Src and Abl as Therapeutic Targets in Lung Cancer: Opportunities for Drug Repurposing
Abstract
1. Introduction
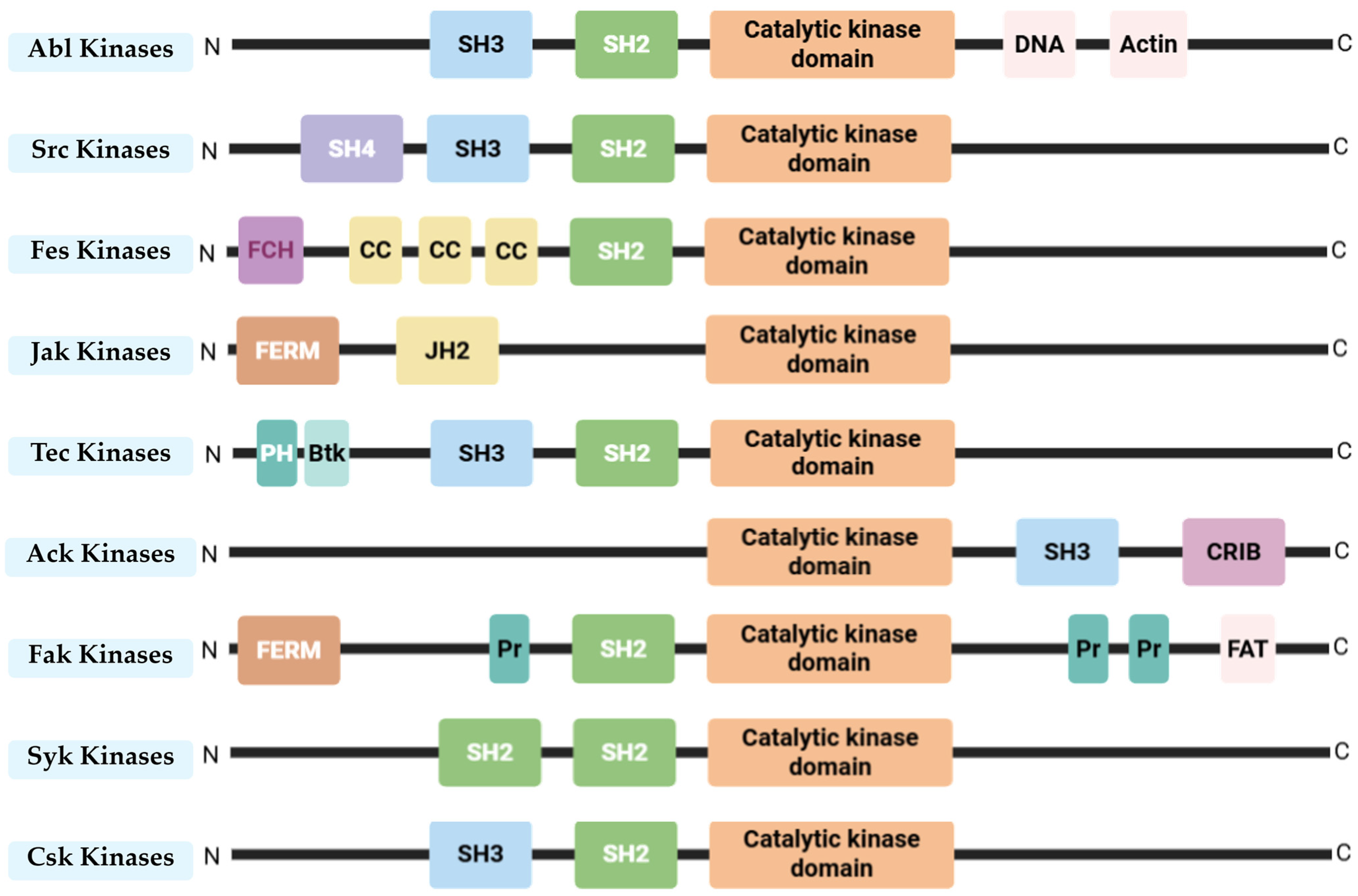
2. Src and Abl—Non-Receptor Tyrosine Kinases
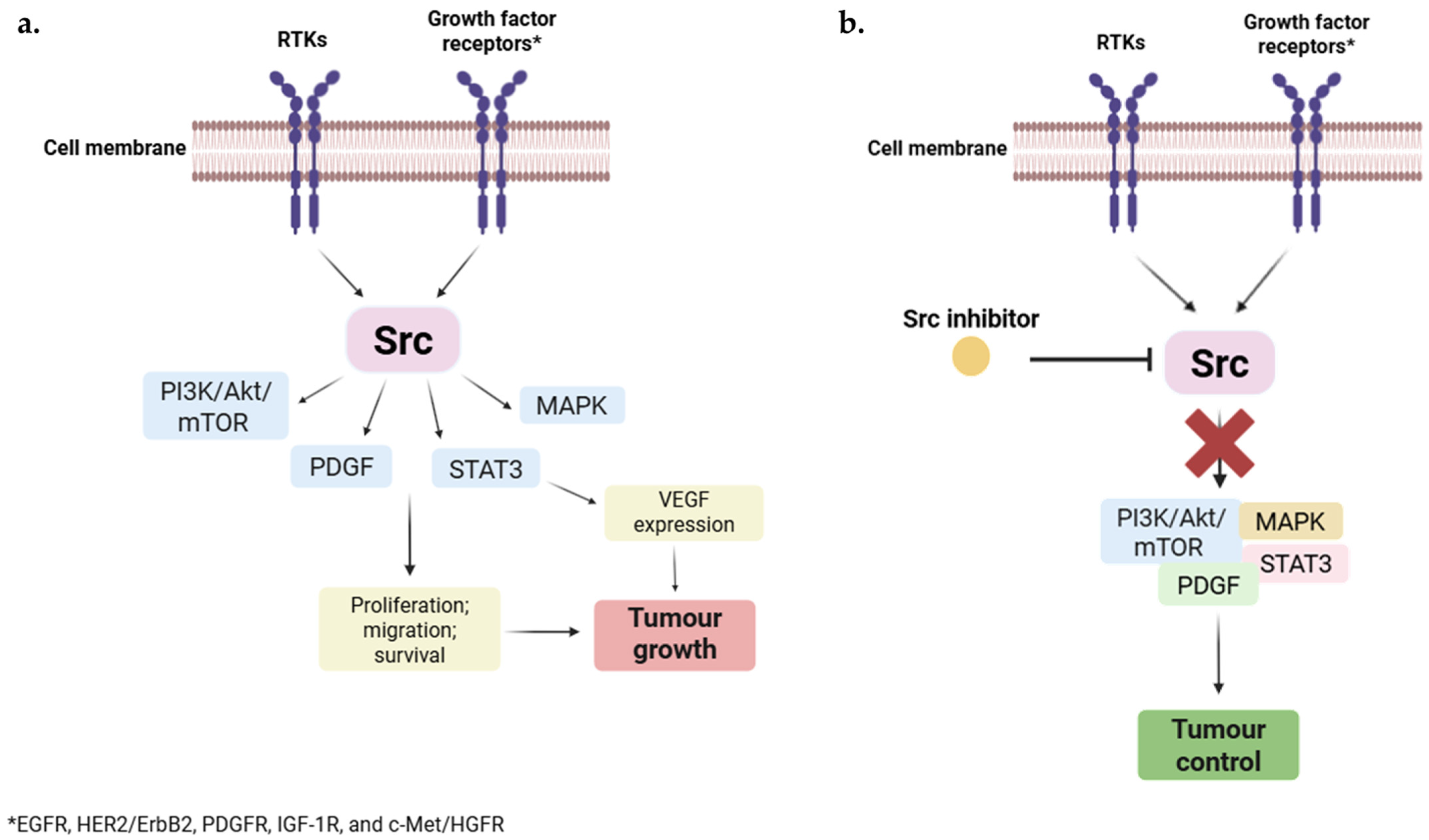
2.1. Src
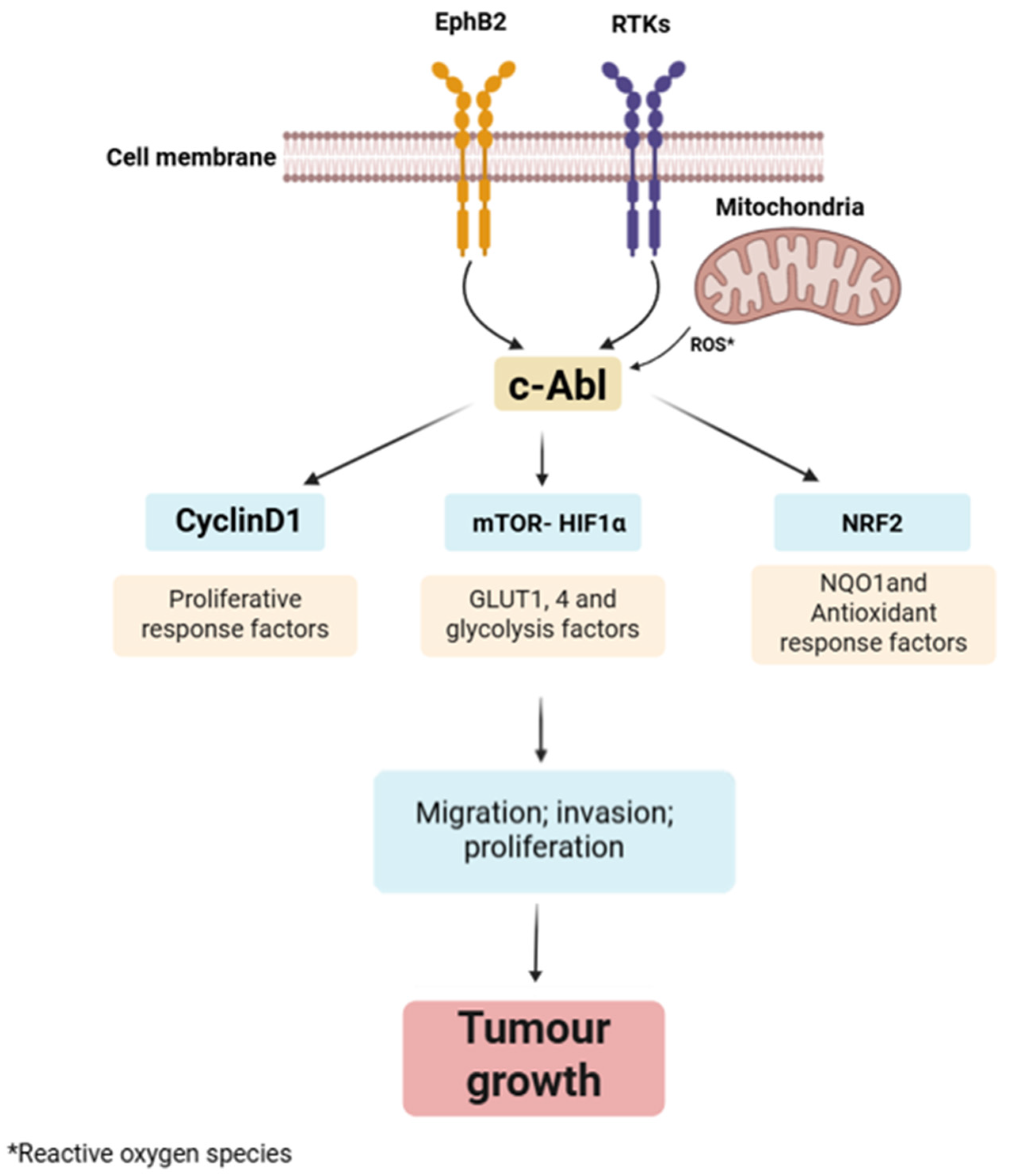
2.2. Abl
3. Src and Abl Inhibitors
3.1. Saracatinib
3.2. Imatinib
3.3. Nilotinib
3.4. PP2
3.5. Tirbanibulin
4. Lung Cancer
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| CML | Chronic myeloid leukemia |
| DDR | DNA damage response |
| EMT | Epithelial–mesenchymal transition |
| FDA | US Food and Drug Administration |
| GI50 | Maximal inhibition of cell growth |
| IC50 | Half maximal inhibitory concentration |
| LDCT | Low-dose computer tomography |
| NRTKs | Non-receptor tyrosine kinases |
| NSCLC | Non-small cell lung cancer |
| PM | Personalized medicine |
| PTKs | Protein tyrosine kinases |
| RTKs | Receptor tyrosine kinases |
| SCLC | Small cell lung cancer |
| SFKs | Src family of protein tyrosine kinases |
References
- Luchini, C.; Pea, A.; Scarpa, A. Artificial intelligence in oncology: Current applications and future perspectives. Br. J. Cancer 2022, 126, 4–9. [Google Scholar] [CrossRef]
- Dharani, S.; Kamaraj, R. A Review of the Regulatory Challenges of Personalized Medicine. Cureus 2024, 16, e67891. [Google Scholar] [CrossRef]
- Singh, D.; Dhiman, V.K.; Pandey, M.; Dhiman, V.K.; Sharma, A.; Pandey, H.; Verma, S.K.; Pandey, R. Personalized medicine: An alternative for cancer treatment. Cancer Treat. Res. Commun. 2024, 42, 100860. [Google Scholar] [CrossRef]
- Hoeben, A.; Joosten, E.A.J.; Van Den Beuken-Van Everdingen, M.H.J. Personalized Medicine: Recent Progress in Cancer Therapy. Cancers 2021, 13, 242. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Fountzilas, E.; Nikanjam, M.; Kurzrock, R. Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat. Rev. 2020, 86, 102019. [Google Scholar] [CrossRef]
- Cohen, P.; Cross, D.; Jänne, P.A. Kinase drug discovery 20 years after imatinib: Progress and future directions. Nat. Rev. Drug Discov. 2021, 20, 551–569. [Google Scholar] [CrossRef]
- Masucci, M.; Karlsson, C.; Blomqvist, L.; Ernberg, I. Bridging the Divide: A Review on the Implementation of Personalized Cancer Medicine. J. Pers. Med. 2024, 14, 561. [Google Scholar] [CrossRef]
- Sha, C.; Lee, P.C. EGFR-Targeted Therapies: A Literature Review. J. Clin. Med. 2024, 13, 6391. [Google Scholar] [CrossRef]
- Lotfi Shahreza, M.; Ghadiri, N.; Mousavi, S.R.; Varshosaz, J.; Green, J.R. A review of network-based approaches to drug repositioning. Brief. Bioinform. 2018, 19, 878–892. [Google Scholar] [CrossRef]
- Kulkarni, V.S.; Alagarsamy, V.; Solomon, V.R.; Jose, P.A.; Murugesan, S. Drug Repurposing: An Effective Tool in Modern Drug Discovery. Russ. J. Bioorg. Chem. 2023, 49, 157–166. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, M.; Huang, H.; Jin, W.-L. Drug repurposing for cancer therapy. Signal Transduct. Target. Ther. 2024, 9, 92. [Google Scholar] [CrossRef]
- Schein, C.H. Repurposing approved drugs for cancer therapy. Br. Med. Bull. 2021, 137, 13–27. [Google Scholar] [CrossRef]
- Hijazi, M.A.; Gessner, A.; El-Najjar, N. Repurposing of Chronically Used Drugs in Cancer Therapy: A Chance to Grasp. Cancers 2023, 15, 3199. [Google Scholar] [CrossRef]
- Mohty, M.; Terpos, E.; Mateos, M.-V.; Cavo, M.; Lejniece, S.; Beksac, M.; Bekadja, M.A.; Legiec, W.; Dimopoulos, M.; Stankovic, S.; et al. Multiple Myeloma Treatment in Real-world Clinical Practice: Results of a Prospective, Multinational, Noninterventional Study. Clin. Lymphoma Myeloma Leuk. 2018, 18, e401–e419. [Google Scholar] [CrossRef]
- Rao, Y.; Li, R.; Zhang, D. A drug from poison: How the therapeutic effect of arsenic trioxide on acute promyelocytic leukemia was discovered. Sci. China Life Sci. 2013, 56, 495–502. [Google Scholar] [CrossRef]
