Abstract
Body weight is a balance between energy intake and energy expenditure. Energy expenditure is mainly governed by physical activity and adaptive thermogenesis. Adaptive dietary thermogenesis in brown and beige adipose tissue occurs through mitochondrial uncoupling protein (UCP-1). Laboratory mice, when housed at an ambient temperature of 22–24 °C, maintain their body temperature by dietary thermogenesis, eating more food compared to thermoneutrality. Humans remain in the thermoneutral zone (TNZ) without expending extra energy to maintain normal body temperature. TRPV1 activation by capsaicin (CAP) inhibited weight gain in mice housed at ambient temperature by activating UCP-1-dependent adaptive thermogenesis. Hence, we evaluated the effect of CAP feeding on WT and UCP-1−/− mice maintained under thermoneutral conditions. Our research presents novel findings that TRPV1 activation by CAP at thermoneutrality counters obesity in WT mice and promotes PRDM-16-dependent UCP-1 transcription. CAP fails to inhibit weight gain in UCP-1−/− mice housed at thermoneutrality and in adipose tissue-specific PRDM-16−/− mice. In vitro, capsaicin treatment increases UCP-1 transcription in PRDM-16 overexpressing cells. Our data indicate for the first time that TRPV1 activation counters obesity at thermoneutrality permissive for UCP-1 and the enhancement of PRDM-16 is not beneficial in the absence of UCP-1.
1. Introduction
Humans are homeothermic organisms that maintain a stable internal body temperature of ~37 °C [1]. Ambient temperature (AT) is the environmental temperature, and the thermoneutral zone is the range of ambient temperatures where the metabolic rate is relatively constant without any heat production or heat loss or regulating dry heat loss by skin blood flow. Obligatory thermogenesis maintains the body at ~37 °C under thermoneutral conditions. Obligatory thermogenesis refers to the heat produced as a byproduct of all the basal metabolic processes such as breathing, pumping of the heart, hormone homeostasis, digestion and storage of nutrients, and functioning of various ion channel proteins, etc. [2]. The thermoneutral zone (TNZ) varies widely between species, for example, 32–33 °C in mice and 26–28 °C in adult humans. When the ambient temperature falls below the lowest point of the thermoneutral zone, the balance is lost between heat loss and the heat produced by obligatory thermogenesis, resulting in a fall in body temperature.
Body weight is determined by an intricate balance between energy intake and energy expenditure [3]. Energy expenditure is governed by four major facets, including the basal metabolic rate (obligatory thermogenesis), physical activity, nonexercised activity thermogenesis (NEAT), and adaptive thermogenesis [4]. NEAT includes daily activities such as walking around the house, gardening, etc. Adaptive thermogenesis occurs due to cold and dietary stimuli in brown and beige adipose tissue and skeletal muscles [5].
Thus, thermal energy is required to keep the body temperature stable. In general, clothing, shelter, and climate control help people maintain their body temperature within comfortable limits, and so thermoregulation is only a very small part of the daily energy expenditure. However, laboratory mice, when housed at an ambient temperature of 22–24 °C, maintain their body temperature by dietary thermogenesis, eating more food compared to thermoneutrality [6].
Mitochondrial uncoupling protein (UCP-1) is a very important component of heat production in brown adipose tissue (BAT) and inguinal white adipose tissue (iWAT) [7]. Fatty acids produced through lipolysis [8] and cold exposure-mediated adrenergic stimulation [9] result in heat generation through mitochondrial respiration via UCP-1-mediated protein leakage. Thus, it can be concluded that UCP-1 is active at ambient temperature and is inactive or less active at thermoneutral temperatures. Mice housed at ambient temperature (22 to 24 °C) experience constant cold stress, which stimulates energy intake to maintain body temperature [10]. However, thermoneutrality (~30 °C for mice) promotes adiposity despite a decrease in energy intake [11]. Surprisingly, mice that lack endogenous UCP-1 (UCP-1−/−) were shown to be resistant to diet-induced obesity at ambient temperature but gain weight and become obese when fed a High Fat Diet (HFD) at thermoneutrality [12]. This indicates that alternate complementary pathways mediate mechanisms of compensation due to the lack of UCP-1 at ambient temperature. However, in the TNZ, those pathways are dormant in UCP-1−/− mice.
We have recently shown that the activation of the transient receptor potential vanilloid subfamily 1 (TRPV1) protein by capsaicin (CAP; a TRPV1 agonist) counters high-fat diet (HFD; 60% of calories from fat)-induced obesity without decreasing energy intake [13,14] in mice housed at ambient temperature. Our preclinical study unequivocally validates that CAP induces the browning of inguinal white adipose tissue (iWAT) and enhances the expression of genes involved in thermogenesis in iWAT and brown adipose tissue (BAT) 13,14]. The results were obtained below the thermoneutral zone in mice but in the thermoneutral zone for adult humans (21 °C) [15,16]. Therefore, the research data obtained from mice housed at 21 °C may not translate to humans, who live in thermoneutrality.
In adipocytes, UCP-1 transcription is tightly regulated by several transcription factors. Specifically, peroxisome proliferator-activated receptor α (PPARα), PPARγ coactivator 1α (PGC-1α), and PR regulatory domain 16 containing protein (PRDM-16) are shown to regulate UCP-1 transcription [17,18,19,20]. We have delineated the mechanism by which CAP activation triggers the signal transduction pathway leading to the activation of SiRT-1, resulting in the deacetylation of two transcription factors, PPARγ and PRDM-16, and their interaction that leads to thermogenesis. Alternately, we have also shown that SiRT-1 also increased PGC-1α and PPARα levels, which increased UCP-1 expression and thermogenesis [13].
Hence, we wanted to know if CAP can induce thermogenesis and weight loss in mice housed in the thermoneutral zone (TNZ). Analyzing this is important since this will help in advancing TRPV1 activation as an effective strategy to promote weight loss in humans because humans remain in a thermal comfort zone. Furthermore, whether the effect of TRPV1 activation is dependent on the UCP-1-dependent thermogenic process remains unexplored. To address this, we evaluated the effect of CAP feeding on WT and UCP-1−/− mice maintained under ambient or thermoneutral conditions and analyzed how TRPV1 activation regulates UCP-1 expression and BAT thermogenesis at thermoneutrality. Our research presents novel findings that demonstrate that TRPV1 activation by CAP at thermoneutrality counters obesity in WT mice and promotes PRDM-16-dependent UCP-1 transcription. CAP fails to inhibit weight gain in UCP-1−/− mice housed at thermoneutrality. In vitro, capsaicin treatment increases UCP-1 transcription in PRDM-16 overexpressing cells. Furthermore, in mice that lack PRDM-16 in the adipose tissues, CAP fails to prevent HFD-induced obesity. Our data indicate for the first time that TRPV1 activation counters obesity at thermoneutrality permissive for UCP-1 and that the enhanced expression of other thermogenic genes and proteins is not beneficial in the absence of UCP-1.
