Folic Acid as a Molecule Protecting Cells from the Negative Effects of Ultraviolet Radiation—An In Vitro Study
Abstract
1. Introduction
2. Results
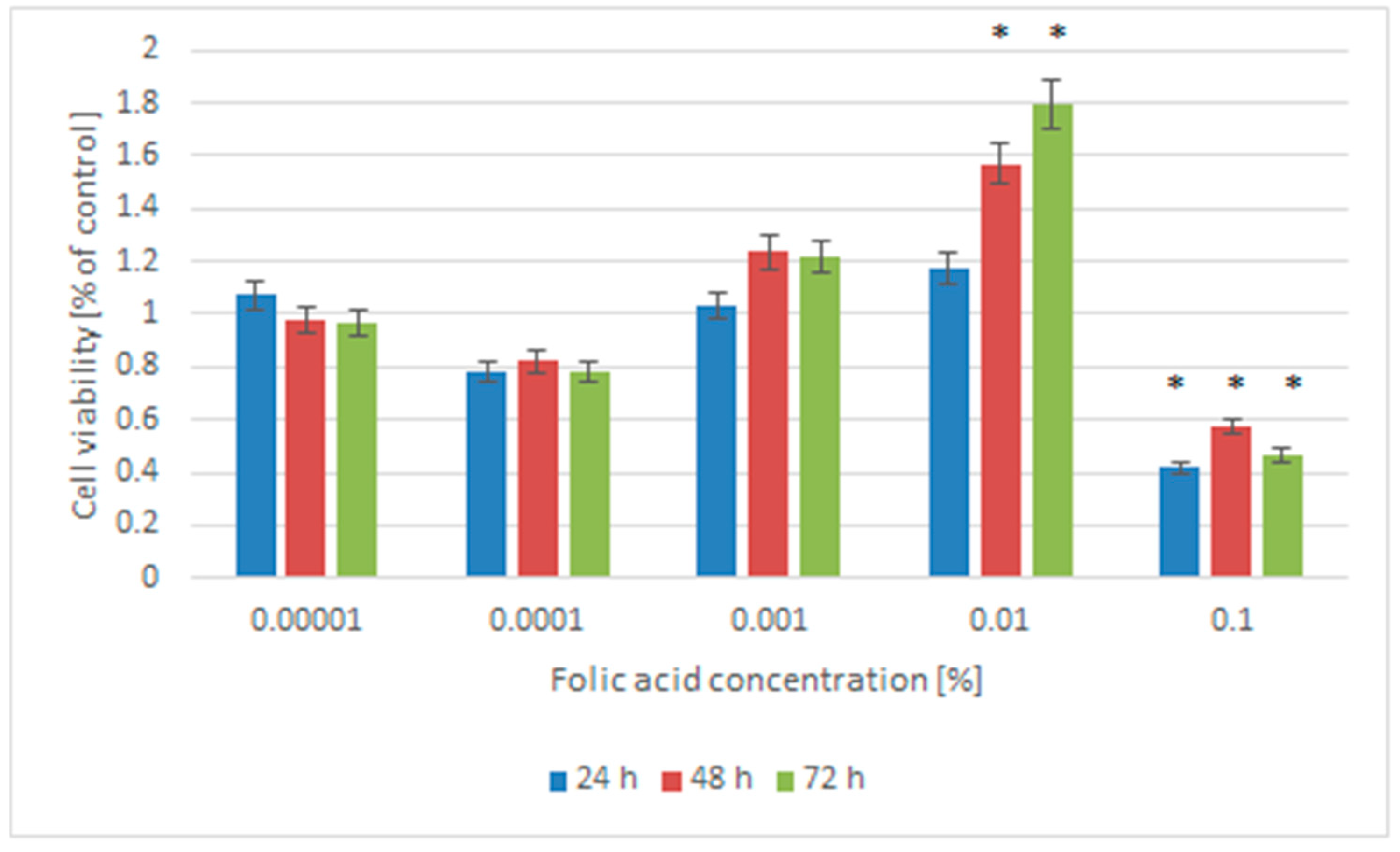
2.1. Cell Viability
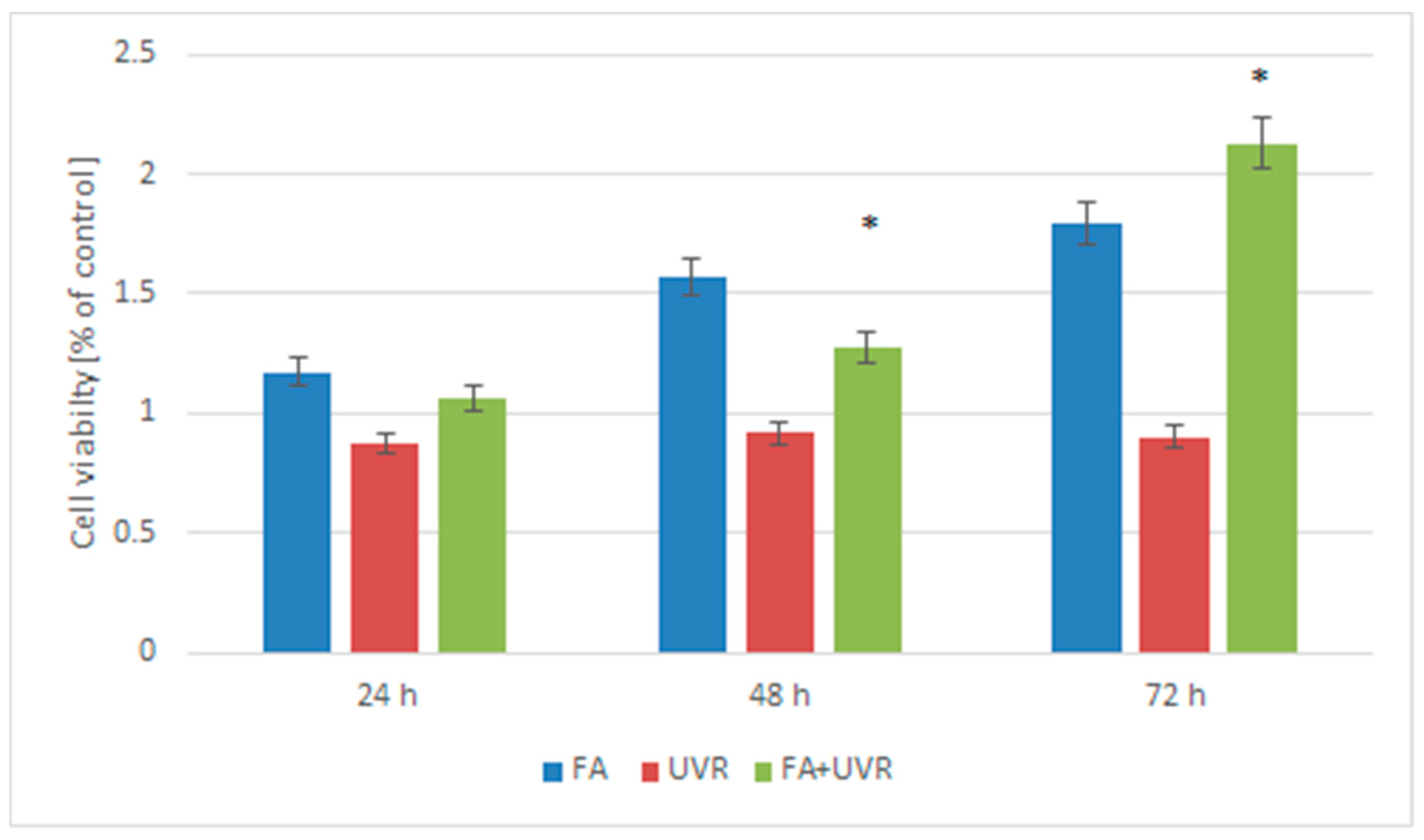
2.2. Viability Measurement of Cells Treated by Folic Acid and UV Irradiation
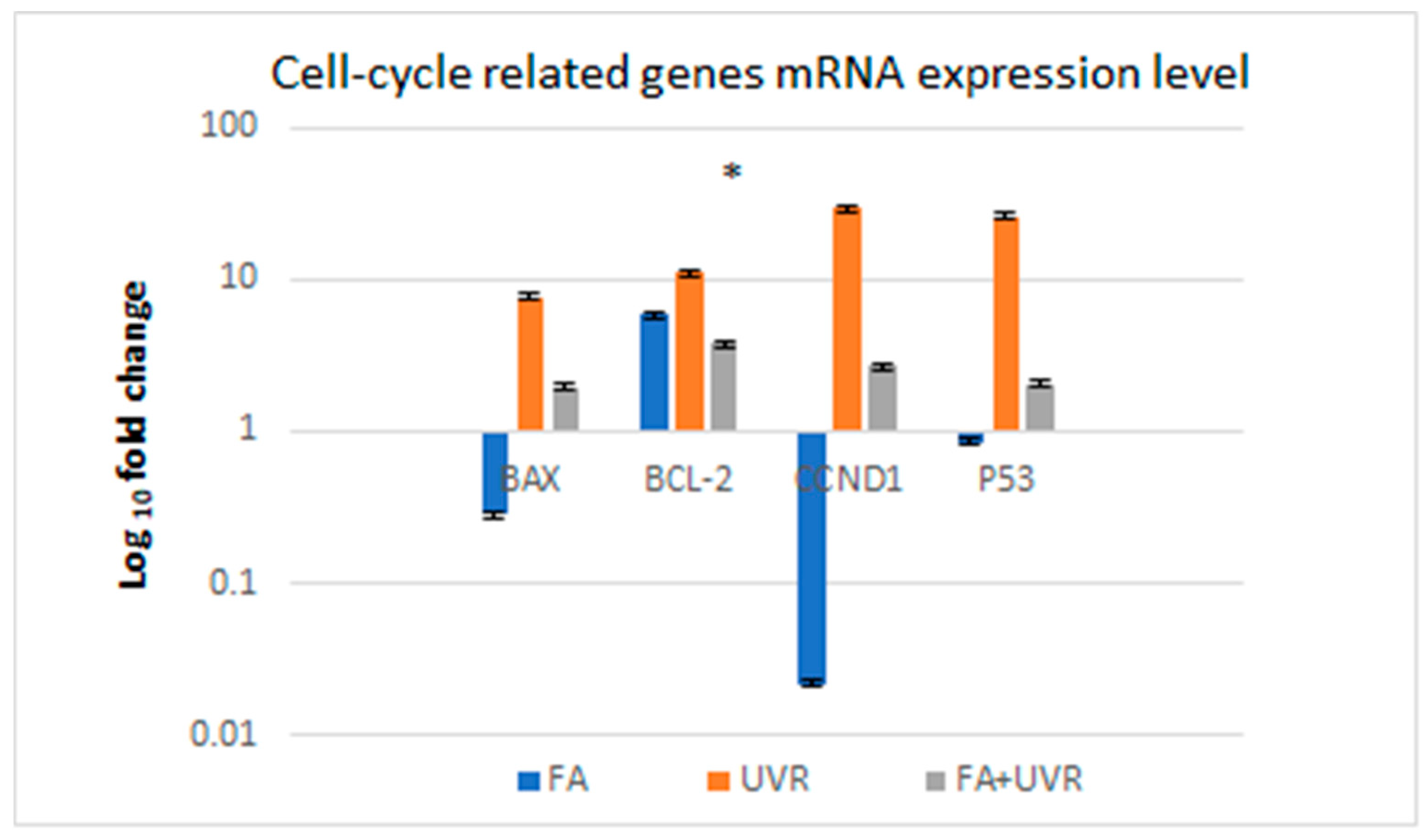
2.3. Influence of UV Radiation on the mRNA Expression of Selected Genes That Regulate the Cell Cycle BAX, BCL-2, CCND1, and P53 in Cells Protected by Folic Acid
2.4. The Effect of UV Radiation on the Interaction Between Folic Acid and Free Radicals Using Electron Paramagnetic Resonance (EPR)
3. Discussion
4. Materials and Methods
4.1. Cell Source and Culture
4.2. Folic Acid (FA)
4.3. Cell Viability Measurement
4.4. Viability Measurement of Cells Treated by Folic Acid and UV Irradiation
4.5. Influence of UV Radiation on the mRNA Expression of Selected Genes That Regulate the Cell Cycle CCND1, P53, BAX, and BCL-2 in Cells Protected by Folic Acid
4.6. Measurement of the Effect of UV Radiation on the Interaction Between Folic Acid and Free Radicals Using Electron Paramagnetic Resonance (EPR)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Balashova, O.A.; Visina, O.; Borodinsky, L.N. Folate action in nervous system development and disease. Dev. Neurobiol. 2018, 78, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Cieślik, E.; Cieślik, I. Occurrence and Significance of Folic Acid. Pteridines 2018, 29, 187–195. [Google Scholar] [CrossRef]
- Kurowska, K.; Kobylinska, M.; Antosik, K. Folic acid-Importane for human health and its role in COVID-19 therapy. Rocz. Państwowego Zakładu Hig. 2023, 74, 131–141. [Google Scholar]
- Witthöft, C.M.; Forssén, K.; Johannesson, L.; Jägerstad, M. Folates—Food sources, analyses, retention and bioavailability. Food Nutr. Res. 1999, 43, 138–146. [Google Scholar] [CrossRef]
- Strickland, K.C.; Krupenko, N.I.; Krupenko, S.A. Molecular mechanisms underlying the potentially adverse effects of folate. Clin. Chem. Lab. Med. 2013, 51, 607–616. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stanger, O. Physiology of folic acid in health and disease. Curr. Drug Metab. 2002, 3, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Crider, K.S.; Yang, T.P.; Berry, R.J.; Bailey, L.B. Folate and DNA methylation: A review of molecular mechanisms and the evidence for folate’s role. Adv. Nutr. 2012, 3, 21–38. [Google Scholar] [CrossRef]
- Safi, J.; Joyeux, L.; Chalouhi, G.E. Periconceptional folate deficiency and implications in neural tube defects. J. Pregnancy 2012, 2012, 295083. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fardous, A.M.; Heydari, A.R. Uncovering the Hidden Dangers and Molecular Mechanisms of Excess Folate: A Narrative Review. Nutrients 2023, 15, 4699. [Google Scholar] [CrossRef]
- Dębowska, R.; Rogiewicz, K.; Iwanenko, T.; Kruszewski, M.; Eris, I. Folic Acid (Folacin)–New application of a cosmetic ingredient. Kosmet. Med. 2005, 3, 16–22. [Google Scholar]
- Eris, I.; Dębowska, R. Kwas foliowy (folacyna) w preparatach do pielęgnacji twarzy—Ocena działania witaminy na komórki skóry w badaniach in vitro. Dermatologica 2003, 6, 13–18. [Google Scholar]
