Synthesis and Anticancer Activity Evaluation of New 5-((5-Nitrofuran-2-yl)allylidene)-2-thioxo-4-thiazolidinones
Abstract
1. Introduction
2. Results
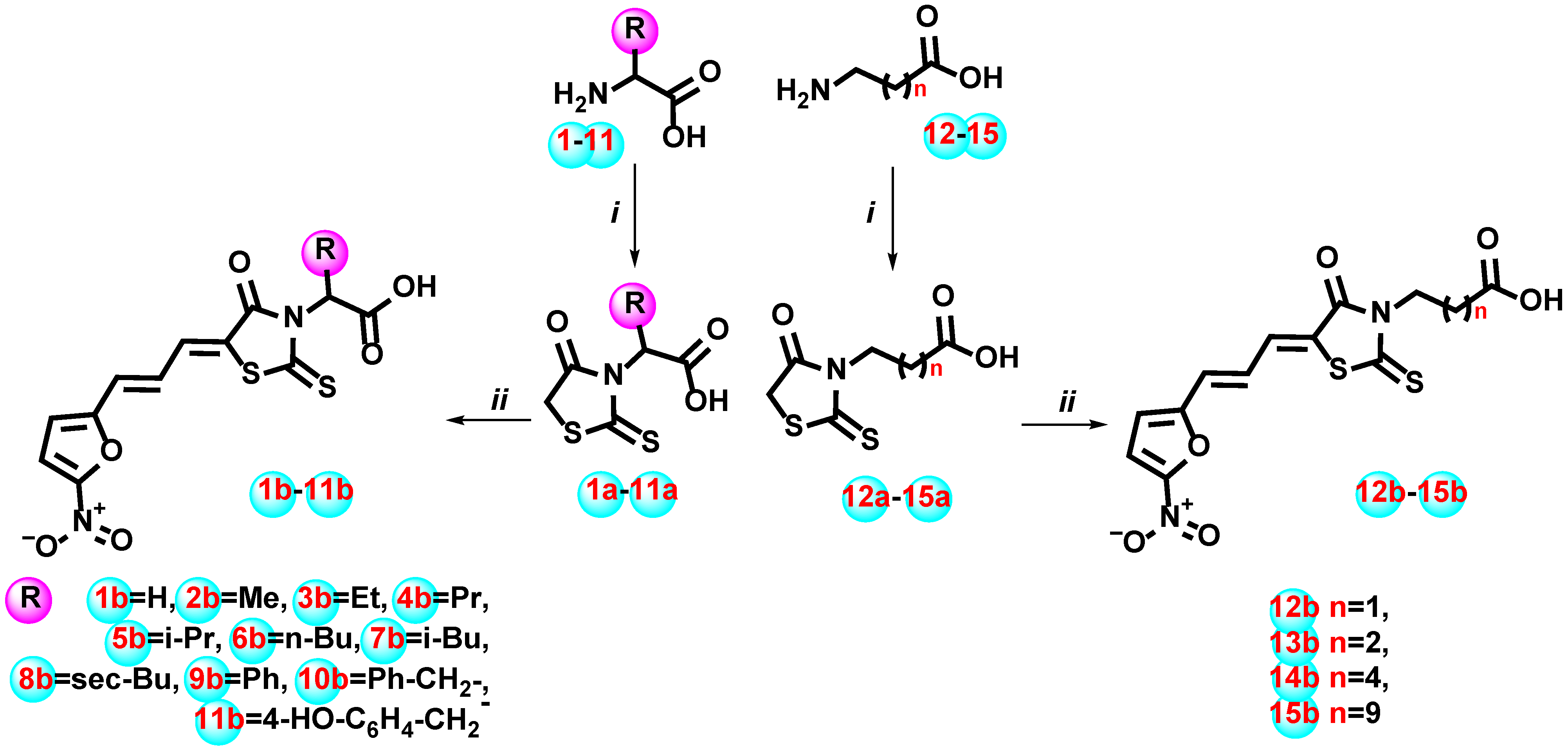
2.1. Chemistry
2.2. Biology
2.2.1. In Vitro Evaluation of the Anticancer Activity According to the DTP NCI Protocol
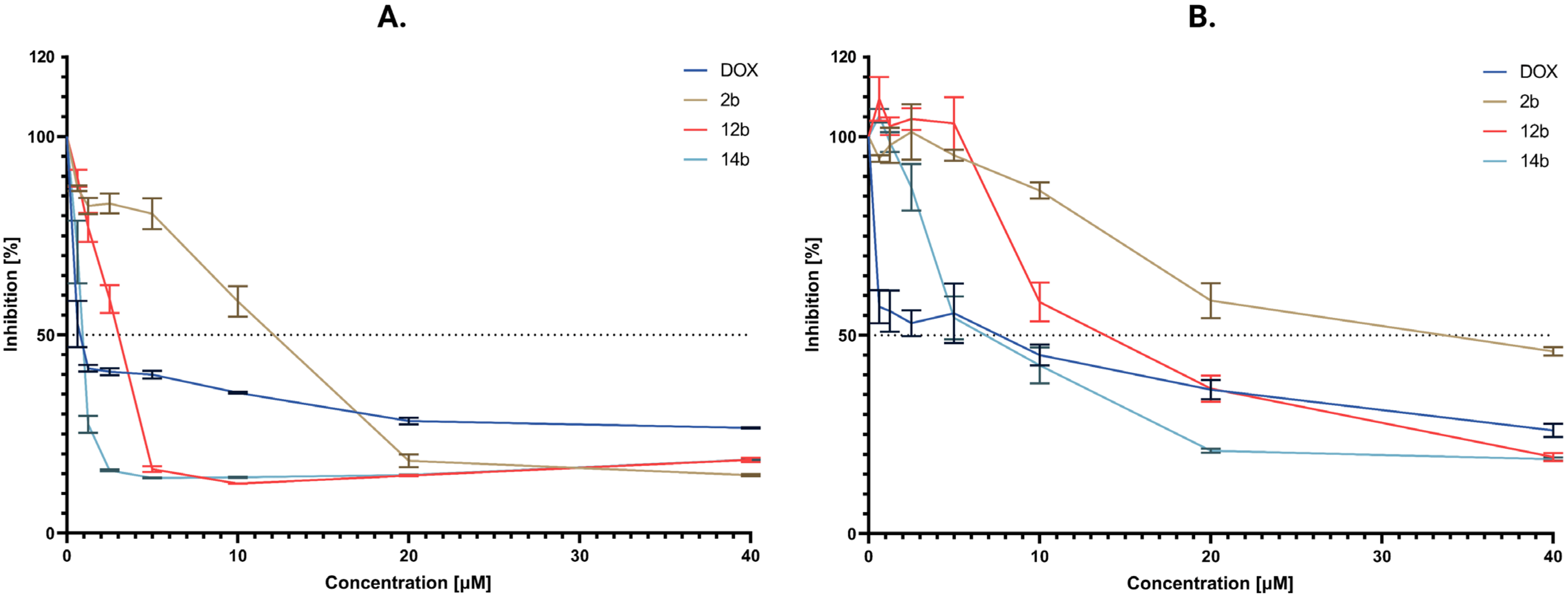
2.2.2. Cytotoxic Activity
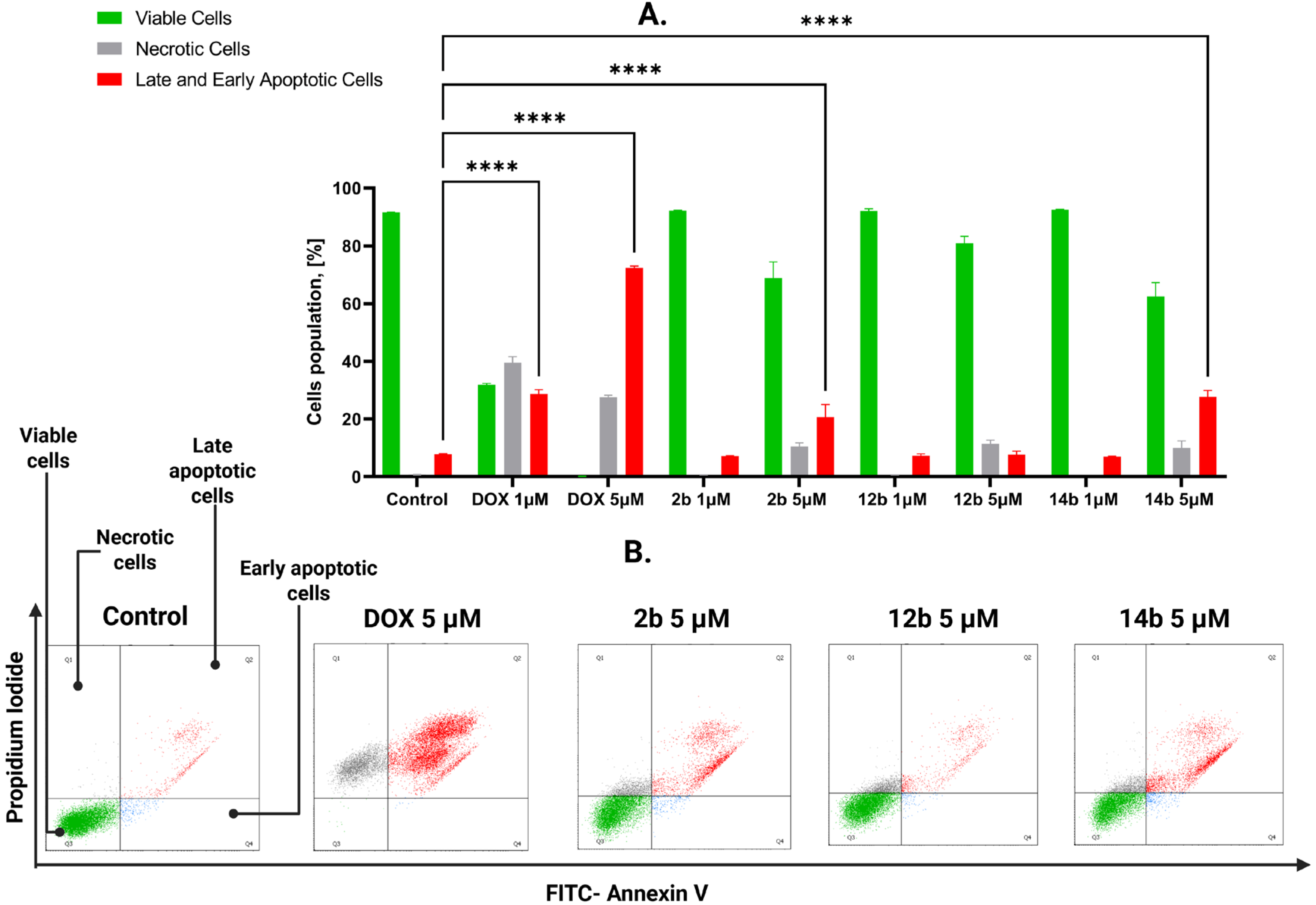
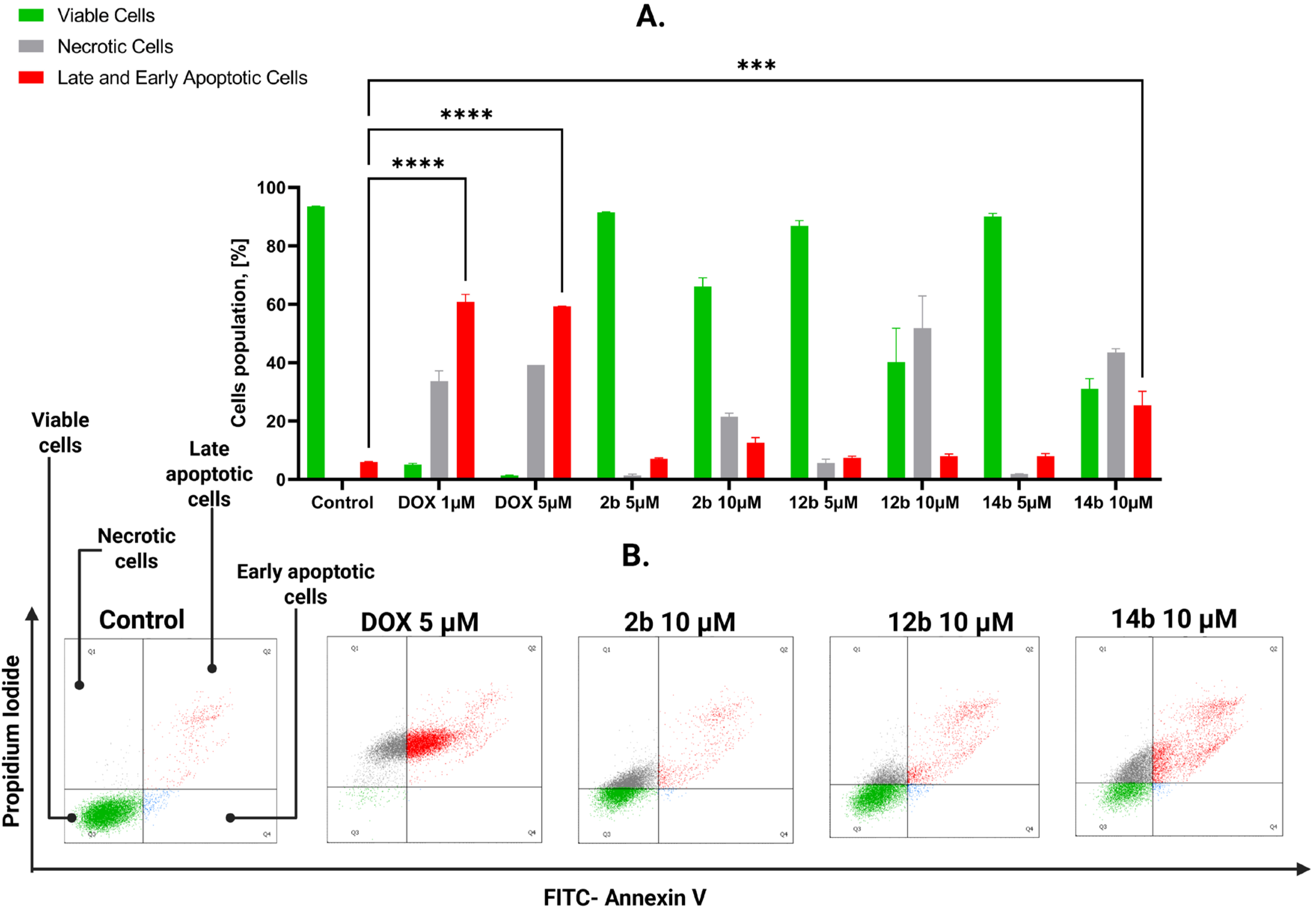
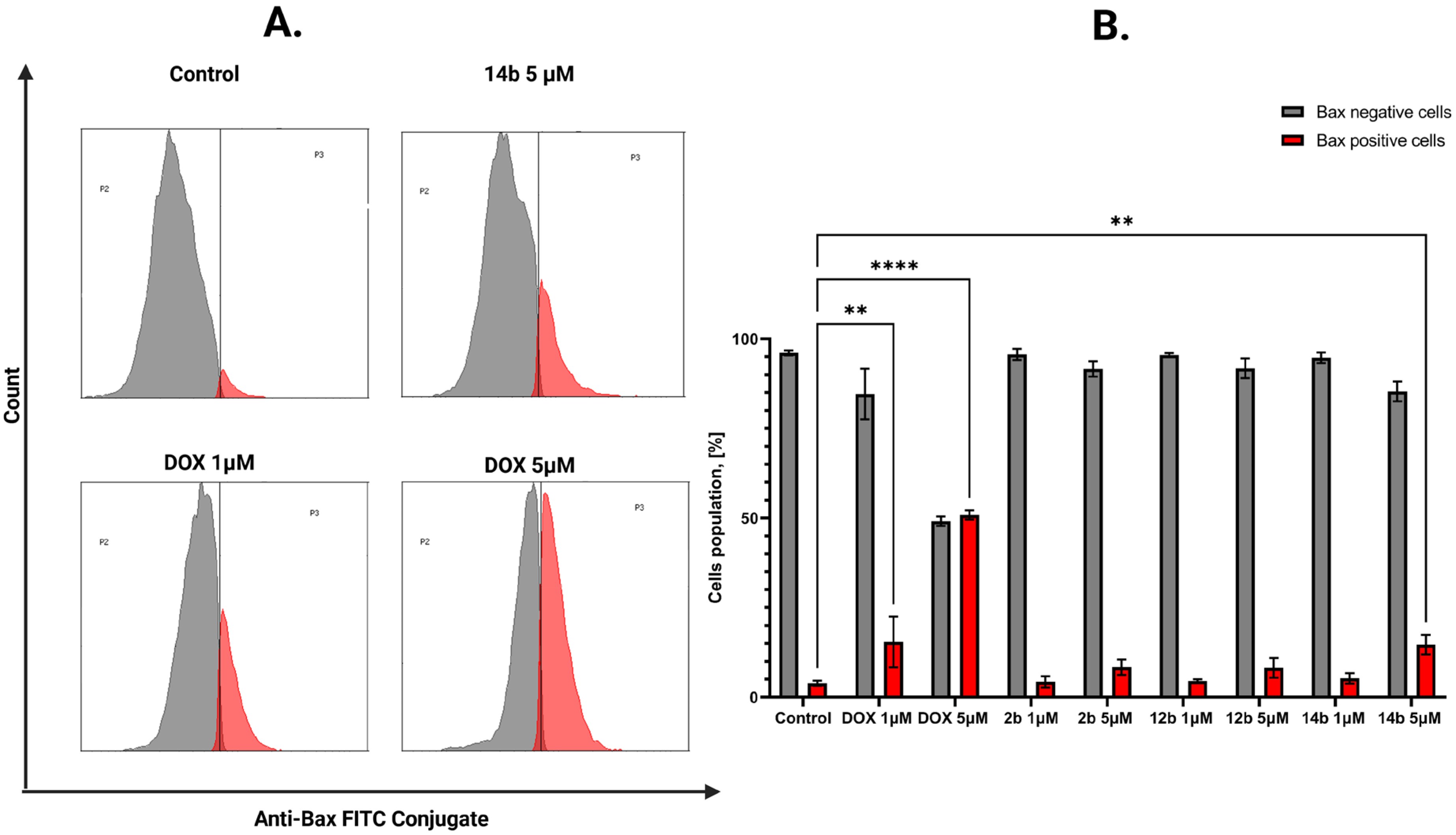
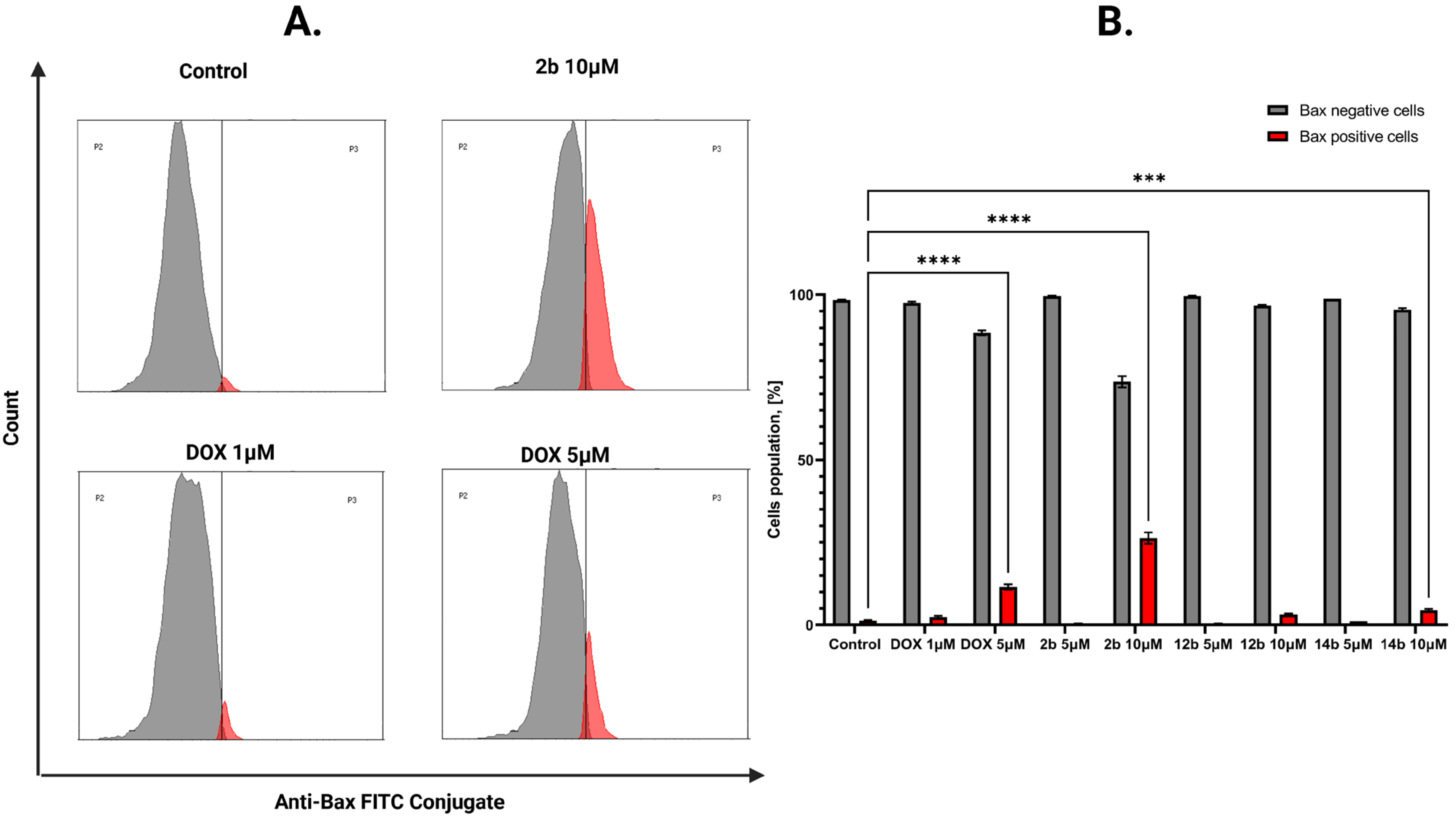
2.2.3. Proapoptotic Effects of Studied Compounds
2.2.4. Generation of Reactive Oxygen Species
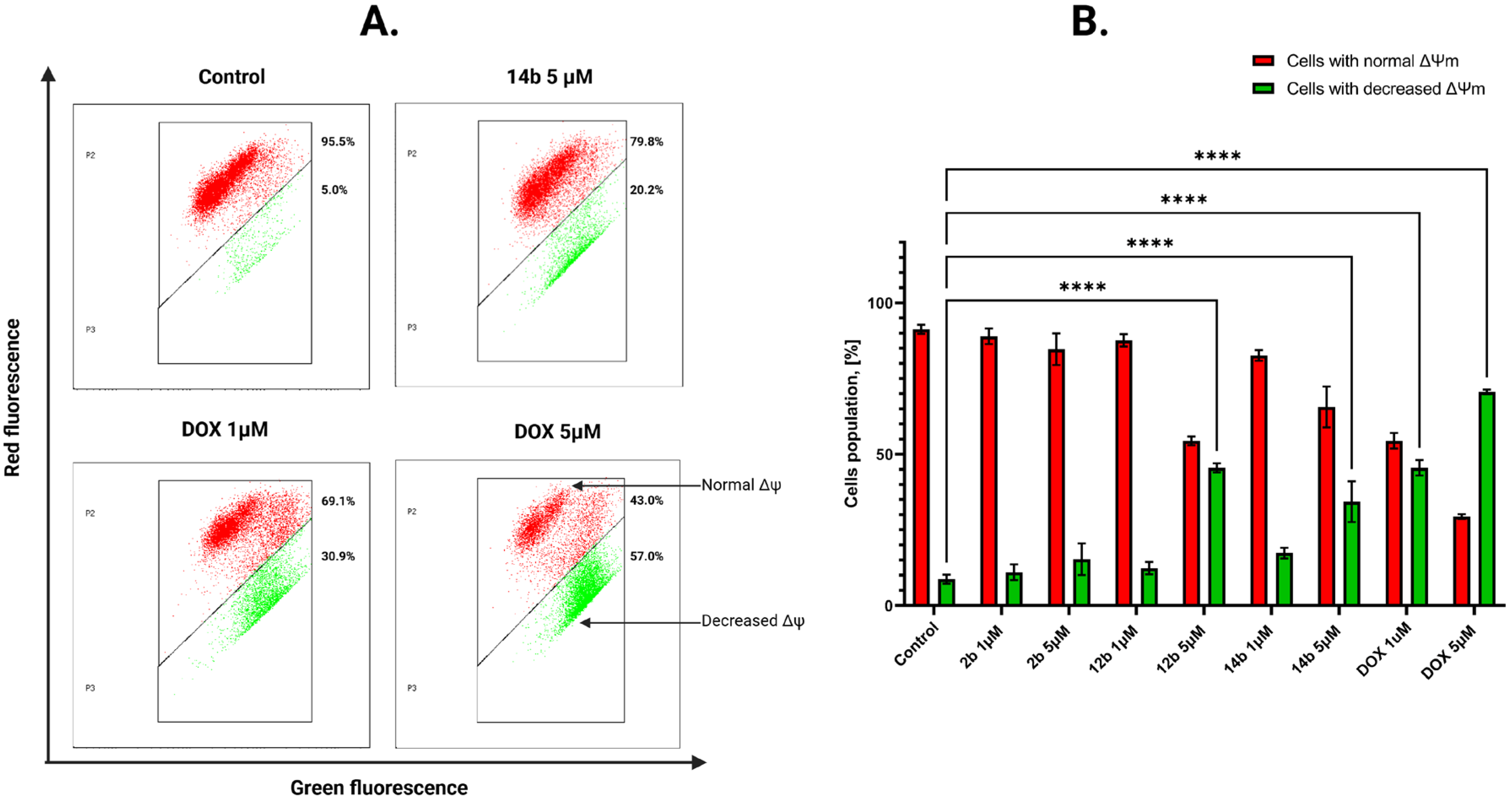
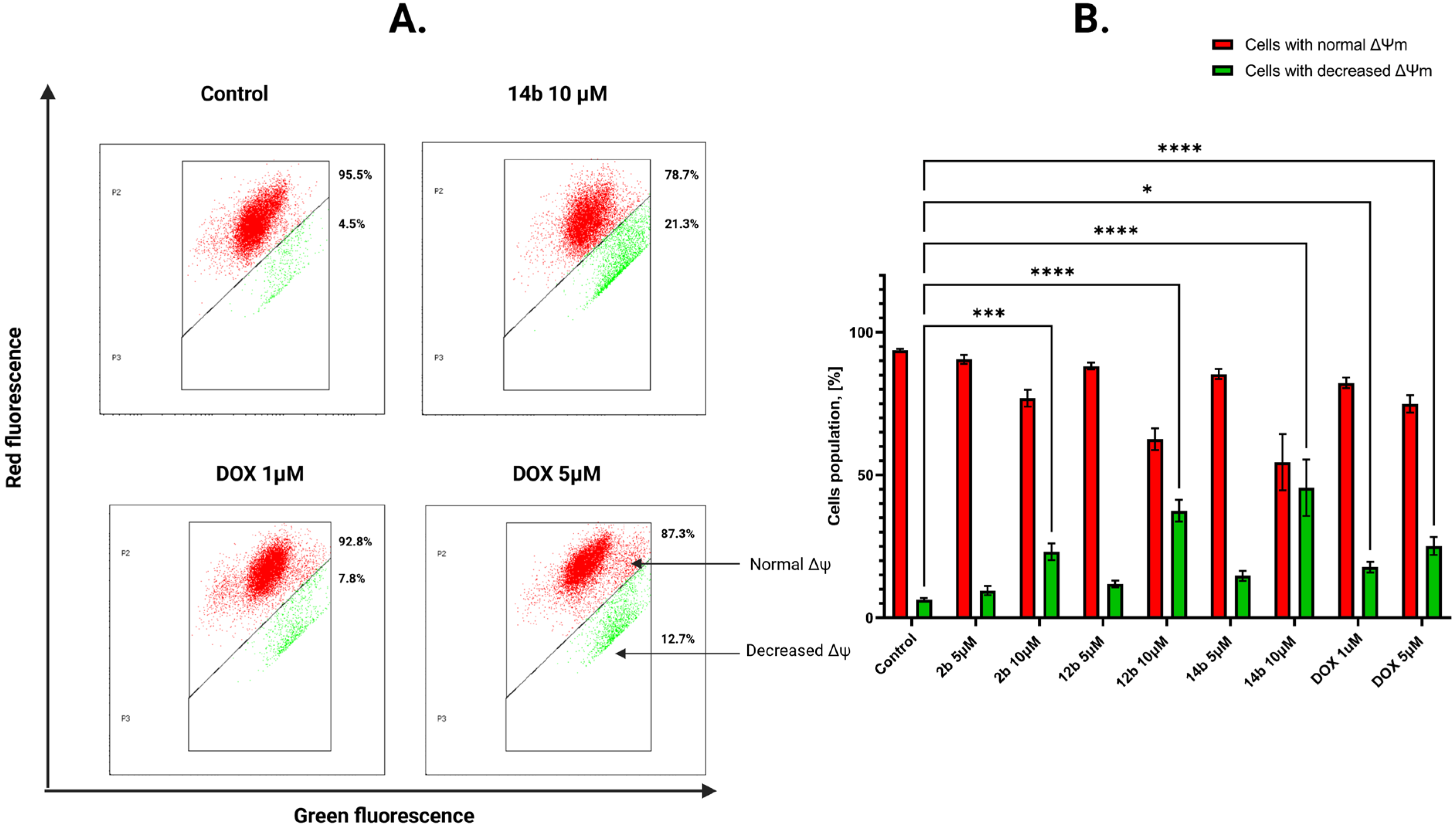
2.2.5. Activation of the Intrinsic Mitochondrial Pathway by the Tested Compounds
3. Discussion
4. Materials and Methods
4.1. Chemistry
- 11-(4-Oxo-2-thioxothiazolidin-3-yl)undecanoic acid (15a)
- 2-((Z)-5-((E)-3-(5-Nitrofuran-2-yl)allylidene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (1b)
- 2-((Z)-5-((E)-3-(5-Nitrofuran-2-yl)allylidene)-4-oxo-2-thioxothiazolidin-3-yl)propanoic acid (2b)
- 2-((Z)-5-((E)-3-(5-Nitrofuran-2-yl)allylidene)-4-oxo-2-thioxothiazolidin-3-yl)butanoic acid (3b)
- 2-((Z)-5-((E)-3-(5-Nitrofuran-2-yl)allylidene)-4-oxo-2-thioxothiazolidin-3-yl)pentanoic acid (4b)
- 3-Methyl-2-((Z)-5-((E)-3-(5-nitrofuran-2-yl)allylidene)-4-oxo-2-thioxothiazolidin-3-yl)butanoic acid (5b)
- 2-((Z)-5-((E)-3-(5-nitrofuran-2-yl)allylidene)-4-oxo-2-thioxothiazolidin-3-yl)hexanoic acid (6b)
- 4-Methyl-2-((Z)-5-((E)-3-(5-nitrofuran-2-yl)allylidene)-4-oxo-2-thioxothiazolidin-3-yl)pentanoic acid (7b)
- 3-Methyl-2-((Z)-5-((E)-3-(5-nitrofuran-2-yl)allylidene)-4-oxo-2-thioxothiazolidin-3-yl)pentanoic acid (8b)
- 2-((Z)-5-((E)-3-(5-Nitrofuran-2-yl)allylidene)-4-oxo-2-thioxothiazolidin-3-yl)-2-phenylacetic acid (9b)
- 2-((Z)-5-((E)-3-(5-Nitrofuran-2-yl)allylidene)-4-oxo-2-thioxothiazolidin-3-yl)-3-phenylpropanoic acid (10b)
- 3-(4-Hydroxyphenyl)-2-((Z)-5-((E)-3-(5-nitrofuran-2-yl)allylidene)-4-oxo-2-thioxothiazolidin-3-yl)propanoic acid (11b)
- 3-((Z)-5-((E)-3-(5-Nitrofuran-2-yl)allylidene)-4-oxo-2-thioxothiazolidin-3-yl)propanoic acid (12b)
- 4-((Z)-5-((E)-3-(5-Nitrofuran-2-yl)allylidene)-4-oxo-2-thioxothiazolidin-3-yl)butanoic acid (13b)
- 6-((Z)-5-((E)-3-(5-nitrofuran-2-yl)allylidene)-4-oxo-2-thioxothiazolidin-3-yl)hexanoic acid (14b)
- 11-((Z)-5-((E)-3-(5-nitrofuran-2-yl)allylidene)-4-oxo-2-thioxothiazolidin-3-yl)undecanoic acid (15b)
