Chia Seed (Salvia hispanica) Attenuates Chemically Induced Lung Carcinomas in Rats through Suppression of Proliferation and Angiogenesis
Abstract
1. Introduction
2. Results
2.1. In Vitro Study
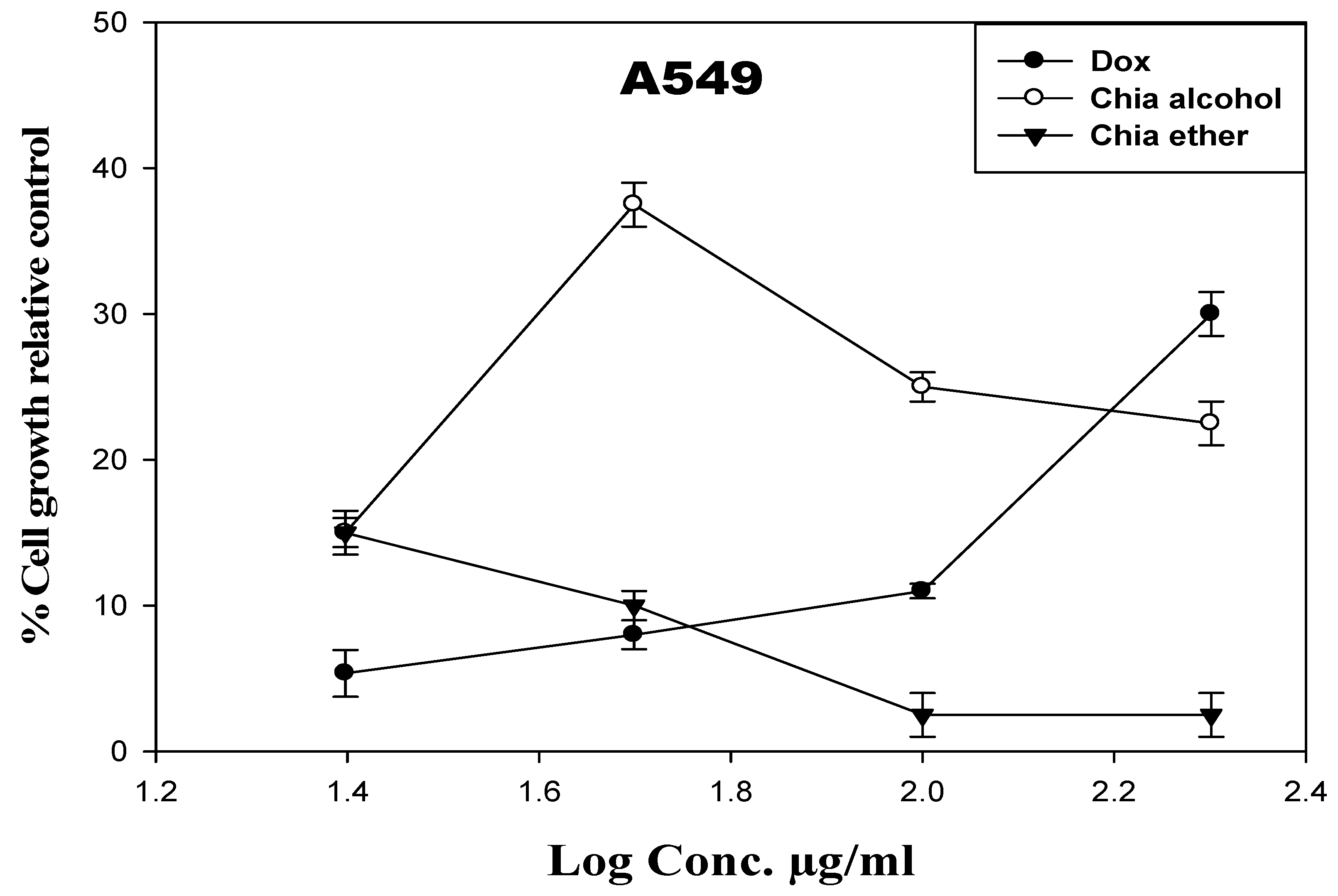
Cytotoxicity Activity of the Chia Extracts against Lung Cancer Cell Line (A549 Cells)
2.2. In Vivo Study on NNK-Induced Lung Cancer in Rat Model
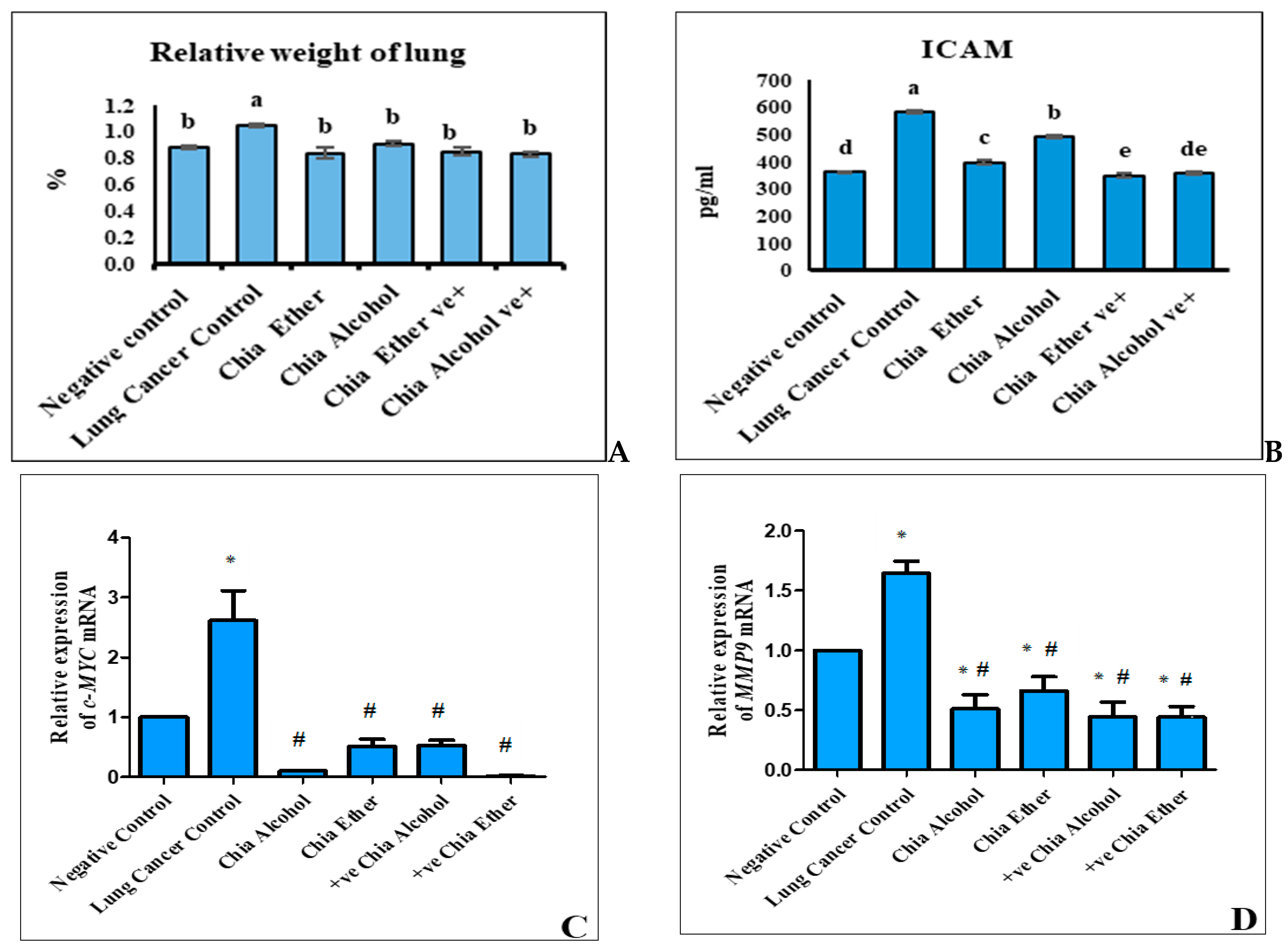
2.2.1. The Effect of Chia Extract on Lung Cancer Biomarkers
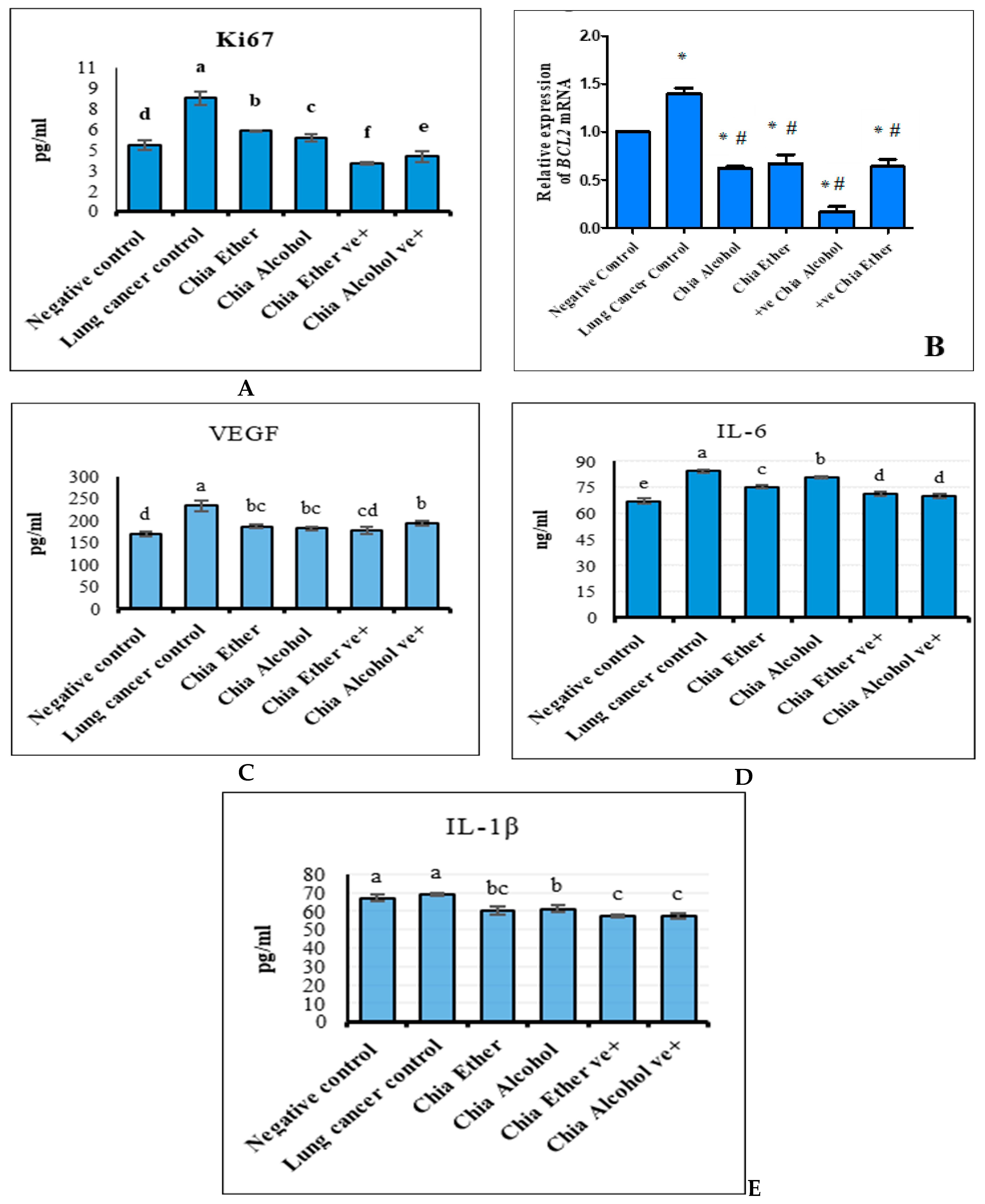
2.2.2. The Effect of Chia Extract on Proliferation
2.2.3. The Effect of Chia Extract on Apoptosis
2.2.4. The Effect of Chia Extract on Angiogenesis
2.2.5. The Effect of Chia Extract on Inflammation
2.2.6. The Effect of Chia Extract on Antioxidants
2.2.7. The Effect of Chia Extract on the Safety Profile of Treated Rats
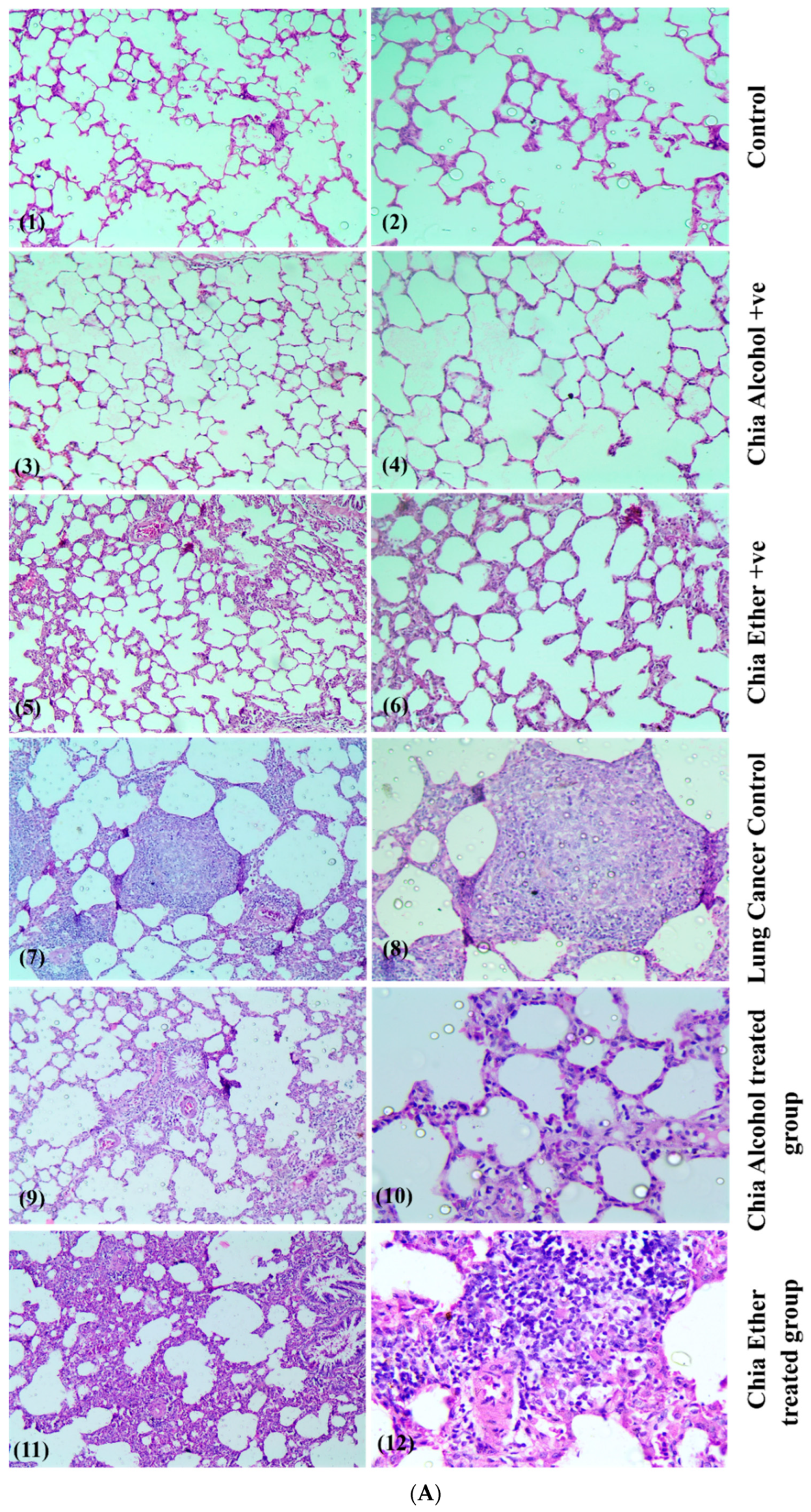
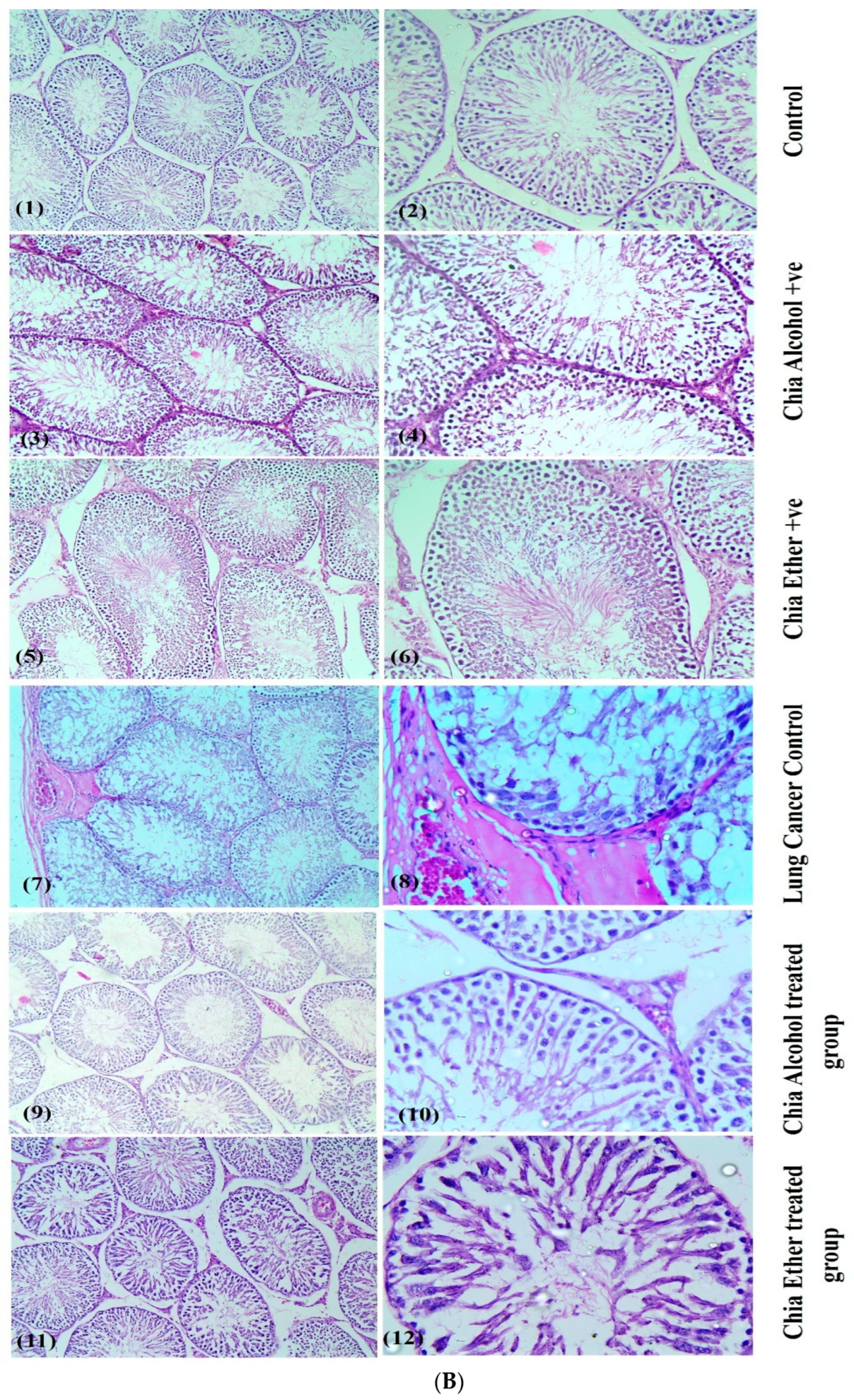
2.2.8. Histopathological Study
2.3. Chemical Profiling of Significant Anticancer Lung Polyphenolics from Ethanol Extract of Egyptian Salvia hispanica Seeds
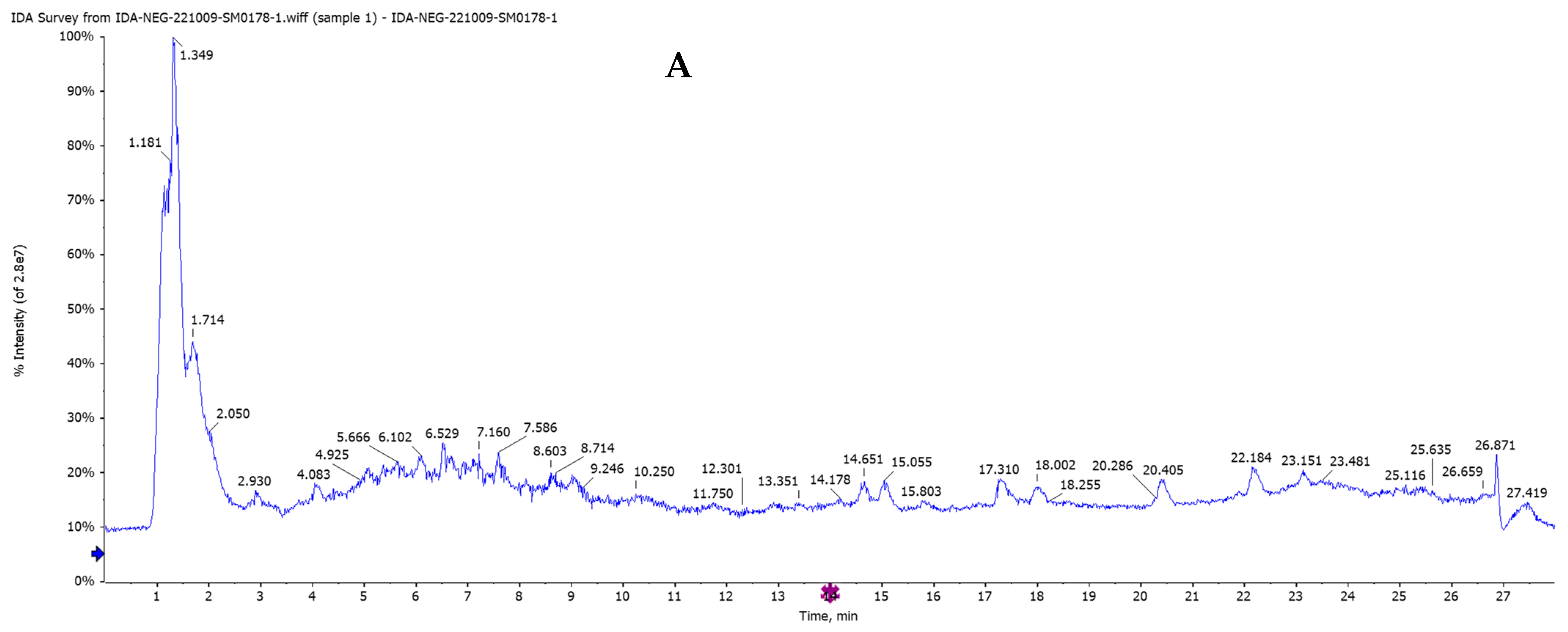
2.4. Characterization of Highly Concentrated Metabolites in Egyptian Chia Salvia hispanica Seeds by HPLC/QTOF-MS/MS Analysis
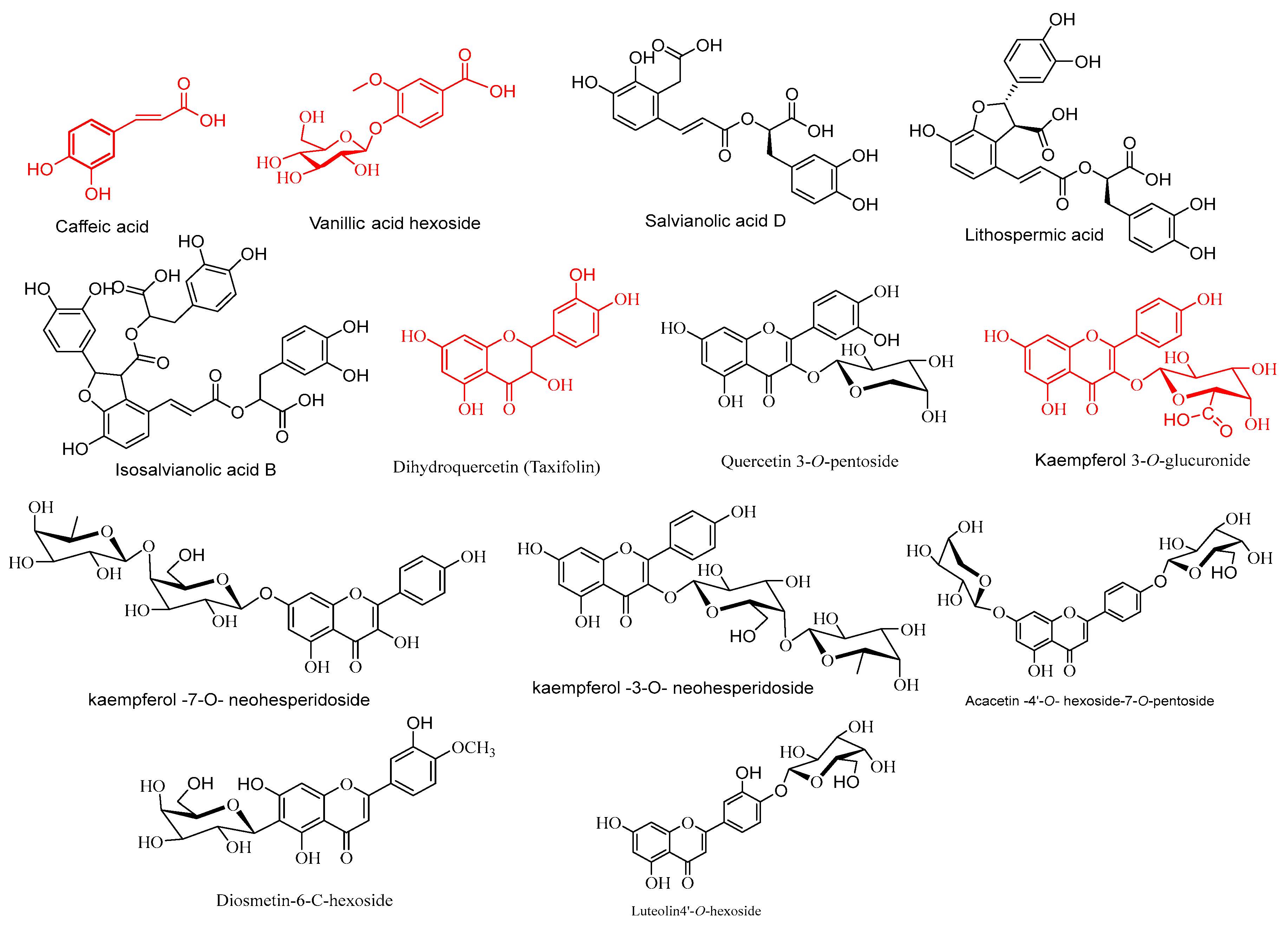
2.4.1. Phenolic Acids
2.4.2. Flavonoids Glycosides
2.4.3. Flavonol Derivatives
2.4.4. Flavone Derivatives
2.4.5. Flavanones
2.4.6. Flavans-3ol
2.4.7. Ellagic Tannins
2.4.8. Anthocyanins
2.4.9. Coumarin
2.4.10. Lignans and Stilbenes
2.4.11. Alkaloids
2.4.12. Diterpenoids
2.5. Investigation of the Chemical Profile of the Petroleum Ether Extract of Salvia hispanica Seeds by GC–MS
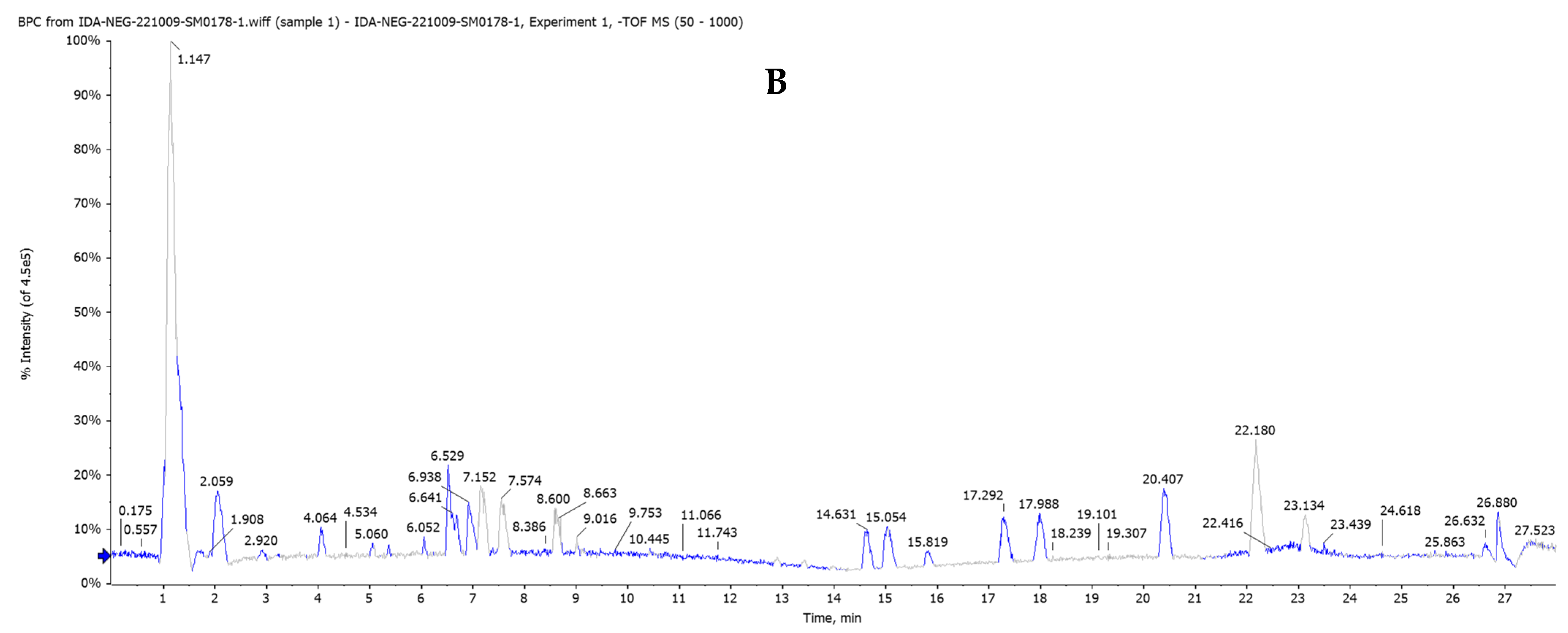
2.6. Investigation of the Chemical Profile of the Petroleum Ether Extract of Salvia hispanica Seeds by HPLC/QTOF-MS/MS in Both Negative (−ve) and Positive (+ve) Ionization Modes
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Authentication of the Plant and Extraction
4.3. Studying the Anti-Lung Cancer of Chia Seed Extracts
4.3.1. In Vitro Cytotoxicity Assay
4.3.2. In Vivo Study
Determination of LD50
Lung Cancer Induction
Experimental Layout
Biochemical Assessments
Gene Expression Assessment
Histopathological Assessments
4.4. Qualitative Analysis of Phytoconstituents in Salvia hispanica (Chia) Seeds Petroleum Ether by GC–MS
4.4.1. Sample Preparation for Lipid Composition GC Analysis
Fatty Acid Methyl Esters (FAME)
Unsaponifiable Matter
4.4.2. Gas Chromatography
4.4.3. Identification of Chemical Composition of Oil Using Gas Chromatography–Mass Spectrometry Analysis (GC–MS)
4.5. Qualitative Analysis of Phytoconstituents in Salvia hispanica (Chia) Seeds by HPLC/HR-QTOF-MS/MS
4.5.1. Sample Preparation
4.5.2. Instruments and Acquisition Method
4.5.3. LC-MS Data Processing
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horn, L.; Iams, W.T. Neoplasms of the Lung. In Harrison’s Principles of Internal Medicine, 21st ed.; Loscalzo, J., Fauci, A., Kasper, D., Jameson, J.L., Hauser, S.L., Longo, D.L., Eds.; McGraw Hill: New York, NY, USA, 2022; ISBN 978-1264268504. [Google Scholar]
- Rivera, P.; Mody, G.N.; Weiner, A.A. Lung Cancer: Treatment. In Murray & Nadel’s Textbook of Respiratory Medicine, 7th ed.; Broaddus, C., Ernst, J.D., King, T.E., Lazarus, S.C., Nadel, J.A., Gotway, M.B., Mason, R.J., Murray, J.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1052–1065. [Google Scholar]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- El-Moselhy, E.A.; Elrifai, A.W. Risk factors of lung cancer worldwide and in Egypt: Current Situation. J. Oncopathol. Clin. Res. 2018, 2, 5. [Google Scholar]
- Akopyan, G.; Bonavida, B. Understanding tobacco smoke carcinogen NNK and lung tumorigenesis. Int. J. Oncol. 2006, 29, 745–752. [Google Scholar] [CrossRef]
- El-Newary, S.A.; Afifi, S.M.; Aly, M.S.; Ahmed, R.F.; El Gendy, A.G.; Abd-ElGawad, A.M.; Farag, M.A.; Elgamal, A.M.; Elshamy, A.I. Chemical profile of Launaea nudicaulis ethanolic extract and its antidiabetic effect in streptozotocin-induced rats. Molecules 2021, 26, 1000. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.Y.; El-Newary, S.A.; Youness, E.R.; Ibrahim, A.M.M.; El Kashak, W.A. Protective and therapeutic effect of Vitex agnus-castusagainst prostate cancer in rat. J. Appl. Pharm. Sci. 2017, 7, 133–143. [Google Scholar] [CrossRef][Green Version]
- El-Newary, S.A.; Youness, E.R.; Ibrahim, A.Y. Vitex berries attenuates chemically-induced mammary carcinomas in rats through modulation of the cancer growth rate-limiting enzymes activities: Aromatase and Na+/K+ ATPase. Egypt. J. Chem. 2024, 67, 235–255. [Google Scholar] [CrossRef]
- Attia, N.A.; Sayed, A.H.; Mahmoud, N.S.; Ahmed, H.H. Phytochemical remedies: A key strategy towards reversing the aggressive murine colon cancer. Med. Chem. Res. 2017, 26, 2614–2623. [Google Scholar] [CrossRef]
- Abd-Rabou, A.A.; Abdalla, A.M.; Ali, N.A.; Zoheir, K.M.A. Moringa oleifera root induces cancer apoptosis more effectively than leave nanocomposites and its free counterpart. Asian Pac. J. Cancer Prev. 2017, 18, 2141–2149. [Google Scholar] [CrossRef]
- Munoz, L.A.; Cobos, A.; Diaz, O.; Aguilera, J.M. Chia seed (Salvia hispanica): An ancient grain and new functional food. Food Res. Int. 2013, 29, 394–408. [Google Scholar] [CrossRef]
- Kulczyński, B.; Kobus-Cisowska, J.; Taczanowski, M.; Kmiecik, D.; Gramza-Michałowska, A. The chemical composition and nutritional value of chia seeds—Current state of knowledge. Nutrients 2019, 11, 1242. [Google Scholar] [CrossRef] [PubMed]
- Marineli, R.; Moura, C.S.; Moraes, É.A.; Lenquiste, S.A.; Lollo, P.C.; Morato, P.N.; Amaya-Farfan, J.; Maróstica, M.R., Jr. Chia (Salvia hispanica L.) enhances HSP, PGC-1α expressions and improves glucose tolerance in diet-induced obese rats. Nutrition 2015, 31, 740–748. [Google Scholar] [CrossRef]
- Fonte-Faria, T.; Citelli, M.; Atella, G.C.; Raposo, H.F.; Zago, L.; de Souza, T.; da Silva, S.V.; Barja-Fidalgo, C. Chia oil supplementation changes body composition and activates insulin signaling cascade in skeletal muscle tissue of obese animals. Nutrition 2019, 58, 167–174. [Google Scholar] [CrossRef]
- Fernandez, I.; Vidueiros, S.M.; Ayerza, R.; Coates, W.; Pallaro, A. Impact of chia (Salvia hispanica L.) on the immune system: Preliminary study. Proc. Nutr. Soc. 2008, 67, E12. [Google Scholar] [CrossRef]
- Liu, A.H.; Lin, Y.H.; Yang, M.; Guo, H.; Guan, S.H.; Sun, J.H.; Guo, D.A. Development of the fingerprints for the quality of the roots of Salvia miltiorrhiza and its related preparations by HPLC-DAD and LC–MSn. J. Chromatogr. B 2007, 846, 32–41. [Google Scholar] [CrossRef]
