Agave-Laurate-Bioconjugated Fructans Decrease Hyperinsulinemia and Insulin Resistance, Whilst Increasing IL-10 in Rats with Metabolic Syndrome Induced by a High-Fat Diet
Abstract
1. Introduction
2. Results
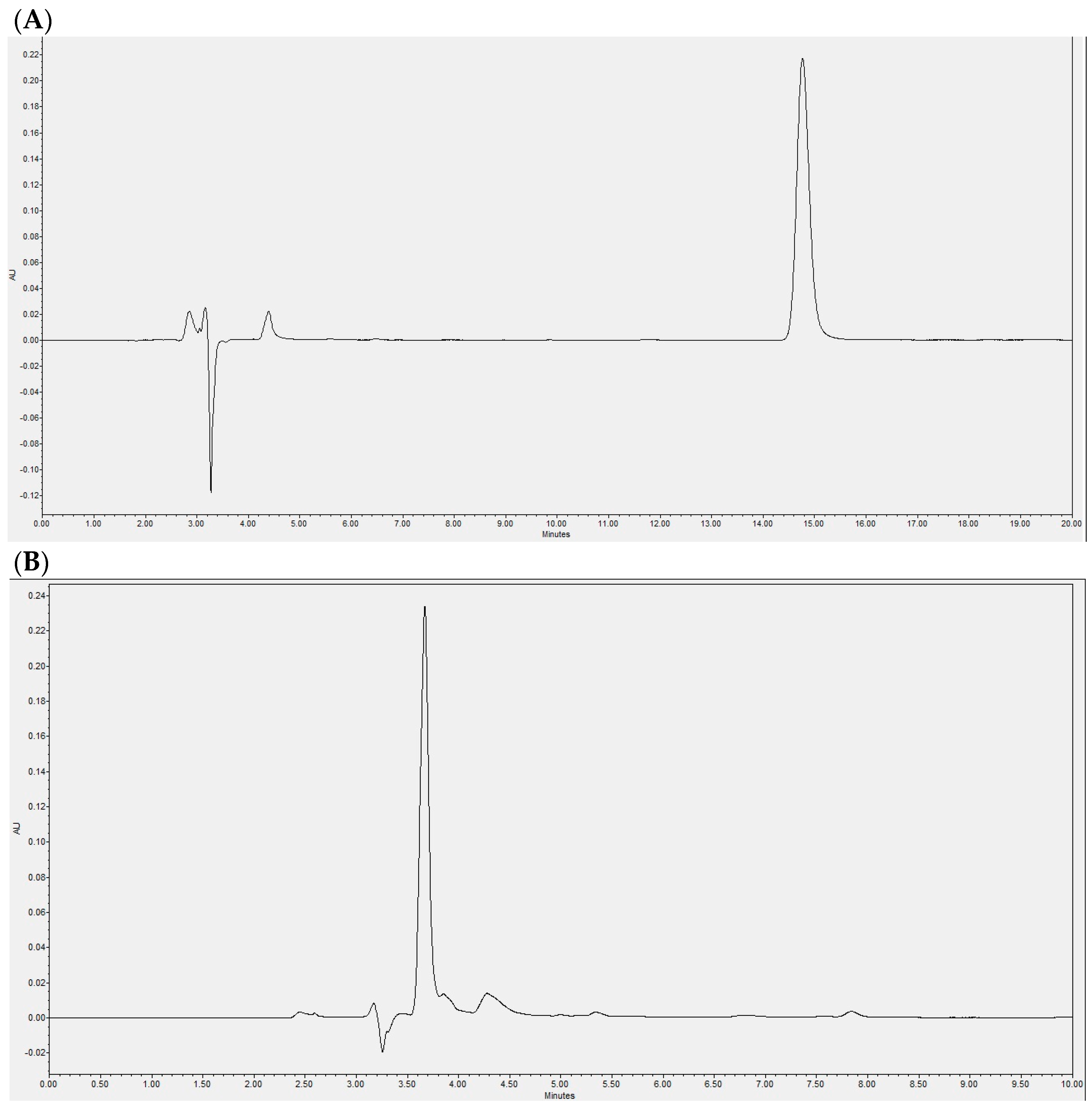
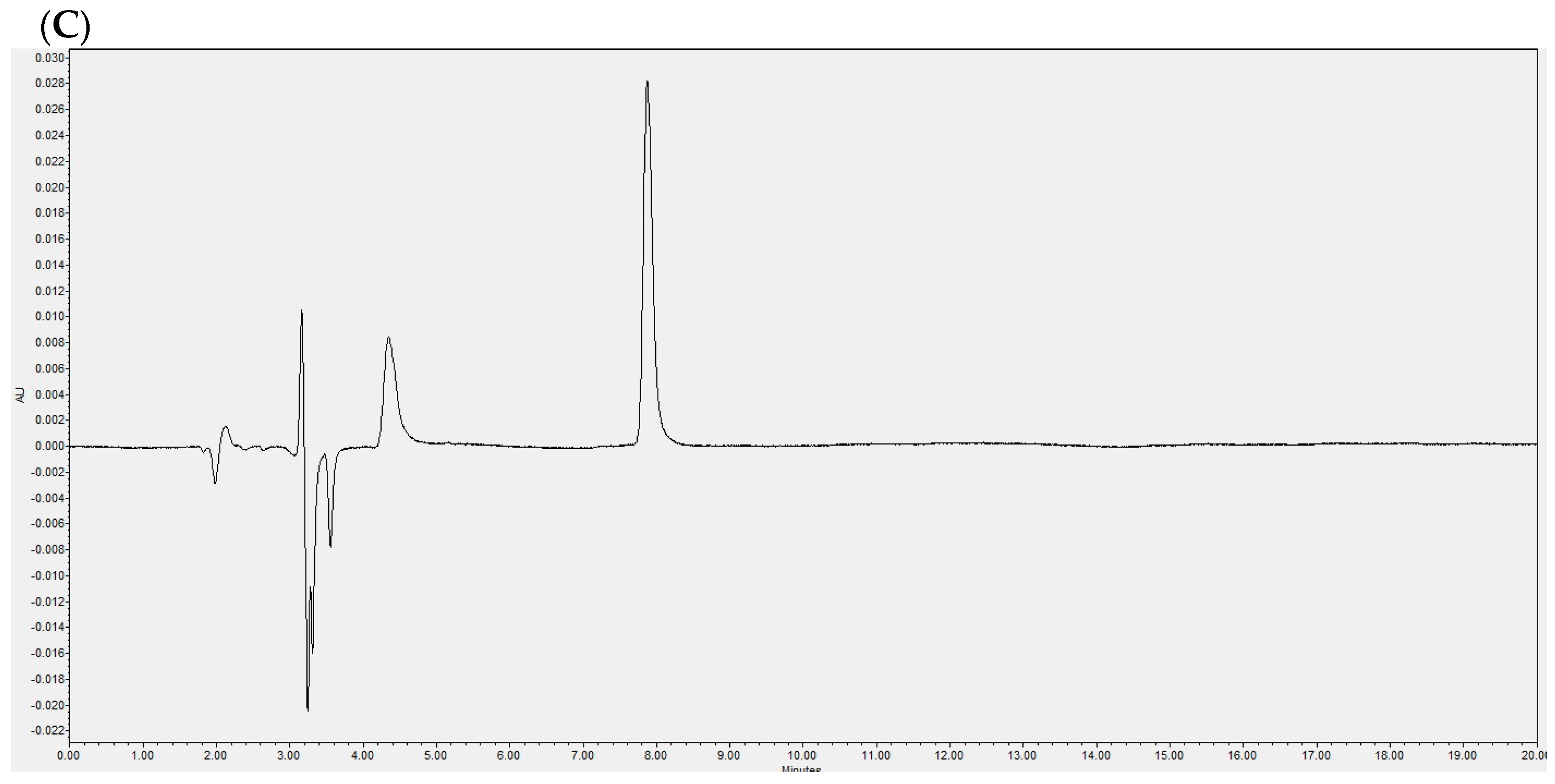
2.1. Laurate-Bioconjugated Fructan Synthesis
2.2. Laurate-Bioconjugated Fructans Reduce Food and Energy Intake
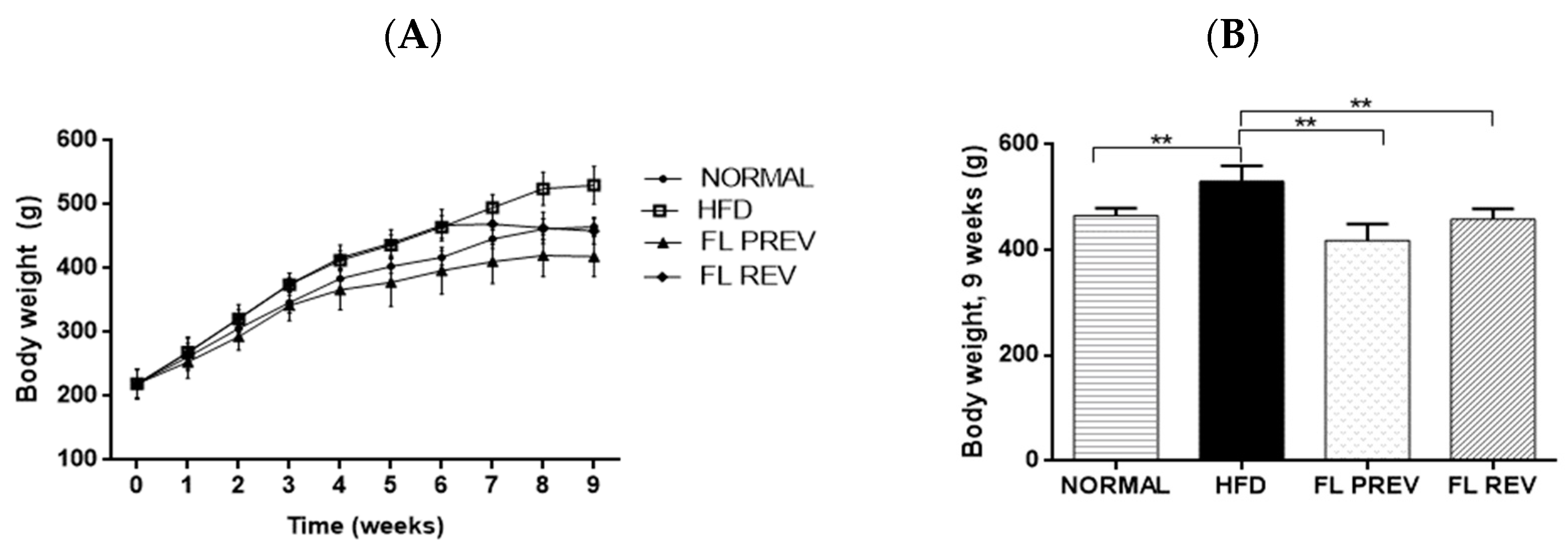
2.3. Laurate-Bioconjugated Fructans Prevent Body Weight Gain, Reverse Obesity, and Improve Metabolic Parameters
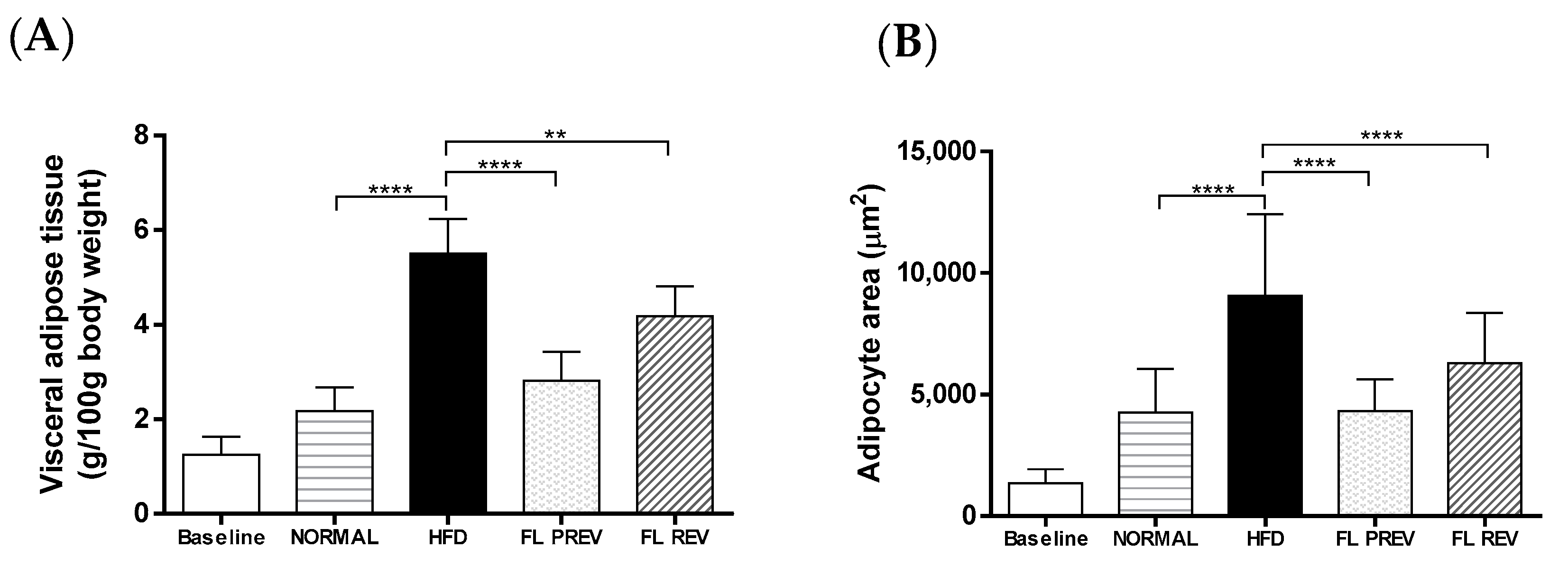
2.4. Laurate-Bioconjugated Fructans Prevent Adipose Tissue Accumulation with No Effect on Liver Histology and Weight
2.5. Laurate-Bioconjugated Fructans Reduce Visceral Adipocyte Area
2.6. Laurate-Bioconjugated Fructans Reduce Systolic Blood Pressure and Insulin Resistance
2.7. Effects of Laurate-Bioconjugated Fructans on Pro- and Anti-Inflammatory Markers
3. Discussion
4. Materials and Methods
4.1. Synthesis of Agave Fructan Bioconjugates
4.2. High-Fat Diet and Food Intake
4.3. Experimental Animals
4.4. Blood Pressure
4.5. Zoometric Measurements, Histological Analysis, and Sample Collection
4.6. Biochemical Determinations
4.7. RNA Isolation and Synthesis of Complementary DNA
4.8. Analysis of Gene Expression
4.9. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, P.L. A Comprehensive Definition for Metabolic Syndrome. Dis. Model Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Lee, D.K.; Liu, M.; Portincasa, P.; Wang, D.Q.H. Novel Insights into the Pathogenesis and Management of the Metabolic Syndrome. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 189–230. [Google Scholar] [CrossRef] [PubMed]
- Fahed, G.; Aoun, L.; Zerdan, M.B.; Allam, S.; Zerdan, M.B.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Niu, M.; Chen, J.; Hou, R.; Sun, Y.; Xiao, Q.; Pan, X.; Zhu, X. Emerging Healthy Lifestyle Factors and All-Cause Mortality among People with Metabolic Syndrome and Metabolic Syndrome-like Characteristics in NHANES. J. Transl. Med. 2023, 21, 239. [Google Scholar] [CrossRef]
- Francisqueti, F.V.; Nascimento, A.F.; Minatel, I.O.; Dias, M.C.; De Azevedo Melo Luvizotto, R.; Berchieri-Ronchi, C.; Ferreira, A.L.A.; Corrêa, C.R. Metabolic Syndrome and Inflammation in Adipose Tissue Occur at Different Times in Animals Submitted to a High-Sugar/Fat Diet. J. Nutr. Sci. 2017, 6, e41. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Li, Y.; Shu, T.; Wang, J. Cytokines and Inflammation in Adipogenesis: An Updated Review. Frontiers of Medicine; Higher Education Press: Beijing, China, 2019; pp. 314–329. [Google Scholar] [CrossRef]
- Yeldu, M.H.; Mus’ab, A. Serum Biochemical and Inflammatory Cytokines in a Rat Model of Metabolic Syndrome. Asian J. Med. Sci. 2020, 11, 46–52. [Google Scholar] [CrossRef]
- Van Der Weerd, K.; Dik, W.A.; Schrijver, B.; Schweitzer, D.H.; Langerak, A.W.; Drexhage, H.A.; Kiewiet, R.M.; Van Aken, M.O.; Van Huisstede, A.; Van Dongen, J.J.M.; et al. Morbidly Obese Human Subjects Have Increased Peripheral Blood CD4+ T Cells with Skewing toward a Treg- and Th2-Dominated Phenotype. Diabetes 2012, 61, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Al-Mansoori, L.; Al-Jaber, H.; Prince, M.S.; Elrayess, M.A. Role of Inflammatory Cytokines, Growth Factors and Adipokines in Adipogenesis and Insulin Resistance. Inflammation 2022, 45, 31–44. [Google Scholar] [CrossRef]
