IL-1β and HMGB1 in Epileptogenesis: Recent Advances and Clinical Translation
Abstract
1. Introduction
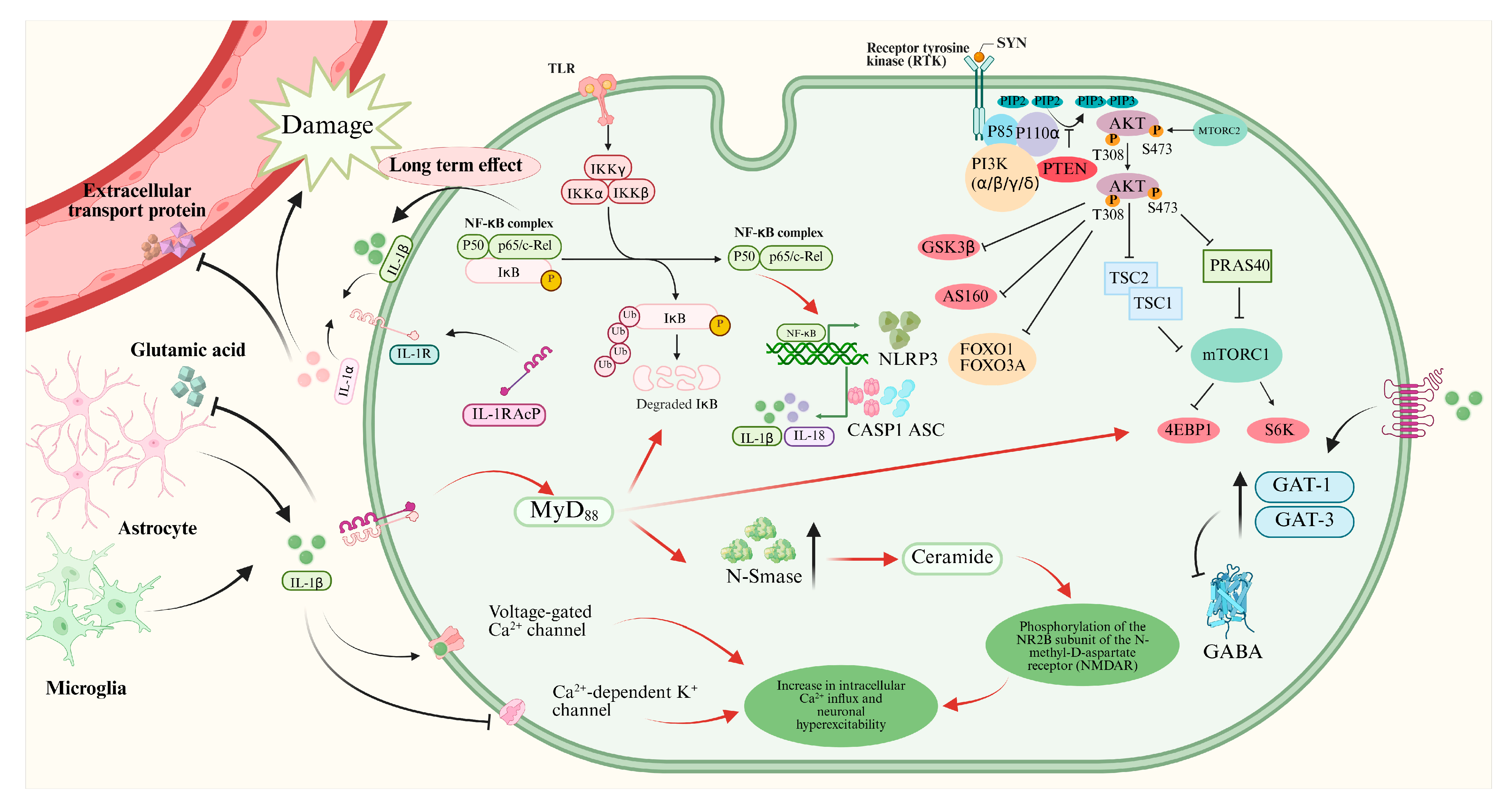
2. IL-1β/IL-1R1/IL-1Ra and Epilepsy
2.1. IL-1β/IL-1R1/IL-1Ra Pathway
2.2. IL-1β/IL-1R1/IL-1Ra in Patients with Epilepsy
2.3. Mechanisms of IL-1β/IL-1R1/IL-1Ra Pathway in Epilepsy
2.4. Substances Targeting IL-1β/IL-1R1/IL-1Ra Pathway
3. HMGB1/TLR4 and Epilepsy
3.1. HMGB1/TLR4 Pathway
3.2. HMGB1/TLR4 Pathway in Patients with Epilepsy
3.3. Substances Targeting HMGB1/TLR4 Pathway
4. Discussion
4.1. Targeting the Shared Mechanisms
4.2. Potential of the Nano Drug Delivery System in Treatment
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bazhanova, E.D.; Kozlov, A.A.; Litovchenko, A.V. Mechanisms of Drug Resistance in the Pathogenesis of Epilepsy: Role of Neuroinflammation. A Literature Review. Brain Sci. 2021, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- Ngugi, A.K.; Bottomley, C.; Kleinschmidt, I.; Sander, J.W.; Newton, C.R. Estimation of the burden of active and life-time epilepsy: A meta-analytic approach. Epilepsia 2010, 51, 883–890. [Google Scholar] [CrossRef]
- Beghi, E. Addressing the burden of epilepsy: Many unmet needs. Pharmacol. Res. 2016, 107, 79–84. [Google Scholar] [CrossRef]
- Singh, G.; Sander, J.W. The global burden of epilepsy report: Implications for low- and middle-income countries. Epilepsy Behav. 2020, 105, 106949. [Google Scholar] [CrossRef]
- Pitkänen, A.; Lukasiuk, K.; Dudek, F.E.; Staley, K.J. Epileptogenesis. Cold Spring Harb. Perspect. Med. 2015, 5, a022822. [Google Scholar] [CrossRef]
- Henshall, D.C.; Hamer, H.M.; Pasterkamp, R.J.; Goldstein, D.B.; Kjems, J.; Prehn, J.H.M.; Schorge, S.; Lamottke, K.; Rosenow, F. MicroRNAs in epilepsy: Pathophysiology and clinical utility. Lancet Neurol. 2016, 15, 1368–1376. [Google Scholar] [CrossRef]
- Dingledine, R.; Coulter, D.A.; Fritsch, B.; Gorter, J.A.; Lelutiu, N.; McNamara, J.; Nadler, J.V.; Pitkänen, A.; Rogawski, M.A.; Skene, P.; et al. Transcriptional profile of hippocampal dentate granule cells in four rat epilepsy models. Sci. Data 2017, 4, 170061. [Google Scholar] [CrossRef]
- Moloney, P.B.; Cavalleri, G.L.; Delanty, N. Epilepsy in the mTORopathies: Opportunities for precision medicine. Brain Commun. 2021, 3, fcab222. [Google Scholar] [CrossRef]
- Librizzi, L.; Noè, F.; Vezzani, A.; de Curtis, M.; Ravizza, T. Seizure-induced brain-borne inflammation sustains seizure recurrence and blood–brain barrier damage. Ann. Neurol. 2012, 72, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Vezzani, A.; O’Brien, T.J.; Jette, N.; Scheffer, I.E.; de Curtis, M.; Perucca, P. Epilepsy. Nat. Rev. Dis. Primers 2018, 4, 18024. [Google Scholar] [CrossRef] [PubMed]
- Lerche, H. Drug-resistant epilepsy—Time to target mechanisms. Nat. Rev. Neurol. 2020, 16, 595–596. [Google Scholar] [CrossRef]
- Vezzani, A.; Balosso, S.; Ravizza, T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat. Rev. Neurol. 2019, 15, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Leung, W.L.; Zamani, A.; O’brien, T.J.; Espinosa, P.M.C.; Semple, B.D. Neuroinflammation in Post-Traumatic Epilepsy: Pathophysiology and Tractable Therapeutic Targets. Brain Sci. 2019, 9, 318. [Google Scholar] [CrossRef]
- Khaboushan, A.S.; Yazdanpanah, N.; Rezaei, N. Neuroinflammation and Proinflammatory Cytokines in Epileptogenesis. Mol. Neurobiol. 2022, 59, 1724–1743. [Google Scholar] [CrossRef]
- Campos-Bedolla, P.; Feria-Romero, I.; Orozco-Suárez, S. Factors not considered in the study of drug-resistant epilepsy: Drug-resistant epilepsy: Assessment of neuroinflammation. Epilepsia Open 2022, 7, S68–S80. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Bandopadhyay, R.; Singh, P.K.; Mishra, P.S.; Sharma, N.; Khurana, N. Neuroinflammation in neurological disorders: Pharmacotherapeutic targets from bench to bedside. Metab. Brain Dis. 2021, 36, 1591–1626. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Musto, A.E. The role of inflammation in the development of epilepsy. J. Neuroinflamm. 2018, 15, 144. [Google Scholar] [CrossRef]
