Optimizing Outcomes: Bevacizumab with Carboplatin and Paclitaxel in 5110 Ovarian Cancer Patients—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection and Data Extraction
2.3. Assessment of Bias Risk and Evidence
2.4. Statistical Analysis
3. Results
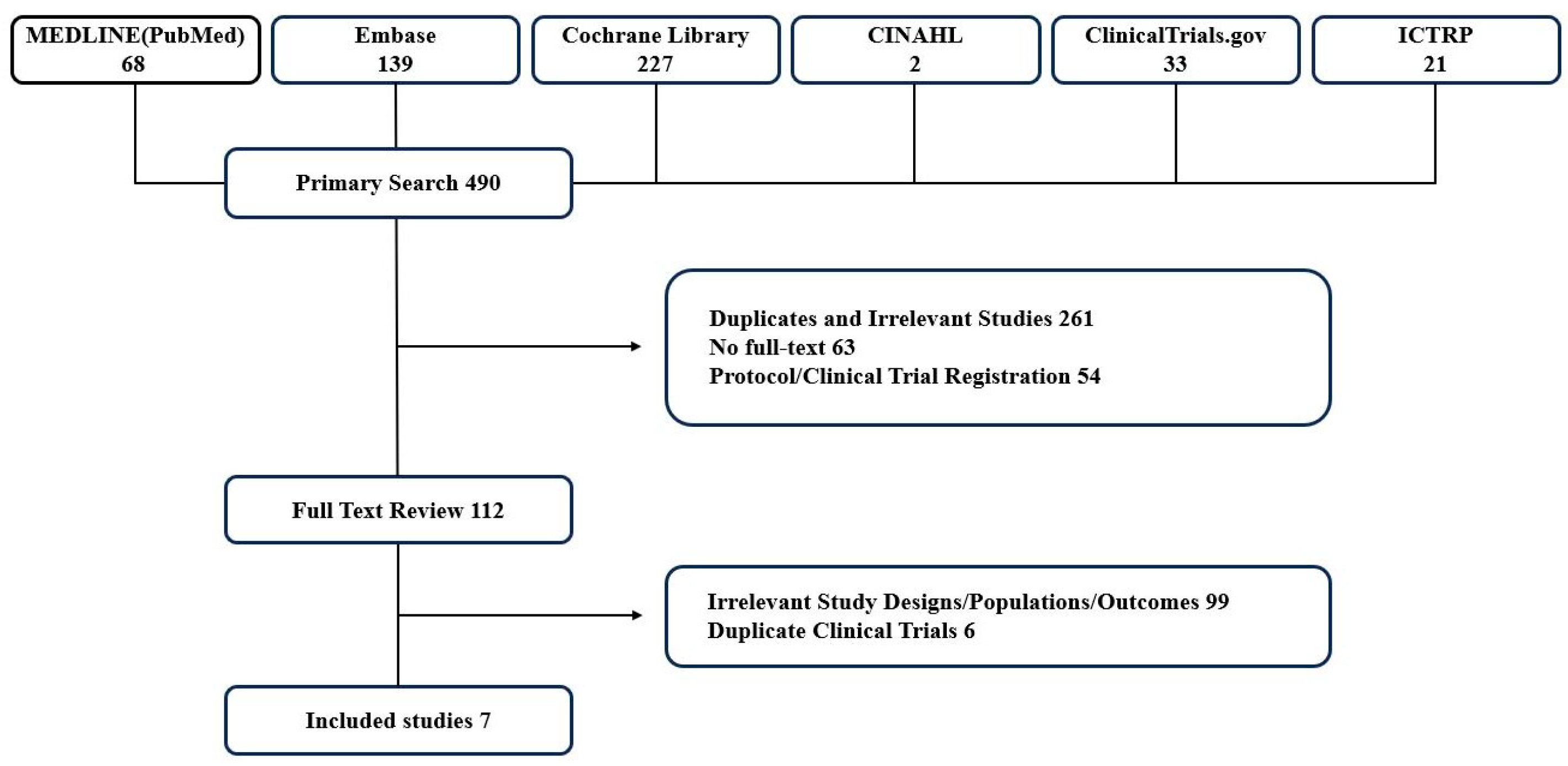
3.1. Study Search and Selection
3.2. Study Characteristics
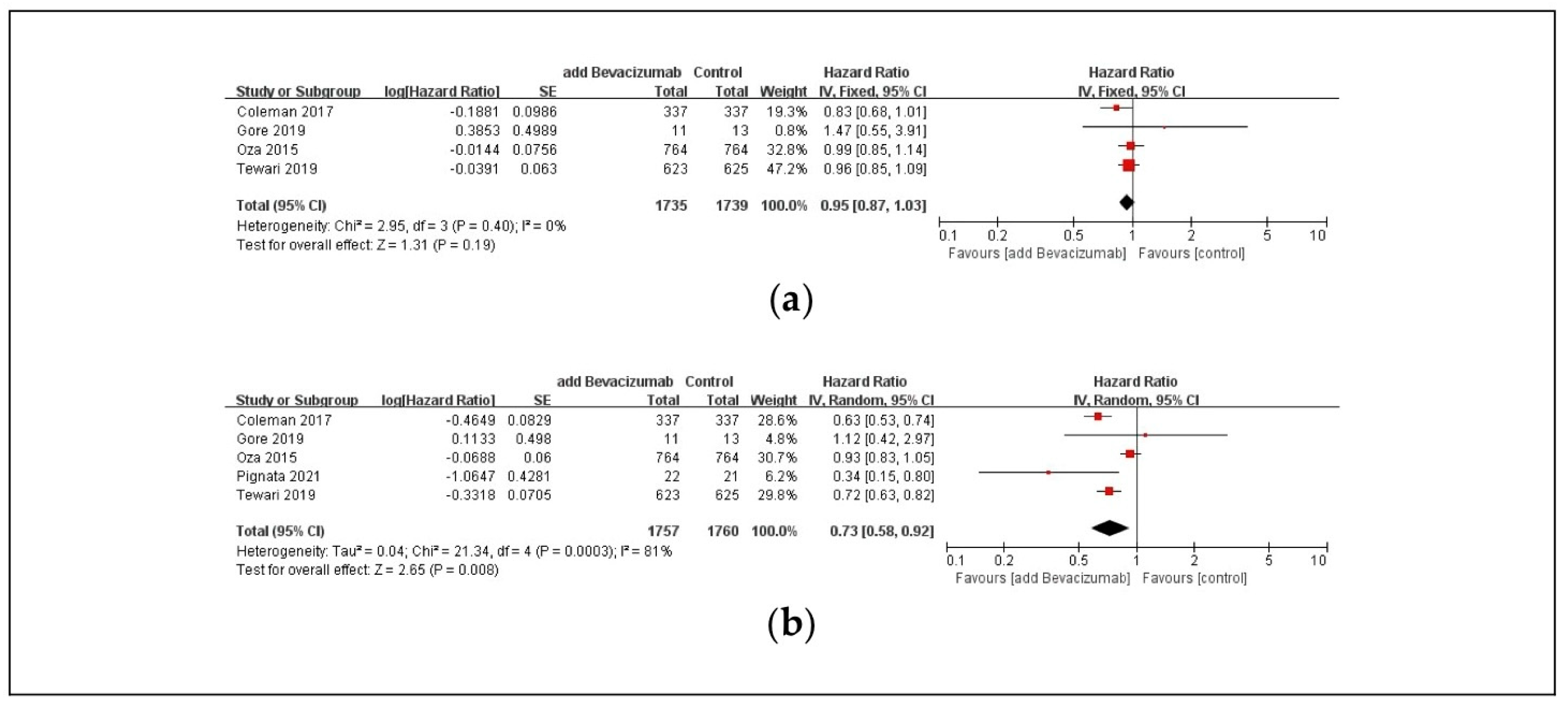
3.3. Efficacy Outcomes
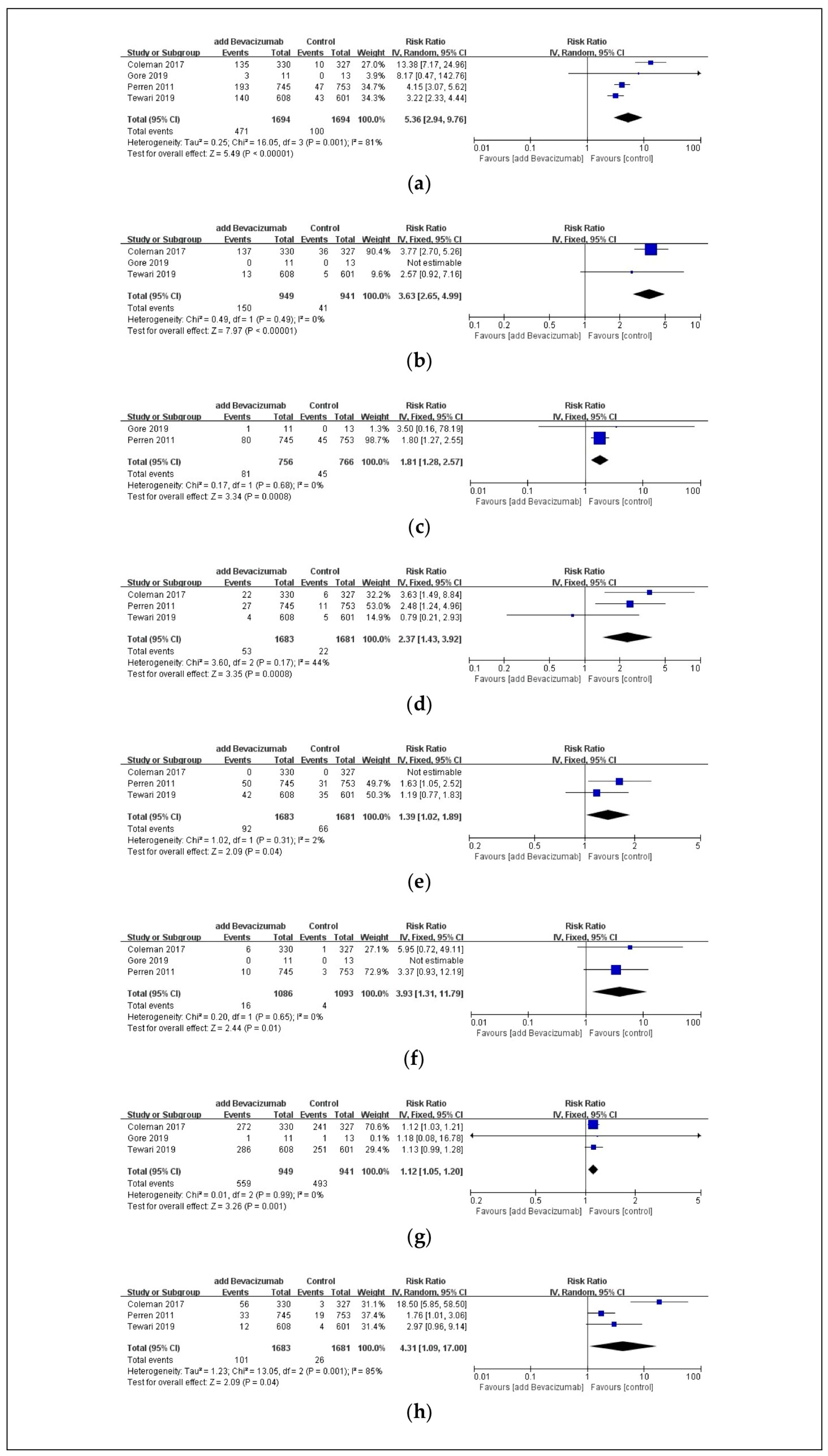
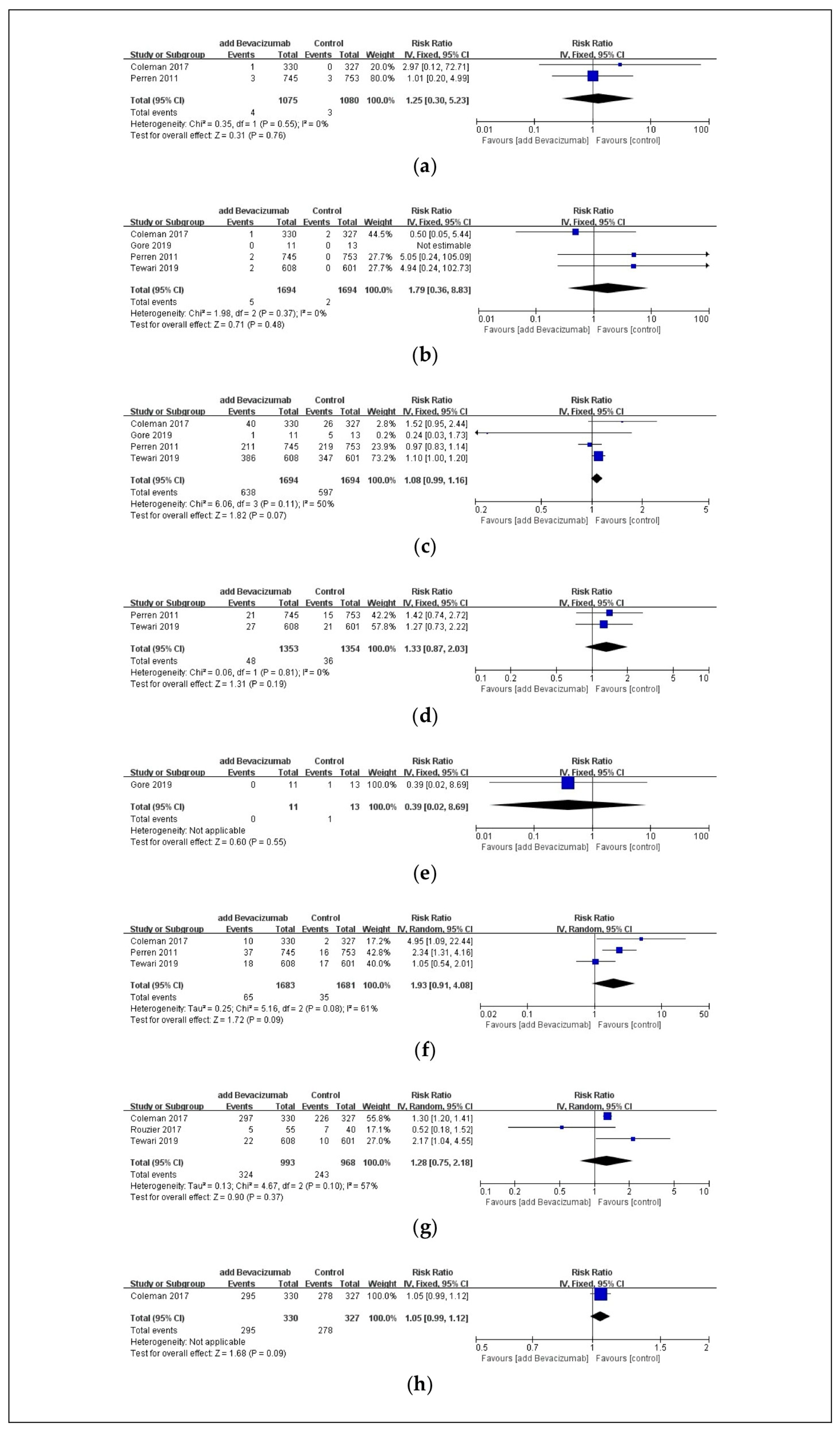
3.4. Safety Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Webb, P.M.; Jordan, S.J. Global epidemiology of epithelial ovarian cancer. Nat. Rev. Clin. Oncol. 2024, 21, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- KOSIS. Relative Survival Rate for 5 Years by 24 Kinds of Cancer, Cancer Occurrence Time and Gender. 2021. Available online: https://kosis.kr/statHtml/statHtml.do?orgId=117&tblId=DT_117N_A00021&conn_path=I2&language=en (accessed on 25 June 2024).