- Jordão-Candido, C.; Silva-Figueiredo, P.; Del Ciampo-Silva, R.; Candeloro-Portugal, L.; Dos Santos-Jaques, J.A.; Alves de Almeida, J.; de Barros-Penteado, B.; Albuquerque-Dias, D.; Marcelino, G.; Pott, A.; et al. Protective Effect of α-Linolenic Acid on Non-Alcoholic Hepatic Steatosis and Interleukin-6 and -10 in Wistar Rats. Nutrients 2020, 12, 9. [Google Scholar] [CrossRef]
- Zahedi, A.S.; Daneshpour, M.S.; Akbarzadeh, M.; Hedayati, M.; Azizi, F.; Zarkesh, M. Association of Baseline and Changes in Adiponectin, Homocysteine, High-Sensitivity C-Reactive Protein, Interleukin-6, and Interleukin-10 Levels and Metabolic Syndrome Incidence: Tehran Lipid and Glucose Study. Heliyon 2023, 9, e19911. [Google Scholar] [CrossRef] [PubMed]
- Graßmann, S.; Wirsching, J.; Eichelmann, F.; Aleksandrova, K. Association between Peripheral Adipokines and Inflammation Markers: A Systematic Review and Meta-Analysis. Obesity 2017, 25, 1776–1785. [Google Scholar] [CrossRef]
- Nilsson, P.M.; Tuomilehto, J.; Rydén, L. The Metabolic Syndrome—What Is It and How Should It Be Man-Aged? Eur. J. Prev. Cardiol. 2019, 26 (Suppl. S2), 33–46. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- González-Garibay, A.S.; López-Vázquez, A.; García-Bañuelos, J.; Sánchez-Enríquez, S.; Sandoval-Rodríguez, A.S.; Del Toro Arreola, S.; Bueno-Topete, M.R.; Muñoz-Valle, J.F.; González Hita, M.E.; Domínguez-Rosales, J.A.; et al. Effect of Ursolic Acid on Insulin Resistance and Hyperinsulinemia in Rats with Diet-Induced Obesity: Role of Adipokines Expression. J. Med. Food 2020, 23, 297–304. [Google Scholar] [CrossRef]
- Fornari-Laurindo, L.; Minniti, G.; José Tofano, R.; Quesada, K.; Federighi Baisi Chagas, E.; MariaBarbalho, S. Detection of Metabolic Syndrome Using Insulin Resistance Indexes: A Cross-Sectional Observational Cohort Study. Endocrines 2023, 4, 257–268. [Google Scholar] [CrossRef]
- Antunes, L.C.; Elkfury, J.L.; Jornada, M.N.; Foletto, K.C.; Bertoluci, M.C. Validation of HOMA-IR in a Model of Insulin-Resistance Induced by a High-Fat Diet in Wistar Rats. Arch. Endocrinol. Metab. 2016, 60, 138–142. [Google Scholar] [CrossRef]
- Anwar, S.; Raut, R.; Alhumaydhi, F.A. A comprehensive investigation on alleviating oxidative stress and inflammation in hyperglycaemic conditions through in vitro experiments and computational analysis. Saudi J. Biol. Sci. 2024, 31, 104003. [Google Scholar] [CrossRef]
- Domingo, E.; Marques, P.; Francisco, V.; Piqueras, L.; Sanz, M.J. Targeting systemic inflammation in metabolic disorders. A therapeutic candidate for the prevention of cardiovascular diseases? Pharmacol. Res. 2024, 200, 107058. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.A.; do Carmo, J.M.; Li, X.; Wang, Z.; Mouton, A.J.; Hall, J.E. Role of Hyperinsulinemia and Insulin Resistance in Hypertension: Metabolic Syndrome Revisited. Can. J. Cardiol. 2020, 36, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Carreres, L.; Jílková, Z.M.; Vial, G.; Marche, P.N.; Decaens, T.; Lerat, H. Modeling Diet-Induced NAFLD and NASH in Rats: A Comprehensive Review. Biomedicines 2021, 9, 378. [Google Scholar] [CrossRef]
- Reddy, P.; Lent-Schochet, D.; Ramakrishnan, N.; McLaughlin, M.; Jialal, I. Metabolic Syndrome Is an Inflammatory Disorder: A Conspiracy between Adipose Tissue and Phagocytes. Clin. Chim. Acta 2019, 496, 35–44. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Hirode, G.; Wong, R.J. Trends in the Prevalence of Metabolic Syndrome in the United States, 2011–2016. JAMA 2020, 323, 2524–2526. [Google Scholar] [CrossRef]
