1. Introduction
Aromatase is the key enzyme responsible for converting androgens to oestrogens in the body [
1]. It is widely expressed in various tissues and organs, such as the central nervous system, placenta, ovaries, testes, mammary glands, bones, liver, uterus, adipose tissue, and skeletal muscle [
2,
3]. Aromatase is the enzyme responsible for oestrogen biosynthesis, and it is known to be overexpressed in various types of tumours. High aromatase expression has been observed in some of the most common cancers, such as endometrial cancer, prostate cancer, and brain tumours [
4,
5,
6,
7,
8]. There are also reports of other diseases related to aromatase, such as Alzheimer’s disease, endometriosis, polycystic ovary syndrome, and depression [
9,
10,
11,
12].
Breast cancer is the most common cancer among women and a leading cause of cancer death, with an incidence rate of 11.6% [
13]. Aromatase is overexpressed in hormone-dependent tumours such as breast and ovarian cancer. This type of cancer is closely associated with hormone levels, particularly in hormone receptor-positive (HR+) breast cancer. In these types of breast cancer, the expression level of aromatase, which is a key enzyme that converts androgens into oestrogens, is often high [
7]. The overexpression of aromatase is closely related to the development, progression, and response of breast cancer to treatment. In breast cancer cells, aromatase is highly expressed in the cytoplasm and endoplasmic reticulum and is crucial for the growth of oestrogen-dependent tumours [
14]. Oestrogen, as a driving force for the growth of these tumours, directly promotes tumour development via increased synthesis. Therefore, aromatase is not only a key enzyme in oestrogen synthesis but also an important target for the treatment of HR+ breast cancer. The MCF-7 cell line is a human breast cancer cell line that is widely used in breast cancer research, and it is considered a typical representative of cells with an oestrogen receptor-positive status [
15]. This cell line has become an important model for studying the pathophysiology of breast cancer and testing new therapies because of its dependence on oestrogen [
16].
Positron emission tomography (PET) is a non-invasive imaging technique widely used in clinical settings, particularly for the detection of tumours and neurological diseases [
17]. PET imaging using radiolabelled aromatase inhibitors can help visualise aromatase expression in vivo, which may be useful for diagnosing and monitoring HR+ breast cancer [
2]. The use of aromatase tracers for positron emission tomography (PET) can be applied to identify highly expressed aromatase tumour locations and diagnose these tumours. During the treatment process, positron emission tomography (PET) can be used to assess the response of highly aromatase-expressing tumours to therapy [
3]. Aromatase tracers can not only be used for imaging but also be designed to carry therapeutic radioisotopes as targeted therapeutic agents. These agents can specifically bind to aromatase on tumour cells, releasing radioactive particles that kill or damage tumour cells. The application of aromatase tracers in PET imaging and therapy may become a research field with the potential to improve disease diagnosis and treatment [
18].
Small-molecule drugs with aromatic or heteroaromatic systems often require the introduction of radionuclides. We reviewed studies reporting that [11C]-vorozole [
2,
19,
20,
21,
22] and [11C]-cetrozole [
23,
24] can function as high-affinity aromatase-binding radiotracers for PET imaging. Owing to the short half-life of [11C], radiopharmaceuticals must be used near the site of production, which limits their delivery and application. In contrast, the longer half-life of [18F] (approximately 110 min) offers a more extended time window for PET imaging. Furthermore, the longer half-life of [18F] allows for the transportation of radioactive pharmaceuticals to more distant locations, enabling a wider range of medical institutions to utilise PET tracers. This is particularly advantageous compared to [11C], which typically requires onsite production. Owing to its superior physical properties, fluorine-18 [18F] has become the predominant radioisotope used in PET imaging [
25].
