Kojic Acid Derivative as an Antimitotic Agent That Selectively Kills Tumour Cells
Abstract
1. Introduction
2. Results
2.1. Investigating the L1 Mechanism of Action
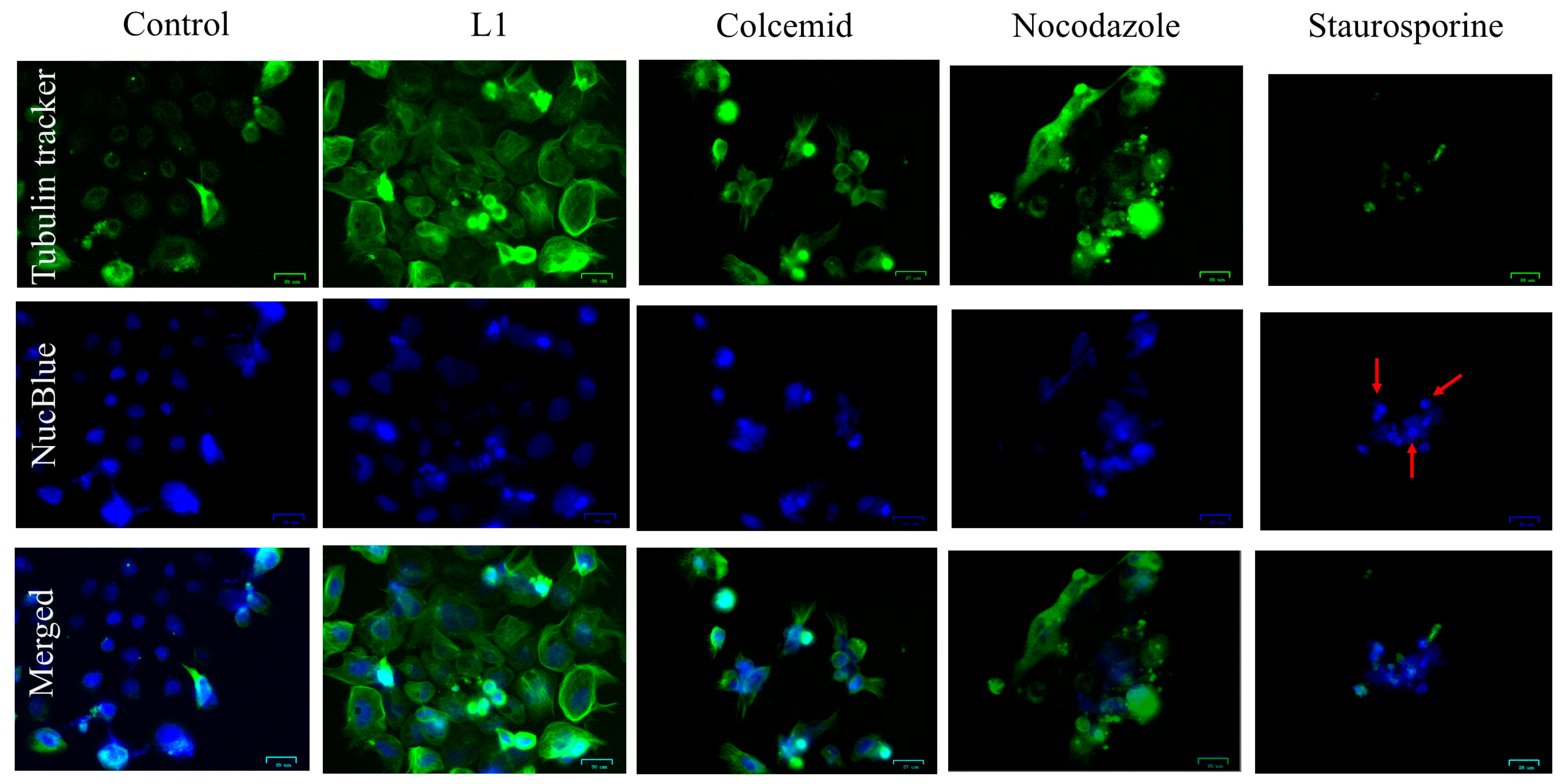
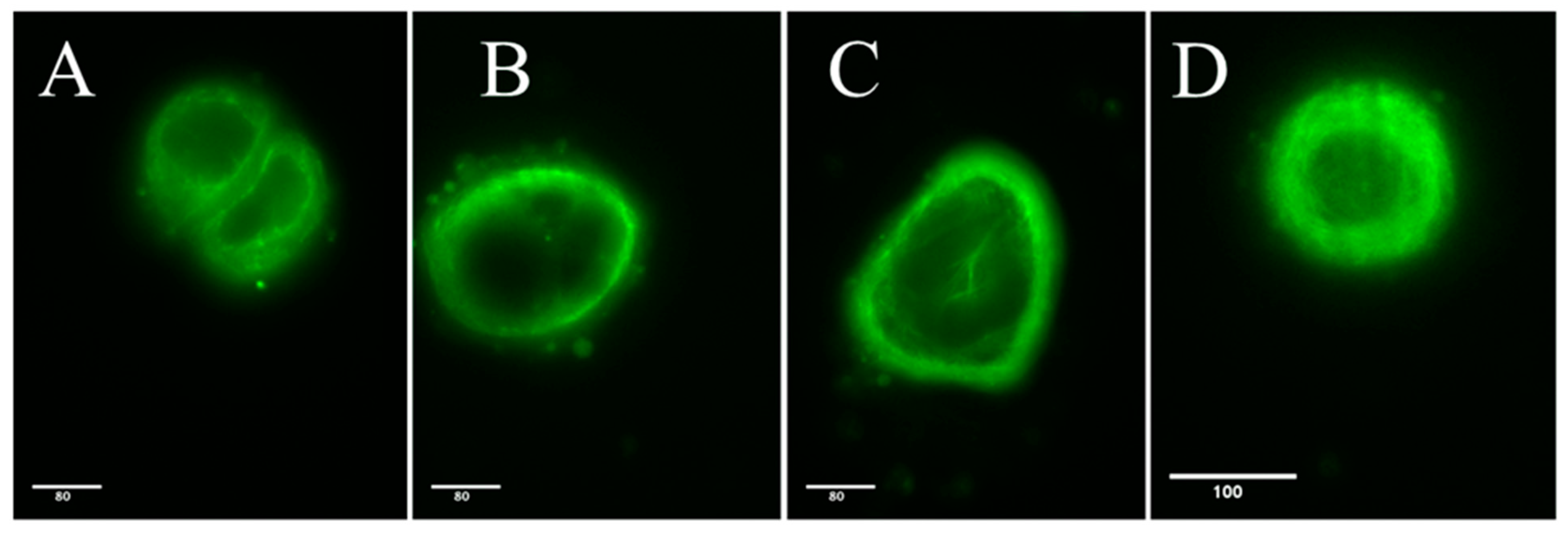
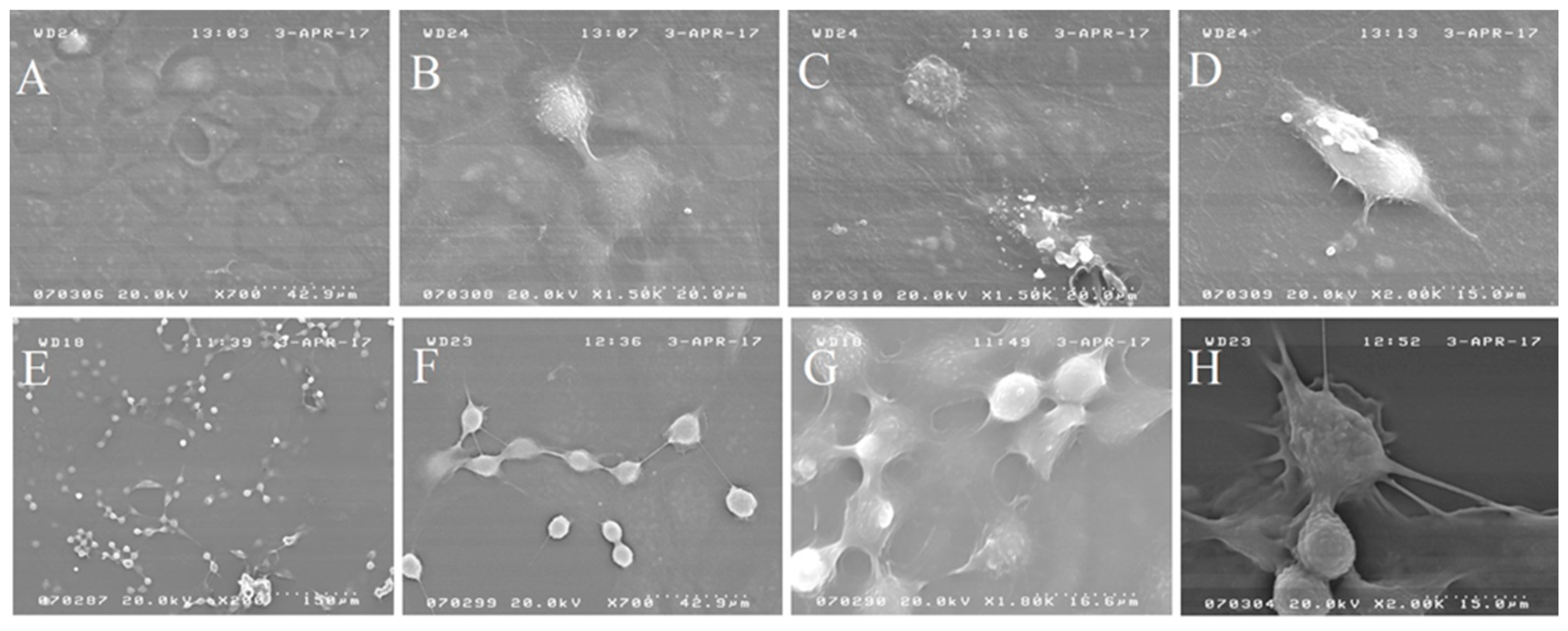
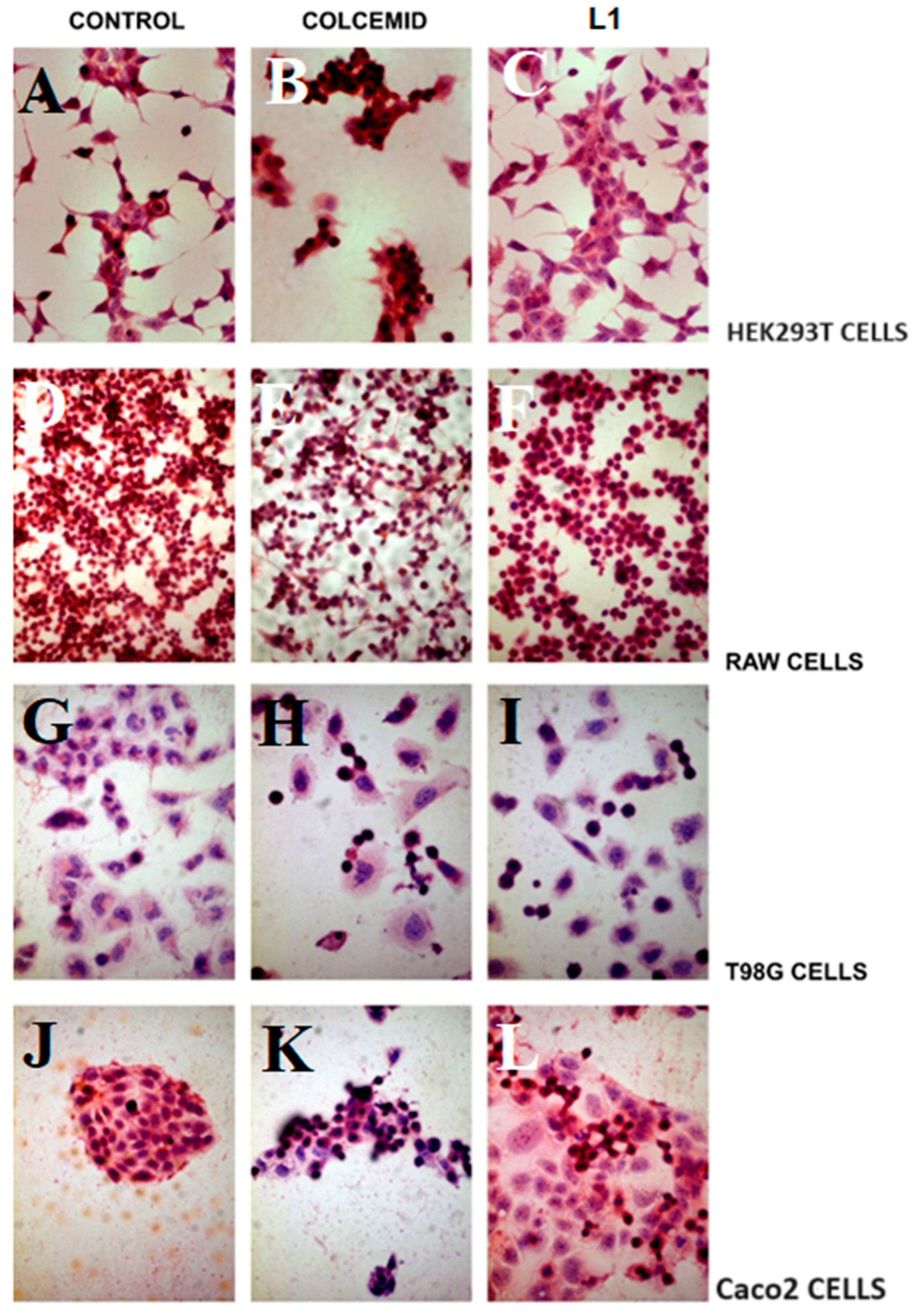
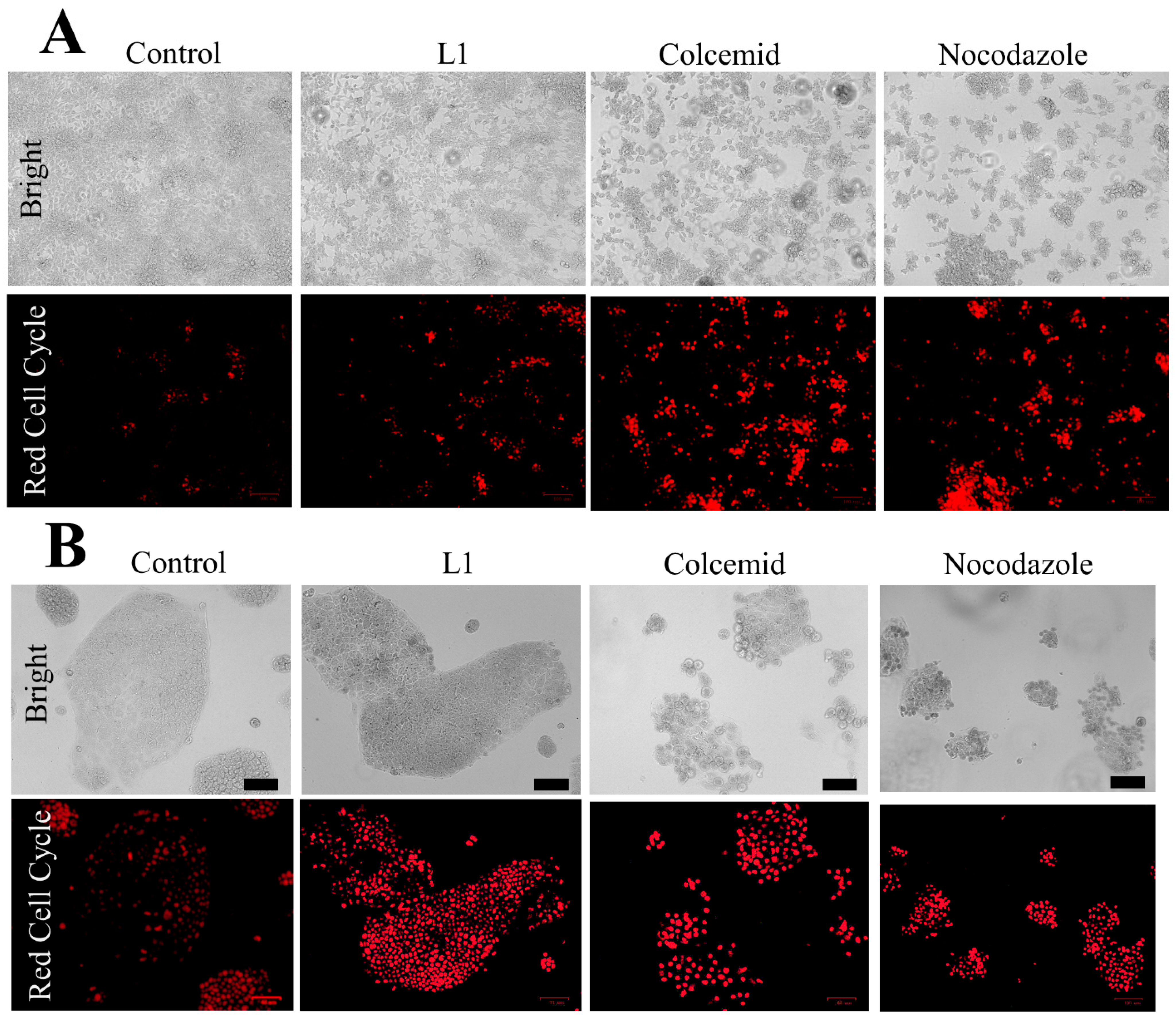
2.1.1. Light and Electron Microscopy Investigations
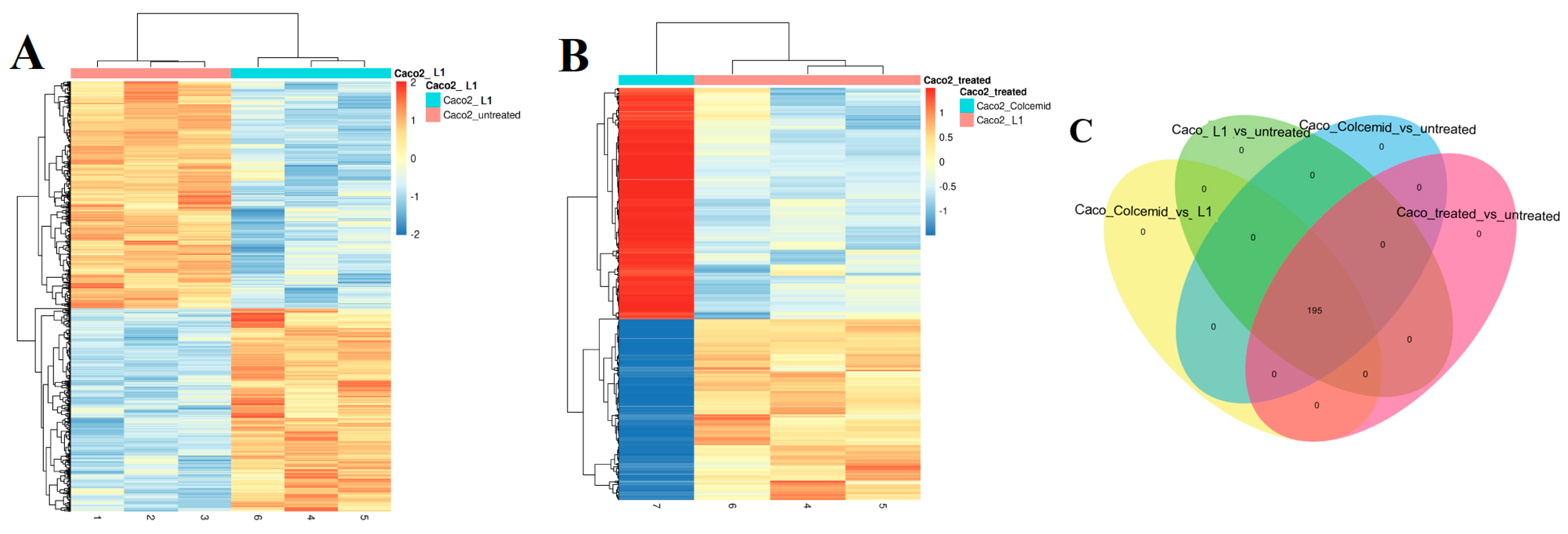
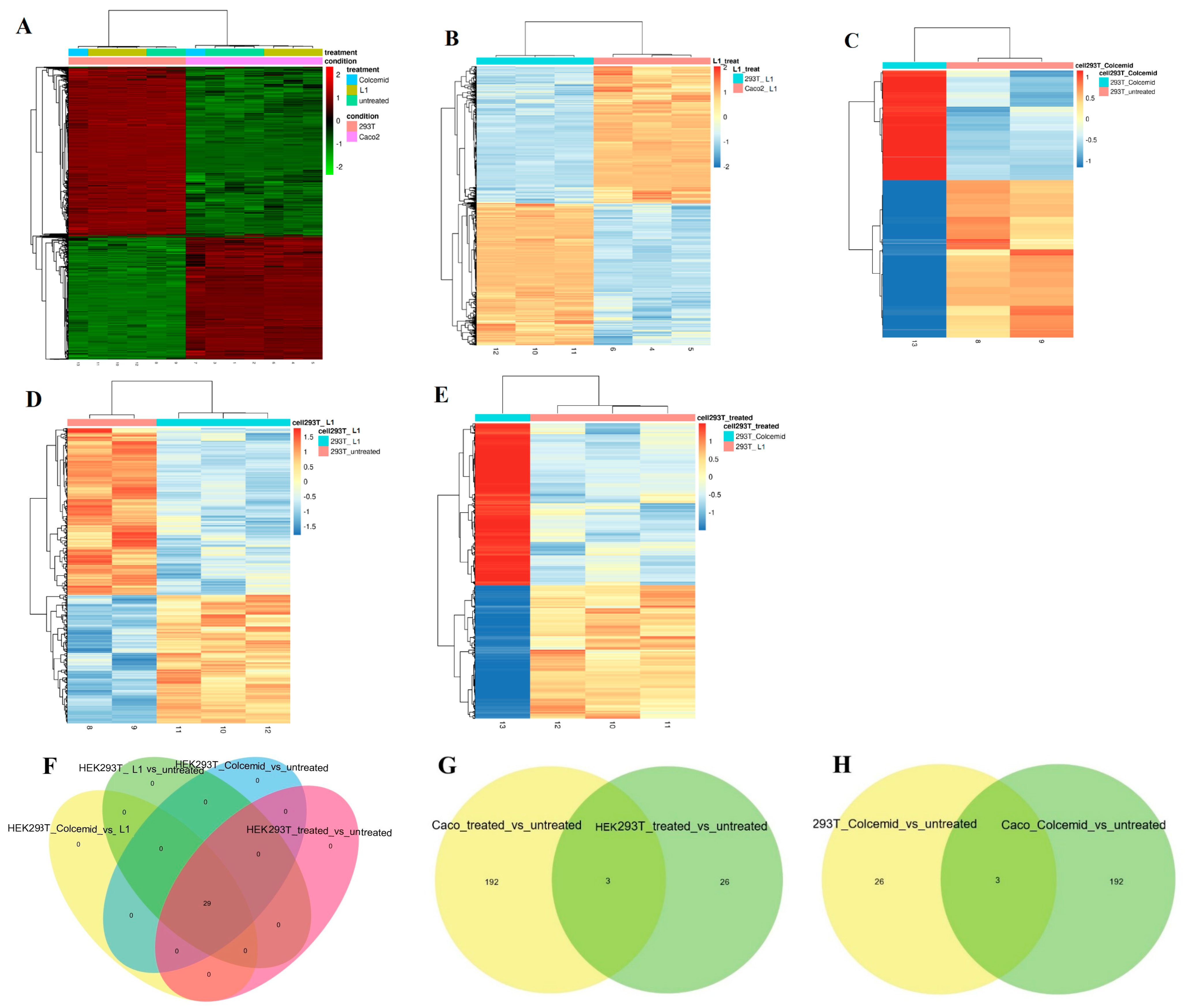
2.1.2. Next Generation Sequencing (NGS) Investigation
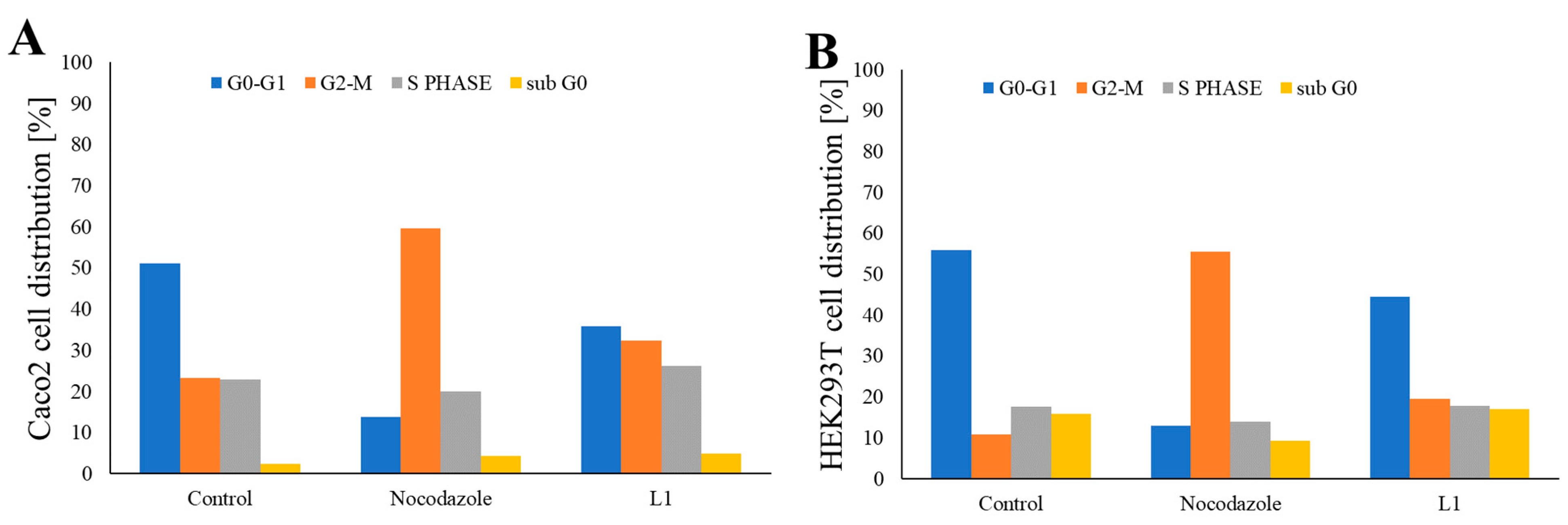
2.2. Investigation of Mitotic Exit into Apoptosis
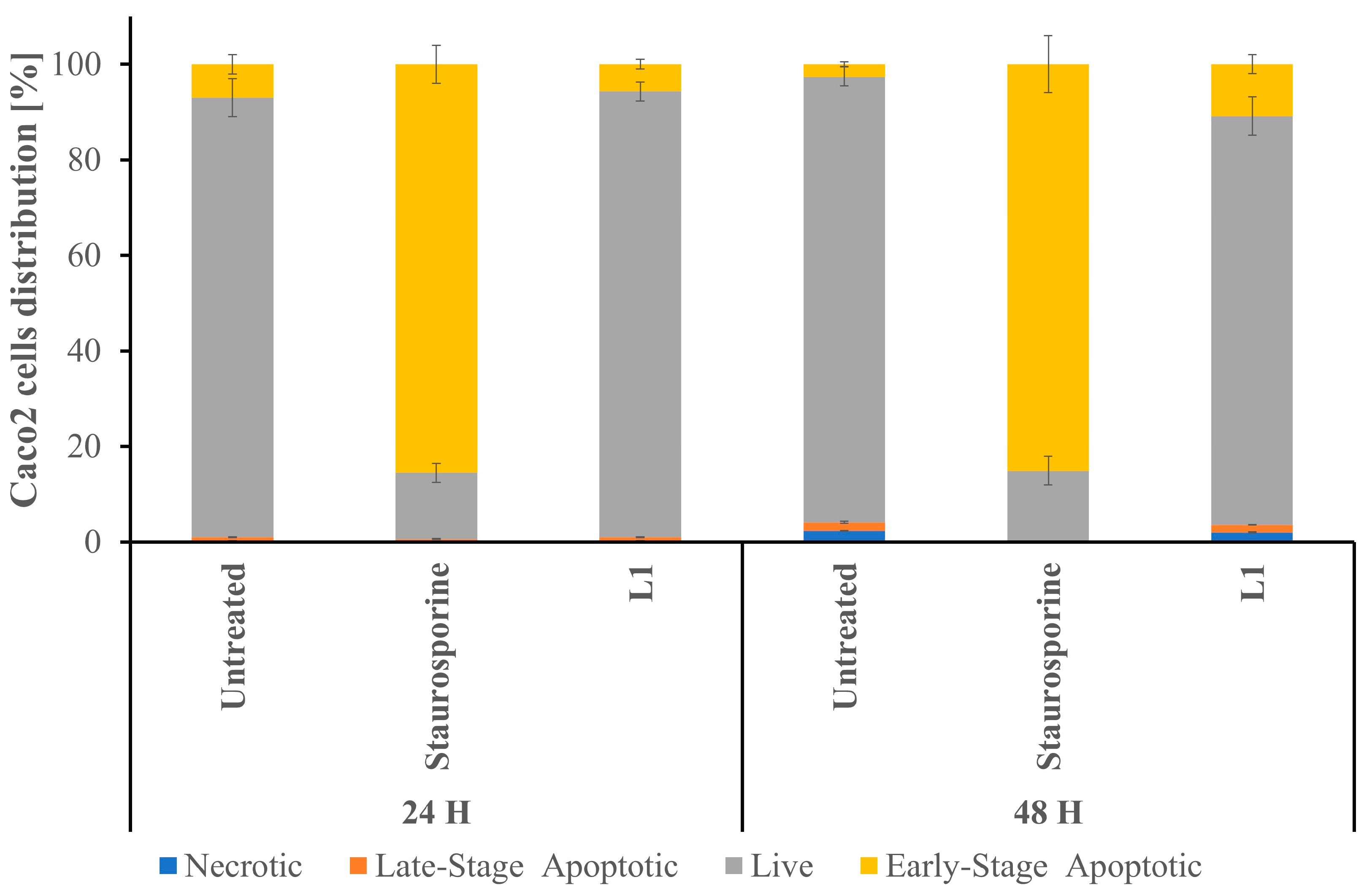
2.3. Studies of L1 Selectivity Versus Tumour Cells
2.3.1. Light Microscopy Investigation of L1 Selectivity
2.3.2. Investigation of L1 Selectivity Using Next Generation Sequencing (NGS)
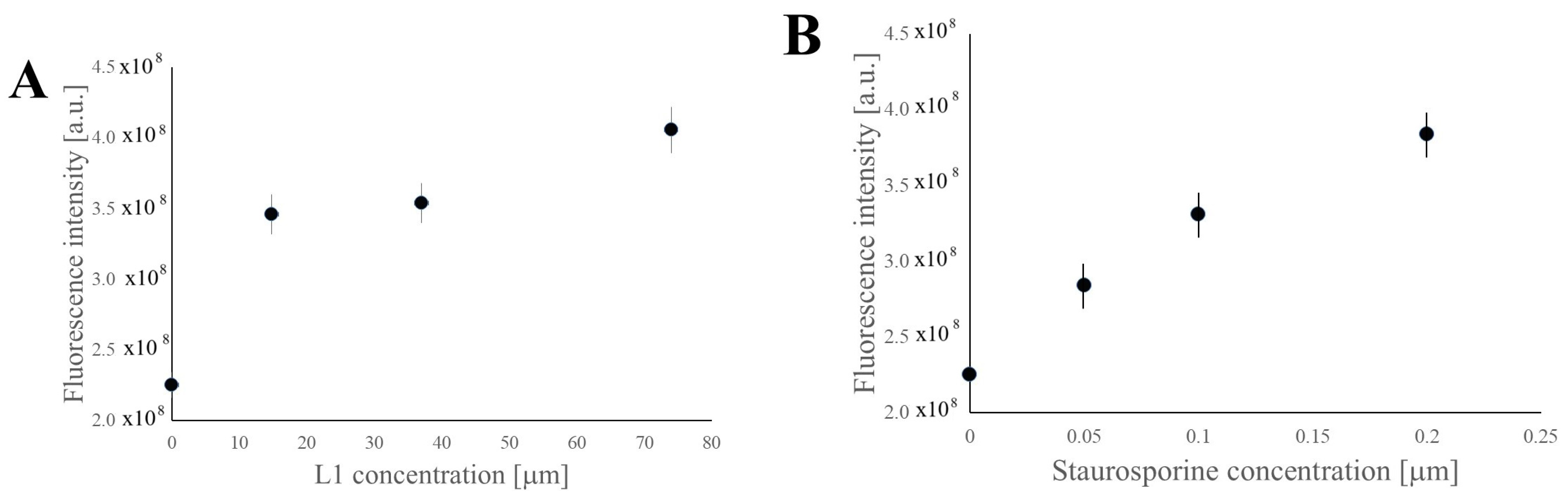
2.3.3. Investigation of Selective Cell Cycle Arrest Using Fluorescence
2.4. Bioavailability and Toxicity Studies of L1 in Different Cell Lines
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Molecules Synthesis and Characterisation
4.3. Cell Cultures
4.4. Cytotoxic Activity
4.4.1. Trypan Blue Staining
4.4.2. MTT Assay
4.5. Determining L1 Bioavailability in the Cells
4.6. Microscopy
4.6.1. Light Microscopy
Hematoxylin and Eosin (H&E) Staining
Fluorescence Microscopy and Tubulin Staining
Toluidine Blue Staining
4.6.2. Electron Microscopy
Transmission Electron Microscopy (TEM)
Scanning Electron Microscopy (SEM)
4.7. Analysis of Cell Cycle Phases
Dose–Response Analysis
4.8. Next Generation Sequencing (NGS)
4.9. Apoptosis/Necrosis Analysis
4.10. Caspase-3 Activity Assay
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rieder, C.L.; Maiato, H. Stuck in division or passing through: What happens when cells cannot satisfy the spindle assembly checkpoint. Dev. Cell 2004, 7, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Brauer, C.; Thu, K.L.; Mason, J.M.; Blaser, H.; Bray, M.R.; Mak, T.W. Targeting mitosis in cancer: Emerging strategies. Mol. Cell 2015, 60, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Novais, P.; Silva, P.M.; Amorim, I.; Bousbaa, H. Second-generation antimitotics in cancer clinical trials. Pharmaceutics 2021, 13, 1011. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093. [Google Scholar] [CrossRef]
- Bennett, M.J.; Barakat, K.; Huzil, J.T.; Tuszynski, J.; Schriemer, D.C. Discovery and characterization of the laulimalide-microtubule binding mode by mass shift perturbation mapping. Chem. Biol. 2010, 17, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Buey, R.M.; Barasoain, I.; Jackson, E.; Meyer, A.; Giannakakou, P.; Paterson, I.; Mooberry, S.; Andreu, J.M.; Díaz, J.F. Microtubule interactions with chemically diverse stabilizing agents: Thermodynamics of binding to the paclitaxel site predicts cytotoxicity. Chem. Biol. 2005, 12, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Shuai, W.; Li, X.; Li, W.; Xu, F.; Lu, L.; Yao, H.; Yang, L.; Zhu, H.; Xu, S.; Zhu, Z. Design, synthesis and anticancer properties of isocombretapyridines as potent colchicine binding site inhibitors. Eur. J. Med. Chem. 2020, 197, 112308. [Google Scholar] [CrossRef] [PubMed]
- González, M.; Ovejero-Sánchez, M.; Vicente-Blázquez, A.; Álvarez, R.; Herrero, A.B.; Medarde, M.; González-Sarmiento, R.; Peláez, R. Microtubule destabilizing sulfonamides as an alternative to taxane-based chemotherapy. Int. J. Mol. Sci. 2021, 22, 1907. [Google Scholar] [CrossRef]
