Paliurus ramosissimus Leaf Extract Inhibits Adipocyte Differentiation In Vitro and In Vivo High-Fat Diet-Induced Obesity Through PPARγ Suppression
Abstract
1. Introduction
2. Results
2.1. PRLE Inhibits Lipid Accumulation in 3T3-L1 Adipocytes
2.2. PRLE Decreases the Expression of Molecular Markers Associated with Adipose Differentiation of 3T3-L1 Cells
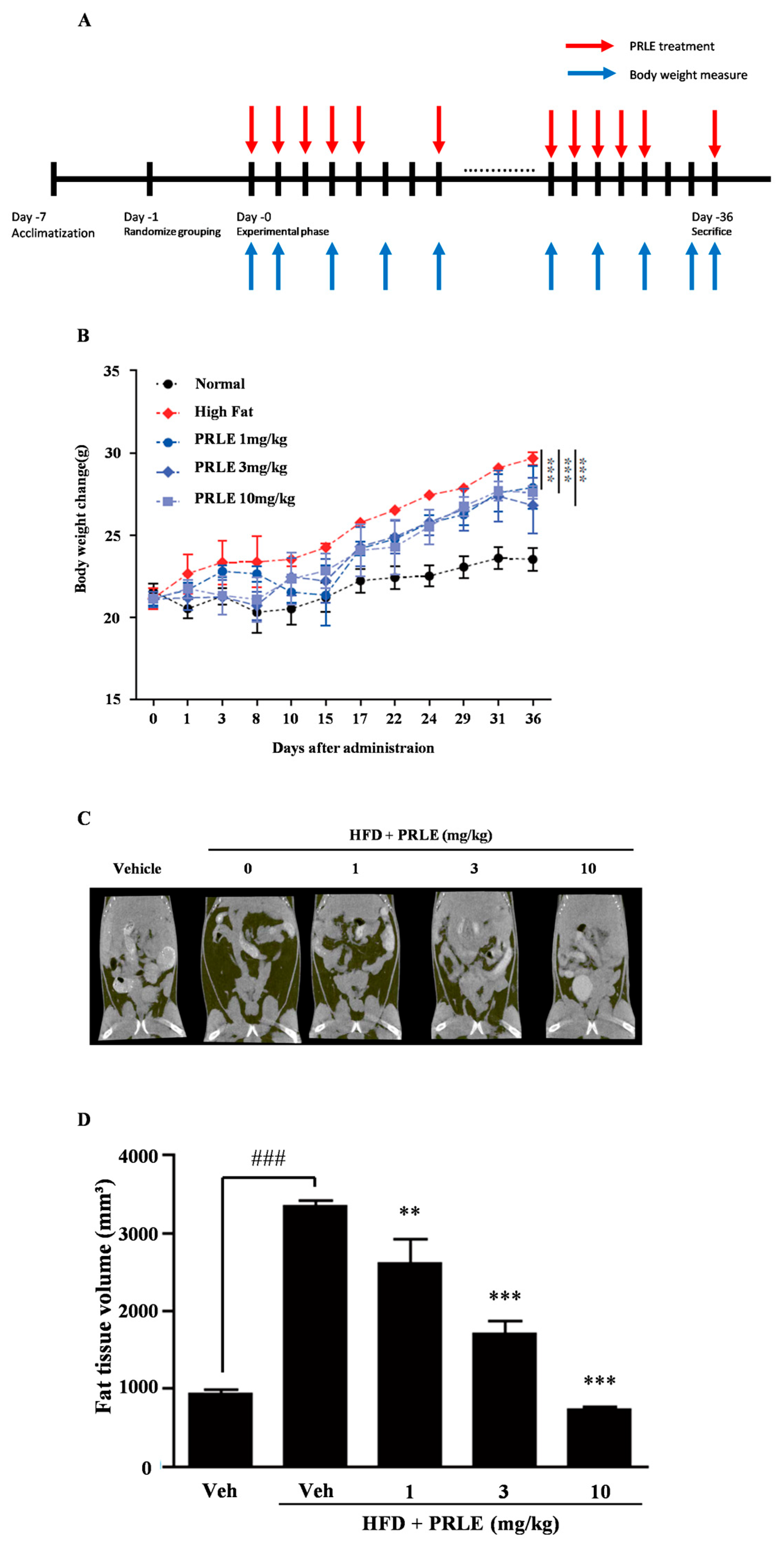
2.3. PRLE Inhibits Body Weight Gain and Reduces Body Fat in HFD-Fed Mice
2.4. Epididymal White Adipose Tissue (WAT) Is Diminished in HFD-Fed Mice
3. Discussion
4. Materials and Methods
4.1. Preparation of PRLE
4.2. Reagents and Antibodies
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Oil Red O Staining
4.6. RNA Isolation and Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
4.7. Western Blotting
4.8. In Vivo High-Fat Diet Obesity Model
4.9. ARRIVE Guidelines
4.10. Morphological Analysis of Adipose Tissue Samples
4.11. Micro-Computed Tomography (Micro-CT)
4.12. Hematoxylin and Eosin Staining
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GLP-1 | glucagon-like peptide 1 |
| HFD | high-fat diet |
| Micro-CT | Micro-Computed Tomography |
| MDI | a cocktail of methylisobutylxanthine, dexamethasone, and insulin |
| PRLE | Paliurus ramosissimus leaf extract |
| RT-qPCR | Reverse Transcription Quantitative Polymerase Chain Reaction |
References
- Stavridou, A.; Kapsali, E.; Panagouli, E.; Thirios, A.; Polychronis, K.; Bacopoulou, F.; Psaltopoulou, T.; Tsolia, M.; Sergentanis, T.N.; Tsitsika, A. Obesity in Children and Adolescents during COVID-19 Pandemic. Children 2021, 8, 135. [Google Scholar] [CrossRef]
- Allabadi, H.; Dabis, J.; Aghabekian, V.; Khader, A.; Khammash, U. Impact of COVID-19 lockdown on dietary and lifestyle behaviours among adolescents in Palestine. Dynam Human. Health 2020, 7, 2170. [Google Scholar]
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 30 April 2024).
- Regan, D.; Morrison, M.A.; Bjornerud, M.; Morrison, T.G.; Kiss, M.J. Anti-Fat Attitudes Towards Weight Gain Caused by the COVID-19 Pandemic or by “Unhealthy” Lifestyle Choices. Obesities 2025, 5, 41. [Google Scholar] [CrossRef]
- Mae, J.; Matsuoka, S.; Tsubota, A.; Nio-Kobayashi, J.; Kimura, K.; Okamatsu-Ogura, Y.; Nagaya, K. Adipocytes and Stromal Cells Regulate Brown Adipogenesis Through Secretory Factors During the Postnatal White-to-Brown Conversion of Adipose Tissue in Syrian Hamsters. Front. Cell Dev. Biol. 2021, 9, 698692. [Google Scholar] [CrossRef] [PubMed]
- Tak, Y.J.; Lee, S.Y. Anti-Obesity Drugs: Long-Term Efficacy and Safety: An Updated Review. World J. Men’s Health 2020, 39, 208. [Google Scholar] [CrossRef] [PubMed]
