Identification of Benzodiazepine Use Based on Dried Blood Stains Analysis
Abstract
1. Introduction
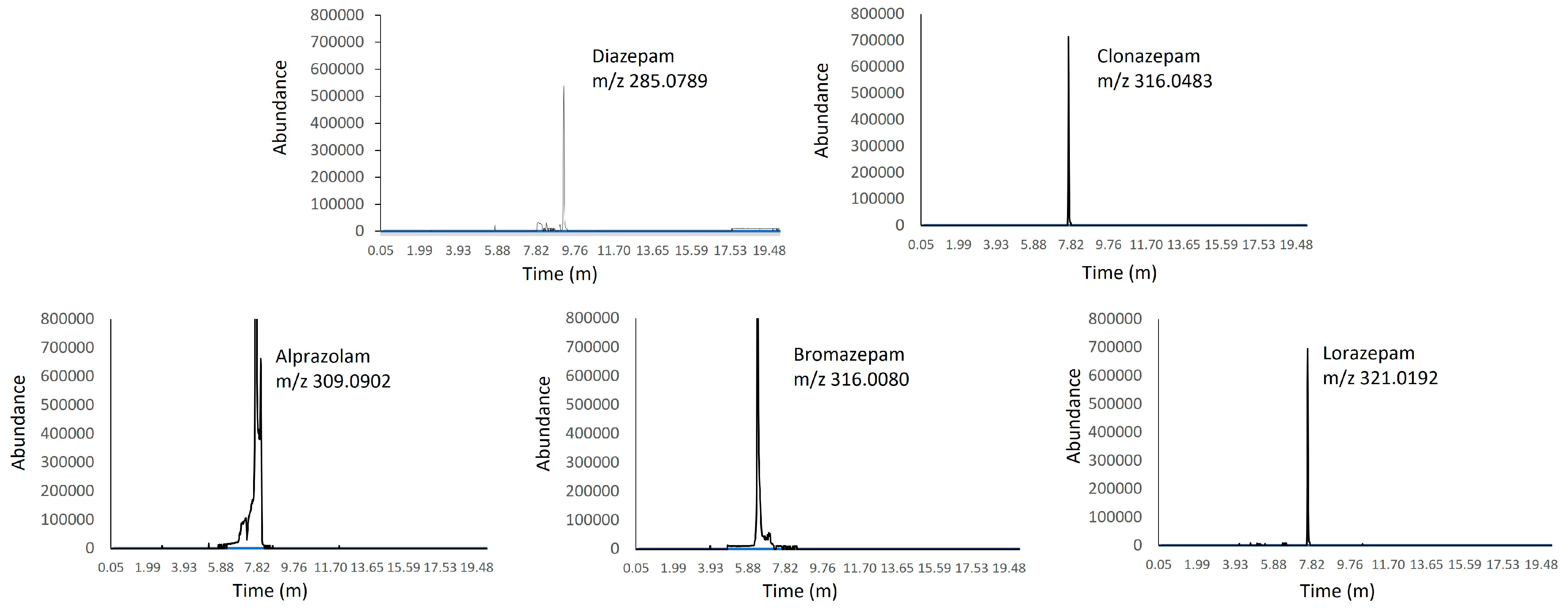
2. Results
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Blood Samples
4.3. Preparation of Stock Standard Solution
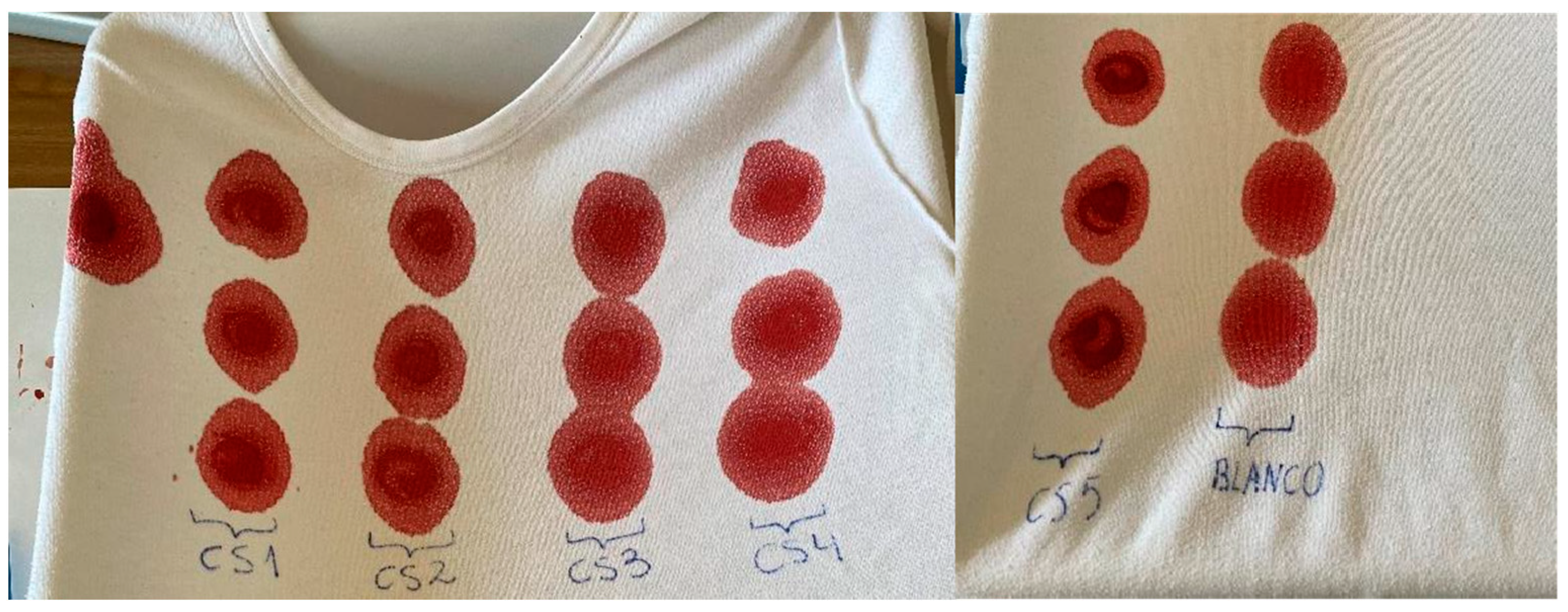
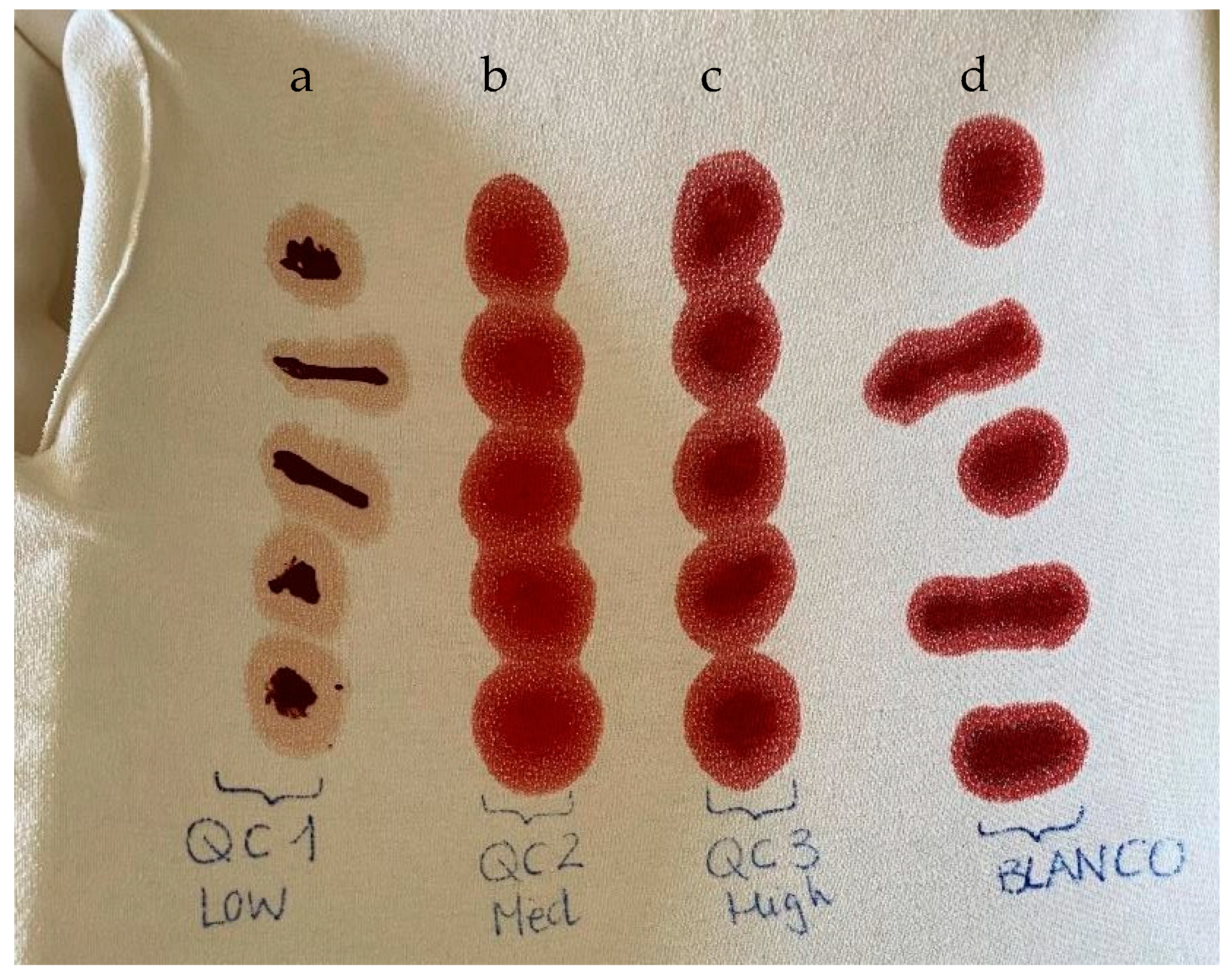
4.4. Preparation of Calibration Standards (CS) and Quality Controls (QC)
4.5. Blood Stains
4.6. Extraction Procedure
4.7. HPLC-MS Conditions
4.8. Validation Procedure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaughn, M.G.; Salas-Wright, C.P.; Reingle-Gonzalez, J.M. Addiction and crime: The importance of asymmetry in offending and the life-course. J. Addict. Dis. 2016, 35, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Diaper, A.M.; Law, F.D.; Melichar, J.K. Pharmacological strategies for detoxification. Br. J. Clin. Pharmacol. 