Anti-Inflammatory, Antinociceptive, and LC-MS Metabolic Profile from Pseudotrimezia juncifolia (Klatt) Lovo & A. Gil
Abstract
1. Introduction
2. Results
2.1. Nociception and Anti-Inflammatory Studies in P. juncifolia
2.1.1. Decoction of P. juncifolia Does Not Present Toxic Effects
2.1.2. P. juncifolia Reduces Chemical-Induced Nociception
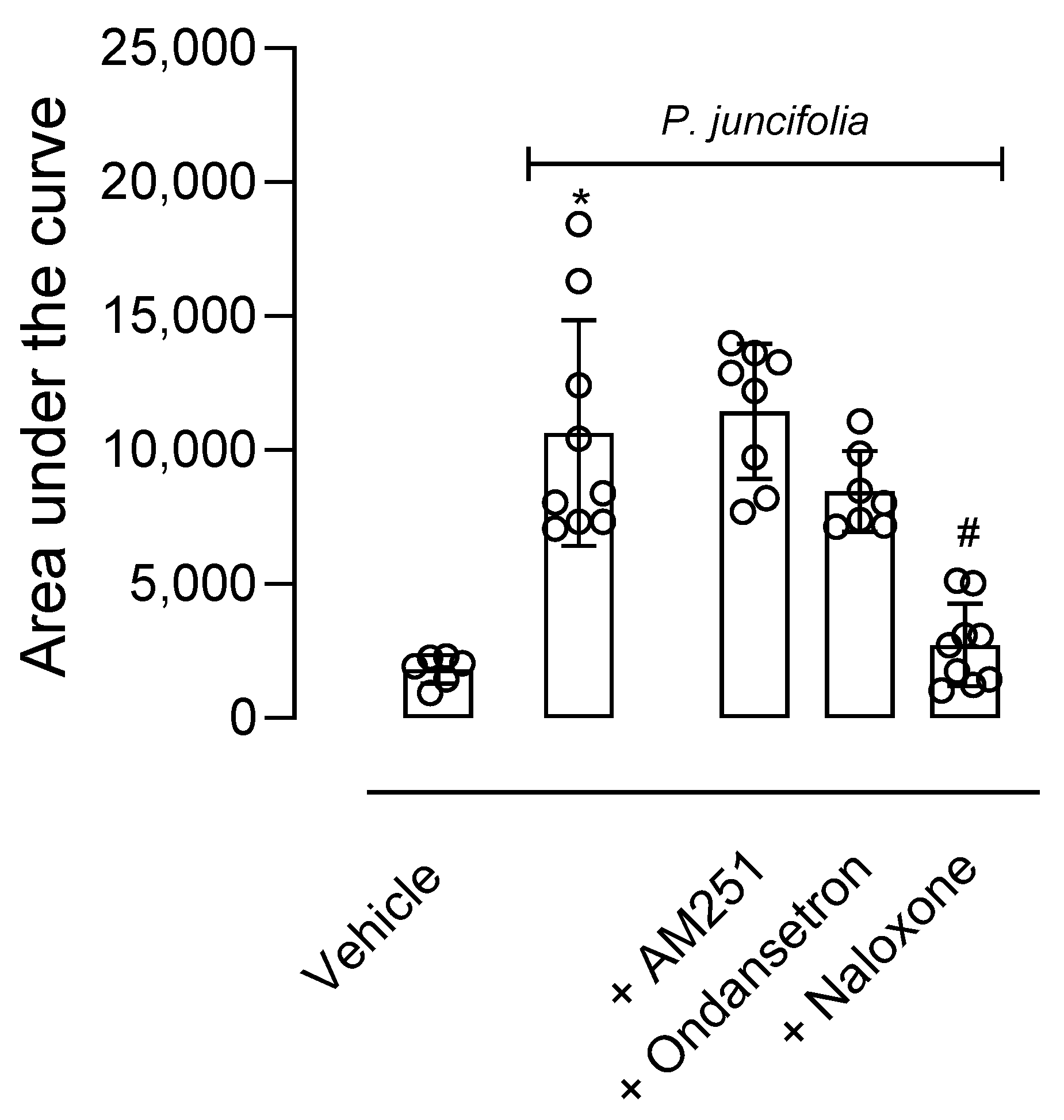
2.1.3. P. juncifolia Reduces Thermal Nociception and Its Possible Mechanism of Action
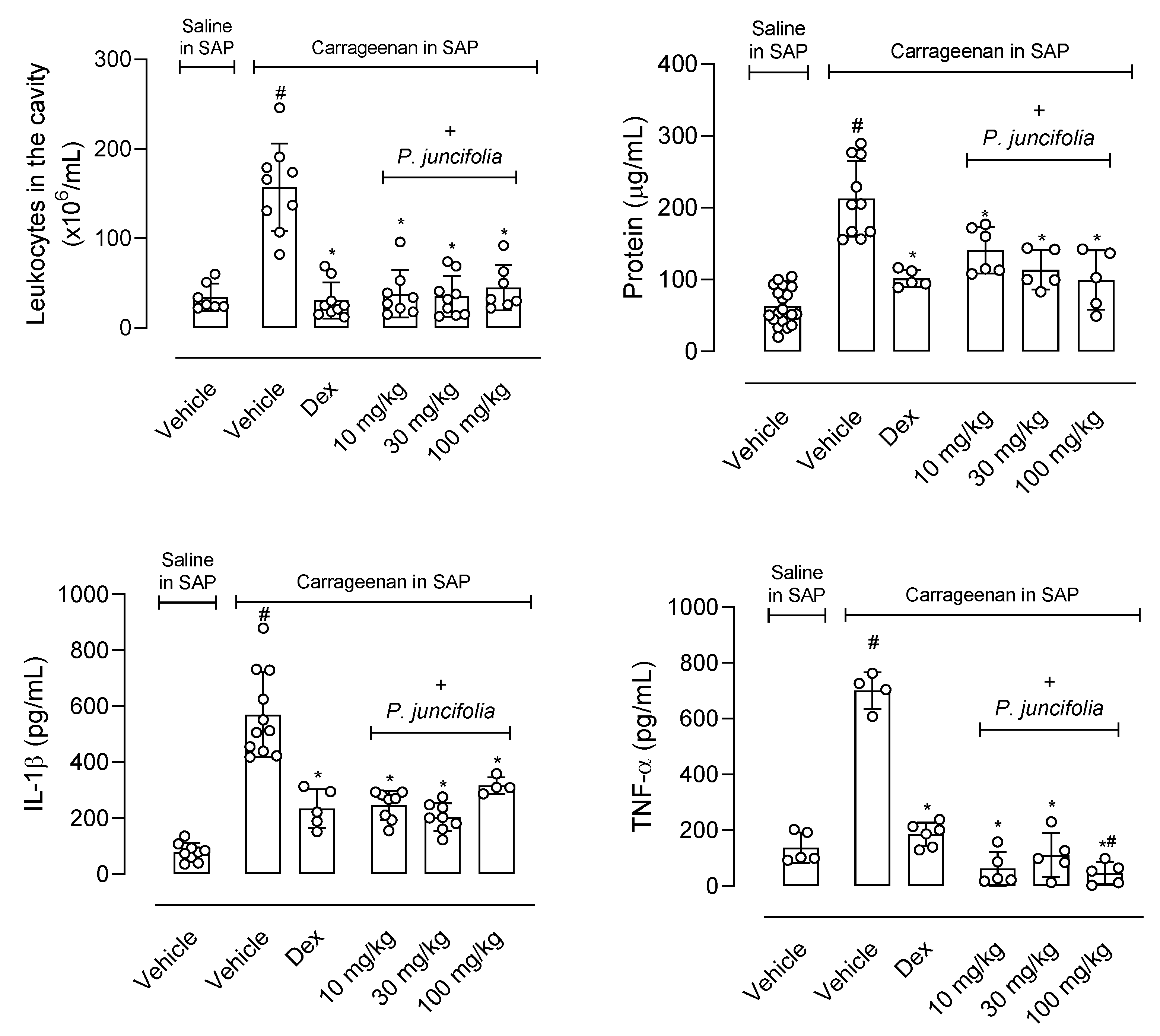
2.1.4. P. juncifolia Presents an Anti-Inflammatory Effect
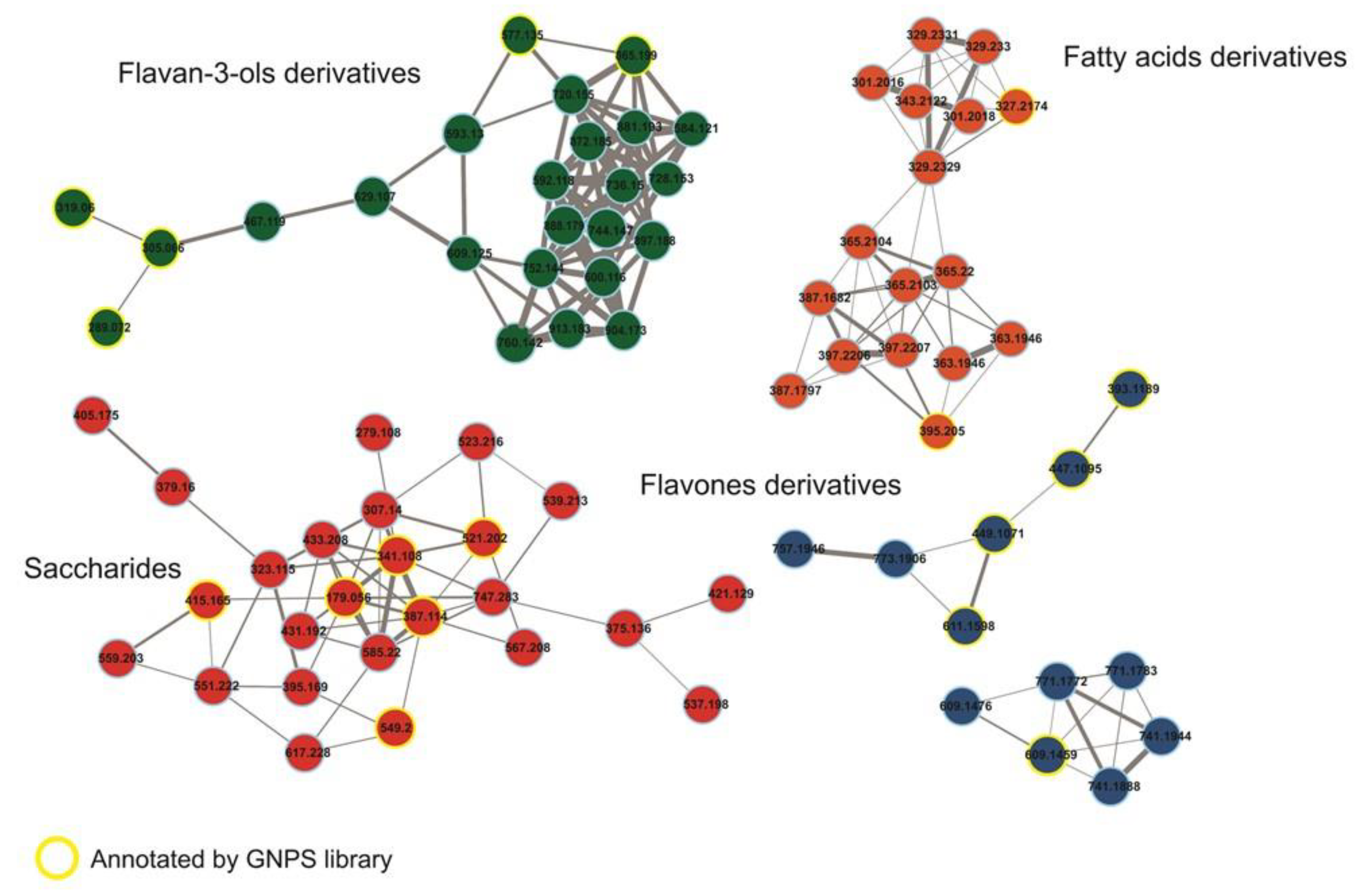
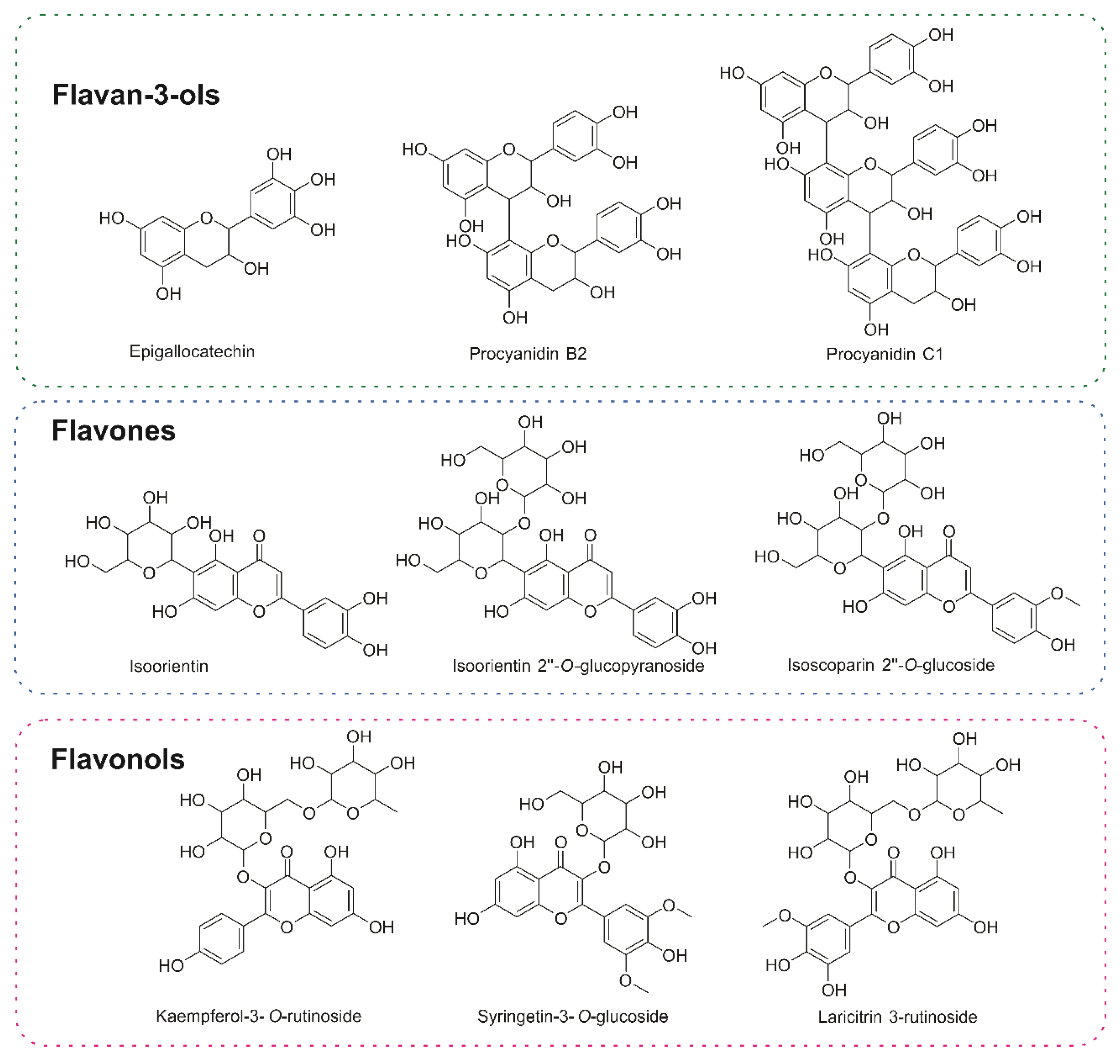
2.2. Metabolomic Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Lyophilized Preparation
4.2. MS Study
4.3. Animals
4.4. Reagents and Laboratories Supplies
4.5. Administration of Pseudotrimezia juncifolia and Drugs
4.6. In Vivo Toxicity Test
4.7. Formalin-Induced Nociception
4.8. Capsaicin-Induced Nociception
4.9. Thermal-Induced Nociception (Hot Plate Test) and Mechanism of Action
4.10. Carrageenan-Induced Leukocyte Migration into the Subcutaneous Air Pouch (SAP)
4.11. Quantification of TNF-α, IL-1β, IFN-γ and Protein
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernstein, N.; Akram, M.; Daniyal, M.; Koltai, H.; Fridlender, M.; Gorelick, J. Anti-inflammatory Potential of Medicinal Plants: A Source for Therapeutic Secondary Metabolites. Adv. Agron. 2018, 150, 131–183. [Google Scholar]
