Pharmacological Actions of Potassium Channel Openers on Voltage-Gated Potassium Channels
Abstract
1. Introduction
2. Results
2.1. Prefrontal Cortex
2.2. Nucleus Accumbens
2.3. Dorsal Striatum
2.4. Hippocampus
2.4.1. Dorsal Hippocampus
2.4.2. Ventral Hippocampus
3. Discussion
3.1. Diazoxide
3.2. Chlorzoxazone
3.3. Flupirtine
4. Materials and Methods
4.1. Potassium Channel Openers
4.2. Ethical Approval
4.3. Animals and Potassium Channel Openers
4.4. Tissue Collection and RNA Extraction
4.5. Quantitative RT-PCR
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| K+ channel, potassium channel; | potassium channel |
| K+ Ag | potassium channel opener |
| V | vehicle control |
| Flu | flupirtine |
| Dia | diazoxide |
| Chl | chlorzoxazone |
| PFC | prefrontal cortex |
| NAc | nucleus accumbens |
| dSTR | dorsal striatum |
| HIP | hippocampus |
| dHIP | dorsal hippocampus |
| vHIP | ventral hippocampus |
| SD | Sprague Dawley |
| PCs | pyramidal cells |
| KO | knockout |
| LOF | loss-of-function |
| GOF | gain-of-function |
| DA | dopamine |
| ANOVA | analysis of variance |
| qRT-PCR | quantitative reverse transcriptase polymerase reaction |
| Kv | voltage-gated potassium channel |
| Kca | calcium-activated potassium channel |
| K2p | two/tandem-pore domain potassium channel |
| Kir | inwardly rectifying potassium channel |
| LC-MS | liquid chromatography–mass spectrometry |
| B2m | beta-2 microglobulin |
| Rps5 | ribosomal protein S5 |
| Tbp | ATA box binding protein |
| Rn18s | 18S ribosomal RNA |
| Tubb2b | tubulin, beta 2B class IIb |
| Ubc | ubiquitin C |
References
- Friedel, H.A.; Fitton, A. Flupirtine. A Review of Its Pharmacological Properties, and Therapeutic Efficacy in Pain States. Drugs 1993, 45, 548–569. [Google Scholar] [CrossRef]
- Krajewska, M.; Szewczyk, A.; Kulawiak, B.; Koprowski, P. Pharmacological Characterization of a Recombinant Mitochondrial ROMK2 Potassium Channel Expressed in Bacteria and Reconstituted in Planar Lipid Bilayers. Membranes 2023, 13, 360. [Google Scholar] [CrossRef]
- Mínguez-Viñas, T.; Prakash, V.; Wang, K.; Lindström, S.H.; Pozzi, S.; Scott, S.A.; Spiteri, E.; Stevenson, D.A.; Ashley, E.A.; Gunnarsson, C.; et al. Two Epilepsy-Associated Variants in KCNA2 (KV 1.2) at Position H310 Oppositely Affect Channel Functional Expression. J. Physiol. 2023, 601, 5367–5389. [Google Scholar] [CrossRef]
- Zhou, Y.-S.; Tao, H.-B.; Lv, S.-S.; Liang, K.-Q.; Shi, W.-Y.; Liu, K.-Y.; Li, Y.-Y.; Chen, L.-Y.; Zhou, L.; Yin, S.-J.; et al. Effects of Kv1.3 Knockout on Pyramidal Neuron Excitability and Synaptic Plasticity in Piriform Cortex of Mice. Acta Pharmacol. Sin. 2024, 45, 2045–2060. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Lacza, Z.; Rajapakse, N.; Horiguchi, T.; Snipes, J.; Busija, D.W. MitoK(ATP) Opener, Diazoxide, Reduces Neuronal Damage after Middle Cerebral Artery Occlusion in the Rat. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1005–H1011. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, L.K.; Aldrich, R.W.; Chandy, K.G.; Grissmer, S.; Wei, A.D.; Wulff, H. International Union of Basic and Clinical Pharmacology. C. Nomenclature and Properties of Calcium-Activated and Sodium-Activated Potassium Channels. Pharmacol. Rev. 2017, 69, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Capera, J.; Serrano-Novillo, C.; Navarro-Pérez, M.; Cassinelli, S.; Felipe, A. The Potassium Channel Odyssey: Mechanisms of Traffic and Membrane Arrangement. Int. J. Mol. Sci. 2019, 20, 734. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, J. Voltage-Gated Potassium Channels and Genetic Epilepsy. Front. Neurol. 2024, 15, 1466075. [Google Scholar] [CrossRef]
- Alfaro-Ruíz, R.; Aguado, C.; Martín-Belmonte, A.; Moreno-Martínez, A.E.; Luján, R. Expression, Cellular and Subcellular Localisation of Kv4.2 and Kv4.3 Channels in the Rodent Hippocampus. Int. J. Mol. Sci. 2019, 20, 246. [Google Scholar] [CrossRef]
- Cui, J. Voltage-Dependent Gating: Novel Insights from KCNQ1 Channels. Biophys. J. 2016, 110, 14–25. [Google Scholar] [CrossRef]
- Ranjan, R.; Logette, E.; Marani, M.; Herzog, M.; Tâche, V.; Scantamburlo, E.; Buchillier, V.; Markram, H. A Kinetic Map of the Homomeric Voltage-Gated Potassium Channel (Kv) Family. Front. Cell. Neurosci. 2019, 13, 358. [Google Scholar] [CrossRef]
- McCoy, M.T.; Jayanthi, S.; Cadet, J.L. Potassium Channels and Their Potential Roles in Substance Use Disorders. Int. J. Mol. Sci. 2021, 22, 1249. [Google Scholar] [CrossRef]
- Beirow, K.; Jedamzik, J.; Schulig, L.; Wurm, K.W.; Lemmerhirt, C.J.; Hofstetter, R.K.; Link, A.; Bednarski, P.J. Structure-Activity Relationships of Flupirtine Analogues for Liver Esterase-Mediated Cleavage of the 4-Fluorobenzylamine Moiety and Its Possible Relevance to Liver Toxicity. ChemMedChem 2023, 18, e202300145. [Google Scholar] [CrossRef]