- Vezzani, A.; Lang, B.; Aronica, E. Immunity and Inflammation in Epilepsy. Cold Spring Harb. Perspect. Med. 2015, 6, a022699. [Google Scholar] [CrossRef]
- Steriade, C.; French, J.; Devinsky, O. Epilepsy: Key experimental therapeutics in early clinical development. Expert Opin. Investig. Drugs 2020, 29, 373–383. [Google Scholar] [CrossRef]
- Wang, S.; Guan, Y.; Li, T. The Potential Therapeutic Role of the HMGB1-TLR Pathway in Epilepsy. Curr. Drug Targets 2021, 22, 171–182. [Google Scholar] [CrossRef]
- Yamanaka, G.; Ishida, Y.; Kanou, K.; Suzuki, S.; Watanabe, Y.; Takamatsu, T.; Morichi, S.; Go, S.; Oana, S.; Yamazaki, T.; et al. Towards a Treatment for Neuroinflammation in Epilepsy: Interleukin-1 Receptor Antagonist, Anakinra, as a Potential Treatment in Intractable Epilepsy. Int. J. Mol. Sci. 2021, 22, 6282. [Google Scholar] [CrossRef]
- Fok, E.T.; Moorlag, S.J.C.F.M.; Negishi, Y.; Groh, L.A.; dos Santos, J.C.; Gräwe, C.; Monge, V.V.; Craenmehr, D.D.D.; van Roosmalen, M.; Jolvino, D.P.d.C.; et al. A chromatin-regulated biphasic circuit coordinates IL-1β-mediated inflammation. Nat. Genet. 2024, 56, 85–99. [Google Scholar] [CrossRef]
- Ford, S.G.; Caswell, P.; Brough, D.; Seoane, P.I. The secretion of interleukin-1β. Cytokine Growth Factor Rev. 2025, 84, 101–113. [Google Scholar] [CrossRef]
- Mendiola, A.S.; Cardona, A.E. The IL-1β phenomena in neuroinflammatory diseases. J. Neural Transm. 2018, 125, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Nordli, D.R.; Alden, T.D.; DiPatri, A.; Laux, L.; Kelley, K.; Rosenow, J.; Schuele, S.U.; Rajaram, V.; Koh, S. Cellular injury and neuroinflammation in children with chronic intractable epilepsy. J. Neuroinflamm. 2009, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Lorigados Pedre, L.; Morales Chacón, L.M.; Pavón Fuentes, N.; Robinson Agramonte, M.D.L.A.; Serrano Sánchez, T.; Cruz-Xenes, R.M.; Hung, M.L.D.; Estupiñán Díaz, B.; Báez Martín, M.M.; Orozco-Suárez, S. Follow-Up of Peripheral IL-1β and IL-6 and Relation with Apoptotic Death in Drug-Resistant Temporal Lobe Epilepsy Patients Submitted to Surgery. Behav. Sci. 2018, 8, 21. [Google Scholar] [CrossRef]
- Aulická, S.; Česká, K.; Šána, J.; Siegl, F.; Brichtová, E.; Ošlejšková, H.; Hermanová, M.; Hendrych, M.; Michu, E.P.; Brázdil, M.; et al. Cytokine-chemokine profiles in the hippocampus of patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Epilepsy Res. 2022, 180, 106858. [Google Scholar] [CrossRef]
- Ravizza, T.; Gagliardi, B.; Noé, F.; Boer, K.; Aronica, E.; Vezzani, A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: Evidence from experimental models and human temporal lobe epilepsy. Neurobiol. Dis. 2008, 29, 142–160. [Google Scholar] [CrossRef]
- Clarkson, B.D.S.; LaFrance-Corey, R.G.; Kahoud, R.J.; Farias-Moeller, R.; Payne, E.T.; Howe, C.L. Functional deficiency in endogenous interleukin-1 receptor antagonist in patients with febrile infection-related epilepsy syndrome. Ann. Neurol. 2019, 85, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Min, H.J.; Shin, J.-S. Increased levels of HMGB1 and pro-inflammatory cytokines in children with febrile seizures. J. Neuroinflamm. 2011, 8, 135. [Google Scholar] [CrossRef]
- Mao, L.; Ding, J.; Peng, W.; Ma, Y.; Zhang, Y.; Fan, W.; Wang, X. Interictal interleukin-17A levels are elevated and correlate with seizure severity of epilepsy patients. Epilepsia 2011, 54, e142–e145. [Google Scholar] [CrossRef] [PubMed]
