Vasorelaxant and Hypotensive Mechanisms of Nelumbo nucifera Seed Extract: Roles of Nitric Oxide, Calcium Channel Blockade and eNOS Interaction with Active Compounds
Abstract
1. Introduction
2. Results
2.1. Phytochemical Screening of LSE by LC-ESI-QTOF/MS
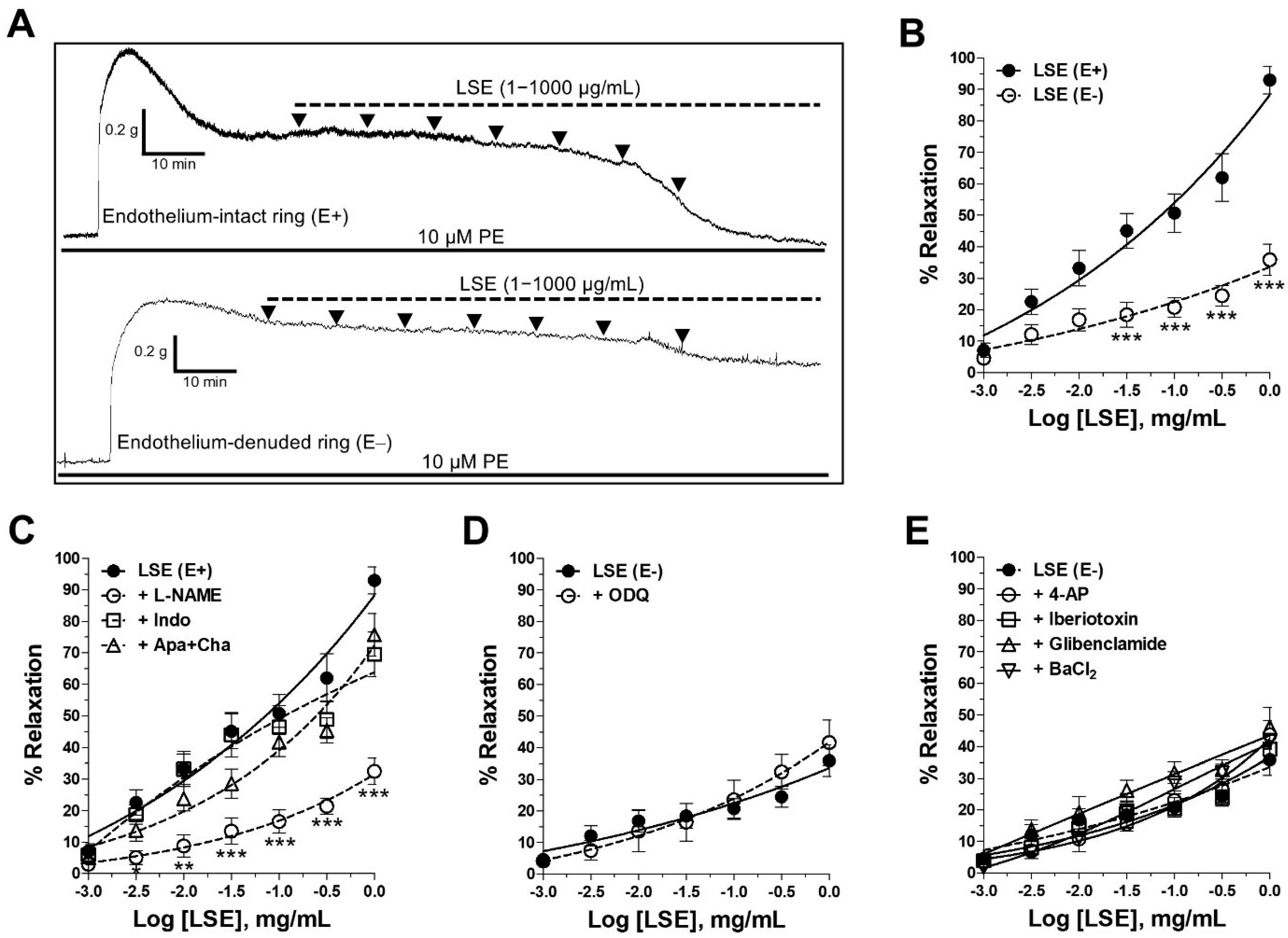
2.2. LSE-Induced Endothelium-Dependent and Endothelium-Independent Vasorelaxant Effects
2.3. Mechanism of Vasorelaxant Action of LSE via NO Pathway and Receptor-Operated Ca2+ Channels (ROCCs) Inhibition
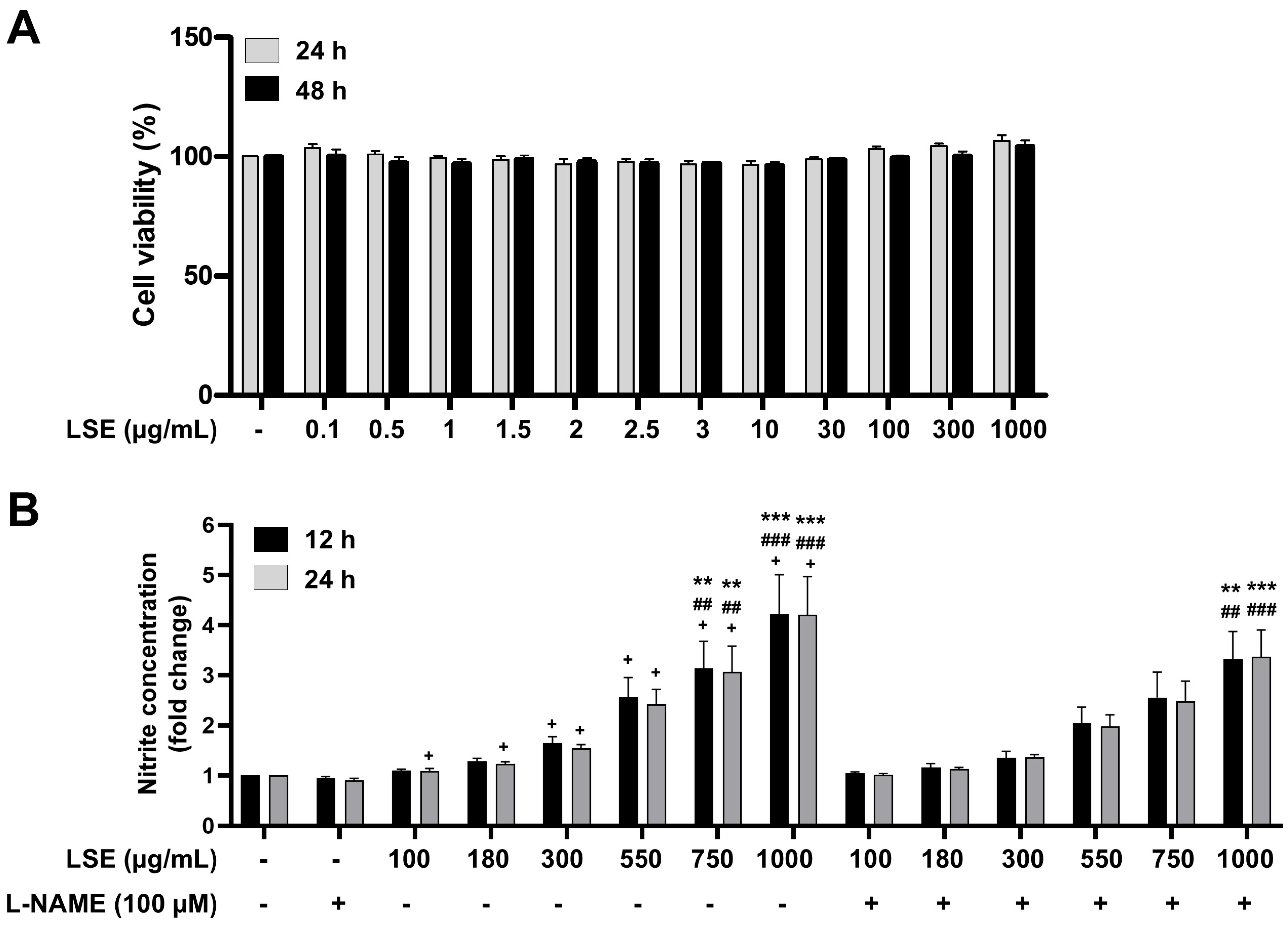
2.4. Cytotoxicity and NO Production in EA.hy926 Human Endothelial Cells Treated with LSE
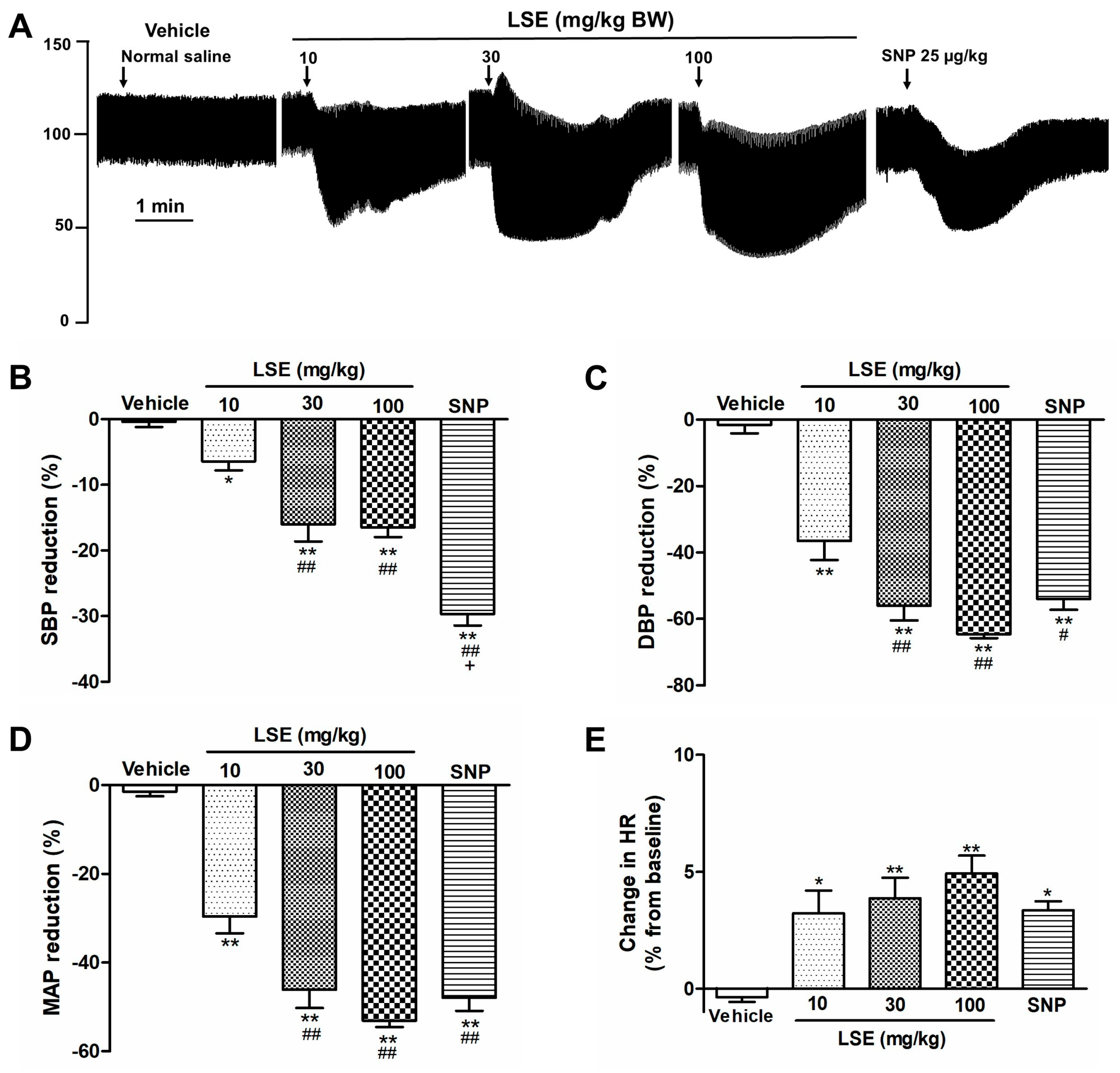
2.5. LSE-Induced Acute Hypotensive Effect
2.6. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Plant Extract Preparation
4.2. Phytochemical Analysis Using LC-ESI-QTOF-MS
4.3. Animals
4.4. Vasorelaxant Effects of LSE on Isolated Rat Thoracic Aorta
4.5. Cytotoxicity and NO Production Effects of LSE on EA.hy926 Human Endothelial Cells
4.6. Acute Hypotensive Effect of LSE
4.7. Molecular Docking
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Vanhoutte, P.M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989, 3, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.M.; da Silva, M.C.; Nascimento, D.V.G.; Lima Silva, E.M.; Gouvêa, F.F.F.; de França Lopes, L.G.; Araújo, A.V.; Ferraz Pereira, K.N.; de Queiroz, T.M. Nitric Oxide as a Central Molecule in Hypertension: Focus on the Vasorelaxant Activity of New Nitric Oxide Donors. Biology 2021, 10, 1041. [Google Scholar] [CrossRef] [PubMed]
- Butnariu, M.; Fratantonio, D.; Herrera-Bravo, J.; Sukreet, S.; Martorell, M.; Ekaterina Robertovna, G.; Les, F.; López, V.; Kumar, M.; Pentea, M.; et al. Plant-food-derived Bioactives in Managing Hypertension: From Current Findings to Upcoming Effective Pharmacotherapies. Curr. Top. Med. Chem. 2023, 23, 589–617. [Google Scholar] [CrossRef] [PubMed]
- Arooj, M.; Imran, S.; Inam-ur-Raheem, M.; Rajoka, M.S.R.; Sameen, A.; Siddique, R.; Sahar, A.; Tariq, S.; Riaz, A.; Hussain, A.; et al. Lotus seeds (Nelumbinis semen) as an emerging therapeutic seed: A comprehensive review. Food Sci. Nutr. 2021, 9, 3971–3987. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Mukherjee, D.; Maji, A.K.; Rai, S.; Heinrich, M. The sacred lotus (Nelumbo nucifera)—Phytochemical and therapeutic profile. J. Pharm. Pharmacol. 2009, 61, 407–422. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Dunno, K.; Kumar, M.; Mostafa, H.; Maqsood, S. A comprehensive review on lotus seeds (Nelumbo nucifera Gaertn.): Nutritional composition, health-related bioactive properties, and industrial applications. J. Funct. Foods 2022, 89, 104937. [Google Scholar] [CrossRef]
