Beta-Myrcene as a Sedative–Hypnotic Component from Lavender Essential Oil in DL-4-Chlorophenylalanine-Induced-Insomnia Mice
Abstract
1. Introduction
2. Results
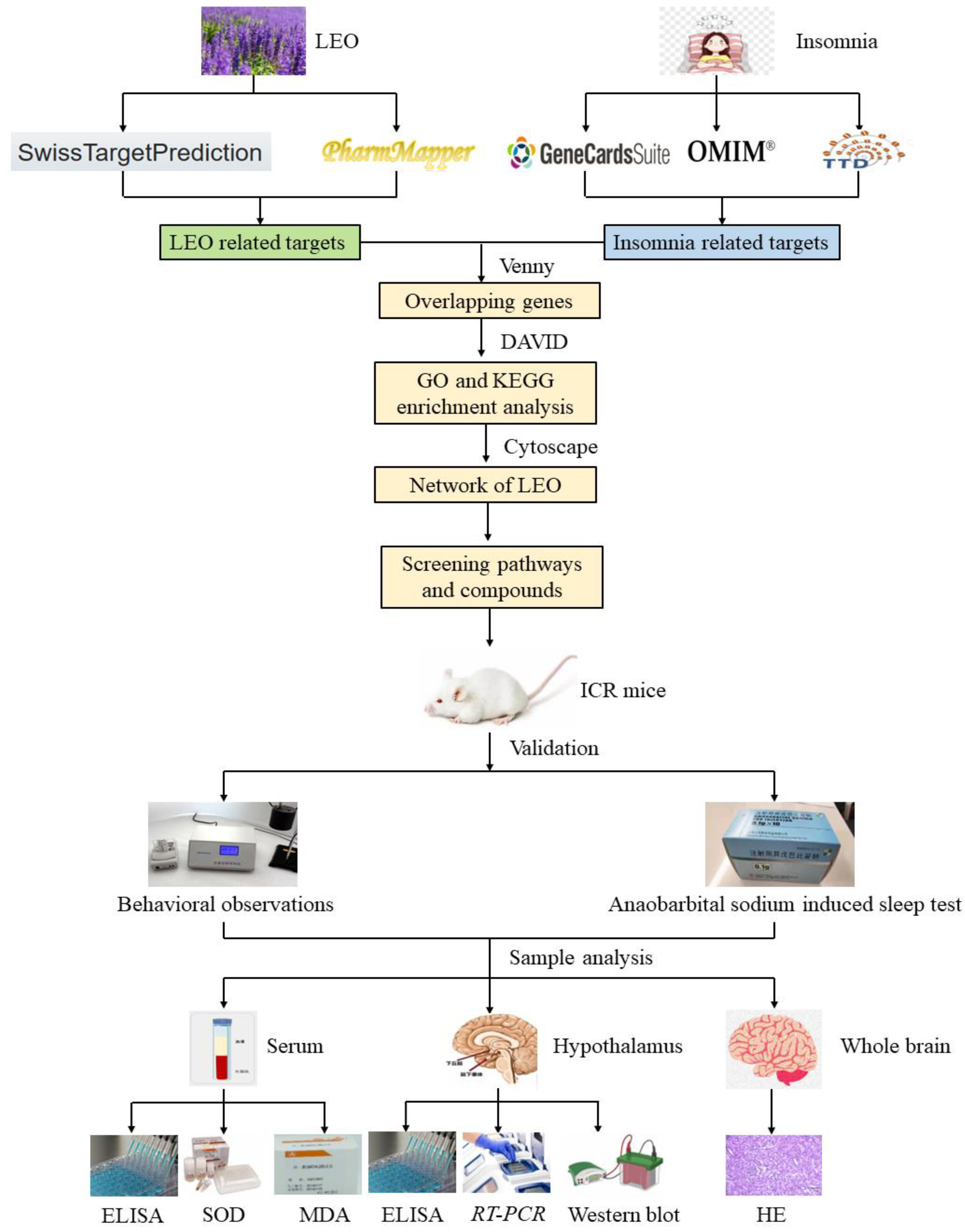
2.1. Selection of Targets and Network Analysis
2.2. GO and KEGG Enrichment Analyses
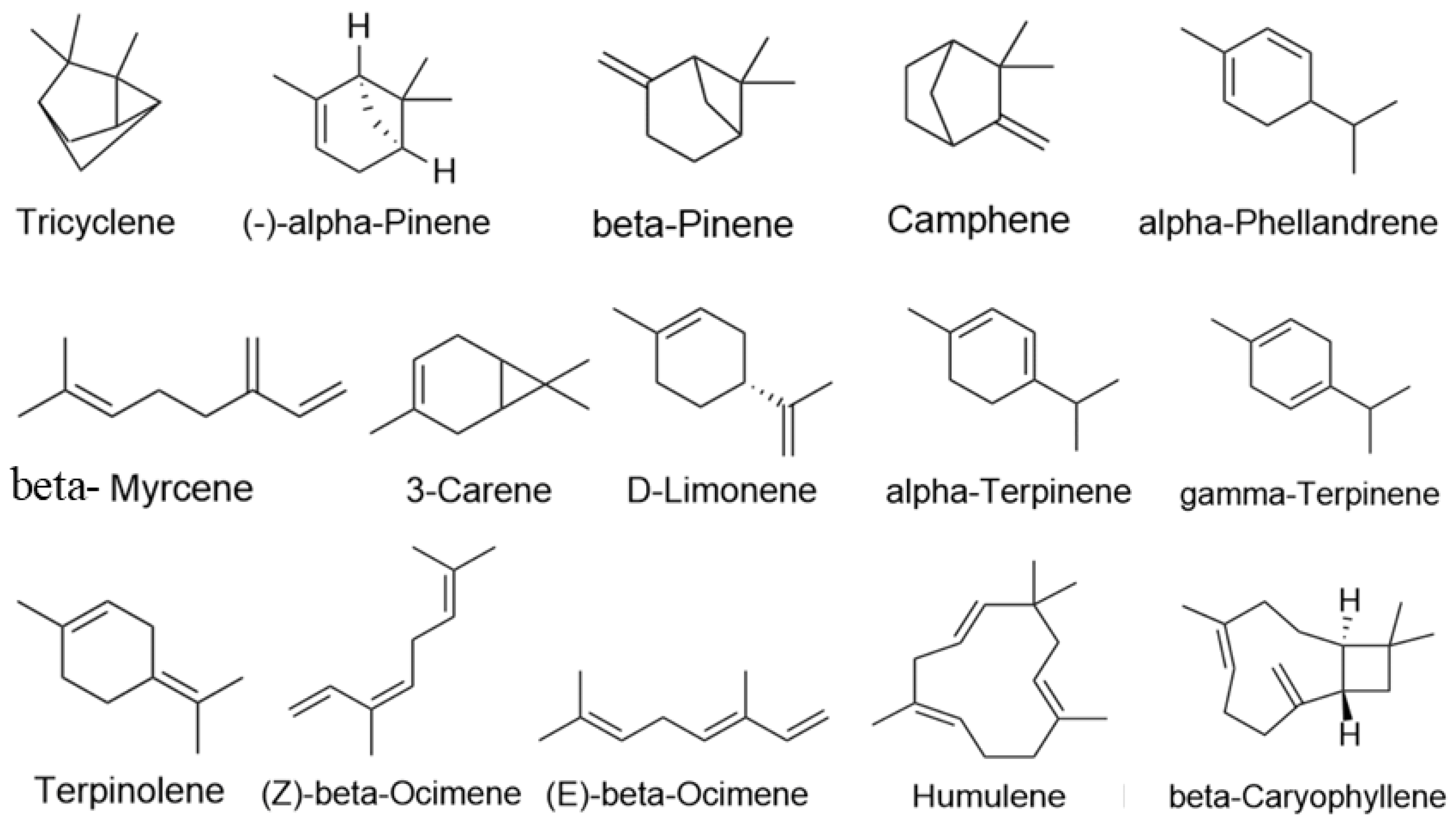
2.3. Screening the Results of the Construction of the “Active Ingredients-Potential Targets-Pathways” Network for LEO
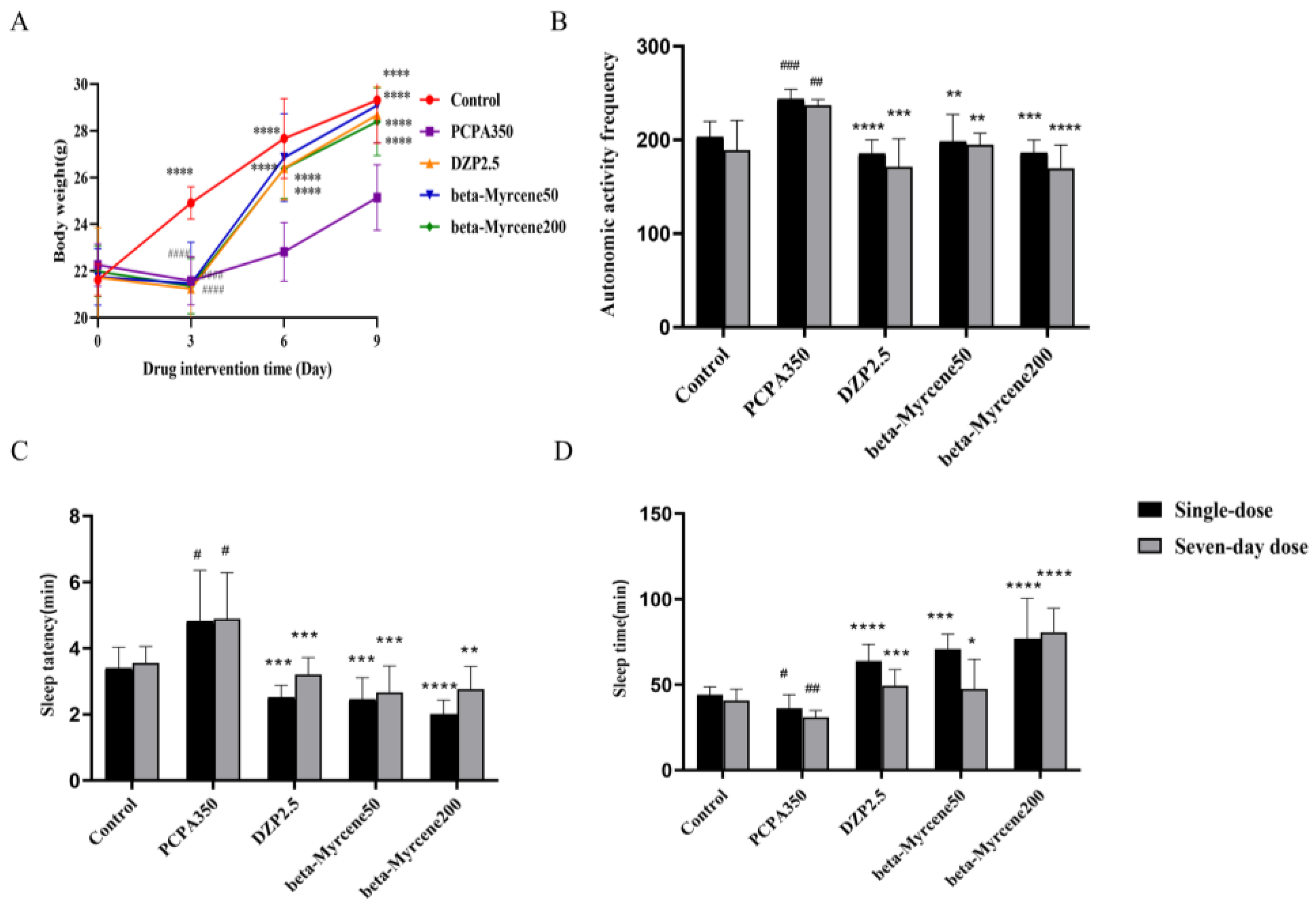
2.4. Ameliorative Effects of Beta-Myrcene in Insomniac Mice
2.5. Effects of Beta-Myrcene on Autonomous Activity in Insomniac Mice
2.6. Effects of Beta-Myrcene on Anaobarbital Sodium-Induced Sleep Test in Mice
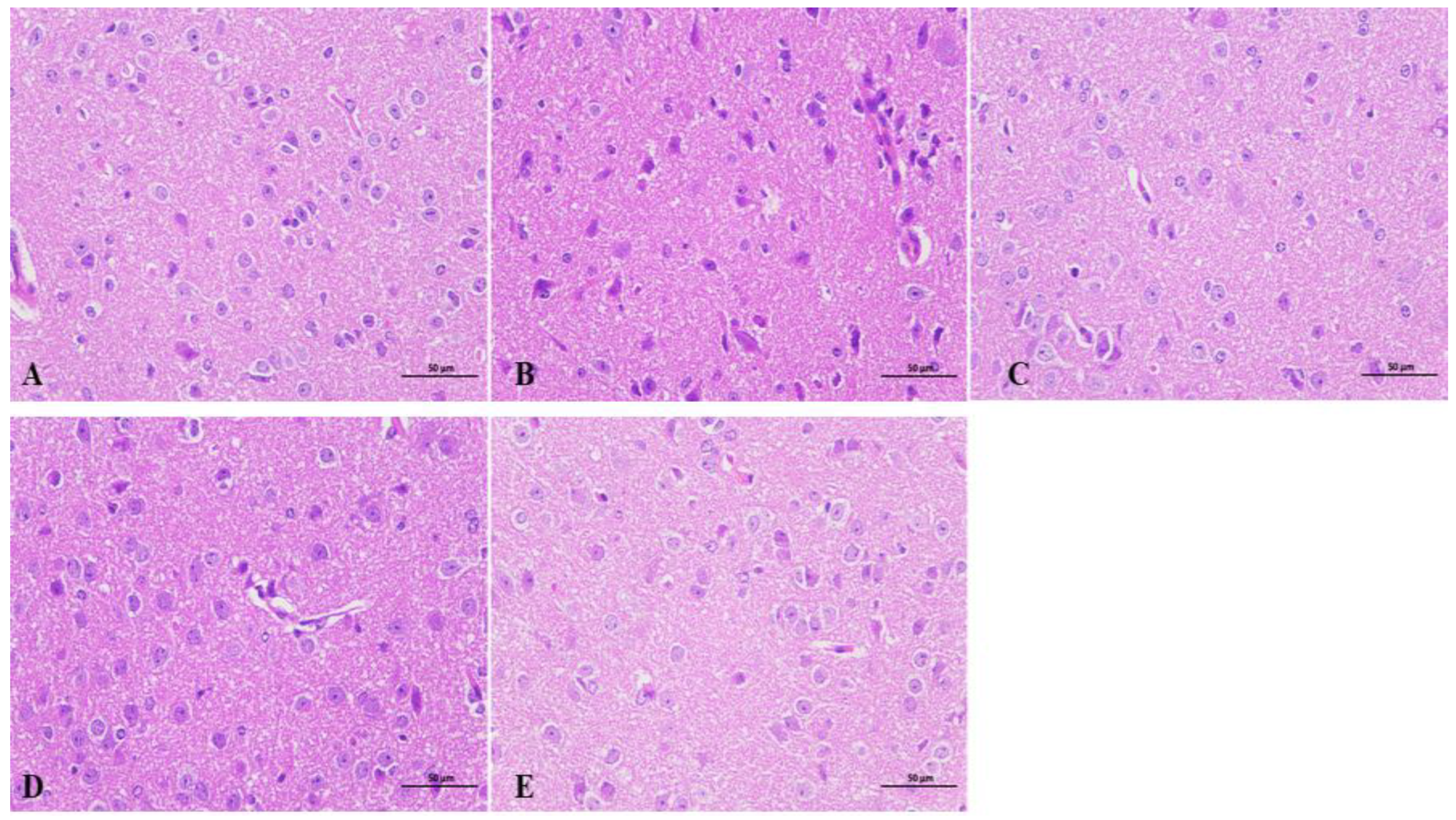
2.7. Effects of Beta-Myrcene on the Hypothalamus of Insomnia Mice
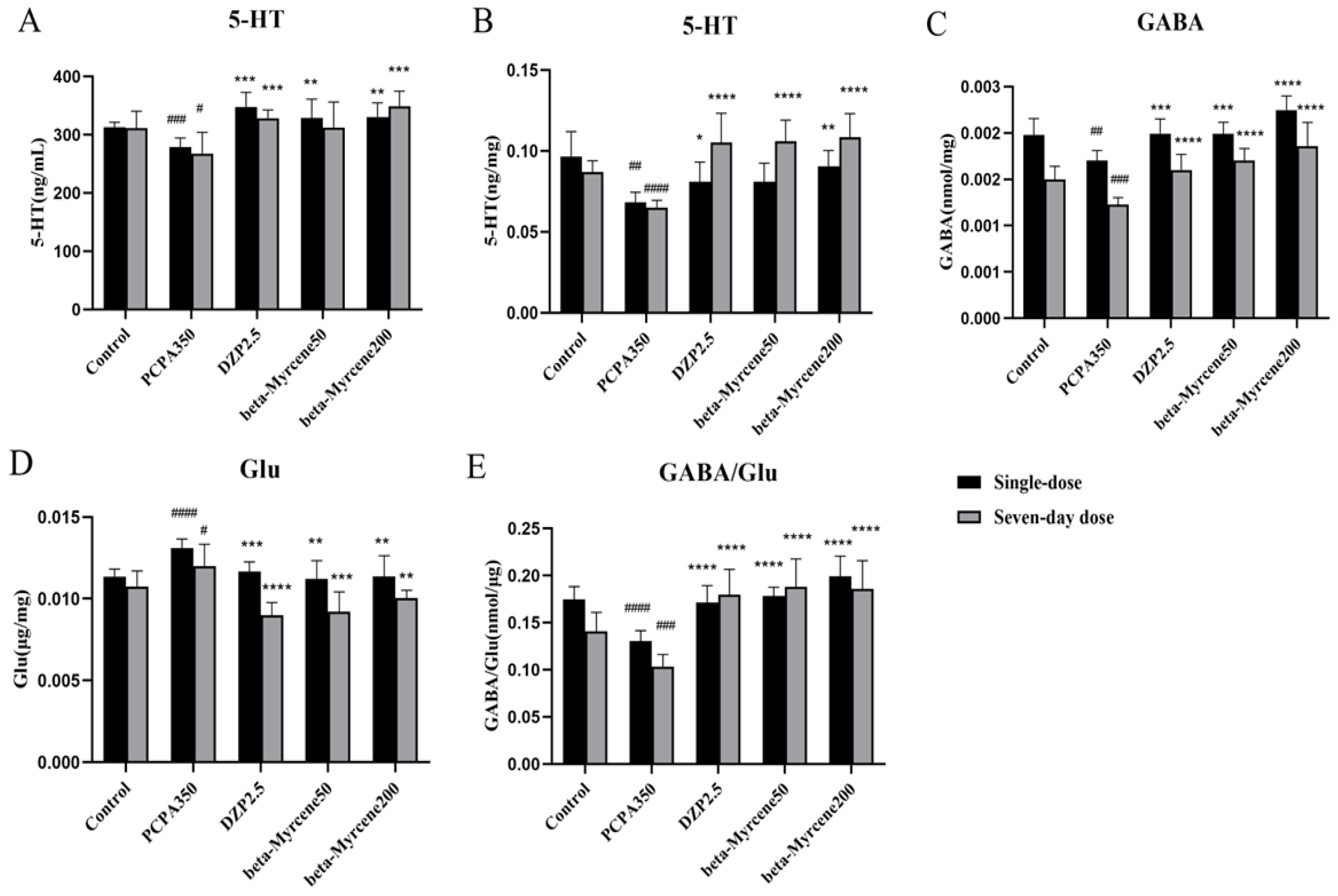
2.8. Effects of Beta-Myrcene on Neurotransmitter Levels in PCPA-Induced Insomnia Mice
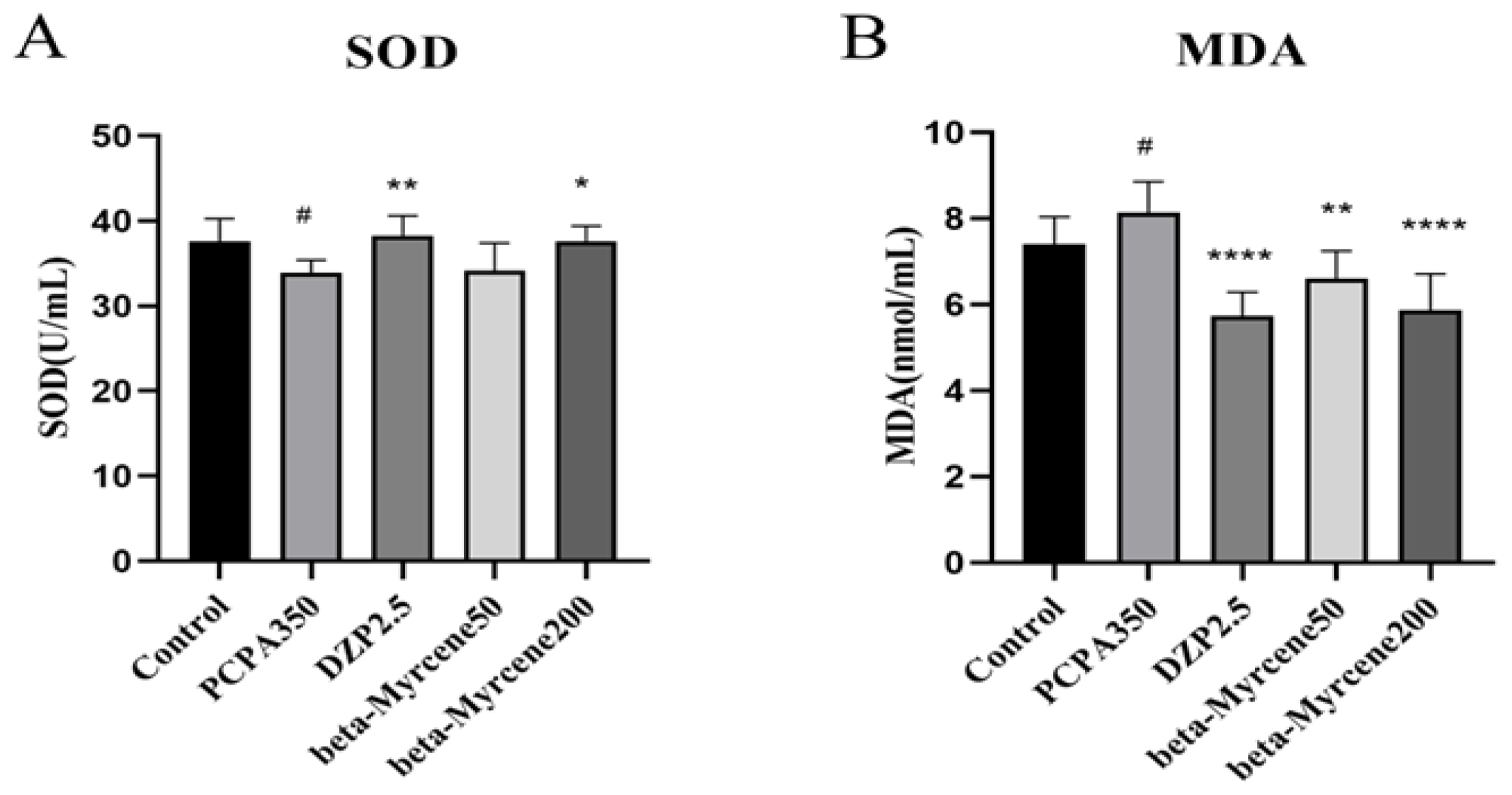
2.9. Effects of Beta-Myrcene on the Levels of SOD and MDA in PCPA-Induced Insomnia Mice Sera
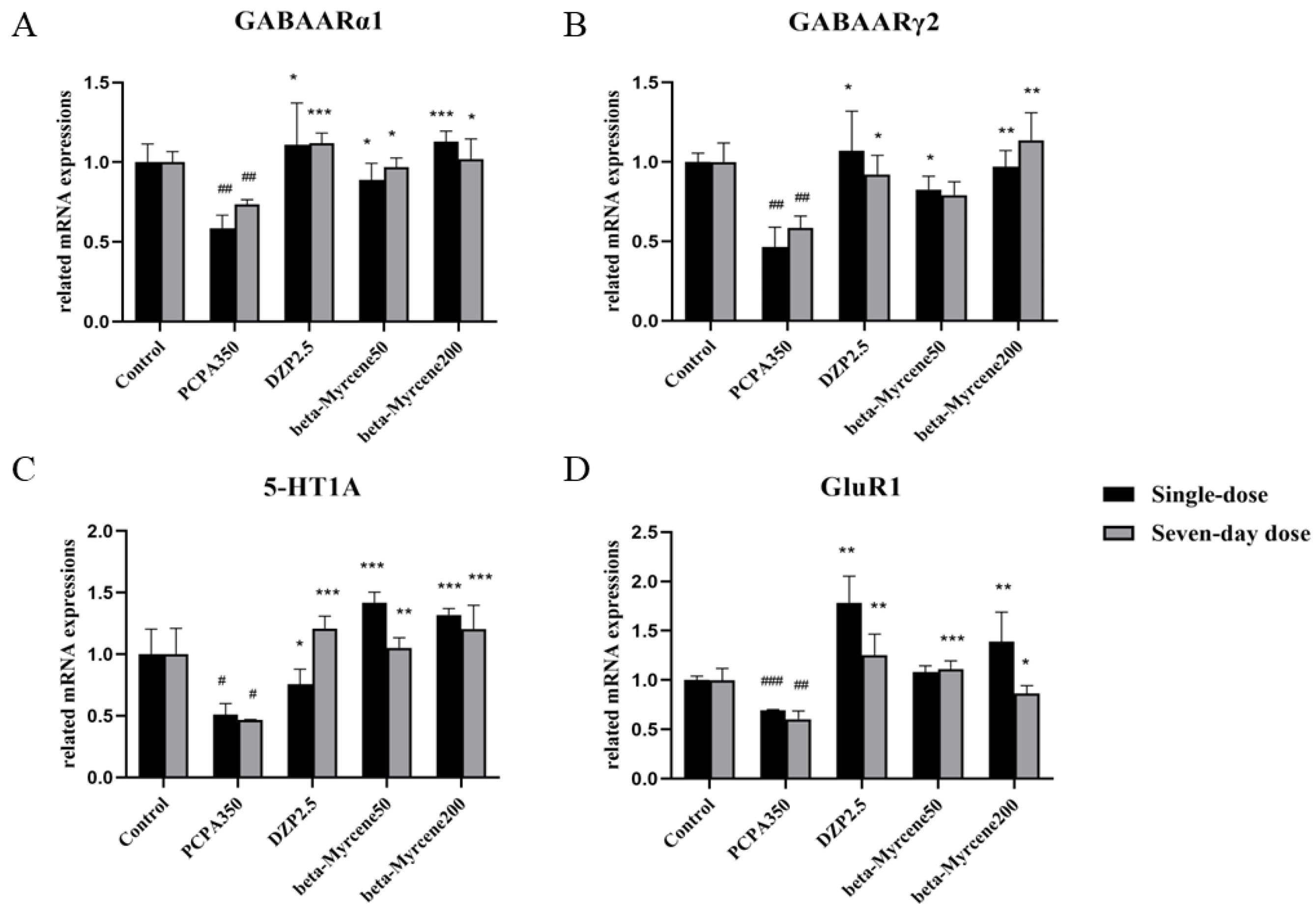
2.10. Effects of Beta-Myrcene on the 5-HT1AR, GABAARα1, GABAARγ2, and GluR1 mRNA Levels
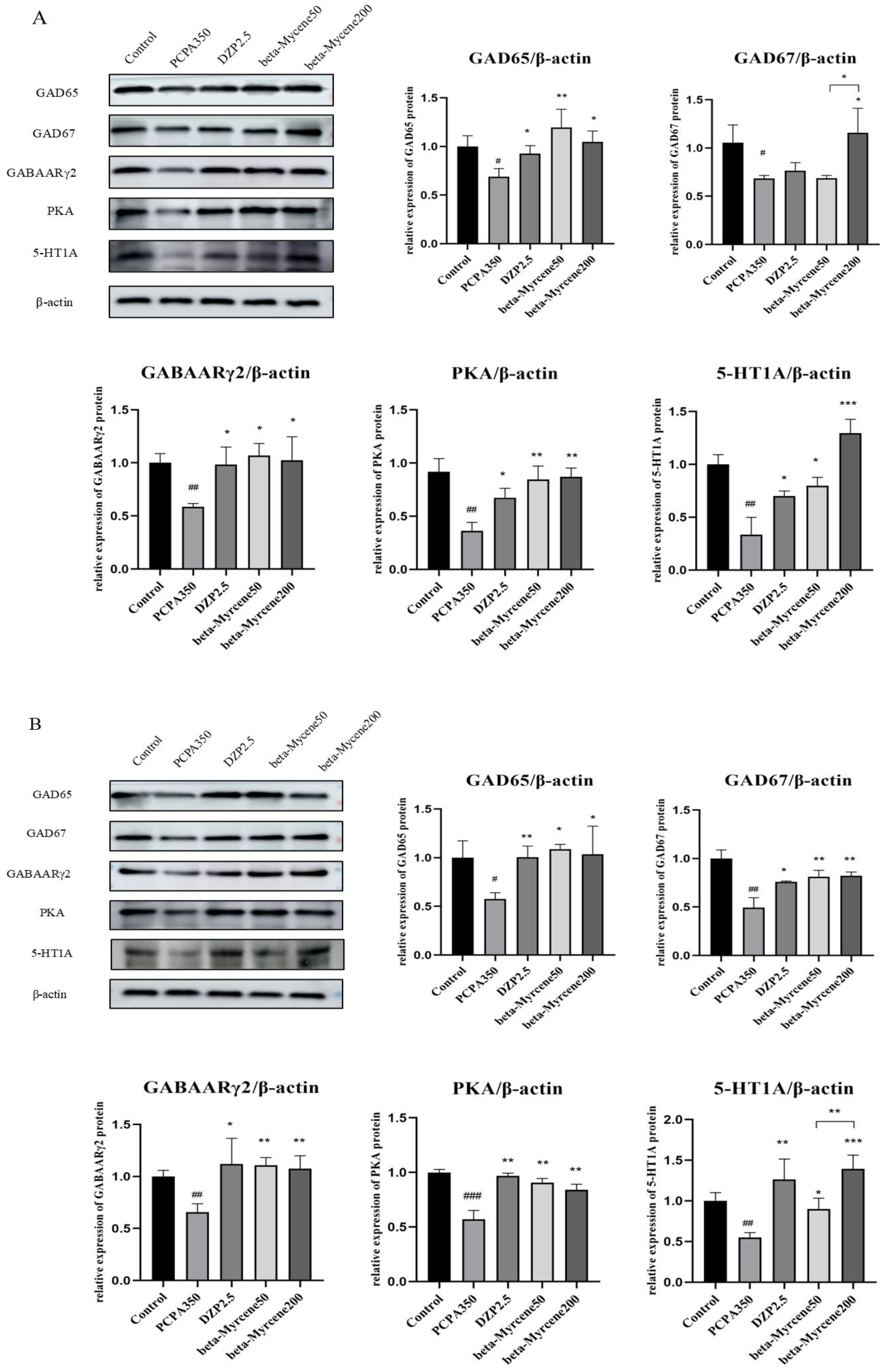
2.11. Effects of Beta-Myrcene on the GAD65, GAD67, GABAARγ2, PKA, and 5-HT1A Proteins Levels
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs and Drug Administration
4.3. Network Pharmacology Analysis Based on “Component-Target”
4.3.1. Acquisition of Active Ingredients of LEO Targets and Insomnia Disease Targets
4.3.2. GO Analysis and KEGG Pathway Enrichment Analysis
4.3.3. Construction of the “Active Ingredient-Potential Target-Pathway” Network of LEO
4.4. Experimental Verification
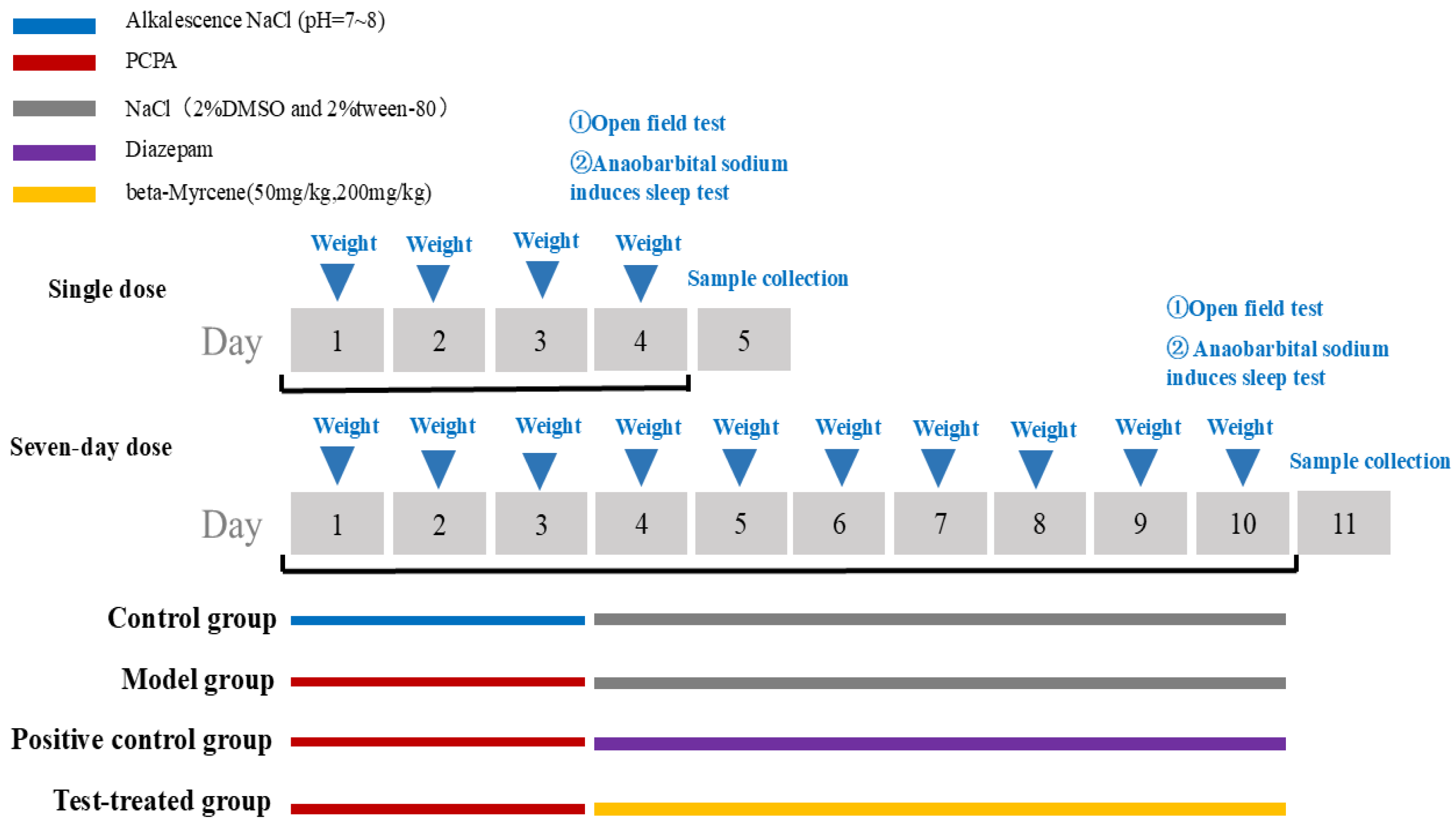
4.4.1. Animal Model Preparation and Grouping
4.4.2. Behavioral Observations in Mice
4.4.3. Anaobarbital Sodium-Induced Sleep Test in Mice
4.4.4. Sample Collection
4.4.5. Histopathological Examinations (HE)
4.4.6. Neurotransmitter Content Was Detected by ELISA
4.4.7. Antioxidant Enzyme Activity Measurements