- Brito, D.A.; Rieder, C.L. Mitotic checkpoint slippage in humans occurs via cyclin B destruction in the presence of an active checkpoint. Curr. Biol. 2006, 16, 1194–1200. [Google Scholar] [CrossRef]
- Akhmanova, A.; Steinmetz, M.O. Control of microtubule organization and dynamics: Two ends in the limelight. Nat. Rev. Mol. Cell Biol. 2015, 16, 711–726. [Google Scholar] [CrossRef]
- Gornstein, E.; Schwarz, T.L. The paradox of paclitaxel neurotoxicity: Mechanisms and unanswered questions. Neuropharmacology 2014, 76, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.; Sloss, O.; Topham, C.; Nelson, L.; Tighe, A.; Taylor, S.S. Inhibition of Bcl-xL sensitizes cells to mitotic blockers, but not mitotic drivers. Open Biol. 2016, 6, 160134. [Google Scholar] [CrossRef]
- Henriques, A.C.; Ribeiro, D.; Pedrosa, J.; Sarmento, B.; Silva, P.M.; Bousbaa, H. Mitosis inhibitors in anticancer therapy: When blocking the exit becomes a solution. Cancer Lett. 2019, 440, 64–81. [Google Scholar] [CrossRef] [PubMed]
- Zilles, J.C.; Dos Santos, F.L.; Kulkamp-Guerreiro, I.C.; Contri, R.V. Biological activities and safety data of kojic acid and its derivatives: A review. Exp. Dermatol. 2022, 31, 1500–1521. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Crisponi, G.; Lachowicz, J.I.; de Guadalupe Jaraquemada-Pelaez, M.; Bretti, C.; Peana, M.; Medici, S.; Zoroddu, M.A. Equilibrium studies of new bis-hydroxypyrone derivatives with Fe3+, Al3+, Cu2+ and Zn2+. J. Inorg. Biochem. 2018, 189, 103–114. [Google Scholar] [CrossRef]
- Lachowicz, J.I.; Mateddu, A.; Coni, P.; Caltagirone, C.; Murgia, S.; Gibson, D.; Dalla Torre, G.; Lopez, X.; Meloni, F.; Pichiri, G. Study of the DNA binding mechanism and in vitro activity against cancer cells of iron(III) and aluminium(III) kojic acid derivative complexes. Dalton Trans. 2022, 51, 6254–6263. [Google Scholar] [CrossRef]
- Rieder, C.L.; Palazzo, R.E. Colcemid and the mitotic cycle. J. Cell Sci. 1992, 102, 387–392. [Google Scholar] [CrossRef]
- El Debs, B.W.; Tschulena, U.; Griffiths, A.D.; Merten, C.A. A competitive co-cultivation assay for cancer drug specificity evaluation. J. Biomol. Screen. 2011, 16, 818–824. [Google Scholar] [CrossRef]
- Skvortsov, D.; Kalinina, M.; Zhirkina, I.; Vasilyeva, L.; Ivanenkov, Y.; Sergiev, P.; Dontsova, O. From Toxicity to Selectivity: Coculture of the Fluorescent Tumor and Non-Tumor Lung Cells and High-Throughput Screening of Anticancer Compounds. Front. Pharmacol. 2021, 12, 713103. [Google Scholar] [CrossRef]
- Cheng, Q.; Yin, H.; Sun, C.; Yue, L.; Ding, Y.; Dehaen, W.; Wang, R. Glutathione-responsive homodithiacalix [4] arene-based nanoparticles for selective intracellular drug delivery. Chem. Commun. 2018, 54, 8128–8131. [Google Scholar] [CrossRef]
- Ndolo, R.A.; Luan, Y.; Duan, S.; Forrest, M.L.; Krise, J.P. Lysosomotropic properties of weakly basic anticancer agents promote cancer cell selectivity in vitro. PLoS ONE 2012, 7, e49366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pagliaricci, N.; Pettinari, R.; Marchetti, F.; Pettinari, C.; Cappellacci, L.; Tombesi, A.; Cuccioloni, M.; Hadiji, M.; Dyson, P.J. Potent and selective anticancer activity of half-sandwich ruthenium and osmium complexes with modified curcuminoid ligands. Dalton Trans. 2022, 51, 13311–13321. [Google Scholar] [CrossRef]
