EZH2 Inhibition to Counteract Oral Cancer Progression through Wnt/β-Catenin Pathway Modulation
Abstract
1. Introduction
2. Results
2.1. GSK343 Treatment Reduces OSCC Cell Viability
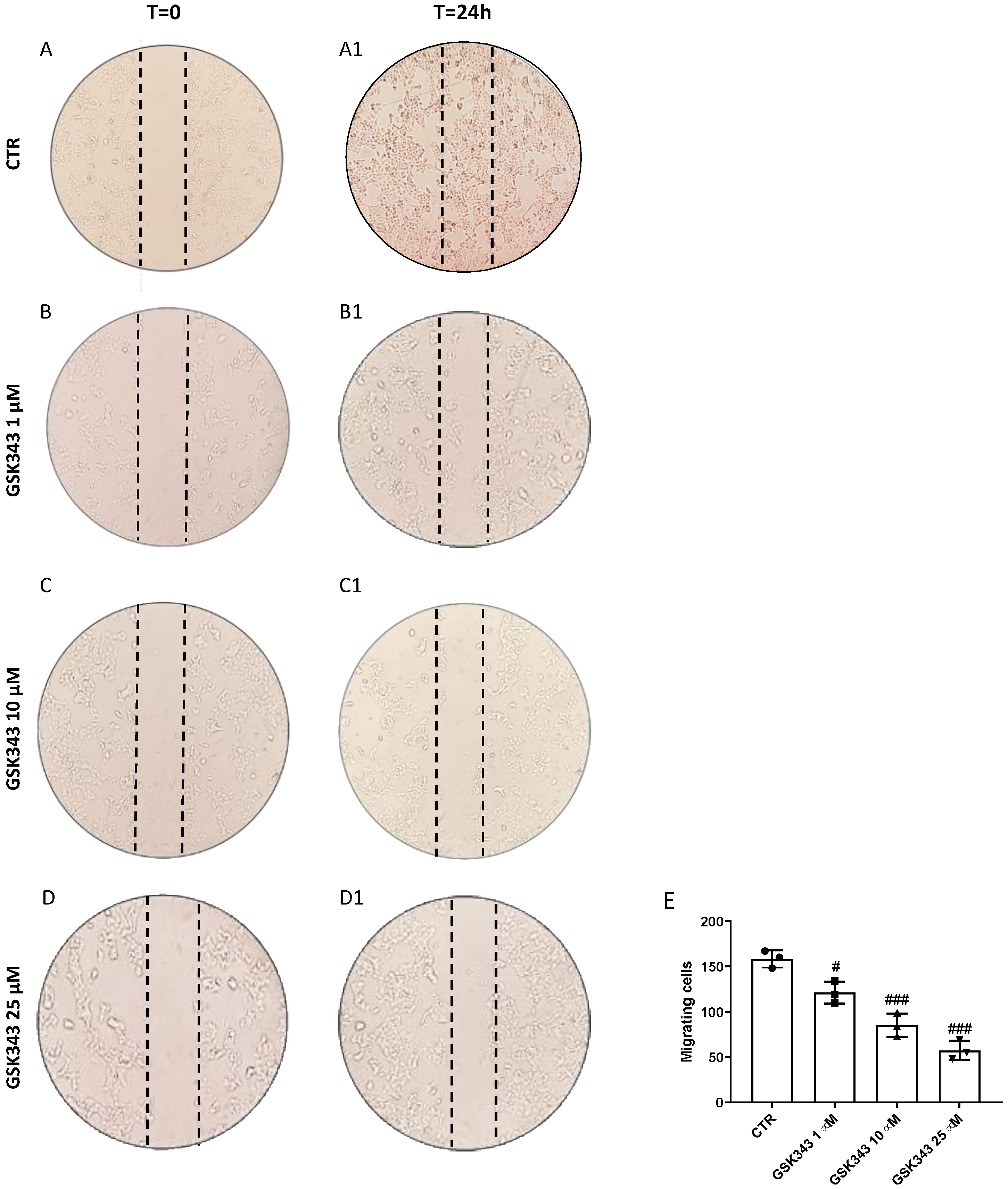
2.2. GSK343 Treatment Reduces CAL27 Cells Migration
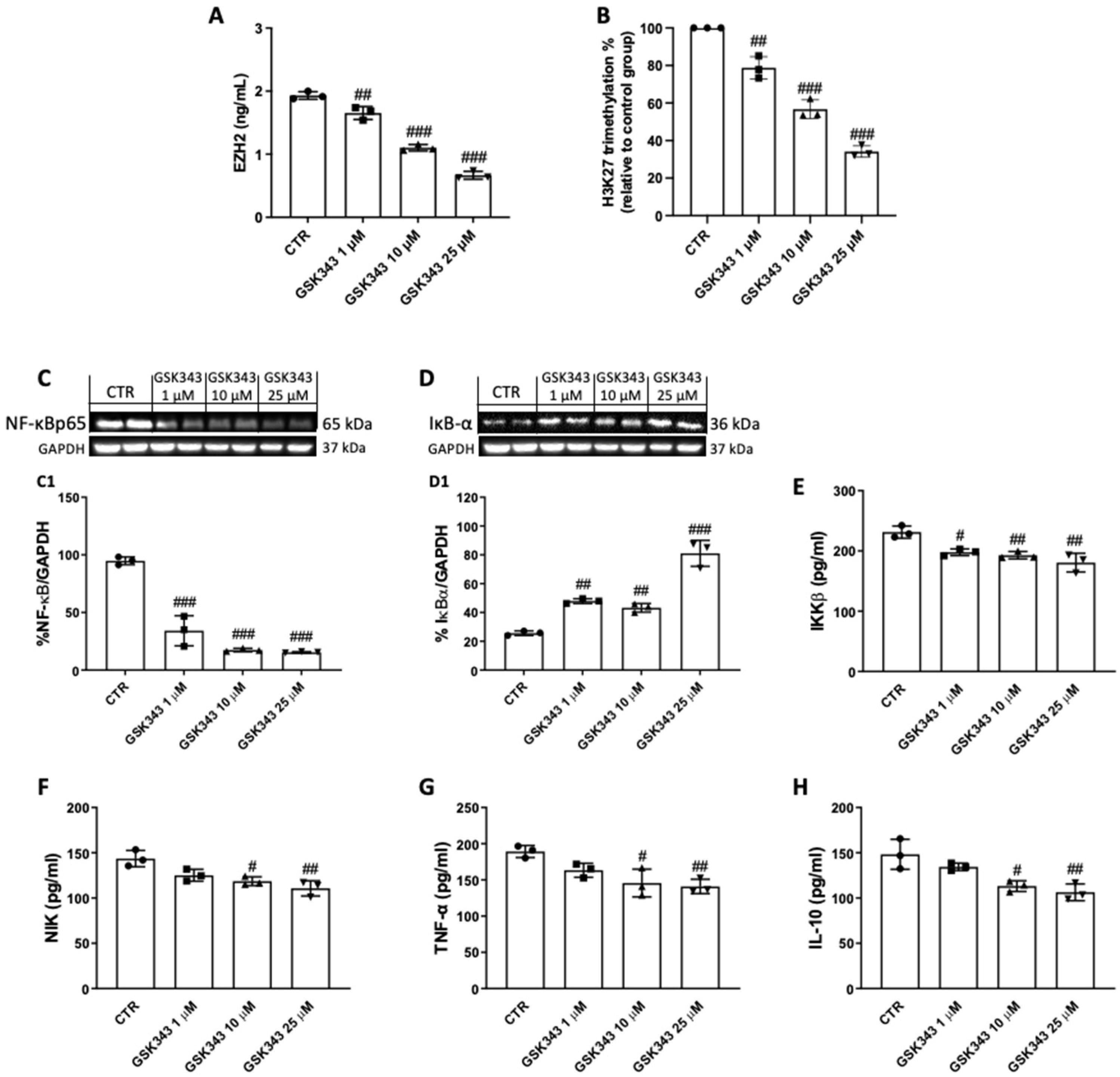
2.3. GSK343 Treatment Reduces EZH2, H3K27me3, and Nf-κB/IκBα Pathway Activation in CAL27 Cells
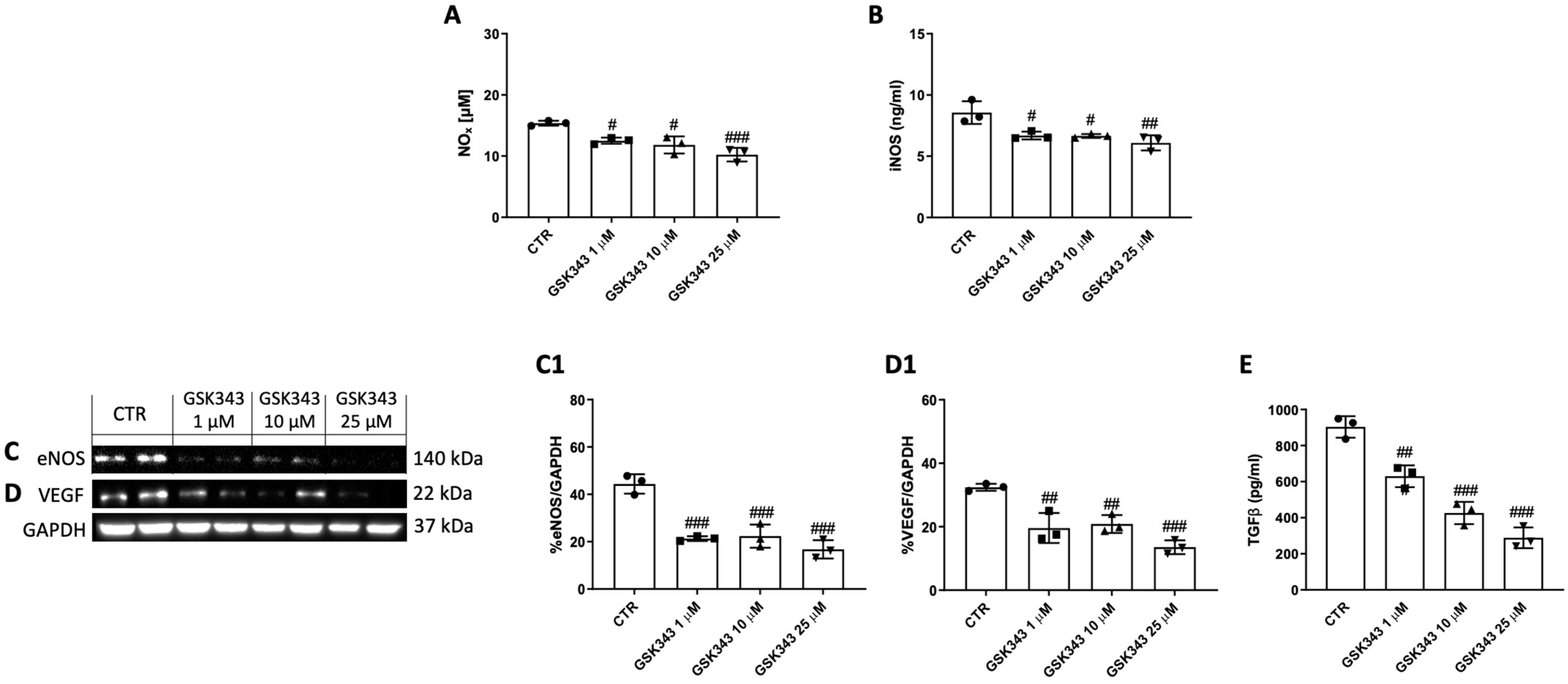
2.4. GSK343 Treatment Reduces NOx, iNOS, eNOS, VEGF, and TGFβ Levels in CAL27 Cells
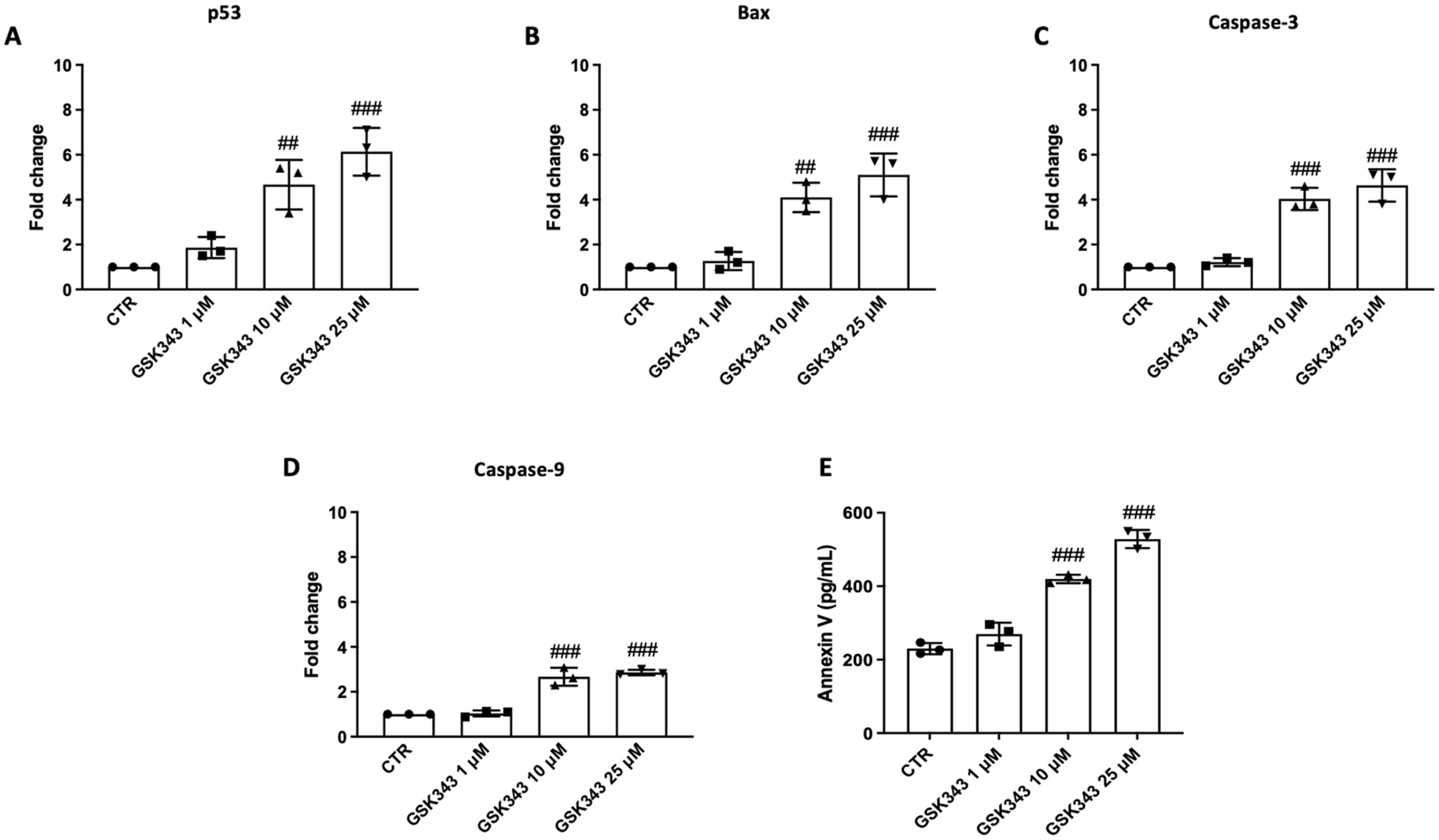
2.5. GSK343 Treatment Increases Apoptosis Process in CAL27 Cells
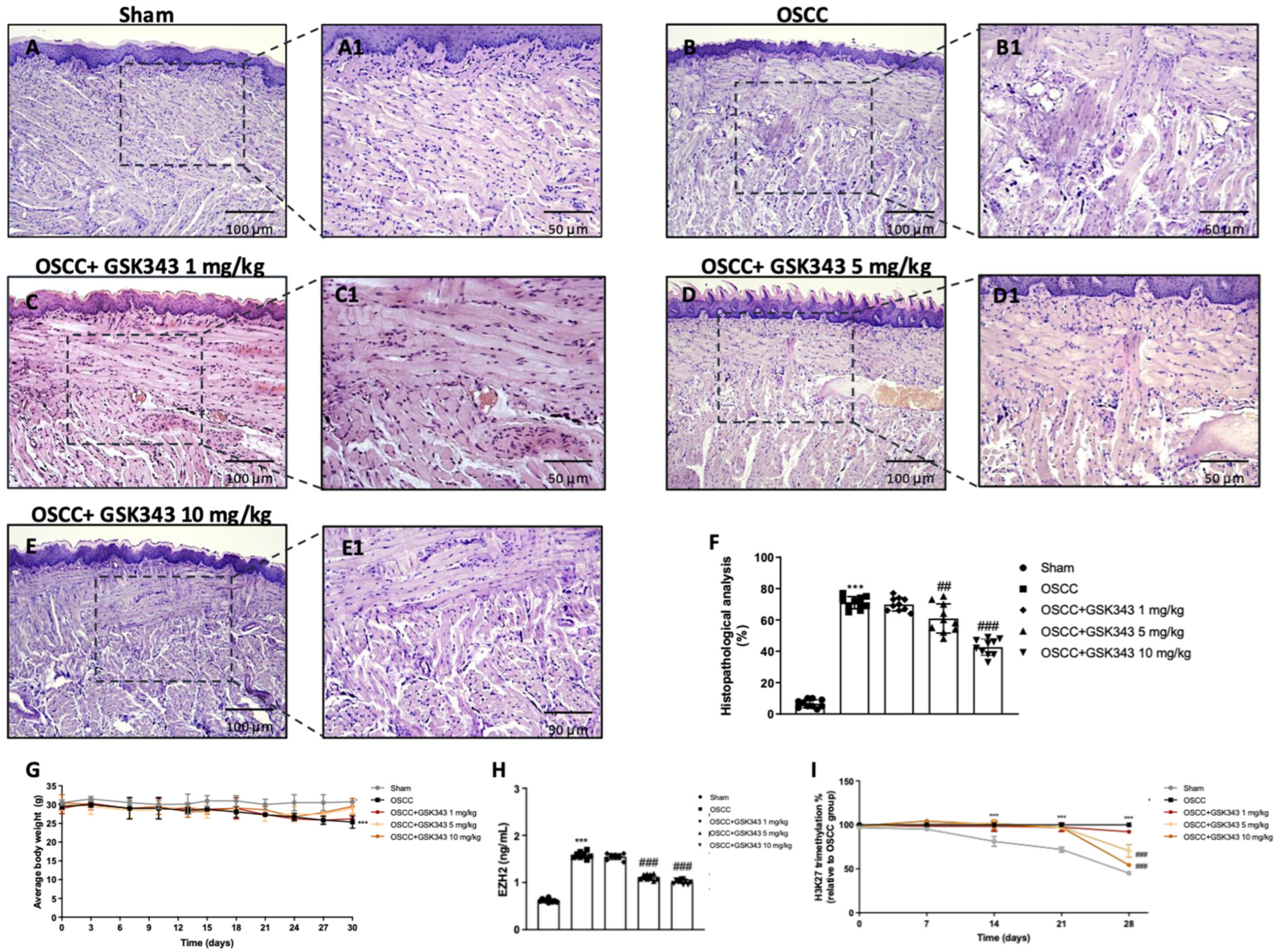
2.6. GSK343 Treatment Reduces In Vivo Tumor Growth through EZH2 Inhibition
2.7. GSK343 Treatment Reduces In Vivo Wnt/β-Catenin Pathway Activation
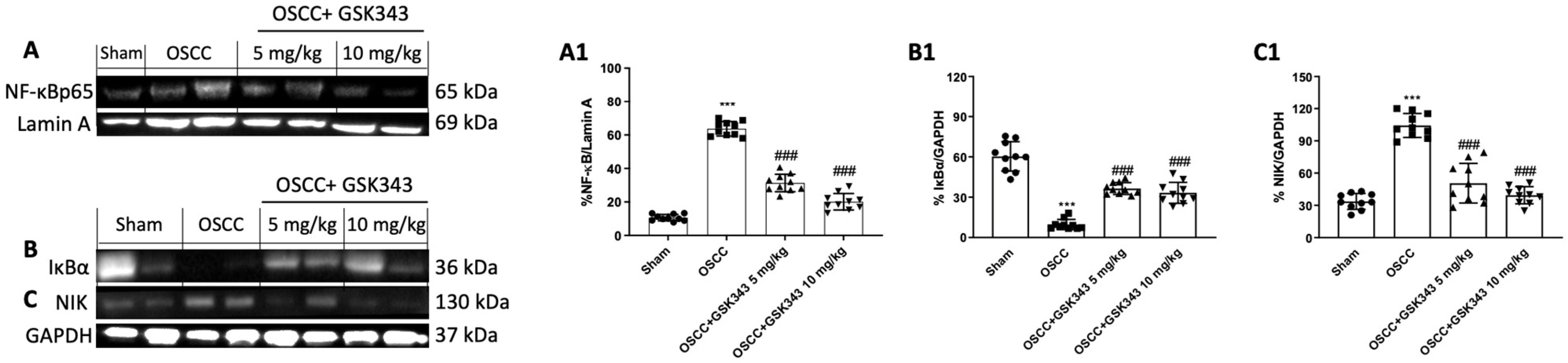
2.8. GSK343 Treatment Decreases In Vivo NF-κB/IκBα Pathway Activation
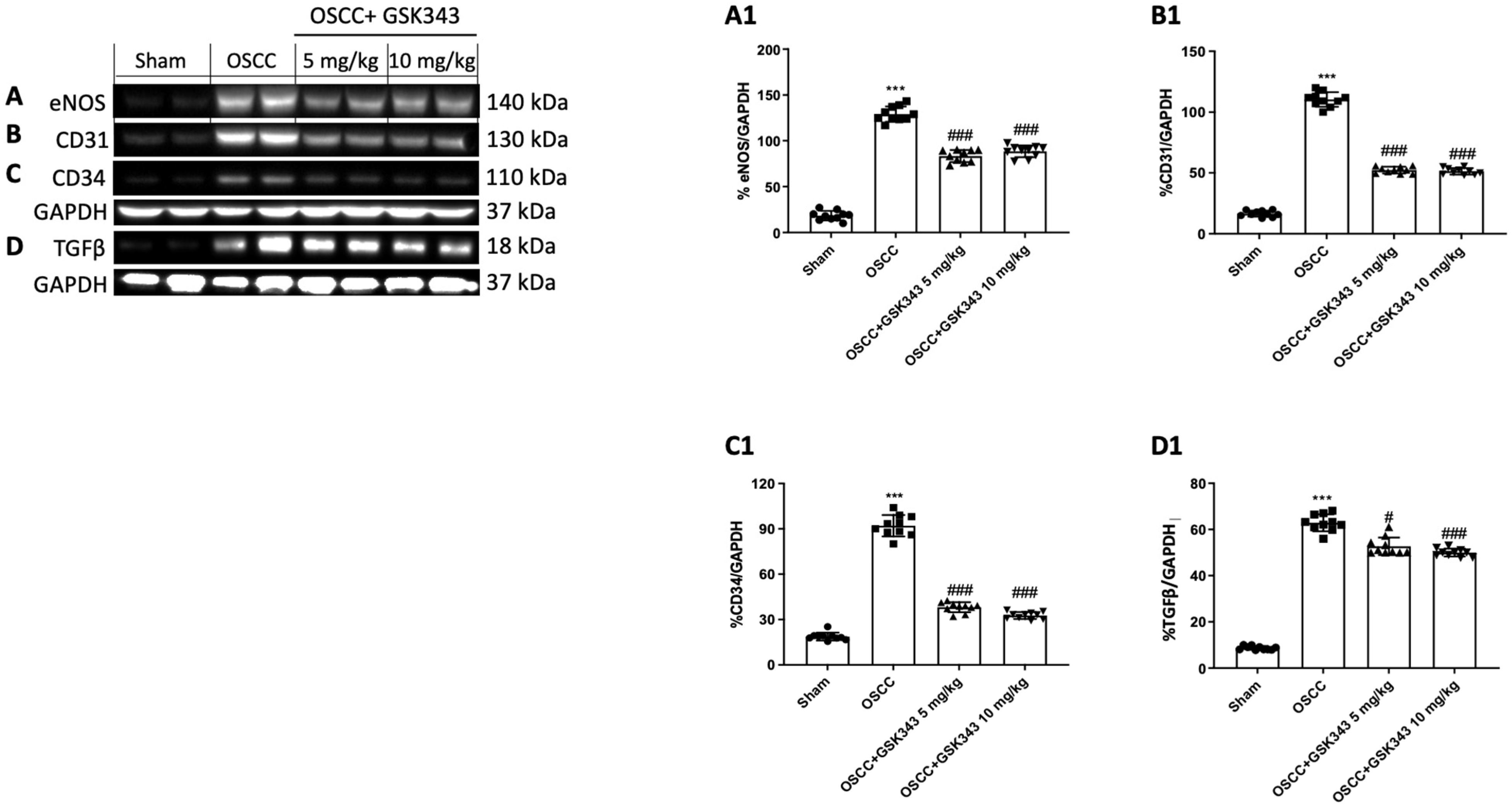
2.9. GSK343 Treatment Reduces In Vivo eNOS, TGFβ, CD31, and CD34 Expression
3. Discussion
4. Materials and Methods
4.1. In Vitro Studies
4.1.1. Cell Culture
4.1.2. MTT Assay
4.1.3. Cell Migration Assay
4.1.4. Immunoblotting
4.1.5. NOx Assay
4.1.6. Enzyme-Linked Immunosorbent Assay (ELISA) for EZH2, H3K27me3, NIK, IKKβ, TNFα, IL-10, TGFβ, iNOS, and Annexin V
4.1.7. RNA Isolation, cDNA Synthesis, and Real-Time Quantitative PCR Amplification
4.2. In Vivo Studies
4.2.1. Cell Line
4.2.2. Animals
4.2.3. OSCC Orthotopic Model
4.2.4. Histological Evaluation
4.2.5. Immunohistochemistry for β-Catenin and p-STAT3
4.2.6. Enzyme-Linked Immunosorbent Assay (ELISA) for EZH2, H3K27me3, Wnt-1, β-Catenin, and STAT3
4.2.7. Immunoblotting
4.3. Materials
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Head and neck squamous cell carcinoma | (HNSCC) |
| Oral squamous cell carcinoma | (OSCC) |
| Enhancer of zeste homolog 2 | (EZH2) |
| Histone H3 lysine27 | (H3K27) |
| Polycomb 2 repressive complex | (PRC2) |
| GlaxoSmithKline 343 | (GSK343) |
| Wnt family member 1 | (Wnt-1) |
| Signal transducer and activator of transcription 3 | (STAT3) |
| Nuclear factor κ-light-chain-enhancer of activated B cells | (NF-κB) |
| Inhibitor nuclear factor of kappa-light-chain-enhancer in B cells α | (IκBα) |
| Tumor necrosis factor-α | (TNF-α) |
| Interleukin-10 | (IL-10) |
| Vascular endothelial growth factor | (VEGF) |
| Endothelial nitric oxide synthase | (eNOS) |
| Transforming growth factor β | (TGFβ) |
References
