Abstract
Metabolic-associated steatotic liver disease (MASLD), the hepatic manifestation of metabolic syndrome, represents a growing global health concern. The intricate pathogenesis of MASLD, driven by genetic, metabolic, epigenetic, and environmental factors, leads to considerable clinical variability. Dysregulation of hepatic lipid metabolism, particularly cholesterol homeostasis, is a critical factor in the progression of MASLD and its more severe form, metabolic dysfunction-associated steatohepatitis (MASH). This review elucidates the multifaceted roles of cholesterol metabolism in MASLD, focusing on its absorption, transportation, biosynthesis, efflux, and conversion. We highlight recent advancements in understanding these processes and explore the therapeutic potential of natural products such as curcumin, berberine, and resveratrol in modulating cholesterol metabolism. By targeting key molecular pathways, these natural products offer promising strategies for MASLD management. Finally, this review also covers the clinical studies of natural products in MASLD, providing new insights for future research and clinical applications.
1. Introduction
Metabolic-associated steatotic liver disease (MASLD), formerly known as non-alcoholic fatty liver disease (NAFLD), has progressively been recognized as a predominant form of chronic liver disease, anticipated to ascend as the primary etiological contributor to end-stage liver diseases in subsequent decades. MASLD encompasses a range from simple hepatic steatosis to metabolic dysfunction-associated steatohepatitis (MASH), which can develop into advanced liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC). Corresponding with global amplification of obesity and type 2 diabetes mellitus (T2DM), epidemiological data suggests that MASLD affects an approximate 32.4% of the global populace [1]. Despite extensive research, the multifactorial etiopathogenesis of MASLD, involving insulin resistance, oxidative stress, pro-inflammatory cytokines, and lipotoxicity, remains incompletely understood [2]. A hallmark of MASLD is the dysregulated accumulation of neutral lipid species in the liver. Recent studies highlight the critical role of disturbances in cholesterol homeostasis in the pathogenesis and progression of MASLD.
Cholesterol plays a dual role in physiological processes, where both its deficiency and excess can lead to significant cellular and systemic issues [3]. Elevated hepatic free cholesterol (FC) has been observed in MASH patients compared to healthy individuals [4], with studies showing progressive increases in hepatic FC from non-MASLD to MASH stages [5]. The intrinsic role of cholesterol in hepatic steatosis and inflammation is underscored by experimental evidence linking high-fat-high-cholesterol (HFHC) diets to MASH development compared to high-fat-non-cholesterol regimens [6]. Furthermore, cholesterol-enrich ox-LDL uptake by hepatic Kupffer cells kindles inflammatory response in MASH [7], underscored by the lysosomal cholesterol crystal formation in macrophages, which serves as a danger signal activating inflammatory response [8]. Understanding the multifaceted roles of cholesterol in MASLD pathophysiology is crucial, as it could reveal novel molecular targets for therapeutic intervention.
Natural products, derived from diverse biological sources, encompassing fauna, flora, and microorganisms, have garnered attention for their purported protective attributes against MASLD. Several such compounds are posited to modulate hepatic cholesterol homeostasis, thereby substantiating their therapeutic potential in MASLD. Nonetheless, the mechanistic pathways through which these natural entities influence cholesterol processes remain to be exhaustively explored. This review aims to delineate the intricate processes of cholesterol absorption, transport, and metabolism under homeostatic conditions, provide a comprehensive overview of recent advances in cholesterol metabolic dysregulation in MASLD, and synthesize insights into the prospective therapeutic implications of natural compounds targeting cholesterol metabolic pathways for improving MASLD management.
2. Cholesterol Homeostasis in Health
2.1. Intestinal Cholesterol Absorption and Blood Release
Numerous dietary sources are rich in cholesterol, such as eggs, butter, seafood, and various meats. The American Cuisine Guidelines in 2015–2020 recommend a daily cholesterol intake not exceeding 300 mg [9,10]. The intestinal absorption of dietary cholesterol predominantly depends on the NPC1-like intracellular cholesterol transporter 1 (NPC1L1). This transporter, noted for its selective N-terminal domain, facilitates the endocytosis of FC into enterocytes, subsequently transporting it to the endoplasmic reticulum (ER) [11]. In the ER, FC undergoes esterification by acyl-CoA acyltransferase (ACAT), participating in the assembly of chylomicron (CM). These CMs are then secreted into the bloodstream via the lymphatic system [12]. In the peripheral blood, triglycerides in CMs are hydrolyzed by the lipoprotein lipase (LPL) on the vascular endothelium. CM remnants bind to the low-density lipoprotein receptor (LDLR) or the LDLR-related protein 1 (LRP) on hepatocyte membranes, facilitating their absorption by hepatocytes [13].
2.2. Reverse Cholesterol Transport (RCT)
RCT delineates the pathway wherein cholesterol from peripheral tissues is transported to the liver by high-density lipoprotein (HDL) and eventually excreted in feces. This mechanism is notably relevant to diseases such as MASLD, atherosclerosis (AS), and coronary heart disease, underscoring the protective function of HDL against elevated plasma lipids and AS. In peripheral serum, immature HDLs from the liver, consisting of phospholipids and apolipoprotein A-I (APOA-I), uptake FC released from adipocytes and muscles via the liver X receptor α (LXRα)-ATP-binding cassette transporter A1 (ABCA1)/ABCG1 pathways, maturing in the process. The maturation of HDL is mediated by lecithin cholesterol acyltransferase (LCAT), which converts cholesterol into cholesteryl esters (CEs) stored within HDL. Additionally, serum HDL levels are regulated by cholesteryl ester transfer protein (CETP), which transfers CEs from HDL to very-low-density lipoprotein (VLDL) and LDL [14]. Therefore, CETP and LCAT play pivotal roles in the RCT process. In the liver, HDL-c is selectively taken up, trafficked transhepatically, and excreted into bile via high-affinity HDL receptors such as scavenger receptor class B type I (SR-BI), thereby enhancing HDL metabolism, lowering plasma HDL-c levels, and promoting macrophage RCT.
2.3. Endogenous Cholesterol De Novo Synthesis
Apart from exogenous sources, cholesterol is synthesized endogenously within mammalian cells. This intricate biosynthetic process involves approximately 30 enzymatic reactions converting acetyl-CoA into cholesterol. Initially, two molecules of acetyl-CoA are condensed and catalyzed to generate mevalonate (MVA) by HMG-CoA synthase (HMGCS) and HMG-CoA reductase (HMGCR). MVA then undergoes a series of phosphorylation, decarboxylation, dehydroxylation, and condensation reactions to generate squalene, which is then catalyzed by ER cyclase and oxygenase to produce lanosterol, eventually yielding 27-carbon cholesterol [11,15]. Cholesterol de novo synthesis is mainly regulated by the sterol regulatory element-binding protein 2 (SREBP2). In low cholesterol conditions, the insulin-induced gene 1/2 (Insig1/2) dissociates from the SREBP cleavage-activating protein (SCAP) in the ER, prompting the SCAP–SREBP2 complex to transfer from the ER to the Golgi apparatus. Proteases S1P and S2P activate SREBP2, which enters the nucleus and activates target genes such as LDLR and HMGCR [16,17]. When cholesterol levels are excessive, cholesterol-derived oxysterols bind to Insig1/2, enhancing the affinity between Insig1/2 and SCAP and preventing SERBP2 transport towards the ER [18].
2.4. Cholesterol Conversion to Bile Acids (BAs)
Cholesterol metabolism primarily occurs in hepatocytes. Remarkably, around 50% of intracellular cholesterol is metabolized into BAs, significantly contributing to the enterohepatic circulation, a key mechanism maintaining intracellular cholesterol homeostasis [19]. Evidence revealed that BAs are synthesized in hepatocytes via cytochrome P450 (CYP)-mediated oxidation of cholesterol, including both ‘classical’ and ‘alternative’ pathways. The ‘classical’ pathway, initiated by CYP7A1, progresses through various enzymatic stages involving CYP8B1 and CYP27A1, resulting in the formation of cholic acid (CA) and chenodeoxycholic acid (CDCA). The ‘alternative’ pathway generates CDCA by the synergistic enzymatic activities of CYP27A1 and CYP7B1 [20]. After their genesis, primary BAs are conjugated and transported from hepatocytes to the upper small intestine via the sphincter of Oddi, where they are extensively metabolized by bacterial enzymes. In the duodenum, BAs solubilize and enhance absorption of dietary lipids and lipid-soluble nutrients [21] (Figure 1).
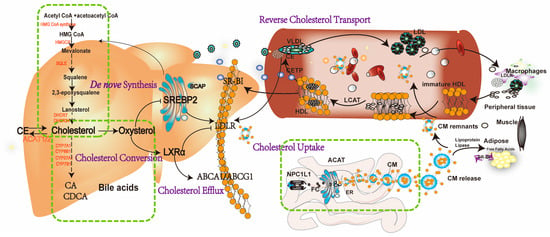
Figure 1.
A schematic representation of cholesterol homeostasis in a host. The intestinal absorption of dietary cholesterol is phagocytosed into enterocytes, which depends on the NPL1L1. Subsequently, free cholesterol (FC) participates in assembling chylomicron (CM) by undergoing esterification via acyl-CoA acyltransferase (ACAT) and is secreted into the blood circulation. After hydrolyzed by the LPL in the circulating to diminish triglycerides, CM remnants are digested by low-density lipoprotein receptor (LDLR) or LDLR-related protein 1 (LRP) on the hepatocyte membrane, leading to their absorption by hepatocytes. In the peripheral serum, immature HDLs from the liver receive FCs from peripheral tissues and become mature by lecithin cholesterol acyltransferase (LCAT), which is responsible for converting cholesterol into cholesteryl esters (CEs) stored within HDL. Cholesteryl Ester Transfer Protein (CETP) transfers CEs from high-density lipoprotein (HDL) to very-low-density lipoprotein (VLDL) and LDL. In the liver, HDL-c are selectively uptake by scavenger receptor class B type I (SR-BI), thus resulting in improving HDL metabolism, lowering plasma HDL-c levels, and promoting macrophage reverse cholesterol transport (RCT). In the liver, cholesterol de novo biosynthesis involves approximately 30 enzymatic reactions, which convert acetyl CoA into cholesterol, such as HMG-CoA reductase (HMGCR), squalene epoxidase (SM), 24-dehydrocholesterol reductase (DHCR24) et al. Cholesterol homeostasis is mainly regulated by transcription factors sterol regulatory element-binding protein 2 (SREBP2) and liver X receptor α (LXRα). In low cholesterol conditions, the SREBP2 pathway activates and then enters the nucleus, activating the target gene such as LDLR and HMGCR. Once excessive cholesterol occurs, oxysterols can activate LXRα, promoting LXR-associated target genes, such as ABCA1 and ABCG1. Additionally, excess FCs are converted to CE stored in lipid droplets by ACAT1/2 or bile acids excreted into the bile duct by cholesterol 7 alpha-hydroxylase (CYP7A1), et al.
3. The Role of Key Targets in Cholesterol Metabolism in MASLD
3.1. Cholesterol Absorption in MASLD
3.1.1. NPC1L1
NPC1L1, a specific cholesterol uptake transporter, is predominantly located in the liver and small intestine. Studies indicate that NPC1L1 facilitates cholesterol absorption via vesicular endocytosis, dependent on microfilaments and the clathrin/AP2 complex [22]. Evidence suggests that dietary cholesterol significantly increases serum total cholesterol (TC) and LDL-c levels [23]. Inhibiting NPC1L1 with Ezetimibe reduces plasma LDL-c and apolipoprotein B (apoB)-containing lipoproteins by inhibiting the intestinal absorption of dietary cholesterol [24]. NPC1L1 knockout (NPC1L1−/−) mice manifested a substantial reduction in intestinal cholesterol uptake and a dramatical decrease in plasma phytosterol levels [25]. Conversely, overexpression of NPC1L1 in the liver results in a significant decrease in biliary cholesterol concentration and an increase in plasma cholesterol [26].
Numerous studies have reported a decline in cholesterol absorption and NPC1L1 expression in MASLD patients [27,28,29]. Recent studies identify hepatic NPC1L1 as a factor exacerbating MASLD. Elevated hepatic NPC1L1 exacerbates high-fat diet (HFD)-induced steatosis and is associated with a diminished hepatic capacity for VLDL-TG secretion [30]. Additionally, hepatic NPC1L1 inhibits hepatocyte autophagy, as indicated by decreased levels of microtubule-associated proteins 1A/1B light chain 3 (LC3) particles and the LC3II/LC3I ratio. Ezetimibe treatment can restore LC3II protein levels and improve liver steatosis [31]. Dietary oxysterols, such as 22(R)-hydroxycholesterol (22R-OHC) and 25-hydroxycholesterol (25-OHC), are believed to be associated with MASLD progression. NPC1L1 uptakes these oxysterols, which correlate positively with hepatic lipid accumulation in humans. Mechanically, these oxysterols contribute to steatosis progression through modulating LXRα and retinoid-related orphan receptor γ (RORγ) [32]. Oxidative stress is critical for MASLD pathogenesis, and the nuclear factor erythroid 2-related factor 2-Kelch-like ECH-associated protein 1 (Nrf2-Keap1) pathway is essential for providing cytoprotection against oxidative stress. Inhibiting NPC1L1 by ezetimibe activates the Nrf2-Keap1 pathway in a p62-dependent manner, decreasing active oxygen species (ROS) levels and hepatic susceptibility to oxidative damage. This underscores the potential antioxidant benefits of NPC1L1 inhibition in combating the pathogenesis of MASH [33]. Additionally, in a guinea pig model, ezetimibe effectively reduced diet-induced circulating cholesterol levels, hepatic lipid accumulation, and inflammatory response [34].
