Multi-Target Therapeutic Potential of Arctii Fructus Lignans in Diabetes Mellitus and Its Complications: A Mechanistic Review
Abstract
1. Introduction
2. Therapeutic Roles of Arctii Fructus Lignans in Diabetes Mellitus
2.1. Efficacy of Arctii Fructus lignans in Different Diabetes Models
| Diabetic Model | Lignan Type | Key Antidiabetic Effects | References |
|---|---|---|---|
| STZ-induced Type 1 DM (mouse/rat) | Arctigenin | ↓ Fasting glucose, ↑ Insulin secretion, ↓ Pancreatic oxidative stress | [7,27] |
| HFD/STZ-induced Type II DM (rat) | Total lignan | ↓ Fasting glucose, ↓ TG/TC, ↑ HDL-C, ↑ GLUT4 expression | [28,30] |
| Spontaneous Type II DM (db/db mouse) | Arctiin | ↓ Postprandial glucose, ↓ Insulin resistance, ↑ Adiponectin | [29,32] |
| Spontaneous Type II DM (GK rat) | Arctigenin | ↓ Fasting glucose, no significant effect on lipid profile | [30,33] |
| Diabetic nephropathy (STZ-induced, rat) | Total lignan | ↓ Urinary microalbumin, ↓ Renal TLR4/NF-κB activation | [34] |
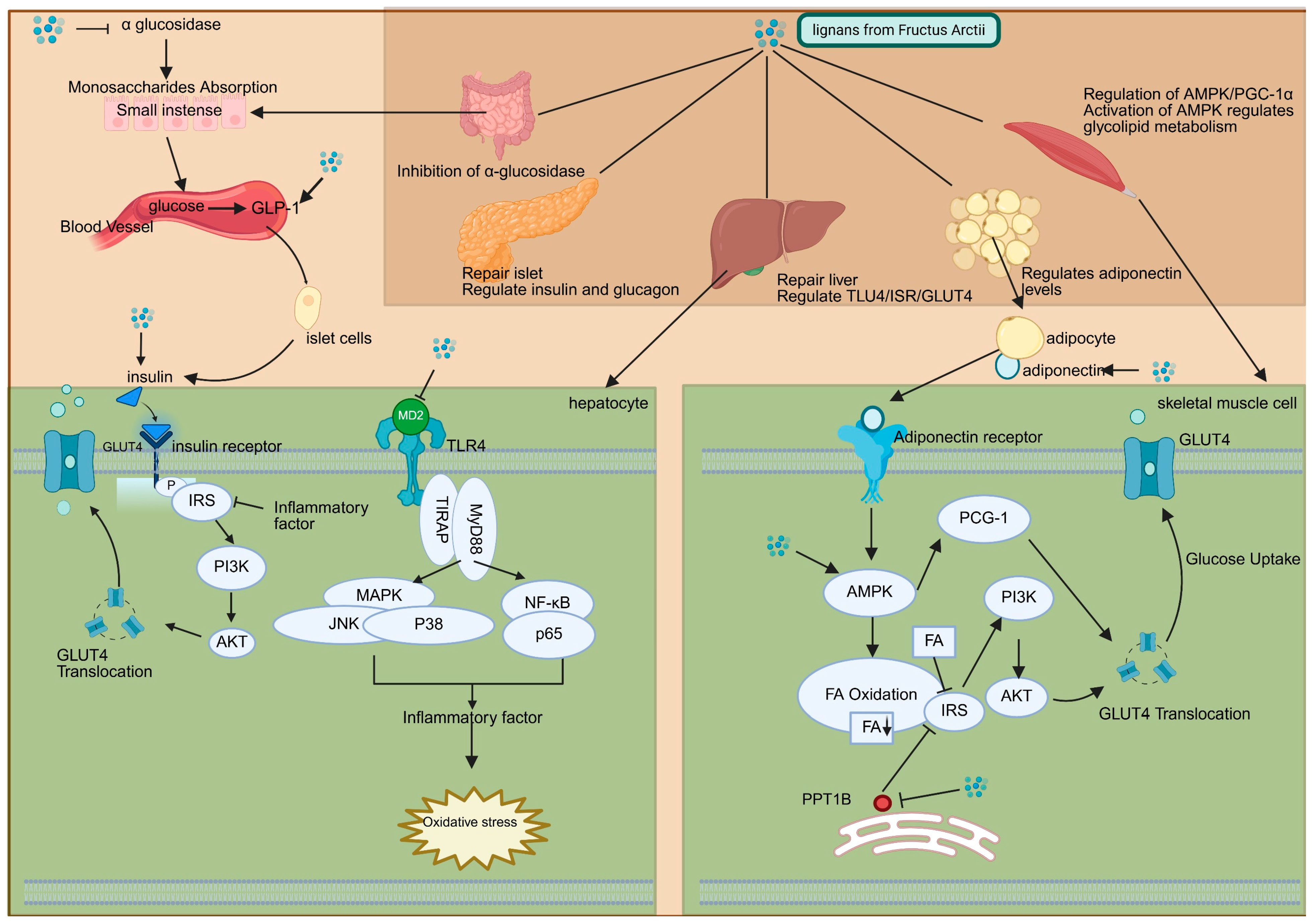
2.2. Mechanisms of Arctii Fructus Lignans Against Diabetes
3. Therapeutic Roles of Arctii Fructus Lignans in Diabetic Complications
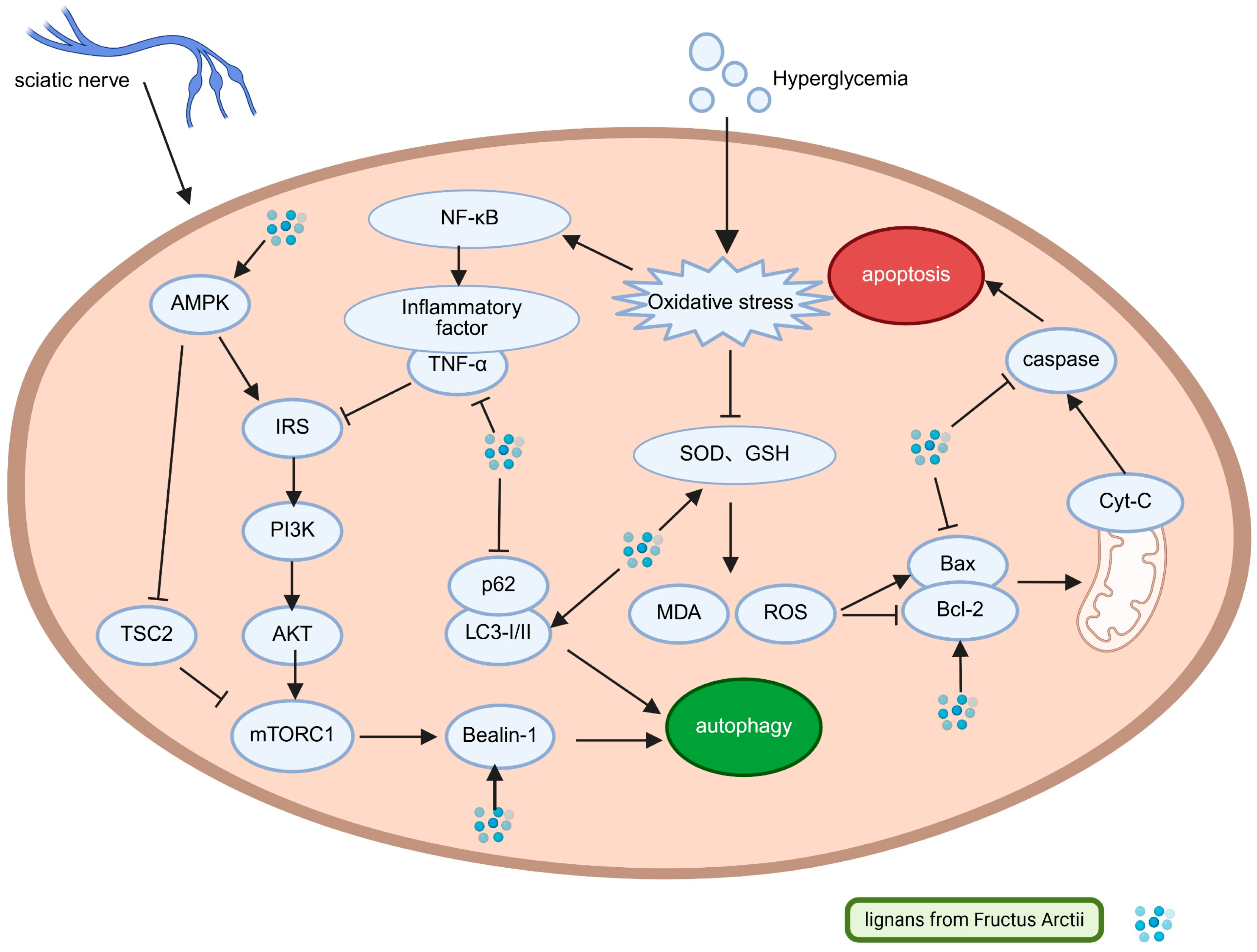
3.1. Arctii Fructus lignans in Diabetic Peripheral Neuropathy
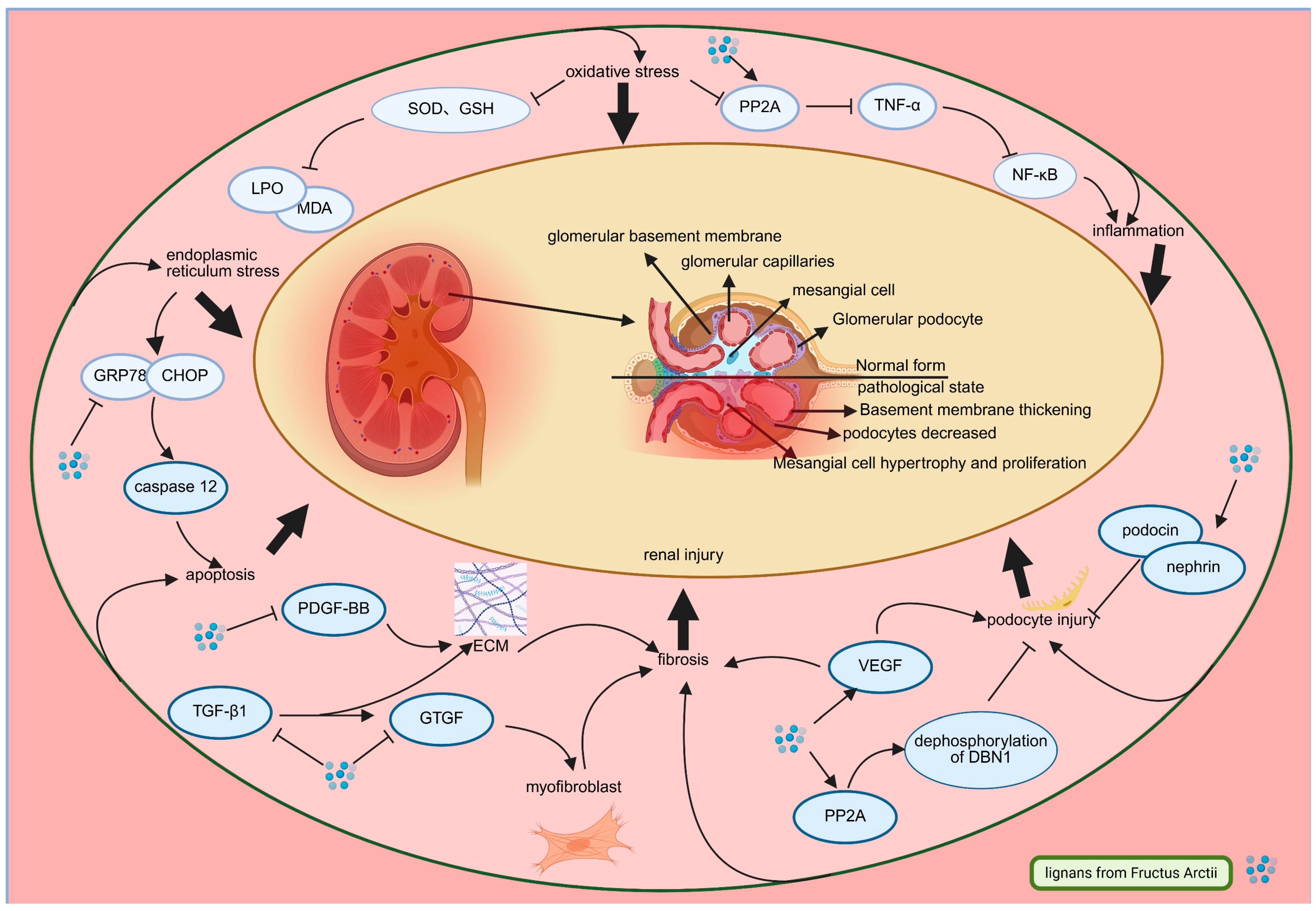
3.2. Arctii Fructus lignans in Diabetic Nephropathy
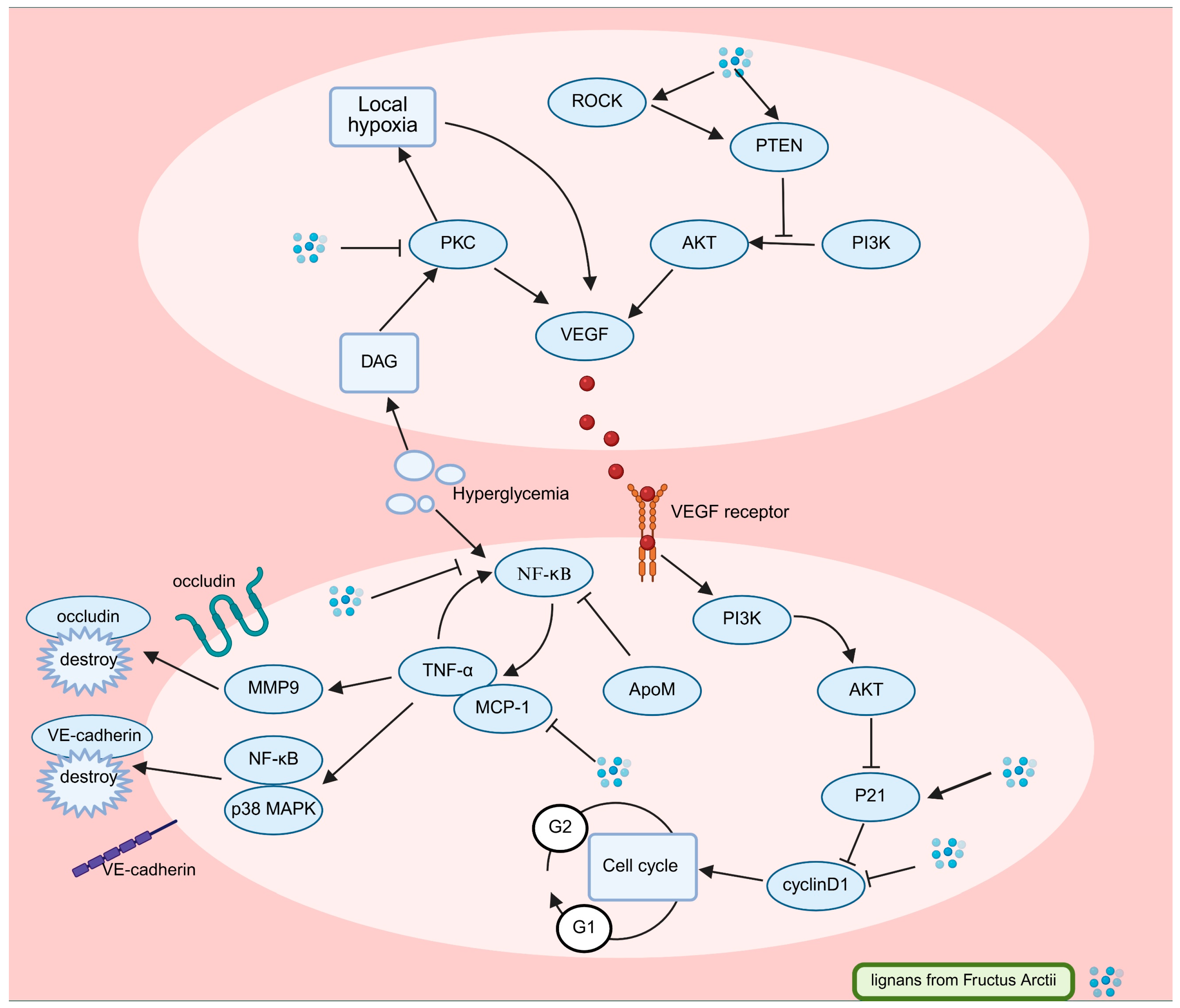
3.3. Arctii Fructus lignans in Diabetic Retinopathy
4. Safety Profile and Pharmacological Basis for Clinical Application of Arctii Fructus Lignans in Diabetes
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zakir, M.; Ahuja, N.; Surksha, M.A.; Sachdev, R.; Kalariya, Y.; Nasir, M.; Kashif, M.; Shahzeen, F.; Tayyab, A.; Khan, M.S.M.; et al. Cardiovascular Complications of Diabetes: From Microvascular to Macrovascular Pathways. Cureus 2023, 15, e45835. [Google Scholar] [CrossRef]
- Jin, X.; Liu, S.; Chen, S.; Wang, L.; Cui, Y.; He, J.; Fang, S.; Li, J.; Chang, Y. A systematic review on botany, ethnopharmacology, quality control, phytochemistry, pharmacology and toxicity of Arctium lappa L. fruit. J. Ethnopharmacol. 2023, 308, 116223. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Q.; Wei, H.; Liang, Y.; Niu, F.; Li, K.; Zhou, S.; Zhou, C. Fructus arctii: An overview on its traditional uses, pharmacology and phytochemistry. J. Pharm. Pharmacol. 2022, 74, 321–336. [Google Scholar] [CrossRef]
- Wu, T. Arctium lappa for type 2 diabetes. J. Tradit. Chin. Med. 1997, 38, 581. [Google Scholar] [CrossRef]
- Cui, B. Arctium lappa for diabetic nephropathy. J. Tradit. Chin. Med. 1997, 10, 582. [Google Scholar] [CrossRef]
- Xu, C.H.; Li, T.; Deng, Y.; Liu, J.W.; Jia, W. Hypoglycemic effect of the extract of Fructus Arctii. Chin. Tradit. Herb. Drugs 2005, 36, 1043–1045. [Google Scholar]
- Xu, Z.; Wang, X.; Zhou, M.; Ma, L.; Deng, Y.; Zhang, H.; Zhao, A.; Zhang, Y.; Jia, W. The antidiabetic activity of total lignan from Fructus Arctii against alloxan-induced diabetes in mice and rats. Phytother. Res. PTR 2008, 22, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Nose, M.; Fujimoto, T.; Takeda, T.; Nishibe, S.; Ogihara, Y. Structural transformation of lignan compounds in rat gastrointestinal tract. Planta Medica 1992, 58, 520–523. [Google Scholar] [CrossRef]
- Wang, D.; Bădărau, A.; Swamy, M.; Shaw, S.; Maggi, F.; da Silva, L.; López, V.; Yeung, A.; Mocan, A.; Atanasov, A. Arctium Species Secondary Metabolites Chemodiversity and Bioactivities. Front. Plant Sci. 2019, 10, 834. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, M.; Zuo, Z. Overview of the anti-inflammatory effects, pharmacokinetic properties and clinical efficacies of arctigenin and arctiin from Arctium lappa L. Acta Pharmacol. Sin. 2018, 39, 787–801. [Google Scholar] [CrossRef]
