Inhibitory Effects of Syringic Acid on Endometrial Cancer Cell Growth and Migration and Its Synergistic Suppression with Doxorubicin
Abstract
1. Introduction
2. Results
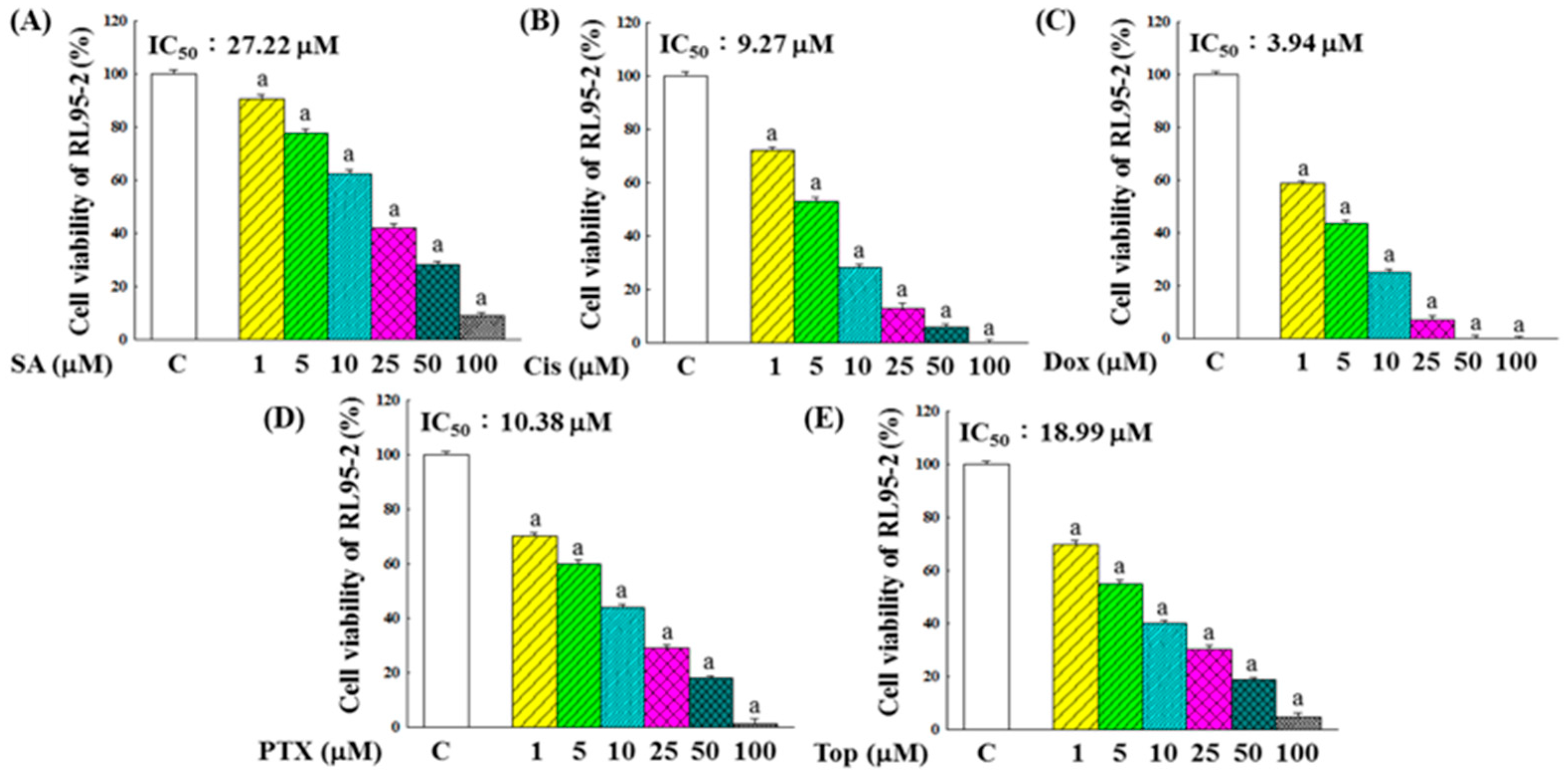
2.1. Inhibitory Effects on the Growth of RL95-2 Cells
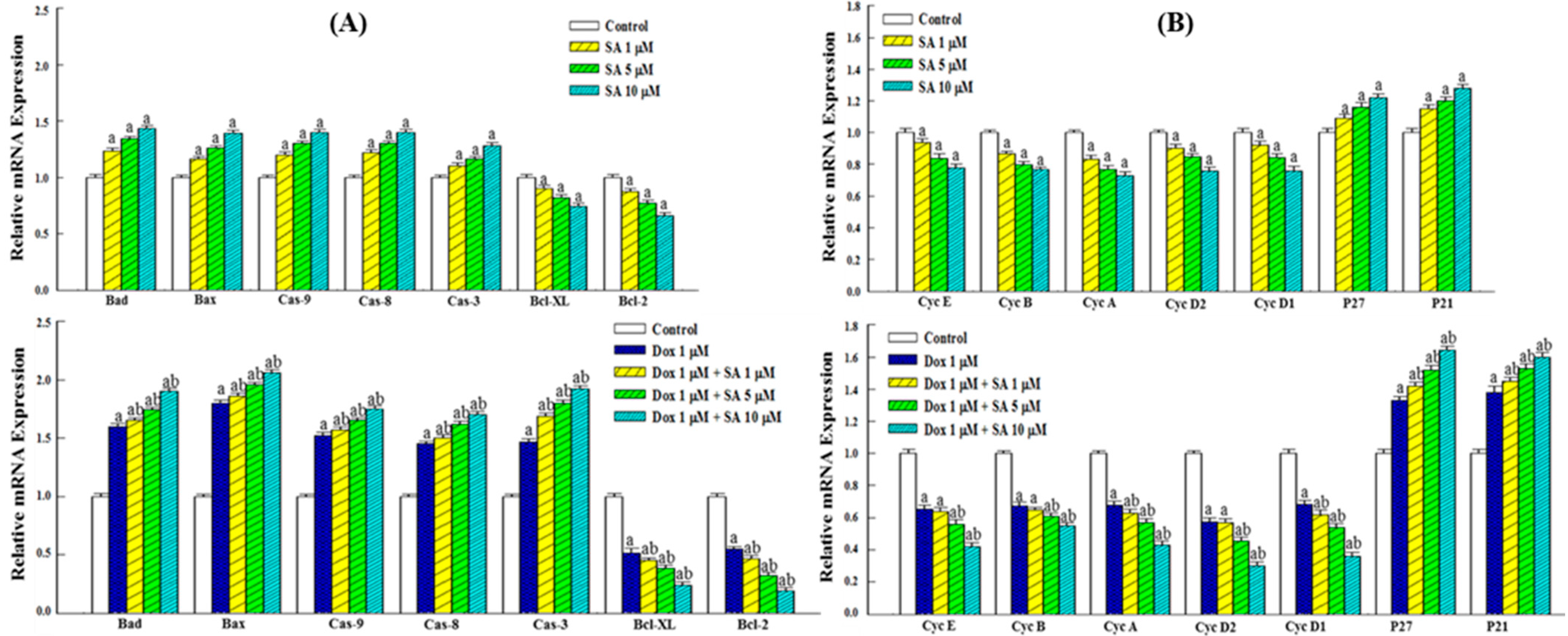
2.2. Synergistic Effects of SA Combined with Anticancer Chemotherapeutic Drugs on the Inhibition of RL95-2 Cell Growth
2.3. Expression of Genes Related to Apoptosis and the Cell Cycle
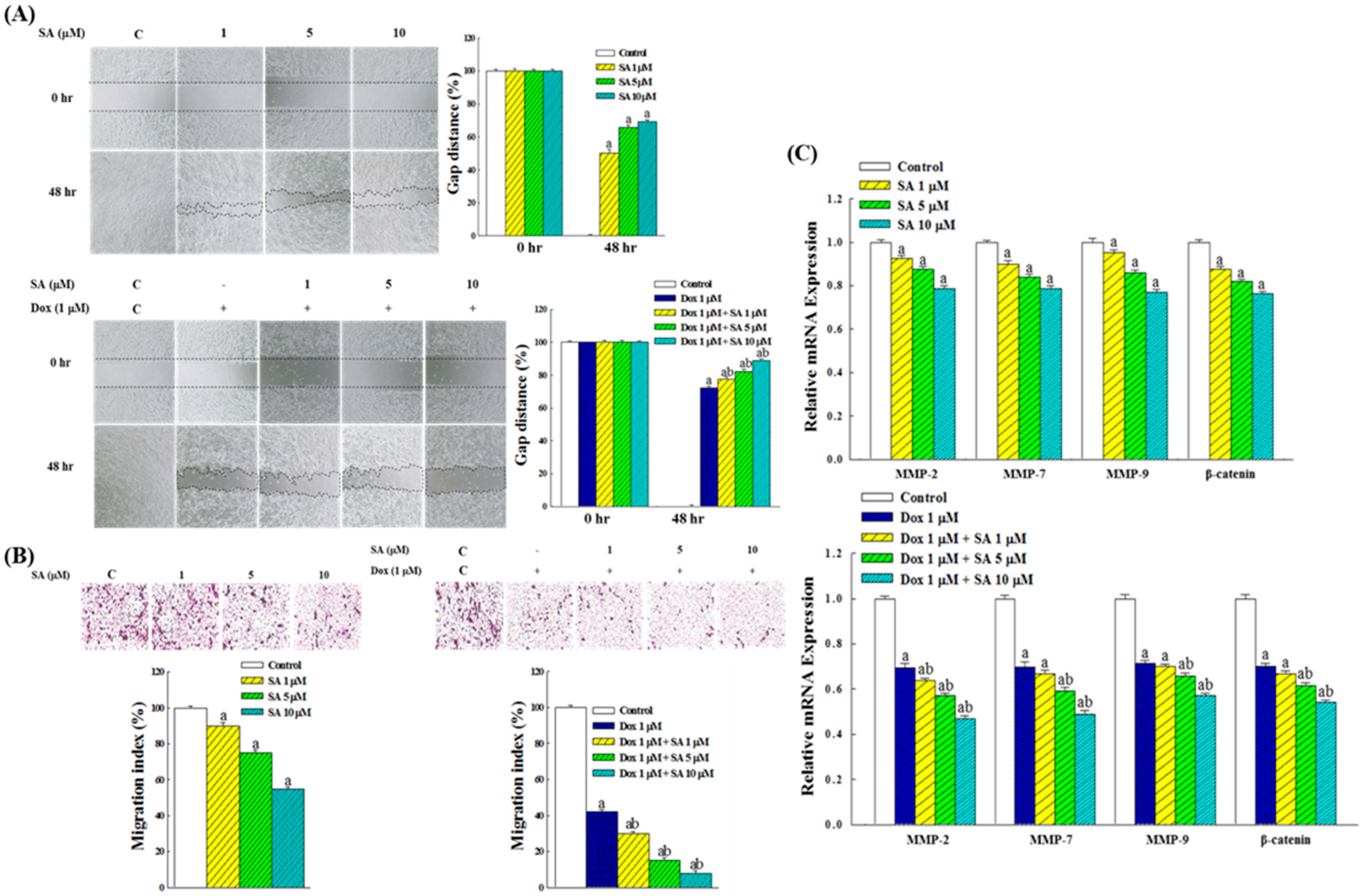
2.4. Inhibitory Effects on RL95-2 Cell Migration
2.5. Expression of Genes Related to Migration in RL95-2 Cells
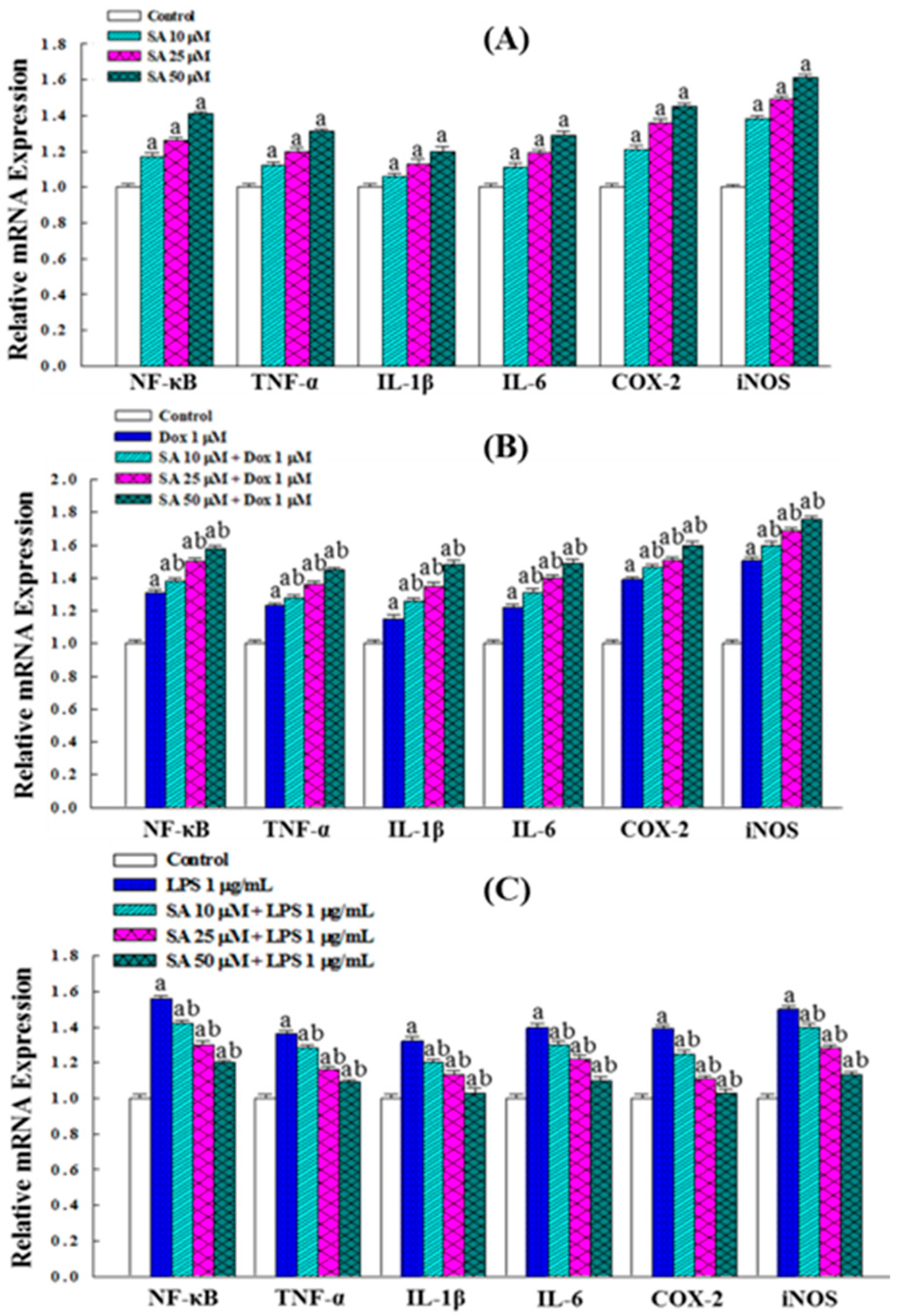
2.6. Inflammatory Responses in RL95-2 Cells Under SA and Dox Treatments
2.7. SA Exhibits Anti-Inflammatory and Cytoprotective Effects in HESC Normal Cells
2.8. Effects on the Expression of Inflammation-Related Genes
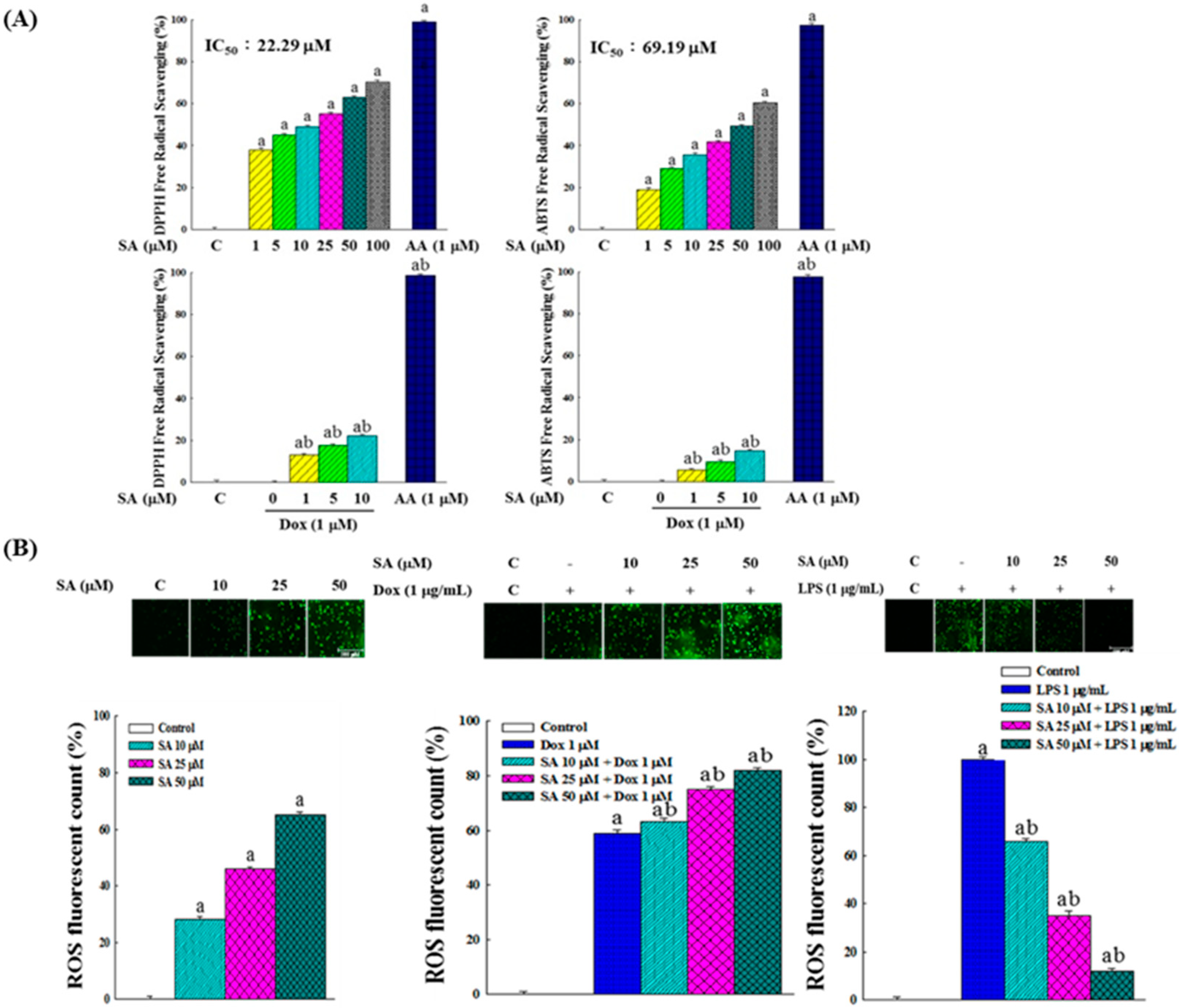
2.9. In Vitro Free Radical Scavenging Activity and Intracellular ROS Production
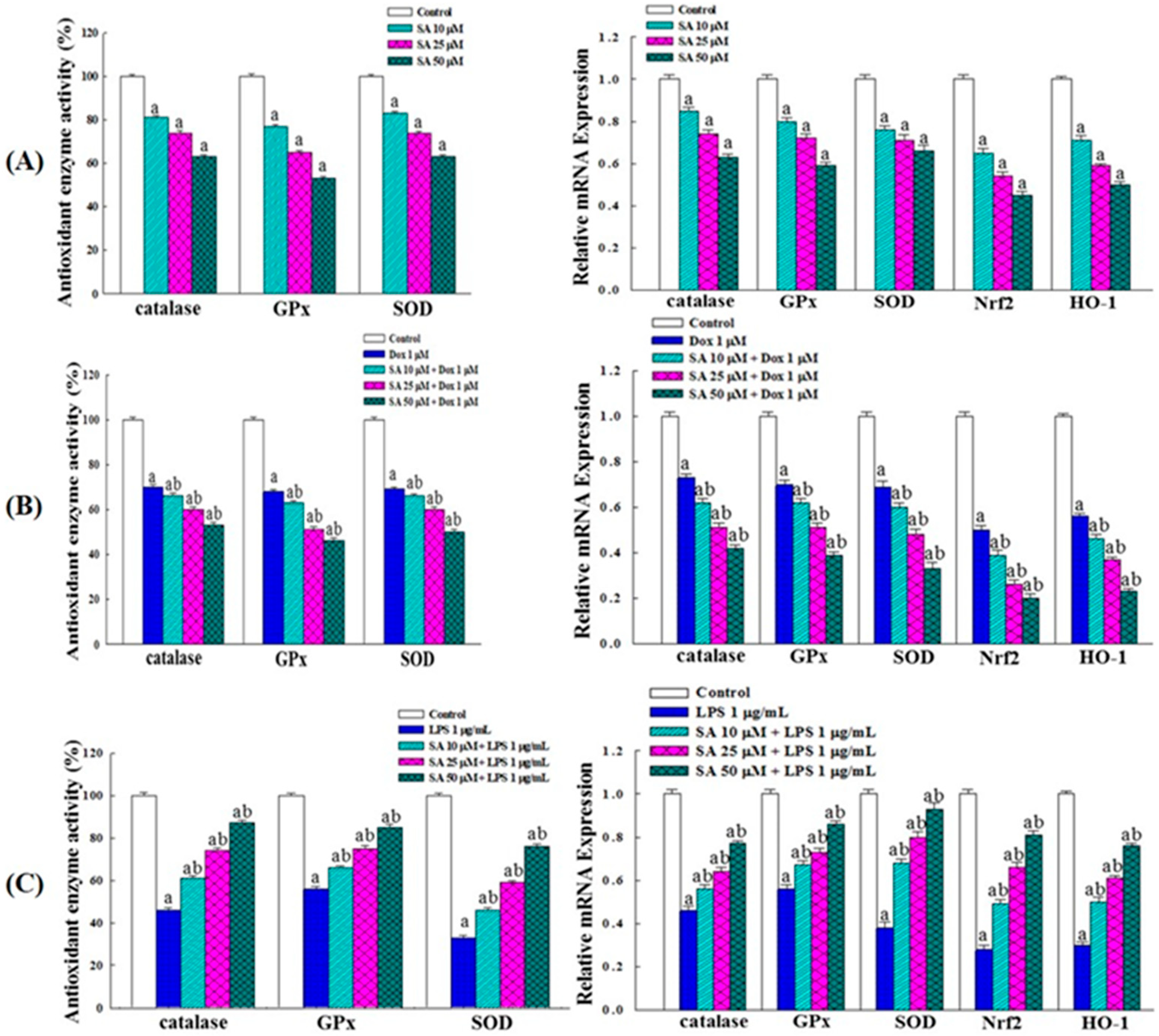
2.10. Effects on Intracellular Antioxidant Enzyme Activity and Gene Expression
3. Discussion
4. Materials and Methods
4.1. Cell Cultivation
4.2. Nuclear Fluorescence Staining
4.3. Cell Viability Assay
4.4. Clonogenic Survival Assay
4.5. Wound Healing Assay
4.6. Transwell Migration Assay
4.7. Intracellular NO Analysis
4.8. Intracellular Cytokine Analysis
4.9. Free Radical Scavenging Capacity Assay
4.10. Intracellular ROS Analysis
4.11. Antioxidant Enzyme Activity Assay
4.12. Gene Expression Assay
4.13. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| ANOVA | One-way analysis of variance |