- Sanz, M.A.; Fenaux, P.; Tallman, M.S.; Estey, E.H.; Löwenberg, B.; Naoe, T.; Lengfelder, E.; Döhner, H.; Burnett, A.K.; Chen, S.-J.; et al. Management of acute promyelocytic leukemia: Updated recommendations from an expert panel of the European LeukemiaNet. Blood 2019, 133, 1630–1643. [Google Scholar] [CrossRef]
- Vargesson, N. The teratogenic effects of thalidomide on limbs. J. Hand Surg. 2019, 44, 88–95. [Google Scholar] [CrossRef]
- Al Khzem, A.H.; Gomaa, M.S.; Alturki, M.S.; Tawfeeq, N.; Sarafroz, M.; Alonaizi, S.M.; Al Faran, A.; Alrumaihi, L.A.; Alansari, F.A.; Alghamdi, A.A. Drug Repurposing for Cancer Treatment: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 12441. [Google Scholar] [CrossRef]
- Greuber, E.K.; Smith-Pearson, P.; Wang, J.; Pendergast, A.M. Role of ABL family kinases in cancer: From leukaemia to solid tumours. Nat. Rev. Cancer 2013, 13, 559–571. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, D. Targeting Src family kinases in anti-cancer therapies: Turning promise into triumph. Trends Pharmacol. Sci. 2012, 33, 122–128. [Google Scholar] [CrossRef]
- Bhanumathy, K.K.; Balagopal, A.; Vizeacoumar, F.S.; Vizeacoumar, F.J.; Freywald, A.; Giambra, V. Protein Tyrosine Kinases: Their Roles and Their Targeting in Leukemia. Cancers 2021, 13, 184. [Google Scholar] [CrossRef]
- Eshaq, A.M.; Flanagan, T.W.; Hassan, S.Y.; Al Asheikh, S.A.; Al-Amoudi, W.A.; Santourlidis, S.; Hassan, S.L.; Alamodi, M.O.; Bendhack, M.L.; Alamodi, M.O.; et al. Non-Receptor Tyrosine Kinases: Their Structure and Mechanistic Role in Tumor Progression and Resistance. Cancers 2024, 16, 2754. [Google Scholar] [CrossRef]
- Siveen, K.S.; Prabhu, K.S.; Achkar, I.W.; Kuttikrishnan, S.; Shyam, S.; Khan, A.Q.; Merhi, M.; Dermime, S.; Uddin, S. Role of Non Receptor Tyrosine Kinases in Hematological Malignances and its Targeting by Natural Products. Mol. Cancer 2018, 17, 31. [Google Scholar] [CrossRef]
- Schenone, S.; Brullo, C.; Musumeci, F.; Botta, M. Novel dual Src/Abl inhibitors for hematologic and solid malignancies. Expert Opin. Investig. Drugs 2010, 19, 931–945. [Google Scholar] [CrossRef]
- Wang, J.; Pendergast, A.M. The Emerging Role of ABL Kinases in Solid Tumors. Trends Cancer 2015, 1, 110–123. [Google Scholar] [CrossRef]
- Portugal, C.C.; Almeida, T.O.; Socodato, R.; Relvas, J.B. Src family kinases (SFKs): Critical regulators of microglial homeostatic functions and neurodegeneration in Parkinson’s and Alzheimer’s diseases. FEBS J. 2022, 289, 7760–7775. [Google Scholar] [CrossRef]
- Chang, J.H.; Gill, S.; Settleman, J.; Parsons, S.J. c-Src regulates the simultaneous rearrangement of actin cytoskeleton, p190RhoGAP, and p120RasGAP following epidermal growth factor stimulation. J. Cell Biol. 1995, 130, 355–368. [Google Scholar] [CrossRef]
- Niu, G.; Bowman, T.; Huang, M.; Shivers, S.; Reintgen, D.; Daud, A.; Chang, A.; Kraker, A.; Jove, R.; Yu, H. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene 2002, 21, 7001–7010. [Google Scholar] [CrossRef]
- Wang, W.; Liu, F.; Wang, C.; Wang, C.; Tang, Y.; Jiang, Z. Src Promotes Metastasis of Human Non-Small Cell Lung Cancer Cells through Fn14-Mediated NF-κB Signaling. Med. Sci. Monit. 2018, 24, 1282–1294. [Google Scholar] [CrossRef]
- Luttman, J.H.; Colemon, A.; Mayro, B.; Pendergast, A.M. Role of the ABL tyrosine kinases in the epithelial–mesenchymal transition and the metastatic cascade. Cell Commun. Signal. 2021, 19, 59. [Google Scholar] [CrossRef]
- Bohio, A.; Wang, R.; Zeng, X.; Ba, X. c-Abl-mediated tyrosine phosphorylation of DNA damage response proteins and implications in important cellular functions (Review). Mol. Med. Rep. 2020, 22, 612–619. [Google Scholar] [CrossRef]
- Wong, S.; Witte, O.N. The BCR-ABL story: Bench to bedside and back. Annu. Rev. Immunol. 2004, 22, 247–306. [Google Scholar] [CrossRef]
