Pyrazole-Imidazoline Derivative Prevents Cardiac Damage and Mortality in Acute Trypanosoma cruzi Infection
Abstract
1. Introduction
2. Results and Discussion
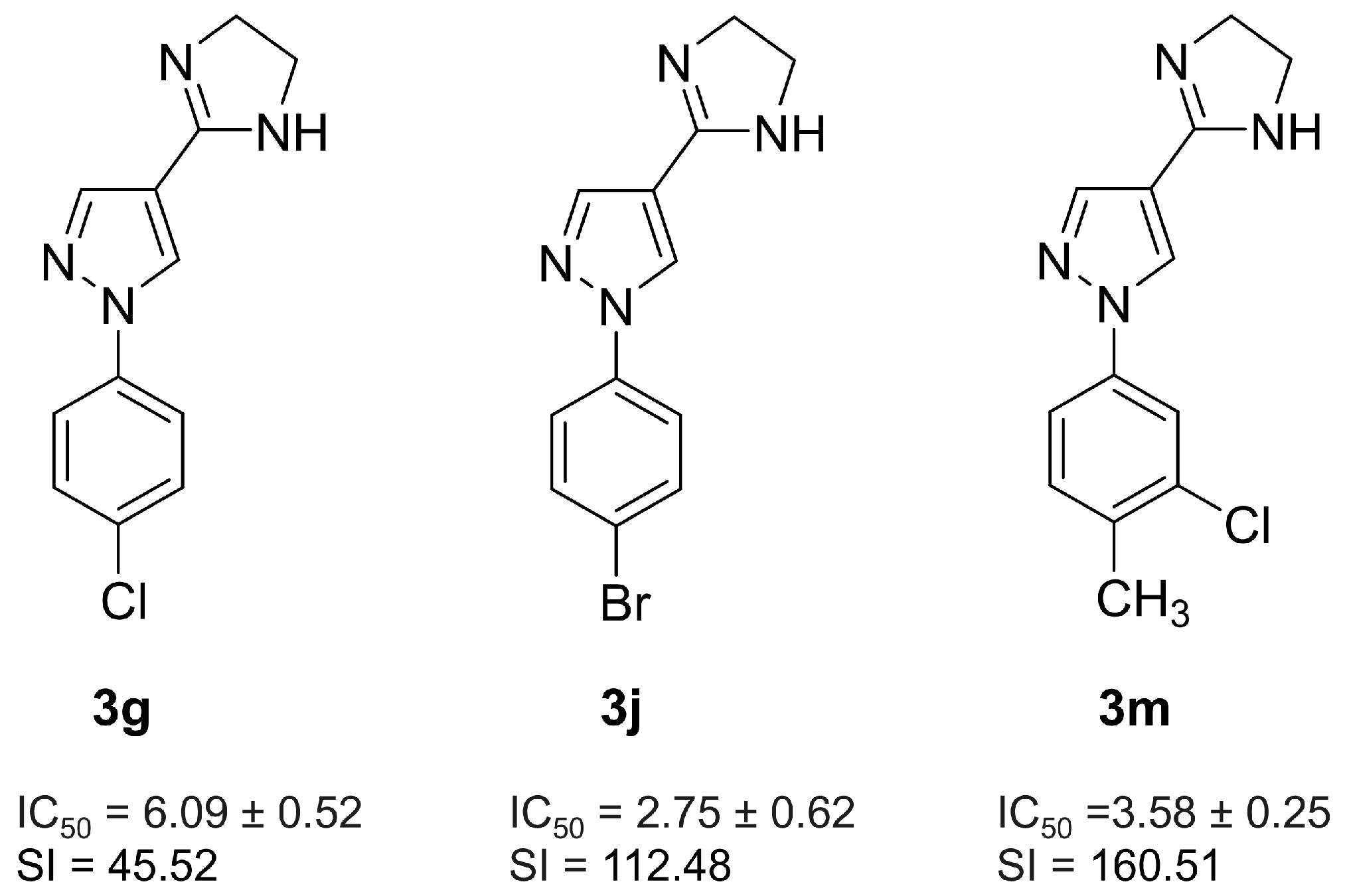
2.1. Effect of 3m Treatment on In Vitro Parasite Resurgence
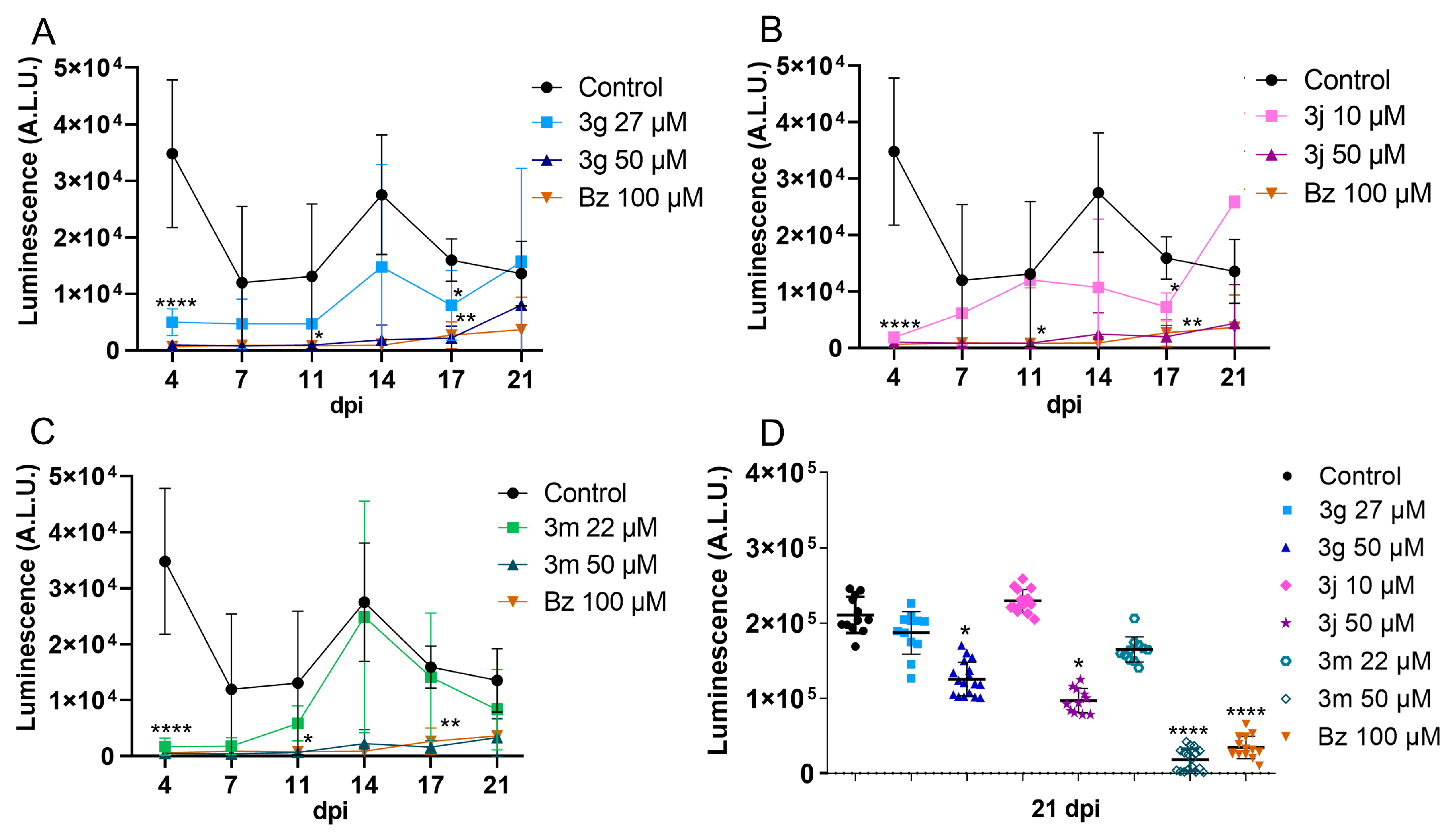
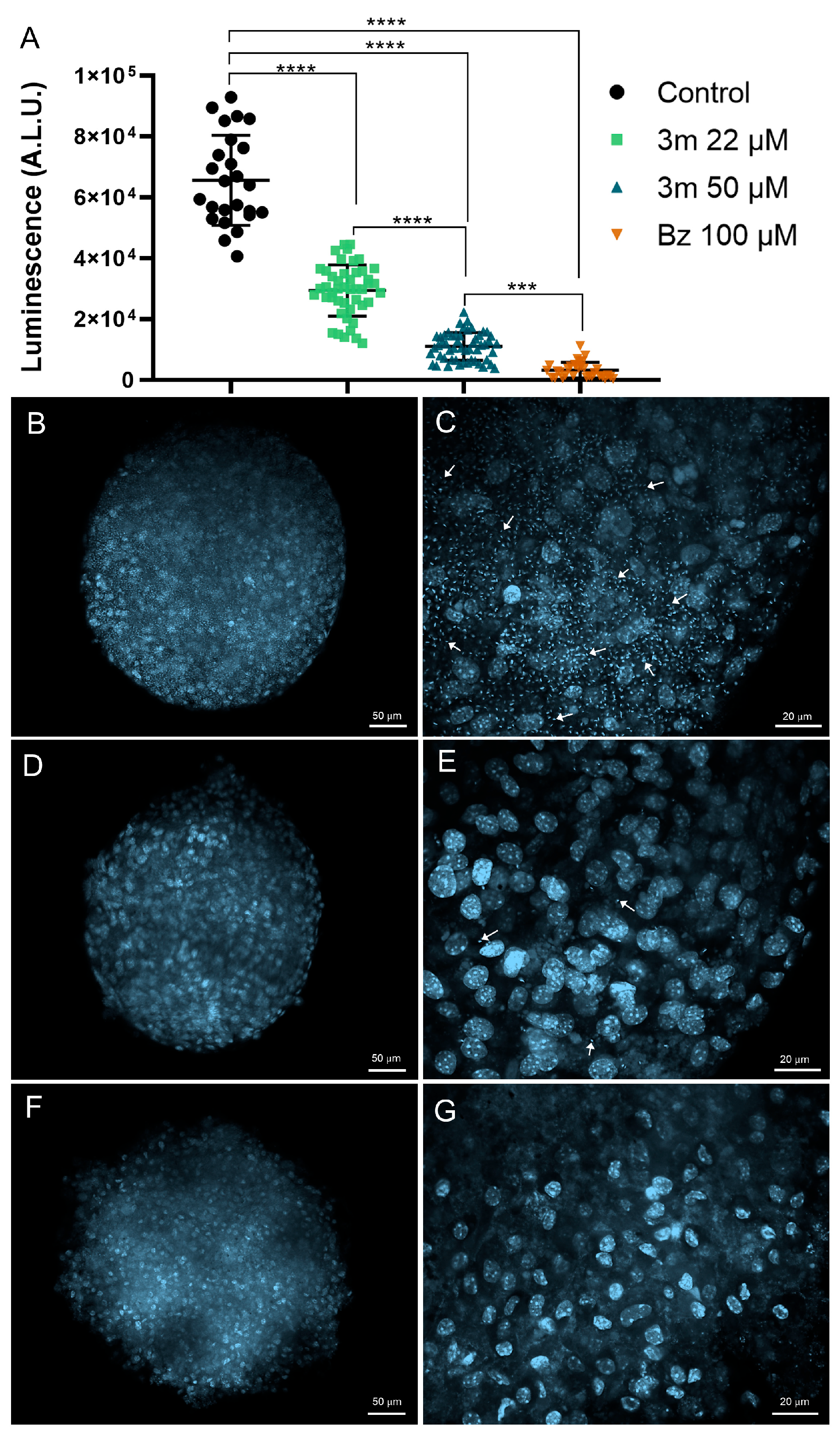
2.2. Drug Toxicity and Efficacy in 3D Cardiac Spheroid
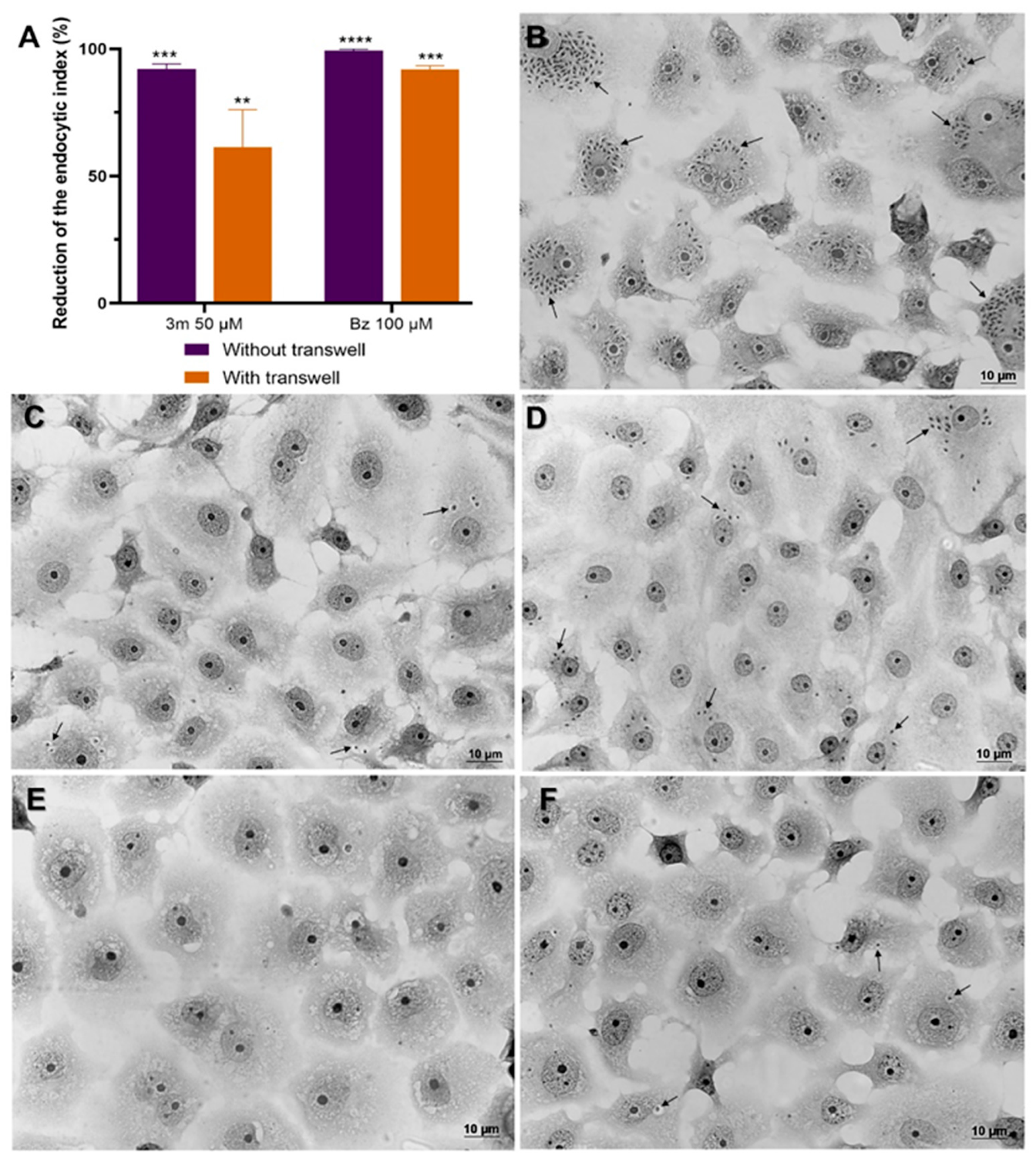