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA A Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef]
- Prat, J. Ovarian carcinomas: Five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012, 460, 237–249. [Google Scholar] [CrossRef]
- Kurman, R. Origin and molecular pathogenesis of ovarian high-grade serous carcinoma. Ann. Oncol. 2013, 24, x16–x21. [Google Scholar] [CrossRef]
- Peres, L.C.; Cushing-Haugen, K.L.; Köbel, M.; Harris, H.R.; Berchuck, A.; Rossing, M.A.; Schildkraut, J.M.; Doherty, J.A. Invasive epithelial ovarian cancer survival by histotype and disease stage. JNCI J. Natl. Cancer Inst. 2019, 111, 60–68. [Google Scholar] [CrossRef]
- Banerjee, S.; Kaye, S.B. New strategies in the treatment of ovarian cancer: Current clinical perspectives and future potential. Clin. Cancer Res. 2013, 19, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Ghirardi, V.; Fagotti, A.; Ansaloni, L.; Valle, M.; Roviello, F.; Sorrentino, L.; Accarpio, F.; Baiocchi, G.; Piccini, L.; De Simone, M. Diagnostic and therapeutic pathway of advanced ovarian cancer with peritoneal metastases. Cancers 2023, 15, 407. [Google Scholar] [CrossRef]
- NCCN. Ovarian Cancer. In NCCN Guidelines for Patients; NCCN: Plymouth Meeting, PA, USA, 2023. [Google Scholar]
- Colomban, O.; Tod, M.; Peron, J.; Perren, T.J.; Leary, A.; Cook, A.D.; Sajous, C.; Freyer, G.; You, B. Bevacizumab for newly diagnosed ovarian cancers: Best candidates among high-risk disease patients (ICON-7). JNCI Cancer Spectr. 2020, 4, pkaa026. [Google Scholar] [CrossRef]
- Genentech. Avastin. Highlights of Prescribing Information. 2022. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125085s340lbl.pdf (accessed on 25 June 2024).
- Aravantinos, G.; Pectasides, D. Bevacizumab in combination with chemotherapy for the treatment of advanced ovarian cancer: A systematic review. J. Ovarian Res. 2014, 7, 57. [Google Scholar] [CrossRef]
- Gaitskell, K.; Rogozińska, E.; Platt, S.; Chen, Y.; Abd El Aziz, M.; Tattersall, A.; Morrison, J. Angiogenesis inhibitors for the treatment of epithelial ovarian cancer. Cochrane Database Syst. Rev. 2023, 4, CD007930. [Google Scholar] [CrossRef]
- Hirte, H.; Poon, R.; Yao, X.; May, T.; Ethier, J.-L.; Petz, L.; Speakman, J.; Elit, L. Neoadjuvant and adjuvant systemic therapy for newly diagnosed stage II-IV epithelial ovary, fallopian tube, or primary peritoneal carcinoma: A systematic review. Crit. Rev. Oncol./Hematol. 2021, 162, 103324. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Y.; Li, J.; Wan, S.; Jiang, N.; Yang, J.; Chiampanichayakul, S.; Tima, S.; Anuchapreeda, S.; Wu, J. A comprehensive comparison of medication strategies for platinum-sensitive recurrent ovarian cancer: A Bayesian network meta-analysis. Front. Pharmacol. 2022, 13, 1010626. [Google Scholar] [CrossRef]
- Petrillo, M.; Nero, C.; Carbone, V.; Bruno, M.; Scambia, G.; Fagotti, A. Systematic review of cytoreductive surgery and bevacizumab-containing chemotherapy in advanced ovarian cancer: Focus on safety. Ann. Surg. Oncol. 2018, 25, 247–254. [Google Scholar] [CrossRef]
- Song, L.; Liu, Y.; Chen, Z.; Li, Z.; Zhu, S.; Zhao, Y.; Li, H. Association of bevacizumab and stroke in ovarian cancer: A systematic review and meta-analysis. Front. Neurosci. 2023, 17, 1187957. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Cochran, W.G. The combination of estimates from different experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Coleman, R.L.; Brady, M.F.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Walker, J.L.; Kim, B.G.; Fujiwara, K.; Tewari, K.S.; O′Malley, D.M.; et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017, 18, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Gore, M.; Hackshaw, A.; Brady, W.E.; Penson, R.T.; Zaino, R.; McCluggage, W.G.; Ganesan, R.; Wilkinson, N.; Perren, T.; Montes, A.; et al. An international, phase III randomized trial in patients with mucinous epithelial ovarian cancer (mEOC/GOG 0241) with long-term follow-up: And experience of conducting a clinical trial in a rare gynecological tumor. Gynecol. Oncol. 2019, 153, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Oza, A.M.; Cook, A.D.; Pfisterer, J.; Embleton, A.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015, 16, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef]