- Musumeci, F.; Schenone, S.; Brullo, C.; Botta, M. An update on dual Src/Abl inhibitors. Future Med. Chem. 2012, 4, 799–822. [Google Scholar] [CrossRef]
- Roskoski, R. Properties of FDA-approved small molecule protein kinase inhibitors: A 2024 update. Pharmacol. Res. 2024, 200, 107059. [Google Scholar] [CrossRef]
- Breen, M.E.; Steffey, M.E.; Lachacz, E.J.; Kwarcinski, F.E.; Fox, C.C.; Soellner, M.B. Substrate Activity Screening with Kinases: Discovery of Small-Molecule Substrate-Competitive c-Src Inhibitors. Angew. Chem. Int. Ed. 2014, 53, 7010–7013. [Google Scholar] [CrossRef]
- Hong, J.-B.; Wu, P.-Y.; Qin, A.; Huang, Y.-W.; Tseng, K.-C.; Lai, C.-Y.; Chan, W.-K.; Fang, J.; Cutler, D.L.; Tsai, T.-F. Topical Tirbanibulin, a Dual Src Kinase and Tubulin Polymerization Inhibitor, for the Treatment of Plaque-Type Psoriasis: Phase I Results. Pharmaceutics 2022, 14, 2159. [Google Scholar] [CrossRef]
- Jones, J.K.; Thompson, E.M. Allosteric Inhibition of ABL Kinases: Therapeutic Potential in Cancer. Mol. Cancer Ther. 2020, 19, 1763–1769. [Google Scholar] [CrossRef]
- Carofiglio, F.; Trisciuzzi, D.; Gambacorta, N.; Leonetti, F.; Stefanachi, A.; Nicolotti, O. Bcr-Abl Allosteric Inhibitors: Where We Are and Where We Are Going to. Molecules 2020, 25, 4210. [Google Scholar] [CrossRef]
- Lahmouad, M.; Rachid, Z.; Bellemrrabet, R.; Zerrouk, J.; Goh, K.W.; Bouyahya, A.; Aboussalah, Y. Mechanisms and signaling pathways of tyrosine kinase inhibitor resistance in chronic myeloid leukemia: A comprehensive review. Leuk. Res. Rep. 2025, 24, 100533. [Google Scholar] [CrossRef]
- Higuchi, M.; Ishiyama, K.; Maruoka, M.; Kanamori, R.; Takaori-Kondo, A.; Watanabe, N. Paradoxical activation of c-Src as a drug-resistant mechanism. Cell Rep. 2021, 34, 108876. [Google Scholar] [CrossRef]
- Kessler, B.E.; Mishall, K.M.; Kellett, M.D.; Clark, E.G.; Pugazhenthi, U.; Pozdeyev, N.; Kim, J.; Tan, A.C.; Schweppe, R.E. Resistance to Src inhibition alters the BRAF-mutant tumor secretome to promote an invasive phenotype and therapeutic escape through a FAK>p130Cas>c-Jun signaling axis. Oncogene 2019, 38, 2565–2579. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zheng, C.; Hou, K.; Zhang, T.; Qu, X.; Liu, Y.; Kang, J.; Hu, X.; Che, X. Tyrosine kinase inhibitor-induced IL-6/STAT3 activation decreases sensitivity of EGFR-mutant non-small cell lung cancer to icotinib. Cell Biol. Int. 2018, 42, 1292–1299. [Google Scholar] [CrossRef]
- Diaz-Jimenez, A.; Ramos, M.; Helm, B.; Chocarro, S.; Frey, D.L.; Agrawal, S.; Somogyi, K.; Klingmüller, U.; Lu, J.; Sotillo, R. Concurrent inhibition of ALK and SRC kinases disrupts the ALK lung tumor cell proteome. Drug Resist. Updat. 2024, 74, 101081. [Google Scholar] [CrossRef]
- Rubio-Cuesta, B.; Carretero-Puche, C.; Llamas, P.; Sarmentero, J.; Gil-Calderon, B.; Lens-Pardo, A.; Antón-Pascual, B.; Rubio-González, E.; Cámara-Jurado, M.; Salamanca, J.; et al. Co-targeting SRC overcomes resistance to BRAF inhibitors in colorectal cancer. Br. J. Cancer 2025, 133, 404–419. [Google Scholar] [CrossRef]
- Sirvent, A.; Boureux, A.; Simon, V.; Leroy, C.; Roche, S. The tyrosine kinase Abl is required for Src-transforming activity in mouse fibroblasts and human breast cancer cells. Oncogene 2007, 26, 7313–7323. [Google Scholar] [CrossRef][Green Version]
- Warmuth, M.; Simon, N.; Mitina, O.; Mathes, R.; Fabbro, D.; Manley, P.W.; Buchdunger, E.; Forster, K.; Moarefi, I.; Hallek, M. Dual-specific Src and Abl kinase inhibitors, PP1 and CGP76030, inhibit growth and survival of cells expressing imatinib mesylate–resistant Bcr-Abl kinases. Blood 2003, 101, 664–672. [Google Scholar] [CrossRef]
- Wang, W.; Sun, Y.; Liu, X.; Kumar, S.K.; Jin, F.; Dai, Y. Dual-Targeted Therapy Circumvents Non-Genetic Drug Resistance to Targeted Therapy. Front. Oncol. 2022, 12, 859455. [Google Scholar] [CrossRef]
- Aleshin, A.; Finn, R.S. SRC: A century of science brought to the clinic. Neoplasia 2010, 12, 599–607. [Google Scholar] [CrossRef]
