Potential Modulation of Polygoni Cuspidati Rhizoma et Radix on Breast Cancer Resistance Protein and Marked Alteration on Methotrexate Pharmacokinetics
Abstract
1. Introduction
2. Results
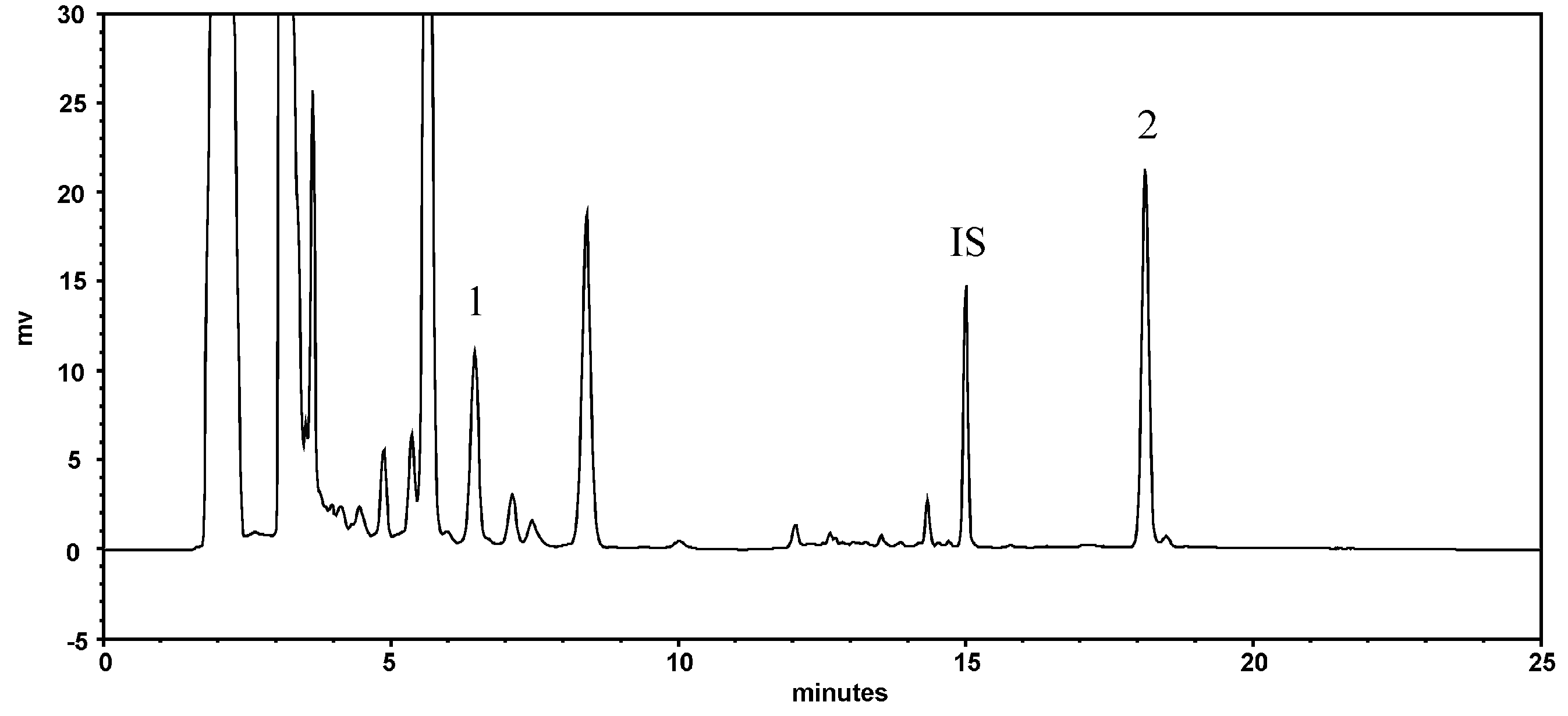
2.1. Quantitation of Resveratrol and Emodin in PCRR Decoction
2.2. PCRR-MTX Pharmacokinetic Interaction Study in Rats
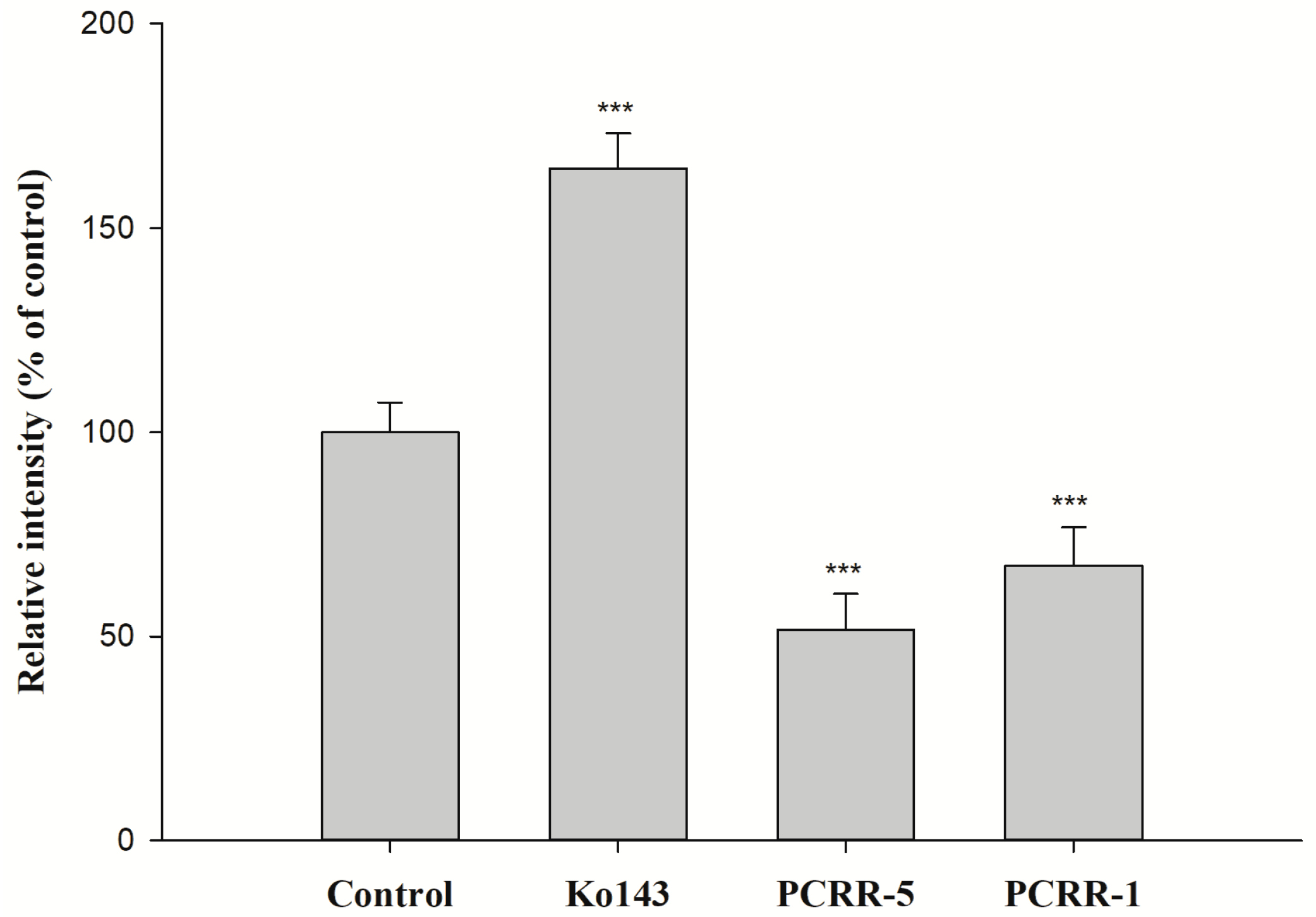
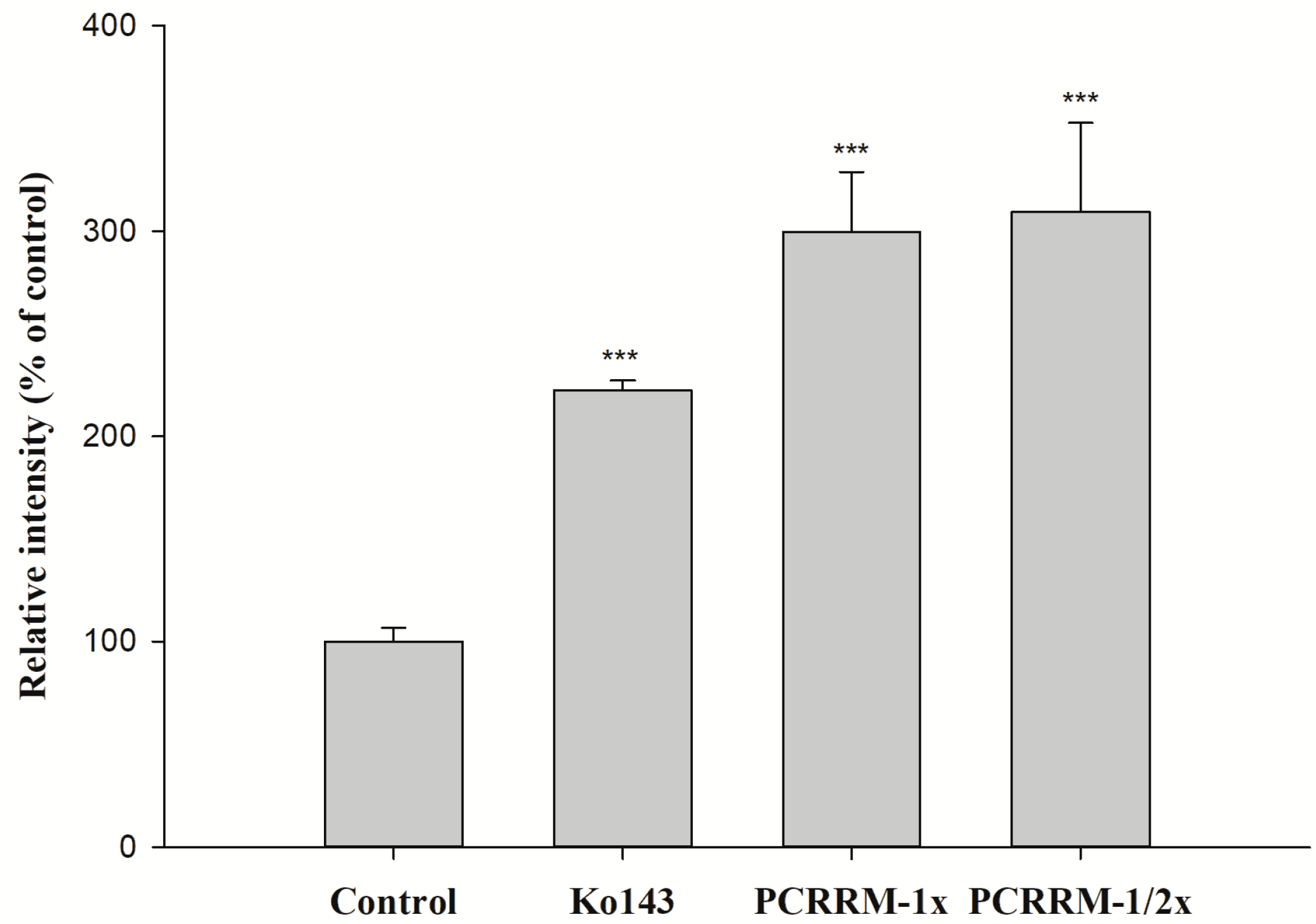
2.3. Function Assay of BCRP After Treatments with PCRR and PCRRM
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals
4.3. Preparation and Characterization of PCRR Decoction and PCRRM
4.4. PCRR-MTX Pharmacokinetic Interaction in Rats
4.5. Cell Culture Condition and Cell Viability Assay
4.6. Function Assay of BCRP After Treatments with PCRR and PCRRM
4.7. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC0-t | Area under the serum level–time curve |
| BCRP | Breast cancer resistance protein |
| Cmax | Peak serum level |
| MDCKII-BCRP MRT | MDCKII transfected cells with overexpression of BCRP Mean residence time |
| MTX | Methotrexate |
| MXR | Mitoxantrone |
| PCRR | Polygoni Cuspidati Rhizoma et Radix |
| PCRRM | The serum metabolites of PCRR |
References
- Chen, B.Y.; Kuo, C.H.; Liu, Y.C.; Ye, L.Y.; Chen, J.H.; Shieh, C.J. Ultrasonic-assisted extraction of the botanical dietary supplement resveratrol and other constituents of Polygonum cuspidatum. J. Nat. Prod. 2012, 75, 1810–1813. [Google Scholar] [CrossRef]
- Lin, J.A.; Kuo, C.H.; Chen, B.Y.; Li, Y.; Liu, Y.C.; Chen, J.H.; Shieh, C.J. A novel enzyme-assisted ultrasonic approach for highly efficient extraction of resveratrol from Polygonum cuspidatum. Ultrason. Sonochem. 2016, 32, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Li, M.T.; Xu, S.; Ma, J.; Liu, M.Y.; Han, Y. Advances for pharmacological activities of Polygonum cuspidatum—A review. Pharm. Biol. 2023, 61, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Kirino, A.; Takasuka, Y.; Nishi, A.; Kawabe, S.; Yamashita, H.; Kimoto, M.; Ito, H.; Tsuji, H. Analysis and functionality of major polyphenolic components of Polygonum cuspidatum (itadori). J. Nutr. Sci. Vitaminol. 2012, 58, 278–286. [Google Scholar] [CrossRef]
