Mechanisms of NMDA Receptor Inhibition by Biguanide Compounds
Abstract
1. Introduction
2. Results
2.1. Screening and Concentration Dependence
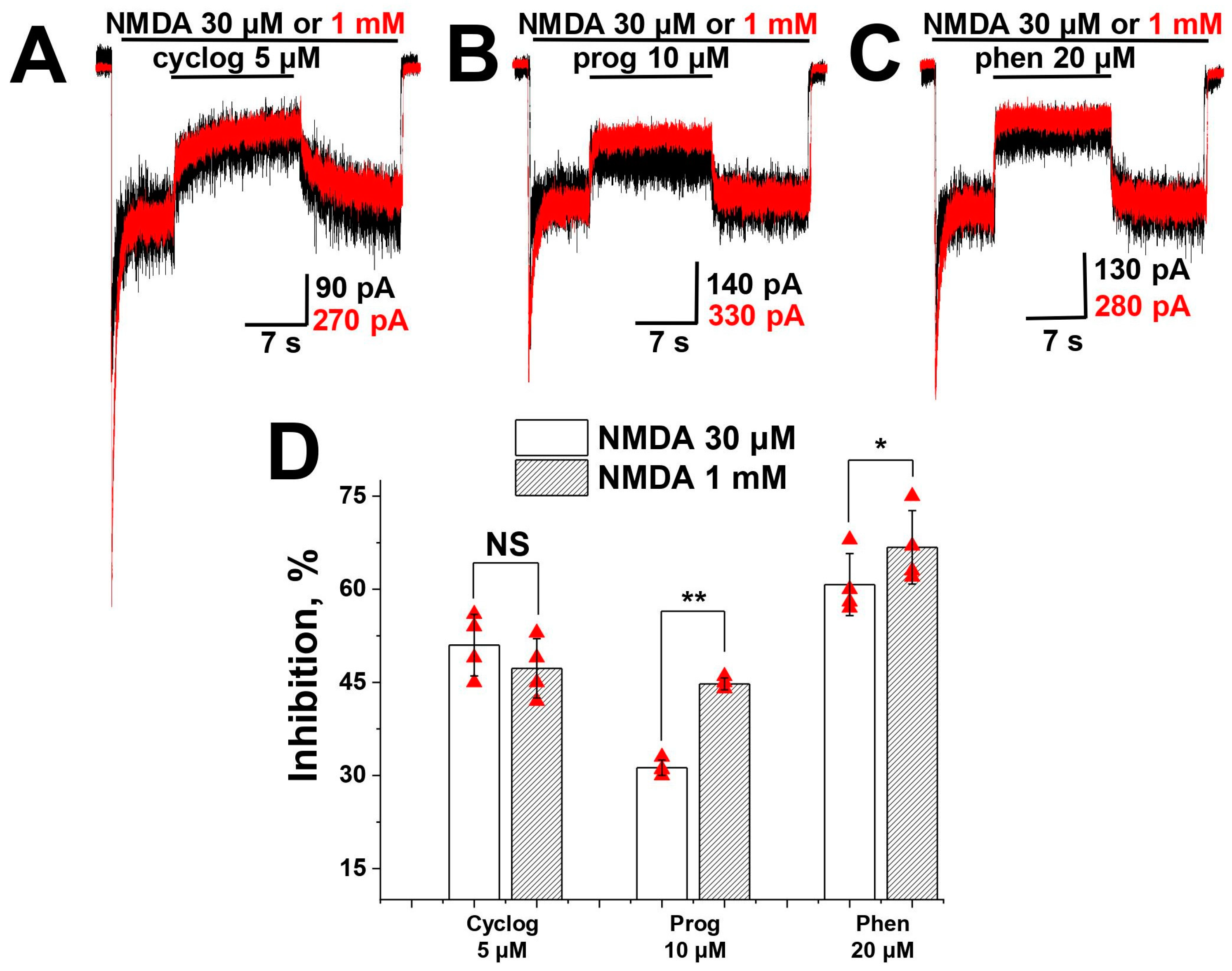
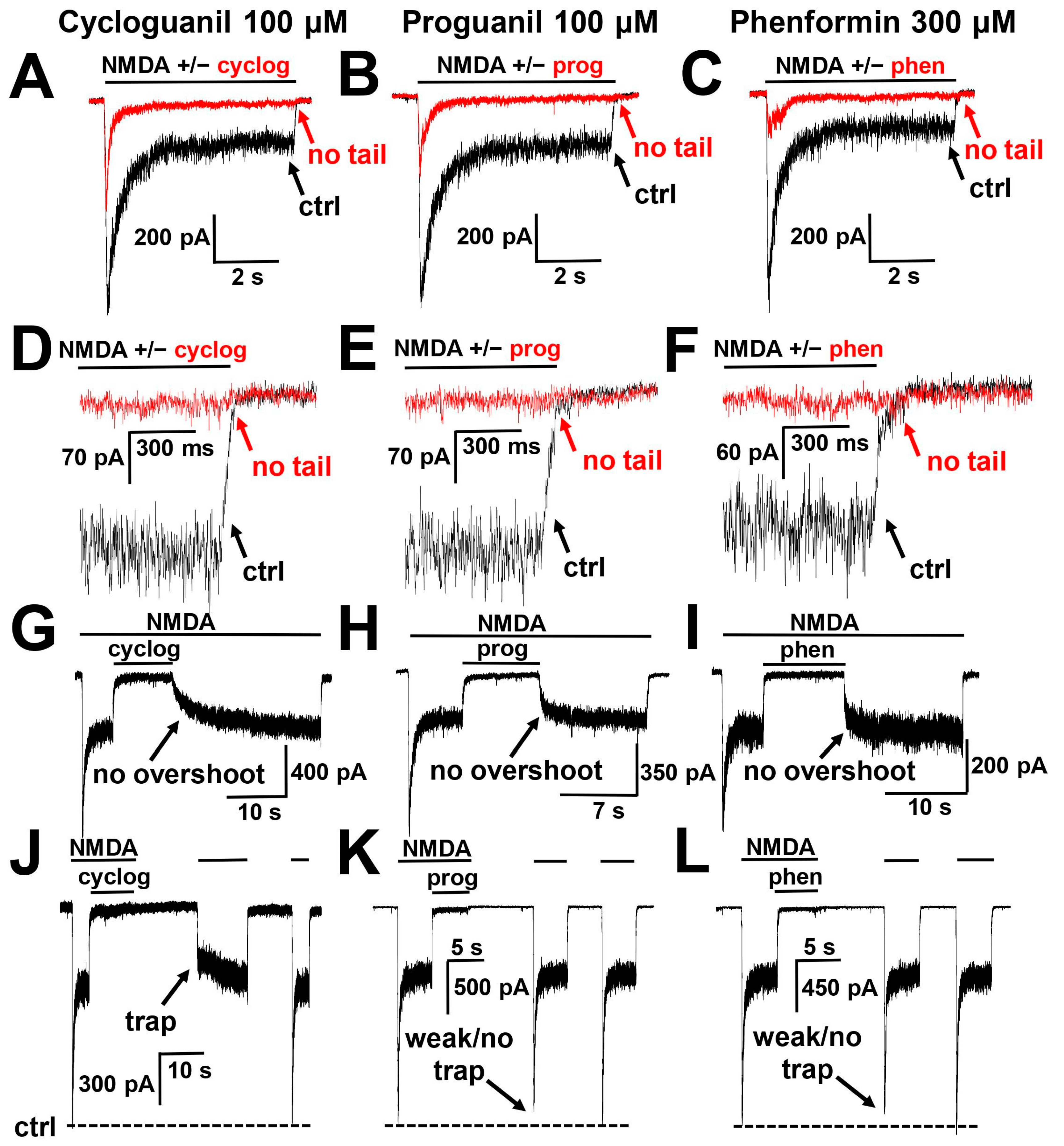
2.2. Agonist Dependence
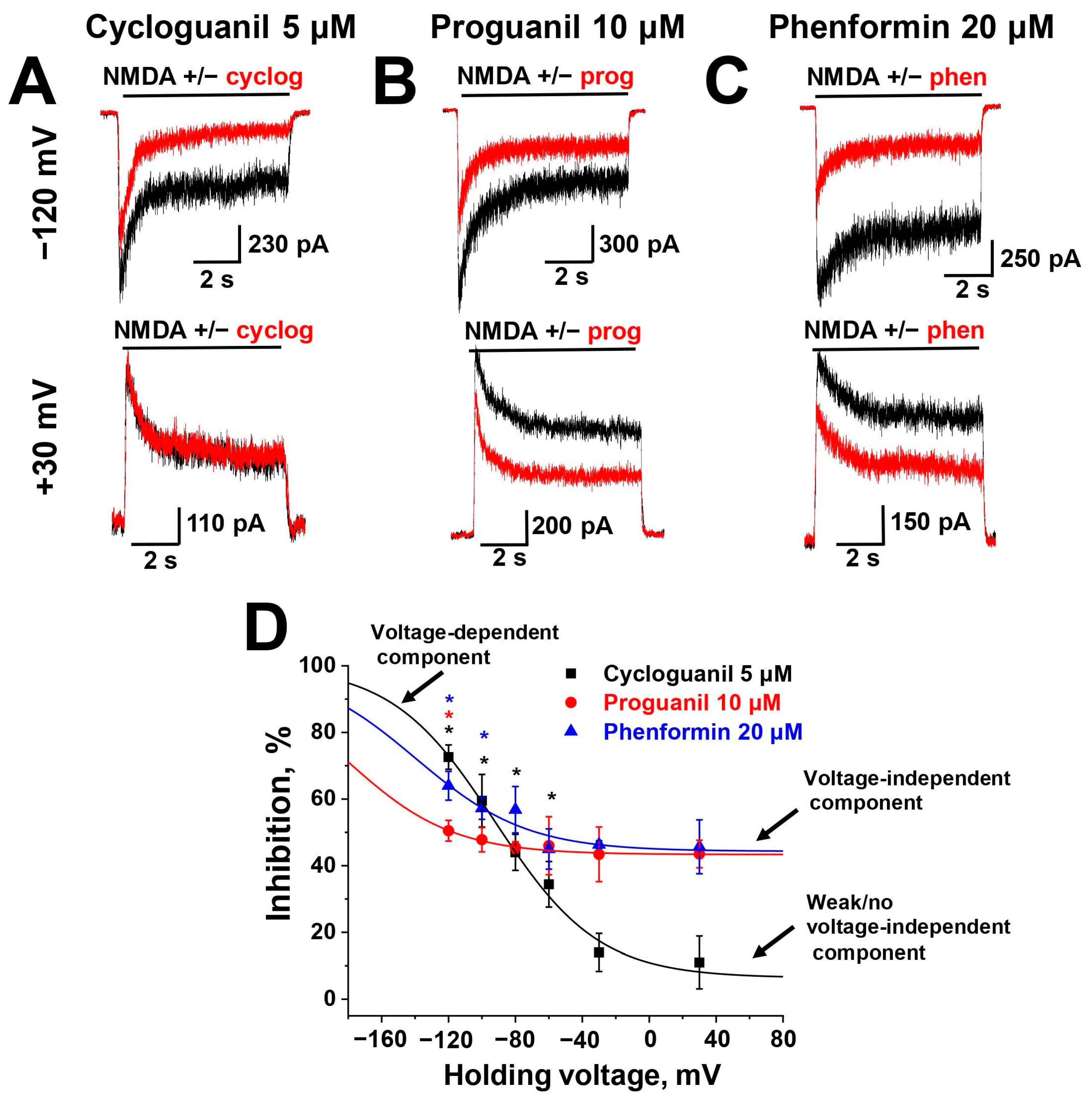
2.3. Voltage Dependence
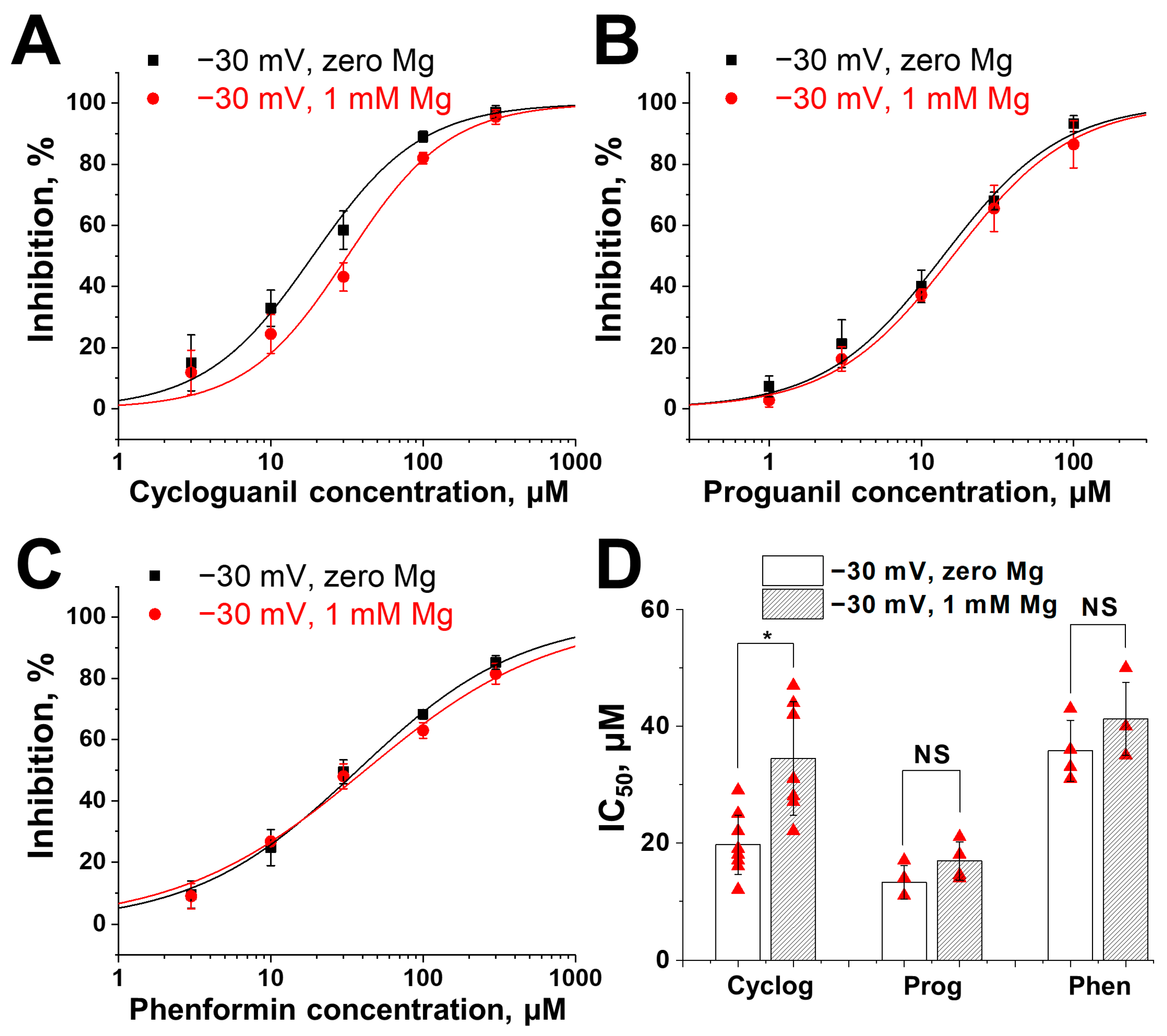
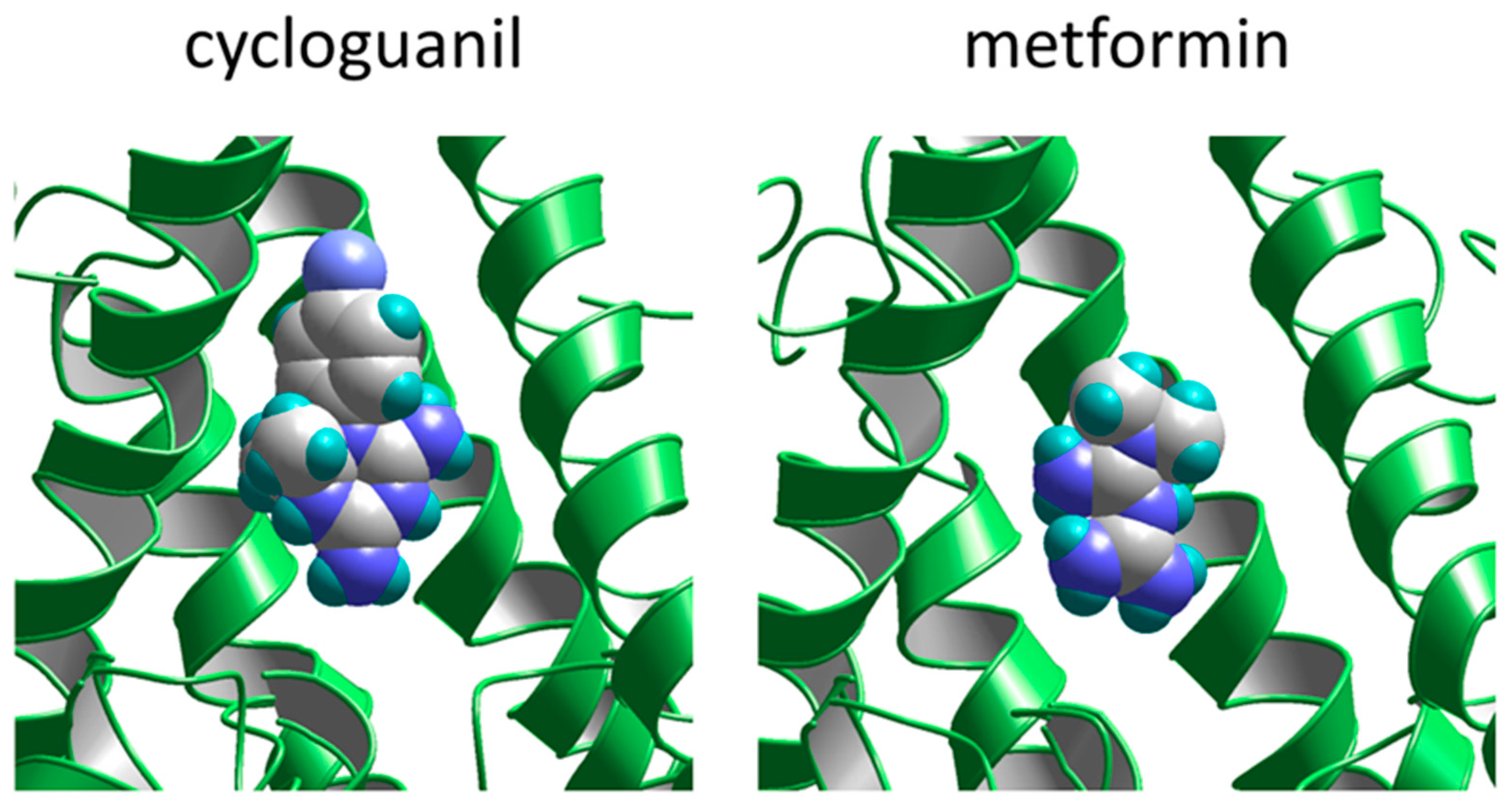
2.4. Competition with Magnesium for Binding Site
2.5. Interaction with the Gating Mechanism of the Channel
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Electrophysiology
4.3. Analysis of Voltage Dependence
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hansen, K.B.; Wollmuth, L.P.; Bowie, D.; Furukawa, H.; Menniti, F.S.; Sobolevsky, A.I.; Swanson, G.T.; Swanger, S.A.; Greger, I.H.; Nakagawa, T.; et al. Structure, Function, and Pharmacology of Glutamate Receptor Ion Channels. Pharmacol. Rev. 2021, 73, 298–487. [Google Scholar] [CrossRef] [PubMed]
- Carles, A.; Freyssin, A.; Perin-Dureau, F.; Rubinstenn, G.; Maurice, T. Targeting N-Methyl-d-Aspartate Receptors in Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 3733. [Google Scholar] [CrossRef] [PubMed]
- Cosman, K.M.; Boyle, L.L.; Porsteinsson, A.P. Memantine in the treatment of mild-to-moderate Alzheimer’s disease. Expert Opin. Pharmacother. 2007, 8, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Chaki, S.; Watanabe, M. Antidepressants in the post-ketamine Era: Pharmacological approaches targeting the glutamatergic system. Neuropharmacology 2023, 223, 109348. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Dextromethorphan/Bupropion: First Approval. CNS Drugs 2022, 36, 1229–1238. [Google Scholar] [CrossRef]