- Huang, W.-C.; Chen, S.-H.; Chiang, W.-H.; Huang, C.-W.; Lo, C.-L.; Chern, C.-S.; Chiu, H.-C. Tumor microenvironment-responsive nanoparticle delivery of chemotherapy for enhanced selective cellular uptake and transportation within tumor. Biomacromolecules 2016, 17, 3883–3892. [Google Scholar] [CrossRef]
- Zieve, G.W.; Turnbull, D.; Mullins, J.M.; McIntosh, J.R. Production of large numbers of mitotic mammalian cells by use of the reversible microtubule inhibitor Nocodazole: Nocodazole accumulated mitotic cells. Exp. Cell Res. 1980, 126, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Robbins, E.; Gonatas, N.K. The ultrastructure of a mammalian cell during the mitotic cycle. J. Cell Biol. 1964, 21, 429–463. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.; Allen, T. Cell surface morphology after trypsinisation depends on initial cell shape. Differentiation 1979, 15, 61–66. [Google Scholar] [CrossRef]
- Sullivan, K.F. Structure and utilization of tubulin isotypes. Annu. Rev. Cell Biol. 1988, 4, 687–716. [Google Scholar] [CrossRef]
- Ludueña, R.F. Multiple forms of tubulin: Different gene products and covalent modifications. Int. Rev. Cytol. 1997, 178, 207–275. [Google Scholar]
- Yeh, I.T.; Ludueña, R.F. The βII isotype of tubulin is present in the cell nuclei of a variety of cancers. Cell Motil. Cytoskelet. 2004, 57, 96–106. [Google Scholar] [CrossRef]
- Walss, C.; Kreisberg, J.I.; Ludueña, R.F. Presence of the βII isotype of tubulin in the nuclei of cultured mesangial cells from rat kidney. Cell Motil. Cytoskelet. 1999, 42, 274–284. [Google Scholar] [CrossRef]
- Kourmouli, N.; Dialynas, G.; Petraki, C.; Pyrpasopoulou, A.; Singh, P.B.; Georgatos, S.D.; Theodoropoulos, P.A. Binding of heterochromatin protein 1 to the nuclear envelope is regulated by a soluble form of tubulin. J. Biol. Chem. 2001, 276, 13007–13014. [Google Scholar] [CrossRef]
- McIntosh, J.R.; Grishchuk, E.L.; Morphew, M.K.; Efremov, A.K.; Zhudenkov, K.; Volkov, V.A.; Cheeseman, I.M.; Desai, A.; Mastronarde, D.N.; Ataullakhanov, F.I. Fibrils connect microtubule tips with kinetochores: A mechanism to couple tubulin dynamics to chromosome motion. Cell 2008, 135, 322–333. [Google Scholar] [CrossRef]
- Ran, Y.; Hu, H.; Zhou, Z.; Yu, L.; Sun, L.; Pan, J.; Liu, J.; Yang, Z. Profiling tumor-associated autoantibodies for the detection of colon cancer. Clin. Cancer Res. 2008, 14, 2696–2700. [Google Scholar] [CrossRef] [PubMed]
- Walss-Bass, C.; Xu, K.; David, S.; Fellous, A.; Ludueña, R.F. Occurrence of nuclear β II-tubulin in cultured cells. Cell Tissue Res. 2002, 308, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Ludueña, R.F. Characterization of nuclear βII-tubulin in tumor cells: A possible novel target for taxol. Cell Motil. Cytoskelet. 2002, 53, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Ruksha, K.; Mezheyeuski, A.; Nerovnya, A.; Bich, T.; Tur, G.; Gorgun, J.; Luduena, R.; Portyanko, A. Over-expression of ΒII-tubulin and especially its localization in cell nuclei correlates with poorer outcomes in colorectal cancer. Cells 2019, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.Y.; Kee, S.H.; Choi, J.W.; Lee, J.H.; Lee, J.H.; Lee, E.S.; Kim, Y.S. Expression of class II β-tubulin in non-melanoma cutaneous tumors. J. Cutan. Pathol. 2007, 34, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S.; Salazar, H.; Benetatos, C.A.; Hudes, G.R. Immunohistochemical analysis of β-tubulin isotypes in human prostate carcinoma and benign prostatic hypertrophy. Prostate 1997, 30, 263–268. [Google Scholar] [CrossRef]