4.4.8. Real-Time Polymerase Chain Reaction (Rt-PCR)
4.4.9. Western Blot Analysis
4.4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OMIM | Online Mendelian Inheritance in Man |
| TTD | Therapeutic Target Database |
| PCPA | DL-4-chlorophenylalanine |
| LEO | Lavender essential oil |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| 5-HT | 5-hydroxytryptamine |
| GABA | gamma-aminobutyric acid |
| PKA | protein kinase A |
| GLU | Glutamic acid |
| CC | cellular components |
| MF | molecular functions |
| BP | biological processes |
| HE | Histopathological examinations |
| ELISA | Enzyme-linked immunosorbent assay |
| SOD | Superoxide dismutase |
| MDA | Malondialdehyde |
| Rt-PCR | Real-time polymerase chain reaction |
| ECL | Electroche miluminescence |
| GAD67 | glutamate decarboxylase 67 |
| GAD65 | glutamate decarboxylase 65 |
| 5-HT1AR | 5-hydroxytryptamine receptor 1A |
| PVDF | polyvinylidene difluoride |
References
- Postuma, R.B.; Iranzo, A.; Hu, M.; Högl, B.; Boeve, B.F.; Manni, R.; Oertel, W.H.; Arnulf, I.; Ferini-Strambi, L.; Puligheddu, M.; et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: A multicentre study. Brain 2019, 142, 744–759. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chen, S.-J.; Ma, M.-Y.; Bao, Y.-P.; Han, Y.; Wang, Y.-M.; Shi, J.; Vitiello, M.V.; Lu, L. Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep Med. Rev. 2018, 40, 4–16. [Google Scholar] [CrossRef]
- Ye, M.; Lee, S.; Yu, H.J.; Kim, K.R.; Park, H.J.; Kang, I.C.; Kang, S.A.; Chung, Y.S.; Shim, I. Sedative-Hypnotic Effects of Glycine max Merr. Extract and Its Active Ingredient Genistein on Electric-Shock-Induced Sleep Disturbances in Rats. Int. J. Mol. Sci. 2023, 24, 7043. [Google Scholar] [CrossRef] [PubMed]
- Monroy, F. Ultrasound-Assisted Extraction of Lavender (Lavandula angustifolia Miller, Cultivar Rosa) Solid By-Products Remaining after the Distillation of the Essential Oil. Appl. Sci. 2021, 11, 5495. [Google Scholar] [CrossRef]