- Solomon, B.; Young, R.J.; Rischin, D. Head and neck squamous cell carcinoma: Genomics and emerging biomarkers for immunomodulatory cancer treatments. Semin. Cancer Biol. 2018, 52 Pt 2, 228–240. [Google Scholar] [CrossRef]
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; O’Sullivan, B. Oral cancer: Current role of radiotherapy and chemotherapy. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e233–e240. [Google Scholar] [CrossRef]
- Ferreira, A.K.; Carvalho, S.; Granville-Garcia, A.; Sarmento, D.; Agripino, G.; Abreu, M.; Melo, M.; Caldas, A., Jr.; Godoy, G. Survival and prognostic factors in patients with oral squamous cell carcinoma. Med. Oral Patol. Oral Cir. Bucal 2021, 26, e387–e392. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yu, Y.; Wu, J.; Bai, J.; Zhao, Y.; Li, C.; Sun, W.; Wang, X. Role of EZH2 in oral squamous cell carcinoma carcinogenesis. Gene 2014, 537, 197–202. [Google Scholar] [CrossRef]
- Cao, W.; Younis, R.H.; Li, J.; Chen, H.; Xia, R.; Mao, L.; Chen, W.; Ren, H. EZH2 promotes malignant phenotypes and is a predictor of oral cancer development in patients with oral leukoplakia. Cancer Prev. Res. 2011, 4, 1816–1824. [Google Scholar] [CrossRef]
- Gan, L.; Yang, Y.; Li, Q.; Feng, Y.; Liu, T.; Guo, W. Epigenetic regulation of cancer progression by EZH2: From biological insights to therapeutic potential. Biomark. Res. 2018, 6, 10. [Google Scholar] [CrossRef]
- Batool, A.; Jin, C.; Liu, Y.X. Role of EZH2 in cell lineage determination and relative signaling pathways. Front. Biosci.-Landmark 2019, 24, 947–960. [Google Scholar] [CrossRef]
- Kim, K.H.; Roberts, C.W. Targeting EZH2 in cancer. Nat. Med. 2016, 22, 128–134. [Google Scholar] [CrossRef]
- Milan, T.M.; Eskenazi, A.P.E.; de Oliveira, L.D.; da Silva, G.; Bighetti-Trevisan, R.L.; Freitas, G.P.; Almeida, L.O. Interplay between EZH2/beta-catenin in stemness of cisplatin-resistant HNSCC and their role as therapeutic targets. Cell. Signal. 2023, 109, 110773. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/beta-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Liu, S.C.; Huang, C.S.; Huang, C.M.; Hsieh, M.S.; Huang, M.S.; Fong, I.H.; Yeh, C.T.; Lin, C.C. Isoorientin inhibits epithelial-to-mesenchymal properties and cancer stem-cell-like features in oral squamous cell carcinoma by blocking Wnt/beta-catenin/STAT3 axis. Toxicol. Appl. Pharmacol. 2021, 424, 115581. [Google Scholar] [CrossRef]