4.2. NCI Anticancer Screening In Vitro
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. [3H]-Thymidine Incorporation Assay
4.6. Flow Cytometry Assessment of Annexin V Binding
4.7. Mitochondrial Membrane Potential (ΔΨm) Analysis
4.8. Caspase-9 Activity Assessment
4.9. Determination of Cytochrome C and Caspase-3 Concentrations
4.10. Bax Concentration Assessment
4.11. Reactive Oxygen Species Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphopshate |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| DCF | 2′,7′-Dichlorofluorescein |
| DOX | Doxorubicin hydrochloride |
| DTP | Developmental Therapeutic Program |
| ELISA | Enzyme-linked immunosorbent assay |
| FBS | Fetal bovine serum |
| FDA | The Food and Drug Administration |
| GI50 | Molar concentration of the compound that inhibits 50% net cell growth |
| IC50 | Half maximal inhibitory concentration |
| LC50 | Molar concentration of the compound that inhibits 50% net cell growth |
| MTT | (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay |
| MP | Melting point |
| NCI | National Cancer Institute |
| PBS | Phosphate-buffered saline |
| PI | Propidium iodide |
| ROS | Reactive oxygen species |
| SAR | Structure-activity relationship analysis |
| SDS | Sodium dodecyl sulfate |
| SRB | Sulforhodamine B |
| TGI | Total growth inhibition |
References
- Roszczenko, P.; Holota, S.; Szewczyk, O.K.; Dudchak, R.; Bielawski, K.; Bielawska, A.; Lesyk, R. 4-Thiazolidinone-Bearing Hybrid Molecules in Anticancer Drug Design. Int. J. Mol. Sci. 2022, 23, 13135. [Google Scholar] [CrossRef]
- Buzun, K.; Kryshchyshyn-Dylevych, A.; Senkiv, J.; Roman, O.; Gzella, A.; Bielawski, K.; Bielawska, A.; Lesyk, R. Synthesis and Anticancer Activity Evaluation of 5-[2-Chloro-3-(4-nitrophenyl)-2-propenylidene]-4-thiazolidinones. Molecules 2021, 26, 3057. [Google Scholar] [CrossRef]
- Szlachcikowska, D.; Tabęcka-Łonczyńska, A.; Holota, S.; Roman, O.; Shepeta, Y.; Lesyk, R.; Szychowski, K.A. Role of Ciminalum-4-Thiazolidinone Hybrids in Molecular NF-ΚB Dependent Pathways. Int. J. Mol. Sci. 2024, 25, 7329. [Google Scholar] [CrossRef] [PubMed]
- Buzun, K.; Gornowicz, A.; Lesyk, R.; Kryshchyshyn-Dylevych, A.; Gzella, A.; Czarnomysy, R.; Latacz, G.; Olejarz-Maciej, A.; Handzlik, J.; Bielawski, K.; et al. 2-{5-[(Z,2Z)-2-Chloro-3-(4-nitrophenyl)-2-propenylidene]-4-oxo-2-thioxothiazolidin-3-yl}-3-methylbutanoic Acid as a Potential Anti-Breast Cancer Molecule. Int. J. Mol. Sci. 2022, 23, 4091. [Google Scholar] [CrossRef] [PubMed]
