Abstract
Although Candida albicans is the most frequently identified Candida species in clinical settings, a significant number of infections related to the non-albicans Candida (NAC) species, Candida krusei, has been reported. Both species are able to produce biofilms and have been an important resistance-related factor to antimicrobial resistance. In addition, the microbial relationship is common in the human body, contributing to the formation of polymicrobial biofilms. Considering the great number of reports showing the increase in cases of resistance to the available antifungal drugs, the development of new and effective antifungal agents is critical. The inhibitory effect of Organoselenium Compounds (OCs) on the development of Candida albicans and Candida krusei was recently demonstrated, supporting the potential of these compounds as efficient antifungal drugs. In addition, OCs were able to reduce the viability and the development of biofilms, a very important step in colonization and infection caused by fungi. Thus, the objective of this study was to investigate the effect of the Organoselenium Compounds (p-MeOPhSe)2, (PhSe)2, and (p-Cl-PhSe)2 on the development of dual-species biofilms of Candida albicans and Candida krusei produced using either RPMI-1640 or Sabouraud Dextrose Broth (SDB) media. The development of dual-species biofilms was evaluated by the determination of both metabolic activity, using a metabolic assay based on the reduction of XTT (2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide sodium salt) assay and identification of either Candida albicans and Candida krusei on CHROMagar Candida medium. Biofilm formation using RPMI-1640 was inhibited in 90, 55, and 20% by 30 µM (p-MeOPhSe)2, (PhSe)2, and (p-Cl-PhSe)2, respectively. However, biofilms produced using SDB presented an inhibition of 62, 30 and 15% in the presence of 30 µM (p-MeOPhSe)2, (PhSe)2, and (p-Cl-PhSe)2, respectively. The metabolic activity of 24 h biofilms was inhibited by 35, 30 and 20% by 30 µM (p-MeOPhSe)2, (PhSe)2, and (p-Cl-PhSe)2, respectively, with RPMI-1640; however, 24 h biofilms formed using SDB were not modified by the OCs. In addition, a great reduction in the number of CFUs of Candida albicans (93%) in biofilms produced using RPMI-1640 in the presence of 30 µM (p-MeOPhSe)2 was observed. However, biofilms formed using SDB and treated with 30 µM (p-MeOPhSe)2 presented a reduction of 97 and 69% in the number of CFUs of Candida albicans and Candida krusei, respectively. These results demonstrated that Organoselenium Compounds, mainly (p-MeOPhSe)2, are able to decrease the metabolic activity of dual-species biofilms by reducing both Candida albicans and Candida krusei cell number during biofilm formation using either RPMI-1640 or SDB. Taken together, these results demonstrated the potential of the OCs to inhibit the development of dual-species biofilms of Candida albicans and Candida krusei.
1. Introduction
Fungi from the Candida genus are generally commensal and constitute the regular human microbiota; however, they have the ability to become opportunistic pathogens, mainly in immunocompromised patients, representing a public health challenge [1,2]. Candida albicans is frequently involved in fungi-related systemic infections due to its ability to transition from planktonic cell form to filamentous form [3]. The presence of filamentous structures represents an advantage for the fungus, contributing to its adhesion to the substrate and, consequently, to the formation of a complex filamentous system called biofilm [4,5]. Although Candida albicans is the most frequently identified Candida species in clinical contexts, a significant number of infections related to the non-albicans Candida (NAC) species, such as Candida krusei (Pichia kudriavzevii), has been reported [6,7]. In addition, mortality levels of infections caused by Candida krusei are alarming, mainly due to its natural resistance to fluconazole—a first-choice antifungal for the treatment of candidiasis [8,9].
Similar to Candida albicans, Candida krusei is also able to produce biofilms, increasing the resistance to conventional antifungal therapy and, consequently, extending the infections [10,11,12]. Biofilm formation is a complex process presenting distinct phases of development: adhesion to an abiotic or biotic surface, cell proliferation and early-stage filamentation of the adhered cells, and, finally, biofilm maturation [4]. Biofilms are communities of polymorphic cells, including hyphal cells, pseudohyphal cells, and yeast cells, protected by an extracellular matrix (ECM) that not only allows fungi to survive in critical conditions but also permits the cells in the biofilm structure to escape the immune system’s defenses [11,13]. In the medical context, biofilms can colonize diverse abiotic surfaces, such as catheters and dentures, although biotic surfaces, such as the gastrointestinal tract, female reproductive tract, oral cavity, and skin, can provide a propitious surface to biofilm development [4,14,15,16].
One of the main causes of nosocomial infections worldwide is those related to Candida species, mainly in immunocompromised patients [17]. In addition, it has been demonstrated that Candidaemia is the principal form of invasive candidiasis, representing the fourth cause of bloodstream infections in intensive care unit settings with high mortality rates [18,19].
Furthermore, an increase in the number of studies demonstrating the increase in rates of antifungal resistance in most fungal infections has been observed [20,21]. The first Fungal Priority Pathogens List (WHO FPPL) was published in 2022 to direct research, development, and public health actions to reinforce the response to antifungal resistance. This list classifies the fungal pathogens into three priority categories: critical, high, and medium. It is very important to highlight that the species Candida albicans and Candida krusei (Pichia kudriavzevii) were included in the WHO FPPL, with Candida albicans categorized into critical groups. In this context, there is an urgent need to develop effective strategies and procedures to control opportunistic human pathogens.
Organoselenium Compounds (OCs) are important intermediates and reagents used in organic syntheses [22] with applications in diverse biological processes, presenting neuroprotective, antitumor, and antimicrobial actions, also acting as cytokine inducers and immunomodulators of the central nervous system [23,24]. The antimicrobial activity of OCs against pathogenic fungi and bacteria has been described by different authors [25,26,27,28]. In the last years, our group has been studying the potential of OCs (p-MeOPhSe)2, (PhSe)2, and (p-Cl-PhSe)2 as promising antifungal drugs against both Candida albicans and Candida [29,30,31,32,33]. These studies demonstrated the ability of OCs (p-MeOPhSe)2, (PhSe)2, and (p-Cl-PhSe)2 to inhibit the development of both Candida albicans and Candida krusei, decreasing biofilm formation and also the viability of formed biofilms.