Professor Zehui Wu’s team discovered radiotracers that target aromatase, [18F]BIBD-069 and [18F]BIBD-071, which exhibit excellent binding affinity for aromatase and good pharmacokinetics; as a result, they have potential for the diagnosis and treatment of aromatase-related diseases [
26]. [18F]BIBD-071 exhibits favourable pharmacokinetic characteristics, allowing rapid clearance from the body as a diagnostic agent. Compared with [11C]-labelled aromatase tracers, [18F]BIBD-071 provides a longer imaging window, facilitating clinical application and patient scheduling. The radiolabelling conditions are relatively simple, and the yield is high, making it convenient for clinical translation. [18F]BIBD-071 has favourable pharmacokinetic properties, including high bioavailability and an appropriate lipid–water partition coefficient. No defluorination of [18F]BIBD-071 was observed in vivo, which helps to reduce nonspecific signals and improve imaging accuracy. Owing to its superior characteristics, [18F]BIBD-071 has the potential to become an important tool in the clinical diagnosis and treatment of aromatase-related diseases, especially in the monitoring and assessment of oestrogen-dependent diseases [
26]. To promote the translation of [18F]BIBD-071 from laboratory research to clinical application and potentially provide new tools for the diagnosis and treatment of aromatase-related diseases, we first applied it to animal models of aromatase-related breast cancer. Moreover, we obtained quantitative biodistribution data of the probe in a breast tumour model through in vivo distribution experiments, which helps us further understand the metabolic behaviour of the radiotracer in important tissues and organs.
In this study, we applied [18F]BIBD-071 in hormone-dependent breast cancer. We plan to conduct further research in other hormone-dependent tumours to explore the diagnostic and therapeutic applications of this aromatase tracer in hormone-dependent diseases. This tracer has the potential to become an important tool for personalised medicine and precision treatment.
3. Discussion
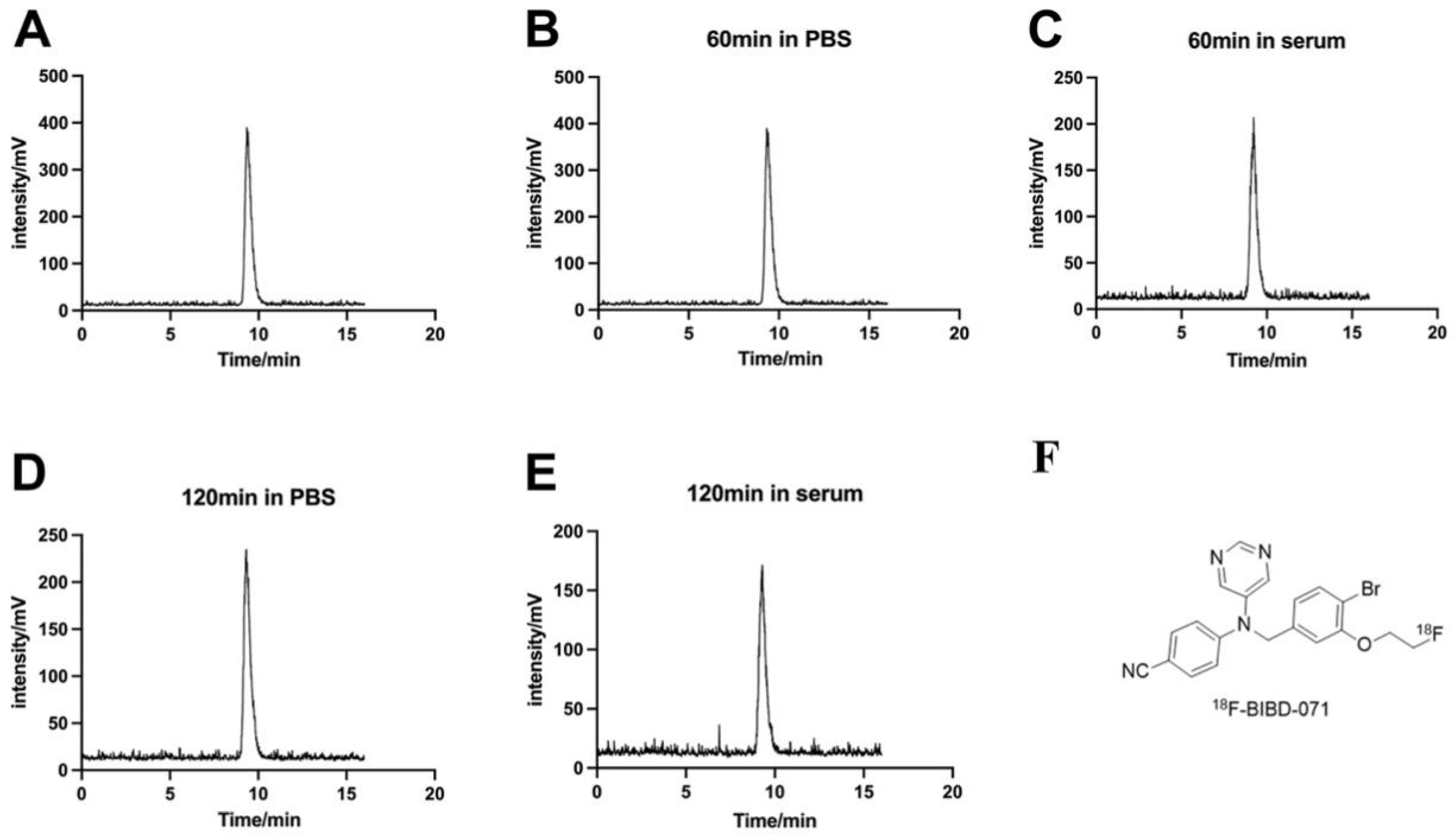
In this study, we introduce [18F]-BIBD-071, a novel [18F]-labelled PET tracer specific to aromatase, which was designed and synthesised by Professor Zehui Wu’s team. That original article mentioned two tracers, [18F]-BIBD-069 and [18F]-BIBD-071. We selected [18F]-BIBD-071 for this study because [18F]BIBD-071 exhibits favourable pharmacokinetic characteristics, allowing rapid clearance from the body as a diagnostic agent [
26]. Additionally, [18F]BIBD-071 could quickly distribute to the target tissue, and we could observe the uptake of the probe by the tumour at 0.5 h. This tracer had previously only undergone design, synthesis, and biological evaluation and has not been applied in an in vivo animal tumour model.