- Bock, C.; Link, A. How to Replace the Lost Keys? Strategies Toward Safer KV7 Channel Openers. Future Med. Chem. 2019, 11, 337–355. [Google Scholar] [CrossRef]
- Laskowski, M.; Augustynek, B.; Bednarczyk, P.; Żochowska, M.; Kalisz, J.; O’Rourke, B.; Szewczyk, A.; Kulawiak, B. Single-Channel Properties of the ROMK-Pore-Forming Subunit of the Mitochondrial ATP-Sensitive Potassium Channel. Int. J. Mol. Sci. 2019, 20, 5323. [Google Scholar] [CrossRef]
- Henn, M.C.; Janjua, M.B.; Kanter, E.M.; Makepeace, C.M.; Schuessler, R.B.; Nichols, C.G.; Lawton, J.S. Adenosine Triphosphate-Sensitive Potassium Channel Kir Subunits Implicated in Cardioprotection by Diazoxide. J. Am. Heart Assoc. 2015, 4, e002016. [Google Scholar] [CrossRef]
- Inagaki, N.; Tsuura, Y.; Namba, N.; Masuda, K.; Gonoi, T.; Horie, M.; Seino, Y.; Mizuta, M.; Seino, S. Cloning and Functional Characterization of a Novel ATP-Sensitive Potassium Channel Ubiquitously Expressed in Rat Tissues, Including Pancreatic Islets, Pituitary, Skeletal Muscle, and Heart. J. Biol. Chem. 1995, 270, 5691–5694. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Chen, L.; Zhao, Y.-F.; Duan, W.-M.; Zhong, L.-M.; Liu, M.-W. Identification of Key Potassium Channel Genes of Temporal Lobe Epilepsy by Bioinformatics Analyses and Experimental Verification. Front. Neurol. 2023, 14, 1175007. [Google Scholar] [CrossRef]
- Thakore, K.N.; Mehendale, H.M. Chlorzoxazone. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 938–939. ISBN 978-0-12-386455-0. [Google Scholar]
- Alotaibi, H.; Alsergani, R.; Alharbi, A.A.; Nagshabandi, K.N.; Almubark, A.A. Chlorzoxazone-Induced Fixed Drug Eruption: A Clinical Case Report. Int. Med. Case Rep. J. 2024, 17, 771–775. [Google Scholar] [CrossRef]
- Ferraguto, C.; Bouleau, Y.; Peineau, T.; Dulon, D.; Pietropaolo, S. Hyperacusis in the Adult Fmr1-KO Mouse Model of Fragile X Syndrome: The Therapeutic Relevance of Cochlear Alterations and BKCa Channels. Int. J. Mol. Sci. 2023, 24, 11863. [Google Scholar] [CrossRef]
- Rinker, J.A.; Fulmer, D.B.; Trantham-Davidson, H.; Smith, M.L.; Williams, R.W.; Lopez, M.F.; Randall, P.K.; Chandler, L.J.; Miles, M.F.; Becker, H.C.; et al. Differential Potassium Channel Gene Regulation in BXD Mice Reveals Novel Targets for Pharmacogenetic Therapies to Reduce Heavy Alcohol Drinking. Alcohol 2017, 58, 33–45. [Google Scholar] [CrossRef]
- Gelernter, J.; Kranzler, H.R.; Sherva, R.; Koesterer, R.; Almasy, L.; Zhao, H.; Farrer, L.A. Genome-Wide Association Study of Opioid Dependence: Multiple Associations Mapped to Calcium and Potassium Pathways. Biol. Psychiatry 2014, 76, 66–74. [Google Scholar] [CrossRef]
- Brown, D.A.; Passmore, G.M. Neural KCNQ (Kv7) Channels. Br. J. Pharmacol. 2009, 156, 1185–1195. [Google Scholar] [CrossRef]
- Diao, Y.; Tian, Y.; Han, S.; Zhang, N.; Li, J.; Yin, Y. Current Insight into the Role of Voltage-Gated Potassiumion Channel 7 (Kv7) Channels: An Emerging Therapy Target against Epilepsy. Neuropsychiatry 2017, 7, 26–31. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Guo, P.; Fu, J.; Mei, L.; Lv, D.; Wang, J.; Lai, D.; Ye, S.; Yang, H.; et al. Molecular Basis for Ligand Activation of the Human KCNQ2 Channel. Cell Res. 2021, 31, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Petykó, Z.; Tóth, A.; Szabó, I.; Gálosi, R.; Lénárd, L. Neuronal Activity in Rat Medial Prefrontal Cortex during Sucrose Solution Intake. Neuroreport 2009, 20, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Horst, N.K.; Laubach, M. Reward-Related Activity in the Medial Prefrontal Cortex Is Driven by Consumption. Front. Neurosci. 2013, 7, 56. [Google Scholar] [CrossRef]
- Jiang, J.; Correa, C.M.; Geerts, J.; van Gaal, S. The Relationship between Conflict Awareness and Behavioral and Oscillatory Signatures of Immediate and Delayed Cognitive Control. Neuroimage 2018, 177, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Niebaum, J.C.; Chevalier, N.; Guild, R.M.; Munakata, Y. Developing Adaptive Control: Age-Related Differences in Task Choices and Awareness of Proactive and Reactive Control Demands. Cogn. Affect. Behav. Neurosci. 2021, 21, 561–572. [Google Scholar] [CrossRef]
- Salatino, A.; Miccolis, R.; Gammeri, R.; Ninghetto, M.; Belli, F.; Nobili, M.; Mouraux, A.; Ricci, R. Improvement of Impulsivity and Decision Making by Transcranial Direct Current Stimulation of the Dorsolateral Prefrontal Cortex in a Patient with Gambling Disorder. J. Gambl. Stud. 2022, 38, 627–634. [Google Scholar] [CrossRef]