- Sharma, B.R.; Gautam, L.N.; Adhikari, D.; Karki, R. A Comprehensive Review on Chemical Profiling of Nelumbo nucifera: Potential for Drug Development. Phytother. Res. 2017, 31, 3–26. [Google Scholar] [CrossRef]
- Zhao, X.; Shen, J.; Chang, K.J.; Kim, S.H. Comparative Analysis of Antioxidant Activity and Functional Components of the Ethanol Extract of Lotus (Nelumbo nucifera) from Various Growing Regions. J. Agric. Food Chem. 2014, 62, 6227–6235. [Google Scholar] [CrossRef]
- Moon, S.W.; Ahn, C.-B.; Oh, Y.; Je, J.-Y. Lotus (Nelumbo nucifera) seed protein isolate exerts anti-inflammatory and antioxidant effects in LPS-stimulated RAW264.7 macrophages via inhibiting NF-κB and MAPK pathways, and upregulating catalase activity. Int. J. Biol. Macromol. 2019, 134, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Feng, X.; Wang, C.; Peng, D.; Zhu, K.; Song, J.L. Anticancer activity of Nelumbo nucifera stamen extract in human colon cancer HCT-116 cells in vitro. Oncol. Lett. 2017, 13, 1470–1478. [Google Scholar] [CrossRef]
- Mutreja, A.; Agarwal, M.; Kushwaha, S.; Chauhan, A. Effect of Nelumbo nucifera seeds on the reproductive organs of female rats. Int. J. Reprod. Biomed. 2008, 6, 7–11. [Google Scholar]
- Liu, C.-P.; Tsai, W.-J.; Lin, Y.-L.; Liao, J.-F.; Chen, C.-F.; Kuo, Y.-C. The extracts from Nelumbo nucifera suppress cell cycle progression, cytokine genes expression, and cell proliferation in human peripheral blood mononuclear cells. Life Sci. 2004, 75, 699–716. [Google Scholar] [CrossRef]
- Inchan, A.; Bualeong, T.; Kaewkong, W.; Nuengchamnong, N.; Apaikawee, P.; Sa-Nguanpong, P.; Sumsakul, W.; Charoenphon, N.; Chatturong, U.; Deetud, W.; et al. Antihypertensive Effects of Lotus Seed (Nelumbo nucifera Gaertn.) Extract via eNOS Upregulation and Oxidative Stress Reduction in L-NAME-Induced Hypertensive Rats. Pharmaceuticals 2025, 18, 1156. [Google Scholar] [CrossRef]
- Balogh, M.P. Interpretation of mass spectra, Part 1: Developing skills. LCGC Eur. 2007, 20. Available online: https://www.chromatographyonline.com/view/interpretation-mass-spectra-part-1-developing-skills (accessed on 24 August 2024).