- World Health Organization. WHO Global Report on Traditional and Complementary Medicine 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Riaz, M.; Khalid, R.; Afzal, M.; Anjum, F.; Fatima, H.; Zia, S.; Rasool, G.; Egbuna, C.; Mtewa, A.G.; Uche, C.Z.; et al. Phytobioactive compounds as therapeutic agents for human diseases: A review. Food Sci. Nutr. 2023, 11, 2500–2529. [Google Scholar] [CrossRef]
- Fagg, C.W.; Lughadha, E.N.; Milliken, W.; Nicholas, H.D.J.; Brandão, M.G. Useful Brazilian plants listed in the manuscripts and publications of the Scottish medic and naturalist George Gardner (1812–1849). J. Ethnopharmacol. 2015, 161, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Godinho, C.C. Caracterização Morfo-Anatômica de Beressol [Trimezia juncifolia (Klatt.) Benth]. Undergraduate Thesis, Universidade Estadual Paulista, São Paulo, Brazil, 2012. [Google Scholar]
- Yazbek, P.B.; Tezoto, J.; Cassas, F.; Rodrigues, E. Plants used during maternity, menstrual cycle and other women's health conditions among Brazilian cultures. J. Ethnopharmacol. 2016, 17, 310–331. [Google Scholar] [CrossRef] [PubMed]
- Almeida, V.O.; Di-Medeiros, M.C.B.; Batista, K.A.; Moraes, M.G.; Fernandes, K.F. Morphological and physicochemical characterization of starches from underground stems of Trimezia juncifolia collected in different phenological stages. Int. J. Biol. Macromol. 