Anti-Biofilm Agents to Overcome Pseudomonas aeruginosa Antibiotic Resistance
Abstract
1. Introduction
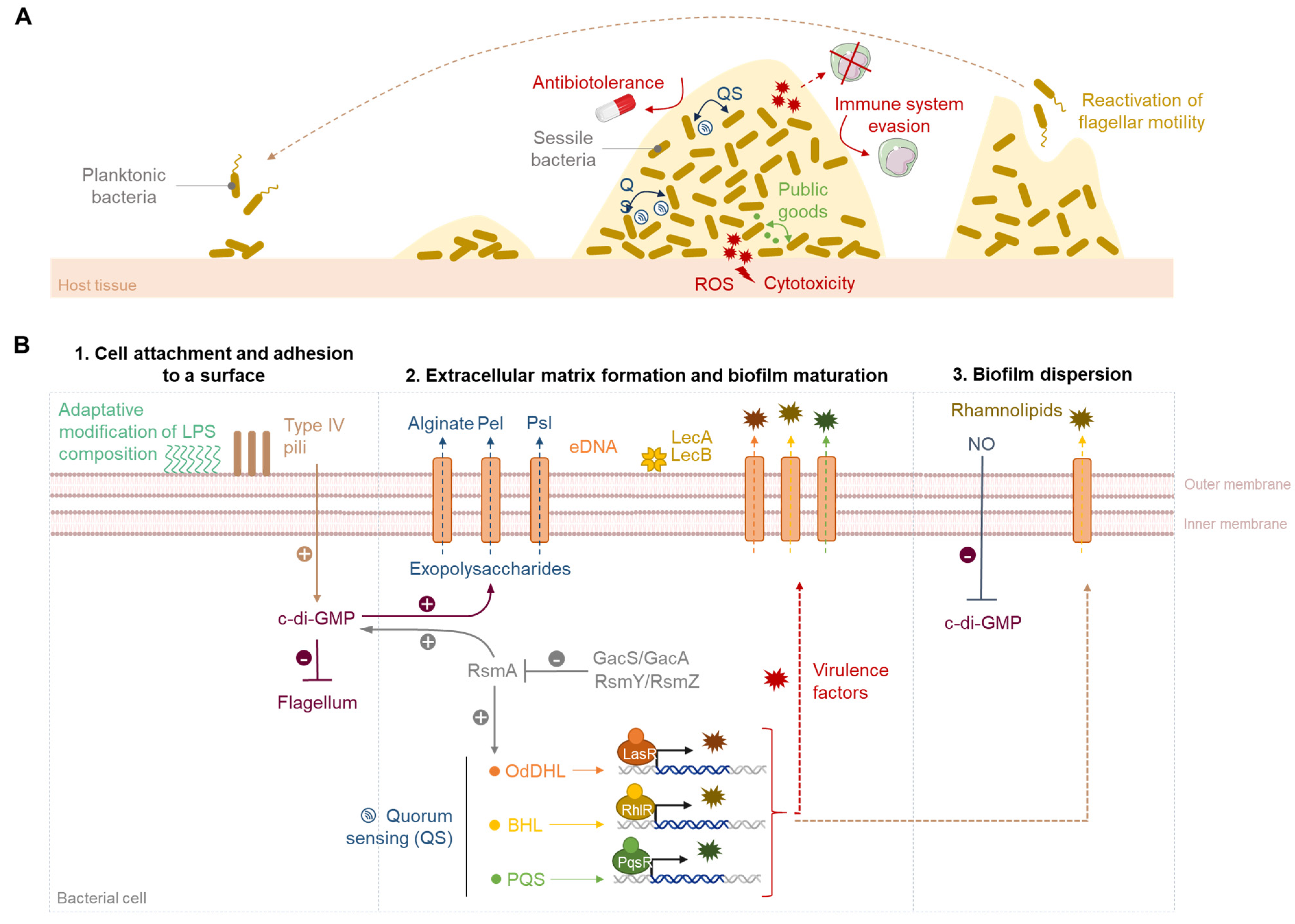
2. The Biofilm Life Cycle
2.1. Cell Attachment
2.2. Irreversible Adhesion and Biofilm Formation
2.3. Biofilm Maturation
2.4. Biofilm Dispersion and Bacterial Dissemination
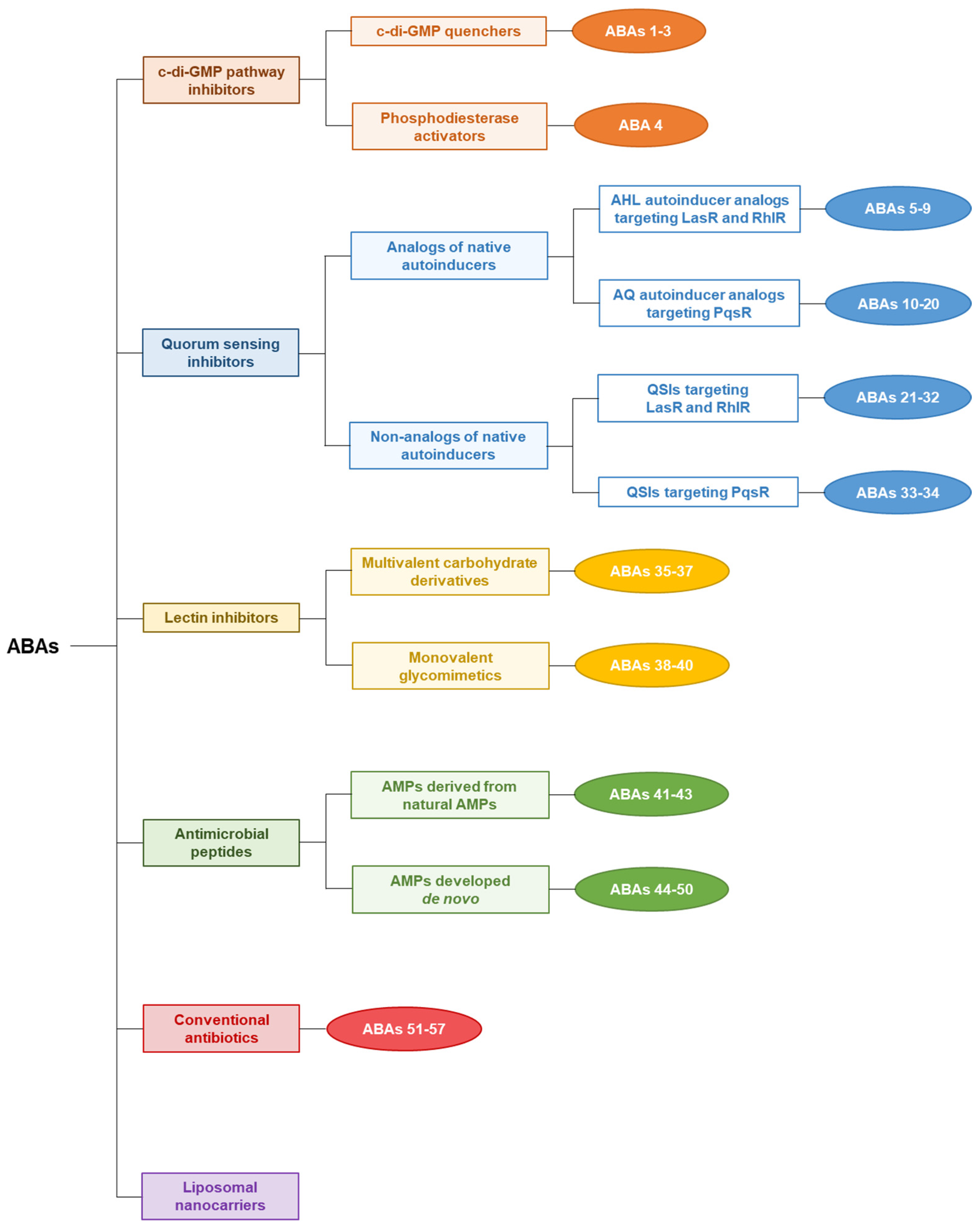
3. Anti-Biofilm Agents to Tackle P. aeruginosa
3.1. Relevant Anti-Virulence Agent Review
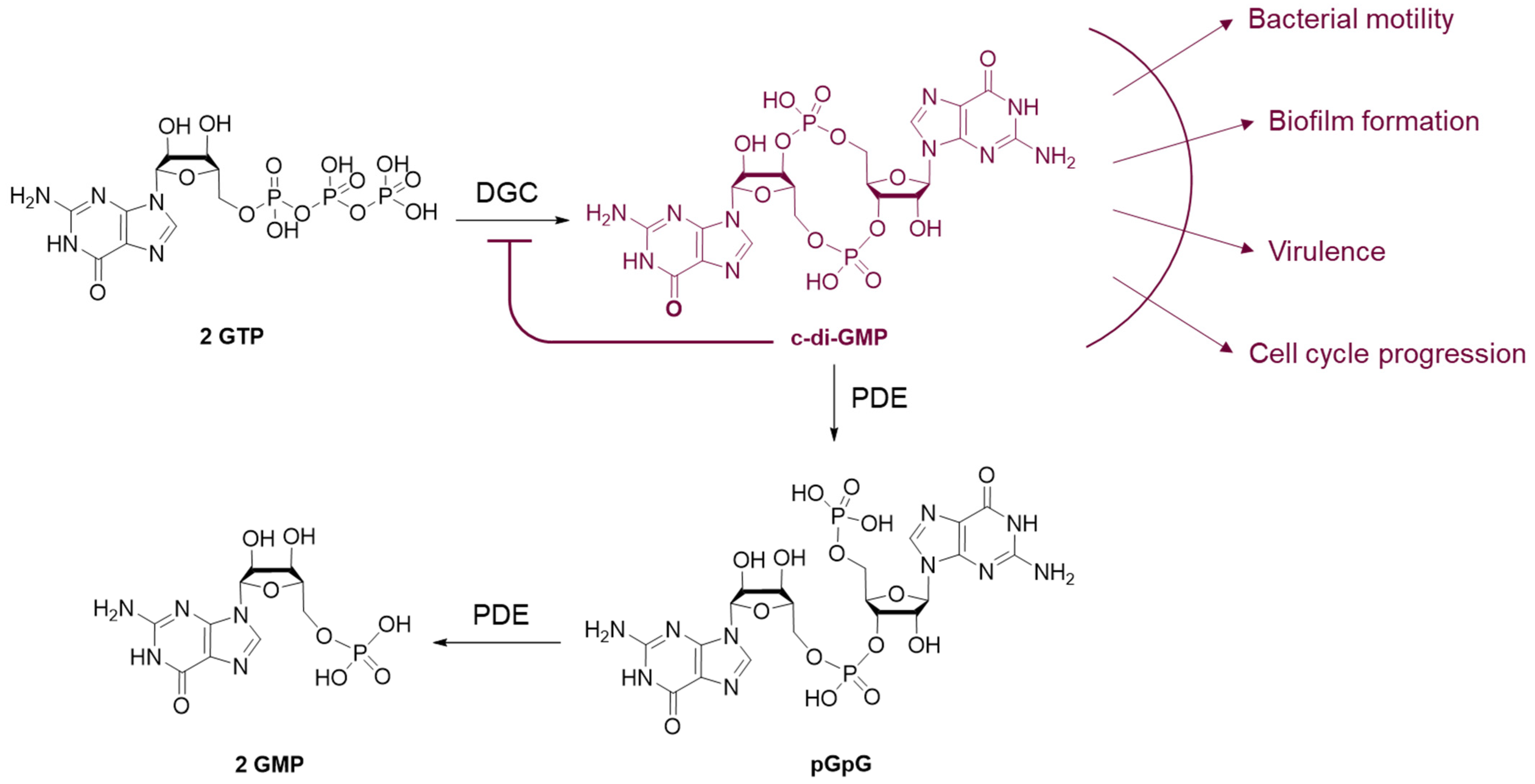
3.1.1. c-di-GMP Signaling Pathway Inhibitors
3.1.2. Quorum Sensing Inhibitors
Analogs of Native Autoinducers
- (1)
- AHL autoinducer analogs targeting LasR and RhlR
- (2)
- AQ autoinducer analogs targeting PqsR
Non-Analogs of Native Autoinducers
- (1)
- QSIs targeting LasR or RhlR
- (2)
- QSIs targeting PqsR
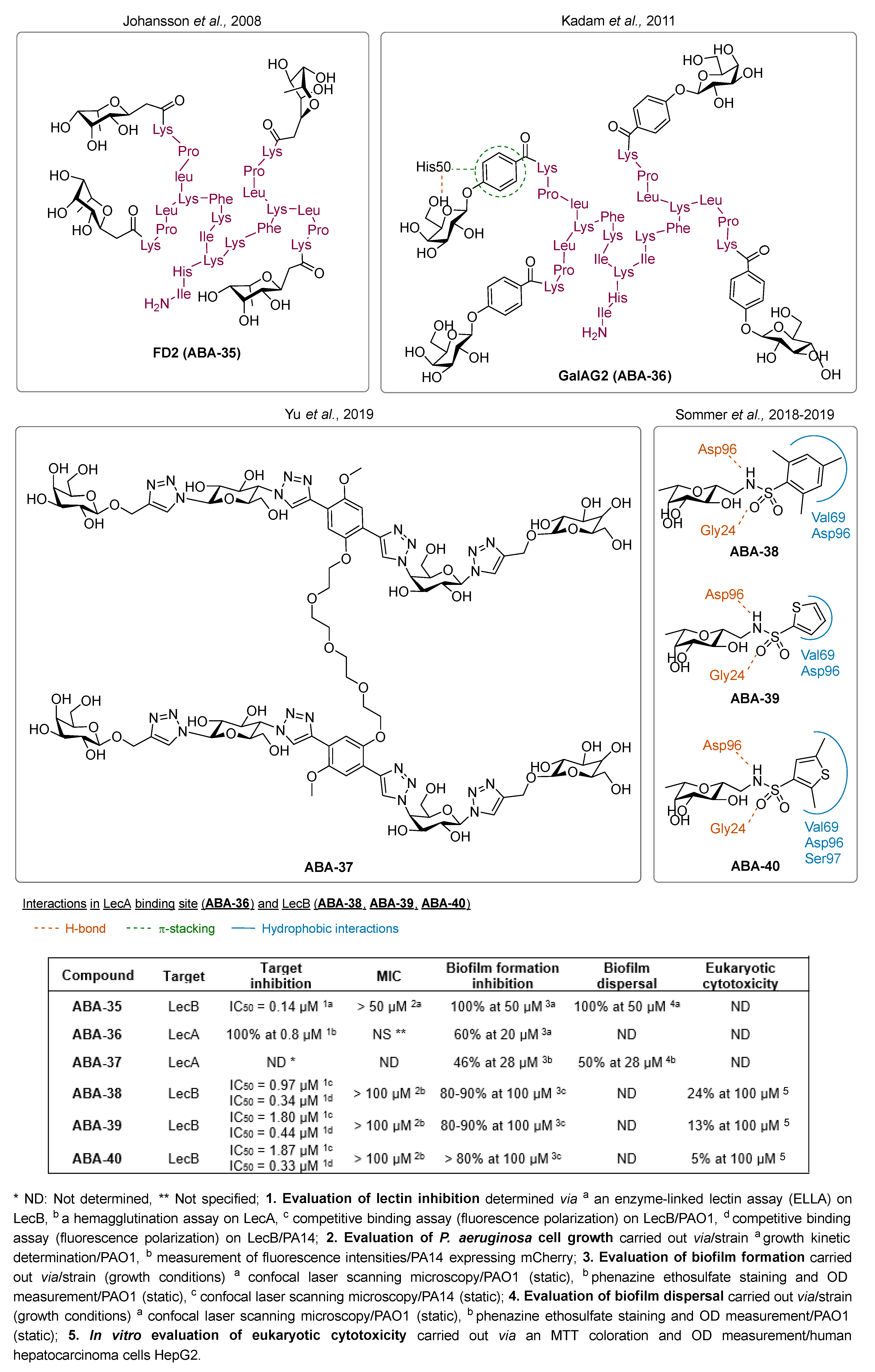
3.1.3. Lectin Inhibitors
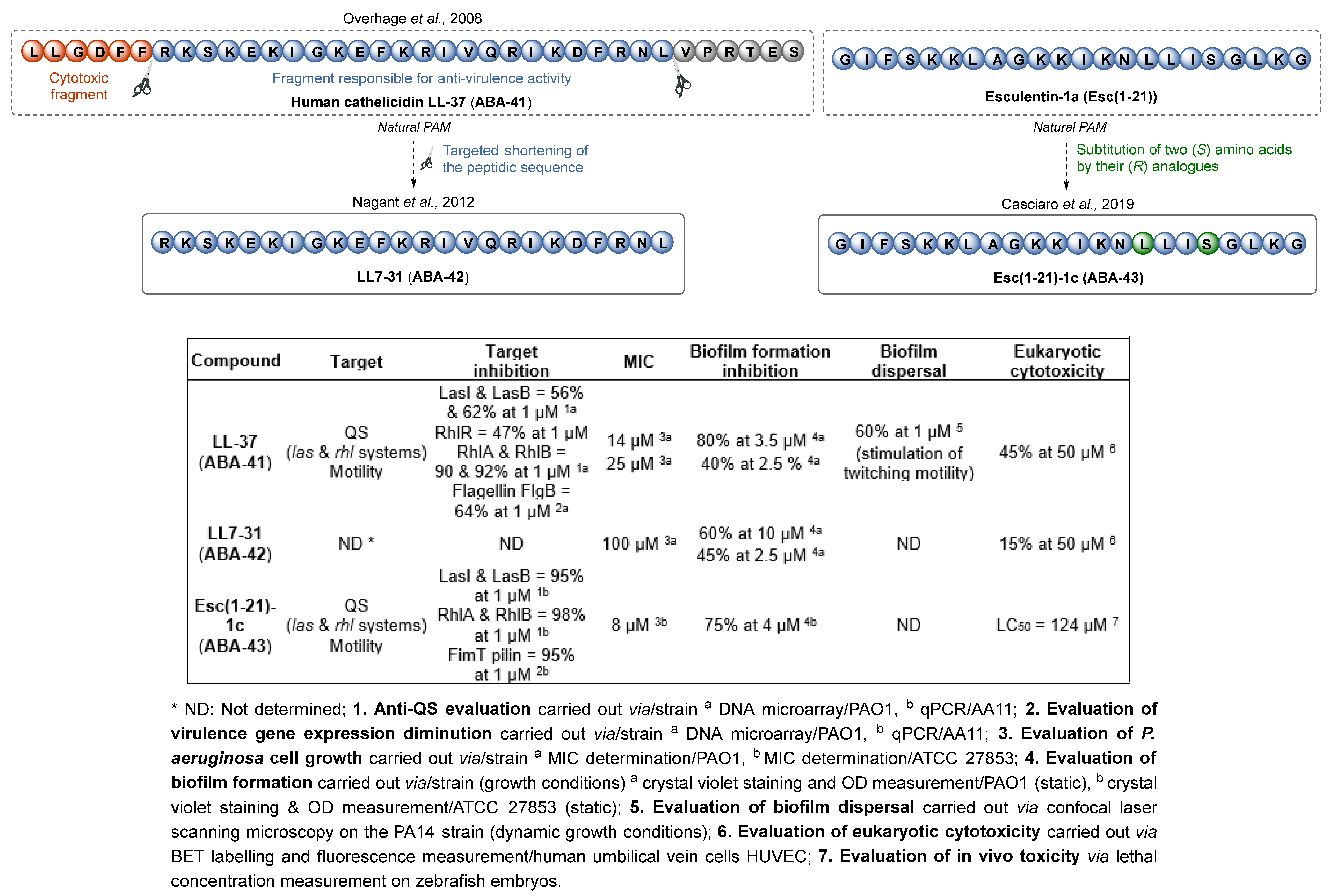
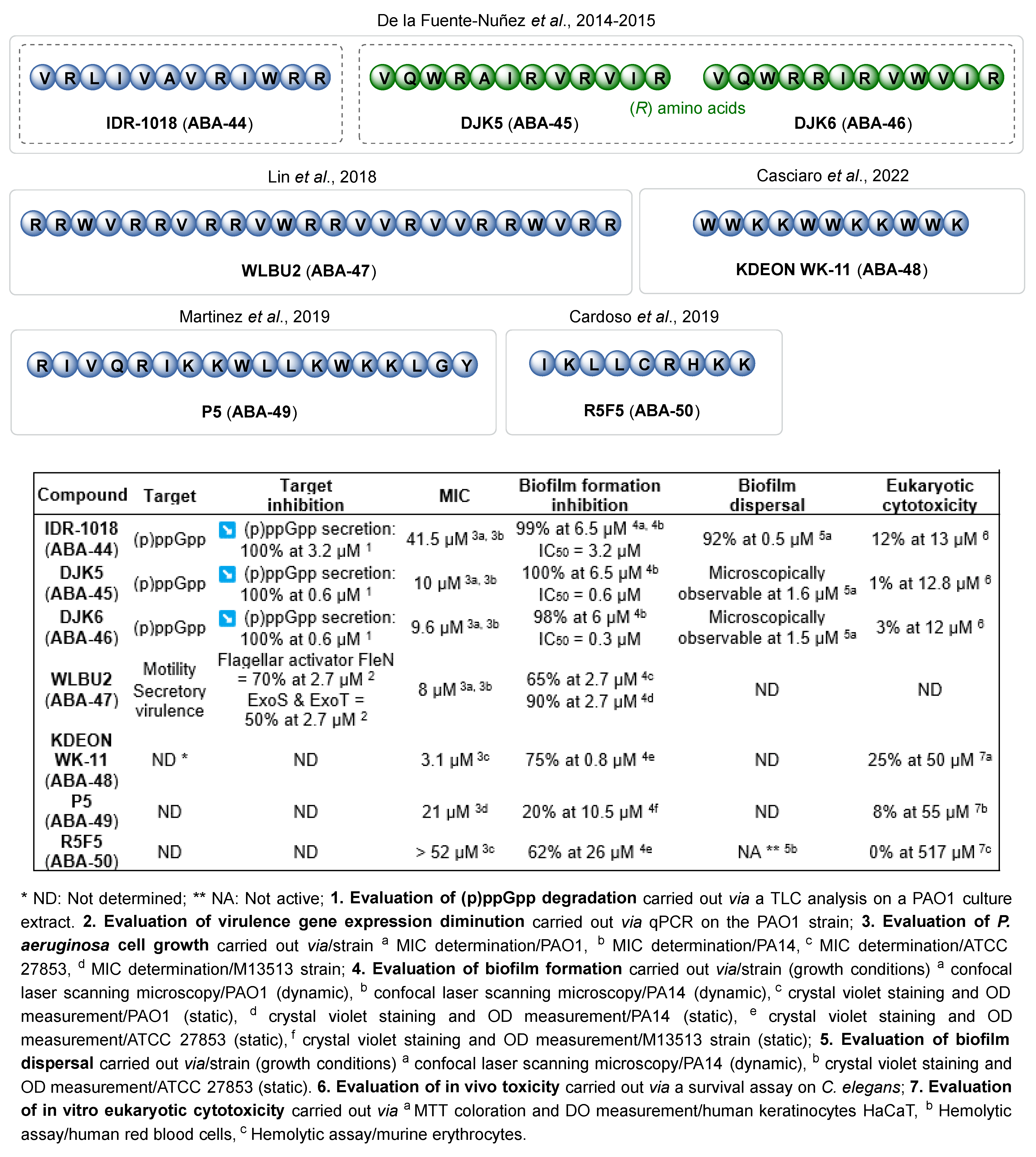
3.1.4. Antimicrobial Peptides
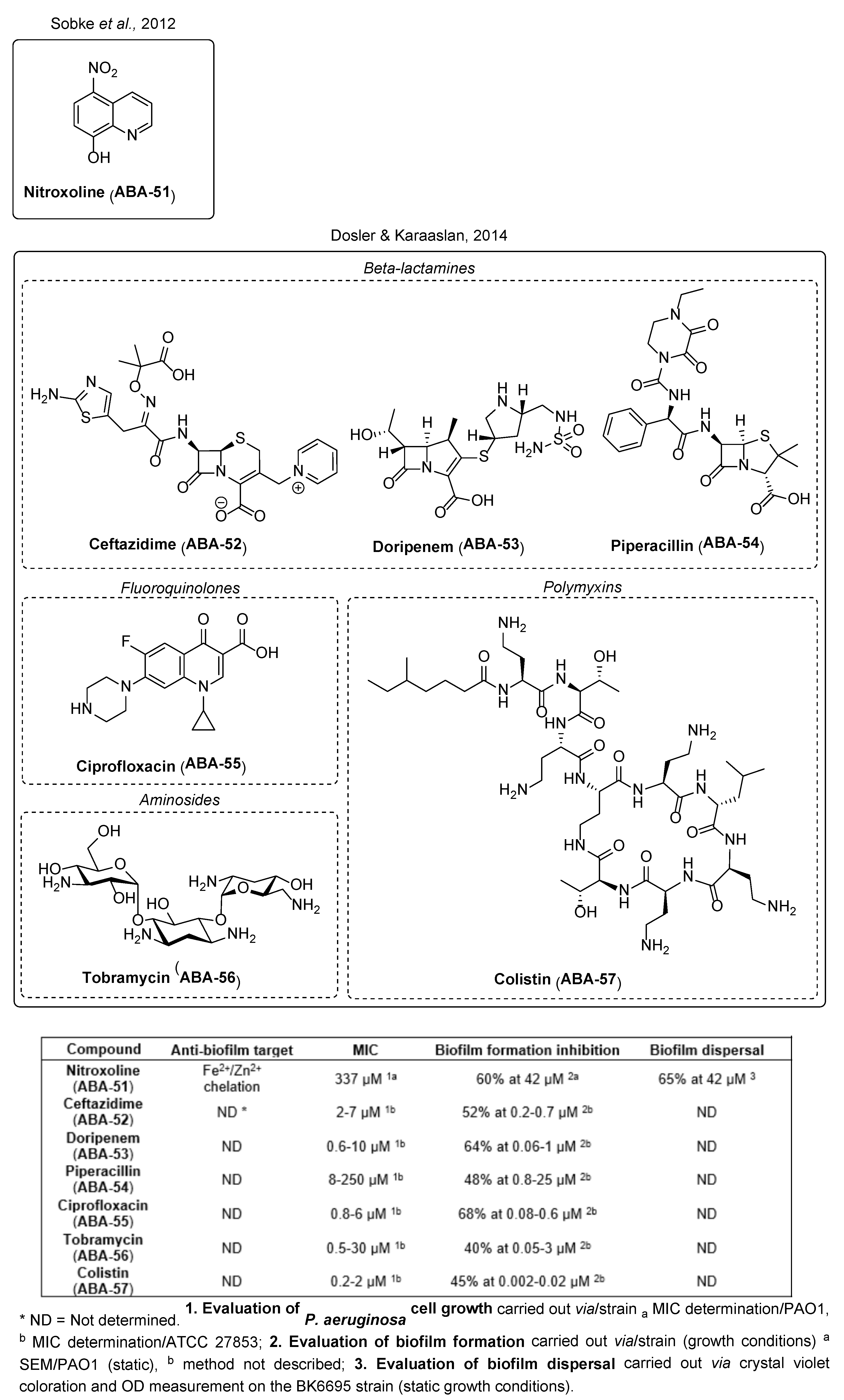
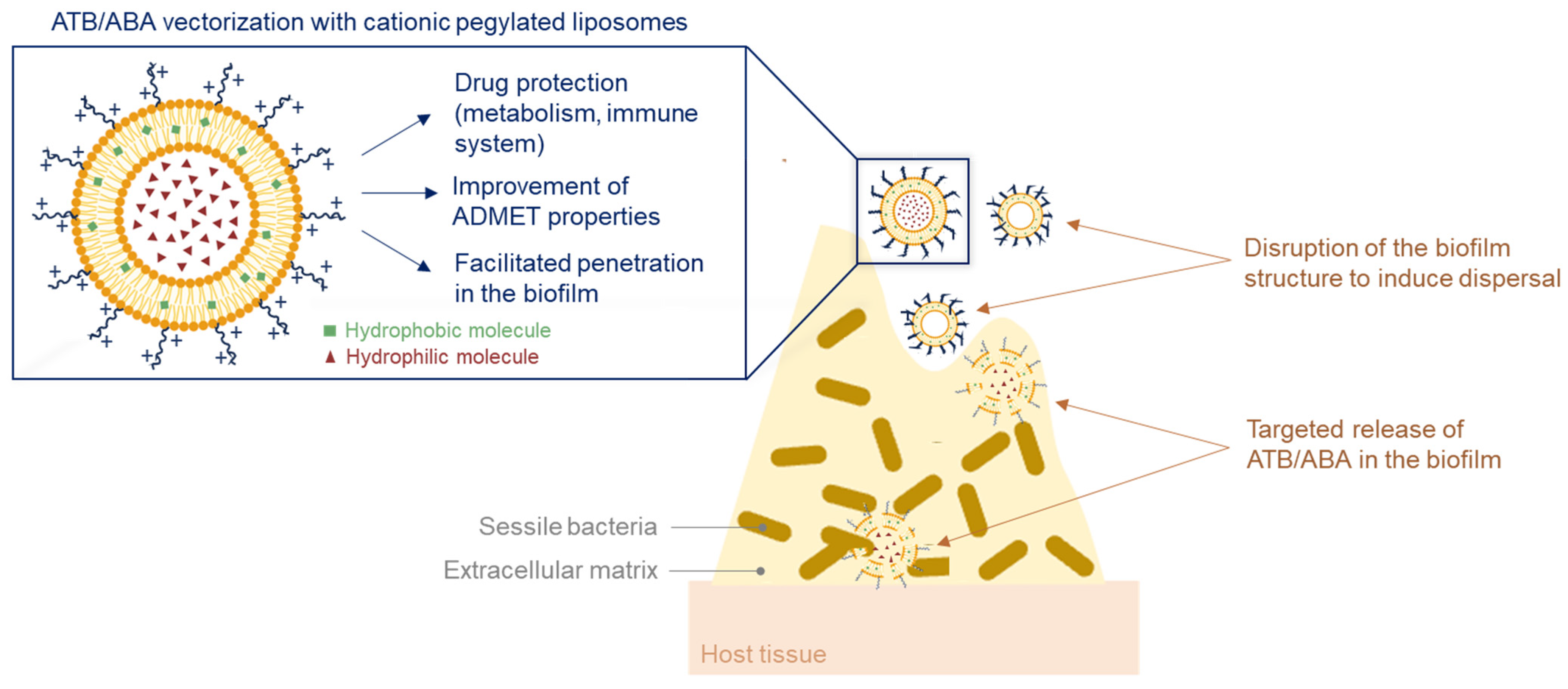
3.1.5. Conventional Antibiotic Repurposing and Anti-Biofilm Nanocarriers
3.2. Eradication of Biofilm-Associated Infections: Combinatorial Approach AVA/ATB
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | anti-biofilm agent |
| ADMET | administration, distribution, metabolism, excretion, toxicity |
| AI | autoinducer |
| AMP | antimicrobial peptide |
| ATB | antibiotic |
| CC50 | cytotoxic concentration 50% |
| CF | cystic fibrosis |
| CFU | colony forming unit |
| HGT | horizontal gene transfer |
| IC50 | inhibitory concentration 50% |
| MIC | minimal inhibitory concentration |
| OD | optical density |
| PDB | protein data bank |
| PDE | phosphodiesterase |
| QS | quorum sensing |
| QSI | quorum sensing inhibitor |
| SAR | structure–activity relationship |
| SCV | small colony variants |
| SEM | scanning electron microscopy |
References