- Law, S.K.; Wang, Y.; Lu, X.; Au, D.C.T.; Chow, W.Y.L.; Leung, A.W.N.; Xu, C. Chinese medicinal herbs as potential prodrugs for obesity. Front. Pharmacol. 2022, 13, 1016004. [Google Scholar] [CrossRef]
- Shaik Mohamed Sayed, U.F.; Ser, H.L.; Dua, K.; Goh, H.P.; Gupta, G.; Singh, S.K.; Moshawih, S.; Goh, B.H.; Chellappan, D.K.; Ming, L.C.; et al. Natural products as novel anti-obesity agents: Insights into mechanisms of action and potential for therapeutic management. Front. Pharmacol. 2023, 14, 1182937. [Google Scholar] [CrossRef]
- Christenhusz, M.J.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Choo, G.C.; Kim, S.I.; Chung, Y.J.; Lee, S.T. A palynotaxonomic study of the Korean Rhamnaceae. Korean J. Plant Taxon. 1993, 23, 175–188. [Google Scholar] [CrossRef]
- Jeong, D.H.; Choi, M.Y.; Park, G.H. Immune-enhancing Activity of Water Extracts for Each Part of 13 Species (Rhamnaceae) in Korea. Korean J. Plant Resour. 2024, 37, 1–10. [Google Scholar] [CrossRef]
- Eo, H.J. Antioxidant and immunoregulatory effects of Korean Rhamnaceae. J. Plant Biotechnol. 2020, 47, 254–259. [Google Scholar] [CrossRef]
- Park, J.; Lee, E.; Nam, J.O. Rubia akane Nakai Fruit Extract Improves Obesity and Insulin Sensitivity in 3T3-L1 Adipocytes and High-Fat Diet-Induced Obese Mice. Int. J. Mol. Sci. 2025, 26, 1833. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.H.; Ard, J.D.; Gudzune, K.A. Phentermine in the Modern Era of Obesity Pharmacotherapy: Does It Still Have a Role in Treatment? Curr. Obes. Rep. 2024, 13, 132–140. [Google Scholar] [CrossRef]
- Lei, X.G.; Yang, X.; Ruan, J.Q.; Sun, Z.; Lai, C. Efficacy and Safety of Phentermine/Topiramate in Adults with Overweight or Obesity: A Systematic Review and Meta-Analysis. Obesity 2021, 29, 985–994. [Google Scholar] [CrossRef]
- Brandfon, S.; Khanna, D.; Eylon, A.; Parmar, M.S. Advances in Anti-obesity Pharmacotherapy: Current Treatments, Emerging Therapies, and Challenges. Cureus 2023, 15, e46623. [Google Scholar] [CrossRef]
- Son, J.W.; Kim, S. Comprehensive Review of Current and Upcoming Anti-Obesity Drugs. Diabetes Metab. J. 2020, 44, 802–818. [Google Scholar] [CrossRef]
- Dębik, M.; Kłos, K.; Żurowska, K.; Ziajor, S.; Krużel, A.; Tomasik, J.; Stodolak, M.; Sajdak, P.; Bednarski, A.; Szydłowski, Ł. GLP-1 analogs: A comparison of new anti diabetic medications—Presenting benefits and risks. J. Educ. Health Sport 2024, 62, 217–239. [Google Scholar] [CrossRef]
