Review of Selected 2-Phenylethylamine Derivatives and Opioids, Systematic Review of Their Effects on Psychomotor Abilities and Driving Performance: Psychopharmacology in the Context of Road Safety
Abstract
1. Introduction
1.1. Characterization of 2-Phenylethylamine, Its Derivatives and Opioids
1.2. Review of PEA: Stimulant Derivatives
1.3. Review of PEA: Hallucinogenic Derivatives
1.4. Review of Opioids
1.5. Overview of DUID Problem
1.6. DUID Problem: Methamphetamine (METH), 3,4-Methylenedioxymethamphetamine (MDMA) and Amphetamine
1.7. DUID Problem: Methylphenidate
1.8. DUID Problem: Opioids
1.8.1. Overview of Fentanyl
1.8.2. Overview of Methadone
1.8.3. Overview of Tramadol
1.9. DUID Problem: Hallucinogens
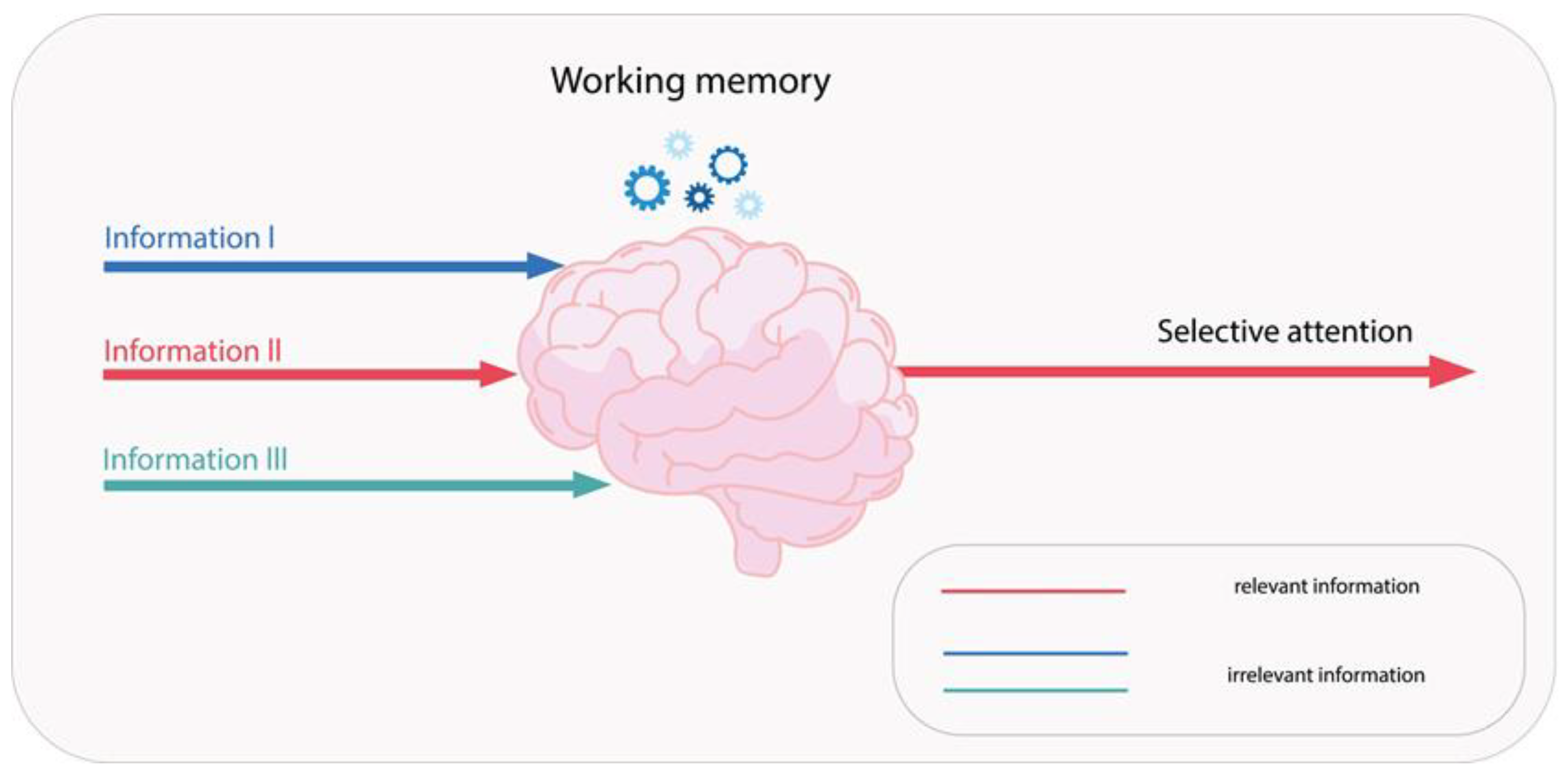
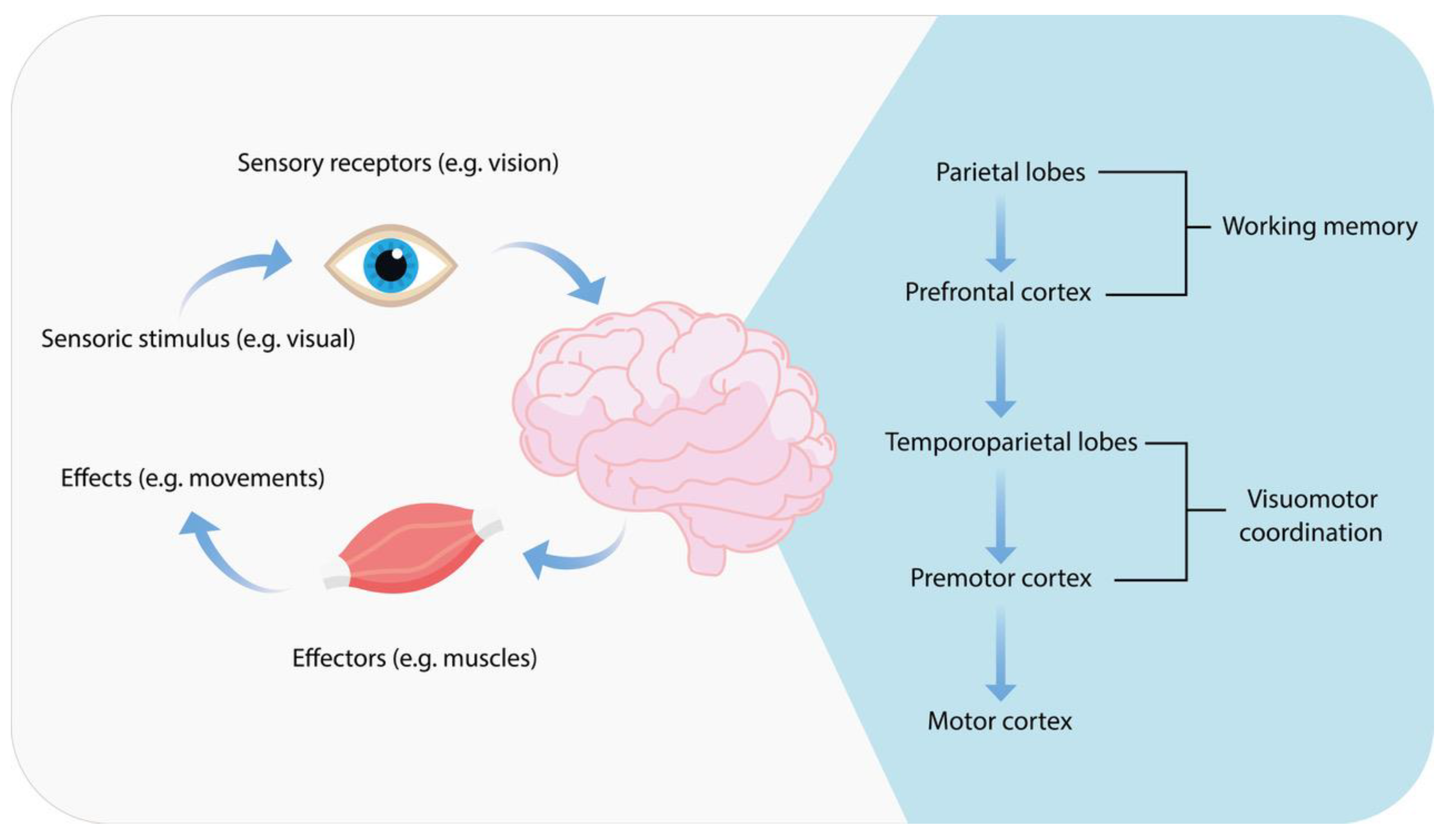
1.10. Definition and Importance of Psychomotor Functions
- −
- perception time-the period from the appearance of a threat in the driver’s field of vision to the moment when attention is drawn to it and it is recognized;
- −
- basic psychological reaction time-this is the period of analyzing an event and making a decision, e.g., whether to brake or to avoid an obstacle. It also includes sending a signal to the motoneurons to initiate a maneuver;
- −
- foot transfer time—the time associated with a reaction, such as transferring the foot from the accelerator to the brake pedal.