3.1.2. LDLR
LDLR is a transmembrane protein primarily expressed in liver cells. It consists of a large extracellular domain with numerous ligand-binding domains and a cytoplasmic tail containing at least one NPxY motif. LDLR plays a crucial role in cholesterol homeostasis by clearing plasma cholesterol via binding circulating Apolipoprotein E (ApoE) and ApoB-containing particles such as chylomicrons, VLDL, and LDL. Approximately 70% of circulating LDL-c is eliminated via hepatic LDLR-mediated endocytosis. LDLR can be subjected to post-translational degradation by proprotein convertase subtilisin/kexin type 9 (PCSK9), a liver-derived solute factor that binds to the extracellular domain of LDLR, reducing LDL internalization and leading to hyperlipidemia. PCSK9 inhibitors like evolocumab and alirocumab are FDA-approved drugs for treating adult heterozygous familial hypercholesterolemia (FH) and clinical atherosclerotic cardiovascular diseases via inhibiting the binding of circulating PCSK9 and LDLR, enhancing the clearance of LDL-c from circulation. FH, an autosomal dominant disease caused by mutations in the LDLR gene, is characterized by high plasma LDL-c levels and accelerated MASLD and AS progression. Additionally, excessive cholesterol levels can activate LXRα, promoting LXR-associated target genes such as inducible degrader of LDLR (IDOL). LDLR can also be ubiquitinated and degraded by the E3 ubiquitin ligase IDOL, reducing intracellular cholesterol transport [35]. Thus, PCSK9 and IDOL serve as potential therapeutic targets for hyperlipidemia [36].
In MASLD, mice exhibit decreased hepatic LDLR and increased serum LDL-c levels [37]. Genetic ablation of LDLR in mice leads to liver injury, inflammatory cytokines releases, and high CD68 expression when fed a high cholesterol diet [38], as well as obesity and insulin resistance under a HFHC diet [39]. It is well-established that dietary cholesterol inhibits hepatic SREBP-mediated LDLR gene transcription, leading to reduced hepatic LDLR mRNA levels in hypercholesterolemic animals, which is a consequence of the feedback mechanism driven by high levels of cellular cholesterol in hepatocytes. However, other studies have shown that high cholesterol specifically accelerates LDLR mRNA degradation by inducing hepatic expression of LDLR mRNA decay-promoting factor heterogeneous nuclear ribonucleoprotein (HNRNP). Depletion of HNRNPD in the liver results in a marked reduction of serum LDL-c and a substantial increase in hepatic LDLR expression in hyperlipidemic mice [40]. In addition, LDLR expression is modulated by the epidermal growth factor receptor (EGFR)-extracellular signal-regulated kinase (ERK1/2) signaling pathway. Activating this pathway could improve MASLD by increasing LDLR in HepG2 cells [37].
Neutrophil infiltration around lipotoxic hepatocytes is a hallmark of MASH. Neutrophil-specific microRNA-223 (miR-223) in extracellular vesicles (EVs) can be preferentially taken up into hepatocytes to inhibit hepatic inflammatory and fibrogenic gene expression. This selective uptake depends on the expression of LDLR on hepatocytes and APOE on neutrophil-derived EVs. In the absence of this LDLR- and APOE-dependent uptake of miR-223-enriched EVs, the progression of steatosis to MASH is accelerated. Administration of the PCSK9 inhibitor Alirocumab enhances LDLR-dependent miR-223 transfer to ameliorate MASH in mice [41]. Although PCSK9 inhibition reduces plasma LDL-c and maintains LDLR expression in hepatocytes, it also increases liver exposure to cholesterol, potentially heightening the risk of MASH and HCC. PCSK9 knockout mice on a HFHC diet developed increased hepatic FC, cholesterol crystals, and fibrosing steatohepatitis, with a higher predisposition to liver cancer compared to wild-type mice. Future studies should evaluate whether patients on long-term anti-PSCK9 monoclonal antibody treatment are at increased risk of hepatic steatosis, MASH, or HCC, considering concurrent use of statins [42].
3.2. RCT in MASLD
3.2.1. LCAT
LCAT is a lipid-modifying enzyme that catalyzes the transfer of the acyl chain from lecithin to the hydroxyl group of cholesterol on plasma lipoproteins, forming cholesteryl acylester and lysolecithin. Predominantly synthesized in the liver and secreted into plasma [43], LCAT plays a pivotal role in the RCT process. However, recent findings indicate that human LCAT overexpression substantially increases plasma HDL-c levels but does not enhance macrophage RCT, even with co-expression of SR-BI or CETP, which promote the transfer of LCAT-derived HDL cholesterol ester to the liver. LCAT-deficient mice show only a 50% reduction in RCT, and their serum promotes ABCA1-mediated cholesterol efflux from macrophages ex vivo. These data suggest that macrophage RCT may not be as dependent on LCAT activity as previously believed [44]. Additionally, LCAT deficiency often leads to reduced plasma HDL-c, corneal opacity, anemia, and renal issues. LCAT enzyme therapy might prevent serious complications, particularly renal dysfunction and corneal opacity [45].
Recent studies have associated higher LCAT activity with elevated fatty liver index (FLI) values, a surrogate marker of MASLD [46]. Specifically, subjects with an FLI ≥ 60 coinciding with type 2 diabetes mellitus (T2DM) and metabolic syndrome (MetS) exhibit an average 12% higher plasma LCAT activity. In age- and sex-adjusted partial linear regression analysis, LCAT activity is positively related to various obesity measures and homeostasis model assessment-insulin resistance (HOMA-IR). Multivariable linear regression analyses adjusted for age and sex show that LCAT activity is associated with an FLI ≥ 60, independent of T2DM and MetS, waist/hip ratio, or HOMA-IR. Therefore, MASLD inferred from an FLI ≥ 60 confers higher plasma LCAT and, to a lesser extent, phospholipid transfer protein activity, even when accounting for T2DM, MetS, central obesity, and insulin resistance [47]. In contrast, LCAT levels are found to be decreased in patients with MASLD and advanced fibrosis [48].
3.2.2. CETP
CETP, predominantly originating from liver Kupffer cells, plays a pivotal role in lipoprotein metabolism by facilitating the transfer of esterified cholesterol from HDL to VLDL and LDL [14]. Clinical studies on CETP inhibitors such as torcetrapid, dalcetrapib, and evacetrapib aimed to curb AS by increasing HDL-c levels [49], though their expected efficacy remains unrealized, necessitating further research into the function of CETP [50,51,52,53]. Genetic CETP deficiency, mimicking pharmacologic CETP inhibition, is associated with a lower risk of cardiovascular morbidity and mortality but a markedly higher risk of age-related macular degeneration [54].
CETP gene expression is upregulated in MASLD induced by a high-cholesterol diet [55]. Patients with MetS with biopsy-proven MASLD (MetS+MASLD) show an increasing tendency of CETP activity. However, as MASLD progresses to severe fibrosis, CETP activity apparently improves [56]. Given that genetic polymorphism is an important factor in the pathogenesis of MASLD, a variant of the CETP gene polymorphism rs1800777 is independently associated with steatosis and lobulillar inflammation in subjects with biopsy-proven MASLD [57]. In addition, the B1B2/B2B2 genotype of CETP and elevated LDL-c serum levels increase the risk of MASLD in women with gallstone disease [58]. Considering that low HDL-c levels are associated with AS and MASH, increased HDL-c levels may reduce the risk of these diseases. A novel CETP vaccine (Fc-CETP6) efficiently elicited antibodies against CETP and reduced susceptibility to both AS and MASH induced by the HFHC diet via increasing plasma HDL-c and ApoA-I levels and decreasing plasma ox-LDL. Therefore, inhibition of CETP activity is considered a promising strategy for increasing HDL-c levels [59].
3.2.3. SB-RI
SR-BI is a class B transmembrane scavenger receptor expressed in the liver, steroidogenic tissues, and adipose tissue [60]. SR-BI facilitates bidirectional cholesterol transport in vivo by the efflux of FC to HDL particles and the selective uptake of CE from HDL. SR-BI is required for insulin-mediated glucose uptake and regulation of energy balance in adipocytes. Loss of SR-BI in adipocytes resulted in inefficient glucose uptake compared to WT adipocytes, suggesting a novel role for SR-BI in glucose uptake and metabolic homeostasis in adipocytes [61]. Additionally, SR-B1 variants might influence adiposity markers in females [62]. SR-B1 deficiency regulates intestinal lipids, amino acids, and neurotransmitter metabolism in mice [63]. However, the crucial role of SR-BI in hepatocytes should also be noted. Research involving mice has shown that overexpression of hepatic SR-BI reduces plasma cholesterol levels but increases cholesterol excretion in feces. Conversely, SR-BI-deficient mice exhibited significantly increased plasma cholesterol with a corresponding decrease in fecal cholesterol excretion [64]. These findings demonstrate the paradoxical yet essential role of hepatic SR-BI. Although it inversely impacts steady-state plasma HDL-c concentrations, SR-BI emerges as an important positive regulator of hepatic RCT.
Generally, low HDL-c is a common feature of patients with MASLD. Hepatic SR-BI protein expression is lower in high-fat/high-sucrose (HFS) diet-induced mice [65]. Other studies report that gene and protein levels of hepatic SR-B1 in MASLD mice increase significantly compared to control mice. Furthermore, SR-B1 immunoreactivity increases and is mainly located in the plasma membrane of hepatocytes, cytoplasm, and the membrane of lipid droplets, with positive expressions associated with the severity of hepatic steatosis [66]. However, MASLD-relevant factors like inflammatory cytokines, lipopolysaccharide, and TGF-β do not affect SR-BI protein levels in primary human hepatocytes. Accordingly, hepatic SR-BI is not changed in human and murine hepatic steatosis and MASH. Therefore, the current study indicates a minor, if any, role of SR-BI in human and murine MASLD [67].
3.3. Cholesterol Biosynthesis in MASLD
HMGCR
HMGCR is a critical enzyme in cholesterol biosynthesis, localized to the ER. This enzyme catalyzes the conversion of 3-hydroxy-3-methylglutarylcoenzyme A (HMG-CoA) to mevalonate, a foundational precursor for cholesterol and other isoprenoids. HMGCR activity is primarily regulated through phosphorylation by AMP-activated protein kinase (AMPK), which inhibits the conversion of HMG-CoA to mevalonate, thereby reducing cholesterol synthesis [68,69]. In its dephosphorylated state, HMGCR is active, whereas phosphorylation by AMPK inhibits its activity. The degradation pathways of HMGCR are influenced by lanosterol, a seminal sterol intermediate in the mevalonate cascade, which facilitates the binding of INSIG to HMGCR [70]. This process triggers HMGCR ubiquitylation, extraction from the membrane, and proteasomal degradation via ER-associated degradation (ERAD) [11].
Empirical studies underscore a linkage between MASLD and perturbed HMGCR functionality. Elevated HMGCR expression, along with decreased phosphorylation of HMGCR, is a hallmark of MASLD and correlates with FC, histopathological severity of MASLD, and LDL-c levels [71]. In HFD-induced MASLD mice, hepatic TC and cholesterol biosynthesis genes such as SREBP2 and HMGCR levels are upregulated [72,73]. Western diet (WD) challenges further support these observations, with hepatic TC levels and HMGCR mRNA expression notably increased [74]. In vitro experiments using free fatty acid (FFA) or oleic acid (OA)-induced hepatic steatosis in HepG2 cells show surges in intracellular TG and TC, concomitant with increased HMGCR and SREBP2 levels [75,76]. Diving deeper into molecular mechanisms, HFDs have been shown to stimulate retinoic acid-inducible gene-I (RIG-I) expression, an RNA virus sensor in innate immune cells. RIG-1 amplifies cholesterol synthesis, precipitating steatosis and its sequelae, including MASH and hepatocarcinogenesis. Mechanistically, RIG-I undergoes constitutive methylation at K18 and K146 sites. In an intriguing twist, demethylase JMJD4 mediates RIG-I demethylation, consequently suppressing IL-6-STAT3 signaling. This methylated RIG-I form interacts with AMPKα, culminating in inhibiting HMGCR phosphorylation, thus promoting HMGCR’s enzymatic activity and fortifying cholesterol synthesis [77].