- Denner, S.S. Lavandula angustifolia miller: English lavender. Holist. Nurs. Pract. 2009, 23, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zheng, Q.; Hu, P.; Huang, X.; Liu, S. Sedative and hypnotic effects of compound Anshen essential oil inhalation for insomnia. BMC Complement. Altern. Med. 2019, 19, 306. [Google Scholar] [CrossRef]
- Yue, X.; Li, M.; Fei, L.; Lei, Y.; Wencui, W.; Suzhen, Y.; Suzhen, Y. Lavender essential oil fractions alleviate sleep disorders induced by the combination of anxiety and caffeine in mice. J. Ethnopharmacol. 2023, 302 Pt A, 115868. [Google Scholar] [CrossRef]
- Xiong, X.; Zhang, L.; Li, X.; Zeng, Q.; Kong, Q. Antifungal mechanisms of lavender essential oil in the inhibition of rot disease caused by Monilinia fructicola in postharvest flat peaches. Can. J. Microbiol. 2021, 67, 724–736. [Google Scholar] [CrossRef]
- Hayani, M.; Benabbouha, T.; Naceiri Mrabti, N.; Eljebri, S.; Sabiri, M.; Zair, T. Bioactive Profiling, Antibacterial Efficacy and Computational Modelling of Myrtus Communis Essential Oil (Morocco). Chem. Biodivers. 2024, 21, e202302114. [Google Scholar] [CrossRef]
- Li, G.; Wang, Z.; Ren, H.; Qi, X.; Han, H.; Ding, X.; Sun, L.; Hafeez, R.; Wang, Q.; Li, B. Ancient bayberry increased stress resistance by enriching tissue-specific microbiome and metabolites. Physiol. Plant 2024, 176, e14314. [Google Scholar] [CrossRef]
- Woo, J.; Yang, H.; Yoon, M.; Gadhe, C.G.; Pae, A.N.; Cho, S.; Lee, C.J. 3-Carene, a Phytoncide from Pine Tree Has a Sleep-enhancing Effect by Targeting the GABAA-benzodiazepine Receptors. Exp. Neurobiol. 2019, 28, 593–601. [Google Scholar] [CrossRef]
- Sadao, Y.; Teruyo, T.; Yoshie, I.; Kazuto, W.; Akikazu, H. Effects of Plant-derived Odors on Sleep–Wakefulness and Circadian Rhythmicity in Rats. Chem. Senses 2005, 30, i264–i265. [Google Scholar] [CrossRef]
- Yang, H.; Woo, J.; Pae, A.N.; Um, M.Y.; Cho, S. α-Pinene, a Major Constituent of Pine Tree Oils, Enhances Non-Rapid Eye Movement Sleep in Mice through GABAA-benzodiazepine Receptors. Mol. Pharmacol. 