- Jackson, W.F. Ion channels and vascular tone. Hypertension 2000, 35, 173–178. [Google Scholar] [CrossRef]
- Kang, K.T. Endothelium-derived Relaxing Factors of Small Resistance Arteries in Hypertension. Toxicol. Res. 2014, 30, 141–148. [Google Scholar] [CrossRef]
- Nava, E.; Llorens, S. The Local Regulation of Vascular Function: From an Inside-Outside to an Outside-Inside Model. Front. Physiol. 2019, 10, 729. [Google Scholar] [CrossRef] [PubMed]
- Negri, S.; Faris, P.; Moccia, F. Reactive Oxygen Species and Endothelial Ca2+ Signaling: Brothers in Arms or Partners in Crime? Int. J. Mol. Sci. 2021, 22, 9821. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Syed, A.U.; Prada, M.P.; Nystoriak, M.A.; Santana, L.F.; Nieves-Cintrón, M.; Navedo, M.F. Calcium Channels in Vascular Smooth Muscle. Adv. Pharmacol. 2017, 78, 49–87. [Google Scholar] [CrossRef]
- Knox, M.; Vinet, R.; Fuentes, L.; Morales, B.; Martínez, J.L. A Review of Endothelium-Dependent and -Independent Vasodilation Induced by Phytochemicals in Isolated Rat Aorta. Animals 2019, 9, 623. [Google Scholar] [CrossRef]
- Qian, J.Q. Cardiovascular pharmacological effects of bisbenzylisoquinoline alkaloid derivatives. Acta Pharmacol. Sin. 2002, 23, 1086–1092. [Google Scholar]
- Bharathi Priya, L.; Huang, C.-Y.; Hu, R.-M.; Balasubramanian, B.; Baskaran, R. An updated review on pharmacological properties of neferine—A bisbenzylisoquinoline alkaloid from Nelumbo nucifera. J. Food Biochem. 2021, 45, e13986. [Google Scholar] [CrossRef]
- Bignon, E.; Rizza, S.; Filomeni, G.; Papaleo, E. Use of Computational Biochemistry for Elucidating Molecular Mechanisms of Nitric Oxide Synthase. Comput. Struct. Biotechnol. J. 2019, 17, 415–429. [Google Scholar] [CrossRef]
- Umar, H.I.; Saliu, T.P.; Josiah, S.S.; Ajayi, A.; Danjuma, J.B. In silico studies of bioactive compounds from selected African plants with inhibitory activity against nitric oxide synthase and arginase implicated in asthma. Egypt. J. Med. Hum. Genet. 2021, 22, 60. [Google Scholar] [CrossRef]
- Shen, Z.-H.; Ye, T.; Chen, B.; Wan, C.; Lu, X.; Chen, T.-H.; Lin, S.; Ye, J.-X.; Xie, L.; Fu, Y.-S. 5,7-Dihydroxyflavone acts on eNOS to achieve hypotensive effects in spontaneously hypertensive rats. Sci. Rep. 2025, 15, 594. [Google Scholar] [CrossRef] [PubMed]
- Rubiolo, J.A.; Lence, E.; González-Bello, C.; Roel, M.; Gil-Longo, J.; Campos-Toimil, M.; Ternon, E.; Thomas, O.P.; González-Cantalapiedra, A.; López-Alonso, H.; et al. Crambescin C1 Acts as A Possible Substrate of iNOS and eNOS Increasing Nitric Oxide Production and Inducing In Vivo Hypotensive Effect. Front. Pharmacol. 2021, 12, 694639. [Google Scholar] [CrossRef]
- Bendall, J.K.; Douglas, G.; McNeill, E.; Channon, K.M.; Crabtree, M.J. Tetrahydrobiopterin in cardiovascular health and disease. Antioxid. Redox Signal 2014, 20, 3040–3077. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, M.J.; Hale, A.B.; Channon, K.M. Dihydrofolate reductase protects endothelial nitric oxide synthase from uncoupling in tetrahydrobiopterin deficiency. Free Radic. Biol. Med. 2011, 50, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Wenceslau, C.F.; McCarthy, C.G.; Earley, S.; England, S.K.; Filosa, J.A.; Goulopoulou, S.; Gutterman, D.D.; Isakson, B.E.; Kanagy, N.L.; Martinez-Lemus, L.A.; et al. Guidelines for the measurement of vascular function and structure in isolated arteries and veins. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H77–H111. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheang, W.S.; Yang, H.; Xiao, L.; Lai, B.; Zhang, M.; Ni, J.; Luo, Z.; Zhang, Z.; Huang, Y.; et al. Nuciferine relaxes rat mesenteric arteries through endothelium-dependent and -independent mechanisms. Br. J. Pharmacol. 2015, 172, 5609–5618. [Google Scholar] [CrossRef] [PubMed]
- Wicha, P.; Onsa-Ard, A.; Chaichompoo, W.; Suksamrarn, A.; Tocharus, C. Vasorelaxant and Antihypertensive Effects of Neferine in Rats: An In Vitro and In Vivo Study. Planta Med. 2020, 86, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Inchan, A.; Chootip, K.; Kongthong, K.; Bualeong, T.; Sumsakul, W.; Apaikawee, P.; Sa-Nguanpong, P.; Senarat, S.; Wongphoom, J.; Charoenphon, N. Lotus seed (Nelumbo nucifera Gaertn.) extract at low dose ameliorates reproductive dysfunction in l-NAME-induced hypertension and oxidative stress in male rats. J. Tradit. Complement. Med. 2025, 15, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Chansriniyom, C.; Nooin, R.; Nuengchamnong, N.; Wongwanakul, R.; Petpiroon, N.; Srinuanchai, W.; Chantarasuwan, B.; Pitchakarn, P.; Temviriyanukul, P.; Nuchuchua, O. Tandem mass spectrometry of aqueous extract from Ficus dubia sap and its cell-based assessments for use as a skin antioxidant. Sci. Rep. 2021, 11, 16899. [Google Scholar] [CrossRef]
- Chatturong, U.; Martin, H.; Totoson, P.; Ingkaninan, K.; Temkitthawon, P.; Sermsenaphorn, S.; Somarin, T.; Konsue, A.; Gleeson, M.P.; Demougeot, C.; et al. Quinazoline-based human phosphodiesterase 5 inhibitors exhibited a selective vasorelaxant effect on rat isolated pulmonary arteries involving NO-sGC-cGMP pathway and calcium inhibitory effects. Vasc. Pharmacol. 2022, 147, 107111. [Google Scholar] [CrossRef]
- Yang, C.-F.; Yu-Chih Chen, M.; Chen, T.-I.; Cheng, C.-F. Dose-dependent effects of isoflurane on cardiovascular function in rats. Tzu Chi Med. J. 2014, 26, 119–122. [Google Scholar] [CrossRef]
- Fischer, T.; Najjar, A.; Totzke, F.; Schächtele, C.; Sippl, W.; Ritter, C.; Hilgeroth, A. Discovery of novel dual inhibitors of receptor tyrosine kinases EGFR and PDGFR-β related to anticancer drug resistance. J. Enzym. Inhib. Med. Chem. 2018, 33, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Sanner, M.F. AutoDock CrankPep: Combining folding and docking to predict protein-peptide complexes. Bioinformatics 2019, 35, 5121–5127. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Suroengrit, A.; Cao, V.; Wilasluck, P.; Deetanya, P.; Wangkanont, K.; Hengphasatporn, K.; Harada, R.; Chamni, S.; Leelahavanichkul, A.; Shigeta, Y.; et al. Alpha and gamma mangostins inhibit wild-type B SARS-CoV-2 more effectively than the SARS-CoV-2 variants and the major target is unlikely the 3C-like protease. Heliyon 2024, 10, e31987. [Google Scholar] [CrossRef] [PubMed]
- Elhinnawi, M.A.; Okita, Y.; Shigematsu, K.; Abdelaziz, M.; Shiratani, R.; Kawanishi, K.; Hengphasatporn, K.; Dang Cao, T.L.; Shigeta, Y.; Kato, M. GPNMB is a novel binding partner of FGFR1 that affects tumorigenic potential through AKT phosphorylation in TNBC. Cancer Sci. 2025, 116, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Panklai, T.; Temkitthawon, P.; Suphrom, N.; Girard, C.; Totoson, P.; Hengphasatporn, K.; Shigeta, Y.; Chootip, K.; Ingkaninan, K. Nymphaea pubescens Willd. extract and its flavonoid constituents vasodilate rat isolated pulmonary artery via NO-sGC-cGMP pathway. Phytomedicine Pus 2025, 5, 100733. [Google Scholar] [CrossRef]
- BIOVIA Discovery Studio Visualizer; Dassault Systèmes: San Diego, CA, USA, 2021.