- Zheng, M.; Cao, M.; Luo, X.; Li, L.; Wang, K.; Wang, S.; Wang, H.; Tang, Y.; Liang, X. EZH2 promotes invasion and tumour glycolysis by regulating STAT3 and FoxO1 signalling in human OSCC cells. J. Cell. Mol. Med. 2019, 23, 6942–6954. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Verma, S.; Kushwaha, P.P.; Gupta, S. EZH2 and NF-kappaB: A context-dependent crosstalk and transcriptional regulation in cancer. Cancer Lett. 2023, 560, 216143. [Google Scholar] [CrossRef]
- Niklander, S.E. Inflammatory Mediators in Oral Cancer: Pathogenic Mechanisms and Diagnostic Potential. Front. Oral Health 2021, 2, 642238. [Google Scholar] [CrossRef] [PubMed]
- Glogauer, J.E.; Sun, C.X.; Bradley, G.; Magalhaes, M.A. Neutrophils Increase Oral Squamous Cell Carcinoma Invasion through an Invadopodia-Dependent Pathway. Cancer Immunol. Res. 2015, 3, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Q. The roles of EZH2 in cancer and its inhibitors. Med. Oncol. 2023, 40, 167. [Google Scholar] [CrossRef]
- Xiong, X.; Zhang, J.; Li, A.; Dai, L.; Qin, S.; Wang, P.; Liu, W.; Zhang, Z.; Li, X.; Liu, Z. GSK343 induces programmed cell death through the inhibition of EZH2 and FBP1 in osteosarcoma cells. Cancer Biol. Ther. 2020, 21, 213–222. [Google Scholar] [CrossRef]
- Yu, T.; Wang, Y.; Hu, Q.; Wu, W.; Wu, Y.; Wei, W.; Han, D.; You, Y.; Lin, N.; Liu, N. The EZH2 inhibitor GSK343 suppresses cancer stem-like phenotypes and reverses mesenchymal transition in glioma cells. Oncotarget 2017, 8, 98348–98359. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, L.; Qian, X.; Zhou, X.; Yan, Y.; Zhou, J.; Ge, W.; Albahde, M.; Wang, W. GSK343 induces autophagy and downregulates the AKT/mTOR signaling pathway in pancreatic cancer cells. Exp. Ther. Med. 2019, 18, 2608–2616. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Qiu, J.; Li, Q.; Yuan, C.; Zhang, W.; Wang, D.; Ye, J.; Jiang, H.; Yang, J.; et al. The polycomb group protein EZH2 is a novel therapeutic target in tongue cancer. Oncotarget 2013, 4, 2532–2549. [Google Scholar] [CrossRef]
- Scuderi, S.A.; Filippone, A.; Basilotta, R.; Mannino, D.; Casili, G.; Capra, A.P.; Chisari, G.; Colarossi, L.; Sava, S.; Campolo, M.; et al. GSK343, an Inhibitor of Enhancer of Zeste Homolog 2, Reduces Glioblastoma Progression through Inflammatory Process Modulation: Focus on Canonical and Non-Canonical NF-kappaB/IkappaBalpha Pathways. Int. J. Mol. Sci. 2022, 23, 13915. [Google Scholar] [CrossRef]