- Finiuk, N.; Kryshchyshyn-Dylevych, A.; Holota, S.; Klyuchivska, O.; Kozytskiy, A.; Karpenko, O.; Manko, N.; Ivasechko, I.; Stoika, R.; Lesyk, R. Novel Hybrid Pyrrolidinedione-Thiazolidinones as Potential Anticancer Agents: Synthesis and Biological Evaluation. Eur. J. Med. Chem. 2022, 238, 114422. [Google Scholar] [CrossRef] [PubMed]
- Finiuk, N.; Kaleniuk, E.; Holota, S.; Stoika, R.; Lesyk, R.; Szychowski, K.A. Pyrrolidinedione-Thiazolidinone Hybrid Molecules with Potent Cytotoxic Effect in Squamous Cell Carcinoma SCC-15 Cells. Bioorg. Med. Chem. 2023, 92, 117442. [Google Scholar] [CrossRef]
- Finiuk, N.; Kozak, Y.; Gornowicz, A.; Czarnomysy, R.; Tynecka, M.; Holota, S.; Moniuszko, M.; Stoika, R.; Lesyk, R.; Bielawski, K.; et al. The Proapoptotic Action of Pyrrolidinedione–Thiazolidinone Hybrids towards Human Breast Carcinoma Cells Does Not Depend on Their Genotype. Cancers 2024, 16, 2924. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Tzeng, C.-C.; Chiu, C.-C.; Hsu, C.-Y.; Chou, C.-K.; Chen, Y.-L. Discovery of 2-[2-(5-Nitrofuran-2-yl)vinyl]quinoline Derivatives as a Novel Type of Antimetastatic Agents. Bioorg. Med. Chem. 2015, 23, 141–148. [Google Scholar] [CrossRef]
- Nelson, E.A.; Walker, S.R.; Kepich, A.; Gashin, L.B.; Hideshima, T.; Ikeda, H.; Chauhan, D.; Anderson, K.C.; Frank, D.A. Nifuroxazide Inhibits Survival of Multiple Myeloma Cells by Directly Inhibiting STAT3. Blood 2008, 112, 5095–5102. [Google Scholar] [CrossRef]
- Ye, T.-H.; Yang, F.-F.; Zhu, Y.-X.; Li, Y.-L.; Lei, Q.; Song, X.-J.; Xia, Y.; Xiong, Y.; Zhang, L.-D.; Wang, N.-Y.; et al. Inhibition of Stat3 Signaling Pathway by Nifuroxazide Improves Antitumor Immunity and Impairs Colorectal Carcinoma Metastasis. Cell Death Dis. 2017, 8, e2534. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, L.; Qiu, J.J.; Sun, X.; Li, S.; Wang, X.; So, C.W.E.; Dong, S. A Novel Application of Furazolidone: Anti-Leukemic Activity in Acute Myeloid Leukemia. PLoS ONE 2013, 8, e72335. [Google Scholar] [CrossRef]
- Elzahhar, P.A.; Nematalla, H.A.; Al-Koussa, H.; Abrahamian, C.; El-Yazbi, A.F.; Bodgi, L.; Bou-Gharios, J.; Azzi, J.; Al Choboq, J.; Labib, H.F.; et al. Inclusion of Nitrofurantoin into the Realm of Cancer Chemotherapy via Biology-Oriented Synthesis and Drug Repurposing. J. Med. Chem. 2023, 66, 4565–4587. [Google Scholar] [CrossRef]