- Pignata, S.; Lorusso, D.; Joly, F.; Gallo, C.; Colombo, N.; Sessa, C.; Bamias, A.; Salutari, V.; Selle, F.; Frezzini, S.; et al. Carboplatin-based doublet plus bevacizumab beyond progression versus carboplatin-based doublet alone in patients with platinum-sensitive ovarian cancer: A randomised, phase 3 trial. Lancet Oncol. 2021, 22, 267–276. [Google Scholar] [CrossRef]
- Rouzier, R.; Gouy, S.; Selle, F.; Lambaudie, E.; Floquet, A.; Fourchotte, V.; Pomel, C.; Colombo, P.E.; Kalbacher, E.; Martin-Francoise, S.; et al. Efficacy and safety of bevacizumab-containing neoadjuvant therapy followed by interval debulking surgery in advanced ovarian cancer: Results from the ANTHALYA trial. Eur. J. Cancer 2017, 70, 133–142. [Google Scholar] [CrossRef]
- Tewari, K.S.; Burger, R.A.; Enserro, D.; Norquist, B.M.; Swisher, E.M.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Huang, H.; Homesley, H.D.; et al. Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. J. Clin. Oncol. 2019, 37, 2317–2328. [Google Scholar] [CrossRef] [PubMed]
- Ruan, G.; Ye, L.; Liu, G.; An, J.; Sehouli, J.; Sun, P. The role of bevacizumab in targeted vascular endothelial growth factor therapy for epithelial ovarian cancer: An updated systematic review and meta-analysis. OncoTargets Ther. 2018, 11, 521–528. [Google Scholar] [CrossRef]
- Poveda, A.M.; Selle, F.; Hilpert, F.; Reuss, A.; Savarese, A.; Vergote, I.; Witteveen, P.; Bamias, A.; Scotto, N.; Mitchell, L. Bevacizumab combined with weekly paclitaxel, pegylated liposomal doxorubicin, or topotecan in platinum-resistant recurrent ovarian cancer: Analysis by chemotherapy cohort of the randomized phase III AURELIA trial. J. Clin. Oncol. 2015, 33, 3836–3838. [Google Scholar] [CrossRef]
- Pfisterer, J.; Joly, F.; Kristensen, G.; Rau, J.; Mahner, S.; Pautier, P.; El-Balat, A.; Kurtz, J.-E.; Canzler, U.; Sehouli, J. Optimal treatment duration of bevacizumab as front-line therapy for advanced ovarian cancer: AGO-OVAR 17 BOOST/GINECO OV118/ENGOT Ov-15 open-label randomized phase III trial. J. Clin. Oncol. 2023, 41, 893–902. [Google Scholar] [CrossRef]
- Herzog, T.J.; Ison, G.; Alvarez, R.D.; Balasubramaniam, S.; Armstrong, D.K.; Beaver, J.A.; Ellis, A.; Tang, S.; Ford, P.; McKee, A. FDA ovarian cancer clinical trial endpoints workshop: A Society of Gynecologic Oncology white paper. Gynecol. Oncol. 2017, 147, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Gutman, S.; Piper, M.; Grant, M.; Basch, E.; Oliansky, D.; Aronson, N. Progression-Free Survival: What Does It Mean for Psychological Well-Being or Quality of Life? [Internet]; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2013. [Google Scholar]
- Hwang, T.J.; Gyawali, B. Association between progression-free survival and patients’ quality of life in cancer clinical trials. Int. J. Cancer 2019, 144, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Plummer, C.; Michael, A.; Shaikh, G.; Stewart, M.; Buckley, L.; Miles, T.; Ograbek, A.; McCormack, T. Expert recommendations on the management of hypertension in patients with ovarian and cervical cancer receiving bevacizumab in the UK. Br. J. Cancer 2019, 121, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Dincer, M.; Altundag, K. Angiotensin-converting enzyme inhibitors for bevacizumab-induced hypertension. Ann. Pharmacother. 2006, 40, 2278–2279. [Google Scholar] [CrossRef]