The human microbiota is composed of a collection of dynamic microbial communities, and it is common to find diverse microorganisms coexisting [34]. Interactions between different microorganisms are complex and can be synergistic, antagonistic, or present neutral relationships [13]. Furthermore, multispecies communities can produce polymicrobial biofilms that can lead to persistent infections in different sites in the human body [34,35]. These polymicrobial infections represent an additional challenge due to the need to administer higher doses of antimicrobial medications, increasing the risks for the patient since it is known that some antifungal drugs can damage kidney and liver cells, mainly when used for extended periods [36]. Consequently, studies related to the development of new and efficient antifungal treatments against multispecies biofilms are crucial. Thus, the objective of this study was to determine the effect of OCs (p-MeOPhSe)2, (PhSe)2, and (p-Cl-PhSe)2 on the development of dual-species biofilms of Candida albicans and Candida krusei.
2. Results
The effect of different Organoselenium Compounds (OCs) was determined on the formation of dual-species biofilm of Candida albicans and Candida krusei, using either RPMI-1640 or SDB (Figure 1). Biofilm formation using RPMI-1640 was inhibited in the presence of (p-MeOPhSe)2 in concentrations varying from 10 to 30 µM (~90%). The compounds (PhSe)2 and (p-Cl-PhSe)2 were also able to reduce biofilm formation but to a minor extent. Inhibition of ~55% and ~20% in the presence of 30 µM (PhSe)2 and (p-Cl-PhSe)2 was observed, respectively (Figure 1A). However, biofilms produced using SDB presented a minor inhibition by OCs. Inhibition of 62, 30, and 15% in the presence of 30 µM (p-MeOPhSe)2, (PhSe)2, and (p-Cl-PhSe)2 was observed, respectively (Figure 1B).
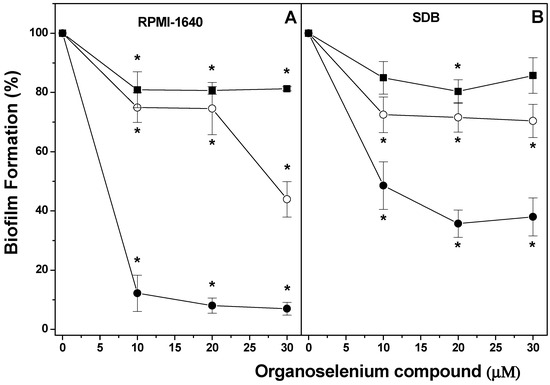
Figure 1.
Effect of (p-MeOPhSe)2 (●), (PhSe)2 (ο), and (p-Cl-PhSe)2 (▄) on the metabolic activity of dual-species biofilm in formation using either RPMI-1640 (A) or SDB (B) medium. Details of the experimental conditions are described in Methods. Yeast-mixed suspension was used to form biofilms for 24 h in the presence of different OC concentrations, with RPMI-1640 or SDB. After this period, the metabolic activity of dual-species biofilms was determined using XTT. The values presented in the figure represent the percentage of the metabolic activity of biofilms, calculated using the control group (biofilms formed in the absence of OCs) as 100% of the metabolic activity of biofilms. The data are mean ± SE (n = 8). * p < 0.05.
Figure 2 shows the effect of 30 µM (p-MeOPhSe)2, (PhSe)2, and (p-Cl-PhSe)2 on 24 h dual-species biofilm produced using either RPMI-1640 or SDB. When studying the effect of the compounds on biofilms produced over 24 h, it was observed that concentrations lower than 30 µM were not able to produce important inhibitory effects on metabolic activity. For this reason, it was chosen the concentration of 30 µM since it is not an exceedingly high concentration, a fact that could not be positive when choosing a medicine. An inhibition of 35, 30, and 20% in the metabolic activity of 24 h dual-species biofilm produced using RPMI-1640 and treated for additional 24 h with 30 µM (p-MeOPhSe)2, (PhSe)2, and (p-Cl-PhSe)2, respectively, was observed. However, the 24 h dual-species biofilm of Candida albicans and Candida krusei formed using SDB was not modified by the OCs tested. Taken together, these results demonstrated the potential of the OCs to inhibit the development of dual-species biofilms of Candida albicans and Candida krusei and that the compounds are more effective on biofilms produced using RPMI-1640.
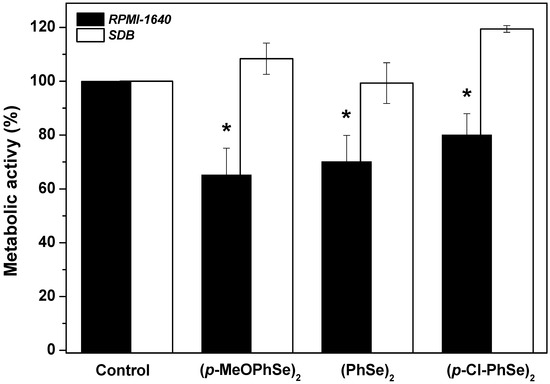
Figure 2.
Effect of (p-MeOPhSe)2, (PhSe)2, and (p-Cl-PhSe)2 on the metabolic activity of 24 h dual-species biofilm. Details of the experimental conditions are described in Methods. Yeast-mixed suspension was used to produce biofilms for 24 h in the presence of RPMI-1640 or SDB. After this period, biofilms were treated and grown for an additional 24 h in the presence of 30 μM OCs with either RPMI-1640 or SDB. The values presented in the figure represent the percentage of the metabolic activity of biofilms, calculated using the control group (biofilms formed in the absence of OCs) as 100% of the metabolic activity of biofilms. The data are mean ± SE (n = 8). * p < 0.05.
The inhibitory effect of OCs can also be observed when observing the morphology of the biofilms produced using RPMI-1640 by light microscopy (Figure 3).