- Stover, C.K.; Pham, X.Q.; Erwin, A.L.; Mizoguchi, S.D.; Warrener, P.; Hickey, M.J.; Brinkman, F.S.L.; Hufnagle, W.O.; Kowalik, D.J.; Lagrou, M.; et al. Complete Genome Sequence of Pseudomonas Aeruginosa PAO1, an Opportunistic Pathogen. Nature 2000, 406, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Mielko, K.A.; Jabłoński, S.J.; Milczewska, J.; Sands, D.; Łukaszewicz, M.; Młynarz, P. Metabolomic Studies of Pseudomonas Aeruginosa. World J. Microbiol. Biotechnol. 2019, 35, 178. [Google Scholar] [CrossRef] [PubMed]
- Sommer, L.M.; Johansen, H.K.; Molin, S. Antibiotic Resistance in Pseudomonas Aeruginosa and Adaptation to Complex Dynamic Environments. Microb. Genom. 2020, 6, e000370. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.D.; Déziel, E.; Gauthier, J.; Levesque, R.C.; Lau, G.W. An Organ System-Based Synopsis of Pseudomonas Aeruginosa Virulence. Virulence 2021, 12, 1469–1507. [Google Scholar] [CrossRef] [PubMed]
- Maurice, N.M.; Bedi, B.; Sadikot, R.T. Pseudomonas Aeruginosa Biofilms: Host Response and Clinical Implications in Lung Infections. Am. J. Respir. Cell Mol. Biol. 2018, 58, 428–439. [Google Scholar] [CrossRef]
- Rodrigo-Troyano, A.; Melo, V.; Marcos, P.J.; Laserna, E.; Peiro, M.; Suarez-Cuartin, G.; Perea, L.; Feliu, A.; Plaza, V.; Faverio, P.; et al. Pseudomonas Aeruginosa in Chronic Obstructive Pulmonary Disease Patients with Frequent Hospitalized Exacerbations: A Prospective Multicentre Study. Respiration 2018, 96, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Ben Haj Khalifa, A.; Moissenet, D.; Vu Thien, H.; Khedher, M. Virulence factors in Pseudomonas aeruginosa: Mechanisms and modes of regulation. Ann. Biol. Clin. 2011, 69, 393–403. [Google Scholar] [CrossRef]
- Guadarrama-Orozco, K.D.; Perez-Gonzalez, C.; Kota, K.; Cocotl-Yañez, M.; Jiménez-Cortés, J.G.; Díaz-Guerrero, M.; Hernández-Garnica, M.; Munson, J.; Cadet, F.; López-Jácome, L.E.; et al. To Cheat or Not to Cheat: Cheatable and Non-Cheatable Virulence Factors in Pseudomonas Aeruginosa. FEMS Microbiol. Ecol. 2023, 99, fiad128. [Google Scholar] [CrossRef]