- Abd Elkarim, A.S.; Ahmed, A.H.; Taie, H.A.A.; Elgamal, A.M.; Abu-elghait, M.; Shabana, S. Synadeniumgrantii hook f.: HPLC/QTOF-MS/MS tentative identification of the phytoconstituents, antioxidant, antimicrobial and antibiofilm evaluation of the aerial parts. Rasayan. J. Chem. 2021, 14, 811–828. [Google Scholar] [CrossRef]
- El-Newary, S.A.; Abd Elkarim, A.S.; Abdelwahed, N.A.; Omer, E.A.; Elgamal, A.M.; Elsayed, W.M. Chenopodium murale Juice shows anti-Fungal efficacy in experimental oral candidiasis in immunosuppressed rats in relation to its chemical profile. Molecules 2023, 28, 4304. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhong, B.; Subbiah, V.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. LC-ESI-QTOF-MS/MS profiling and antioxidant activity of phenolics from custard apple fruit and by-products. Separations 2021, 8, 62. [Google Scholar] [CrossRef]
- Ali, A.; Bashmil, Y.M.; Cottrell, J.J.; Suleria, H.A.; Dunshea, F.R. LC-MS/MS-QTOF screening and identification of phenolic compounds from Australian grown herbs and their antioxidant potential. Antioxidants 2021, 10, 1770. [Google Scholar] [CrossRef]
- Nagy, A.M.; Abdelhameed, M.F.; Abd Elkarim, A.S.; Sarker, T.C.; Abd-ElGawad, A.M.; Elshamy, A.I.; Hammam, A.M. Enhancement of Female Rat Fertility via Ethanolic Extract from Nigella sativa L. (Black Cumin) Seeds Assessed via HPLC-ESI-MS/MS and Molecular Docking. Molecules 2024, 29, 735. [Google Scholar] [CrossRef]
- Oliveira-Alves, S.C.; Vendramini-Costa, D.B.; Cazarin, C.B.B.; Júnior, M.R.M.; Ferreira, J.P.B.; Silva, A.B.; Prado, M.A.; Bronze, M.R. Characterization of phenolic compounds in chia (Salvia hispanica L.) seeds, fiber flour and oil. Food Chem. 2017, 232, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, Y.P.; Wu, Y.C.; Hua, W.P.; Chen, C.; Ge, Q.; Wang, Z.Z. Pathway engineering for phenolic acid accumulations in Salvia miltiorrhiza by combinational genetic manipulation. Metab. Eng. 2014, 21, 71–80. [Google Scholar] [CrossRef]
- Nuengchamnong, N.; Krittasilp, K.; Ingkaninan, K. Characterisation of phenolic antioxidants in aqueous extract of Orthosiphon grandifloras tea by LC-ESI-MS/MS coupled to DPPH assay. Food Chem. 2011, 127, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, Z.; Li, M.; Yuan, Y.; Cui, S.; Chen, J.; Li, R. An integrated strategy for profiling the chemical components of Scutellariae Radix and their exogenous substances in rats by ultra-high-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2020, 34, e8823. [Google Scholar] [CrossRef] [PubMed]
- Abd Elkarim, A.S.; Taie, H.A. Characterization of Flavonoids from Combretum indicum L. Growing in Egypt as Antioxidant and Antitumor Agents. Egypt. J. Chem. 2023, 66, 1519–1543. [Google Scholar]
- Goufo, P.; Singh, R.K.; Cortez, I. A reference list of phenolic compounds (including stilbenes) in grapevine (Vitis vinifera L.) roots, woods, canes, stems, and leaves. Antioxidants 2020, 9, 398. [Google Scholar] [CrossRef]
- Li, Z.H.; Guo, H.; Xu, W.B.; Ge, J.; Li, X.; Alimu, M.; He, D.J. Rapid identification of flavonoid constituents directly from PTP1B inhibitive extract of raspberry (Rubus idaeus L.) leaves by HPLC–ESI–QTOF–MS-MS. J. Chromatogr. Sci. 2016, 54, 805–810. [Google Scholar] [CrossRef]
- Al-Jaber, H.I.; Shakya, A.K.; Elagbar, Z.A. HPLC profiling of selected phenolic acids and flavonoids in Salvia eigii, Salvia hierosolymitana and Salvia viridis growing wild in Jordan and their in vitro antioxidant activity. PeerJ 2020, 8, e9769. [Google Scholar] [CrossRef] [PubMed]
- Waridel, P.; Wolfender, J.L.; Ndjoko, K.; Hobby, K.R.; Major, H.J.; Hostettmann, K. Evaluation of quadrupole time-of-flight tandem mass spectrometry and ion-trap multiple-stage mass spectrometry for the differentiation of C-glycosidic flavonoid isomers. J. Chromatogr. A 2001, 926, 29–41. [Google Scholar] [CrossRef]
- Jaiswal, R.; Jayasinghe, L.; Kuhnert, N. Identification and characterization of proanthocyanidins of 16 members of the Rhododendron genus (Ericaceae) by tandem LC–MS. J. Mass Spectrom. 2012, 47, 502–515. [Google Scholar] [CrossRef]
- Tourino, S.; Fuguet, E.; Jáuregui, O.; Saura-Calixto, F.; Cascante, M.; Torres, J.L. High-resolution liquid chromatography/electrospray ionization time-of-flight mass spectrometry combined with liquid chromatography/electrospray ionization tandem mass spectrometry to identify polyphenols from grape antioxidant dietary fiber. Rapid Commun. Mass Spectrom. 2008, 22, 3489–3500. [Google Scholar] [CrossRef] [PubMed]
- Flamini, R. Recent applications of mass spectrometry in the study of grape and wine polyphenols. Int. Sch. Res. Not. 2013, 2013, 813563. [Google Scholar] [CrossRef]
- Ren, Z.; Nie, B.; Liu, T.; Yuan, F.; Feng, F.; Zhang, Y.; Zhou, W.; Xu, X.; Yao, M.; Zhang, F. Simultaneous determination of coumarin and its derivatives in tobacco products by liquid chromatography-tandem mass spectrometry. Molecules 2016, 21, 1511. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.A.; Chen, H.; Chen, X.L.; Zhang, X.M.; Lei, L.G.; Chen, J.J. Rapid characterization of chemical constituents in Saniculiphyllum guangxiense by ultra fast liquid chromatography with diode array detection and electrospray ionization tandem mass spectrometry. Int. J. Mass Spectrom. 2014, 361, 9–22. [Google Scholar] [CrossRef]
- Jiao, Q.S.; Xu, L.L.; Zhang, J.Y.; Wang, Z.J.; Jiang, Y.Y.; Liu, B. Rapid characterization and identification of non-diterpenoid constituents in Tinospora sinensis by HPLC-ltq-orbitrap msn. Molecules 2018, 23, 274. [Google Scholar] [CrossRef]
- Gong, L.; Haiyu, X.; Wang, L.; Xiaojie, Y.; Huijun, Y.; Songsong, W.; Cheng, L.; Ma, X.; Gao, S.; Liang, R. Identification and evaluation of the chemical similarity of yindanxinnaotong samples by ultra high performance liquid chromatography with quadrupole time-of-flight mass spectrometry fingerprinting. J. Sep. Sci. 2016, 39, 611–622. [Google Scholar] [CrossRef]
- Ozarowski, M.; Piasecka, A.; Gryszczy’nska, A.; Sawikowska, A.; Pietrowiak, A.; Opala, B.; Mikołajczak, P.Ł.; Kujawski, R.; Kachlicki, P.; Buchwald, W. Determination of phenolic compounds and diterpenes in roots of Salvia miltiorrhiza and Salvia przewalskiiby two LC-MS tools: Multi-stage and high resolution tandem mass spectrometry with assessment of antioxidant capacity. Phytochem. Lett. 2017, 20, 331–338. [Google Scholar] [CrossRef]
- Martin-Arjol, I.; Bassas-Galia, M.; Bermudo, E.; Garcia, F.; Manresa, A. Identification of oxylipins with antifungal activity by LC–MS/MS from the supernatant of Pseudomonas 42A2. Chem. Phys. Lipids 2010, 163, 341–346. [Google Scholar] [CrossRef]
