Direct Targeting of CXCR2 Receptor Inhibits Neuroblastoma Growth: An In Vitro Assessment
Abstract
1. Introduction
2. Results
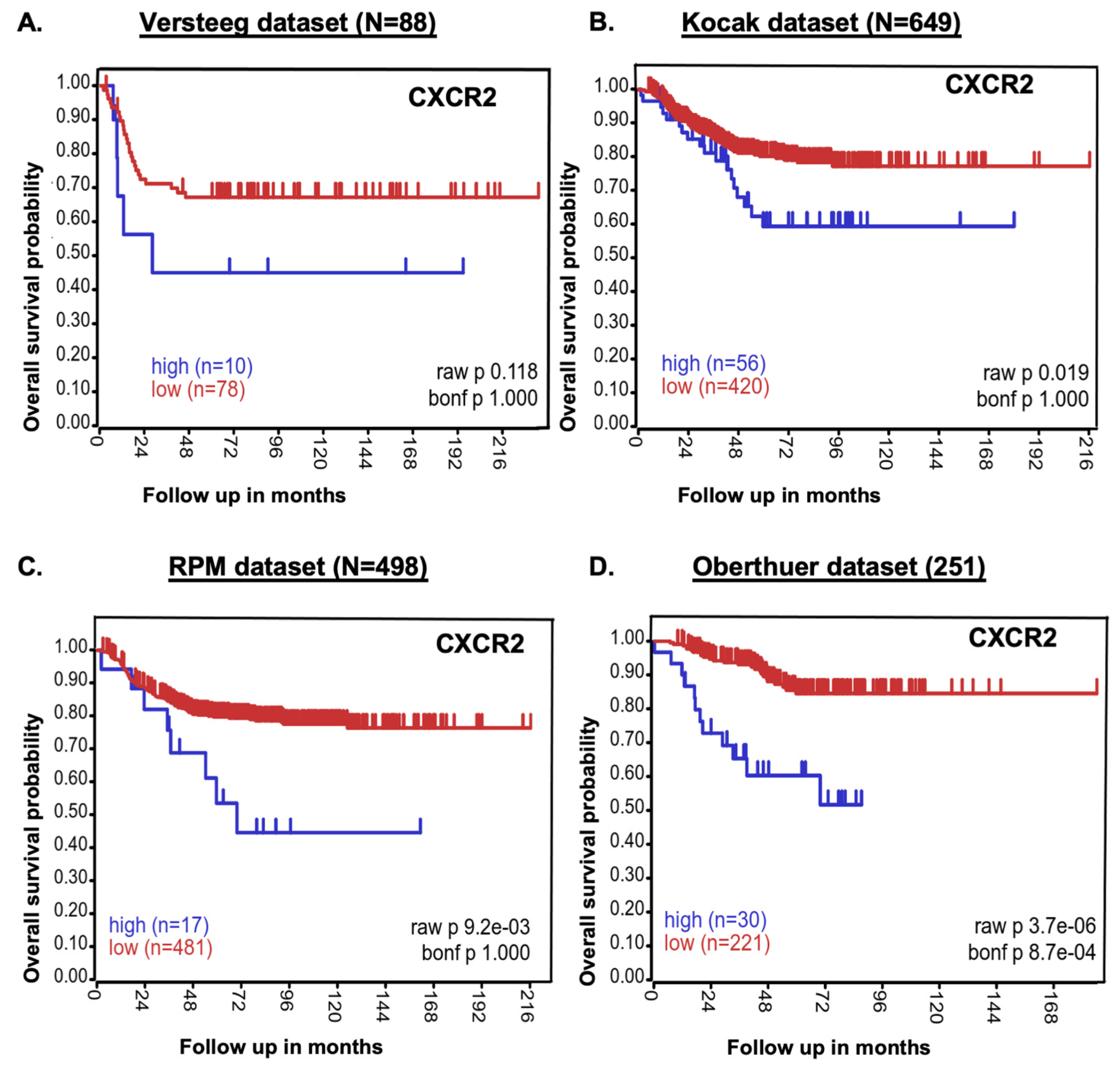
2.1. CXCR2 Expression Inversely Correlates with NB Patients’ Survival
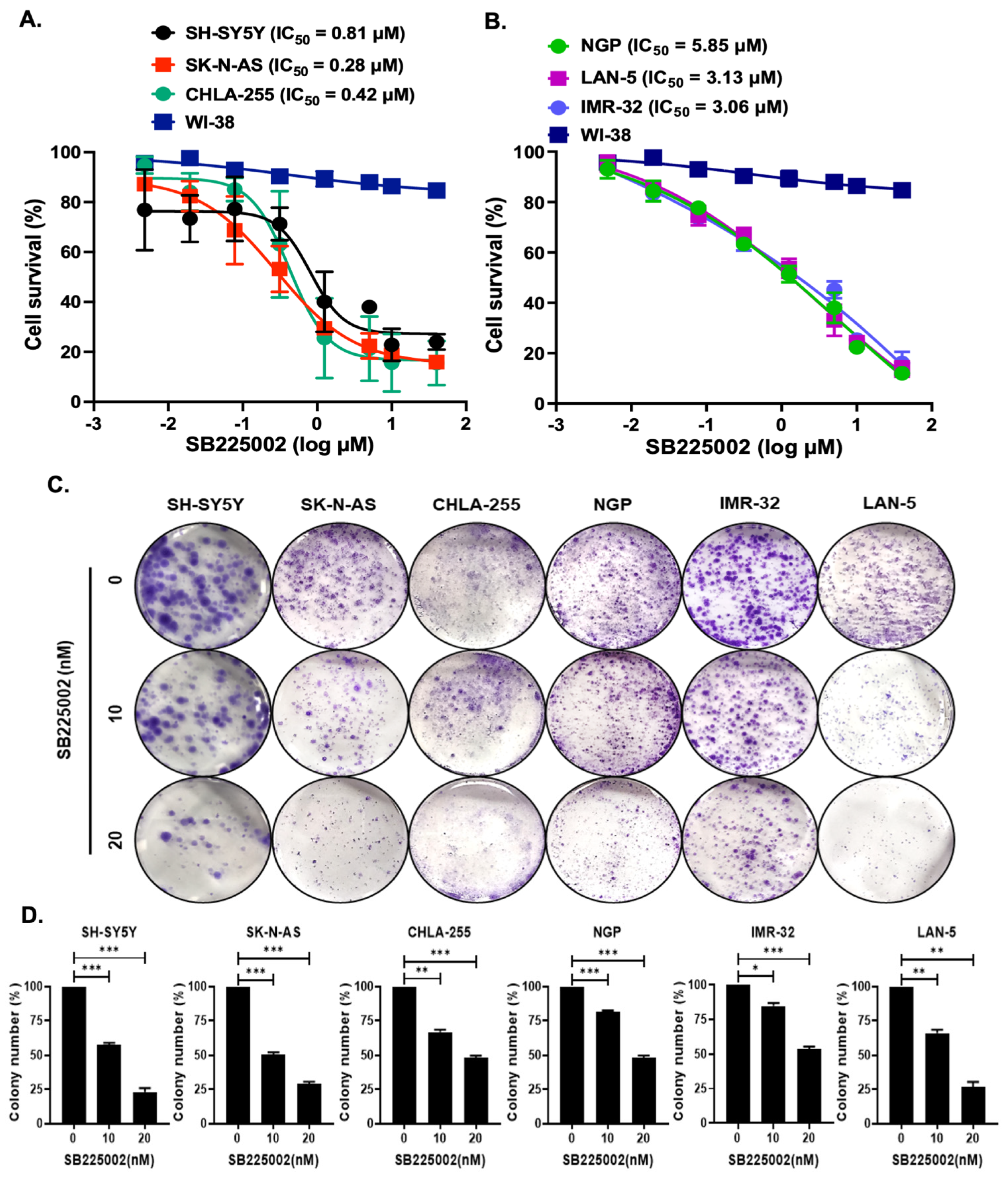
2.2. SB225002 Inhibits NB Cell Proliferation
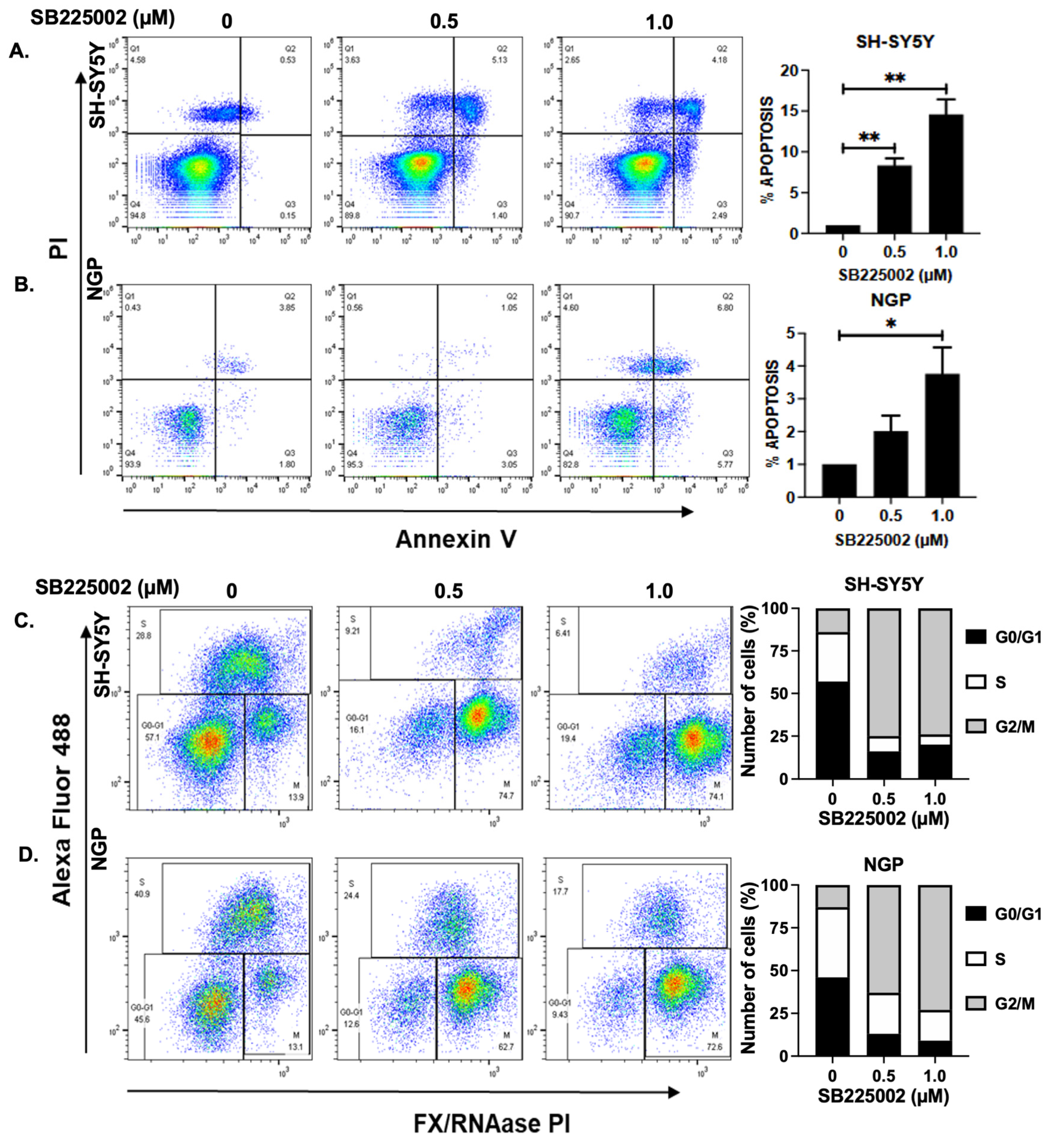
2.3. SB225002 Induces Apoptosis and Blocks Cell Cycle Progression in NB
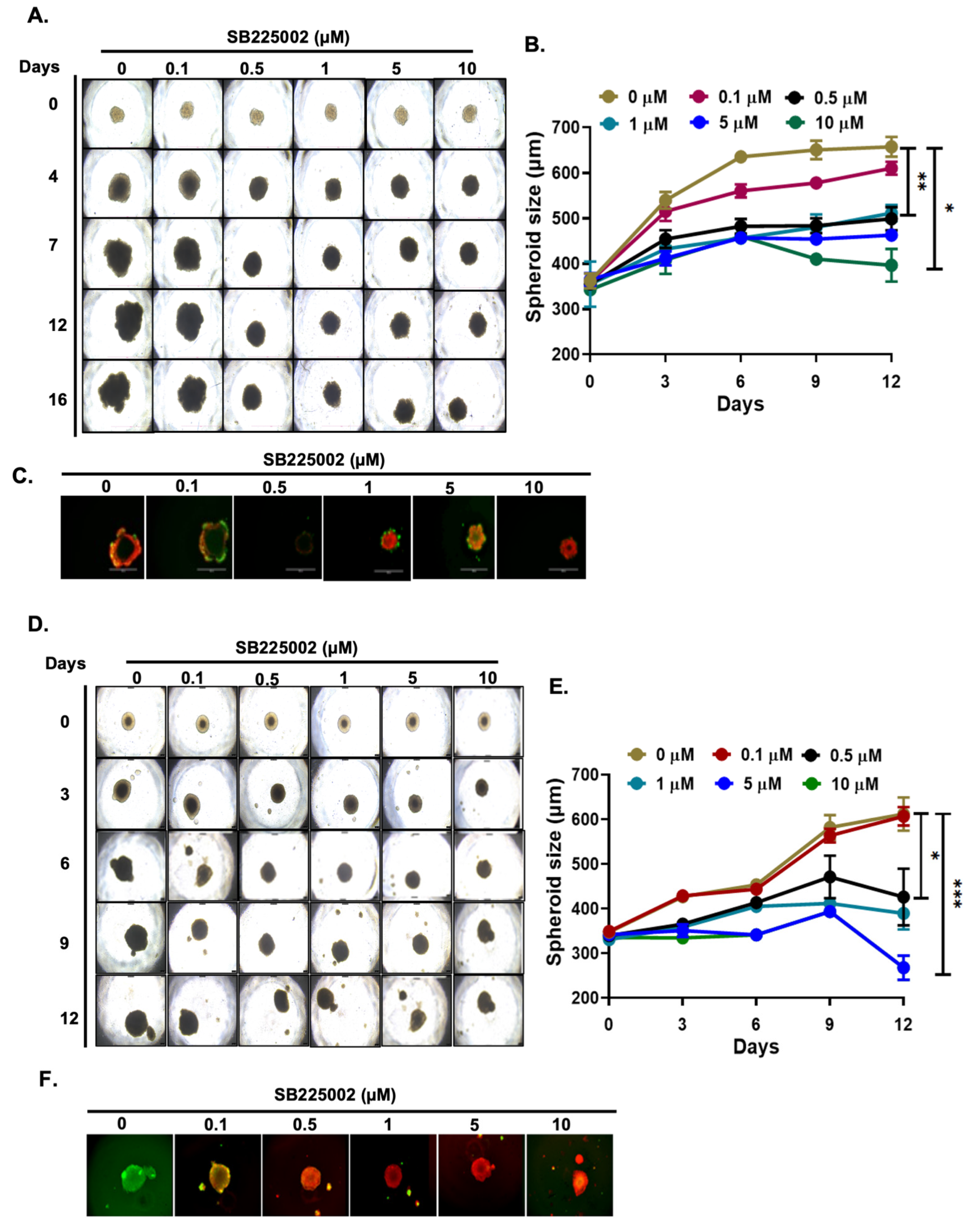
2.4. SB225002 Inhibits NB 3D Spheroid Growth
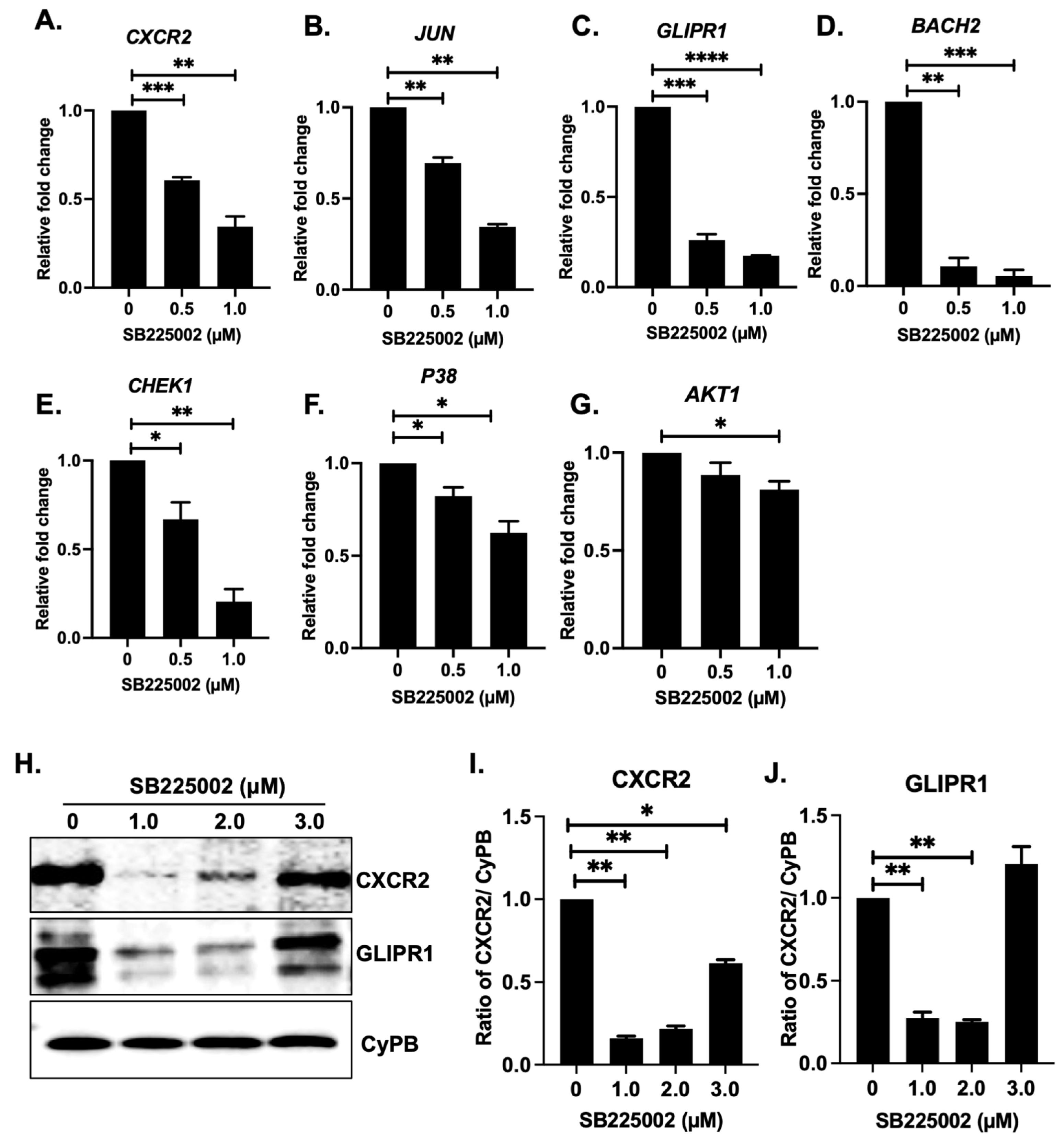
2.5. SB225002 Inhibits the CXCR2 Receptor Pathway
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Patient Dataset
4.2. Clinical Patient Dataset
4.3. Cell Proliferation and 3D Spheroid Assays
4.4. Apoptosis and Cell Cycle Assay
4.5. Gene Expression and Immunoblotting Assays
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korbecki, J.; Kupnicka, P.; Chlubek, M.; Goracy, J.; Gutowska, I.; Baranowska-Bosiacka, I. CXCR2 Receptor: Regulation of Expression, Signal Transduction, and Involvement in Cancer. Int. J. Mol. Sci. 2022, 23, 2168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, A.; Tian, Y.; Wu, J.D.; Liu, Y.; Li, T.; Chen, Y.; Han, X.; Wu, K. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 2016, 31, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Capucetti, A.; Albano, F.; Bonecchi, R. Multiple Roles for Chemokines in Neutrophil Biology. Front. Immunol. 2020, 11, 1259. [Google Scholar] [CrossRef] [PubMed]
- Safarulla, S.; Madan, A.; Xing, F.; Chandrasekaran, A. CXCR2 Mediates Distinct Neutrophil Behavior in Brain Metastatic Breast Tumor. Cancers 2022, 14, 515. [Google Scholar] [CrossRef]
- Sawant, K.V.; Sepuru, K.M.; Lowry, E.; Penaranda, B.; Frevert, C.W.; Garofalo, R.P.; Rajarathnam, K. Neutrophil recruitment by chemokines Cxcl1/KC and Cxcl2/MIP2: Role of Cxcr2 activation and glycosaminoglycan interactions. J. Leukoc. Biol. 2021, 109, 777–791. [Google Scholar] [CrossRef]
