Therapeutic Potential of Cajanus cajan (L.) Millsp. Leaf Extract in Modulating Gut Microbiota and Immune Response for the Treatment of Inflammatory Bowel Disease
Abstract
1. Introduction
2. Results
2.1. The Identification of Bioactive Compounds
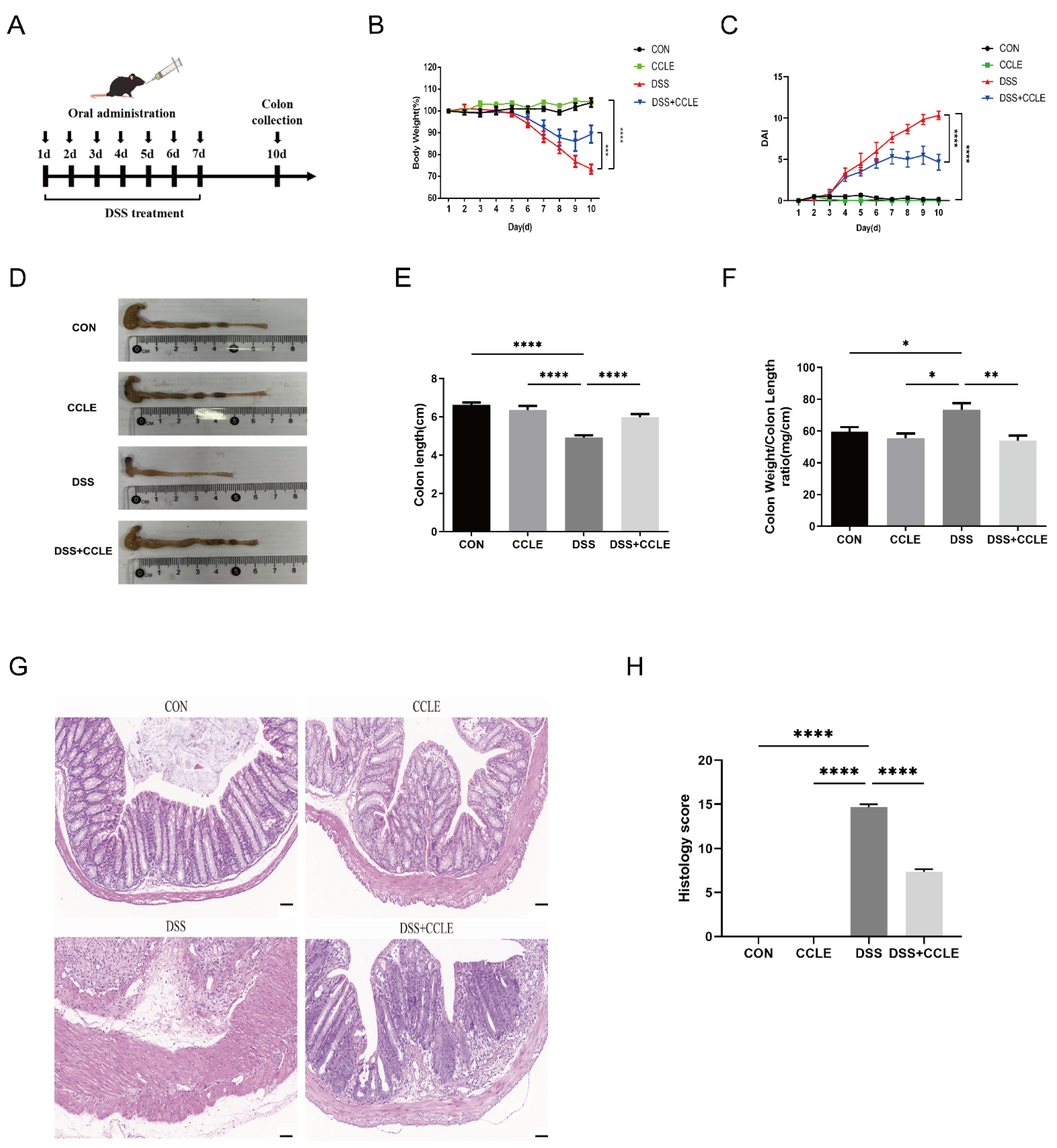
2.2. Therapeutic Efficacy of CCLE Against IBD
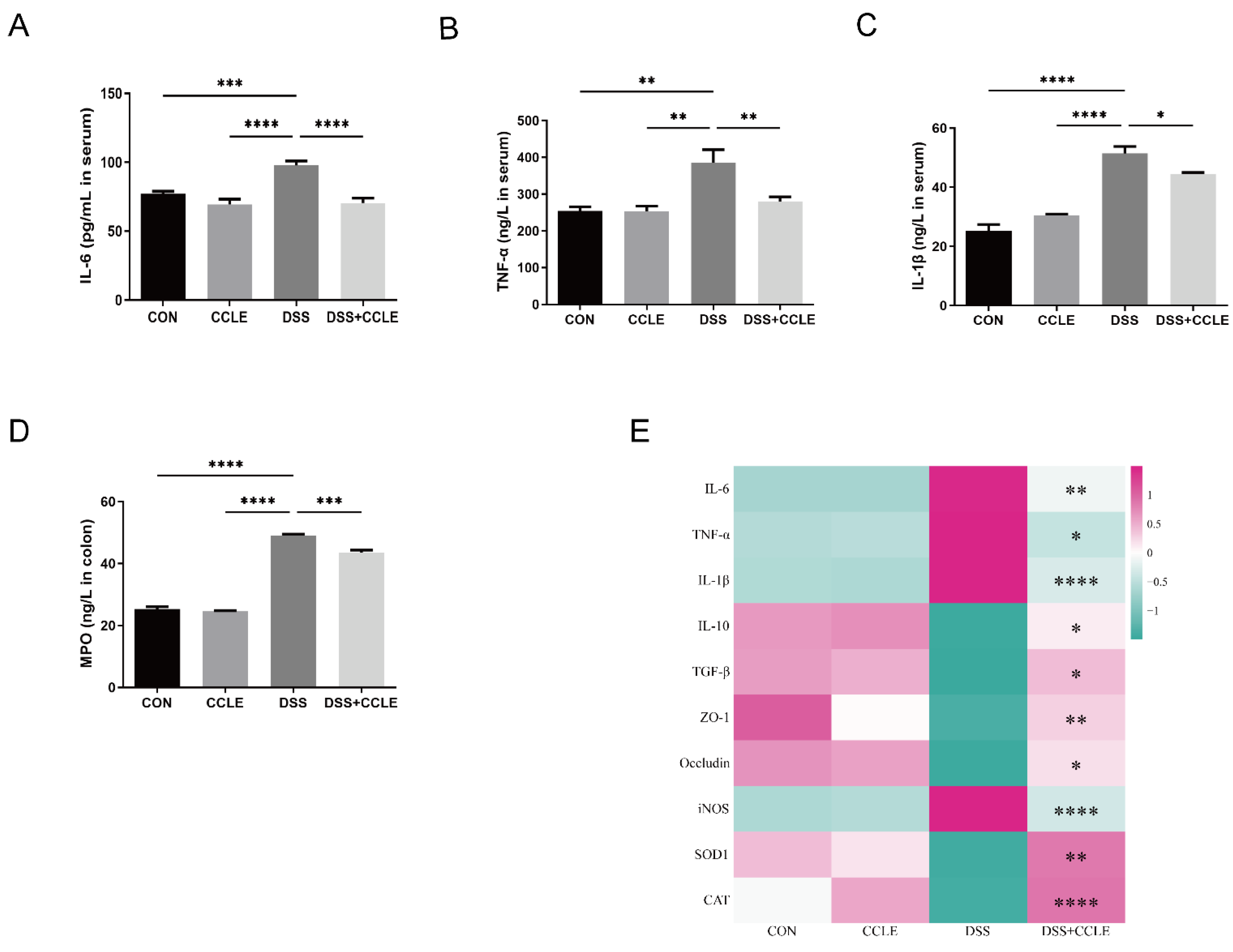
2.3. Effect of CCLE Administration on Inflammatory Cytokines
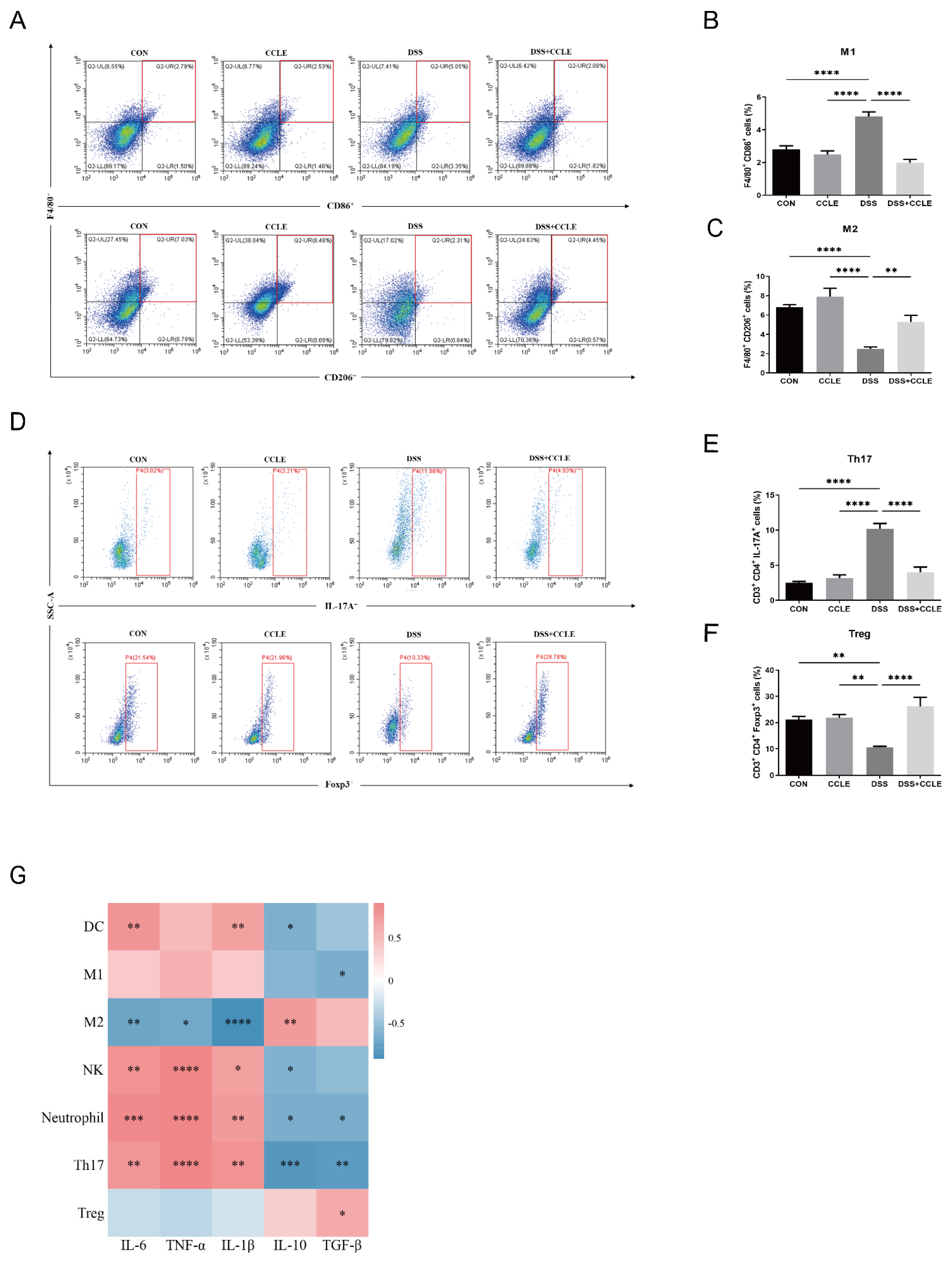
2.4. Effect of CCLE Administration on Splenic Immune Response
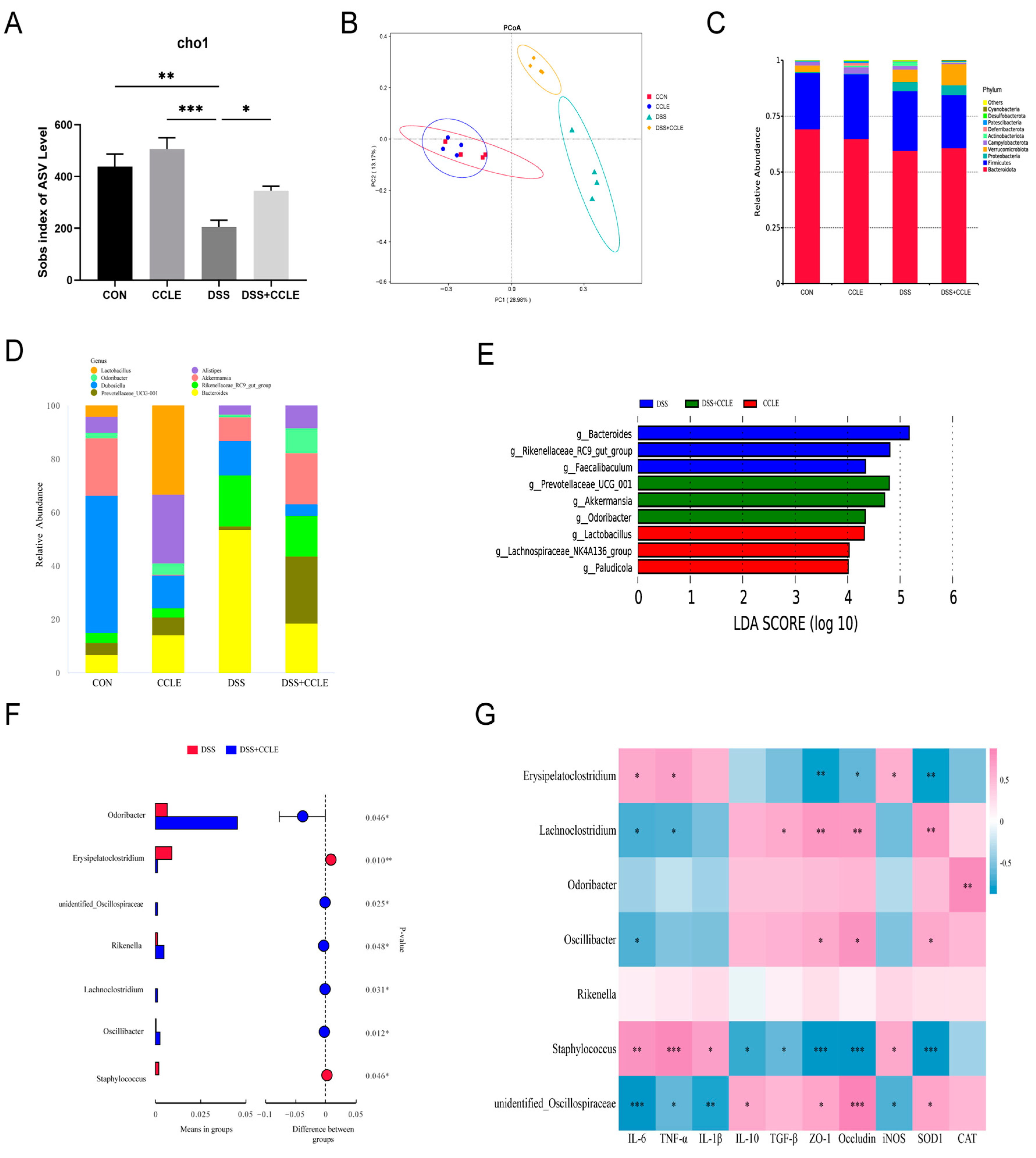
2.5. Effect of CCLE Administration on Gut Microbiota
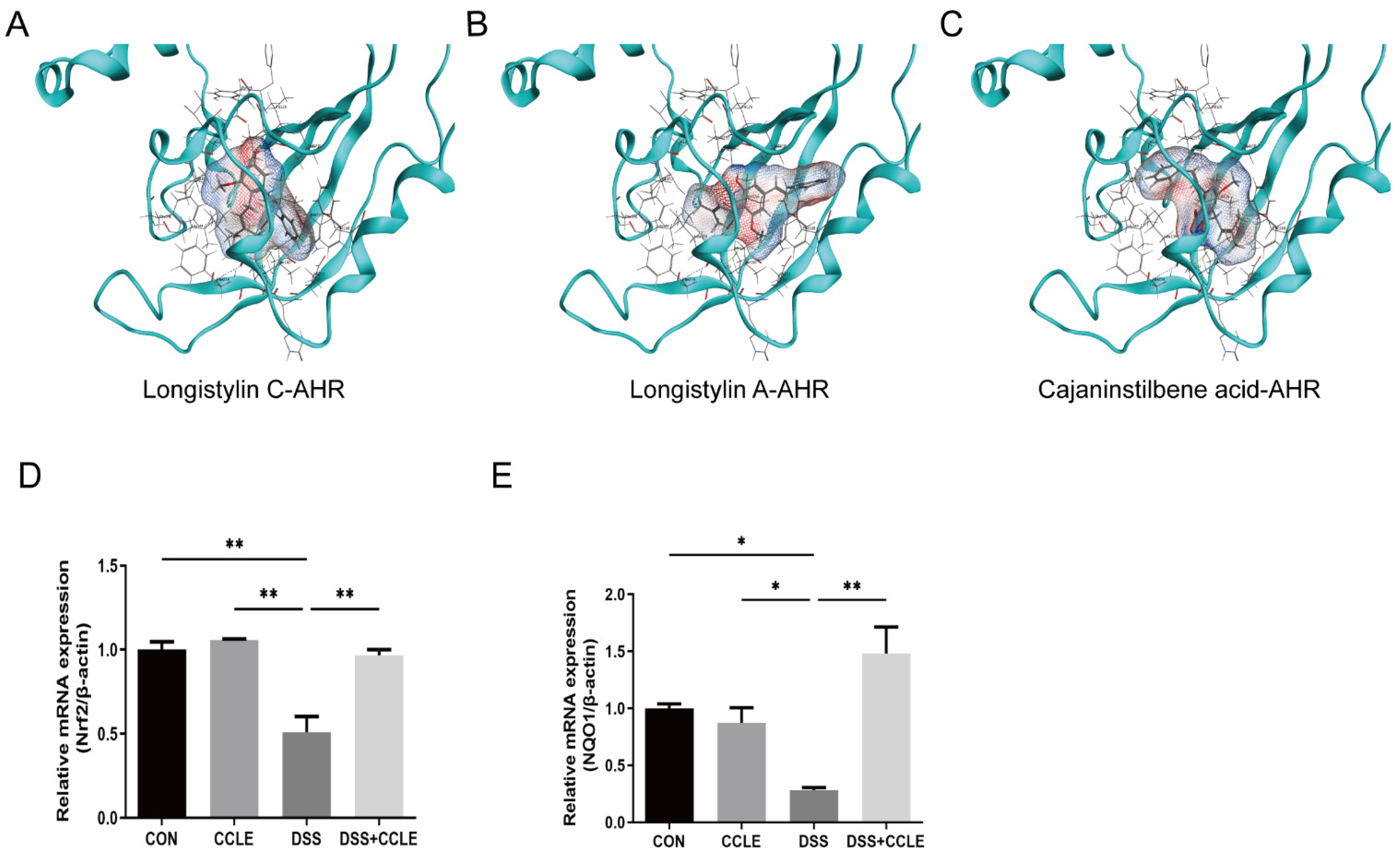
2.6. CCLE Administration Activates AHR-Nrf2-NQO1 Axis
3. Discussion
4. Materials and Methods
4.1. Preparation of Plant Extracts
4.2. Identification Compounds in CCLE by LCMS-IT-TOF
4.3. Induction of IBD Mice Model and Drug Administration
4.4. Disease Activity Index (DAI)
4.5. Histological Analysis (H&E Staining)
4.6. Enzyme-Linked Immunosorbent Assay (ELISA) Analysis
4.7. Quantitative PCR Analysis
4.8. Flow Cytometry Analysis
4.9. Gut Microbiota Analysis
4.10. Molecular Docking
4.11. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Mao, Q.; Pan, H.; Zhang, Y.; Zhang, Y.; Zhu, Q.; Hong, Y.; Huang, Z.; Li, Y.; Feng, X.; Fang, Y.; et al. GelNB molecular coating as a biophysical barrier to isolate intestinal irritating metabolites and regulate intestinal microbial homeostasis in the treatment of inflammatory bowel disease. Bioact. Mater. 