2.3. Influence of 3m Absorption on Drug Efficacy
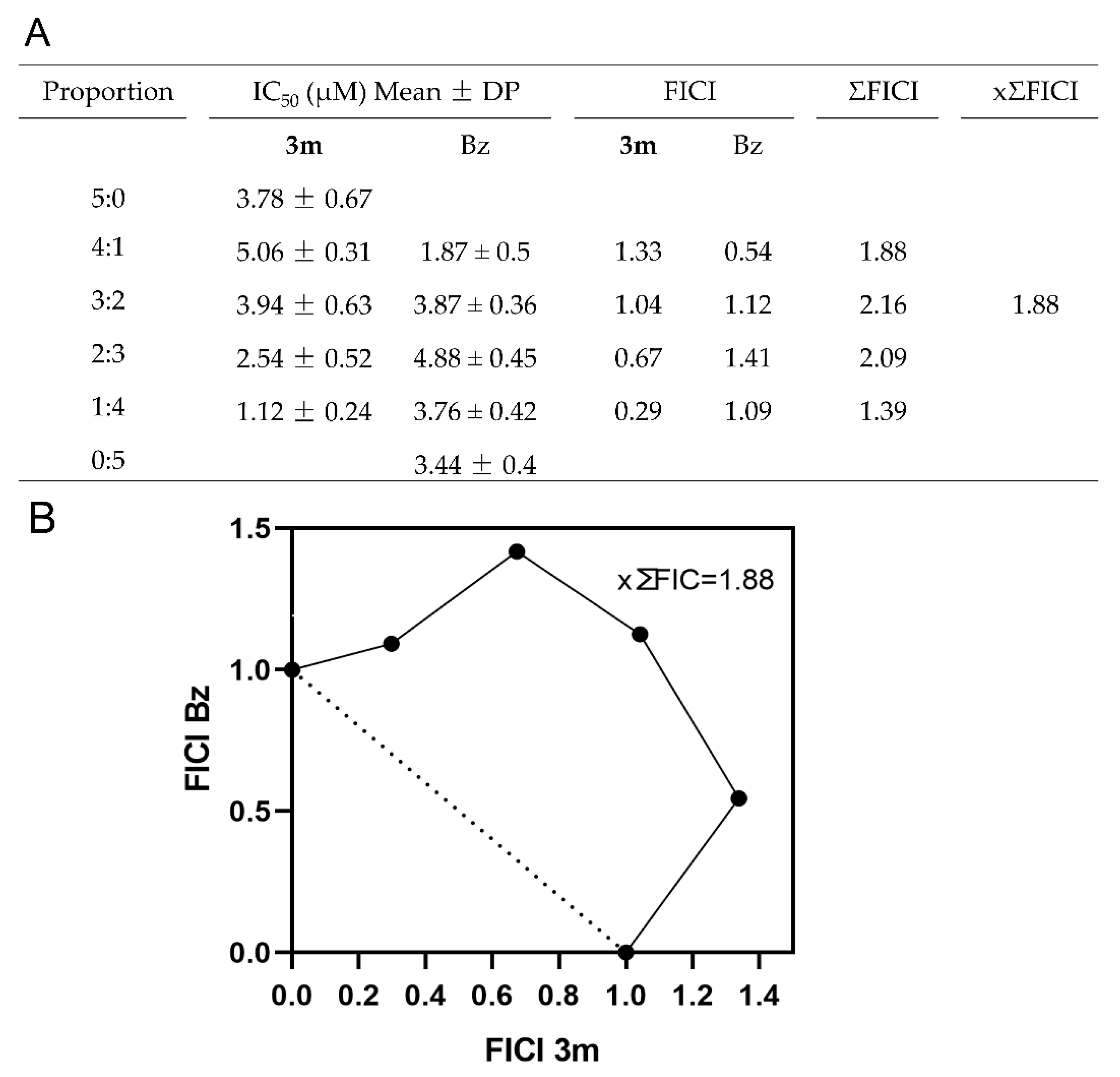
2.4. Impact of the Combination on Drug Interaction
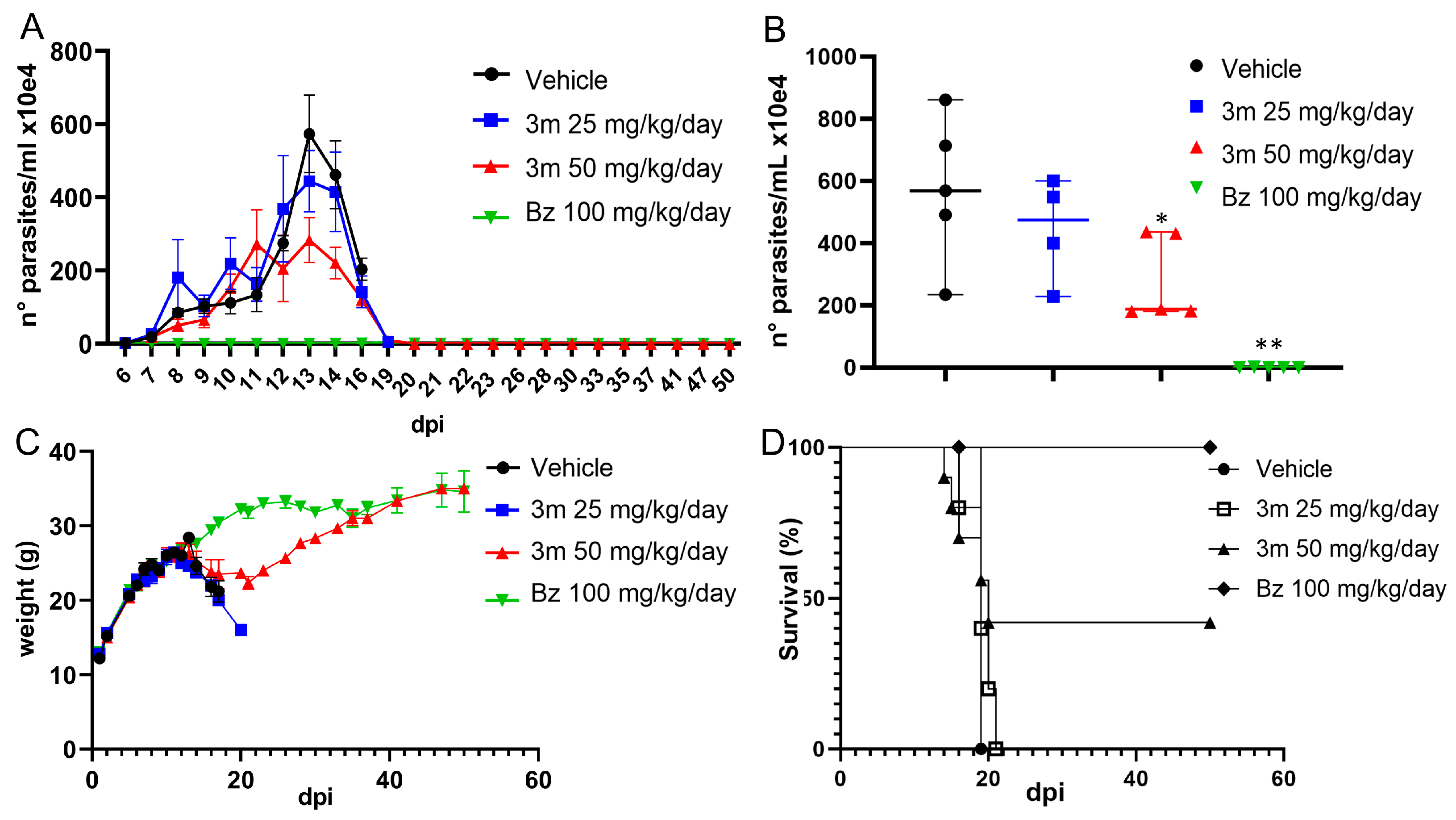
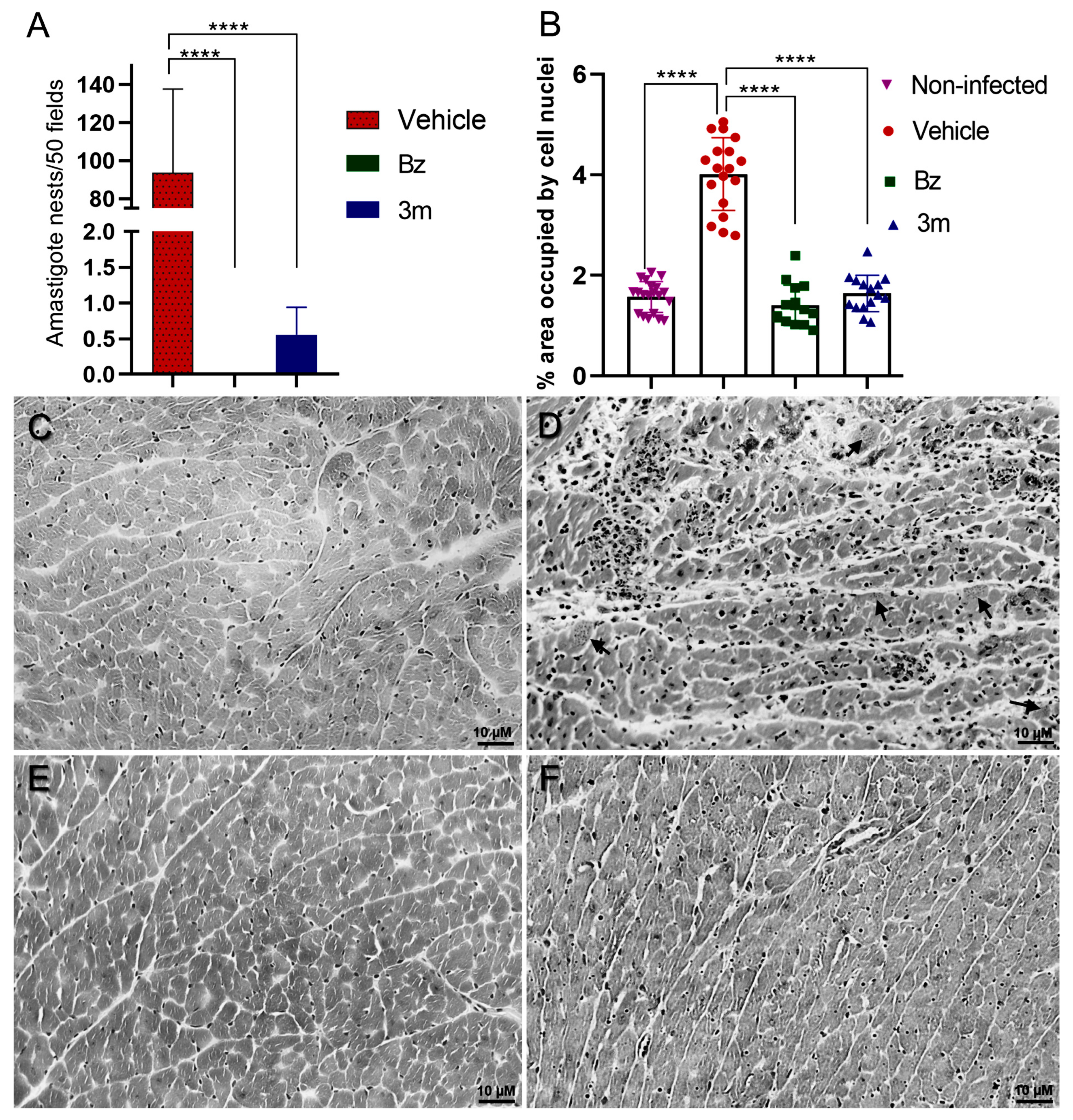
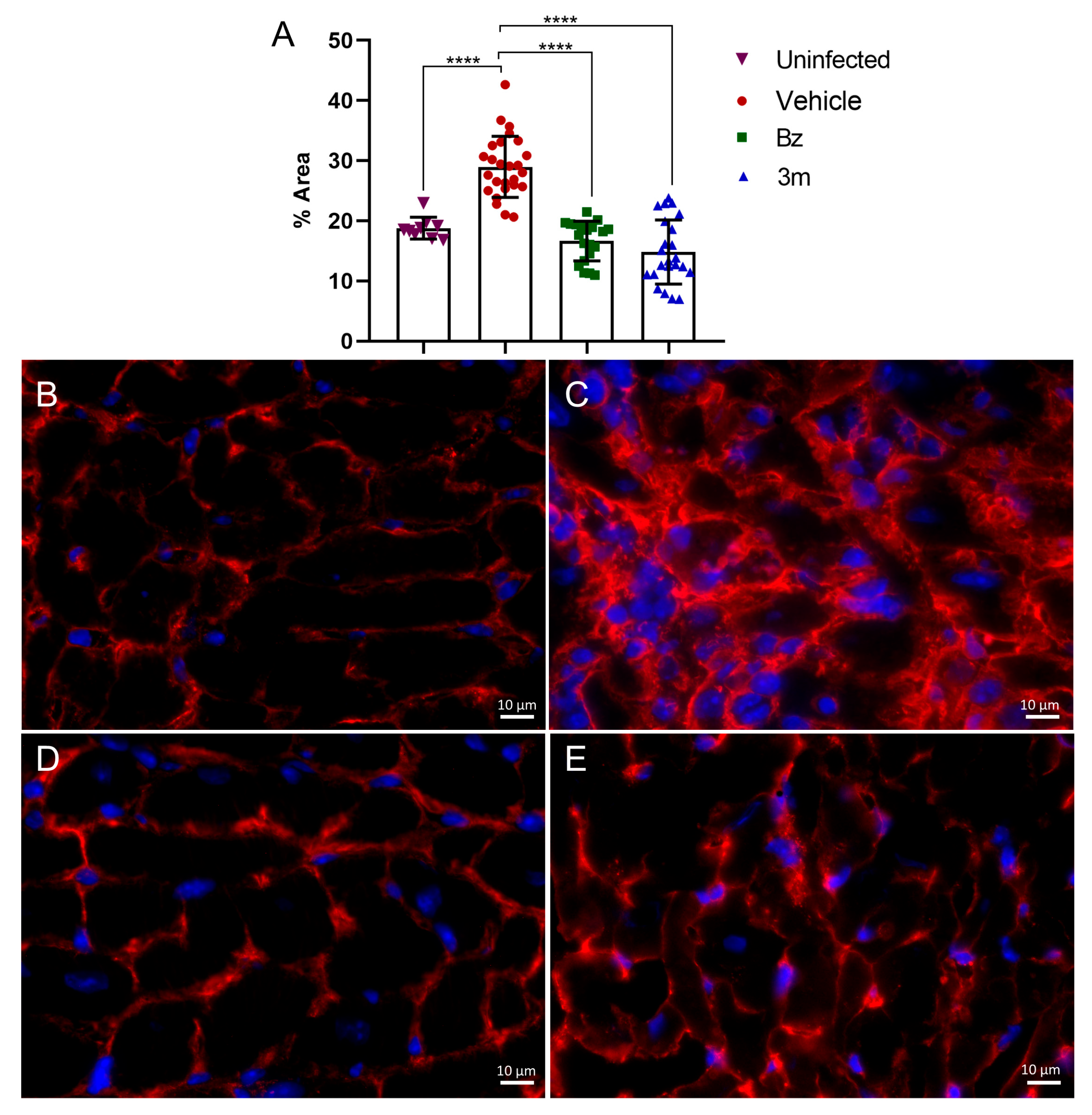
2.5. T. cruzi Acute Experimental Infection
3. Materials and Methods
3.1. Chemistry
3.2. Parasites
3.3. Cell Culture
3.4. Cytotoxicity in Cardiac Muscle Cells
3.5. Evaluating the Drug’s Effectiveness on T. cruzi-Infected 3D Cardiac Spheroids