- Camilleri, M.; Acosta, A. Newer pharmacological interventions directed at gut hormones for obesity. Br. J. Pharmacol. 2023, 181, 1153–1164. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P. Anti-obesity drugs: A review about their effects and their safety. Expert. Opin. Drug Saf. 2012, 11, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Kim Tae, W.; Kim Kyoung, K.; Im Jae, C.; Lee Hye, R.; Kim Jung, M. Effects of Geranium wilfordii Maxim. Ethanol Extract of on Adipogenesis and Lipogenesis. Korean J. Plant Resour. 2024, 37, 307–313. [Google Scholar] [CrossRef]
- Savova, M.S.; Vasileva, L.V.; Mladenova, S.G.; Amirova, K.M.; Ferrante, C.; Orlando, G.; Wabitsch, M.; Georgiev, M.I. Ziziphus jujuba Mill. leaf extract restrains adipogenesis by targeting PI3K/AKT signaling pathway. Biomed. Pharmacother. 2021, 141, 111934. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-M.; Ye, J.; Yang, Y.-F.; Shen, Z.-F.; Lian, C.-F.; Liu, Y.-Y.; Sun, Q.-W.; Gao, L.-L.; Liu, Y.-L.; Liu, S.-N.; et al. Ramulus Mori (Sangzhi) Alkaloids Alleviate High-Fat Diet-Induced Obesity and Nonalcoholic Fatty Liver Disease in Mice. Antioxidants 2022, 11, 905. [Google Scholar] [CrossRef]
- Cave, E.; Crowther, N.J. The Use of 3T3-L1 Murine Preadipocytes as a Model of Adipogenesis. Methods Mol. Biol. 2019, 1916, 263–272. [Google Scholar] [CrossRef]
- Kong, L.; Liao, M.; Xu, M.; Yang, L.; Zhang, Q.; Qiu, Y.; Zheng, G. Chlorogenic acid and caffeine combination attenuates adipogenesis by regulating fat metabolism and inhibiting adipocyte differentiation in 3T3-L1 cells. J. Food Biochem. 2021, 45, e13795. [Google Scholar] [CrossRef]
- Kalamkar, S.D.; Bose, G.S.; Ghaskadbi, S.; Mittal, S. Andrographolide and pterostilbene inhibit adipocyte differentiation by downregulating PPARγ through different regulators. Nat. Product. Res. 2022, 37, 3145–3151. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Shan, J.; Wu, H.; Che, L.; Jia, Y. The effects of Formoterol in preventing adipogenesis and obesity are mediated by PPARγ/C/EBPα axis and AMPK/PGC-1α pathway. Biosci. Biotechnol. Biochem. 2022, zbac103. [Google Scholar] [CrossRef]
- Furuhashi, M.; Saitoh, S.; Shimamoto, K.; Miura, T. Fatty Acid-Binding Protein 4 (FABP4): Pathophysiological Insights and Potent Clinical Biomarker of Metabolic and Cardiovascular Diseases. Clin. Med. Insights Cardiol. 2014, 8, 23–33. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and obesity: Role and clinical implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Qian, H.; An, Y.; Chu, L.; Tan, D.; Qin, C.; Sun, Q.; Wang, Y.; Qi, W. PPARγ and C/EBPα enable adipocyte differentiation upon inhibition of histone methyltransferase PRC2 in malignant tumors. J. Biol. Chem. 2024, 300, 107765. [Google Scholar] [CrossRef]