- Uludag, I.F.; Duksal, T.; Tiftikcioglu, B.I.; Zorlu, Y.; Ozkaya, F.; Kirkali, G. IL-1β, IL-6 and IL1Ra levels in temporal lobe epilepsy. Seizure 2015, 26, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Kamaşak, T.; Dilber, B.; Yaman, S.Ö.; Durgut, B.D.; Kurt, T.; Çoban, E.; Arslan, E.A.; Şahin, S.; Karahan, S.C.; Cansu, A. HMGB-1, TLR4, IL-1R1, TNF-α, and IL-1β: Novel epilepsy markers? Epileptic Disord. 2020, 22, 183–193. [Google Scholar] [CrossRef]
- Gallentine, W.B.; Shinnar, S.; Hesdorffer, D.C.; Epstein, L.; Nordli, D.R.; Lewis, D.V.; Frank, L.M.; Seinfeld, S.; Shinnar, R.C.; Cornett, K.; et al. Plasma cytokines associated with febrile status epilepticus in children: A potential biomarker for acute hippocampal injury. Epilepsia 2017, 58, 1102–1111. [Google Scholar] [CrossRef]
- Xiao, Z.; Peng, J.; Wu, L.; Arafat, A.; Yin, F. The effect of IL-1β on synaptophysin expression and electrophysiology of hippocampal neurons through the PI3K/Akt/mTOR signaling pathway in a rat model of mesial temporal lobe epilepsy. Neurol. Res. 2017, 39, 640–648. [Google Scholar] [CrossRef]
- Cai, M.; Lin, W. The Function of NF-Kappa B During Epilepsy, a Potential Therapeutic Target. Front. Neurosci. 2020, 16, 851394. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, H.; Tian, S.; Yi, W.; Zhou, Y.; Yang, H.; Li, X.; Wu, B.; Li, X.; Wu, J.; et al. Critical roles of NLRP3 inflammasome in IL-1β secretion induced by Corynebacterium pseudotuberculosis in vitro. Mol. Immunol. 2019, 116, 11–17. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms Underlying Inflammation in Neurodegeneration. Cell 2019, 140, 918–934. [Google Scholar] [CrossRef]
- Zhand, A.; Sayad, A.; Ghafouri-Fard, S.; Arsang-Jang, S.; Mazdeh, M.; Taheri, M. Expression analysis of GRIN2B, BDNF, and IL-1β genes in the whole blood of epileptic patients. Neurol. Sci. 2018, 39, 1945–1953. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, F.; Zhai, F.; Liang, S. Role of HMGB1/TLR4 and IL-1β/IL-1R1 Signaling Pathways in Epilepsy. Front. Neurol. 2022, 13, 904225. [Google Scholar] [CrossRef]
- Maroso, M.; Balosso, S.; Ravizza, T.; Liu, J.; Bianchi, M.E.; Vezzani, A. Interleukin-1 type 1 receptor/Toll-like receptor signalling in epilepsy: The importance of IL-1beta and high-mobility group box 1. J. Intern. Med. 2011, 270, 319–326. [Google Scholar] [CrossRef]
- Li, G.; Bauer, S.; Nowak, M.; Norwood, B.; Tackenberg, B.; Rosenow, F.; Knake, S.; Oertel, W.H.; Hamer, H.M. Cytokines and epilepsy. Seizure 2010, 20, 249–256. [Google Scholar] [CrossRef]
- Su, J.; Yin, J.; Qin, W.; Sha, S.; Xu, J.; Jiang, C. Role for Pro-inflammatory Cytokines in Regulating Expression of GABA Transporter Type 1 and 3 in Specific Brain Regions of Kainic Acid-Induced Status Epilepticus. Neurochem. Res. 2015, 40, 621–627. [Google Scholar] [CrossRef]
- Roseti, C.; van Vliet, E.A.; Cifelli, P.; Ruffolo, G.; Baayen, J.C.; Di Castro, M.A.; Bertollini, C.; Limatola, C.; Aronica, E.; Vezzani, A.; et al. GABAA currents are decreased by IL-1β in epileptogenic tissue of patients with temporal lobe epilepsy: Implications for ictogenesis. Neurobiol. Dis. 2015, 82, 311–320. [Google Scholar] [CrossRef]
- Huang, X.; Hussain, B.; Chang, J. Peripheral inflammation and blood–brain barrier disruption: Effects and mechanisms. CNS Neurosci. Ther. 2021, 27, 36–47. [Google Scholar] [CrossRef]
- Poller, B.; Drewe, J.; Krähenbühl, S.; Huwyler, J.; Gutmann, H. Regulation of BCRP (ABCG2) and P-Glycoprotein (ABCB1) by Cytokines in a Model of the Human Blood–Brain Barrier. Cell. Mol. Neurobiol. 2010, 30, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Klement, W.; Garbelli, R.; Zub, E.; Rossini, L.; Tassi, L.; Girard, B.; Blaquiere, M.; Bertaso, F.; Perroy, J.; de Bock, F.; et al. Seizure progression and inflammatory mediators promote pericytosis and pericyte-microglia clustering at the cerebrovasculature. Neurobiol. Dis. 2018, 113, 70–81. [Google Scholar] [CrossRef]
- Bialer, M.; Johannessen, S.I.; Levy, R.H.; Perucca, E.; Tomson, T.; White, H.S. Progress report on new antiepileptic drugs: A summary of the Eleventh Eilat Conference (EILAT XI). Epilepsy Res. 2013, 103, 2–30. [Google Scholar] [CrossRef] [PubMed]