- Ahmed, M.; Kumari, N.; Mirgani, Z.; Saeed, A.; Ramadan, A.; Ahmed, M.H.; Almobarak, A.O. Metabolic Syndrome; Definition, Pathogenesis, Elements, and the Effects of Medicinal Plants on It’s Elements. J. Diabetes Metab. Disord. 2022, 21, 1011–1022. [Google Scholar] [CrossRef]
- Julibert, A.; Bibiloni, M.d.M.; Tur, J.A. Dietary Fat Intake and Metabolic Syndrome in Adults: A Systematic Review. Nutr. Metab. Cardiovasc. Dis. 2019, 19, 887–905. [Google Scholar] [CrossRef]
- Harrison, S.; Couture, P.; Lamarche, B. Diet Quality, Saturated Fat and Metabolic Syndrome. Nutrients 2020, 12, 3232. [Google Scholar] [CrossRef]
- Castro-Barquero, S.; Ruiz-León, A.M.; Sierra-Pérez, M.; Estruch, R.; Casas, R. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef]
- Lopez, M.G.; Mancilla-Margalli, N.A.; Mendoza-Diaz, G. Molecular Structures of Fructans from Agave tequilana Weber Var. Azul. J. Agric. Food Chem. 2003, 51, 7835–7840. [Google Scholar] [CrossRef]
- López-Romero, J.C.; Ayala-Zavala, J.F.; González-Aguilar, G.A.; Peña-Ramos, E.A.; González-Ríos, H. Biological Activities of Agave By-Products and Their Possible Applications in Food and Pharmaceuticals. J. Sci. Food Agric. 2018, 98, 2461–2474. [Google Scholar] [CrossRef]
- Secretariat of Economy. Secretaría de Economía Alimentos-Fructanos de Agave-Especificaciones, Etiquetado y Métodos de ensayo (Prueba). Foods-Agave Fructans-Specifications, Labelling and Testing Methods. 2009. Available online: http://www.economia-nmx.gob.mx/normas/nmx/2010/nmx-f-591-scfi-2010.pdf (accessed on 3 May 2024).
- Espinosa-Andrews, H.; Urías-Silvas, J.E.; Morales-Hernández, N. The Role of Agave Fructans in Health and Food Applications: A Review. Trends Food Sci. Technol. 2021, 114, 585–598. [Google Scholar] [CrossRef]
- Shinoki, A.; Hara, H. Dietary Fructo-Oligosaccharides Improve Insulin Sensitivity along with the Suppression of Adipocytokine Secretion from Mesenteric Fat Cells in Rats. Br. J. Nutr. 2011, 106, 1190–1197. [Google Scholar] [CrossRef]
- Urías-Silvas, J.E.; Cani, P.D.; Delmée, E.; Neyrinck, A.; López, M.G.; Delzenne, N.M. Physiological Effects of Dietary Fructans Extracted from Agave tequilana Gto. and Dasylirion spp. Br. J. Nutr. 2008, 99, 254–261. [Google Scholar] [CrossRef]
- Santiago-García, P.A.; López, M.G. Agavins from Agave angustifolia and Agave potatorum Affect Food Intake, Body Weight Gain and Satiety-Related Hormones (GLP-1 and Ghrelin) in Mice. Food Funct. 2014, 5, 3311–3319. [Google Scholar] [CrossRef]
- Alves, N.F.B.; de Queiroz, T.M.; de Almeida Travassos, R.; Magnani, M.; de Andrade Braga, V. Acute Treatment with Lauric Acid Reduces Blood Pressure and Oxidative Stress in Spontaneously Hypertensive Rats. Basic Clin. Pharmacol. Toxicol. 2017, 120, 348–353. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, J.; Liu, T.; Wang, J.; Cai, H.; Zhang, X.; Quay Huai Xia, D.; Feng, F.; Tang, J. Differential Modulations of Lauric Acid and Its Glycerides on High Fat Diet-Induced Metabolic Disorders and Gut Microbiota Dysbiosis. Food Res. Int. 2022, 157, 111437. [Google Scholar] [CrossRef]