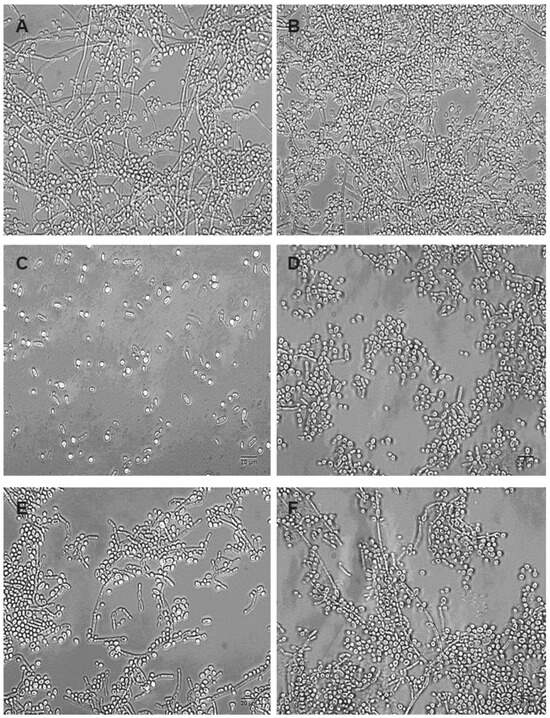
Figure 3.
Morphological characterization of dual-species biofilms of Candida albicans and Candida krusei. Yeast-mixed suspensions were used to form biofilms in the absence (A,B) and in the presence of 30 µM (p-MeOPhSe)2 (C,D) or 30 µM (PhSe)2 (E,F). (A,C,E) represents biofilms formed during 24 h in the presence of OCs. (B,D,F) represents 24 h biofilms treated with OCs. Bar 20 µm.
Dual-species biofilms produced using RPMI-1640 presented a large amount of cells in the filamentous form, and cells were observed in both spherical and cylindrical forms, typical of Candida albicans and Candida krusei, respectively (Figure 3A). Compared to the control group, biofilms produced in the presence of 30 µM (p-MeOPhSe)2 and (PhSe)2 presented an evident decrease in the number of cells in both yeast and filamentous form (compare Figure 3C,E with Figure 3A). However, the total absence of cells presenting filamentous form was observed in dual-species biofilms produced in the presence of 30 µM (p-MeOPhSe)2. Despite having a smaller effect than (p-MeOPhSe)2, the compound (PhSe)2 was able to reduce the number of cells in either yeast or filamentous form in biofilm formation. In addition, a reduction in the length of the filaments was observed in biofilms formed in the presence of 30 µM (PhSe)2 (Figure 3E). However, 24 h dual-species biofilms were less affected by OCs than biofilms in formation. Biofilms produced and posteriorly treated with 30 µM (p-MeOPhSe)2 or (PhSe)2 for an additional 24 h presented a reduction in the number of cells (compare Figure 3D,F with Figure 3B). The number of cells presenting filamentous forms was greatly reduced after treatment with 30 µM (p-MeOPhSe)2 (Figure 3D). These results support the data presented in Figure 1 and Figure 2, showing the reduction in both metabolic activity and cell number in dual-species biofilms.
In order to understand in which of the biofilm formation stages the OCs act, the effect of OCs was determined on cell adhesion (Figure 4). Following the period of cell adhesion, biofilm formation was produced using either RPMI-1640 or SDB. A significant reduction in biofilm formation using RPMI-1640 when cell adhesion was formed in the presence of 30 µM (p-MeOPhSe)2 was observed. The compounds (PhSe)2 e (p-Cl-PhSe)2 were also able to reduce the cell adhesion but to a minor extent. Biofilms formed using RPMI-1640 after a period of adhesion in the presence of 30 µM (p-MeOPhSe)2, (PhSe)2, and (p-Cl-PhSe)2 presented a decrease of 86, 37 and 31% in metabolic activity, respectively. However, after the period of adhesion in the presence of 30 µM (p-MeOPhSe)2 or (PhSe)2, biofilms formed using SDB presented a decrease of 35 and 20% in the metabolic activity, respectively. The presence of (p-Cl-PhSe)2 during the adhesion period did not modify the metabolic activity of biofilms formed using SDB.
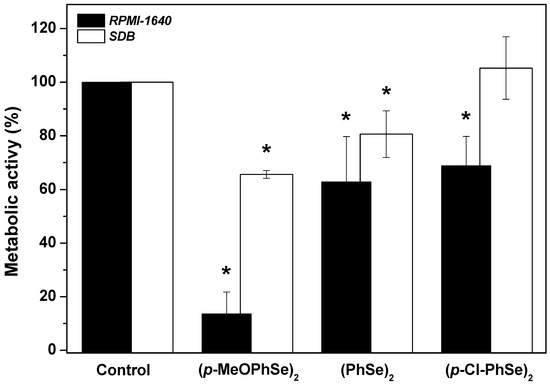
Figure 4.
Effect of (p-MeOPhSe)2, (PhSe)2, and (p-Cl-PhSe)2 on cell adhesion to polystyrene surfaces. Details of the experimental conditions are described in Methods. Values presented in the figure represent the percentage of the metabolic activity of biofilms, calculated using the control group (biofilms formed in the absence of OCs) as 100% of the metabolic activity of biofilms. The data are mean ± SE (n = 8). * p < 0.05.
Following, to determine the contribution of each species in metabolic activity obtained, the number of CFUs of both Candida albicans and Candida krusei was determined after different treatments. A high Candida albicans/Candida krusei ratio in biofilms formed using RPMI-1640 was observed, while a repeated predominance of Candida krusei was observed in biofilms formed using SDB (Figure 5).
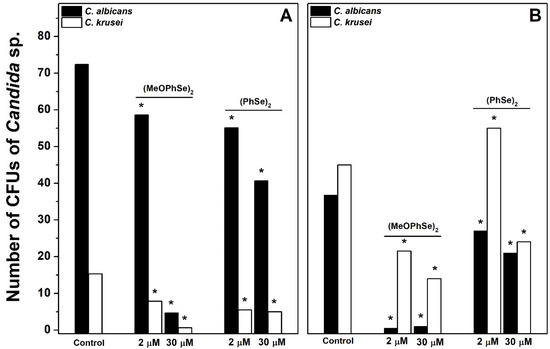
Figure 5.
