Integration of Network Pharmacology, Molecular Docking, and In Vitro Nitric Oxide Inhibition Assay to Explore the Mechanism of Action of Thai Traditional Polyherbal Remedy, Mo-Ha-Rak, in the Treatment of Prolonged Fever
Abstract
1. Introduction
| Botanical Name | Part Used | Ratio (% w/w) | Pharmacological Activities |
|---|---|---|---|
| Azadirachta indica A.Juss. | Petiole | 4 | - |
| Bridelia ovata Decne. | Leaf | 4 | - |
| Capparis micracantha DC. | Root | 4 | Antipyretic [19,20], Anti-inflammatory [21] |
| Cassia fistula L. | Pulp | 12 | Antipyretic [22], Anti-inflammatory [23] |
| Clerodendrum indicum (L.) Kuntze | Root | 4 | Antipyretic [20], Anti-inflammatory [24] |
| Dracaena cochinchinensis (Lour.) S.C.Chen | Wood | 4 | Antipyretic [25], Anti-inflammatory [26,27] |
| Ficus racemosa L. | Root | 4 | Antipyretic [19,20], Anti-inflammatory [28,29] |
| Gymnopetalum chinense (Lour.) Merr. | Fruit | 4 | Anti-inflammatory [30] |
| Harrisonia perforata (Blanco) Merr. | Root | 4 | Antipyretic [19,20], Anti-inflammatory [31,32,33] |
| Ligusticum sinense Oliv. | Rhizome | 1 | Anti-inflammatory [34] |
| Mesua ferrea L. | Flower | 4 | Anti-inflammatory [35] |
| Nelumbo nucifera Gaertn. | Stamen | 2 | Anti-inflammatory [36] |
| Phyllanthus emblica L. | Fruit | 8 | Anti-inflammatory [37] |
| Pinus kesiya Royle ex Gordon | Wood | 1 | - |
| Tarenna hoaensis Pit. | Wood | 4 | - |
| Terminalia bellirica (Gaertn.) Roxb. | Fruit | 8 | Antipyretic [38], Anti-inflammatory [39,40] |
| Terminalia chebula Retz. | Fruit | 8 | Antipyretic [41], Anti-inflammatory [42] |
| Terminalia sp. “Samo Thet in Thai” | Fruit | 8 | - |
| Tiliacora triandra (Colebr.) Diels | Root | 4 | Antipyretic [19,20], Anti-inflammatory [32,43,44] |
| Tinospora crispa (L.) Hook. f. & Thomson | Stem | 4 | Antipyretic [45], Anti-inflammatory [46,47] |
| Vetiveria zizanioides (L.) Nash | Root | 4 | Antipyretic [48], Anti-inflammatory [48,49] |
2. Results
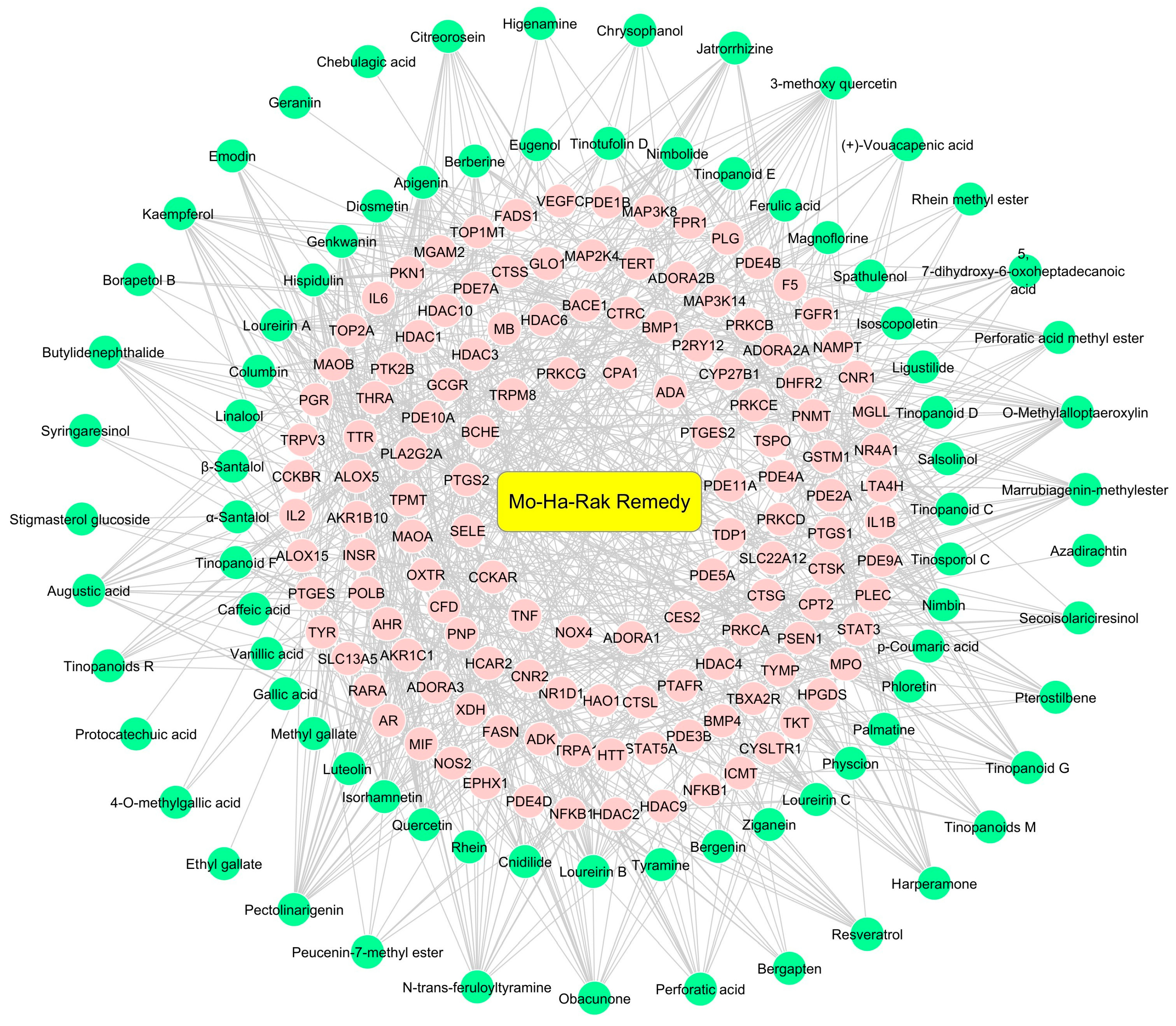
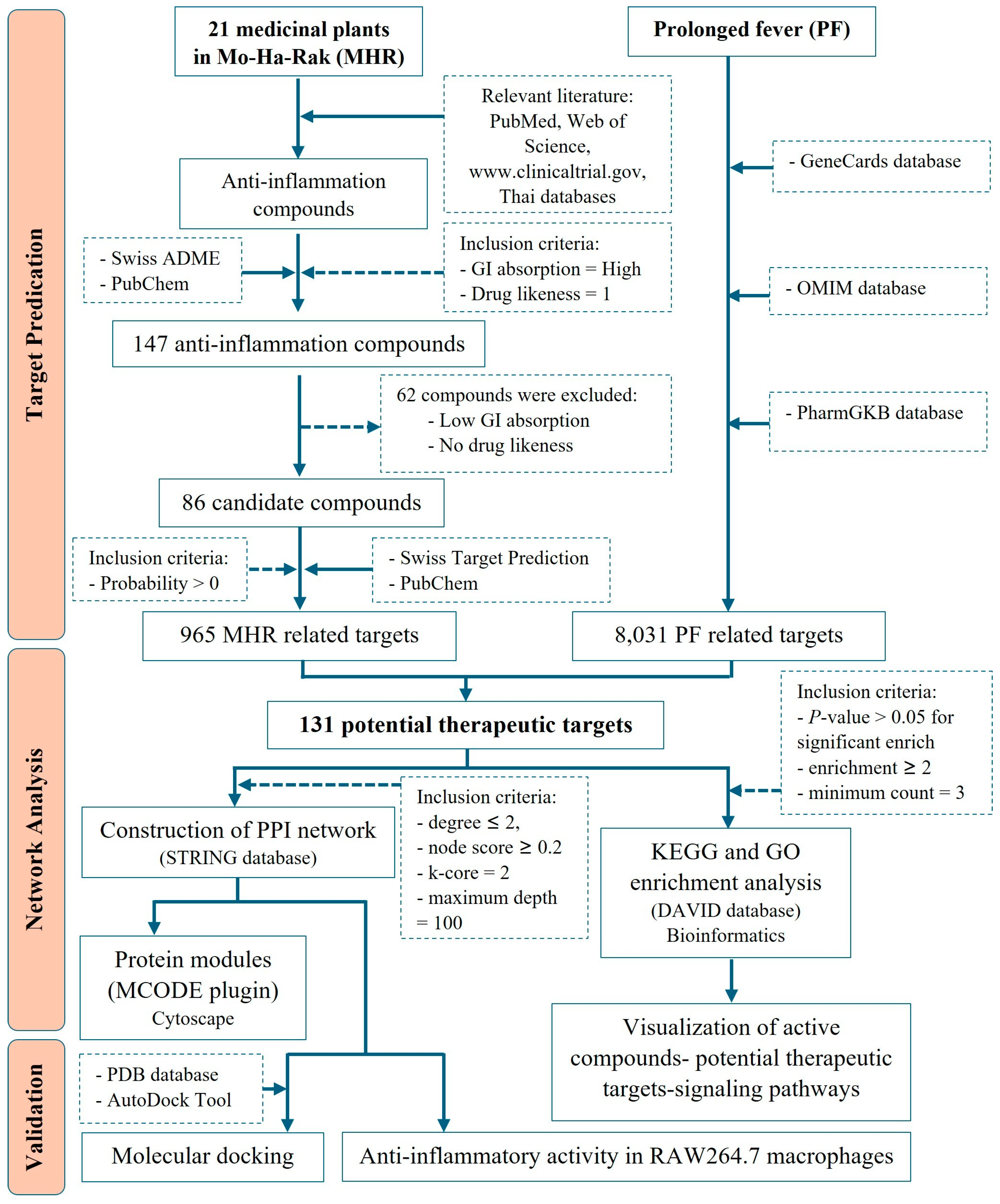
2.1. Screening of Active Compounds and MHR-Related Targets
2.2. Potential Therapeutic Targets of MHR Used in PF Treatment
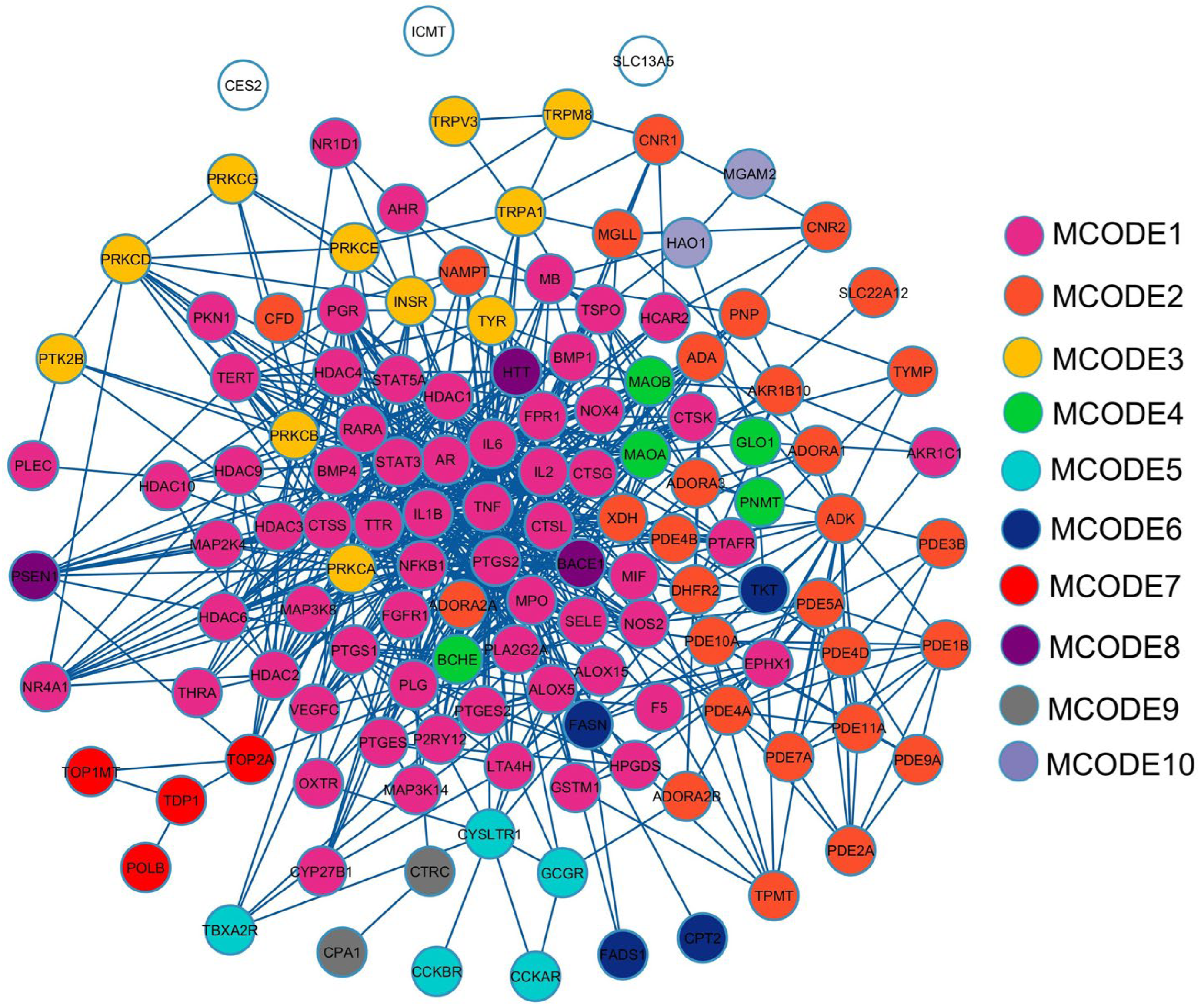
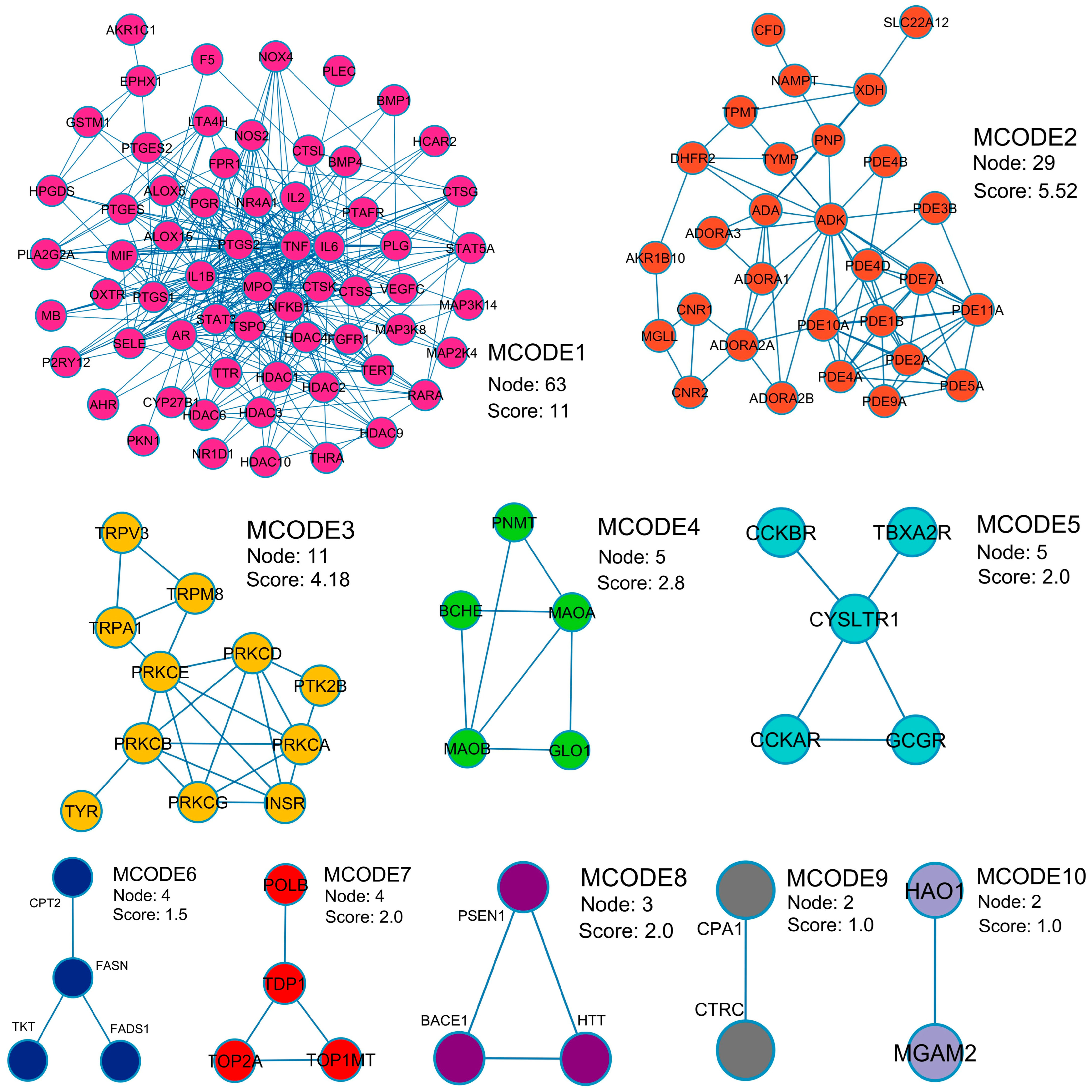
2.3. Protein–Protein Interaction (PPI) Visualization and Modular Analysis
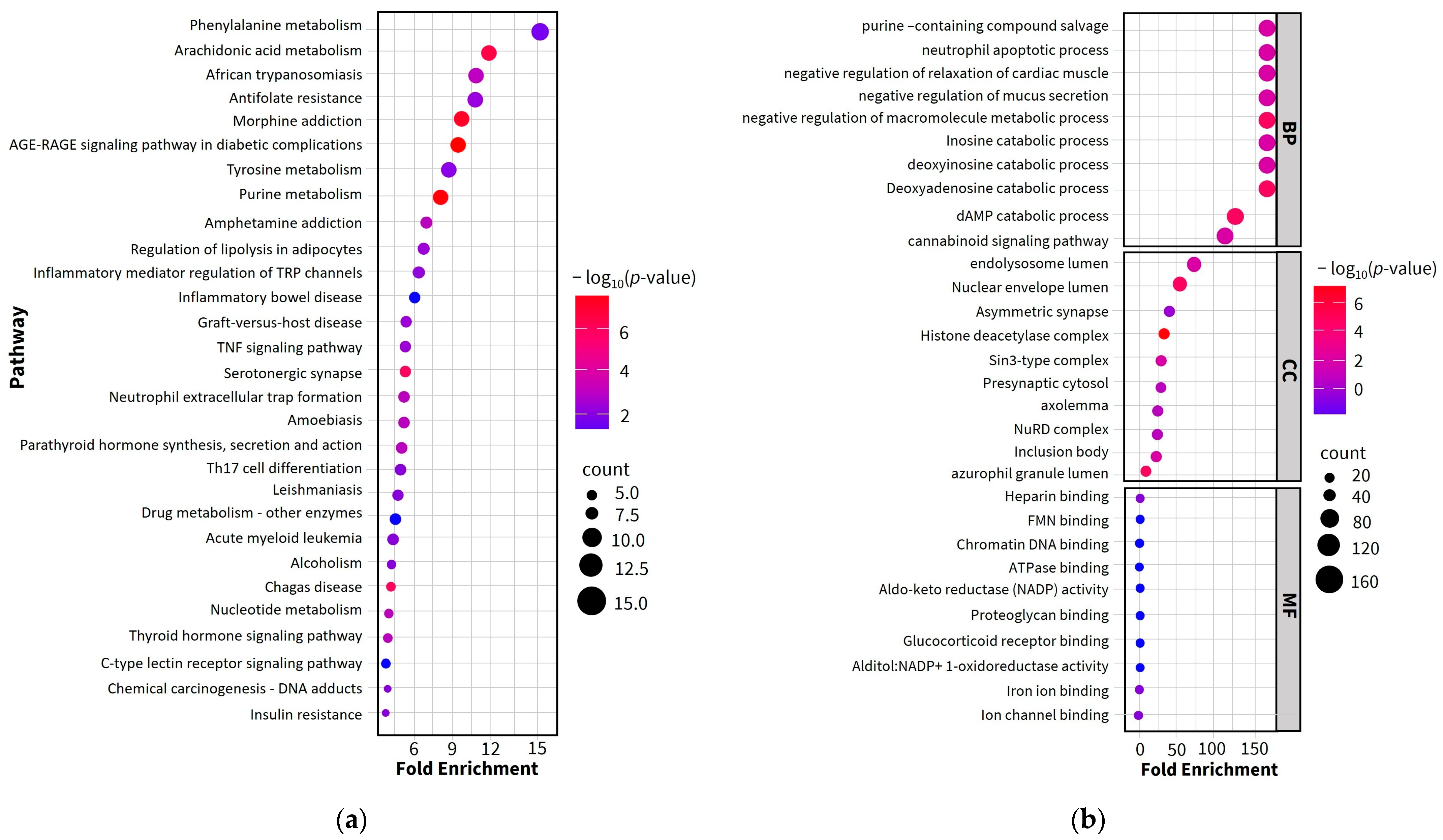
2.4. Gene Ontology (GO) and Enriched Pathway Analysis
2.5. Investigation of Possible Therapeutic Targets of MHR for PF Treatment
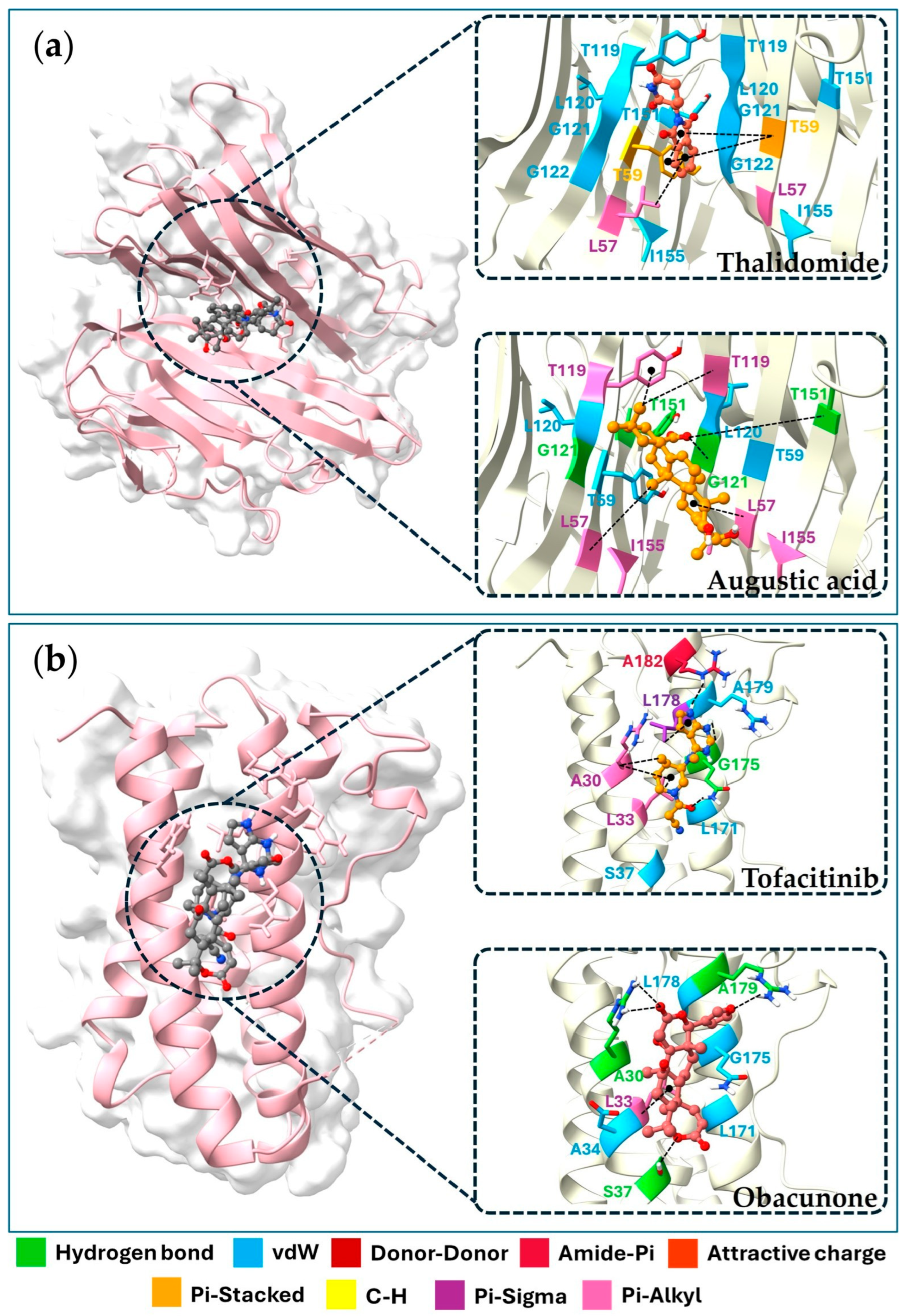
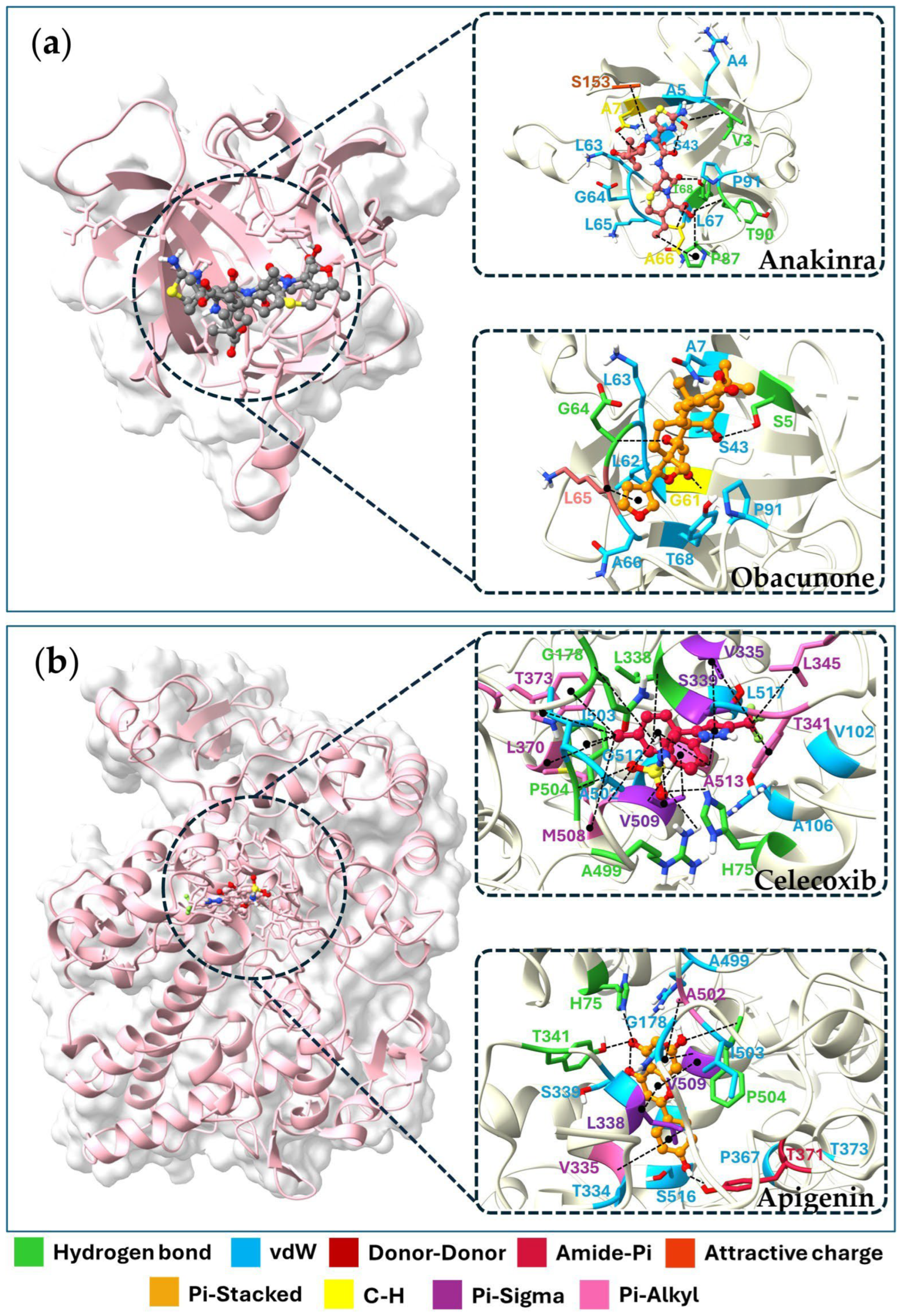
2.6. Molecular Docking of Key Targets
2.7. Preparation of MHR Extract
2.8. Characterization of MHR Extract
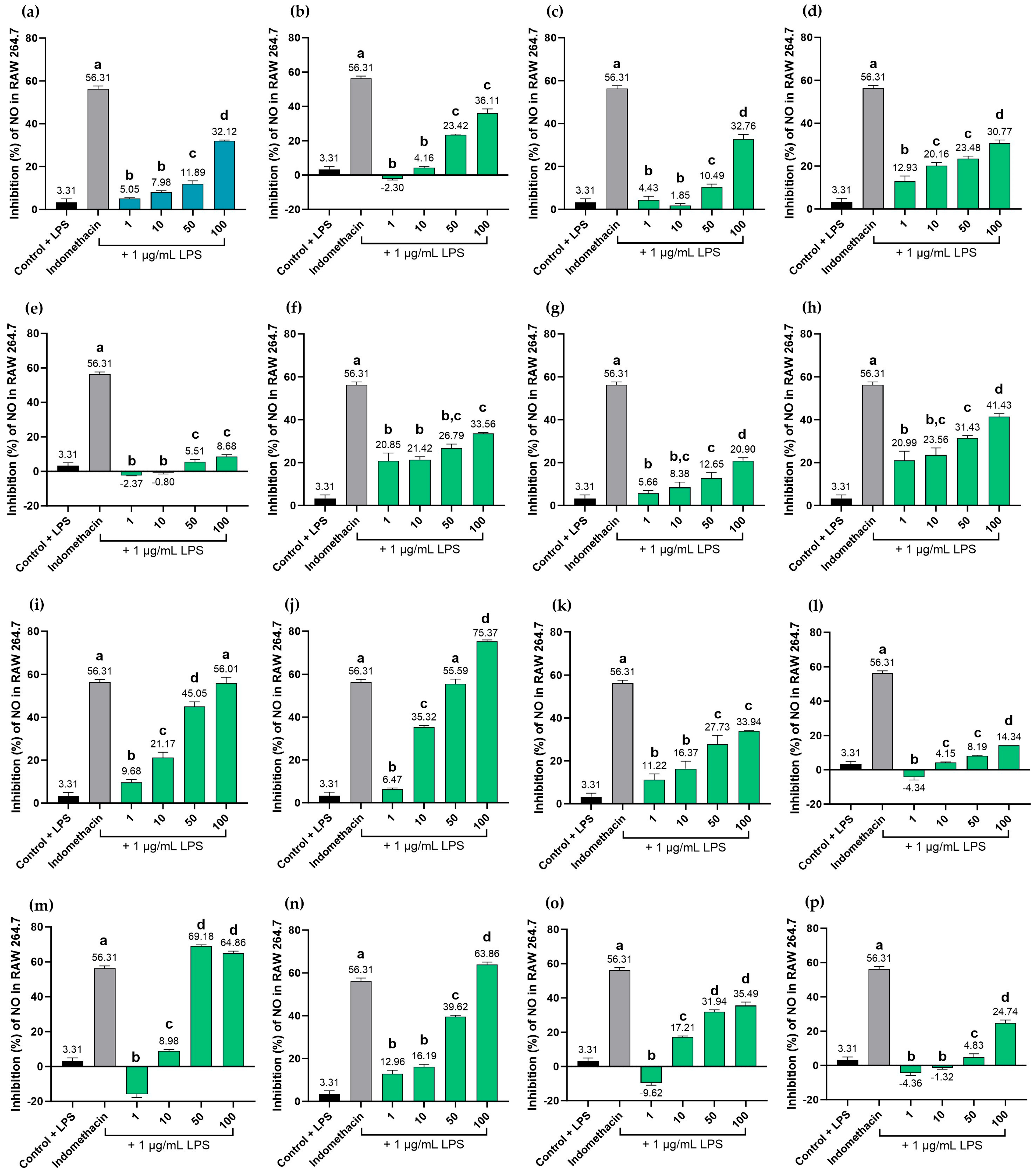
2.9. Effects of MHR Extract and Biomarker Compounds on NO Production in LPS-Induced RAW264.7 Macrophages
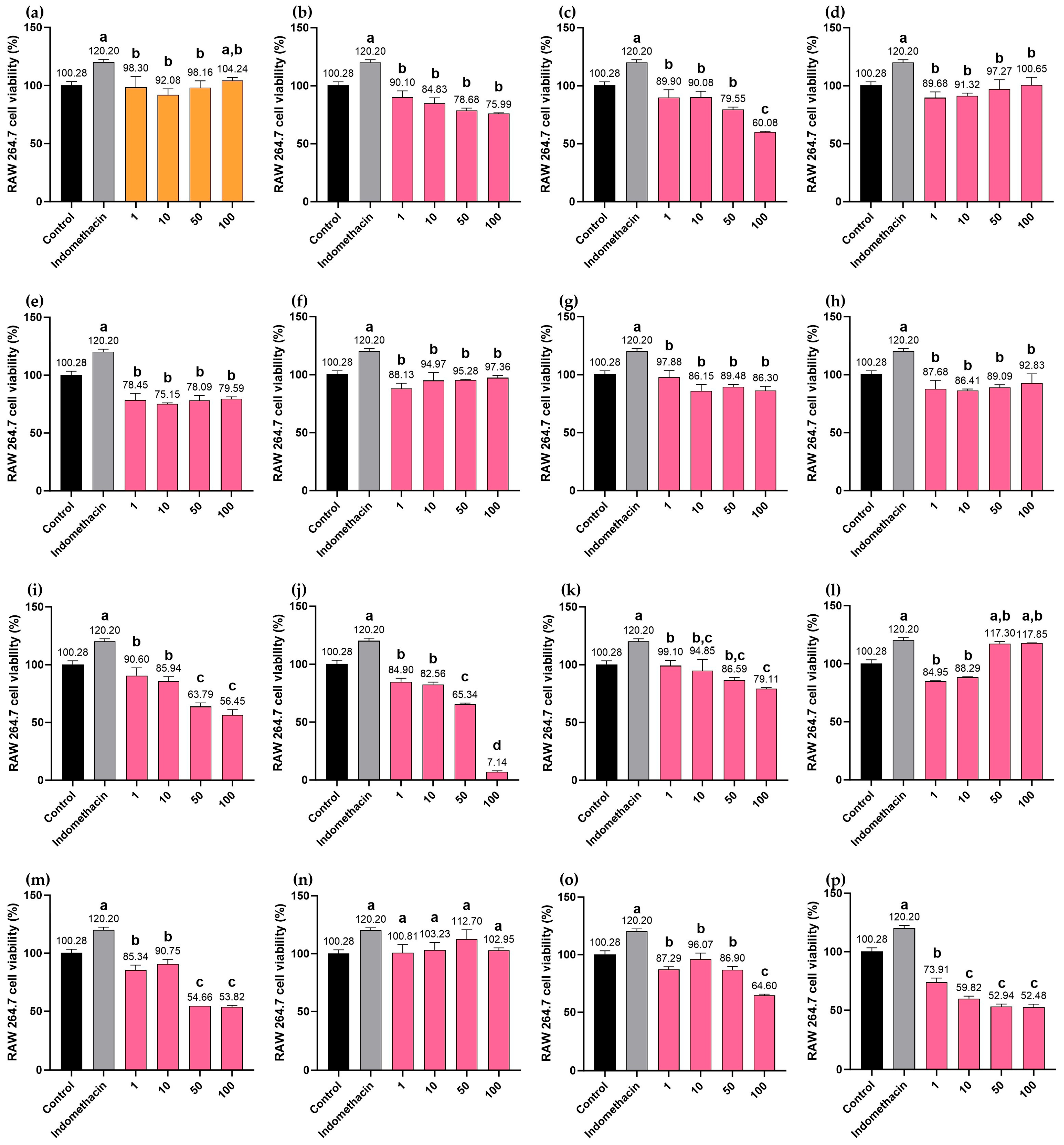
2.10. Cytotoxicity Effects of MHR Extract and Biomarker Compounds on RAW264.7 Macrophages
3. Discussion
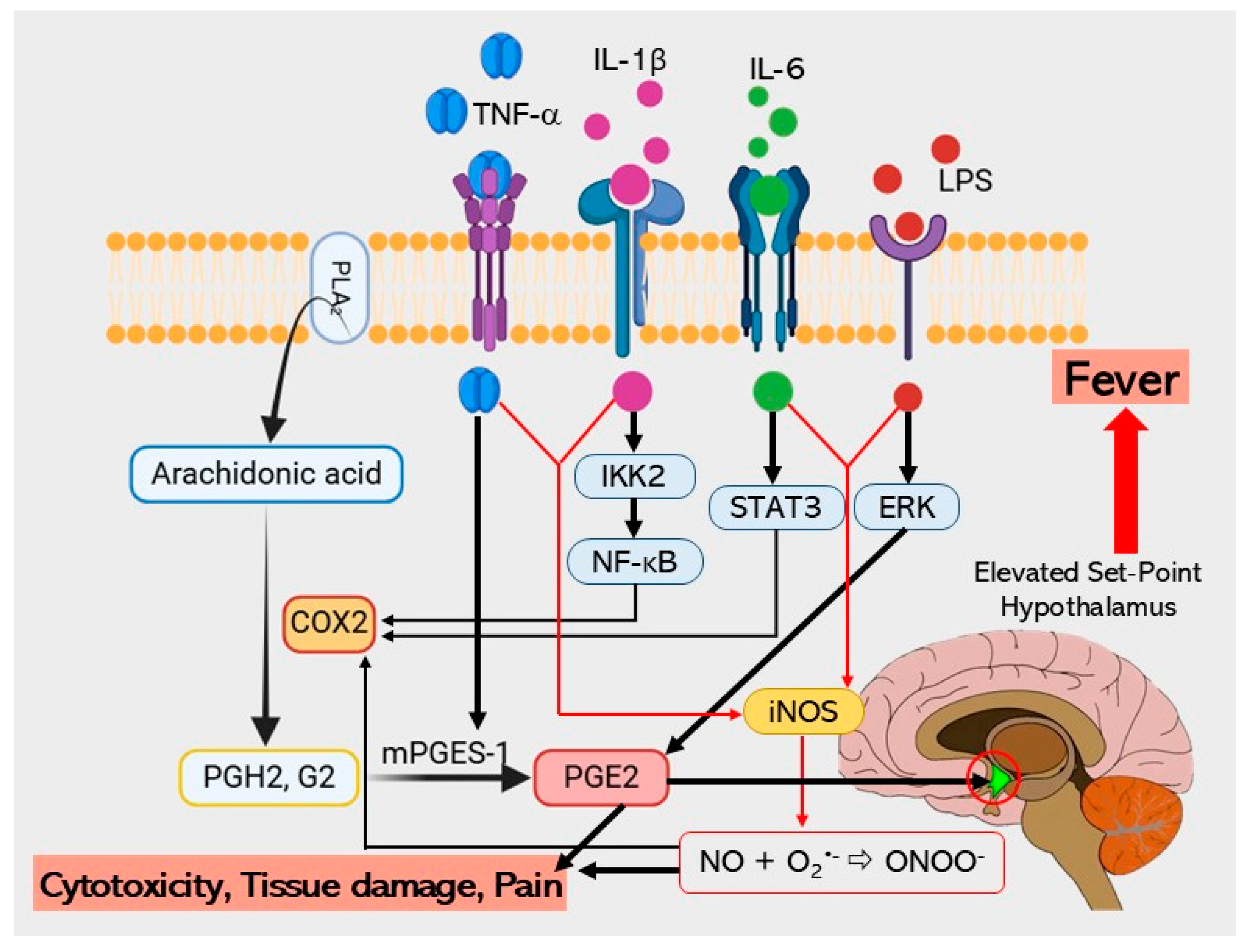
4. Materials and Methods
4.1. Screening for Potential Active Compounds and MHR-Related Targets
4.2. Identification of PF-Related Targets
4.3. Protein-Protein Interaction (PPI) Network and Modular Identification
4.4. Functional Enrichment and Pathway Analysis
4.5. Verification with Molecular Docking
4.5.1. Protein Structures and Modeling of Ligands Preparation
4.5.2. Protein-Ligand Docking
4.5.3. Docking Validation
4.6. Preparation of MHR Extract
4.7. Chemicals and Reagents
4.8. HPLC Analysis
4.9. Nitric Oxide (NO) Inhibitory Activity and Cytotoxicity of MHR Extract and Biomarkers on RAW 264.7 Macrophages
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BP | Biological Process |
| CC | Cellular Component |
| GI | Gastrointestinal |
| GO | Gene Ontology |
| HDAC1 | Histone Deacetylase 1 |
| IL1B | Interleukin-1β |
| IL6 | Interleukin-6 |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MF | Molecular Function |
| MHR | Mo-Ha-Rak |
| MPO | Myeloperoxidase |
| NFKB1 | Nuclear factor-kappa-B p105 subunit |
| PF | Prolonged Fever |
| PPI | Protein-protein interaction |
| PRKCA | Protein Kinase C Alpha |