- Hou, R.; Yu, Y.; Sluter, M.N.; Li, L.; Hao, J.; Fang, J.; Yang, J.; Jiang, J. Targeting EP2 receptor with multifaceted mechanisms for high-risk neuroblastoma. Cell Rep. 2022, 39, 111000. [Google Scholar] [CrossRef]
- Brat, D.J.; Bellail, A.C.; Van Meir, E.G. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005, 7, 122–133. [Google Scholar] [CrossRef]
- Luppi, F.; Longo, A.M.; de Boer, W.I.; Rabe, K.F.; Hiemstra, P.S. Interleukin-8 stimulates cell proliferation in non-small cell lung cancer through epidermal growth factor receptor transactivation. Lung Cancer 2007, 56, 25–33. [Google Scholar] [CrossRef]
- Duckworth, C.; Zhang, L.; Carroll, S.L.; Ethier, S.P.; Cheung, H.W. Overexpression of GAB2 in ovarian cancer cells promotes tumor growth and angiogenesis by upregulating chemokine expression. Oncogene 2016, 35, 4036–4047. [Google Scholar] [CrossRef]
- PDQ Pediatric Treatment Editorial Board. Neuroblastoma Treatment (PDQ®): Health Professional Version. In PDQ Cancer Information Summaries; National Cancer Institute: Bethesda, MD, USA, 2002. [Google Scholar] [PubMed]
- Hashimoto, O.; Yoshida, M.; Koma, Y.; Yanai, T.; Hasegawa, D.; Kosaka, Y.; Nishimura, N.; Yokozaki, H. Collaboration of cancer-associated fibroblasts and tumour-associated macrophages for neuroblastoma development. J. Pathol. 2016, 240, 211–223. [Google Scholar] [CrossRef]
- Liu, K.X.; Joshi, S. “Re-educating” Tumor Associated Macrophages as a Novel Immunotherapy Strategy for Neuroblastoma. Front. Immunol. 2020, 11, 1947. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Thirant, C.; Kramdi, A.; Pierre-Eugene, C.; Louis-Brennetot, C.; Blanchard, O.; Surdez, D.; Gruel, N.; Lapouble, E.; Pierron, G.; et al. Single-cell transcriptomics reveals shared immunosuppressive landscapes of mouse and human neuroblastoma. J. Immunother. Cancer 2022, 10, e004807. [Google Scholar] [CrossRef] [PubMed]