- Wolf, J.A.; Moyer, J.T.; Lazarewicz, M.T.; Contreras, D.; Benoit-Marand, M.; O’Donnell, P.; Finkel, L.H. NMDA/AMPA Ratio Impacts State Transitions and Entrainment to Oscillations in a Computational Model of the Nucleus Accumbens Medium Spiny Projection Neuron. J. Neurosci. 2005, 25, 9080–9095. [Google Scholar] [CrossRef]
- Marti-Prats, L.; Giuliano, C.; Domi, A.; Puaud, M.; Peña-Oliver, Y.; Fouyssac, M.; McKenzie, C.; Everitt, B.J.; Belin, D. The Development of Compulsive Coping Behavior Depends on Dorsolateral Striatum Dopamine-Dependent Mechanisms. Mol. Psychiatry 2023, 28, 4666–4678. [Google Scholar] [CrossRef]
- Martínez-Coria, H.; Serrano-García, N.; López-Valdés, H.E.; López-Chávez, G.S.; Rivera-Alvarez, J.; Romero-Hernández, Á.; Valverde, F.F.; Orozco-Ibarra, M.; Torres-Ramos, M.A. Morin Improves Learning and Memory in Healthy Adult Mice. Brain Behav. 2024, 14, e3444. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Hu, S.; Yang, P.; Long, H.-C.; Ma, Q.-H.; Yin, D.-M.; Xu, G.-Y. HDAC9-Mediated Calmodulin Deacetylation Induces Memory Impairment in Alzheimer’s Disease. CNS Neurosci. Ther. 2024, 30, e14573. [Google Scholar] [CrossRef]
- Klinger, F.; Bajric, M.; Salzer, I.; Dorostkar, M.M.; Khan, D.; Pollak, D.D.; Kubista, H.; Boehm, S.; Koenig, X. δ Subunit-Containing GABAA Receptors Are Preferred Targets for the Centrally Acting Analgesic Flupirtine. Br. J. Pharmacol. 2015, 172, 4946–4958. [Google Scholar] [CrossRef]
- Dong, Z.; Xiang, S.; Pan, C.; Jiang, C.; Bao, S.; Shangguan, W.; Zeng, R.; Li, J.; Lian, Q.; Wu, B. The Excitatory Transmission from Basolateral Nucleus of Amygdala to Nucleus Accumbens Shell Regulates Propofol Self-Administration through AMPA Receptors. Addict. Biol. 2023, 28, e13310. [Google Scholar] [CrossRef]
- Vieitas-Gaspar, N.; Soares-Cunha, C.; Rodrigues, A.J. From Valence Encoding to Motivated Behavior: A Focus on the Nucleus Accumbens Circuitry. Neurosci. Biobehav. Rev. 2025, 172, 106125. [Google Scholar] [CrossRef]
- O’Donovan, B.; Adeluyi, A.; Anderson, E.L.; Cole, R.D.; Turner, J.R.; Ortinski, P.I. Altered Gating of Kv1.4 in the Nucleus Accumbens Suppresses Motivation for Reward. Elife 2019, 8, e47870. [Google Scholar] [CrossRef]
- Haber, S.N.; Knutson, B. The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology 2010, 35, 4–26. [Google Scholar] [CrossRef]
- Balleine, B.W.; Delgado, M.R.; Hikosaka, O. The Role of the Dorsal Striatum in Reward and Decision-Making. J. Neurosci. 2007, 27, 8161–8165. [Google Scholar] [CrossRef]
- Hyder, S.K.; Lazarini-Lopes, W.; Toib, J.; Williams, G.; Sukharev, A.; Forcelli, P.A. Optogenetic Stimulation of the Dorsal Striatum Bidirectionally Controls Seizures. Proc. Natl. Acad. Sci. USA 2025, 122, e2419178122. [Google Scholar] [CrossRef]
- Al-Redouan, A.; Busch, A.; Salaj, M.; Kubova, H.; Druga, R. Dorsal Striatum Is Compromised by Status Epilepticus Induced in Immature Developing Animal Experimental Model of Mesial Temporal Lobe Epilepsy. Int. J. Mol. Sci. 2025, 26, 3349. [Google Scholar] [CrossRef]
- McCutcheon, R.A.; Abi-Dargham, A.; Howes, O.D. Schizophrenia, Dopamine and the Striatum: From Biology to Symptoms. Trends Neurosci. 2019, 42, 205–220. [Google Scholar] [CrossRef]
- Barceló, A.C.; Filippini, B.; Pazo, J.H. The Striatum and Pain Modulation. Cell. Mol. Neurobiol. 2012, 32, 1–12. [Google Scholar] [CrossRef]
- Babiec, W.E.; Jami, S.A.; Guglietta, R.; Chen, P.B.; O’Dell, T.J. Differential Regulation of NMDA Receptor-Mediated Transmission by SK Channels Underlies Dorsal-Ventral Differences in Dynamics of Schaffer Collateral Synaptic Function. J. Neurosci. 2017, 37, 1950–1964. [Google Scholar] [CrossRef]
- Beletskiy, A.; Positselskaya, E.; Vinarskaya, A.K.; Spivak, Y.S.; Dobryakova, Y.V.; Tyulenev, I.; Markevich, V.A.; Bolshakov, A.P. Detailed Analysis of Dorsal-Ventral Gradients of Gene Expression in the Hippocampus of Adult Rats. Int. J. Mol. Sci. 2022, 23, 9948. [Google Scholar] [CrossRef]
- Henke, P.G. Hippocampal Pathway to the Amygdala and Stress Ulcer Development. Brain Res. Bull. 1990, 25, 691–695. [Google Scholar] [CrossRef]
- Lee, A.-R.; Kim, J.-H.; Cho, E.; Kim, M.; Park, M. Dorsal and Ventral Hippocampus Differentiate in Functional Pathways and Differentially Associate with Neurological Disease-Related Genes during Postnatal Development. Front. Mol. Neurosci. 2017, 10, 331. [Google Scholar] [CrossRef]
- Moser, E.; Moser, M.B.; Andersen, P. Spatial Learning Impairment Parallels the Magnitude of Dorsal Hippocampal Lesions, but Is Hardly Present Following Ventral Lesions. J. Neurosci. 1993, 13, 3916–3925. [Google Scholar] [CrossRef]
- Moser, M.B.; Moser, E.I.; Forrest, E.; Andersen, P.; Morris, R.G. Spatial Learning with a Minislab in the Dorsal Hippocampus. Proc. Natl. Acad. Sci. USA 1995, 92, 9697–9701. [Google Scholar] [CrossRef]
