Analysis of Secretory Structures, Chemical Composition, and Anti-Inflammatory Properties of Allophylus edulis (A. St.-Hil., A. Juss. & Cambess.) Radlk Leaves
Abstract
1. Introduction
2. Results
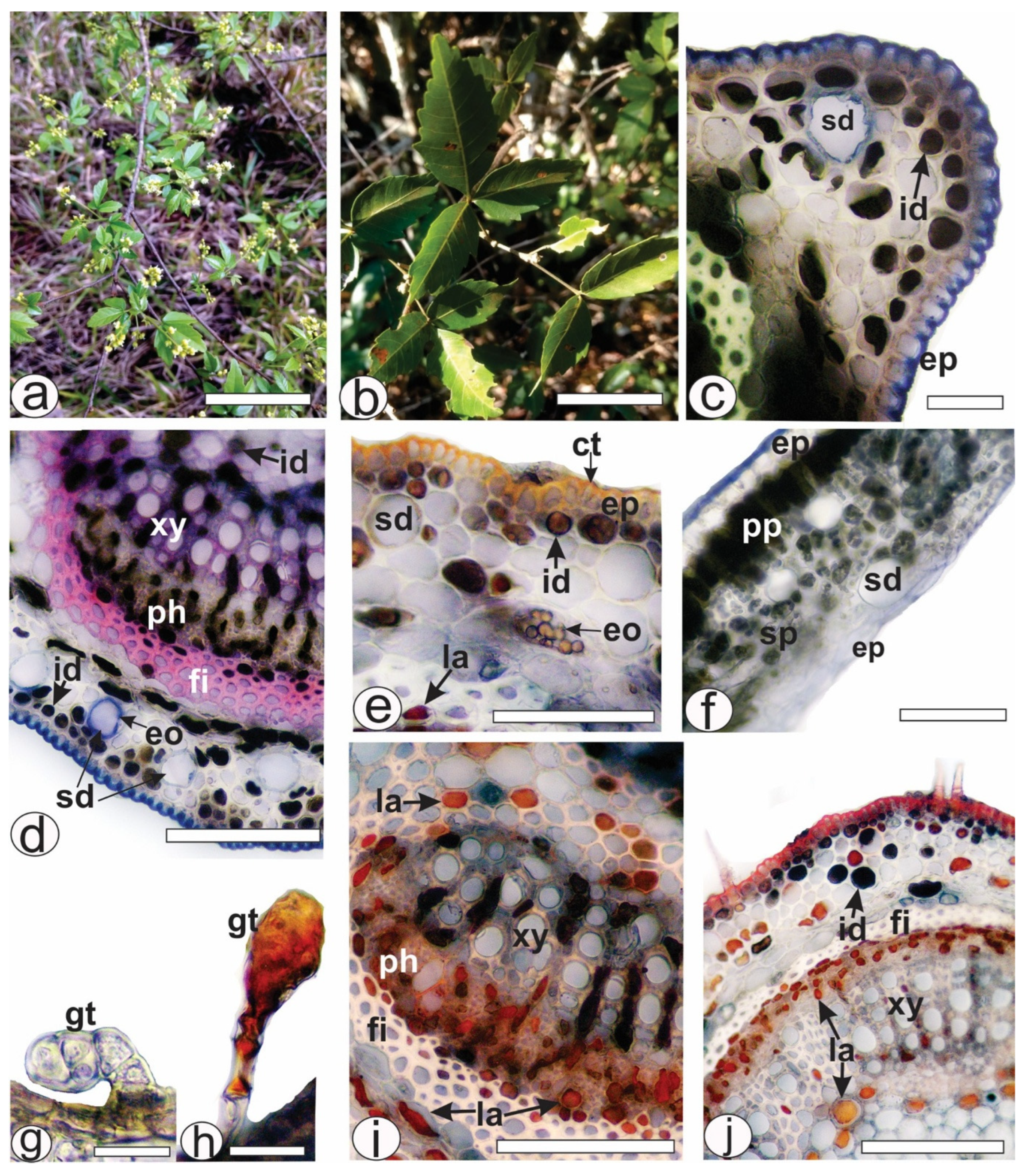
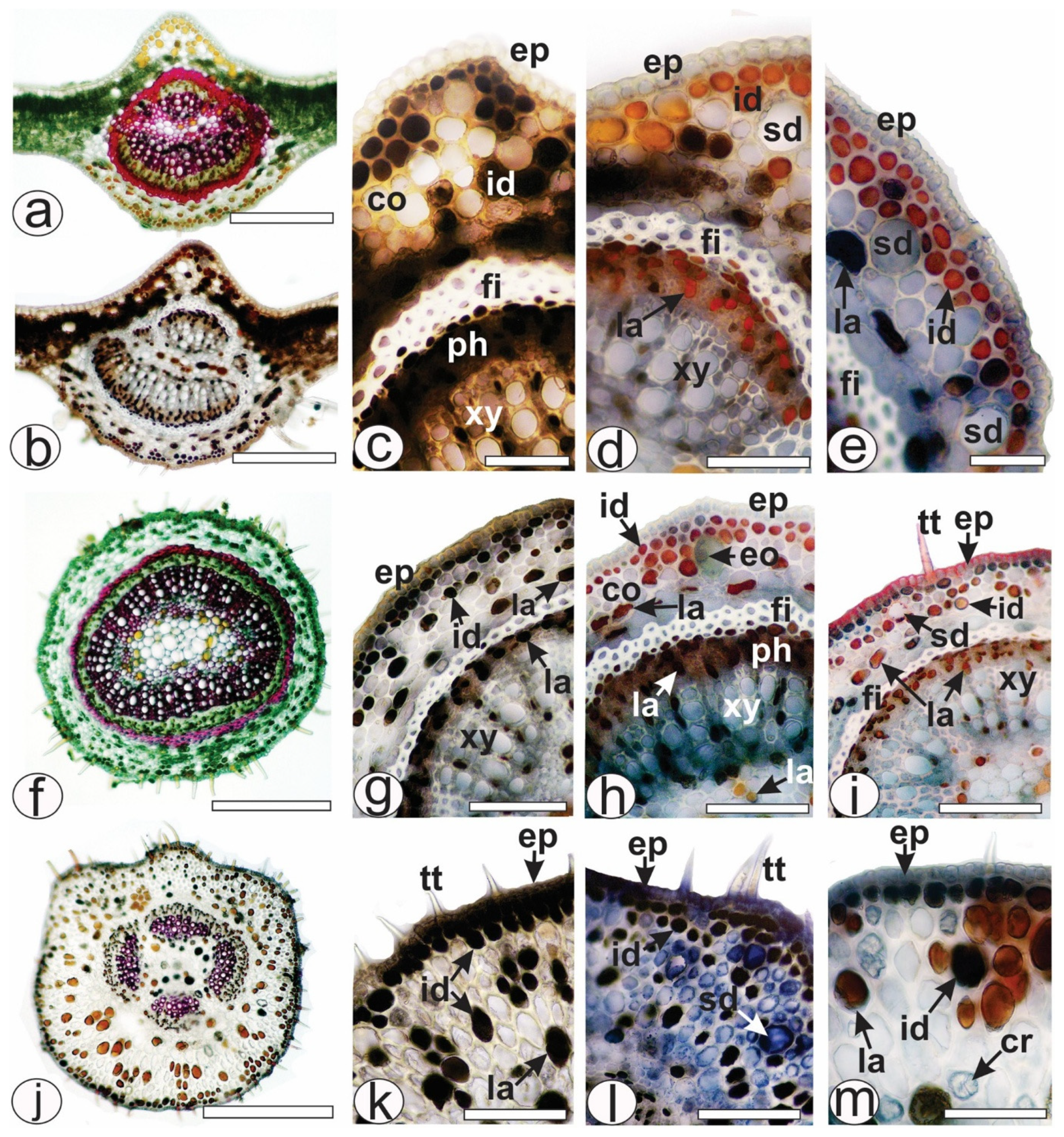
2.1. Histochemical Analysis of Leaf Secretory Structures
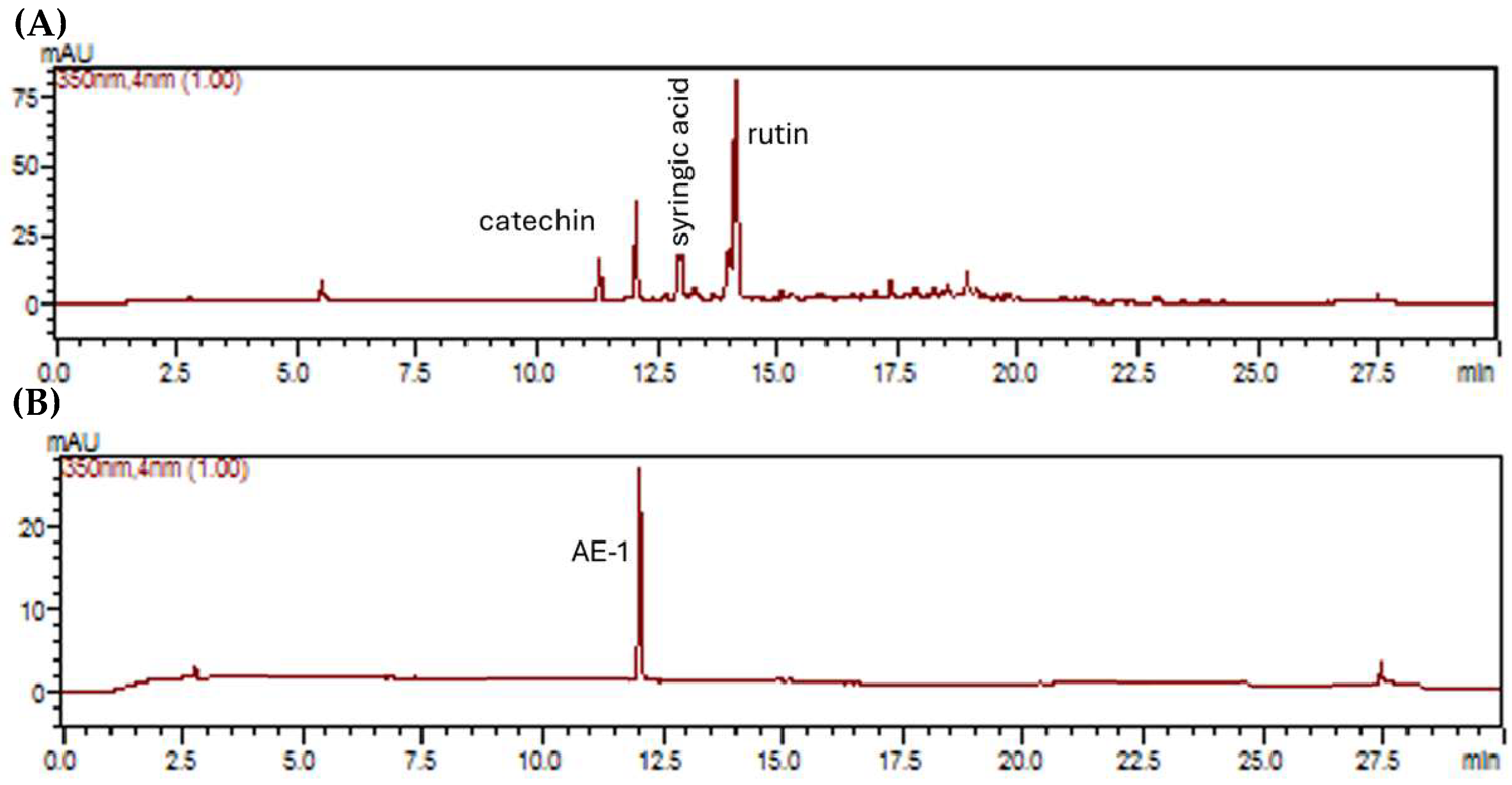
2.2. Chemical Study
2.3. In Silico Toxicity Prediction of AE-1
2.4. Antioxidant Activity
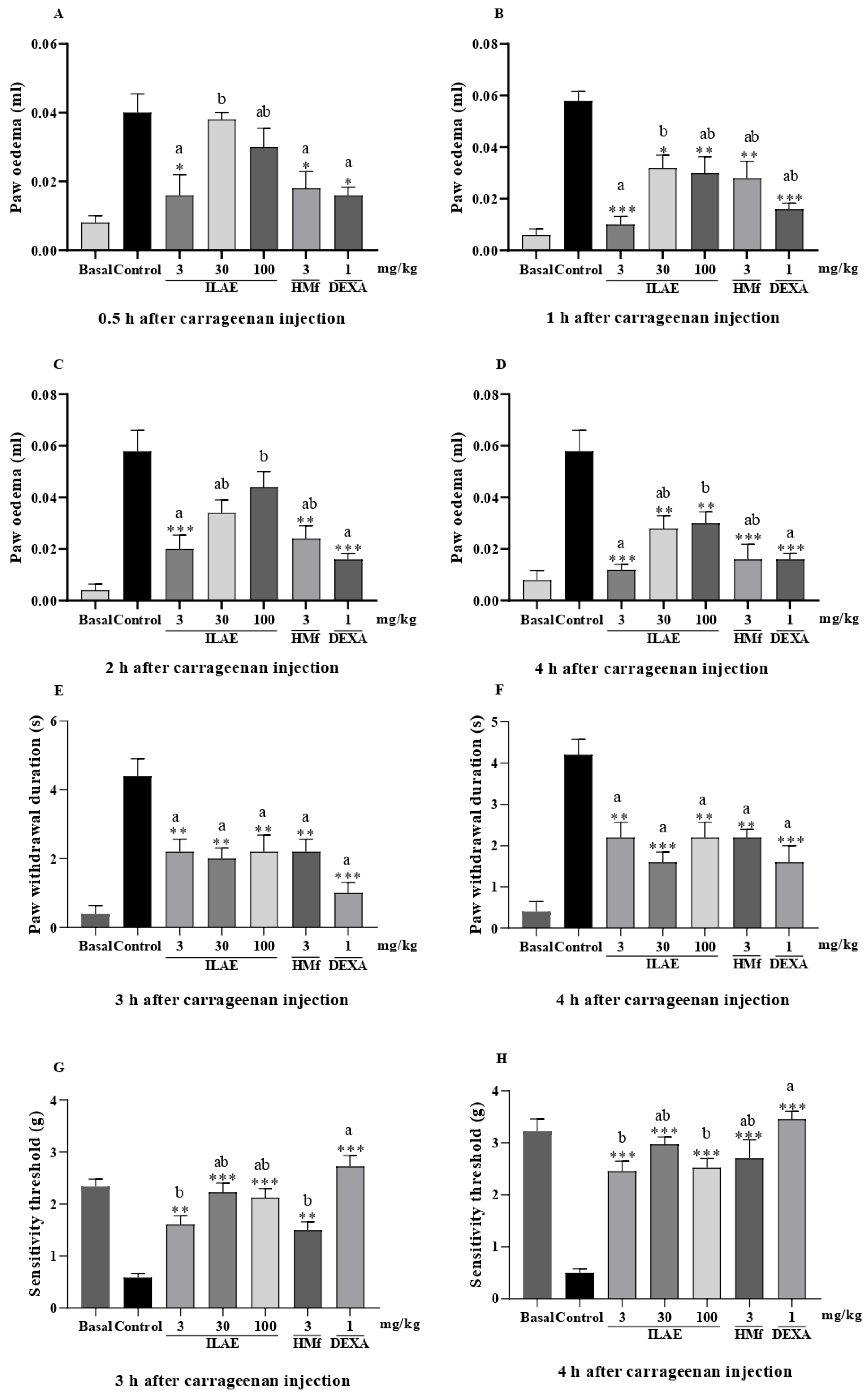
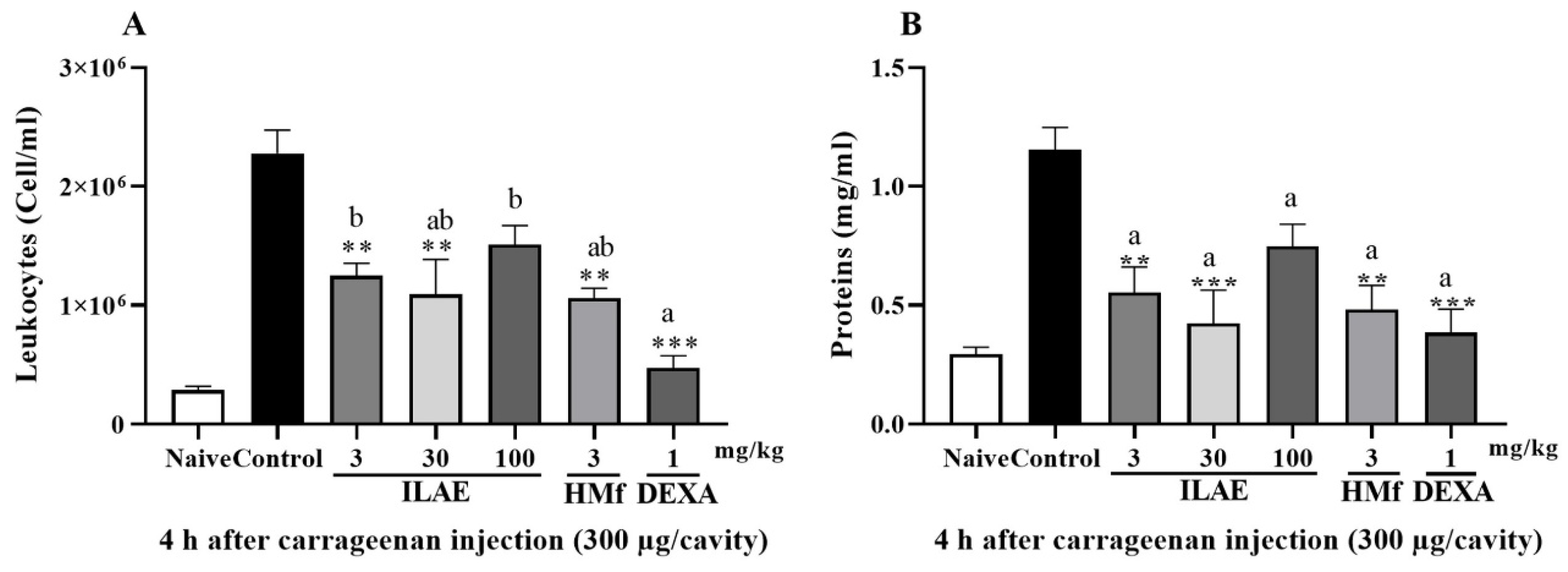
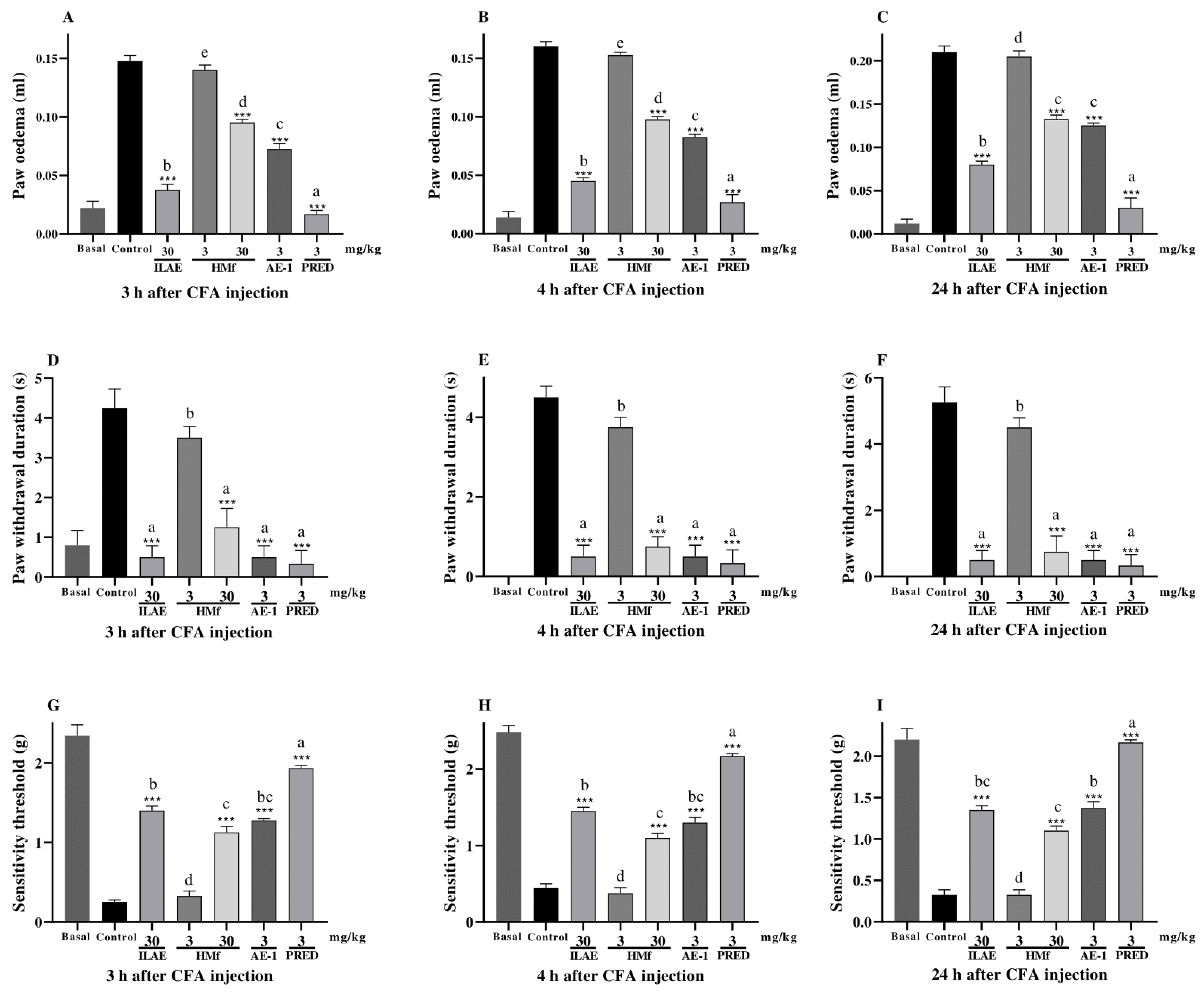
2.5. Anti-Inflammatory and Anti-Hyperalgesic Activity of ILAE, HMf and/or AE-1
3. Discussion
4. Materials and Methods
4.1. Drugs and Solvents
4.2. Plant Material
4.3. Histochemical Analyses
4.4. Infusion Preparation, Fractioning, Isolation, and NMR Analysis
4.5. Liquid Chromatographic Analysis of ILAE
4.6. Quantification of Constituents: Total Phenols, Flavonoids, Flavonols, and Condensed Tannins
4.7. In Silico Bioactivity and Toxicity Prediction of Vitexin 2″-O-Rhamnoside
4.8. Antioxidant Activity
4.8.1. Radical Scavenging Activity
4.8.2. Lipid Peroxidation Assay
4.9. Pharmacology Studies
4.9.1. Animals and Ethical Clearance
4.9.2. Paw Inflammatory Model Induced by Carrageenan
4.9.3. Pleurisy Induced by Carrageenan
4.9.4. Inflammatory Paw Model Induced by CFA
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt |
| BHT | Butylated hydroxytoluene |
| CE | Catechin equivalent |