- Hennequin, L.F.; Allen, J.; Breed, J.; Curwen, J.; Fennell, M.; Green, T.P.; Lambert-van der Brempt, C.; Morgentin, R.; Norman, R.A.; Olivier, A.; et al. N-(5-chloro-1,3-benzodioxol-4-yl)-7-[2-(4-methylpiperazin-1-yl)ethoxy]-5- (tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine, a novel, highly selective, orally available, dual-specific c-Src/Abl kinase inhibitor. J. Med. Chem. 2006, 49, 6465–6488. [Google Scholar] [CrossRef]
- Jha, V.; Macchia, M.; Tuccinardi, T.; Poli, G. Three-Dimensional Interactions Analysis of the Anticancer Target c-Src Kinase with Its Inhibitors. Cancers 2020, 12, 2327. [Google Scholar] [CrossRef]
- Folch, J.; Petrov, D.; Ettcheto, M.; Abad, S.; Sánchez-López, E.; García, M.L.; Olloquequi, J.; Beas-Zarate, C.; Auladell, C.; Camins, A. Current Research Therapeutic Strategies for Alzheimer’s Disease Treatment. Neural Plast. 2016, 2016, 8501693. [Google Scholar] [CrossRef]
- Kaufman, A.C.; Salazar, S.V.; Haas, L.T.; Yang, J.; Kostylev, M.A.; Jeng, A.T.; Robinson, S.A.; Gunther, E.C.; Van Dyck, C.H.; Nygaard, H.B.; et al. Fyn inhibition rescues established memory and synapse loss in Alzheimer mice. Ann. Neurol. 2015, 77, 953–971. [Google Scholar] [CrossRef]
- Ramos, R.; Vale, N. Dual Drug Repurposing: The Example of Saracatinib. Int. J. Mol. Sci. 2024, 25, 4565. [Google Scholar] [CrossRef]
- Puls, L.N.; Eadens, M.; Messersmith, W. Current Status of Src Inhibitors in Solid Tumor Malignancies. Oncologist 2011, 16, 566–578. [Google Scholar] [CrossRef]
- Formisano, L.; D’Amato, V.; Servetto, A.; Brillante, S.; Raimondo, L.; Di Mauro, C.; Marciano, R.; Orsini, R.C.; Cosconati, S.; Randazzo, A.; et al. Src inhibitors act through different mechanisms in Non-Small Cell Lung Cancer models depending on EGFR and RAS mutational status. Oncotarget 2015, 6, 26090–26103. [Google Scholar] [CrossRef]
- Laurie, S.A.; Goss, G.D.; Shepherd, F.A.; Reaume, M.N.; Nicholas, G.; Philip, L.; Wang, L.; Schwock, J.; Hirsh, V.; Oza, A.; et al. A phase II trial of saracatinib, an inhibitor of src kinases, in previously-treated advanced non-small-cell lung cancer: The princess margaret hospital phase II consortium. Clin. Lung Cancer 2014, 15, 52–57. [Google Scholar] [CrossRef]
- Capdeville, R.; Silberman, S.; Dimitrijevic, S. Imatinib: The first 3 years. Eur. J. Cancer 2002, 38 (Suppl. S5), S77–S82. [Google Scholar] [CrossRef]
- Seeliger, M.A.; Nagar, B.; Frank, F.; Cao, X.; Henderson, M.N.; Kuriyan, J. c-Src Binds to the Cancer Drug Imatinib with an Inactive Abl/c-Kit Conformation and a Distributed Thermodynamic Penalty. Structure 2007, 15, 299–311. [Google Scholar] [CrossRef]
- Amouei, A.; Daeian, N.; Khezrnia, S.S.; Mansouri, A.; Hadjibabaie, M. Imatinib Efficacy, Safety and Resistance in Iranian Patients with Chronic Myeloid Leukemia: A Review of Literature. Int. J. Hematol. Oncol. Stem Cell Res. 2021, 15, 114–131. [Google Scholar] [CrossRef]
- Gafter-Gvili, A.; Leader, A.; Gurion, R.; Vidal, L.; Ram, R.; Shacham-Abulafia, A.; Ben-Bassat, I.; Lishner, M.; Shpilberg, O.; Raanani, P. High-dose imatinib for newly diagnosed chronic phase chronic myeloid leukemia patients--systematic review and meta-analysis. Am. J. Hematol. 2011, 86, 657–662. [Google Scholar] [CrossRef]
- Stavrou, N.; Memos, N.; Filippatos, C.; Sergentanis, T.N.; Zagouri, F.; Gavriatopoulou, M.; Ntanasis-Stathopoulos, I. Neoadjuvant Imatinib in Recurrent/Metastatic Gastrointestinal Stromal Tumors: A Systematic Review and Meta-analysis of Proportions. J. Gastrointest. Cancer 2025, 56, 88. [Google Scholar] [CrossRef]
- Puri, S.; Kaur, G.; Piplani, H.; Sanyal, S.N.; Vaish, V. Imatinib modulates pro-inflammatory microenvironment with angiostatic effects in experimental lung carcinogenesis. Inflammopharmacology 2020, 28, 231–252. [Google Scholar] [CrossRef]