2016, 90, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Galdino, P.M.; Nascimento, M.V.M.; Florentino, I.F.; Lino, R.C.; Fajemiroye, J.O.; Chaibub, B.A.; Paula, J.R.d.; Lima, T.C.M.D.; Costa, E.A. The anxiolytic-like effect of an essential oil derived from Spiranthera odoratissima A. St. Hil. leaves and its major component, β-caryophyllene, in male mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 38, 276–284. [Google Scholar] [CrossRef]
- Lai, Y.; Hua, L.; Yang, J.; Xu, J.; Chen, J.; Zhang, S.; Zhu, S.; Li, J.; Shi, S. The Effect of Chinese Agarwood Essential Oil with Cyclodextrin Inclusion against PCPA-Induced Insomnia Rats. Molecules 2023, 28, 635. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, J.; Jia, Y.; Liang, Y.; Zhang, X.; Wang, C.L.; Wang, X.; Guo, D.; Shi, Y.; Yang, M. A Study on the Mechanism of Lavender in the Treatment of Insomnia Based on Network Pharmacology. Comb. Chem. High. Throughput Screen. 2020, 23, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.L.; Park, D.; Zhu, L.; Palmer, M.R.; Broadhurst, R.Y.; Arrigoni, E. Regulation of Lateral Hypothalamic Orexin Activity by Local GABAergic Neurons. J. Neurosci. 2018, 38, 1588–1599. [Google Scholar] [CrossRef]
- Ferreri, M.C.; Gutiérrez, M.L.; Gravielle, M.C. Tolerance to the sedative and anxiolytic effects of diazepam is associated with different alterations of GABAA receptors in rat cerebral cortex. Neuroscience 2015, 310, 152–162. [Google Scholar] [CrossRef]
- Yan, M.; Chang, Q.; Zhong, Y.; Xiao, B.; Feng, L.; Cao, F.; Pan, R.L.; Zhang, Z.; Liao, Y.H.; Liu, X. Lotus Leaf Alkaloid Extract Displays Sedative-Hypnotic and Anxiolytic Effects through GABAA Receptor. J. Agric. Food Chem. 2015, 63, 9277–9285. [Google Scholar] [CrossRef]
- Hassan, S.H.; El-Nashar, H.A.S.; Rahman, M.A.; Polash, J.I.; Bappi, M.H.; Mondal, M.; Abdel-Maksoud, M.A.; Malik, A.; Aufy, M.; El-Shazly, M.; et al. Sclareol antagonizes the sedative effect of diazepam in thiopental sodium-induced sleeping animals: In vivo and in silico studies. Biomed. Pharmacother. 2024, 176, 116939. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Peng, D.; Liu, X.; Wu, C.; Guo, P.; Wei, J. Agarwood Essential Oil Displays Sedative-Hypnotic Effects through the GABAergic System. Molecules 2017, 22, 2190. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, X.; Du, J.; Yang, W.; Fu, R.; Li, Y.; Zhao, W.; Wang, H. Propofol mitigates brain injury and oxidative stress, and enhances GABAA receptor α1 subunit expression in a rat model of lithium chloride-pilocarpine induced status epilepticus. Turk. J. Med. Sci. 2023, 53, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Lv, J.; Duan, C.; An, X.; Zhang, C.; Li, D.; Li, C.; Liu, S. Armillaria mellea fermentation liquor ameliorates p-chlorophenylalanine-induced insomnia associated with the modulation of serotonergic system and gut microbiota in rats. J. Food Biochem. 2022, 46, e14075. [Google Scholar] [CrossRef]
- Sheng, J.A.; Bales, N.J.; Myers, S.A.; Bautista, A.I.; Handa, R.J. The Hypothalamic-Pituitary-Adrenal Axis: Development, Programming Actions of Hormones, and Maternal-Fetal Interactions. Front. Behav. Neurosci. 2021, 14, 601939. [Google Scholar] [CrossRef]
- Bao, Y.; Zhou, H.; Fu, Y.; Wang, C.; Huang, Q. Zhumian Granules improves PCPA-induced insomnia by regulating the expression level of neurotransmitters and reducing neuronal apoptosis. J. Ethnopharmacol. 2024, 327, 118048. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Wei, W.; Chen, X.; Xie, X.; Guo, T.; Sasaki, Y.; Zhang, Y.; Wang, L.; Zhang, F.; Feng, S. A comprehensive study on the relieving effect of Lilium brownii on the intestinal flora and metabolic disorder in p-chlorphenylalanine induced insomnia rats. Pharm. Biol. 2022, 60, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yu, H.; Si, L.; Meng, H.; Chen, W.; Wang, Z.; Gula, A. Influence of warm acupuncture on gut microbiota and metabolites in rats with insomnia induced by PCPA. PLoS ONE 2022, 17, e0267843. [Google Scholar] [CrossRef]
- Li, Y.; Deng, Y.; Zhang, Y.; Xu, D.; Zhang, X.; Li, Y.; Li, Y.; Chen, M.; Wang, Y.; Zhang, J.; et al. Distinct glutamatergic projections of the posteroventral medial amygdala play different roles in arousal and anxiety. JCI Insight 2024, 9, e176329. [Google Scholar] [CrossRef]