3.6. Washout Assay
3.7. Drug Absorption and Efficacy
3.8. Drug Combination Assay
3.9. In Vivo Assay
3.10. Histological Analysis
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMD | Amiodarone |
| BSA | Bovine Serum Albumin |
| Bz | Benznidazole |
| CD | Chagas disease |
| DABCO | 1,4-diazabicyclo[2.2.2]octane |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMEM | Dulbecco’s Modified Eagle Medium |
| dpi | Days post-infection |
| ECM | Extracellular Matrix |
| DMSO | Dimethyl sulfoxide |
| EI | Endocytic Index |
| FBS | Fetal Bovine Serum |
| FICI | Fractional Inhibitory Concentration Index |
| FT-IR | Fourier Transform Infrared |
| H&E | Hematoxylin & Eosin |
| HRMS | High-Resolution Mass Spectrometry |
| Nif | Nifurtimox |
| NOAEL | No-oberserved-adverse-effect level |
| NMR | Nuclear Magnetic Resonance |
| PDB | Protein Data Bank |
| pIC50 | Negative log of IC50 in molar |
| PTX | Pentoxifyline |
| SI | Selectivity index |
| WHO | World Health Organization |
References
- Gonzalez-Sanz, M.; Crespillo-Andújar, C.; Chamorro-Tojeiro, S.; Monge-Maillo, B.; Perez-Molina, J.A.; Norman, F.F. Chagas Disease in Europe. Trop. Med. Infect. Dis. 2023, 8, 513. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, Chagas Disease (Also Known as American Trypanosomiasis). Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 24 March 2025).