2023, 19, 251–267. [Google Scholar] [CrossRef]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Xue, J.C.; Yuan, S.; Meng, H.; Hou, X.T.; Li, J.; Zhang, H.M.; Chen, L.L.; Zhang, C.H.; Zhang, Q.G. The role and mechanism of flavonoid herbal natural products in ulcerative colitis. Biomed. Pharmacother. 2023, 158, 114086. [Google Scholar] [CrossRef] [PubMed]
- Saeid Seyedian, S.; Nokhostin, F.; Dargahi Malamir, M. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef]
- Wei, Y.-y.; Fan, Y.-m.; Ga, Y.; Zhang, Y.-n.; Han, J.-c.; Hao, Z.-h. Shaoyao decoction attenuates DSS-induced ulcerative colitis, macrophage and NLRP3 inflammasome activation through the MKP1/NF-κB pathway. Phytomedicine 2021, 92, 153743. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, L.; Xu, Q.; Yang, W.; Zhao, J.; Ren, Y.; Yu, Z.; Ma, L. Taxifolin Alleviates DSS-Induced Ulcerative Colitis by Acting on Gut Microbiome to Produce Butyric Acid. Nutrients 2022, 14, 1069. [Google Scholar] [CrossRef]
- Yang, S.; Li, F.; Lu, S.; Ren, L.; Bian, S.; Liu, M.; Zhao, D.; Wang, S.; Wang, J. Ginseng root extract attenuates inflammation by inhibiting the MAPK/NF-kappaB signaling pathway and activating autophagy and p62-Nrf2-Keap1 signaling in vitro and in vivo. J. Ethnopharmacol. 2022, 283, 114739. [Google Scholar] [CrossRef]