- de Vasconcellos, J.F.; Laranjeira, A.B.; Leal, P.C.; Bhasin, M.K.; Zenatti, P.P.; Nunes, R.J.; Yunes, R.A.; Nowill, A.E.; Libermann, T.A.; Zerbini, L.F.; et al. SB225002 Induces Cell Death and Cell Cycle Arrest in Acute Lymphoblastic Leukemia Cells through the Activation of GLIPR1. PLoS ONE 2015, 10, e0134783. [Google Scholar] [CrossRef] [PubMed]
- White, J.R.; Lee, J.M.; Young, P.R.; Hertzberg, R.P.; Jurewicz, A.J.; Chaikin, M.A.; Widdowson, K.; Foley, J.J.; Martin, L.D.; Griswold, D.E.; et al. Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J. Biol. Chem. 1998, 273, 10095–10098. [Google Scholar] [CrossRef]
- Yu, M.; Berk, R.; Kosir, M.A. CXCL7-Mediated Stimulation of Lymphangiogenic Factors VEGF-C, VEGF-D in Human Breast Cancer Cells. J. Oncol. 2010, 2010, 939407. [Google Scholar] [CrossRef]
- Du, M.; Qiu, Q.; Gruslin, A.; Gordon, J.; He, M.; Chan, C.C.; Li, D.; Tsang, B.K. SB225002 promotes mitotic catastrophe in chemo-sensitive and -resistant ovarian cancer cells independent of p53 status in vitro. PLoS ONE 2013, 8, e54572. [Google Scholar] [CrossRef]
- Lo, M.C.; Yip, T.C.; Ngan, K.C.; Cheng, W.W.; Law, C.K.; Chan, P.S.; Chan, K.C.; Wong, C.K.; Wong, R.N.; Lo, K.W.; et al. Role of MIF/CXCL8/CXCR2 signaling in the growth of nasopharyngeal carcinoma tumor spheres. Cancer Lett. 2013, 335, 81–92. [Google Scholar] [CrossRef]
- Wang, B.; Hendricks, D.T.; Wamunyokoli, F.; Parker, M.I. A growth-related oncogene/CXC chemokine receptor 2 autocrine loop contributes to cellular proliferation in esophageal cancer. Cancer Res. 2006, 66, 3071–3077. [Google Scholar] [CrossRef]
- Grepin, R.; Guyot, M.; Giuliano, S.; Boncompagni, M.; Ambrosetti, D.; Chamorey, E.; Scoazec, J.Y.; Negrier, S.; Simonnet, H.; Pages, G. The CXCL7/CXCR1/2 axis is a key driver in the growth of clear cell renal cell carcinoma. Cancer Res. 2014, 74, 873–883. [Google Scholar] [CrossRef]
- Sueoka, H.; Hirano, T.; Uda, Y.; Iimuro, Y.; Yamanaka, J.; Fujimoto, J. Blockage of CXCR2 suppresses tumor growth of intrahepatic cholangiocellular carcinoma. Surgery 2014, 155, 640–649. [Google Scholar] [CrossRef]