- Güzel, S.; Ülger, M.; Özay, Y. Antimicrobial and antiproliferative activities of Chia (Salvia hispanica L.) seeds. Int. J. Second. Metab. 2020, 7, 174–180. [Google Scholar] [CrossRef]
- El Makawy, A.I.; Abdel-Aziem, S.H.; Mohammed, S.E.; Ibrahim, F.M.; Abd El-Kader, H.A.; Sharaf, H.A.; Youssef, D.A.; Mabrouk, D.M. Exploration of tumor growth regression of quinoa and chia oil nanocapsules via the control of PIK3CA and MYC expression, anti-inflammation and cell proliferation inhibition, and their hepatorenal safety in rat breast cancer model. Bull. Natl. Res. Cent. 2024, 48, 7. [Google Scholar] [CrossRef]
- El Makawy, A.I.; Mabrouk, D.M.; Mohammed, S.E.; Abdel-Aziem, S.H.; Abd El-Kader, H.A.; Sharaf, H.A.; Youssef, D.A.; Ibrahim, F.M. The suppressive role of nanoencapsulated chia oil against DMBA-induced breast cancer through oxidative stress repression and tumor genes expression modulation in rats. Mol. Biol. Rep. 2022, 49, 10217–10228. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.M.M.; Campos, M.R.S. Effect of Chia Seed Oil (Salvia hispanica L.) on Cell Viability in Breast Cancer Cell MCF-7. Proceedings 2020, 53, 18. [Google Scholar] [CrossRef]
- Shaer, N.A.; Al-Abbas, N.S. Potential effect of Chia Seeds Crude Extract Nanoparticles on Mcf-7 Breast Cancer Cell. Al-Azhar Int. Med. J. 2022, 22, 123–127. [Google Scholar] [CrossRef]
- Ahmadi, S.E.; Rahimi, S.; Zarandi, B. MYC: A multipurpose oncogenewith prognostic and therapeutic implications in blood malignancies. J. Hematol. Oncol. 2021, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, H.; Qing, G. Targeting oncogenic Myc as a strategy for cancer treatment. Sig. Transduct. Target Ther. 2018, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Han, Z.; Li, J.; Du, Y. c-MYC and HIF1α promoter G-quadruplexes dependent metabolic regulation mechanism of berberine in colon cancer. J. Gastrointest. Oncol. 2022, 13, 1152–1168. [Google Scholar] [CrossRef]
- Wallbillich, N.J.; Lu, H. Role of c-Myc in lung cancer: Progress, challenges, and prospects. Chin. Med. J. Pulm. Crit. Care Med. 2023, 1, 129–138. [Google Scholar] [CrossRef]
- Li, Z.; Owonikoko, T.K.; Sun, S.Y. c-Myc suppression of DNA double-strand break repair. Neoplasia 2012, 14, 1190–1202. [Google Scholar] [CrossRef]
- Brindle, N.R.; Joyce, J.A.; Rostker, F. Deficiency for the cysteine protease cathepsin L impairs Myc-induced tumorigenesis in a mouse model of pancreatic neuroen-docrine cancer. PLoS ONE 2015, 10, e0120348. [Google Scholar] [CrossRef]
- Hu, T.; Lu, Y.R. BCYRN1, a c-Myc-activated long non-coding RNA, regulates cell metastasis of non-small-cell lung cancer. Cancer Cell Int. 2015, 15, 36. [Google Scholar] [CrossRef]
- Pello, O.M.; Chèvre, R.; Laoui, D. In vivo inhibition of c-MYC in myeloid cells impairs tumor-associated macrophage maturation and pro-tumoral activities. PLoS ONE 2012, 7, e45399. [Google Scholar] [CrossRef]
- Saman, H.; Raza, S.S.; Uddinm, S.; Rasulm, K. Inducing Angiogenesis, a Key Step in Cancer Vascularization, and Treatment Approaches. Cancers 2023, 15, 4648. [Google Scholar] [CrossRef] [PubMed]
- Ngaha, T.Y.S.; Zhilenkova, A.V.; Essogmo, F.E.; Uchendu, I.K.; Abah, M.O.; Fossa, L.T.; Sangadzhieva, Z.D.D.; Sanikovich, V.; Rusanov, A.S.; Pirogova, Y.N. Angiogenesis in Lung Cancer: Understanding the Roles of Growth Factors. Cancers 2023, 15, 4648. [Google Scholar] [CrossRef]
- Andreeff, M.; Goodrich, D.W.; Pardee, A.B. Proliferation. In Holland-Frei Cancer Medicine, 6th ed.; Kufe, D.W., Pollock, R.E., Weichselbaum, R.R., Bast, R.C., Gansler, T.S., Holland, J.F., Frei, E., Eds.; BC Decker: Hamilton, ON, Canada, 2003. [Google Scholar]
- Tabata, T.; Tanaka, K.; Hayashi, T.; Hori, T.; Nunomura, S.; Yonezawa, S.; Fukuoka, J. Ki-67 is a strong prognostic marker of non-small cell lung cancer when tissue heterogeneity is considered. BMC Clin. Pathol. 2014, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Sonnenblick, A.; Francis, P.A.; Azim, H.A.; de Azambuja, E.; Nordenskjöld, B.; Gutiérez, J.; Quinaux, E.; Mastropasqua, M.G.; Ameye, L.; Anderson, M.; et al. Final 10-year results of the Breast International Group 2-98 phase III trial and the role of Ki67 in predicting benefit of adjuvant docetaxel in patients with oestrogen receptor positive breast cancer. Eur. J. Cancer 2015, 51, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhou, M.; Liao, T.; Kuang, W.; Xia, H.; Yin, Z.; Tan, Q.; Li, Y.; Song, S.; Zhou, E.; et al. Targeting cancer cell ferroptosis to reverse immune checkpoint inhibitor therapy resistance. Front. Cell Dev. Biol. 2022, 10, 818453. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Hsiao, H.H.; Wu, T.C.; Tsai, Y.H.; Kuo, C.H.; Huang, R.H.; Hong, Y.H.; Huang, C.Y. Effect of Oversulfation on the Composition, Structure, and In Vitro Anti-Lung Cancer Activity of Fucoidans Extracted from Sargassum aquifolium. Mar. Drugs 2021, 19, 215. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Rajasegaran, T.; How, C.W.; Saud, A.; Ali, A.; Lim, J.C.W. Targeting Inflammation in Non-Small Cell Lung Cancer through Drug Repurposing. Pharmaceuticals 2023, 16, 451. [Google Scholar] [CrossRef]
- Sionov, R.V.; Fridlender, Z.G.; Granot, Z. The Multifaceted Roles Neutrophils Play in the Tumor Microenvironment. Cancer Microenviron. 2015, 8, 125–158. [Google Scholar] [CrossRef]
- Bekaert, S.; Fillet, M.; Detry, B.; Pichavant, M.; Marée, R.; Noel, A.; Rocks, N.; Cataldo, D. Inflammation-generated extracellular matrix fragments drive lung metastasis. Cancer Growth Metastasis 2017, 10, 5539. [Google Scholar] [CrossRef]
- Bent, R.; Moll, L.; Grabbe, S.; Bros, M. Interleukin-1 Beta-A Friend or Foe in Malignancies? Int. J. Mol. Sci. 2018, 19, 2155. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, V.; Vajagathali, M. Pathogenesis of oxidative stress in lung cancer and its therapeutic aspects. In Handbook of Oxidative Stress in Cancer: Herapeutic Aspects; Chakraborti, S., Ed.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, I.; Pasupuleti, V.R. Therapeutic implications of caffeic acid in cancer and neurological diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Chen, R.F.; Chen, J.Y.F.; Chu, Y.C.; Wang, H.M.; Chou, H.L.; Chang, W.C.; Fong, Y.; Chang, W.T.; Wu, C.Y. Protective effect of caffeic acid on paclitaxel induced anti-proliferation and apoptosis of lung cancer cells involves NF-κB Pathway. Int. J. Mol. Sci. 2012, 13, 6236–6245. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Li, S.T.; Liu, X.Y.; An, H.L.; Kang, X.J.; Guo, S. Caffeic acid, an active ingredient in coffee, combine with dox for multitarget combination therapy of lung cancer. J. Agric. Food Chem. 2022, 70, 8326–8337. [Google Scholar] [CrossRef]