| PTGS2 | Prostaglandin-Endoperoxide Synthase 2 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| STRING | Search Tool for the Retrieval of Interacting Genes |
| TNF | Tumor Necrosis Factor-alpha |
References
- Vanderschueren, S.; Knockaert, D.; Adriaenssens, T.; Demey, W.; Durnez, A.; Blockmans, D.; Bobbaers, H. From Prolonged Febrile Illness to Fever of Unknown Origin: The Challenge Continues. Arch. Intern. Med. 2003, 163, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Gargarella, E.R.; Vocaturo, F.; Guarneri, A.; Calcagni, M.L.; Leccisotti, L. Role of 18F FDG-PET-CT in fever and inflammation of unknown origin. J. Clin. Med. 2025, 14, 5861. [Google Scholar] [CrossRef]
- Petersdorf, R.G.; Beeson, P.B. Fever of unexplained origin: Report on 100 cases. Medicine 1961, 40, 1–30. [Google Scholar] [CrossRef]
- Knopp, B.W.; Parmar, J. Fever of unknown origin and atrial fibrillation: A case report. Cureus. 2022, 14, e32472. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K. Fever of Unknown Origin. Med. Clin. N. Am. 2024, 108, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Kawana, E.; Rahmani, R.; Turnbull, S.; Khattak, A.; Do, K.H.; Singh, A. A rare presentation of systemic lupus erythematosus in a patient with fever of unknown origin. Cureus 2024, 16, e59286. [Google Scholar] [CrossRef]
- Shoman, W.; Galal, A.; Elshishiny, A.M.; Hamza, E. Fever of unknown origin in pediatrics: Causes and clinical characteristics in a single centre experience. Gaz. Egypt. Paediatr. Assoc. 2024, 72, 65. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, W.; Tian, F.; Chen, J.; Zhang, W.; Ruan, Q.; Song, J. Clinical analysis of histiocytic necrotizing lymphadenitis in adults with fever of unknown origin: A retrospective study. Diagn. Pathol. 2025, 20, 92. [Google Scholar] [CrossRef]
- Topiwala, M.; Medisetty, M.K.; Verma, D.; Yadav, S. Unresolved fever in methicillin-sensitive Staphylococcus aureus bacteremia: Insights from a case without an identifiable source. IDCases 2025, 39, e02183. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, W.; Tian, F.; Sun, W.; Xing, M.; Ruan, Q.; Song, J. Fever of unknown origin: Clinical significance of the etiology and common inflammatory parameters. Diagn. Microbiol. Infect. Dis. 2025, 112, 116801. [Google Scholar] [CrossRef]
- Someko, H.; Kataoka, Y.; Obara, T. Drug fever: A narrative review. Ann. Clin. Epidemiol. 2023, 5, 95–106. [Google Scholar] [CrossRef]
- Bogossian, E.G.; Salvagno, M.; Fiore, M.; Talamonti, M.; Prezioso, C.; Montanaro, F.; Fratino, S.; Schuind, S.; Taccone, F.S. Impact of fever on the outcome non-anoxic acute brain injury patients: A systematic review and meta-analysis. Crit. Care 2024, 28, 367. [Google Scholar] [CrossRef]
- Nualkaew, S. Applied Thai Traditional Pharmacy, 1st ed.; KKU Printing House: Khon Kaen, Thailand, 2020; pp. 178–229. [Google Scholar]
- Zhou, W.; Zhang, H.; Wang, X.; Kang, J.; Guo, W.; Zhou, L.; Liu, H.; Wang, M.; Jia, R.; Du, X.; et al. Network pharmacology to unveil the mechanism of Moluodan in the treatment of chronic atrophic gastritis. Phytomedicine 2022, 95, 153837. [Google Scholar] [CrossRef]
- Chen, W.; Li, Y.; Zhang, C.; Zhou, H.; Ma, J.; Vaishnani, D.K.; Zeng, B.; Yu, J.; Mao, H.; Zheng, J. Multi-omics and experimental validation reveal anti-HCC mechanisms of Tibetan liuwei muxiang pill and quercetin. Pharmaceuticals 2025, 18, 900. [Google Scholar] [CrossRef]
- Wang, X.-H.; Li, Y.-H.; Zhang, J.-C.; Li, Z.; Liu, G.-X.; Zhang, T.; Zhang, M.-Y. Analysis of the effect of Bupleurum on fever in Xiaochaihu Decoction based on network pharmacology. J. Hainan Med. Univ. 2021, 27, 55–59. [Google Scholar]
- Pei, K.; Wang, Y.; Guo, W.; Lin, H.; Lin, Z.; Lv, G. Antipyretic mechanism of Bai Hu Tang on LPS-induced fever in rat: A network pharmacology and metabolomics analysis. Pharmaceuticals 2025, 18, 610. [Google Scholar] [CrossRef]
- Zhang, H.; Ge, S.; Diao, F.; Song, W.; Zhang, Y.; Zhuang, P.; Zhang, Y. Network pharmacology integrated with experimental verification reveals the antipyretic characteristics and mechanism of Zi Xue powder. Pharm. Biol. 2023, 61, 1512–1524. [Google Scholar] [CrossRef]