2021, 166, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Pilon, A.C.; Selegato, D.M.; Fernandes, R.P.; Bueno, P.C.P.; Pinho, D.R.; Carnevale Neto, F.; Freire, R.T.; Castro-Gamboa, I.; Bolzani, V.B.; Lopes, N.P. Plant metabolomics: Methods and challenges. Quim. Nova 2020, 43, 329–354. [Google Scholar]
- Lopes, N.P.; Stark, C.B.W.; Gates, P.J.; Staunton, J. Fragmentation studies on monensin A by sequential electrospray mass spectrometry. Analyst 2002, 127, 503–506. [Google Scholar] [CrossRef]
- Crotti, A.E.M.; Lopes, J.L.C.; Lopes, N.P. Triple quadrupole tandem mass spectrometry of sesquiterpene lactones: A study of goyazensolide and its congeners. J. Mass. Spectrom. 2005, 40, 1030–1034. [Google Scholar] [CrossRef]
- Misra, B.B. New software tools, databases, and resources in metabolomics: Updates from 2020. Metabolomics 2021, 17, 49. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828. [Google Scholar] [CrossRef]
- De Villiers, A.; Venter, P.; Pasch, H. Recent advances and trends in the liquid-chromatography-mass spectrometry analysis of flavonoids. J. Chromat. 2015, 1430, 16–78. [Google Scholar] [CrossRef] [PubMed]
- Pilon, A.C.; Gu, H.; Raftery, D.; Bolzani, V.d.S.; Lopes, N.P.; Castro-Gamboa, I.; Carnevale Neto, F. Mass Spectral Similarity Networking and Gas-Phase Fragmentation Reactions in the Structural Analysis of Flavonoid Glycoconjugates. Anal. Chem. 2019, 91, 10413–10423. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R.; Wall, P.D. Evolution of pain theories. Int. Anesthesiol. Clin. 1970, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Martins, D. Serotonin and nociception: From nociceptive transduction at the periphery to pain modulation from the brain. In The Serotonin System: History, Neuropharmacology, and Pathology; Tricklebank, M.D., Daly, E., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2019; pp. 207–224. [Google Scholar]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1986, 30, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Woller, S.A.; Eddinger, K.A.; Corr, M.; Yaksh, T.L. An overview of pathways encoding nociception. Clin. Exp. Rheumatol. 2017, 107, 40–46. [Google Scholar]
- Raymundo, L.J.R.P.; Guilhon, C.C.; Alviano, D.S.; Matheus, M.E.; Antoniolli, A.R.; Cavalcanti, S.C.H.; Alves, P.B.; Alviano, C.S.; Fernandes, P.D. Characterisation of the anti-inflammatory and antinociceptive activities of the Hyptis pectinata (L.) Poit essential oil. J. Ethnopharmacol. 2011, 134, 725–732. [Google Scholar] [CrossRef]
- Keles, L.C.; Melo, N.I.; Aguiar, G.D.; Wakabayashi, K.A.; Carvalho, C.E.; Cunha, W.R.; Crotti, A.E.; Lopes, J.L.; Lopes, N.P. Lychnophorinae (asteraceae): A survey of its chemical constituents and biological activities. Quim. Nova 2010, 33, 2245. [Google Scholar] [CrossRef]