| CFA | Complete Freund’s Adjuvant |
| DEXA | Dexamethasone |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| EAf | Ethyl acetate fraction |
| GAE | Gallic acid equivalent |
| Hf | n-Hexane fraction |
| HMf | Hydromethanol fraction |
| ILAE | Lyophilized infusion of A. edulis |
| NMR | Nuclear Magnetic Resonance |
| PBS | Phosphate-buffered saline |
| PRED | Prednisolone |
| QE | Quercetin equivalent |
| SisGen | National System for the Genetic Heritage and Associated Traditional Knowledge Management |
| TLC | Thin-layer chromatography |
| UFGD | Federal University of Grande Dourados |
References
- Arisawa, M.; Morinaga, Y.; Nishi, Y.; Ueno, H.; Suzuki, S.; Hayashi, T.; Shimizu Yoshizaki, M.; Morita, N.; Berganza, L.H. Chemical and Pharmaceutical Studies on Medicinal Plants in Paraguay. Constituents of Angiotensin Converting Enzyme Inhibitory Fraction from “Cocu”, Allophylus edulis Radlk. Jpn. J. Pharmacol. 1989, 43, 78–80. [Google Scholar]
- Körbes, V.C. Plantas Medicinais; Associação de Estudo, Orientação e Assistência Social: Francisco Beltrão, Brazil, 1995. [Google Scholar]
- Franco, I.J.; Fontana, V.L. Ervas & Plantas: A Medicina dos Simples; Erexim: Edelbra, Brazil, 2001. [Google Scholar]
- Kujawska, M.; Pieroni, A. Plants Used as Food and Medicine by Polish Migrants in Misiones, Argentina. Ecol. Food Nutr. 2015, 54, 255–279. [Google Scholar] [CrossRef]