- Economopoulou, P.; Kotsakis, A.; Kapiris, I.; Kentepozidis, N. Cancer therapy and cardiovascular risk: Focus on bevacizumab. Cancer Manag. Res. 2015, 7, 133–143. [Google Scholar] [CrossRef]
| Component | Definition |
|---|---|
| P (patients) | Patients with ovarian cancer |
| I (intervention) | Combination therapy of carboplatin, paclitaxel, and bevacizumab |
| C (comparator) | Combination therapy of carboplatin and paclitaxel |
| O (outcomes) | Efficacy: OS and PFS; safety (adverse events): hypertension, heart failure, CNS bleeding, non-CNS bleeding, thromboembolic events (any, arterial, or venous), neutropenia, febrile neutropenia, anemia, wound complications, GI disorders, GI perforation, pain, dermatologic disorders, and proteinuria |
| S (study design) | RCTs |
| Study Name | Study Period | Country | Study Design | Patient Population | Intervention | Comparator | OS Outcome | PFS Outcome | Safety Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Coleman 2017 [24]/GOG-0213 study | December 2007–November 2014 | United States, Japan, and South Korea | Multi-center, open-label, randomized phase 3 trial | Adult females (aged ≥ 18 years) with recurrent measurable or evaluable epithelial ovarian, primary peritoneal, or fallopian tube cancer (n = 674) | Standard chemotherapy regimen plus bevacizumab (15 mg/kg) every 3 weeks, which was then continued as maintenance every 3 weeks until disease progression or unacceptable toxicity | Standard chemotherapy (six 3-weekly cycles of paclitaxel [175 mg/m2] and carboplatin [AUC 5]) | HR: 0.829 [0.683–1.005], p = 0.056 | HR: 0.628 [0.534–0.739], p < 0.0001 | Hypertension, heart failure, CNS bleeding, non-CNS bleeding, arterial thromboembolic events, venous thromboembolic events, neutropenia, wound complications, GI disorders, GI perforation, pain, dermatologic disorders, and proteinuria |
| Gore 2019 [25]/GOG-0241 study | March 2010–August 2013 | United Kingdom and the United States | Multi-center phase 3 factorial trial | Patients with histological diagnosis of primary mEOC; aged ≥ 18 years; newly diagnosed FIGO stage II–IV, or recurrence after stage I disease; no previous chemotherapy; ECOG performance status 0–2 (n = 24) | Carboplatin, paclitaxel, and bevacizumab (15 mg/kg intravenous every 3 weeks); then, bevacizumab maintenance (15 mg/kg on day 1, every 3 weeks) | Carboplatin (AUC 5/6) and paclitaxel (175 mg/m2), both intravenous, day 1 | HR: 1.47, p = 0.44 | HR: 1.12, p = 0.82 | Hypertension, CNS bleeding, non-CNS bleeding, any thromboembolic events, neutropenia, anemia, GI perforation, and pain |
| Oza 2015 [26]/ICON7 study | April 2006–March 2013 | 11 countries across Europe, Canada, Australia, and New Zealand | International, phase 3, open-label, randomized trial | Patients aged ≥ 18 years; with newly diagnosed epithelial ovarian, fallopian tube, or primary peritoneal cancer; an ECOG performance status of 0–2; FIGO stage IIb–IV or high-risk (grade 3 or clear cell histology) stage I–IIa disease (n = 1528) | Standard chemotherapy plus intravenous bevacizumab 7.5 mg/kg every 3 weeks given concurrently and continued with up to 12 further 3-weekly cycles of maintenance therapy | Standard chemotherapy (six 3-weekly cycles of intravenous carboplatin [AUC 5 or 6] and paclitaxel 175 mg/m2) | HR: 0.99 [0.85–1.14], p = 0.85 | HR: 0.93 [0.83–1.05], p = 0.25 | Not available |