- Rabe, J.H.; Mamelak, A.J.; McElgunn, P.J.; Morison, W.L.; Sauder, D.N. Photoaging: Mechanisms and repair. J. Am. Acad. Dermatol. 2006, 55, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bickers, D.R.; Athar, M. Oxidative Stress in the Pathogenesis of Skin Disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef]
- Ittycheri, A.; Lipsky, Z.W.; Hookway, T.A.; German, G.K. Ultraviolet light induces mechanical and structural changes in full thickness human skin. J. Mech. Behav. Biomed. Mater. 2023, 143, 105880. [Google Scholar] [CrossRef] [PubMed]
- Svobodova, A.; Walterova, D.; Vostalova, J. Ultraviolet Light Induced Alteration to the Skin. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2006, 150, 25–38. [Google Scholar] [CrossRef]
- Palmer, D.M.; Kitchin, J.S. Oxidative damage, skin aging, antioxidants and a novel antioxidant rating system. J. Drugs Dermatol. 2010, 9, 11–15. [Google Scholar]
- Pinnell, S.R. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J. Am. Acad. Dermatol. 2003, 48, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Borradale, D.C.; Kimlin, M.G. Folate degradation due to ultraviolet radiation: Possible implications for human health and nutrition. Nutr. Rev. 2012, 70, 414–422. [Google Scholar] [CrossRef]
- Hirakawa, K.; Suzuki, H.; Oikawa, S.; Kawanishi, S. Sequence-specific DNA damage induced by ultraviolet A-irradiated folic acid via its photolysis product. Arch. Biochem. Biophys. 2003, 410, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.J.; Khan, M.A.; Ahmad, I. Identification of photoproducts of folic acid and its degradation pathways in aqueous solution. J. Pharm. Biomed. Anal. 2003, 31, 579–588. [Google Scholar] [CrossRef]
- Thomas, A.H.; Suarez, G.; Cabrerizo, F.M.; Martino, R.; Capparelli, A.L. Study of the photolysis of folic acid and 6-formylpterin in acid aqueous solutions. J. Photochem. Photobiol. 2000, 135, 147–154. [Google Scholar] [CrossRef]
- Off, M.K.; Steindal, A.E.; Porojnicu, A.C.; Juzeniene, A.; Vorobey, A.; Johnsson, A.; Moan, J. Ultraviolet photodegradation of folic acid. J. Photochem. Photobiol. B 2005, 80, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Goossens, J.F.; Thuru, X.; Bailly, C. Properties and reactivity of the folic acid and folate photoproduct 6-formylpterin. Free Radic. Biol. Med. 2021, 171, 1–10. [Google Scholar] [CrossRef]
- Carvalho, C.; Silva, R.; Melo, T.M.V.D.P.e.; Inga, A.; Saraiva, L. P53 and the Ultraviolet Radiation-Induced Skin Response: Finding the Light in the Darkness of Triggered Carcinogenesis. Cancers 2024, 16, 3978. [Google Scholar] [CrossRef]
- Wiman, K.G.; Zhivotovsky, B. Understanding cell cycle and cell death regulation provides novel weapons against human diseases. J. Intern. Med. 2017, 281, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, C.L.; Ullrich, S.E.; Kripke, M.L.; Ananthaswamy, H.N. P53 Tumor Suppressor Gene: A Critical Molecular Target for UV Induction and Prevention of Skin Cancer. Photochem. Photobiol. 2008, 84, 55–62. [Google Scholar] [CrossRef]
- Feroz, W.; Sheikh, A.M.A. Exploring the multiple roles of guardian of the genome: P53. Egypt. J. Med. Hum. Genet. 2020, 21, 49. [Google Scholar] [CrossRef]