2. Results
2.1. TRPV1 Expression and Activity in BAT and iWAT
TRPV1 expression was detected in iWAT and BAT by immunoblotting and qRT-PCR. To compare the relative expression of TRPV1 in adipose tissues, immunoblotting was performed to show that the molecular weight of TRPV1 in adipose fat corresponded to that of TRPV1 found in the dorsal root ganglion and the HEK TRPV1 stable cell line. The mRNA of TRPV1 was quantified in iWAT, BAT, DRG, and in TRPV1 overexpressing cells. Immunohistochemistry for TRPV1 in primary adipocytes was maximum under normal diet-fed conditions and was downregulated under HFD-fed conditions. CAP prevented the downregulation of TRPV1 activity under HFD-fed conditions. Furthermore, HFD suppressed the CAP-activated TRPV1 currents, and this was restored in the primary brown adipocytes of HFD + CAP-fed WT mice, as shown in Figure 1
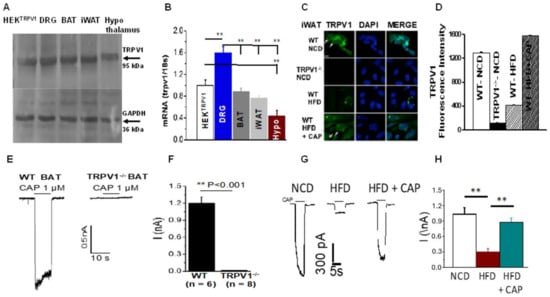
Figure 1.
TRPV1 expression and activity in WAT and BAT. (A). Western blot showing TRPV1 expression in mouse tissues. HEKTRPV1 is the positive control. (B). TRPV1 mRNA expression normalized to the 18s RNA in these tissues (n = 5). (C). Representative micrograph showing the immunohistochemical detection of TRPV1 expression in the iWAT preadipocytes of WT and TRPV1−/− mice fed various diets. (D). Quantification of the fluorescence intensity (arbitrary units). (E). Representative traces of CAP-stimulated TRPV1 currents in the primary brown preadipocytes of WT and TRPV1−/− mice at −60 mV. (F). Average currents ± S.E.M. in these cells (n = 6 to 8). (G). CAP-stimulated TRPV1 currents in NCD- or HFD (±CAP)-fed primary brown preadipocytes from WT mice. (H). Average currents ± S.E.M. in these cells (n = 9 to 11). ** p < 0.01, significantly different.
2.2. Decreased Body Temperature in Diet-Induced Obese WT Mice at Ambient Temperature and in the Thermoneutral Zone
At ambient temperature, adaptive thermogenesis is triggered by increasing food intake and heat production. However, in obese mice, a downregulation of the thermogenic machinery occurs [13,14]. So, we wanted to see the body temperature of WT mice housed at ambient temperature and in the TNZ. As shown in Figure 2, HFD-fed obese mice had a lower body temperature compared to NCD mice at both ambient temperature and in the TNZ. CAP-fed mice had body temperatures like those of NCD mice, indicating effective thermogenesis. There was no difference in body temperature of the WT mice fed the NCD and HFD + CAP at ambient temperature or in the TNZ.
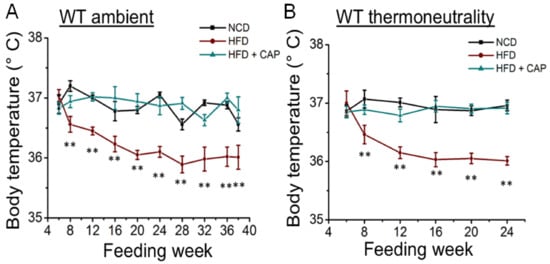
Figure 2.
Rectal temperatures of WT mice at ambient temperature (A) and in the TNZ (B). Rectal temperatures of WT mice fed the NCD or HFD or HFD + CAP at ambient temperature and in the TNZ measured every week from 6 weeks till 38 weeks of age using a thermometer. Average mean temperatures ± S.E.Ms. of these mice (n = 8 for each condition). ** p < 0.01, significantly different.
2.3. CAP Inhibited Weight Gain in WT and UCP1−/− Mice at Ambient Temperature but Inhibited HFD-Induced Weight Gain Only in WT Mice and Not in UCP−/− Mice in the TNZ
Our earlier data show that dietary CAP significantly antagonizes diet-induced obesity at ambient temperature by activating UCP-1 [10]. As shown in Figure 2, there was no difference in body temperature between CAP-fed mice at ambient temperature and in the TNZ. So, we wanted to study the body weight gain of WT mice and UCP-1−/− mice at ambient temperature and in the TNZ. The body weights were the same between all the cohorts at the beginning of the feeding experiments at 6 weeks of age (Figure 3A,B,E,F). The weight gain became statistically different when the WT mice gained weight at a faster rate compared to UCP-1−/− mice. WT mice fed the HFD weighed twice as much as UCP-1−/− mice fed the HFD at ambient temperature (Figure 3A,B). CAP-fed mice weighed the same as their NCD-fed counterparts. There was no difference in food or water intake between the different cohorts. The HFD increased weight gain in UCP-1−/− mice housed in the TNZ, and CAP failed to inhibit the weight gain of UCP-1−/− mice in the TNZ (Figure 3F). There was no difference in food or water intake between WT and UCP-1−/− mice fed the respective diets at ambient temperature (Figure 3C) or in the TNZ (Figure 3G). The food intake of all cohorts was slightly higher at ambient temperature compared to the TNZ (Figure 3C,G) and water intake was slightly higher in the TNZ (Figure 3D,H) compared to ambient temperature. Weight gain was less in mice with pair feeding compared to the mice that were fed ad libitum (Figure 3A,I).