- Fiebiger, E.; Hirsch, C.; Vyas, J.M.; Gordon, E.; Ploegh, H.L.; Tortorella, D. Dissection of the Dislocation Pathway for Type I Membrane Proteins with a New Small Molecule Inhibitor, Eeyarestatin. Mol. Biol. Cell. 2004, 15, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, G.; Martinotti, S.; Ranzato, E. Endoplasmic Reticulum Stress and Cancer: Could Unfolded Protein Response Be a Druggable Target for Cancer Therapy? Int. J. Mol. Sci. 2023, 24, 1566. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Zhang, T.; Xie, J.; Williams, J.; Ye, Y.; Chen, L. Eeyarestatin I Derivatives with Improved Aqueous Solubility. Bioorg. Med. Chem. Lett. 2016, 26, 5177–5181. [Google Scholar] [CrossRef]
- Gamayun, I.; O’Keefe, S.; Pick, T.; Klein, M.-C.; Nguyen, D.; McKibbin, C.; Piacenti, M.; Williams, H.M.; Flitsch, S.L.; Whitehead, R.C.; et al. Eeyarestatin Compounds Selectively Enhance Sec61-Mediated Ca2+ Leakage from the Endoplasmic Reticulum. Cell Chem. Biol. 2019, 26, 571–583.e6. [Google Scholar] [CrossRef]
- Peng, J.; Wei, C.-I.; Lee, S.-H. Eeyarestatin I (ESI)-Induced ERAD Inhibition Exhibits Anti-Cancer Activity through Multiple Mechanisms in Human Colorectal Cancer Cells. Eur. J. Pharmacol. 2025, 997, 177623. [Google Scholar] [CrossRef]
- Guillon, C.; Vetrano, A.M.; Saxena, J.; Hunter, A.; Verderone, G.; Finetti, T.M.; Wisnoski, J.; DeMatteo, P.W.; Rapp, R.D.; Heindel, N.D.; et al. Derivatives of 1,2,4-Triazole Imines Acting as Dual INOS and Tumor Cell Growth Inhibitors. Bioorg. Chem. 2020, 103, 104128. [Google Scholar] [CrossRef]
- Grant, E.B.; Guiadeen, D.; Baum, E.Z.; Foleno, B.D.; Jin, H.; Montenegro, D.A.; Nelson, E.A.; Bush, K.; Hlasta, D.J. The Synthesis and SAR of Rhodanines as Novel Class C β-Lactamase Inhibitors. Bioorganic. Med. Chem. Lett. 2000, 10, 2179–2182. [Google Scholar] [CrossRef]
- Aguirre, G.; Boiani, M.; Cabrera, E.; Cerecetto, H.; Di Maio, R.; González, M.; Denicola, A.; Sant’Anna, C.M.R.; Barreiro, E.J. New Potent 5-Nitrofuryl Derivatives as Inhibitors of Trypanosoma Cruzi Growth. 3D-QSAR (CoMFA) Studies. Eur. J. Med. Chem. 2006, 41, 457–466. [Google Scholar] [CrossRef]
- Rodrigues, A.P.C.; Costa, L.M.M.; Santos, B.L.R.; Maia, R.C.; Miranda, A.L.P.; Barreiro, E.J.; Fraga, C.A.M. Novel Furfurylidene N-Acylhydrazones Derived from Natural Safrole: Discovery of LASSBio-1215, a New Potent Antiplatelet Prototype. J. Enzym. Inhib. Med. Chem. 2012, 27, 101–109. [Google Scholar] [CrossRef]