| cDNA | Complementary DNA |
| Cis | Cisplatin |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| Dox | Doxorubicin |
| EC | Endometrial cancer |
| ECM | Extracellular matrix |
| EMT | Epithelial–mesenchymal transition |
| ER | Endoplasmic reticulum |
| FBS | Fetal bovine serum |
| GPx | Glutathione peroxidase |
| HESC | Human endometrial stromal cells |
| LPS | Lipopolysaccharide |
| MMPs | Matrix metalloproteinases |
| MTT | (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NO | Nitric oxides |
| PBS | Phosphate-buffered saline |
| PGE2 | Prostaglandin E2 |
| PTX | Paclitaxel |
| ROS | Reactive oxygen species |
| RT-PCR | Real-time polymerase chain reaction |
| SA | Syringic acid |
| SD | Standard deviation |
| SOD | Superoxide dismutase |
| TME | Tumor microenvironment |
| Top | Topotecan |
References
- Zaim, S.N.M.; Nafi, S.N.M.; Zawawi, N.; Adnan, W.F.W. Clinical and histopathological evaluation of patients with endometrial cancer in a University Hospital: Seven-year experience. Malays. J. Pathol. 2024, 46, 413–421. [Google Scholar] [PubMed]
- Raglan, O.; Kalliala, I.; Markozannes, G.; Cividini, S.; Gunter, M.J.; Nautiyal, J.; Gabra, H.; Paraskevaidis, E.; Martin-Hirsch, P.; Tsilidis, K.K.; et al. Risk factors for endometrial cancer: An umbrella review of the literature. Int. J. Cancer 2019, 145, 1719–1730. [Google Scholar] [CrossRef]
- Thrastardottir, T.O.; Copeland, V.J.; Constantinou, C. The association between nutrition, obesity, inflammation, and endometrial cancer: A scoping review. Curr. Nutr. Rep. 2023, 12, 98–121. [Google Scholar] [CrossRef]