- Lee, J.; Zhang, L. The Hierarchy Quorum Sensing Network in Pseudomonas Aeruginosa. Protein Cell 2015, 6, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.W.; Asfahl, K.L.; Dandekar, A.A.; Greenberg, E.P. Pseudomonas aeruginosa Quorum Sensing. Advances in Experimental Medicine and Biology; Filloux, A., Ramos, J.-L., Eds.; Springer International Publishing: Cham, Switzerland, 2022; Volume 1386, pp. 95–115. ISBN 978-3-031-08490-4. [Google Scholar]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Bennett, P.M. Plasmid Encoded Antibiotic Resistance: Acquisition and Transfer of Antibiotic Resistance Genes in Bacteria. Br. J. Pharmacol. 2008, 153. [Google Scholar] [CrossRef]
- Ciofu, O.; Moser, C.; Jensen, P.Ø.; Høiby, N. Tolerance and Resistance of Microbial Biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente-Núñez, C.; Reffuveille, F.; Fernández, L.; Hancock, R.E. Bacterial Biofilm Development as a Multicellular Adaptation: Antibiotic Resistance and New Therapeutic Strategies. Curr. Opin. Microbiol. 2013, 16, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Tseng, B.S.; Zhang, W.; Harrison, J.J.; Quach, T.P.; Song, J.L.; Penterman, J.; Singh, P.K.; Chopp, D.L.; Packman, A.I.; Parsek, M.R. The Extracellular Matrix Protects Pseudomonas Aeruginosa Biofilms by Limiting the Penetration of Tobramycin. Environ. Microbiol. 2013, 15, 2865–2878. [Google Scholar] [CrossRef]
- Goltermann, L.; Tolker-Nielsen, T. Importance of the Exopolysaccharide Matrix in Antimicrobial Tolerance of Pseudomonas Aeruginosa Aggregates. Antimicrob. Agents Chemother. 2017, 61, e02696-16. [Google Scholar] [CrossRef]
- Nguyen, D.; Joshi-Datar, A.; Lepine, F.; Bauerle, E.; Olakanmi, O.; Beer, K.; McKay, G.; Siehnel, R.; Schafhauser, J.; Wang, Y.; et al. Active Starvation Responses Mediate Antibiotic Tolerance in Biofilms and Nutrient-Limited Bacteria. Science 2011, 334, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, K.S.; Swetschinski, L.R.; Robles Aguilar, G.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Davis Weaver, N.; Wool, E.E.; Han, C.; Gershberg Hayoon, A.; et al. Global Mortality Associated with 33 Bacterial Pathogens in 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2019. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2019 (accessed on 18 November 2020).