- Boyd, M.R. The NCI In Vitro Anticancer Drug Discovery Screen. In Anticancer Drug Development Guide; Humana Press: Totowa, NJ, USA, 1997; pp. 23–42. [Google Scholar]
- Boyd, M.R.; Paull, K.D. Some Practical Considerations and Applications of the National Cancer Institute in Vitro Anticancer Drug Discovery Screen. Drug Dev. Res. 1995, 34, 91–109. [Google Scholar] [CrossRef]
- Shoemaker, R.H. The NCI60 Human Tumour Cell Line Anticancer Drug Screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; He, G.; Yan, S.; Chen, C.; Song, L.; Rosol, T.J.; Deng, X. Triple-Negative Breast Cancer: Is There a Treatment on the Horizon? Oncotarget 2017, 8, 1913–1924. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, B.; Fichtner, I.; Killian, P.H.; Kronski, E.; Pfeffer, U.; Efferth, T. Development of Resistance towards Artesunate in MDA-MB-231 Human Breast Cancer Cells. PLoS ONE 2011, 6, e20550. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Naggar, M.; Eldehna, W.M.; Almahli, H.; Elgez, A.; Fares, M.; Elaasser, M.M.; Abdel-Aziz, H.A. Novel Thiazolidinone/Thiazolo[3,2-a]benzimidazolone-Isatin Conjugates as Apoptotic Anti-Proliferative Agents Towards Breast Cancer: One-Pot Synthesis and In Vitro Biological Evaluation. Molecules 2018, 23, 1420. [Google Scholar] [CrossRef]
- Sevinc, S.K.; Çıkla-Süzgün, P.; Tiber, P.M.; Güniz Küçükgüzel, Ş.; Orun, O. Biological Activities of Etodolac-Based Hydrazone, Thiazolidinone and Triazole Derivatives on Breast Cancer Cell Lines MCF-7 and MDA-MB-231. J. Biochem. Mol. Toxicol. 2025, 39, e70428. [Google Scholar] [CrossRef]
- Fu, H.; Hou, X.; Wang, L.; Dun, Y.; Yang, X.; Fang, H. Design, Synthesis and Biological Evaluation of 3-Aryl-rhodanine Benzoic Acids as Anti-Apoptotic Protein Bcl-2 Inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 5265–5269. [Google Scholar] [CrossRef]
- Ramesh, V.; Ananda Rao, B.; Sharma, P.; Swarna, B.; Thummuri, D.; Srinivas, K.; Naidu, V.G.M.; Jayathirtha Rao, V. Synthesis and Biological Evaluation of New Rhodanine Analogues Bearing 2-Chloroquinoline and Benzo[h]quinoline Scaffolds as Anticancer Agents. Eur. J. Med. Chem. 2014, 83, 569–580. [Google Scholar] [CrossRef]
- Pilco-Ferreto, N.; Calaf, G.M. Influence of Doxorubicin on Apoptosis and Oxidative Stress in Breast Cancer Cell Lines. Int. J. Oncol. 2016, 49, 753–762. [Google Scholar] [CrossRef]