- Matsuo, Y.; Campbell, P.M.; Brekken, R.A.; Sung, B.; Ouellette, M.M.; Fleming, J.B.; Aggarwal, B.B.; Der, C.J.; Guha, S. K-Ras promotes angiogenesis mediated by immortalized human pancreatic epithelial cells through mitogen-activated protein kinase signaling pathways. Mol. Cancer Res. 2009, 7, 799–808. [Google Scholar] [CrossRef]
- Bakshi, P.; Jin, C.; Broutin, P.; Berhane, B.; Reed, J.; Mullan, M. Structural optimization of a CXCR2-directed antagonist that indirectly inhibits gamma-secretase and reduces Abeta. Bioorg. Med. Chem. 2009, 17, 8102–8112. [Google Scholar] [CrossRef]
- Zhou, Z.; Xia, G.; Xiang, Z.; Liu, M.; Wei, Z.; Yan, J.; Chen, W.; Zhu, J.; Awasthi, N.; Sun, X.; et al. A C-X-C Chemokine Receptor Type 2-Dominated Cross-talk between Tumor Cells and Macrophages Drives Gastric Cancer Metastasis. Clin. Cancer Res. 2019, 25, 3317–3328. [Google Scholar] [CrossRef]
- Dong, Y.L.; Kabir, S.M.; Lee, E.S.; Son, D.S. CXCR2-driven ovarian cancer progression involves upregulation of proinflammatory chemokines by potentiating NF-κB activation via EGFR-transactivated Akt signaling. PLoS ONE 2013, 8, e83789. [Google Scholar] [CrossRef]
- Jeong, Y.; Yoon, S.Y.; Jung, S.P.; Nam, S.J.; Lee, J.E.; Kim, S. Inhibition of Interleukin-8/C-X-C Chemokine Receptor 2 Signaling Axis Prevents Tumor Growth and Metastasis in Triple-Negative Breast Cancer Cells. Pharmacology 2025, 110, 178–190. [Google Scholar] [CrossRef]
- Erin, N.; Nizam, E.; Tanriover, G.; Koksoy, S. Autocrine control of MIP-2 secretion from metastatic breast cancer cells is mediated by CXCR2: A mechanism for possible resistance to CXCR2 antagonists. Breast Cancer Res. Treat. 2015, 150, 57–69. [Google Scholar] [CrossRef]
- Song, X.; Wang, Z.; Jin, Y.; Wang, Y.; Duan, W. Loss of miR-532-5p in vitro promotes cell proliferation and metastasis by influencing CXCL2 expression in HCC. Am. J. Transl. Res. 2015, 7, 2254–2261. [Google Scholar] [PubMed]