- Chaichian, S.; Nikfar, B.; Arbabi Bidgoli, S.; Moazzami, B. The role of quercetin for the treatment of endometriosis and endometrial cancer: A comprehensive review. Curr. Med. Chem. 2025, 32, 74–86. [Google Scholar] [CrossRef]
- Mhandire, D.Z.; Goey, A.K.L. The value of pharmacogenetics to reduce drug-related toxicity in cancer patients. Mol. Diagn. Ther. 2022, 26, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zeng, Y.; Zheng, R.; Wang, Y.; Li, T.; Song, S.; Zhang, S.; Huang, J.; Ren, Y. Natural products modulate cell apoptosis: A promising way for treating endometrial cancer. Front. Pharmacol. 2023, 14, 1209412. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, W.; Liu, S. The key role of natural products in the fight against endometrial cancer. Int. Immunopharmacol. 2025, 151, 114344. [Google Scholar] [CrossRef]
- Singh, R.; Manna, P.P. Reactive oxygen species in cancer progression and its role in therapeutics. Explor. Med. 2022, 3, 43–57. [Google Scholar] [CrossRef]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh Kumar, C. Syringic acid (SA)—A review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef]
- Ogut, E.; Armagan, K.; Gül, Z. The role of syringic acid as a neuroprotective agent for neurodegenerative disorders and future expectations. Metab. Brain Dis. 2022, 37, 859–880. [Google Scholar] [CrossRef] [PubMed]
- Bartel, I.; Mandryk, I.; Horbańczuk, J.O.; Wierzbicka, A.; Koszarska, M. Nutraceutical properties of syringic acid in civilization diseases—Review. Nutrients 2023, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Abaza, M.S.; Al-Attiyah, R.; Bhardwaj, R.; Abbadi, G.; Koyippally, M.; Afzal, M. Syringic acid from Tamarix aucheriana possesses antimitogenic and chemo-sensitizing activities in human colorectal cancer cells. Pharm. Biol. 2013, 51, 1110–1124. [Google Scholar] [CrossRef] [PubMed]