- Díaz-Ramos, D.I.; Ortiz-Basurto, R.I.; García-Barradas, O.; Chacón-López, M.A.; Montalvo-González, E.; Pascual-Pineda, L.A.; Valenzuela-Vázquez, U.; Jiménez-Fernández, M. Lauroylated, Acetylated, and Succinylated Agave tequilana Fructans Fractions: Structural Characterization, Prebiotic, Antibacterial Activity and Their Effect on Lactobacillus paracasei under Gastrointestinal Conditions. Polymers 2023, 15, 3115. [Google Scholar] [CrossRef]
- Casas-Godoy, L.; Arrizon, J.; Arrieta-Baez, D.; Plou, F.J.; Sandoval, G. Synthesis and Emulsifying Properties of Carbohydrate Fatty Acid Esters Produced from Agave tequilana Fructans by Enzymatic Acylation. Food Chem. 2016, 204, 437–443. [Google Scholar] [CrossRef]
- Padilla-Camberos, E.; Arrizon, J.; Sandoval, G. Effect of Agave Fructan Bioconjugates on Metabolic Syndrome Parameters in a Murine Model. Pharmaceuticals 2023, 16, 412. [Google Scholar] [CrossRef]
- Hernández-Pérez, S.; Oliart-Ros, R.M.; Casas-Godoy, L.; Sandoval, G.; Guarner-Lans, V.; Castrejón-Téllez, V.; Quevedo-Corona, L.; Peña-Montes, C.; Ramírez-Higuera, A. Beneficial Effects of Fructooligosaccharides Esterified with Lauric Acid in a Metabolic Syndrome Model Induced by a High-Fat and High-Carbohydrate Diet in Wistar Rats. J. Med. Food 2022, 25, 828–835. [Google Scholar] [CrossRef]
- Moreno-Fernández, S.; Garcés-Rimón, M.; Vera, G.; Astier, J.; Landrier, J.F.; Miguel, M. High Fat/High Glucose Diet Induces Metabolic Syndrome in an Experimental Rat Model. Nutrients 2018, 10, 1502. [Google Scholar] [CrossRef]
- Marques, C.; Meireles, M.; Norberto, S.; Leite, J.; Freitas, J.; Pestana, D.; Faria, A.; Calhau, C. High-Fat Diet-Induced Obesity Rat Model: A Comparison between Wistar and Sprague-Dawley Rat. Adipocyte 2016, 5, 11–21. [Google Scholar] [CrossRef]
- Gancheva, S.; Zhelyazkova-Savova, M.; Galunska, B.; Chervenkov, T. Experimental models of metabolic syndrome in Rats. Scr. Sci. Medica 2015, 47, 14–21. [Google Scholar] [CrossRef]
- Gunawan, S.; Aulia, A.; Soetikno, V. Development of Rat Metabolic Syndrome Models: A Review. Vet. World 2021, 14, 1774–1783. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, M.; Zhao, M.; Ge, A.; Guo, F.; Zhang, M.; Yang, Y.; Liu, L.; Yang, N. Differential Effects of High-Fat-Diet Rich in Lard Oil or Soybean Oil on Osteopontin Expression and Inflammation of Adipose Tissue in Diet-Induced Obese Rats. Eur. J. Nutr. 2013, 52, 1181–1189. [Google Scholar] [CrossRef]
- Schaafsma, G.; Slavin, J.L. Significance of Inulin Fructans in the Human Diet. Compr. Rev. Food Sci. Food Saf. 2015, 14, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Frontiers in Endocrinology. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef]
- Luong, Q.; Huang, J.; Lee, K.Y. Deciphering White Adipose Tissue Heterogeneity. Biology 2019, 8, 23. [Google Scholar] [CrossRef]
- Rabadán-Chávez, G.M.; Reyes-Maldonado, E.; Quevedo-Corona, L.; Paniagua-Castro, N.; Escalona-Cardoso, G.; Jaramillo-Flores, M.E. The Prothrombotic State Associated with Obesity-Induced Hypertension Is Reduced by Cocoa and Its Main Flavanols. Food Funct. 2016, 7, 4880–4888. [Google Scholar] [CrossRef]
- Spoto, B.; Di Betta, E.; Pizzini, P.; Lonardi, S.; Mallamaci, F.; Tripepi, G.; Kanbay, M.; Cancarini, G.; Zoccali, C. Inflammation Biomarkers and Inflammatory Genes Expression in Metabolically Healthy Obese Patients. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 584–591. [Google Scholar] [CrossRef]