- Bazzichetto, C.; Di Martile, M.; Del Bufalo, D.; Milella, M.; Conciatori, F. Induction of cell death by the CXCR2 antagonist SB225002 in colorectal cancer and stromal cells. Biomed. Pharmacother. 2025, 188, 118203. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, Y.; Butler, W.; Xu, L.; Chang, Y.; Lei, K.; Zhang, H.; Zhou, Y.; Gao, A.C.; Zhang, Q.; et al. Targeting cellular heterogeneity with CXCR2 blockade for the treatment of therapy-resistant prostate cancer. Sci. Transl. Med. 2019, 11, eaax0428. [Google Scholar] [CrossRef] [PubMed]
- Mestas, J.; Burdick, M.D.; Reckamp, K.; Pantuck, A.; Figlin, R.A.; Strieter, R.M. The role of CXCR2/CXCR2 ligand biological axis in renal cell carcinoma. J. Immunol. 2005, 175, 5351–5357. [Google Scholar] [CrossRef]
- Stofas, A.; Levidou, G.; Piperi, C.; Adamopoulos, C.; Dalagiorgou, G.; Bamias, A.; Karadimou, A.; Lainakis, G.A.; Papadoukakis, S.; Stravodimos, K.; et al. The role of CXC-chemokine receptor CXCR2 and suppressor of cytokine signaling-3 (SOCS-3) in renal cell carcinoma. BMC Cancer 2014, 14, 149. [Google Scholar] [CrossRef]
- Takikawa, T.; Hamada, S.; Matsumoto, R.; Tanaka, Y.; Kataoka, F.; Sasaki, A.; Masamune, A. Senescent Human Pancreatic Stellate Cells Secrete CXCR2 Agonist CXCLs to Promote Proliferation and Migration of Human Pancreatic Cancer AsPC-1 and MIAPaCa-2 Cell Lines. Int. J. Mol. Sci. 2022, 23, 9275. [Google Scholar] [CrossRef]
- Sun, J.; Yuan, J. Chemokine (C-X-C motif) ligand 1/chemokine (C-X-C motif) receptor 2 autocrine loop contributes to cellular proliferation, migration and apoptosis in cervical cancer. Bioengineered 2022, 13, 7579–7591. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, G.; Shi, Z.; Guo, J.; Yu, S.; Yu, C.; Shen, C. Interleukin-8 Regulates the Autophagy and Apoptosis in Gastric Cancer Cells via Regulating PI3K/Akt Signaling Pathway. Dis. Markers 2022, 2022, 7300987. [Google Scholar] [CrossRef] [PubMed]