- Jelen, L.A.; Stone, J.M. Ketamine for depression. Int. Rev. Psychiatry 2021, 33, 207–228. [Google Scholar] [CrossRef]
- Nguyen, L.; Thomas, K.L.; Lucke-Wold, B.P.; Cavendish, J.Z.; Crowe, M.S.; Matsumoto, R.R. Dextromethorphan: An update on its utility for neurological and neuropsychiatric disorders. Pharmacol. Ther. 2016, 159, 1–22. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Kakoti, B.B.; Bezbaruah, R.; Ahmed, N. Therapeutic drug repositioning with special emphasis on neurodegenerative diseases: Threats and issues. Front. Pharmacol. 2022, 13, 1007315. [Google Scholar] [CrossRef]
- Shukla, H.; John, D.; Banerjee, S.; Tiwari, A.K. Drug repurposing for neurodegenerative diseases. Prog. Mol. Biol. Transl. Sci. 2024, 207, 249–319. [Google Scholar] [CrossRef]
- Das, J. Repurposing of Drugs-The Ketamine Story. J. Med. Chem. 2020, 63, 13514–13525. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Jain, K.; Saini, A.; Mani, I.; Singh, V. Exploring the potential of drug repurposing for treating depression. Prog. Mol. Biol. Transl. Sci. 2024, 207, 79–105. [Google Scholar] [CrossRef]
- Stroebel, D.; Buhl, D.L.; Knafels, J.D.; Chanda, P.K.; Green, M.; Sciabola, S.; Mony, L.; Paoletti, P.; Pandit, J. A Novel Binding Mode Reveals Two Distinct Classes of NMDA Receptor GluN2B-selective Antagonists. Mol. Pharmacol. 2016, 89, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.B.; Yi, F.; Perszyk, R.E.; Furukawa, H.; Wollmuth, L.P.; Gibb, A.J.; Traynelis, S.F. Structure, function, and allosteric modulation of NMDA receptors. J. Gen. Physiol. 2018, 150, 1081–1105. [Google Scholar] [CrossRef] [PubMed]