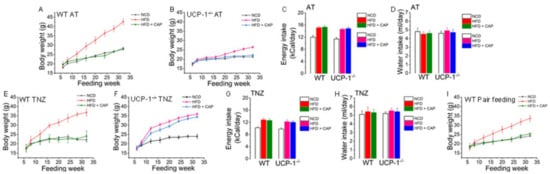
Figure 3.
CAP counters HFD-induced weight gain in WT mice at ambient temperature and at thermoneutrality. Weight gain plotted against the feeding week for NCD- or HFD (±CAP, 0.01% in diet)-fed WT and UCP-1−/− mice at ambient temperature (A,B) and at thermoneutrality (E,F). Mean energy and water intake (±S.E.M.) of these mice at ambient temperature (C,D) and at thermoneutrality (G,H). (I). Weight gain plotted against the feeding week for NCD- or HFD (±CAP, 0.01% in diet)-fed WT mice that received the same number of calories that WT mice received at ambient temperature.
2.4. Metabolic Activity
The respiratory coefficient was used to determine the basal metabolic rate. We have shown earlier that CAP increases the respiratory rate and energy expenditure of WT mice housed at ambient temperature [14,21]. So, we wanted to determine if CAP increases the respiratory rate in WT mice housed in the TNZ. The metabolic activity was determined using the Comprehensive Laboratory Animal Monitoring System at ambient temperature. The HFD inhibited the RER, energy expenditure, and locomotion. CAP revived the RER, mean energy expenditure, and locomotion in WT mice similar to the mice under the NCD-fed condition (Figure 4A,B,E,F,I,J,M,N). CAP failed to revive the respiratory coefficient, energy expenditure, and locomotion in UCP1−/− mice housed in the TNZ (Figure 4C,D,G,H,K,L,O,P).
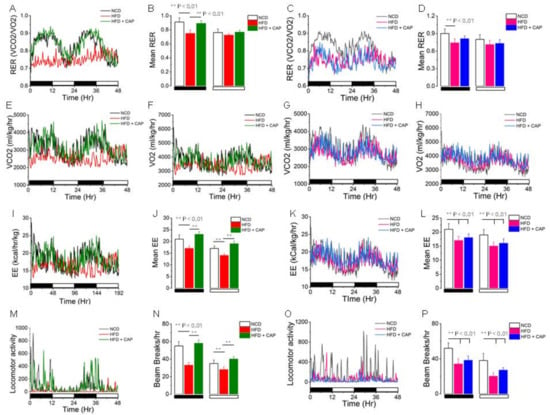
Figure 4.
CAP feeding increases the respiratory quotient (respiratory exchange ratio, RER= VCO2/VO2) and energy expenditure (EE) in WT mice maintained at thermoneutrality. RER (A,C), VCO2 (E,G), VO2 (F,H), EE (I,K), and locomotor activity (M,O) of NCD- or HFD (± CAP, 0.01% in diet)-fed WT and UCP-1−/− mice at thermoneutrality. Means ± S.E.Ms. for the RER (B,D), EE (J,L), and locomotor activities (N,P) of these mice. ** represents statistical significance at p < 0.01 for n = 8 mice for each condition.
2.5. White Adipogenic and Thermogenic Gene Expression
Since CAP inhibited the weight gain in WT mice in the TNZ similar to ambient temperature, white adipogenic and thermogenic gene expression were characterized by quantitative RT-PCR [14]. A gene expression analysis was also performed in BAT from UCP-1−/− mice housed in the TNZ. Mitochondrial thermogenic genes such as BMP4, Cidea, CoxII, Foxc2 and Dio2 (Figure 5A,D,E,F,G), the mitochondrial quality control protein PGC-1alpha (Figure 5H), and energy sensor and transcriptional regulator of PGC-1alpha SiRT-1 (Figure 5I) were upregulated by CAP in WT mice, and a significant upregulation compared to WT mice was not found in UCP-1−/− mice. However, the expression of BMP8a (Figure 5B), another regulator of adipocyte differentiation, was enhanced in BAT from UCP-1−/− mice compared to BAT from WT mice.
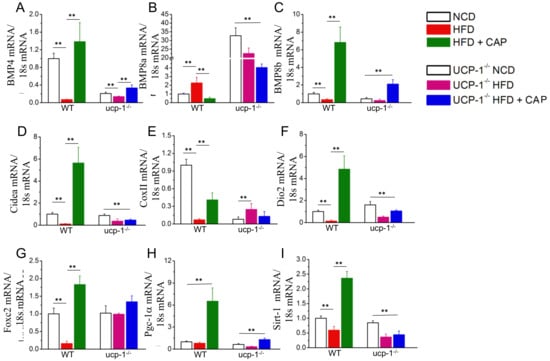
Figure 5.
Effect of CAP feeding on the mRNA levels of adipogenic and thermogenic genes in the BAT of NCD- or HFD (±CAP)-fed WT and UCP-1−/− mice at thermoneutrality. Mean mRNA levels ± S.E.Ms. for Bmp4 (A), Bmp8a (B), Bmp8b (C), Cidea (D), CoxII (E), Dio2 (F), Foxc2 (G), Pgc-1α (H), and Sirt-1 (I) in the BAT of these mice. For quantitative RT-PCR experiments, 18s ribosomal RNA was used as control. ** represents statistical significance at p < 0.01 for n = 4 experiments.
2.6. UCP-1 and PRDM-16 Gene Expression
Our earlier studies have shown that CAP inhibits weight gain in diet-induced obese mice by upregulating UCP-1. To examine the involvement of UCP-1 and its transcriptional regulator PRDM16, quantitative RT-PCR was performed in the BAT of WT and UCP-1−/− mice housed in the TNZ. Figure 6A shows UCP1 levels less in the TNZ compared to ambient temperature. CAP increased UCP-1 levels at both ambient temperature and in the TNZ. The UCP-1 mRNA was undetected in UCP-1−/− BAT. Figure 6B shows similar PRDM16 mRNA levels in WT and UCP-1−/− BAT at ambient temperature, as well as in the TNZ. PRDM16 was downregulated under HFD-fed conditions across the conditions and CAP increased the mRNA levels in WT and UCP-1−/− BAT at both AT and in the TNZ.
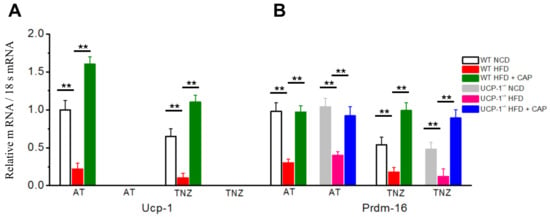
Figure 6.