- Wei, X.; Centeno, M.V.; Ren, W.; Borruto, A.M.; Procissi, D.; Xu, T.; Jabakhanji, R.; Mao, Z.; Kim, H.; Li, Y.; et al. Activation of the Dorsal, but Not the Ventral, Hippocampus Relieves Neuropathic Pain in Rodents. PAIN 2021, 162, 2865. [Google Scholar] [CrossRef]
- Cembrowski, M.S.; Bachman, J.L.; Wang, L.; Sugino, K.; Shields, B.C.; Spruston, N. Spatial Gene-Expression Gradients Underlie Prominent Heterogeneity of CA1 Pyramidal Neurons. Neuron 2016, 89, 351–368. [Google Scholar] [CrossRef]
- Zhu, Y.; Wendler, C.C.; Shi, O.; Rivkees, S.A. Diazoxide Promotes Oligodendrocyte Differentiation in Neonatal Brain in Normoxia and Chronic Sublethal Hypoxia. Brain Res. 2014, 1586, 64–72. [Google Scholar] [CrossRef]
- Martínez-Moreno, M.; Batlle, M.; Ortega, F.J.; Gimeno-Bayón, J.; Andrade, C.; Mahy, N.; Rodríguez, M.J. Diazoxide Enhances Excitotoxicity-Induced Neurogenesis and Attenuates Neurodegeneration in the Rat Non-Neurogenic Hippocampus. Neuroscience 2016, 333, 229–243. [Google Scholar] [CrossRef][Green Version]
- Calvo, M.; Richards, N.; Schmid, A.B.; Barroso, A.; Zhu, L.; Ivulic, D.; Zhu, N.; Anwandter, P.; Bhat, M.A.; Court, F.A.; et al. Altered Potassium Channel Distribution and Composition in Myelinated Axons Suppresses Hyperexcitability Following Injury. Elife 2016, 5, e12661. [Google Scholar] [CrossRef]
- Peck, L.J.; Patel, R.; Diaz, P.; Wintle, Y.M.; Dickenson, A.H.; Todd, A.J.; Calvo, M.; Bennett, D.L.H. Studying Independent Kcna6 Knock-out Mice Reveals Toxicity of Exogenous LacZ to Central Nociceptor Terminals and Differential Effects of Kv1.6 on Acute and Neuropathic Pain Sensation. J. Neurosci. 2021, 41, 9141–9162. [Google Scholar] [CrossRef]
- Perucca, E.; Taglialatela, M. Targeting Kv7 Potassium Channels for Epilepsy. CNS Drugs 2025, 39, 263–288. [Google Scholar] [CrossRef]
- Elenbaas, J.K. Centrally Acting Oral Skeletal Muscle Relaxants. Am. J. Hosp. Pharm. 1980, 37, 1313–1323. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Lo, Y.-K.; Wu, S.-N. Stimulatory Effects of Chlorzoxazone, a Centrally Acting Muscle Relaxant, on Large Conductance Calcium-Activated Potassium Channels in Pituitary GH3 Cells. Brain Res. 2003, 959, 86–97. [Google Scholar] [CrossRef]
- Ferraguto, C.; Piquemal-Lagoueillat, M.; Lemaire, V.; Moreau, M.M.; Trazzi, S.; Uguagliati, B.; Ciani, E.; Bertrand, S.S.; Louette, E.; Bontempi, B.; et al. Therapeutic Efficacy of the BKCa Channel Opener Chlorzoxazone in a Mouse Model of Fragile X Syndrome. Neuropsychopharmacology 2024, 49, 2032–2041. [Google Scholar] [CrossRef]
- Lemaire-Mayo, V.; Piquemal, M.; Crusio, W.E.; Louette, E.; Pietropaolo, S. Therapeutic Effects of Chlorzoxazone, a BKCa Channel Agonist, in a Mouse Model of Fragile X Syndrome. bioRxiv 2020. [Google Scholar] [CrossRef]
- Piquemal, M.; Abdulkarim-Abdalla, N.; Ortiz-Romero, P.; Lemaire-Mayo, V.; Crusio, W.E.; Louette, E.; Campuzano, V.; Pietropaolo, S. Chlorzoxazone, A BKCa Channel Agonist, Rescues The Pathological Phenotypes Of Williams-Beuren Syndrome In A Preclinical Model. bioRxiv 2020. [Google Scholar] [CrossRef]
- Marinina, K.S.; Bezprozvanny, I.B.; Egorova, P.A. A Combination of Chlorzoxazone and Folic Acid Improves Recognition Memory, Anxiety and Depression in SCA3-84Q Mice. Hum. Mol. Genet. 2024, 33, 1406–1419. [Google Scholar] [CrossRef]
- Marinina, K.S.; Bezprozvanny, I.B.; Egorova, P.A. A Chlorzoxazone-Folic Acid Combination Improves Cognitive Affective Decline in SCA2-58Q Mice. Sci. Rep. 2023, 13, 12588. [Google Scholar] [CrossRef]
- Hopf, F.W.; Simms, J.A.; Chang, S.-J.; Seif, T.; Bartlett, S.E.; Bonci, A. Chlorzoxazone, an SK-Type Potassium Channel Activator Used in Humans, Reduces Excessive Alcohol Intake in Rats. Biol. Psychiatry 2011, 69, 618–624. [Google Scholar] [CrossRef]
- Xie, C.; Kessi, M.; Yin, F.; Peng, J. Roles of KCNA2 in Neurological Diseases: From Physiology to Pathology. Mol. Neurobiol. 2024, 61, 8491–8517. [Google Scholar] [CrossRef]
- Soldovieri, M.V.; Ambrosino, P.; Mosca, I.; Servettini, I.; Pietrunti, F.; Belperio, G.; KCNA3 study group; Syrbe, S.; Taglialatela, M.; Lemke, J.R. De Novo Variants in KCNA3 Cause Developmental and Epileptic Encephalopathy. Ann. Neurol. 2024, 95, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Salpietro, V.; Galassi Deforie, V.; Efthymiou, S.; O’Connor, E.; Marcé-Grau, A.; Maroofian, R.; Striano, P.; Zara, F.; Morrow, M.M.; SYNAPS Study Group; et al. De Novo KCNA6 Variants with Attenuated KV 1.6 Channel Deactivation in Patients with Epilepsy. Epilepsia 2023, 64, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Flupirtine-Containing Medicinal Products—Referral|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/referrals/flupirtine-containing-medicinal-products (accessed on 24 June 2025).