- Mihanfar, A.; Darband, S.G.; Sadighparvar, S.; Kaviani, M.; Mirza-Aghazadeh-Attari, M.; Yousefi, B.; Majidinia, M. In vitro and in vivo anticancer effects of syringic acid on colorectal cancer: Possible mechanistic view. Chem. Biol. Interact. 2021, 337, 109337. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Qiu, F.; Zhu, F.; Qi, L. Therapeutic potential of zinc oxide-loaded syringic acid against in vitro and in vivo model of lung cancer. Int. J. Nanomed. 2020, 15, 8249–8260. [Google Scholar] [CrossRef]
- Abijeth, B.; Ezhilarasan, D. Syringic acid induces apoptosis in human oral squamous carcinoma cells through mitochondrial pathway. J. Oral Maxillofac. Pathol. 2020, 24, 40–45. [Google Scholar] [CrossRef]
- Pei, J.; Velu, P.; Zareian, M.; Feng, Z.; Vijayalakshmi, A. Effects of syringic acid on apoptosis, inflammation, and AKT/mTOR signaling pathway in gastric cancer cells. Front. Nutr. 2021, 14, 788929. [Google Scholar] [CrossRef]
- Yang, L.; Qu, C.; Jin, J.; Yang, H.; Pei, L. Syringic acid regulates suppression of the STAT3/JNK/AKT pathway via inhibition of human ovarian teratoma cancer cell (PA-1) growth—In Vitro study. J. Biochem. Mol. Toxicol. 2021, 35, 1–9. [Google Scholar] [CrossRef]
- Ismail, T.; Kim, Y.; Lee, H.; Lee, D.S.; Lee, H.S. Interplay between mitochondrial peroxiredoxins and ROS in cancer development and progression. Int. J. Mol. Sci. 2019, 20, 4407. [Google Scholar] [CrossRef]
- Margul, D.; Yu, C.; AlHilli, M.M. Tumor immune microenvironment in gynecologic cancers. Cancers 2023, 15, 3849. [Google Scholar] [CrossRef]