1.11. Current Regulations on Driving Under the Influence of Selected Substances in Poland
- −
- Group I-P psychotropic substances–MDMA;
- −
- Group II-P psychotropic substances–methylphenidate and amphetamine;
- −
- Group I-N narcotic drugs-Fentanyl and methadone [90].
- −
- Operating a vehicle under the influence of an intoxicating substance carries a penalty of imprisonment for up to three years [88];
- −
2. Materials and Methods
2.1. Literature Research
2.2. PICO Framework and Risk of Bias Assessment
- Patient: adults taking selected 2-phenylethylamine derivatives (2-phenylethylamine, L-phenylalanine, phenylacetic acid, methylphenidate, amphetamine, methamphetamine (METH), mescaline, methylenedioxyamphetamine (3,4-methylenedioxymethamphetamine, MDMA, ecstasy), 2,5-dimethoxy-4-methylamphetamine (DOM) or selected opioids (methadone, tramadol, fentanyl).
- Intervention: exposure to selected 2-phenylethylamine derivatives or opioids.
- Comparison: population not taking 2-phenylethylamine derivatives or opioids, placebo, the same population with and without 2-phenylethylamine derivatives or opioids.
- Outcomes: changes in psychomotor functioning, affect, cognitive impairment, influence on driving safety, reaction time, engaging in reckless driving, lane-keeping ability.
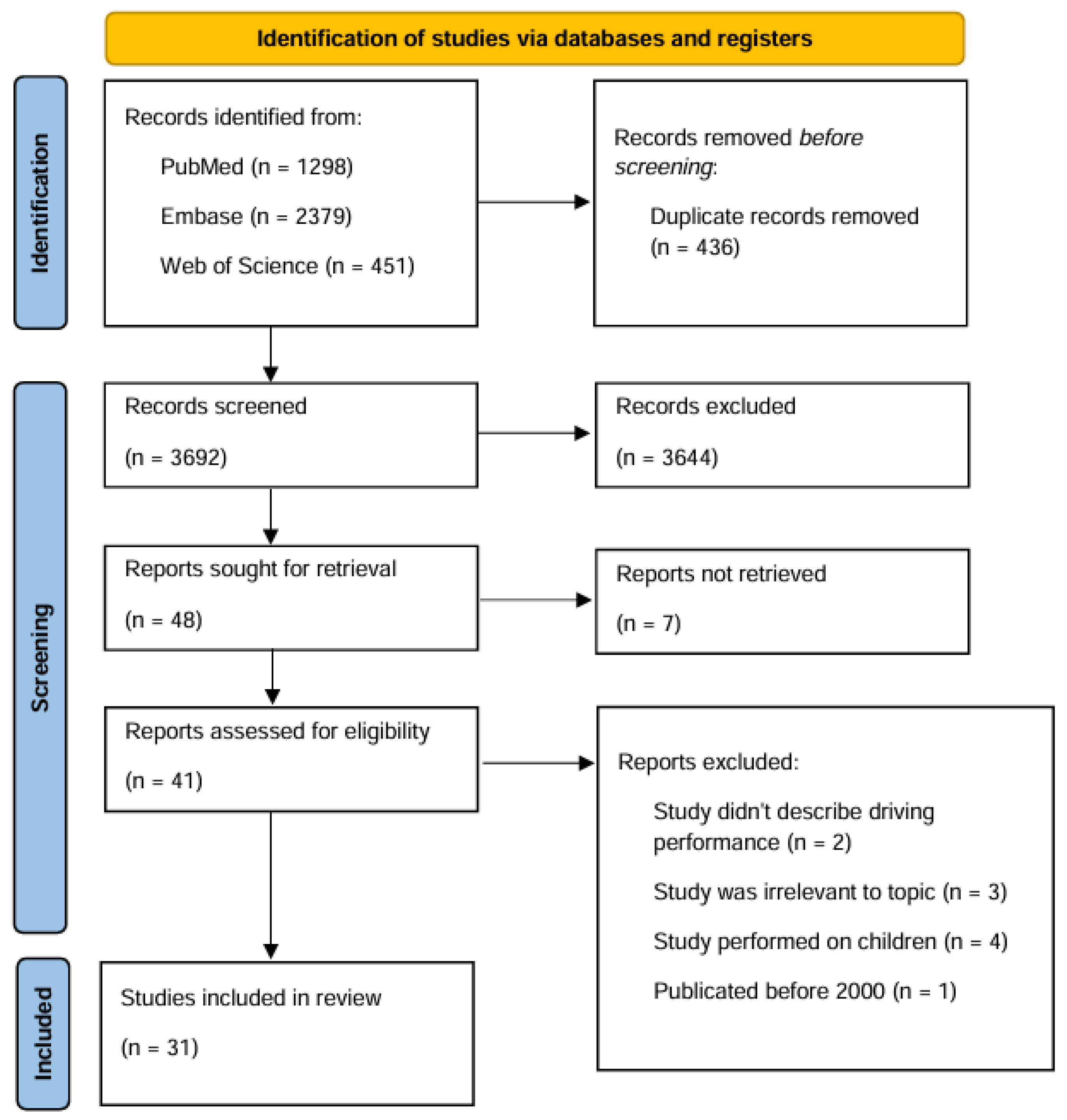
3. Results
3.1. Results: Amphetamine
3.2. Results: Methamphetamine
3.3. Results: Methylphenidate
3.4. Results: MDMA
3.5. Results: Fentanyl
3.6. Results: Methadone
3.7. Other Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PEA | 2-phenylethylamine |
| ADHD | Attention-Deficit/Hyperactivity Disorder |
| DUID | Driving under the influence of drugs |
| L-AAD | L-aromatic amino acid decarboxylase |
| MAO-B | Monoamine oxygenase B |
| TAAR1 | Trace amine-related receptor 1 |
| MDMA | 3,4-Methylenedioxymethamphetamine |
| DOM | 2,5-dimethoxy-4-methylamphetamine |
| PTSD | Post-traumatic stress disorder |
| 25 H-NBOMe | 2-(4-iodo-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine |
| 25 I–NBOMe | 2-(4-iodo-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine |
| 25 B–NBOMe | 2-(4-bromo-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine |
| WMC | Working memory capacity |
| OxyHb | Oxyhemoglobin |
| DeoxyHb | Deoxyhemoglobin |
References
- Blandino, A.; Cotroneo, R.; Tambuzzi, S.; Candia, D.D.; Genovese, U.; Zoja, R. Driving under the influence of drugs: Correlation between blood psychoactive drug concentrations and cognitive impairment. A narrative review taking into account forensic issues. Forensic Sci. Int. Synerg. 2022, 4, 100224. [Google Scholar] [CrossRef]
- Ryu, I.S.; Kim, O.-H.; Kim, J.S.; Sohn, S.; Choe, E.S.; Lim, R.-N.; Kim, T.W.; Seo, J.-W.; Jang, E.Y. Effects of β-Phenylethylamine on Psychomotor, Rewarding, and Reinforcing Behaviors and Affective State: The Role of Dopamine D1 Receptors. Int. J. Mol. Sci. 2021, 22, 9485. [Google Scholar] [CrossRef] [PubMed]
- Krombholz, S.; Thomas, A.; Piper, T.; Lagojda, A.; Kühne, D.; Thevis, M. Urinary phenylethylamine metabolites as potential markers for sports drug testing purposes. Biomed. Chromatogr. 2021, 36, e5274. [Google Scholar] [CrossRef] [PubMed]
- Żełabowski, K.; Petrov, W.; Ślebioda, D.; Rusinek, M.; Biedka, K.; Błaszczyk, K.; Wesołowski, M.; Wojtysiak, K.; Sroka, M.; Ratka, Z.; et al. Holistic Management of Adult ADHD with a History of Addiction: Emphasis on Low-Addiction-Risk Psychopharmacotherapy. J. Clin. Med. 2025, 14, 6470. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.; Shover, C.L. Charting the fourth wave: Geographic, temporal, race/ethnicity and demographic trends in polysubstance fentanyl overdose deaths in the United States, 2010–2021. Addiction 2023, 118, 2477–2485. [Google Scholar] [CrossRef]
- Ciccarone, D. The rise of illicit fentanyls, stimulants and the fourth wave of the opioid overdose crisis. Curr. Opin. Psychiatry 2021, 34, 344–350. [Google Scholar] [CrossRef]
- Rudisill, T.M.; Smith, G.S. Risk factors associated with driving under the influence of drugs in the USA. Inj. Prev. 2021, 27, 514–520. [Google Scholar] [CrossRef]
- Sabelli, H.C.; Javaid, J.I. Phenylethylamine modulation of affect: Therapeutic and diagnostic implications. J. Neuropsychiatry Clin. Neurosci. 2006, 7, 6–14. [Google Scholar] [CrossRef]
- Sengupta, T.; Mohanakumar, K.P. 2-Phenylethylamine, a constituent of chocolate and wine, causes mitochondrial complex-I inhibition, generation of hydroxyl radicals and depletion of striatal biogenic amines leading to psycho-motor dysfunctions in Balb/c mice. Neurochem. Int. 2010, 57, 637–646. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, Y.; Wang, Y.; Wang, Y.; He, X.; Xu, P.; Huang, S.; Yuan, Q.; Zhang, X.; Wang, L.; et al. Recognition of methamphetamine and other amines by trace amine receptor TAAR1. Nature 2023, 624, 663–671. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, H.R.; Lee, C.Y.; Hyun, S.A.; Ko, M.Y.; Lee, B.S.; Hwang, D.Y.; Ka, M. 2-Phenylethylamine (PEA) Ameliorates Corticosterone-Induced Depression-Like Phenotype via the BDNF/TrkB/CREB Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 9103. [Google Scholar] [CrossRef]
- Kitanaka, J.; Kitanaka, N.; Tatsuta, T.; Takemura, M. 2-Phenylethylamine in combination with l-deprenyl lowers the striatal level of dopamine and prolongs the duration of the stereotypy in mice. Pharmacol. Biochem. Behav. 2005, 82, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Miller, G.M. Beta-phenylethylamine alters monoamine transporter function via trace amine-associated receptor 1: Implication for modulatory roles of trace amines in brain. J. Pharmacol. Exp. Ther. 2008, 325, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Steingard, R.; Taskiran, S.; Connor, D.F.; Markowitz, J.S.; Stein, M.A. New Formulations of Stimulants: An Update for Clinicians. J. Child Adolesc. Psychopharmacol. 2019, 29, 324–339. [Google Scholar] [CrossRef] [PubMed]