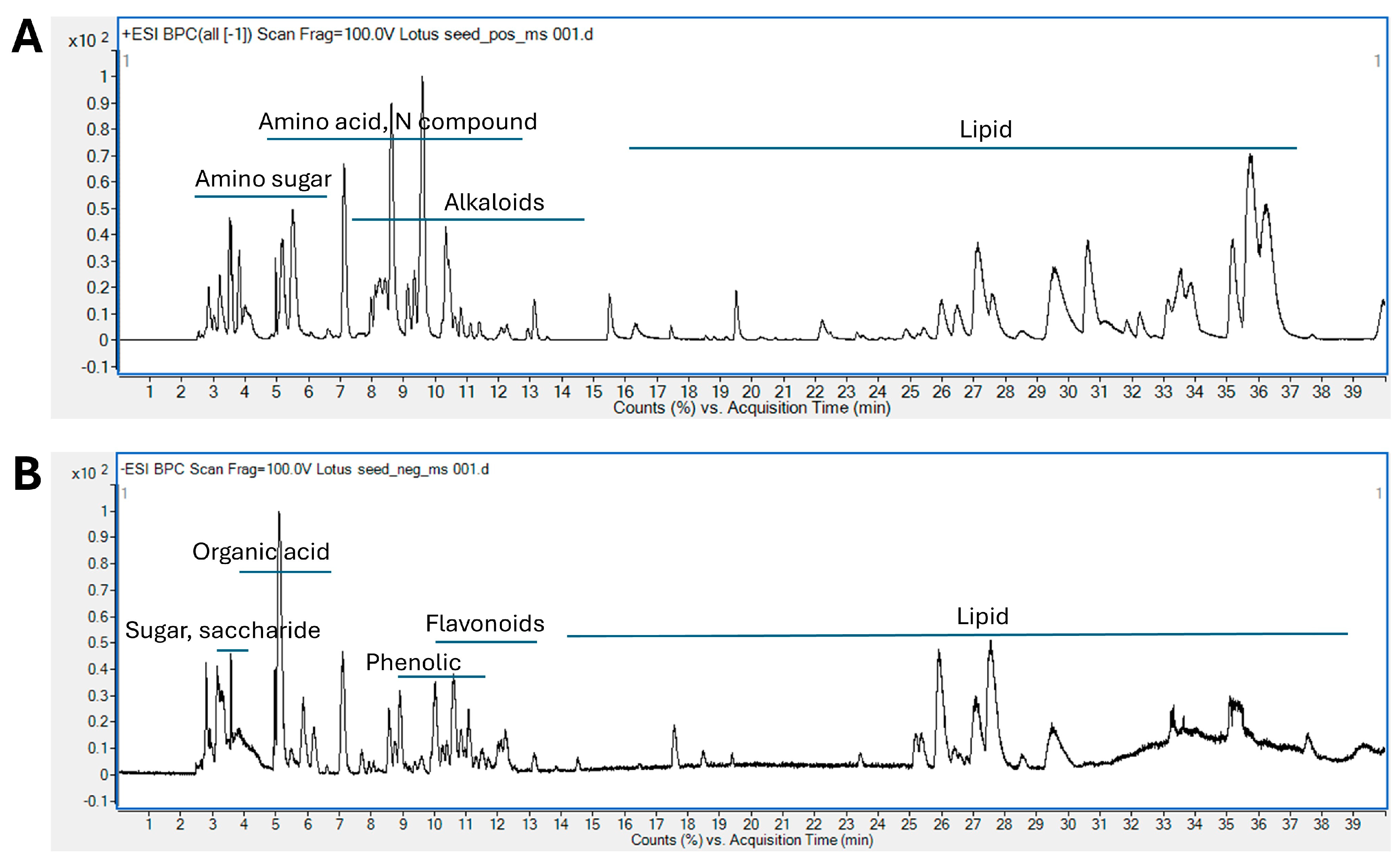


| No | RT(min) | m/z | Adduct | MS/MS | Tentative Identification | Formula | Error (ppm) |
|---|---|---|---|---|---|---|---|
| Amino sugars | |||||||
| 1 | 2.811 | 264.1086 | [M−H]− | 174.0740,102.0543 | N-fructosyl-GABA | C10H19NO7 | −2.74 |
| 266.1239 | [M+H]+ | 248.1135,182.0816,116.0709, 104.0706,98.0602 | N-fructosyl-GABA | C10H19NO7 | −1.77 | ||
| 2 | 2.988 | 250.0935 | [M−H]− | 130.0490,88.0393 | N-fructosyl alanine | C9H17NO7 | −1.1 |
| 252.1085 | [M+H]+ | 234.0979,216.0868,188.0919, 170.0818,90.0552 | N-fructosyl alanine | C9H17NO7 | −2.86 | ||
| 3 | 3.554 | 280.1392 | [M+H]+ | 216.1238,118.0866,72.0810 | N-fructosyl valine | C11H21NO7 | −0.43 |
| 4 | 4.971 | 290.0898 | [M−H]− | 200.0538,128.0332 | N-Fructosyl pyroglutamate | C11H17NO8 | −2.96 |
| 5 | 5.054 | 344.1343 | [M+H]+ | 326.1246,308.1133,280.1185, 182.0805 | N-fructosyl tyrosine | C15H21NO8 | −0.89 |
| 6 | 5.249 | 294.1551 | [M+H]+ | 276.1451,230.1394,132.1020, 86.0966 | N-fructosyl isoleucine | C12H23NO7 | −1.26 |
| 7 | 7.112 | 328.1396 | [M+H]+ | 310.1294,292.1179,264.1242, 166.0883,120.0813 | N-fructosyl phenylalanine | C15H21NO7 | −1.59 |
| 8 | 8.362 | 365.136 | [M−H]− | 203.0800,101.0236 | N-Fructosyl-tryptophan | C17H22N2O7 | −1.58 |
| 8.408 | 367.1504 | [M+H]+ | 349.1395,229.0980,188.0717, 146.0606,118.0650,85.0283 | N-Fructosyl-tryptophan | C17H22N2O7 | −1.15 | |
| Sugar | |||||||
| 9 | 3.047 | 665.2082 | [M−H]− | 503.1555,383.1115,179.0520, 89.0225 | Tetraglucoside | C24H42O21 | 9.59 |
| 10 | 3.102 | 181.0723 | [M−H]− | 89.0228,59.0137 | D-manitol | C6H14O6 | −2.97 |
| 11 | 3.144 | 549.1579 | [M+HCOO]− | 503.1623,341.1019,323.0906, 179.0535,89.0231 | Triglucose (Maltotriose) | C18H32O16 | −1.08 |
| 12 | 3.22 | 537.167 | [M−H]− | 503.1521,341.1032,195.0472, 129.0185,75.0080 | Gluconic acid glycoside | C18H34O18 | −2.35 |
| 13 | 3.295 | 683.2262 | [2M−H]− | 341.1028,179.0633,89.0230 | Sucrose | C12H22O11 | −1.54 |
| 14 | 3.493 | 179.0568 | [M−H]− | 89.0230,59.0135 | Fructose | C6H12O6 | −3.84 |
| Organic acids | |||||||
| 15 | 3.572 | 133.0146 | [M−H]− | 115.0021,71.0138 | Malic acid | C4H6O5 | 0.35 |
| 16 | 5.01 | 191.0202 | [M−H]− | 111.0075,87.0079,57.0346 | Citric acid | C6H8O7 | −2.48 |
| 17 | 5.868 | 117.0236 | [M−H]− | 73.0292 | Succinic acid | C4H6O4 | −0.58 |
| 18 | 6.042 | 115.004 | [M−H]− | 71.0135 | Maleic acid | C4H4O4 | −2.76 |
| 19 | 6.09 | 133.0509 | [M−H]− | 71.0134 | 4,5-Dihydroxypentanoic acid | C5H10O4 | −2.01 |
| 20 | 6.21 | 161.0461 | [M−H]− | 99.0441,57.0345 | 3-Hydroxy-2-methylglutaric acid | C6H10O5 | −1.57 |
| 21 | 9.467 | 117.0557 | [M−H]− | 73.0281,71.049 | 5-Hydroxypentanoic acid | C5H10O3 | 0.15 |
| 22 | 10.269 | 175.0616 | [M−H]− | 157.0489,115.0326 | 2-Isopropylmalic acid | C7H12O5 | −2.3 |
| Amino acids & N-compounds | |||||||
| 23 | 2.528 | 156.0772 | [M+H]+ | 110.0713 | Histidine | C6H9N3O2 | −2.86 |
| 24 | 2.531 | 175.1197 | [M+H]+ | 158.0927,130.0975,116.0710 | Arginine | C6H14N4O2 | −4.27 |
| 25 | 2.762 | 207.2075 | [2M−H]+ | 104.1073 | 2-Amino-3-methyl-1-butanol | C5H13NO | −3.84 |
| 26 | 2.812 | 174.0772 | [M−H]− | 145.0613,102.0546,59.0129 | N-Carboxyethyl-gramma-aminobutyric acid | C7H13NO4 | −0.11 |
| 27 | 3.228 | 138.0549 | [M+H]+ | 94.0662,92.0496 | p-Aminobenzoic acid | C7H7NO2 | 0.4 |
| 28 | 3.258 | 116.0709 | [M+H]+ | 70.0654 | Proline | C5H9NO2 | |
| 29 | 3.514 | 118.086 | [M+H]+ | 72.0810 | Valine | C5H11NO2 | 2.16 |
| 30 | 3.62 | 182.0816 | [M+H]+ | 165.0639,147.0441,136.0759, 107.0490,91.0543 | Tyrosine | C9H11NO3 | −2.36 |
| 31 | 3.815 | 132.1019 | [M+H]+ | 86.0967 | Beta leucine | C6H13NO2 | 0.04 |
| 32 | 5.077 | 130.05 | [M+H]+ | 84.0448 | Pyroglutamic acid | C5H7NO3 | −1.0 |