- Ichihara, A.; Oda, K.; Numata, Y.; Sakamura, S. Lappaol A and B, novel lignans from Arctium lappa L. Tetrahedron Lett. 1976, 17, 3961–3964. [Google Scholar] [CrossRef]
- Tezuka, Y.; Yamamoto, K.; Awale, S.; Lia, F.; Yomoda, S.; Kadota, S. Anti-austeric activity of phenolic constituents of seeds of Arctium lappa. Nat. Prod. Commun. 2013, 8, 463–466. [Google Scholar] [CrossRef]
- Park, S.; Hong, S.; Han, X.; Hwang, J.; Lee, D.; Ro, J.; Hwang, B. Lignans from Arctium lappa and their inhibition of LPS-induced nitric oxide production. Chem. Pharm. Bull. 2007, 55, 150–152. [Google Scholar] [CrossRef]
- Ichihara, A.; Numata, Y.; Kanai, S.; Sakamura, S. New Sesquilignans from Arctium lappa L. The Structure of Lappaol C, D and E. Agric. Biol. Chem. 1977, 41, 1813–1814. [Google Scholar] [CrossRef]
- Suzuki, S.; Umezawa, T.; Shimada, M. Stereochemical Difference in Secoisolariciresinol Formation between Cell-free Extracts from Petioles and from Ripening Seeds of Arctium lappa L. Biosci. Biotechnol. Biochem. 1998, 62, 1468–1470. [Google Scholar] [CrossRef]
- Ichihara, A.; Kanai, S.; Nakamura, Y.; Sakamura, S. Structures of lappaol F and H, dilignans from Arctium lappa L. Tetrahedron Lett. 1978, 19, 3035–3038. [Google Scholar] [CrossRef]
- Yong, M.; Kun, G.; Qiu, M. A new lignan from the seeds of Arctium lappa. J. Asian Nat. Prod. Res. 2007, 9, 541–544. [Google Scholar] [CrossRef]
- Wang, H.; Yang, J. Studies on the chemical constituents of Arctium lappa L. Yao Xue Xue Bao = Acta Pharm. Sin. 1993, 28, 911–917. [Google Scholar]
- Ichikawa, K.; Kinoshita, T.; Nishibe, S.; Sankawa, U. The Ca2+ antagonist activity of lignans. Chem. Pharm. Bull. 1986, 34, 3514–3517. [Google Scholar] [CrossRef]
- Han, B.; Kang, Y.; Yang, H.; Park, M. A butyrolactone lignan dimer from Arctium lappa. Phytochemistry 1994, 37, 1161–1163. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, F.; Feng, Z.; Jiang, J.; Zhang, P. Two new neolignan glucosides from Arctii Fructus. Asian Nat. Prod. Res. 2012, 14, 981–985. [Google Scholar] [CrossRef]
- Su, S.; Wink, M. Natural lignans from Arctium lappa as antiaging agents in Caenorhabditis elegans. Phytochemistry 2015, 117, 340–350. [Google Scholar] [CrossRef]
- Ferracane, R.; Graziani, G.; Gallo, M.; Fogliano, V.; Ritieni, A. Metabolic profile of the bioactive compounds of burdock (Arctium lappa) seeds, roots and leaves. J. Pharm. Biomed. Anal. 2010, 51, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Attele, A.; Zhou, Y.; Xie, J.; Wu, J.; Zhang, L.; Dey, L.; Pugh, W.; Rue, P.; Polonsky, K.; Yuan, C. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes 2002, 51, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Rydin, A.; Spiegel, G.; Frohnert, B.; Kaess, A.; Oswald, L.; Owen, D.; Simmons, K. Medical management of children with type 1 diabetes on low-carbohydrate or ketogenic diets. Pediatr. Diabetes 2021, 22, 448–454. [Google Scholar] [CrossRef]
- Ferrannini, E. Insulin resistance versus insulin deficiency in non-insulin-dependent diabetes mellitus: Problems and prospects. Endocr. Rev. 1998, 19, 477–490. [Google Scholar] [CrossRef]
- Sun, W.; Li, M.; Lin, Q.; Jin, X.; Zhao, B.; Jiang, Z.; Zhang, R.; Li, X. Arctiin Inhibits Hyperglycemia-Induced Oxidative Stress by Activating the Nrf2/HO-1 Signaling Pathway to Treat Type 2 Diabetic Osteoporosis. Mol. Nutr. Food Res. 2025, 69, e70053. [Google Scholar] [CrossRef] [PubMed]
- An, Y. Effect of arctiin on glucose and lipid metabolism in diabetic mice. Henan Tradit. Chin. Med. 2013, 33, 193–195. [Google Scholar] [CrossRef]
