Antioxidative and Antiglycative Stress Activities of Selenoglutathione Diselenide
Abstract
1. Introduction
2. Results and Discussion
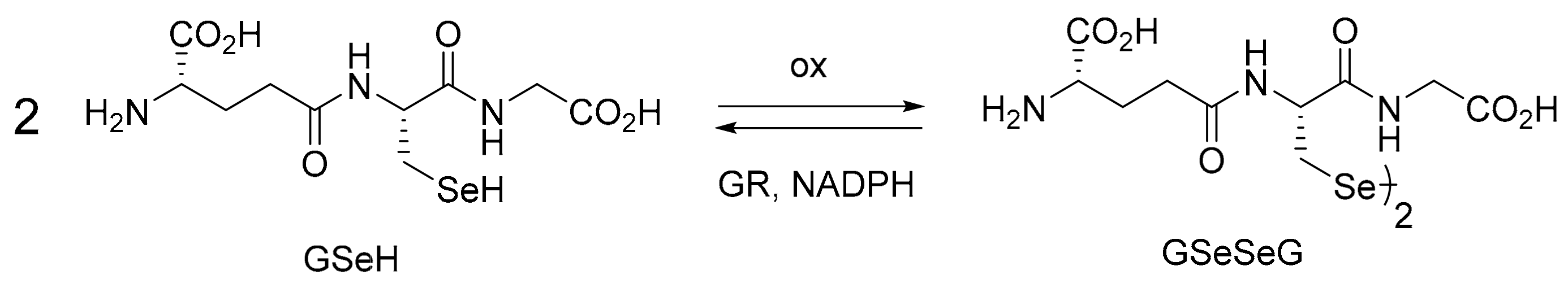
2.1. GPx-like Antioxidant Activity of GSeSeG