- Kenney-Jung, D.L.; Vezzani, A.; Kahoud, R.J.; LaFrance-Corey, R.G.; Ho, M.-L.L.; Muskardin, T.W.; Wirrell, E.C.; Howe, C.L.; Payne, E.T. Febrile infection-related epilepsy syndrome treated with anakinra. Ann. Neurol. 2016, 80, 939–945. [Google Scholar] [CrossRef]
- Westbrook, C.; Subramaniam, T.; Seagren, R.M.; Tarula, E.; Co, D.; Furstenberg-Knauff, M.; Wallace, A.; Hsu, D.; Payne, E. Febrile Infection-Related Epilepsy Syndrome Treated Successfully with Anakinra in a 21-Year-Old Woman. WMJ 2019, 118, 135–139. [Google Scholar]
- Choi, C.; Ma, S.; Ma, K.K.; Leung, H.; Mok, V.C. Super-refractory status epilepticus in autoimmune encephalitis treated with interleukin-1 receptor antagonist, anakinra. Epileptic Disord. 2021, 23, 500–505. [Google Scholar] [CrossRef]
- Jyonouchi, H. Intractable epilepsy (IE) and responses to anakinra, a human recombinant IL-1 receptor antagonist (IL-1Ra): Case reports. J. Clin. Cell. Immunol. 2016, 7, 456–460. [Google Scholar] [CrossRef]
- Lai, Y.; Muscal, E.; Wells, E.; Shukla, N.; Eschbach, K.; Lee, K.H.; Kaliakatsos, M.; Desai, N.; Wickström, R.; Viri, M.; et al. Anakinra usage in febrile infection related epilepsy syndrome: An international cohort. Ann. Clin. Transl. Neurol. 2020, 7, 2467–2474. [Google Scholar] [CrossRef]
- Bialer, M.; Johannessen, S.I.; Koepp, M.J.; Levy, R.H.; Perucca, E.; Perucca, P.; Tomson, T.; White, H.S. Progress report on new antiepileptic drugs: A summary of the Fifteenth Eilat Conference on New Antiepileptic Drugs and Devices (EILAT XV). I. Drugs in preclinical and early clinical development. Epilepsia 2020, 61, 2340–2364. [Google Scholar] [CrossRef]
- Mazumder, A.G.; Patial, V.; Singh, D. Mycophenolate mofetil contributes to downregulation of the hippocampal interleukin type 2 and 1β mediated PI3K/AKT/mTOR pathway hyperactivation and attenuates neurobehavioral comorbidities in a rat model of temporal lobe epilepsy. Brain Behav. Immun. 2019, 75, 84–93. [Google Scholar] [CrossRef]
- Zhang, H.; Tao, J.; Zhang, S.; Lv, X. LncRNA MEG3 Reduces Hippocampal Neuron Apoptosis via the PI3K/AKT/mTOR Pathway in a Rat Model of Temporal Lobe Epilepsy. Neuropsychiatr. Dis. Treat. 2020, 16, 2519–2528. [Google Scholar] [CrossRef]
- Ye, M.; Bi, Y.-F.; Ding, L.; Zhu, W.-W.; Gao, W. Saikosaponin a functions as anti-epileptic effect in pentylenetetrazol induced rats through inhibiting mTOR signaling pathway. Biomed. Pharmacother. 2016, 81, 281–287. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, R.M.; Fawzy, M.N.; Zaki, H.F.; El-Haleim, E.A.A. Neuroprotection impact of biochanin A against pentylenetetrazol-kindled mice: Targeting NLRP3 inflammasome/TXNIP pathway and autophagy modulation. Int. Immunopharmacol. 2023, 115, 109711. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yang, J.; Yu, W.; Cao, J.; Li, X. Rhein attenuates PTZ-induced epilepsy and exerts neuroprotective activity via inhibition of the TLR4-NFκB signaling pathway. Neurosci. Lett. 2021, 758, 136002. [Google Scholar] [CrossRef]
- Tao, Z.; Chun-Yan, H.; Hua, P.; Bin-Bin, Y.; Xiaoping, T. Phyllathin From Phyllanthus Amarus Ameliorates Epileptic Convulsion and Kindling Associated Post-Ictal Depression in Mice via Inhibition of NF-κB/TLR-4 Pathway. Dose-Response 2020, 18, 1559325820946914. [Google Scholar] [CrossRef]
- Singh, S.; Singh, T.G.; Singh, M.; Najda, A.; Nurzyńska-Wierdak, R.; Almeer, R.; Kamel, M.; Abdel-Daim, M.M. Anticonvulsive Effects of Chondroitin Sulfate on Pilocarpine and Pentylenetetrazole Induced Epileptogenesis in Mice. Molecules 2021, 26, 6773. [Google Scholar] [CrossRef]