- Takigawa, N.; Takeyama, M.; Kozuki, T.; Shibayama, T.; Hisamoto, A.; Kiura, K.; Tada, A.; Hotta, K.; Umemura, S.; Ohashi, K.; et al. Combination of SN-38 with gefitinib or imatinib overcomes SN-38-resistant small-cell lung cancer cells. Oncol. Rep. 2007, 17, 983–987. [Google Scholar] [CrossRef][Green Version]
- Yokoyama, T.; Miyazawa, K.; Yoshida, T.; Ohyashiki, K. Combination of vitamin K2 plus imatinib mesylate enhances induction of apoptosis in small cell lung cancer cell lines. Int. J. Oncol. 2005, 26, 33–40. [Google Scholar] [CrossRef]
- Litz, J.; Krystal, G.W. Imatinib inhibits c-Kit-induced hypoxia-inducible factor-1alpha activity and vascular endothelial growth factor expression in small cell lung cancer cells. Mol. Cancer Ther. 2006, 5, 1415–1422. [Google Scholar] [CrossRef]
- Wolff, N.C.; Randle, D.E.; Egorin, M.J.; Minna, J.D.; Ilaria, R.L., Jr. Imatinib mesylate efficiently achieves therapeutic intratumor concentrations in vivo but has limited activity in a xenograft model of small cell lung cancer. Clin. Cancer Res. 2004, 10, 3528–3534. [Google Scholar] [CrossRef]
- Dy, G.K.; Miller, A.A.; Mandrekar, S.J.; Aubry, M.C.; Langdon, R.M., Jr.; Morton, R.F.; Schild, S.E.; Jett, J.R.; Adjei, A.A. A phase II trial of imatinib (ST1571) in patients with c-kit expressing relapsed small-cell lung cancer: A CALGB and NCCTG study. Ann. Oncol. 2005, 16, 1811–1816. [Google Scholar] [CrossRef]
- Johnson, B.E.; Fischer, T.; Fischer, B.; Dunlop, D.; Rischin, D.; Silberman, S.; Kowalski, M.O.; Sayles, D.; Dimitrijevic, S.; Fletcher, C.; et al. Phase II study of imatinib in patients with small cell lung cancer. Clin. Cancer Res. 2003, 9, 5880–5887. [Google Scholar]
- Soria, J.C.; Johnson, B.E.; Chevalier, T.L. Imatinib in small cell lung cancer. Lung Cancer 2003, 41 (Suppl. S1), S49–S53. [Google Scholar] [CrossRef]
- Spigel, D.R.; Hainsworth, J.D.; Simons, L.; Meng, C.; Burris, H.A., III; Yardley, D.A.; Grapski, R.; Schreeder, M.; Mallidi, P.V.; Greco, F.A. Irinotecan, carboplatin, and imatinib in untreated extensive-stage small-cell lung cancer: A phase II trial of the Minnie Pearl Cancer Research Network. J. Thorac. Oncol. 2007, 2, 854–861. [Google Scholar] [CrossRef]
- Kellar, A.; Egan, C.; Morris, D. Preclinical Murine Models for Lung Cancer: Clinical Trial Applications. Biomed Res. Int. 2015, 2015, 621324. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, J.; Zhang, B.; Liang, G.; Chen, X.; Yao, F.; Xu, X.; Wu, H.; He, Q.; Ding, L.; et al. Imatinib prevents lung cancer metastasis by inhibiting M2-like polarization of macrophages. Pharmacol. Res. 2018, 133, 121–131. [Google Scholar] [CrossRef]
- Oliveira, A.; Moura, S.; Pimentel, L.; Neto, J.; Dantas, R.; Silva-Jr, F.; Bastos, M.; Boechat, N. New Imatinib Derivatives with Antiproliferative Activity against A549 and K562 Cancer Cells. Molecules 2022, 27, 750. [Google Scholar] [CrossRef]
- Kinoshita, K.; Nakagawa, K.; Hamada, J.; Hida, Y.; Tada, M.; Kondo, S.; Moriuchi, T. Imatinib mesylate inhibits the proliferation-stimulating effect of human lung cancer-associated stromal fibroblasts on lung cancer cells. Int. J. Oncol. 2010, 37, 869–877. [Google Scholar] [CrossRef]
- Bauman, J.E.; Eaton, K.D.; Wallace, S.G.; Carr, L.L.; Lee, S.J.; Jones, D.V.; Arias-Pulido, H.; Cerilli, L.A.; Martins, R.G. A Phase II study of pulse dose imatinib mesylate and weekly paclitaxel in patients aged 70 and over with advanced non-small cell lung cancer. BMC Cancer 2012, 12, 449. [Google Scholar] [CrossRef]