- Konsue, A.; Sattayasai, J.; Puapairoj, P.; Picheansoonthon, C. Antipyretic effects of Bencha-Loga-Wichien herbal drug in rats. Thai J. Pharmacol. 2008, 29, 79–82. [Google Scholar]
- Jongchanapong, A.; Singharachai, C.; Palanuvej, C.; Ruangrungsi, N.; Towiwat, P. Antipyretic and Antinociceptive effects of Ben-Cha-Lo-Ka-Wi-Chian Remedy. J. Health Res. 2010, 24, 15–22. [Google Scholar]
- Juckmeta, T.; Pipatrattanaseree, W.; Jaidee, W.; Dechayont, B.; Chunthorng-Orn, J.; Andersen, R.J.; Itharat, A. Cytotoxicity to five cancer cell lines of the respiratory tract system and anti-inflammatory activity of Thai traditional remedy. Nat. Prod. Commun. 2019, 14, 1934578X19845815. [Google Scholar] [CrossRef]
- Singh, A.; Singh, M.P.; Alam, G.; Patel, R.; Datt, N. Antipyretic activity of Cassia fistula Linn. pods. J. Pharm. Res. 2012, 5, 2593–2594. [Google Scholar]
- Zubairi, M.N.; Afzal, M.K.; Akhtar, S.; Noman, A.M.; Musarrat, A.; Fayyaz, H.M.; Sultan, M.T.; Rao, N.; Khalid, M.U.; Saeed, W.; et al. Phytochemicals analysis via GC-MS, assessment of anti-inflammatory, anti-bacterial, and anti-fungal properties of Cassia fistula pod-based functional tea. J. Popul. Ther. Clin. Pharmacol. 2024, 31, 1021–1031. [Google Scholar]
- Sushma, M.; Lahari, S.; Mounika, A.; Sailaja, K.E. Phytochemical screening & in vitro evaluation of anti-inflammatory activity of Cerodendrum indicum roots. World J. Curr. Med. Pharm. Res. 2021, 3, 140–143. [Google Scholar]
- Reanmongkol, W.; Subhadhirasakul, S.; Bouking, P. Antinociceptive and antipyretic activities of extracts and fractions from Dracaena loureiri in experimental animals. Songklanakarin J. Sci. Technol. 2003, 25, 467–476. [Google Scholar]
- Likhitwitayawuid, K.; Sawasdee, K.; Kirtikara, K. Flavonoids and stilbenoids with COX-1 and COX-2 inhibitory activity from Dracaena loureiri. Planta Med. 2002, 68, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-Y.; Yi, T.; Sze-To, C.-M.; Zhu, L.; Peng, W.-L.; Zhang, Y.-Z.; Zhao, Z.-Z.; Chen, H.-B. A Systematic Review of the Botanical, Phytochemical and Pharmacological Profile of Dracaena cochinchinensis, a Plant Source of the Ethnomedicine “Dragon’s Blood”. Molecules 2014, 19, 10650–10669. [Google Scholar] [CrossRef]
- Li, R.W.; Myers, S.P.; Leach, D.N.; Lin, G.D.; Leach, G. A cross-cultural study: Anti-inflammatory activity of Australian and Chinese plants. J. Ethnopharmacol. 2003, 85, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Li, R.W.; Leach, D.N.; Myers, S.P.; Lin, G.D.; Leach, G.J.; Waterman, P.G. A new anti-inflammatory glucoside from Ficus racemosa L. Planta Med. 2004, 70, 421–426. [Google Scholar] [PubMed]
- Triet, N.T. Phytochemical and Biological Investigations of Vietnamese Medicinal Plants with a Special Focus on Anti-Cancer and Anti-Inflammatory Activities. Ph.D. Thesis, Karl-Franzens University of Graz, Styria, Austria, December 2018. [Google Scholar]
- Somsil, P.; Ruangrungsi, N.; Limpanasitikula, W.; Itthipanichpong, C. In vivo and in vitro anti-inflammatory activity of Harrisonia perforata root extract. Phcog. J. 2012, 4, 38–44. [Google Scholar] [CrossRef]
- Juckmeta, T.; Itharat, A. Anti-inflammatory and antioxidant activities of Thai traditional remedy called “Ya-ha-rak”. J. Health Res. 2012, 26, 205–210. [Google Scholar]
- Choodej, S.; Sommit, D.; Pudhom, K. Rearranged limonoids and chromones from Harrisonia perforata and their anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2013, 23, 3896–3900. [Google Scholar] [CrossRef] [PubMed]
- Itharat, A.; Makchuchit, S.; Tewtrakul, S. Anti-inflammatory activity of Thai traditional medicine preparation called Prasaprohyai. Planta Med. 2009, 75, PJ55. [Google Scholar] [CrossRef]
- Mokmued, K.; Ruangnoo, S.; Itharat, A. Anti-inflammatory of the ethanolic extract of Thai traditional post-partum remedy (Sa-Tri-Lhang-Klod) and plant ingredients. Thammasat Med. J. 2017, 17, 557–564. [Google Scholar]
- Chen, R.; Li, Y.; Zhang, N.; Zhang, L.; Zhang, P.; Wu, T. Anti-inflammatory oleanane triterpenoids with mono-, di-, and tri-palmitoyl chains from the stamen of Nelumbo nucifera Gaertn. Ind. Crops Prod. 2024, 216, 118698. [Google Scholar] [CrossRef]