- Dinoi, G.; Morin, M.; Conte, E.; Mor Shaked, H.; Coppola, M.A.; D’Adamo, M.C.; Elpeleg, O.; Liantonio, A.; Hartmann, I.; De Luca, A.; et al. Clinical and Functional Study of a De Novo Variant in the PVP Motif of Kv1.1 Channel Associated with Epilepsy, Developmental Delay and Ataxia. Int. J. Mol. Sci. 2022, 23, 8079. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.C. State-of-the-Art Gene Therapy in Epilepsy. Curr. Opin. Neurol. 2025, 38, 128–134. [Google Scholar] [CrossRef]
- Smart, S.L.; Lopantsev, V.; Zhang, C.L.; Robbins, C.A.; Wang, H.; Chiu, S.Y.; Schwartzkroin, P.A.; Messing, A.; Tempel, B.L. Deletion of the K(V)1.1 Potassium Channel Causes Epilepsy in Mice. Neuron 1998, 20, 809–819. [Google Scholar] [CrossRef]
- Peng, H.; Bian, X.-L.; Ma, F.-C.; Wang, K.-W. Pharmacological Modulation of the Voltage-Gated Neuronal Kv7/KCNQ/M-Channel Alters the Intrinsic Excitability and Synaptic Responses of Pyramidal Neurons in Rat Prefrontal Cortex Slices. Acta Pharmacol. Sin. 2017, 38, 1248–1256. [Google Scholar] [CrossRef]
- Alaimo, A.; Etxeberria, A.; Gómez-Posada, J.C.; Gomis-Perez, C.; Fernández-Orth, J.; Malo, C.; Villarroel, A. Lack of Correlation between Surface Expression and Currents in Epileptogenic AB-Calmodulin Binding Domain Kv7.2 Potassium Channel Mutants. Channels 2018, 12, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Nappi, M.; Barrese, V.; Carotenuto, L.; Lesca, G.; Labalme, A.; Ville, D.; Smol, T.; Rama, M.; Dieux-Coeslier, A.; Rivier-Ringenbach, C.; et al. Gain of Function Due to Increased Opening Probability by Two KCNQ5 Pore Variants Causing Developmental and Epileptic Encephalopathy. Proc. Natl. Acad. Sci. USA 2022, 119, e2116887119. [Google Scholar] [CrossRef]
- Chokvithaya, S.; Caengprasath, N.; Buasong, A.; Jantasuwan, S.; Santawong, K.; Leela-Adisorn, N.; Tongkobpetch, S.; Ittiwut, C.; Saengow, V.E.; Kamolvisit, W.; et al. Nine Patients with KCNQ2-Related Neonatal Seizures and Functional Studies of Two Missense Variants. Sci. Rep. 2023, 13, 3328. [Google Scholar] [CrossRef]
- De Wachter, M.; Millevert, C.; Nicolai, J.; Cats, E.; Kluger, G.; Milh, M.; Cloarec, R.; Syrbe, S.; Arts, K.; Jansen, K.; et al. Amitriptyline Use in Individuals with KCNQ2/3 Gain-of-Function Variants: A Retrospective Cohort Study. Epilepsia 2025, 66, 1628–1640. [Google Scholar] [CrossRef] [PubMed]
- Maljevic, S.; Lerche, H. Potassium Channel Genes and Benign Familial Neonatal Epilepsy. Prog. Brain Res. 2014, 213, 17–53. [Google Scholar] [CrossRef] [PubMed]
- Abidi, A.; Devaux, J.J.; Molinari, F.; Alcaraz, G.; Michon, F.-X.; Sutera-Sardo, J.; Becq, H.; Lacoste, C.; Altuzarra, C.; Afenjar, A.; et al. A Recurrent KCNQ2 Pore Mutation Causing Early Onset Epileptic Encephalopathy Has a Moderate Effect on M Current but Alters Subcellular Localization of Kv7 Channels. Neurobiol. Dis. 2015, 80, 80–92. [Google Scholar] [CrossRef]