3.4. Cholesterol Efflux in MASLD
LXRα
LXRα, a pivotal member of the nuclear receptor superfamily, forms a dimer with retinoid X receptors, creating the RXR/LXR complex. This complex is modulated by rexinoids and oxysterol, such as 22(R)-hydroxycholesterol, 24(S)-hydroxycholesterol, and 24,25(S)-epoxycholesterol. Serving as a sophisticated sterol sensor, LXRα orchestrates a balance of sterol catabolism, storage, efflux, and elimination. For instance, LXRα regulates ABCA1, which is crucial for cholesterol absorption, leading to increased expression in jejunal enterocytes upon LXR agonist exposure. Furthermore, the ABCG5 and ABCG8 genes, under the regulation of LXR, enhance biliary cholesterol excretion [78]. Notably, a LXRα deficiency culminates in the liver’s inability to transcribe CYP7A, pivotal for cholesterol-to-BA conversion, leading to rapid hepatic cholesterol accumulation [79]. Consequently, liver-specific deletion of LXRα in mice impedes reverse cholesterol transport, catabolism, and excretion [80], emphasizing the paramount significance of LXRα in maintaining cholesterol homeostasis.
Accumulating evidence demonstrates the involvement of LXRα in MASH pathogenesis, particularly through the SREBP1c pathway [81]. MASLD patients exhibit increased expression of LXR and SREBP1c, with LXR overexpression closely linked to SREBP-1c augmentation [82]. Similarly, HFD-induced MASLD mice show increased LXRα mRNA expression [81,83], correlating with intrahepatic lipid accumulation and inflammation markers [28]. In both MASLD and hepatitis C virus (HCV)-afflicted patients with steatosis, hepatic LXRα expression, alongside lipogenic (e.g., PPAR-γ, SREBP-1c, SREBP-2, and FAS) and inflammatory genes (e.g., TNF-α, IL-6, and iNOS), is abnormally increased, pointing to the conceivable role of LXRα in these liver conditions’ pathogenesis [84]. Additionally, LXRα-knockout mice on HFHC diet demonstrated a surge in F4/80+CD68+CD11b+ macrophages, converging with hepatic cholesterol accumulation [85]. In conclusion, LXR expression reflects hepatic lipid deposition and hepatic inflammation in MASLD patients, highlighting LXR as a promising therapeutic target for hepatic inflammation and fibrosis [28].
3.5. Cholesterol Conversion in MASLD
CYP7A1
CYP7A1 stands at the biochemical crossroads of cholesterol metabolism, catalyzing the conversion of cholesterol into 7α-hydroxycholesterol, a pivotal precursor in BA synthesis. Deficiency in CYP7A1 in humans leads to significant metabolic aberrations, including impaired conversion of cholesterol to BAs, accumulation of hepatic cholesterol, reduction of LDL receptor expression, exacerbated hypercholesterolemia, and diminished statin response. Furthermore, this deficiency is associated with an increased risk of gallstone disease due to reduced bile acid secretion [86]. In CYP7A1−/− mice, significantly elevated hepatic TC concentrations are observed compared to WT counterparts [87]. Conversely, upregulation of CYP7A1 can counteract cholesterol diet-induced hypercholesterolemia by preventing hepatic cholesterol accumulation and reducing the proliferation of apoB-containing lipoproteins in plasma, facilitated by augmented SREBP-mediated LDL receptor transcription [88].
In the context of MASLD, notable perturbations in BA metabolic profiles are evident, especially in individuals with MASH. These patients exhibit elevated total fecal and serum BAs, along with a reduced secondary-to-primary BA ratio compared to healthy individuals [89,90]. Within the MASLD/MASH spectrum, increased CYP7A1 expression is commonly observed, particularly when compared to control cohorts [91]. Therapeutically, modulating BA synthesis by downregulating CYP7A1 presents a potential approach to mitigate MASLD progression [92,93]. However, divergent data indicates a marked suppression of CYP7A1 in HFD-induced MASLD [72]. This discrepancy highlights the complex interplay between the farnesoid X receptor (FXR) signaling pathway and MASLD-associated BA dysregulation. FXR exerts an inhibitory effect on CYP7A1 transcription, mediated through the intestinal fibroblast growth factor 19 (FGF19)/FGFR4/β-Klotho axis or the hepatic small heterodimer partner (SHP). A therapeutic strategy that involves counteracting FXR signaling while upregulating CYP7A1 may offer a promising remedy for MASLD [94,95]. Additionally, the cholesterol metabolic enzyme 25-hydroxylase (Ch25H) and its product 25-hydroxycholesterol (25-HC) offer another therapeutic proposition. Their role in alleviating HFD-induced hepatic steatosis, mediated via the LXRα–CYP7A1 axis, suggests a novel strategy to address the multifaceted challenges of MASLD [96].
4. Natural Product Targeting Cholesterol Metabolism for MASLD
As mentioned above, the dysregulation of cholesterol metabolism and key targets is a critical factor in the progression of MASLD. Despite the lack of approved specific therapeutic drugs, numerous natural products have exhibited therapeutic potential for MASLD in vitro and in vivo. Herein, we summarize the hepatoprotective effects of natural products targeting crucial cholesterol metabolic pathways (Figure 2), including inhibiting cholesterol absorption (NPL1L1 and LDLR), enhancing RCT, inhibiting cholesterol biosynthesis, modulating the LXRα pathway, and promoting BA excretion via CYP7A1.
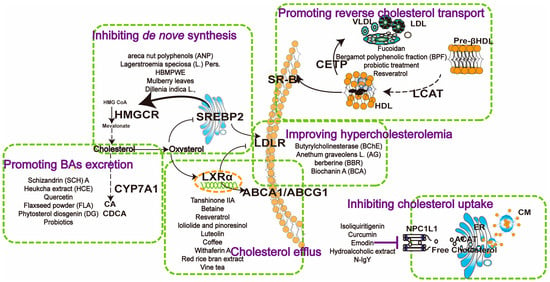
Figure 2.
Mechanisms of natural products in regulating cholesterol metabolism in MASLD. NPC1L1 inhibitors such as isoliquiritigenin, curcumin, and emodin reduce intestinal cholesterol uptake. Enhancing reverse cholesterol transport by compounds such as fucoidan, bergamot polyphenolic fraction, and probiotics, which enhance CETP, SR-B1, and LCAT expression, facilitating cholesterol transfer from peripheral tissues to the liver. Inhibiting de novo synthesis involves compounds like areca nut polyphenols, Lagerstroemia speciosa, and HBMPWE, mulberry leaves, which suppress HMGCR activity, reducing cholesterol synthesis. Promoting bile acid excretion through Schizandrin A, Heuchka extract, and quercetin upregulates CYP7A1, enhancing cholesterol conversion to bile acids. Butyrylcholinesterase, Anethum graveolens L., and berberine improve hypercholesterolemia by lowering cholesterol levels at various metabolic points. Cholesterol efflux is promoted by compounds like tanshinone IIA, betaine, and resveratrol, which enhance ABC transporter activity (ABCA1/ABCG1), promoting cholesterol removal from cells to HDL. These pathways collectively maintain cholesterol homeostasis, offering potential therapeutic strategies for MASLD.
4.1. Inhibiting Cholesterol Absorption
4.1.1. NPCL1L Inhibitors
Recent studies have demonstrated that various natural products can reduce the cellular uptake of cholesterol by inhibiting the NPC1L1 protein. For example, isoliquiritigenin, a flavonoid with a chalcone structure extracted from the natural herb glycyrrhiza glabra, has been shown to downregulate NPC1L1 expression and competitively inhibit cellular cholesterol uptake by binding to NPC1L1 in a concentration-dependent manner in vitro [97]. A systematic review and meta-analysis evaluating the therapeutic efficacy of oral dietary polyphenols in patients with MASLD indicated that curcumin significantly decreases AST, ALT, TG, TC, and HOMA-IR compared to placebo [98]. In vitro, curcumin inhibits cholesterol uptake in Caco-2 cells by down-regulating NPC1L1 expression [99]. Additionally, in vivo supplementation with curcumin reduces intestinal cholesterol absorption and improves HFD-induced dyslipidemia by downregulating the expression of NPC1L1 [100].
Emodin, an active component of the traditional Chinese medicine rhubarb, also demonstrates cholesterol-lowering properties. Mechanically, studies showed that NBD-cholesterol uptake in human HepG2 cells decreased significantly after treatment with various concentrations of emodin, compared to control and ezetimibe treatments. Thus, emodin inhibited cholesterol uptake by HepG2 and Caco-2 cells more effectively than ezetimibe. This inhibition occurs via NPC1L1 in an anti-competitive manner [101]. The hydroalcoholic extract obtained from the açai seed (ASE), can reduce NPC1L1 and increase the expression of ABCG5 and PPAR-α to improve lipid profile and attenuate hepatic steatosis in HFD-induced MASLD mice [102]. Moreover, N-IgY, a chicken egg yolk-derived IgY specific for NPC1L1, has been shown to block cholesterol transport efficiently in HepG2 and Caco-2 cells, with an effect comparable to ezetimibe. N-IgY combined with omega-3 polyunsaturated fatty acids led to significant upregulation of genes involved in cholesterol uptake (LDLR), reverse cholesterol transport (ABCG5/ABCG8), and BA metabolism (CYP7A1), representing a promising treatment strategy to prevent HFD-induced MASLD through the activation of cholesterol catabolism to BAs and by decreasing cholesterol-induced fibrosis [103].
4.1.2. LDLR Upregulation
The ERK signaling cascade is a central regulator of LDLR expression in MASLD. Caffeine markedly improved HFD-induced MASLD by activating EGFR-ERK1/2 signaling and promoting LDLR expression in ApoE KO mice [37]. Liver butyrylcholinesterase (BChE) governs LDL-c levels and LDL uptake capacity through the MEK-ERK signaling cascade to promote LDLR transcription. These findings suggest that targeting liver LDLR is an effective therapeutic strategy to treat MASLD and hypercholesterolemia [104]. Anethum graveolens L. (AG), an annual plant known for its hypolipidemic effects, has shown promise in normalizing liver histopathological changes in high-cholesterol diet-fed animals by significantly increasing liver antioxidants and upregulating LDLR expression. This modulation leads to improved circulating cholesterol and LDL-c clearance [105]. Berberine (BBR) has effectively ameliorated MASLD via lowering glucose levels, reducing inflammatory factors, and inhibiting endotoxin release. Furthermore, BBR administration could reverse the abnormal expression of MTTP and LDLR to improve HFD-induced MASLD. Mechanistically, berberine stabilizes LDLR mRNA and increases LDLR expression, leading to increased cholesterol catabolism [106]. Additional evidence indicates that the stabilization of LDLR mRNA induced by berberine is dependent on ERK activation [107]. Biochanin A (BCA), an ox-methylated isoflavone found in soybeans, red clover, alfalfa, peanuts, and chickpeas, delays liver damage of MAFLD, though significantly increasing CYP7A1, LDLR, and PPAR-α protein expression while downregulating HMGCR, SREBP-1c, and PPAR-γ protein expression. This regulation promotes cholesterol absorption and metabolism, thereby protecting against MASLD progression [72].
4.2. Enhancing RCT
RCT is a physiological process where excess peripheral cholesterol is transported to the liver for excretion into the bile and subsequently the feces. Fucoidan, derived from Ascophyllum nodosum, has been shown to lower lipid levels by modulating RCT-related protein expression. Specifically, fucoidan enhances lipid transfer from plasma to the liver by activating SR-B1 and LDLR while inactivating PCSK9. It also upregulates lipid metabolism by activating ABCA1, ABCG8, and CYP7A1 [108]. Accumulating evidence indicates that bergamot polyphenolic fraction (BPF) can improve histopathological and serum biomarkers of MASLD. This suggests a correlation between BPF and improved biochemical modulation in liver tissues. BPF restores the dysregulated lipid transfer protein system via normalizing serum concentrations of lipemic biomarkers and the activity of ACAT, LCAT, and CETP. This normalization leads to a decrease in hepatic cholesterol content and improved lipoprotein trafficking in the liver, contributing to the hypolipemic response observed in MASLD patients [109]. Probiotic treatment has also been found to significantly protect against MASLD by restoring gut flora, improving liver functions, and reducing inflammatory cytokines. For example, Lactobacillus acidophilus supplementation can normalize the expression of lipid-related genes affected by a high-cholesterol diet. This is achieved by upregulating CETP, lipoprotein lipase (LPL), and hepatic lipase (HL), while downregulating LDLR [55]. Resveratrol has been shown to protect against HFHS-induced decreases in hepatic LDLR and SR-BI gene and protein expressions, providing new insights into its pharmacological targets in MASLD prevention [65]. Considering hypercholesterolemia as a leading cause of MASLD, many natural products can attenuate hypercholesterolemia by upregulating LCAT and SR-BI expression and downregulating CEPT expression, thereby accelerating RCT. Examples include curcuminoids, Desmodium gyrans methanolic extract, a 5% ethanol extract of unripe Rubus coreanus, and the ethanol extract of Edgeworthia gardneri (Wall.) Meisn [110,111,112,113].