- Gimenes, A.D.; Andrade, B.F.D.; Pinotti, J.V.P.; Oliani, S.M.; Galvis-Alonso, O.Y.; Gil, C.D. Annexin A1-derived peptide Ac2-26 in a pilocarpine-induced status epilepticus model: Anti-inflammatory and neuroprotective effects. J. Neuroinflamm. 2019, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Cvetkovic, R.S.; Keating, G. Anakinra. BioDrugs 2002, 16, 303–311; discussion 313–304. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.; Cañete, J.D. Anakinra for the treatment of rheumatoid arthritis: A safety evaluation. Expert Opin. Drug Saf. 2018, 17, 727–732. [Google Scholar] [CrossRef]
- Chakrabarti, K.; Frame, D.; Al Abbas, M.; McCune, W.J. The use of mycophenolate mofetil area under the curve. Curr. Opin. Rheumatol. 2021, 33, 221–232. [Google Scholar] [CrossRef]
- Volpi, N. Chondroitin Sulfate Safety and Quality. Molecules 2019, 24, 1447. [Google Scholar] [CrossRef]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu. Rev. Pathol. Mech. Dis. 2019, 15, 493–518. [Google Scholar] [CrossRef] [PubMed]
- Leitner, G.R.; Wenzel, T.J.; Marshall, N.; Gates, E.J.; Klegeris, A. Targeting toll-like receptor 4 to modulate neuroinflammation in central nervous system disorders. Expert Opin. Ther. Targets 2019, 23, 865–882. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Andersson, U. Targeting Inflammation Driven by HMGB1. Front. Immunol. 2020, 11, 484. [Google Scholar] [CrossRef]
- Li, J.; Zhu, C.S.; He, L.; Qiang, X.; Chen, W.; Wang, H. A two-decade journey in identifying high mobility group box 1 (HMGB1) and procathepsin L (pCTS-L) as potential therapeutic targets for sepsis. Expert Opin. Ther. Targets 2023, 27, 575–591. [Google Scholar] [CrossRef]
- Qi, S.; Wu, Q.; Xiang, P.; Hou, C.; Kang, Z.; Chen, M.; Yi, C.; Bai, X.; Li, T.; Li, Z.; et al. HMGB1 in Septic Muscle Atrophy: Roles and Therapeutic Potential for Muscle Atrophy and Regeneration. J. Cachex- Sarcopenia Muscle 2025, 16, e13711. [Google Scholar] [CrossRef]
- Mercado-Gómez, O.F.; Córdova-Dávalos, L.; García-Betanzo, D.; Rocha, L.; Alonso-Vanegas, M.A.; Cienfuegos, J.; Guevara-Guzmán, R. Overexpression of inflammatory-related and nitric oxide synthase genes in olfactory bulbs from frontal lobe epilepsy patients. Epilepsy Res. 2018, 148, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Wang, S.; Tang, C.-Y.; Ma, H.-W.; Cheng, Z.-Z.; Zhao, M.; Sun, W.-J.; Wang, X.-F.; Wang, M.-Y.; Li, T.-F.; et al. Translocation of High Mobility Group Box 1 From the Nucleus to the Cytoplasm in Depressed Patients With Epilepsy. ASN Neuro 2022, 14, 17590914221136662. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.A.; Erin, N.; Bozkurt, O.; Erkek, N.; Duman, O.; Haspolat, S. Changes of HMGB-1 and sTLR4 levels in cerebrospinal fluid of patients with febrile seizures. Epilepsy Res. 2021, 169, 106516. [Google Scholar] [CrossRef]
- Nass, R.D.; Wagner, M.; Surges, R.; Holdenrieder, S. Time courses of HMGB1 and other inflammatory markers after generalized convulsive seizures. Epilepsy Res. 2020, 162, 106301. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, J.; Shi, Z.; Zhu, X. Correlation of MMP-9 and HMGB1 expression with the cognitive function in patients with epilepsy and factors affecting the prognosis. Cell. Mol. Biol. 2020, 66, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chen, J.; Guo, H.; Ding, L.; Zhang, Y.; Xu, Y. High Mobility Group Protein B1 (HMGB1) and Interleukin-1β as Prognostic Biomarkers of Epilepsy in Children. J. Child Neurol. 2018, 33, 909–917. [Google Scholar] [CrossRef]
- Kan, M.; Song, L.; Zhang, X.; Zhang, J.; Fang, P. Circulating high mobility group box-1 and toll-like receptor 4 expressions increase the risk and severity of epilepsy. Braz. J. Med Biol. Res. 2019, 52, e7374. [Google Scholar] [CrossRef]