- Yang, S.-E.; Lin, Y.-F.; Liao, J.-W.; Chen, J.-T.; Chen, C.-L.; Chen, C.-I.; Hsu, S.-L.; Song, T.-Y. Insulin Sensitizer and Antihyperlipidemic Effects of Cajanus Cajan (L.) Millsp. Root in Methylglyoxal-Induced Diabetic Rats. Chin. J. Physiol. 2022, 65, 125–135. [Google Scholar] [CrossRef]
- Zhong, X. The Value in Use of Semen Cajani. Mod. Chin. Med. 2001, 3, 47. [Google Scholar] [CrossRef]
- Chen, D.H.; Li, H.Y.; Lin, H. Studies on chemical constituents in pigeonpea leaves. Chin. Tradit. Herb Drugs 1985, 16, 134–136. [Google Scholar]
- Sun, S.M.; Song, Y.M.; Liu, J. Studies on the pharmacology of Cajanin preparation. Chin. Tradit. Herb Drugs 1995, 26, 147–148. [Google Scholar]
- Cai, J.z.; Dai, W.; Zhang, N.l. Advance on chemical constituents and pharmacological activities of Cajanus cajan (L.) Millsp. Nat. Prod. Res. Dev. 2020, 32, 11. [Google Scholar] [CrossRef]
- Li, M.; Lu, H.; Xu, Z. Research progress on inflammatory bowel disease alleviation by flavonoid natural products through regulating intesti-nal microbiota. J. Henan Univ. Technol. Nat. Sci. Ed. 2020, 41, 12. [Google Scholar] [CrossRef]
- Batovska, D.I.; Todorova, I.T. Trends in utilization of the pharmacological potential of chalcones. Curr. Clin. Pharmacol. 2010, 5, 1–29. [Google Scholar] [CrossRef]