- Li, P.-H.; Wang, C.-W.; Lu, W.-C.; Song, T.-Y.; Wang, C.-C.R. Antioxidant, anti-inflammatory activities, and neuroprotective behaviors of Phyllanthus emblica L. fruit extracts. Agriculture 2022, 12, 588. [Google Scholar] [CrossRef]
- Takuathung, M.N.; Jaijoy, K.; Soonthornchareonnon, N.; Sireeratawong, S. Anti-inflammatory, antinociceptive, and antitumorigenesis activities of Terminalia bellerica (Gaertn.) Roxb. in animal models. Nat. Prod. Commun. 2022, 17, 1934578X221089996. [Google Scholar] [CrossRef]
- Bharathi, R.P.; Manohar, V.R.; Rai, M.; Athiyamaan, M.S. Evaluation of the antipyretic and anti-inflammatory potential of aqueous fruit pulp extract of Terminalia bellirica. Biomed. Pharmacol. J. 2023, 16, 295–304. [Google Scholar] [CrossRef]
- Winidmanokul, P.; Panwong, S.; Panya, A. Terminalia bellirica extract suppresses SARS-Cov-2 nucleocapsid-induced inflammation in A549 cells. Nat. Life Sci. Commun. 2024, 23, e2024026. [Google Scholar] [CrossRef]
- Afsar, S.K.; Reddy, V.P.; Priyanka, B.; Srilakshmi, A.; SaiSaran, S.; Rajaram, C. Pharmacological evaluation of anti-pyretic activity of ethanolic fruit extract of Terminalia chebula in Wistar rats. IJAPR Int. J. Adv. Pharm. Res. 2012, 3, 692–695. [Google Scholar]
- Wang, C.; Zhang, H.; Wang, X.; Wang, X.; Li, X.; Li, C.; Wang, Y.; Zhang, M. Comprehensive review on fruit of Terminalia chebula: Traditional uses, phytochemistry, pharmacology, toxicity, and pharmacokinetics. Molecules 2024, 29, 5547. [Google Scholar] [CrossRef]
- Pradubyat, N.; Wunnakup, T.; Praparatana, R.; Wongwiwatthananukit, S.; Jongrungruangchok, S.; Songsak, T.; Madaka, F.; Sudsai, T. Evaluation of antioxidant and anti-inflammatory properties, bioactive compound profiling, and molecular mechanisms of a multicomponent Thai herbal formulation. Phytomed. Plus 2024, 4, 100662. [Google Scholar] [CrossRef]
- Pradubyat, N.; Madaka, F.; Songsak, T.; Jongrungruangchok, S. In vitro biological activity of Tiliacora triandra (Colebr.) Diels root extract. J. Curr. Sci. Technol. 2025, 15, 77. [Google Scholar] [CrossRef]
- Supakitjaroenkul, P.; Yama, S.; Photichai, K.; Arunorat, J.; Yeewa, R.; Sirilun, S.; Yossawimolwat, S.; Sirisa-ard, P.; Charumanee, S. Effect of Tinospora crispa (L.) extracts on anti-pyretic activity, platelet amount and blood biochemistry in Wistar rats. Health Sci. Tech. Rev. 2023, 16, 15–29. [Google Scholar]
- Llamasares-Castillo, A.; Uclusin-Bolibol, R.; Rojsitthisak, P.; Alcantara, K.P. In vitro and in vivo studies of the therapeutic potential of Tinospora crispa extracts in osteoarthritis: Targeting oxidation, inflammation, and chondroprotection. J. Ethnopharmacol. 2024, 333, 118446. [Google Scholar] [CrossRef]
- Arshad, L.; Haque, M.A.; Harikrishnan, H.; Ibrahim, S.; Jantan, I. Syringin from Tinospora crispa downregulates pro-inflammatory mediator production through MyD88-dependent pathways in lipopolysaccharide (LPS)-induced U937 macrophages. Mol. Biol. Rep. 2024, 51, 789. [Google Scholar] [CrossRef]
- Narkhede, M.B.; Ajmire, P.V.; Wagh, A.E.; Bhise, M.R.; Mehetre, G.D.; Patil, H.J. An evaluation of anti-pyretic potential of Vetiveria zizanioides (Linn.) root. Res. J. Pharmacog. Phytochem. 2012, 4, 11–13. [Google Scholar]
- Keerthana, B.; Karthikeyan, M. Anti-inflammatory activity of Vetiveria zizanioides mediated silver nanoparticles. Cuest. Fisioter. 2025, 54, 90–102. [Google Scholar] [CrossRef]
- Lai, J.; Wu, H.; Qin, A. Cytokines in Febrile Diseases. J. Interferon Cytokine Res. 2021, 41, 1–11. [Google Scholar] [CrossRef]
- Ma, L.L.; Liu, H.M.; Luo, C.H.; He, Y.N.; Wang, F.; Huang, H.Z.; Han, L.; Yang, M.; Xu, R.C.; Zhang, D.K. Fever and antipyretic supported by Traditional Chinese medicine: A multi-pathway regulation. Front. Pharmacol. 2021, 12, 583279. [Google Scholar] [CrossRef]
- Sundgren-Andersson, A.K.; Östlund, P.; Bartfai, T. IL-6 is essential in TNF-α-induced fever. Am. J. Physiol. 1998, 275, R2028–R2034. [Google Scholar] [CrossRef]