- Tsao, A.S.; Liu, S.; Fujimoto, J.; Wistuba, I.I.; Lee, J.J.; Marom, E.M.; Charnsangavej, C.; Fossella, F.V.; Tran, H.T.; Blumenschein, G.R.; et al. Phase II trials of imatinib mesylate and docetaxel in patients with metastatic non-small cell lung cancer and head and neck squamous cell carcinoma. J. Thorac. Oncol. 2011, 6, 2104–2111. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, H.; Heimbach, T.; He, H.; Buchbinder, A.; Aghoghovbia, M.; Hourcade-Potelleret, F. Clinical Pharmacokinetic and Pharmacodynamic Overview of Nilotinib, a Selective Tyrosine Kinase Inhibitor. J. Clin. Pharmacol. 2018, 58, 1533–1540. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Hughes, T.P.; Larson, R.A.; Kim, D.W.; Issaragrisil, S.; le Coutre, P.; Etienne, G.; Boquimpani, C.; Pasquini, R.; Clark, R.E.; et al. Long-term outcomes with frontline nilotinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase: ENESTnd 10-year analysis. Leukemia 2021, 35, 440–453. [Google Scholar] [CrossRef]
- Jabbour, E.; Kantarjian, H. Chronic myeloid leukemia: 2025 update on diagnosis, therapy, and monitoring. Am. J. Hematol. 2024, 99, 2191–2212. [Google Scholar] [CrossRef]
- Demetri, G.D.; Casali, P.G.; Blay, J.Y.; von Mehren, M.; Morgan, J.A.; Bertulli, R.; Ray-Coquard, I.; Cassier, P.; Davey, M.; Borghaei, H.; et al. A phase I study of single-agent nilotinib or in combination with imatinib in patients with imatinib-resistant gastrointestinal stromal tumors. Clin. Cancer Res. 2009, 15, 5910–5916. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, T.M.; Kim, Y.J.; Jang, K.T.; Lee, H.J.; Lee, S.N.; Ahn, M.S.; Hwang, I.G.; Lee, S.; Lee, M.H.; et al. Phase II Trial of Nilotinib in Patients With Metastatic Malignant Melanoma Harboring KIT Gene Aberration: A Multicenter Trial of Korean Cancer Study Group (UN10-06). Oncologist 2015, 20, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, P.; Montemurro, M. Clinical experience to date with nilotinib in gastrointestinal stromal tumors. Semin. Oncol. 2011, 38 (Suppl. S1), S20–S27. [Google Scholar] [CrossRef]
- Weisberg, E.; Nonami, A.; Griffin, J.D. Combination therapy with nilotinib for drug-sensitive and drug-resistant BCR-ABL-positive leukemia and other malignancies. Arch. Toxicol. 2014, 88, 2233–2242. [Google Scholar] [CrossRef]
- Watanabe, K.; Kage, H.; Nagoshi, S.; Toyama, K.; Ohno, Y.; Shinozaki-Ushiku, A.; Nakazaki, K.; Suzuki, H.; Kurokawa, M.; Nagase, T. Dual EGFR and ABL Tyrosine Kinase Inhibitor Treatment in a Patient with Concomitant EGFR-Mutated Lung Adenocarcinoma and BCR-ABL1-Positive CML. Case Rep. Oncol. Med. 2020, 2020, 4201727. [Google Scholar] [CrossRef]
- Shin, S.J.; O’Sullivan Coyne, G.; Kummar, S.; Miller, S.B.; Johnson, B.C.; Anderson, L.; Rubinstein, L.; Miller, B.; Wilsker, D.F.; Ferry-Galow, K.V.; et al. A Phase I Study of Nilotinib in Combination with Paclitaxel in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2025, 31, 2124–2133. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.; Ryu, J.S.; Kang, J.; Kim, I.; Son, S.; Lee, B.S.; Kim, C.H.; Kim, Y.S. c-Src inhibitor PP2 inhibits head and neck cancer progression through regulation of the epithelial-mesenchymal transition. Exp. Biol. Med. 2023, 248, 492–500. [Google Scholar] [CrossRef]
- Vu, A.T.; Akingunsade, L.; Hoffer, K.; Petersen, C.; Betz, C.S.; Rothkamm, K.; Rieckmann, T.; Bussmann, L.; Kriegs, M. Src family kinase targeting in head and neck tumor cells using SU6656, PP2 and dasatinib. Head. Neck 2023, 45, 147–155. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, C.; Chen, W.; Li, X.; Wang, J.; Wang, T.; Zhao, Q.; Huang, H.; Li, Y.; Yang, C.; et al. PP2 alleviates the progression of osteoarthritis by inhibiting Wnt/β-catenin and activating TGF-β/Smad signaling. Int. Immunopharmacol. 2023, 124, 110948. [Google Scholar] [CrossRef]
- Nam, J.S.; Ino, Y.; Sakamoto, M.; Hirohashi, S. Src family kinase inhibitor PP2 restores the E-cadherin/catenin cell adhesion system in human cancer cells and reduces cancer metastasis. Clin. Cancer Res. 2002, 8, 2430–2436. [Google Scholar]