- Devaux, J.; Abidi, A.; Roubertie, A.; Molinari, F.; Becq, H.; Lacoste, C.; Villard, L.; Milh, M.; Aniksztejn, L. A Kv7.2 Mutation Associated with Early Onset Epileptic Encephalopathy with Suppression-Burst Enhances Kv7/M Channel Activity. Epilepsia 2016, 57, e87–e93. [Google Scholar] [CrossRef]
- Weckhuysen, S.; Mandelstam, S.; Suls, A.; Audenaert, D.; Deconinck, T.; Claes, L.R.F.; Deprez, L.; Smets, K.; Hristova, D.; Yordanova, I.; et al. KCNQ2 Encephalopathy: Emerging Phenotype of a Neonatal Epileptic Encephalopathy. Ann. Neurol. 2012, 71, 15–25. [Google Scholar] [CrossRef]
- Huang, P.; Li, C.; Fu, T.; Zhao, D.; Yi, Z.; Lu, Q.; Guo, L.; Xu, X. Flupirtine Attenuates Chronic Restraint Stress-Induced Cognitive Deficits and Hippocampal Apoptosis in Male Mice. Behav. Brain Res. 2015, 288, 1–10. [Google Scholar] [CrossRef]
- Mooney, J.; Rawls, S.M. KCNQ2/3 Channel Agonist Flupirtine Reduces Cocaine Place Preference in Rats. Behav. Pharmacol. 2017, 28, 405–407. [Google Scholar] [CrossRef]
- Block, F.; Pergande, G.; Schwarz, M. Flupirtine Reduces Functional Deficits and Neuronal Damage after Global Ischemia in Rats. Brain Res. 1997, 754, 279–284. [Google Scholar] [CrossRef]
- Schmidt, W.J.; Schuster, G.; Wacker, E.; Pergande, G. Antiparkinsonian and Other Motor Effects of Flupirtine Alone and in Combination with Dopaminergic Drugs. Eur. J. Pharmacol. 1997, 327, 1–9. [Google Scholar] [CrossRef]
- Schwarz, M.; Nolden-Koch, M.; Purr, J.; Pergande, G.; Block, F. Antiparkinsonian Effect of Flupirtine in Monoamine-Depleted Rats. J. Neural Transm. 1996, 103, 581–590. [Google Scholar] [CrossRef]
- Authier, N.; Gillet, J.-P.; Fialip, J.; Eschalier, A.; Coudore, F. Assessment of Neurotoxicity Following Repeated Cremophor/Ethanol Injections in Rats. Neurotox. Res. 2001, 3, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Farkas, E.; Institóris, A.; Domoki, F.; Mihály, A.; Bari, F. The effect of pre- and posttreatment with diazoxide on the early phase of chronic cerebral hypoperfusion in the rat. Brain Res. 2006, 1087, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Mayanagi, K.; Gáspár, T.; Katakam, P.V.; Busija, D.W. Systemic administration of diazoxide induces delayed preconditioning against transient focal cerebral ischemia in rats. Brain Res. 2007, 1168, 106–111. [Google Scholar] [CrossRef] [PubMed]
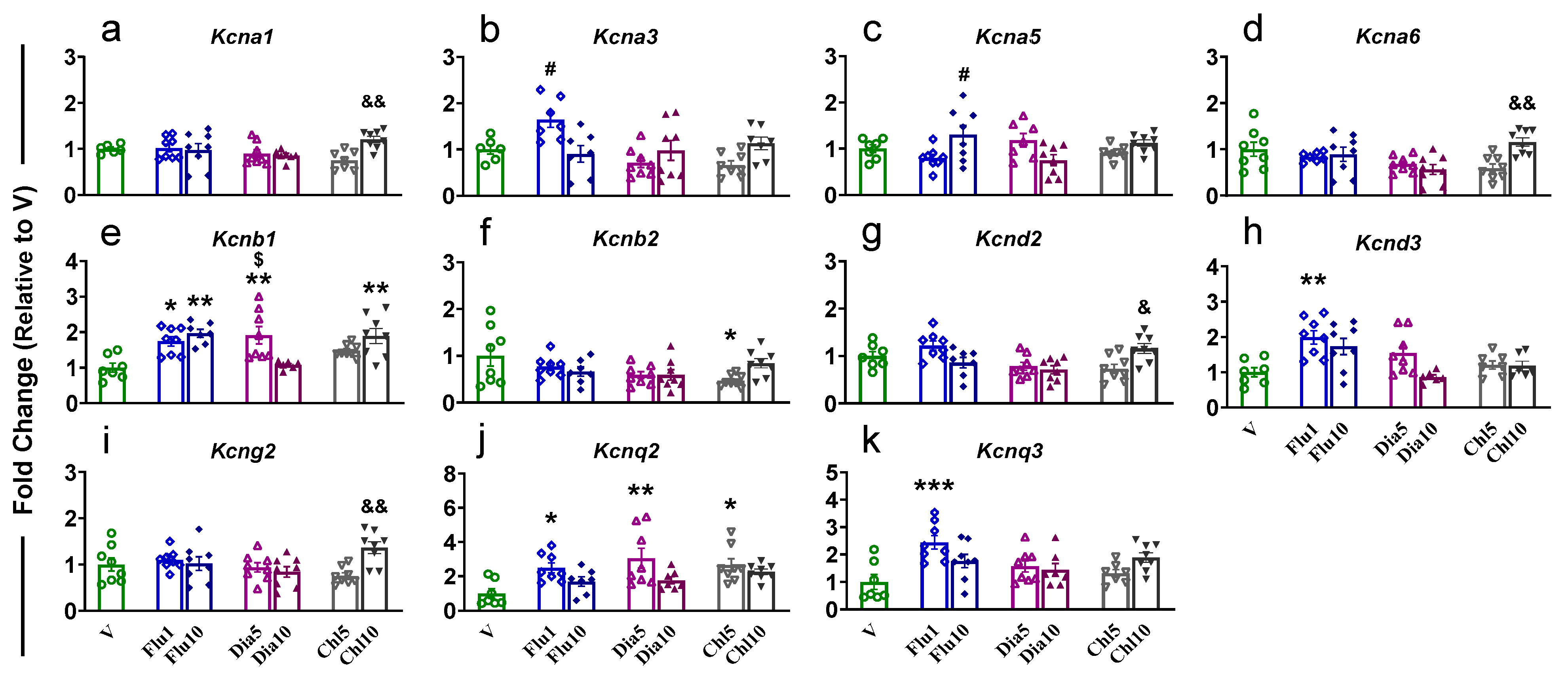
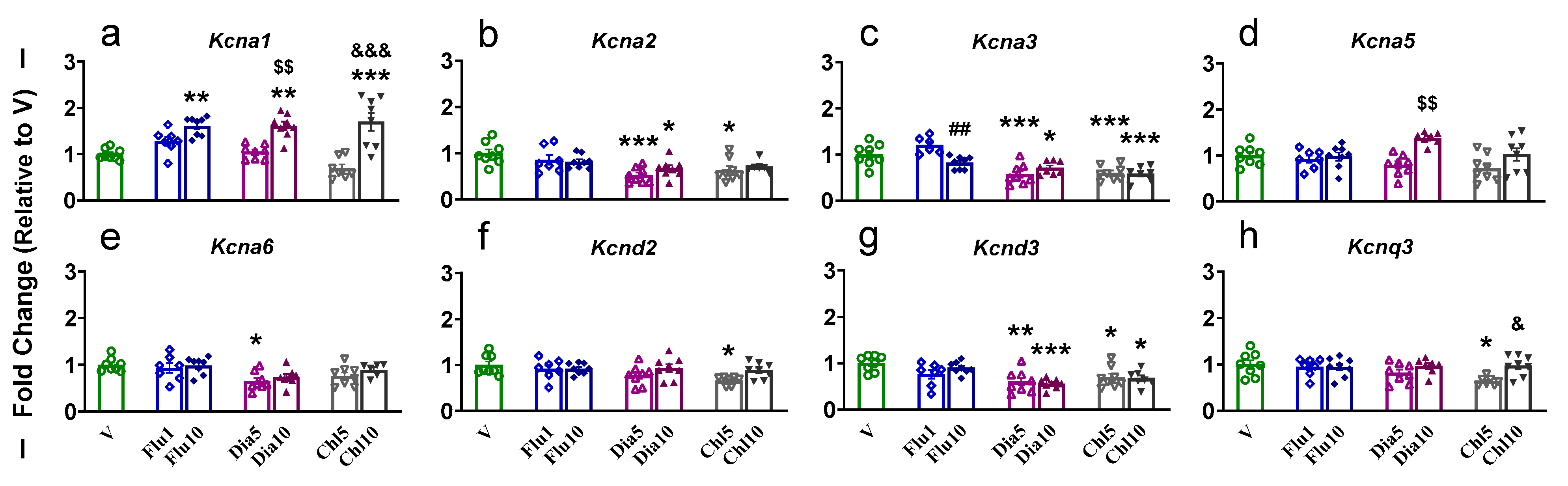

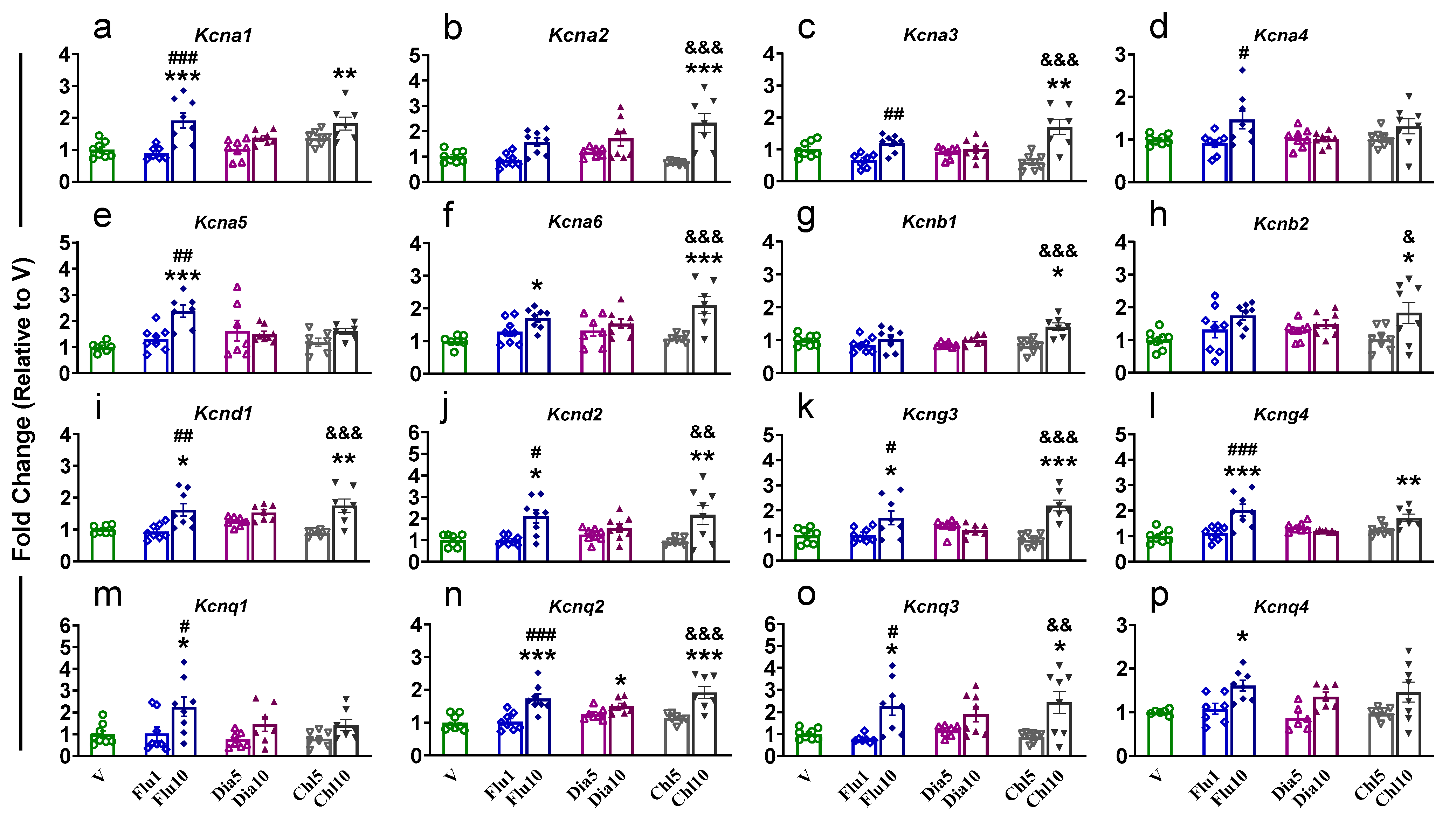