- Silva, N.L.; Saldanha, A.A.; Silva, D.B.; Carollo, C.A.; Sartori, A.L.B.; Soares, A.C.; de Siqueira, J.M. Anti-inflammatory, antinociceptive and antioxidant activities of the hydromethanolic fraction from Annona nutans leaves. Biosci. J. 2019, 35, 1599–1613. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Moreira, E.A.; Pilon, A.C.; Andrade, L.E.; Lopes, N.P. New Perspectives on Chlorogenic Acid Accumulation in Harvested Leaf Tissue: Impact on Traditional Medicine Preparations. ACS Omega 2018, 3, 18380–18386. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.D.; Gobbo-Neto, L.; Albarella, L.; de Souza, G.E.; Lopes, N.P. Analgesic activity of di-caffeoylquinic acids from roots of Lychnophora ericoides (Arnica da serra). J. Ethnopharmacol. 2005, 96, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.D.; Almeida, M.C.; Lopes, N.P.; Souza, G.E.P. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol. Pharm. Bull. 2004, 29, 2236–2240. [Google Scholar] [CrossRef]
- Santos, M.D.; Chen, G.; Almeida, M.C.; Soares, D.M.; Souza, G.E.P.; Lopes, M.P.; Lantz, L.C. Effects of caffeoylquinic acid derivatives and C-flavonoid from Lychnophora ericoides on in vitro inflammatory mediator production. Nat. Prod. Commun. 2010, 5, 733–740. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Ysrafil, Y.; Sapiun, Z.; Slamet, N.S.; Mohamad, F.; Hartati, H.; Damiti, S.A.; Alexandra, F.D.; Rahman, S.; Masyeni, S.; Harapan, H.; et al. Anti-inflammatory activities of flavonoid derivates. ADMET DMPK 2023, 11, 331–359. [Google Scholar] [CrossRef] [PubMed]