Effect of (p-MeOPhSe)2 and (PhSe)2 on the number of CFUs of Candida albicans and Candida krusei in biofilm formation. The experimental conditions are described under Materials and Methods. Yeast-mixed suspension was used to produce biofilms for 24 h in the presence of RPMI-1640 (A) or SDB (B). The number of CFUs of both Candida albicans and Candida krusei was determined after different treatments using CHROMagar Candida medium. The data are mean ± SE (n = 8). * p < 0.05.
This result demonstrates that different Candida albicans/Candida krusei ratios are present in biofilms formed using either RPMI-1640 or SDB. In biofilms formed using RPMI-1640, the number of CFUs of Candida albicans was 72, 59, and 5 in the absence and in the presence of 2 and 30 µM (p-MeOPhSe)2, respectively (Figure 5A). Furthermore, the number of CFUs of Candida albicans was 37, 0.5, and 1 in the absence and in the presence of 2 and 30 µM (p-MeOPhSe)2, respectively, in biofilms formed using SDB (Figure 5B). A decrease in the number of CFUs of Candida albicans by 30 µM (PhSe)2 was observed, but this effect was smaller than that obtained with 30 µM (p-MeOPhSe)2, using either RPMI-1640 (43%) or SDB (42%). (p-MeOPhSe)2 was also able to reduce the number of CFUs of Candida krusei; however, the impact of this inhibition in total metabolic activity was lower, probably due to the limited Candida krusei collaboration in the structure of biofilm produced using RPMI-1640. Furthermore, biofilms formed using SDB and treated with 30 µM (p-MeOPhSe)2 presented a reduction of 97 and 69% in the number of CFUs of Candida albicans and Candida krusei, respectively. These results demonstrated that (p-MeOPhSe)2 was able to decrease the metabolic activity of dual-species biofilms by reducing the cell number of both Candida albicans and Candida krusei during biofilm formation using either RPMI-1640 or SDB. Figure 6 shows the number of CFUs of Candida albicans and Candida krusei in 24 h dual-species biofilms produced using either RPMI-1640 or SDB and treated for an additional 24 h with 30 µM (p-MeOPhSe)2 or (PhSe)2. Similar to what is shown in Figure 5, different Candida albicans/Candida krusei ratios were obtained using either RPMI-1640 or SDB. A Candida albicans/Candida krusei ratio of 6.3 and 0.2 was observed in biofilms produced using RPMI-1640 or SDB, respectively. In 24 h dual-species biofilms produced using RPMI-1640 it was observed a reduction in the number of CFUs of Candida albicans of 16 and 27% in the presence of 30 µM (p-MeOPhSe)2 and (PhSe)2, respectively; however, the number of CFUs of Candida krusei was reduced around 64% in the presence of either 30 µM (p-MeOPhSe)2 or (PhSe)2 (Figure 6A).
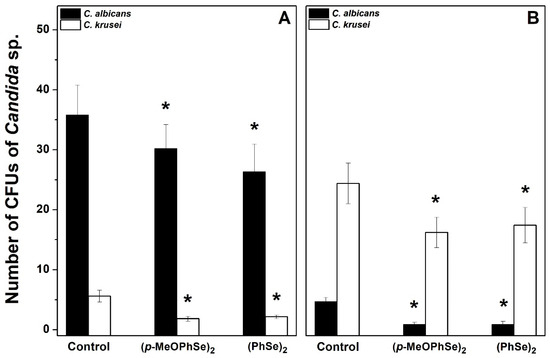
Figure 6.
Effect of (p-MeOPhSe)2 and (PhSe)2 on the number of CFUs of Candida albicans and Candida krusei in 24 h dual-species biofilms cultivated on either RPMI-1640 (A) or SDB (B). Experimental conditions are described in Methods. The number of CFUs of both Candida albicans and Candida krusei was determined after different treatments using CHROMagar Candida medium. The data are mean ± SE (n = 8). * p < 0.05.
Biofilms formed using SDB presented a decrease in the number of CFUs of Candida albicans and Candida krusei of 78 and 31%, respectively, by either 30 µM (p-MeOPhSe)2 or (PhSe)2 (Figure 6B). Taken together, these results demonstrated the potential of the Organoselenium Compounds as promisor new antifungal drugs.
3. Discussions
Despite its lower frequency, invasive and/or systemic candidiasis present higher mortality (35 to 80%), producing around 1.5 million deaths per year in the world [37]. Furthermore, an increase in the incidence of Candidemias has been described by different authors [38,39]. This problem became more evident after the COVID-19 pandemic because of the increase in the cases of COVID-19-associated candidiasis worldwide [37,40]. In this context, and considering the great number of reports showing the increase in the cases of resistance to the available antifungal drugs, the development of new and effective antifungal agents is critical. The potential of different Organoselenium Compounds as promising antifungal drugs has been demonstrated. The ability of different OCs to reduce the development of biofilms mono-species of either Candida albicans or Candia krusei was recently demonstrated [30,31,32,33]. In this paper, we demonstrated, for the first time, the inhibitory effect of (p-MeOPhSe)2, (PhSe)2, and (p-Cl-PhSe)2 on dual-species biofilm of Candida albicans and Candida krusei. Although, the inhibitory effect was clearly dependent on the medium used to form biofilms. The results presented here showed that dual-species biofilms produced using RPMI-1640 present a predominance of Candida albicans on Candida krusei cells and, consequently, a high Candida albicans/Candida krusei ratio, while biofilms formed with SDB showed the greater number of Candida krusei than Candida albicans cells. This alteration in biofilm structure was also observed when analyzing the morphology of cells in biofilms by light microscopy. Thus, the differences observed in the inhibitory effect seem to be related to different Candida albicans/Candida krusei ratios obtained when using different media. It was recently demonstrated that the inhibitory effect of antimicrobial photodynamic therapy (aPDT) on dual-species biofilms of Candida albicans and Candida krusei can be modified by Candida albicans/Candida krusei ratio and that Candida krusei, when present in the structure of dual-species biofilms can be resistant to aPDT [41]. Furthermore, it was demonstrated that the medium and environment could regulate interactions between both yeast species, including the response to Voriconazole [42]. These results indicate that the inhibitory effects of antifungal drugs may be different depending on the medium tested, representing a new challenge in the study of antifungal compounds. A similar effect was demonstrated in this paper since dual-species biofilms formed using RPMI-1640, presenting a high Candida albicans/Candida krusei ratio, seem to be more sensitive to OCs than biofilms produced using SDB, suggesting a higher resistance of Candida krusei when associated with Candida albicans in the biofilm structure. However, the inhibitory effect of (p-MeOPhSe)2 on mono-species biofilms of Candida albicans is similar to that observed on Candida krusei biofilm formation (~80%) [30,32], indicating that Candida krusei when associated with Candida albicans in biofilms present tolerance to (p-MeOPhSe)2. Increased OC concentrations were able to reduce both biofilm formation and viability of 24 h dual-species biofilms, being (p-MeOPhSe)2 more efficient than (PhSe)2 and (p-Cl-PhSe)2, a similar result to that obtained when studying biofilms mono-species of Candida albicans and Candida krusei.