- Sobolevsky, A.I.; Koshelev, S.G.; Khodorov, B.I. Probing of NMDA channels with fast blockers. J. Neurosci. 1999, 19, 10611–10626. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.W.; Glasgow, N.G.; Povysheva, N.V. Recent insights into the mode of action of memantine and ketamine. Curr. Opin. Pharmacol. 2015, 20, 54–63. [Google Scholar] [CrossRef]
- Parsons, C.G.; Quack, G.; Bresink, I.; Baran, L.; Przegalinski, E.; Kostowski, W.; Krzascik, P.; Hartmann, S.; Danysz, W. Comparison of the potency, kinetics and voltage-dependency of a series of uncompetitive NMDA receptor antagonists in vitro with anticonvulsive and motor impairment activity in vivo. Neuropharmacology 1995, 34, 1239–1258. [Google Scholar] [CrossRef]
- Lipton, S.A. Paradigm shift in neuroprotection by NMDA receptor blockade: Memantine and beyond. Nat. Rev. Drug Discov. 2006, 5, 160–170. [Google Scholar] [CrossRef]
- Kalia, L.V.; Kalia, S.K.; Salter, M.W. NMDA receptors in clinical neurology: Excitatory times ahead. Lancet Neurol. 2008, 7, 742–755. [Google Scholar] [CrossRef]
- Rogawski, M.A.; Wenk, G.L. The neuropharmacological basis for the use of memantine in the treatment of Alzheimer’s disease. CNS Drug Rev. 2003, 9, 275–308. [Google Scholar] [CrossRef]
- Kathuria, D.; Raul, A.D.; Wanjari, P.; Bharatam, P.V. Biguanides: Species with versatile therapeutic applications. Eur. J. Med. Chem. 2021, 219, 113378. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Velazquez, E.D.; Alba-Betancourt, C.; Alonso-Castro, A.J.; Ortiz-Alvarado, R.; Lopez, J.A.; Meza-Carmen, V.; Solorio-Alvarado, C.R. Metformin, a biological and synthetic overview. Bioorg. Med. Chem. Lett. 2023, 86, 129241. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Swanson, K.D.; Zheng, B. Therapeutic Repurposing of Biguanides in Cancer. Trends Cancer 2021, 7, 714–730. [Google Scholar] [CrossRef] [PubMed]