Effect of CAP feeding on the Ucp-1 (A) and Prdm-16 (B) mRNAs normalized to 18s RNA in NCD- or HFD (± CAP)-fed WT and UCP-1−/− mice. Mean Ucp-1 and Prdm-16 mRNA levels normalized to 18s RNA ± S.E.Ms in the BAT of these mice. ** represents statistical significance at p < 0.01 for n = 4 experiments.
2.7. CAP Fails to Counter HFD-Induced Weight Gain in Adipose Tissue-Specific PRDM16−/− Mice
To confirm that CAP exerts its thermogenic activity through the PRDM16-UCP-1 pathway, adipose tissue-specific PARDm16−/− mice were generated through the Cre-lox system by crossing PRDM16 lox mice with FABP4-cre mice. The adipose tissue-specific PRDM16 knockout mice gained weight with the HFD, which was not inhibited by feeding CAP at ambient temperature, as shown in Figure 7A. There was no difference in food or water intake between the HFD and HFD + CAP cohorts. Figure 7C shows the UCP-1 mRNA levels in BAT of adipose tissue-specific PRDM16−/− mice. UCP-1 mRNA levels were low in ADPRDM16−/− mice fed different diets in comparison to the respective WT controls.
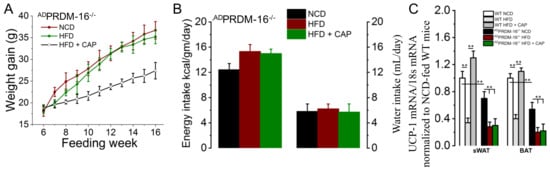
Figure 7.
CAP does not counter obesity in ADPRDM-16−/− mice. (A) Mean body weight gain in NCD- or HFD (± CAP, 0.01% in diet)-fed ATPRDM-16−/− mice (n = 4). (B) Average daily energy and water intake in these mice (n = 6). (C) UCP-1 mRNA levels in the sWAT and BAT of these mice (n = 6 experiments). ** p < 0.01.
2.8. PRDM-16 Overexpression Upregulates UCP-1 Transcription in HEK TRPV1 Cells
As the knockout of PRDM16 resulted in a decrease in UCP-1, the effect of overexpression of PRDM16 on UCP-1 was analyzed in stable TRPV1-expressing HEK293 cells. As shown in Figure 8, CAP stimulated the GFP reporter-driven UCP-1 promoter. PRDM16 co-transfection increased the expression of GFP, which was further stimulated by CAP and inhibited by the TRPV1 antagonist capsazepine.
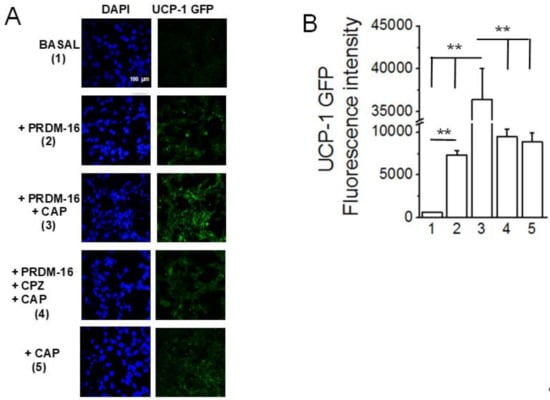
Figure 8.
PRDM-16 overexpression increases UCP-1 transcription and CAP treatment enhances it further. (A) Micrographs showing UCP-1-GFP expression in HEK TRPV1 cells from the control (basal; 1), PRDM-16 (2), PRDM-16 + CAP (1 μM; 3), PRDM-16, CPZ (10 μM; TRPV1 inhibitor) + CAP (1 μM), or CAP (1 μM) treatment groups. The magnification is 10x, and the scale bar is 100 μm. (B) Mean intensity of UCP-1-GFP normalized to the control (basal) group ± S.E.M. for n = 3 independent experiments. ** p < 0.01.
3. Discussion
Obesity is excess adiposity and plays a central role in cellular metabolic dysregulation that accounts for insulin resistance, type 2 diabetes, dyslipidemia, hypertension, atherosclerosis, fatty liver, etc. [22]. Obesity is a serious public health problem affecting more than two of five adults in the United States [23]. Recent research has undoubtedly shown the presence of BAT in humans, and its activation inhibits insulin resistance and obesity[24]. BAT is active in mice housed at ambient temperature. Humans generally live in the TNZ without expending extra energy to maintain normal body temperature. This work systematically analyzed the role of TRPV1 activation in countering obesity in WT mice housed in the TNZ and delineated the mechanism of UCP1 activation in energy expenditure in the TNZ using WT and UCP-1−/− mice.
This research demonstrates that TRPV1 is expressed in both iWAT and BAT and its expression is inhibited by HFD (Figure 1). Furthermore, CAP counters this, which is consistent with previous findings [13,14]. In addition, we have previously shown that TRPV1 activation in BAT and iWAT triggers signal transduction pathways involving SiRT-1, which causes deacetylation of PPAR gamma and PRDM-16 and stimulates their interaction in iWAT to cause the browning of iWAT [13,14]. However, these experiments were performed by housing mice at ambient temperature. These mice experienced mild cold stress at ambient temperature. However, chronic HFD feeding resulted in a defective thermogenic capacity, leading to obesity. In the HFD + CAP-fed group, TRPV1 activation by CAP increased UCP-1 expression and activity and converted fat to heat. However, this research did not show how TRPV1 activation enhanced UCP-1 expression and activation.
We hypothesized that CAP stimulates PRDM-16 to enhance UCP-1 expression transcriptionally. Consistent with this, CAP failed to increase UCP-1 expression in the BAT of adipose-specific PRDM16 KO mice, while it enhanced UCP-1 mRNA expression in the BAT of WT mice (Figure 7). Furthermore, overexpression of PRDM-16 in stable TRPV1-expressing HEK293 cells induced an increase in UCP-1 transcription, which was further enhanced by CAP treatment (Figure 8) These data unequivocally demonstrate that TRPV1 activation by CAP enhances UCP-1 expression via PRDM-16.