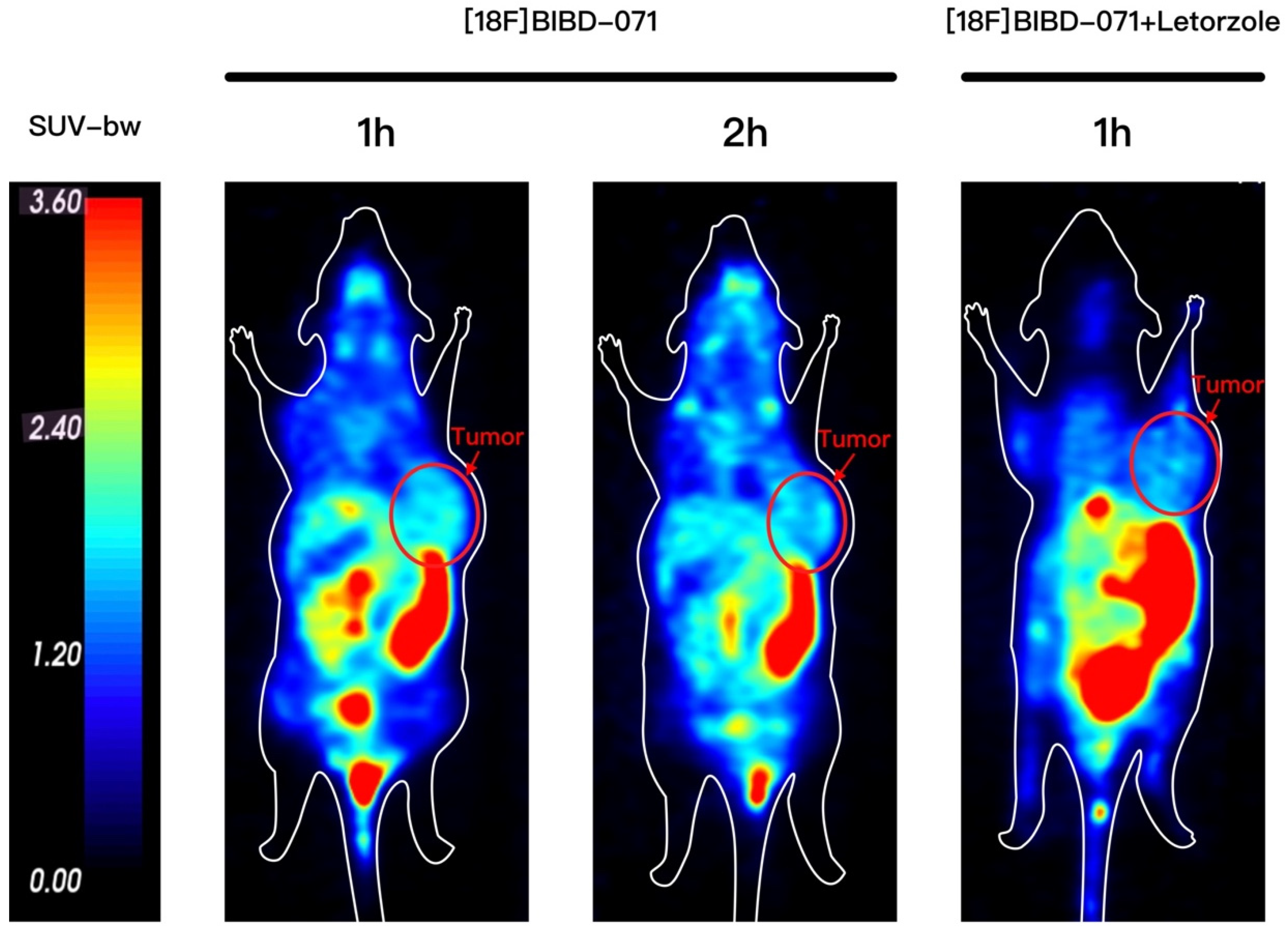
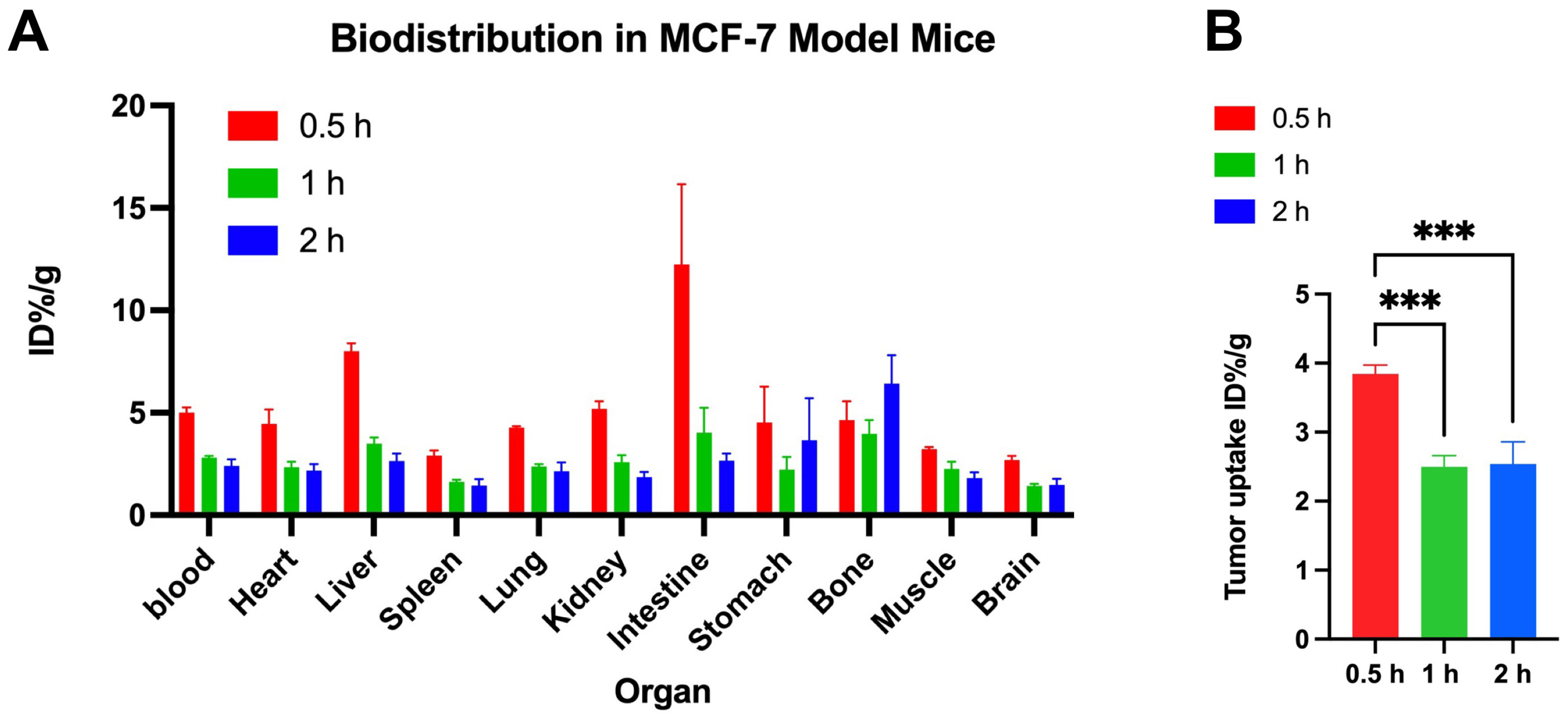
This study introduces [18F]BIBD-071, a novel PET tracer with a high degree of specificity for visualising aromatase expression, particularly in breast cancer. We conducted our investigation using the widely recognised MCF-7 xenograft model, which is a standard for evaluating the efficacy of new cancer imaging agents. Our research findings demonstrate that [18F]BIBD-071 possesses remarkable in vitro stability, a key factor for reliable PET imaging. Moreover, it shows significant tumour uptake and an advantageous tumour-to-background ratio in PET/CT imaging studies. We injected tumours in the right axillary region and the contralateral axilla as a background for comparison. Through PET/CT imaging, a clear contrast could be visually observed. As shown by the biodistribution results, the uptake of the probe in nontargeted tissues such as the liver, small intestine, and stomach significantly decreased. These attributes are paramount for the precise detection and monitoring of cancerous tissues, which are fundamental components of personalised medicine and precision therapy in oncology. The successful application of [18F]BIBD-071 in the MCF-7 model suggests its potential for clinical translation, offering a promising tool for the management of hormone-dependent breast cancer.
Currently, there are reports on the application of [11C]-labelled aromatase tracers [
19,
20,
21,
22,
23,
24]. However, one of the most significant advantages of [18F]BIBD-071 is that [18F] has a longer half-life than [11C]-labelled tracers do, allowing for a longer imaging window [
25]. These advantages could increase the accessibility of PET imaging with this tracer for a broader range of patients and medical centres. When selecting the imaging time points, we referred to the most widely used [18F]-labelled positron diagnostic reagent [18F]FDG (fluorodeoxyglucose) [
27]. At present, [18F]FDG imaging in our nuclear medicine is usually performed 40 min to an hour after injection. Therefore, for [18F] BIBD-071, a small-molecule diagnostic probe labelled with [18F], we selected imaging time points of 0.5, 1, and 2 h in our design. We hope that the [18F]-labelled tracers are relatively stable; our study confirmed that [18F]BIBD-071 is sufficiently stable both in PBS and in FBS by using HPLC at the 1 h and 2 h time points, and PET imaging was performed at these intervals. This is what is observed for [18F]-FDG, a commonly used PET imaging tracer, where the optimal imaging time is 40 min to 60 min after the injection of the tracer [
27].