- Urbantat, R.M.; Jelgersma, C.; Vajkoczy, P.; Brandenburg, S.; Acker, G. Combining TMZ and SB225002 induces changes of CXCR2 and VEGFR signalling in primary human endothelial cells in vitro. Oncol. Rep. 2022, 48, 158. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, X.; Zhao, S.; Jing, J.; Wang, Q.; Dang, Y. HOXC10 Promotes Metastasis in Colorectal Cancer by Recruiting Myeloid-derived Suppressor Cells. J. Cancer 2022, 13, 3308–3317. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Fang, D.; Liu, H.; Ou, X.; Zhang, C.; Zhao, Z.; Zhao, S.; Peng, J.; Cai, S.; He, Y.; et al. PMN-MDSCs accumulation induced by CXCL1 promotes CD8+ T cells exhaustion in gastric cancer. Cancer Lett. 2022, 532, 215598. [Google Scholar] [CrossRef]
- Li, Y.; Liu, A.; Liu, S.; Yan, L.; Yuan, Y.; Xu, Q. Involvement of CXCL17 and GPR35 in Gastric Cancer Initiation and Progression. Int. J. Mol. Sci. 2022, 24, 615. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Lan, B.; Wang, Y.; Lin, W.; Jiang, X.; Ye, J.; Shang, B.; Feng, C.; Liu, J.; et al. Heterogeneity of tyrosine-based melanin anabolism regulates pulmonary and cerebral organotropic colonization microenvironment of melanoma cells. Theranostics 2022, 12, 2063–2079. [Google Scholar] [CrossRef]
- Sun, B.; Ross, S.M.; Rowley, S.; Adeleye, Y.; Clewell, R.A. Contribution of ATM and ATR kinase pathways to p53-mediated response in etoposide and methyl methanesulfonate induced DNA damage. Environ. Mol. Mutagen. 2017, 58, 72–83. [Google Scholar] [CrossRef]
- Liu, Z.; Rader, J.; He, S.; Phung, T.; Thiele, C.J. CASZ1 inhibits cell cycle progression in neuroblastoma by restoring pRb activity. Cell Cycle 2013, 12, 2210–2218. [Google Scholar] [CrossRef]
- Nie, S.; Wan, Y.; Wang, H.; Liu, J.; Yang, J.; Sun, R.; Meng, H.; Ma, X.; Jiang, Y.; Cheng, W. CXCL2-mediated ATR/CHK1 signaling pathway and platinum resistance in epithelial ovarian cancer. J. Ovarian Res. 2021, 14, 115. [Google Scholar] [CrossRef]