- Jirásek, T.; Cipro, Š.; Musilova, A.; Kubecova, M.; Mandys, V. Expression of class III β-tubulin in colorectal carcinomas: An immunohistochemical study using TU-20 & TuJ-1 antibody. Indian J. Med. Res. 2009, 129, 89–94. [Google Scholar] [PubMed]
- Giarnieri, E.; De Francesco, G.P.; Carico, E.; Midiri, G.; Amanti, C.; Giacomelli, L.; Tucci, G.; Gidaro, S.; Stroppa, I.; Gidaro, G. α-and β-tubulin expression in rectal cancer development. Anticancer. Res. 2005, 25, 3237–3241. [Google Scholar] [PubMed]
- Hiser, L.; Aggarwal, A.; Young, R.; Frankfurter, A.; Spano, A.; Correia, J.J.; Lobert, S. Comparison of β-tubulin mRNA and protein levels in 12 human cancer cell lines. Cell Motil. Cytoskelet. 2006, 63, 41–52. [Google Scholar] [CrossRef]
- Bouras, G.; Nakanishi, T.; Fujita, Y.; Tsunemi, S.; Takubo, T.; Tanigawa, N. Identification of β-tubulin as a common immunogen in gastrointestinal malignancy by mass spectrometry of colorectal cancer proteome: Implications for early disease detection. Anal. Bioanal. Chem. 2012, 403, 1801–1809. [Google Scholar] [CrossRef]
- Yabe, D.; Brown, M.S.; Goldstein, J.L. Insig-2, a second endoplasmic reticulum protein that binds SCAP and blocks export of sterol regulatory element-binding proteins. Proc. Natl. Acad. Sci. USA 2002, 99, 12753–12758. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, A.; Kaminishi, M.; Nakanishi, Y.; Sugimura, T.; Ushijima, T. Reduced expression of the insulin-induced protein 1 and p41 Arp2/3 complex genes in human gastric cancers. Int. J. Cancer 2002, 100, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Pardee, A.B. Suberoylanilide hydroxamic acid as a potential therapeutic agent for human breast cancer treatment. Mol. Med. 2000, 6, 849–866. [Google Scholar] [CrossRef]
- Gaviraghi, M.; Vivori, C.; Pareja Sanchez, Y.; Invernizzi, F.; Cattaneo, A.; Santoliquido, B.M.; Frenquelli, M.; Segalla, S.; Bachi, A.; Doglioni, C. Tumor suppressor PNRC 1 blocks r RNA maturation by recruiting the decapping complex to the nucleolus. EMBO J. 2018, 37, e99179. [Google Scholar] [CrossRef] [PubMed]
- Mugridge, J.S.; Gross, J.D. Decapping enzymes STOP “cancer” ribosomes in their tracks. EMBO J. 2018, 37, e100801. [Google Scholar] [CrossRef]
- Liang, G.; Li, Q.; Tang, Y.; Kokame, K.; Kikuchi, T.; Wu, G.; Chen, X.-Z. Polycystin-2 is regulated by endoplasmic reticulum-associated degradation. Hum. Mol. Genet. 2008, 17, 1109–1119. [Google Scholar] [CrossRef][Green Version]
- Marutani, T.; Maeda, T.; Tanabe, C.; Zou, K.; Araki, W.; Kokame, K.; Michikawa, M.; Komano, H. ER-stress-inducible Herp, facilitates the degradation of immature nicastrin. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2011, 1810, 790–798. [Google Scholar] [CrossRef]
- Segawa, T.; Nau, M.E.; Xu, L.L.; Chilukuri, R.N.; Makarem, M.; Zhang, W.; Petrovics, G.; Sesterhenn, I.A.; McLeod, D.G.; Moul, J.W. Androgen-induced expression of endoplasmic reticulum (ER) stress response genes in prostate cancer cells. Oncogene 2002, 21, 8749–8758. [Google Scholar] [CrossRef]
- Erzurumlu, Y.; Doganlar, Y.; Dogan, H.K.; Catakli, D.; Aydogdu, E. HERPUD1, a member of the Endoplasmic Reticulum Protein Quality Control Mechanism, may be a Good Target for Suppressing Tumorigenesis in Breast Cancer Cells. Turk. J. Pharm. Sci. 2022, 20, 157. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, P.J.; Dits, N.F.; Kokame, K.; Veldhoven, A.; van Weerden, W.M.; Bangma, C.H.; Trapman, J.; Jenster, G. Evolution of the androgen receptor pathway during progression of prostate cancer. Cancer Res. 2006, 66, 5012–5020. [Google Scholar] [CrossRef]