- Jiang, L.; Ji, N.; Zhou, Y.; Li, J.; Liu, X.; Wang, Z.; Chen, Q.; Zeng, X. CAL 27 is an oral adenosquamous carcinoma cell line. Oral Oncol. 2009, 45, e204–e207. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hu, H.; Miao, S.; Zheng, J.; Xie, Z.; Zhao, H. Anti-tumor effect of cisplatin in human oral squamous cell carcinoma was enhanced by andrographolide via upregulation of phospho-p53 in vitro and in vivo. Tumour Biol. 2017, 39, 1010428317705330. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, S.A.; Casili, G.; Basilotta, R.; Lanza, M.; Filippone, A.; Raciti, G.; Puliafito, I.; Colarossi, L.; Esposito, E.; Paterniti, I. NLRP3 Inflammasome Inhibitor BAY-117082 Reduces Oral Squamous Cell Carcinoma Progression. Int. J. Mol. Sci. 2021, 22, 11108. [Google Scholar] [CrossRef] [PubMed]
- Pflug, K.M.; Sitcheran, R. Targeting NF-kappaB-Inducing Kinase (NIK) in Immunity, Inflammation, and Cancer. Int. J. Mol. Sci. 2020, 21, 8470. [Google Scholar] [CrossRef]
- Lu, C.; Han, H.D.; Mangala, L.S.; Ali-Fehmi, R.; Newton, C.S.; Ozbun, L.; Armaiz-Pena, G.N.; Hu, W.; Stone, R.L.; Munkarah, A.; et al. Regulation of tumor angiogenesis by EZH2. Cancer Cell 2010, 18, 185–197. [Google Scholar] [CrossRef]
- Schluter, A.; Weller, P.; Kanaan, O.; Nel, I.; Heusgen, L.; Höing, B.; Haßkamp, P.; Zander, S.; Mandapathil, M.; Dominas, N.; et al. CD31 and VEGF are prognostic biomarkers in early-stage, but not in late-stage, laryngeal squamous cell carcinoma. BMC Cancer 2018, 18, 272. [Google Scholar] [CrossRef]
- Liu, L.; Shi, G.P. CD31: Beyond a marker for endothelial cells. Cardiovasc. Res. 2012, 94, 3–5. [Google Scholar] [CrossRef]
- Chabowski, M.; Nowak, A.; Grzegrzolka, J.; Piotrowska, A.; Janczak, D.; Dziegiel, P. Comparison of Microvessel Density Using Nestin and CD34 in Colorectal Cancer. Anticancer Res. 2018, 38, 3889–3895. [Google Scholar] [CrossRef] [PubMed]
- Pammar, C.; Nayak, R.S.; Kotrashetti, V.S.; Hosmani, J. Comparison of microvessel density using CD34 and CD105 in oral submucous fibrosis and its correlation with clinicopathological features: An immunohistochemical study. J. Cancer Res. Ther. 2018, 14, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Kademani, D.; Lewis, J.T.; Lamb, D.H.; Rallis, D.J.; Harrington, J.R. Angiogenesis and CD34 expression as a predictor of recurrence in oral squamous cell carcinoma. J. Oral Maxillofac. Surg. 2009, 67, 1800–1805. [Google Scholar] [CrossRef]
- Aggarwal, S.; Singh, B.; Sharma, S.C.; Das, S.N. Circulating Vimentin Over-Expression in Patients with Oral Sub Mucosal Fibrosis and Oral Squamous Cell Carcinoma. Indian J. Otolaryngol. Head Neck Surg. 2022, 74, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Miranda, T.B.; Cortez, C.C.; Yoo, C.B.; Liang, G.; Abe, M.; Kelly, T.K.; Marquez, V.E.; Jones, P.A. DZNep is a global histone methylation inhibitor that reactivates developmental genes not silenced by DNA methylation. Mol. Cancer Ther. 2009, 8, 1579–1588. [Google Scholar] [CrossRef]