- Walker, L.E.; Sills, G.J.; Jorgensen, A.; Alapirtti, T.; Peltola, J.; Brodie, M.J.; Marson, A.G.; Vezzani, A.; Pirmohamed, M. High-mobility group box 1 as a predictive biomarker for drug-resistant epilepsy: A proof-of-concept study. Epilepsia 2022, 63, E1–E6. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, L.; Teng, J.; Miao, W. HMGB1 mediates microglia activation via the TLR4/NF-κB pathway in coriaria lactone induced epilepsy. Mol. Med. Rep. 2018, 17, 5125–5131. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Shaikh, M.F.; Chakraborti, A.; Kumari, Y.; Aledo-Serrano, Á.; Aleksovska, K.; Alvim, M.K.M.; Othman, I. HMGB1: A Common Biomarker and Potential Target for TBI, Neuroinflammation, Epilepsy, and Cognitive Dysfunction. Front. Neurosci. 2018, 12, 628. [Google Scholar] [CrossRef]
- Maroso, M.; Balosso, S.; Ravizza, T.; Liu, J.; Aronica, E.; Iyer, A.M.; Rossetti, C.; Molteni, M.; Casalgrandi, M.; A Manfredi, A.; et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat. Med. 2010, 16, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Terrone, G.; Balosso, S.; Pauletti, A.; Ravizza, T.; Vezzani, A. Inflammation and reactive oxygen species as disease modifiers in epilepsy. Neuropharmacology 2020, 167, 107742. [Google Scholar] [CrossRef]
- Kim, S.Y.; Senatorov, V.V.; Morrissey, C.S.; Lippmann, K.; Vazquez, O.; Milikovsky, D.Z.; Gu, F.; Parada, I.; Prince, D.A.; Becker, A.J.; et al. TGFβ signaling is associated with changes in inflammatory gene expression and perineuronal net degradation around inhibitory neurons following various neurological insults. Sci. Rep. 2017, 7, 7711. [Google Scholar] [CrossRef] [PubMed]
- Nishibori, M.; Wang, D.; Ousaka, D.; Wake, H. High Mobility Group Box-1 and Blood–Brain Barrier Disruption. Cells 2020, 9, 2650. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Liu, K.; Wake, H.; Teshigawara, K.; Yoshino, T.; Takahashi, H.; Mori, S.; Nishibori, M. Therapeutic effects of anti-HMGB1 monoclonal antibody on pilocarpine-induced status epilepticus in mice. Sci. Rep. 2017, 7, 1179. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Othman, I.; Shaikh, M.F. Anti-High Mobility Group Box-1 Monoclonal Antibody Attenuates Seizure-Induced Cognitive Decline by Suppressing Neuroinflammation in an Adult Zebrafish Model. Front. Pharmacol. 2020, 11, 613009. [Google Scholar] [CrossRef]
- Li, Y.-J.; Wang, L.; Zhang, B.; Gao, F.; Yang, C.-M. Glycyrrhizin, an HMGB1 inhibitor, exhibits neuroprotective effects in rats after lithium-pilocarpine-induced status epilepticus. J. Pharm. Pharmacol. 2019, 71, 390–399. [Google Scholar] [CrossRef]
- Alsaegh, H.; Eweis, H.; Kamel, F.; Alrafiah, A. Celecoxib Decrease Seizures Susceptibility in a Rat Model of Inflammation by Inhibiting HMGB1 Translocation. Pharmaceuticals 2021, 14, 380. [Google Scholar] [CrossRef]
- Morales-Sosa, M.; Orozco-Suárez, S.; Vega-García, A.; Caballero-Chacón, S.; Feria-Romero, I.A. Immunomodulatory effect of Celecoxib on HMGB1/TLR4 pathway in a recurrent seizures model in immature rats. Pharmacol. Biochem. Behav. 2018, 170, 79–86. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Qi, X.; Li, K.; Xu, W.; Wang, Y.; Chen, X.; Sha, S.; Wu, C.; Du, Y.; Chen, L. Blockage of TRPV4 Downregulates the Nuclear Factor-Kappa B Signaling Pathway to Inhibit Inflammatory Responses and Neuronal Death in Mice with Pilocarpine-Induced Status Epilepticus. Cell. Mol. Neurobiol. 2023, 43, 1283–1300. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, S.; Agarwal, N.B.; Samim, M.; Alam, O. Neuroprotective Effect of Fisetin Through Suppression of IL-1R/TLR Axis and Apoptosis in Pentylenetetrazole-Induced Kindling in Mice. Front. Neurol. 2021, 12, 689069. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Asad, M.; Akhter, J.; Hoda, U.; Rastogi, S.; Arora, I.; Aggarwal, N.B.; Samim, M. Resveratrol-Loaded Glutathione-Coated Collagen Nanoparticles Attenuate Acute Seizures by Inhibiting HMGB1 and TLR-4 in the Hippocampus of Mice. ACS Chem. Neurosci. 2022, 13, 1342–1354. [Google Scholar] [CrossRef]
- Celecoxib: Drugs and Lactation Database (LactMed®); National Institute of Child Health and Human Development: Bethesda, MD, USA, 2016.