Uncoupling proteins are anchored in the inner membrane of mitochondria and take part in the maintenance of the energy balance by diverting energy from ATP synthesis to thermogenesis [25]. They are highly expressed in brown and beige adipocytes, which are rich in mitochondria. UCP-1, also called thermogenin, is responsible for BAT thermogenesis and systemic energy homeostasis through excessive energy intake [26] and the generation of body heat through uncoupling oxidative phosphorylation during adaptive thermogenesis [27]. Thus, this indicates the significance of UCP-1 in preventing excessive adiposity in mice. Accordingly, UCP-1 KO mice develop hypothermia when exposed to cold temperatures [28]. However, in contrast, UCP-1 KO mice did not gain weight on the HFD when housed at ambient temperature. They also showed increased locomotion and oxygen consumption and a reduced respiratory coefficient, indicating alternate pathways for the metabolism of lipids and maintaining body temperature [29]. However, in the thermoneutral zone, UCP-1 activation is not required to maintain body temperature, and hence UCP-1 and alternate pathways of lipid metabolism remain dormant. Thus, UCP-1 has to be activated to produce heat [30]. Consistently, UCP-1 KO mice gained body weight after feeding on the HFD in the TNZ, as the alternate pathways for thermogenesis were not triggered (Figure 3). Thus, UCP-1 activation is required to maintain energy homeostasis under high energy intake conditions at thermoneutrality.
We have earlier shown that the HFD diet inhibited the expression and activity of thermogenic genes, including UCP-1, in both BAT and iWAT. Dietary CAP inhibited diet-induced obesity by increasing thermogenesis. Thus, HFD diet-induced obese mice showed a reduced body temperature at both ambient temperature, as well as in the TNZ, where normal chow diet-fed mice and mice with dietary CAP treatment maintained a body temperature of 37 °C (Figure 2). CAP inhibited weight gain in UCP1−/− mice at ambient temperature. This was not significant, as the HFD did not cause weight gain in UCP1−/− mice at ambient temperature (Figure 3A,B). However, CAP inhibited weight gain in WT mice in the TNZ but not in UCP1−/− mice (Figure 3E,F). This shows that CAP increases diet-induced thermogenesis by activating mitochondrial UCP-1 in BAT and iWAT. Similarly, UCP-1 is required for CAP-mediated increases in oxygen consumption, RER, and energy expenditure (Figure 4).
Thermogenic genes such as BMP4, BMP8b, Cidea, COX II, Dio2, FoxC2, PGC1-α and SiRT-1 are either directly or indirectly involved in the transcription of UCP-1 and therefore thermogenesis [31,32,33,34,35,36,37,38]. There was no or a modest upregulation of these thermogenic genes by CAP in UCP-1−/− mice in the TNZ (Figure 5). This suggests that the lack of UCP-1 in the BAT of UCP-1 KO mice suppresses these thermogenic genes through unknown mechanisms. Interestingly, CAP increases PRDM-16 expression in the UCP-1 KO mice (Figure 6). However, this does not enhance metabolic activity or heat production (Figure 4). Thus, our results confirm that the mechanisms of action of CAP to counter HFD-induced obesity are permissive of UCP-1 in adipose tissues.
PRDM16 is a critical regulator of UCP-1 transcription [20]. PRDM16 suppresses the expression of white fat-specific genes in BAT and maintains the brown fat-specific profile in iWAT. Therefore, mice lacking PRDM16 specifically in adipose tissues will have substantially less heat production [39,40]. Dietary CAP upregulated the PRDM16 mRNA to the same extent in WT and UCP-1−/− mice at ambient temperature and in the TNZ (Figure 6B). The HFD inhibited the PRDM 16 in both WT and UCP1−/− mice at ambient temperature and in the TNZ. However, the UCP-1 mRNA was detected only in WT mice, and it was higher at AT than in the TNZ (Figure 6A). These data collectively suggest a regulatory role for PRDM 16 in UCP-1 transcription and therefore energy homeostasis. The regulatory role of PRDM 16-mediated UCP-1 transcription was further confirmed in Figure 7. In adipose tissue-specific PRDM16 knockout mice, CAP failed to inhibit HFD-induced weight gain and did not increase UCP-1 mRNA levels. Similarly, CAP stimulation in PRDM16-overexpressing cells increased UCP-1 gene expression (Figure 8).
Thus, our data unambiguously demonstrate that CAP counters diet-induced obesity in WT mice robustly at ambient temperature and in the TNZ through the PRDM16-mediated enhancement of UCP-1 transcription, as shown in Figure 9B. The HFD inhibits TRPV1, prevents Ca2+ influx and activation of kinases, and activates SIRT-1. This results in the acetylation of PRDM-16 and its downregulation, as shown in Figure 9A. HFD-induced obesity is associated with a diminished ability to expend energy as heat due to the downregulation of UCP-1, as PRDM16 is downregulated. As shown in Figure 9C, the CAP-mediated signal transduction mechanism maintains PRDM-16 in a deacetylated state but energy is not expended, as UCP-1 is not present in UCP-1−/− mice. Our data provide strong evidence that the TRPV1/PRDM-16/UCP-1 axis inhibits weight gain at ambient temperature, as well as in the TNZ (model shown in Figure 9). These data have a significant translational impact since humans protect themselves from external variations in temperatures and remain in a thermoneutral zone, and targeting and activating TRPV1 at thermoneutrality in humans would be a viable strategy to counter diet-induced obesity. Thus, this research reveals novel and promising findings to support the activation of TRPV1 in humans.
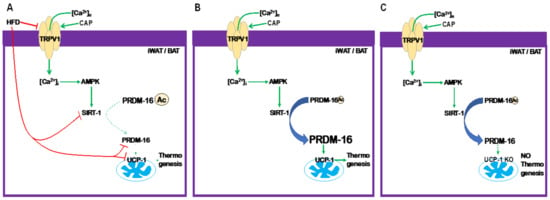
Figure 9.
Model describing the effect of TRPV1 activation by CAP on PRDM-16-dependent UCP-1 expression and therefore thermogenesis. (A). The HFD inhibits TRPV1 [10], SiRT-1, and PRDM-16 [9]. Inhibition of SiRT-1 suppresses the deacetylation of PRDM-16 [9] and PRDM-16-dependent UCP-1 transcription. (B). CAP counters the effect of the HFD. (C). In UCP-1 KO mice, CAP activates the TRPV1-SIRT-1-PRDM-16-dependent signaling axis, but it fails to stimulate thermogenesis.