In Professor Wu’s article, the biodistribution results revealed greater uptake of the tracer in the stomach, ovaries, and adrenal glands than in other analysed tissues. Our study, however, demonstrated increased uptake in the small intestine, liver, and kidneys. Professor Wu’s experiments were conducted on Sprague–Dawley rats [
26], whereas our experiments utilised nude mice. There may be some differences resulting from the different species being used in the studies. Like rats and mice, for the same probe ([18F]BIBD-071), the same organs had varying uptake, and further experiments are needed to explore the distinct organ distributions of this tracer. The biodistribution data revealed high initial blood and tumour uptake, with a subsequent decline, suggesting rapid metabolic clearance. The relatively low uptake in the brain is likely due to the blood–brain barrier and the low expression of aromatase in the brain, which is consistent with the findings of Professor Kayo Takahashi and colleagues [
24].
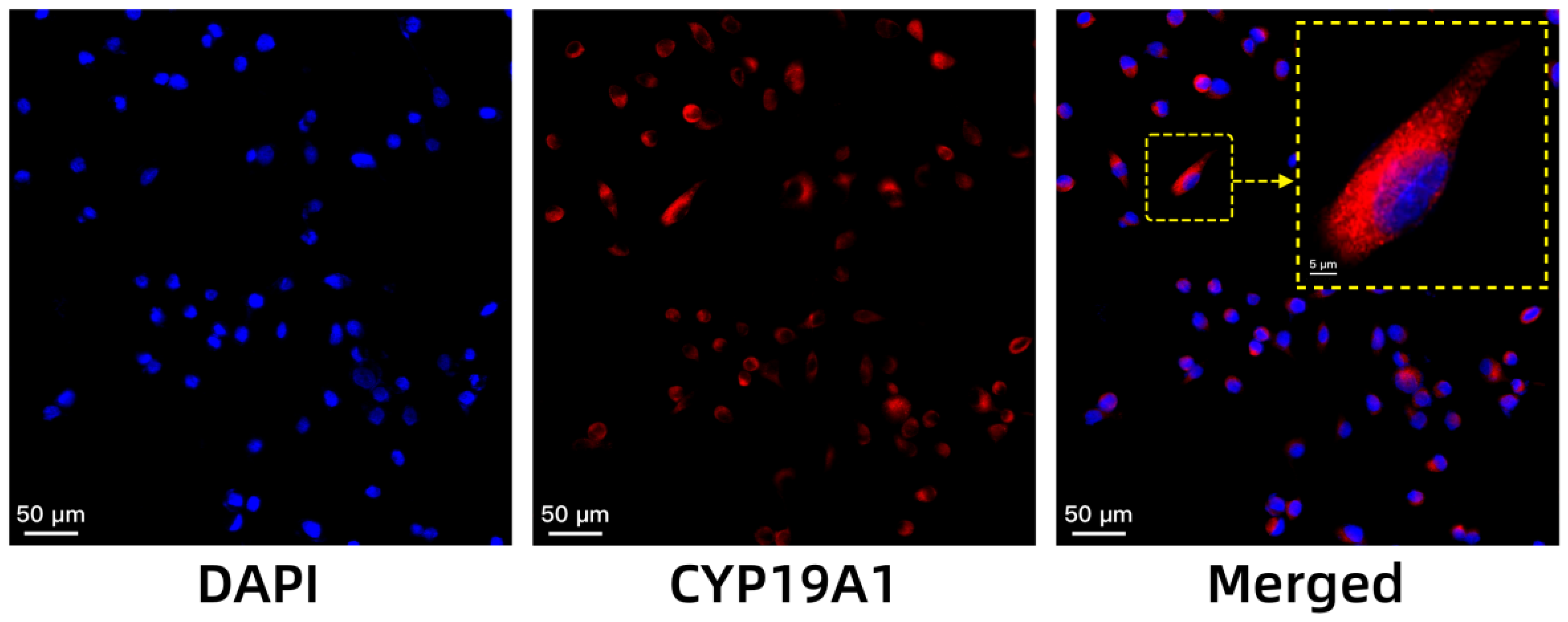
The high expression of aromatase in breast cancer has been well-documented in the literature, highlighting its importance as a therapeutic target [
28]. Aromatase, the enzyme responsible for the conversion of androgens to oestrogens, is notably elevated in the cytoplasm and endoplasmic reticulum of breast cancer cells and is crucial for oestrogen-dependent tumour growth [
14]. Our immunofluorescence results align with previous findings, confirming that MCF-7 cells, a widely recognised model for HR+ breast cancer, express high levels of
CYP19A1, the gene encoding aromatase. This high expression validates the choice of the MCF-7 cell line for our experiments, as it provides a relevant and representative model for studying the role of aromatase in breast cancer [
15].