- Stefferl, A.; Hopkins, S.J.; Rothwell, N.J.; Luheshi, G.N. The role of TNF-α in fever: Opposing actions of human and murine TNF-α and interactions with IL-β in the rat. Br. J. Pharmacol. 1996, 118, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Nadjar, A.; Bluthé, R.M.; May, M.; Dantzer, R.; Parnet, P. Inactivation of the cerebral NFκB pathway inhibits interleukin-1β-induced sickness behavior and c-fos expression in various brain nuclei. Neuropsychopharmacology 2005, 30, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Yang, X.; Zhang, W.X.; Zhou, Y.; Wei, T.T.; Cui, N.; Du, J.; Liu, W.; Lu, Q.B. Metabolic alterations in urine among the patients with severe fever with thrombocytopenia syndrome. Virol. J. 2024, 21, 11. [Google Scholar] [CrossRef]
- Luporini, R.L.; Pott-Junior, H.; Di Medeiros Leal, M.C.B.; Castro, A.; Ferreira, A.G.; Cominetti, M.R.; de Freitas Anibal, F. Phenylalanine and COVID-19: Tracking disease severity markers. Int. Immunopharmacol. 2021, 101, 108313. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Tredicine, M.; Mucci, M.; Recchiuti, A.; Mattoscio, D. Immunoregulatory mechanisms of the arachidonic acid pathway in cancer. FEBS Lett. 2025, 599, 927–951. [Google Scholar] [CrossRef] [PubMed]
- Omeje, E.; Nworu, C.; Osadebe, P.; Onugwu, L.; Maurya, R.; Chattopadhyay, N. 3-methoxy quercetin isolated from mistletoe parasitic on Garcinia kola exhibits potent anti-inflammatory activities in vitro. Planta Med. 2014, 80, LP29. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.T.; Xiao, L.; Zhu, L.; Wang, Q.; Yan, T. Anti-inflammatory effects of apigenin in lipopolysaccharide-induced inflammatory in acute lung injury by suppressing COX-2 and NF-kB pathway. Inflammation 2014, 37, 2085–2090. [Google Scholar] [CrossRef]
- Funakoshi-Tago, M.; Nakamura, K.; Tago, K.; Mashino, T.; Kasahara, T. Anti-inflammatory activity of structurally related flavonoids, Apigenin, Luteolin and Fisetin. Int. Immunopharmacol. 2011, 11, 1150–1159. [Google Scholar] [CrossRef]
- Bao, Y.; Sun, Y.-W.; Gan, L.; Zhang, C.-F.; Wang, C.-Z.; Yuan, C.-S. Genkwanin ameliorates adjuvant-induced arthritis in rats through inhibiting JAK/STAT and NF-κB signaling pathways. Phytomedicine 2019, 63, 153036. [Google Scholar] [CrossRef]
- Yu, C.I.; Cheng, C.I.; Kang, Y.F.; Chang, P.C.; Lin, I.P.; Kuo, Y.H.; Jhou, A.J.; Lin, M.Y.; Chen, C.Y.; Lee, C.H. Hispidulin inhibits neuroinflammation in lipopolysaccharide-activated BV2 microglia and attenuates the activation of Akt, NF-κB, and STAT3 pathway. Neurotox. Res. 2020, 38, 163–174. [Google Scholar] [CrossRef]
- Seo, K.; Yang, J.H.; Kim, S.C.; Ku, S.K.; Ki, S.H.; Shin, S.M. The antioxidant effects of isorhamnetin contribute to inhibit COX-2 expression in response to inflammation: A potential role of HO-1. Inflammation 2014, 37, 712–722. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 45673. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wen, K.; Xu, Z.; He, Z.; Wu, B.; Yang, X.; Wang, X. Effect of Loureirin B on Crohn’s disease rat model induced by TNBS via IL-6/STAT3/NF-κB signaling pathway. Chin. Med. 2020, 15, 2. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, L.; Wang, M.-H. N-trans-feruloyltyramine inhibits LPS-induced NO and PGE2 production in RAW 264.7 macrophages: Involvement of AP-1 and MAP kinase signalling pathways. Chem. Biol. Interact. 2015, 235, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Juckmeta, T. Biological Activities of the Ethanolic Extracts from Pikut Benjalokawichian (ha-rak) and Its Isolate Compounds. Master’s Thesis, Thammasat University, Bangkok, Thailand, 22 June 2011. [Google Scholar]
- Feng, Y.; Bhandari, R.; Li, C.; Shu, P.; Shaikh, I.I. Pectolinarigenin suppresses LPS-induced inflammatory response in macrophages and attenuates dss-induced colitis by modulating the NF-κB/Nrf2 signaling pathway. Inflammation 2022, 45, 2529–2543. [Google Scholar] [CrossRef]
- Kleemann, R.; Verschuren, L.; Morrison, M.; Zadelaar, S.; van Erk, M.J.; Wielinga, P.Y.; Kooistra, T. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis 2011, 218, 44–52. [Google Scholar] [CrossRef]
- Seguin, P.; Roquilly, A.; Mimoz, O.; Maguet, P.L.; Asehnoune, K.; Biederman, S.; Carise, E.; Malledant, Y.; The AtlanRea Group. Risk factors and outcomes for prolonged versus brief fever: A prospective cohort study. Crit. Care 2012, 16, R150. [Google Scholar] [CrossRef]