- Xu, M.; Jiang, H.; Wang, H.; Liu, J.; Liu, B.; Guo, Z. SB225002 inhibits prostate cancer invasion and attenuates the expression of BSP, OPN and MMP-2. Oncol. Rep. 2018, 40, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Lepsenyi, M.; Algethami, N.; Al-Haidari, A.A.; Algaber, A.; Syk, I.; Rahman, M.; Thorlacius, H. CXCL2-CXCR2 axis mediates alphaV integrin-dependent peritoneal metastasis of colon cancer cells. Clin. Exp. Metastasis 2021, 38, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, H.; Wu, S.; Tan, H.; Sun, Y.; Liu, X.; Si, S.; Xu, L.; Huang, J.; Zhou, W.; et al. IL-17A promotes CXCR2-dependent angiogenesis in a mouse model of liver cancer. Mol. Med. Rep. 2019, 20, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Park, G.Y.; Pathak, H.B.; Godwin, A.K.; Kwon, Y. Epithelial-stromal communication via CXCL1-CXCR2 interaction stimulates growth of ovarian cancer cells through p38 activation. Cell. Oncol. 2021, 44, 77–92. [Google Scholar] [CrossRef]
- Lazennec, G.; Rajarathnam, K.; Richmond, A. CXCR2 chemokine receptor—A master regulator in cancer and physiology. Trends Mol. Med. 2024, 30, 37–55. [Google Scholar] [CrossRef]
- Stip, M.C.; Teeuwen, L.; Dierselhuis, M.P.; Leusen, J.H.W.; Krijgsman, D. Targeting the myeloid microenvironment in neuroblastoma. J. Exp. Clin. Cancer Res. 2023, 42, 337. [Google Scholar] [CrossRef]
- Zhou, Y.; Shen, G.; Zhou, X.; Li, J. Therapeutic potential of tumor-associated neutrophils: Dual role and phenotypic plasticity. Signal Transduct. Target. Ther. 2025, 10, 178. [Google Scholar] [CrossRef]
- Lv, Y.; Chen, C.; Han, M.; Tian, C.; Song, F.; Feng, S.; Xu, M.; Zhao, Z.; Zhou, H.; Su, W.; et al. CXCL2: A key player in the tumor microenvironment and inflammatory diseases. Cancer Cell Int. 2025, 25, 133. [Google Scholar] [CrossRef]
- Chilamakuri, R.; Rouse, D.C.; Yu, Y.; Kabir, A.S.; Muth, A.; Yang, J.; Lipton, J.M.; Agarwal, S. BX-795 inhibits neuroblastoma growth and enhances sensitivity towards chemotherapy. Transl. Oncol. 2022, 15, 101272. [Google Scholar] [CrossRef]