Our study has several limitations that cause our findings to warrant further investigation: (1) The high uptake in the liver and small intestine may necessitate further strategies to reduce background signals and enhance tumour visualisation. Subsequent studies should design more imaging time points to find a better balance between a lower background signal and a more suitable tumour signal. Alternatively, adjusting the injection dose may reduce uptake in nontarget organs while maintaining tumour visualisation by reducing the dose. (2) Professor Wu’s team chose Sprague–Dawley rats for drug stability experiments [
26], whereas we selected nude mice for the establishment of tumour models. This is because nude mice have an incomplete immune system, which facilitates the establishment of xenograft tumour models. Because different tumour models may lead to different results in biodistribution data, the uptake of biodistribution in the same organs at the same time point may vary. (3) The current study provides valuable insights into the biodistribution and PET imaging of [18F]BIBD-071 in an HR+ breast cancer xenograft model. The next step should be to further investigate its application in other oestrogen-dependent tumour models.
In conclusion, the present study demonstrates the promising potential of the novel [18F]-labelled radiotracer [18F]BIBD-071 in PET imaging for HR+ breast cancer. Our findings revealed that [18F]BIBD-071 exhibited excellent in vitro stability and selective uptake in the MCF-7 xenograft tumour model, with favourable tumour visualisation and a significant tumour-to-background ratio observed via PET/CT imaging at 1 and 2 h postinjection. The biodistribution studies confirmed high tracer uptake in the liver, small intestine, and stomach, with a notable decrease over time. Aromatase expression in MCF-7 cells, as confirmed by immunofluorescence, is correlated with the tumour uptake of [18F]BIBD-071. These results suggest that [18F]BIBD-071 is a valuable PET tracer for diagnosing and monitoring HR+ breast cancer and warrants further investigation into its application in hormone-dependent cancers. Future research should focus on optimising the biodistribution profile of [18F]BIBD-071, exploring its application in other cancer types, and conducting clinical trials.
4. Materials and Methods
4.1. Synthesis and Radiolabelling of [18F]BIBD-071
[18F]BIBD-071 was synthesised as described previously [
26]. The precursor was synthesised by us, and the [18F]-fluoride solution in [18O]H
2O was purchased from China Beijing HTA Co., Ltd. (Beijing, China). Briefly, an activated Sep-Pak Light QMA Carb was loaded with 740 MBq (20 mCi) of [18F]fluoride and eluted with K2.2.2/K
2CO
3. In total, 1 milligram of precursor was dissolved in 1 mL of DMSO and added to [18F] fluoride. The mixture was heated at 80 °C for 10 min and added to water after cooling. The mixture was loaded onto an activated Oasis HLB 3 cm
3 cartridge, pushed through, and washed with water. The crude product [18F]BIBD-071 was eluted with ethanol, diluted with water, and separated by HPLC with a mobile phase of CH
3CN/H
2O (40/60) at a flow rate of 2 mL/min. The fraction containing [18F]BIBD-071 was collected, diluted with water and concentrated using a C18 Sep-Pak cartridge. The product was passed through a sterile membrane filter (0.22 μm) and afforded a formulated solution that was ready for administration.
4.2. In Vitro Stability Testing
The quality control of [18F]BIBD-071 used HPLC with a mobile phase of CH3CN/H2O (40/60) at a flow rate of 2 mL/min. The radiochemical purity was greater than 98%. The stability of [18F]BIBD-071 was assessed over various time points after incubation by conducting radio-HPLC to determine its purity. A total of 0.74 MBq of [18F]BIBD-071 was separately added to 100 μL solutions of phosphate-buffered saline (PBS) or foetal bovine serum (FBS) provided by China Zhejiang Jiangsu Biotechnology Co., Ltd. (Suzhou, China). The radiochemical purity was measured via high-performance liquid chromatography (HPLC) at 60 and 120 min with a mobile phase of CH3CN/H2O (40/60) at a flow rate of 2 mL/min.