- Ray, I.; Michael, A.; Meira, L.B.; Ellis, P.E. The role of cytokines in epithelial-mesenchymal transition in gynaecological cancers: A systematic review. Cells 2023, 12, 416. [Google Scholar] [CrossRef]
- Hong, Y.H.; Weng, L.W.; Chang, C.C.; Hsu, H.F.; Wang, C.P.; Wang, S.W.; Houng, J.Y. Anti-inflammatory effects of Siegesbeckia orientalis ethanol extract in in vitro and in vivo models. BioMed Res. Int. 2014, 2014, 329712. [Google Scholar] [CrossRef]
- Padežnik, T.; Oleksy, A.; Cokan, A.; Takač, I.; Sobočan, M. Changes in the extracellular matrix in endometrial and cervical cancer: A systematic review. Int. J. Mol. Sci. 2023, 24, 5463. [Google Scholar] [CrossRef] [PubMed]
- Mahecha, A.M.; Wang, H. The influence of vascular endothelial growth factor-A and matrix metalloproteinase-2 and -9 in angiogenesis, metastasis, and prognosis of endometrial cancer. Onco. Targets Ther. 2017, 10, 4617–4624. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, J.; Zhuang, J.; Ma, X.; Zheng, N.; Song, Y.; Xia, W. P4HB modulates epithelial-mesenchymal transition and the β-catenin/Snail pathway influencing chemoresistance in liver cancer cells. Oncol. Lett. 2020, 20, 257–265. [Google Scholar] [CrossRef] [PubMed]
- McMellen, A.; Woodruff, E.R.; Corr, B.R.; Bitler, B.G.; Moroney, M.R. Wnt signaling in gynecologic malignancies. Int. J. Mol. Sci. 2020, 21, 4272. [Google Scholar] [CrossRef]
- Tian, Y.; Lai, T.; Li, Z.; Mao, M.; Jin, Y.; Liu, Y.; Guo, R. Role of non-coding RNA intertwined with the Wnt/β-catenin signaling pathway in endometrial cancer (Review). Mol. Med. Rep. 2023, 28, 150. [Google Scholar] [CrossRef]