- Kulsoom, B.; Shamsi, T.S.; Afsar, N.A.; Memon, Z.; Ahmed, N.; Hasnain, S.N. Bax, Bcl-2, and Bax/Bcl-2 as prognostic markers in acute myeloid leukemia: Are we ready for Bcl-2-directed therapy? Cancer Manag. Res. 2018, 10, 403–416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Georgakopoulou, E.; Evangelou, K.; Havaki, S.; Townsend, P.; Kanavaros, P.; Gorgoulis, V.G. Apoptosis or senescence? Which exit route do epithelial cells and fibroblasts preferentially follow? Mech. Ageing Dev. 2016, 156, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Solaki, M.; Ewald, J.C. Fueling the Cycle: CDKs in Carbon and Energy Metabolism. Front. Cell Dev. Biol. 2018, 6, 93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fajas, L. Re-thinking cell cycle regulators: The cross-talk with metabolism. Front. Oncol. 2013, 3, 4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alao, J.P.; Gamble, S.C.; Stavropoulou, A.V.; Pomeranz, K.M.; Lam, E.; Coombes, R.C.; Vigushin, D.M. The cyclin D1 proto-oncogene is sequestered in the cytoplasm of mammalian cancer cell lines. Mol. Cancer 2006, 5, 7. [Google Scholar] [CrossRef]
- Hao, Q.; Chen, J.; Lu, H.; Zhou, X.; Yao, X. The ARTS of P53-dependent mitochondrial apoptosis. J. Mol. Cell Biol. 2022, 14, mjac074. [Google Scholar] [CrossRef]
- Yang, K.; Hitomi, M.; Stacey, D.W. Variations in cyclin D1 levels through the cell cycle determine the proliferative fate of a cell. Cell Div. 2006, 1, 32. [Google Scholar] [CrossRef]
- Smith, J.; Rai, V. Novel Factors Regulating Proliferation, Migration, and Differentiation of Fibroblasts, Keratinocytes, and Vascular Smooth Muscle Cells during Wound Healing. Biomedicines 2024, 12, 1939. [Google Scholar] [CrossRef] [PubMed]
- Pondeljak, N.; Lugović-Mihić, L.; Tomić, L.; Parać, E.; Pedić, L.; Lazić-Mosler, E. Key Factors in the Complex and Coordinated Network of Skin Keratinization: Their Significance and Involvement in Common Skin Conditions. Int. J. Mol. Sci. 2023, 25, 236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moltrasio, C.; Romagnuolo, M.; Marzano, A.V. Epigenetic Mechanisms of Epidermal Differentiation. Int. J. Mol. Sci. 2022, 23, 4874. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eckert, R.L.; Efimova, T.; Dashti, S.R.; Balasubramanian, S.; Deucher, A.; Crish, J.F.; Sturniolo, M.; Bone, F. Keratinocyte survival, differentiation, and death: Many roads lead to mitogen-activated protein kinase. J. Investig. Dermatol. Symp. Proc. 2002, 7, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Eckhart, L.; Declercq, W.; Ban, J.; Rendl, M.; Lengauer, B.; Mayer, C.; Lippens, S.; Vandenabeele, P.; Tschachler, E. Terminal differentiation of human keratinocytes and stratum corneum formation is associated with caspase-14 activation. J. Investig. Dermatol. 2000, 115, 1148–1151. [Google Scholar] [CrossRef] [PubMed]
- Eckhart, L.; Lippens, S.; Tschachler, E.; Declercq, W. Cell death by cornification. Biochim. Biophys. Acta. 2013, 1833, 3471–3480. [Google Scholar] [CrossRef] [PubMed]
- Bito, T.; Nishigori, C. Impact of reactive oxygen species on keratinocyte signaling pathways. J. Dermatol. Sci. 2012, 68, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Errico, M.; Lemma, T.; Calcagnile, A.; Proietti De Santis, L.; Dogliotti, E. Cell type and DNA damage specific response of human skin cells to environmental agents. Mutat. Res. 2007, 614, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Badr-Eldin, S.M.; Aldawsari, H.M.; Kotta, S.; Deb, P.K.; Venugopala, K.N. Three-Dimensional In Vitro Cell Culture Models for Efficient Drug Discovery: Progress So Far and Future Prospects. Pharmaceuticals 2022, 15, 926. [Google Scholar] [CrossRef]