| 5.119 | 128.0351 | [M−H]− | 71.0129,52.0188 | Pyroglutamic acid | C5H7NO3 | 0.13 | |
| 33 | 5.138 | 268.1046 | [M+H]+ | 136.0623,57.0337 | Adenosine | C9H17NO8 | −7.11 |
| 34 | 5.173 | 132.1021 | [M+H]+ | 86.0968,56.0496 | Leucine | C6H13NO2 | 0.04 |
| 35 | 5.513 | 132.1016 | [M+H]+ | 86.0968 | Isoleucine | C6H13NO2 | 2.31 |
| 5.501 | 130.0875 | [M−H]− | 71.0138,59.0396 | Isoeucine | C6H13NO2 | 3.82 | |
| 36 | 6.089 | 160.0763 | [M+H]+ | 132.0810,115.0544,86.0964, 72.0444,61.0283 | Indoleacetaldehyde | C10H9NO | −3.81 |
| 37 | 7.131 | 166.0863 | [M+H]+ | 120.0810,77.0389 | Phenylalanine | C9H11NO2 | −0.27 |
| 7.142 | 164.0722 | [M−H]− | 147.0430,103.0547,77.0388 | Phenylalanine | C9H11NO2 | 2.14 | |
| 38 | 7.687 | 220.1184 | [M+H]+ | 202.1077,184.0968,90.0551 | Pantothenic acid | C9H17NO5 | −2.05 |
| 39 | 8.583 | 203.083 | [M−H]− | 116.0496,74.0244 | Tryptophan | C11H12N2O2 | 2.03 |
| 8.593 | 205.0976 | [M+H]+ | 188.0712,146.0606,118.0653, 74.0231 | Tryptophan | C11H12N2O2 | −2.17 | |
| 40 | 11.092 | 210.0776 | [M−H]− | 179.0343,124.0390,94.0288 | 3-Methoxy-DL-tyrosine | C10H13NO4 | −1.99 |
| 41 | 12.53 | 289.0834 | [M−H]− | 173.0688,132.0275,88.0394 | L-N-(1H-Indol-3-yl acetylaspartic acid) | C14H14N2O5 | −1.4 |
| Phenolic compounds | |||||||
| 42 | 4.978 | 180.1024 | [M+NH4]+ | 163.0757,145.0652,117.0700, 91.0543 | 4-Methylcinnamic acid | C10H10O2 | −2.75 |
| 43 | 6.662 | 194.1183 | [M+NH4]+ | 177.0788,91.0534 | Cinnamyl acetate | C11H12O2 | −3.84 |
| 44 | 8.766 | 371.0929 | [M+HCOO]− | 163.0382,119.0496 | 6-O-p-Coumaroyl-D-glucose | C15H18O8 | −1.97 |
| 45 | 9.088 | 153.0194 | [M−H]− | 109.0285,81.0331 | Protocatechuic acid | C7H6O4 | −0.44 |
| 46 | 9.595 | 421.0909 | [M+Cl]− | 385.1084,265.0660,223.0586, 179.0635 | 1-O-Sinapoylglucose | C17H22O10 | −0.48 |
| 47 | 10.986 | 137.0245 | [M−H]− | 93.0334,65.0387 | 3-Hydroxybenzoic acid | C7H6O3 | −0.6 |
| 48 | 11.265 | 179.035 | [M−H]− | 135.0432,107.0491 | Caffeic acid | C9H8O4 | −0.1 |
| 49 | 11.511 | 165.0194 | [M−H]− | 121.0279,77.0392 | 1,4-benzendicarboxylic acid | C8H6O4 | −0.41 |
| 50 | 13.164 | 163.0382 | [M−H]− | 119.0486 | p-coumaric acid | C9H8O3 | 11.46 |
| Flavonoids | |||||||
| 51 | 9.601 | 577.1349 | [M−H]− | 451.0956,425.0812,407.0716, 289.0675,245.0412,125.0230 | Procyanidin B2 | C30H26O12 | 0.43 |
| 52 | 9.924 | 593.1522 | [M−H]− | 503.1130,473.1004,383.0708, 353.0591,289.0672,139.0036 | Apigenin-6,8-di-C-glycopyranoside (Vicenin-2) | C27H30O15 | −1.7 |
| 53 | 10.03 | 289.0722 | [M−H]− | 245.0781,203.0684,125.0229, 109.0283 | Catechin | C15H14O6 | −1.52 |
| 54 | 10.615 | 563.1411 | [M−H]− | 503.1100,473.1010,443.0908, 383.0712,353.0618,325.0660, 297.0707 | Apigenin 6-C-glucosyl-8-C-arabinoside (Schaftoside) | C26H28O14 | −0.84 |
| 10.621 | 565.1565 | [M+H]+ | 529.1347,481.1134,427.1032, 379.0830,325.0712 | Apigenin 6-C-glucoside 8-C-arabinoside (Schaftoside) | C26H28O14 | −2.33 | |
| 55 | 10.768 | 449.1087 | [M−H]− | 357.0542,329.0636,287.0511, 259.0581,125.0229 | Eriodictyol 7-O-glucoside | C21H22O11 | 0.52 |
| 56 | 10.847 | 563.142 | [M−H]− | 503.1000,443.0886,353.0612, 125.0224 | Apigenin 6-C-arabinoside 8-C-glucoside (Isoschaftoside) | C26H28O14 | −0.84 |
| 10.851 | 565.1563 | [M+H]+ | 427.1033,379.0814,325.0699 | Apigenin 6-C-arabinoside 8-C-glucoside (Isoschaftoside) | C26H28O14 | −2.33 | |
| 57 | 10.963 | 447.0939 | [M−H]− | 357.0556,327.0451,285.0361 | Luteolin 6-C-glucoside (Isorientin) | C21H20O11 | −1.38 |
| 449.108 | [M+H]+ | 413.0869,329.0653,243.0290 | Luteolin 6-C-glucoside (Isorientin) | C21H20O11 | −0.36 | ||
| 58 | 11.517 | 577.1559 | [M−H]− | 487.1164,457.1079,383.0706, 353.0605,325.0670,289.0655, 179.0522,89.0231 | Apigenin 6-C-glucosyl-8-C-rhamnoside | C27H30O14 | 0.66 |