- Clemow, D.B.; Walker, D.J. The Potential for Misuse and Abuse of Medications in ADHD: A Review. Postgrad. Med. 2014, 126, 64–81. [Google Scholar] [CrossRef]
- Lee, C.W.; Chen, J.Y.; Ko, C.C.; Chuang, M.H.; Tsai, W.W.; Sun, C.K.; Hung, K.C. Efficacy of methylphenidate for the treatment of apathy in patients with Alzheimer’s disease: A systematic review and meta-analysis of randomized controlled studies. Psychopharmacology 2022, 239, 3743–3753. [Google Scholar] [CrossRef]
- Panušková, K.; Voděrová, L.; Vaculín, Š.; Vaculin, S. Methylphenidate Attenuates Signs of Evoked Neuropathic Pain in Animal Model. Physiol. Res. 2023, 72, S551–S558. [Google Scholar] [CrossRef]
- Iv, H.W.; Tocci, D.; Prashar, S.; Boldt, E.; Khalil, A.; Arora, S.; Matthews, T.; Wahid, T.; Fernandez, R.; Ram, D.; et al. Role of vesicular monoamine transporter-2 for treating attention deficit hyperactivity disorder: A review. Psychopharmacology 2024, 241, 2191–2203. [Google Scholar] [CrossRef]
- Parlatini, V.; Bellato, A.; Murphy, D.; Cortese, S. From neurons to brain networks, pharmacodynamics of stimulant medication for ADHD. Neurosci. Biobehav. Rev. 2024, 164, 105841. [Google Scholar] [CrossRef]
- Solhjoo, S.; Punjabi, N.M.; Ivanescu, A.E.; Crainiceanu, C.; Gaynanova, I.; Wicken, C.; Buckenmaier, C.; Haigney, M.C. Methadone Destabilizes Cardiac Repolarization During Sleep. Clin. Pharmacol. Ther. 2021, 110, 1066–1074. [Google Scholar] [CrossRef]
- Ewald, A.H.; Puetz, M.; Maurer, H.H. Designer drug 2,5-dimethoxy-4-methyl-amphetamine (DOM, STP): Involvement of the cytochrome P450 isoenzymes in formation of its main metabolite and detection of the latter in rat urine as proof of a drug intake using gas chromatography–mass spectrometry. J. Chromatogr. B 2008, 862, 252–256. [Google Scholar] [CrossRef]
- Papaseit, E.; Pérez-Mañá, C.; Torrens, M.; Farré, A.; Poyatos, L.; Hladun, O.; Sanvisens, A.; Muga, R.; Farré, M. MDMA interactions with pharmaceuticals and drugs of abuse. Expert Opin. Drug Metab. Toxicol. 2020, 16, 357–369. [Google Scholar] [CrossRef]
- Smith, K.W.; Sicignano, D.J.; Hernandez, A.V.; White, C.M. MDMA-Assisted Psychotherapy for Treatment of Posttraumatic Stress Disorder: A Systematic Review with Meta-Analysis. J. Clin. Pharmacol. 2022, 62, 463–471. [Google Scholar] [CrossRef]
- Latimer, D.; Stocker, M.D.; Sayers, K.; Green, J.; Kaye, A.M.; Abd-Elsayed, A.; Cornett, E.M.; Kaye, A.D.; Varrassi, G.; Viswanath, O.; et al. MDMA to Treat PTSD in Adults. Psychopharmacol. Bull. 2021, 51, 125. [Google Scholar]
- Thorarinsdottir, H.; Gudmundsdottir, B.; Sigurdsson, E. [MDMA-assisted therapy for PTSD]. Laeknabladid 2024, 110, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Feduccia, A.A.; Mithoefer, M.C. MDMA-assisted psychotherapy for PTSD: Are memory reconsolidation and fear extinction underlying mechanisms? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 84, 221–228. [Google Scholar] [CrossRef]
- Johnson, M.W.; Richards, W.A.; Griffiths, R.R. Human hallucinogen research: Guidelines for safety. J. Psychopharmacol. 2008, 22, 603–620. [Google Scholar] [CrossRef] [PubMed]
- Vamvakopoulou, I.A.; Narine, K.A.D.; Campbell, I.; Dyck, J.R.B.; Nutt, D.J. Mescaline: The forgotten psychedelic. Neuropharmacology 2023, 222, 109294. [Google Scholar] [CrossRef] [PubMed]
- Calder, A.E.; Hasler, G. Towards an understanding of psychedelic-induced neuroplasticity. Neuropsychopharmacology 2022, 48, 104–112. [Google Scholar] [CrossRef]
- Cassels, B.K.; Sáez-Briones, P. Dark Classics in Chemical Neuroscience: Mescaline. ACS Chem. Neurosci. 2018, 9, 2448–2458. [Google Scholar] [CrossRef]
- Gennaro, R.J. Synesthesia, hallucination, and autism. Front. Biosci.-Landmark 2021, 26, 797–809. [Google Scholar] [CrossRef]
- Stein, C. Opioid receptors. Annu. Rev. Med. 2016, 67, 433–451. [Google Scholar] [CrossRef]
- Cherny, N.I. Opioid Analgesics: Comparative Features and Prescribing Guidelines. Drugs 1996, 51, 713–737. [Google Scholar] [CrossRef]
- Ragaban, F.; Purohit, O.; Fabbro, E.D. Methadone in Cancer-Related Neuropathic Pain: A Narrative Review. Curr. Oncol. 2024, 31, 7613–7624. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.; Kraus, C.; Fleming, M.; Reddy, S. Methadone: Applied pharmacology and use as adjunctive treatment in chronic pain. Postgrad. Med. J. 2004, 80, 654. [Google Scholar] [CrossRef] [PubMed]
- Miotto, K.; Cho, A.K.; Khalil, M.A.; Blanco, K.; Sasaki, J.D.; Rawson, R. Trends in Tramadol: Pharmacology, Metabolism, and Misuse. Anesth. Analg. 2017, 124, 44–51. [Google Scholar] [CrossRef]
- Barakat, A. Revisiting Tramadol: A Multi-Modal Agent for Pain Management. CNS Drugs 2019, 33, 481–501. [Google Scholar] [CrossRef] [PubMed]
- Grond, S.; Sablotzki, A. Clinical pharmacology of tramadol. Clin. Pharmacokinet. 2004, 43, 879–923. [Google Scholar] [CrossRef]
- Hassamal, S.; Miotto, K.; Dale, W.; Danovitch, I. Tramadol: Understanding the Risk of Serotonin Syndrome and Seizures. Am. J. Med. 2018, 131, 1382.e1–1382.e6. [Google Scholar] [CrossRef]
- Patocka, J.; Wu, W.; Oleksak, P.; Jelinkova, R.; Nepovimova, E.; Spicanova, L.; Springerova, P.; Alomar, S.; Long, M.; Kuca, K. Fentanyl and its derivatives: Pain-killers or man-killers? Heliyon 2024, 10, e28795. [Google Scholar] [CrossRef] [PubMed]
- Stanley, T.H. Fentanyl. J. Pain Symptom Manag. 2005, 29, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Stanley, T.H. The fentanyl story. J. Pain 2014, 15, 1215–1226. [Google Scholar] [CrossRef]
- Heilig, M.; Petrella, M. Neural pathways for reward and relief promote fentanyl addiction. Nature 2024, 630, 38–39. [Google Scholar] [CrossRef] [PubMed]