- Potron, A.; Poirel, L.; Nordmann, P. Emerging Broad-Spectrum Resistance in Pseudomonas Aeruginosa and Acinetobacter Baumannii: Mechanisms and Epidemiology. Int. J. Antimicrob. Agents 2015, 45, 568–585. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Wang-Kan, X.; Neuberger, A.; Van Veen, H.W.; Pos, K.M.; Piddock, L.J.V.; Luisi, B.F. Multidrug Efflux Pumps: Structure, Function and Regulation. Nat. Rev. Microbiol. 2018, 16, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, A.B.; Carrara, J.A.; Barroso, C.D.N.; Tuon, F.F.; Faoro, H. Role of Efflux Pumps on Antimicrobial Resistance in Pseudomonas Aeruginosa. Int. J. Mol. Sci. 2022, 23, 15779. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, C.; Grohmann, E. Horizontal Gene Transfer of Antibiotic Resistance Genes in Biofilms. Antibiotics 2023, 12, 328. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Yu, S.; Li, D.; Gillings, M.R.; Ren, H.; Mao, D.; Guo, J.; Luo, Y. Inter-Plasmid Transfer of Antibiotic Resistance Genes Accelerates Antibiotic Resistance in Bacterial Pathogens. ISME J. 2024, 18, wrad032. [Google Scholar] [CrossRef]
- Oliver, A.; Rojo-Molinero, E.; Arca-Suarez, J.; Beşli, Y.; Bogaerts, P.; Cantón, R.; Cimen, C.; Croughs, P.D.; Denis, O.; Giske, C.G.; et al. Pseudomonas Aeruginosa Antimicrobial Susceptibility Profiles, Resistance Mechanisms and International Clonal Lineages: Update from ESGARS-ESCMID/ISARPAE Group. Clin. Microbiol. Infect. 2024, 30, 469–480. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Kolter, R. Flagellar and Twitching Motility Are Necessary for Pseudomonas aeruginosa Biofilm Development. Mol. Microbiol. 1998, 30, 295–304. [Google Scholar] [CrossRef]
- Schniederberend, M.; Williams, J.F.; Shine, E.; Shen, C.; Jain, R.; Emonet, T.; Kazmierczak, B.I. Modulation of Flagellar Rotation in Surface-Attached Bacteria: A Pathway for Rapid Surface-Sensing after Flagellar Attachment. PLoS Pathog. 2019, 15, e1008149. [Google Scholar] [CrossRef] [PubMed]
- Webster, S.S.; Lee, C.K.; Schmidt, W.C.; Wong, G.C.L.; O’Toole, G.A. Interaction between the Type 4 Pili Machinery and a Diguanylate Cyclase Fine-Tune c-Di-GMP Levels during Early Biofilm Formation. Proc. Natl. Acad. Sci. USA 2021, 118, e2105566118. [Google Scholar] [CrossRef] [PubMed]
- Valentini, M.; Filloux, A. Biofilms and Cyclic Di-GMP (c-Di-GMP) Signaling: Lessons from Pseudomonas Aeruginosa and Other Bacteria. J. Biol. Chem. 2016, 291, 12547–12555. [Google Scholar] [CrossRef] [PubMed]
- Makin, S.A.; Beveridge, T.J. The Influence of A-Band and B-Band Lipopolysaccharide on the Surface Characteristics and Adhesion of Pseudomonas Aeruginosa to Surfaces. Microbiology 1996, 142, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Huszczynski, S.M.; Lam, J.S.; Khursigara, C.M. The Role of Pseudomonas Aeruginosa Lipopolysaccharide in Bacterial Pathogenesis and Physiology. Pathogens 2019, 9, 6. [Google Scholar] [CrossRef]
- Reichhardt, C.; Parsek, M.R. Confocal Laser Scanning Microscopy for Analysis of Pseudomonas Aeruginosa Biofilm Architecture and Matrix Localization. Front. Microbiol. 2019, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Gheorghita, A.A.; Wozniak, D.J.; Parsek, M.R.; Howell, P.L. Pseudomonas Aeruginosa Biofilm Exopolysaccharides: Assembly, Function, and Degradation. FEMS Microbiol. Rev. 2023, 47, fuad060. [Google Scholar] [CrossRef]
- Liang, Z.; Rybtke, M.; Kragh, K.N.; Johnson, O.; Schicketanz, M.; Zhang, Y.E.; Andersen, J.B.; Tolker-Nielsen, T. Transcription of the Alginate Operon in Pseudomonas Aeruginosa Is Regulated by C-Di-GMP. Microbiol. Spectr. 2022, 10, e00675-22. [Google Scholar] [CrossRef] [PubMed]
- May, T.B.; Shinabarger, D.; Maharaj, R.; Kato, J.; Chu, L.; DeVault, J.D.; Roychoudhury, S.; Zielinski, N.A.; Berry, A.; Rothmel, R.K. Alginate Synthesis by Pseudomonas Aeruginosa: A Key Pathogenic Factor in Chronic Pulmonary Infections of Cystic Fibrosis Patients. Clin. Microbiol. Rev. 1991, 4, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, X.; Liu, H.; Zhang, L.; Guo, Y.; Yu, S.; Wozniak, D.J.; Ma, L.Z. The Exopolysaccharide Psl–eDNA Interaction Enables the Formation of a Biofilm Skeleton in Pseudomonas Aeruginosa. Environ. Microbiol. Rep. 2015, 7, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Chemani, C.; Imberty, A.; De Bentzmann, S.; Pierre, M.; Wimmerová, M.; Guery, B.P.; Faure, K. Role of LecA and LecB Lectins in Pseudomonas Aeruginosa -Induced Lung Injury and Effect of Carbohydrate Ligands. Infect. Immun. 2009, 77, 2065–2075. [Google Scholar] [CrossRef]
- Jenal, U.; Reinders, A.; Lori, C. Cyclic Di-GMP: Second Messenger Extraordinaire. Nat. Rev. Microbiol. 2017, 15, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Pouget, C.; Dunyach-Remy, C.; Magnan, C.; Pantel, A.; Sotto, A.; Lavigne, J.-P. Polymicrobial Biofilm Organization of Staphylococcus Aureus and Pseudomonas Aeruginosa in a Chronic Wound Environment. Int. J. Mol. Sci. 2022, 23, 10761. [Google Scholar] [CrossRef]
- Li, B.; Qiu, Y.; Zhang, J.; Huang, X.; Shi, H.; Yin, H. Real-Time Study of Rapid Spread of Antibiotic Resistance Plasmid in Biofilm Using Microfluidics. Environ. Sci. Technol. 2018, 52, 11132–11141. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Lee, J.-H. Biofilm Dispersion in Pseudomonas Aeruginosa. J. Microbiol. 2016, 54, 71–85. [Google Scholar] [CrossRef]
- Barraud, N.; Schleheck, D.; Klebensberger, J.; Webb, J.S.; Hassett, D.J.; Rice, S.A.; Kjelleberg, S. Nitric Oxide Signaling in Pseudomonas Aeruginosa Biofilms Mediates Phosphodiesterase Activity, Decreased Cyclic Di-GMP Levels, and Enhanced Dispersal. J. Bacteriol. 2009, 191, 7333–7342. [Google Scholar] [CrossRef]