2014, 77, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Votaw, V.R.; Geyer, R.; Rieselbach, M.M.; McHugh, R.K. The epidemiology of benzodiazepine misuse: A systematic review. Drug Alcohol. Depend. 2019, 200, 95–114. [Google Scholar] [CrossRef] [PubMed]
- Nafti, M.; Sirois, C.; Kröger, E.; Carmichael, P.H.; Laurin, D. Is Benzodiazepine Use Associated With the Risk of Dementia and Cognitive Impairment-Not Dementia in Older Persons? The Canadian Study of Health and Aging. Ann. Pharmacother. 2020, 54, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Olfson, M.; King, M.; Schoenbaum, M. Benzodiazepine use in the United States. JAMA Psychiatry 2015, 72, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Morford, K.L.; Levander, X.A. Benzodiazepines and Related Sedatives. Med. Clin. N. Am. 2022, 106, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S. Benzodiazepines. Curr. Top. Behav. Neurosci. 2017, 34, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Kroll, D.S.; Nieva, H.R.; Barsky, A.J.; Linder, J.A. Benzodiazepines are Prescribed More Frequently to Patients Already at Risk for Benzodiazepine-Related Adverse Events in Primary Care. J. Gen. Intern. Med. 2016, 31, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Uzun, S.; Kozumplik, O.; Jakovljević, M.; Sedić, B. Side effects of treatment with benzodiazepines. Psychiatr. Danub. 2010, 22, 90–93. [Google Scholar]
- de Souza Costa, Y.R.; Lavorato, S.N.; de Campos, J.J.C.M. Violence against women and drug-facilitated sexual assault (DFSA): A review of the main drugs. J. Forensic Leg Med. 2020, 74, 102020. [Google Scholar]
- International Narcotics Control Board. 2022 Annual Report. Available online: https://www.incb.org/incb/es/publications/annual-reports/annual-report.html (accessed on 6 April 2023).