- Masud, M.; Chan, H.; Erdelyi, S.; Yuan, Y.; Brubacher, J.R. Epidemiology of drug driving: Protocol from a national Canadian study measuring levels of cannabis, alcohol and other substances in injured drivers. BMC Public Health 2020, 20, 1070. [Google Scholar] [CrossRef]
- Aglan, M.; Adawi, A. Incidence of substance abuse among cab-drivers involved in non fatal accidents. Trends Med. Res. 2016, 11, 20–27. [Google Scholar] [CrossRef][Green Version]
- Thomas, F.D.; Darrah, J.; Graham, L.; Berning, A.; Blomberg, R.; Finstad, K.; Griggs, C.; Crandall, M.; Schulman, C.; Kozar, R.; et al. Alcohol and Drug Prevalence Among Seriously or Fatally Injured Road Users; National High-way Traffic Safety Administration (NHTSA); U.S. Department of Transportation; Washington, DC, USA, 2022; pp. 10–11. Available online: https://www.nhtsa.gov/sites/nhtsa.gov/files/2022-12/Alcohol-Drug-Prevalence-Among-Road-Users-Report_112922-tag.pdf (accessed on 27 July 2025).[Green Version]
- Musshoff, F.; Madea, B. Driving Under the Influence of Amphetamine-Like Drugs. J. Forensic Sci. 2012, 57, 413–419. [Google Scholar] [CrossRef]
- Pelletti, G.; Boscolo-Berto, R.; Barone, R.; Giorgetti, A.; Fiorentini, C.; Pascali, J.P.; Fais, P.; Pelotti, S. Gender differences in driving under the influence of psychoactive drugs: Evidence mapping of real case studies and meta-analysis. Forensic Sci. Int. 2022, 341, 111479. [Google Scholar] [CrossRef]
- McGrane, I.R.; Ramsbacher, N.C.; Rook, W.C.; Omar, F.A. Effects of 3,4-methylenedioxymethamphetamine and methamphetamine on motor vehicle driving performance: A systematic review of experimental and observational studies. J. Forensic Sci. 2022, 68, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Parrott, A.C.; Gibbs, A.; Scholey, A.B.; King, R.; Owens, K.; Swann, P.; Ogden, E.; Stough, C. MDMA and methamphetamine: Some paradoxical negative and positive mood changes in an acute dose laboratory study. Psychopharmacology 2011, 215, 527–536. [Google Scholar] [CrossRef]
- Hayley, A.C.; Ogeil, R.P.; Faulkner, A.; Beard, N.; Downey, L.A.; Smith, K.; Lubman, D.I.; Scott, D. The Incidence and Temporal Patterns of Use of Amphetamine-Type Stimulant Use in Traffic-Related Ambulance Attendances From 2015 to 2020 in Victoria, Australia. J. Stud. Alcohol Drugs 2023, 84, 128–136. [Google Scholar] [CrossRef]
- Nowicki, P.; Klos, J.; Kokot, Z.J. Amphetamines in wastewater of the city Poznań (Poland)–estimation of drug abuse. Acta Pol. Pharm. 2014, 71, 25–33. [Google Scholar]
- Kirkpatrick, M.G.; Johanson, C.E.; Wit, H.D. Personality and the acute subjective effects of d-amphetamine in humans. J. Psychopharmacol. Oxf. Engl. 2013, 27, 256. [Google Scholar] [CrossRef]
- Heide, G.; Jamt, R.E.G.; Fainberg-Sandbu, J.; Øiestad, Å.M.L.; Høiseth, G. Driving under the influence of cocaine and MDMA: Relationship between blood concentrations and results from clinical test of impairment. J. Anal. Toxicol. 2024, 48, 380–387. [Google Scholar] [CrossRef]
- Brookhuis, K.A.; de Waard, D.; Samyn, N. Effects of MDMA (ecstasy), and multiple drugs use on (simulated) driving performance and traffic safety. Psychopharmacology 2004, 173, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Steinkellner, T.; Freissmuth, M.; Sitte, H.H.; Montgomery, T. The ugly side of amphetamines: Short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”), methamphetamine and d-amphetamine. Biol. Chem. 2011, 392, 103. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.; Toyang, N.; Steele, B.; Grant, J.; Ali, A.; Gordon, L.; Ngwa, W. Psychedelics: Alternative and Potential Therapeutic Options for Treating Mood and Anxiety Disorders. Molecules 2022, 27, 2520. [Google Scholar] [CrossRef]
- Bazin, B.; Duroy, D.; Lejoyeux, M. MDMA Use by Paris Medical Students: Prevalence and Characteristics. Subst. Use Misuse 2021, 56, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Lake, S.; Gaddis, A.; Tupper, K.W.; Nosova, E.; DeBeck, K. 3,4-Methylenedioxymethamphetamine (MDMA; ecstasy) use and transitions to injection drug use among street-involved youth. Subst. Abus. 2019, 40, 350–355. [Google Scholar] [CrossRef]
- Madaan, V.; Bhaskar, S.; Donnelly, G.A.E.; Cox, D.J. A randomized, phase 3, double-blind, crossover comparison of multilayer, extended-release methylphenidate (PRC-063), and lisdexamfetamine in the driving performance of young adults with ADHD. J. Atten. Disord. 2024, 28, 947–956. [Google Scholar] [CrossRef]
- Randell, N.J.S.; Charlton, S.G.; Starkey, N.J. Driving With ADHD: Performance Effects and Environment Demand in Traffic. J. Atten. Disord. 2016, 24, 1570–1580. [Google Scholar] [CrossRef]
- Groom, M.J.; Cortese, S. Current Pharmacological Treatments for ADHD. Curr. Top. Behav. Neurosci. 2022, 57, 19–50. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.J.; Merkel, R.L.; Moore, M.; Thorndike, F.; Muller, C.; Kovatchev, B. Relative benefits of stimulant therapy with OROS methylphenidate versus mixed amphetamine salts extended release in improving the driving performance of adolescent drivers with attention-deficit/hyperactivity disorder. Pediatrics 2006, 118, e704-10. [Google Scholar] [CrossRef]
- Louw, W.A.N.; Davids, R.A. Prevalence of methylphenidate use by Master of Medicine students at a South African university. Postgrad. Med. J. 2022, 98, 925–929. [Google Scholar] [CrossRef]
- Gobbo, M.A.; Louzã, M.R. Influence of stimulant and non-stimulant drug treatment on driving performance in patients with attention deficit hyperactivity disorder: A systematic review. Eur. Neuropsychopharmacol. 2014, 24, 1425–1443. [Google Scholar] [CrossRef]
- Chan-Hosokawa, A.; Bierly, J.J. 11-Year Study of Fentanyl in Driving Under the Influence of Drugs Casework. J. Anal. Toxicol. 2021, 46, 337–341. [Google Scholar] [CrossRef]