- Bissell, M.J.; Rizki, A.; Mian, I.S. Tissue architecture: The ultimate regulator of breast epithelial function. Curr. Opin. Cell Biol. 2003, 15, 753. [Google Scholar] [CrossRef]
- Riabinin, A.; Pankratova, M.; Rogovaya, O.; Vorotelyak, E.; Terskikh, V.; Vasiliev, A. Ideal Living Skin Equivalents, from Old Technologies and Models to Advanced Ones: The Prospects for an Integrated Approach. BioMed Res. Int. 2024, 2024, 9947692. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ncbi.nlm.nih.gov/books/NBK304366/ (accessed on 28 September 2025).
- Tang, X.; Yang, T.; Yu, D.; Xiong, H.; Zhang, S. Current insights and future perspectives of ultraviolet radiation (UV) exposure: Friends and foes to the skin and beyond the skin. Environ. Int. 2024, 185, 108535. [Google Scholar] [CrossRef] [PubMed]
- Battie, C.; Verschoore, M. Cutaneous solar ultraviolet exposure and clinical aspects of photodamage. Indian J. Dermatol. Venereol. Leprol. 2012, 78 (Suppl. 1), S9–S14. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.E.; Kennelley, G.E.; Amaye-Obu, T.; Jowdy, P.F.; Ghadersohi, S.; Nasir-Moin, M.; Paragh, G.; Berman, H.A.; Huss, W.J. The phenomenon of phototoxicity and long-term risks of commonly prescribed and structurally diverse drugs. J. Photochem. Photobiol. 2024, 19, 100221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Basu-Modak, S.; Tyrrell, R.M. Modulation of gene expression by solar ultraviolet radiation. In Comprehensive Series in Photosciences; Giacomoni, P.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Li, Q.; Qian, W.; Zhang, Y.; Hu, L.; Chen, S.; Xia, Y. A new wave of innovations within the DNA damage response. Signal Transduct. Target. Ther. 2023, 8, 338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Heemst, D.; den Reijer, P.M.; Westendorp, R.G. Ageing or cancer: A review on the role of caretakers and gatekeepers. Eur. J. Cancer. 2007, 43, 2144–2152. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Marciniak, B.; Mojzych, M.; Kontek, R. Focus on UV-Induced DNA Damage and Repair—Disease Relevance and Protective Strategies. Int. J. Mol. Sci. 2020, 21, 7264. [Google Scholar] [CrossRef]
- Averill-Bates, D. Reactive oxygen species and cell signaling. Review. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119573. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://dermnetnz.org/cme/phototherapy/uva-photochemotherapy (accessed on 26 September 2025).
- Young, A.R. Biophysical and Physiological Effects of Solar Radiation on Human Skin; Giacomoni, P.U., Jori, G., Hader, D., Eds.; The Royal Society of Chemistry: London, UK, 2007; Volume 10, Chapter 1; pp. 3–23. [Google Scholar]
- Godar, D.E. UV doses worldwide. Photochem. Photobiol. 2005, 81, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://ec.europa.eu/health/scientific_committees/scheer/docs/scheer_o_003.pdf (accessed on 26 September 2025).
- Available online: https://www.ncbi.nlm.nih.gov/books/NBK321117/ (accessed on 26 September 2025).