| Perren 2011 [27]/ICON7 study | April 2006–February 2010 | United Kingdom, Germany, France, Canada, Australia, New Zealand, Denmark, Finland, Norway, Sweden, and Spain | Phase 3, open-label, randomized trial | Females with histologically confirmed, high-risk, early-stage disease (FIGO stage I or IIA and clear-cell or grade 3 tumors) or advanced (FIGO stage IIB to IV) epithelial ovarian cancer, primary peritoneal cancer, or fallopian tube cancer (n = 1498) | Standard-chemotherapy group plus bevacizumab (7.5 mg/kg) given concurrently every 3 weeks for 5 or 6 cycles and continued for 12 additional cycles or until disease progression | Carboplatin (AUC 5 or 6) and paclitaxel (175 mg/m2) given every 3 weeks for 6 cycles (standard-chemotherapy group) | Not available | Not available | Hypertension, heart failure, CNS bleeding, any thromboembolic events, arterial thromboembolic events, venous thromboembolic events, neutropenia, febrile neutropenia, wound complications, GI perforation, and proteinuria |
| Pignata 2021 [28]/NCT01802749 study | December 2013–February 2018 | France, Greece, Italy, Monaco, and Switzerland | Academic, multi-center, open-label, randomized phase 3 trial | Females aged ≥ 18 years with histologically confirmed FIGO stage IIIB–IV ovarian cancer, fallopian tube carcinoma, or peritoneal carcinoma (including mixed Mullerian tumors) (n = 43) | Carboplatin-based doublet plus bevacizumab | Intravenous carboplatin-based doublet (carboplatin AUC 5 on day 1 plus paclitaxel 175 mg/m2 on day 1, every 21 days | Not available | HR: 0.34 [0.15–0.80] | Not available |
| Rouizer 2017 [29]/ANTHALYA study | January 2013–August 2016 | France | Multi-center, open-label, non-comparative phase 2 study | Females aged ≥ 18 years with histologically confirmed, chemotherapy-naive, high-risk FIGO stage IIIC/IV epithelial ovarian carcinoma, fallopian tube carcinoma, or primary peritoneal carcinoma (ineligible for primary complete debulking surgery) (n = 95) | Four cycles of neoadjuvant carboplatin-paclitaxel + 3 concomitant cycles of bevacizumab 15 mg/kg | Four cycles of neoadjuvant carboplatin-paclitaxel | Not available | Not available | GI disorders |
| Tewari 2019 [30]/GOG-0218 study | October 2005–January 2018 | United States, Canada, Japan, and South Korea | International, multi-center, double-blind, placebo-controlled, phase 3 trial | Females with stage III or IV epithelial ovarian, primary peritoneal, or fallopian tube carcinoma (n = 1248) | Six 21-day cycles of intravenous carboplatin (AUC 6) plus paclitaxel (175 mg/m2), followed by bevacizumab (15 mg/kg) in cycles 7 to 22 | Six 21-day cycles of intravenous carboplatin (AUC 6) plus paclitaxel (175 mg/m2), followed by 16 21-day cycles of placebo | HR: 0.96 [0.85–1.09], p = 0.53 | HR: 0.717 [0.625–0.824], p < 0.001 | Hypertension, CNS bleeding, non-CNS bleeding, arterial thromboembolic events, venous thromboembolic events, neutropenia, febrile neutropenia, wound complications, GI disorders, pain, and proteinuria |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.J.; Lee, H.M.; Lee, G.E.; Yoo, J.H.; Lee, H.J.; Rhie, S.J. Optimizing Outcomes: Bevacizumab with Carboplatin and Paclitaxel in 5110 Ovarian Cancer Patients—A Systematic Review and Meta-Analysis. Pharmaceuticals 2024, 17, 1095. https://doi.org/10.3390/ph17081095
Kim YJ, Lee HM, Lee GE, Yoo JH, Lee HJ, Rhie SJ. Optimizing Outcomes: Bevacizumab with Carboplatin and Paclitaxel in 5110 Ovarian Cancer Patients—A Systematic Review and Meta-Analysis. Pharmaceuticals. 2024; 17(8):1095. https://doi.org/10.3390/ph17081095
Chicago/Turabian StyleKim, Yu Jin, Hee Min Lee, Ga Eun Lee, Jin Hui Yoo, Hwa Jeong Lee, and Sandy Jeong Rhie. 2024. "Optimizing Outcomes: Bevacizumab with Carboplatin and Paclitaxel in 5110 Ovarian Cancer Patients—A Systematic Review and Meta-Analysis" Pharmaceuticals 17, no. 8: 1095. https://doi.org/10.3390/ph17081095
APA StyleKim, Y. J., Lee, H. M., Lee, G. E., Yoo, J. H., Lee, H. J., & Rhie, S. J. (2024). Optimizing Outcomes: Bevacizumab with Carboplatin and Paclitaxel in 5110 Ovarian Cancer Patients—A Systematic Review and Meta-Analysis. Pharmaceuticals, 17(8), 1095. https://doi.org/10.3390/ph17081095