- Ozturk, G.; Ginis, Z.; Akyol, S.; Erden, G.; Gurel, A.; Akyol, O. The anticancer mechanism of caffeic acid phenethyl ester (cape): Review of melanomas, lung and prostate cancers. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 2064–2068. [Google Scholar]
- Ulasli, S.S.; Celik, S.; Gunay, E.; Ozdemir, M.; Hazman, O.; Ozyurek, A.; Koyuncu, T.; Unlu, M. Anticancer effects of thymoquinone, caffeic acid phenethyl ester and resveratrol on A549 non-small cell lung cancer cells exposed to benzo(a)pyrene. Asian Pac. J. Cancer Prev. 2013, 14, 6159–6164. [Google Scholar] [CrossRef]
- Gong, J.; Zhou, S.; Yang, S. Vanillic Acid Suppresses HIF-1α Expression via Inhibition of mTOR/p70S6K/4E-BP1 and Raf/MEK/ERK Pathways in Human Colon Cancer HCT116 Cells. Int. J. Mol. Sci. 2019, 20, 465. [Google Scholar] [CrossRef]
- Jinbong, P.; Cho, S.Y.; Kang, J.W.; Park, W.Y.; Lee, S.; Jung, Y.; Kang, M.W.; Kwak, H.J.; Um, J.Y. Vanillic acid improves comorbidity of cancer and obesity through STAT3 regulation in high-fat-diet-induced obese and B16BL6 melanoma-injected mice. Biomolecules 2020, 10, 1098. [Google Scholar] [CrossRef]
- Punvittayagul, C.; Chariyakornkul, A.; Jarukamjorn, K.; Wongpoomchai, R. Protective role of vanillic acid against diethylnitrosamine- and 1,2-dimethylhydrazine-induced hepatocarcinogenesis in rats. Molecules 2021, 26, 2718. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhu, X.; Wang, Q.; Li, X.; Wang, E.; Zhao, Q.; Wang, Q.; Cao, H. The anti-tumor effect of taxifolin on lung cancer via suppressing stemness and epithelial-mesenchymal transition in vitro and oncogenesis in nude mice. Ann. Transl. Med. 2020, 8, 590. [Google Scholar] [CrossRef]
- Razak, S.; Afsar, T.; Ullah, A.; Almajwal, A.; Alkholief, M.; Alshamsan, A.; Jahan, S. Taxifolin, a natural flavonoid interacts with cell cycle regulators causes cell cycle arrest and causes tumor regression by activating Wnt/β-catenin signaling pathway. BMC Cancer 2018, 18, 1043. [Google Scholar] [CrossRef]
- Butt, S.S.; Khan, K.; Badshah, Y.; Rafiq, M.; Shabbir, M. Evaluation of pro-apoptotic potential of taxifolin against liver cancer. PeerJ 2021, 9, e11276. [Google Scholar] [CrossRef] [PubMed]
- Uto, T.; Tung, N.H.; Ohta, T.; Juengsanguanpornsuk, W.; Hung, L.Q.; Hai, N.T.; Long, D.D.; Thuong, P.T.; Okubo, S.; Hirata, S.; et al. Antiproliferative activity and apoptosis induction by trijuganone C isolated from the root of Salvia miltiorrhizaBunge (Danshen). Phytother. Res. 2018, 32, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Chen-Yu, L.; Sher, H.-F.; Chen, H.-W.; Liu, C.-C.; Chen, C.-H.; Lin, C.-S.; Yang, P.-C.; Tsay, H.-S.; Jeremy, J.W. Chen. Anticancer effects of tanshinone I in human non-small cell lung cancer. Mol. Cancer Ther. 2008, 7, 3527–3538. [Google Scholar]
- Li, Q.; Zhang, J.; Liang, Y.; Mu, W.; Hou, X.; Ma, X.; Cao, Q. Tanshinone l exhibits anticancer effects in human endometrial carcinoma HEC-1-A cells via mitochondrial mediated apoptosis, cell cycle arrest and inhibition of JAK/STAT signalling pathway. J. BUON 2018, 23, 1092–1096. [Google Scholar]
- Wang, L.; Sun, Y.; Liu, H.; Yang, X.; Wen, Z.; Tian, X. β-Sitosterol attenuates anlotinib resistance in non-small cell lung cancer cells by inhibiting miR-181a-3p/SHQ1 signaling. Chem. Biol. Drug Des. 2024, 103, e14493. [Google Scholar] [CrossRef]
- Rajavel, T.; Packiyaraj, P.; Suryanarayanan, V.; Singh, S.K.; Ruckmani, K.; Devi, K.P. β-Sitosterol targets Trx/Trx1 reductase to induce apoptosis in A549 cells via ROS mediated mitochondrial dysregulation and p53 activation. Sci. Rep. 2018, 8, 2071. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, S.; Yang, L.; Jiang, M.; Xin, Y.; Liao, X.; Li, Y.; Lu, J. The Antitumor Effects of α-Linolenic Acid. J. Pers. Med. 2024, 14, 260. [Google Scholar] [CrossRef]
- Repetto, G.; Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- OECD. OECD Guidelines for the Testing of Chemicals No. 423: Acute Oral Toxicity—Acute Toxic Class Method; OECD: Paris, France, 1996. [Google Scholar]
- Chung, F.L.; Wang, M.; Rivenson, A.; Iatropoulos, M.J.; Reinhardt, J.C.; Pittman, B.; Ho, C.T.; Amin, S.G. Inhibition of lung carcinogenesis by black tea in Fischer rats treated with a tobacco-specific carcinogen: Caffeine as an important constituent. Cancer Res. 1998, 58, 4096–4101. [Google Scholar] [PubMed]
- Van Pelt, L. Ketamine and xylazine for surgical anesthesia in rats. J. Am. Vet. Med. Assoc. 1977, 171, 842–844. [Google Scholar]
- Henry, R. Clinical Chemistry. Principles and Techniques; Harper & Row, Publishers: New York, NY, USA, 1964. [Google Scholar]
- Doumas, B.T.; Watson, W.A.; Biggs, H.G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta 1971, 31, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Rettman, S.; Frankel, L.S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Tabacco, A.; Meiattini, F.; Moda, E.; Tarli, P. Simplified enzymic/colorimetric serum urea nitrogen determination. Clin. Chem. 1979, 25, 336–337. [Google Scholar] [CrossRef]
- Gochman, N.; Schmitz, J.M. Automated determination of uric acid, with use of auricase—Peroxidase system. Clin. Chem. 1971, 17, 1154–1159. [Google Scholar] [CrossRef]
- Reinhold, J.G. Standard Methods in Clinical Chemistry; Academic Press: New York, NY, USA, 1953. [Google Scholar]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Spooner, R.J. Glutathione reductase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Bergmeyer, J., GraBI, M., Eds.; Verlag Chemie: Basel, Switzerland, 1983; Volume 3, pp. 258–265. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 22−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, S.K.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier: Oxford, UK, 2019. [Google Scholar]



| Groups | GSH (mmm/L) | GR (U/g) | GST (U/g) | GPx (U/g) |
|---|---|---|---|---|
| Negative control | 4.76 ± 0.15 d | 3.89 ± 0.24 e | 3.02 ± 0.41 e | 2.26 ± 0.31 c |
| NNK-control | 3.20 ± 0.11 e | 2.80 ± 0.07 f | 2.44 ± 0.05 f | 1.66 ± 0.04 d |
| Chia Ether-treated | 5.73 ± 0.41 b | 4.84 ± 0.38 b | 3.95 ± 0.38 b | 3.08 ± 0.30 a |
| Chia Alcohol-treated | 4.81 ± 0.14 d | 4.14 ± 0.05 d | 3.47 ± 0.17 d | 2.67 ± 0.13 b |
| Ether ve+ | 6.75 ± 0.21 c | 5.57 ± 0.17 c | 4.40 ± 0.15 c | 3.08 ± 0.10 a |
| Alcoholic ve+ | 5.25 ± 0.05 a | 4.48 ± 0.05 a | 3.72 ± 0.11 a | 2.90 ± 0.09 a |
| Groups | Total Protein (g/dL) | Albumin (g/dL) | Globulin (g/dL) | Albumin/Globulin | GPT (U/L) | GOT (U/L) | GOT/GPT Ratio |
|---|---|---|---|---|---|---|---|