- Magenau, J.M.; Jaglowski, S.M.; Uberti, J.; Farag, S.S.; Mansour Riwes, M.; Pawarode, A.; Anand, S.; Ghosh, M.; Maciejewski, J.; Braun, T.M.A. Phase 2 Trial of CD24Fc for Prevention of Graft-vs-Host Disease. Blood J. 2023, 2023, 020250. [Google Scholar]
- Fields, J.K.; Günther, S.; Sundberg, E.J. Structural Basis of IL-1 Family Cytokine Signaling. Front. Immunol. 2019, 10, 1412. [Google Scholar] [CrossRef] [PubMed]
- Verstrepen, L.; Bekaert, T.; Chau, T.-L.; Tavernier, J.; Chariot, A.; Beyaert, R. TLR-4, IL-1R and TNF-R signaling to NF-κB: Variations on a common theme. Cell. Mol. Life Sci. 2008, 65, 2964–2978. [Google Scholar] [CrossRef]
- Liu, J.-T.; Wu, S.-X.; Zhang, H.; Kuang, F. Inhibition of MyD88 Signaling Skews Microglia/Macrophage Polarization and Attenuates Neuronal Apoptosis in the Hippocampus After Status Epilepticus in Mice. Neurotherapeutics 2018, 15, 1093–1111. [Google Scholar] [CrossRef]
- Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef]
- Tang, C.; Jin, Y.; Wu, M.; Jia, F.; Lu, X.; Li, J.; Wu, J.; Zhu, S.; Wang, Z.; An, D.; et al. A biomimic anti-neuroinflammatory nanoplatform for active neutrophil extracellular traps targeting and spinal cord injury therapy. Mater. Today Bio 2024, 28, 101218. [Google Scholar] [CrossRef]
- Ran, W.; Xue, X. Theranostical application of nanomedicine for treating central nervous system disorders. Sci. China Life Sci. 2018, 61, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Sun, R.; Wu, X.; Chu, X.; Zhou, S.; Hu, X.; Gao, L.; Kong, Q. The treatment value of IL-1β monoclonal antibody under the targeting location of alpha-methyl-l-tryptophan and superparamagnetic iron oxide nanoparticles in an acute temporal lobe epilepsy model. J. Transl. Med. 2018, 16, 337. [Google Scholar] [CrossRef]
- Wu, D.; Fei, F.; Zhang, Q.; Wang, X.; Gong, Y.; Chen, X.; Zheng, Y.; Tan, B.; Xu, C.; Xie, H.; et al. Nanoengineered on-demand drug delivery system improves efficacy of pharmacotherapy for epilepsy. Sci. Adv. 2022, 8, eabm3381. [Google Scholar] [CrossRef]
- Ji, X.; Walczak, P.; Boltze, J. Exploring novel experimental treatments for major neurodegenerative disorders. Neuroprotection 2023, 1, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, H.; Xue, S.; Xiao, L.; Xu, J.; Tong, S.; Wei, X. MiR129-5p-loaded exosomes suppress seizure-associated neurodegeneration in status epilepticus model mice by inhibiting HMGB1/TLR4-mediated neuroinflammation. Mol. Biol. Rep. 2024, 51, 292. [Google Scholar] [CrossRef] [PubMed]
- Xian, P.; Hei, Y.; Wang, R.; Wang, T.; Yang, J.; Li, J.; Di, Z.; Liu, Z.; Baskys, A.; Liu, W.; et al. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics 2019, 9, 5956–5975. [Google Scholar] [CrossRef] [PubMed]

| Drug | Structure | Target and Mechanisms | Latest Status | Outcome | Ref. |
|---|---|---|---|---|---|
| VX09-765 |  | Caspase 1 IL-1β synthesis inhibitor | Clinical: Phase IIa for drug-resistant focal-onset epilepsy in adults (Phase IIb enrolled but stopped for business reasons) | A ≥50% reduction in seizures in 18.8% of subjects in the VX-765 group versus 8.3% in the placebo group (p > 0.05); 31.3% in the VX-765 group compared with 8.3% in the placebo group in the post hoc analysis, suggesting a delayed effect | [48] |
| Anakinra |  | IL-1 receptor IL-1 receptor antagonist | Clinical: Retrospective cohort for febrile infection-related epilepsy syndrome (FIRES) in children case reports for FIRES (adult), super-refractory status epilepticus in autoimmune encephalitis in adults, and drug-resistant epilepsy in children | FIRES: Seizure reduction was associated with the use of anakinra in both children and adults. Super-refractory status epilepticus in autoimmune encephalitis: This patient made a recovery with effective communication and the ability to walk with assistance after 16 weeks of coma. Drug-resistant epilepsy: reduction in seizures and improvement of cognition. | [49,50,51,52,53] |