- Pedersen, C.; Lefevre, S.; Peters, V.; Patterson, M.; Ghatei, M.A.; Morgan, L.M.; Frost, G.S. Gut hormone release and appetite regulation in healthy non-obese participants following oligofructose intake. A dose-escalation study. Appetite 2013, 66, 44–53. [Google Scholar] [CrossRef]
- Daud, N.M.; Ismail, N.A.; Thomas, E.L.; Fitzpatrick, J.A.; Bell, J.D.; Swann, J.R.; Costabile, A.; Childs, C.E.; Pedersen, C.; Goldstone, A.P.; et al. The impact of oligofructose on stimulation of gut hormones, appetite regulation and adiposity. Obesity 2014, 22, 1430–1438. [Google Scholar] [CrossRef]
- Ignot-Gutiérrez, A.; Ortiz-Basurto, R.I.; García-Barradas, O.; Díaz-Ramos, D.I.; Jiménez-Fernández, M. Physicochemical and Functional Properties of Native and Modified Agave Fructans by Acylation. Carbohydr. Polym. 2020, 245, 116529. [Google Scholar] [CrossRef]
- Rendón-Huerta, J.A.; Juárez-Flores, B.; Pinos-Rodríguez, J.M.; Aguirre-Rivera, J.R.; Delgado-Portales, R.E. Effects of Different Sources of Fructans on Body Weight, Blood Metabolites and Fecal Bacteria in Normal and Obese Non-Diabetic and Diabetic Rats. Plant Foods Hum. Nutr. 2012, 67, 64–70. [Google Scholar] [CrossRef]
- Hosoo, S.; Koyama, M.; Watanabe, A.; Ishida, R.; Hirata, T.; Yamaguchi, Y.; Yamasaki, H.; Wada, K.; Higashi, Y.; Nakamura, K. Preventive Effect of Eucommia Leaf Extract on Aortic Media Hypertrophy in Wistar-Kyoto Rats Fed a High-Fat Diet. Hypertens. Res. 2017, 40, 546–551. [Google Scholar] [CrossRef]
- Aslam, M.; Madhu, S.V. Development of Metabolic Syndrome in High-Sucrose Diet Fed Rats Is Not Associated with Decrease in Adiponectin Levels. Endocrine 2017, 58, 59–65. [Google Scholar] [CrossRef]
- Cano, P.; Cardinali, D.P.; Ríos-Lugo, M.J.; Fernández-Mateos, M.P.; Reyes Toso, C.F.; Esquifino, A.I. Effect of a High-Fat Diet on 24-Hour Pattern of Circulating Adipocytokines in Rats. Obesity 2009, 17, 1866–1871. [Google Scholar] [CrossRef]
- Szydełko, J.; Trojanowska, P.; Dąbrowska, I.; Szydełko-Gorzkowicz, M.; Litwińczuk, M. Adiponectin as Novel Biomarker of Endothelial Dysfunction in Insulin Resistance and Obesity—A Narrative Review. J. Educ. Health Sport 2020, 10, 591–606. [Google Scholar] [CrossRef]
- Costa, G.T.; Vasconcelos, Q.D.J.S.; Aragão, G.F. Fructooligosaccharides on Inflammation, Immunomodulation, Oxidative Stress, and Gut Immune Response: A Systematic Review. Nutr. Rev. 2022, 80, 709–722. [Google Scholar] [CrossRef]
- Da Silva Borges, D.; Fernandes, R.; Thives Mello, A.; Da Silva Fontoura, E.; Soares Dos Santos, A.R.; Santos De Moraes Trindade, E.B. Prebiotics May Reduce Serum Concentrations of C-Reactive Protein and Ghrelin in Overweight and Obese Adults: A Systematic Review and Meta-Analysis. Nutr. Rev. 2020, 78, 235–248. [Google Scholar] [CrossRef]
- Stanimirovic, J.; Radovanovic, J.; Banjac, K.; Obradovic, M.; Essack, M.; Zafirovic, S.; Gluvic, Z.; Gojobori, T.; Isenovic, E.R. Role of C-Reactive Protein in Diabetic Inflammation. Mediat. Inflamm. 2022, 2022, 3706508. [Google Scholar] [CrossRef]
- Hallberg, S.J.; Gershuni, V.M.; Athinarayanan, S.J. Reversing Type 2 Diabetes: A Narrative Review of the Evidence. Nutrients 2019, 11, 766. [Google Scholar] [CrossRef]
- Galaviz, K.I.; Weber, M.B.; Suvada, K.; Gujral, U.P.; Wei, J.; Merchant, R.; Dharanendra, S.; Haw, J.S.; Narayan, K.M.V.; Ali, M.K. Interventions for Reversing Prediabetes: A Systematic Review and Meta-Analysis. Am. J. Prev. Med. 2022, 62, 614–625. [Google Scholar] [CrossRef]