- Kujawska, M.; Schmeda-Hirschmann, G. The Use of Medicinal Plants by Paraguayan Migrants in the Atlantic Forest of Misiones, Argentina, Is Based on Guaraní Tradition, Colonial and Current Plant Knowledge. J. Ethnopharmacol. 2022, 283, 114702. [Google Scholar] [CrossRef] [PubMed]
- Arruda, G.; Vardânega Périco, L.G.; Parpinelli, R.; Peretti, R.F.; Scur, M.C.; Caovilla Follador, F.A. Phytochemical Prospecting, Antimicrobial Activity, and Acute Toxicity of Aqueous Plant Extract of Two Plant Species Allophylus edulis (A. St. Hilaire, Cambessedes & A. Jussieu) RADLK Ex WARM and Matayba elaeagnoides RADLK. Int. J. New Technol. Res. 2019, 5, 10–13. [Google Scholar] [CrossRef]
- Tirloni, C.A.S.; Macorini, L.F.B.; dos Santos, U.P.; da Rocha, P.S.; Barros, S.V.; de Melo, A.M.M.F.; Vieira, M.C.; Souza, K.P.; dos Santos, E.L. Evaluation of the Antioxidant Activity, Antimicrobial Effect and Acute Toxicity from Leaves of Allophylus edulis (A. St.-Hil., A. Juss. Cambess.) Hieron. Ex Niederl. Afr. J. Pharm. Pharmacol. 2015, 9, 353–362. [Google Scholar] [CrossRef]
- Hoffmann-Bohm, K.; Lotter, H.; Seligmann, O.; Wagner, H. Antihepatotoxic C-Glycosylflavones from the Leaves of Allophyllus edulis Var. edulis and gracilis. Planta Med. 1992, 58, 544–548. [Google Scholar] [CrossRef]
- Matsunaga, K.; Sasaki, S.; Ohizumi, Y. Excitatory and Inhibitory Effects of Paraguayan Medicinal Plants Equisetum giganteum, Acanthospermum australe, Allophylus edulis and Cordia salicifolia on Contraction of Rabbit Aorta and Guinea-Pig Left Atrium. Nat. Med. 1997, 51, 478–481. [Google Scholar]