4.3. Inhibiting Cholesterol Biosynthesis
Inhibiting cholesterol biosynthesis is a key strategy in managing MASLD, as it helps reduce hepatic cholesterol levels and prevent lipid accumulation. Areca nut polyphenols (ANP), derived from the areca nut, have been found to reduce WD-induced TC and non-high-density lipoprotein (non-HDL) levels by increasing the abundance of beneficial bacteria in the gut microbiota and reducing the expressions of SREBP2 and HMGCR. The mechanism involves increasing the expression of phosphorylated AMPKα, which inhibits cholesterol synthesis in MASLD mice [114]. DLBS3733, a bioactive fraction of Lagerstroemia speciosa (L.) Pers., shows potential in treating hepatic steatosis-related diseases through downregulating the expression of HMGCR and SREBP and upregulating CPT-1 in HepG2 cells. SREBP directly activates the expression of genes involved in cholesterol synthesis, and its downregulation by DLBS3733 helps repress hepatic steatosis [115]. In high-fat, high-fructose, high-cholesterol diet (HFFCD)-fed MASLD hamsters, treatments with HBMPWE—a product fermented by inoculating Monascus purpureus on highland barley fruit—significantly inhibited lipid accumulation. This was achieved by downregulating proteins related to fatty acid synthesis and cholesterol synthesis (SREBP-1/ACC/FAS/AceS1 and HMGCR) and upregulating cholesterol clearance (CYP7A1) [116]. Mulberry leaves (MLF) exhibit regulatory effects on abnormal cholesterol metabolism, improving hepatic injury in MASLD. In vivo studies show that MLF treatment significantly downregulates the hepatic expression levels of SREBP2, HMGCR, and miR-33a. In vitro, quercetin, an active metabolite of MLF, significantly decreases lipid accumulation in HepG2 cells by inhibiting the gene and protein expression level of SREBP2 and HMGCR [117]. Dillenia indica L., an edible plant from the Dilleniaceae family present in India and other Asian countries, significantly reduced the expression of SREBP-1c, SREBP-2, HMGCR, FAS, and CD36 in oleic acid-treated cells, thereby alleviating lipid accumulation [76].
4.4. Modulating LXRα Pathways
Abnormal and excessive accumulation of lipid droplets within hepatic cells is the main feature of MASLD. The LXRα–SREBP1 pathway plays a crucial role in hepatic steatosis and the pathological progression of MASLD. Tanshinone IIA, a bioactive phytochemical from Salvia miltiorrhiza Bunge, attenuates lipid accumulation by modulating the LXRα–SREBP1 pathway to treat MASLD [118]. Loliolide and pinoresinol, identified in the dichloromethane fraction, significantly accelerate the protein degradation of LXRs by enhanced ubiquitination. This reduces the expression levels of lipogenic factors including SREBP-1, SCD1, FAS, and ACC, thus inhibiting lipogenesis and improving MASLD [119]. Luteolin can abolish lipid accumulation induced by LXR-SREBP-1c activation both in vivo and in vitro, indicating its potential as a therapeutic agent for treating MASLD [120]. Additionally, betaine prevents MASLD by reversing the expressions of LXRα and PPARα in the liver and promoting the expression of genes related to fatty acid oxidation [121].
As a dual LXR/FXR receptor activator, withaferin A activates both LXR-α and FXR, inducing their canonical target genes (ABCA1 and ABCB11) and inhibiting diet-induced hepatic steatosis, steatohepatitis, and fibrosis [122]. Red rice bran extract (RRBE) attenuates markers of inflammation such as NF-κB and iNOS genes in the liver and alleviates the expression of key genes involved in cholesterol metabolism. Specifically, RRBE decreases CD36 and HMGCR levels while increasing LXRα expression in HFD-induced MASH, demonstrating its anti-inflammatory properties [123]. Emerging evidence suggests AMPK-LXRα participates in the development of MASH [124]. AMPK activators have also been shown to inactivate LXRα. Chinese vine tea (VTE) alleviated the progression of MASH via enhancing AMPK and blocking LXRα signaling in mouse livers [125]. Diosgenin is another potential agent for preventing the development of MASLD through the AMPK and LXR signaling pathways [126]. Current studies have identified that coffee intake reduces HFD-induced liver macrovesicular steatosis and serum cholesterol levels. Coffee supplementation prevents HFD-induced MASLD in mice by modulating liver LXRα expressions. LXRα regulates systemic cholesterol homoeostasis by increasing biliary cholesterol excretion through the regulation of the intestinal membrane transporters ABCA1 and ABCGl, thereby impacting intestinal cholesterol efflux and improving MASLD [127]. Combining caffeine with chlorogenic acid exerts collaborative effects in HFD-fed mice via the AMPKα–LXRα/SREBP-1c pathway [128]. Resveratrol and atorvastatin administration elevated ABCA1 and ABCG1 and reduced LXRα protein expression to improve MASLD by targeting genes involved in cholesterol metabolism [129].
4.5. Promoting BA Excretion via CYP7A1
CYP7A1 is a rate-limiting enzyme for the conversion of cholesterol to BAs. Enhancing CYP7A1 expression through natural products has shown potential in improving MASLD by promoting bile acid excretion. Schizandrin A, a lignan found in the fruits of the Schisandra genus, significantly alleviates HFHC diet-induced MASLD. This is achieved by markedly increasing the expression of CYP7A1 and ABCA1, facilitating biliary cholesterol excretion and cholesterol efflux to HDL in the liver [130]. Heukcha extract (HCE), a naturally post-fermented green tea extract, has been shown to suppress diet-induced MASLD. This effect is achieved by significantly increasing the protein level of CYP7A1 in the liver [131]. Quercetin, known for its hepatoprotective effect on T2DM-associated MASLD, down-regulates nuclear YY1, which directly binds to the CYP7A1 promoter and activates its transcription. This results in the restoration of cholesterol homeostasis via the conversion of cholesterol to BAs [132].
Previous studies show that BA imbalance in MASLD is closely associated with the FXR signaling pathway. FXR negatively regulates CYP7A1 transcription by fibroblast growth factor 19 (FGF19)/FGF4/klothoβ in the intestine or by small heterodimer partner (SHP) in the liver. Phytosterol diosgenin (DG) notably decreases hepatic cholesterol through increasing hepatic CYP7A1 and prohibiting FXR-mediated signaling, thus contributing to cholesterol elimination and alleviating HFD-induced hypercholesterolemia [94]. Flaxseed powder (FLA) is rich in α-linolenic acid, dietary fiber, lignans, and other active ingredients. Animal experiments reveal that FLA intervention significantly activates the intestinal FXR-FGFR4-CYP7A1 to lower lipid and modulate TGR5-TLR4-TNFα pathways to alleviate inflammation in HFD-induced MASLD [133]. Probiotics are prospective for the prevention and treatment of hypercholesterolemia and MASLD. For example, L. plantarum WLPL21 showed the best mitigatory effect on HCD-induced hypercholesterolemia, including upregulating cholesterol metabolism (CYP27A1, CYP7B1, CYP7A1, and CYP8B1) levels in the liver, cholesterol transportation (ABCA1, ABCG5, and ABCG8) in the ileum or liver, and downregulating NPC1L1 [134] (Table 1).

Table 1.
Natural products targeting cholesterol dysfunction in MASLD.
5. Clinical Trials of Natural Products for the Treatment of MASLD
The promising preclinical efficacy of natural products targeting cholesterol metabolism for MASLD has led to their clinical evaluation. Although only a few of the 25 natural products mentioned have been tested in clinical trials for MASLD, the results are encouraging (Table 2). (1) Curcumin: Systematic review and meta-analysis show curcumin reduces liver enzymes (AST and ALT), body weight, waist circumference, body fat percentage, and body mass index (BMI) compared to placebo [136,137,138]. A randomized double-blind placebo-controlled trial (80 mg/day for 3 months) in overweight/obese MASLD patients showed improvements in various metabolic parameters, indicating anti-inflammation, antidiabetic, and lipid-lowering effects [139]. Another trial (500 mg/day for 8 weeks) confirmed benefits, though some patients experienced stomachache and nausea, leading to three drop-outs [140]. (2) Berberine: A meta-analysis of 10 randomized controlled trials involving 811 patients demonstrated its efficacy in improving liver function, serum lipid profile, and insulin sensitivity in MASLD patients [141]. Treatment with Berberis integerrima extract (750 mg twice/day for 2 months) significantly decreased BMI, serum lipid profile, fasting blood glucose (FBG), and liver enzymes, while increasing HDL-c, glutathione peroxidase enzyme, and total antioxidant capacity [142]. Another trial found berberine (0.5 g three times/day for 16 weeks) more effective than pioglitazone (15 mg/day) in reducing body weight and improving serum lipid profile, despite some digestive side effects [143]. (3) Resveratrol: Clinical trials on the effects of resveratrol on liver enzymes in MASLD are mixed [144]. Five trials involving 216 patients showed significant changes in ALT and AST levels [145]. However, a randomized, double-blind, placebo-controlled trial (600 mg/day for 12 weeks) showed significant reductions in body weight, BMI, and waist circumference [146]. Another trial found resveratrol (500 mg/day for 12 weeks) with lifestyle modification superior to lifestyle modification alone in reducing inflammation and hepatocellular apoptosis [147]. Further studies are needed to confirm the effect of resveratrol on liver enzymes. (4) Quercetin: A randomized clinical trial combining rosuvastatin with quercetin (40 mg three times/day for 3 months) showed significant reductions in oxidative stress, enhanced antioxidant protection activity, and decreased hepatocyte apoptosis [148]. (5) Hesperidin and flaxseed: In a randomized, controlled clinical trial, hesperidin (1 g/day) and flaxseed (30 g/day) for 12 weeks improved glucose and lipid metabolism while reducing inflammation and hepatic steatosis in MASLD patients, showing synergistic effects on FBG and HOMA-IR [149].

Table 2.
Clinical trial efficacy and safety of natural products on MASLD.
Despite these promising results, most other natural products have been evaluated primarily in animal models rather than clinical trials, necessitating further validation. Adequate subject numbers and balanced sex ratios are essential for objective results in clinical trials. Existing human clinical trials often focus on simple biomarkers, lacking a comprehensive multi-indicator evaluation system associated with the functional targets of natural products.
6. Concluding Remarks and Future Perspectives
MASLD represents a significant global health issue, being the leading cause of hepatogenic mortality and affecting approximately one-fourth of the global population. Previous studies have identified that various natural products can mitigate MASLD by reducing intestinal cholesterol absorption and hepatic cholesterol levels. This review emphasizes the hepatoprotective effects of natural products targeting crucial metabolic pathways, including uptake (NPL1L1 and LDLR), transport (CETP, LCAT, and SRBI), biosynthesis (HMGCR), efflux (LXRα), and conversion (CYP7A1). Despite these advancements, several challenges remain in utilizing natural molecules for MASLD therapy. Many natural compounds in the biological world remain undiscovered. With the rapid development of bioinformatics and artificial intelligence, the discovery of more efficient and low-toxicity natural products is anticipated. These advancements could address poor clinical efficacy and side effects associated with some natural molecule drugs. Moreover, small molecules often exhibit poor water solubility and low oral availability, necessitating further experiments to better understand their absorption and conversion in vivo. Additionally, the complex and dynamic nature of cholesterol-related metabolic homeostasis requires further exploration. These limitations pose significant challenges in developing and practically applying natural therapeutic molecules for treating MASLD. A deeper understanding of how natural products modulate cholesterol homeostasis is fundamentally important for establishing novel therapeutic approaches to improve MASLD.