- Peng, W.; Qin, R.; Li, X.; Zhou, H. Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb.et Zucc.: A review. J. Ethnopharmacol. 2013, 148, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Welfare Taiwan (Ed.) Taiwan Herbal Pharmacopeia, 4th ed.; Department of Chinese Medicine and Pharmacy, Ministry of Health and Welfare: Taipei, Taiwan, 2022. [Google Scholar]
- Zhang, H.; Li, C.; Kwok, S.T.; Zhang, Q.W.; Chan, S.W. A review of the pharmacological effects of the dried root of Polygonum cuspidatum (Hu Zhang) and its constituents. Evid.-Based Complement. Altern. Med. 2013, 2013, 208349. [Google Scholar] [CrossRef]
- Chen, X.; Song, X.; Zhao, X.; Zhang, Y.; Wang, Y.; Jia, R.; Zou, Y.; Li, L.; Yin, Z. Insights into the anti-inflammatory and antiviral mechanisms of resveratrol. Mediat. Inflamm. 2022, 2022, 7138756. [Google Scholar] [CrossRef]
- Sall, C.; Argikar, U.; Fonseca, K.; Hilgendorf, C.; Lopes, F.; Riedel, J.; Schiller, H.; Sonesson, A.; Umehara, K.; Wang, K. Industry perspective on therapeutic peptide drug-drug interaction assessments during drug development: A European Federation of Pharmaceutical Industries and Associations white paper. Clin. Pharmacol. Ther. 2023, 113, 1199–1216. [Google Scholar] [CrossRef]
- Alam, A.; Locher, K.P. Structure and mechanism of human ABC transporters. Annu. Rev. Biophys. 2023, 52, 275–300. [Google Scholar] [CrossRef]
- Schaller, S.; Michon, I.; Baier, V.; Martins, F.S.; Nolain, P.; Taneja, A. Evaluation of BCRP-Related DDIs Between Methotrexate and Cyclosporin A Using Physiologically Based Pharmacokinetic Modelling. Drugs RD 2025, 25, 1–17. [Google Scholar] [CrossRef]