- Shahbazi, J.; Lock, R.; Liu, T. Tumor protein 53-induced nuclear protein 1 enhances p53 function and represses tumorigenesis. Front. Genet. 2013, 4, 80. [Google Scholar] [CrossRef]
- Ivanova, S.; Zorzano, A. TP53INP2 at the crossroad of apoptosis and autophagy in death receptor signaling. Mol. Cell. Oncol. 2019, 6, e1632687. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Liu, S.; Zhang, J.; Ren, X.; Chen, X.; Cheng, B.; Xia, J. Integrative analysis of TP53INP2 in head and neck squamous cell carcinoma. Front. Genet. 2021, 12, 630794. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, X.; Xue, W.; Pang, J.; Meng, Y.; Shen, Y.; Xu, Q. TP53INP2-related basal autophagy is involved in the growth and malignant progression in human liposarcoma cells. Biomed. Pharmacother. 2017, 88, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Guardavaccaro, D.; Corrente, G.; Covone, F.; Micheli, L.; D’Agnano, I.; Starace, G.; Caruso, M.; Tirone, F. Arrest of G1-S progression by the p53-inducible gene PC3 is Rb dependent and relies on the inhibition of cyclin D1 transcription. Mol. Cell. Biol. 2000, 20, 1797–1815. [Google Scholar] [CrossRef]
- Ikematsu, N.; Yoshida, Y.; Kawamura-Tsuzuku, J.; Ohsugi, M.; Onda, M.; Hirai, M.; Fujimoto, J.; Yamamoto, T. Tob2, a novel anti-proliferative Tob/BTG1 family member, associates with a component of the CCR4 transcriptional regulatory complex capable of binding cyclin-dependent kinases. Oncogene 1999, 18, 7432–7441. [Google Scholar] [CrossRef]
- Rouault, J.-P.; Rimokh, R.; Tessa, C.; Paranhos, G.; Ffrench, M.; Duret, L.; Garoccio, M.; Germain, D.; Samarut, J.; Magaud, J.-P. BTG1, a member of a new family of antiproliferative genes. EMBO J. 1992, 11, 1663–1670. [Google Scholar] [CrossRef]
- Matsuda, S.; Kawamura-Tsuzuku, J.; Ohsugi, M.; Yoshida, M.; Emi, M.; Nakamura, Y.; Onda, M.; Yoshida, Y.; Nishiyama, A.; Yamamoto, T. Tob, a novel protein that interacts with p185erbB2, is associated with anti-proliferative activity. Oncogene 1996, 12, 705–713. [Google Scholar]
- O’Malley, S.; Su, H.; Zhang, T.; Ng, C.; Ge, H.; Tang, C.K. TOB suppresses breast cancer tumorigenesis. Int. J. Cancer 2009, 125, 1805–1813. [Google Scholar] [CrossRef]
- Yanagie, H.; Tanabe, T.; Sumimoto, H.; Sugiyama, H.; Matsuda, S.; Nonaka, Y.; Ogiwara, N.; Sasaki, K.; Tani, K.; Takamoto, S. Tumor growth suppression by adenovirus-mediated introduction of a cell growth suppressing gene tob in a pancreatic cancer model. Biomed. Pharmacother. 2009, 63, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Suzuki, T.; Yoshida, H.; Tomoda, C.; Uruno, T.; Takamura, Y.; Miya, A.; Kobayashi, K.; Matsuzuka, F.; Kuma, K. Phosphorylation and inactivation of Tob contributes to the progression of papillary carcinoma of the thyroid. Cancer Lett. 2005, 220, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, P.; Cui, X.; Sui, Y.; Ji, G.; Guan, R.; Sun, D.; Ji, W.; Liu, F.; Liu, A. Identification of novel subregions of LOH in gastric cancer and analysis of the HIC1 and TOB1 tumor suppressor genes in these subregions. Mol. Cells 2011, 32, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Wahab, S.; Kundu, J.K.; Choi, Y.-L.; Erkin, O.C.; Lee, H.S.; Park, S.G.; Shin, Y.K. Tob1 induces apoptosis and inhibits proliferation, migration and invasion of gastric cancer cells by activating Smad4 and inhibiting β-catenin signaling. Int. J. Oncol. 2012, 41, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Bossy-Wetzel, E.; Bakiri, L.; Yaniv, M. Induction of apoptosis by the transcription factor c-Jun. EMBO J. 1997, 16, 1695–1709. [Google Scholar] [CrossRef]