- Sabatowski, R.; Schwalen, S.; Rettig, K.; Herberg, K.W.; Kasper, S.M.; Radbruch, L. Driving ability under long-term treatment with transdermal fentanyl. J. Pain Symptom Manag. 2003, 25, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Rohrig, T.P.; Nash, E.; Osawa, K.A.; Shan, X.; Scarneo, C.; Youso, K.B.; Miller, R.; Tiscione, N.B. Fentanyl and Driving Impairment. J. Anal. Toxicol. 2020, 45, 389–396. [Google Scholar] [CrossRef]
- Menefee, L.A.; Frank, E.D.; Crerand, C.; Jalali, S.; Park, J.; Sanschagrin, K.; Besser, M. The effects of transdermal fentanyl on driving, cognitive performance, and balance in patients with chronic nonmalignant pain conditions. Pain Med. 2004, 5, 42–49. [Google Scholar] [CrossRef]
- Kiely, E.; Juhascik, M. Fentanyl, Acetylfentanyl and Carfentanil in Impaired Driving Cases: A Review of 270 Cases. J. Anal. Toxicol. 2021, 45, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Specka, M.; Finkbeiner, T.; Lodemann, E.; Leifert, K.; Kluwig, J.; Gastpar, M. Cognitive-Motor Performance of Methadone-Maintained Patients. Eur. Addict. Res. 2000, 6, 8–19. [Google Scholar] [CrossRef]
- Rass, O.; Schacht, R.L.; Buckheit, K.; Johnson, M.W.; Strain, E.C.; Mintzer, M.Z. A randomized controlled trial of the effects of working memory training in methadone maintenance patients. Drug Alcohol Depend. 2015, 156, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Veldhuijzen, D.S.; van Wijck, A.J.M.; Wille, F.; Verster, J.C.; Kenemans, J.L.; Kalkman, C.J.; Olivier, B.; Volkerts, E.R. Effect of chronic nonmalignant pain on highway driving performance. PAIN 2006, 122, 28. [Google Scholar] [CrossRef] [PubMed]
- Strumpf, M.; Köhler, A.; Zenz, M.; Willweber-Strumpf, A.; Dertwinkel, R.; Donner, B. Opioide und fahrtuchtigkeit. Schmerz 1997, 11, 233–240. [Google Scholar] [CrossRef]
- Salas-Wright, C.P.; Cano, M.; Hodges, J.; Oh, S.; Hai, A.H.; Vaughn, M.G. Driving while under the influence of hallucinogens: Prevalence, correlates, and risk profiles. Drug Alcohol Depend. 2021, 228, 109055. [Google Scholar] [CrossRef]
- Tirri, M.; Bilel, S.; Arfè, R.; Corli, G.; Marchetti, B.; Bernardi, T.; Boccuto, F.; Serpelloni, G.; Botrè, F.; De-Giorgio, F.; et al. Effect of -NBOMe Compounds on Sensorimotor, Motor, and Prepulse Inhibition Responses in Mice in Comparison With the 2C Analogs and Lysergic Acid Diethylamide: From Preclinical Evidence to Forensic Implication in Driving Under the Influence of Drugs. Front. Psychiatry 2022, 13, 875722. [Google Scholar] [CrossRef]
- Harvey, P.D. Domains of cognition and their assessment. Dialogues Clin. Neurosci. 2019, 21, 227–237. [Google Scholar] [CrossRef] [PubMed]
- McMullan, R.D.; Aryal, N.; Li, L.; Wiggins, M.; Clive, J.; Westbrook, J.I. Working memory capacity improves checking performance for errors on a simulated rail control task. Appl. Ergon. 2025, 125, 104482. [Google Scholar] [CrossRef]
- Ledger, S.; Bennett, J.M.; Chekaluk, E.; Batchelor, J.; Meco, A.D. Cognitive function and driving in middle adulthood: Does age matter? Transp. research. Part F Traffic Psychol. Behav. 2019, 66, 471–484. [Google Scholar] [CrossRef]
- Branch, S.; Espinosa, N.; McKinnon, A.C.; Bennett, J.M. The relationship between brain structure and function and driving performance in older adults with and without cognitive impairment: A systematic review. Transp. research. Part F Traffic Psychol. Behav. 2025, 109, 1523–1541. [Google Scholar] [CrossRef]
- Zhu, Y.; Yue, L.; Zhang, Q.; Sun, J. Modeling distracted driving behavior considering cognitive processes. Accid. Anal. Prev. 2024, 202, 107602. [Google Scholar] [CrossRef]
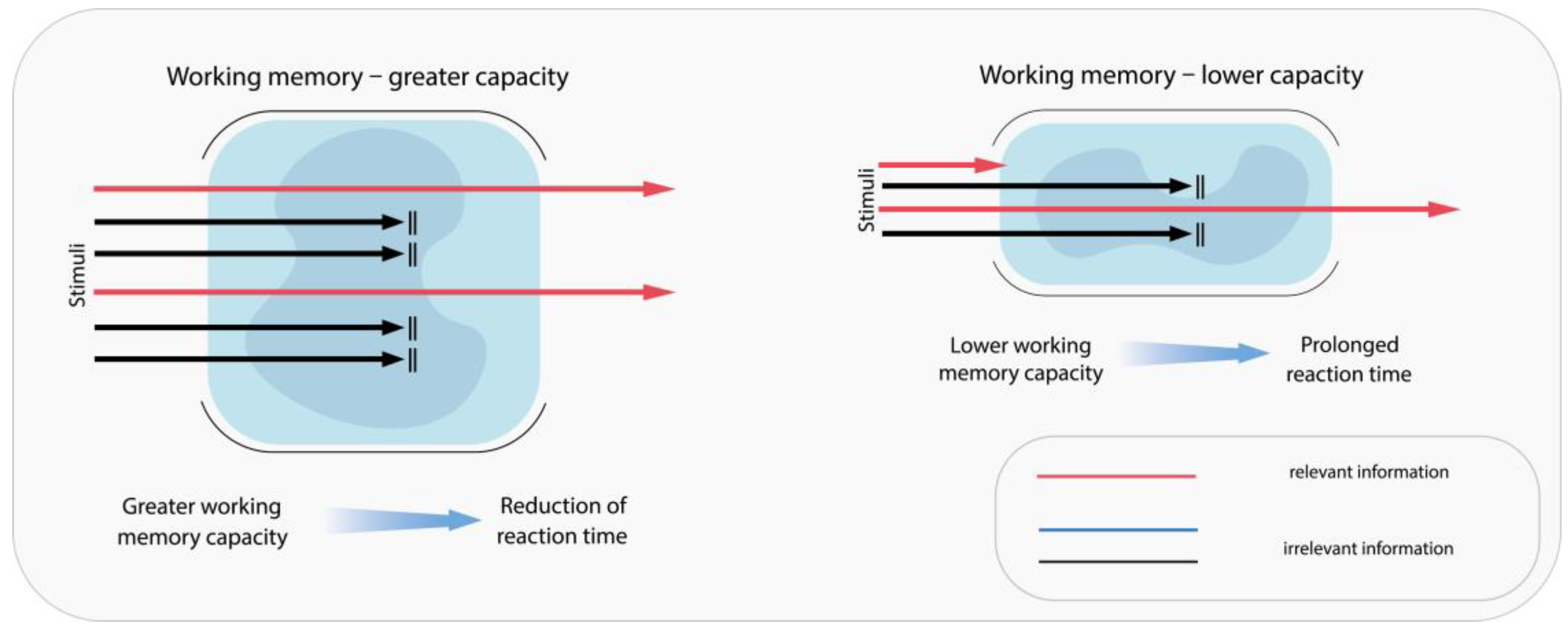
- Zhang, H.; Guo, Y.; Yuan, W.; Li, K. On the importance of working memory in the driving safety field: A systematic review. Accid. Anal. Prev. 2023, 187, 107071. [Google Scholar] [CrossRef]
- Ben-Artzi, I.; Luria, R.; Shahar, N. Working memory capacity estimates moderate value learning for outcome-irrelevant features. Sci. Rep. 2022, 12, 19677. [Google Scholar] [CrossRef]
- Ross, V.; Jongen, E.M.M.; Wang, W.; Brijs, T.; Brijs, K.; Ruiter, R.A.C.; Wets, G. Investigating the influence of working memory capacity when driving behavior is combined with cognitive load: An LCT study of young novice drivers. Accid. Anal. Prev. 2014, 62, 377–387. [Google Scholar] [CrossRef]
- Droździel, P.; Tarkowski, S.; Rybicka, I.; Wrona, R. Drivers ’reaction time research in the conditions in the real traffic. Open Eng. 2020, 10, 35–47. [Google Scholar] [CrossRef]
- Jeong, M.; Tashiro, M.; Singh, L.N.; Yamaguchi, K.; Horikawa, E.; Miyake, M.; Watanuki, S.; Iwata, R.; Fukuda, H.; Takahashi, Y.; et al. Functional brain mapping of actual car-driving using [18F]FDG-PET. Ann. Nucl. Med. 2006, 20, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.W.; Chatziastros, A.; von der Heyde, M.; Bülthoff, H.H. Driving in the future: Temporal visuomotor adaptation and generalization. J. Vis. 2001, 1, 88–98. [Google Scholar] [CrossRef]
- United Nations. Single Convention on Narcotic Drugs (1961) 1961. Available online: https://libr.sejm.gov.pl/tek01/txt/onz/1961b.html (accessed on 11 March 2025).
- Regulation of the Minister of Health. Rozporządzenie Ministra Zdrowia z dnia 17 Kwietnia 2023 r. w Sprawie Wykazu Substancji Psychotropowych, środków Odurzających oraz Nowych Substancji Psychoaktywnych. Dziennik Ustaw. 2023. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20230000744 (accessed on 11 March 2025).
- Polska, S.N.R. Supreme Court of Poland. Uchwała Sądu Najwyższego z dnia 27 lutego 2007 r., sygn. akt I KZP 36/06. Sąd Najwyższy. 2007. Available online: https://www.sn.pl/sites/orzecznictwo/Orzeczenia1/I%20KZP%2036-06.pdf (accessed on 11 March 2025).
- Act on the Professions of Physician and Dentist. Ustawa z dnia 5 Grudnia 1996 r. o Zawodach Lekarza i Lekarza Dentysty. Dz.U. 1997 nr 28 poz. 152. Dziennik Ustaw. 1996. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu19970280152 (accessed on 11 March 2025).