| PFC | NAc | dSTR | dHIP | vHIP | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Flu 1 | Flu 10 | Dia5 | Dia10 | Chl5 | Chl10 | Flu 1 | Flu 10 | Dia5 | Dia10 | Chl5 | Chl10 | Flu 1 | Flu 10 | Dia5 | Dia10 | Chl5 | Chl10 | Flu 1 | Flu 10 | Dia5 | Dia10 | Chl5 | Chl10 | Flu 1 | Flu 10 | Dia5 | Dia10 | Chl5 | Chl10 |
| Kcna1 | − | − | − | − | − | ↑d | − | ↑a | − | ↑ac | − | ↑ad | − | ↑b | − | ↑c | − | − | − | ↑ab | − | − | − | ↑a | − | − | − | − | − | − |
| Kcna2 | − | − | − | − | − | − | − | − | ↓a | ↓a | ↓a | − | ↓a | − | ↓a | ↓a | − | − | − | − | − | − | − | ↑ad | − | ↑a | ↑a | − | − | ↑a |
| Kcna3 | ↑b | − | − | − | − | − | − | ↓b | ↓a | ↓a | ↓a | ↓a | − | − | − | − | − | − | − | ↓b | − | − | − | ↑ad | − | ↑a | − | − | − | ↑ad |
| Kcna4 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ↑b | − | − | − | − | − | ↑b | − | − | − | − |
| Kcna5 | ↑b | − | − | − | − | − | − | − | − | ↑c | − | − | − | − | − | − | − | − | − | ↑ab | − | − | − | − | − | − | − | − | − | − |
| Kcna6 | − | − | − | − | − | ↑d | − | − | ↓a | − | − | − | − | ↑b | ↓a | ↓a | − | − | − | ↑a | − | − | − | ↑ad | − | ↑a | ↑a | ↑a | ↑a | ↑a |
| Kcnb1 | ↑a | ↑a | ↑ac | − | − | ↑a | − | − | − | − | − | − | − | − | − | ↓a | − | − | − | − | − | − | − | ↑ad | − | ↑a | − | − | − | − |
| Kcnb2 | − | − | − | − | ↓a | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ↑ad | − | − | − | − | − | − |
| Kcnb3 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ↑a | ↑ac | − | − | ↑d |
| Kcnd1 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ↑ab | − | − | − | ↑ad | − | ↑a | − | − | ↑a | − |
| Kcnd2 | − | − | − | − | − | ↑d | − | − | − | − | ↓a | − | − | − | − | − | − | − | − | ↑ab | − | − | − | ↑ad | − | − | − | − | − | − |
| Kcnd3 | ↑a | − | − | − | − | − | − | − | ↓a | ↓a | ↓a | ↓a | ↓a | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Kcng1 | − | − | − | − | − | − | − | − | − | − | − | − | − | ↓a | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Kcng2 | − | − | − | − | − | ↑d | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Kcng3 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ↑ab | − | − | − | ↑ad | − | − | ↑a | − | − | ↑a |
| Kcng4 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ↑ab | − | − | − | ↑a | − | ↑ab | − | − | − | ↑a |
| Kcnq1 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ↑ab | − | − | − | − | − | − | − | − | − | − |
| Kcnq2 | ↑a | − | ↑a | − | ↑a | − | − | − | − | − | − | − | − | − | − | ↓a | − | − | − | ↑ab | − | ↑a | − | ↑ad | − | ↑ab | − | − | − | − |
| Kcnq3 | ↑a | − | − | − | − | − | − | − | − | − | ↓ad | − | − | − | − | − | − | − | − | ↑ab | − | − | − | ↑ad | − | ↑b | − | − | − | − |
| Kcnq4 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ↑a | − | − | − | − | − | − | − | − | − | ↑a |
| Primers | Seq Up | Seq Down |
|---|---|---|
| Kcna1 | TTG GTA AGG GTG TTC AGA AT | GCA AAG TAC ACT GCA CTA GA |