- Xu, Z.; Ju, J.; Wang, K.; Gu, C.; Feng, Y. Evaluation of hypoglycemic activity of total lignans from Fructus Arctii in the spontaneously diabetic Goto-Kakizaki rats. J. Ethnopharmacol. 2014, 151, 548–555. [Google Scholar] [CrossRef]
- Gao, Y.; Gu, C.; Wang, K.; Wang, H.; Ruan, K.; Xu, Z.; Feng, Y. The effects of hypoglycemia and weight loss of total lignans from Fructus Arctii in KKAy mice and its mechanisms of the activity. Phytother. Res. PTR 2018, 32, 631–642. [Google Scholar] [CrossRef]
- Ferhatbegović, L.; Mršić, D.; Kušljugić, S.; Pojskić, B. LDL-C: The Only Causal Risk Factor for ASCVD. Why Is It Still Overlooked and Underestimated? Curr. Atheroscler. Rep. 2022, 24, 635–642. [Google Scholar] [CrossRef]
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [CrossRef]
- Pyner, A.; Nyambe-Silavwe, H.; Williamson, G. Inhibition of Human and Rat Sucrase and Maltase Activities To Assess Antiglycemic Potential: Optimization of the Assay Using Acarbose and Polyphenols. J. Agric. Food Chem. 2017, 65, 8643–8651. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Run, K.; Xu, Z. Study on α-glucosidase Inhibitors in Arctii Fructus. Tradit. Chin. Drug Res. Clin. Pharmacol. 2020, 31, 163–168. [Google Scholar] [CrossRef]
- Nose, M.; Fujimoto, T.; Nishibe, S.; Ogihara, Y. Structural transformation of lignan compounds in rat gastrointestinal tract; II. Serum concentration of lignans and their metabolites. Planta Medica 1993, 59, 131–134. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, L.; Xiang, R.; Bu, X.; Qin, G.; Dai, J.; Zhao, Z.; Fang, X.; Yang, S.; Han, J.; et al. Arctigenin mitigates insulin resistance by modulating the IRS2/GLUT4 pathway via TLR4 in type 2 diabetes mellitus mice. Int. Immunopharmacol. 2023, 114, 109529. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Hardie, D.G. New insights into activation and function of the AMPK. Nat. Rev. Mol. Cell Biol. 2023, 24, 255–272. [Google Scholar] [CrossRef]
- Balkrishna, A.; Dobhal, V.; Verma, S.; Srivastava, D.; Singh, S.; Arya, V. Arctigenin: A Potential Component with Multifaceted Therapeutic Properties. Phytopharmacology 2022, 11, 414–420. [Google Scholar] [CrossRef]
- Huang, S.; Yu, R.; Gong, J.; Feng, Y.; Dai, Y.; Hu, F.; Hu, Y.; Tao, Y.; Leng, Y. Arctigenin, a natural compound, activates AMP-activated protein kinase via inhibition of mitochondria complex I and ameliorates metabolic disorders in ob/ob mice. Diabetologia 2012, 55, 1469–1481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lazzarini, P.; McPhail, S.; van Netten, J.; Armstrong, D.; Pacella, R. Global Disability Burdens of Diabetes-Related Lower-Extremity Complications in 1990 and 2016. Diabetes Care 2020, 43, 964–974. [Google Scholar] [CrossRef]
- Preston, F.G.; Riley, D.R.; Azmi, S.; Alam, U. Painful Diabetic Peripheral Neuropathy: Practical Guidance and Challenges for Clinical Management. Diabetes Metab. Syndr. Obes. 2023, 16, 1595–1612. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, Z.; Luo, Y.; Liu, Y.; Luo, W.; Du, X.; Luo, Z.; Hu, J.; Peng, S. Diabetic peripheral neuropathy: Pathogenetic mechanisms and treatment. Front. Endocrinol. 2023, 14, 1265372. [Google Scholar] [CrossRef]
- Yang, K.; Wang, Y.; Li, Y.W.; Chen, Y.G.; Xing, N.; Lin, H.B.; Zhou, P.; Yu, X.P. Progress in the treatment of diabetic peripheral neuropathy. Biomed. Pharmacother. 2022, 148, 112717. [Google Scholar] [CrossRef]
- Huang, S.S.; Wang, X.R.; Yuan, J.S.; Du, L. Experimental research progress in traditional Chinese medicine prevention and treatment of diabetic peripheral neuropathy based on autophagy. China J. Chin. Mater. Med. 2023, 48, 6315–6323. [Google Scholar] [CrossRef]