- Allkja, J.; Bjarnsholt, T.; Coenye, T.; Cos, P.; Fallarero, A.; Harrison, J.J.; Lopes, S.P.; Oliver, A.; Pereira, M.O.; Ramage, G.; et al. Minimum Information Guideline for Spectrophotometric and Fluorometric Methods to Assess Biofilm Formation in Microplates. Biofilm 2020, 2, 100010. [Google Scholar] [CrossRef] [PubMed]
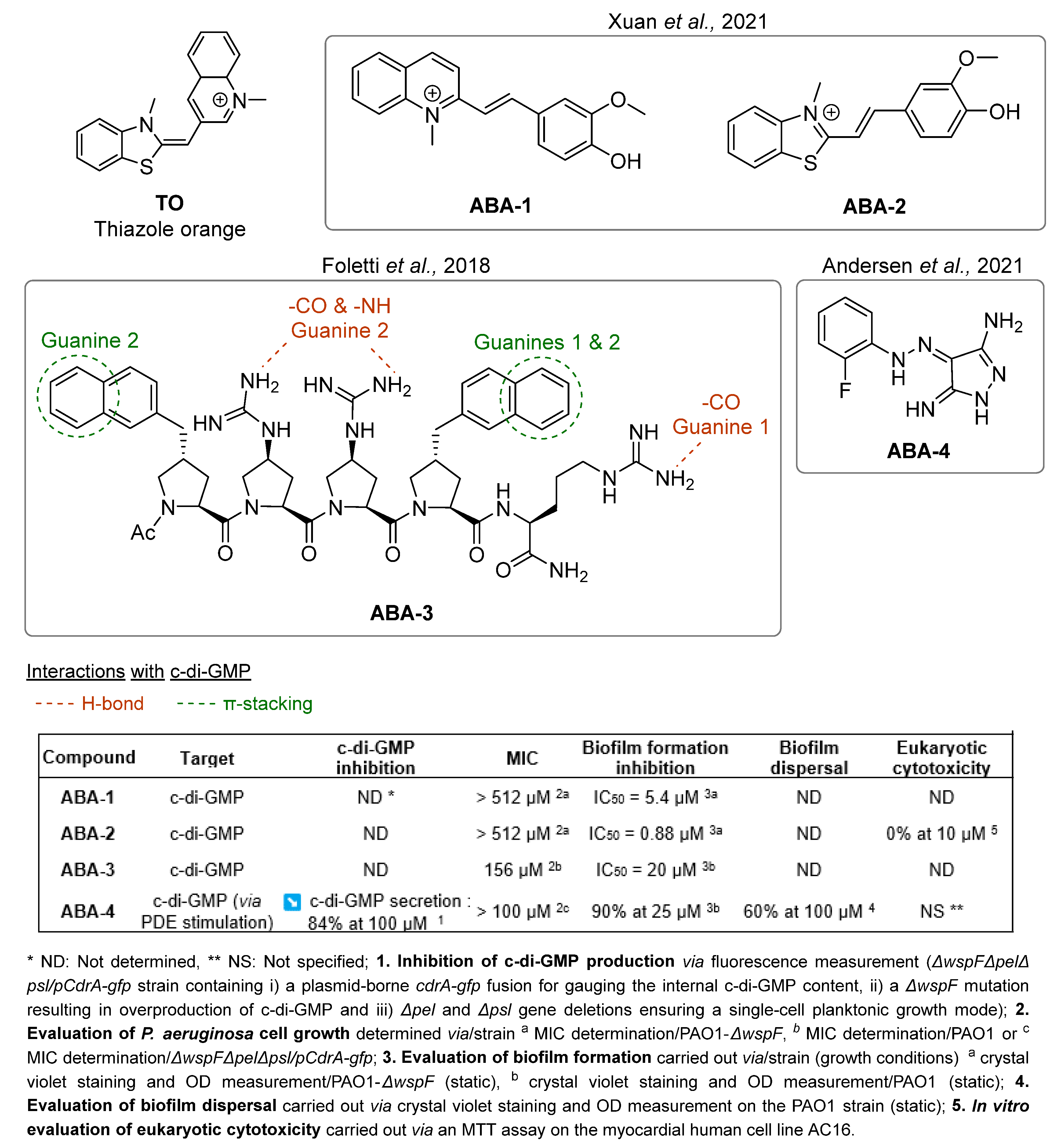
- Xuan, T.-F.; Wang, Z.-Q.; Liu, J.; Yu, H.-T.; Lin, Q.-W.; Chen, W.-M.; Lin, J. Design and Synthesis of Novel C-Di-GMP G-Quadruplex Inducers as Bacterial Biofilm Inhibitors. J. Med. Chem. 2021, 64, 11074–11089. [Google Scholar] [CrossRef] [PubMed]
- Foletti, C.; Kramer, R.A.; Mauser, H.; Jenal, U.; Bleicher, K.H.; Wennemers, H. Functionalized Proline-Rich Peptides Bind the Bacterial Second Messenger c-di-GMP. Angew. Chem. Int. Ed. 2018, 57, 7729–7733. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.B.; Hultqvist, L.D.; Jansen, C.U.; Jakobsen, T.H.; Nilsson, M.; Rybtke, M.; Uhd, J.; Fritz, B.G.; Seifert, R.; Berthelsen, J.; et al. Identification of Small Molecules That Interfere with C-Di-GMP Signaling and Induce Dispersal of Pseudomonas Aeruginosa Biofilms. npj Biofilms Microbiomes 2021, 7, 59. [Google Scholar] [CrossRef]
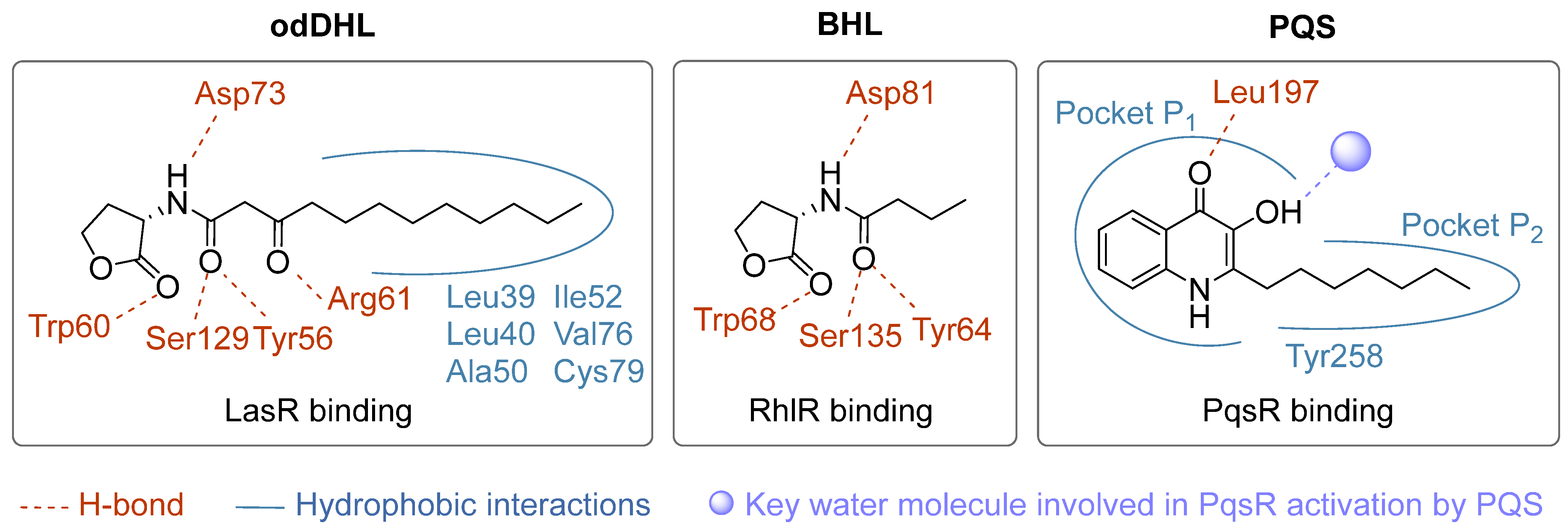
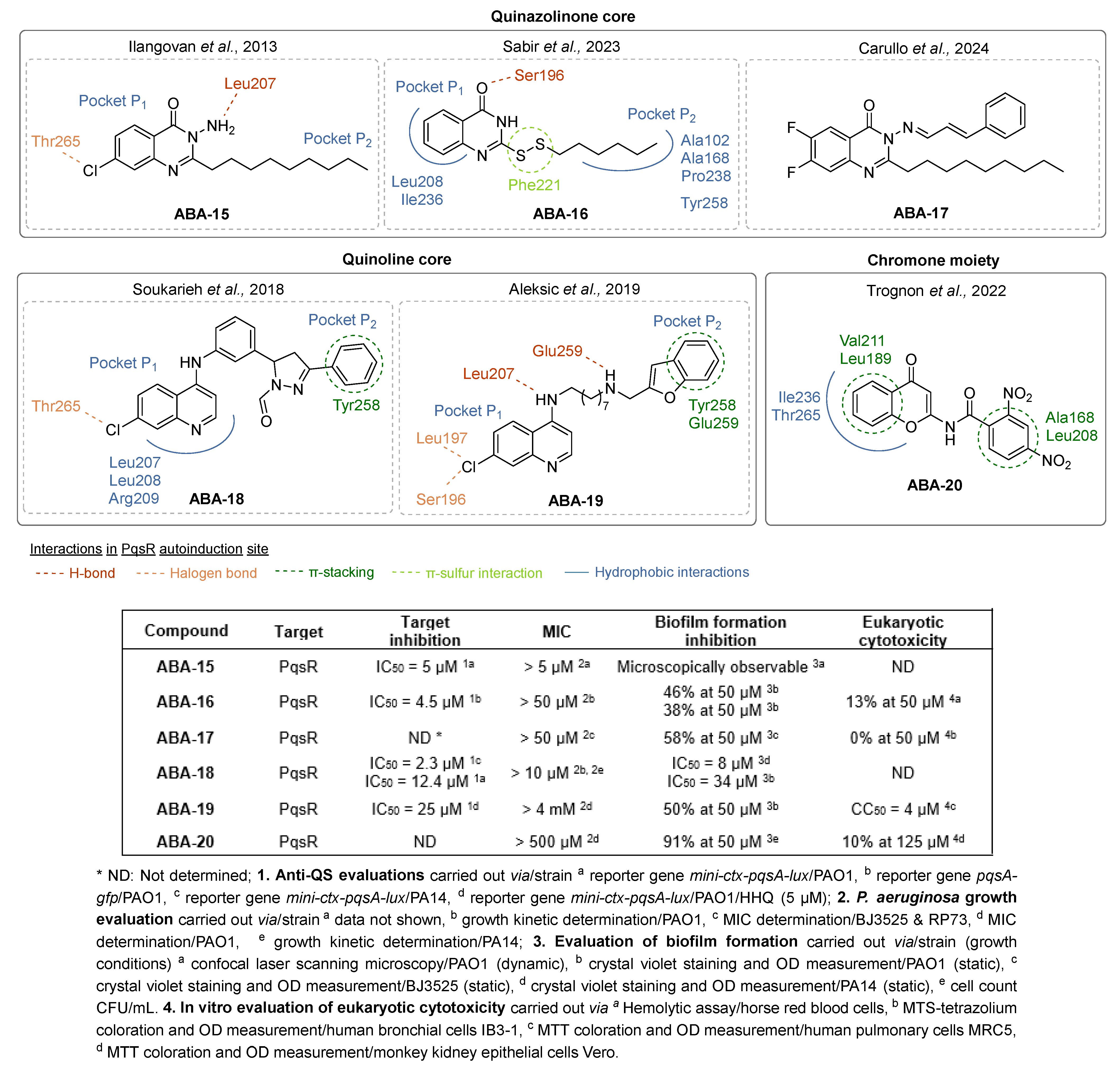
- Ilangovan, A.; Fletcher, M.; Rampioni, G.; Pustelny, C.; Rumbaugh, K.; Heeb, S.; Cámara, M.; Truman, A.; Chhabra, S.R.; Emsley, J.; et al. Structural Basis for Native Agonist and Synthetic Inhibitor Recognition by the Pseudomonas Aeruginosa Quorum Sensing Regulator PqsR (MvfR). PLoS Pathog. 2013, 9, e1003508. [Google Scholar] [CrossRef] [PubMed]
- Bottomley, M.J.; Muraglia, E.; Bazzo, R.; Carfì, A. Molecular Insights into Quorum Sensing in the Human Pathogen Pseudomonas Aeruginosa from the Structure of the Virulence Regulator LasR Bound to Its Autoinducer. J. Biol. Chem. 2007, 282, 13592–13600. [Google Scholar] [CrossRef] [PubMed]
- Borgert, S.R.; Henke, S.; Witzgall, F.; Schmelz, S.; Zur Lage, S.; Hotop, S.-K.; Stephen, S.; Lübken, D.; Krüger, J.; Gomez, N.O.; et al. Moonlighting Chaperone Activity of the Enzyme PqsE Contributes to RhlR-Controlled Virulence of Pseudomonas Aeruginosa. Nat. Commun. 2022, 13, 7402. [Google Scholar] [CrossRef]
- Shandil, S.; Yu, T.T.; Sabir, S.; Black, D.S.; Kumar, N. Synthesis of Novel Quinazolinone Analogues for Quorum Sensing Inhibition. Antibiotics 2023, 12, 1227. [Google Scholar] [CrossRef] [PubMed]
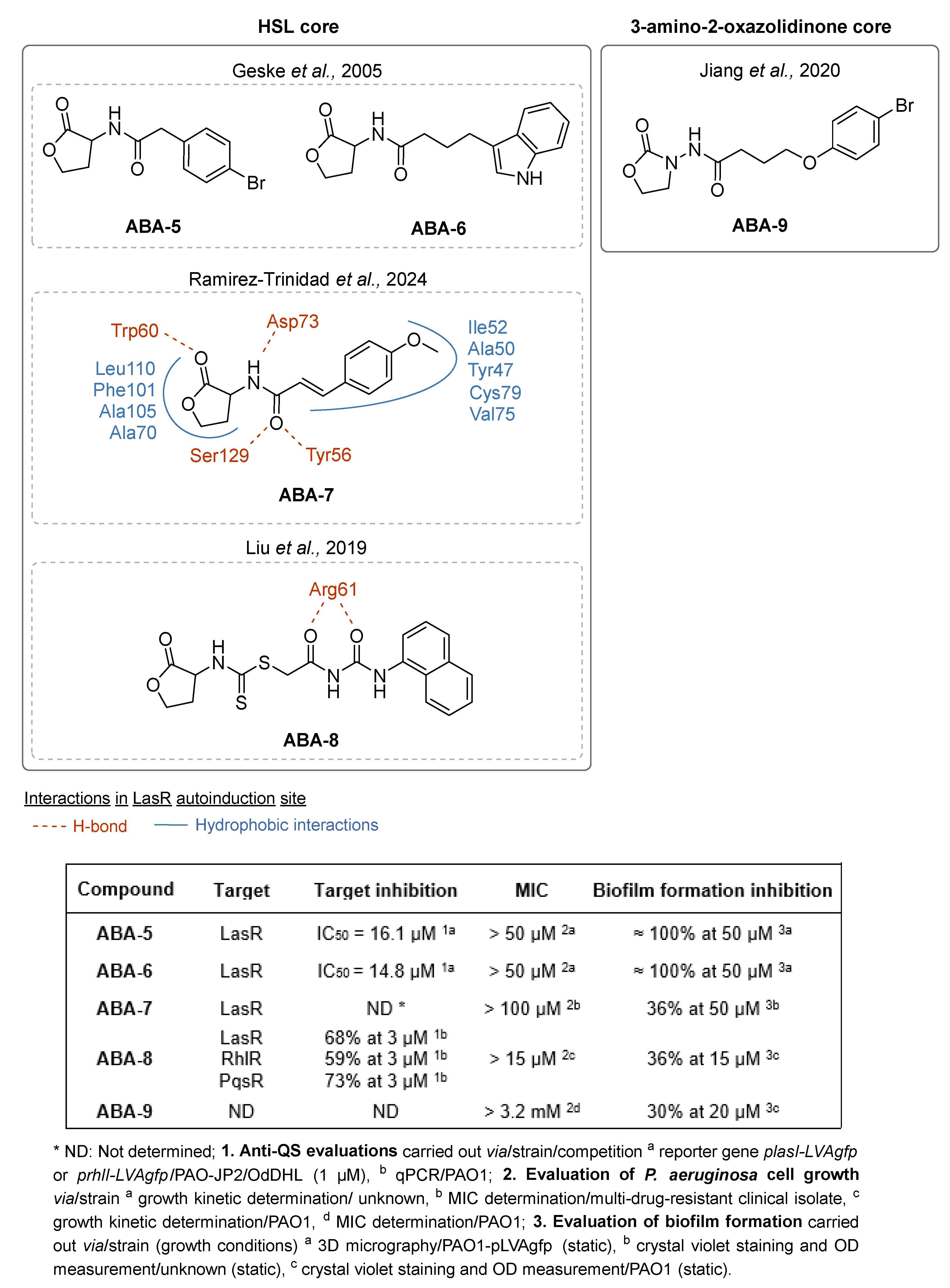
- Geske, G.D.; Wezeman, R.J.; Siegel, A.P.; Blackwell, H.E. Small Molecule Inhibitors of Bacterial Quorum Sensing and Biofilm Formation. J. Am. Chem. Soc. 2005, 127, 12762–12763. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Trinidad, Á.; Martínez-Solano, E.; Tovar-Roman, C.E.; García-Guerrero, M.; Rivera-Chávez, J.A.; Hernández-Vázquez, E. Synthesis, Antibiofilm Activity and Molecular Docking of N-Acylhomoserine Lactones Containing Cinammic Moieties. Bioorg. Med. Chem. Lett. 2024, 98, 129592. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gong, Q.; Luo, C.; Liang, Y.; Kong, X.; Wu, C.; Feng, P.; Wang, Q.; Zhang, H.; Wireko, M.A. Synthesis and Biological Evaluation of Novel L-Homoserine Lactone Analogs as Quorum Sensing Inhibitors of Pseudomonas aeruginosa. Chem. Pharm. Bull. 2019, 67, 1088–1098. [Google Scholar] [CrossRef]
- Jiang, K.; Yan, X.; Yu, J.; Xiao, Z.; Wu, H.; Zhao, M.; Yue, Y.; Zhou, X.; Xiao, J.; Lin, F. Design, Synthesis, and Biological Evaluation of 3-Amino-2-Oxazolidinone Derivatives as Potent Quorum-Sensing Inhibitors of Pseudomonas Aeruginosa PAO1. Eur. J. Med. Chem. 2020, 194, 112252. [Google Scholar] [CrossRef] [PubMed]
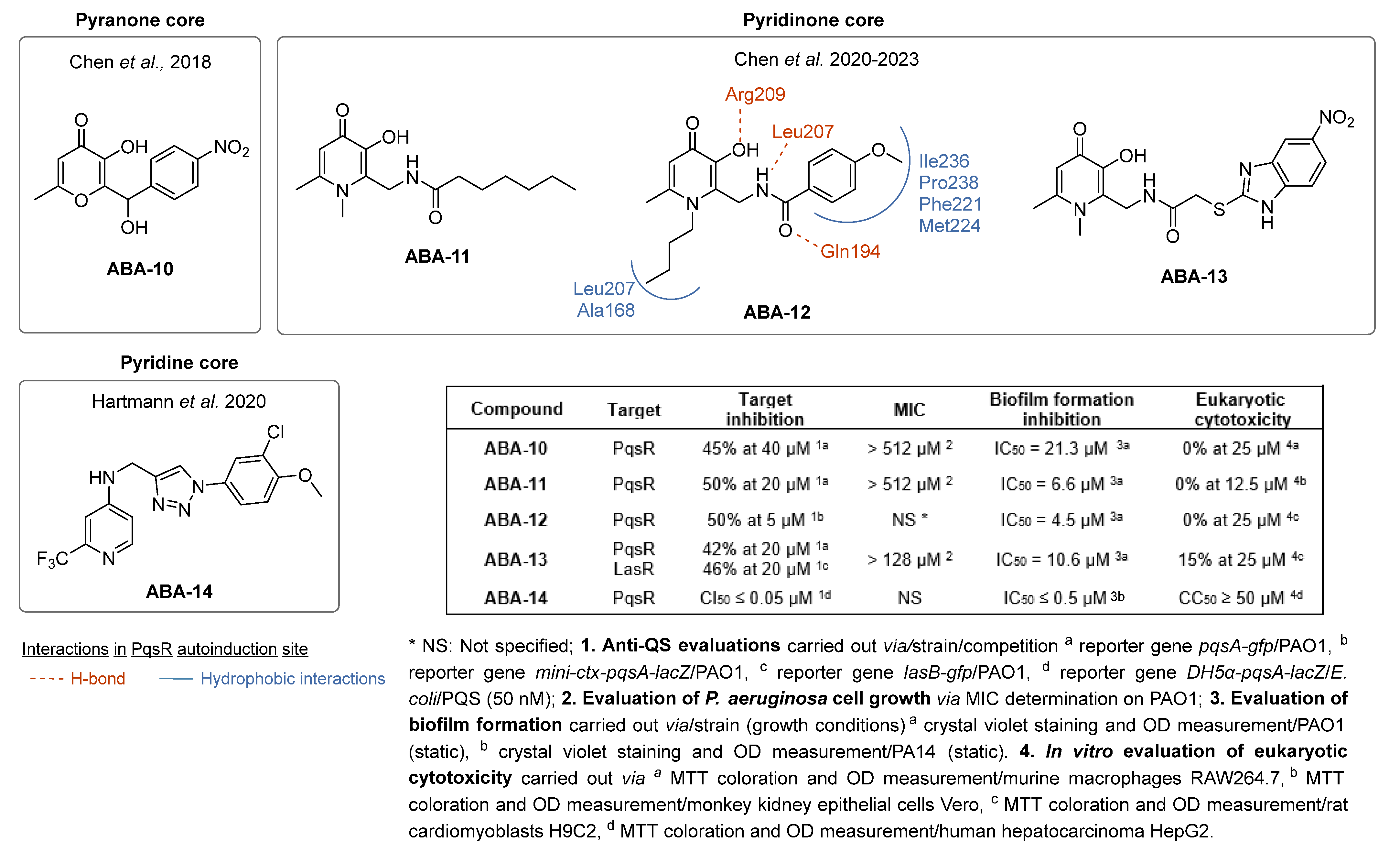
- Li, Y.-B.; Liu, J.; Huang, Z.-X.; Yu, J.-H.; Xu, X.-F.; Sun, P.-H.; Lin, J.; Chen, W.-M. Design, Synthesis and Biological Evaluation of 2-Substituted 3-Hydroxy-6-Methyl-4H-Pyran-4-One Derivatives as Pseudomonas Aeruginosa Biofilm Inhibitors. Eur. J. Med. Chem. 2018, 158, 753–766. [Google Scholar] [CrossRef]
- Liu, J.; Hou, J.-S.; Li, Y.-B.; Miao, Z.-Y.; Sun, P.-H.; Lin, J.; Chen, W.-M. Novel 2-Substituted 3-Hydroxy-1,6-Dimethylpyridin-4(1 H )-Ones as Dual-Acting Biofilm Inhibitors of Pseudomonas aeruginosa. J. Med. Chem. 2020, 63, 10921–10945. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hou, J.-S.; Chang, Y.-Q.; Peng, L.-J.; Zhang, X.-Y.; Miao, Z.-Y.; Sun, P.-H.; Lin, J.; Chen, W.-M. New Pqs Quorum Sensing System Inhibitor as an Antibacterial Synergist against Multidrug-Resistant Pseudomonas Aeruginosa. J. Med. Chem. 2022, 65, 688–709. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Meng, Y.; Yang, M.-H.; Zhang, X.-Y.; Zhao, J.-F.; Sun, P.-H.; Chen, W.-M. Design, Synthesis and Biological Evaluation of Novel 3-Hydroxypyridin-4(1H)-Ones Based Hybrids as Pseudomonas Aeruginosa Biofilm Inhibitors. Eur. J. Med. Chem. 2023, 259, 115665. [Google Scholar] [CrossRef]
- Ahmed, A.S.A.; Empting, M.; Hamed, M.; Hartmann, R.W. Preparation of Five- and Six-Membered Nitrogen Heterocycles as PQSR Inverse Agonists for the Treatment and Prevention of Bacterial Infections. WO 2020/007938 A1 3 July 2019. [Google Scholar]
- Sabir, S.; Das, T.; Kuppusamy, R.; Yu, T.T.; Willcox, M.D.; Black, D.S.; Kumar, N. Novel Quinazolinone Disulfide Analogues as Pqs Quorum Sensing Inhibitors against Pseudomonas Aeruginosa. Bioorganic Chem. 2023, 130, 106226. [Google Scholar] [CrossRef]