Author Contributions
Writing—original draft preparation, X.L.; writing—review and editing, M.L.; visualization, X.L., and M.L.; supervision, M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China, Grant No. 82104479.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ACAT | Acyl-CoA acyltransferase |
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| AMPK | AMP-activated protein kinase |
| APOA-I | Apolipoprotein A-I |
| AST | Aspartate aminotransferase |
| BMI | Body mass index |
| CA | Cholic acid |
| CDCA | Chenodeoxycholic acid |
| CE | Cholesteryl ester |
| CETP | Cholesteryl ester transfer protein |
| CM | Chylomicron |
| CYP7A1 | Cholesterol 7 alpha-hydroxylase |
| ER | Endoplasmic reticulum |
| ERK | Extracellular regulated protein kinase |
| Ev | Extracellular vesicle |
| FBG | Fasting blood glucose |
| FBI | Fasting blood insulin |
| FC | Free cholesterol |
| FH | Familial hypercholesterolemia |
| FFA | Free fatty acid |
| HCV | Hepatitis C virus |
| HCC | Hepatocellular carcinoma |
| HDL | High-density lipoprotein |
| HFD | High-fat diet |
| HOMA-IR | Homeostasis model assessment-insulin resistance |
| HMG-CoA | 3-hydroxy-3-methylglutarylcoenzyme A |
| Insig1/2 | Insulin-induced gene 1/2 |
| LCAT | Lecithin cholesterol acyltransferase |
| LDLR | Low-density lipoprotein receptor |
| LPL | Lipoprotein lipase |
| LXRα | Liver X receptor α |
| MASLD | Metabolic-associated steatotic liver disease |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| MetS | Metabolic syndrome |
| RIG-I | Retinoic acid-inducible gene-I |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9 |
| RCT | Reverse cholesterol transport |
| SCAP | SREBP cleavage-activating protein |
| SHP | Small heterodimer partner |
| SR-BI | Scavenger receptor class B type I |
| SREBP2 | Sterol regulatory element-binding protein 2 |
| TC | Total cholesterol |
| T2DM | Type 2 diabetes mellitus |
| VLDL | Very-low-density lipoprotein |
| WD | Western diet |
References
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; Petta, S. Nafld/Nash. J. Hepatol. 2022, 77, 549–550. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zheng, L.; Jiang, S.; Yang, H.; Guo, J.; Jiang, L.Y.; Li, T.; Zhang, H.; Bai, Y.; Lou, Y.; et al. Exhaustion- associated cholesterol deficiency dampens the cytotoxic arm of antitumor immunity. Cancer Cell 2023, 41, 1276–1293. [Google Scholar] [CrossRef] [PubMed]
- Caballero, F.; Fernandez, A.; De Lacy, A.M.; Fernandez-Checa, J.C.; Caballeria, J.; Garcia-Ruiz, C. Enhanced free cholesterol, SREBP-2 and StAR expression in human NASH. J. Hepatol. 2009, 50, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Baillie, R.A.; Wiest, M.M.; Mirshahi, F.; Choudhury, J.; Cheung, O.; Sargeant, C.; Contos, M.J.; Sanyal, A.J. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 2007, 46, 1081–1090. [Google Scholar] [CrossRef]
- Liang, J.Q.; Teoh, N.; Xu, L.; Pok, S.; Li, X.; Chu, E.S.H.; Chiu, J.; Dong, L.; Arfianti, E.; Haigh, W.G.; et al. Dietary cholesterol promotes steatohepatitis related hepatocellular carcinoma through dysregulated metabolism and calcium signaling. Nat. Commun. 2018, 9, 4490. [Google Scholar] [CrossRef]
- Walenbergh, S.M.; Koek, G.H.; Bieghs, V.; Shiri-Sverdlov, R. Non-alcoholic steatohepatitis: The role of oxidized low-density lipoproteins. J. Hepatol. 2013, 58, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nunez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef]
- Clayton, Z.S.; Fusco, E.; Kern, M. Egg consumption and heart health: A review. Nutrition 2017, 37, 79–85. [Google Scholar] [CrossRef]
- Soliman, G.A. Dietary Cholesterol and the Lack of Evidence in Cardiovascular Disease. Nutrients 2018, 10, 780. [Google Scholar] [CrossRef]
- Luo, J.; Yang, H.; Song, B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell. Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Stahel, P.; Lewis, G.F. Regulation of Chylomicron Secretion: Focus on Post-Assembly Mechanisms. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 487–501. [Google Scholar] [CrossRef]
- Donetti, E.; Hultin, M.; Soma, M.R.; Olivecrona, T. Barberi Conversion of chylomicrons into remnants. Atherosclerosis 1998, 141, S25–S29. [Google Scholar]
- Shrestha, S.; Wu, B.J.; Guiney, L.; Barter, P.J.; Rye, K.A. Cholesteryl ester transfer protein and its inhibitors. J. Lipid. Res. 2018, 59, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Howe, V.; Sharpe, L.J.; Prabhu, A.V.; Brown, A.J. New insights into cellular cholesterol acquisition: Promoter analysis of human HMGCR and SQLE, two key control enzymes in cholesterol synthesis. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2017, 1862, 647–657. [Google Scholar] [CrossRef]
- Brown, M.S.; Radhakrishnan, A.; Goldstein, J.L. Retrospective on Cholesterol Homeostasis: The Central Role of Scap. Annu. Rev. Biochem. 2018, 87, 783–807. [Google Scholar] [CrossRef]
- Goldstein, J.L.; DeBose-Boyd, R.A.; Brown, M.S. Protein sensors for membrane sterols. Cell 2006, 124, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Gelissen, I.C.; Brown, A.J. An Overview of Cholesterol Homeostasis. Methods Mol. Biol. 2017, 1583, 1–6. [Google Scholar]
- Redinger, R.N. Nuclear receptors in cholesterol catabolism: Molecular biology of the enterohepatic circulation of bile salts and its role in cholesterol homeostasis. J. Lab. Clin. Med. 2003, 142, 7–20. [Google Scholar] [CrossRef]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef]
- Cai, J.; Rimal, B.; Jiang, C.; Chiang, J.Y.L.; Patterson, A.D. Bile acid metabolism and signaling, the microbiota, and metabolic disease. Pharmacol. Ther. 2022, 237, 108238. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Wang, J.; Qi, W.; Miao, H.H.; Cao, J.; Qu, Y.X.; Li, B.L.; Song, B.L. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 2008, 7, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Raman, G.; Vishwanathan, R.; Jacques, P.F.; Johnson, E.J. Dietary cholesterol and cardiovascular disease: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2015, 102, 276–294. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, M.; Pisciotta, L.; Madeo, A.; Bertamino, M.; Bertolini, S. Long term substrate reduction therapy with ezetimibe alone or associated with statins in three adult patients with lysosomal acid lipase deficiency. Orphanet J. Rare Dis. 2018, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.R., Jr.; Zhu, L.J.; Hoos, L.M.; Tetzloff, G.; Maguire, M.; Liu, J.; Yao, X.; Iyer, S.P.; Lam, M.H.; Lund, E.G.; et al. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 2004, 279, 33586–33592. [Google Scholar] [CrossRef] [PubMed]
- Temel, R.E.; Tang, W.; Ma, Y.; Rudel, L.L.; Willingham, M.C.; Ioannou, Y.A.; Davies, J.P.; Nilsson, L.M.; Yu, L. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J. Clin. Investig. 2007, 117, 1968–1978. [Google Scholar] [CrossRef] [PubMed]
- Simonen, P.; Kotronen, A.; Hallikainen, M.; Sevastianova, K.; Makkonen, J.; Hakkarainen, A.; Lundbom, N.; Miettinen, T.A.; Gylling, H.; Yki-Jarvinen, H. Cholesterol synthesis is increased and absorption decreased in non-alcoholic fatty liver disease independent of obesity. J. Hepatol. 2011, 54, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.B.; Jang, K.; Jun, D.W.; Lee, B.H.; Shin, K.J. Expression of liver X receptor correlates with intrahepatic inflammation and fibrosis in patients with nonalcoholic fatty liver disease. Dig. Dis. Sci. 2014, 59, 2975–2982. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.B.; Jun, D.W.; Jang, K.; Lee, B.H.; Shin, K.J. Duodenal Niemann-Pick C1-like 1 expression was negatively correlated with liver X receptor expression in nonalcoholic fatty liver disease. Korean J. Intern. Med. 2019, 34, 777–784. [Google Scholar] [CrossRef]
- Toyoda, Y.; Takada, T.; Yamanashi, Y.; Suzuki, H. Pathophysiological importance of bile cholesterol reabsorption: Hepatic NPC1L1-exacerbated steatosis and decreasing VLDL-TG secretion in mice fed a high-fat diet. Lipids Health Dis. 2019, 18, 234. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, P.; Zhang, B.; Ding, Y.; Lei, S.; Hou, Y.; Guan, X.; Li, Q. Hepatic NPC1L1 overexpression attenuates alcoholic autophagy in mice. Mol. Med. Rep. 2019, 20, 3224–3232. [Google Scholar] [CrossRef]
- Yamanashi, Y.; Takada, T.; Tanaka, Y.; Ogata, Y.; Toyoda, Y.; Ito, S.M.; Kitani, M.; Oshida, N.; Okada, K.; Shoda, J.; et al. Hepatic Niemann-Pick C1-Like 1 exacerbates non-alcoholic fatty liver disease by re-absorbing specific biliary oxysterols. Biomed. Pharmacother. 2022, 156, 113877. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Han, D.H.; Nam, K.T.; Park, J.S.; Kim, S.H.; Lee, M.; Kim, G.; Min, B.S.; Cha, B.S.; Lee, Y.S.; et al. Ezetimibe, an NPC1L1 inhibitor, is a potent Nrf2 activator that protects mice from diet-induced nonalcoholic steatohepatitis. Free Radic. Biol. Med. 2016, 99, 520–532. [Google Scholar] [CrossRef]
- Fraunberger, P.; Gröne, E.; Gröne, H.J.; Drexel, H.; Walli, A.K. Ezetimibe reduces cholesterol content and NF-kappaB activation in liver but not in intestinal tissue in guinea pigs. J. Inflamm. 2017, 14, 3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, H.X.; Zhang, M.; Long, S.Y.; Tuo, Q.H.; Tian, Y.; Chen, J.X.; Zhang, C.P.; Liao, D.F. Cholesterol in LDL receptor recycling and degradation. Clin. Chim. Acta 2020, 500, 81–86. [Google Scholar] [CrossRef]
- Yu, Q.; Zheng, H.; Zhang, Y. Inducible degrader of LDLR: A potential novel therapeutic target and emerging treatment for hyperlipidemia. Vascul. Pharmacol. 2021, 140, 106878. [Google Scholar] [CrossRef]
- Huang, Y.W.; Wang, L.T.; Zhang, M.; Nie, Y.; Yang, J.B.; Meng, W.L.; Wang, X.J.; Sheng, J. Caffeine can alleviate non-alcoholic fatty liver disease by augmenting LDLR expression via targeting EGFR. Food Funct. 2023, 14, 3269–3278. [Google Scholar] [CrossRef]
- Wang, F.; Yao, W.; Yu, D.; Hao, Y.; Wu, Y.; Zhang, X. Protective role of thymoquinone in hyperlipidemia-induced liver injury in LDL-R(−/−)mice. BMC Gastroenterol. 2023, 23, 276. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Mahmood, S.; Morrice, N.; Kamli-Salino, S.; Dekeryte, R.; Hoffmann, P.A.; Doherty, M.K.; Whitfield, P.D.; Delibegović, M.; Mody, N. Fenretinide inhibits obesity and fatty liver disease but induces Smpd3 to increase serum ceramides and worsen atherosclerosis in LDLR(−/−) mice. Sci. Rep. 2023, 13, 3937. [Google Scholar] [CrossRef]
- Singh, A.B.; Kan, C.F.; Shende, V.; Dong, B.; Liu, J. A novel posttranscriptional mechanism for dietary cholesterol-mediated suppression of liver LDL receptor expression. J. Lipid Res. 2014, 55, 1397–1407. [Google Scholar] [CrossRef]