| Negative control | 7.86 ± 0.05 a | 3.38 ± 0.02 ab | 4.48 ± 0.05 a | 0.75 ± 0.01 c | 31.08 ± 1.37 c | 67.42 ± 2.13 c | 2.17 ± 0.03 b |
| Lung cancer control | 5.84 ± 0.20 c | 2.54 ± 0.06 c | 3.30 ± 0.24 d | 0.78 ± 0.07 c | 40.93 ± 1.36 a | 81.71 ± 1.98 a | 2.00 ± 0.02 d |
| Chia Ether-treated | 7.11 ± 0.22 b | 3.43 ± 0.14 ab | 3.69 ± 0.11 c | 0.93 ± 0.03 a | 32.94 ± 1.29 b | 70.11 ± 1.87 b | 2.13 ± 0.03 c |
| Chia Alcohol-treated | 7.14 ± 0.21 b | 3.44 ± 0.18 ab | 3.70 ± 0.19 c | 0.93 ± 0.08 a | 30.15 ± 1.09 c | 66.07 ± 1.59 c | 2.19 ± 0.03 ab |
| Chia Ether ve+ | 8.00 ± 0.05 a | 3.54 ± 0.02 ab | 4.46 ± 0.05 a | 0.79 ± 0.01 c | 31.08 ± 0.88 c | 67.42 ± 1.28 c | 2.17 ± 0.02 b |
| Chia Alcohol ve+ | 7.76 ± 0.30 a | 3.57 ± 0.09 a | 4.19 ± 0.28 b | 0.86 ± 0.06 b | 29.25 ± 1.00 c | 64.76 ± 1.45 c | 2.21 ± 0.03 a |
| Groups | Uric Acid (mg/dL) | Urea (mg/dL) |
|---|---|---|
| Negative control | 5.65 ± 0.11 bc | 54.68 ± 2.11 c |
| Lung cancer control | 7.14 ± 0.16 a | 62.77 ± 0.72 a |
| Chia Ether | 5.86 ± 0.11 b | 58.76 ± 0.24 b |
| Chia Alcohol | 5.75 ± 0.41 bc | 56.08 ± 1.19 c |
| Chia Ether ve+ | 5.90 ± 0.11 b | 52.01 ± 2.11 d |
| Chia Alcohol ve+ | 5.41 ± 0.39 c | 54.34 ± 1.16 c |
| No. | RT (min) | Name | Class | [M-H]− m/z | [M+H]+ m/z | Diff (ppm) | MF | MS2 |
|---|---|---|---|---|---|---|---|---|
| Phenolics | ||||||||
| 1 | 0.949 | Isocitric acid | Organic acid | 191.0198 | 193.2103 | 1.19 | C6H8O7 | 173.0078 [M-H-H2O]−, 111.0085, 87.0089, 85.0298, |
| 2 | 0.976 | Citric acid | Organic acid | 191.0213 | 193.1380 | 0.42 | C6H8O7 | 173.0085 [M-H-H2O]−, 129.0204 [M-H-H2O-CO2]− 85.0295, 87.0089, 175.0100, |
| 3 | 0.988 | Succinic acid | Organic acid | 117.0223 | 0.63 | C4H6O4 | 99.0094, 73.0309 | |
| 4 | 1.016 | * Quinic acid | Organic acid | 191.0200 | 193.1018 | 0.11 | C7H12O6 | 173.0100 [M-H-H2O]−, 146.0619 [M-H-COOH]− 85.0305, 87.0095, 114.0561, |
| 5 | 1.093 | *** Caffeic acid | Hydroxy-cinnamic acids | 179.0551 | 181.1023 | 2.56 | C9H8O4 | 161.0420 [M-H-H2O]−, 135.0402 [M-H-CO2]−, 75.0085, 163.0899, 137.0720 |
| 6 | 1.259 | ** Ferulic acid | Hydroxy-cinnamic acids | 193.717 | 195.1176 | 1.70 | C10H10O4 | 177.0700, 175.0609 [M-H-H2O]−, 157.0389 [M-H-2H2O]−, 149.0555 [M-H-CO2]−, 151.0749 |
| 7 | 1.352 | ** Rosmarinic acid | Hydroxy-cinnamic acids | 359.2063 | 361.0489 | 0.72 | C18H16O8 | 315.1075 [M-H-CO2]−, 311.1118, 299.0021, 271.1119, 179.0569 [M-H-(C9H9O4)•]−,181.0706, 301.1221 |
| 8 | 1.390 | ** Coumaric acid | Hydroxy-cinnamic acids | 163.0413 | 165.0405 | 0.32 | C9H8O3 | 119.0504 [M-H-CO2]−, 147.0315 [M-H-H2O]−, 149.1924, 121.0315 |
| 9 | 1.583 | * Protocatechueic acid | Hydroxy-benzoic acids | 153.0200 | 155.1495 | 2.71 | C7H6O4 | 135.000 [M-H-H2O]−, 137.0261, 109.0315 [M-H-CO2]−, 111.0347, 84.9906 |
| 10 | 1.606 | * Przewalskinic acid | Hydroxy-cinnamic acids | 356.9589 | 359.1562 | 2.10 | C18H14O8 | 313.1408 [M-H-COOH]−, 313.9870, 179.1068 [M-H-C9H8O4]−, 197.1156, 189.9389, 135.1153 |
| 11 | 1.761 | * Danshensu | Hydroxy-cinnamic acids | 197.09175 | 199.1730 | 1.93 | C9H10O5 | 152.9751 [M-H-CO2]−, 112.9854, 181.1058 [M-H-H2O]− |
| 12 | 2.470 | Vanillic acid | Hydroxy-benzoic acids | 167.0340 | 169.0840 | 0.22 | C8H8O4 | 149.0223 [M-H-H2O]−, 122.9672 [M-H-CO2]−, 151.1080, 125.0663, 93.0666 |
| 13 | 2.548 | P-hydroxy benzoic acid | Hydroxy-benzoic acids | 137.0249 | 139.1209 | 0.15 | C7H6O3 | 121.0388 [M-H-H2O]−, 93.0345, 97.0660, |
| 14 | 4.064 | * Protocatechuic aldehyde | 137.0235 | 0.30 | C7H6O3 | 108.0211 [M-H-CHO]−, 109.0284 [M-H-CO]− | ||
| 15 | 8.0540 | D-(+)-Galacturonic acid | Hydroxy-cinnamic acids | 193.0469 | 4.71 | C6H10O7 | 161.0206, 149.0924 | |
| 16 | 15.008 | Salvianolic acid A | Hydroxy-cinnamic acids | 493.2001 | 6.34 | C26H22O10 | 431.1568 [M-H-H2O-CO2]−, 356.9344 [M-H-2H2O]−, 294.9048 [M-H-198(C9H9O5•]−, 248.9587, 288.9440, 256.9281, 384.9308, 364.9154, 325.1803, 256.9281, | |
| 17 | 15.781 | * Salvianolic acid F | Hydroxy-cinnamic acids | 313.0713 | 3.62 | C17H14O6 | 294.903, 269.0936 [M-H-CO2]−,267.2695, 255.2312, 188.9408, 153,0566, 147.0476, 135.9427, 228.9303 | |
| 18 | 16.728 | * Salvianolic acid L | Hydroxy-cinnamic acids | 717.2300 | 1 | C36H30O16 | 672.8041 [M-H-COOH]−, 518.8446 [M-H-198]−, 501.5309 [M-H-198-18]−, 549.2213, 617.1651, 573.1695, 586.8670 | |
| 19 | 17.279 | * Isosalvianolic acid B | Hydroxy-cinnamic acids | 717.2853 | 1.83 | C20H18O10 | 671.2583 [M-H-COOH]−, 581.2956, 518.8367 [M-H-198]−, 481.1706, 345.2053 | |
| 20 | 17.898 | * Salvianolic acid D | Hydroxy-cinnamic acids | 417.2852 | 0.52 | C20H18O10 | 373.2140 [M-H-CO2]−, 354.9166 [M-H-H2O-CO2]−, 349.2291, 326.9340, 286.9384, 248.9610 | |
| 21 | 20.368 | * Lithospermic acid | Hydroxy-cinnamic acids | 537.3337 | 2.36 | C27H22O12 | 492.8111 [M-H-44]−, 316.9444, 248.9591, 180.9715, 112.9853 | |
| Phenolic acid hexosides | ||||||||
| 22 | 1.044 | * Protocatechueic acid-C-hexoside | Hydroxy-benzoic acids | 315.0049 | 1.7 | C13H16O9 | 195.0514 [M-H-120]−, 225.0023 [M-H-90]− 271.0386 [M-H-CO2]−, 75.0092 | |
| 23 | 1.210 | *** Vanillic acid O-hexoside | Hydroxy-benzoic acids | 329.0383 | 331.0652 | 0.75 | C14H18O9 | 285.0926 [M-H-CO2]−, 167.0361. [M-H-162(hex)]−, 123.0461, 122.0390 |
| 24 | 1.288 | ** Ferulic acid O-hexoside | Hydroxy-cinnamic acids | 355.0736 | 1.43 | C16H20O9 | 193.0508 [M-H-162(hex)]−, 178.0272, 163.0407, 85.0295 | |
| 25 | 1.326 | ** Proto- catechueic acid-O-hexoside | Hydroxy-benzoic acids | 315.0720 | 3.00 | C13H16O9 | 153.0196 [M-H-162(hex)]−, 123.0437, 109,0290, 108.0217 | |
| 26 | 1.352 | ** Caffeic acid O-hexoside | Hydroxy-cinnamic acids | 341.0981 | 343.1373 | 1.12 | C15H18O9 | 179.0345 [M-H-162(hex)]−, 135.0447 [M-H-162(hex)-CO2]−, 181.0703, 164.0762 [M-H-caffiec]− |
| 27 | 1.431 | * Hydroxy- benzoic acid O-hexoside | Hydroxy-benzoic acids | 299.0774 | 301.1236 | 3.02 | C13H16O8 | 137.0236 [M-H-162(hex)]−, 139,0505 239.1370, 241.1230, 39.0348 |
| 28 | 1.803 | Caffeoylquinic acid | Hydroxy-cinnamic acids | 353.0816 | 355.0741 | 0.22 | C16H18O9 | 191.0497 [M-H-162]− 193.0686, 145.0970 |
| 29 | 1.977 | Quinic acid di hexose | Organic acid glycoside | 515.1584 | 0.85 | C19H32O16 | 352.9196 [M-H-162(hex)]−, 191.1060 [M-H-324 (di hex)]−, 173.0922 [M-H-324 (di hex)-H2O]−, 139.0749 | |
| 30 | 2.049 | Caffeic acid di rhamnoside | Hydroxy-cinnamic acids | 471.1412 | 3.54 | C21H28O12 | 427.4572 [M-H-CO2]−, 324.9018 [M-H-146(rham)]−, 145.9292 [M-H-146(rham)-179(Caffoyl]− | |
| Flavonoids (flavonols) | ||||||||
| Kaempferol derivatives | ||||||||
| 31 | 1.122 | *** Kaempferol-3-O-Glucuronide | Flavonol | 461.0726 | 0.99 | C21H18O12 | 284.9790 [M-H-176(gluc)]− | |