- Choi, A.; Ryter, S.; Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 2013, 368, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Medras, Z.; Mostafa, Y.; Ahmed, A.; El-Sayed, N. Arctigenin improves neuropathy via ameliorating apoptosis and modulating autophagy in streptozotocin-induced diabetic mice. CNS Neurosci. Ther. 2023, 29, 3068–3080. [Google Scholar] [CrossRef]
- Shen, X.; Zhuang, J.; Chen, Y.; Lei, M.; Chen, J.; Shen, X.; Hu, L. Synthesis and biological evaluation of arctigenin ester and ether derivatives as activators of AMPK. Bioorg. Med. Chem. 2013, 21, 3882–3893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, D.; Zhang, Z.; Tian, J.; An, J.; Zhang, W.; Ben, Y. Alpha-lipoic acid activates AMPK to protect against oxidative stress and apoptosis in rats with diabetic peripheral neuropathy. Hormones 2023, 22, 95–105. [Google Scholar] [CrossRef]
- Wu, R.; Sun, Y.; Zhou, T.; Zhu, Z.; Zhuang, J.; Tang, X.; Chen, J.; Hu, L.; Shen, X. Arctigenin enhances swimming endurance of sedentary rats partially by regulation of antioxidant pathways. Acta Pharmacol. Sin. 2014, 35, 1274–1284. [Google Scholar] [CrossRef]
- Min, B.; Lee, H.; Song, J.H.; Han, M.J.; Chung, J. Arctiin inhibits adipogenesis in 3T3-L1 cells and decreases adiposity and body weight in mice fed a high-fat diet. Nutr. Res. Pract. 2014, 8, 655–661. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, Y.; Chen, T.; Lei, J.; Jiang, X. AMPK/SIRT1 Pathway is Involved in Arctigenin-Mediated Protective Effects Against Myocardial Ischemia-Reperfusion Injury. Front. Pharmacol. 2020, 11, 616813. [Google Scholar] [CrossRef]
- Zhao, M.; Klionsky, D.J. AMPK-Dependent Phosphorylation of ULK1 Induces Autophagy. Cell Metab. 2011, 13, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Oshima, M.; Shimizu, M.; Yamanouchi, M.; Toyama, T.; Hara, A.; Furuichi, K.; Wada, T. Trajectories of kidney function in diabetes: A clinicopathological update. Nat. Rev. Nephrol. 2021, 17, 740–750. [Google Scholar] [CrossRef]
- Barutta, F.; Bellini, S.; Gruden, G. Mechanisms of podocyte injury and implications for diabetic nephropathy. Clin. Sci. 2022, 136, 493–520. [Google Scholar] [CrossRef]
- Wang, H. Experimental study on the mechanism of Arctium lappa and its extract to ameliorate kidney lesions in diabetic rats. J. Chin. Med. Sci. Tech. 2003, 10, 223–225. [Google Scholar]
- Ma, S.; Liu, D.; Niu, R.; Liu, R.; Ji, Q.; ZHan, J.; Shi, W.; Zhang, L. A randomized, double-blind, multicenter, placebo Phase III trial of arctiin in the treatment of diabetic nephropathy. Chin. J. Clin. Pharm 2011, 27, 15–18. [Google Scholar] [CrossRef]
- Zhao, H.; Jia, L.; Yuan, L.; Wang, W.; Li, L. Effect of Arctigenin on expression of VEGF and PDGF-BB in rat mesangial cells stimulated by high glucose. J. Hebei Unit Univ. 2013, 15, 633–634. [Google Scholar] [CrossRef]
- Zhong, Y.; Lee, K.; Deng, Y.; Ma, Y.; Chen, Y.; Li, X.; Wei, C.; Yang, S.; Wang, T.; Wong, N.; et al. Arctigenin attenuates diabetic kidney disease through the activation of PP2A in podocytes. Nat. Commun. 2019, 10, 4523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, P.; Gui, J.; Wang, X.; Han, J.; Wang, Y.; Wang, G. Arctigenin ameliorates renal impairment and inhibits endoplasmic reticulum stress in diabetic db/db mice. Life Sci. 2019, 223, 194–201. [Google Scholar] [CrossRef]
- Ma, S.; Liu, D.; Deng, J.; Niu, R.; Liu, R. Effect of arctiin on glomerular filtration barrier damage in STZ-induced diabetic nephropathy rats. Phytother. Res. PTR 2013, 27, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chen, A.; Dong, Y.; Dou, D. Fructus arctii mitigates diabetic nephropathy via the Apoh/PPAR-γ pathway. Mol. Immunol. 2025, 181, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.-H.; Chen, D.-Q.; Wang, Y.-N.; Feng, Y.-L.; Cao, G.; Vaziri, N.D.; Zhao, Y.-Y. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef]