- Polska, R. Polish Penal Code. Kodeks Karny. Dziennik Ustaw. Dz.U. 1997 nr 88 poz. 553, June 1997. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU1997088055 (accessed on 11 March 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021, 372, n71. [Google Scholar] [CrossRef]
- Möhring, L.; Gläscher, J. Prediction errors drive dynamic changes in neural patterns that guide behavior. Cell Rep. 2023, 42, 112931. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Hjälmdahl, M.; Vadeby, A.; Forsman, A.; Fors, C.; Ceder, G.; Woxler, P.; Kronstrand, R. Effects of d-amphetamine on simulated driving performance before and after sleep deprivation. Psychopharmacology 2012, 222, 401–411. [Google Scholar] [CrossRef]
- Kay, G.G.; Michaels, M.A.; Pakull, B. Simulated driving changes in young adults with ADHD receiving mixed amphetamine salts extended release and atomoxetine. J. Atten. Disord. 2009, 12, 316–329. [Google Scholar] [CrossRef]
- Silber, B.Y.; Papafotiou, K.; Croft, R.J.; Ogden, E.; Swann, P.; Stough, C. The effects of dexamphetamine on simulated driving performance. Psychopharmacology 2005, 179, 536–543. [Google Scholar] [CrossRef]
- Silber, B.Y.; Croft, R.J.; Papafotiou, K.; Stough, C. The acute effects of d-amphetamine and methamphetamine on attention and psychomotor performance. Psychopharmacology 2006, 187, 154–169. [Google Scholar] [CrossRef]
- Bosanquet, D.; Macdougall, H.G.; Rogers, S.J.; Starmer, G.A.; McKetin, R.; Blaszczynski, A.; McGregor, I.S. Driving on ice: Impaired driving skills in current methamphetamine users. Psychopharmacology 2013, 225, 161–172. [Google Scholar] [CrossRef]
- Stough, C.; Downey, L.A.; King, R.; Papafotiou, K.; Swann, P.; Ogden, E. The acute effects of 3,4-methylenedioxymethamphetamine and methamphetamine on driving: A simulator study. Accid. Anal. Prev. 2012, 45, 493–497. [Google Scholar] [CrossRef]
- Barkley, R.A.; Murphy, K.R.; O’Connell, T.; Connor, D.F. Effects of two doses of methylphenidate on simulator driving performance in adults with attention deficit hyperactivity disorder. J. Saf. Res. 2005, 36, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Sobanski, E.; Sabljic, D.; Alm, B.; Skopp, G.; Kettler, N.; Mattern, R.; Strohbeck-Kühner, P. Driving-related risks and impact of methylphenidate treatment on driving in adults with attention-deficit/hyperactivity disorder (ADHD). J. Neural Transm. 2008, 115, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.J.; Davis, M.; Mikami, A.Y.; Singh, H.; Merkel, R.L.; Burket, R. Long-acting methylphenidate reduces collision rates of young adult drivers with attention-deficit/hyperactivity disorder. J. Clin. Psychopharmacol. 2012, 32, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Verster, J.C.; Roth, T. Methylphenidate significantly reduces lapses of attention during on-road highway driving in patients with ADHD. J. Clin. Psychopharmacol. 2014, 34, 633–636. [Google Scholar] [CrossRef][Green Version]
- Aitken, B.; Downey, L.A.; Rose, S.; Arkell, T.R.; Shiferaw, B.; Hayley, A.C. Driving performance and ocular activity following acute administration of 10 mg methylphenidate: A randomised, double-blind, placebo-controlled study. J. Psychopharmacol. 2024, 38, 998–1006. [Google Scholar] [CrossRef]
- Samyn, N.; Boeck, G.D.; Verstraete, A. The use of oral fluid and sweat wipes for the detection of drugs of abuse in drivers. J. Forensic Sci. 2002, 47, 1380–1387. [Google Scholar] [CrossRef]
- Bosker, W.M.; Kuypers, K.P.C.; Conen, S.; Kauert, G.F.; Toennes, S.W.; Skopp, G.; Ramaekers, J.G. MDMA (ecstasy) effects on actual driving performance before and after sleep deprivation, as function of dose and concentration in blood and oral fluid. Psychopharmacology 2012, 222, 367–376. [Google Scholar] [CrossRef]
- Kuypers, K.P.C.; Samyn, N.; Ramaekers, J.G. MDMA and alcohol effects, combined and alone, on objective and subjective measures of actual driving performance and psychomotor function. Psychopharmacology 2006, 187, 467–475. [Google Scholar] [CrossRef]
- Dastrup, E.; Lees, M.N.; Bechara, A.; Dawson, J.D.; Rizzo, M. Risky car following in abstinent users of MDMA. Accid. Anal. Prev. 2010, 42, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, D.R.; Chung, F.; Smiley, A. General anesthesia does not impair simulator driving skills in volunteers in the immediate recovery period—A pilot study. J. Can. D’anesthesie [Can. J. Anaesth.] 2003, 50, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Hayley, A.C.; Downey, L.A.; Green, M.; Shiferaw, B.; Kenneally, M.; Keane, M.; Adams, M.; Shehabi, Y. Driving Simulator Performance After Administration of Analgesic Doses of Ketamine With Dexmedetomidine or Fentanyl. J. Clin. Psychopharmacol. 2019, 39, 446. [Google Scholar] [CrossRef]
- Kosten, T.R.; George, T.P. The Neurobiology of Opioid Dependence: Implications for Treatment. Sci. Pract. Perspect. 2002, 1, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Yarotskyy, V.; Nass, S.R.; Hahn, Y.-K.; Contois, L.; McQuiston, A.R.; Knapp, P.E.; Hauser, K.F. Sustained fentanyl exposure inhibits neuronal activity in dissociated striatal neuronal-glial cocultures through actions independent of opioid receptors. J. Neurophysiol. 2024, 132, 1056–1073. [Google Scholar] [CrossRef]
- Lam, D.; Sebastian, A.; Bogguri, C.; Hum, N.R.; Ladd, A.; Cadena, J.; Valdez, C.A.; Fischer, N.O.; Loots, G.G.; Enright, H.A. Dose-dependent consequences of sub-chronic fentanyl exposure on neuron and glial co-cultures. Front. Toxicol. 2022, 4, 983415. [Google Scholar] [CrossRef]
- Collin, E.; Cesselin, F. Neurobiological Mechanisms of Opioid Tolerance and Dependence. Clin. Neuropharmacol. 1991, 14, 465. [Google Scholar] [CrossRef]
- Feng, J.; Xu, N.; Wang, L.; Wang, H.; Zhou, Y.; Shen, Q. Synaptic Structure and Transcriptomic Profiling of Reward and Sensory Brain Areas in Male Mice of Fentanyl Addiction. Subst. Abus. Rehabil. 2024, 15, 233–245. [Google Scholar] [CrossRef]
- Bramness, J.G.; Gundersen, Ø.H.; Guterstam, J.; Rognli, E.B.; Konstenius, M.; Løberg, E.M.; Medhus, S.; Tanum, L.; Franck, J. Amphetamine-induced psychosis—A separate diagnostic entity or primary psychosis triggered in the vulnerable? BMC Psychiatry 2012, 12, 221. [Google Scholar] [CrossRef]
- Baewert, A.; Gombas, W.; Schindler, S.-D.; Peternell-Moelzer, A.; Eder, H.; Jagsch, R.; Fischer, G. Influence of peak and trough levels of opioid maintenance therapy on driving aptitude. Eur. Addict. Res. 2007, 13, 127–135. [Google Scholar] [CrossRef]
- Schindler, S.-D.; Ortner, R.; Peternell, A.; Eder, H.; Opgenoorth, E.; Fischer, G. Maintenance therapy with synthetic opioids and driving aptitude. Eur. Addict. Res. 2004, 10, 80–87. [Google Scholar] [CrossRef]