- Maksimovic, V.; Pavlovic-Popovic, Z.; Vukmirovic, S.; Cvejic, J.; Mooranian, A.; Al-Salami, H.; Mikov, M.; Golocorbin-Kon, S. Molecular mechanism of action and pharmacokinetic properties of methotrexate. Mol. Biol. Rep. 2020, 47, 4699–4708. [Google Scholar] [CrossRef]
- Hamed, K.M.; Dighriri, I.M.; Baomar, A.F.; Alharthy, B.T.; Alenazi, F.E.; Alali, G.H.; Alenazy, R.H.; Alhumaidi, N.T.; Alhulayfi, D.H.; Alotaibi, Y.B.; et al. Overview of methotrexate toxicity: A comprehensive literature review. Cureus 2022, 14, e29518. [Google Scholar] [CrossRef]
- Ouellette, S.; Shah, R.; Razi, S.; Ashforth, G.; Wassef, C. Fatal low-dose methotrexate toxicity: A case report and literature review. Dermatol. Ther. 2022, 35, e15945. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mo, J.; Yang, Z.; Zhao, Z.; Mei, S. Risk factors associated with high-dose methotrexate induced toxicities. Expert Opin. Drug Metab. Toxicol. 2024, 20, 263–274. [Google Scholar] [CrossRef]
- Feltrin, C.; Oliveira Simoes, C.M. Reviewing the mechanisms of natural product-drug interactions involving efflux transporters and metabolic enzymes. Chem. Biol. Interact. 2019, 314, 108825. [Google Scholar] [CrossRef]
- Vimalavathini, R.; Shri Hari Subhashri, R.; Kavimani, S. Herb-Drug Interactions. In Evidence Based Validation of Traditional Medicines; Mandal, S.C., Chakraborty, R., Sen, S., Eds.; Springer: Singapore, 2021; pp. 649–658. [Google Scholar]
- Yu, C.P.; Li, P.Y.; Chen, S.Y.; Lin, S.P.; Chen, Y.C.; Ho, L.C.; Hsieh, Y.W.; Hou, Y.C. An acute herb-drug interaction of Magnoliae Officinalis Cortex with methotrexate via inhibiting multidrug resistance-associated protein 2. J. Food Drug Anal. 2024, 32, 103–111. [Google Scholar] [CrossRef]
- Yu, C.P.; Hsieh, Y.C.; Shia, C.S.; Hsu, P.W.; Chen, J.Y.; Hou, Y.C.; Hsieh, Y.W. Increased systemic exposure of methotrexate by a polyphenol-rich herb via modulation on efflux transporters multidrug resistance-associated protein 2 and breast cancer resistance protein. J. Pharm. Sci. 2016, 105, 343–349. [Google Scholar] [CrossRef]