- Fujita, K.; Kumazawa, S.; Kojima-Yuasa, A.; Sonoda, T.; Honda, S.; Matsui-Yuasa, I.; Norikura, T. Carob pod polyphenols suppress the differentiation of adipocytes through posttranscriptional regulation of C/EBPβ. PLoS ONE 2021, 16, e0248073. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.; Sousa, A.P.; Santos, I.; Rocha, A.C.; Alencastre, I.; Pereira, A.C.; Martins-Mendes, D.; Barata, P.; Baylina, P.; Fernandes, R. Enhanced 3T3-L1 Differentiation into Adipocytes by Pioglitazone Pharmacological Activation of Peroxisome Proliferator Activated Receptor-Gamma (PPAR-gamma). Biology 2022, 11, 806. [Google Scholar] [CrossRef]
- Mohseni, R.; Teimouri, M.; Safaei, M.; Arab Sadeghabadi, Z. AMP-activated protein kinase is a key regulator of obesity-associated factors. Cell Biochem. Funct. 2023, 41, 20–32. [Google Scholar] [CrossRef]
- Goransson, O.; Kopietz, F.; Rider, M.H. Metabolic control by AMPK in white adipose tissue. Trends Endocrinol. Metab. 2023, 34, 704–717. [Google Scholar] [CrossRef]
- Venetos, N.M.; Premont, R.T.; Stomberski, C.T.; Stamler, J.S.; Qian, Z. Activation of hepatic acetyl-CoA carboxylase by S-nitrosylation in response to diet. J. Lipid Res. 2024, 65, 100542. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; An, M.; Kim, J.-K.; Lim, Y.-H. Antiobesity effect of ethanolic extract of Ramulus mori in differentiated 3T3-L1 adipocytes and high-fat diet-induced obese mice. J. Ethnopharmacol. 2020, 251, 112542. [Google Scholar] [CrossRef]
- Liu, H.; Sathiaseelan, R.; Agbaga, M.P.; Brush, R.S.; Zhu, S.; Witzigreuter, L.; Stout, M.B. Obesity promotes lipid accumulation in mouse cartilage-A potential role of acetyl-CoA carboxylase (ACC) mediated chondrocyte de novo lipogenesis. J. Orthop. Res. 2022, 40, 2771–2779. [Google Scholar] [CrossRef]
- Shimizu, T.; Shuto, S.; Nakajima, Y.; Aoki-Saito, H.; Yamada, S.; Nakakura, T.; Fukuda, H.; Hisada, T.; Fujiwara, K.; Saito, T.; et al. Resolvin E3 ameliorates high-fat diet-induced insulin resistance via the phosphatidylinositol-3-kinase/Akt signaling pathway in adipocytes. FASEB J. 2022, 36, e22188. [Google Scholar] [CrossRef]
- Martins, T.; Castro-Ribeiro, C.; Lemos, S.; Ferreira, T.; Nascimento-Gonçalves, E.; Rosa, E.; Oliveira, P.A.; Antunes, L.M. Murine Models of Obesity. Obesities 2022, 2, 127–147. [Google Scholar] [CrossRef]
- Stapleton, S.; Welch, G.; DiBerardo, L.; Freeman, L.R. Sex differences in a mouse model of diet-induced obesity: The role of the gut microbiome. Biol. Sex. Differ. 2024, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- de Moura e Dias, M.; dos Reis, S.A.; da Conceição, L.L.; Sediyama, C.M.N.d.O.; Pereira, S.S.; de Oliveira, L.L.; Gouveia Peluzio, M.d.C.; Martinez, J.A.; Milagro, F.I. Diet-induced obesity in animal models: Points to consider and influence on metabolic markers. Diabetol. Metab. Syndr. 2021, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Xu, J.; Li, X.; Huang, L.; Hopkins, C.; Wang, H.; Yao, H.; Mi, J.; Zheng, L.; Wang, J.; et al. Macrophages in epididymal adipose tissue secrete osteopontin to regulate bone homeostasis. Nat. Commun. 2022, 13, 427. [Google Scholar] [CrossRef]