- Zhang, X.H.; Zhang, X.; Liu, X.W.; Lai, P.H.; Cao, C.C.; Wang, Y.L.; Ren, L.J. Examining the Role of GLU/GABA to GLN Metabolic Cycle in the Pathogenesis of Post-Stroke Depressive Disorder and Insomnia. Neuropsychiatr. Dis. Treat. 2023, 19, 2833–2840. [Google Scholar] [CrossRef]
- JM, M. Serotonin control of sleep-wake behavior. Sleep Med. Rev. 2011, 15, 269–281. [Google Scholar] [CrossRef]
- Bernabe, C.S.; Caliman, I.F.; de Abreu, A.R.R.; Molosh, A.I.; Truitt, W.A.; Shekhar, A.; Johnson, P.L. Identification of a novel perifornical-hypothalamic-area-projecting serotonergic system that inhibits innate panic and conditioned fear responses. Transl. Psychiatry 2024, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Kushikata, T.; Fang, J.; Krueger, J.M. Interleukin-10 inhibits spontaneous sleep in rabbits. J. Interferon Cytokine Res. 1999, 19, 1025–1030. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, X.; Peng, Q.; Tang, L. Electroacupuncture of the cymba concha alleviates p-chlorophenylalanine-induced insomnia in mice. Acupunct. Med. 2023, 41, 345–353. [Google Scholar] [CrossRef]
- Krueger, J.M. The role of cytokines in sleep regulation. Curr. Pharm. Des. 2008, 14, 3408–3416. [Google Scholar] [CrossRef]
- Uusi-Oukari, M.; Korpi, E.R. Regulation of GABA(A) receptor subunit expression by pharmacological agents. Pharmacol. Rev. 2010, 62, 97–135. [Google Scholar] [CrossRef]
- Sheethal, S.; Ratheesh, M.; Jose, S.P.; Sandya, S.; Samuel, S.; Madhavan, J. Anti-insomnia Effect of a Polyherbal Formulation on P-chlorophenyalanine Induced Experimental Animal Model. Neurochem. Res. 2024, 49, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Martella, N.; Colardo, M.; Sergio, W.; Petraroia, M.; Varone, M.; Pensabene, D.; Russo, M.; Di Bartolomeo, S.; Ranalli, G.; Saviano, G.; et al. Lavender Essential Oil Modulates Hepatic Cholesterol Metabolism in HepG2 Cells. Curr. Issues Mol. Biol. 2023, 45, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017, 45, W356–W360. [Google Scholar] [CrossRef]