- Banno, N.; Akihisa, T.; Tokuda, H.; Yasukawa, K.; Higashihara, H.; Ukiya, M.; Watanabe, K.; Kimura, Y.; Hasegawa, J.; Nishino, H. Triterpene acids from the leaves of Perilla frutescens and their anti-inflammatory and antitumor-promoting effects. Biosci. Biotechnol. Biochem. 2004, 68, 85–90. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, K.; Shi, C.; Gao, Y.; Kong, D.Q. Obacunone acts as a histone deacetylase 1 inhibitor to limit p38MAPK signaling and alleviate osteoarthritis progression. J. Orthop. Surg. Res. 2025, 20, 441. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, R.; Liu, Y.; Cong, Y.; Zhang, D.; Zhang, Y.; Yang, X.; Lu, C.; Shen, Y. Anti-virulence activities of biflavonoids from Mesua ferrea L. flower. Drug Discov. Ther. 2019, 13, 222–227. [Google Scholar] [CrossRef]
- Jain, R.; Rawat, S.; Jain, S.C. Phytochemicals and antioxidant evaluation of Ficus racemosa root bark. Pharm. Res. 2013, 6, 615–619. [Google Scholar] [CrossRef]
- Boonsong, N.; Preeprame, S.; Putalun, W. Quantitative determination of bergenin in callus, twig and root of Ficus racemosa L. Extract by High Performance Liquid Chromatography. KKU Res. J. (Grad. Stud.) 2022, 22, 87–98. [Google Scholar]
- Zheng, Q.A.; Li, H.Z.; Zhang, Y.J.; Yang, C.R. Dracaenogenins A and B, new spirostanols from the red resin of Dracaena cochinchinensis. Steroids 2006, 71, 160–164. [Google Scholar] [CrossRef]
- Lee, C.-K.; Lee, P.-H.; Kuo, Y.-H. The chemical constituents from the aril of Cassia fistula L. J. Chin. Chem. Soc. 2001, 48, 1053–1058. [Google Scholar] [CrossRef]
- Stitmannaitham, M. Isolation and Structural Determination of Compounds from Roots of Harrisonia perforata Merr. Master’s Thesis, Chulalongkorn University, Bangkok, Thailand, 1992. [Google Scholar]
- Thadaniti, S.; Archakunakorn, W.; Tuntiwachwuttikul, P.; Bremner, J.B. Chromones from Harrisonia perforata (Blanco.) Merr. J. Sci. Soc. Thailand 1994, 20, 183–187. [Google Scholar] [CrossRef]
- Chunthorng-Orn, J.; Pipatrattanaseree, W.; Juckmeta, T.; Dechayont, B.; Phuaklee, P.; Itharat, A. Quality evaluation and pectolinarigenin contents analysis of Harak remedy in Thailand. J. Health Sci. Altern. Med. 2019, 1, 25–33. [Google Scholar]
- Sakpakdeejaroen, I.; Juckmeta, T.; Itharat, A. Development and validation of RP-HPLC method to determine anti-allergic compound in Thai traditional remedy called Benjalokawichien. J. Med. Assoc. Thail. 2014, 97, S76–S80. [Google Scholar]
- Ryszkiewicz, P.; Schlicker, E.; Malinowska, B. Is inducible nitric oxide synthase (iNOS) promising as a new target against pulmonary hypertension? Antioxidants 2025, 14, 377. [Google Scholar] [CrossRef]
- Soufli, I.; Toumi, R.; Rafa, H.; Touil-Boukoffa, C. Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 353–360. [Google Scholar] [CrossRef]
- Pal, P.P.; Begum, A.S.; Basha, S.A.; Araya, H.; Fujimoto, Y. New natural pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β) and iNOS inhibitors identified from Penicillium polonicum through in vitro and in vivo studies. Int. Immunopharmacol. 2023, 117, 109940. [Google Scholar]
- Pratama, R.R.; Sari, R.A.; Sholikhah, I.; Mansor, H.; Chang, H.-I.; Sukardiman; Widyowati, R. Inhibition of nitric oxide production in RAW 264.7 cells and cytokines IL-1β in osteoarthritis rat models of 70% ethanol extract of Arcangelisia flava (L.) merr stems. Heliyon 2024, 10, e35730. [Google Scholar] [CrossRef]
- Huang, L.; Hou, L.; Xue, H.; Wang, C. Gallic acid inhibits inflammatory response of RAW264.7 macrophages by blocking the activation of TLR4/NF-κB induced by LPS. Chin. J. Microbiol. Immunol. 2016, 32, 1610–1614. [Google Scholar]
- Wang, H.Y.; Wang, H.; Wang, J.H.; Wang, Q.; Ma, Q.F.; Chen, Y.Y. Protocatechuic acid inhibits inflammatory responses in LPS-stimulated BV2 microglia via NF-κB and MAPKs signaling pathways. Neurochem. Res. 2015, 40, 1655–1660. [Google Scholar] [CrossRef]
- Gao, X.J.; Guo, M.Y.; Zhang, Z.C.; Wang, T.C.; Cao, Y.G.; Zhang, N.S. Bergenin plays an anti-inflammatory role via the modulation of MAPK and NF-κB signaling pathways in a mouse model of LPS-induced mastitis. Inflammation 2015, 38, 1142–1150. [Google Scholar] [CrossRef]