- Dron, M.Y.; Zhigulin, A.S.; Barygin, O.I. Mechanisms of NMDA receptor inhibition by diarylamidine compounds. Eur. J. Neurosci. 2020, 51, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, I.J.; Aizenman, E. Pentamidine is an N-methyl-D-aspartate receptor antagonist and is neuroprotective in vitro. J. Neurosci. 1992, 12, 970–975. [Google Scholar] [CrossRef]
- Williams, K.; Dattilo, M.; Sabado, T.N.; Kashiwagi, K.; Igarashi, K. Pharmacology of delta2 glutamate receptors: Effects of pentamidine and protons. J. Pharmacol. Exp. Ther. 2003, 305, 740–748. [Google Scholar] [CrossRef]
- Zhigulin, A.S.; Barygin, O.I. Mechanisms of NMDA receptor inhibition by nafamostat, gabexate and furamidine. Eur. J. Pharmacol. 2022, 919, 174795. [Google Scholar] [CrossRef]
- Zhigulin, A.S.; Barygin, O.I. Mechanisms of NMDA Receptor Inhibition by Sepimostat-Comparison with Nafamostat and Diarylamidine Compounds. Int. J. Mol. Sci. 2023, 24, 15685. [Google Scholar] [CrossRef]
- Lee, J.; Chan, S.L.; Lu, C.; Lane, M.A.; Mattson, M.P. Phenformin suppresses calcium responses to glutamate and protects hippocampal neurons against excitotoxicity. Exp. Neurol. 2002, 175, 161–167. [Google Scholar] [CrossRef]
- Foster, K.A.; McLaughlin, N.; Edbauer, D.; Phillips, M.; Bolton, A.; Constantine-Paton, M.; Sheng, M. Distinct roles of NR2A and NR2B cytoplasmic tails in long-term potentiation. J. Neurosci. 2010, 30, 2676–2685. [Google Scholar] [CrossRef]