- Parrish, A.B.; Freel, C.D.; Kornbluth, S. Cellular Mechanisms Controlling Caspase Activation and Function. Cold Spring Harb. Perspect. Biol. 2013, 5, a008672. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, X. Cytochrome C-Mediated Apoptosis. Annu. Rev. Biochem. 2004, 73, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Trayes, K.P.; Cokenakes, S.E.H. Breast Cancer Treatment. Am. Fam. Physician 2021, 104, 171–178. [Google Scholar] [PubMed]
- Burguin, A.; Diorio, C.; Durocher, F. Breast Cancer Treatments: Updates and New Challenges. J. Pers. Med. 2021, 11, 808. [Google Scholar] [CrossRef]
- Kinnel, B.; Singh, S.K.; Oprea-Ilies, G.; Singh, R. Targeted Therapy and Mechanisms of Drug Resistance in Breast Cancer. Cancers 2023, 15, 1320. [Google Scholar] [CrossRef]
- Abouzeid, H.A.; Kassem, L.; Liu, X.; Abuelhana, A. Paclitaxel Resistance in Breast Cancer: Current Challenges and Recent Advanced Therapeutic Strategies. Cancer Treat. Res. Commun. 2025, 43, 100918. [Google Scholar] [CrossRef]
- Yde, C.; Issinger, O.-G. Enhancing Cisplatin Sensitivity in MCF-7 Human Breast Cancer Cells by down-Regulation of Bcl-2 and Cyclin D1. Int. J. Oncol. 2006, 29, 1397–1404. [Google Scholar] [CrossRef]
- Wu, C.-C.; Bratton, S.B. Regulation of the Intrinsic Apoptosis Pathway by Reactive Oxygen Species. Antioxid. Redox Signal. 2013, 19, 546–558. [Google Scholar] [CrossRef]
- Bratton, S.B.; Lewis, J.; Butterworth, M.; Duckett, C.S.; Cohen, G.M. XIAP Inhibition of Caspase-3 Preserves Its Association with the Apaf-1 Apoptosome and Prevents CD95- and Bax-Induced Apoptosis. Cell Death Differ. 2002, 9, 881–892. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial Membrane Potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of Cytochrome c Release from Mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef]
- Griffiths, M.; Sundaram, H. Drug Design and Testing: Profiling of Antiproliferative Agents for Cancer Therapy Using a Cell-Based Methyl-[3H]-thymidine Incorporation Assay. In Cancer Cell Culture: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2011; pp. 451–465. [Google Scholar]
- Bernardo, P.H.; Sivaraman, T.; Wan, K.-F.; Xu, J.; Krishnamoorthy, J.; Song, C.M.; Tian, L.; Chin, J.S.F.; Lim, D.S.W.; Mok, H.Y.K.; et al. Synthesis of a Rhodanine-Based Compound Library Targeting Bcl-XL and Mcl-1. Pure Appl. Chem. 2011, 83, 723–731. [Google Scholar] [CrossRef][Green Version]
- Slepikas, L.; Chiriano, G.; Perozzo, R.; Tardy, S.; Kranjc, A.; Patthey-Vuadens, O.; Ouertatani-Sakouhi, H.; Kicka, S.; Harrison, C.F.; Scrignari, T.; et al. In Silico Driven Design and Synthesis of Rhodanine Derivatives as Novel Antibacterials Targeting the Enoyl Reductase InhA. J. Med. Chem. 2016, 59, 10917–10928. [Google Scholar] [CrossRef] [PubMed]