- Verma, S.K.; Tian, X.; LaFrance, L.V.; Duquenne, C.; Suarez, D.P.; Newlander, K.A.; Romeril, S.P.; Burgess, J.L.; Grant, S.W.; Brackley, J.A.; et al. Identification of Potent, Selective, Cell-Active Inhibitors of the Histone Lysine Methyltransferase EZH2. ACS Med. Chem. Lett. 2012, 3, 1091–1096. [Google Scholar] [CrossRef]
- Roskelley, C.D.; Williams, D.E.; McHardy, L.M.; Leong, K.G.; Troussard, A.; Karsan, A.; Andersen, R.J.; Dedhar, S.; Roberge, M. Inhibition of tumor cell invasion and angiogenesis by motuporamines. Cancer Res. 2001, 61, 6788–6794. [Google Scholar] [PubMed]
- Chien, Y.C.; Liu, L.C.; Ye, H.Y.; Wu, J.Y.; Yu, Y.L. EZH2 promotes migration and invasion of triple-negative breast cancer cells via regulating TIMP2-MMP-2/-9 pathway. Am. J. Cancer Res. 2018, 8, 422–434. [Google Scholar]
- Furuta, H.; Osawa, K.; Shin, M.; Ishikawa, A.; Matsuo, K.; Khan, M.; Aoki, K.; Ohya, K.; Okamoto, M.; Tominaga, K.; et al. Selective inhibition of NF-kappaB suppresses bone invasion by oral squamous cell carcinoma in vivo. Int. J. Cancer 2012, 131, E625–E635. [Google Scholar] [CrossRef]
- Dardis, G.J.; Wang, J.; Simon, J.M.; Wang, G.G.; Baldwin, A.S. An EZH2-NF-kappaB regulatory axis drives expression of pro-oncogenic gene signatures in triple negative breast cancer. iScience 2023, 26, 107115. [Google Scholar] [CrossRef]
- Chiamulera, M.M.A.; Zancan, C.B.; Remor, A.P.; Cordeiro, M.F.; Gleber-Netto, F.O.; Baptistella, A.R. Salivary cytokines as biomarkers of oral cancer: A systematic review and meta-analysis. BMC Cancer 2021, 21, 205. [Google Scholar] [CrossRef] [PubMed]
- Hamzavi, M.; Tadbir, A.A.; Rezvani, G.; Ashraf, M.J.; Fattahi, M.J.; Khademi, B.; Sardari, Y.; Jeirudi, N. Tissue expression, serum and salivary levels of IL-10 in patients with head and neck squamous cell carcinoma. Asian Pac. J. Cancer Prev. 2013, 14, 1681–1685. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Sung, W.-W.; Su, T.-C.; Chen, M.-K.; Wu, P.-R.; Yeh, K.-T.; Ko, J.-L.; Lee, H. High expression of interleukin 10 might predict poor prognosis in early stage oral squamous cell carcinoma patients. Clin. Chim. Acta 2013, 415, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, X.; Luo, H.; Guo, H.; Zhang, J.; Chen, Z.; Lin, F.; Zhao, G. EZH2 can be used as a therapeutic agent for inhibiting endothelial dysfunction. Biochem. Pharmacol. 2023, 213, 115594. [Google Scholar] [CrossRef]