- He, Y.; Rodrigues, R.M.; Wang, X.; Seo, W.; Ma, J.; Hwang, S.; Fu, Y.; Trojnár, E.; Mátyás, C.; Zhao, S.; et al. Neutrophil-to-hepatocyte communication via LDLR-dependent miR-223-enriched extracellular vesicle transfer ameliorates nonalcoholic steatohepatitis. J. Clin. Investig. 2021, 131, e141513. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Lee, S.P.; Linsley, P.S.; Gersuk, V.; Yeh, M.M.; Chen, Y.Y.; Peng, Y.J.; Dutta, M.; Mascarinas, G.; Molla, B.; et al. Pcsk9 Deletion Promotes Murine Nonalcoholic Steatohepatitis and Hepatic Carcinogenesis: Role of Cholesterol. Hepatol. Commun. 2022, 6, 780–794. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, L.; Franceschini, G. Lecithin:cholesterol acyltransferase, high-density lipoproteins, and atheroprotection in humans. Trends Cardiovasc. Med. 2010, 20, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Tanigawa, H.; Billheimer, J.T.; Tohyama, J.; Fuki, I.V.; Ng, D.S.; Rothblat, G.H.; Rader, D.J. Lecithin: Cholesterol acyltransferase expression has minimal effects on macrophage reverse cholesterol transport in vivo. Circulation 2009, 120, 160–169. [Google Scholar] [CrossRef]
- Kuroda, M.; Bujo, H.; Yokote, K.; Murano, T.; Yamaguchi, T.; Ogura, M.; Ikewaki, K.; Koseki, M.; Takeuchi, Y.; Nakatsuka, A.; et al. Current Status of Familial LCAT Deficiency in Japan. J. Atheroscler. Thromb. 2021, 28, 679–691. [Google Scholar] [CrossRef]
- Janac, J.; Zeljkovic, A.; Jelic-Ivanovic, Z.; Dimitrijevic-Sreckovic, V.; Miljkovic, M.; Stefanovic, A.; Munjas, J.; Vekic, J.; Kotur-Stevuljevic, J.; Spasojević-Kalimanovska, V. The association between lecithin-cholesterol acyltransferase activity and fatty liver index. Ann. Clin. Biochem. 2019, 56, 583–592. [Google Scholar] [CrossRef]
- Nass, K.J.; van den Berg, E.H.; Gruppen, E.G.; Dullaart, R.P.F. Plasma lecithin:cholesterol acyltransferase and phospholipid transfer protein activity independently associate with nonalcoholic fatty liver disease. Eur. J. Clin. Investig. 2018, 48, e12988. [Google Scholar] [CrossRef] [PubMed]
- Bril, F.; Pearce, R.W.; Collier, T.S.; McPhaul, M.J. Differences in HDL-Bound Apolipoproteins in Patients with Advanced Liver Fibrosis Due to Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2022, 108, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; van der Tuin, S.; Tjeerdema, N.; van Dam, A.D.; Rensen, S.S.; Hendrikx, T.; Berbee, J.F.; Atanasovska, B.; Fu, J.; Hoekstra, M.; et al. Plasma cholesteryl ester transfer protein is predominantly derived from Kupffer cells. Hepatology 2015, 62, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Barkowski, R.S.; Frishman, W.H. HDL metabolism and CETP inhibition. Cardiol. Rev. 2008, 16, 154–162. [Google Scholar] [CrossRef]
- Doggrell, S.A. The failure of torcetrapib: Is there a case for independent preclinical and clinical testing? Expert Opin. Pharmacother. 2008, 9, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Rhainds, D.; Arsenault, B.J.; Brodeur, M.R.; Tardif, J.C. An update on the clinical development of dalcetrapib (RO4607381), a cholesteryl ester transfer protein modulator that increases HDL cholesterol levels. Future Cardiol. 2012, 8, 513–531. [Google Scholar] [CrossRef]
- Eyvazian, V.A.; Frishman, W.H. Evacetrapib: Another CETP Inhibitor for Dyslipidemia with No Clinical Benefit. Cardiol. Rev. 2017, 25, 43–52. [Google Scholar] [CrossRef]
- Nordestgaard, L.T.; Christoffersen, M.; Lauridsen, B.K.; Afzal, S.; Nordestgaard, B.G.; Frikke-Schmidt, R.; Tybjaerg-Hansen, A. Long-term Benefits and Harms Associated with Genetic Cholesteryl Ester Transfer Protein Deficiency in the General Population. JAMA Cardiol. 2022, 7, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Hemeda, S.A.; Albadrani, G.M.; Fadl, S.E.; Elgendey, F. Ameliorating effect of probiotic on nonalcoholic fatty liver disease and lipolytic gene expression in rabbits. Sci. Rep. 2023, 13, 6312. [Google Scholar] [CrossRef]
- Lucero, D.; Miksztowicz, V.; Gualano, G.; Longo, C.; Landeira, G.; Álvarez, E.; Zago, V.; Brites, F.; Berg, G.; Fassio, E.; et al. Nonalcoholic fatty liver disease associated with metabolic syndrome: Influence of liver fibrosis stages on characteristics of very low-density lipoproteins. Clin. Chim. Acta 2017, 473, 1–8. [Google Scholar] [CrossRef]
- Aller, R.; Izaola, O.; Primo, D.; de Luis, D. Cholesteryl Ester Transfer Protein Variant (RS1800777) with Liver Histology in Non-Alcoholic Fatty Liver Disease Patients. Ann. Nutr. Metab. 2018, 73, 265–270. [Google Scholar] [CrossRef]
- Perez-Robles, M.; Campos-Perez, W.; Rivera-Valdés, J.J.; Franco-Topete, R.A.; Navarrete-Medina, E.M.; Maldonado-González, M.; Ruíz-Madrigal, B.; Rodríguez-Reyes, S.C.; Martinez-Lopez, E. Elevated Serum Low-Density Lipoproteins-Cholesterol Levels and B1B2/B2B2 CETP Genotype Are Positively Associated with Nonalcoholic Fatty Liver Disease in Women with Gallstone Disease. Metab. Syndr. Relat. Disord. 2023, 21, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Liaw, Y.W.; Lin, C.Y.; Lai, Y.S.; Yang, T.C.; Wang, C.J.; Whang-Peng, J.; Liu, L.F.; Lin, C.P.; Nieh, S.; Lu, S.C.; et al. A vaccine targeted at CETP alleviates high fat and high cholesterol diet-induced atherosclerosis and non-alcoholic steatohepatitis in rabbit. PLoS ONE 2014, 9, e111529. [Google Scholar] [CrossRef] [PubMed]
- Rigotti, A.; Miettinen, H.E.; Krieger, M. The role of the high-density lipoprotein receptor SR-BI in the lipid metabolism of endocrine and other tissues. Endocr. Rev. 2003, 24, 357–387. [Google Scholar] [CrossRef]
- Knaack, D.A.; Chang, J.; Thomas, M.J.; Sorci-Thomas, M.G.; Chen, Y.; Sahoo, D. Scavenger receptor class B type I is required for efficient glucose uptake and metabolic homeostasis in adipocytes. bioRxiv 2023. [Google Scholar]
- Zago, V.H.S.; Scherrer, D.Z.; Parra, E.S.; Vieira, I.C.; Marson, F.A.L.; de Faria, E.C. Effects of SNVs in ABCA1, ABCG1, ABCG5, ABCG8, and SCARB1 Genes on Plasma Lipids, Lipoproteins, and Adiposity Markers in a Brazilian Population. Biochem. Genet. 2022, 60, 822–841. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, L.; Chen, J.; Song, H.; Xing, W.; Wang, Z.; Song, X.; Yang, H.; Zhao, W. Analysis of Intestinal Metabolites in SR-B1 Knockout Mice via Ultra-Performance Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry. Molecules 2023, 28, 610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Da Silva, J.R.; Reilly, M.; Billheimer, J.T.; Rothblat, G.H.; Rader, D.J. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J. Clin. Investig. 2005, 115, 2870–2874. [Google Scholar] [CrossRef] [PubMed]
- Xin, P.; Han, H.; Gao, D.; Cui, W.; Yang, X.; Ying, C.; Sun, X.; Hao, L. Alleviative effects of resveratrol on nonalcoholic fatty liver disease are associated with up regulation of hepatic low density lipoprotein receptor and scavenger receptor class B type I gene expressions in rats. Food Chem. Toxicol. 2013, 52, 12–18. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, S.; Chen, H.T.; Yu, C.H.; Teng, X.D.; Yao, H.T.; Xu, G.Q. Upregulation of caveolin-1 and SR-B1 in mice with non-alcoholic fatty liver disease. Hepatobiliary Pancreat. Dis. Int. 2013, 12, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Rein-Fischboeck, L.; Krautbauer, S.; Eisinger, K.; Pohl, R.; Meier, E.M.; Weiss, T.S.; Buechler, C. Hepatic scavenger receptor BI is associated with type 2 diabetes but unrelated to human and murine non-alcoholic fatty liver disease. Biochem. Biophys. Res. Commun. 2015, 467, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Soto-Acosta, R.; Bautista-Carbajal, P.; Cervantes-Salazar, M.; Angel-Ambrocio, A.H.; Del Angel, R.M. DENV up-regulates the HMG-CoA reductase activity through the impairment of AMPK phosphorylation: A potential antiviral target. PLoS Pathog. 2017, 13, e1006257. [Google Scholar] [CrossRef] [PubMed]
- Clarke, P.R.; Hardie, D.G. Regulation of HMG-CoA reductase: Identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 1990, 9, 2439–2446. [Google Scholar] [CrossRef]
- Song, B.L.; Javitt, N.B.; DeBose-Boyd, R.A. Insig-mediated degradation of HMG CoA reductase stimulated by lanosterol, an intermediate in the synthesis of cholesterol. Cell Metab. 2005, 1, 179–189. [Google Scholar] [CrossRef]
- Kerr, T.A.; Davidson, N.O. Cholesterol and nonalcoholic fatty liver disease: Renewed focus on an old villain. Hepatology 2012, 56, 1995–1998. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yan, L.T.; Yao, Z.; Xiong, G.Y. Biochanin A Regulates Cholesterol Metabolism Further Delays the Progression of Nonalcoholic Fatty Liver Disease. Diabetes Metab. Syndr. Obes. 2021, 14, 3161–3172. [Google Scholar] [CrossRef] [PubMed]
- Shatoor, A.S.; Al Humayed, S.; Almohiy, H.M. Astaxanthin attenuates hepatic steatosis in high-fat diet-fed rats by suppressing microRNA-21 via transactivation of nuclear factor erythroid 2-related factor 2. J. Physiol. Biochem. 2022, 78, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Cominguez, D.C.; Park, Y.J.; Kang, Y.M.; Nugroho, A.; Kim, S.; An, H.J. Clitorin ameliorates western diet-induced hepatic steatosis by regulating lipogenesis and fatty acid oxidation in vivo and in vitro. Sci. Rep. 2022, 12, 4154. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Li, Y.; Fu, Q.; Wang, J.; Lin, Y.; Qiu, L.; Ran, L.; Yang, J.; Yang, C. Bio-Assay-Guided Isolation of Fractions and Constituents with Antioxidant and Lipid-lowering Activity from Allium cepa. Antioxidants 2023, 12, 1448. [Google Scholar] [CrossRef] [PubMed]
- Poornima, M.S.; Sindhu, G.; Billu, A.; Sruthi, C.R.; Nisha, P.; Gogoi, P.; Baishya, G.; Raghu, K.G. Pretreatment of hydroethanolic extract of Dillenia indica L. attenuates oleic acid induced NAFLD in HepG2 cells via modulating SIRT-1/p-LKB-1/AMPK, HMGCR & PPAR-α signaling pathways. J. Ethnopharmacol. 2022, 292, 115237. [Google Scholar]
- Li, Z.; Zhou, Y.; Jia, K.; Yang, Y.; Zhang, L.; Wang, S.; Dong, Y.; Wang, M.; Li, Y.; Lu, S.; et al. JMJD4-demethylated RIG-I prevents hepatic steatosis and carcinogenesis. J. Hematol. Oncol. 2022, 15, 161. [Google Scholar] [CrossRef] [PubMed]
- Repa, J.J.; Berge, K.E.; Pomajzl, C.; Richardson, J.A.; Hobbs, H.; Mangelsdorf, D.J. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J. Biol. Chem. 2002, 277, 18793–18800. [Google Scholar] [CrossRef] [PubMed]
- Peet, D.J.; Turley, S.D.; Ma, W.; Janowski, B.A.; Lobaccaro, J.M.; Hammer, R.E.; Mangelsdorf, D.J. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell 1998, 93, 693–704. [Google Scholar] [CrossRef]
- Zhang, Y.; Breevoort, S.R.; Angdisen, J.; Fu, M.; Schmidt, D.R.; Holmstrom, S.R.; Kliewer, S.A.; Mangelsdorf, D.J.; Schulman, I.G. Liver LXRalpha expression is crucial for whole body cholesterol homeostasis and reverse cholesterol transport in mice. J. Clin. Investig. 2012, 122, 1688–1699. [Google Scholar] [CrossRef]
- Ai, Z.L.; Zhu, C.H.; Min, M.; Wang, J.; Lan, C.H.; Fan, L.L.; Sun, W.J.; Chen, D.F. The role of hepatic liver X receptor α- and sterol regulatory element binding protein-1c-mediated lipid disorder in the pathogenesis of non-alcoholic steatohepatitis in rats. .J. Int. Med. Res. 2011, 39, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Nakamuta, M.; Fujino, T.; Yada, R.; Yada, M.; Yasutake, K.; Yoshimoto, T.; Harada, N.; Higuchi, N.; Kato, M.; Kohjima, M.; et al. Impact of cholesterol metabolism and the LXRalpha-SREBP-1c pathway on nonalcoholic fatty liver disease. Int. J. Mol. Med. 2009, 23, 603–608. [Google Scholar] [PubMed]