- Geng, X.; Ma, A.; He, J.; Wang, L.; Jia, Y.; Shao, G.; Li, M.; Zhou, H.; Lin, S.; Ran, J.; et al. Ganoderic acid hinders renal fibrosis via suppressing the TGF-β/Smad and MAPK signaling pathways. Acta Pharmacol. Sin. 2020, 41, 670–677. [Google Scholar] [CrossRef]
- Kim, H.; Son, S.; Ko, Y.; Shin, I. CTGF regulates cell proliferation, migration, and glucose metabolism through activation of FAK signaling in triple-negative breast cancer. Oncogene 2021, 40, 2667–2681. [Google Scholar] [CrossRef] [PubMed]
- Rogier, E.; Durrbach, A.; Abecassis, L.; Ferlicot, S.; Snanoudj, R.; Baudreuil, S.; Arzouk, N.; Vazquez, A.; Charpentier, B.; Bourgeade, M.-F. A novel biological assay to detect the active form of TGF-β in urine to monitor renal allograft rejection. Kidney Int. 2005, 68, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Roestenberg, P.; van Nieuwenhoven, F.A.; Wieten, L.; Boer, P.; Diekman, T.; Tiller, A.M.; Wiersinga, W.M.; Oliver, N.; Usinger, W.; Weitz, S.; et al. Connective Tissue Growth Factor Is Increased in Plasma of Type 1 Diabetic Patients With Nephropathy. Diabetes Care 2004, 27, 1164–1170. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; He, D. Diverse effects of platelet-derived growth factor-BB on cell signaling pathways. Cytokine 2019, 113, 13–20. [Google Scholar] [CrossRef]
- Rustiasari, U.J.; Roelofs, J.J. The Role of Platelets in Diabetic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 8270. [Google Scholar] [CrossRef]
- Donate-Correa, J.; Tagua, V.G.; Ferri, C.; Martín-Núñez, E.; Hernández-Carballo, C.; Ureña-Torres, P.; Ruiz-Ortega, M.; Ortiz, A.; Mora-Fernández, C.; Navarro-González, J.F. Pentoxifylline for Renal Protection in Diabetic Kidney Disease. A Model of Old Drugs for New Horizons. J. Clin. Med. 2019, 8, 287. [Google Scholar] [CrossRef]
- Leeuwis, J.; Nguyen, T.; Dendooven, A.; Kok, R.; Goldschmeding, R. Targeting podocyte-associated diseases. Adv. Drug Deliv. Rev. 2010, 62, 1325–1336. [Google Scholar] [CrossRef]
- Shin, E.; Sorenson, C.; Sheibani, N. Diabetes and retinal vascular dysfunction. J. Ophthalmic Vis. Res. 2014, 9, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Skaat, A.; Chetrit, A.; Belkin, M.; Kinori, M.; Kalter-Leibovici, O. Time trends in the incidence and causes of blindness in Israel. Am. J. Ophthalmol. 2012, 153, 214–221.e211. [Google Scholar] [CrossRef]
- Zheng, Y.; He, M.; Congdon, N. The worldwide epidemic of diabetic retinopathy. Indian J. Ophthalmol. 2012, 60, 428–431. [Google Scholar] [CrossRef]
- Tang, J.; Kern, T. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011, 30, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Mitchell, P.; Wong, T. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Díaz-Coránguez, M.; Ramos, C.; Antonetti, D.A. The inner blood-retinal barrier: Cellular basis and development. Vis. Res. 2017, 139, 123–137. [Google Scholar] [CrossRef]
- Song, J.; Li, N.; Xia, Y.; Gao, Z.; Zou, S.; Kong, L.; Yao, Y.; Jiao, Y.; Yan, Y.; Li, S.; et al. Arctigenin Treatment Protects against Brain Damage through an Anti-Inflammatory and Anti-Apoptotic Mechanism after Needle Insertion. Front. Pharmacol. 2016, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, P. Study on the mechanism of arctiin inhibiting diabetic retinopathy by regulating vascular endothelial growth factor. Chin. Med. Bio. 2020, 15, 40–47. [Google Scholar]