- Bursavich, M.G.; Gilbert, A.M.; Lombardi, S.; Georgiadis, K.E.; Reifenberg, E.; Flannery, C.R.; Morris, E.A. Synthesis and Evaluation of Aryl Thioxothiazolidinone Inhibitors of ADAMTS-5 (Aggrecanase-2). Bioorg. Med. Chem. Lett. 2007, 17, 1185–1188. [Google Scholar] [CrossRef] [PubMed]
- Prashantha Kumar, B.R.; Baig, N.R.; Sudhir, S.; Kar, K.; Kiranmai, M.; Pankaj, M.; Joghee, N.M. Discovery of Novel Glitazones Incorporated with Phenylalanine and Tyrosine: Synthesis, Antidiabetic Activity and Structure–Activity Relationships. Bioorg.Chem. 2012, 45, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Prashantha Kumar, B.R.; Basu, P.; Adhikary, L.; Nanjan, M.J. Efficient Conversion of N -Terminal of L-Tyrosine, DL-Phenyl Alanine, and Glycine to Substituted 2-Thioxo-thiazolidine-4-ones: A Stereospecific Synthesis. Synth. Commun. 2012, 42, 3089–3096. [Google Scholar] [CrossRef]
- Tejchman, W.; Korona-Glowniak, I.; Malm, A.; Zylewski, M.; Suder, P. Antibacterial Properties of 5-Substituted Derivatives of Rhodanine-3-carboxyalkyl Acids. Med. Chem. Res. 2017, 26, 1316–1324. [Google Scholar] [CrossRef]


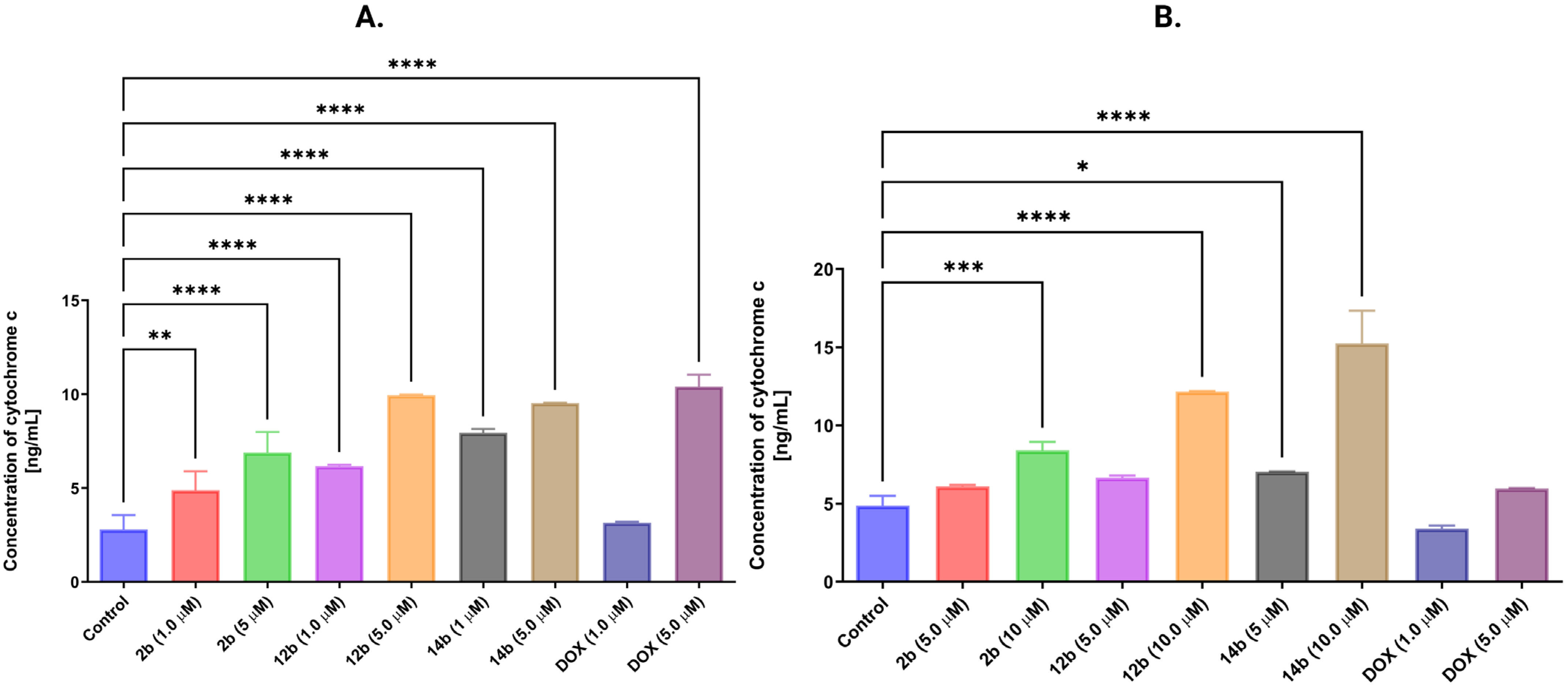

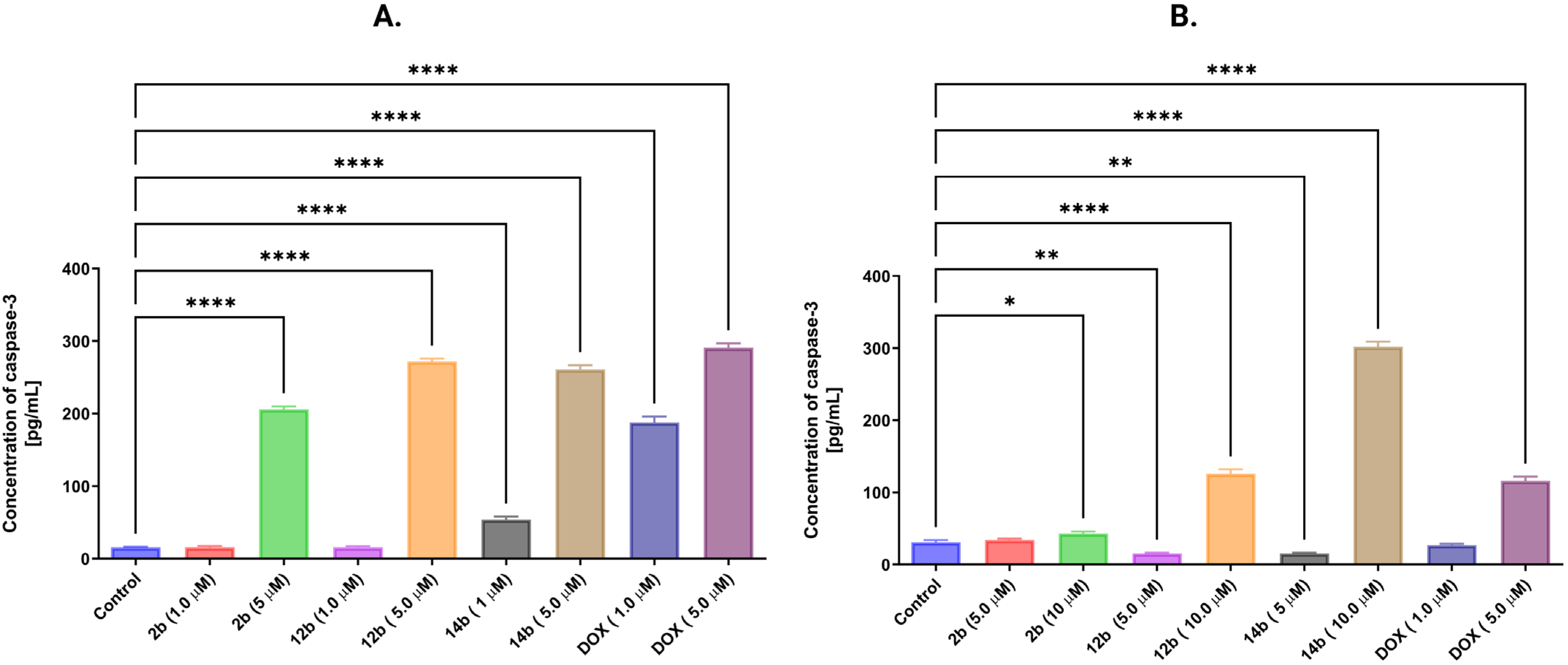

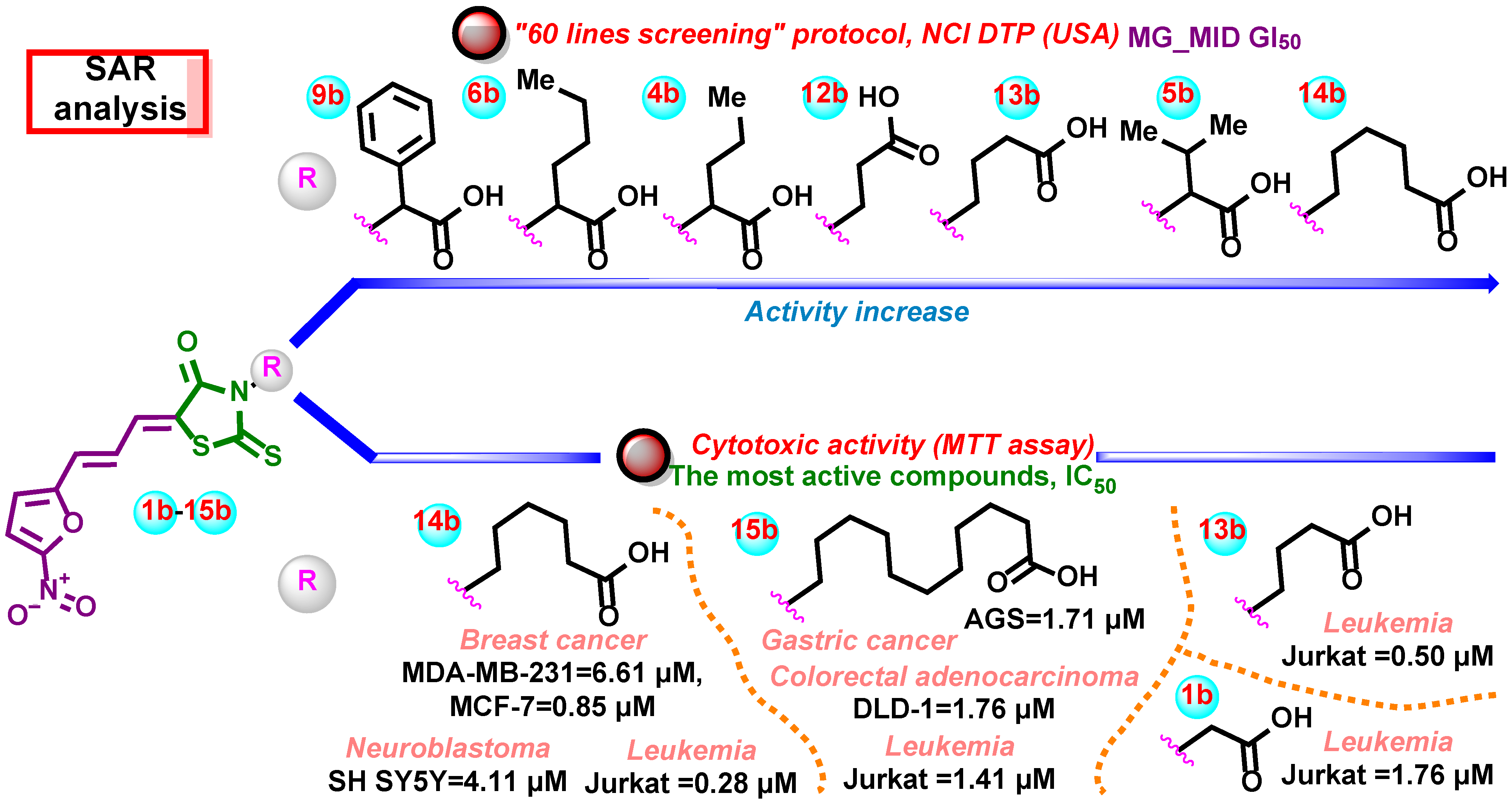
| Comp./ Cell Lines | 1b | 2b | 3b | 4b | 5b | 6b | 7b | 8b | 9b | 10b | 11b | 12b | 13b | 14b | 15b | DOX |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breast cancer | ||||||||||||||||
| MCF-7 | >40 | 11.32 | 7.74 | 5.87 | 3.32 | 6.39 | 7.13 | 2.38 | 14.1 | 9.23 | 33.13 | 3.01 | 1.63 | 0.85 | 4.05 | 0.73 |
| MDA-MB-231 | >40 | 25.36 | 18.51 | 17.46 | 9.12 | 10.35 | 16.7 | 8.34 | 36.72 | 22.9 | >40 | 12.91 | 8.73 | 6.61 | >40 | 7.53 |
| HCC-1954 | 25.31 | 12.54 | 17.57 | 7.07 | 6.42 | 4.18 | 6.28 | 4.9 | 20.57 | 19.13 | >40 | 4.86 | 3.12 | 3.3 | 6.23 | 3.14 |
| Gastric cancer | ||||||||||||||||
| AGS | 36.23 | 12.05 | 6.53 | 6.54 | 2.65 | 3.99 | 5.55 | 3.38 | 12.93 | 8.57 | 30.56 | 3.65 | 2.1 | 2.57 | 1.71 | 1.09 |
| Colorectal cancer | ||||||||||||||||
| DLD-1 | 28.77 | 9.98 | 8.03 | 7.61 | 3.86 | 4.58 | 7.67 | 3.68 | 12.33 | 9.29 | 20.28 | 3.31 | 3.18 | 1.8 | 1.76 | 2.83 |
| HCT-116 | >40 | 38.99 | 14.65 | 7.89 | 4.21 | 17.55 | 7.98 | 4.15 | 11.69 | 13.48 | 36.09 | 3.68 | 3.97 | 2.11 | 10.53 | 16.76 |
| HT-29 | 22.31 | 24.47 | 30.4 | 13.23 | 7.74 | 8.01 | 13.61 | 8.04 | 19.99 | 28.63 | >40 | 8.56 | 4.39 | 4.52 | 9.61 | 0.73 |