4. Materials and Methods
4.1. Mouse Model of High-Fat Diet-Induced Obesity
Adult male and female UCP-1−/− (129S-Ucp1tm1Kz/J) mice and their genetically unaltered control (wild type, WT) mice were purchased from the Jackson Laboratory, Maine, USA. Mice were housed in the research animal facility at the School of Pharmacy, University of Wyoming, and used for experiments as per approved IACUC protocols. UCP-1−/− mice were genotyped three weeks after birth using primers specified by Jackson Laboratories.
For experiments, groups of WT and UCP-1−/− mice were fed a normal chow diet (NCD) until six weeks of age and then fed ad libitum either the NCD (Labdiet Rodent 5001; gross energy kcal/g is 4.07) or were switched to a high-fat diet (HFD; 60% calories from fat; D 12492; Research Diet Inc., New Brunswick, NJ, USA; 5.21 kcal/g and fed until 38 weeks of age. Mice were randomly assigned into feeding groups and were housed in groups of four in separate cages. Mice were maintained either at ambient temperature (22–24 °C) or at thermoneutrality (29 to 31 °C) on a twelve-hour dark–light cycle and their weekly weight gain and food and water intake were recorded in a blinded fashion. For energy/water intake, the average quantity consumed per mouse per day was calculated, and the mean values of data were pooled from week 6 through week 38 of feeding. At the end of 32 weeks of feeding (mice were 38 weeks of age), a metabolic study was performed with a group of mice. Two days after the completion of the metabolic study, epididymal and brown adipose fat pad tissues (EF and BF, respectively) were isolated and used for quantitative RT–PCR experiments.
4.2. Adipose Tissue-Specific PRDM-16 Knockout Mice
Adipose tissue-specific PRDM16 knockout mice were generated by crossing PRDM16lox/lox mice with Fabp4-cre mice. Genotyping was carried out using primers specified by Jackson Laboratories (Bar Harbor, ME, USA) for generic Cre.
4.3. Measurement of Rectal Temperature in Mice
The rectal temperature was determined by inserting a petroleum jelly-lubricated thermometer into the rectum of the mice. The temperature was recorded when it was stable, and the probe was removed.
4.4. Metabolic Activity Measurement
Metabolic activity and the respiratory quotient were determined for NCD- or HFD-fed WT and TRPV1−/− mice at the end of 32 weeks of feeding using the Comprehensive Laboratory Animal Monitoring System [CLAMS™], Columbus Instruments, Columbus, OH, USA [41,42]. Mice were individually placed in the CLAMS metabolic cages with ad libitum access to food and water. After acclimatization for 48 hr., metabolic parameters, including the volume of carbon dioxide produced (VCO2), the volume of oxygen consumed (VO2), the respiratory quotient (RQ), and respiratory exchange ratio (RER = VCO2/VO2), and ambulatory/locomotor activity were determined as described previously.
4.5. Fat Tissue Isolation [43]
Following euthanization, mice were placed on a surgical pad with the dorsal surface facing up and cut open along the back to the neck. The intrascapular BF found right under the skin between the shoulders, seen as two lobes, was dissected.
For inguinal subcutaneous fat pad isolation, a cut was made through the skin using a scalpel just below the rib cage across the dorsal surface joining the two lateral incisions. After peeling the skin, the fat underlying the muscle and fascia was dissected using a pair of scissors.
4.6. Isolation and Primary Culture of Brown Adipose Tissue Preadipocytes
The primary preadipocyte culture from BAT was performed in the same way as from SCF, as described previously [14].
4.7. Immunohistochemistry
Immunostaining for TRPV1 and DAPI was performed in primary adipocytes obtained from the iWAT of NCD-, HFD-, or HFD + CAP-fed WT mice, as described previously [14].
4.8. Whole-Cell Patch Clamp of Primary Brown Adipocytes
TRPV1 activity was measured at -60 mV in the primary brown preadipocytes by measuring CAP (1 μM)-stimulated currents using patch clamping, as described previously [44].
4.9. Quantitative RT-PCR Measurements (qRT-PCR)
The isolation of total RNA was performed using Tri-reagent (Sigma, St. Louis, MO, USA) as per the manufacturer’s protocol. cDNA was synthesized using a QuantiTect reverse transcription kit (Qiagen, Valencia, CA, USA) using a Q5plex PCR system (Qiagen, Valencia, CA, USA). Real-time PCR was performed using a QuantiTect SYBR Green PCR kit on the Q5plex system. 18s RNA was used as the internal control. Amplifications were performed in a 25 µL reaction volume. All the primers used for quantitative RT-PCR experiments are given in Table 1.

Table 1.
Quantitative RT-PCR primers used for the research study.
4.10. Immunoblotting
Isolated fat pads were washed with chilled PBS, lysed in lysis buffer (50 mM, Tris pH 7.5, 250 mM sodium chloride, 0.5% NP40, 0.5% sodium deoxycholate, 2 mM EDTA, 0.5 mM dithiothreitol, 1 mM sodium orthovanadate, and protease inhibitor cocktail), and centrifuged at 14000 rpm for 20 min. to remove tissue debris. The concentration of protein was determined by the Bradford method and equal amounts of protein were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and immunoblotted with a TRPV1 antibody at a dilution of 1:100, (SC-28759, Santacruz Biotechnologies, Dallas, TX, USA) and a GAPDH antibody at a dilution of 1:1000, (SC-365062, Santacruz Biotechnologies, Dallas, TX, USA).
4.11. PRDM16 and GFP-UCP1 Overexpression and Stimulation in the HEK TRPV1 Cell Line
The HEK TRPV1 cells were grown to 70% confluence in poly-lysine-coated plates and were transfected with UCP-1-GFP alone (#104585, Addgene, Watertown, MA 02472, USA) or UCP-1-GFP and PRDM16 (Origene, #MC229536, Rockville, MD 20850, USA) using Lipofectamine transfection reagent. UCP-1-GFP-transfected cells were treated with vehicle or CAP (1 μM) overnight. UCP-1-GFP- and PRDM16-transfected cells were treated with vehicle or CAP (1 μM) or capsazepine (10 μM) for 1 h followed by CAP and measured for GFP fluorescence using a confocal microscope.
4.12. Drugs and Chemicals
The normal chow diet was obtained from Lab Diets, Inc., USA. Other special diets were obtained from Research Diets Inc., New Brunswick, NJ, USA. All chemicals were obtained from Sigma, USA. Quantitative RT-PCR kits were obtained from Qiagen, USA.