- Tang, H.; Luo, G.; Yao, S.; Wang, M.; Pan, L.; Yu, M.; Yu, Y.; Liu, Y. Inhibitory effects of apolipoprotein M on inflammatory factors induced by high glucose in human retinal vascular endothelial cells. J. Exp. Ophthalmol. 2018, 36, 194–198. [Google Scholar] [CrossRef]
- Yuan, Q.; Cheng, Y.; Yang, Y.; Sun, L.; Guo, J. Effect on cell proliferation of mouse dorsal root ganglion cells coculture with human microvascular endothelial cells. Chin. J. Exp. Surg. 2015, 32, 135–138. [Google Scholar] [CrossRef]
- Rangasamy, S.; McGuire, P.; Das, A. Diabetic retinopathy and inflammation: Novel therapeutic targets. Middle East Afr. J. Ophthalmol. 2012, 19, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, Y.; Liu, Q.; Oh, J.; Woo, E.; Kang, K. 4-Hydroxykobusin inhibits the induction of nitric oxide synthase by inhibiting NF-kappaB and AP-1 activation. Biol. Pharm. Bull. 2007, 30, 1097–1101. [Google Scholar] [CrossRef]
- Vingolo, E.; Fragiotta, S.; Mafrici, M.; Cutini, A.; Marinelli, C.; Concistrè, A.; Iannucci, G.; Petramala, L.; Letizia, C. Vitreous and plasma changes of endothelin-1, adrenomedullin and vascular endothelium growth factor in patients with proliferative diabetic retinopathy. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 662–668. [Google Scholar]
- Fogli, S.; Mogavero, S.; Egan, C.; Del Re, M.; Danesi, R. Pathophysiology and pharmacological targets of VEGF in diabetic macular edema. Pharmacol. Res. 2016, 103, 149–157. [Google Scholar] [CrossRef]
- Le, Y. VEGF production and signaling in Müller glia are critical to modulating vascular function and neuronal integrity in diabetic retinopathy and hypoxic retinal vascular diseases. Vis. Res. 2017, 139, 108–114. [Google Scholar] [CrossRef]
- Zhang, Z.; Qin, X.; Zhang, J. MicroRNA-183 inhibition exerts suppressive effects on diabetic retinopathy by inactivating BTG1-mediated PI3K/Akt/VEGF signaling pathway. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E1050–E1060. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhou, J.; Budhraja, A.; Hu, X.; Chen, Y.; Cheng, Q.; Liu, L.; Zhou, T.; Li, P.; Liu, E.; et al. Mitochondrial translocation and interaction of cofilin and Drp1 are required for erucin-induced mitochondrial fission and apoptosis. Oncotarget 2015, 6, 1834–1849. [Google Scholar] [CrossRef]
- Deng, M.; Luo, Y.; Li, Y.; Yang, Q.; Deng, X.; Wu, P.; Ma, H. Klotho gene delivery ameliorates renal hypertrophy and fibrosis in streptozotocin-induced diabetic rats by suppressing the Rho-associated coiled-coil kinase signaling pathway. Mol. Med. Rep. 2015, 12, 45–54. [Google Scholar] [CrossRef]
- Furukawa, N.; Ongusaha, P.; Jahng, W.; Araki, K.; Choi, C.; Kim, H.; Lee, Y.; Kaibuchi, K.; Kahn, B.; Masuzaki, H.; et al. Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metab. 2005, 2, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Keniry, M.; Parsons, R. The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene 2008, 27, 5477–5485. [Google Scholar] [CrossRef]
- Pal, A.; Barber, T.; BM, V.; Rudge, S.; Zhang, Q.; Lachlan, K.; Cooper, N.; Linden, H.; Levy, J.; Wakelam, M.; et al. PTEN mutations as a cause of constitutive insulin sensitivity and obesity. N. Engl. J. Med. 2012, 367, 1002–1011. [Google Scholar] [CrossRef]
- Kar, S.; Samii, A.; Bertalanffy, H. PTEN/PI3K/Akt/VEGF signaling and the cross talk to KRIT1, CCM2, and PDCD10 proteins in cerebral cavernous malformations. Neurosurg. Rev. 2015, 38, 229–236; discussion 227–236. [Google Scholar] [CrossRef]
- Zhou, M.; Li, G.; Zhu, L.; Zhou, H.; Lu, L. Arctiin attenuates high glucose-induced human retinal capillary endothelial cell proliferation by regulating ROCK1/PTEN/PI3K/Akt/VEGF pathway in vitro. J. Cell. Mol. Med. 2020, 24, 5695–5706. [Google Scholar] [CrossRef]