- Lee, Y.; Sugihara, K.; Gillilland, M.G.; Jon, S.; Kamada, N.; Moon, J.J. Hyaluronic acid–bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat. Mater. 2020, 19, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ding, S.; Jiang, H.; Liu, G. Roles of Macrophages in the Development and Treatment of Gut Inflammation. Front. Cell Dev. Biol. 2021, 9, 625423. [Google Scholar] [CrossRef] [PubMed]
- Fakharian, F.; Thirugnanam, S.; Welsh, D.A.; Kim, W.-K.; Rappaport, J.; Bittinger, K.; Rout, N. The Role of Gut Dysbiosis in the Loss of Intestinal Immune Cell Functions and Viral Pathogenesis. Microorganisms 2023, 11, 1849. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Haileselassie, Y.; Ji, A.R.; Maecker, H.T.; Sinha, S.R.; Brim, H.; Habtezion, A.; Ashktorab, H. Protective Effect of Saffron in Mouse Colitis Models Through Immune Modulation. Dig. Dis. Sci. 2022, 67, 2922–2935. [Google Scholar] [CrossRef]
- Athapaththu, A.; Lee, K.T.; Kavinda, M.H.D.; Lee, S.; Kang, S.; Lee, M.H.; Kang, C.H.; Choi, Y.H.; Kim, G.Y. Pinostrobin ameliorates lipopolysaccharide (LPS)-induced inflammation and endotoxemia by inhibiting LPS binding to the TLR4/MD2 complex. Biomed. Pharmacother. 2022, 156, 113874. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, B.; Xiao, W.; Hu, J.; Xie, J.; Yuan, J.; Wang, L. Longistylin A, a natural stilbene isolated from the leaves of Cajanus cajan, exhibits significant anti-MRSA activity. Int. J. Antimicrob. Agents 2020, 55, 105821. [Google Scholar] [CrossRef]