4.13. Statistical Analyses
Data for all figures are expressed as means ± S.E.Ms. Student’s t-test and ANOVA evaluated the statistical significance of population means. Sample sizes were set to determine whether the mean value of an outcome variable in one group differed significantly from that in another group. A p value < 0.05 was considered statistically significant. Comparisons between groups were analyzed using one-way ANOVA, and post hoc analyses were performed using Tukey’s test. Figures from analyzed data were plotted using Microcal Origin 2020 software (OriginLab, Northampton, MA, USA), and figures were generated using the same program and then converted into image files using the software Adobe Photoshop CS5 Extended (version 12.1; www.adobe.com).
Author Contributions
P.B.: conceptualization, methodology, experiments, analysis, writing—original draft preparation, supervision. N.G.: maintain the mouse colony—breeding, weaning, genotyping, maintain mice at TNZ, and monitor weight gain and food and water intake. N.C.: maintain the mouse colony—breeding of PRDM16 Lox/FABP4-cre mice, weaning, genotyping, maintain mice, and monitor weight gain and food and water intake. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from the American Association Colleges of Pharmacy New Investigator Award to PB and the Howard University College of Pharmacy Start-up Grant.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of the University of Wyoming (protocol codes 20150114BT0013701 and 02, and 01/14/2016-01/13/2017) for studies involving animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Troynikov, O.; Watson, C.G.; Nawaz, N. Sleep environments and sleep physiology: A review. J. Therm. Biol. 2018, 78, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Someren, E.J.W.V. Age-Related Changes in Thermoreception and Thermoregulation. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780123786388000221 (accessed on 9 August 2024).
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. The Importance of Energy Balance. Eur. Endocrinol. 2013, 9, 111–115. [Google Scholar]
- Manini, T.M. Energy expenditure and aging. Ageing Res. Rev. 2010, 9, 1. [Google Scholar]
- Tremblay, A.; Royer, M.M.; Chaput, J.P.; Doucet, E. Adaptive thermogenesis can make a difference in the ability of obese individuals to lose body weight. Int. J. Obes. (Lond.) 2013, 37, 759–764. [Google Scholar] [PubMed]
- Ravussin, Y.; LeDuc, C.A.; Watanabe, K.; Leibel, R.L. Effects of ambient temperature on adaptive thermogenesis during maintenance of reduced body weight in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R438–R448. [Google Scholar] [PubMed]
- Klingenspor, M.; Bast, A.; Bolze, F.; Li, Y.; Maurer, S.; Schweizer, S.; Willershäuser, M.; Fromme, T. Brown Adipose Tissue. 2017. Available online: https://portal.fis.tum.de/en/publications/brown-adipose-tissue-2 (accessed on 9 August 2024).
- Fedorenko, A.; Lishko, P.V.; Kirichok, Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 2012, 151, 400–413. [Google Scholar] [CrossRef]
- Razzoli, M.; Emmett, M.J.; Lazar, M.A.; Bartolomucci, A. β-Adrenergic receptors control brown adipose UCP-1 tone and cold response without affecting its circadian rhythmicity. FASEB J. 2018, 32, 5640–5646. [Google Scholar] [CrossRef]
- John, L.M.; Petersen, N.; Gerstenberg, M.K.; Torz, L.; Pedersen, K.; Christoffersen, B.; Kuhre, R.E. Housing-temperature reveals energy intake counter-balances energy expenditure in normal-weight, but not diet-induced obese, male mice. Commun. Biol. 2022, 5, 946. [Google Scholar] [CrossRef]
- Cui, X.; Nguyen, N.L.; Zarebidaki, E.; Cao, Q.; Li, F.; Zha, L.; Bartness, T.; Shi, H.; Xue, B. Thermoneutrality decreases thermogenic program and promotes adiposity in high-fat diet-fed mice. Physiol. Rep. 2016, 4, e12799. [Google Scholar] [CrossRef]
- Dieckmann, S.; Strohmeyer, A.; Willershäuser, M.; Maurer, S.F.; Wurst, W.; Marschall, S.; de Angelis, M.H.; Kühn, R.; Worthmann, A.; Fuh, M.M.; et al. Susceptibility to diet-induced obesity at thermoneutral conditions is independent of UCP1. Am. J. Physiol. Endocrinol. Metab. 2022, 322, e85–e100. [Google Scholar] [CrossRef]
- Baskaran, P.; Krishnan, V.; Fettel, K.; Gao, P.; Zhu, Z.; Ren, J.; Thyagarajan, B. TRPV1 activation counters diet-induced obesity through sirtuin-1 activation and PRDM-16 deacetylation in brown adipose tissue. Int. J. Obes. (Lond.) 2017, 41, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, P.; Krishnan, V.; Ren, J.; Thyagarajan, B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br. J. Pharmacol. 2016, 173, 2369–2389. [Google Scholar] [CrossRef] [PubMed]
- Kingma, B.R.; Frijns, A.J.; Schellen, L.; van Marken Lichtenbelt, W.D. Beyond the classic thermoneutral zone: Including thermal comfort. Temperature 2014, 1, 142–149. [Google Scholar] [CrossRef]
- Kingma, B.; Frijns, A.; van Marken Lichtenbelt, W. The thermoneutral zone: Implications for metabolic studies. Front. Biosci. 2012, 4, 1975–1985. [Google Scholar] [CrossRef]
- Lehr, L.; Canola, K.; Asensio, C.; Jimenez, M.; Kuehne, F.; Giacobino, J.P.; Muzzin, P. The control of UCP1 is dissociated from that of PGC-1alpha or of mitochondriogenesis as revealed by a study using beta-less mouse brown adipocytes in culture. FEBS Lett. 2006, 580, 4661–4666. [Google Scholar] [CrossRef]
- Teruel, T.; Clapham, J.C.; Smith, S.A. PPARalpha activation by Wy 14643 induces transactivation of the rat UCP-1 promoter without increasing UCP-1 mRNA levels and attenuates PPARgamma-mediated increases in UCP-1 mRNA levels induced by rosiglitazone in fetal rat brown adipocytes. Biochem. Biophys. Res. Commun. 1999, 264, 311–315. [Google Scholar] [CrossRef]
- Barbera, M.J.; Schluter, A.; Pedraza, N.; Iglesias, R.; Villarroya, F.; Giralt, M. Peroxisome proliferator-activated receptor alpha activates transcription of the brown fat uncoupling protein-1 gene. A link between regulation of the thermogenic and lipid oxidation pathways in the brown fat cell. J. Biol. Chem. 2001, 276, 1486–1493. [Google Scholar] [CrossRef]
- Iida, S.; Chen, W.; Nakadai, T.; Ohkuma, Y.; Roeder, R.G. PRDM16 enhances nuclear receptor-dependent transcription of the brown fat-specific Ucp1 gene through interactions with Mediator subunit MED1. Genes. Dev. 2015, 29, 308–321. [Google Scholar] [CrossRef]
- Baskaran, P.; Markert, L.; Bennis, J.; Zimmerman, L.; Fox, J.; Thyagarajan, B. Assessment of Pharmacology, Safety, and Metabolic activity of Capsaicin Feeding in Mice. Sci. Rep. 2019, 9, 8588. [Google Scholar] [CrossRef]
- Redinger, R.N. The pathophysiology of obesity and its clinical manifestations. Gastroenterol. Hepatol. (NY) 2007, 3, 856–863. [Google Scholar]
- NIDDK. Overweight & Obesity Statistics. Available online: https://www.niddk.nih.gov/health-information/health-statistics/overweight-obesity (accessed on 9 August 2024).