- Schreiber, M.; Kolbus, A.; Piu, F.; Szabowski, A.; Möhle-Steinlein, U.; Tian, J.; Karin, M.; Angel, P.; Wagner, E.F. Control of cell cycle progression by c-Jun is p53 dependent. Genes Dev. 1999, 13, 607–619. [Google Scholar] [CrossRef]
- Ou, Y.; Wang, S.-J.; Li, D.; Chu, B.; Gu, W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc. Natl. Acad. Sci. USA 2016, 113, E6806–E6812. [Google Scholar] [CrossRef] [PubMed]
- Salomé, M.; Hopcroft, L.; Keeshan, K. Inverse and correlative relationships between TRIBBLES genes indicate non-redundant functions during normal and malignant hemopoiesis. Exp. Hematol. 2018, 66, 63–78. e13. [Google Scholar] [CrossRef]
- Naiki, T.; Saijou, E.; Miyaoka, Y.; Sekine, K.; Miyajima, A. TRB2, a mouse Tribbles ortholog, suppresses adipocyte differentiation by inhibiting AKT and C/EBPβ. Journal of Biological Chemistry 2007, 282, 24075–24082. [Google Scholar] [CrossRef]
- McMillan, H.D.; Keeshan, K.; Dunbier, A.K.; Mace, P.D. Structure vs. Function of TRIB1—Myeloid Neoplasms and Beyond. Cancers 2021, 13, 3060. [Google Scholar] [CrossRef]
- Mariño, G.; Kroemer, G. Mechanisms of apoptotic phosphatidylserine exposure. Cell Res. 2013, 23, 1247–1248. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alcázar, J.A.; Rodríguez-Hernández, Á.; Cordero, M.D.; Fernández-Ayala, D.J.; Brea-Calvo, G.; Garcia, K.; Navas, P. The apoptotic microtubule network preserves plasma membrane integrity during the execution phase of apoptosis. Apoptosis 2007, 12, 1195–1208. [Google Scholar] [CrossRef]
- Povea-Cabello, S.; Oropesa-Ávila, M.; de la Cruz-Ojeda, P.; Villanueva-Paz, M.; De la Mata, M.; Suárez-Rivero, J.M.; Sánchez-Alcázar, J.A. Dynamic Reorganization of the Cytoskeleton during Apoptosis: The Two Coffins Hypothesis. Int. J. Mol. Sci. 2017, 18, 2393. [Google Scholar] [CrossRef] [PubMed]
- Nurchi, V.M.; de Guadalupe Jaraquemada-Pelaez, M.; Crisponi, G.; Lachowicz, J.I.; Cappai, R.; Gano, L.; Santos, M.A.; Melchior, A.; Tolazzi, M.; Peana, M. A new tripodal kojic acid derivative for iron sequestration: Synthesis, protonation, complex formation studies with Fe3+, Al3+, Cu2+ and Zn2+, and in vivo bioassays. J. Inorg. Biochem. 2019, 193, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Nurchi, V.M.; Crisponi, G.; Arca, M.; Crespo-Alonso, M.; Lachowicz, J.I.; Mansoori, D.; Toso, L.; Pichiri, G.; Santos, M.A.; Marques, S.M. A new bis-3-hydroxy-4-pyrone as a potential therapeutic iron chelating agent. Effect of connecting and side chains on the complex structures and metal ion selectivity. J. Inorg. Biochem. 2014, 141, 132–143. [Google Scholar] [CrossRef]




| External Gene Name | External Gene Source | Description | Base Mean | log2 Fold Change | lfcSE | Stat | p-Value | Padj |
|---|---|---|---|---|---|---|---|---|
| TUBB2B | HGNC Symbol | tubulin beta 2B class IIb[Source:HGNCSymbol;Acc:HGNC:30829] | 4300 | 1.2 | 0.05 | 23 | 8.70 × 10−119 | 1.20 × 10−114 |
| INSIG1 | HGNC Symbol | insulin induced gene 1[Source:HGNCSymbol;Acc:HGNC:6083] | 2300 | 1.3 | 0.063 | 21 | 6.10 × 10−95 | 4.30 × 10−91 |
| IDI1 | HGNC Symbol | isopentenyl-diphosphatedelta isomerase 1[Source:HGNCSymbol;Acc:HGNC:5387] | 7700 | 0.78 | 0.04 | 20 | 1.10 × 10−86 | 5.00 × 10−83 |