- Tanveer, M.A.; Rashid, H.; Tasduq, S.A. Molecular basis of skin photoaging and therapeutic interventions by plant-derived natural product ingredients: A comprehensive review. Heliyon 2023, 9, e13580. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Young, O.; Ngo, N.; Lin, L.; Stanbery, L.; Creeden, J.F.; Hamouda, D.; Nemunaitis, J. Folate Receptor as a Biomarker and Therapeutic Target in Solid Tumors. Curr. Probl. Cancer. 2023, 47, 100917. [Google Scholar] [CrossRef] [PubMed]
- Elnakat, H.; Ratnam, M. Distribution, functionality and gene regulation of folate receptor isoforms: Implications in targeted therapy. Adv. Drug Deliv. Rev. 2004, 56, 1067–1084. [Google Scholar] [CrossRef]
- Salazar, M.D.; Ratnam, M. The folate receptor: What does it promise in tissue-targeted therapeutics? Cancer Metastasis Rev. 2007, 26, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Scaranti, M.; Cojocaru, E.; Banerjee, S.; Banerji, U. Exploiting the folate receptor α in oncology. Nat. Rev. Clin. Oncol. 2020, 17, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.S. Folate Receptor-Targeted Diagnostics and Therapeutics for Inflammatory Diseases. Immune Netw. 2016, 16, 337–343. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hsueh, M.F.; Lu, Y.; Wheeler, L.; Wellman, S.S.; Bolognesi, M.P.; Kraus, V.B. Functional folate receptor cells within synovium and fluid as therapeutic targets for osteoarthritis. Osteoarthr. Cartil. 2017, 25, S42–S43. [Google Scholar] [CrossRef]
- Halik, P.K.; Koźmiński, P.; Gniazdowska, E. Perspectives of Methotrexate-Based Radioagents for Application in Nuclear Medicine. Mol. Pharm. 2021, 18, 33–43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, W.D.; Pirona, A.C.; Sarvin, B.; Stern, A.; Nevo-Dinur, K.; Besser, E.; Sarvin, N.; Lagziel, S.; Mukha, D.; Raz, S.; et al. Tumor Reliance on Cytosolic versus Mitochondrial One-Carbon Flux Depends on Folate Availability. Cell Metab. 2021, 33, 190–198.e6. [Google Scholar] [CrossRef] [PubMed]
- Valerio, H.P.; Ravagnani, F.G.; Ronsein, G.E.; Di Mascio, P. A Single Dose of Ultraviolet-A Induces Proteome Remodeling and Senescence in Primary Human Keratinocytes. Sci. Rep. 2021, 11, 23355. [Google Scholar] [CrossRef]
- Yang, T.T.; Lan, C.E. Photocarcinogenesis of the skin: Current status and future trends. Kaohsiung J. Med. Sci. 2025, 41, e12946. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rastogi, R.P.; Richa Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucl. Acids. 2010, 2010, 592980. [Google Scholar] [CrossRef]
- Cadet, J.; Grand, A.; Douki, T. Solar UV radiation-induced DNA bipyrimidine photoproducts: Formation and mechanistic insights. Top. Curr. Chem. 2015, 356, 249–275. [Google Scholar] [PubMed]
- Wong, H.Y.; Lee, R.; Chong, S.; Kapadia, S.; Freeman, M.; Murigneux, V.; Brown, S.; Soyer, H.P.; Roy, E.; Khosrotehrani, K.; et al. Epidermal mutation accumulation in photodamaged skin is associated with skin cancer burden and can be targeted through ablative therapy. Sci. Adv. 2023, 9, eadf2384. [Google Scholar] [CrossRef] [PubMed]
- Rosette, C.; Karin, M. Ultraviolet Light and Osmotic Stress: Activation of the JNK Cascade Through Multiple Growth Factor and Cytokine Receptors. Science 1996, 274, 1194–1197. [Google Scholar] [CrossRef]
- Rehemtulla, A.; Hamilton, C.A.; Chinnaiyan, A.M.; Dixit, V.M. Ultraviolet radiation-induced apoptosis is mediated by activation of CD-95 (Fas/APO-1). J. Biol. Chem. 1997, 272, 25783–25786. [Google Scholar] [CrossRef] [PubMed]
- Batista, L.F.Z.; Kaina, B.; Meneghini, R.; Menck, C.F.M. How DNA lesions are turned into powerful killing structures: Insights from UV-induced apoptosis. Mutat. Res. 2009, 681, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Wu, S.B.; Hong, C.H.; Yu, H.S.; Wei, Y.H. Molecular Mechanisms of UV-Induced Apoptosis and Its Effects on Skin Residential Cells: The Implication in UV-Based Phototherapy. Int. J. Mol. Sci. 2013, 14, 6414–6435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Rosenstein, B.; Goldwyn, S.; Zhang, X.; Lebwohl, M.; Wei, H. Differential regulation of P53 and Bcl-2 expression by ultraviolet A and B. J. Investig. Dermatol. 1998, 111, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Susnow, N.; Zeng, L.; Margineantu, D.; Hockenbery, D.M. Bcl-2 family proteins as regulators of oxidative stress. Semin. Cancer Biol. 2009, 19, 42–49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Longoni, B.; Boschi, E.; Demontis, G.C.; Marchiafava, P.L.; Mosca, F. Regulation of Bcl-2 protein expression during oxidative stress in neuronal and in endothelial cells. Biochem. Biophys. Res. Commun. 1999, 260, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Su, J.H.; Ivins, K.J.; Van Houten, B.; Cotman, C.W. Bcl-2 facilitates recovery from DNA damage after oxidative stress. Exp. Neurol. 1999, 159, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Kowaltowski, A.J.; Fenton, R.G.; Fiskum, G. Bcl-2 family proteins regulate mitochondrial reactive oxygen production and protect against oxidative stress. Free Radic. Biol. Med. 2004, 37, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Rieger, K.E.; Chu, G. Portrait of transcriptional responses to ultraviolet and ionizing radiation in human cells. Nucleic Acids Res. 2004, 32, 4786–4803. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alamro, A.A.; Al-Malky, M.M.; Ansari, M.G.A.; Amer, O.E.; Alnaami, A.M.; Hussain, S.D.; Barhoumi, T.A.; Alghamdi, A.A.; Haq, S.H.; Sabico, S.; et al. The effects of melatonin and vitamin D3 on the gene expression of Bcl-2 and BAX in MCF-7 breast cancer cell line. J. King Saud. Univ. Sci. 2021, 33, 101287. [Google Scholar] [CrossRef]
- Montalto, F.I.; De Amicis, F. Cyclin D1 in Cancer: A Molecular Connection for Cell Cycle Control, Adhesion and Invasion in Tumor and Stroma. Cells 2020, 9, 2648. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balagula, Y.; Kang, S.; Patel, M.J. Synergism between mTOR pathway and ultraviolet radiation in the pathogenesis of squamous cell carcinoma and its implication for solid-organ transplant recipients. Photodermatol. Photoimmunol. Photomed. 2015, 31, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.cdc.gov/radiation-health/features/uv-radiation.html (accessed on 26 September 2025).