- Dreger, H.; Ludwig, A.; Weller, A.; Baumann, G.; Stangl, V.; Stangl, K. Epigenetic suppression of iNOS expression in human endothelial cells: A potential role of Ezh2-mediated H3K27me3. Genomics 2016, 107, 145–149. [Google Scholar] [CrossRef]
- Sangle, V.; Chaware, S.; Kulkarni, M.; Ingle, Y.; Singh, P.; Pooja, V. Elevated tissue nitric oxide in oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2018, 22, 35–39. [Google Scholar] [CrossRef]
- Augustine, D.; Sekar, B.; Murali, S.; Ramesh, M.; Madhavan, R.N.; Patil, S.G.; Rao, R.S. Expression of inducible nitric oxide synthase in carcinomas and sarcomas affecting the oral cavity. South Asian J. Cancer 2015, 4, 78–82. [Google Scholar] [CrossRef]
- Ucuzian, A.A.; Gassman, A.A.; East, A.T.; Greisler, H.P. Molecular mediators of angiogenesis. J. Burn Care Res. 2010, 31, 158–175. [Google Scholar] [CrossRef]
- Lu, S.L.; Reh, D.; Li, A.G.; Woods, J.; Corless, C.L.; Kulesz-Martin, M.; Wang, X.J. Overexpression of transforming growth factor beta1 in head and neck epithelia results in inflammation, angiogenesis, and epithelial hyperproliferation. Cancer Res. 2004, 64, 4405–4410. [Google Scholar] [CrossRef]
- Zhang, L.; Qu, J.; Qi, Y.; Duan, Y.; Huang, Y.W.; Zhou, Z.; Li, P.; Yao, J.; Huang, B.; Zhang, S.; et al. EZH2 engages TGFbeta signaling to promote breast cancer bone metastasis via integrin beta1-FAK activation. Nat. Commun. 2022, 13, 2543. [Google Scholar] [CrossRef]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-beta-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef] [PubMed]
- Paterniti, I.; Scuderi, S.A.; Casili, G.; Lanza, M.; Mare, M.; Giuffrida, R.; Colarossi, C.; Portelli, M.; Cuzzocrea, S.; Esposito, E. Poly (ADP-Ribose) Polymerase Inhibitor, ABT888, Improved Cisplatin Effect in Human Oral Cell Carcinoma. Biomedicines 2021, 9, 771. [Google Scholar] [CrossRef] [PubMed]
- Verzella, D.; Pescatore, A.; Capece, D.; Vecchiotti, D.; Ursini, M.V.; Franzoso, G.; Alesse, E.; Zazzeroni, F. Life, death, and autophagy in cancer: NF-kappaB turns up everywhere. Cell Death Dis. 2020, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Padi, S.K.R.; Tindall, D.J.; Guo, B. Polycomb protein EZH2 suppresses apoptosis by silencing the proapoptotic miR-31. Cell Death Dis. 2014, 5, e1486. [Google Scholar] [CrossRef]
- Dasgupta, M.; Dermawan, J.K.T.; Willard, B.; Stark, G.R. STAT3-driven transcription depends upon the dimethylation of K49 by EZH2. Proc. Natl. Acad. Sci. USA 2015, 112, 3985–3990. [Google Scholar] [CrossRef]
- Kim, E.; Kim, M.; Woo, D.-H.; Shin, Y.; Shin, J.; Chang, N.; Oh, Y.T.; Kim, H.; Rheey, J.; Nakano, I.; et al. Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells. Cancer Cell 2013, 23, 839–852. [Google Scholar] [CrossRef]