- Rebhan, M.; Chalifa-Caspi, V.; Prilusky, J.; Lancet, D. GeneCards: A novel functional genomics compendium with automated data mining and query reformulation support. Bioinformatics 1998, 14, 656–664. [Google Scholar] [CrossRef]
- Ada, H.; Scott, A.F.; Joanna, A.; Carol, B.; David, V.; Mckusick, V.A. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005, 33, 514–517. [Google Scholar] [CrossRef]
- Chen, X.; Ji, Z.L.; Chen, Y.Z. TTD: Therapeutic Target Database. Nucleic Acids Res. 2002, 30, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2008, 44, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Li, J.C.; Gu, Y.F.; Qiu, R.H.; Huang, J.Y.; Xue, R.; Li, S.; Zhang, Y.; Zhang, K.; Zhang, Y.Z. Cannabidiol Exerts Sedative and Hypnotic Effects in Normal and Insomnia Model Mice Through Activation of 5-HT1A Receptor. Neurochem. Res. 2024, 49, 1150–1165. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zheng, Q.; Hu, P.; Huang, X.; Zhang, M. Sedative and hypnotic effects of Perilla frutescens essential oil through GABAergic system pathway. J. Ethnopharmacol. 2020, 279, 113627. [Google Scholar] [CrossRef]
- Sarangi, L.N.; Reddy, R.V.C.; Rana, S.K.; Naveena, T.; Ponnanna, N.M.; Sharma, G.K. Sero-diagnostic efficacy of various ELISA kits for diagnosis of infectious bovine rhinotracheitis (IBR) in cattle and buffaloes in India. Vet. Immunol. Immunopathol. 2021, 241, 110324. [Google Scholar] [CrossRef]


| Single Dose | Seven-Day Dose | |||||
|---|---|---|---|---|---|---|
| Group | Dose (mg/kg) | Total Number | Sleeping Mice | Sleeping Rate (%) | Sleeping Mice | Sleeping Rate (%) |
| Control | - | 12 | 3 | 25 | 3 | 25 |
| PCPA | 350 | 12 | 1 | 8.33 | 1 | 8.33 |
| Diazepam | 2.5 | 12 | 8 | 66.67 | 5 | 41.67 |
| beta-Myrcene | 50 | 12 | 6 | 50 | 6 | 50 |
| beta-Myrcene | 200 | 12 | 12 | 100 | 11 | 91.67 |
| Active Ingredients | CAS | Component Type |
|---|---|---|
| Tricyclene | 508-32-7 | Monoterpene |
| (-)-alpha-Pinene | 7785-26-4 | Monoterpene |
| beta-Pinene | 127-91-3 | Monoterpene |
| Camphene | 79-92-5 | Monoterpene |
| alpha-Phellandrene | 99-83-2 | Monoterpene |