- Carullo, G.; Di Bonaventura, G.; Rossi, S.; Lupetti, V.; Tudino, V.; Brogi, S.; Butini, S.; Campiani, G.; Gemma, S.; Pompilio, A. Development of Quinazolinone Derivatives as Modulators of Virulence Factors of Pseudomonas Aeruginosa Cystic Fibrosis Strains. Molecules 2023, 28, 6535. [Google Scholar] [CrossRef] [PubMed]
- Soukarieh, F.; Vico Oton, E.; Dubern, J.-F.; Gomes, J.; Halliday, N.; de Pilar Crespo, M.; Ramírez-Prada, J.; Insuasty, B.; Abonia, R.; Quiroga, J.; et al. In Silico and in Vitro-Guided Identification of Inhibitors of Alkylquinolone-Dependent Quorum Sensing in Pseudomonas Aeruginosa. Molecules 2018, 23, 257. [Google Scholar] [CrossRef] [PubMed]
- Aleksic, I.; Jeremic, J.; Milivojevic, D.; Ilic-Tomic, T.; Šegan, S.; Zlatović, M.; Opsenica, D.M.; Senerovic, L. N-Benzyl Derivatives of Long-Chained 4-Amino-7-Chloro-Quionolines as Inhibitors of Pyocyanin Production in Pseudomonas Aeruginosa. ACS Chem. Biol. 2019, 14, 2800–2809. [Google Scholar] [CrossRef] [PubMed]
- Aleksić, I.; Šegan, S.; Andrić, F.; Zlatović, M.; Moric, I.; Opsenica, D.M.; Senerovic, L. Long-Chain 4-Aminoquinolines as Quorum Sensing Inhibitors in Serratia Marcescens and Pseudomonas aeruginosa. ACS Chem. Biol. 2017, 12, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Trognon, J.; Vera, G.; Rima, M.; Stigliani, J.-L.; Amielet, L.; El Hage, S.; Lajoie, B.; Roques, C.; El Garah, F. Investigation of Direct and Retro Chromone-2-Carboxamides Based Analogs of Pseudomonas Aeruginosa Quorum Sensing Signal as New Anti-Biofilm Agents. Pharmaceuticals 2022, 15, 417. [Google Scholar] [CrossRef] [PubMed]
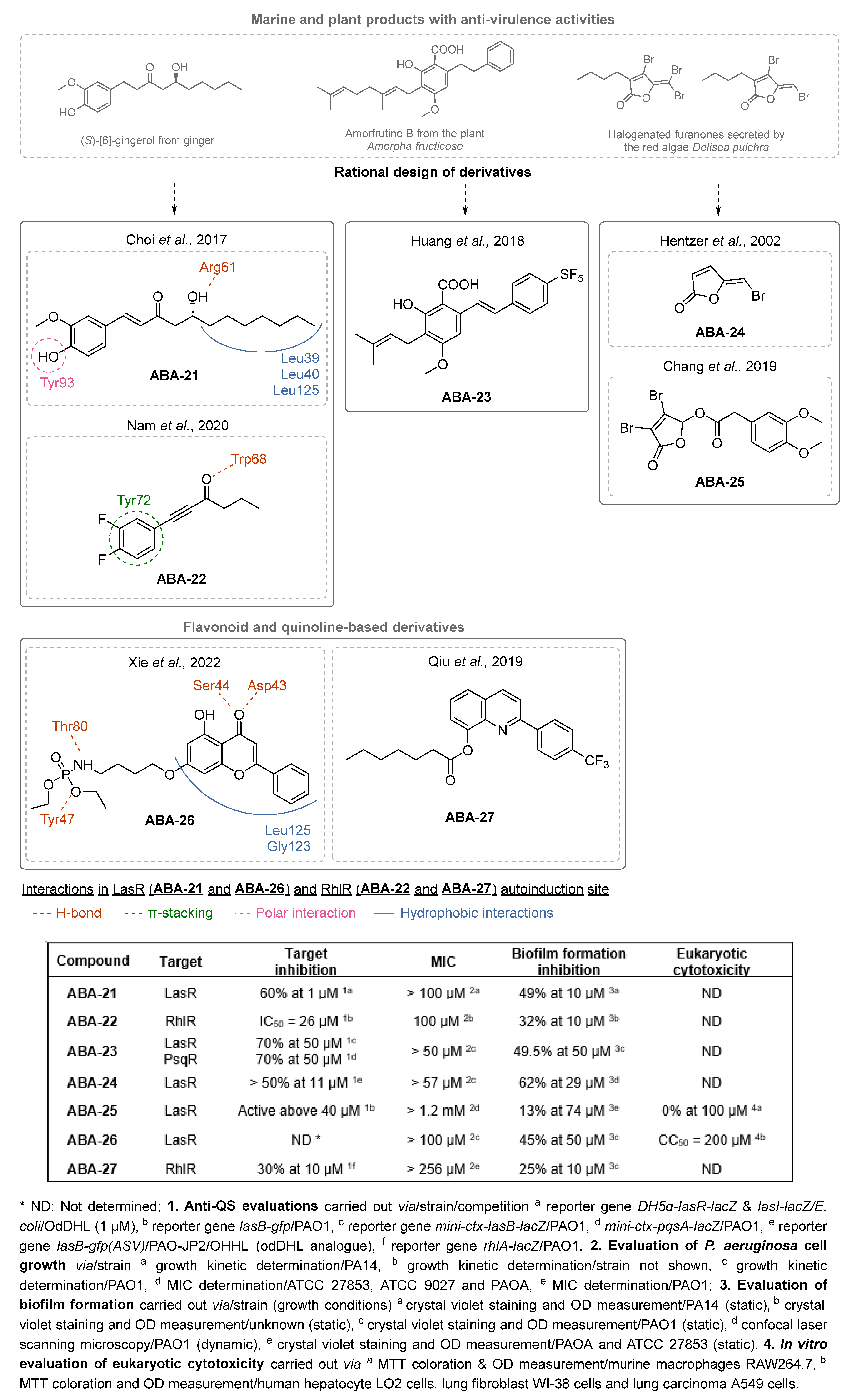
- Choi, H.; Ham, S.-Y.; Cha, E.; Shin, Y.; Kim, H.-S.; Bang, J.K.; Son, S.-H.; Park, H.-D.; Byun, Y. Structure–Activity Relationships of 6- and 8-Gingerol Analogs as Anti-Biofilm Agents. J. Med. Chem. 2017, 60, 9821–9837. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Ham, S.-Y.; Kwon, H.; Kim, H.-S.; Moon, S.; Lee, J.-H.; Lim, T.; Son, S.-H.; Park, H.-D.; Byun, Y. Discovery and Characterization of Pure RhlR Antagonists against Pseudomonas Aeruginosa Infections. J. Med. Chem. 2020, 63, 8388–8407. [Google Scholar] [CrossRef]
- Xu, X.-J.; Zeng, T.; Huang, Z.-X.; Xu, X.-F.; Lin, J.; Chen, W.-M. Synthesis and Biological Evaluation of Cajaninstilbene Acid and Amorfrutins A and B as Inhibitors of the Pseudomonas Aeruginosa Quorum Sensing System. J. Nat. Prod. 2018, 81, 2621–2629. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-X.; Yu, J.-H.; Xu, X.-J.; Xu, X.-F.; Zeng, T.; Lin, J.; Chen, W.-M. Cajaninstilbene Acid Analogues as Novel Quorum Sensing and Biofilm Inhibitors of Pseudomonas Aeruginosa. Microb. Pathog. 2020, 148, 104414. [Google Scholar] [CrossRef]
- Hentzer, M.; Riedel, K.; Rasmussen, T.B.; Heydorn, A.; Andersen, J.B.; Parsek, M.R.; Rice, S.A.; Eberl, L.; Molin, S.; Høiby, N.; et al. Inhibition of Quorum Sensing in Pseudomonas Aeruginosa Biofilm Bacteria by a Halogenated Furanone Compound. Microbiology 2002, 148, 87–102. [Google Scholar] [CrossRef]
- Chang, Y.; Wang, P.-C.; Ma, H.-M.; Chen, S.-Y.; Fu, Y.-H.; Liu, Y.-Y.; Wang, X.; Yu, G.-C.; Huang, T.; Hibbs, D.E.; et al. Design, Synthesis and Evaluation of Halogenated Furanone Derivatives as Quorum Sensing Inhibitors in Pseudomonas Aeruginosa. Eur. J. Pharm. Sci. 2019, 140, 105058. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, J.; Wang, B.; Peng, A.-Y.; Mao, Z.-W.; Xia, W. Inhibition of Quorum-Sensing Regulator from Pseudomonas Aeruginosa Using a Flavone Derivative. Molecules 2022, 27, 2439. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.-N.; Wang, F.; Chen, S.-Y.; Wang, P.-C.; Fu, Y.-H.; Liu, Y.-Y.; Wang, X.; Wang, F.-B.; Wang, C.; Yang, H.-W.; et al. Novel 2, 8-Bit Derivatives of Quinolines Attenuate Pseudomonas Aeruginosa Virulence and Biofilm Formation. Bioorg. Med. Chem. Lett. 2019, 29, 749–754. [Google Scholar] [CrossRef]
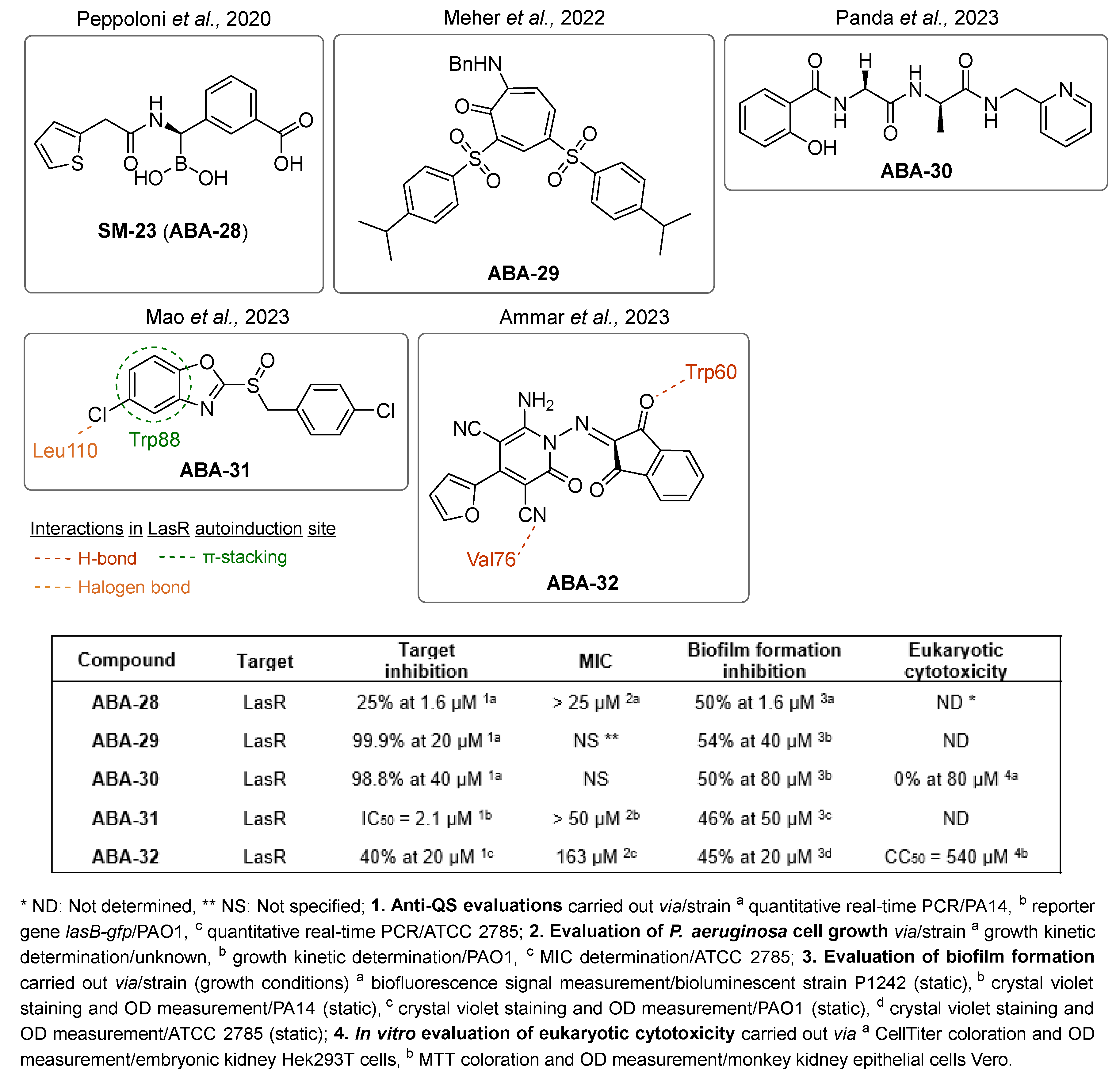
- Peppoloni, S.; Pericolini, E.; Colombari, B.; Pinetti, D.; Cermelli, C.; Fini, F.; Prati, F.; Caselli, E.; Blasi, E. The β-Lactamase Inhibitor Boronic Acid Derivative SM23 as a New Anti-Pseudomonas Aeruginosa Biofilm. Front. Microbiol. 2020, 11, 35. [Google Scholar] [CrossRef]
- Meher, S.; Kumari, S.; Dixit, M.; Sharma, N.K. Cu-Catalyzed Synthesis of Alkylaminotroponyl Sulfones as Pseudomonas Aeruginosa Quorum Sensing Inhibitors Targeting lasI/R QS Circuitry. Chem. Asian J. 2022, 17, e202200866. [Google Scholar] [CrossRef]
- Panda, S.S.; Kumari, S.; Dixit, M.; Sharma, N.K. N-Salicyl-AAn-Picolamide Foldameric Peptides Exhibit Quorum Sensing Inhibition of Pseudomonas Aeruginosa (PA14). ACS Omega 2023, 8, 30349–30358. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Li, Q.; Yang, Z.; Li, Y.; Ye, X.; Wang, H. Design, Synthesis, and Biological Evaluation of Benzoheterocyclic Sulfoxide Derivatives as Quorum Sensing Inhibitors in Pseudomonas Aeruginosa. J. Enzyme Inhib. Med. Chem. 2023, 38, 2175820. [Google Scholar] [CrossRef] [PubMed]
- Ammar, Y.A.; Ragab, A.; Migahed, M.A.; Al-Sharbasy, S.; Salem, M.A.; Riad, O.K.M.; Selim, H.M.R.M.; Abd-elmaksoud, G.A.; Abusaif, M.S. Design, Green Synthesis, and Quorum Sensing Quenching Potential of Novel 2-Oxo-Pyridines Containing a Thiophene/Furan Scaffold and Targeting a Las R Gene on P. Aeruginosa. RSC Adv. 2023, 13, 27363–27384. [Google Scholar] [CrossRef] [PubMed]
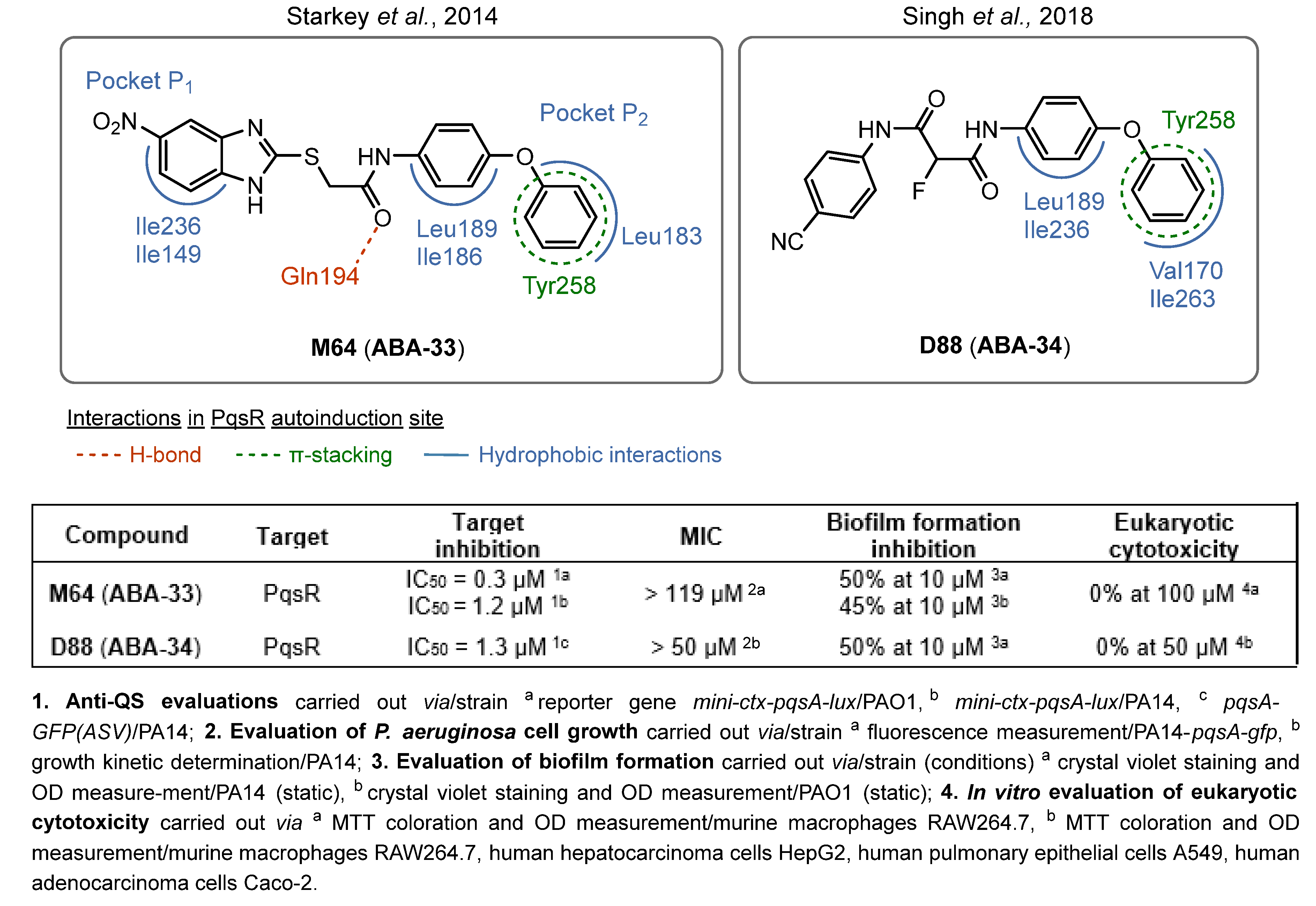
- Starkey, M.; Lepine, F.; Maura, D.; Bandyopadhaya, A.; Lesic, B.; He, J.; Kitao, T.; Righi, V.; Milot, S.; Tzika, A.; et al. Identification of Anti-Virulence Compounds That Disrupt Quorum-Sensing Regulated Acute and Persistent Pathogenicity. PLoS Pathog. 2014, 10, e1004321. [Google Scholar] [CrossRef]