- Trevizan, L.N.F.; do Nascimento, K.F.; Santos, J.A.; Kassuya, C.A.L.; Cardoso, C.A.L.; do Carmo Vieira, M.; Moreira, F.M.F.; Croda, J.; Formagio, A.S.N. Anti-Inflammatory, Antioxidant and Anti-Mycobacterium Tuberculosis Activity of Viridiflorol: The Major Constituent of Allophylus edulis (A. St.-Hil., A. Juss. & Cambess.) Radlk. J. Ethnopharmacol. 2016, 192, 510–515. [Google Scholar] [CrossRef]
- dos Santos, S.M.; de Oliveira Junior, P.C.; de Matos Balsalobre, N.; Kassuya, C.A.L.; Cardoso, C.A.L.; Pereira, Z.V.; Silva, R.M.M.F.; Formagio, A.S.N. Variation in Essential Oil Components and Anti-Inflammatory Activity of Allophylus edulis Leaves Collected in Central-Western Brazil. J. Ethnopharmacol. 2021, 267, 113495. [Google Scholar] [CrossRef]
- Balsalobre, N.M.; dos Santos, E.; Mariano dos Santos, S.; Arena, A.C.; Konkiewitz, E.C.; Ziff, E.B.; Nazari Formagio, A.S.; Leite Kassuya, C.A. Potential Anti-Arthritic and Analgesic Properties of Essential Oil and Viridiflorol Obtained from Allophylus edulis Leaves in Mice. J. Ethnopharmacol. 2023, 301, 115785. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Li, C.; Liu, G. Recent Advances in the Extraction, Purification and Analytical Techniques for Flavonoids from Plants: Taking Hawthorn as an Example. J. Food Compos. Anal. 2025, 141, 107372. [Google Scholar] [CrossRef]