| Neuroblastoma cell line | ||||||||||||||||
| SH SY5Y | >40 | 30.63 | 28.54 | 16.32 | 9.37 | 8.39 | 15.55 | 6.6 | 20.11 | 18.89 | >40 | 13.13 | 8.41 | 4.11 | 3.76 | 39.92 |
| Leukemia | ||||||||||||||||
| K562 | >40 | >40 | 25.80 | 16.12 | 16.14 | 36.18 | 18.51 | 7.35 | 37.56 | 25.5 | >40 | 7.15 | 3.57 | 1.53 | 15.54 | 0.43 |
| Jurkat | 1.76 | 4.78 | 14.15 | 5.85 | 3.73 | 13.94 | 7.72 | 3.19 | 2.51 | 6.7 | 17.09 | 1.77 | 0.5 | 0.28 | 1.41 | 0.72 |
| RPMI 8866 | >40 | 28.99 | 35.18 | 19.57 | 13.52 | 15.23 | 16.81 | 14.15 | 5.54 | 16.46 | 27.76 | 7.78 | 3.94 | 3.13 | 10.95 | 0.38 |
| Comp./Cell Lines | 1b | 2b | 3b | 4b | 5b | 6b | 7b | 8b | 9b | 10b | 11b | 12b | 13b | 14b | 15b | DOX |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breast Cancer | ||||||||||||||||
| MCF-7 | >10 | 6.61 | 7.79 | 6.13 | 1.93 | 5.81 | 6.69 | 2.21 | 7.98 | 7.87 | >10 | 6.66 | 3.97 | 2.6 | 3.13 | 1.78 |
| MDA-MB-231 | >30 | 5.56 | 2.65 | 3.66 | 6.01 | 2.01 | 2.19 | 1.84 | 7.53 | 5.99 | >30 | 1.82 | 1.77 | 1.6 | >30 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podolak, M.; Horishny, V.; Dudchak, R.; Gornowicz, A.; Czarnomysy, R.; Mural, D.; Holota, S.; Bielawski, K.; Lesyk, R.; Bielawska, A. Synthesis and Anticancer Activity Evaluation of New 5-((5-Nitrofuran-2-yl)allylidene)-2-thioxo-4-thiazolidinones. Pharmaceuticals 2025, 18, 1598. https://doi.org/10.3390/ph18111598
Podolak M, Horishny V, Dudchak R, Gornowicz A, Czarnomysy R, Mural D, Holota S, Bielawski K, Lesyk R, Bielawska A. Synthesis and Anticancer Activity Evaluation of New 5-((5-Nitrofuran-2-yl)allylidene)-2-thioxo-4-thiazolidinones. Pharmaceuticals. 2025; 18(11):1598. https://doi.org/10.3390/ph18111598
Chicago/Turabian StylePodolak, Magdalena, Volodymyr Horishny, Rostyslav Dudchak, Agnieszka Gornowicz, Robert Czarnomysy, Dmytro Mural, Serhii Holota, Krzysztof Bielawski, Roman Lesyk, and Anna Bielawska. 2025. "Synthesis and Anticancer Activity Evaluation of New 5-((5-Nitrofuran-2-yl)allylidene)-2-thioxo-4-thiazolidinones" Pharmaceuticals 18, no. 11: 1598. https://doi.org/10.3390/ph18111598
APA StylePodolak, M., Horishny, V., Dudchak, R., Gornowicz, A., Czarnomysy, R., Mural, D., Holota, S., Bielawski, K., Lesyk, R., & Bielawska, A. (2025). Synthesis and Anticancer Activity Evaluation of New 5-((5-Nitrofuran-2-yl)allylidene)-2-thioxo-4-thiazolidinones. Pharmaceuticals, 18(11), 1598. https://doi.org/10.3390/ph18111598