- Schuster, R.; Holzer, W.; Doerfler, H.; Weckwerth, W.; Viernstein, H.; Okonogi, S.; Mueller, M. Cajanus cajan—A source of PPARγ activators leading to anti-inflammatory and cytotoxic effects. Food Funct. 2016, 7, 3798–3806. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Fu, K.; Fu, Y.J.; Zu, Y.G.; Chang, F.R.; Chen, Y.H.; Liu, X.L.; Kong, Y.; Liu, W.; Gu, C.B. Antioxidant activities of extracts and main components of Pigeonpea [Cajanus cajan (L.) Millsp.] leaves. Molecules 2009, 14, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.K.; Bhutani, K.K. Pinostrobin and Cajanus lactone isolated from Cajanus cajan (L.) leaves inhibits TNF-α and IL-1β production: In vitro and in vivo experimentation. Phytomedicine 2014, 21, 946–953. [Google Scholar] [CrossRef]
- Mahapatro, M.; Erkert, L.; Becker, C. Cytokine-Mediated Crosstalk between Immune Cells and Epithelial Cells in the Gut. Cells 2021, 10, 111. [Google Scholar] [CrossRef]
- Curciarello, R.; Canziani, K.E.; Docena, G.H.; Muglia, C.I. Contribution of Non-immune Cells to Activation and Modulation of the Intestinal Inflammation. Front. Immunol. 2019, 10, 647. [Google Scholar] [CrossRef]
