trzaskoma P, Dziopa A, Moeini A, Soczawa M, Krasiński Z, Cielecka-Piontek J. Innovative Strategies to Enhance the Bioavailability of Cannabidiol: Nanotechnology and Advanced Delivery Systems. Pharmaceuticals. 2025; 18(11):1637. https://doi.org/10.3390/ph18111637
Chicago/Turabian StylePaczkowska-Walendowska, Magdalena, Piotr Trzaskoma, Aleksandra Dziopa, Arash Moeini, Michał Soczawa, Zbigniew Krasiński, and Judyta Cielecka-Piontek. 2025. "Innovative Strategies to Enhance the Bioavailability of Cannabidiol: Nanotechnology and Advanced Delivery Systems" Pharmaceuticals 18, no. 11: 1637. https://doi.org/10.3390/ph18111637
APA StylePaczkowska-Walendowska, M., Trzaskoma, P., Dziopa, A., Moeini, A., Soczawa, M., Krasiński, Z., & Cielecka-Piontek, J. (2025). Innovative Strategies to Enhance the Bioavailability of Cannabidiol: Nanotechnology and Advanced Delivery Systems. Pharmaceuticals, 18(11), 1637. https://doi.org/10.3390/ph18111637