- Strand, M.C.; Ramaekers, J.G.; Gjerde, H.; Mørland, J.; Vindenes, V. Pharmacokinetics of single doses of methadone and buprenorphine in blood and oral fluid in healthy volunteers and correlation with effects on psychomotor and cognitive functions. J. Clin. Psychopharmacol. 2019, 39, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-H.; Ho, P.-S.; Wu, T.-N.; Wang, P.-W.; Lin, C.-H.R.; Tsai, J.-H.; Guo, Y.L.; Chuang, H.-Y. Risk of motor vehicle collisions after methadone use. eLife 2021, 10, e63954. [Google Scholar] [CrossRef] [PubMed]
- Lenné, M.G.; Dietze, P.; Rumbold, G.R.; Redman, J.R.; Triggs, T.J. The effects of the opioid pharmacotherapies methadone, LAAM and buprenorphine, alone and in combination with alcohol, on simulated driving. Drug Alcohol Depend. 2003, 72, 271–278. [Google Scholar] [CrossRef]
- Brubacher, J.R.; Erdelyi, S.; Chan, H.; Simmons, S.; Atkinson, P.; Besserer, F.; Clarke, D.B.; Davis, P.; Daoust, R.; Émond, M.; et al. Prevalence of impairing substance use in injured drivers. JAMA Netw. Open 2025, 8, e256379. [Google Scholar] [CrossRef]
- Silber, B.Y.; Croft, R.J.; Downey, L.A.; Papafotiou, K.; Camfield, D.A.; Stough, C. The effect of d-methamphetamine on simulated driving performance. Hum. Psychopharmacol. 2012, 27, 139–144. [Google Scholar] [CrossRef]
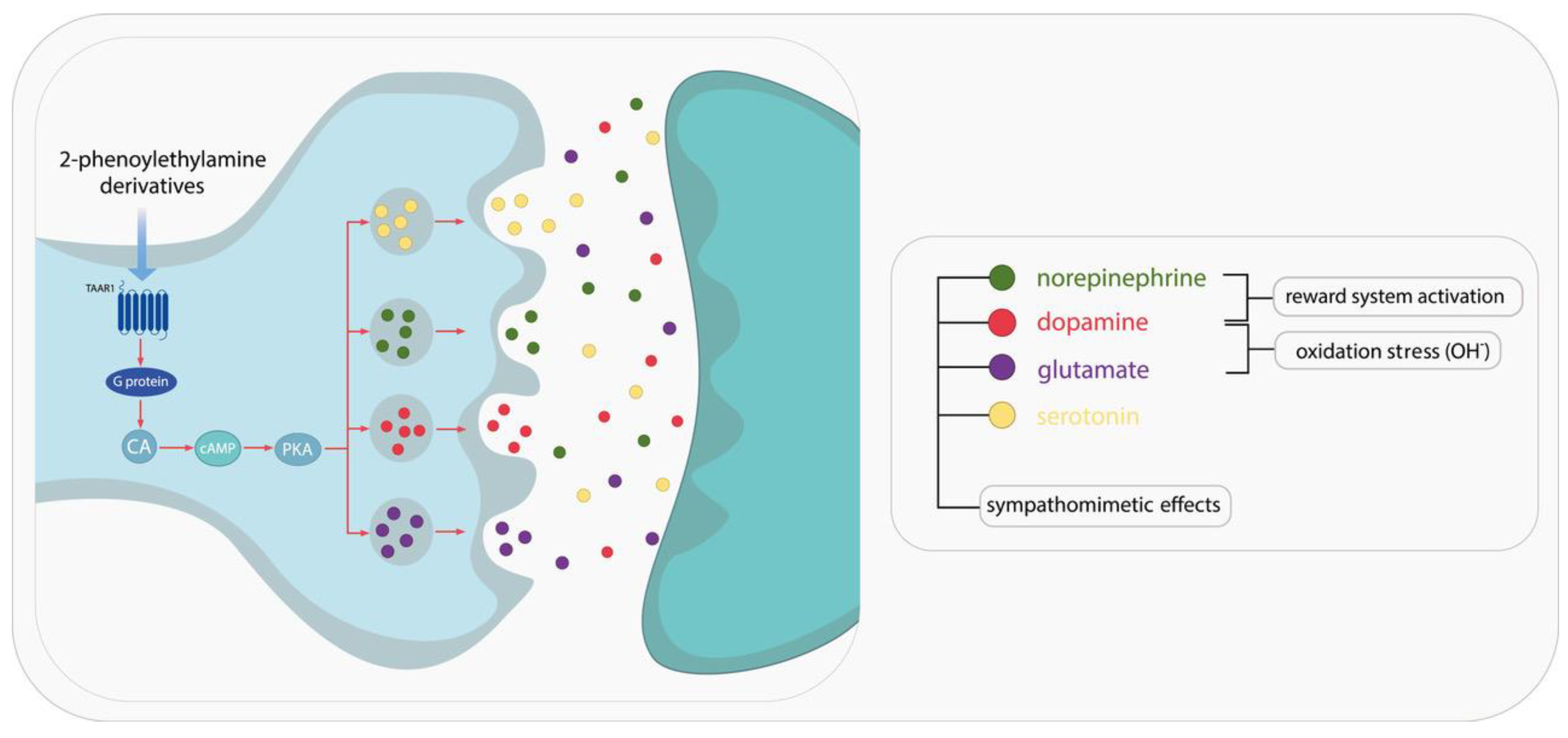


| Chemical Compound | Chemical Structure | Mode of Action | Medical Properties, Adverse Effects |
|---|---|---|---|
| 2-Phenylethylamine (PEA, β-phenylethylamine) | 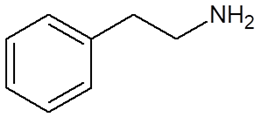 |
|
|
| Methamphetamine (METH) | 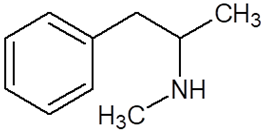 |
|
|
| Methylphenidate | 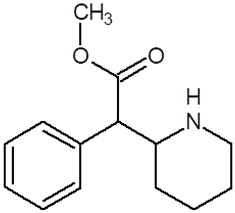 |
|
|
| Amphetamine | 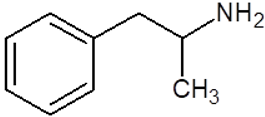 |
|
|
| Methylenedioxyamphetamine (3,4-methylenedioxymethamphetamine, MDMA, ecstasy) | 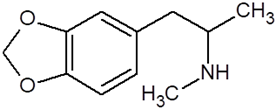 |
|
|
| 2,5-dimethoxy-4-methylamphetamine (DOM) | 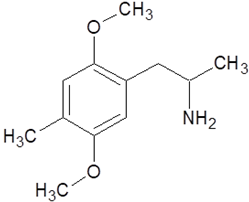 |
| |
| Mescaline | 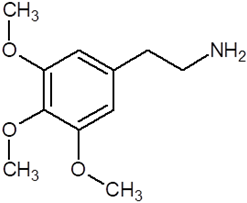 |
|
|
| Methadone | 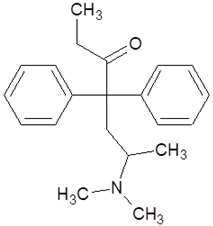 |
|
|
| Tramadol | 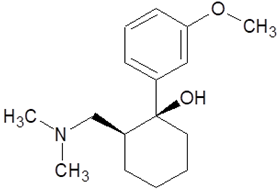 |
|
|
| Fentanyl | 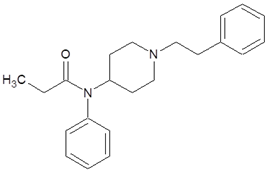 |
|
|
| References: Author, Year | Patients | Intervention | Comparison | Outcome | Limitations | Risk of Bias |
|---|---|---|---|---|---|---|
| Hayley, 2023 [51] | n = 20 Healthy individuals reporting recreational stimulant use | 0.42 mg/kg methamphetamine, with or without alcohol (0.38 g/kg) | RCT with placebo | Deterioration in methamphetamine group and alcohol-methamphetamine group compared to placebo, with approximately 19 times higher risk of collision | Not stated | L |
| Brookhuis, 2004 [55] | n = 20 Healthy MDMA users | MDMA (avg. 59 mg) with or without other substances (marijuana, alcohol, LSD) | simulation study Same population sober and after using substances | 2 times higher simulated collision rate after MDMA, 2.5 times higher after polysubstance use | A quasi-experimental design | M |
| Madaan, 2024 [60] | n = 44 Individuals diagnosed with ADHD | Mixed-layer extended-release methylphenidate, in dose optimized individually during 21 day period | RCT Individuals treated with lisdexamphetamine dimesylate in therapeutic dose | No significant differences between lisdexamphetamine dimesylate and mixed-layer extended-release methylphenidate groups | Omission of a placebo group | L |
| Cox, 2006 [63] | n = 35 individuals with diagnosed ADHD | OSMOS methylphenidate, followed by extended-release mixed amphetamine salts | RCT with placebo | Participants and researchers reported noticeable driving improvements, with association in fewer past collisions | Difference in potency of MPH and amps Few participants with hyperactive subtype | L |
| Menefee, 2004 [69] | n = 23 Patients with chronic pain | Transdermal fentanyl, dosage 25 µg/h increasing to 50 µg/h | observational study Comparison to the same group before starting therapy | No significant deterioration in driving abilities compared to placebo group | Small sample | M |
| Hjälmdahl, 2012 [96] | n = 18 Healthy individuals | 10 mg or 40 mg amphetamine | RCT with placebo | Improved performance after 10 mg, no enhanced or sustained effects after 40 mg | Gender inequality (only male subjects); risk of learning effect in the study | L |
| Kay, 2009 [97] | n = 19 Adults with diagnosed ADHD | Single-dose mixed amphetamine salts – extended release (MAS XR) 50 mg/d | simulation study Placebo, 80 mg atomoxetine | improved performance in 80% individuals on MAS XR, 40% in individuals on atomoxetine compared to placebo | Young, inexpirienced in driving population (Age: 19–25); patients with ADHD | L |
| Silber, 2005 [98] | n = 20 Healthy individuals | 30 mg dexamphetamine | RCT with placebo | Significant deterioration compared to placebo | Not randomized tests | L |
| Silber, 2006 [99] | n = 20 Healthy individuals with stimulant experience | 0.42 mg/kg of d-amphetamine, d,l-methamphetamine or d-methamphetamine | RCT with placebo | Low doses generally improved attention, psychomotor functions and perception speed | Not assessed baseline; brief practice sessions | M |
| Bosanquet, 2013 [100] | n = 30 Individuals reporting using methamphetamine at least once a week over the past three months | Participants tested for presence of psychoactive substances | observational study Individuals who reported no chronic or episodic drug use thorough their lifetime | Methamphetamine users exhibited riskier driving behavior compared to control group | Not stated | M |
| Stough, 2012 [101] | n = 61 | MDMA in 100 mg dose or 0,42 mg/kg methamphetamine | RCT with placebo | Decrease in performance in methamphetamine and MDMA group compared to placebo | Not stated | M |
| Barkley, 2005 [102] | n = 52 Adults with diagnosed ADHD | One dose of 10 mg or 20 mg methylphenidate | RCT Crossover type placebo | Significant reduction in the number of impulsive errors in high dose group compared to placebo | Using a single acute dose of medication Using relatively low doses Using immediate release methylphenidate | L |
| Sobanski, 2008 [103] | n = 27 Individuals diagnosed with ADHD | Methylphenidate in extended release form in range: 30–60 mg | observational study Individuals with ADHD without treatment, healthy individuals without treatment | Increase in driving performance after 6-week methylphenidate treatment compared to no treatment in adults with or without ADHD | Small sample size No multiple testing | M |
| Cox, 2012 [104] | n = 17 Individuals with ADHD not routinely using ADHD medications | Participants randomly assigned to one of two sequences: mo treatment for 3 months followed by 3 months transdermal methylphenidate in dose 10–30 mg, or reverse order | Patients driving vehicles for 3 months without medication | During the use of transdermal methylphenidate system patients exhibited significantly fewer ADHD symptoms and experienced fewer traffic accidents compared to the period without medication | Small sample Open study | M |