- Woodhull, A.M. Ionic blockage of sodium channels in nerve. J. Gen. Physiol. 1973, 61, 687–708. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.L.; Westbrook, G.L.; Guthrie, P.B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 1984, 309, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Nowak, L.; Bregestovski, P.; Ascher, P.; Herbet, A.; Prochiantz, A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature 1984, 307, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Burnashev, N.; Schoepfer, R.; Monyer, H.; Ruppersberg, J.P.; Gunther, W.; Seeburg, P.H.; Sakmann, B. Control by asparagine residues of calcium permeability and magnesium blockade in the NMDA receptor. Science 1992, 257, 1415–1419. [Google Scholar] [CrossRef]
- Kuner, T.; Schoepfer, R. Multiple structural elements determine subunit specificity of Mg2+ block in NMDA receptor channels. J. Neurosci. 1996, 16, 3549–3558. [Google Scholar] [CrossRef]
- Kupper, J.; Ascher, P.; Neyton, J. Probing the pore region of recombinant N-methyl-D-aspartate channels using external and internal magnesium block. Proc. Natl. Acad. Sci. USA 1996, 93, 8648–8653. [Google Scholar] [CrossRef]
- Chou, T.H.; Epstein, M.; Michalski, K.; Fine, E.; Biggin, P.C.; Furukawa, H. Structural insights into binding of therapeutic channel blockers in NMDA receptors. Nat. Struct. Mol. Biol. 2022, 29, 507–518. [Google Scholar] [CrossRef]
- Song, X.; Jensen, M.O.; Jogini, V.; Stein, R.A.; Lee, C.H.; McHaourab, H.S.; Shaw, D.E.; Gouaux, E. Mechanism of NMDA receptor channel block by MK-801 and memantine. Nature 2018, 556, 515–519. [Google Scholar] [CrossRef]
- Kotermanski, S.E.; Johnson, J.W. Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer’s drug memantine. J. Neurosci. 2009, 29, 2774–2779. [Google Scholar] [CrossRef]