- Huang, T.Y.; Yu, C.P.; Hsieh, Y.W.; Lin, S.P.; Hou, Y.C. Resveratrol stereoselectively affected (+/−)warfarin pharmacokinetics and enhanced the anticoagulation effect. Sci. Rep. 2020, 10, 15910. [Google Scholar] [CrossRef]
- da Silva Zanzarini, I.; Kita, D.H.; Scheiffer, G.; Dos Santos, K.K.; de Paula Dutra, J.; Pastore, M.A.; de Moraes Rego, F.G.; Picheth, G.; Ambudkar, S.V.; Pulvirenti, L. Magnolol derivatives as specific and noncytotoxic inhibitors of breast cancer resistance protein (BCRP/ABCG2). Bioorganic Chem. 2024, 146, 107283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M.; Hu, G.; Zhang, Z.; Song, R. Elevated system exposures of baicalin after combinatory oral administration of rhein and baicalin: Mainly related to breast cancer resistance protein (ABCG2), not UDP-glucuronosyltransferases. J. Ethnopharmacol. 2020, 250, 112528. [Google Scholar] [CrossRef] [PubMed]
- Jarvinen, E.; Deng, F.; Kiander, W.; Sinokki, A.; Kidron, H.; Sjostedt, N. The role of uptake and efflux transporters in the disposition of glucuronide and sulfate conjugates. Front. Pharmacol. 2021, 12, 802539. [Google Scholar] [CrossRef]
- Planas, J.M.; Alfaras, I.; Colom, H.; Juan, M.E. The bioavailability and distribution of trans-resveratrol are constrained by ABC transporters. Arch. Biochem. Biophys. 2012, 527, 67–73. [Google Scholar] [CrossRef]
- Chi, Y.C.; Lin, S.P.; Hou, Y.C. A new herb-drug interaction of Polygonum cuspidatum, a resveratrol-rich nutraceutical, with carbamazepine in rats. Toxicol. Appl. Pharmacol. 2012, 263, 315–322. [Google Scholar] [CrossRef]
- Muanda, F.T.; Blake, P.G.; Weir, M.A.; Ahmadi, F.; McArthur, E.; Sontrop, J.M.; Urquhart, B.L.; Kim, R.B.; Garg, A.X. Low-dose methotrexate and serious adverse events among older adults with chronic kidney disease. JAMA Netw. Open 2023, 6, e2345132. [Google Scholar] [CrossRef]