- Maura, D.; Rahme, L.G. Pharmacological Inhibition of the Pseudomonas aeruginosa MvfR Quorum-Sensing System Interferes with Biofilm Formation and Potentiates Antibiotic-Mediated Biofilm Disruption. Antimicrob. Agents Chemother. 2017, 61, e01362-17. [Google Scholar] [CrossRef] [PubMed]
- Kitao, T.; Lepine, F.; Babloudi, S.; Walte, F.; Steinbacher, S.; Maskos, K.; Blaesse, M.; Negri, M.; Pucci, M.; Zahler, B.; et al. Molecular Insights into Function and Competitive Inhibition of Pseudomonas Aeruginosa Multiple Virulence Factor Regulator. mBio 2018, 9, e02158-17. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Gahane, A.; Singh, V.; Ghosh, S.; Thakur, A. Antibiofilm Activity of Fmoc-Phenylalanine against Gram-Positive and Gram-Negative Bacterial Biofilms. J. Antibiot. 2021, 74, 407–416. [Google Scholar] [CrossRef]
- Johansson, E.M.V.; Crusz, S.A.; Kolomiets, E.; Buts, L.; Kadam, R.U.; Cacciarini, M.; Bartels, K.-M.; Diggle, S.P.; Cámara, M.; Williams, P.; et al. Inhibition and Dispersion of Pseudomonas Aeruginosa Biofilms by Glycopeptide Dendrimers Targeting the Fucose-Specific Lectin LecB. Chem. Biol. 2008, 15, 1249–1257. [Google Scholar] [CrossRef]
- Kadam, R.U.; Bergmann, M.; Hurley, M.; Garg, D.; Cacciarini, M.; Swiderska, M.A.; Nativi, C.; Sattler, M.; Smyth, A.R.; Williams, P.; et al. A Glycopeptide Dendrimer Inhibitor of the Galactose-Specific Lectin LecA and of Pseudomonas Aeruginosa Biofilms. Angew. Chem. Int. Ed. 2011, 50, 10631–10635. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Thies-Weesie, D.M.E.; Pieters, R.J. Tetravalent Pseudomonas Aeruginosa Adhesion Lectin LecA Inhibitor for Enhanced Biofilm Inhibition. Helv. Chim. Acta 2019, 102, e1900014. [Google Scholar] [CrossRef]
- Sommer, R.; Wagner, S.; Rox, K.; Varrot, A.; Hauck, D.; Wamhoff, E.-C.; Schreiber, J.; Ryckmans, T.; Brunner, T.; Rademacher, C.; et al. Glycomimetic, Orally Bioavailable LecB Inhibitors Block Biofilm Formation of Pseudomonas Aeruginosa. J. Am. Chem. Soc. 2018, 140, 2537–2545. [Google Scholar] [CrossRef] [PubMed]
- Sommer, R.; Rox, K.; Wagner, S.; Hauck, D.; Henrikus, S.S.; Newsad, S.; Arnold, T.; Ryckmans, T.; Brönstrup, M.; Imberty, A.; et al. Anti-Biofilm Agents against Pseudomonas Aeruginosa: A Structure–Activity Relationship Study of C -Glycosidic LecB Inhibitors. J. Med. Chem. 2019, 62, 9201–9216. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial Peptides: Pore Formers or Metabolic Inhibitors in Bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.W.; Rehm, B.H.A.; Hancock, R.E.W. Human Host Defense Peptide LL-37 Prevents Bacterial Biofilm Formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef] [PubMed]
- Nagant, C.; Pitts, B.; Nazmi, K.; Vandenbranden, M.; Bolscher, J.G.; Stewart, P.S.; Dehaye, J.-P. Identification of Peptides Derived from the Human Antimicrobial Peptide LL-37 Active against Biofilms Formed by Pseudomonas Aeruginosa Using a Library of Truncated Fragments. Antimicrob. Agents Chemother. 2012, 56, 5698–5708. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, M.R.; Ghosh, A.; Harmouche, N.; Casciaro, B.; Luca, V.; Bortolotti, A.; Cappiello, F.; Stella, L.; Bhunia, A.; Bechinger, B.; et al. Membrane Perturbing Activities and Structural Properties of the Frog-Skin Derived Peptide Esculentin-1a(1-21)NH2 and Its Diastereomer Esc(1-21)-1c: Correlation with Their Antipseudomonal and Cytotoxic Activity. Biochim. Biophys. Acta BBA-Biomembr. 2017, 1859, 2327–2339. [Google Scholar] [CrossRef]
- Casciaro, B.; Lin, Q.; Afonin, S.; Loffredo, M.R.; De Turris, V.; Middel, V.; Ulrich, A.S.; Di, Y.P.; Mangoni, M.L. Inhibition of Pseudomonas Aeruginosa Biofilm Formation and Expression of Virulence Genes by Selective Epimerization in the Peptide Esculentin-1a(1-21) NH2. FEBS J. 2019, 286, 3874–3891. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente-Núñez, C.; Reffuveille, F.; Haney, E.F.; Straus, S.K.; Hancock, R.E.W. Broad-Spectrum Anti-Biofilm Peptide That Targets a Cellular Stress Response. PLoS Pathog. 2014, 10, e1004152. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente-Núñez, C.; Reffuveille, F.; Mansour, S.C.; Reckseidler-Zenteno, S.L.; Hernández, D.; Brackman, G.; Coenye, T.; Hancock, R.E.W. D-Enantiomeric Peptides That Eradicate Wild-Type and Multidrug-Resistant Biofilms and Protect against Lethal Pseudomonas Aeruginosa Infections. Chem. Biol. 2015, 22, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Deslouches, B.; Gonzalez, I.A.; DeAlmeida, D.; Islam, K.; Steele, C.; Montelaro, R.C.; Mietzner, T.A. De Novo-Derived Cationic Antimicrobial Peptide Activity in a Murine Model of Pseudomonas Aeruginosa Bacteraemia. J. Antimicrob. Chemother. 2007, 60, 669–672. [Google Scholar] [CrossRef]
- Lin, Q.; Deslouches, B.; Montelaro, R.C.; Di, Y.P. Prevention of ESKAPE Pathogen Biofilm Formation by Antimicrobial Peptides WLBU2 and LL37. Int. J. Antimicrob. Agents 2018, 52, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Casciaro, B.; Loffredo, M.R.; Cappiello, F.; O’Sullivan, N.; Tortora, C.; Manzer, R.; Karmakar, S.; Haskell, A.; Hasan, S.K.; Mangoni, M.L. KDEON WK-11: A Short Antipseudomonal Peptide with Promising Potential. Front. Chem. 2022, 10, 1000765. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Gonçalves, S.; Felício, M.R.; Maturana, P.; Santos, N.C.; Semorile, L.; Hollmann, A.; Maffía, P.C. Synergistic and Antibiofilm Activity of the Antimicrobial Peptide P5 against Carbapenem-Resistant Pseudomonas Aeruginosa. Biochim. Biophys. Acta BBA-Biomembr. 2019, 1861, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Faccone, D.; Veliz, O.; Corso, A.; Noguera, M.; Martínez, M.; Payes, C.; Semorile, L.; Maffía, P.C. Antimicrobial Activity of de Novo Designed Cationic Peptides against Multi-Resistant Clinical Isolates. Eur. J. Med. Chem. 2014, 71, 31–35. [Google Scholar] [CrossRef]