| PNRC1 | HGNC Symbol | proline rich nuclearreceptor coactivator 1[Source:HGNCSymbol;Acc:HGNC:17278] | 2700 | 0.93 | 0.052 | 18 | 8.00 × 10−71 | 2.80 × 10−67 |
| HERPUD1 | HGNC Symbol | homocysteine inducible ERprotein with ubiquitin likedomain 1 [Source:HGNCSymbol;Acc:HGNC:13744] | 4800 | 0.83 | 0.047 | 18 | 9.80 × 10−70 | 2.80 × 10−66 |
| TP53INP2 | HGNC Symbol | tumour protein p53inducible nuclear protein 2[Source:HGNCSymbol;Acc:HGNC:16104] | 2000 | 1.2 | 0.067 | 17 | 1.40 × 10−66 | 3.20 × 10−63 |
| TOB1 | HGNC Symbol | transducer of ERBB2, 1[Source:HGNCSymbol;Acc:HGNC:11979] | 8700 | 0.66 | 0.039 | 17 | 5.10 × 10−66 | 1.00 × 10−62 |
| JUN | HGNC Symbol | Jun proto-oncogene, AP-1transcription factorsubunit [Source:HGNCSymbol;Acc:HGNC:6204] | 1700 | 1.2 | 0.072 | 17 | 6.40 × 10−65 | 1.10 × 10−61 |
| SAT1 | HGNC Symbol | spermidine/spermine N1-acetyltransferase 1[Source:HGNCSymbol;Acc:HGNC:10540] | 3800 | 0.79 | 0.047 | 17 | 1.40 × 10−64 | 2.20 × 10−61 |
| TRIB1 | HGNC Symbol | tribbles pseudokinase 1[Source:HGNCSymbol;Acc:HGNC:16891] | 2000 | 1.1 | 0.066 | 17 | 2.90 × 10−62 | 4.20 × 10−59 |
| External Gene Name | External Gene Source | Description | Base Mean | log2 Fold Change | lfcSE | Stat | p-Value | Padj |
|---|---|---|---|---|---|---|---|---|
| TUBB | HGNC Symbol | tubulin beta class I[Source:HGNCSymbol;Acc:HGNC:20778] | 42,000 | −2.1 | 0.072 | −29 | 3.00 × 10−191 | 4.10 × 10−187 |
| TUBA1B | HGNC Symbol | tubulin alpha 1b[Source:HGNCSymbol;Acc:HGNC:18809] | 73,000 | −1.9 | 0.074 | −25 | 4.40 × 10−142 | 3.00 × 10−138 |
| TUBB4B | HGNC Symbol | tubulin beta 4B class IVb[Source:HGNCSymbol;Acc:HGNC:20771] | 24,000 | −1.8 | 0.076 | −23 | 9.40 × 10−122 | 4.30 × 10−118 |
| ANKRD1 | HGNC Symbol | ankyrin repeat domain 1[Source:HGNCSymbol;Acc:HGNC:15819] | 6700 | 2.1 | 0.1 | 20 | 3.70 × 10−93 | 1.30 × 10−89 |
| TAGLN | HGNC Symbol | transgelin [Source:HGNCSymbol;Acc:HGNC:11553] | 14,000 | 1.9 | 0.094 | 20 | 1.30 × 10−91 | 3.60 × 10−88 |
| ITGA2 | HGNC Symbol | integrin subunit alpha 2[Source:HGNCSymbol;Acc:HGNC:6137] | 3400 | 1.9 | 0.095 | 20 | 3.70 × 10−88 | 8.50 × 10−85 |
| TUBB2B | HGNC Symbol | tubulin beta 2B class IIb[Source:HGNCSymbol;Acc:HGNC:30829] | 4800 | −1.4 | 0.094 | −15 | 1.20 × 10−51 | 2.30 × 10−48 |
| LAMA1 | HGNC Symbol | laminin subunit alpha 1[Source:HGNCSymbol;Acc:HGNC:6481] | 18,000 | −1.1 | 0.074 | −15 | 2.00 × 10−50 | 3.40 × 10−47 |
| TUBA1C | HGNC Symbol | tubulin alpha 1c[Source:HGNCSymbol;Acc:HGNC:20768] | 27,000 | −1.2 | 0.082 | −15 | 5.60 × 10−49 | 8.50 × 10−46 |
| DKK1 | HGNC Symbol | dickkopf WNT signalingpathway inhibitor 1[Source:HGNCSymbol;Acc:HGNC:2891] | 16,000 | 1 | 0.077 | 13 | 4.60 × 10−39 | 6.40 × 10−36 |
| External_gene_name | External_gene_source | Description | BaseMean | log2FoldChange | lfcSE | Stat | p-Value | Padj |
|---|---|---|---|---|---|---|---|---|
| TUBB | HGNC Symbol | tubulin beta class I[Source:HGNCSymbol;Acc:HGNC:20778] | 56,000 | −1.9 | 0.057 | −33 | 1.4 × 10−243 | 1.7 × 10−239 |
| TUBA1B | HGNC Symbol | tubulin alpha 1b[Source:HGNCSymbol;Acc:HGNC:18809] | 70,000 | −1.7 | 0.057 | −30 | 3.6 × 10−195 | 2.2 × 10−191 |
| FLNA | HGNC Symbol | filamin A [Source:HGNCSymbol;Acc:HGNC:3754] | 45,000 | 1.3 | 0.057 | 23 | 1.3 × 10−116 | 5.1 × 10−113 |
| IRS4 | HGNC Symbol | insulin receptor substrate4 [Source:HGNCSymbol;Acc:HGNC:6128] | 23,000 | −1.4 | 0.063 | −22 | 3.7 × 10−103 | 1.1 × 10−99 |
| AL035425.2 | Clone-based (Ensembl)gene | insulin receptor substrate4 [Source:NCBIgene;Acc:8471] | 9800 | −1.7 | 0.084 | −20 | 4.8 × 10−93 | 1.2 × 10−89 |