- Iqbal, K.; Chaudhry, S.R.; Khaliq, S.; Imran, B.; Saeed, M. Effect of Different Prebiotics (Galacto Oligosaccharide, Fructo Oligosaccharide, and Manno Oligosaccharide) on the Inflammatory Markers of High Fat Fed Rats. Pak. J. Med. Health Sci. 2020, 15, 389–393. [Google Scholar]
- Dehghan, P.; Pourghassem Gargari, B.; Asghari Jafar-abadi, M. Oligofructose-Enriched Inulin Improves Some Inflammatory Markers and Metabolic Endotoxemia in Women with Type 2 Diabetes Mellitus: A Randomized Controlled Clinical Trial. Nutrition 2014, 30, 418–423. [Google Scholar] [CrossRef]
- Malik, R.; Sadewa, A.H.; Sunarti. High Fiber Diets Enhance Il-10 Gene Expression and Itslevelin Hyperlipidemic Rats Model. Curr. Res. Nutr. Food Sci. 2020, 8, 471–478. [Google Scholar] [CrossRef]
- Lindsay, J.O.; Whelan, K.; Stagg, A.J.; Gobin, P.; Al-Hassi, H.O.; Rayment, N.; Kamm, M.A.; Knight, S.C.; Forbes, A. Clinical, Microbiological, and Immunological Effects of Fructo-Oligosaccharide in Patients with Crohn’s Disease. Gut 2006, 55, 348–355. [Google Scholar] [CrossRef]
- Gomez, E.; Tuohy, K.M.; Gibson, G.R.; Klinder, A.; Costabile, A. In Vitro Evaluation of the Fermentation Properties and Potential Prebiotic Activity of Agave Fructans. J. Appl. Microbiol. 2010, 108, 2114–2121. [Google Scholar] [CrossRef]
- Dayrit, F.M. The Properties of Lauric Acid and Their Significance in Coconut Oil. J. Am. Oil Chem. Soc. 2015, 92, 1–15. [Google Scholar] [CrossRef]
- Saraswathi, V.; Kumar, N.; Gopal, T.; Bhatt, S.; Ai, W.; Ma, C.; Talmon, G.A.; Desouza, C. Lauric Acid versus Palmitic Acid: Effects on Adipose Tissue Inflammation, Insulin Resistance, and Non-Alcoholic Fatty Liver Disease in Obesity. Biology 2020, 9, 346. [Google Scholar] [CrossRef]
- Buettner, R.; Parhofer, K.G.; Woenckhaus, M.; Wrede, C.E.; Kunz-Schughart, L.A.; Schölmerich, J.; Bollheimer, L.C. Defining high-fat-diet rat models: Metabolic and molecular effects of different fat types. J. Mol. Endocrinol. 2006, 36, 485–501. [Google Scholar] [CrossRef]
- Turner, N.; Hariharan, K.; TidAng, J.; Frangioudakis, G.; Beale, S.M.; Wright, L.E.; Zeng, X.Y.; Leslie, S.J.; Li, J.Y.; Kraegen, E.W.; et al. Enhancement of muscle mitochondrial oxidative capacity and alterations in insulin action are lipid species dependent: Potent tissue-specific effects of medium-chain fatty acids. Diabetes 2009, 58, 2547–2554. [Google Scholar] [CrossRef]
- Lundsgaard, A.M.; Fritzen, A.M.; Sjøberg, K.A.; Kleinert, M.; Richter, E.A.; Kiens, B. Small Amounts of Dietary Medium-Chain Fatty Acids Protect against Insulin Resistance during Caloric Excess in Humans. Diabetes 2021, 70, 91–98. [Google Scholar] [CrossRef]
- Sedik, A.A.; Elgohary, R.; Khalifa, E.; Khalil, W.K.B.; Shalaby, M.B. Lauric Acid Attenuates Hepato-Metabolic Complications and Molecular Alterations in High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease in Rats. Toxicol. Mech. Methods 2024, 34, 454–467. [Google Scholar] [CrossRef]
- Sandoval, G.; Arrizon, J.; González-Avila, M.; Padilla-Camberos, E.; Martínez-Velázquez, M.; Villanueva-Rodríguez, S.; Casas-Godoy, L. Bioconjugate Molecules with Biological and Techno-Functional Activity, Method for the Production Thereof and Use. Thereof. Patent Number MX 358789, 18 December 2013. [Google Scholar]
- Secretariat of Agriculture, Livestock, Rural Development, Fisheries and Food. Official Mexican Standard NOM-062-ZOO-1999. 2001. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 3 January 2024).