| 59 | 11.712 | 609.1469 | [M−H]− | 300.0222,301.0296,151.0014 | Rutin | C27H30O16 | −1.3 |
| 11.71 | 611.1619 | [M+H]+ | 465.1035,303.0505129.0546, 85.0283 | Rutin | C27H30O16 | −2.03 | |
| 60 | 11.943 | 593.1511 | [M−H]− | 285.0361,151.0018 | Kaempferol 3-O-rutinoside | C27H30O15 | 0.16 |
| 11.958 | 595.1661 | [M+H]+ | 541.2672,449.1087,287.0551, 249.1143,192.1003 | Kaempferol 3-O-rutinoside | C27H30O15 | −0.59 | |
| 61 | 12.029 | 431.098 | [M−H]− | 311.0510,283.0564,223.0922 | Apigenin 8-C-glucoside | C21H20O10 | 0.86 |
| 12.03 | 433.1136 | [M+H]+ | 313.0711,283.0605 | Apigenin 8-C-glucoside (Vitexin) | C21H20O10 | −1.56 | |
| 62 | 12.369 | 463.0877 | [M−H]− | 301.0287,151.0006 | Quercetin 3′-O-glucoside | C21H20O12 | 1.08 |
| 12.431 | 465.1031 | [M+H]+ | 303.0502,145.0496,85.0284 | Quercetin 3′-O-glucoside | C21H20O12 | −0.75 | |
| 63 | 12.642 | 625.1765 | [M+H]+ | 479.1185,317.0658,129.0541 | Isorhamnetin-3-O-b-D- rutinoside (Narcissoside) | C28H32O16 | −0.3 |
| 12.576 | 623.1618 | [M−H]− | 315.0456,271.0187,151.0355 | Isorhamnetin-3-O-b-D- rutinoside (Narcissoside) | C28H32O16 | −0.07 | |
| 64 | 13.463 | 447.0863 | [M−H]− | 285.0369,151.0001,137.0245, 59.0131 | Kaempferol 3-O-glucoside | C21H20O11 | 15.62 |
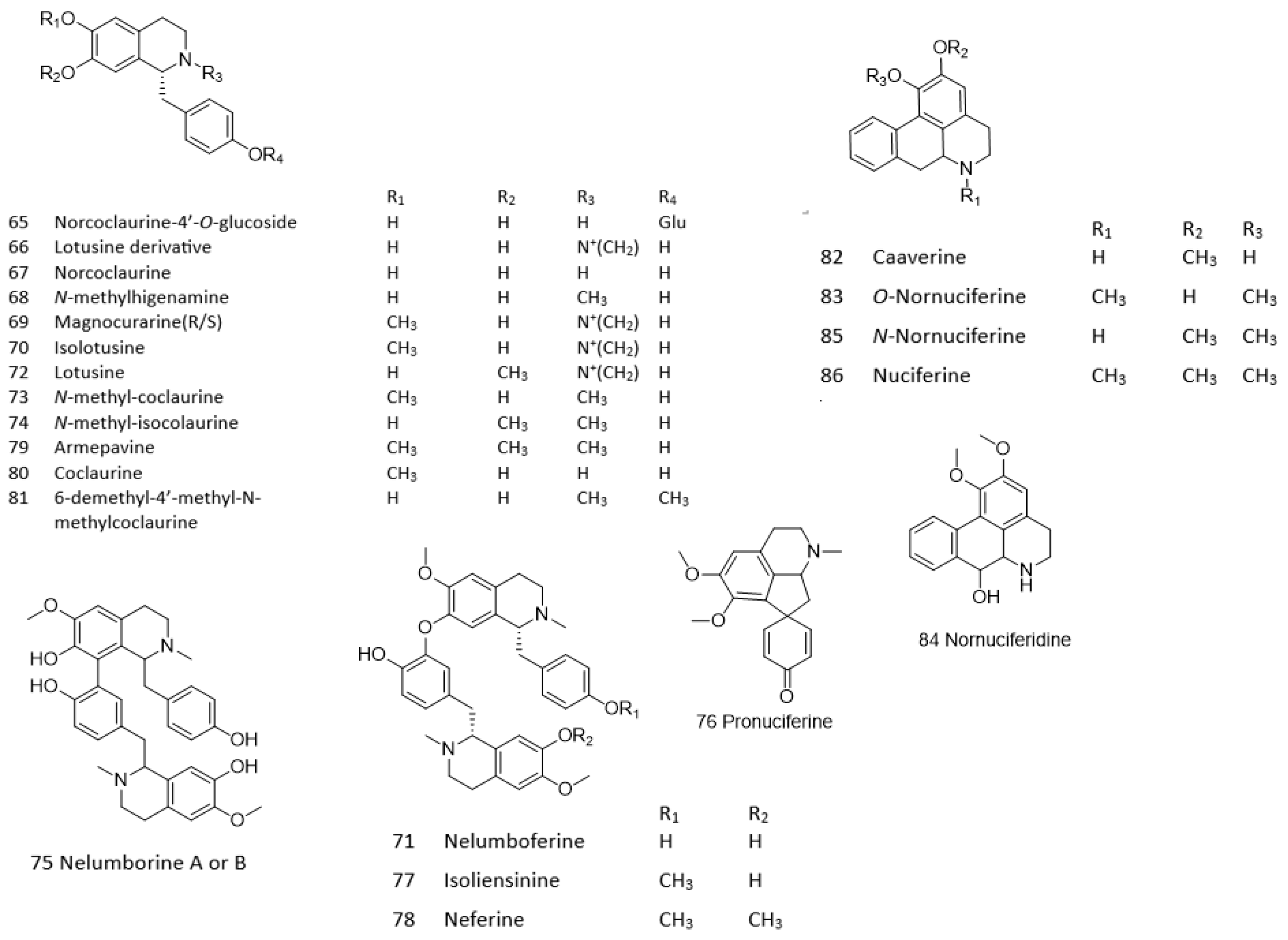
| Alkaloids | |||||||
| 65 | 6.614 | 432.1666 | [M−H]− | 270.1102,162.0540,96.9579 | Norcoclaurine-4′-O-glucoside | C12H27NO8 | −0.49 |
| 6.614 | 434.1816 | [M+H]+ | 272.1282,255.1025,194.1170, 161.0603,107.0494 | Norcoclaurine-4′-O-glucoside | C12H27NO8 | −1.51 | |
| 66 | 7.753 | 300.1603 | M+ | 255.1006,107.0493,58.0653 | Lotusine derivative | C18H21NO3 | −2.93 |
| 67 | 7.976 | 272.1289 | [M+H]+ | 255.1023,209.0968,161.0699, 107.0495 | Norcoclaurine | C16H17NO3 | −2.87 |
| 7.961 | 270.1151 | [M−H]− | 249.1187,162.0546,135.0425, 114.0439,59.0139 | Norcoclaurine | C16H17NO3 | −5.68 | |
| 68 | 8.082 | 284.1296 | [M−H]− | 219.0605,176.0699,107.0494 | N-methylhigenamine | C17H19NO3 | −1.35 |
| 8.111 | 286.144 | [M+H]+ | 255.1017,107.0493,58.0653 | N-methylhigenamine | C17H19NO3 | −0.8 | |
| 69 | 8.235 | 314.1745 | M+ | 269.1181,237.0914,175.0756, 107.0493,58.0654 | Magnocurarine | C19H24NO3+ | 1.81 |
| 70 | 8.409 | 314.1756 | M+ | 269.1180,237.0917,209.0945, 175.0757,145.0652,107.0494, 58.0653 | Isolotusine | C19H24NO3+ | −1.69 |
| 8.56 | 312.1624 | [M−H]− | 297.1327,239.0680,119.0490 | Isolotusine | C19H24NO3 | −6.03 | |
| 71 | 8.525 | 299.1526 | [M+2H]+ | 269.1181,237.0917,192.1025, 107.0495,58.0653 | Nelumboferine | C36H40N2O6 | −3.29 |
| 597.2969 | [M+H]+ | Nelumboferine | C36H40N2O6 | −3.83 | |||
| 72 | 8.573 | 312.1609 | [M−H]− | 297.1327,239.0680,163.0383, 119.0490,93.0341,59.0127 | Lotusine | C19H23NO3 | −1.23 |
| 8.625 | 314.1755 | M+ | 269.1177,237.0911,209.0966, 175.0759,107.0493,58.0654 | Lotusine | C19H23NO3 | −1.37 | |
| 73 | 9.135 | 300.1598 | [M+H]+ | 269.1178,237.0915,175.0758, 137.0599,107.0495,58.0653 | N-methyl-coclaurine | C18H21NO3 | −1.27 |
| 74 | 9.346 | 300.1597 | [M+H]+ | 269.1181,237.0918,209.0968, 175.0759,137.0594,107.0494, 77.0387,58.0652 | N-methyl-isococlaurine | C18H21NO3 | −0.93 |
| 75 | 9.431 | 299.1526 | [M+2H]+ | 269.1185,192.1023,137.0596, 107.0491,58.0647 | Nelumborine A or B | C36H40N2O6 | −3.29 |
| 597.2982 | [M+H]+ | 597.2982,405.1902,300.1586, 269.1168,192.1023,107.0500 | Nelumborine A or B | C36H40N2O6 | −3.83 | ||
| 76 | 9.533 | 312.1601 | [M+H]+ | 283.1346,269.1185,206.1173, 149.0226,107.0492,58.0652 | Pronuciferine | C19H21NO3 | 2.18 |
| 77 | 9.6 | 306.1602 | [M+2H]+ | 192.1025,158.0734,121.0653, 91.0546 | Isoliensinine | C37H42N2O6 | −2.32 |