- Lima, S.F.; Gogokhia, L.; Viladomiu, M.; Chou, L.; Putzel, G.; Jin, W.B.; Pires, S.; Guo, C.J.; Gerardin, Y.; Crawford, C.V.; et al. Transferable Immunoglobulin A-Coated Odoribacter splanchnicus in Responders to Fecal Microbiota Transplantation for Ulcerative Colitis Limits Colonic Inflammation. Gastroenterology 2022, 162, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Wang, P.Y.; Wang, X.; Wan, Y.L.; Liu, Y.C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottiere, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Zha, Z.; Lv, Y.; Tang, H.; Li, T.; Miao, Y.; Cheng, J.; Wang, G.; Tan, Y.; Zhu, Y.; Xing, X.; et al. An orally administered butyrate-releasing xylan derivative reduces inflammation in dextran sulphate sodium-induced murine colitis. Int. J. Biol. Macromol. 2020, 156, 1217–1233. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, H.; Wu, Y.; Hu, N.; Lei, M.; Zhang, Y.; Wang, S. Intervention with the crude polysaccharides of Physalis pubescens L. mitigates colitis by preventing oxidative damage, aberrant immune responses, and dysbacteriosis. J. Food Sci. 2020, 85, 2596–2607. [Google Scholar] [CrossRef] [PubMed]
- Dorofeyev, A.E.; Vasilenko, I.V.; Rassokhina, O.A. Joint Extraintestinal Manifestations in Ulcerative Colitis. Dig. Dis. 2009, 27, 502–510. [Google Scholar] [CrossRef]