- Salamone, F.; Li Volti, G.; Titta, L.; Puzzo, L.; Barbagallo, I.; La Delia, F.; Zelber-Sagi, S.; Malaguarnera, M.; Pelicci, P.G.; Giorgio, M.; et al. Moro orange juice prevents fatty liver in mice. World J. Gastroenterol. 2012, 18, 3862–3868. [Google Scholar] [CrossRef] [PubMed]
- Lima-Cabello, E.; García-Mediavilla, M.V.; Miquilena-Colina, M.E.; Vargas-Castrillón, J.; Lozano-Rodríguez, T.; Fernández-Bermejo, M.; Olcoz, J.L.; González-Gallego, J.; García-Monzón, C.; Sánchez-Campos, S. Enhanced expression of pro-inflammatory mediators and liver X-receptor-regulated lipogenic genes in non-alcoholic fatty liver disease and hepatitis C. Clin. Sci. 2011, 120, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Endo-Umeda, K.; Makishima, M. Liver X Receptors Regulate Cholesterol Metabolism and Immunity in Hepatic Nonparenchymal Cells. Int. J. Mol. Sci. 2019, 20, 5045. [Google Scholar] [CrossRef] [PubMed]
- Beigneux, A.; Hofmann, A.F.; Young, S.G. Human CYP7A1 deficiency: Progress and enigmas. J. Clin. Investig. 2002, 110, 29–31. [Google Scholar] [CrossRef]
- Jones, R.D.; Lopez, A.M.; Tong, E.Y.; Posey, K.S.; Chuang, J.C.; Repa, J.J.; Turley, S.D. Impact of physiological levels of chenodeoxycholic acid supplementation on intestinal and hepatic bile acid and cholesterol metabolism in Cyp7a1-deficient mice. Steroids 2015, 93, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, E.P.; Gutierrez, A.; Davis, R.A. Transgenic expression of CYP7A1 in LDL receptor-deficient mice blocks diet-induced hypercholesterolemia. J. Lipid Res. 2006, 47, 1513–1520. [Google Scholar] [CrossRef]
- Mouzaki, M.; Wang, A.Y.; Bandsma, R.; Comelli, E.M.; Arendt, B.M.; Zhang, L.; Fung, S.; Fischer, S.E.; McGilvray, I.G.; Allard, J.P. Bile Acids and Dysbiosis in Non-Alcoholic Fatty Liver Disease. PLoS ONE 2016, 11, e0151829. [Google Scholar] [CrossRef]
- Luo, Y.; Decato, B.E.; Charles, E.D.; Shevell, D.E.; McNaney, C.; Shipkova, P.; Apfel, A.; Tirucherai, G.S.; Sanyal, A.J. Pegbelfermin selectively reduces secondary bile acid concentrations in patients with non-alcoholic steatohepatitis. JHEP Rep. 2021, 4, 100392. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Lin, Y.; Wang, Q.; Li, Y.; Zhao, Y.; Chen, L.; Wu, Q.; Xu, C.; Zhou, C.; Sun, Y.; et al. Integrated Multichip Analysis Identifies Potential Key Genes in the Pathogenesis of Nonalcoholic Steatohepatitis. Front. Endocrinol. 2020, 11, 601745. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhao, W.; Yu, L.; Hu, X.; Zhao, Y.; Guo, Q.; Wang, X.; Wu, X. Dihydroflavonoids as Bioactive Components of Penthorum chinense, a Miao Ethnomedicine, against NAFLD through Bile Acid Metabolism Pathway. Chem. Biodivers. 2022, 19, e202200146. [Google Scholar] [CrossRef] [PubMed]
- Kouno, T.; Liu, X.; Zhao, H.; Kisseleva, T.; Cable, E.E.; Schnabl, B. Selective PPARδ agonist seladelpar suppresses bile acid synthesis by reducing hepatocyte CYP7A1 via the fibroblast growth factor 21 signaling pathway. J. Biol. Chem. 2022, 298, 102056. [Google Scholar] [CrossRef]
- Yu, L.; Lu, H.; Yang, X.; Li, R.; Shi, J.; Yu, Y.; Ma, C.; Sun, F.; Zhang, S.; Zhang, F. Diosgenin alleviates hypercholesterolemia via SRB1/CES-1/CYP7A1/FXR pathway in high-fat diet-fed rats. Toxicol. Appl. Pharmacol. 2021, 412, 115388. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Ferrell, J.M. Discovery of farnesoid X receptor and its role in bile acid metabolism. Mol. Cell. Endocrinol. 2022, 548, 111618. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; He, F.; Yan, X.; Xing, Y.; Lei, Y.; Gao, J.; He, M.; Li, D.; Bai, L.; Yuan, Z.; et al. Hepatic Reduction in Cholesterol 25-Hydroxylase Aggravates Diet-induced Steatosis. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 1161–1179. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Liu, W.; Liang, B.; Shi, L.; Yang, S.; Meng, J.; Chang, J.; Hu, X.; Zhang, R.; Xing, D. Inhibitory Effect of Isoliquiritigenin in Niemann-Pick C1-Like 1-Mediated Cholesterol Uptake. Molecules 2022, 27, 7494. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Chen, J.; Zhang, T.; Yuan, X.; Ge, A.; Wang, S.; Xu, H.; Zeng, L.; Ge, J. Efficacy and safety of dietary polyphenol supplementation in the treatment of non-alcoholic fatty liver disease: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 949746. [Google Scholar]
- Feng, D.; Ohlsson, L.; Duan, R.D. Curcumin inhibits cholesterol uptake in Caco-2 cells by down-regulation of NPC1L1 expression. Lipids Health Dis. 2010, 9, 40. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, S.; Li, P.; Zheng, X.; Feng, D. Supplementation with curcumin inhibits intestinal cholesterol absorption and prevents atherosclerosis in high-fat diet-fed apolipoprotein E knockout mice. Nutr. Res. 2018, 56, 32–40. [Google Scholar] [CrossRef]
- Meng, J.; Xu, J.; Yang, S.; Liu, W.; Zeng, J.; Shi, L.; Chang, J.; Zhang, R.; Xing, D. Emodin lows NPC1L1-mediated cholesterol absorption as an uncompetitive inhibitor. Bioorg. Med. Chem. Lett. 2022, 75, 128974. [Google Scholar] [CrossRef]
- Tavares, T.B.; Santos, I.B.; de Bem, G.F.; Ognibene, D.T.; da Rocha, A.P.M.; de Moura, R.S.; Resende, A.C.; Daleprane, J.B.; da Costa, C.A. Therapeutic effects of açaí seed extract on hepatic steatosis in high-fat diet-induced obesity in male mice: A comparative effect with rosuvastatin. J. Pharm. Pharmacol. 2020, 72, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Park, J.M.; Lee, J.; Oh, B.C.; Jang, S.H.; Lee, Y.B.; Han, Y.M.; Ock, C.Y.; Cha, J.Y.; Hahm, K.B. Amelioration of non-alcoholic fatty liver disease with NPC1L1-targeted IgY or n-3 polyunsaturated fatty acids in mice. Metabolism 2017, 66, 32–44. [Google Scholar] [CrossRef]
- Wang, D.; Tan, K.S.; Zeng, W.; Li, S.; Wang, Y.; Xu, F.; Tan, W. Hepatocellular BChE as a therapeutic target to ameliorate hypercholesterolemia through PRMT5 selective degradation to restore LDL receptor transcription. Life Sci. 2022, 293, 120336. [Google Scholar] [CrossRef] [PubMed]
- Abbasi-Oshaghi, E.; Khodadadi, I.; Tavilani, H.; Mirzaei, F.; Goodarzi, M.T. Dill-normalized liver lipid accumulation, oxidative stress, and low-density lipoprotein receptor levels in high cholesterol fed hamsters. ARYA Atheroscler. 2018, 14, 218–224. [Google Scholar]
- Chen, P.; Li, Y.; Xiao, L. Berberine ameliorates nonalcoholic fatty liver disease by decreasing the liver lipid content via reversing the abnormal expression of MTTP and LDLR. Exp. Ther. Med. 2021, 22, 1109. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Wei, J.; Abidi, P.; Lin, M.; Inaba, S.; Li, C.; Wang, Y.; Wang, Z.; Si, S.; Pan, H.; et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 2004, 10, 1344–1351. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, G.; Wang, Y.; Yin, J.; Wang, J.; Xia, B.; Li, T.; Yang, X.; Hou, P.; Hu, S.; et al. Fucoidan A2 from the Brown Seaweed Ascophyllum nodosum Lowers Lipid by Improving Reverse Cholesterol Transport in C57BL/6J Mice Fed a High-Fat Diet. J. Agric. Food. Chem. 2019, 67, 5782–5791. [Google Scholar] [CrossRef] [PubMed]
- Musolino, V.; Gliozzi, M.; Nucera, S.; Carresi, C.; Maiuolo, J.; Mollace, R.; Paone, S.; Bosco, F.; Scarano, F.; Scicchitano, M.; et al. The effect of bergamot polyphenolic fraction on lipid transfer protein system and vascular oxidative stress in a rat model of hyperlipemia. Lipids Health Dis. 2019, 18, 115. [Google Scholar] [CrossRef]
- Elseweidy, M.M.; Younis, N.N.; Elswefy, S.E.; Abdallah, F.R.; El-Dahmy, S.I.; Elnagar, G.; Kassem, H.M. Atheroprotective potentials of curcuminoids against ginger extract in hypercholesterolaemic rabbits. Nat. Prod. Res. 2015, 29, 961–965. [Google Scholar] [CrossRef]
- Indu, M.S.; Narayanankutty, A.; Ramavarma, S.K.; Manalil, J.J.; Padikkala, J.; Raghavamenon, A.C. Desmodium gyrans dc modulates lipid trafficking in cultured macrophages and improves functional high-density lipoprotein in male wistar rats. Indian J. Pharmacol. 2021, 53, 286–293. [Google Scholar] [PubMed]
- Lee, K.H.; Jeong, E.S.; Jang, G.; Na, J.R.; Park, S.; Kang, W.S.; Kim, E.; Choi, H.; Kim, J.S.; Kim, S. Unripe Rubus coreanus Miquel Extract Containing Ellagic Acid Regulates AMPK, SREBP-2, HMGCR, and INSIG-1 Signaling and Cholesterol Metabolism In Vitro and In Vivo. Nutrients 2020, 12, 610. [Google Scholar] [CrossRef]
- Tang, L.; Kuang, C.; Shan, D.; Shi, M.; Li, J.; Qiu, L.; Yu, J. The ethanol extract of Edgeworthia gardneri (Wall.) Meisn attenuates macrophage foam cell formation and atherogenesis in ApoE(−/−) mice. Front. Cardiovasc. Med. 2022, 9, 1023438. [Google Scholar] [CrossRef]
- Yi, S.; Chen, K.; Sakao, K.; Ikenaga, M.; Wang, Y.; Hou, D.X. Assessment of Areca Nut Bioactivities in Western Diet-Induced Mice NAFLD Model. Nutrients 2023, 15, 2403. [Google Scholar] [CrossRef]
- Tandrasasmita, O.M.; Berlian, G.; Tjandrawinata, R.R. Molecular mechanism of DLBS3733, a bioactive fraction of Lagerstroemia speciosa (L.) Pers., on ameliorating hepatic lipid accumulation in HepG2 cells. Biomed. Pharmacother. 2021, 141, 111937. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Z.; Jiang, W.; Zhu, Y.Y.; Wang, C.Z.; Zhong, W.H.; Wu, G.; Chen, J.; Zhu, M.N.; Wu, Q.L.; Du, X.L.; et al. Highland barley Monascus purpureus Went extract ameliorates high-fat, high-fructose, high-cholesterol diet induced nonalcoholic fatty liver disease by regulating lipid metabolism in golden hamsters. J. Ethnopharmacol. 2022, 286, 114922. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xu, J.; Chen, Q.; Liu, M.; Wang, S.; Yu, H.; Zhang, Y.; Wang, T. Regulation effects of total flavonoids in Morus alba L. on hepatic cholesterol disorders in orotic acid induced NAFLD rats. BMC Complement. Med. Ther. 2020, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.Y.; Chen, P.Y.; Hsu, H.J.; Lin, C.Y.; Wu, M.J.; Yen, J.H. Tanshinone IIA Downregulates Lipogenic Gene Expression and Attenuates Lipid Accumulation through the Modulation of LXRα/SREBP1 Pathway in HepG2 Cells. Biomedicines 2021, 9, 326. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, J.Y.; Jhin, C.; Shin, J.M.; Kim, M.; Ahn, H.R.; Yoo, G.; Son, Y.J.; Jung, S.H.; Nho, C.W. Reduction of Hepatic Lipogenesis by Loliolide and Pinoresinol from Lysimachia vulgaris via Degrading Liver X Receptors. J. Agric. Food. Chem. 2019, 67, 12419–12427. [Google Scholar] [CrossRef]
- Yin, Y.; Gao, L.; Lin, H.; Wu, Y.; Han, X.; Zhu, Y.; Li, J. Luteolin improves non-alcoholic fatty liver disease in db/db mice by inhibition of liver X receptor activation to down-regulate expression of sterol regulatory element binding protein 1c. Biochem. Biophys. Res. Commun. 2017, 482, 720–726. [Google Scholar] [CrossRef]
- Wang, C.; Ma, C.; Gong, L.; Dai, S.; Li, Y. Preventive and therapeutic role of betaine in liver disease: A review on molecular mechanisms. Eur. J. Pharmacol. 2021, 912, 174604. [Google Scholar] [CrossRef] [PubMed]