The exact mechanism by which OCs decrease the development of both mono and dual-species biofilms of Candida albicans and Candida krusei is still unknown, although the results obtained in our study suggest that the substituent groups in symmetric diselenides significantly influence their chemical activity. Our experiments demonstrate that deactivating groups, such as chloride, reduce chemical activity, whereas activating groups, such as methoxy, enhance it. Multiple observations indicate that the substituent groups on the aromatic ring play a crucial role in modulating the chemical reactivity of the organoselenium compound [43]. This modulation is mainly attributed to variations in electronegativity relative to carbon, along with differences in atomic volume and spatial arrangements [44].
Furthermore, dual-species biofilms previously formed were more resistant to OCs than biofilms in formation, similar to that observed in mono-species of either Candida albicans or Candida krusei [30,32]. Žiemytė and collaborators (2023) demonstrated that micafungin and caspofungin failed to eradicate 24 h mono-species biofilms of Candida albicans and Candida glabrata, corroborating that Candida spp. biofilms previously formed are extremely difficult to eliminate [45]. In addition, our results demonstrated that biofilms presenting low Candida albicans/Candida krusei ratio were refractory to OC treatment. Comparing Figure 1 and Figure 4, it is possible to observe a similarity, suggesting that the inhibitory effect of OCs could be related to a decrease in cell adherence. A significant decrease in Candida spp. adhesion to both polystyrene plates and epithelial cells by (PhSe)2 and (p-Cl-PhSe)2 was recently demonstrated [31,33]. Considering that adherence of yeast cells to the epithelial cell surface is the first step for colonization, tissue invasion, and spread of systemic infection [4], drugs able to reduce/eliminate yeast–surface interaction can represent a very important step in the fight against systemic candidiasis. Furthermore, the determination of the number of CFUs of Candida albicans and Candida krusei in biofilms dual-species demonstrated that the composition of biofilms is a determinant factor in antifungal therapy. In biofilms formed with RPMI-1640, (p-MeOPhSe)2 decreased the metabolic activity of biofilm formation (~90%) by reducing both Candida albicans and Candida krusei development, however considering that Candida albicans was present in a higher number, the participation of Candida krusei in metabolic activity is probably lower. In addition, during biofilm formation with SDB, the participation of Candida krusei is higher, and consequently, the inhibition of metabolic activity by (p-MeOPhSe)2 is lower. Analyzing the number of CFUs of Candida albicans and Candida krusei in 24 h dual-species biofilms, a similar profile was observed, although inhibition by OCs was significantly reduced. When we reflect on the fight against diseases caused by fungi in the world, some points are most relevant: (1) the increase in the frequency of fungal infections; (2) the increase in the number of infections refractory to medicines available; (3) the toxic effects associated with antifungal drugs. Although amphotericin B (AmB) is a life-saving medicine in the treatment of serious systemic fungal infections and is the most used antifungal medicine in intensive care, the acute and chronic toxicity induced by this medicine represents a limitation in its use [46]. Reactions such as fever, rigors, headache, arthralgia, nausea, vomiting, and hypotension have been related to the AmB administration. Furthermore, the nephrotoxic effect of AmB has been extensively demonstrated [47]. These factors highlighted above make clear the urgency of new treatments against pathogenic fungi. The results present here demonstrate the potential of OCs, mainly (p-MeOPhSe)2, as promising antifungal drugs by reducing biofilm formation, viability of biofilm produced, and adhesion to a surface.
4. Materials and Methods
4.1. Yeast Strain and Growth Conditions
Candida albicans (Manassas, VA, USA; ATCC #1023) and Candida krusei (Manassas, VA, USA; ATCC #6258) were purchased in American Type Culture Collection and plated on Sabouraud dextrose agar (Merck, Darmstadt, Hesse, Germany). Cultures were incubated in ambient air for 48 h at 37 °C. After this period, a sample of the cultures was removed from the surface of the agar and suspended in a physiological solution at a cell density of 107 cell.mL−1, determined using the Neubauer chamber.
4.2. Organoselenium Compounds (OCs)
Diphenyl diselenide-(PhSe)2 was purchased from Sigma-Aldrich (St. Louis, MO, USA). The compounds p-chloro diphenyl diselenide-(p-Cl-PhSe)2 and p,p′-methoxyl-diphenyl diselenide-(p-MeOPhSe)2 were obtained according to Paulmier (1986) [48] and were kindly provided by Prof. João Batista Teixeira da Rocha (Universidade Federal de Santa Maria, Brazil). Analysis of the 1H NMR and 13C NMR spectra showed that compounds presented analytical and spectroscopic data in full agreement with their assigned structure. The chemical purity of all compounds was higher than 99.9%, as determined by GC/HPLC. The chemical structures of the OCs are shown in Figure 7. OC stock solutions were prepared in 100% Dimethylsulphoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) and diluted in ultrapure water to reach the test concentration of 1%. The control group was included in all experiments and represented the treatment condition with DMSO 1%, which did not modify the studied parameter in Candida albicans and Candida krusei.
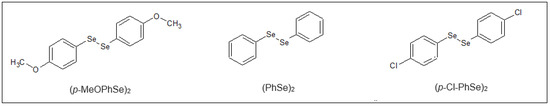
Figure 7.