- Stockinger, B.; Shah, K.; Wincent, E. AHR in the intestinal microenvironment: Safeguarding barrier function. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Samsamikor, M.; Daryani, N.E.; Asl, P.R.; Hekmatdoost, A. Resveratrol Supplementation and Oxidative/Anti-Oxidative Status in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch. Med. Res. 2016, 47, 304–309. [Google Scholar] [CrossRef] [PubMed]
- SamsamiKor, M.; Daryani, N.E.; Asl, P.R.; Hekmatdoost, A. Anti-Inflammatory Effects of Resveratrol in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch. Med. Res. 2015, 46, 280–285. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, J.; Jiang, B.; Miao, M. Resveratrol and inflammatory bowel disease. Ann. N. Y. Acad. Sci. 2017, 1403, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ocansey, D.K.W.; Pei, B.; Zhang, Y.; Wang, N.; Wang, Z.; Mao, F. Resveratrol alleviates DSS-induced IBD in mice by regulating the intestinal microbiota-macrophage-arginine metabolism axis. Eur. J. Med. Res. 2023, 28, 319. [Google Scholar] [CrossRef]
- Wang, K.; Lv, Q.; Miao, Y.M.; Qiao, S.M.; Dai, Y.; Wei, Z.F. Cardamonin, a natural flavone, alleviates inflammatory bowel disease by the inhibition of NLRP3 inflammasome activation via an AhR/Nrf2/NQO1 pathway. Biochem. Pharmacol. 2018, 155, 494–509. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, J.; Long, G.; Xia, X.H.; Liu, M. 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside, a major bioactive component from Polygoni multiflori Radix (Heshouwu) suppresses DSS induced acute colitis in BALb/c mice by modulating gut microbiota. Biomed. Pharmacother. 