- Lou, L.; Yu, Z.; Wang, Y.; Wang, S.; Zhao, Y. c-Src inhibitor selectively inhibits triple-negative breast cancer overexpressed Vimentin in vitro and in vivo. Cancer Sci. 2018, 109, 1648–1659. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, N.; Liu, Y.; Choi, W.; Kim, J.H.; Kim, H.G.; Yu, T.; Cho, J.Y. PP2 suppresses proliferation and migration of C6 Glioma and MDA-MB-231 cells by targeting both fibroblast growth factor receptor 1 and Src. Chem.-Biol. Interact. 2024, 403, 111252. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.D.; Wang, H.; Avila, H.; Parikh, N.U.; Kessler, H.; Magdolen, V.; Gallick, G.E. Combination of an SRC kinase inhibitor with a novel pharmacological antagonist of the urokinase receptor diminishes in vitro colon cancer invasiveness. Clin. Cancer Res. 2004, 10, 1545–1555. [Google Scholar] [CrossRef]
- Jung, Y.S.; Cheong, H.J.; Kim, S.J.; Kim, K.H.; Lee, N.; Park, H.S.; Won, J.H. Src family kinase inhibitor PP2 enhances differentiation of acute promyelocytic leukemia cell line induced by combination of all-trans-retinoic acid and arsenic trioxide. Leuk. Res. 2014, 38, 977–982. [Google Scholar] [CrossRef]
- Eom, K.Y.; Cho, B.J.; Choi, E.J.; Kim, J.H.; Chie, E.K.; Wu, H.G.; Kim, I.H.; Paek, S.H.; Kim, J.S.; Kim, I.A. The Effect of Chemoradiotherapy with SRC Tyrosine Kinase Inhibitor, PP2 and Temozolomide on Malignant Glioma Cells In Vitro and In Vivo. Cancer Res. Treat. 2016, 48, 687–697. [Google Scholar] [CrossRef]
- Dai, X.; Wang, L.J.; Wu, J.; Shi, Y.X.; Li, G.P.; Yang, X.Q. Src kinase inhibitor PP2 regulates the biological characteristics of A549 cells via the PI3K/Akt signaling pathway. Oncol. Lett. 2018, 16, 5059–5065. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Y.; Zhao, W.; Wang, R.; Tong, X.; Jiang, G. SRC kinase inhibitor PP2 inhibits invasion and metastasis of lung cancer A549 cells by upregulating connexin43 expression. Nan Fang. Yi Ke Da Xue Xue Bao 2019, 39, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Gilchrest, B.A. Tirbanibulin: A New Topical Therapy for Actinic Keratoses With a Novel Mechanism of Action and Improved Ease of Use. Clin. Pharmacol. Drug Dev. 2021, 10, 1126–1129. [Google Scholar] [CrossRef]
- Patel, P.; Wang, J.; Bitterman, D.; Mineroff, J.; Austin, E.; Jagdeo, J. Systematic review of randomized controlled trials of topicals for actinic keratosis field therapy. Arch. Dermatol. Res. 2024, 316, 108. [Google Scholar] [CrossRef]
- Schlesinger, T.; Stockfleth, E.; Grada, A.; Berman, B. Tirbanibulin for Actinic Keratosis: Insights into the Mechanism of Action. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2495–2506. [Google Scholar] [CrossRef]
- Markham, A.; Duggan, S. Tirbanibulin: First Approval. Drugs 2021, 81, 509–513. [Google Scholar] [CrossRef]
- Smolinski, M.P.; Bu, Y.; Clements, J.; Gelman, I.H.; Hegab, T.; Cutler, D.L.; Fang, J.W.S.; Fetterly, G.; Kwan, R.; Barnett, A.; et al. Discovery of Novel Dual Mechanism of Action Src Signaling and Tubulin Polymerization Inhibitors (KX2-391 and KX2-361). J. Med. Chem. 2018, 61, 4704–4719. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.; Cohen, R.; Dy, G.K.; Hong, D.S.; Dyster, L.; Hangauer, D.G.; Kwan, R.; Fetterly, G.; Kurzrock, R.; Adjei, A.A. A phase I trial of KX2-391, a novel non-ATP competitive substrate-pocket- directed SRC inhibitor, in patients with advanced malignancies. Investig. New Drugs 2013, 31, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. 2024. Available online: https://gco.iarc.who.int/today (accessed on 7 August 2025).
- Ruano-Raviña, A.; Provencio, M.; Calvo de Juan, V.; Carcereny, E.; Moran, T.; Rodriguez-Abreu, D.; López-Castro, R.; Cuadrado Albite, E.; Guirado, M.; Gómez González, L.; et al. Lung cancer symptoms at diagnosis: Results of a nationwide registry study. ESMO Open 2020, 5, e001021. [Google Scholar] [CrossRef] [PubMed]
- Bhopal, A.; Peake, M.D.; Gilligan, D.; Cosford, P. Lung cancer in never-smokers: A hidden disease. J. R. Soc. Med. 2019, 112, 269–271. [Google Scholar] [CrossRef]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef]