- Li, T.; Liang, M.; Li, Z.; Gu, F.; Guo, Q.; Wang, Q. Synthesis of Novel Resveratrol Nervonic Acid Ester Using a Solvent-Free Mechanochemical Method: Improved Lipophilicity, Thermostability, and Oxidation Stability. Food Chem. 2025, 480, 143958. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, J.; Li, H.; Zhao, D.; Liu, Z.; Zhu, L.; Zhang, Z.; Peng, W. Quercetin: A Promising Therapy for Diabetic Encephalopathy through Inhibition of Hippocampal Ferroptosis. Phytomedicine 2024, 126, 154887. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.F.; Li, J.Y.; Wei, X.Y.; Ma, S.Q.; Wang, Q.G.; Qi, Z.; Duan, Z.C.; Tan, L.; Tang, H. Preclinical Evidence of Reno-Protective Effect of Quercetin on Acute Kidney Injury: A Meta-Analysis of Animal Studies. Front. Pharmacol. 2023, 14, 1310023. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, H.; Matsubara, Y.; Iizuka, Y.; Okamoto, K.; Yokoi, K. Structure and Hypotensive Effect of Flavonoid Glycosides in Kinkan (Fortunella japonica) Peelings. Agric. Biol. Chem. 1985, 49, 2613–2618. [Google Scholar] [CrossRef]
- Dutra, L.M.; Henrique Vieira Teles, P.; Diego da Conceição Santos, A.; Franklin de Melo, N.; Nagata, N.; Roberto Guedes da Silva Almeida, J. 1H NMR-Based Metabolic Profile and Chemometric Analysis for the Discrimination of Passiflora Species Genotypic Variations. Food Res. Int. 2023, 164, 112441. [Google Scholar] [CrossRef]
- Forzza, R.C. Catálogo de Plantas e Fungos do Brasil; Andrea Jakobsson Estúdio and Instituto de Pesquisa Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brazil, 2010.