- Piotrowski, I.; Kulcenty, K.; Suchorska, W. Interplay between inflammation and cancer. Rep. Pract. Oncol. Radiother. 2020, 25, 422–427. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Srivastava, P.; Paladhi, A.; Singh, R.; Srivastava, D.N.; Singh, R.A.; Hira, S.K.; Manna, P.P. Targeting PD-1 in CD8+ T cells with a biomimetic bilirubin-5-fluoro-2-deoxyuridine-bovine serum albumin nanoconstruct for effective chemotherapy against experimental lymphoma. Mol. Pharm. 2021, 18, 2053–2065. [Google Scholar] [CrossRef]
- Pan, F.; Luo, Y.; Wang, L.; Cheng, Y.; Bu, W.; Zhao, X.; Xu, Y.; Chen, L.; Wang, H. Identification of immunogenic cell death-associated subtypes and characterization of the tumor microenvironment in endometrial cancer. J. Gene Med. 2023, 25, e3495. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed]
- Gheena, S.; Ezhilarasan, D. Syringic acid triggers reactive oxygen species-mediated cytotoxicity in HepG2 cells. Hum. Exp. Toxicol. 2019, 38, 694–702. [Google Scholar] [CrossRef]
- Ito-Hagiwara, K.; Hagiwara, J.; Endo, Y.; Becker, L.B.; Hayashida, K. Cardioprotective strategies against doxorubicin-induced cardiotoxicity: A review from standard therapies to emerging mitochondrial transplantation. Biomed. Pharmacother. 2025, 189, 118315. [Google Scholar] [CrossRef]
- Morelli, M.B.; Bongiovanni, C.; Da Pra, S.; Miano, C.; Sacchi, F.; Lauriola, M.; D’Uva, G. Cardiotoxicity of anticancer drugs: Molecular mechanisms and strategies for cardioprotection. Front. Cardiovasc. Med. 2022, 9, 847012. [Google Scholar] [CrossRef] [PubMed]
- Syahputra, R.A.; Harahap, U.; Dalimunthe, A.; Nasution, M.P.; Satria, D. The role of flavonoids as a cardioprotective strategy against doxorubicin-induced cardiotoxicity: A review. Molecules 2022, 27, 1320. [Google Scholar] [CrossRef] [PubMed]