| 611.3131 | [M+H]+ | 537.2224,475.2244,312.1597, 192.1027,121.0631,58.0652 | Isoliensinine | C37H42N2O6 | −2.51 | ||
| 78 | 10.275 | 313.1679 | [M+2H]+ | Neferine | C38H44N2O6 | −1.7 | |
| 625.3295 | [M+H]+ | 594.2868,582.2857,489.2401, 206.1178 | Neferine | C38H44N2O6 | −3.66 | ||
| 79 | 10.371 | 314.1752 | [M+H]+ | 283.1338,252.1163,107.0496, 77.0389,58.0652 | Armepavine | C19H23NO3 | −0.41 |
| 80 | 10.815 | 286.1444 | [M+H]+ | 269.1179,175.0754,143.0495, 107.0495 | Coclaurine | C17H19NO3 | −2.2 |
| 81 | 10.839 | 300.1601 | [M+H]+ | 269.1171,237.0912,192.1021, 163.0737,143.0490,107.0494, 77.0386,58.0652 | 6-demethyl-4′-methyl-N-methylcoclaurine | C18H21NO3 | −1.53 |
| 82 | 11.117 | 268.1339 | [M+H]+ | 251.1074,219.0808,191.0864, 149.0236 | Caaverine | C17H17NO2 | −2.59 |
| 83 | 11.394 | 282.1489 | [M+H]+ | 251.1490,219.0808,191.0859, 149.0234 | O-Nornuciferine | C18H19NO2 | −0.16 |
| 84 | 11.913 | 298.1447 | [M+H]+ | 251.1076,219.0806,191.0863, 121.0664,74.0957 | Nornuciferidine | C18H19NO3 | −3.12 |
| 85 | 12.925 | 282.1497 | [M+H]+ | 265.1228,250.0992,234.1041, 219.0815,149.0234,74.0965 | N-Nornuciferine | C18H19NO2 | −2.99 |
| 86 | 13.135 | 296.165 | [M+H]+ | 265.1229,250.0990,234.1040, 219.0802 | Nuciferine | C19H21NO2 | −1.67 |
| Lipid compounds | |||||||
| 87 | 17.585 | 327.2184 | [M−H]− | 211.1315,171.1008 | 9,12,13-Trihydroxy- 10(E),15(Z)-octadeca dienoic acid | C18H32O5 | −2.15 |
| 88 | 18.493 | 329.2336 | [M−H]− | 211.1317,171.1004,99.0807 | 9,10,13-Trihydroxy-11-octadecenoic acid | C18H34O5 | −0.77 |
| 89 | 19.193 | 298.2746 | [M+H]+ | 280.2632,250.2519 | Palmitoleoyl Ethanolamine | C18H35NO2 | −1.82 |
| 90 | 19.512 | 318.3011 | [M+H]+ | 282.2787,219.1741,60.0443 | Phytosphingosine | C18H39NO3 | −2.61 |
| 91 | 22.476 | 366.3375 | M+ | 307.2627,129.0546,81.0699 | Linoleoylcholine | C23H44NO2 | −0.81 |
| 92 | 23.33 | 342.3372 | M+ | 283.2632,95.0843 | N,N,N-trimethyl-sphingosine | C21H44NO2 | 0.01 |
| 93 | 23.448 | 711.3362 | [M+Cl]− | 595.2801,397.1294,277.2134 | 3′-O-Linolenoylglyceryl 6-O-galactopyranosyl-galactopyranoside | C33H56O14 | 0.29 |
| 94 | 25.432 | 478.2938 | M+ | 337.2737,263.2370,184.0728, 81.0699 | 1-Linoleoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine | C23H44NO7P | −2.06 |
| 25.945 | 476.2768 | [M−H]− | 279.2280,196.0340,140.0087, 78.9580 | LysoPE(0:0/18:2(9Z,12Z)) | C23H44NO7P | 3.07 | |
| 95 | 26.421 | 554.3006 | [M+Cl]− | 504.3001,433.2276,279.2274, 78.9569 | 1-Linoleoyl-glycero-3-phosphocholine | C26H50NO7P | 2.33 |
| 26.489 | 520.3407 | M+ | 337.2734,258.1098,184.0734, 86.0964 | 1-Linoleoyl-glycero-3-phosphocholine | C26H51NO7P | −0.74 | |
| 96 | 26.594 | 689.3499 | [M+Cl]− | 653.3615,397.1276,279.2263, 255.2270,179.0541 | 1-palmitoyl-2-azeloyl-sn-glycero-3-phospho-(1′-sn-glycerol) | C31H59O12P | −8.83 |
| 97 | 27.112 | 554.3009 | [M+Cl]− | 504.2997,279.2281,168.0394, 78.9575 | 1-(2E,4E-octadecadienoyl)-sn-glycero-3-phosphocholine | C26H50NO7P | 2.33 |
| 27.156 | 520.3405 | M+ | 443.2518,337.2736,258.1096, 184.0735,104.1068 | 1-(2E,4E-octadecadienoyl)-sn-glycero-3-phosphocholine | C26H51NO7P | −0.36 | |
| 98 | 27.565 | 452.2767 | [M−H]− | 255.2281,196.0345,140.0090, 78.9579 | 2-Palmitoyl-sn-glycero-3-phosphoethanolamine | C21H44NO7P | 3.46 |
| 27.613 | 454.2934 | M+ | 436.2824,393.2403,313.2741, 155.0098,62.0600 | 2-Palmitoyl-sn-glycero-3-phosphoethanolamine | C21H44NO7P | −1.29 | |
| 99 | 28.532 | 496.3408 | [M+H]+ | 184.0735,104.1068 | 1-tetradecyl-2-acetyl-sn- glycero-3-phosphocholine | C24H50NO7P | −2.08 |
| 100 | 28.571 | 478.2928 | [M−H]− | 281.2428,152.9916,78.9582 | 1-Oleoyl-2-hydroxy-sn-glycero-3-PE | C23H46NO7P | 2.33 |
| 28.629 | 480.3091 | [M+H]+ | 419.2544,339.2893,184.0736 | 1-Oleoyl-2-hydroxy-sn-glycero-3-PE | C23H46NO7P | −1.32 | |