- Adamowicz, P.; Ziora, B. Can scene bloodstains be used to quantify drug concentration at the moment of injury? Forensic Sci. Int. 2022, 341, 111498. [Google Scholar] [CrossRef] [PubMed]
- Jantos, R.; Veldstra, J.L.; Mattern, R.; Brookhuis, K.A.; Skopp, G. Analysis of 3,4-Methylenedioxymetamphetamine: Whole Blood Versus Dried Blood Spots. J. Anal. Toxicol. 2011, 35, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Sadler Simões, S.; Castañera Ajenjo, A.; Dias, M.J. Dried blood spots combined to an UPLC-MS/MS method for the simultaneous determination of drugs of abuse in forensic toxicology. J. Pharm. Biomed. Anal. 2018, 147, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, C.A.; Casapao, A.M.; Siller, S.; Isache, C.; Cani, K.V.; Claudio, A.M.; Brown, M.; Milstid, B.; Feldhammer, M. Preanalytical Challenges During Capillary Fingerstick Sampling Preclude Its Widespread Use in Adult Hospitalized Patients. Am. J. Clin. Pathol. 2021, 155, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Malsagova, K.; Kopylov, A.; Stepanov, A.; Butkova, T.; Izotov, A.; Kaysheva, A. Dried blood spot in laboratory: Directions and prospects. Diagnostics 2020, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Meesters, R.J.W.; Hoof, G.P. State-of-the-art dried blood spot analysis: An overview of recent advances and future trend. Bioanalysis 2013, 5, 2187–2208. [Google Scholar] [PubMed]
- Li, W.; Tse, F.L.S. Dried blood spot sampling in combination with LC–MS/MS for quantitative analysis of small molecules. Biomed. Chromatogr. 2010, 24, 49–65. [Google Scholar] [PubMed]
- Stove, C.P.; Ingels, A.S.; De Kesel, P.M.; Lambert, W.E. Dried blood spots in toxicology: From the cradle to the grave? Crit. Rev. Toxicol. 2012, 42, 230–243. [Google Scholar]
- Lee, H.; Park, Y.; Jo, J.; In, S.; Park, Y.; Kim, E.; Pyo, J.; Choe, S. Analysis of benzodiazepines and their metabolites using DBS cards and LC-MS/MS. Forensic Sci. Int. 2015, 255, 137–145. [Google Scholar]
- Moretti, M.; Manfredi, A.; Freni, F.; Previderé, C.; Osculati, A.M.M.; Grignani, P.; Tronconi, L.; Carelli, C.; Vignali, C.; Morini, L. A comparison between two different dried blood substrates in determination of psychoactive substances in postmortem samples. Forensic Toxicol. 2021, 39, 385–393. [Google Scholar]
- la Marca, G.; Carling, R.S.; Moat, S.J.; Yahyaoui, R.; Ranieri, E.; Bonham, J.R.; Schielen, P.C.J.I. Current State and Innovations in Newborn Screening: Continuing to Do Good and Avoid Harm. Int. J. Neonatal Screen. 2023, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Willigenburg, H.V.; Domburg, B.V.; Ambagtsheer, G.; Brandt, R.M.; Hesselink, D.A.; de Bruin, R.W.; de Winter, B.C. Validation of a dried blood spot method to measure tacrolimus concentrations in small volumes of mouse blood. Bioanalysis 2022, 14, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Garcia Boy, R.; Henseler, R.; Mattern, R.; Skopp, G. Determination of morphine and 6-acetylmorphine in blood with use of dried blood spots. Ther. Drug Monit. 2008, 30, 733–739. [Google Scholar] [PubMed]
- Majda, A.; Wietecha-Posłuszny, R.; Świądro, M.; Mrochem, K.; Kościelniak, P. Dried blood spots sampling in case samples deprived of hematocrit level information—Investigation and calculation strategy. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1124, 308–312. [Google Scholar] [CrossRef]