2021, 137, 111420. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.; Piao, L.; Zhao, Z.; Liao, L.; Wang, D.; Zhang, H.; Liu, X. Therapeutic Potential of Cajanus cajan (L.) Millsp. Leaf Extract in Modulating Gut Microbiota and Immune Response for the Treatment of Inflammatory Bowel Disease. Pharmaceuticals 2025, 18, 67. https://doi.org/10.3390/ph18010067
Lin M, Piao L, Zhao Z, Liao L, Wang D, Zhang H, Liu X. Therapeutic Potential of Cajanus cajan (L.) Millsp. Leaf Extract in Modulating Gut Microbiota and Immune Response for the Treatment of Inflammatory Bowel Disease. Pharmaceuticals. 2025; 18(1):67. https://doi.org/10.3390/ph18010067
Chicago/Turabian StyleLin, Mingzhang, Linghua Piao, Zhendong Zhao, Li Liao, Dayong Wang, Haiwen Zhang, and Xiande Liu. 2025. "Therapeutic Potential of Cajanus cajan (L.) Millsp. Leaf Extract in Modulating Gut Microbiota and Immune Response for the Treatment of Inflammatory Bowel Disease" Pharmaceuticals 18, no. 1: 67. https://doi.org/10.3390/ph18010067
APA StyleLin, M., Piao, L., Zhao, Z., Liao, L., Wang, D., Zhang, H., & Liu, X. (2025). Therapeutic Potential of Cajanus cajan (L.) Millsp. Leaf Extract in Modulating Gut Microbiota and Immune Response for the Treatment of Inflammatory Bowel Disease. Pharmaceuticals, 18(1), 67. https://doi.org/10.3390/ph18010067