- Nikolaev, M.V.; Magazanik, L.G.; Tikhonov, D.B. Influence of external magnesium ions on the NMDA receptor channel block by different types of organic cations. Neuropharmacology 2012, 62, 2078–2085. [Google Scholar] [CrossRef]
- Blanpied, T.A.; Boeckman, F.A.; Aizenman, E.; Johnson, J.W. Trapping channel block of NMDA-activated responses by amantadine and memantine. J. Neurophysiol. 1997, 77, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Bolshakov, K.V.; Gmiro, V.E.; Tikhonov, D.B.; Magazanik, L.G. Determinants of trapping block of N-methyl-d-aspartate receptor channels. J. Neurochem. 2003, 87, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Huettner, J.E.; Bean, B.P. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: Selective binding to open channels. Proc. Natl. Acad. Sci. USA 1988, 85, 1307–1311. [Google Scholar] [CrossRef]
- Lerma, J.; Zukin, R.S.; Bennett, M.V. Interaction of Mg2+ and phencyclidine in use-dependent block of NMDA channels. Neurosci. Lett. 1991, 123, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Mealing, G.A.; Lanthorn, T.H.; Murray, C.L.; Small, D.L.; Morley, P. Differences in degree of trapping of low-affinity uncompetitive N-methyl-D-aspartic acid receptor antagonists with similar kinetics of block. J. Pharmacol. Exp. Ther. 1999, 288, 204–210. [Google Scholar] [PubMed]
- Barygin, O.I.; Grishin, E.V.; Tikhonov, D.B. Argiotoxin in the closed AMPA receptor channel: Experimental and modeling study. Biochemistry 2011, 50, 8213–8220. [Google Scholar] [CrossRef]
- Gresch, A.; Hurtado, H.N.; Wormeyer, L.; De Luca, V.; Wiggers, R.; Seebohm, G.; Wunsch, B.; Dufer, M. Selective Inhibition of N-Methyl-d-aspartate Receptors with GluN2B Subunit Protects beta Cells against Stress-Induced Apoptotic Cell Death. J. Pharmacol. Exp. Ther. 2021, 379, 235–244. [Google Scholar] [CrossRef]