- Cardoso, M.H.; Santos, V.P.M.; Costa, B.O.; Buccini, D.F.; Rezende, S.B.; Porto, W.F.; Santos, M.J.; Silva, O.N.; Ribeiro, S.M.; Franco, O.L. A Short Peptide with Selective Anti-Biofilm Activity against Pseudomonas Aeruginosa and Klebsiella Pneumoniae Carbapenemase-Producing Bacteria. Microb. Pathog. 2019, 135, 103605. [Google Scholar] [CrossRef] [PubMed]
- Porto, W.F.; Fensterseifer, I.C.M.; Ribeiro, S.M.; Franco, O.L. Joker: An Algorithm to Insert Patterns into Sequences for Designing Antimicrobial Peptides. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2043–2052. [Google Scholar] [CrossRef] [PubMed]
- Sobke, A.; Klinger, M.; Hermann, B.; Sachse, S.; Nietzsche, S.; Makarewicz, O.; Keller, P.M.; Pfister, W.; Straube, E. The Urinary Antibiotic 5-Nitro-8-Hydroxyquinoline (Nitroxoline) Reduces the Formation and Induces the Dispersal of Pseudomonas Aeruginosa Biofilms by Chelation of Iron and Zinc. Antimicrob. Agents Chemother. 2012, 56, 6021–6025. [Google Scholar] [CrossRef]
- Dosler, S.; Karaaslan, E. Inhibition and Destruction of Pseudomonas Aeruginosa Biofilms by Antibiotics and Antimicrobial Peptides. Peptides 2014, 62, 32–37. [Google Scholar] [CrossRef]
- Panthi, V.K.; Fairfull-Smith, K.E.; Islam, N. Liposomal Drug Delivery Strategies to Eradicate Bacterial Biofilms: Challenges, Recent Advances, and Future Perspectives. Int. J. Pharm. 2024, 655, 124046. [Google Scholar] [CrossRef]
- Ibaraki, H.; Kanazawa, T.; Chien, W.-Y.; Nakaminami, H.; Aoki, M.; Ozawa, K.; Kaneko, H.; Takashima, Y.; Noguchi, N.; Seta, Y. The Effects of Surface Properties of Liposomes on Their Activity against Pseudomonas Aeruginosa PAO-1 Biofilm. J. Drug Deliv. Sci. Technol. 2020, 57, 101754. [Google Scholar] [CrossRef]
- Thomann, A.; de Mello Martins, A.G.G.; Brengel, C.; Empting, M.; Hartmann, R.W. Application of Dual Inhibition Concept within Looped Autoregulatory Systems toward Antivirulence Agents against Pseudomonas Aeruginosa Infections. ACS Chem. Biol. 2016, 11, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.J.; Dubern, J.-F.; Chan, W.C.; Chhabra, S.R.; Williams, P. A Pseudomonas Aeruginosa PQS Quorum-Sensing System Inhibitor with Anti-Staphylococcal Activity Sensitizes Polymicrobial Biofilms to Tobramycin. Cell Chem. Biol. 2022, 29, 1187–1199.e6. [Google Scholar] [CrossRef]
- Bernabè, G.; Marzaro, G.; Di Pietra, G.; Otero, A.; Bellato, M.; Pauletto, A.; Scarpa, M.; Sut, S.; Chilin, A.; Dall’Acqua, S.; et al. A Novel Phenolic Derivative Inhibits AHL-Dependent Quorum Sensing Signaling in Pseudomonas Aeruginosa. Front. Pharmacol. 2022, 13, 996871. [Google Scholar] [CrossRef] [PubMed]
- Reffuveille, F.; de la Fuente-Núñez, C.; Mansour, S.; Hancock, R.E.W. A Broad-Spectrum Antibiofilm Peptide Enhances Antibiotic Action against Bacterial Biofilms. Antimicrob. Agents Chemother. 2014, 58, 5363–5371. [Google Scholar] [CrossRef] [PubMed]
| Combination | Biofilm Eradication | Biofilm Dispersal | Ref | |||||
|---|---|---|---|---|---|---|---|---|
| ATB | AVA | ATB | ATB + AVA | ATB | ATB + AVA | |||
| Structure | Posology | Structure | Posology | |||||
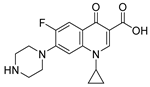 Ciprofloxacin | 1.5 µM |  ABA-4 | 100 µM | 33% 1a | 84% 1a | ND * | [46] | |
| 1 µM | 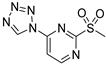 ABA-58 | 50 µM | 28% 1b | 62% 1b | ND | [106] | ||
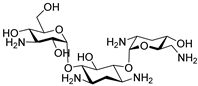 Tobramycin Tobramycin | 64 µM | 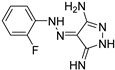 ABA-4 | 100 µM | 8% 1a | 57% 1a | ND | [46] | |
| 21 µM |  ABA-36 | 10 µM | 24% 1c | 66% 1c | ND | [80] | ||
| 214 µM | 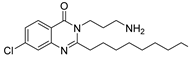 ABA-59 | 100 µM | 60% 1d | 95% 1d | ND | [107] | ||
| 214 µM | 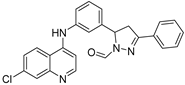 ABA-18 | 8 µM | 75% 1e | 95% 1e | ND | [62] | ||
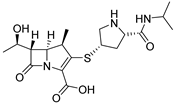 Meropenem | 26 µM | 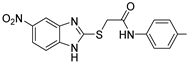 ABA-36 | 10 µM | 3% 1c | 34% 1c | ND | [80] | |
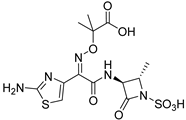 Aztreonam | 9 µM | 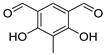 ABA-60 | 100 µM | ND | 40% 2 | 60% 2 | [108] | |
| Combination | Biofilm Formation Inhibition | Biofilm Dispersal | Biofilm Eradication | Ref | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ATB | AMP | ATB | ATB + AMP | ATB | ATB + AMP | ATB | ATB + AMP | |||
| Structure | Posology | Structure | Posology | |||||||
 Ciprofloxacin | 0.12 µM | ABA-44 (1018) | 0.52 µM | ND * | ND | ND | ND | NS ** | 90% 3a | [109] |
| ABA-45 (DJK5) | 0.064 µM | ND | 100% 1a | ND 2a | Weak 2a | ND 3b | 100% 3b | [94] | ||
| ABA-46 (DJK6) | 0.6 µM | ND | 100% 1a | ND 2a | 100% 2a | ND 3b | 100% 3b | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanot, M.; Lohou, E.; Sonnet, P. Anti-Biofilm Agents to Overcome Pseudomonas aeruginosa Antibiotic Resistance. Pharmaceuticals 2025, 18, 92. https://doi.org/10.3390/ph18010092
Hanot M, Lohou E, Sonnet P. Anti-Biofilm Agents to Overcome Pseudomonas aeruginosa Antibiotic Resistance. Pharmaceuticals. 2025; 18(1):92. https://doi.org/10.3390/ph18010092
Chicago/Turabian StyleHanot, Marie, Elodie Lohou, and Pascal Sonnet. 2025. "Anti-Biofilm Agents to Overcome Pseudomonas aeruginosa Antibiotic Resistance" Pharmaceuticals 18, no. 1: 92. https://doi.org/10.3390/ph18010092
APA StyleHanot, M., Lohou, E., & Sonnet, P. (2025). Anti-Biofilm Agents to Overcome Pseudomonas aeruginosa Antibiotic Resistance. Pharmaceuticals, 18(1), 92. https://doi.org/10.3390/ph18010092