| Kcna2 | TCT CCA TGA CAA CTG TAG GCT ATG | GAC TGG TAA GGC AAT GGT TAA CAC |
| Kcna3 | TCA TCT TCT GCT TGG AGA CA | TAT TTC TGG AGA AGG TGG CTT TA |
| Kcna4 | CAT GAC AAC TGT GGG CTA CGG | CGG GCA AAG CAA TGG TTA AGA |
| Kcna5 | TTC TCT AGT ATC CCA GAT GC | CCC GAT GAT AGA AGT AAT TAA AG |
| Kcna6 | TAT GGA AGA GAT TCG CTT CTA | GAA CTC TCC GGA TAC TCA AA |
| Kcnb1 | CTC CAT CTA CAC CAC AGC AAG T | CTG AAC TTG GGA CTG GTA CTC C |
| Kcnb2 | CAC AAC TGT AGG CAA GAC ATT TA | TCC TGG GTT AGA ATG AAT TTC TG |
| Kcnb3 | AGC TTA ATC CAA ATA GCA AGT G | AGC CAT ATA TTA GGA CAA GGG |
| Kcnd1 | GAA TCT TCA AGT TCT CCA GGC A | AAA GAT GAT GAT AGC CAT GGT |
| Kcnd2 | TTG TGA ATG AGC ACA ATG AAA | ATT CAA CTG GCA CAT TAT GTC |
| Kcnd3 | TCT TGT GGA TGA TCC CCT GTT G | GGG TAG TTC TGC ATT GAG CTC T |
| Kcng1 | AAA GGA TCT GTG TCT CTT AGT | CTT AAA GGT CTG TCT GTT TGC |
| Kcng2 | GTA GCC TGG AGG AGA TCG CAA | GGA ATT TCT GGG ACT CAA TTT T |
| Kcng3 | CTC TCC GCT GAG TTC CTG AAT T | CCC AGG GAG AAA CAC GTG AAT A |
| Kcng4 | TGT CCA CAT ATC CAT GTG TTC | GGT CAC TTT ATT TCA GAT TCG TC |
| Kcnq1 | GTG ATG TTG ACC ACT TCC GAA TAC | TCA CTT TAG GGG AGA AGT TGT CAG |
| Kcnq2 | CTC TAC TCT GGT GAG GAA TAA TC | AAC CGA GGG CTC TAT TAT ATC |
| Kcnq3 | CCA GGA TGA GGA ATG CAA ATT AGT C | TCA GGA GGA GTA AAA ATG GGT GAT T |
| Kcnq4 | ATA GGC AGA AAC ACT TTG AGA | TAG TAA TAC CAG GTG GCT GTC |
| Rps5 | CTC CAT GAT GAT GCA CGG | CCA ATG CGT GTG GAG TC |
| Ubc | CAA CAT CCA GAA GGA GTC | GTA CGA GTA TCT TCC TGT TT |
| Tbp | ACT CTT CCA TTC TCA AAC TCT A | GTC AAG TTT ACA GCC AAG AT |
| Tubb2b | TAC AAC GAA GCA ACT GGT AAT | AGC TTT CTG ACT CCT TCC TAA |
| Rn18s | GCG CAA ATT ACC CAC T | ATC CAA CTA CGA GCT T |
| B2m | GAT CTT TCT GGT GCT TGT | AGC TCA ATT TCT ATT TGA GGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCoy, M.T.; Ladenheim, B.; Cadet, J.L.; Daiwile, A.P. Pharmacological Actions of Potassium Channel Openers on Voltage-Gated Potassium Channels. Pharmaceuticals 2025, 18, 1446. https://doi.org/10.3390/ph18101446
McCoy MT, Ladenheim B, Cadet JL, Daiwile AP. Pharmacological Actions of Potassium Channel Openers on Voltage-Gated Potassium Channels. Pharmaceuticals. 2025; 18(10):1446. https://doi.org/10.3390/ph18101446
Chicago/Turabian StyleMcCoy, Michael T., Bruce Ladenheim, Jean Lud Cadet, and Atul P. Daiwile. 2025. "Pharmacological Actions of Potassium Channel Openers on Voltage-Gated Potassium Channels" Pharmaceuticals 18, no. 10: 1446. https://doi.org/10.3390/ph18101446
APA StyleMcCoy, M. T., Ladenheim, B., Cadet, J. L., & Daiwile, A. P. (2025). Pharmacological Actions of Potassium Channel Openers on Voltage-Gated Potassium Channels. Pharmaceuticals, 18(10), 1446. https://doi.org/10.3390/ph18101446