| Gene | Forward (5′−3′) | Reverse (5′−3′) |
|---|---|---|
| GAPDH | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG |
| Bad | CAGTGATCTGCTCCACATTC | TCCAGCTAGGATGATAGGAC |
| Bax | CGGCGAATTGGAGATGAACTGG | CTAGCAAAGTAGAAGAGGGCAACC |
| Caspase-9 | TCAGTGACGTCTGTGTTCAGGAGA | TTGTTGATGATGAGGCAGTAGCCG |
| Caspase-8 | ACAAGGGCATCATCTATGGCTCTGA | CCAGTGAAGTAAGAGGTCAGCTCAT |
| Caspase-3 | GTGGAACTGACGATGATATGGC | CGCAAAGTGACTGGATGAACC |
| Bcl-xL | AACATCCCAGCTTCACATAACCCC | GCGACCCCAGTTTACTCCATCC |
| Bcl-2 | CTGAGTACCTGAACCGGCA | GAGAAATCAAACAGAGGCCG |
| Cyclin E | GGAAGAGGAAGGCAAACG | GCAATAATCCGAGGCTTG |
| Cyclin B | AAGGTGCCTGTGTGTGAACC | GTCAGCCCCATCATCTGCG |
| Cyclin A | AACGATGAGCACGTCCCTAC | CAGCTGGCCTCTTCTGAGTC |
| Cyclin D2 | TGTGGATTGTCTCAAAGCCTG | CAACATCCCGCACGTCTGTA |
| Cyclin D1 | CAGAAGTGCGAAGAGGAGGTC | TCATCTTAGAGGCCACGAACAT |
| p27 | GGTGCCTTCAATTGGGTCTC | GCTTCCTCATCCCTGGACAC |
| p21 | GAGCAGTGCCCGAGTTAAGG | TGGAACAGGTCGGACATCAC |
| MMP-2 | AGAACTTCCGATTATCCCATGATGA | TGACAGGTCCCAGTGTTGGTG |
| MMP-7 | GGCGGAGATGCTCACTTTGAC | AATTCATGGGTGGCAGCAAAC |
| MMP-9 | GCCCTGGAACTCACACGACA | TTGGAAACTCACACGCCAGAAG |
| β-Catenin | ATTGATTCGAAACCTTGCCC | AGCTCCAGTACACCCTTCTA |
| NF-κB | GAAATTCCTGATCCAGACAAAAAC | ATCACTTCAATGGCCTCTGTGTAG |
| TNF-α | CAGGTTCTGTCCCTTTCACTCACT | GTTCAGTAGACAGAAGAGCGTGGT |
| IL-1β | GGTCAAAGGTTTGGAAGCAG | TGTGAAATGCCACCTTTTGA |
| IL-6 | TGGAGTACCATAGCTACCTGGAGT | TCCTTAGCCACTCCTTCTGTGACT |
| COX-2 | CCGGGTACAATCGCACTTAT | GGCGCTCAGCCATACAG |
| iNOS | CTCAGCCCAACAATACAAGATGACCCTAAG | AGAGTGAGCTGGTAGGTTCCTGTTGTTTCT |