- Pacheco, L.V.; Santana, L.S.; Barreto, B.C.; Santos, E.S.; Meira, C.S. Oral transmission of Chagas disease: A literature review. Res. Soc. Dev. 2021, 10, e31910212636. [Google Scholar] [CrossRef]
- Franco-Paredes, C.; Villamil-Gómez, W.E.; Schultz, J.; Henao-Martínez, A.F.; Parra-Henao, G.; Rassi, A.; Rodríguez-Morales, A.J.; Suarez, J.A. A deadly feast: Elucidating the burden of orally acquired acute Chagas disease in Latin America—Public health and travel medicine importance. Travel Med. Infect. Dis. 2020, 36, 101565. [Google Scholar] [CrossRef] [PubMed]
- Carlier, Y.; Altcheh, J.; Angheben, A.; Freilij, H.; Luquetti, A.O.; Schijman, A.G.; Segovia, M.; Wagner, N.; Albajar, V.P. Congenital Chagas disease: Updated recommendations for prevention, diagnosis, treatment, and follow-up of newborns and siblings, girls, women of childbearing age, and pregnant women. PLoS Negl. Trop. Dis. 2019, 13, e0007694. [Google Scholar] [CrossRef]
- Chadalawada, S.; Rassi, A., Jr.; Samara, O.; Monzon, A.; Gudapati, D.; Vargas Barahona, L.; Vargas, D.; Hyson, P.S.S.; Mestroni, L.; Taylor, M.; et al. Mortality risk in chronic Chagas cardiomyopathy: A systematic review and meta-analysis. ESC Heart Fail. 2021, 8, 5466–5481. [Google Scholar] [CrossRef]
- de Sousa, A.S.; Vermeij, D.; Ramos, A.N., Jr.; Luquetti, A.O. Chagas disease. Lancet 2024, 403, 203–218. [Google Scholar] [CrossRef]
- Pérez-Molina, J.A.; Crespillo-Andújar, C.; Bosch-Nicolau, P.; Molina, I. Trypanocidal treatment of Chagas disease. Enferm. Infecc. Microbiol. Clin. 2021, 39, 458–470. [Google Scholar] [CrossRef]
- Molina, I.; Prat, J.G.; Salvador, F.; Treviño, B.; Sulleiro, E.; Serre, N.; Pou, D.; Roure, S.; Cabezos, J.; Valerio, L.; et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N. Engl. J. Med. 2014, 370, 1899–1908. [Google Scholar] [CrossRef]
- Pinazo, M.J.; Forsyth, C.; Losada, I.; Esteban, E.T.; García-Rodríguez, M.; Villegas, M.L.; Molina, I.; Crespillo-Andújar, C.; Gállego, M.; Ballart, C.; et al. FEXI-12 Study Team, Efficacy and safety of fexinidazole for treatment of chronic indeterminate Chagas disease (FEXI-12): A multicentre, randomised, double-blind, phase 2 trial. Lancet Infect. Dis. 2024, 24, 395–403. [Google Scholar] [CrossRef]
- Torrico, F.; Gascón, J.; Ortiz, L.; Pinto, J.; Rojas, G.; Palacios, A.; Barreira, F.; Blum, B.; Schijman, A.G.; Vaillant, M.; et al. On behalf of the FEXI-CHAGAS Study Group, A Phase 2, Randomized, Multicenter, Placebo-Controlled, Proof-of-Concept Trial of Oral Fexinidazole in Adults with Chronic Indeterminate Chagas Disease. Clin. Infect. Dis. 2023, 76, e1186–e1194. [Google Scholar] [CrossRef]
- Morillo, C.A.; Waskin, H.; Sosa-Estani, S.; Del Carmen Bangher, M.; Cuneo, C.; Milesi, R.; Mallagray, M.; Apt, W.; Beloscar, J.; Gascon, J.; et al. STOP-CHAGAS Investigators, Benznidazole and posaconazole in eliminating parasites in asymptomatic T. cruzi carriers. J. Am. Coll. Cardiol. 2017, 69, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Torrico, F.; Gascón, J.; Barreira, F.; Blum, B.; Almeida, I.C.; Alonso-Vega, C.; Barboza, T.; Bilbe, G.; Correia, E.; Garcia, W.; et al. BENDITA study group, New regimens of benznidazole monotherapy and in combination with fosravuconazole for treatment of Chagas disease (BENDITA): A phase 2, double-blind, randomised trial. Lancet Infect. Dis. 2021, 21, 1129–1140. [Google Scholar] [CrossRef]
- Bosch-Nicolau, P.; Fernández, M.L.; Sulleiro, E.; Villar, J.C.; Perez-Molina, J.A.; Correa-Oliveira, R.; Sosa-Estani, S.; Sánchez-Montalvá, A.; Del Carmen Bangher, M.; Moreira, O.C.; et al. MULTIBENZ Study Group, Efficacy of three benznidazole dosing strategies for adults living with chronic Chagas disease (MULTIBENZ): An international, randomised, double-blind, phase 2b trial. Lancet Infect. Dis. 2024, 24, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Padilla, A.M.; Wang, W.; Jacobs, R.T.; Tarleton, R.L. Discovery of an orally active benzoxaborole prodrug effective in the treatment of Chagas disease in non-human primates. Nat. Microbiol. 2022, 7, 1536–1546. [Google Scholar] [CrossRef]
- Nagle, A.; Biggart, A.; Be, C.; Srinivas, H.; Hein, A.; Caridha, D.; Sciotti, R.J.; Pybus, B.; Kreishman-Deitrick, M.; Bursulaya, B.; et al. Discovery and Characterization of Clinical Candidate LXE408 as a Kinetoplastid-Selective Proteasome Inhibitor for the Treatment of Leishmaniases. J. Med. Chem. 2020, 63, 10773–10781. [Google Scholar] [CrossRef]