| AMOT | HGNC Symbol | angiomotin [Source:HGNCSymbol;Acc:HGNC:17810] | 51,000 | −1.1 | 0.057 | −20 | 3.4 × 10−88 | 6.8 × 10−85 |
| TPM4 | HGNC Symbol | tropomyosin 4[Source:HGNCSymbol;Acc:HGNC:12013] | 11,000 | 1.2 | 0.073 | 17 | 1.1 × 10−63 | 1.9 × 10−60 |
| GLA | HGNC Symbol | galactosidase alpha[Source:HGNCSymbol;Acc:HGNC:4296] | 10,000 | −1.3 | 0.076 | −17 | 2.1 × 10−62 | 3.2 × 10−59 |
| TUBB4B | HGNC Symbol | tubulin beta 4B class IVb[Source:HGNCSymbol;Acc:HGNC:20771] | 16,000 | −1.2 | 0.072 | −16 | 5.8 × 10−60 | 7.8 × 10−57 |
| JUN | HGNC Symbol | Jun proto-oncogene, AP-1transcription factorsubunit [Source:HGNCSymbol;Acc:HGNC:6204] | 9500 | 1.2 | 0.076 | 15 | 8.8 × 10−53 | 1.1 × 10−49 |
| External_gene_name | External_gene_source | Description | BaseMean | log2FoldChange | lfcSE | Stat | p-Value | Padj |
|---|---|---|---|---|---|---|---|---|
| IRS4 | HGNC Symbol | insulin receptor substrate4 [Source:HGNCSymbol;Acc:HGNC:6128] | 23,000 | −0.69 | 0.032 | −22 | 8.8 × 10−106 | 1.2 × 10−101 |
| NEFM | HGNC Symbol | neurofilament medium[Source:HGNCSymbol;Acc:HGNC:7734] | 15,000 | −0.61 | 0.035 | −18 | 2.4 × 10−70 | 1.7 × 10−66 |
| BMP2 | HGNC Symbol | bone morphogeneticprotein 2 [Source:HGNCSymbol;Acc:HGNC:1069] | 4100 | −0.88 | 0.051 | −17 | 6.8 × 10−66 | 3.1 × 10−62 |
| AL035425.2 | Clone-based (Ensembl)gene | insulin receptor substrate4 [Source:NCBIgene;Acc:8471] | 9400 | −0.86 | 0.055 | −16 | 6.3 × 10−55 | 2.2 × 10−51 |
| ID2 | HGNC Symbol | inhibitor of DNA binding2 [Source:HGNCSymbol;Acc:HGNC:5361] | 7200 | −0.74 | 0.048 | −16 | 2.9 × 10−54 | 8 × 10−51 |
| FAM84B | HGNC Symbol | family with sequencesimilarity 84 member B[Source:HGNCSymbol;Acc:HGNC:24166] | 4500 | −0.86 | 0.059 | −15 | 1 × 10−48 | 2.3 × 10−45 |
| DFFA | HGNC Symbol | DNA fragmentation factorsubunit alpha[Source:HGNCSymbol;Acc:HGNC:2772] | 12,000 | −0.54 | 0.037 | −14 | 2.2 × 10−47 | 4.3 × 10−44 |
| TFRC | HGNC Symbol | transferrin receptor[Source:HGNCSymbol;Acc:HGNC:11763] | 15,000 | 0.5 | 0.037 | 14 | 8.7 × 10−42 | 1.5 × 10−38 |
| AMOT | HGNC Symbol | angiomotin[Source:HGNCSymbol;Acc:HGNC:17810] | 56,000 | −0.34 | 0.026 | −13 | 1 × 10−40 | 1.6 × 10−37 |
| CA2 | HGNC Symbol | carbonic anhydrase 2[Source:HGNCSymbol;Acc:HGNC:1373] | 15,000 | −0.5 | 0.038 | −13 | 6 × 10−38 | 8.3 × 10−35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pichiri, G.; Piludu, M.; Congiu, T.; Grandi, N.; Coni, P.; Piras, M.; Jaremko, M.; Lachowicz, J.I. Kojic Acid Derivative as an Antimitotic Agent That Selectively Kills Tumour Cells. Pharmaceuticals 2025, 18, 11. https://doi.org/10.3390/ph18010011
Pichiri G, Piludu M, Congiu T, Grandi N, Coni P, Piras M, Jaremko M, Lachowicz JI. Kojic Acid Derivative as an Antimitotic Agent That Selectively Kills Tumour Cells. Pharmaceuticals. 2025; 18(1):11. https://doi.org/10.3390/ph18010011
Chicago/Turabian StylePichiri, Giuseppina, Marco Piludu, Terenzio Congiu, Nicole Grandi, Pierpaolo Coni, Monica Piras, Mariusz Jaremko, and Joanna Izabela Lachowicz. 2025. "Kojic Acid Derivative as an Antimitotic Agent That Selectively Kills Tumour Cells" Pharmaceuticals 18, no. 1: 11. https://doi.org/10.3390/ph18010011
APA StylePichiri, G., Piludu, M., Congiu, T., Grandi, N., Coni, P., Piras, M., Jaremko, M., & Lachowicz, J. I. (2025). Kojic Acid Derivative as an Antimitotic Agent That Selectively Kills Tumour Cells. Pharmaceuticals, 18(1), 11. https://doi.org/10.3390/ph18010011