- Betz, M.J.; Enerbäck, S. Human Brown Adipose Tissue: What We Have Learned So Far. Diabetes 2015, 64, 2352–2360. [Google Scholar] [CrossRef] [PubMed]
- Abete, I. Genetic Regulation of Energy Homeostasis: Obesity Implications. Available online: https://www.biorxiv.org/content/10.1101/2020.07.27.223529v1.full (accessed on 9 August 2024).
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef]
- Kim, K.; Wann, J.; Kim, H.G.; So, J.; Rosen, E.D.; Roh, H.C. Uncoupling protein 1-driven Cre (Ucp1-Cre) is expressed in the epithelial cells of mammary glands and various non-adipose tissues. bioRxiv 2023. [Google Scholar] [CrossRef]
- Enerbäck, S.; Jacobsson, A.; Simpson, E.M.; Guerra, C.; Yamashita, H.; Harper, M.E.; Kozak, L.P. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 1997, 387, 90–94. [Google Scholar] [CrossRef]
- Liu, X.; Rossmeisl, M.; McClaine, J.; Riachi, M.; Harper, M.E.; Kozak, L.P. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J. Clin. Investig. 2003, 111, 399–407. [Google Scholar] [CrossRef]
- Li, Y.; Fromme, T. Uncoupling Protein 1 Does Not Produce Heat without Activation. Int. J. Mol. Sci. 2022, 23, 2406. [Google Scholar] [CrossRef]
- Qian, S.W.; Tang, Y.; Li, X.; Liu, Y.; Zhang, Y.Y.; Huang, H.Y.; Xue, R.D.; Yu, H.Y.; Guo, L.; Gao, H.D.; et al. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc. Natl. Acad. Sci. USA 2013, 110, E798–E807. [Google Scholar] [CrossRef]
- Whittle, A.J.; Carobbio, S.; Martins, L.; Slawik, M.; Hondares, E.; Vázquez, M.J.; Morgan, D.; Csikasz, R.I.; Gallego, R.; Rodriguez-Cuenca, S.; et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 2012, 149, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Jash, S.; Banerjee, S.; Lee, M.J.; Farmer, S.R.; Puri, V. CIDEA Transcriptionally Regulates UCP1 for Britening and Thermogenesis in Human Fat Cells. iScience 2019, 20, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Banhos Danneskiold-Samsøe, N.; Sonne, S.B.; Larsen, J.M.; Hansen, A.N.; Fjære, E.; Isidor, M.S.; Petersen, S.; Henningsen, J.; Severi, I.; Sartini, L.; et al. Overexpression of cyclooxygenase-2 in adipocytes reduces fat accumulation in inguinal white adipose tissue and hepatic steatosis in high-fat fed mice. Sci. Rep. 2019, 9, 8979. [Google Scholar] [CrossRef]
- Tabuchi, C.; Sul, H.S. Signaling Pathways Regulating Thermogenesis. Front. Endocrinol. (Lausanne) 2021, 12, 595020. [Google Scholar] [CrossRef]
- Gan, L.; Liu, Z.; Chen, Y.; Dan, L.; Feng, F.; Liu, G.; Sun, C. α-MSH and Foxc2 promote fatty acid oxidation through C/EBPβ negative transcription in mice adipose tissue. Sci. Rep. 2016, 6, 36661. [Google Scholar] [CrossRef]
- Uldry, M.; Yang, W.; St-Pierre, J.; Lin, J.; Seale, P.; Spiegelman, B.M. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006, 3, 333–341. [Google Scholar] [CrossRef]
- Qiang, L.; Wang, L.; Kon, N.; Zhao, W.; Lee, S.; Zhang, Y.; Rosenbaum, M.; Zhao, Y.; Gu, W.; Farmer, S.R.; et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell 2012, 150, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.J.; Ishibashi, J.; Wang, W.; Lim, H.W.; Goyama, S.; Sato, T.; Kurokawa, M.; Won, K.J.; Seale, P. Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell Metab. 2014, 19, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Levy, J.D.; Zhang, Y.; Frontini, A.; Kolodin, D.P.; Svensson, K.J.; Lo, J.C.; Zeng, X.; Ye, L.; Khandekar, M.J.; et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 2014, 156, 304–316. [Google Scholar]
- Turdi, S.; Kandadi, M.R.; Zhao, J.; Huff, A.F.; Du, M.; Ren, J. Deficiency in AMP-activated protein kinase exaggerates high fat diet-induced cardiac hypertrophy and contractile dysfunction. J. Mol. Cell Cardiol. 2011, 50, 712–722. [Google Scholar] [CrossRef]
- Ren, J. Leptin and hyperleptinemia—From friend to foe for cardiovascular function. J. Endocrinol. 2004, 181, 1–10. [Google Scholar]
- Bagchi, D.P.; MacDougald, O.A. Identification and Dissection of Diverse Mouse Adipose Depots. J. Vis. Exp. 2019. [Google Scholar] [CrossRef]
- Thyagarajan, B.; Lukacs, V.; Rohacs, T. Hydrolysis of phosphatidylinositol 4,5-bisphosphate mediates calcium-induced inactivation of TRPV6 channels. J. Biol. Chem. 2008, 283, 14980–14987. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).