- Chilamakuri, R.; Rouse, D.C.; Agarwal, S. Inhibition of Polo-like Kinase 1 by HMN-214 Blocks Cell Cycle Progression and Inhibits Neuroblastoma Growth. Pharmaceuticals 2022, 15, 523. [Google Scholar] [CrossRef]
- Chilamakuri, R.; Agarwal, S. Direct Targeting of the Raf-MEK-ERK Signaling Cascade Inhibits Neuroblastoma Growth. Curr. Oncol. 2022, 29, 6508–6522. [Google Scholar] [CrossRef]
| S.No. | Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|
| 1 | CXCR2 | CTCCAATAACAGCAGGTCAC | GGCTCAGCAGGAATACCA |
| 2 | JUN | CCCCAAGATCCTGAAACAGA | CCGTTGCTGGACTGGATTAT |
| 3 | GLIPR1 | AGCTGCACCCAAACTTCACT | ATCTGCCCAAACAACCTGAG |
| 4 | BACH2 | GAAAACGATGCTGCCATTTT | TTGGTGCACACTTCTGCTTC |
| 5 | CHEK1 | GACTGGGACTTGGTGCAAAC | TGCCATGAGTTGATGGAAGA |
| 6 | P38 | CGCAAGGTCACTGGAGGAAT | CTGGGCTTTAGGTCCCTGTG |
| 7 | AKT1 | GCACAAACGAGGGGAGTACA | AAGGTGCGTTCGATGACAGT |
| 8 | GAPDH | CACCATCTTCCAGGAGCGAG | TGATGACCCTTTTGGCTCCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chilamakuri, R.; Godugu, D.; Agarwal, S. Direct Targeting of CXCR2 Receptor Inhibits Neuroblastoma Growth: An In Vitro Assessment. Pharmaceuticals 2025, 18, 1547. https://doi.org/10.3390/ph18101547
Chilamakuri R, Godugu D, Agarwal S. Direct Targeting of CXCR2 Receptor Inhibits Neuroblastoma Growth: An In Vitro Assessment. Pharmaceuticals. 2025; 18(10):1547. https://doi.org/10.3390/ph18101547
Chicago/Turabian StyleChilamakuri, Rameswari, Deepika Godugu, and Saurabh Agarwal. 2025. "Direct Targeting of CXCR2 Receptor Inhibits Neuroblastoma Growth: An In Vitro Assessment" Pharmaceuticals 18, no. 10: 1547. https://doi.org/10.3390/ph18101547
APA StyleChilamakuri, R., Godugu, D., & Agarwal, S. (2025). Direct Targeting of CXCR2 Receptor Inhibits Neuroblastoma Growth: An In Vitro Assessment. Pharmaceuticals, 18(10), 1547. https://doi.org/10.3390/ph18101547