- Mitić, T.; Caporali, A.; Floris, I.; Meloni, M.; Marchetti, M.; Urrutia, R.; Angelini, G.D.; Emanueli, C. EZH2 modulates angiogenesis in vitro and in a mouse model of limb ischemia. Mol. Ther. 2015, 23, 32–42. [Google Scholar] [CrossRef]
- Yodavudh, S.; Tangjitgamol, S.; Puangsa-art, S. Prognostic significance of microvessel density and mast cell density for the survival of Thai patients with primary colorectal cancer. J. Med. Assoc. Thai. 2008, 91, 723–732. [Google Scholar]
- Maqsood, A.; Ali, A.; Zaffar, Z.; Mokeem, S.; Mokeem, S.S.; Ahmed, N.; Al-Hamoudi, N.; Vohra, F.; Javed, F.; Abduljabbar, T. Expression of CD34 and alpha-SMA Markers in Oral Squamous Cell Carcinoma Differentiation. A Histological and Histo-Chemical Study. Int. J. Environ. Res. Public Health 2020, 18, 192. [Google Scholar] [CrossRef]
- Monteiro-Amado, F.; Castro-Silva, I.I.; Lima, C.J.D.; Soares, F.A.; Kowalski, L.P.; Granjeiro, J.M. Immunohistochemical evaluation of MMP-2, MMP-9 and CD31/microvascular density in squamous cell carcinomas of the floor of the mouth. Braz. Dent. J. 2013, 24, 3–9. [Google Scholar] [CrossRef]
- Scuderi, S.A.; Casili, G.; Ardizzone, A.; Forte, S.; Colarossi, L.; Sava, S.; Paterniti, I.; Esposito, E.; Cuzzocrea, S.; Campolo, M. KYP-2047, an Inhibitor of Prolyl-Oligopeptidase, Reduces GlioBlastoma Proliferation through Angiogenesis and Apoptosis Modulation. Cancers 2021, 13, 3444. [Google Scholar] [CrossRef]
- Budi, H.S.; Anitasari, S.; Ulfa, N.M.; Setiabudi, M.A.; Ramasamy, R.; Wu, C.-Z.; Shen, Y.-K. Palmitic acid of Musa Paradisiaca induces apoptosis through caspase-3 in human oral squamous cell carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 7099–7114. [Google Scholar]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Sun, J. RASSF-1A modulates proliferation-mediated oral squamous cell carcinoma progression. Cancer Cell Int. 2019, 19, 213. [Google Scholar] [CrossRef]
- Chen, H.; Wang, M.; Qi, M.; Tian, Z.; Wu, W.; Yang, J.; Zhang, M.; Tang, L.; Tang, X. The antilymphatic metastatic effect of hyaluronic acid in a mouse model of oral squamous cell carcinoma. Cancer Biol. Ther. 2020, 21, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Su, Y.; Chen, S.; Wen, J.; Gao, F.; Wu, Y.; Zhang, X. MMP-9 Knockdown Inhibits Oral Squamous Cell Carcinoma Lymph Node Metastasis in the Nude Mouse Tongue-Xenografted Model through the RhoC/Src Pathway. Anal. Cell Pathol. 2021, 2021, 6683391. [Google Scholar] [CrossRef] [PubMed]