- Backes, P.; Irgang, B. Mata Atlântica: As Árvores e a Paisagem; Paisagem do Sul: Porto Alegre, Brazil, 2004. [Google Scholar]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil; Instituto Plantarum: Nova Odessa, Brazil, 2016. [Google Scholar]
- Arambarri, A.M.; Freire, S.E.; Colares, M.N.; Bayón, N.D.; Novoa, M.C.; Monti, C.; Stenglein, S.A. Leaf Anatomy of Medicinal Shrubs and Trees from Gallery Forests of the Paranaense Province (Argentina). Bol. Soc. Argent. Bot. 2006, 41, 233–268. [Google Scholar]
- Cunha Neto, I.L.; Martins, F.M.; Somner, G.V.; Tamaio, N. Secretory structures in stems of five lianas of Paullinieae (Sapindaceae): Morphology and histochemistry. Flora 2017, 235, 29–40. [Google Scholar] [CrossRef]
- Medina, M.C.; Sousa-Baena, M.S.; Prado, E.; Acevedo-Rodríguez, P.; Dias, P.; Demarco, D. Laticifers in Sapindaceae: Structure, Evolution and Phylogenetic Importance. Front. Plant Sci. 2021, 11, 612985. [Google Scholar] [CrossRef]
- Ibrahim, F.S.; Mohammed, Z.; Nuhu, A.; Shehu, S.; Ilyas, N. Acute Toxicity and Anti-Inflammatory Activity of Hydromethanol Extract of Allophylus africanus Beauv in rats. J. Herbmed Pharmacol. 2018, 7, 119–123. [Google Scholar] [CrossRef]
- Dos Santos, S.M.; Cardoso, C.A.L.; de Oliveira Junior, P.C.; da Silva, M.E.; Pereira, Z.V.; Silva, R.M.M.F.; Formagio, A.S.N. Seasonal and Geographical Variation in the Chemical Composition of Essential Oil from Allophylus edulis Leaves. S. Afr. J. Bot. 2023, 154, 41–45. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Ferreres, F.; Gomes, N.G.M.; Valentão, P.; Pereira, D.M.; Gil-Izquierdo, A.; Araújo, L.; Silva, T.C.; Andrade, P.B. Leaves and Stem Bark from Allophylus Africanus P. Beauv: An Approach to Anti-Inflammatory Properties and Characterization of Their Flavonoid Profile. Food Chem. Toxicol. 2018, 118, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Strada, C.L.; Lima, K.d.C.; da Silva, V.C.; Ribeiro, R.V.; Dores, E.F.G.d.C.; Dall’Oglio, E.L.; Schmeda-Hirschmann, G.; Carollo, C.A.; Martins, D.T.O.; de Sousa Júnior, P.T. Isovitexin as Marker and Bioactive Compound in the Antinociceptive Activity of the Brazilian Crude Drug Extracts of Echinodorus scaber and E. grandiflorus. Rev. Bras. Farmacogn. 2017, 27, 619–626. [Google Scholar] [CrossRef]
- Hong, I.-T.; Kim, B.-J.; Yu, D.-C.; Kim, J.-H.; Kim, J.-H.; Heo, M.-Y.; Lee, S.-J.; Kim, H.-P. Antioxidative and Anti-Inflammatory Activities of Phaseolus aureus. J. Soc. Cosmet. Sci. Korea 1996, 22, 41–51. [Google Scholar]
- Rosa, S.I.G.; Rios-Santos, F.; Balogun, S.O.; Martins, D.T.D.O. Vitexin Reduces Neutrophil Migration to Inflammatory Focus by Down-Regulating pro-Inflammatory Mediators via Inhibition of P38, ERK1/2 and JNK Pathway. Phytomedicine 2016, 23, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, R.d.P.d.; Machado, A.P.d.F.; Lima, V.S.; Moya, A.M.T.M.; Reguengo, L.M.; Bogusz Junior, S.; Leal, R.F.; Cao-Ngoc, P.; Rossi, J.C.; Leclercq, L.; et al. Chemoprevention with a Tea from Hawthorn (Crataegus oxyacantha) Leaves and Flowers Attenuates Colitis in Rats by Reducing Inflammation and Oxidative Stress. Food Chem. X 2021, 12, 100139. [Google Scholar] [CrossRef]
- Li, S.; Liang, T.; Zhang, Y.; Huang, K.; Yang, S.; Lv, H.; Chen, Y.; Zhang, C.; Guan, X. Vitexin Alleviates High-Fat Diet Induced Brain Oxidative Stress and Inflammation via Anti-Oxidant, Anti-Inflammatory and Gut Microbiota Modulating Properties. Free Radic. Biol. Med. 2021, 171, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Li, H.; Xiong, Z.; Sun, Z.; Cai, S.; Zhu, W.; Bi, Y.; Li, F. LC determination of malondialdehyde concentrations in the human umbilical vein endothelial cell culture medium: Application to the antioxidant effect of vitexin-2″-O-rhamnoside. Chromatographia 2008, 67, 679–686. [Google Scholar] [CrossRef]
- Wei, W.; Ying, X.; Zhang, W.; Chen, Y.; Leng, A.; Jiang, C.; Liu, J. Effects of Vitexin-2″-O-Rhamnoside and Vitexin-4″-O-Glucoside on Growth and Oxidative Stress-Induced Cell Apoptosis of Human Adipose-Derived Stem Cells. J. Pharm. Pharmacol. 2014, 66, 988–997. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Li, M.F.; Yang, Y.S.; Li, R.; Tan, J.; Tang, S.H.; Jiang, Z.T. Composition, cytotoxicity and antioxidant activities of polyphenols in the leaves of star anise (Illicium verum Hook. F.). Sci. Asia 2019, 45, 532–537. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, W.; Jin, X.; Li, J.; Yu, Y. Vitexin-2-O-Rhamnoside Improves Immunosuppression, Oxidative Stress, and Phosphorylation of PI3K/Akt Signal Pathway in Cyclophosphamide Treated Mice. Eur. J. Pharmacol. 2022, 925, 174999. [Google Scholar] [CrossRef] [PubMed]