4.3. Cell Lines and Culture Conditions
The human breast cancer cell line MCF-7 was purchased from the Institution of Biophysics, Chinese Academy of Sciences. MCF-7 cells were cultured in high-glucose DMEM (GIBCO, Grand Island, NY, USA) supplemented with 10% FBS and 1% penicillin–streptomycin (Procell Life Science & Technology Co., Ltd., Wuhan, China). The cells were grown in a humid incubator at 37 °C with 5% CO2.
4.4. Animal Models
BALB/c nude mice (female, 4–5 weeks) were purchased from Vital River Laboratory (Animal Technology Co., Ltd., Beijing, China). All animal experiments were performed in accordance with the instructions and permissions of the ethical committee of Beijing Tiantan Hospital (ethics approval number: BNI202404007). Subcutaneous tumour models were established by injecting a suspension of 6 × 106 cells in 100 μL of PBS into the right shoulder of nude mice. The mice were kept in the specific pathogen-free (SPF) degree facility under the following conditions: room temperature maintained at 20 to 26 °C, humidity at 40% to 70%, and cycle of 12 h light and 12 h dark. The average daily feed consumption was 5 g for 8-week-old animals, and the average daily water consumption was 6 to 7 mL for 8-week-old animals. After 2–3 weeks, when the tumour volume reached 200–300 mm3, the mice bearing tumours were used for biodistribution and microPET/CT imaging.
4.5. Micro PET/CT Imaging
The mice that had been injected with MCF-7 cells were injected with 4.74 MBq (100 μL, 128 μCi) [18F]BIBD-071 via the tail vein. After the tracer injection, the mice were anaesthetised with 2% isoflurane in 1 L/min oxygen and then placed in the prone position for scanning. Representative PET/CT images of mice bearing MCF-7 tumours were collected at 1 and 2 h after intravenous injection of [18F]BIBD-071 via the tail vein. All PET/CT imaging was performed on a dedicated small animal PET/CT scanner (SuperNova®, Preclinical, Shanghai, China). The images were reconstructed via the median root prior to correction and were converted to standardised uptake value images. For the blocking experiment, the inhibitor agent letrozole (5 mg/kg, 100 μL per mouse) was injected via the tail vein half an hour before the tracer was injected. Representative PET/CT images of mice bearing MCF-7 tumours were collected at 1 h after intravenous injection of [18F]BIBD-071.
4.6. Biodistribution in an MCF-7 Xenograft Tumour Mouse Model
Mice bearing MCF-7 tumours were injected with approximately 0.65 MBq (100 μL, 17.5 μCi) [18F]BIBD-071 via the tail vein. Nine mice were randomly divided into three groups and sacrificed by cervical dislocation at 0.5, 1, and 2 h postinjection (n = 3). Organs and tissues of interest, including the blood, heart, liver, spleen, lung, kidney, intestine, stomach, bone, muscle, brain, and tumour, were collected and weighed. The radioactivity of these samples was measured with a gamma counter, and the results are expressed as the percent uptake of the injected dose per gram (%ID/g).
4.7. Immunofluorescence for Cells
A total of 2 × 105 cells were seeded onto coverslips that had been placed in 6-well plates. Following overnight culture, the cells were fixed with paraformaldehyde at room temperature and then washed with PBS. The cells were subsequently incubated with the primary antibody overnight in a humid chamber at 4 °C. The following day, the corresponding secondary antibody was added, and the samples were incubated for 50 min at room temperature. Following rinsing three times with PBS, the samples were stained with a DAPI solution to label the nuclei, and the outcome was examined via ortho-fluorescence microscopy.
4.8. Statistical Analysis
All the quantitative data are presented as the means ± standard deviations. Statistical tests were performed with GraphPad Prism version 10.0. A p value of less than 0.05 was considered statistically significant.