- Rao, S.P.S.; Gould, M.K.; Noeske, J.; Saldivia, M.; Jumani, R.S.; Ng, P.S.; René, O.; Chen, Y.L.; Kaiser, M.; Ritchie, R.; et al. Cyanotriazoles are selective topoisomerase II poisons that rapidly cure trypanosome infections. Science 2023, 380, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.E.; Lechuga, G.C.; Lara, L.S.; Souto, B.A.; Viganó, M.G.; Bourguignon, S.C.; Calvet, C.M.; Oliveira, F.O.R., Jr.; Alves, C.R.; Souza-Silva, F.; et al. Synthesis, structure-activity relationship and trypanocidal activity of pyrazole-imidazoline and new pyrazole-tetrahydropyrimidine hybrids as promising chemotherapeutic agents for Chagas disease. Eur. J. Med. Chem. 2019, 182, 111610–111623. [Google Scholar] [CrossRef] [PubMed]
- Ebenezer, O.; Shapi, M.; Tuszynski, J.A. A Review of the Recent Development in the Synthesis and Biological Evaluations of Pyrazole Derivatives. Biomedicines 2022, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Ravindar, L.; Hasbullah, S.A.; Rakesh, K.P.; Hassan, N.I. Pyrazole and pyrazoline derivatives as antimalarial agents: A key review. Eur. J. Pharm. Sci. 2023, 183, 106365. [Google Scholar] [CrossRef] [PubMed]
- Tochowicz, A.; McKerrow, J.H.; Craik, C.S. Crystal Structure Analysis of Cruzain with Fragment 1 (N-(1Hbenzimidazol-2-yl)-1,3-dimethyl-pyrazole-4-carboxamide). Available online: https://www.wwpdb.org/pdb?id=pdb_00004w5b (accessed on 30 March 2025).
- Orlando, L.M.R.; Lechuga, G.C.; Lara, L.S.; Ferreira, B.S.; Pereira, C.N.; Silva, R.C.; Dos Santos, M.S.; Pereira, M.C.S. Structural Optimization and Biological Activity of Pyrazole Derivatives: Virtual Computational Analysis, Recovery Assay and 3D Culture Model as Potential Predictive Tools of Effectiveness against Trypanosoma cruzi. Molecules 2021, 26, 6742. [Google Scholar] [CrossRef] [PubMed]
- Gabaldón-Figueira, J.C.; Martinez-Peinado, N.; Escabia, E.; Ros-Lucas, A.; Chatelain, E.; Scandale, I.; Gascon, J.; Pinazo, M.J.; Alonso-Padilla, J. State-of-the-Art in the Drug Discovery Pathway for Chagas Disease: A Framework for Drug Development and Target Validation. Res. Rep. Trop. Med. 2023, 14, 1–19. [Google Scholar] [CrossRef]
- Soeiro, M.N.C.; Sales-Junior, P.A.; Pereira, V.R.A.; Vannier-Santos, M.A.; Murta, S.M.F.; Sousa, A.S.; Sangenis, L.H.C.; Moreno, A.M.H.; Boechat, N.; Branco, F.S.C.; et al. Drug screening and development cascade for Chagas disease: An update of in vitro and in vivo experimental models. Mem. Inst. Oswaldo Cruz 2024, 119, e240057. [Google Scholar]
- Abdelsayed, G.; Ali, D.; Malone, A.; Saidi, J.; Myneni, M.; Rajagopal, K.; Faisal, H.; Cheema, F.H.; Hameed, A. 2D and 3D in vitro models for mimicking cardiac physiology. Appl. Eng. Sci. 2022, 12, 100115. [Google Scholar] [CrossRef]
- Mamoshina, P.; Rodriguez, B.; Bueno-Orovio, A. Toward a broader view of mechanisms of drug cardiotoxicity. Cell Rep. Med. 2021, 2, 100216. [Google Scholar] [CrossRef]
- Arai, K.; Kitsuka, T.; Nakayama, K. Scaffold-based and scaffold-free cardiac constructs for drug testing. Biofabrication 2021, 13, 042001. [Google Scholar] [CrossRef]
- Garzoni, L.R.; Adesse, D.; Soares, M.J.; Rossi, M.I.; Borojevic, R.; de Meirelles, M.N. Fibrosis and hypertrophy induced by Trypanosoma cruzi in a three-dimensional cardiomyocyte-culture system. J. Infect. Dis. 2008, 197, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Alagga, A.A.; Pellegrini, M.V.; Gupta, V. Drug Absorption; StatPearls Publishing: Treasure Island, FL, USA, 2025; p. 32491337. [Google Scholar]
- Fedi, A.; Vitale, C.; Ponschin, G.; Ayehunie, S.; Fato, M.; Scaglione, S. In vitro models replicating the human intestinal epithelium for absorption and metabolism studies: A systematic review. J. Control. Release 2021, 335, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Mazzeti, A.L.; Capelari-Oliveira, P.; Bahia, M.T.; Mosqueira, V.C.F. Review on Experimental Treatment Strategies Against Trypanosoma cruzi. J. Exp. Pharmacol. 2021, 13, 409–432. [Google Scholar] [CrossRef] [PubMed]
- Murta, S.M.F.; Lemos Santana, P.A.; Jacques Dit Lapierre, T.J.W.; Penteado, A.B.; El Hajje, M.; Navarro Vinha, T.C.; Liarte, D.B.; de Souza, M.L.; Goulart Trossini, G.H.; de Oliveira Rezende Júnior, C.; et al. New drug discovery strategies for the treatment of benznidazole-resistance in Trypanosoma cruzi, the causative agent of Chagas disease. Expert Opin. Drug Discov. 2024, 19, 741–753. [Google Scholar] [CrossRef]
- Fivelman, Q.L.; Adagu, I.S.; Warhurst, D.C. Modified fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisinin against drug-resistant strains of Plasmodium falciparum. Antimicrob. Agents Chemother. 2004, 48, 4097–4102. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Nogueira, J.R.; Cardoso-Sousa, L.; Gomes, L.H.F.; Moraes, C.B.; Ferreira, R.S., Jr. Drug associations as alternative and complementary therapy for neglected tropical diseases. Eur. J. Med. Chem. Rep. 2021, 3, 100021. [Google Scholar]