- Demarque, D.P.; Crotti, A.E.M.; Vissechi, E.; Lopes, J.L.C.; Lopes, N.P. Fragmentation reactions using electrospray ionization mass spectrometry: An important tool for the structural elucidation and characterization of synthetic and natural products. Nat. Prod. Rep. 2016, 33, 432–455. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical guidelines for investigation of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Sakurada, T.; Wako, K.; Sugiyama, A.; Sakurada, C.; Tan-NO, K.; Kisara, K. Involvement of spinal NMDA receptors in capsaicin-induced nociception. Pharmacol. Biochem. Behav. 1998, 59, 339–345. [Google Scholar] [CrossRef]
- Matheus, M.E.; Berrondo, L.F.; Vieitas, E.C.; Menezes, F.S.; Fernandes, P.D. Evaluation of the antinociceptive properties from Brillantaisia palisotii Lindau stems extracts. J. Ethnopharmacol. 2005, 102, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Sahley, T.L.; Berntson, G.G. Antinociceptive effects of central and systemic administration of nicotine in the rat. Psychopharmacology 1979, 65, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Meckling, K.A.; Marcone, M.F.; Kakuda, Y.; Tsao, R. Synergistic, additive, and antagonistic effects of food mixtures on total antioxidant capacities. J. Agric. Food Chem. 2011, 59, 960–968. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minho, A.S.; Almeida, P.G.d.; Kato, N.N.; Brand, A.L.M.; Vieira, R.F.; Garrett, R.; Lopes, N.P.; Rezende, C.M.; Fernandes, P.D. Anti-Inflammatory, Antinociceptive, and LC-MS Metabolic Profile from Pseudotrimezia juncifolia (Klatt) Lovo & A. Gil. Pharmaceuticals 2024, 17, 1101. https://doi.org/10.3390/ph17081101
Minho AS, Almeida PGd, Kato NN, Brand ALM, Vieira RF, Garrett R, Lopes NP, Rezende CM, Fernandes PD. Anti-Inflammatory, Antinociceptive, and LC-MS Metabolic Profile from Pseudotrimezia juncifolia (Klatt) Lovo & A. Gil. Pharmaceuticals. 2024; 17(8):1101. https://doi.org/10.3390/ph17081101
Chicago/Turabian StyleMinho, Alan Silva, Pamela Gomes de Almeida, Natália Naomi Kato, Ana Laura Macedo Brand, Roberto Fontes Vieira, Rafael Garrett, Norberto Peporine Lopes, Claudia Moraes Rezende, and Patricia Dias Fernandes. 2024. "Anti-Inflammatory, Antinociceptive, and LC-MS Metabolic Profile from Pseudotrimezia juncifolia (Klatt) Lovo & A. Gil" Pharmaceuticals 17, no. 8: 1101. https://doi.org/10.3390/ph17081101
APA StyleMinho, A. S., Almeida, P. G. d., Kato, N. N., Brand, A. L. M., Vieira, R. F., Garrett, R., Lopes, N. P., Rezende, C. M., & Fernandes, P. D. (2024). Anti-Inflammatory, Antinociceptive, and LC-MS Metabolic Profile from Pseudotrimezia juncifolia (Klatt) Lovo & A. Gil. Pharmaceuticals, 17(8), 1101. https://doi.org/10.3390/ph17081101