| 32 | 1.314 | * Dihydro kaempferol-3-O-hexoside | Flavonol | 449.201 | 1.87 | C21H12O11 | 431.2058 [M-H-H2O]−, 287.0200 [M-H-162(gl)]−, 269.1382, 251.1277 | |
| 33 | 1.978 | Kaempferol-3-O-α-L-rhamnoside | Flavonol | 431.1766 | 2.12 | C21H20O10 | 285.0432 [M-H-146(rham)]− | |
| 34 | 6.475 | * kaempferol-3-O-neohesperido- side | Flavonol | 593.1432 | 3.66 | C28H32O15 | 447.1335 [M-H-146(rham]−, 431.0731 [M-H-162(gl)]−, 285.0410, 284.0324 | |
| 35 | 6.530 | * kaempferol-7-O-neohesperdo- side | Flavonol | 593.1189 | 595.1794 | 1.15 | C28H32O15 | 447.0907 [M-H-146(rham]−, 431.1450 [M-H-162(gl)]−, 284.0359 [M-H-162(gl)-146(rham]−, 285.3020, 433.1600, 287.0339 |
| 36 | 6.698 | Kaempferol-3-O-hexoside | Flavonol | 447.0922 | 449.2290 | 0.33 | C21H20O11 | 284.0303 [M-H-162(gl)]−, 285.0370, 255.0929, 287.0866 |
| 37 | 7.149 | * Kaempferol-3-O-α-L- arabinoside | Flavonol | 417.0794 | 0.94 | C20H18O10 | 284.0307, 285.0494 [M-H-132(ara)]− | |
| 38 | 8.628 | * Kaempferol 3-O-pentoside-7-O-glucuronide | Flavonol | 593.3003 | 1.50 | C26H26O16 | 461.2058 [M-H-132(pent)], 285.0268 [M-H-132(pent)176(gluc)]− | |
| 39 | 8.600 | Kaempferol 3,7-O-bis-alpha-L-rhamnoside | Flavonol | 577.2154 | 2.84 | C27H30O14 | 430.0999 [M-H-146(rham)]−, 285.0310 [M-H-292(2rham)]− | |
| 40 | 9.876 | Kaempferol 4′-O-methoxy | Flavonol | 299.0969 | 301.1364 | 0 | C16H12O6 | 284.0445 [M-H-CH3]−, 286.0133, 153.0716 |
| 41 | 14.577 | * Kaempferol 3-O-glucuronide-7-O-pentoside | Flavonol | 593.2150 | 0 | C26H26O16 | 416.8290 [M-H-176(gluc)]−, 285.0348 [M-H-176(gluc)-132(pent)]− | |
| 42 | 17.0317 | Kaempferol 3-O-robinoside-7-O-rhamnoside | Flavonol | 739.3776 | 3.65 | C33H40O19 | 593.3730 [M-H-146(rham)]−, 431.1500 [M-H-162(gl)-146(rham]−, 285.0530 [M-H-146(rham)-308] | |
| Quercetin derivatives | ||||||||
| 43 | 6.073 | Quercetin-3-O- hexoside | Flavonol | 463.0866 | 3.22 | C21H20O12 | 300.0251, 301.0265 [M-H-162(gl)]−, 271.0208 | |
| 44 | 7.099 | * Quercetin-3-O-pentoside | Flavonol | 433.2067 | 0.61 | C20H18O11 | 301.1034 [M-H-132(pent)]−, 261.1120, 228.9288 | |
| 45 | 7.353 | * Isorhamentin-3-O-hexoside | Flavonol | 447.2007 | 3.10 | C22H22O12 | 314.0445, 315.0410 [M-H-162(gl)]−, 301.3301 [M-H-162(gl)-CH3]−, 355.1398, 285.0468 [M-H-162(gl)-CH3-OH]−, 294.4905 | |
| 46 | 7.392 | * Quercetin-7-O-hexoside | Flavonol | 463.0358 | 0.28 | C21H20O12 | 301.0333, 300.0265, 285.0301 | |
| 47 | 7.573 | * Isorhamnetin-3-O-rhamnoside-7-O-hexoside | Flavonol | 623.2701 | 625.3426 | 7.30 | C28H32O16 | 577.2863 [M-H-146(rham)]−, 461.1439 [M-H-162(gl)]−, 317.1066 [M-H-146(rham)-162(gl)]−, 463.2891, 256.9902 |
| 48 | 11.414 | Isorhamentin-3-O-hexoside-7-O-pentoside | Flavonol | 609.2966 | 3.62 | C16H30O16 | 446.9234 [M-H-162(gl)]−, 314.0422 [M-H-162(gl)-132(pen)]−, 301.516 [M-H-162(gl)-132(pen)-CH3]− | |
| 49 | 14.108 | Isorhamentine-3-O-pentoside-7-O-glucuronide | Flavonol | 623.2062 | 4.44 | C27H28O17 | 491.2102 [M-H-132(pent)]−,447.2551 [M-H-176]− | |
| 50 | 14.168 | Quercetin-3-O- rhamnoside-7-O-glucuronide | Flavonol | 623.2340 | 1.61 | C27H28O17 | 477.2551 [M-H-146(rham)]−, 447.1668 [M-H-176(glu)]−, 301.0022 | |
| 51 | 14.438 | * 3′,4′-di methyl quercetin | Flavonol | 329.1042 | 1.49 | C17H14O7 | 314.0796 [M-H-CH3]−, 299.0562 [M-H-2CH3]−, 283.2662, 255.0794 | |
| 52 | 14.629 | * Isorhamnetin 3-O-pentoside-7-O- rhamnoside | Flavonol | 593.2672 | 1.25 | C27H30O15 | 460.9255 [M-H-132(pent)]−,447.1633 [M-H-146(rham)]−, 299.5170 [M-H-132(pent)-146(rham)-CH3]−, 284.5016 | |
| 53 | 15.105 | Axillarin | Flavonol | 345.0562 | 0.55 | C17H14O8 | 327.1058 [M-H-H2O]−, 315.0480 [M-H-2CH3]−, 301.1271, 299.0208 [M-H-OCH3-CH3]−, 283.1136 [M-H-2OCH3]−, 329.1173, 311.0982, 241.0972, 329.1811, 312.1949, 285.2878 | |
| Myricetin derivatives | ||||||||
| 54 | 1.379 | *** Myricitrin | Flavonol | 463.1352 | 0.91 | C21H20O12 | 317.0900 [M-H-146(rham)]+, 283.1069 | |
| 55 | 4.241 | Syringetin-3-O-hexoside | Flavonol | 507.1362 | 509.1349 | 0.53 | C23H24O13 | 461.1482 [M-H-CH3-H2O]−, 345.1182 [M-H-162(gl)]−, 347.5120, 435.0228, 286.9434, 294.8986, 400.8672, 283.1105, 218.9472, 327.1020, 315.1098 |
| 56 | 5.811 | Syringetin-3-O-hexoside-7-O- glucuronide | Flavonol | 683.2351 | 0.12 | C29H32O19 | 521.1075 [M-H-gl (162)]−, 345.1395 [M-H-gl (162)-gluc(176)]−, 314.8951 | |
| Flavonoids (flavones) | ||||||||
| 57 | 4.040 | Diosmetin 7-O-pentoside | Flavone | 431.1327 | 0.81 | C21H20O10 | 301.1066, 299.0884 [M-H-132(pent)]−, 267.9964 [M-H-132(pent)-OCH3]− | |
| 58 | 5.048 | Apigenin7-O- hexoside | Flavone | 431.1439 | 433.1628 | 0.13 | C21H20O10 | 269.1038 [M-H-162(gl)]−, 271.1914, 283.1165, 227.1060 |
| 59 | 4.102 | Diosmetin 8-C-hexoside-7-O- pentoside | Flavone | 593.2098 | −5.2 | C27H30O15 | 473.1159 [M-H-120]−, 431.1121 [M-H-120-132(pent]−, 298.0425, 284.4091 | |
| 60 | 5.550 | Diosmetin 6-C-pentoside | Flavone | 433.1143 | 0.67 | C21H20O10 | 373.1115 [M-H-60]−, 343.1513, [M-H-90]− 301.1092, 271.1412 | |
| 61 | 5.930 | Diosmetin 5-O-robinoside-7-O-pentoside | Flavone | 739.2645 | 0.96 | C33H40O19 | 607.2067 [M-H-132(pent)]−, 577.2339 [M-H-162(gl)]−, 298.9051 [M-H-132-308]− | |
| 62 | 6.052 | Vitexin-7-O-hexoside | Flavone | 593.2286 | 3.15 | C27H30O1 | 473.2267 [M-H-120]−, 269.1150 [M-H-324(digl)]− | |
| 63 | 6.422 | Acacetin di-O-hexoside | Flavone | 607.2552 | 4.33 | C28H32O15 | 444.9950 [M-H-162(pent)]−, 282.9815 | |
| 64 | 6.638 | * Acacetin-4′-O-hexoside-7-O- pentoside | Flavone | 577.1778 | 579.2121 | 0.49 | C27H30O14 | 415.1565 [M-H-162(gl)]−, 282.9325 [M-H-162(gl)-pent(132)]−, 179.0547, 417.1486, 285.2756, 342.0983 |
| 65 | 6.940 | * Luteolin-4′-O- hexoside | Flavone | 447.2077 | 449.1687 | 0.80 | C21H20O11 | 287.0590 [M+H-162(gl)]+, 270.1509, 262.0928, 269.1466, 284.0303, 285.0370 [M-H-162(gl)]−, 334.8963, 255.0292 |
| 66 | 7.274 | * Diosmetin-7-O-hexoside | Flavone | 461.0213 | 1.89 | C22H22O11 | 430.4532 [M-H-OCH3)]−, 299.0960 [M-H-162(gl)]−, 276.7211 [M-H-162(gl)-OCH3]− | |
| 67 | 7.642 | * Luteolin-7-O- hexoside | Flavone | 447.0972 | 449.0168 | 2.51 | C15H12O4 | 285.0461 [M-H-162(gl)]−, 284.0372, 355.1246, 334.8923, 227.0360, 256.9271, 243.0273, 287.0590, 270.1509, 251.1326 |
| 68 | 7.495 | * Diosmetin-6-C-hexoside | Flavone | 461.1483 | 463.1213 | 0.76 | C22H22O11 | 341.0640 [M-H-120]−, 370.8851 [M-H-90]−, 299.0790, 286.8904, 301.1072, 343.1250, 283.1101 |
| 69 | 7.788 | * Pinocembrin | Flavone | 255.2329 | 0.28 | C15H12O4 | 237.0635, 210.9630, 155.0319 | |
| 70 | 8.690 | * 5,7, 3′-Trihydroxy-6, 4′, 5′-trimethoxy flavone | Flavone | 359.2780 | 361.1542 | 2 | C81H16O8 | 344.421 [M-H-CH3]−, 329.1391 [M-H-2CH3]−, 314.1921 [M-H-3CH3]−, 331.1248, 315.1230, 343.1713 |