- Gouveia, M.J.; Brindley, P.J.; Gärtner, F.; Costa, J.M.C.D.; Vale, N. Drug Repurposing for Schistosomiasis: Combinations of Drugs or Biomolecules. Pharmaceuticals 2018, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Mazzeti, A.L.; Gonçalves, K.R.; Mota, S.L.; Pereira, D.E.; Diniz, L.D.F.; Bahia, M.T. Combination Therapy Using Nitro Compounds Improves the Efficacy of Experimental Chagas Disease Treatment. Parasitology 2021, 148, 1320–1327. [Google Scholar] [CrossRef]
- de Araújo, J.S.; França, S.C.; Batista, D.D.G.J.; Nefertiti, A.; Fiuza, L.F.D.A.; Fonseca-Berzal, C.R.; Bernardino, S.P.; Batista, M.M.; Sijm, M.; Kalejaiye, T.D.; et al. Efficacy of novel pyrazolone phosphodiesterase inhibitors in experimental mouse models of Trypanosoma cruzi. Antimicrob. Agents Chemother. 2020, 64, 414–420. [Google Scholar] [CrossRef]
- Calvet, C.M.; Choi, J.Y.; Thomas, D.; Suzuki, B.; Hirata, K.; Lostracco-Johnson, S.; de Mesquita, L.B.; Nogueira, A.; Meuser-Batista, M.; Silva, T.A.; et al. 4-aminopyridyl-based lead compounds targeting CYP51 prevent spontaneous parasite relapse in a chronic model and improve cardiac pathology in an acute model of Trypanosoma cruzi infection. PLoS Negl. Trop. Dis. 2017, 11, e0006132. [Google Scholar] [CrossRef]
- Barbosa, J.M.C.; Rezende, Y.P.; de Melo, T.G.; de Oliveira, G.; Cascabulho, C.M.; Pereira, E.N.G.D.S.; Daliry, A.; Salem, K.S. Experimental Combination Therapy with Amiodarone and Low-Dose Benznidazole in a Mouse Model of Trypanosoma cruzi Acute Infection. Microbiol. Spectr. 2022, 10, e0185221. [Google Scholar] [CrossRef]
- Lannes-Vieira, J. Multi-therapeutic strategy targeting parasite and inflammation-related alterations to improve prognosis of chronic Chagas cardiomyopathy: A hypothesis-based approach. Mem. Inst. Oswaldo Cruz 2022, 117, e220019. [Google Scholar] [CrossRef] [PubMed]
- Henriques, C.; Castro, D.P.; Gomes, L.H.F.; Garcia, E.S.; de Souza, W. Bioluminescent imaging of Trypanosoma cruzi infection in Rhodnius prolixus, Parasit. Vectors 2012, 5, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, M.N.; de Araujo-Jorge, T.C.; Miranda, C.F.; de Souza, W.; Barbosa, H.S. Interaction of Trypanosoma cruzi with heart muscle cells: Ultrastructural and cytochemical analysis of endocytic vacuole formation and effect upon myogenesis in vitro. Eur. J. Cell Biol. 1986, 41, 198–206. [Google Scholar]
- de Oliveira, E.C.; Lara, L.D.S.; Orlando, L.M.R.; Lanera, S.D.C.; de Souza, T.P.; Figueiredo, N.D.S.; Paes, V.B.; Mazzochi, A.C.; Fernandes, P.H.M.; Dos Santos, M.S.; et al. Anti-Trypanosoma cruzi Potential of New Pyrazole-Imidazoline Derivatives. Molecules 2025, 30, 3082. [Google Scholar] [CrossRef] [PubMed]
- Organization for Economic Co-operation and Development. OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 2002; Available online: https://www.oecd.org/en/publications/test-no-408-repeated-dose-90-day-oral-toxicity-study-in-rodents_9789264070707-en.html (accessed on 13 October 2025). [CrossRef]
- Brener, Z. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Ver. Inst. Med. Trop. 1962, 4, 389–396. [Google Scholar]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008, 2008, pdb.prot4986. [Google Scholar] [CrossRef] [PubMed]
| Compounds | Toxicity (CC50 µM) | |
| 3m | Bz | |
| 229.59 ± 2.93 | >500 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlando, L.M.R.; Lara, L.d.S.; de Souza, T.P.; Paes, V.B.; Calvet, C.M.; de Mesquita, L.B.; Lechuga, G.C.; Pereira, C.N.; dos Santos, M.S.; Pereira, M.C.d.S. Pyrazole-Imidazoline Derivative Prevents Cardiac Damage and Mortality in Acute Trypanosoma cruzi Infection. Pharmaceuticals 2025, 18, 1552. https://doi.org/10.3390/ph18101552
Orlando LMR, Lara LdS, de Souza TP, Paes VB, Calvet CM, de Mesquita LB, Lechuga GC, Pereira CN, dos Santos MS, Pereira MCdS. Pyrazole-Imidazoline Derivative Prevents Cardiac Damage and Mortality in Acute Trypanosoma cruzi Infection. Pharmaceuticals. 2025; 18(10):1552. https://doi.org/10.3390/ph18101552
Chicago/Turabian StyleOrlando, Lorraine Martins Rocha, Leonardo da Silva Lara, Thamyris Pérez de Souza, Vitoria Barbosa Paes, Claudia Magalhães Calvet, Liliane Batista de Mesquita, Guilherme Cury Lechuga, Cynthia Nathália Pereira, Maurício Silva dos Santos, and Mirian Claudia de Souza Pereira. 2025. "Pyrazole-Imidazoline Derivative Prevents Cardiac Damage and Mortality in Acute Trypanosoma cruzi Infection" Pharmaceuticals 18, no. 10: 1552. https://doi.org/10.3390/ph18101552
APA StyleOrlando, L. M. R., Lara, L. d. S., de Souza, T. P., Paes, V. B., Calvet, C. M., de Mesquita, L. B., Lechuga, G. C., Pereira, C. N., dos Santos, M. S., & Pereira, M. C. d. S. (2025). Pyrazole-Imidazoline Derivative Prevents Cardiac Damage and Mortality in Acute Trypanosoma cruzi Infection. Pharmaceuticals, 18(10), 1552. https://doi.org/10.3390/ph18101552