- Shiragannavar, V.D.; Sannappa Gowda, N.G.; Puttahanumantharayappa, L.D.; Karunakara, S.H.; Bhat, S.; Prasad, S.K.; Kumar, D.P.; Santhekadur, P.K. The ameliorating effect of withaferin A on high-fat diet-induced non-alcoholic fatty liver disease by acting as an LXR/FXR dual receptor activator. Front. Pharmacol. 2023, 14, 1135952. [Google Scholar] [CrossRef]
- Munkong, N.; Somnuk, S.; Jantarach, N.; Ruxsanawet, K.; Nuntaboon, P.; Kanjoo, V.; Yoysungnoen, B. Red Rice Bran Extract Alleviates High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease and Dyslipidemia in Mice. Nutrients 2023, 15, 246. [Google Scholar] [CrossRef]
- Tai, T.S.; Tien, N.; Shen, H.Y.; Chu, F.Y.; Wang, C.C.N.; Lu, C.H.; Yu, H.I.; Kung, F.P.; Chuang, H.H.; Lee, Y.R.; et al. Sesamin, a Naturally Occurring Lignan, Inhibits Ligand-Induced Lipogenesis through Interaction with Liver X Receptor Alpha (LXRα) and Pregnane X Receptor (PXR). Evid. Based Complement. Altern. Med. 2019, 2019, 9401648. [Google Scholar] [CrossRef]
- Chen, Y.J.; Song, H.Y.; Zhang, Z.W.; Chen, Q.; Tang, Z.P.; Gu, M. Extracts of Vine Tea Improve Diet-Induced Non-Alcoholic Steatohepatitis Through AMPK-LXRα Signaling. Front. Pharmacol. 2021, 12, 711763. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Liang, S.; Liu, Q.; Deng, Z.; Zhang, Y.; Du, J.; Zhang, Y.; Li, S.; Cheng, B.; Ling, C. Diosgenin prevents high-fat diet-induced rat non-alcoholic fatty liver disease through the AMPK and LXR signaling pathways. Int. J. Mol. Med. 2018, 41, 1089–1095. [Google Scholar] [CrossRef]
- Vitaglione, P.; Mazzone, G.; Lembo, V.; D‘Argenio, G.; Rossi, A.; Guido, M.; Savoia, M.; Salomone, F.; Mennella, I.; De Filippis, F.; et al. Coffee prevents fatty liver disease induced by a high-fat diet by modulating pathways of the gut-liver axis. J. Nutr. Sci. 2019, 8, e15. [Google Scholar] [CrossRef]
- Xu, M.; Yang, L.; Zhu, Y.; Liao, M.; Chu, L.; Li, X.; Lin, L.; Zheng, G. Collaborative effects of chlorogenic acid and caffeine on lipid metabolism via the AMPKα-LXRα/SREBP-1c pathway in high-fat diet-induced obese mice. Food Funct. 2019, 10, 7489–7497. [Google Scholar] [CrossRef] [PubMed]
- Yarahmadi, S.; Farahmandian, N.; Fadaei, R.; Koushki, M.; Bahreini, E.; Karima, S.; Barzin Tond, S.; Rezaei, A.; Nourbakhsh, M.; Fallah, S. Therapeutic Potential of Resveratrol and Atorvastatin Following High-Fat Diet Uptake-Induced Nonalcoholic Fatty Liver Disease by Targeting Genes Involved in Cholesterol Metabolism and miR33. DNA Cell Biol. 2023, 42, 82–90. [Google Scholar] [CrossRef]
- Jeong, M.J.; Kim, S.R.; Jung, U.J. Schizandrin A supplementation improves nonalcoholic fatty liver disease in mice fed a high-fat and high-cholesterol diet. Nutr. Res. 2019, 64, 64–71. [Google Scholar] [CrossRef]
- Kang, M.; Kim, E.H.; Jeong, J.; Ha, H. Heukcha, naturally post-fermented green tea extract, ameliorates diet-induced hypercholesterolemia and NAFLD in hamster. J. Food Sci. 2021, 86, 5016–5025. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, Y.; Cao, X.; Peng, Y.; Huang, J.; Chen, L.; Pang, J.; Jiang, Z.; Qian, S.; Liu, Y.; et al. Targeting mTOR/YY1 signaling pathway by quercetin through CYP7A1-mediated cholesterol-to-bile acids conversion alleviated type 2 diabetes mellitus induced hepatic lipid accumulation. Phytomedicine 2023, 113, 154703. [Google Scholar] [CrossRef]
- Yang, C.; Yang, L.; Yang, Y.; Wan, M.; Xu, D.; Pan, D.; Sun, G. Effects of flaxseed powder in improving non-alcoholic fatty liver by regulating gut microbiota-bile acids metabolic pathway through FXR/TGR5 mediating. Biomed. Pharmacother. 2023, 163, 114864. [Google Scholar] [CrossRef]
- Zhao, K.; Qiu, L.; He, Y.; Tao, X.; Zhang, Z.; Wei, H. Alleviation Syndrome of High-Cholesterol-Diet-Induced Hypercholesterolemia in Mice by Intervention with Lactiplantibacillus plantarum WLPL21 via Regulation of Cholesterol Metabolism and Transportation as Well as Gut Microbiota. Nutrients 2023, 15, 2600. [Google Scholar] [CrossRef]
- Mu, J.; Tan, F.; Zhou, X.; Zhao, X. Lactobacillus fermentum CQPC06 in naturally fermented pickles prevents non-alcoholic fatty liver disease by stabilizing the gut-liver axis in mice. Food Funct. 2020, 11, 8707–8723. [Google Scholar] [CrossRef]
- Vajdi, M.; Hassanizadeh, S.; Hassanizadeh, R.; Bagherniya, M. Curcumin supplementation effect on liver enzymes in patients with nonalcoholic fatty liver disease: A GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Nutr. Rev. 2024. [Google Scholar] [CrossRef]
- Ebrahimzadeh, A.; Mohseni, S.; Safargar, M.; Mohtashamian, A.; Niknam, S.; Bakhoda, M.; Afshari, S.; Jafari, A.; Ebrahimzadeh, A.; Fooladshekan, S.; et al. Curcumin effects on glycaemic indices, lipid profile, blood pressure, inflammatory markers and anthropometric measurements of non-alcoholic fatty liver disease patients: A systematic review and meta-analysis of randomized clinical trials. Complement. Ther. Med. 2024, 80, 103025. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Malik, M. Effects of curcumin in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. Can. Liver J. 2024, 7, 299–315. [Google Scholar] [CrossRef]
- Jazayeri-Tehrani, S.A.; Rezayat, S.M.; Mansouri, S.; Qorbani, M.; Alavian, S.M.; Daneshi-Maskooni, M.; Hosseinzadeh-Attar, M.J. Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): A double-blind randomized placebo-controlled clinical trial. Nutr. Metab. 2019, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of Non-alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-controlled Trial. Phytother. Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef]
- Nie, Q.; Li, M.; Huang, C.; Yuan, Y.; Liang, Q.; Ma, X.; Qiu, T.; Li, J. The clinical efficacy and safety of berberine in the treatment of non-alcoholic fatty liver disease: A meta-analysis and systematic review. J. Transl. Med. 2024, 22, 225. [Google Scholar] [CrossRef]
- Afsharinasab, M.; Mohammad-Sadeghipour, M.; Reza Hajizadeh, M.; Khoshdel, A.; Mirzaiey, V.; Mahmoodi, M. The effect of hydroalcoholic Berberis integerrima fruits extract on the lipid profile, antioxidant parameters and liver and kidney function tests in patients with nonalcoholic fatty liver disease. Saudi J. Biol. Sci. 2020, 27, 2031–2037. [Google Scholar] [CrossRef]
- Yan, H.M.; Xia, M.F.; Wang, Y.; Chang, X.X.; Yao, X.Z.; Rao, S.X.; Zeng, M.S.; Tu, Y.F.; Feng, R.; Jia, W.P.; et al. Efficacy of Berberine in Patients with Non-Alcoholic Fatty Liver Disease. PLoS ONE 2015, 10, e0134172. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Skonieczna-Żydecka, K.; Kałduńska, J.; Stachowska, E.; Gutowska, I.; Janda, K. Effects of Resveratrol Supplementation in Patients with Non-Alcoholic Fatty Liver Disease-A Meta-Analysis. Nutrients 2020, 12, 2435. [Google Scholar] [CrossRef]
- Wei, S.; Yu, X. Efficacy of resveratrol supplementation on liver enzymes in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. Complement. Ther. Med. 2021, 57, 102635. [Google Scholar] [CrossRef] [PubMed]
- Farzin, L.; Asghari, S.; Rafraf, M.; Asghari-Jafarabadi, M.; Shirmohammadi, M. No beneficial effects of resveratrol supplementation on atherogenic risk factors in patients with nonalcoholic fatty liver disease. Int. J. Vitam. Nutr. Res. 2020, 90, 279–289. [Google Scholar]
- Faghihzadeh, F.; Adibi, P.; Rafiei, R.; Hekmatdoost, A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr. Res. 2014, 34, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Kravchenko, L.; Unhurian, L.; Tiuzhinska, S.K.; Ivanova, Y.; Obrazenko, M.; Zahorodnya, L.; Yamilova, T. Increasing the efficiency of hypolipidemic therapy with the combined use of quercetin in patients with non-alcoholic fatty liver disease on the background of the metabolic syndrome. Ceska Slov. Farm. 2024, 72, 297–303. [Google Scholar] [PubMed]
- Yari, Z.; Cheraghpour, M.; Alavian, S.M.; Hedayati, M.; Eini-Zinab, H.; Hekmatdoost, A. The efficacy of flaxseed and hesperidin on non-alcoholic fatty liver disease: An open-labeled randomized controlled trial. Eur. J. Clin. Nutr. 2021, 75, 99–111. [Google Scholar] [CrossRef]
- Koperska, A.; Moszak, M.; Seraszek-Jaros, A.; Bogdanski, P.; Szulinska, M. Does berberine impact anthropometric, hepatic, and metabolic parameters in patients with metabolic dysfunction-associated fatty liver disease? Randomized, double-blind placebo-controlled trial. J. Physiol. Pharmacol. 2024, 75, 291–302. [Google Scholar]
- Safari, Z.; Bagherniya, M.; Khoram, Z.; Ebrahimi Varzaneh, A.; Heidari, Z.; Sahebkar, A.; Askari, G. The effect of curcumin on anthropometric indices, blood pressure, lipid profiles, fasting blood glucose, liver enzymes, fibrosis, and steatosis in non-alcoholic fatty livers. Front. Nutr. 2023, 10, 1163950. [Google Scholar] [CrossRef] [PubMed]
- Beheshti Namdar, A.; Ahadi, M.; Hoseini, S.M.; Vosoghinia, H.; Rajablou, H.; Farsi, S.; Zangouei, A.; Rahimi, H.R. Effect of nano-micelle curcumin on hepatic enzymes: A new treatment approach for non-alcoholic fatty liver disease (NAFLD). Avicenna J. Phytomed. 2023, 13, 615–625. [Google Scholar] [PubMed]
- Mirhafez, S.R.; Rezai, A.; Dehabeh, M.; Nobakht, M.G.B.F.; Bidkhori, M.; Sahebkar, A.; Hariri, M. Efficacy of phytosomal curcumin among patients with non-alcoholic fatty liver disease. Int. J. Vitam. Nutr. Res. 2021, 91, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Mirhafez, S.R.; Dehabeh, M.; Hariri, M.; Farimani, A.R.; Movahedi, A.; Naderan, R.D.; Jamialahmadi, T.; Simental-Mendía, L.E.; Sahebkar, A. Curcumin and Piperine Combination for the Treatment of Patients with Non-alcoholic Fatty Liver Disease: A Double-Blind Randomized Placebo-Controlled Trial. Adv. Exp. Med. Biol. 2021, 1328, 11–19. [Google Scholar]
- Saadati, S.; Hatami, B.; Yari, Z.; Shahrbaf, M.A.; Eghtesad, S.; Mansour, A.; Poustchi, H.; Hedayati, M.; Aghajanpoor-Pasha, M.; Sadeghi, A.; et al. The effects of curcumin supplementation on liver enzymes, lipid profile, glucose homeostasis, and hepatic steatosis and fibrosis in patients with non-alcoholic fatty liver disease. Eur. J. Clin. Nutr. 2019, 73, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Valizadegan, G.; Ahamdi, N.; Ganjali, S.; Majeed, M.; Sahebkar, A. Curcuminoids plus piperine improve nonalcoholic fatty liver disease: A clinical trial. J. Cell. Biochem. 2019, 120, 15989–15996. [Google Scholar] [CrossRef]
- Mansour, A.; Mohajeri-Tehrani, M.R.; Karimi, S.; Sanginabadi, M.; Poustchi, H.; Enayati, S.; Asgarbeik, S.; Nasrollahzadeh, J.; Hekmatdoost, A. Short term effects of coffee components consumption on gut microbiota in patients with non-alcoholic fatty liver and diabetes: A pilot randomized placebo-controlled, clinical trial. EXCLI J. 2020, 19, 241–250. [Google Scholar]
- Theodotou, M.; Fokianos, K.; Moniatis, D.; Kadlenic, R.; Chrysikou, A.; Aristotelous, A.; Mouzouridou, A.; Diakides, J.; Stavrou, E. Effect of resveratrol on non-alcoholic fatty liver disease. Exp. Ther. Med. 2019, 18, 559–565. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).