| GAO-3-02 | N/A | None declared | Preclinical phase | Action on IL-1β: GAO-3-02 concentration-dependently resolved the inflammatory response induced by IL-1β in immortalized human microglial cells Action on epilepsy: reduced seizures and possible benefits on cognition | [54] |
| Substance | Targeted Mechanism | Model | Outcome | Ref. |
|---|---|---|---|---|
| Mycophenolate mofetil | IL-1β-mediated PI3K/AKT/mTOR signaling pathway hyperactivation | Lithium pilocarpine induced recurrent seizure in rats | Decreased recurrent seizures and improved neurobehavioral comorbidities | [55] |
| LncRNA MEG3 | IL-1β-mediated PI3K/AKT/mTOR signaling pathway hyperactivation | Lithium chloride and pilocarpine induced seizures in rats | Decreased proinflammatory cytokines, oxidative stress, and apoptosis rate of hippocampal neurons, and enhanced cell viability | [56] |
| Rhein | IL-1β-mediated NF-κB signaling | Pentylenetetrazole-induced seizure in mice | Delayed onset of seizure, decreased severity, duration, and frequency of seizures | [59] |
| Saikosaponin a | IL-1β-mediated PI3K/AKT/mTOR signaling pathway hyperactivation | Pentylenetetrazole-Induced seizure in rats | Reduced seizure severity and duration, elevated seizure latency | [57] |
| Phyllathin | IL-1β-mediated NF-κB signaling | Pentylenetetrazole-induced seizure in mice | Delayed onset of seizure, decreased severity, duration, and frequency of seizures | [60] |
| Chondroitin Sulfate | IL-1β-mediated NF-κB signaling | Pentylenetetrazole induced epilepsy and pilocarpine induced status epilepticus in mice | Reduced seizure severity and frequency | [61] |
| ANXA1-derived peptide Ac2-26 | Modulation of the levels of formyl peptide receptor 2 and phosphorylated extracellular signal-regulated kinase | Pilocarpine-induced status epilepticus in rats | Reduced neuronal injury and inflammation related to status epilepticus | [62] |
| Biochanin A | NLRP3 inflammasome, IL-1β-mediated PI3K/AKT/mTOR signaling pathway hyperactivation | Pentylenetetrazole-induced seizure in mice | Reduced seizure severity and duration | [58] |
| Substance | Targeted Mechanism | Model | Outcome | Ref. |
|---|---|---|---|---|
| Celecoxib | HMGB1 translocation | Lipopolysaccharides and pilocarpine induced seizures in rats | Reduced Racine score and delayed latency to generalized tonic–clonic seizures onset | [89] |
| Kainic acid induced recurrent seizures in immature rats | Reduced recurrent seizures | [90] | ||
| TAK-242 | HMGB1/TLR4/IκK/κBα/NF-κB signaling | Pilocarpine-induced status epilepticus in mice | Alleviates hippocampal neuronal injury, reduces levels of proinflammatory cytokines | [91] |
| Anti-HMGB1 monoclonal | HMGB1 | Pilocarpine and methylscopolamine induced acute seizures in mice | Prolonged onset and latency of the Racine stage five | [86] |
| Pentylenetetrazole-induced seizure in zebrafish | Prolonged onset of seizure and attenuated memory impairment | [87] | ||
| Fisetin | HMGB1-mediated AkT/mTOR pathway and NF-κB signaling | Pentylenetetrazole induced seizure in mice | Increased the latency for myoclonic jerks and generalized seizures | [92] |
| Glycyrrhizin | HMGB1 | Lithium–pilocarpine-induced status epilepticus in rats | Decreased mortality and hippocampal neuronal damage | [86] |
| Resveratrol | HMGB1/TLR4 signaling | Pentylenetetrazole induced seizure in mice | Prolonged onset and latency of seizure | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, H.; Sha, L.; Chen, L. IL-1β and HMGB1 in Epileptogenesis: Recent Advances and Clinical Translation. Pharmaceuticals 2025, 18, 1522. https://doi.org/10.3390/ph18101522
Geng H, Sha L, Chen L. IL-1β and HMGB1 in Epileptogenesis: Recent Advances and Clinical Translation. Pharmaceuticals. 2025; 18(10):1522. https://doi.org/10.3390/ph18101522
Chicago/Turabian StyleGeng, Huali, Leihao Sha, and Lei Chen. 2025. "IL-1β and HMGB1 in Epileptogenesis: Recent Advances and Clinical Translation" Pharmaceuticals 18, no. 10: 1522. https://doi.org/10.3390/ph18101522
APA StyleGeng, H., Sha, L., & Chen, L. (2025). IL-1β and HMGB1 in Epileptogenesis: Recent Advances and Clinical Translation. Pharmaceuticals, 18(10), 1522. https://doi.org/10.3390/ph18101522