| 101 | 28.64 | 551.2976 | [M+Cl]− | 515.3118,279.2281,152.9920 | 3′-O-Linoleoyglyceryl-6-O-galactopyranoside | C27H48O9 | 2.96 |
| 102 | 28.701 | 615.3032 | [M+Cl]− | 579.3273,341.1049,255.2275, 179.0542 | 1-palmitoyl-2-azeloyl-sn-glycero-3-phosphate | C28H53O10P | 6.23 |
| 103 | 29.58 | 496.3405 | [M+H]+ | 419.2551,184.0736,104.1069 | 2-Palmitoyl-sn-glycero-3-phosphocholine | C24H50NO7P | −1.48 |
| 29.725 | 530.3014 | [M+Cl]− | 480.3013,409.2263,339.3201, 255.2289,168.0405,78.9566 | 2-Palmitoyl-sn-glycero-3-phosphocholine | C24H50NO7P | 0.93 | |
| 104 | 31.798 | 366.3001 | [M+NH4]+ | 261.2201,86.0599 | 2-hydroxy-6-pentadecyl benzoic acid | C22H36O3 | 0.47 |
| 105 | 31.823 | 358.2518 | [M+Cl]− | Linoleoyl ethanolamide | C20H37NO2 | 0.09 | |
| 31.856 | 324.2896 | [M+H]+ | 245.2240,62.0598 | Linoleoyl ethanolamide | C20H37NO2 | 0.33 | |
| 106 | 32.266 | 403.2329 | [M+H]+ | 365.1930,259.1541,185.0808, 129.0180,61.0282 | 5S-HETE di-endoperoxide | C20H34O8 | −0.63 |
| 107 | 33.635 | 389.2463 | [M+Cl]− | 279.2268,152.9958 | 2,3-dihydroxypropyl (9Z,12Z)-octadeca-9,12-dienoate | C21H38O4 | 0.29 |
| 108 | 33.635 | 699.5029 | [M−H]− | 437.2563,279.2274,181.0258 | Octadecanoyl-(9Z,12Z-octadecadienoyl)-sn-glycero-3-phosphate | C39H73O8P | −8.39 |
| 109 | 33.649 | 280.2631 | [M+H]+ | 263.237,245.2265,198.1855, 149.0229,95.0833,81.0699, 69.0698 | Linoleamide | C18H33NO | 1.4 |
| 110 | 35.189 | 256.2641 | [M+H]+ | 116.1070,102.0913,88.0757 | Palmitamide | C16H33NO | −2.38 |
| 111 | 35.242 | 699.4941 | [M−H]− | 437.2562,279.2280,255.2289, 134.8921 | 1-(9Z,12Z-octadecadienoyl)-2-octadecanoyl-glycero-3-phosphate | C39H73O8P | 4.19 |
| 112 | 35.734 | 282.2805 | [M+H]+ | 256.2634,97.1010,83.0856 69.0699,55.0541 | Oleamide | C18H35NO | −4.81 |
| 113 | 36.239 | 282.2812 | [M+H]+ | 83.0855,69.0698,55.0542 | Elaidamide | C18H35NO | −7.29 |
| 114 | 39.9 | 284.2955 | [M+H]+ | 130.1220,102.0914,88.0754 | Steramide | C18H37NO | −2.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatturong, U.; Nuengchamnong, N.; Inchan, A.; To-On, K.; Bualeong, T.; Sumsakul, W.; Atipimonpat, A.; Meekarn, K.; Shigeta, Y.; Hengphasatporn, K.; et al. Vasorelaxant and Hypotensive Mechanisms of Nelumbo nucifera Seed Extract: Roles of Nitric Oxide, Calcium Channel Blockade and eNOS Interaction with Active Compounds. Pharmaceuticals 2025, 18, 1500. https://doi.org/10.3390/ph18101500
Chatturong U, Nuengchamnong N, Inchan A, To-On K, Bualeong T, Sumsakul W, Atipimonpat A, Meekarn K, Shigeta Y, Hengphasatporn K, et al. Vasorelaxant and Hypotensive Mechanisms of Nelumbo nucifera Seed Extract: Roles of Nitric Oxide, Calcium Channel Blockade and eNOS Interaction with Active Compounds. Pharmaceuticals. 2025; 18(10):1500. https://doi.org/10.3390/ph18101500
Chicago/Turabian StyleChatturong, Usana, Nitra Nuengchamnong, Anjaree Inchan, Kittiwoot To-On, Tippaporn Bualeong, Wiriyaporn Sumsakul, Anyapat Atipimonpat, Kittiphum Meekarn, Yasuteru Shigeta, Kowit Hengphasatporn, and et al. 2025. "Vasorelaxant and Hypotensive Mechanisms of Nelumbo nucifera Seed Extract: Roles of Nitric Oxide, Calcium Channel Blockade and eNOS Interaction with Active Compounds" Pharmaceuticals 18, no. 10: 1500. https://doi.org/10.3390/ph18101500
APA StyleChatturong, U., Nuengchamnong, N., Inchan, A., To-On, K., Bualeong, T., Sumsakul, W., Atipimonpat, A., Meekarn, K., Shigeta, Y., Hengphasatporn, K., Kumphune, S., & Chootip, K. (2025). Vasorelaxant and Hypotensive Mechanisms of Nelumbo nucifera Seed Extract: Roles of Nitric Oxide, Calcium Channel Blockade and eNOS Interaction with Active Compounds. Pharmaceuticals, 18(10), 1500. https://doi.org/10.3390/ph18101500