- Liu, Y.; Bao, L.; Xuan, L.; Song, B.; Lin, L.; Han, H. Chebulagic acid inhibits the LPS-induced expression of TNF-α and IL-1β in endothelial cells by suppressing MAPK activation. Exp. Ther. Med. 2015, 10, 263–268. [Google Scholar] [CrossRef]
- Reddy, D.B.; Reddanna, P. Chebulagic acid (CA) attenuates LPS-induced inflammation by suppressing NF-kappaB and MAPK activation in RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 2009, 381, 112–117. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem substance and compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef] [PubMed]
- Gfeller, D.; Grosdidier, A.; Wirth, W.; Daina, A.; Michielin, O.; Zoete, V. Swisstarget prediction: A web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014, 42, W32–W38. [Google Scholar] [CrossRef]
- Safran, M.; Dalah, I.; Alexander, J.; Rosen, N.; Stein, T.I.; Shmoish, M.; Nativ, N.; Bahir, I.; Doniger, T.; Krug, H.; et al. GeneCards version 3: The human gene integrator. Database 2010, 2010, baq020. [Google Scholar] [CrossRef] [PubMed]
- Amberger, J.S.; Bocchini, C.A.; Schiettecatte, F.; Scott, A.F.; Hamosh, A. OMIM.org: Online mendelian inheritance in man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015, 43, D789–D798. [Google Scholar] [CrossRef] [PubMed]
- Whirl-Carrillo, M.; Huddart, R.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Whaley, R.; Klein, T.E. An evidence-based framework for evaluating pharmacogenomics knowledge for personalized medicine. Clin. Pharm. Therap. 2021, 110, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Salman, H.A.; Yaakop, A.S.; Aladaileh, S.; Mustafa, M.; Gharaibeh, M.; Kahar, U.M. Inhibitory effects of Ephedra alte on IL-6, hybrid TLR4, TNF-α, IL-1β, and extracted TLR4 receptors: In silico molecular docking. Heliyon 2023, 9, e12730. [Google Scholar] [CrossRef]
- Shrivastava, N.; Joshi, J.; Sehgal, N.; Kumar, I.P. Cyclooxygenase-2 identified as a potential target for novel radiomodulator scopolamine methyl bromide: An in silico study. Inform. Med. Unlocked 2017, 9, 18–25. [Google Scholar] [CrossRef]
- Wang, S.-Q.; Shi, M.; Fang, L.; Xu, S.-M.; Wang, C.; Yu, Z.-X. Design of dual inhibitors of human TNF-α and IL-6 with potentials for the treatment of rheumatoid arthritis. Trop. J. Pharm. Res. 2019, 18, 2305–2312. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Shivaleela, B.; Srushti, S.C.; Shreedevi, S.J.; Babu, R.L. Thalidomide-based inhibitor for TNF-α: Designing and in silico evaluation. Future J. Pharm. Sci. 2022, 8, 5. [Google Scholar] [CrossRef]
- Chiu, Y.-S.; Bamodu, O.A.; Fong, I.H.; Lee, W.-H.; Lin, C.-C.; Lu, C.-H.; Yeh, C.-T. The JAK inhibitor Tofacitinib inhibits structural damage in osteoarthritis by modulating JAK1/TNF-alpha/IL-6 signaling through Mir-149-5p. Bone 2021, 151, 116024. [Google Scholar] [CrossRef]
- Kary, S.; Burmester, G.R. Anakinra: The first interleukin-1 inhibitor in the treatment of rheumatoid arthritis. Int. J. Clin. Pract. 2023, 57, 231–234. [Google Scholar] [CrossRef]
- Antoniou, K.; Malamas, M.; Drosos, A.A. Clinical pharmacology of celecoxib, a COX-2 selective inhibitor. Expert Opin. Pharmacother. 2007, 8, 1719–1732. [Google Scholar] [CrossRef]
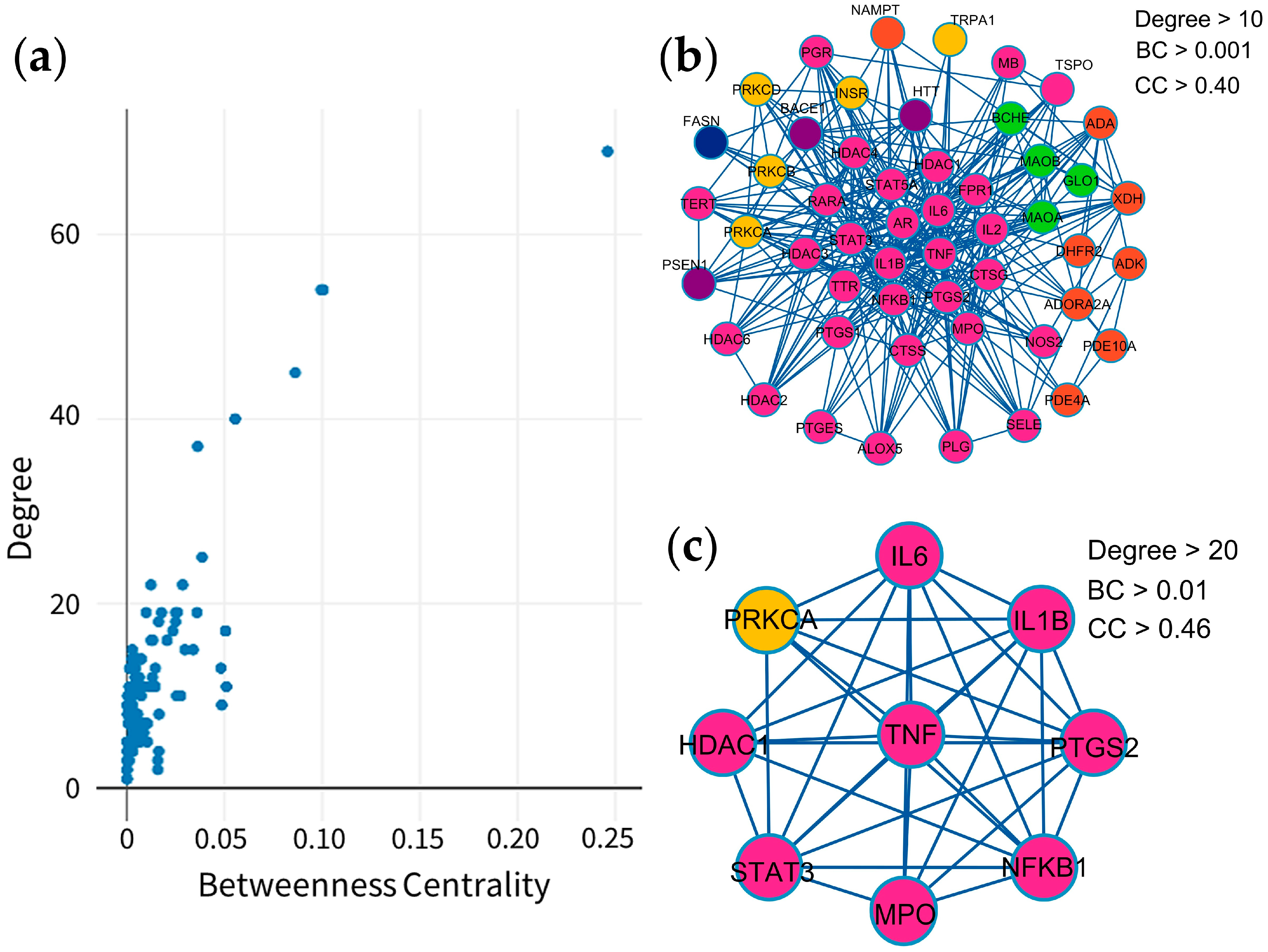

| Target | Degree | Betweenness Centrality | Closeness Centrality | Type |
|---|---|---|---|---|
| TNF | 69 | 0.246113 | 0.658031 | Cytokines |
| IL6 | 54 | 0.099402 | 0.604762 | Cytokines |
| IL1B | 54 | 0.100554 | 0.604762 | Cytokines |
| PTGS2 | 45 | 0.086126 | 0.561947 | Oxidoreductase |
| STAT3 | 40 | 0.055452 | 0.531381 | Transcription |
| NFKB1 | 37 | 0.036233 | 0.516260 | Transcription |
| HDAC1 | 25 | 0.038500 | 0.484733 | Cell cycle |
| PRKCA | 22 | 0.028542 | 0.463504 | Transferase |
| MPO | 22 | 0.012275 | 0.482890 | Oxidoreductase |
| MCODE | Pathway | Description | Fold Enrichment |
|---|---|---|---|
| MCODE1 | hsa00590 | Arachidonic acid metabolism | 21.3 |
| MCODE1 | hsa01523 | Antifolate resistance | 19.2 |
| MCODE1 | hsa05143 | African trypanosomiasis | 15.6 |
| MCODE2 | hsa00230 | Purine metabolism | 36.3 |
| MCODE2 | hsa05032 | Morphine addiction | 34.0 |