| 71 | 10.892 | Baicalein-7-O- glucuronide | Flavone | 445.3198 | 0 | C21H18O11 | 401.2819 [M-H-COO]−, 399 [M-H-COOH]−, 269.1280 [M-H-176(gluc)]− | |
| 72 | 11.041 | Sorbifolin | Flavone | 301.1468 | 0.40 | C16H12O6 | 286.0480 [M-H-CH3]−, 268.0123 [M-H-CH3-H2O]−, 283.1083, 255.0924 | |
| 73 | 14.549 | * Diosmetin-5-O-rhamnoside-7-O-pentoside | Flavone | 577.2592 | 3.57 | C27H30O14 | 431.7884 [M-H-132(pent)], 445.0339 [M-H-146(rham)]−, 299.0445 [M-H-132(pent)-146(rham)]− | |
| 74 | 17.010 | 3,5, 7-trihydroxy-4′-methoxy-flavone (diosmetin) | Flavone | 299.2039 | 301.1468 | 4.18 | C16H12O6 | 284.0333 [M-H-CH3]−, 271.0174, 256.0266, 153.0532, 117.0362, 286.0480, 301.1468, 286.0480 |
| Flavanones | ||||||||
| 75 | 8.263 | Isosakuranetin-7-O-neohesperido-side | Flavanone | 593.1828 | 1 | C28H34O14 | 447.0959, 285.2212 | |
| 76 | 4.930 | Daidzein-8-C- hexoside (Puerarin) | Isoflavonoid C-glycosides | 415.0411 | 417.1523 | 0.83 | C21H20O9 | 352.8659, 327.1397, 303.1324, 329.1250, 253.1495, 237.0435, 255.1210 |
| Flavan-3-ol | ||||||||
| 77 | 1.458 | (+)-Catechin | Flavan-3-ol | 289.1634 | 291.0704 | 1.7 | C15H14O6 | 271.1141 [M-H-H2O]−, 243.0639, 245.0942 [M-H-CO2]−, 203.0835, 153.315, 273.0993 |
| 78 | 1.363 | (+)-Gallocatechin | Flavan-3-ol | 305.0738 | 7.5 | C15H14O7 | 179.0606 [M-H-125(C6H5O3•)]−, 261.1294, 287.0953, 225.1206 | |
| Tannins | ||||||||
| 79 | 5.976 | Ellagic acid-pentoside | 433.2188 | 435.1711 | −3.6 | C19H14O12 | 301.0137 [M-H-132(pent)]−, 256.9320, 228.9299, 418.1720, 372.1430, 347.2093, 238.1145 | |
| 80 | 4.226 | Ellagic acid-hexoside | 463.6302 | −5.1 | C20H16O13 | 299.9887, 258.9403, 256.9124 | ||
| Anthocyanins | ||||||||
| 81 | 6.237 | Delphinidin-3-O-glucopyranoside | Anthocyanin | 463.0816 | 4.24 | C21H21O+12 | 300.276, 301.0343 [M-H-162(gl)]−, 294.9091, 271.0208 | |
| 82 | 8.700 | Delphinidin-3-O-(6″-O-α-rhamno-pyranosyl-β- glucopyranosid) | Anthocyanin | 609.3502 | 2.65 | C27H31O+16 | 563.3040 [M-H-146(rham)]−, 447.1565 [M-H-162(gl)]− | |
| 83 | 7.376 | * Phloretin | Dihydro chalcones | 272.9206 | 5.21 | C15H14O5 | 228.9333, 188.9393 | |
| 84 | 9.953 | Phloretin 2′-glucoside | Phloretin-hexoside (Phlorizin) | 435.1531 | 0.36 | C21H24O10 | 372.8508, 310.8799, 374.8417, 273.1094 | |
| Stilbenes | ||||||||
| 85 | 8.163 | E-3,4,5′-Trihydroxy-3′-glucopyranosylstilbene | Stilbene | 405.1245 | 0.12 | C20H22O9 | 336.8967 [M-H-4OH]−, 296.9058 [M-H-C6H5O2•]−, 272.0836 [M-H-C8H7O2•]−, 243.1549 [M-H-162(gl)]− | |
| 86 | 13.580 | * Pinoresinol | Tetrahydro furan lignan | 359.2283 | 0.57 | C20H22O6 | 315.12159810 [M+H-CH3-H2O]+, 329.00109810 [M+H-2CH3]+, 3132013. 155.0931, 297.11529810 [M+H-2OCH3]+ | |
| Aglycones | ||||||||
| 87 | 1.160 | *** Dihydro-quercetin (taxifolin) | Flavonol | 303.1398 | 305.1232 | 0.29 | C15H12O7 | 285.1118 [M-H-H2O]−, 267.0891 [M-H-2H2O]−, 243.0154, 287.1253, 257.0932 |
| 88 | 6.327 | Isorhamentin | Flavonol | 315.0540 | 317.1157 | 0.54 | C16H12O7 | 300.0216 [M-H-CH3)]−, 284.0247, 255.0316, 302.0400, 299.9887, 269.2421, 285.0205 [M-H-OCH3)]−, 287.0205, 271.0941, 257.0857 |
| 89 | 7.181 | * Naringenin | Flavone | 270.9701 | 273.1741 | 0.43 | C15H12O5 | 225.1045, 255.1765, 159.0904, 129.0704 |
| 90 | 8.655 | * Querecetin | Flavonol | 301.0004 | 303.1149 | 0.93 | C15H10O7 | 283.9982, 255.2302, 229.0132, 151.0066, 285.1116,257.1038, 288.0892, 177.1100 |
| 91 | 9.017 | Kaempferol | Flavonol | 285.0368 | 287.1367 | 0.06 | C15H11O6 | 241.0761, 211.0494, 197.0584, 271.1000, 244.0265, 231.0230, 170.0761, 145.0853 |
| 92 | 9.061 | Hesperetin | 301.0345 | 303.1613 | 0.28 | C16H14O6 | 283.0573, 285.1972, 255.2383, 245.0498, 179.0347, 164.0099, 151.0041, 191.1036, | |
| 93 | 14.440 | * Apigenin (Genistein) | Flavone | 269.2099 | 271.3207 | 0.53 | C15H10O5 | 254.1110, 251.2067, 225.2285, 134.0233, 253.54302, 229.2165, |
| 94 | 15.088 | *** Acacetin (Linarigenin) | Flavone | 283.0626 | 0.06 | C16H12O5 | 268.0371 [M-H-CH3)]−, 240.0426, 239.0331, 225.0188 | |
| 95 | 15.025 | ** Myricetin | Flavonol | 317.0236 | 319.3023 | 0.77 | C15H10O8 | 301.6302, 283.2832, 302.2432, 272.9571, 248.9191 |
| 96 | 15.526 | Luteolin | Flavone | 285.0395 | 0.72 | C15H10O6 | 271.0354, 257.0208, 241.0452, 229.0522, 211.0665 | |
| Coumarins | ||||||||
| 97 | 2.776 | Esculin | Coumarin glycoside | 339.0652 | 0.93 | C15H16O9 | 177.0174 [M-H-162(gl))]−, | |
| 98 | 6.748 | * (Daphnetin)7, 8-dihydroxy coumarins | Dihydroxy coumarins | 177.0187 | 179.1027 | 6.43 | C9H6O4 | 133.0271, 149.0247 [M-H-CO]− 105.0363 [M-H-2H2O-CO]−, 91.0500, 123.0975 |
| Alkaloids | ||||||||
| 99 | 13.579 | * Tembetarine | Alkaloid | 345.1332 | 0.19 | C20H26 NO4 | 327.1227 [M-H-H2O]−, 297.0772 [M-H-2CH3-H2O]−, 209.0451, 107.0483 | |
| 100 | 15.398 | * Jatrorrhizine | Alkaloid | 337.2053 | 339.1709 | 0.62 | C20H20 NO4 | 309.1431 [M-H-OCH3]−, 293.2282 [M-H-OCH3-CH3]− 291.2018, 275.2087 [M-H-3CH3-H2O]−, 274.9289 [M-H-2OCH3]−, 279.2315, 321.2729, 276. 9100 [M-H-2OCH3]− |
| 101 | 15.413 | * Menisperine | Alkaloid | 357.1111 | 1.70 | C21H26 NO4 | 311.0995 [M-H-OCH3-CH3]− | |
| Diterpene | ||||||||
| 102 | 8.357 | 6-Hydroxy-7- Methoxy- tremetone | Member of benzofurans | 249.1230 | 1.27 | C14H16O4 | 234.0590 [M+H-CH3]+, 231.1038 [M+H-H2O]+, 187.0729 [M+H-2OCH3]+, 141.0696 [M+H-C7H8O2−]+ | |
| 103 | 13.963 | Tanshinone IIb | Diterpene | 311.0561 | 1.51 | C19H18O4 | 293.2092 [M + H-H2O]+, 283.1309 [M + H-CO]+, 268.388 [M + H-CO-CH3]+ | |
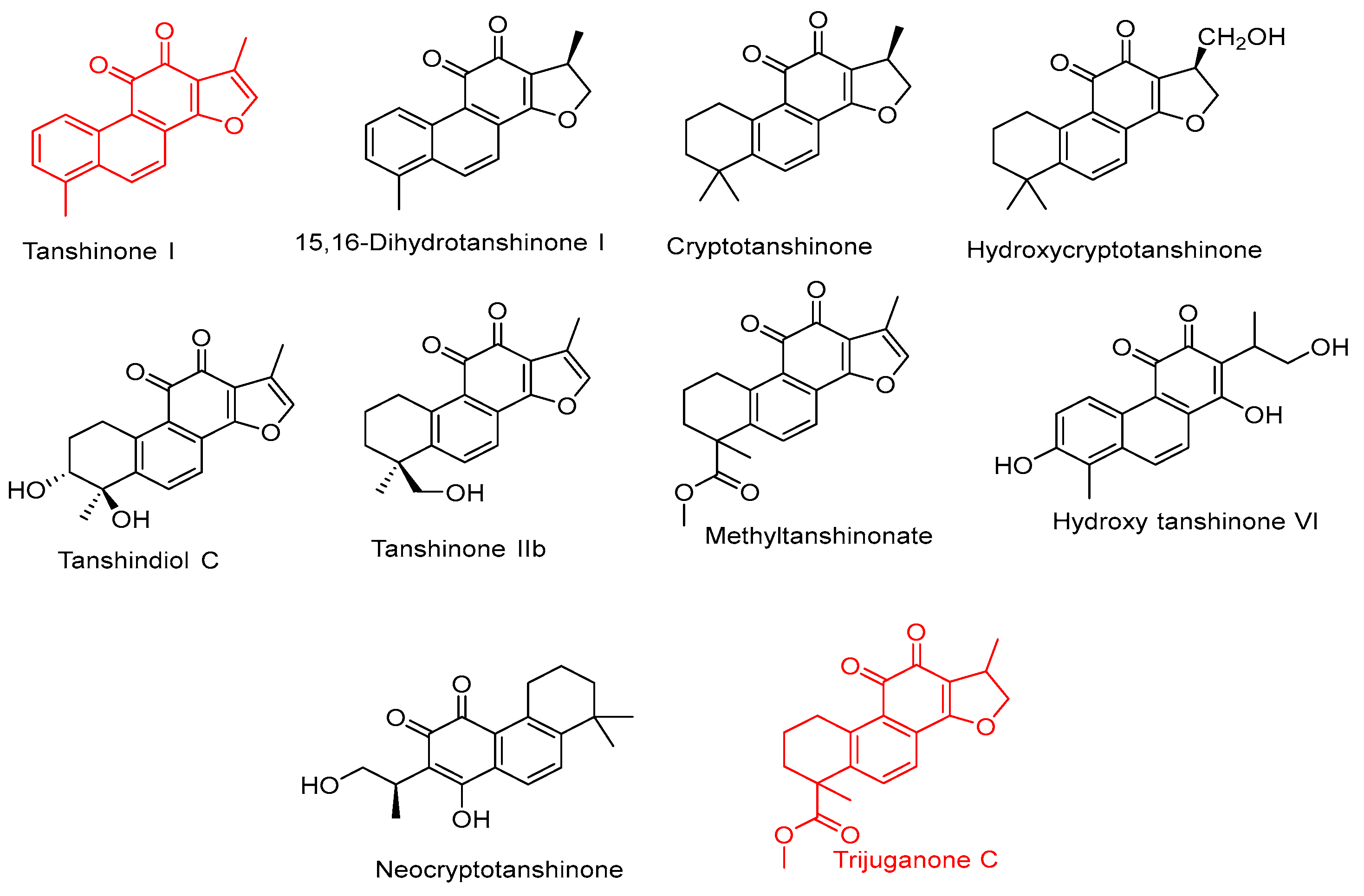
| 104 | 15.108 | *** Trijuganone C | Diterpene | 341.0988 | 0.14 | C20H20O5 | 309.0861 [M+H-32]+, 281.5246 [M+H-32-18]+, 295.0876 [M+H-COOH]+, 311.1120 [M+H-2CH3]+, 282.1352 [M+H-COOCH3]+ | |
| 105 | 15.169 | *** Tanshinone I | Diterpene | 277.1856 | 0.40 | C18H12O3 | 259.9341 [M+H-CH3]+, 246.9221 [M+H-2CH3]+, 137.0590 [M-H-C11H82•]−, 161.1012 [M+H-CH3-C9H82•]+ | |
| 106 | 15.206 | * Tanshinone IIA | Diterpene | 295.0962 | 0.91 | C19H18O3 | 277.1797 [M+H-H2O]+, 249.1854 [M + H-H2O-CO]+, 231.1707, [M+H-H2O-CO-H2O]+, 161.0965, 137.0587 | |
| 107 | 15.238 | Tanshindiol C | Diterpene | 313.1525 | 0.84 | C18H16O5 | 295.0780 [M + H-H2O]+, 267.2577 [M + H-H2O-CO]+ | |
| 108 | 14.743 | Hydroxycrypto-tanshinone | Diterpene | 313.1068 | 0.51 | C19H20O4 | 295.0947 [M+H-H2O]+, 281.1056 [M+H-CH2OH]+ , 277.2162 | |
| 109 | 15.324 | * Neocrypto- tanshinone | Diterpene | 315.1334 | 0.14 | C19H22O4 | 297.1187 [M+H-H2O]+, 279 [M+H-2H2O]+, 255.1144 [M+H-C3H7O•]+, 163.1406 | |
| 110 | 15.740 | Methyl- tanshinonate | Diterpene | 339.1617 | 0.27 | C20H18O5 | 307.1825 [M + H-32]+, 289.0550 [M + H-32-H2O]+ | |