| Verster, 2014 [105] | n = 18 Individuals diagnosed with ADHD | Individually tailored dose of methylphenidate | RCT with placebo | Methylphenidate significantly improved driving ability in adults with ADHD | Not stated | M |
| Aitken, 2024 [106] | n = 25 Healthy individuals | Single dose of 10 mg methylphenidate | RCT with placebo | Methylphenidate significantly improved driving ability | Low complexity of the simulated highway drive, the demands on gaze behaviour, the necessity for frequent and varied eye movements, possible ceiling effect | L |
| Samyn, 2002 [107] | n = 31 Healthy MDMA users | MDMA in 75 mg dose | simulation study Same population before and after administration | No significant change in performance | Not stated | L |
| Bosker, 2012 [108] | n = 16 Healthy individuals | Single-dose MDMA, 25 mg, 50 mg or 100 mg | RCT with placebo | No significant deterioration in driving abilities compared to placebo group | Not stated | L |
| Kuypers, 2006 [109] | n = 18 Healthy individuals | Single-dose MDMA, 75 mg or 100 mg, the same doses combined with alcohol | RCT with placebo | MDMA group was comparable to placebo group, MDMA + alcohol gropu performance was significantly deteriorated | Not stated | L |
| Dastrup, 2010 [110] | n = 16 Healthy individuals, MDMA-polisubstance users during abstinence | No MDMA administration | observational study Comparison to group never using MDMA | No significant deterioration in driving abilities in MDMA-abstinent group compared to never using group | “Nature-nurture” issues regarding patients | L |
| Sinclair, 2003 [111] | n = 12 Healthy individuals | General anesthesia induced by 2,5 mg/kg propofol and 1 μg/kg fentanyl or alcohol in dose inducing 0,08% BAC | simulation study The same group not recieving treatment, driving efficiency was tested in 2, 3, 4, and 24 h after inducing general anesthesia | No significant difference was observed in general anesthesia group after 2, 3, 4 and 24 h, with significant impairment in alcohol group | Results obtained without premedication and other adjuvants | L |
| Hayley, 2019 [112] | n = 41 Healthy individuals | 0.15 mg/kg/h of ketamine for 3 h, followed by three boluses of 25 μg fentanyl | simulation study Same population before and after administration | Driving simulator results after administration of analgesic doses of ketamine and fentanyl were comparable to baseline | Not stated | M |
| Bramness, 2012 [118] | All Norvegians aged 18 to 69 years | Patients exposed to varying durations of methadone treatment in various form | observational study People not exposed to methadone in the same group | Exposure to methadone was associated with an approximately twice as high increase in risk of involvement in road traffic accidents resulting in bodily injury | Not stated | M |
| Baewert, 2007 [119] | n = 40 Opioid dependent individuals, maintained on buprenorphine or methadone for at least 2 months | Average dose of methadone 52.7 mg, and 13.4 mg buprenorphine | observational study Selected from a sample of 14,500 individuals who had previously completed 2020 standard tests for the Austrian Road Safety Board | Participants undergoing maintenence therapy performed worse in tests compared to control group | Not stated | M |
| Schindler, 2004 [120] | n = 30 Individuals addicted to opiates in substitutional treatment with methadone or buprenorphine | Standard doses of methadone or buprenorphine | observational study Healthy individuals without opiate-addiction history | Significantly increased errors rate and reaction time in methadone group | Not stated | M |
| Strand, 2019 [121] | n = 22 Healthy individuals | Two single doses of methadone (5 mg and 10 mg) and buprenorphine (0.2 mg and 0.4 mg) | RCT with placebo | Increased driving impairment in both groups compared to placebo | Not stated | L |
| Yang, 2021 [122] | n = 1012 Individuals with opiate use history | Undergoing methadone substitution treatment | observational study Adult opiate users not undergoing methadone substitution treatment | increase in colision risk in population undergoing methadone substitution treatment | The lack of information on road exposure Unreported or minor motor vehicle collisions not be included in the study | M |
| Lenné, 2003 [123] | n = 10 Patients treated with methadone | Methadone treatment with or without alcohol | observational study Individuals not using drugs | No significant differences in driving performance between methadone and control group | Not stated | L |
| Brubacher, 2025 [124] | n = 8328 Individuals with blood drawn within 6 h after crash | Blood obtained for research toxicology testing | observational study Drivers in whom no substances impairing the ability to drive were detected. | 10.9% drivers were found driving under the influence of opiates, psychoactive substances were found in 56.% men and 52.4% women | No interview or examination of drivers Unavailability of excess blood | M |
| Silber, 2012 [125] | n = 20 Healthy recreational users of illicit stimulants | 0.42 mg/kg of d,l-methamphetamine | RCT with placebo | No significant impairment in simulated driving performance | Not stated | L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żełabowski, K.; Biedka, K.; Pichowicz, W.; Sterkowicz, M.; Radzka, I.; Ilski, I.; Wesołowski, M.; Wojtysiak, K.; Petrov, W.; Ślebioda, D.; et al. Review of Selected 2-Phenylethylamine Derivatives and Opioids, Systematic Review of Their Effects on Psychomotor Abilities and Driving Performance: Psychopharmacology in the Context of Road Safety. Pharmaceuticals 2025, 18, 1555. https://doi.org/10.3390/ph18101555
Żełabowski K, Biedka K, Pichowicz W, Sterkowicz M, Radzka I, Ilski I, Wesołowski M, Wojtysiak K, Petrov W, Ślebioda D, et al. Review of Selected 2-Phenylethylamine Derivatives and Opioids, Systematic Review of Their Effects on Psychomotor Abilities and Driving Performance: Psychopharmacology in the Context of Road Safety. Pharmaceuticals. 2025; 18(10):1555. https://doi.org/10.3390/ph18101555
Chicago/Turabian StyleŻełabowski, Kacper, Kamil Biedka, Wojciech Pichowicz, Maria Sterkowicz, Izabela Radzka, Ignacy Ilski, Michał Wesołowski, Kacper Wojtysiak, Wiktor Petrov, Dawid Ślebioda, and et al. 2025. "Review of Selected 2-Phenylethylamine Derivatives and Opioids, Systematic Review of Their Effects on Psychomotor Abilities and Driving Performance: Psychopharmacology in the Context of Road Safety" Pharmaceuticals 18, no. 10: 1555. https://doi.org/10.3390/ph18101555
APA StyleŻełabowski, K., Biedka, K., Pichowicz, W., Sterkowicz, M., Radzka, I., Ilski, I., Wesołowski, M., Wojtysiak, K., Petrov, W., Ślebioda, D., Rząca, M., & Chłopaś-Konowałek, A. (2025). Review of Selected 2-Phenylethylamine Derivatives and Opioids, Systematic Review of Their Effects on Psychomotor Abilities and Driving Performance: Psychopharmacology in the Context of Road Safety. Pharmaceuticals, 18(10), 1555. https://doi.org/10.3390/ph18101555