- Parlee, S.D.; Lentz, S.I.; Mori, H.; MacDougald, O.A. Quantifying Size and Number of Adipocytes in Adipose Tissue. In Methods in Enzymology; Academic Press Inc.: Cambridge, MA, USA, 2014; Volume 537, pp. 93–122. [Google Scholar] [CrossRef]
- Muniyappa, R.; Chen, H.; Muzumdar, R.H.; Einstein, F.H.; Yan, X.; Yue, L.Q.; Barzilai, N.; Quon, M.J. Comparison between Surrogate Indexes of Insulin Sensitivity/Resistance and Hyperinsulinemic Euglycemic Clamp Estimates in Rats. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1023–E1029. [Google Scholar] [CrossRef]
- Chirgwin, J.; Przybyla, A.; MacDonald, R.; Rutter, W. Isolation of Biologically Active Ribonucleic Acid from Sources Enriched in Ribonuclease. Biochemistry 1979, 18, 5294–5299. [Google Scholar] [CrossRef]
- Vogelstein, B.; Gillespiet, D. Preparative and Analytical Purification of DNA from Agarose. Proc. Natl. Acad. Sci. USA 1979, 76, 615–619. [Google Scholar] [CrossRef]
- Alvarez, M.L.; Cotta-Doné, S. SYBR ® Green and TaqMan ® Quantitative PCR Arrays: Expression profile of genes relevant to a pathway or a disease state. In RNA Mapping: Methods and Protocols; Alvarez, M.L., Nourbakhsh, M., Eds.; Humana Press: New York, NY, USA, 2014; pp. 321–358. [Google Scholar]
- Perez, L.J.; Rios, L.; Trivedi, P.; D’Souza, K.; Cowie, A.; Nzirorera, C.; Webster, D.; Brunt, K.; Legare, J.F.; Hassan, A.; et al. Validation of Optimal Reference Genes for Quantitative Real Time PCR in Muscle and Adipose Tissue for Obesity and Diabetes Research. Sci. Rep. 2017, 7, 3612. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
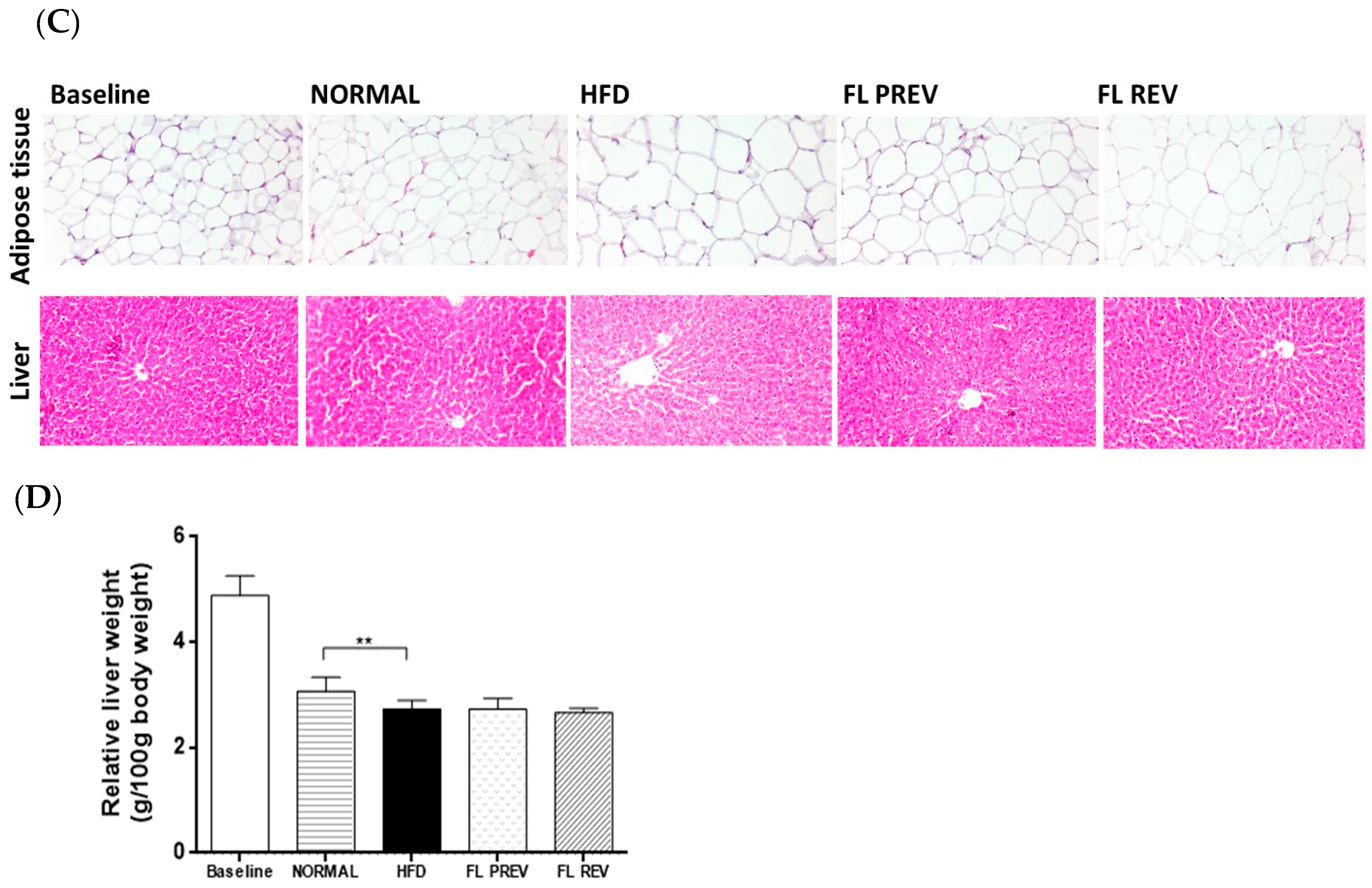
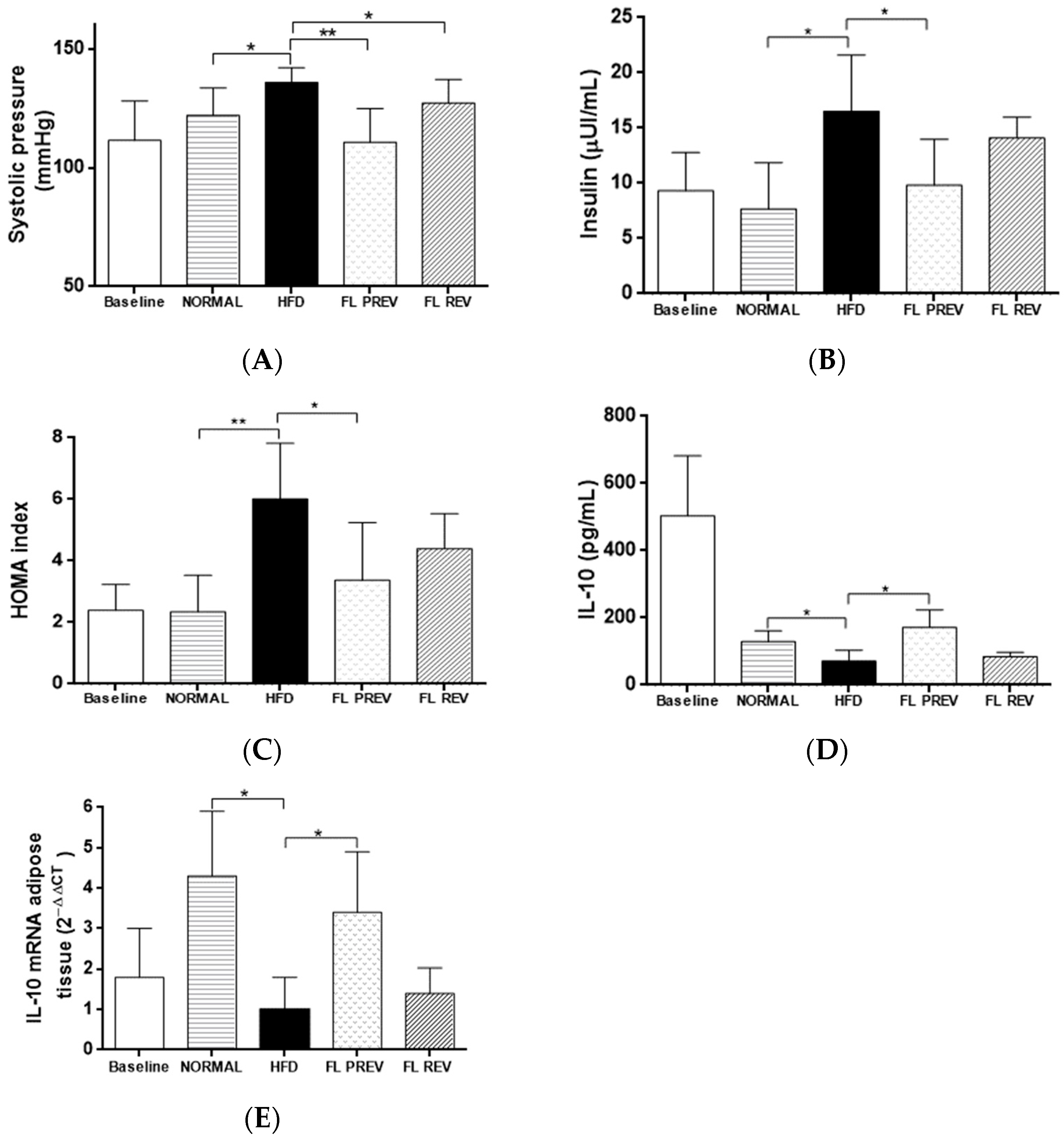
| Baseline | NORMAL | HFD | FL PREV | FL REV | |
|---|---|---|---|---|---|
| Food intake (g/day) | 11.8 ± 2.5 | 24.6 ± 2.4 | 18.4 ± 2.1 | 14.8 ± 2.5 **** | 16.5 ± 3.6 **** |
| Energy intake (kcal/day) | 40.0 ± 8.4 | 83.8 ± 8.2 | 84.7 ± 9.5 | 68.2 ± 11.6 **** #### | 76.0 ± 16.7 **** ### |
| Energy intake from fat (kcal/day) | 5.6 ± 1.2 | 11.8 ± 1.2 | 38.7 ± 4.3 #### | 31.2 ± 5.3 **** | 34.7 ± 7.6 **** |
| Energy intake from fat (%kcal) | 14.1 | 14.1 | 45.7 | 45.7 | 45.7 |
| Baseline | NORMAL | HFD | FL PREV | FL REV | |
|---|---|---|---|---|---|
| Glucose (mg/dL) | 105.1 ± 14.6 | 126 ± 22.8 | 148.3 ± 12.4 | 134.3 ± 14.6 | 125.8 ± 22.2 |
| Total cholesterol (mg/dL) | 64.2 ± 6.4 | 57.4 ± 6.5 | 67.7 ± 7.3 | 63.9 ± 10.8 | 70.7 ± 10.6 |
| Triglycerides (mg/dL) | 49.8 ± 12.5 | 68.2 ± 13.4 | 99.1 ± 15.2 # | 66.7 ± 24.9 | 60 ± 28.8 |
| Adiponectin (μg/mL) | 9.8 ± 1.7 | 6.1 ± 0.8 | 7.4 ± 0.8 | 6.9 ± 0.5 | 7.7 ± 0.9 |