- Lu, L.; Zhou, W.; Li, Z.; Yu, C.; Li, C.; Luo, M.; Xie, H. Effects of arctiin on streptozotocin-induced diabetic retinopathy in Sprague-Dawley rats. Planta Medica 2012, 78, 1317–1323. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y.; Zhang, J.; Wang, K.; Jin, T.; Wang, H.; Ruan, K.; Wu, F.; Xu, Z. The effect of total lignans from Fructus Arctii on Streptozotocin-induced diabetic retinopathy in Wistar rats. J. Ethnopharmacol. 2020, 255, 112773. [Google Scholar] [CrossRef]
- Aiello, L. The potential role of PKC beta in diabetic retinopathy and macular edema. Surv. Ophthalmol. 2002, 47 (Suppl. 2), S263–S269. [Google Scholar] [CrossRef]
- Antonetti, D.; Barber, A.; Khin, S.; Lieth, E.; Tarbell, J.; Gardner, T. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: Vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes 1998, 47, 1953–1959. [Google Scholar] [CrossRef]
- Kowluru, R. Diabetes-induced elevations in retinal oxidative stress, protein kinase C and nitric oxide are interrelated. Acta Diabetol. 2001, 38, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Dejana, E.; Orsenigo, F.; Lampugnani, M. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 2008, 121, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- van der Wijk, A.E.; Vogels, I.; van Noorden, C.; Klaassen, I.; Schlingemann, R. TNFα-Induced Disruption of the Blood-Retinal Barrier In Vitro Is Regulated by Intracellular 3′,5′-Cyclic Adenosine Monophosphate Levels. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3496–3505. [Google Scholar] [CrossRef]
- Lee, I.; Lin, C.; Wu, Y.; Yang, C. TNF-alpha induces matrix metalloproteinase-9 expression in A549 cells: Role of TNFR1/TRAF2/PKCalpha-dependent signaling pathways. J. Cell. Physiol. 2010, 224, 454–464. [Google Scholar] [CrossRef]
- Giebel, S.; Menicucci, G.; McGuire, P.; Das, A. Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood-retinal barrier. Lab. Investig. 2005, 85, 597–607. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, H.; Zhou, M.; Feng, Y.; Jia, W. Inhibitory effect of total lignan from Fructus Arctii on aldose reductase. Phytother. Res. PTR 2010, 24, 472–473. [Google Scholar] [CrossRef]
- Daruich, A.; Matet, A.; Moulin, A.; Kowalczuk, L.; Nicolas, M.; Sellam, A.; Rothschild, P.; Omri, S.; Gélizé, E.; Jonet, L.; et al. Mechanisms of macular edema: Beyond the surface. Prog. Retin. Eye Res. 2018, 63, 20–68. [Google Scholar] [CrossRef]
- Vinores, S.A.; Xiao, W.; Shen, J.; Campochiaro, P. TNF-alpha is critical for ischemia-induced leukostasis, but not retinal neovascularization nor VEGF-induced leakage. J. Neuroimmunol. 2007, 182, 73–79. [Google Scholar] [CrossRef]
- Cox, J.; Eliott, D.; Sobrin, L. Inflammatory Complications of Intravitreal Anti-VEGF Injections. J. Clin. Med. 2021, 10, 981. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Ren, Y.-S.; Lv, Y.-Y.; Zhang, J.-S.; Xu, X.-L.; Wang, X.-Z.; Yao, J.-C.; Zhang, G.-M.; Liu, Z. Elucidation of Arctigenin Pharmacokinetics and Tissue Distribution after Intravenous, Oral, Hypodermic and Sublingual Administration in Rats and Beagle Dogs: Integration of In Vitro and In Vivo Findings. Front. Pharmacol. 2017, 8, 376. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, Y.; Wo, S.; Zuo, Z. Extensive intestinal first-pass metabolism of arctigenin: Evidenced by simultaneous monitoring of both parent drug and its major metabolites. J. Pharm. Biomed. Anal. 2014, 91, 60–67. [Google Scholar] [CrossRef]