- He, M.Q.; Wang, J.Y.; Wang, Y.; Sui, J.; Zhang, M.; Ding, X.; Zhao, Y.; Chen, Z.Y.; Ren, X.X.; Shi, B.Y. High-fat diet-induced adipose tissue expansion occurs prior to insulin resistance in C57BL/6J mice. Chronic Dis. Transl. Med. 2020, 6, 198–207. [Google Scholar] [CrossRef]
- Berger, E.; Géloën, A. FABP4 Controls Fat Mass Expandability (Adipocyte Size and Number) through Inhibition of CD36/SR-B2 Signalling. Int. J. Mol. Sci. 2023, 24, 1032. [Google Scholar] [CrossRef] [PubMed]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000, 132, 365–386. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]



| Target Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| C/EBPα | GAA CAG CAA CGA GTA CCG GGT | GCC ATG GCC TTG ACC AAG GAG |
| PPARγ | CCA GAG CAT GGT GCC TTC GCT | CAG CAA CCA TTG GGT CAG CTC |
| FABP4 | GGA TGG AAA GTC GAC CAC AA | TGG AAG TCA CGC CTT TCA TA |
| Leptin | CCT CAT CAA GAC CAT TGT CAC C | TCT CCA GGT CAT TGG CTA TCT G |
| HPRT1 | TGC TCG AGA TGT CAT GAA GG | AGA GGT CCT TTT CAC CAG CA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Son, T.H.; Shin, H.-L.; Kim, D.; Park, G.H.; Seo, J.W.; Kim, H.-G.; Choi, S.-W. Paliurus ramosissimus Leaf Extract Inhibits Adipocyte Differentiation In Vitro and In Vivo High-Fat Diet-Induced Obesity Through PPARγ Suppression. Pharmaceuticals 2025, 18, 1515. https://doi.org/10.3390/ph18101515
Kim S-H, Son TH, Shin H-L, Kim D, Park GH, Seo JW, Kim H-G, Choi S-W. Paliurus ramosissimus Leaf Extract Inhibits Adipocyte Differentiation In Vitro and In Vivo High-Fat Diet-Induced Obesity Through PPARγ Suppression. Pharmaceuticals. 2025; 18(10):1515. https://doi.org/10.3390/ph18101515
Chicago/Turabian StyleKim, Shin-Hye, Tae Hyun Son, Hye-Lim Shin, Dongsoo Kim, Gwang Hun Park, Jeong Won Seo, Hwan-Gyu Kim, and Sik-Won Choi. 2025. "Paliurus ramosissimus Leaf Extract Inhibits Adipocyte Differentiation In Vitro and In Vivo High-Fat Diet-Induced Obesity Through PPARγ Suppression" Pharmaceuticals 18, no. 10: 1515. https://doi.org/10.3390/ph18101515
APA StyleKim, S.-H., Son, T. H., Shin, H.-L., Kim, D., Park, G. H., Seo, J. W., Kim, H.-G., & Choi, S.-W. (2025). Paliurus ramosissimus Leaf Extract Inhibits Adipocyte Differentiation In Vitro and In Vivo High-Fat Diet-Induced Obesity Through PPARγ Suppression. Pharmaceuticals, 18(10), 1515. https://doi.org/10.3390/ph18101515