- Umeo, S.H.; Ito, T.M.; Yokota, M.E.; Romagnolo, M.B.; Junior, A.L. Avaliação das propriedades antioxidantes, anticolinesterásicas e citotóxicas dos frutos de Allophylus edulis (A.ST.-HIL., Cambess. & A. Juss.) Radlk. (Sapindaceae). Arq. Ciênc. Saúde UNIPAR 2011, 15, 167–171. [Google Scholar]
- Schmeda-Hirschmann, G.; Feresin, G.; Tapia, A.; Hilgert, N.; Theoduloz, C. Proximate Composition and Free Radical Scavenging Activity of Edible Fruits from the Argentinian Yungas. J. Sci. Food Agric. 2005, 85, 1357–1364. [Google Scholar] [CrossRef]
- Arora, A.; Nair, M.G.; Strasburg, G.M. Structure–activity relationships for antioxidant activities of a series of flavonoids in a liposomal system. Free Radic. Biol. Med. 1998, 24, 1355–1363. [Google Scholar] [CrossRef]
- Lorizola, I.M.; Furlan, C.P.B.; Portovedo, M.; Milanski, M.; Botelho, P.B.; Bezerra, R.M.N.; Sumere, B.R.; Rostagno, M.A.; Capitani, C.D. Beet Stalks and Leaves (Beta vulgaris L.) Protect Against High-Fat Diet-Induced Oxidative Damage in the Liver in Mice. Nutrients 2018, 10, 872. [Google Scholar] [CrossRef]
- Myers, M.J.; Deaver, C.M.; Lewandowski, A.J. Molecular Mechanism of Action Responsible for Carrageenan-Induced Inflammatory Response. Mol. Immunol. 2019, 109, 38–42. [Google Scholar] [CrossRef]
- Pober, J.S.; Sessa, W.C. Inflammation and the Blood Microvascular System. Cold Spring Harb. Perspect. Biol. 2015, 7, a016345. [Google Scholar] [CrossRef]
- Li, L.; Wang, B.; Gao, T.; Zhang, X.; Hao, J.-X.; Vlodavsky, I.; Wiesenfeld-Hallin, Z.; Xu, X.-J.; Li, J.-P. Heparanase Overexpression Reduces Carrageenan-Induced Mechanical and Cold Hypersensitivity in Mice. Neurosci. Lett. 2012, 511, 4–7. [Google Scholar] [CrossRef]
- Suyenaga, E.S.; Klein, L.C.; Passos, C.D.S.; Marin, R.; Santin, J.R.; Machado, I.D.; Farsky, S.H.P.; Henriques, A.T. Beyond Organoleptic Characteristics: The Pharmacological Potential of Flavonoids and Their Role in Leukocyte Migration and in L-Selectin and Β2-Integrin Expression during Inflammation. Phytother. Res. 2014, 28, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Werner, I.; Guo, F.; Kiessling, A.H.; Juengel, E.; Relja, B.; Lamm, P.; Stock, U.A.; Moritz, A.; Beiras-Fernandez, A. Treatment of Endothelial Cell with Flavonoids Modulates Transendothelial Leukocyte Migration. Phlebology 2014, 30, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Lotito, S.B.; Frei, B. Dietary Flavonoids Attenuate Tumor Necrosis Factor α-Induced Adhesion Molecule Expression in Human Aortic Endothelial Cells. J. Biol. Chem. 2006, 281, 37102–37110. [Google Scholar] [CrossRef]
- Zhu, X.X.; Li, L.D.; Liu, J.X.; Liu, Z.Y.; Ma, X.Y. Effect of vitexia-rhamnoside (V-R) on vasomotor factors expression of endothelial cell. Zhongguo Zhong Yao Za Zhi 2006, 31, 566–569. [Google Scholar]
- Billiau, A.; Matthys, P. Modes of Action of Freund’s Adjuvants in Experimental Models of Autoimmune Diseases. J. Leukoc. Biol. 2001, 70, 849–860. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Artero, N.A.; Rasquel-Oliveira, F.S.; Fattori, V.; Casagrande, R.; Verri, W.A. Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef]
- Iannitti, T.; Di Cerbo, A.; Loschi, A.R.; Rea, S.; Suzawa, M.; Morales-Medina, J.C. Repeated Administration of a Flavonoid-based Formulated Extract from Citrus Peels Significantly Reduces Peripheral Inflammation-induced Pain in the Rat. Food Sci. Nutr. 2020, 8, 3173. [Google Scholar] [CrossRef]
- Zhang, W.; Lyu, J.; Xu, J.; Zhang, P.; Zhang, S.; Chen, Y.; Wang, Y.; Chen, G. The Related Mechanism of Complete Freund’s Adjuvant-Induced Chronic Inflammation Pain Based on Metabolomics Analysis. Biomed. Chromatogr. 2021, 35, e5020. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- Pearse, A.G.E. Histochemistry: Theoretical and Applied: Volume 2, 3rd ed.; Churchill Livingston: London, UK, 1972. [Google Scholar]
- David, R.; Carde, J.P. Coloration différentielle des inclusions lipidiques et terpéniques des pseudophylles du Pin maritime au moyen du réactif Nadi. C. R. Acad. Sci. D 1964, 258, 1338–1340. [Google Scholar]
- Johansen, D.A. Plant Microtechnique; McGraw Hill Book: New York, NY, USA, 1940. [Google Scholar]
- Gabe, M. Techniques Histologiques; Masson & Cie: Paris, France, 1968. [Google Scholar]
- Mace, M.E.; Howell, C.R. Histochemistry and identification of condensed tannin precursor in roots of cotton seedlings. Can. J. Bot. 1974, 52, 2423–2426. [Google Scholar] [CrossRef]
- Svendsen, A.B.; Verpoorte, R. Cromatography of Alkaloids; Elsevier Scientific Publishing Company: New York, NY, USA, 1983. [Google Scholar]
- Pearse, A.G.E. Histochemistry: Theoretical and Applied: Volume 1, 3rd ed.; Churchill Livingston: London, UK, 1968. [Google Scholar]