- Valentim, A.M.; Guedes, S.R.; Pereira, A.M.; Antunes, L.M. Euthanasia using gaseous agents in laboratory rodents. Lab. Anim. 2016, 50, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Hu, Q.; Chen, X.; Wang, C.; Zhang, J.; Ren, X.; Wang, Y.; Tao, X.; Li, H.; Song, M.; et al. Diet-induced obesity accelerates oral carcinogenesis by recruitment and functional enhancement of myeloid-derived suppressor cells. Cell Death Dis. 2021, 12, 946. [Google Scholar] [CrossRef]
- Scuderi, S.A.; Casili, G.; Filippone, A.; Lanza, M.; Basilotta, R.; Giuffrida, R.; Munaò, S.; Colarossi, L.; Capra, A.P.; Esposito, E.; et al. Beneficial effect of KYP-2047, a propyl-oligopeptidase inhibitor, on oral squamous cell carcinoma. Oncotarget 2021, 12, 2459–2473. [Google Scholar] [CrossRef]


| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| p53 | -AGAGTCTATAGGCCCACCCC- | -GCTCGACGCTAGGATCTGAC- |
| Bax | -GGACGAACTGGACAGTAACATG- | -GCAAAGTAGAAAAGGGCGACA- |
| Caspase-3 | -CTGAGGCATGGTGAAGAAGGA- | -GTCCAGTTCTGTACCACGGCA- |
| Caspase-9 | -TGCGAACTAACAGGCAAGCA- | -GTCTGAACCTCTCTGGTTTGC- |
| β-actin | -GACTTCGAGCAAGAGATGG- | -AGCACTGTGTGGCGTACAG- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campolo, M.; Scuderi, S.A.; Filippone, A.; Bova, V.; Lombardo, S.P.; Colarossi, L.; Sava, S.; Capra, A.P.; De Gaetano, F.; Portelli, M.; et al. EZH2 Inhibition to Counteract Oral Cancer Progression through Wnt/β-Catenin Pathway Modulation. Pharmaceuticals 2024, 17, 1102. https://doi.org/10.3390/ph17081102
Campolo M, Scuderi SA, Filippone A, Bova V, Lombardo SP, Colarossi L, Sava S, Capra AP, De Gaetano F, Portelli M, et al. EZH2 Inhibition to Counteract Oral Cancer Progression through Wnt/β-Catenin Pathway Modulation. Pharmaceuticals. 2024; 17(8):1102. https://doi.org/10.3390/ph17081102
Chicago/Turabian StyleCampolo, Michela, Sarah Adriana Scuderi, Alessia Filippone, Valentina Bova, Sofia Paola Lombardo, Lorenzo Colarossi, Serena Sava, Anna Paola Capra, Federica De Gaetano, Marco Portelli, and et al. 2024. "EZH2 Inhibition to Counteract Oral Cancer Progression through Wnt/β-Catenin Pathway Modulation" Pharmaceuticals 17, no. 8: 1102. https://doi.org/10.3390/ph17081102
APA StyleCampolo, M., Scuderi, S. A., Filippone, A., Bova, V., Lombardo, S. P., Colarossi, L., Sava, S., Capra, A. P., De Gaetano, F., Portelli, M., Militi, A., Esposito, E., & Paterniti, I. (2024). EZH2 Inhibition to Counteract Oral Cancer Progression through Wnt/β-Catenin Pathway Modulation. Pharmaceuticals, 17(8), 1102. https://doi.org/10.3390/ph17081102