- Sterk, M.; Krizancic Bombek, L.; Skelin Klemen, M.; Slak Rupnik, M.; Marhl, M.; Stozer, A.; Gosak, M. NMDA receptor inhibition increases, synchronizes, and stabilizes the collective pancreatic beta cell activity: Insights through multilayer network analysis. PLoS Comput. Biol. 2021, 17, e1009002. [Google Scholar] [CrossRef]
- Welters, A.; Kluppel, C.; Mrugala, J.; Wormeyer, L.; Meissner, T.; Mayatepek, E.; Heiss, C.; Eberhard, D.; Lammert, E. NMDAR antagonists for the treatment of diabetes mellitus-Current status and future directions. Diabetes Obes. Metab. 2017, 19 (Suppl. S1), 95–106. [Google Scholar] [CrossRef]
- Wormeyer, L.; Nortmann, O.; Hamacher, A.; Uhlemeyer, C.; Belgardt, B.; Eberhard, D.; Mayatepek, E.; Meissner, T.; Lammert, E.; Welters, A. The N-Methyl-D-Aspartate Receptor Antagonist Dextromethorphan Improves Glucose Homeostasis and Preserves Pancreatic Islets in NOD Mice. Horm. Metab. Res. 2024, 56, 223–234. [Google Scholar] [CrossRef]
- Marquard, J.; Otter, S.; Welters, A.; Stirban, A.; Fischer, A.; Eglinger, J.; Herebian, D.; Kletke, O.; Klemen, M.S.; Stozer, A.; et al. Characterization of pancreatic NMDA receptors as possible drug targets for diabetes treatment. Nat. Med. 2015, 21, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.B.; Cuzon Carlson, V.; Ikeda, S.; Lovinger, D. Vibrodissociation of neurons from rodent brain slices to study synaptic transmission and image presynaptic terminals. J. Vis. Exp. 2011, 51, e2752. [Google Scholar] [CrossRef]