| MCODE2 | hsa01232 | Nucleotide metabolism | 18.2 |
| MCODE3 | hsa04960 | Aldosterone-regulated sodium reabsorption | 85.1 |
| MCODE3 | hsa04750 | Inflammatory mediator regulation of TRP channels | 64.3 |
| MCODE3 | hsa05143 | African trypanosomiasis | 63.8 |
| MCODE4 | hsa00360 | Phenylalanine metabolism | 270.7 |
| MCODE4 | hsa00340 | Histidine metabolism | 196.9 |
| MCODE4 | hsa00350 | Tyrosine metabolism | 180.5 |
| MCODE5 | hsa04020 | Calcium signaling pathway | 27.4 |
| MCODE5 | hsa04080 | Neuroactive ligand-receptor interaction | 23.6 |
| MCODE6 | hsa01212 | Fatty acid metabolism | 114.0 |
| MCODE7 | hsa03410 | Base excision repair | 131.2 |
| MCODE8 | hsa05010 | Alzheimer disease | 15.0 |
| Compounds | Binding Energy (ΔGbind, kcal/mol) | |||
|---|---|---|---|---|
| TNF (PDB: 2AZ5) | IL6 (PDB: 1ALU) | IL1B (PDB: 5I1B) | PTGS2 (PDB: 3LN1) | |
| Phytochemicals | ||||
| (+)-Vouacapenic acid | −8.9 | −7.3 | −6.4 | −7.8 |
| Apigenin | −7.5 | −6.8 | −7.1 | −9.8 |
| Augustic acid | −9.1 | −7.4 | −7.4 | −8.5 |
| Berberine | −8.7 | −7.4 | −6.9 | −7.9 |
| Chrysophanol | −8.0 | −6.4 | −7.4 | −9.4 |
| Columbin | −8.7 | −7.1 | −7.1 | −7.4 |
| Diosmetin | −7.6 | −6.9 | −7.2 | −8.9 |
| Emodin | −7.6 | −6.7 | −7.2 | −9.1 |
| Genkwanin | −7.6 | −6.5 | −7.1 | −9.1 |
| Hispidulin | −7.4 | −6.9 | −7.0 | −9.4 |
| Isorhamnetin | −7.6 | −6.7 | −7.4 | −9.7 |
| Jatrorrhizine | −7.7 | −6.9 | −6.8 | −7.0 |
| Kaempferol | −7.3 | −6.5 | −7.2 | −9.5 |
| Luteolin | −7.7 | −7.2 | −7.5 | −9.7 |
| Nimbolide | −9.1 | −7.3 | −7.0 | −8.2 |
| Obacunone | −9.1 | −7.7 | −8.2 | −8.8 |
| Palmatine | −8.0 | −6.6 | −6.5 | −6.7 |
| Perforatic acid methyl ester | −7.4 | −6.4 | −6.5 | −9.3 |
| Quercetin | −7.2 | −7.1 | −7.5 | −9.6 |
| Rhein | −8.3 | −6.6 | −7.3 | −9.2 |
| Stigmasterol glucoside | −8.9 | −6.9 | −7.4 | −8.5 |
| Standard Drug | ||||
| Thalidomide | −7.4 | - | - | - |
| Tofacitinib | - | −6.4 | - | - |
| Anakinra | - | - | −6.6 | - |
| Celecoxib | - | - | - | −12.1 |
| Marker Compounds | Content of Biomarkers in MHR Extract (mg/g Extract) |
|---|---|
| Chebulic acid (1) | 9.07 ± 0.16 a |
| Gallic acid (2) | 19.79 ± 0.00 b |
| Protocatechuic acid (3) | 2.78 ± 0.02 c |
| Bergenin (4) | 0.03 ± 0.00 d |
| Chebulanin (5) | 15.94 ± 0.34 be |
| Corilagin (6) | 11.27 ± 0.23 f |
| Chebulagic acid (7) | 36.85 ± 0.02 g |
| Ellagic acid (8) | 10.60 ± 0.01 aef |
| Resveratrol (9) | 0.62 ± 0.00 h |
| Perforatic acid (10) | 8.86 ± 0.02 af |
| O-Methyllaloptaeroxyrin (11) | 1.47 ± 0.05 i |
| Rhein (12) | 0.31 ± 0.00 j |
| Loureirin A (13) | 3.20 ± 0.01 k |
| Pectolinarigenin (14) | 0.11 ± 0.00 l |
| Peucenin-7-methyl ether (15) | 1.26 ± 0.01 i |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaloemram, C.; Rattarom, R.; Kijjoa, A.; Nualkaew, S. Integration of Network Pharmacology, Molecular Docking, and In Vitro Nitric Oxide Inhibition Assay to Explore the Mechanism of Action of Thai Traditional Polyherbal Remedy, Mo-Ha-Rak, in the Treatment of Prolonged Fever. Pharmaceuticals 2025, 18, 1541. https://doi.org/10.3390/ph18101541
Chaloemram C, Rattarom R, Kijjoa A, Nualkaew S. Integration of Network Pharmacology, Molecular Docking, and In Vitro Nitric Oxide Inhibition Assay to Explore the Mechanism of Action of Thai Traditional Polyherbal Remedy, Mo-Ha-Rak, in the Treatment of Prolonged Fever. Pharmaceuticals. 2025; 18(10):1541. https://doi.org/10.3390/ph18101541
Chicago/Turabian StyleChaloemram, Chinnaphat, Ruchilak Rattarom, Anake Kijjoa, and Somsak Nualkaew. 2025. "Integration of Network Pharmacology, Molecular Docking, and In Vitro Nitric Oxide Inhibition Assay to Explore the Mechanism of Action of Thai Traditional Polyherbal Remedy, Mo-Ha-Rak, in the Treatment of Prolonged Fever" Pharmaceuticals 18, no. 10: 1541. https://doi.org/10.3390/ph18101541
APA StyleChaloemram, C., Rattarom, R., Kijjoa, A., & Nualkaew, S. (2025). Integration of Network Pharmacology, Molecular Docking, and In Vitro Nitric Oxide Inhibition Assay to Explore the Mechanism of Action of Thai Traditional Polyherbal Remedy, Mo-Ha-Rak, in the Treatment of Prolonged Fever. Pharmaceuticals, 18(10), 1541. https://doi.org/10.3390/ph18101541