Chemical structures of the Organoselenium Compounds. p-chloro-diphenyl diselenide-(p-Cl-PhSe)2; p-methoxy-diphenyl diselenide-(p-MeOPhSe)2; diphenyl diselenide-(PhSe)2.
4.3. Effect of OCs on Biofilm Formation
A mixture of suspensions of Candida albicans (107 cell·mL−1) and Candida krusei (107 cell·mL−1) was performed in equal proportion (1:1) and used to produce biofilms. This yeast-mixed suspension (25 µL) was seeded in a 96-well polystyrene plate containing either RPMI-1640 medium (Sigma, St. Louis, MO, USA) or Sabouraud Dextrose Broth (SDB) medium (Merck, Darmstadt, Hesse, Germany) in the presence of different concentrations of OCs (varying from 10 to 30 μΜ) in a final volume of 200 μL, for 24 h at 37 °C. After this period, the medium was removed, and each well was washed twice with PBS. Biofilms produced using RPMI-1640 medium were washed with 200 µL, although biofilms produced using SDB medium were washed with 100 µL. Biofilm formation was monitored by XTT assay based on the reduction of XTT (2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide sodium salt; Molecular Probes, Eugene, OR, USA) assay, according to the literature (da Silva et al., 2021). The reduced formazan-colored product was determined at 490 nm (OD490) in a Synergy HT Multi-Detection Microplate Reader (Bio-Tek, Winooski, VT, USA). Data obtained were expressed as a percentage (%) of the metabolic activity of biofilms produced relative to the control group (100%).
4.4. Effect of OCs on Dual-Species Biofilm Produced during 24 h
Yeast-mixed suspension (25 µL) was seeded in a 96-well plate containing either RPMI-1640 or SDB in a final volume of 200 μL in the absence of OCs. Plates were incubated for 24 h at 37 °C to produce biofilms. After biofilm formation, the media were removed, the wells were washed three times with PBS solution, and a fresh medium containing 30 µM OCs was added to the wells (final volume = 200 μL). Biofilms were produced for an additional 24 h at 37 °C. Next, the wells were washed twice with PBS, and the metabolic activity of the biofilm produced was monitored by XTT assay.
4.5. Effect of OCs on Cell Adhesion to Polystyrene Surfaces
Aliquots of yeast-mixed suspension (25 µL) were added in 96-well plates with saline solution and 30 µM OCs for 90 min at 37 °C. After this period of cell adhesion, the medium was removed, and the wells were washed with PBS (100 µL). Following, either RPMI-1640 or SDB was added to the wells in a final volume of 200 μL, and the plates were incubated for 24 h at 37 °C. Next, the biofilms produced were washed twice with PBS, and the metabolic activity of the biofilm produced was monitored by XTT assay.
4.6. Effect of OCs on Number of Colony Forming Units (CFUs) of Either Candida albicans or Candida krusei
In parallel with carrying out the experiments to determine metabolic activity using XTT assay, the number of CFUs of Candida albicans and Candida krusei present in biofilms structure was determined using CHROMagar Candida® (BD, Sparks, NV, USA). The cells of the biofilms produced after the different treatments using OCs were washed twice with PBS, harvested using a scraper, and diluted 800 times. An aliquot of this biofilm suspension (25 µL) was seeded on CHROMagar Candida medium to determine the number of CFUs of both Candida albicans and Candida krusei after 48 h at 37 °C. The number of colonies was determined visually.
4.7. Morphological Analyses of Dual-Species Biofilms
The morphology of dual-species biofilms was observed by light microscopy before cells in biofilms were harvested. Biofilms were examined using a microscope by light microscopy (Axioskop 2, Zeiss, Jena, Germany), and the images were captured with a Pixera digital camera system (Pixera Corporation, Santa Clara, CA, USA) attached to the photomicroscope and a microcomputer (IntelVR PentiumVR, Santa Clara, CA, USA) using the software Adobe Photoshop version 7.0.1 (Adobe Systems, Atlanta, GA, USA).
4.8. Statistical Analysis
Values were expressed as means ± standard deviation (SD) of different and independent experiments (n = 8), performed in triplicate. Statistical analyses used was the one-way analysis of variance (ANOVA) followed by the Tukey–Kramer post hoc test for multiple comparisons. p values < 0.05 were considered significant. Graphics were presented, and statistical analysis was performed using OriginPro 8.5 (OriginLab Corporation, Northampton, MA, USA).
5. Conclusions
The results presented here demonstrated, for the first time, the inhibitory effect of Organoselenium Compounds on the development of dual-species biofilms of Candida albicans and Candida krusei, suggesting the potential of these compounds as promising antifungal medicine.
Author Contributions
Conceptualization, M.S.C.; Methodology, G.d.S.C., G.N.J.C., Q.L.C. and A.L.M.d.S.; Software, G.N.J.C. and D.N.d.M.; Formal analysis, Q.L.C.; Investigation, G.d.S.C., G.N.J.C. and A.L.M.d.S.; Writing—original draft, G.d.S.C. and M.S.C.; Writing—review & editing, D.N.d.M., J.G.d.C.P. and M.S.C.; Project administration, M.S.C.; Funding acquisition, J.G.d.C.P. and M.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001. Compliance with Ethical Standards.
Institutional Review Board Statement
In this study, all experiments were performed using Cultures of Candida albicans (ATCC 10231) and Candida krusei strain ATCC 6258; therefore, it is not necessary to have approval from local authorities.
Informed Consent Statement
We have obtained permission from all the authors. We declare that the material has not been published in whole or in part elsewhere, and the paper is not currently being considered for publication elsewhere.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors would like to thank FAPESP for its financial support.
Conflicts of Interest
The authors have no financial, personal, or other conflicts of interest related to this work.