- He, B.; Zhang, H.-J.; Yang, W.-H.; Shao, Z.-Y.; Wu, L.-J.; Chen, X.-B.; Chen, J.; Liu, W.; Ran, Z.-P.; Jin, R.-G.; et al. Pharmacokinetics of Arctigenin and Fructus Arctii Powder in Piglets. Front. Vet. Sci. 2019, 6, 235. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Sato, M.; Awazuhara, T.; Nukui, Y.; Yoshida, A.; Terashima, T.; Watanabe, K.; Fujioka, R.; Tsuchihara, K.; Kishino, S.; et al. Simultaneous quantification of arctigenin and its glucuronide conjugate in mouse plasma using ultra-high performance liquid chromatography coupled to tandem mass spectrometry. J. Sep. Sci. 2021, 44, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Pan, Q.; Han, X.-Y.; Wang, J.; Tan, R.-Q.; He, F.; Dou, D.-Q.; Kang, T.-G. Simultaneous determination of arctiin and its metabolites in rat urine and feces by HPLC. Fitoterapia 2013, 86, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lv, Y.-g.; Pan, L.-h.; Yao, F.-f.; Peng, T.; Tan, Y.-j.; Zhang, G.-M.; Liu, Z.; Yao, J.-c.; Ren, Y.-s. Toxicity Study of 28-Day Subcutaneous Injection of Arctigenin in Beagle Dogs. Front. Pharmacol. 2019, 10, 1218. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.-j.; Ren, Y.-s.; Gao, L.; Li, L.-f.; Cui, L.-j.; Li, B.; Li, X.; Yang, J.; Wang, M.-z.; Lv, Y.-y.; et al. 28-Day Oral Chronic Toxicity Study of Arctigenin in Rats. Front. Pharmacol. 2018, 9, 1077. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Mousavi*, Z.; Rastegar, T.; Amin, G. Safety Assessment of Arctium lappa L. Fruit Extract in Female Wistar Rats: Acute and Repeated Oral Toxicity Studies. Res. J. Pharmacogn. 2019, 6, 39–48. [Google Scholar] [CrossRef]
- He, F.; Dou, D.-Q.; Hou, Q.; Sun, Y.; Kang, T.-G. Pharmacokinetic study of arctigenin in rat plasma and organ tissue by RP-HPLC method. Nat. Prod. Res. 2013, 27, 903–906. [Google Scholar] [CrossRef] [PubMed]

| Number | Name | References |
|---|---|---|
| 1 | lappaol A | [11] |
| 2 | lappaol B | [12] |
| 3 | lappaol C | [12] |
| 4 | lappaol D | [13] |
| 5 | lappaol E | [14] |
| 6 | (+)-secoisolariciresinol | [15] |
| 7 | lappaol F | [16] |
| 8 | lappaol H | [16] |
| 9 | neoarctin A | [17] |
| 10 | neoarctin B | [18] |
| 11 | trachelogenin | [19] |
| 12 | diarctigenin | [20] |
| 13 | arctiinoside A | [21] |
| 14 | arctiinoside B | [21] |
| 15 | pinoresinol | [15] |
| 16 | matairesinol | [22] |
| 17 | arctignan A | [21] |
| 18 | arctignan G | [21] |
| 19 | arctignan H | [21] |
| 20 | (+)-7,8-didebydroarctigenin | [23] |
| 21 | Arctiin | [23] |
| 22 | Arctigenin | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, S.; Li, J.; Hu, Y.; Cai, J.; Nan, G.; Kong, Y.; Shen, X.; Zhu, L.; Yang, S.; Dong, C. Multi-Target Therapeutic Potential of Arctii Fructus Lignans in Diabetes Mellitus and Its Complications: A Mechanistic Review. Pharmaceuticals 2025, 18, 1569. https://doi.org/10.3390/ph18101569
Lv S, Li J, Hu Y, Cai J, Nan G, Kong Y, Shen X, Zhu L, Yang S, Dong C. Multi-Target Therapeutic Potential of Arctii Fructus Lignans in Diabetes Mellitus and Its Complications: A Mechanistic Review. Pharmaceuticals. 2025; 18(10):1569. https://doi.org/10.3390/ph18101569
Chicago/Turabian StyleLv, Shuaiyi, Jieming Li, Yulong Hu, Juntao Cai, Guanglei Nan, Yuanfang Kong, Xu Shen, Lifeng Zhu, Shaohua Yang, and Chunhong Dong. 2025. "Multi-Target Therapeutic Potential of Arctii Fructus Lignans in Diabetes Mellitus and Its Complications: A Mechanistic Review" Pharmaceuticals 18, no. 10: 1569. https://doi.org/10.3390/ph18101569
APA StyleLv, S., Li, J., Hu, Y., Cai, J., Nan, G., Kong, Y., Shen, X., Zhu, L., Yang, S., & Dong, C. (2025). Multi-Target Therapeutic Potential of Arctii Fructus Lignans in Diabetes Mellitus and Its Complications: A Mechanistic Review. Pharmaceuticals, 18(10), 1569. https://doi.org/10.3390/ph18101569