- Nguyen, L.B.L.; Soumah, A.A.; Hoang, V.T.; Nguyen, A.T.; Pham, T.H.; Royer-Devaux, S.; Madec, Y. Performances of Dried Blood Spots and Point-of-Care Devices to Identify Virological Failure in HIV-Infected Patients: A Systematic Review and Meta-Analysis. AIDS Patient Care STDS 2023, 37, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Freni, F.; Tomaciello, I.; Vignali, C.; Groppi, A.; Visonà, S.D.; Tajana, L.; Osculati, A.M.M.; Morini, L. Determination of benzodiazepines in blood and in dried blood spots collected from post-mortem samples and evaluation of the stability over a three-month period. Drug Test. Anal. 2019, 11, 1403–1411. [Google Scholar] [CrossRef]
- Hammond, M.D.; Osselton, M.D.; Moffat, A.C. The Extraction and Analysis of Benzodiazepine Drugs in Bloodstains. J. Forensic Sci. Soc. 1979, 19, 193–198. [Google Scholar] [CrossRef]
- Antelo-Domínguez, A.; Cocho, J.A.; Tabernero, M.J.; Bermejo, A.M.; Bermejo-Barrera, P.; Moreda-Pineiro, A. Simultaneous determination of cocaine and opiates in dried blood spots by electrospray ionization tandem mass spectrometry. Talanta 2013, 117, 235–241. [Google Scholar] [CrossRef]
- Alfazil, A.A.; Anderson, R.A. Stability of benzodiazepines and cocaine in blood spots stored on filter paper. J. Anal. Toxicol. 2008, 32, 511–515. [Google Scholar] [CrossRef]
- Not, A.; Saludes, V.; Gálvez, M.; Miralpeix, A.; Bordoy, A.E.; González, N.; González-Gómez, S.; Muntané, L.; Reyes-Urueña, J.; Majó, X.; et al. Usefulness of dried blood spot samples for monitoring hepatitis C treatment outcome and reinfection among people who inject drugs in a test-and-treat program. J. Med. Virol. 2023, 95, e28544. [Google Scholar] [CrossRef]
- Pujol-Hodge, E.; Salazar-Gonzalez, J.F.; Ssemwanga, D.; Charlebois, E.D.; Ayieko, J.; Grant, H.E.; Liegler, T.; Atkins, K.E.; Kaleebu, P.; Kamya, M.R.; et al. Detection of HIV-1 Transmission Clusters from Dried Blood Spots within a Universal Test-and-Treat Trial in East Africa. Viruses 2022, 14, 1673. [Google Scholar] [CrossRef] [PubMed]
- Chaouachi, L. Chemical submission and GHB: What we continue to ignore? Arch. Women’s Ment. Health 2023, 26, 417–418. [Google Scholar] [CrossRef]
- Tobe, S.S.; Watson, N.; Daeid, N.N. Evaluation of six presumptive tests for blood, their specificity, sensitivity, and effect on high molecular-weight DNA. J. Forensic Sci. 2007, 52, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Freni, F.; Valentini, B.; Vignali, C.; Groppi, A.; Visonà, S.D.; Marco, A.; Osculati, M.; Morini, L. Determination of antidepressants and antipsychotics in dried blood spots (DBSs) collected from post-mortem samples and evaluation of the stability over a three-month period. Molecules 2019, 24, 3636. [Google Scholar] [CrossRef] [PubMed]
- Nudelman, N.S.; Cabrera, C.G. Isolation and structural elucidation of degradation products of alprazolam: Photostability studies of alprazolam tablets. J. Pharm. Sci. 2002, 91, 1274–1286. [Google Scholar] [CrossRef]
- Nudelman, N.S.; de Waisbaum, R.G. Acid hydrolysis of diazepam. Kinetic study of the reactions of 2-(N-methylamino)-5-chlorobenzophenone, with HCl in MeOH-H2O. J. Pharm. Sci. 1995, 84, 998–1004. [Google Scholar]
- Ambach, L.; Redondo, A.H.; König, S.; Weinmann, W. Rapid and simple LC–MS/MS screening of 64 novel psychoactive substances using dried blood spots. Drug Test. Anal. 2013, 6, 367–375. [Google Scholar] [CrossRef]
- Wille, S.M.R.; Coucke, W.; De Baere, T.; Peters, F.T. Update of Standard Practices for New Method Validation in Forensic Toxicology. Curr. Pharm. Des. 2017, 23, 5442–5454. [Google Scholar] [CrossRef]
- Peters, F.T.; Wissenbach, D.K.; Busardò, F.P.; Marchei, E.; Pichini, S. Method Development in Forensic Toxicology. Curr. Pharm. Des. 2017, 23, 5455–5467. [Google Scholar] [CrossRef] [PubMed]