- Available online: https://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_oc03_019.pdf (accessed on 26 September 2025).
- Ishii, H.; Arai, T.; Mori, H.; Yamada, H.; Endo, N.; Makino, K.; Fukuda, K. Protective effects of intracellular reactive oxygen species generated by 6-formylpterin on tumor necrosis factor-alpha-induced apoptotic cell injury in cultured rat hepatocytes. Life Sci. 2005, 77, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, T.; Miyata, H.; Imoto, T.; Arai, T.; Endo, N.; Makino, K.; Yang, C.H.; Ohama, E. 6-Formylpterin protects retinal neurons from transient ischemia-reperfusion injury in rats: A morphological and immunohistochemical study. Neuropathology 2003, 23, 161–168. [Google Scholar] [CrossRef]
- Kim, H.J.; Jin, S.P.; Kang, J.; Bae, S.H.; Son, J.B.; Oh, J.H.; Youn, H.; Kim, S.K.; Kang, K.W.; Chung, J.H. Uncovering the impact of UV radiation on mitochondria in dermal cells: A STED nanoscopy study. Sci. Rep. 2024, 14, 8675. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sabetghadam Moghadam, M.; Wiens, E.; Gauvrit, S.; Sammynaiken, R.; Collins, M.M. Electron paramagnetic resonance spectroscopy for analysis of free radicals in zebrafish. PLoS ONE 2025, 20, e0318212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakanishi, I.; Shoji, Y.; Ohkubo, K.; Ito, H.; Fukuzumi, S. Water-Induced Regeneration of a 2,2-Diphenyl-1-picrylhydrazyl Radical after Its Scandium Ion-Promoted Electron-Transfer Disproportionation in an Aprotic Medium. Molecules 2023, 28, 5002. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Khan, M.A.; Ahmad, I. Photodegradation of folic acid in aqueous solution. J. Pharm. Biomed. Anal. 1999, 19, 269–275. [Google Scholar] [CrossRef]
- Fukuwatari, T.; Fujita, M.; Shibata, K. Effects of UVA irradiation on the concentration of folate in human blood. Biosci. Biotechnol. Biochem. 2009, 73, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Rashed, A. High-Performance Liquid Chromatography (HPLC): Principles, Applications, Versatality, Efficiency, Innovation and Comparative Analysis in Modern Analytical Chemistry and In Pharmaceutical Sciences. Clin. Investig. 2024, 14, 524–535. [Google Scholar]