- Jafari, F.; Arasteh, O.; Hosseinjani, H.; Allahyari, A.; Ataei Azimi, S.; Askari, V.R. A critical review of methotrexate clinical interactions: Role of transporters. Expert Opin. Drug Met. 2023, 19, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Shitara, Y.; Maeda, K.; Ikejiri, K.; Yoshida, K.; Horie, T.; Sugiyama, Y. Clinical significance of organic anion transporting polypeptides (OATPs) in drug disposition: Their roles in hepatic clearance and intestinal absorption. Biopharm. Drug Dispos. 2013, 34, 45–78. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.; Sulaiman, A.A.; Alhaddad, H.; Alhadidi, Q. Natural polyphenols: Influence on membrane transporters. J. Intercult. Ethnopharmcol. 2016, 5, 97. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, Z.; Wang, C.; Meng, Q.; Huo, X.; Liu, Q.; Sun, H.; Sun, P.; Yang, X.; Ma, X. P-gp, MRP2 and OAT1/OAT3 mediate the drug-drug interaction between resveratrol and methotrexate. Toxicol. Appl. Pharmacol. 2016, 306, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Riha, J.; Brenner, S.; Bohmdorfer, M.; Giessrigl, B.; Pignitter, M.; Schueller, K.; Thalhammer, T.; Stieger, B.; Somoza, V.; Szekeres, T.; et al. Resveratrol and its major sulfated conjugates are substrates of organic anion transporting polypeptides (OATPs): Impact on growth of ZR-75-1 breast cancer cells. Mol. Nutr. Food Res. 2014, 58, 1830–1842. [Google Scholar] [CrossRef]
- So, E.L.; Ruggles, K.H.; Cascino, G.D.; Ahmann, P.A.; Weatherford, K.W. Seizure exacerbation and status epileptics related to carbamazepine-10, 11-epoxide. Ann. Neurol. 1994, 35, 743–746. [Google Scholar] [CrossRef]
- Singh, J.; Elbarbry, F.; Lan, K.; Grabowski, T. Animal pharmacokinetic/pharmacodynamic studies (APPS) reporting guidelines. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 483–494. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; Center for Drug Evaluation and Research (CDER): Rockville, MD, USA, 2005.