- Formagio, A.S.N.; Volobuff, C.R.F.; Santiago, M.; Cardoso, C.A.L.; Vieira, M.C.; Pereira, Z.V. Evaluation of Antioxidant Activity, Total Flavonoids, Tannins and Phenolic Compounds in Psychotria Leaf Extracts. Antioxidants 2014, 3, 745–757. [Google Scholar] [CrossRef]
- Hayat, J.; Akodad, M.; Moumen, A.; Baghour, M.; Skalli, A.; Ezrari, S.; Belmalha, S. Phytochemical Screening, Polyphenols, Flavonoids and Tannin Content, Antioxidant Activities and FTIR Characterization of Marrubium Vulgare L. from 2 Different Localities of Northeast of Morocco. Heliyon 2020, 6, e05609. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Marco, G.J. A Rapid Method for Evaluation of Antioxidants. J. Am. Oil Chem. Soc. 1968, 45, 594–598. [Google Scholar] [CrossRef]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef]
- Decosterd, I.; Woolf, C.J. Spared Nerve Injury: An Animal Model of Persistent Peripheral Neuropathic Pain. Pain 2000, 87, 149–158. [Google Scholar] [CrossRef]
- Deuis, J.R.; Lim, Y.L.; De Sousa, S.R.; Lewis, R.J.; Alewood, P.F.; Cabot, P.J.; Vetter, I. Analgesic Effects of Clinically Used Compounds in Novel Mouse Models of Polyneuropathy Induced by Oxaliplatin and Cisplatin. Neuro-Oncology 2014, 16, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Vinegar, R.; Truax, J.F.; Selph, J.L. Some Quantitative Temporal Characteristics of Carrageenin-Induced Pleurisy in the Rat. Proc. Soc. Exp. Biol. Med. 1973, 143, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.A.; Brown, D.R.; El-Atrash, S.; Walser, M.M. Pain Threshold Changes in Adjuvant-Induced Inflammation: A Possible Model of Chronic Pain in the Mouse. Pharmacol. Biochem. Behav. 1986, 24, 49–53. [Google Scholar] [CrossRef]

| Metabolites | ILAE | EAf | HMf |
|---|---|---|---|
| Total phenols (mg GAE/g) | 177.56 ± 6.16 | 155.92 ± 4.59 | 159.56 ± 4.74 |
| Flavonoids (mg QE/g) | 64.29 ± 0.18 | 46.37 ± 0.18 | 63.10 ± 0.20 |
| Flavonols (mg QE/g) | 55.22 ± 0.11 | 23.71 ± 0.09 | 49.63 ± 0.42 |
| Condensed tannins (mg CE/g) | 43.78 ± 1.12 | 37.53 ± 1.31 | 44.88 ± 1.50 |
| Toxicity | |||
|---|---|---|---|
| Target | Prediction | Probability | |
| Hepatotoxicity | Inactive | 0.81 a | |
| Carcinogenicity | Inactive | 0.90 a | |
| Immunotoxicity | Active | 0.98 a | |
| Mutagenicity | Inactive | 0.73 a | |
| Cytotoxicity | Inactive | 0.66 a | |
| Cytochrome inhibitors | CYP1A2 | Inactive | 0.99 b |
| CYP2C19 | Inactive | 0.99 b | |
| CYP2C9 | Inactive | 0.90 b | |
| CYP2D6 | Inactive | 0.95 b | |
| CYP3A4 | Inactive | 0.99 b | |
| CYP2E1 | Inactive | 0.98 b | |
| LD50 (mg/kg) | 5000 (Class 5) | 67.38 c | |
| Assays | ILAE | EAf | HMf | BHT |
|---|---|---|---|---|
| IC50 (µg/mL) | ||||
| DPPH | 27.88 ± 0.002 | 28.07 ± 0.003 | 15.17 ± 0.004 | 9.89 ± 0.002 |
| ABTS | 40.55 ± 0.029 | 61.80 ± 0.013 | 25.15 ± 0.039 | 9.75 ± 0.004 |
| β-carotene/linoleic acid | 117.9 ± 0.50 | 195.60 ± 0.09 | 55.44 ± 0.72 | 13.03 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, S.M.d.; Faoro, J.A.M.; Junior, P.C.d.O.; Santos, E.d.; Kassuya, C.A.L.; Pereira, Z.V.; Almeida, V.P.d.; Machado, C.D.; Manfron, J.; Souza, N.L.B.; et al. Analysis of Secretory Structures, Chemical Composition, and Anti-Inflammatory Properties of Allophylus edulis (A. St.-Hil., A. Juss. & Cambess.) Radlk Leaves. Pharmaceuticals 2025, 18, 1479. https://doi.org/10.3390/ph18101479
Santos SMd, Faoro JAM, Junior PCdO, Santos Ed, Kassuya CAL, Pereira ZV, Almeida VPd, Machado CD, Manfron J, Souza NLB, et al. Analysis of Secretory Structures, Chemical Composition, and Anti-Inflammatory Properties of Allophylus edulis (A. St.-Hil., A. Juss. & Cambess.) Radlk Leaves. Pharmaceuticals. 2025; 18(10):1479. https://doi.org/10.3390/ph18101479
Chicago/Turabian StyleSantos, Sidney Mariano dos, Janaine Alberto Marangoni Faoro, Pedro Cruz de Oliveira Junior, Elisangela dos Santos, Candida Aparecida Leite Kassuya, Zefa Valdevina Pereira, Valter Paes de Almeida, Camila Dias Machado, Jane Manfron, Nadia Laiz Benites Souza, and et al. 2025. "Analysis of Secretory Structures, Chemical Composition, and Anti-Inflammatory Properties of Allophylus edulis (A. St.-Hil., A. Juss. & Cambess.) Radlk Leaves" Pharmaceuticals 18, no. 10: 1479. https://doi.org/10.3390/ph18101479
APA StyleSantos, S. M. d., Faoro, J. A. M., Junior, P. C. d. O., Santos, E. d., Kassuya, C. A. L., Pereira, Z. V., Almeida, V. P. d., Machado, C. D., Manfron, J., Souza, N. L. B., Lima Cardoso, C. A., Mussury, R. M., & Formagio, A. S. N. (2025). Analysis of Secretory Structures, Chemical Composition, and Anti-Inflammatory Properties of Allophylus edulis (A. St.-Hil., A. Juss. & Cambess.) Radlk Leaves. Pharmaceuticals, 18(10), 1479. https://doi.org/10.3390/ph18101479