- Hardavella, G.; Frille, A.; Sreter, K.B.; Atrafi, F.; Yousaf-Khan, U.; Beyaz, F.; Kyriakou, F.; Bellou, E.; Mullin, M.L.; Janes, S.M. Lung cancer screening: Where do we stand? Breathe 2024, 20, 230190. [Google Scholar] [CrossRef]
- Wolf, A.M.D.; Oeffinger, K.C.; Shih, T.Y.; Walter, L.C.; Church, T.R.; Fontham, E.T.H.; Elkin, E.B.; Etzioni, R.D.; Guerra, C.E.; Perkins, R.B.; et al. Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA Cancer J. Clin. 2024, 74, 50–81. [Google Scholar] [CrossRef]
- Zito Marino, F.; Bianco, R.; Accardo, M.; Ronchi, A.; Cozzolino, I.; Morgillo, F.; Rossi, G.; Franco, R. Molecular heterogeneity in lung cancer: From mechanisms of origin to clinical implications. Int. J. Med. Sci. 2019, 16, 981–989. [Google Scholar] [CrossRef]
- Doumat, G.; Daher, D.; Zerdan, M.B.; Nasra, N.; Bahmad, H.F.; Recine, M.; Poppiti, R. Drug Repurposing in Non-Small Cell Lung Carcinoma: Old Solutions for New Problems. Curr. Oncol. 2023, 30, 704–719. [Google Scholar] [CrossRef]
- Jamal-Hanjani, M.; Quezada, S.A.; Larkin, J.; Swanton, C. Translational Implications of Tumor Heterogeneity. Clin. Cancer Res. 2015, 21, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.; Moura, C.S.; Costa, M.; Lamas, N.J.; Correia, R.; Garcez, D.; Pereira, J.M.; Lindahl, T.; Sousa, C.; Vale, N. Lung Cancer Therapy: The Role of Personalized Medicine. Cancers 2025, 17, 725. [Google Scholar] [CrossRef]
- Johnson, F.M.; Gallick, G.E. SRC family nonreceptor tyrosine kinases as molecular targets for cancer therapy. Anticancer Agents Med. Chem. 2007, 7, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.J.; Rouse, C.; Xu, X.; Wang, J.; Onaitis, M.W.; Pendergast, A.M. Inactivation of ABL kinases suppresses non-small cell lung cancer metastasis. JCI Insight 2016, 1, e89647. [Google Scholar] [CrossRef] [PubMed][Green Version]
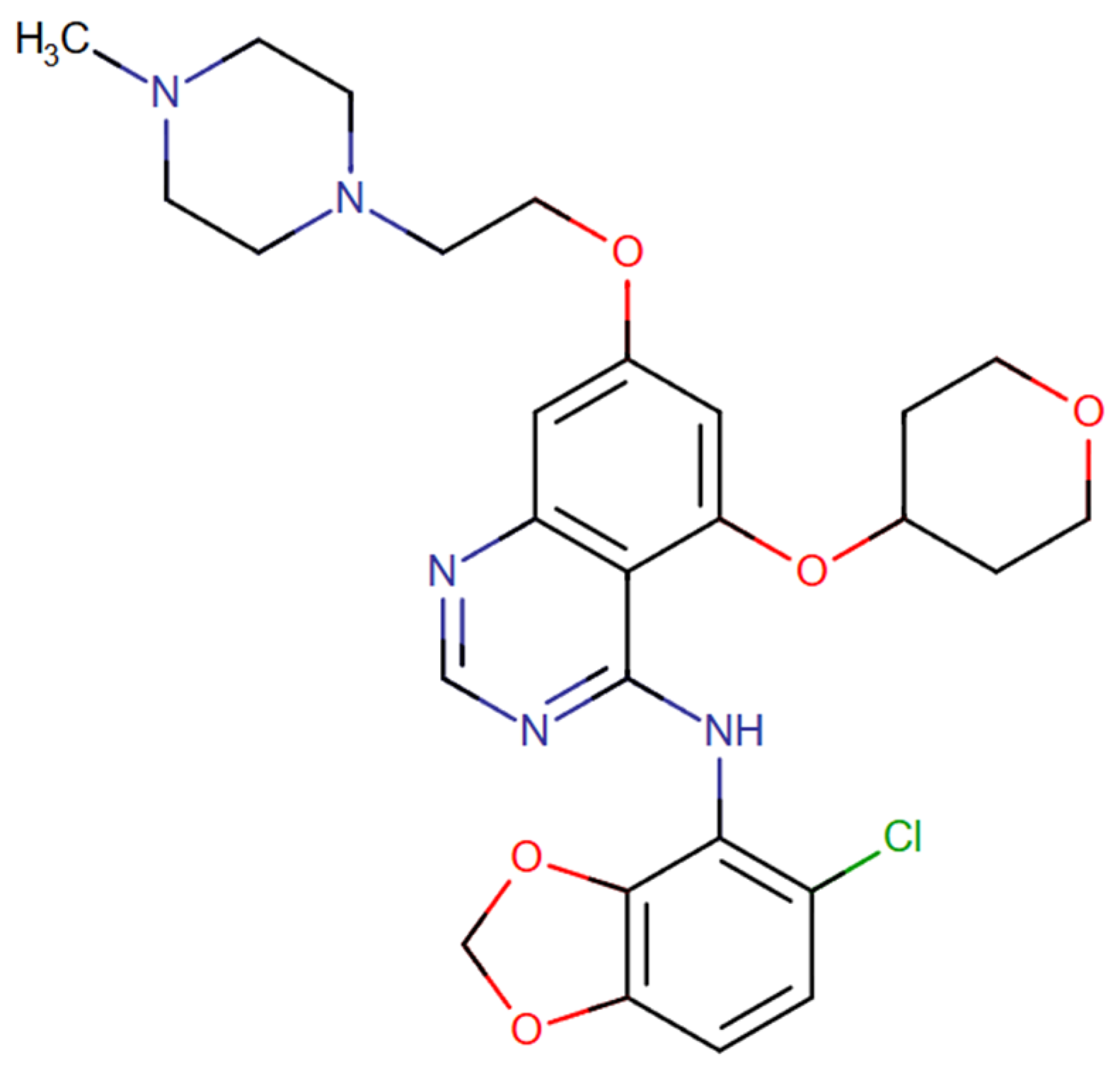
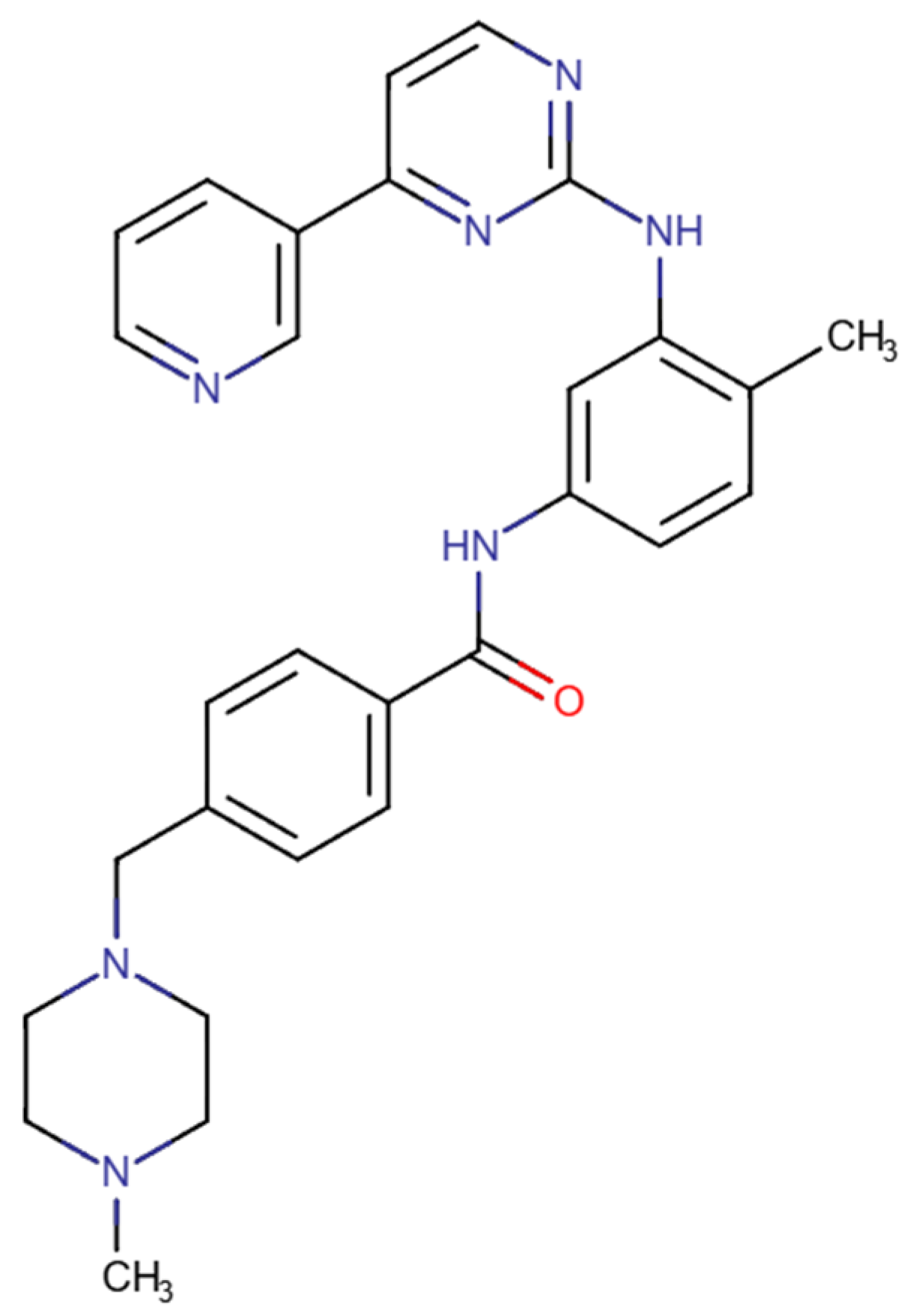

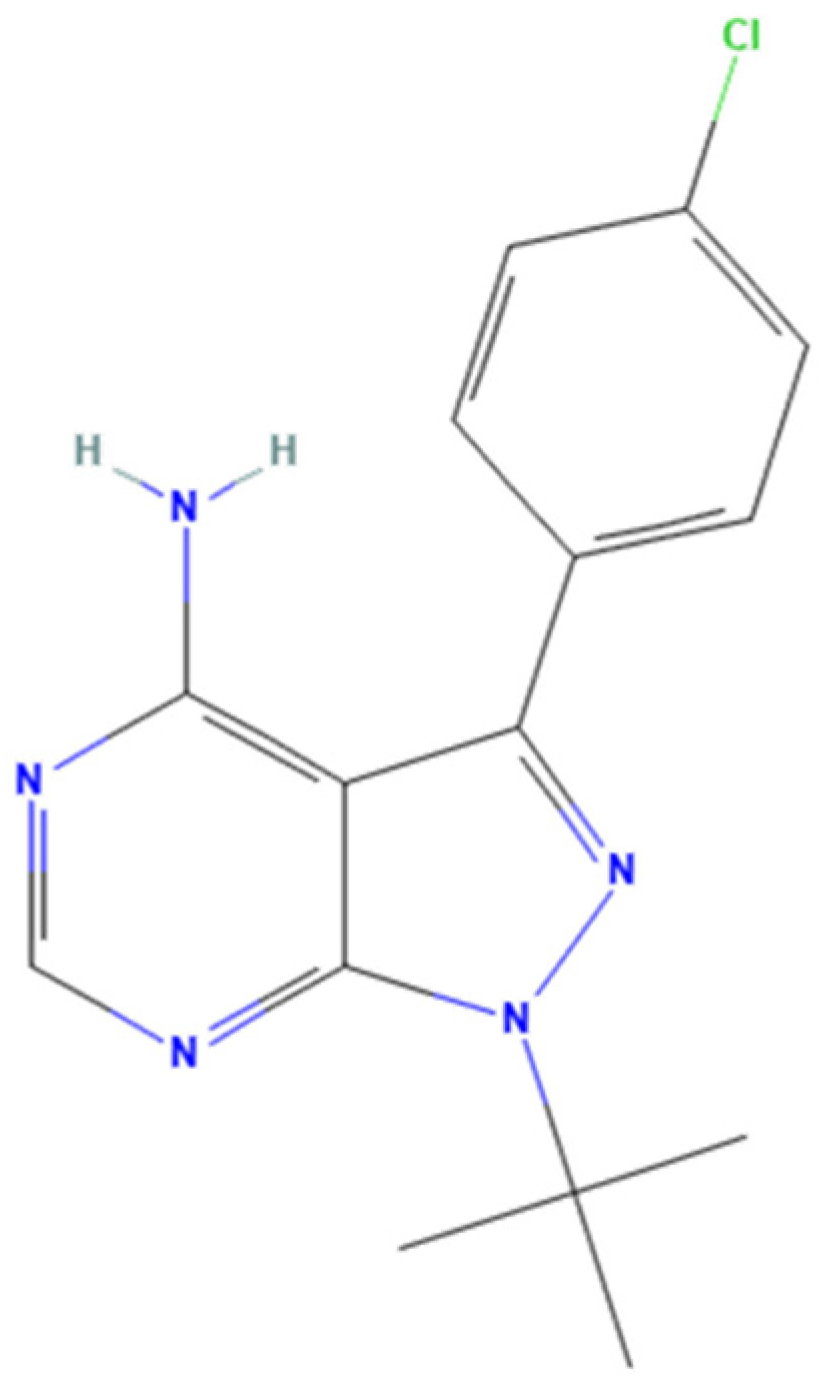
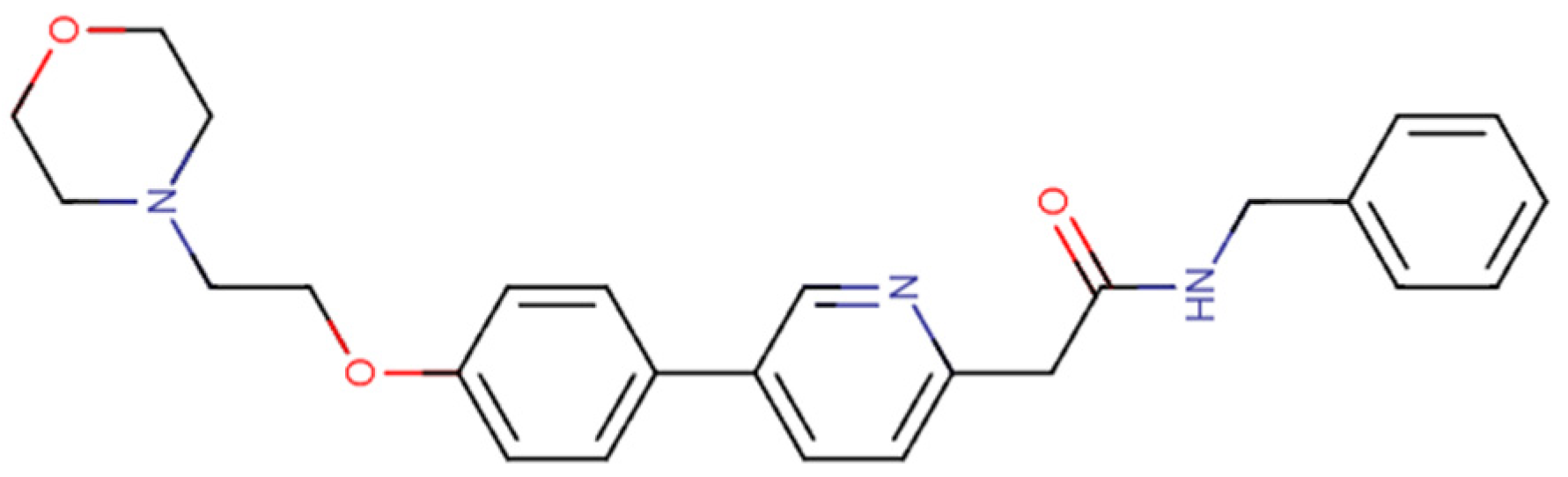
| Pharmacological Class | Drug | Repurposing Target (Cancer Type) | Clinical Trial Phase |
|---|---|---|---|
| Anti-Platelet | Aspirin | Colon Cancer | Phase III (NCT02301286) |
| Anti-Diabetic | Metformin | Glioma | Phase II (NCT05929495) |
| Pioglitazone | Head and Neck Cancer | Phase II (NCT00099021) | |
| Vasodilator | Minoxidil | Epithelial Ovarian Cancer | Phase II (NCT05272462) |
| Anti-Viral | Ribavirin | Acute Myelocytic Leukemia | Phase II (NCT00559091) |
| Angiotensin Receptor Blocker | Losartan | Pancreatic Cancer | Phase II (NCT01821729) |
| Beta Blockers | Propranolol | Breast Cancer | Phase II (NCT02596867) |
| Calcium Channel Blockers | Mibefradil | High-Grade Glioma | Phase I (NCT01480050) |
| Antibiotic | Minocycline (Tetracycline) | Recurrent Glioma | Phase Ib/II (NCT01580969) |
| Tigecycline (Tetracycline) | Acute Myeloid Leukemia | Phase I (NCT01332786) | |
| Disease-Modifying Antirheumatic Drug | Auranofin | Non-Small-Cell Lung Cancer or Small-Cell Lung Cancer | Phase I/II (NCT01737502) |
| Chronic Lymphocytic Leukemia | Phase I/II (NCT01419691) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, R.; Sousa, C.; Vale, N. Src and Abl as Therapeutic Targets in Lung Cancer: Opportunities for Drug Repurposing. Pharmaceuticals 2025, 18, 1426. https://doi.org/10.3390/ph18101426
Ramos R, Sousa C, Vale N. Src and Abl as Therapeutic Targets in Lung Cancer: Opportunities for Drug Repurposing. Pharmaceuticals. 2025; 18(10):1426. https://doi.org/10.3390/ph18101426
Chicago/Turabian StyleRamos, Raquel, Carlos Sousa, and Nuno Vale. 2025. "Src and Abl as Therapeutic Targets in Lung Cancer: Opportunities for Drug Repurposing" Pharmaceuticals 18, no. 10: 1426. https://doi.org/10.3390/ph18101426
APA StyleRamos, R., Sousa, C., & Vale, N. (2025). Src and Abl as Therapeutic Targets in Lung Cancer: Opportunities for Drug Repurposing. Pharmaceuticals, 18(10), 1426. https://doi.org/10.3390/ph18101426