- Boulgakov, A.A.; Moor, S.R.; Jo, H.H.; Metola, P.; Joyce, L.A.; Marcotte, E.M.; Welch, C.J.; Anslyn, E.V. Next-Generation TLC: A Quantitative Platform for Parallel Spotting and Imaging. J. Org. Chem. 2020, 85, 9447–9453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lefeuvre, S.; Bois-Maublanc, J.; Mongeois, E.; Policarpo, V.; Formaux, L.; Francia, T.; Billaud, E.M.; Got, L. Quantitation using HRMS: A new tool for rapid, specific and sensitive determination of catecholamines and deconjugated methanephrines metanephrines in urine. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2021, 1166, 122391. [Google Scholar] [CrossRef] [PubMed]
- Juzeniene, A.; Thu Tam, T.T.; Iani, V.; Moan, J. The action spectrum for folic acid photodegradation in aqueous solutions. J. Photochem. Photobiol. B. 2013, 126, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Adhikari, S.; Patro, B.S.; Chattopadhyay, S.; Mukherjee, T. Free radical scavenging behavior of folic acid: Evidence for possible antioxidant activity. Free Radic. Biol. Med. 2001, 12, 1390–1399. [Google Scholar] [CrossRef]
- Gliszczyńska-Świgło, A. Folates as antioxidants. Food Chem. 2007, 101, 1480–1483. [Google Scholar] [CrossRef]
- Tejchman, K.; Kotfis, K.; Sieńko, J. Biomarkers and Mechanisms of Oxidative Stress—Last 20 Years of Research with an Emphasis on Kidney Damage and Renal Transplantation. Int. J. Mol. Sci. 2021, 22, 8010. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Ghanavati, M.; Ashtary-Larky, D.; Bagheri, R.; Rezaei Kelishadi, M.; Nazarian, B.; Nordvall, M.; Wong, A.; Dutheil, F.; Suzuki, K.; et al. Effects of Folic Acid Supplementation on Oxidative Stress Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Antioxidants 2021, 10, 871. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Eaton, G.R.; Eaton, S.S.; Salikhov, K.M. Foundations of Modern EPR; World Scientific: Singapore, 1998. [Google Scholar]