- Vorobjev, V.S. Vibrodissociation of sliced mammalian nervous tissue. J. Neurosci. Methods 1991, 38, 145–150. [Google Scholar] [CrossRef] [PubMed]
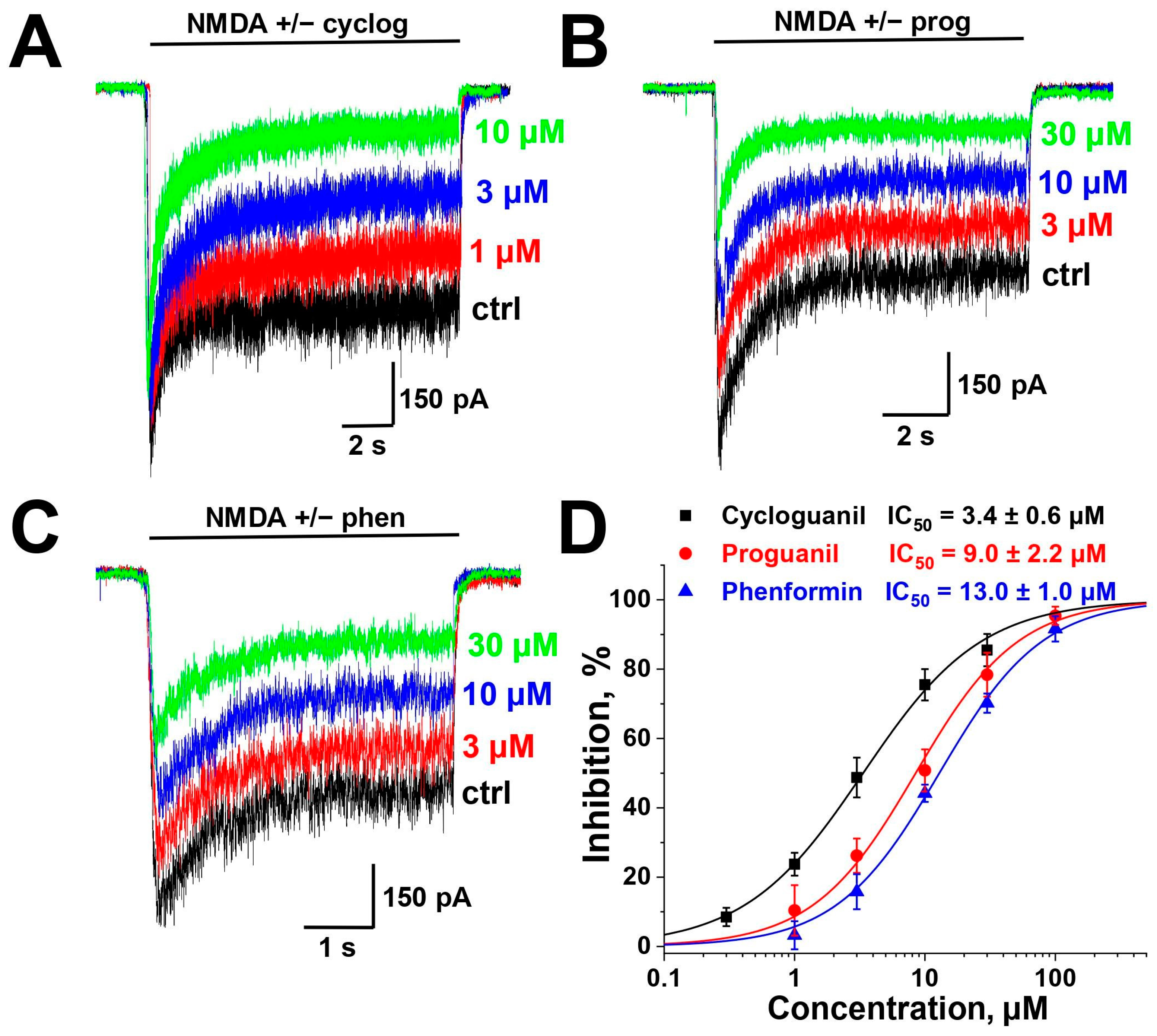

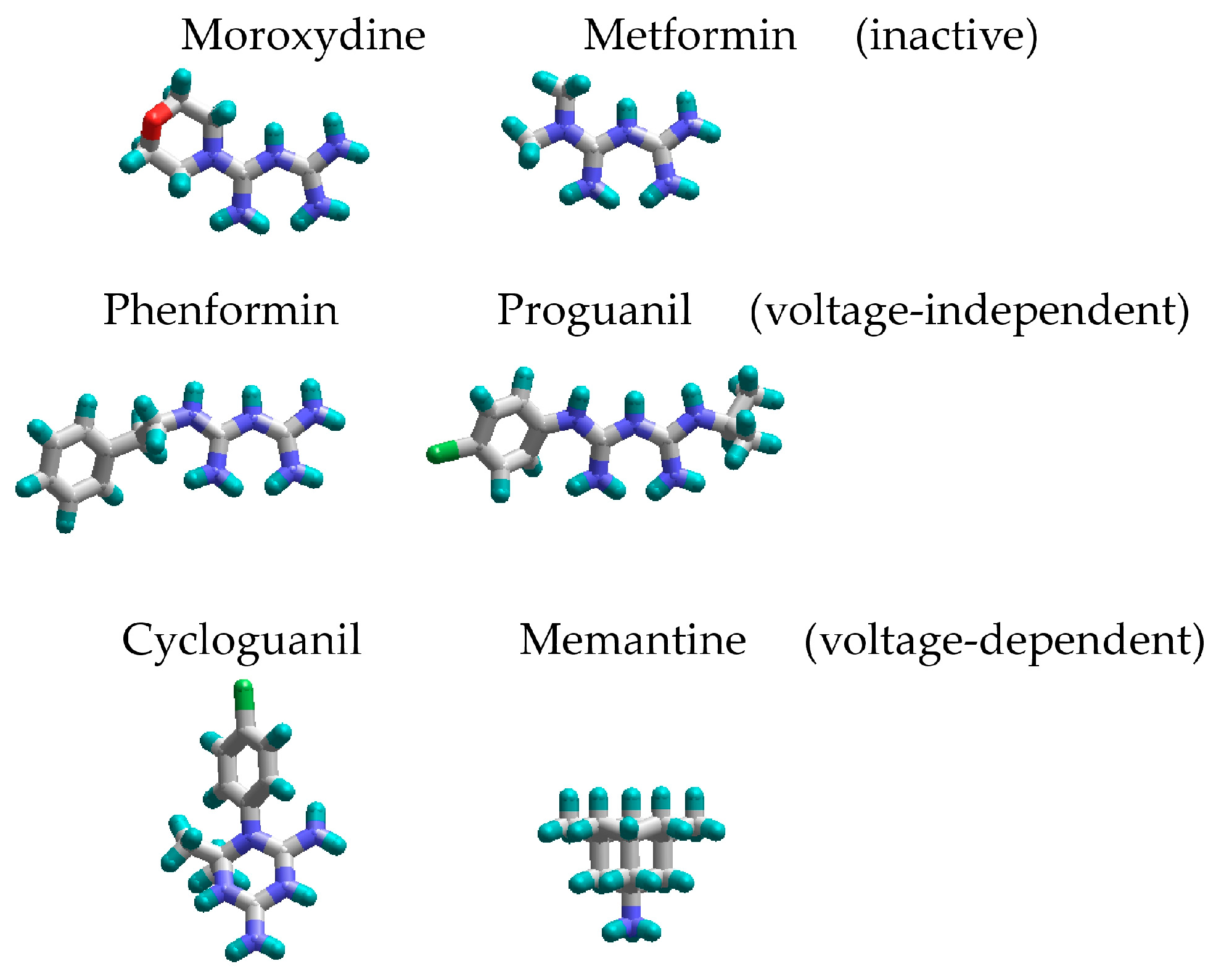
| Compound | Chemical Structure | % of Inhibition (10 µM) −80 mV | IC50, µM −80 mV | Hill Coeff. |
|---|---|---|---|---|
| Metformin | 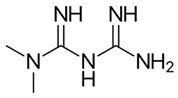 | 6 ± 5 (n = 4) | - | - |
| Phenformin |  | 44 ± 2 (n = 5) | 13.0 ± 1.0 (n = 5) | 1.1 ± 0.1 |
| Proguanil | 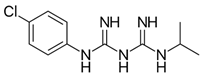 | 51 ± 6 (n = 5) | 9.0 ± 2.2 (n = 5) | 1.1 ± 0.2 |
| Cycloguanil |  | 76 ± 5 (n = 4) | 3.4 ± 0.6 (n = 4) | 1.0 ± 0.1 |
| Moroxydine | 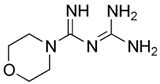 | 5 ± 4 (n = 4) | - | - |
| Compound | Kvi, µM | Kvd, µM | δ |
|---|---|---|---|
| Cycloguanil | >500 | 55 ± 11 | 0.7 ± 0.1 |
| Proguanil | 14 ± 1 | >500 | 0.7 * |
| Phenformin | 22 ± 2 | >500 | 0.7 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhigulin, A.S.; Novikova, A.O.; Barygin, O.I. Mechanisms of NMDA Receptor Inhibition by Biguanide Compounds. Pharmaceuticals 2024, 17, 1234. https://doi.org/10.3390/ph17091234
Zhigulin AS, Novikova AO, Barygin OI. Mechanisms of NMDA Receptor Inhibition by Biguanide Compounds. Pharmaceuticals. 2024; 17(9):1234. https://doi.org/10.3390/ph17091234
Chicago/Turabian StyleZhigulin, Arseniy S., Anastasiya O. Novikova, and Oleg I. Barygin. 2024. "Mechanisms of NMDA Receptor Inhibition by Biguanide Compounds" Pharmaceuticals 17, no. 9: 1234. https://doi.org/10.3390/ph17091234
APA StyleZhigulin, A. S., Novikova, A. O., & Barygin, O. I. (2024). Mechanisms of NMDA Receptor Inhibition by Biguanide Compounds. Pharmaceuticals, 17(9), 1234. https://doi.org/10.3390/ph17091234