| TNF-α (pg/mL) | 9.8 ± 2.4 | 19.5 ± 7.5 | 19.1 ± 9.9 | 19.4 ± 5.5 | 18.5 ± 3.6 |
| IL-6 (ng/mL) | 1.5 ± 0.2 | 3.2 ± 0.8 | 4.7 ± 1.7 | 3.3 ± 0.7 | 3.9 ± 0.9 |
| IL-7 (ng/mL) | 89.7 ± 49.3 | 175.3 ± 47.9 | 179.1 ± 88.9 | 157.9 ± 57.4 | 290.2 ± 33.6 |
| CRP (μg/mL) | 292 ± 30.4 | 320.3 ± 8.1 | 335.6 ± 24.2 | 266 ± 49.5 * | 290.2 ± 33.6 |
| Abdominal circumference (cm) | 16.8 ± 0.3 | 19.1 ± 0.3 | 19.7 ± 1.1 | 17.8 ± 0.7 ** | 18.8 ± 0.9 |
| Length (cm) | 18.9 ± 0.3 | 26.3 ± 0.3 | 27.2 ± 0.4 ## | 26.4 ± 0.5 * | 26.8 ± 0.3 |
| BMI (g/cm2) | 0.56 ± 0.0 | 0.67 ± 0.0 | 0.72 ± 0.0 # | 0.60 ± 0.0 **** | 0.64 ± 0.0 ** |
| Grade | Baseline | NORMAL | HFD | FL PREV | FL REV | |
|---|---|---|---|---|---|---|
| Steatosis | 0/1/2/3 | 6/0/0/0 | 6/0/0/0 | 2/2/2/0 | 2/2/2/0 | 2/2/2/0 |
| Ballooning | 0/1/2 | 6/0/0 | 6/0/0 | 0/6/0 | 1/5/0 | 0/6/0 |
| Lobular inflammation | 0/1/2/3 | 6/0/0/0 | 6/0/0/0 | 6/0/0/0 | 6/0/0/0 | 6/0/0/0 |
| NAS score | 0 ± 0.0 | 0 ± 0.0 | 2 ± 0.9 ## | 1.8 ± 1.0 | 1.8 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Garibay, A.S.; Sandoval, G.; Torres-González, O.R.; Bastidas-Ramírez, B.E.; Sánchez-Hernández, I.M.; Padilla-Camberos, E. Agave-Laurate-Bioconjugated Fructans Decrease Hyperinsulinemia and Insulin Resistance, Whilst Increasing IL-10 in Rats with Metabolic Syndrome Induced by a High-Fat Diet. Pharmaceuticals 2024, 17, 1036. https://doi.org/10.3390/ph17081036
González-Garibay AS, Sandoval G, Torres-González OR, Bastidas-Ramírez BE, Sánchez-Hernández IM, Padilla-Camberos E. Agave-Laurate-Bioconjugated Fructans Decrease Hyperinsulinemia and Insulin Resistance, Whilst Increasing IL-10 in Rats with Metabolic Syndrome Induced by a High-Fat Diet. Pharmaceuticals. 2024; 17(8):1036. https://doi.org/10.3390/ph17081036
Chicago/Turabian StyleGonzález-Garibay, Angélica Sofía, Georgina Sandoval, Omar Ricardo Torres-González, Blanca Estela Bastidas-Ramírez, Iván Moisés Sánchez-Hernández, and Eduardo Padilla-Camberos. 2024. "Agave-Laurate-Bioconjugated Fructans Decrease Hyperinsulinemia and Insulin Resistance, Whilst Increasing IL-10 in Rats with Metabolic Syndrome Induced by a High-Fat Diet" Pharmaceuticals 17, no. 8: 1036. https://doi.org/10.3390/ph17081036
APA StyleGonzález-Garibay, A. S., Sandoval, G., Torres-González, O. R., Bastidas-Ramírez, B. E., Sánchez-Hernández, I. M., & Padilla-Camberos, E. (2024). Agave-Laurate-Bioconjugated Fructans Decrease Hyperinsulinemia and Insulin Resistance, Whilst Increasing IL-10 in Rats with Metabolic Syndrome Induced by a High-Fat Diet. Pharmaceuticals, 17(8), 1036. https://doi.org/10.3390/ph17081036