References
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Araújo, D.; Henriques, M.; Silva, S. Portrait of Candida Species Biofilm Regulatory Network Genes. Trends Microbiol. 2017, 25, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; dos Santos Fontenelle, R.O.; de Brito, E.H.S.; de Morais, S.M. Biofilm of Candida albicans: Formation, regulation and resistance. J. Appl. Microbiol. 2021, 131, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, X.; Ren, B.; Cheng, L. The regulation of hyphae growth in Candida albicans. Virulence 2020, 11, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Guinea, J. Global trends in the distribution of Candida species causing candidemia. Clin. Microbiol. Infect. 2014, 20, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Ebbers, J.; Geurtz, L.; Stefanik, D.; Major, Y.; Edmond, M.B.; Wenzel, R.P.; Seifert, H. Nosocomial bloodstream infections due to Candida sin the USA: Species distribution, clinical features and antifungal susceptibilities. Int. J. Antimicrob. Agents 2014, 43, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole antifungal resistance in Candida albicans and emerging non-albicans Candida Species. Front. Microbiol. 2017, 7, 2173. [Google Scholar] [CrossRef] [PubMed]
- Jamiu, A.T.; Albertyn, J.; Sebolai, O.M.; Pohl, C.H. Update on Candida krusei, a potential multidrug-resistant pathogen. Med. Mycol. 2021, 59, 14–30. [Google Scholar] [CrossRef]
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef]
- Kaur, J.; Nobile, C.J. Antifungal drug-resistance mechanisms in Candida biofilms. Curr. Opin. Microbiol. 2023, 71, 102237. [Google Scholar] [CrossRef] [PubMed]
- Malinovská, Z.; Čonková, E.; Váczi, P. Biofilm Formation in Medically Important Candida Species. J. Fungi 2023, 9, 955. [Google Scholar] [CrossRef] [PubMed]
- Ponde, N.O.; Lortal, L.; Ramage, G.; Naglik, J.R.; Richardson, J.P. Candida albicans biofilms and polymicrobial interactions. Crit. Rev. Microbiol. 2021, 47, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Mitchell, A.P. Mucosal biofilms of Candida albicans. Curr. Opin. Microbiol. 2011, 14, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Tsui, C.; Kong, E.F.; Jabra-Rizk, M.A. Pathogenesis of Candida albicans biofilm. Pathog. Dis. 2016, 74, ftw018. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.P.; Lionakis, M.S. Pathogenesis and virulence of Candida albicans. Virulence 2022, 13, 89–121. [Google Scholar] [CrossRef]
- Suleyman, G.; Alangaden, G.J. Nosocomial Fungal Infections: Epidemiology, Infection Control, and Prevention. Infect. Dis. Clin. N. Am. 2021, 35, 1027–1053. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and multi-national prevalence of fungal diseases—Estimate precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Timsit, J.F.; Ruppé, E.; Barbier, F.; Tabah, A.; Bassetti, M. Bloodstream infections in critically ill patients: An expert statement. Intensive Care Med. 2020, 46, 266–284. [Google Scholar] [CrossRef]
- Fisher, M.C.; Denning, D.W. The WHO fungal priority pathogens list as a game-changer. Nat. Rev. Microbiol. 2023, 21, 211–212. [Google Scholar] [CrossRef]
- Garvey, M.; Rowan, N.J. Pathogenic Drug Resistant Fungi: A Review of Mitigation Strategies. Int. J. Mol. Sci. 2023, 24, 1584. [Google Scholar] [CrossRef]
- Braga, D.; Costa, A.L.; Grepioni, F.; Scaccianoce, L.; Tagliavini, E. Organic-Organometallic Crystal Synthesis. 1. HostingParamagnetic [(η6-Arene)2 Cr] + (Arene) Benzene, Toluene) in Organic Anion Frameworks via OsH‚‚‚O andCsH‚‚‚O Hydrogen Bonds. Organometallics 1997, 16, 2070–2079. [Google Scholar] [CrossRef]
- Machado, M.S.; Villela, I.V.; Moura, D.J.; Rosa, R.M.; Salvador, M.; Lopes, N.P.; Braga, A.L.; Roesler, R.; Saffi, J.; Henriques, J.A.P. 3′3-ditrifluoromethyldiphenyl diselenide: A new organoselenium compound with interesting antigenotoxic and antimutagenic activities. Mutat. Res. 2009, 17, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Giurg, M.; Golab, A.; Suchodolski, J.; Kaleta, R.; Krasowska, A.; Piasecki, E.; Piętka-Ottlik, M. Reaction of bis[(2-chlorocarbonyl)phenyl] Diselenide with Phenols, Aminophenols, and Other Amines towards Diphenyl Diselenides with Antimicrobial and Antiviral Properties. Molecules 2017, 12, 974. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and Organotellurium Compounds: Toxicology and Pharmacology. Chem. Rev. 2004, 104, 6255–6285. [Google Scholar] [CrossRef]
- Rosseti, I.B.; Wagner, C.; Fachinetto, R.; Taube Junior, P.; Costa, M.S. Candida albicans growth and germ tube formation can be inhibited by simple diphenyl diselenides [(PhSe) 2, (MeOPhSe) 2, (p-Cl-PhSe) 2, (F 3CPhSe) 2] and diphenyl ditelluride. Mycoses 2011, 54, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Di Leo, I.; Messina, F.; Nascimento, V.; Nacca, F.G.; Pietrella, D.; Perin, G.; Sancineto, L. Synthetic Approaches to Organoselenium Derivatives with Antimicrobial and Anti-Biofilm Activity. Mini-Rev. Org.-Chem. 2019, 16, 589–601. [Google Scholar] [CrossRef]
- Wall, G.; Lopez-Ribot, J.L. Screening Repurposing Libraries for Identification of Drugs with Novel Antifungal Activity. Antimicrob. Agents Chemother. 2020, 64, 10–1128. [Google Scholar] [CrossRef]