- Lin, S.P.; Chu, P.M.; Tsai, S.Y.; Wu, M.H.; Hou, Y.C. Pharmacokinetics and tissue distribution of resveratrol, emodin and their metabolites after intake of Polygonum cuspidatum in rats. J. Ethnopharmacol. 2012, 144, 671–676. [Google Scholar] [CrossRef] [PubMed]

| Treatments | MTX Alone | MTX + PCRR (1.0 g/kg) (n = 6) | MTX + PCRR (2.0 g/kg) (n = 5) | |
|---|---|---|---|---|
| Parameters | ||||
| Tmax | 25.0 ± 3.2 | 25.0 ± 3.2 | 18.0 ± 3.0 | |
| Cmax | 0.35 ± 0.05 a | 0.23 ± 0.01 ab | 0.19 ± 0.02 b (−46%) | |
| AUC0~2880 | 143.2 ± 21.2 ab | 106.8 ± 7.9 a | 181.8 ± 17.2 b | |
| AUC0~240 | 51.4 ± 6.6 a | 35.4 ± 1.5 b (−31%) | 21.8 ± 1.2 b (−58%) | |
| AUC240~2880 | 91.8 ± 17.7 a | 71.4 ± 7.6 a | 160.1 ± 17.4 b (+39%) | |
| MRT0~2880 | 690.9 ± 79.7 a | 681.4 ± 79.4 a | 1134.3 ± 56.5 b (+74%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.-C.; Li, P.-Y.; Lin, S.-P.; Hsu, P.-W.; Wu, M.-H.; Yu, C.-P. Potential Modulation of Polygoni Cuspidati Rhizoma et Radix on Breast Cancer Resistance Protein and Marked Alteration on Methotrexate Pharmacokinetics. Pharmaceuticals 2025, 18, 1636. https://doi.org/10.3390/ph18111636
Hou Y-C, Li P-Y, Lin S-P, Hsu P-W, Wu M-H, Yu C-P. Potential Modulation of Polygoni Cuspidati Rhizoma et Radix on Breast Cancer Resistance Protein and Marked Alteration on Methotrexate Pharmacokinetics. Pharmaceuticals. 2025; 18(11):1636. https://doi.org/10.3390/ph18111636
Chicago/Turabian StyleHou, Yu-Chi, Pei-Ying Li, Shiuan-Pey Lin, Pei-Wen Hsu, Meng-Hao Wu, and Chung-Ping Yu. 2025. "Potential Modulation of Polygoni Cuspidati Rhizoma et Radix on Breast Cancer Resistance Protein and Marked Alteration on Methotrexate Pharmacokinetics" Pharmaceuticals 18, no. 11: 1636. https://doi.org/10.3390/ph18111636
APA StyleHou, Y.-C., Li, P.-Y., Lin, S.-P., Hsu, P.-W., Wu, M.-H., & Yu, C.-P. (2025). Potential Modulation of Polygoni Cuspidati Rhizoma et Radix on Breast Cancer Resistance Protein and Marked Alteration on Methotrexate Pharmacokinetics. Pharmaceuticals, 18(11), 1636. https://doi.org/10.3390/ph18111636