| beta-Myrcene | 123-35-3 | Monoterpene |
| 3-Carene | 13466-78-9 | Monoterpene |
| D-Limonene | 5989-27-5 | Monoterpene |
| alpha-Terpinene | 99-86-5 | Monoterpene |
| gamma-Terpinene | 99-85-4 | Monoterpene |
| Terpinolene | 586-62-9 | Monoterpene |
| (Z)-beta-Ocimene | 3338-55-4 | Monoterpene |
| (E)-beta-Ocimene | 3779-61-1 | Monoterpene |
| Humulene | 6753-98-6 | Sesquiterpene |
| beta-Caryophyllene | 87-44-5 | Sesquiterpene |
| Mouse | Gene | Primers | Size (bps) |
| GABAARγ2 | F 5′ AGAATATGGCTATGAGTGTTTGGATGG 3′ | 27 | |
| R 5′ GGCTCCTGTTCGGCAATCTTC 3′ | 21 | ||
| GABAARα1 | F 5′ CCGTTCAGTGGTTGTAGCAGAAG 3′ | 23 | |
| R 5′ TTCAAGTGGAAGTGAGTCGTCATAAC 3′ | 26 | ||
| 5-HT1A | F 5′ TTCTATATTCCGCTGCTGCTCATG 3′ | 24 | |
| R 5′ CCACCTTCTTGACCGTCTTGC 3′ | 21 | ||
| GLUR1 | F 5′ ACAACTCAAGCGTCCAGAATAGAAC 3′ | 25 | |
| R 5′ CCTCATAGCGGTCATTGCCTTC 3′ | 22 | ||
| β-actin | F 5′ GAGGGAAATCGTGCGTGAC 3′ | 19 | |
| R 5′ GCTGGAAGGTGGACAGTGAG 3′ | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Liu, Y.; Xu, D.; Zhang, N.; Chen, Y.; Yang, J.; Sun, L. Beta-Myrcene as a Sedative–Hypnotic Component from Lavender Essential Oil in DL-4-Chlorophenylalanine-Induced-Insomnia Mice. Pharmaceuticals 2024, 17, 1161. https://doi.org/10.3390/ph17091161
Chen L, Liu Y, Xu D, Zhang N, Chen Y, Yang J, Sun L. Beta-Myrcene as a Sedative–Hypnotic Component from Lavender Essential Oil in DL-4-Chlorophenylalanine-Induced-Insomnia Mice. Pharmaceuticals. 2024; 17(9):1161. https://doi.org/10.3390/ph17091161
Chicago/Turabian StyleChen, Luge, Yingwei Liu, Dawei Xu, Na Zhang, Yong Chen, Jin Yang, and Lijuan Sun. 2024. "Beta-Myrcene as a Sedative–Hypnotic Component from Lavender Essential Oil in DL-4-Chlorophenylalanine-Induced-Insomnia Mice" Pharmaceuticals 17, no. 9: 1161. https://doi.org/10.3390/ph17091161
APA StyleChen, L., Liu, Y., Xu, D., Zhang, N., Chen, Y., Yang, J., & Sun, L. (2024). Beta-Myrcene as a Sedative–Hypnotic Component from Lavender Essential Oil in DL-4-Chlorophenylalanine-Induced-Insomnia Mice. Pharmaceuticals, 17(9), 1161. https://doi.org/10.3390/ph17091161