- Raikos, N.; Tsoukali, H.; Njau, S. Determination of opiates in postmortem bone and bone marrow. Forensic Sci. Int. 2001, 123, 140–141. [Google Scholar] [CrossRef]
- Wiebe, T.R.; Watterson, J.H. Analysis of tramadol and O-desmethyltramadol in decomposed skeletal tissues following acute and repeated tramadol exposure by gas chromatography mass spectrometry. Forensic Sci. Int. 2014, 242, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Mancini, R.; Fernandez-Lopez, L.; Falcon, M.; Pellegrini, M.; Luna, A.; Rotolo, M. Postmortem Analysis of Benzodiazepines in Human Bone by Gas Chromatography-Mass Spectrometry. J. Anal. Toxicol. 2020, 44, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rabie, P.; Denniff, P.; Spooner, N.; Brynjolffssen, J.; Galluzzo, P.; Sanders, G. Method of applying internal standard to dried matrix spot samples for use in quantitative bioanalysis. Anal. Chem. 2011, 83, 8779–8786. [Google Scholar] [CrossRef] [PubMed]
| Analyte | Calibration Parameters | |||
|---|---|---|---|---|
| Equation | Determination Coefficients (r2) | LOD (ng/mL) | LOQ (ng/mL) | |
| Alprazolam | y = 1850.3x + 663,736 | 0.991 | 0.5 | 1.5 |
| Bromazepam | y = 739.49x + 103,379 | 0.999 | 0.5 | 1.7 |
| Clonazepam | y = 145.17x + 51,295 | 0.997 | 0.2 | 0.6 |
| Diazepam | y = 357.78x + 163,114 | 0.996 | 0.3 | 1.0 |
| Lorazepam | y = 162.86x + 89,121 | 0.994 | 0.5 | 1.5 |
| Analyte | RSD (%) | Accuracy (%) | ||
|---|---|---|---|---|
| QCM | QCH | QCM | QCH | |
| Alprazolam | 15.1 | 1.4 | 85.1 | 89.0 |
| Bromazepam | 23.7 | 4.5 | 88.8 | 93.0 |
| Clonazepam | 7.9 | 15.8 | 89.3 | 94.9 |
| Diazepam | 34.7 | 45.3 | 92.0 | 88.1 |
| Lorazepam | 1.1 | 13.4 | 107.3 | 109.5 |
| Calibration Standard (CS) | Concentration (ng/mL) | SSS, WS1, WS2 Volume (mL) | Blood Volume (mL) |
|---|---|---|---|
| CS1 | 1 | 0.5 WS2 | 4.5 |
| CS2 | 10 | 0.5 WS1 | 4.5 |
| CS3 | 100 | 0.1 SSS | 4.9 |
| CS4 | 1000 | 1 SSS | 4 |
| CS5 | 5000 | 0.25 of each 100 µg/mL solution | 3.75 |
| Benzodiazepines | Precursor Ion [M + H]+ (m/z) | MS/MS (m/z) | Retention Time (min) |
|---|---|---|---|
| Alprazolam | 309.0902 | 281.0721 | 7.72 |
| Bromazepam | 316.0080 | 182.0838 | 6.36 |
| Clonazepam | 316.0483 | 270.0554 | 7.67 |
| Diazepam | 285.0789 | 154.0418 | 9.12 |
| Lorazepam | 321.0192 | 275.0133 | 7.60 |
| 7-aminoclonazepam-d4 | 290.1055 | 274.0805 | 2.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-López, L.; Rodríguez, S.; Cánovas-Cabanes, A.; Teruel-Fernández, F.-J.; Almela, P.; del Rincón, J.-P.H.; Navarro-Zaragoza, J.; Falcón, M. Identification of Benzodiazepine Use Based on Dried Blood Stains Analysis. Pharmaceuticals 2024, 17, 799. https://doi.org/10.3390/ph17060799
Fernández-López L, Rodríguez S, Cánovas-Cabanes A, Teruel-Fernández F-J, Almela P, del Rincón J-PH, Navarro-Zaragoza J, Falcón M. Identification of Benzodiazepine Use Based on Dried Blood Stains Analysis. Pharmaceuticals. 2024; 17(6):799. https://doi.org/10.3390/ph17060799
Chicago/Turabian StyleFernández-López, Lucía, Sandra Rodríguez, Alberto Cánovas-Cabanes, Francisco-Javier Teruel-Fernández, Pilar Almela, Juan-Pedro Hernández del Rincón, Javier Navarro-Zaragoza, and María Falcón. 2024. "Identification of Benzodiazepine Use Based on Dried Blood Stains Analysis" Pharmaceuticals 17, no. 6: 799. https://doi.org/10.3390/ph17060799
APA StyleFernández-López, L., Rodríguez, S., Cánovas-Cabanes, A., Teruel-Fernández, F.-J., Almela, P., del Rincón, J.-P. H., Navarro-Zaragoza, J., & Falcón, M. (2024). Identification of Benzodiazepine Use Based on Dried Blood Stains Analysis. Pharmaceuticals, 17(6), 799. https://doi.org/10.3390/ph17060799