- Wertz, J.E.; Bolton, J.R. Electron Spin Resonance: Elementary Theory and Practical Applications; Chapman and Hall: London, UK, 1986. [Google Scholar]
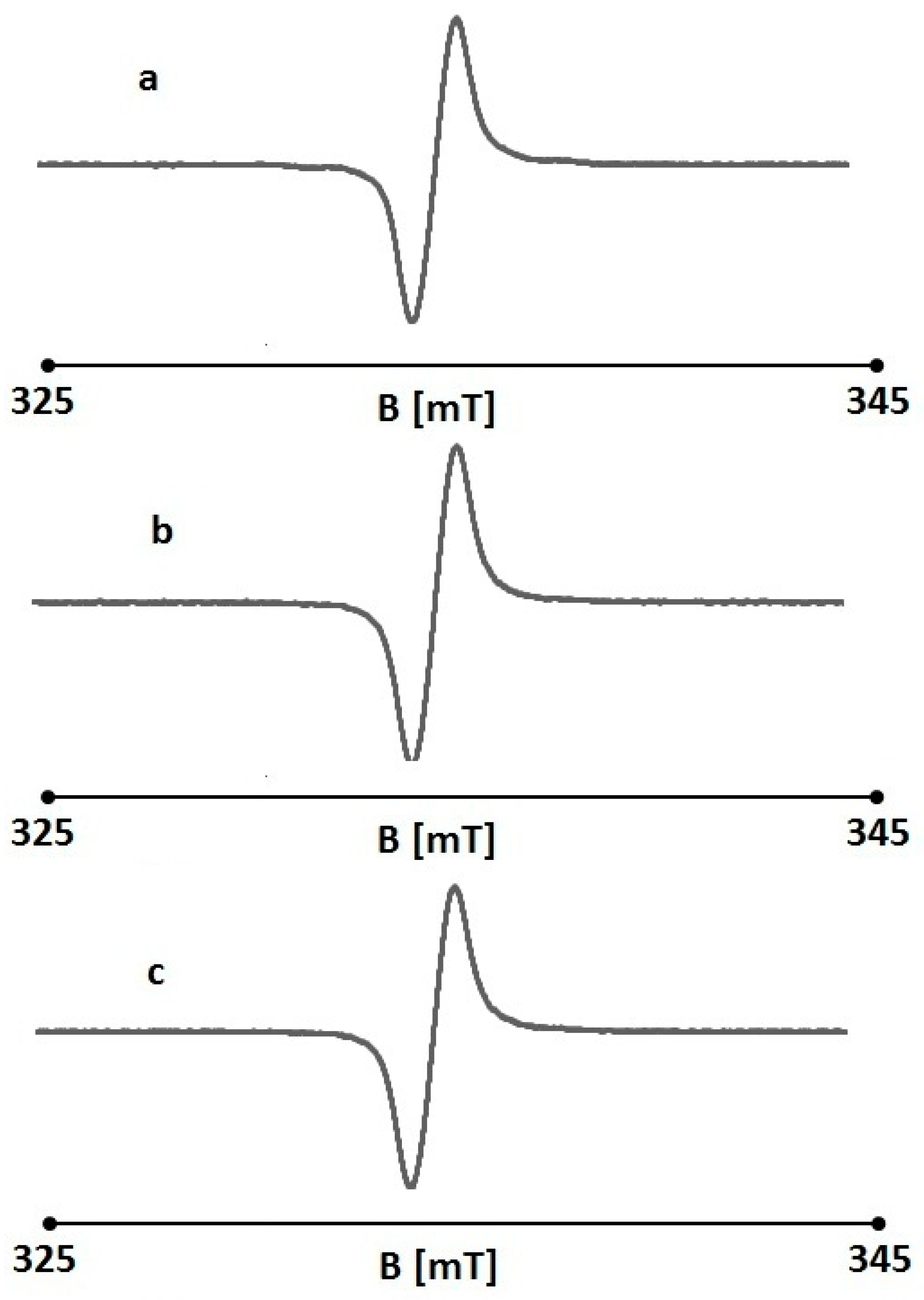
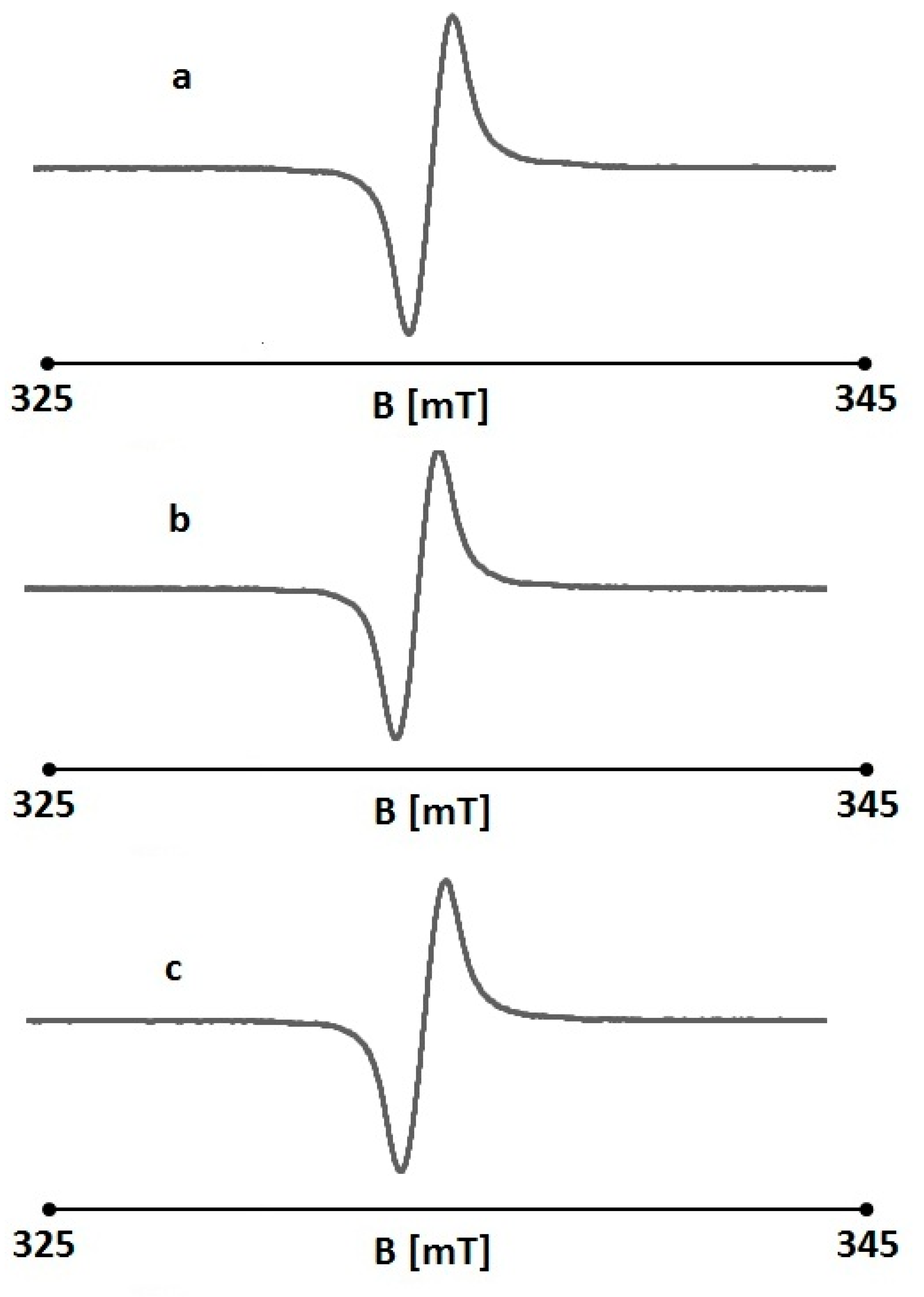
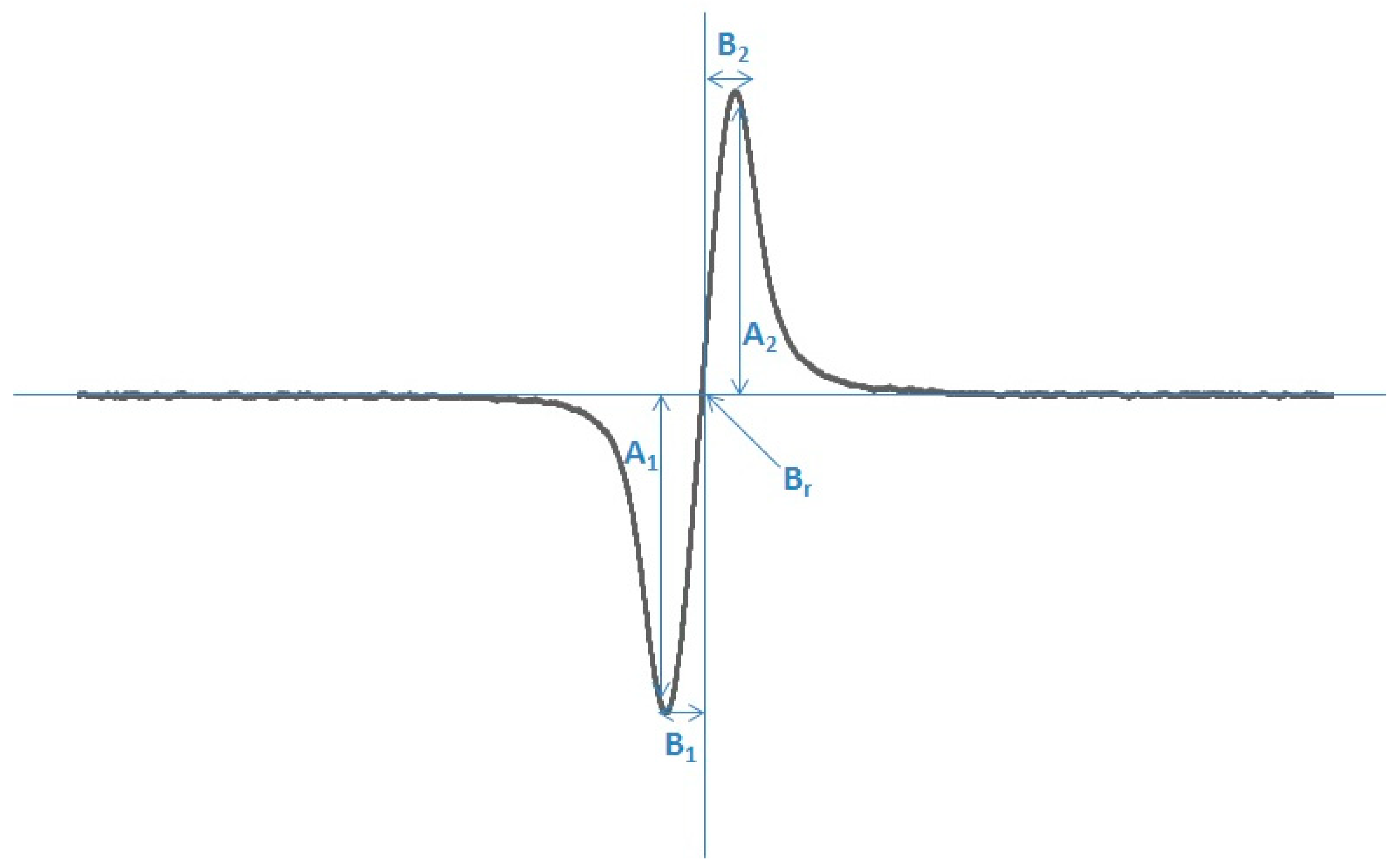
| Sample | A [a.u.] [+0.01] | ΔBpp [mT] [+0.02] | g [+0.0002] | A1/A2 [+0.02] | B1/B2 [+0.02] |
|---|---|---|---|---|---|
| DPPH | 0.40 | 0.48 | 2.0036 | 1.00 | 1.25 |
| 0.1% FA | 0.27 | 0.48 | 2.0036 | 0.93 | 1.15 |
| 0.01% | 0.24 | 0.47 | 2.0036 | 0.72 | 1.32 |
| 0.001% | 0.27 | 0.47 | 2.0036 | 0.98 | 1.25 |
| 0.0001% | 0.27 | 0.47 | 2.0036 | 0.98 | 1.25 |
| 0.00001% | 0.33 | 0.45 | 2.0036 | 0.99 | 1.17 |
| Sample | A [a.u.] [+0.01] | ΔBpp [mT] [+0.02] | g [+0.0002] | A1/A2 [+0.02] | B1/B2 [+0.02] |
|---|---|---|---|---|---|
| DPPH | 0.40 | 0.48 | 2.0036 | 1.00 | 1.25 |
| 0.1% | 0.77 | 0.47 | 2.0036 | 0.90 | 1.02 |
| 0.01% | 0.41 | 0.48 | 2.0036 | 0.98 | 1.15 |
| 0.001% | 0.45 | 0.46 | 2.0036 | 0.87 | 1.06 |
| 0.0001% | 0.50 | 0.47 | 2.0036 | 0.92 | 1.34 |
| 0.00001% | 0.47 | 0.47 | 2.0036 | 0.89 | 1.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurzak, M.; Ramos, P.; Pilawa, B.; Bednarek, I.A. Folic Acid as a Molecule Protecting Cells from the Negative Effects of Ultraviolet Radiation—An In Vitro Study. Pharmaceuticals 2025, 18, 1497. https://doi.org/10.3390/ph18101497
Jurzak M, Ramos P, Pilawa B, Bednarek IA. Folic Acid as a Molecule Protecting Cells from the Negative Effects of Ultraviolet Radiation—An In Vitro Study. Pharmaceuticals. 2025; 18(10):1497. https://doi.org/10.3390/ph18101497
Chicago/Turabian StyleJurzak, Magdalena, Paweł Ramos, Barbara Pilawa, and Ilona Anna Bednarek. 2025. "Folic Acid as a Molecule Protecting Cells from the Negative Effects of Ultraviolet Radiation—An In Vitro Study" Pharmaceuticals 18, no. 10: 1497. https://doi.org/10.3390/ph18101497
APA StyleJurzak, M., Ramos, P., Pilawa, B., & Bednarek, I. A. (2025). Folic Acid as a Molecule Protecting Cells from the Negative Effects of Ultraviolet Radiation—An In Vitro Study. Pharmaceuticals, 18(10), 1497. https://doi.org/10.3390/ph18101497