- Rosseti, I.B.; Rocha, J.B.T.; Costa, M.S. Diphenyl diselenide (PhSe)2 inhibits biofilm formation by Candida albicans, increasing both ROS production and membrane permeability. J. Trace Elem. Med. Biol. 2015, 29, 289–295. [Google Scholar] [CrossRef]
- de Amorim, L.M.M.; Braga, M.T.; Carvalho, M.L.; de Oliveira, I.R.; Querobino, S.M.; Alberto-Silva, C.; da Rocha, J.B.T.; Costa, M.S. The Organochalcogen Compound (MeOPhSe)2 Inhibits Both Formation and the Viability of the Biofilm Produced by Candida albicans, at Different Stages of Development. Curr. Pharm. Des. 2018, 24, 3964–3971. [Google Scholar] [CrossRef]
- da Silva, B.M.; Braga, M.T.; da Silva Passos, J.C.; Carvalho, M.L.; Rosseti, I.B.; de Amorim, L.M.M.; da Rocha, J.B.T.; Alberto-Silva, C.; Costa, M.S. (PhSe)2 and (pCl-PhSe)2 organochalcogen compounds inhibit Candida albicans adhesion to human endocervical (HeLa) cells and show anti-biofilm activities. Biofouling 2021, 37, 235–245. [Google Scholar] [CrossRef] [PubMed]
- de Siqueira, V.M.; da Silva, B.G.M.; Passos, J.C.d.S.; Pinto, A.P.; da Rocha, J.B.T.; Alberto-Silva, C.; Costa, M.S. (MeOPhSe)2, a synthetic organic selenium compound, inhibits virulence factors of Candida krusei: Adherence to cervical epithelial cells and biofilm formation. J. Trace Elem. Med. Biol. 2022, 73, 127019. [Google Scholar] [CrossRef]
- da Silva, B.G.M.; Pinto, A.P.; Passos, J.C.d.S.; da Rocha, J.B.T.; Alberto-Silva, C.; Costa, M.S. Diphenyl diselenide suppresses key virulence factors of Candida krusei, a neglected fungal pathogen. Biofouling 2022, 38, 427–440. [Google Scholar] [CrossRef]
- Aggarwal, N.; Kitano, S.; Puah, G.R.Y.; Kittelmann, S.; Hwang, I.Y.; Chang, M.W. Microbiome and Human Health: Current Understanding, Engineering, and Enabling Technologies. Chem. Rev. 2023, 123, 31–72. [Google Scholar] [CrossRef]
- Orazi, G.; O’Toole, G.A. “It takes a village”: Mechanisms underlying antimicrobial recalcitrance of polymicrobial biofilms. J. Bacteriol. 2020, 202, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Tits, J.; Cammue, B.P.A.; Thevissen, K. Combination therapy to treat fungal biofilm-based infections. Int. J. Mol. Sci. 2020, 21, 8873. [Google Scholar] [CrossRef] [PubMed]
- Lass-Flörl, C.; Steixner, S. The changing epidemiology of fungal infections. Mol. Asp. Med. 2023, 94, 101215. [Google Scholar] [CrossRef]
- Zilberberg, M.D.; Shorr, A.F.; Kollef, M.H. Secular Trends in Candidemia-Related Hospitalization in the United States, 2000–2005. Infect. Control. Hosp. Epidemiol. 2008, 29, 978–980. [Google Scholar] [CrossRef]
- Koehler, P.; Stecher, M.; Cornely, O.A.; Koehler, D.; Vehreschild, M.J.G.T.; Bohlius, J.; Wisplinghoff, H.; Vehreschild, J.J. Morbidity and mortality of candidaemia in Europe: An epidemiologic meta-analysis. Clin. Microbiol. Infect. 2019, 25, 1200–1212. [Google Scholar] [CrossRef]
- Salehi, M.; Ahmadikia, K.; Mahmoudi, S.; Kalantari, S.; Jamalimoghadamsiahkali, S.; Izadi, A.; Kord, M.; Dehghan Manshadi, S.A.; Seifi, A.; Ghiasvand, F.; et al. Oropharyngeal candidiasis in hospitalised COVID-19 patients from Iran: Species identification and antifungal susceptibility pattern. Mycoses 2020, 63, 771–778. [Google Scholar] [CrossRef]
- Passos, J.C.D.S.; Calvi, G.S.; Rodrigues, A.B.F.; Costa, M.S. The inhibitory effect of photodynamic therapy on dual-species biofilms of Candida albicans and Candida krusei can be determined by Candida albicans/Candida krusei ratio. Photodiagnosis Photodyn Ther. 2023, 44, 103787. [Google Scholar] [CrossRef]
- Passos, J.C.D.S.; Rodrigues, A.B.F.; Alberto-Silva, C.; Costa, M.S. The arrangement of dual-species biofilms of Candida albicans and Issatchenkia orientalis can be modified by the medium: Effect of Voriconazole. Biofouling 2024, 8, 1–11. [Google Scholar] [CrossRef]
- Rosa, R.M.; Guecheva, T.N.; Oliveira, I.M.; Braga, A.L.; Henriques, J.A.P. Genetic toxicity of three symmetrical diselenides in yeast. J. Braz. Chem. Soc. 2010, 21, 2119–2124. [Google Scholar] [CrossRef][Green Version]
- Giles, N.M.; Watts, A.B.; Giles, G.I.; Fry, F.H.; Littlechild, J.A.; Jacob, C. Metal and redox modulation of cysteine protein function. Chem. Biol. 2003, 10, 677–693. [Google Scholar] [CrossRef]
- Žiemytė, M.; Rodríguez-Díaz, J.C.; Ventero-Martín, M.P.; Mira, A.; Ferrer, M.D. Real-time monitoring of biofilm growth identifies andrographolide as a potent antifungal compound eradicating Candida biofilm. Biofilm 2023, 5, 100134. [Google Scholar] [CrossRef] [PubMed]
- Cavassin, F.B.; Baú-Carneiro, J.L.; Vilas-Boas, R.R.; Queiroz-Telles, F. Sixty years of Amphotericin B: An Overview of the Main Antifungal Agent Used to Treat Invasive Fungal Infections. Infect. Dis. Ther. 2021, 10, 115–147. [Google Scholar] [CrossRef] [PubMed]
- Adler-Moore, J.; Lewis, R.E.; Brüggemann, R.J.M.; Rijnders, B.J.A.; Groll, A.H.; Walsh, T.J. Preclinical Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Antifungal Activity of Liposomal Amphotericin B. Clin. Infect. Dis. 2019, 68, S244–S259. [Google Scholar] [CrossRef] [PubMed]
- Paulmier, C. Selenium Reagents and Intermediates in Organic Synthesis; Elsevier: Amsterdam, The Netherlands, 1986. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).