| Cat | GCCATTGCCACAGGAAAGTA | CCTTGGTGAGATCGAATGGA |
| GPx | CCAAGCTCATCACCTGGTCT | TCGATGTCAATGGTCTGGAA |
| SOD | TGGCCGATGTGTCTATTGAA | CACCTTTGCCCAAGTCATCT |
| Nrf2 | CAGCGACGGAAAGAGTATGA | TGGGCAACCTGGGAGTAG |
| HO-1 | CATGACACCAAGGACCAGAG | AGTGTAAGGACCCATCGGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, Y.-T.; Chang, C.-C.; Chang, Y.; Hsuan, C.-F.; Chang, T.-H.; Chen, Y.-L.; Houng, H.-Y.; Su, Y.-C.; Houng, J.-Y. Inhibitory Effects of Syringic Acid on Endometrial Cancer Cell Growth and Migration and Its Synergistic Suppression with Doxorubicin. Pharmaceuticals 2025, 18, 1596. https://doi.org/10.3390/ph18111596
Kuo Y-T, Chang C-C, Chang Y, Hsuan C-F, Chang T-H, Chen Y-L, Houng H-Y, Su Y-C, Houng J-Y. Inhibitory Effects of Syringic Acid on Endometrial Cancer Cell Growth and Migration and Its Synergistic Suppression with Doxorubicin. Pharmaceuticals. 2025; 18(11):1596. https://doi.org/10.3390/ph18111596
Chicago/Turabian StyleKuo, Yi-Ting, Chi-Chang Chang, Yu Chang, Chin-Feng Hsuan, Tzu-Hsien Chang, Ya-Ling Chen, Hsin-Ya Houng, Yu-Chieh Su, and Jer-Yiing Houng. 2025. "Inhibitory Effects of Syringic Acid on Endometrial Cancer Cell Growth and Migration and Its Synergistic Suppression with Doxorubicin" Pharmaceuticals 18, no. 11: 1596. https://doi.org/10.3390/ph18111596
APA StyleKuo, Y.-T., Chang, C.-C., Chang, Y., Hsuan, C.-F., Chang, T.-H., Chen, Y.-L., Houng, H.-Y., Su, Y.-C., & Houng, J.-Y. (2025). Inhibitory Effects of Syringic Acid on Endometrial Cancer Cell Growth and Migration and Its Synergistic Suppression with Doxorubicin. Pharmaceuticals, 18(11), 1596. https://doi.org/10.3390/ph18111596