| 111 | 19.678 | * Carnosol | Diterpene | 331.2072 | 0.32 | C20H26O4 | 316.5390 [M+H-CH3]+, 313.2737, 301.0655 [M+H-2CH3]+, 271.1711 [M+H-4CH3]+, 253.1120 [M+H-4CH3-18]+, | |
| 112 | 19.841 | * β-Caryophyllene | Sesqui-terpenes | 204.1768 | 0.36 | C15H24 | 175.6093, 149.0242, 109.952, 93.0347 | |
| 113 | 19.905 | Crypto- tanshinone | Diterpene | 297.1134 | 1.55 | C19H20O3 | 279.2280 [M + H-18]+, 251.2321 [M + H-18-28]+, 149.226 | |
| 114 | 20.021 | * Miltirone | Abietane-type diterpene quinine | 283.1572 | 0.34 | C19H22O2 | 268.3310 [M+H-CH3]+, 240 [M + H-CH3-CO]+ | |
| 115 | 23.697 | 15,16-Di-hydro tanshinoneI | Diterpene | 279.2340 | 0.72 | C18H14O3 | 264.0875 [M+H-CH3]+, 170.9375, 233.5410 | |
| 116 | 23.598 | Salviacoccin | Diterpene | 357.2975 | 3.35 | C20H20O6 | 339.2889, 290.5238 [M+H-C4H3O•]+, 265.2520, 181.1620 | |
| 117 | 24.132 | Hydroxy-tanshinone VI | Diterpene | 313.2731 | 0.12 | C18H16O5 | 297.9810 [M+H-CH3]+, 254.4611 [M+H-C3H7O•]+, 149.0223, 95.0850 [M+H-C12H11O4•]+ | |
| Amino acids | ||||||||
| 118 | 22.809 | * Norvaline | L-alpha-amino acids | 115.9319 | −1.00 | C5H11NO2 | 72.0441 [M-H-COOH]−, 99.0255 [M-H-NH2]− | |
| Organic compounds | ||||||||
| 119 | 7.919 | P-Nitrophenol | 138.0231 | 0.20 | C6H5NO3 | Not fragments | ||
| 120 | 8.630 | N-trans-Feruloyl-tyramine | 314.1380 | 1.34 | C18H19NO4 | 177.0539, 145.0279, 121.0649 | ||
| Fatty Acids | ||
| Rt | %Rel. | Identified Fatty Acid |
| 28.62 | 16.95 | Palmitic acid, methyl ester |
| 30.44 | 2.56 | Palmitic acid |
| 32.81 | 67.48 | Linolenic acid, methyl ester |
| 33.42 | 10.47 | Stearic acid, methyl ester |
| 37.20 | 0.51 | 11-Eicosenoic acid, methyl ester |
| 37.82 | 1.27 | Eicosanoic acid, methyl ester |
| Unsaponifiable matter | ||
| Rt | %Rel. | Identified Compounds |
| 4.12 | 6.59 | n-Decane |
| 6.70 | 0.53 | n-Dodecane |
| 11.36 | 6.44 | n-Pentadecane |
| 12.52 | 0.19 | n-Hexadecane |
| 15.14 | 0.33 | n-Octadecane |
| 18.08 | 0.25 | n-Heneicosane |
| 18.97 | 0.45 | n-Docosane |
| 20.79 | 0.26 | n-Tricosane |
| 21.83 | 0.54 | n-Tetracosane |
| 22.74 | 0.34 | n-Pentacosane |
| 23.65 | 0.24 | n-Hexacosane |
| 24.55 | 1.28 | n-Heptacosane |
| 27.87 | 3.87 | Cholesterol |
| 29.35 | 6.76 | Stigmasterol |
| 30.66 | 43.10 | B-Sitosterol |
| 31.49 | 7.05 | B-Sitosterol |
| 32.40 | 11.32 | alpha-amyrine |
| No. | RT (min) | Name | Class | [M-H]− m/z | [M+H]+ m/z | Diff (ppm) | MF | MS2 |
|---|---|---|---|---|---|---|---|---|
| Fatty acids | ||||||||
| 1 | 13.028 | * L-Arginine | Amino acid | 173.0814 | 3.8 | C6H14N4O2 | 155.0105 [M-H-H2O]−, 129.0188 [M-H-CO2], 111.0097, 85.0310 | |
| 2 | 14.041 | Glutaric acid | Fatty acid | 133.0138 | 7.3 | C5H8O4 | 115.030 [M-H-CO2]−, 87.0078 [M-H-H2O]− | |
| 3 | 15.136 | D-3-Phenyllactic acid | Fatty acid | 167.1143 | 11.8 | C9H10O3 | 149.0187 [M-H-H2O]−, 122.10040 [M-H-COOH]− | |
| 4 | 16.028 | Suberic acid | di-carboxylic acid | 173.0747 | 6.1 | C8H14O4 | 154.9605 [M-H-H2O]−, 145.0279 [M-H-CO]−, 128.0585 [M-H-COOH]− | |
| 5 | 20.079 | Citraconic acid | Unsaturated di-carboxylic acid | 128.0354 | 130.0383 | 4.3 | C5H6O4 | 84.0460 [M-H-CO2]− |
| 6 | 21.593 | Octadecenoic acid | Fatty acid | 281.2466 | −5.2 | C18H33O2 | 266.0416 [M-H-CH3]−, 237.18785 [M-H-CO2]−, 212.9265, 172.9433 | |
| 7 | 22.492 | *** Stearic acid | Fatty acid | 283.2637 | 0.3 | C18H35O2 | 239.246 [M-H-CO2]−, 215.170, 146.9090 | |
| 8 | 23.061 | Dihydroxy-octadecenoic acid | Fatty acid | 313.2400 | −2.4 | C18H33O4 | 298.447, 295.2256 [M-H-H2O]−, 283.0374, 267.2174, 183.0006 | |
| 9 | 24.116 | Eicosadienoic acid | Fatty acid | 307.2700 | −0.4 | C20H35O2 | 262.1038 [M-H-COOH]−, 238.9305, 170.9435, 112.9843 | |
| 10 | 24.572 | Hydroxy-octadecadienoic acid | Fatty acid | 295.2616 | −1.5 | C18H31O3 | 277.043 1 [M-H-H2O]−, 195.1195, 183.1019, 230.9773, 165.0840 | |
| 11 | 25.696 | Hydroxy-octadecatrienoic acid | Fatty acid | 293.1663 | −0.8 | C18H29O3 | 275.1851 [M-H-H2O]−, 236.1109, 221.15125, 220.1454, 193.1615, 177.0985, 183.0152, 162.0521 | |
| 12 | 25.891 | Dihydroxy-octadecadienoic acid | Fatty acid | 311.1680 | −6.5 | C18H32O4 | 293.221 [M-H-H2O]−, 275.000 [M-H-2H2O]−, 264.457, 239.0827 | |
| 13 | 26.207 | Octadecatrienoic acid | Fatty acid | 277.2132 | 0.1 | C18H29O2 | 233.1572, 205.1610, 145.0773 | |
| 14 | 26.320 | Hydroxy-octadecenoic acid | Fatty acid | 297.2771 | 0.8 | C18H33O3 | 278.9026 [M-H-H2O]−, 183.0129, 92.9278 | |
| 15 | 26.671 | ** Hydroxy-oxohexadecanoic acid | Fatty acid | 285.2703 | −3.2 | C16H29O4 | Not fragment | |
| 16 | 26.720 | ** Palmitic acid | Fatty acid | 255.2306 | −1.6 | C16H31O2 | 237.108 [M-H-H2O]−, 211.0392 [M-H-CO2]−, 186.9306 | |
| 17 | 26.822 | ** Eicosaenoic acid | Fatty acid | 309.1000 | −0.2 | C20H37O2 | 265.1532 [M-H-CO2]−, 247.1215, 240.9238, 172.9363, 104.9527 | |
| 18 | 27.515 | Octadecadienoic acid | Fatty acid | 278.9182 | −7.1 | C18H31O2 | 261.0170 [M-H-H2O]−, 234.9942 [M-H-CO2]−, 220.1481, 210.9172 | |
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| β-actin | CACGTGGGCCGCTCTAGGCACCAA | CTCTTTGATGTCACGCACGATTTC |
| c-MYC | CTGTCCATTCAAGCAGACGA | TCCAGCTCCTCCTCGAGTTA |
| MMP9 | CGGCACGCCTTGGTGTAGCA | AGGCAGAGTAGGAGCGGCCC |
| BCL2 | CTC AGTCATCCACAGGGCGA | AGAGGGGCTACGAGTGGGAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, N.A.; Elsayed, G.H.; Mohamed, S.H.; Abd Elkarim, A.S.; Aly, M.S.; Elgamal, A.M.; Elsayed, W.M.; El-Newary, S.A. Chia Seed (Salvia hispanica) Attenuates Chemically Induced Lung Carcinomas in Rats through Suppression of Proliferation and Angiogenesis. Pharmaceuticals 2024, 17, 1129. https://doi.org/10.3390/ph17091129
Ali NA, Elsayed GH, Mohamed SH, Abd Elkarim AS, Aly MS, Elgamal AM, Elsayed WM, El-Newary SA. Chia Seed (Salvia hispanica) Attenuates Chemically Induced Lung Carcinomas in Rats through Suppression of Proliferation and Angiogenesis. Pharmaceuticals. 2024; 17(9):1129. https://doi.org/10.3390/ph17091129
Chicago/Turabian StyleAli, Naglaa A., Ghada H. Elsayed, Safaa H. Mohamed, Asmaa S. Abd Elkarim, Mohamed S. Aly, Abdelbaset M. Elgamal, Wael M. Elsayed, and Samah A. El-Newary. 2024. "Chia Seed (Salvia hispanica) Attenuates Chemically Induced Lung Carcinomas in Rats through Suppression of Proliferation and Angiogenesis" Pharmaceuticals 17, no. 9: 1129. https://doi.org/10.3390/ph17091129
APA StyleAli, N. A., Elsayed, G. H., Mohamed, S. H., Abd Elkarim, A. S., Aly, M. S., Elgamal, A. M., Elsayed, W. M., & El-Newary, S. A. (2024). Chia Seed (Salvia hispanica) Attenuates Chemically Induced Lung Carcinomas in Rats through Suppression of Proliferation and Angiogenesis. Pharmaceuticals, 17(9), 1129. https://doi.org/10.3390/ph17091129