2.2. Glyoxalase 1 (GLO 1)-like Antiglycative Stress Activity of GSeSeG
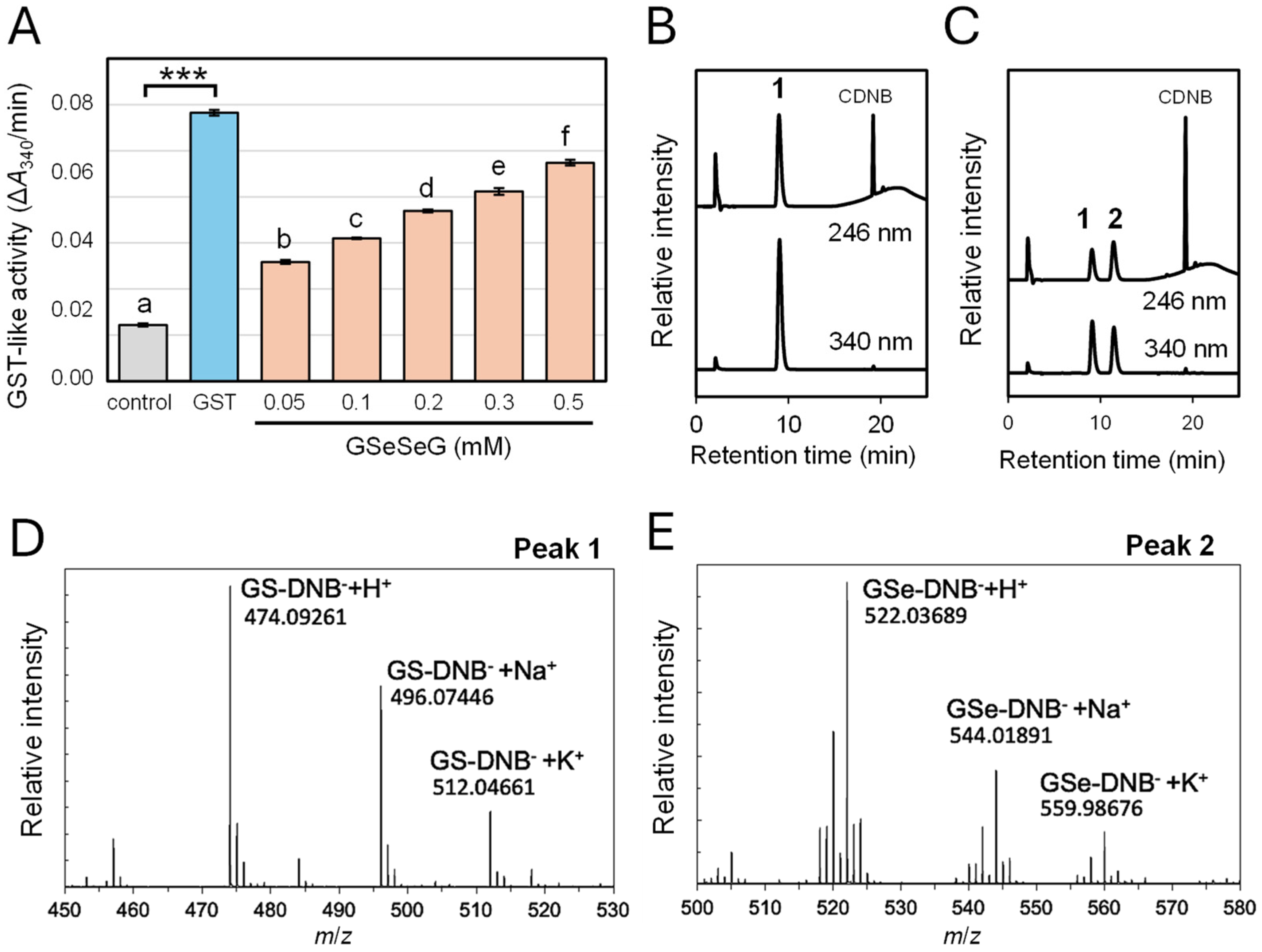
2.3. Glutathione S-Transferase-like Activity of GSeSeG
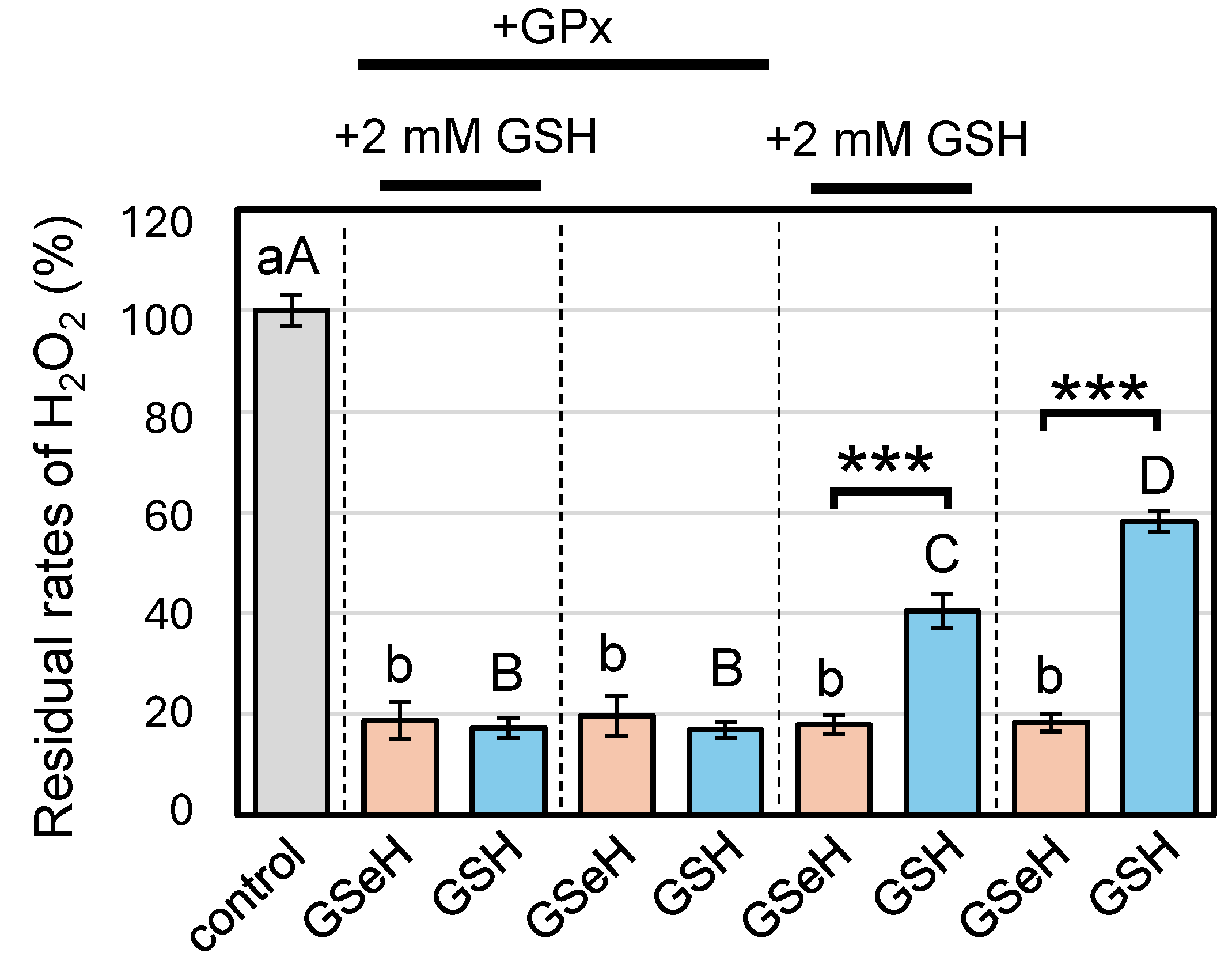
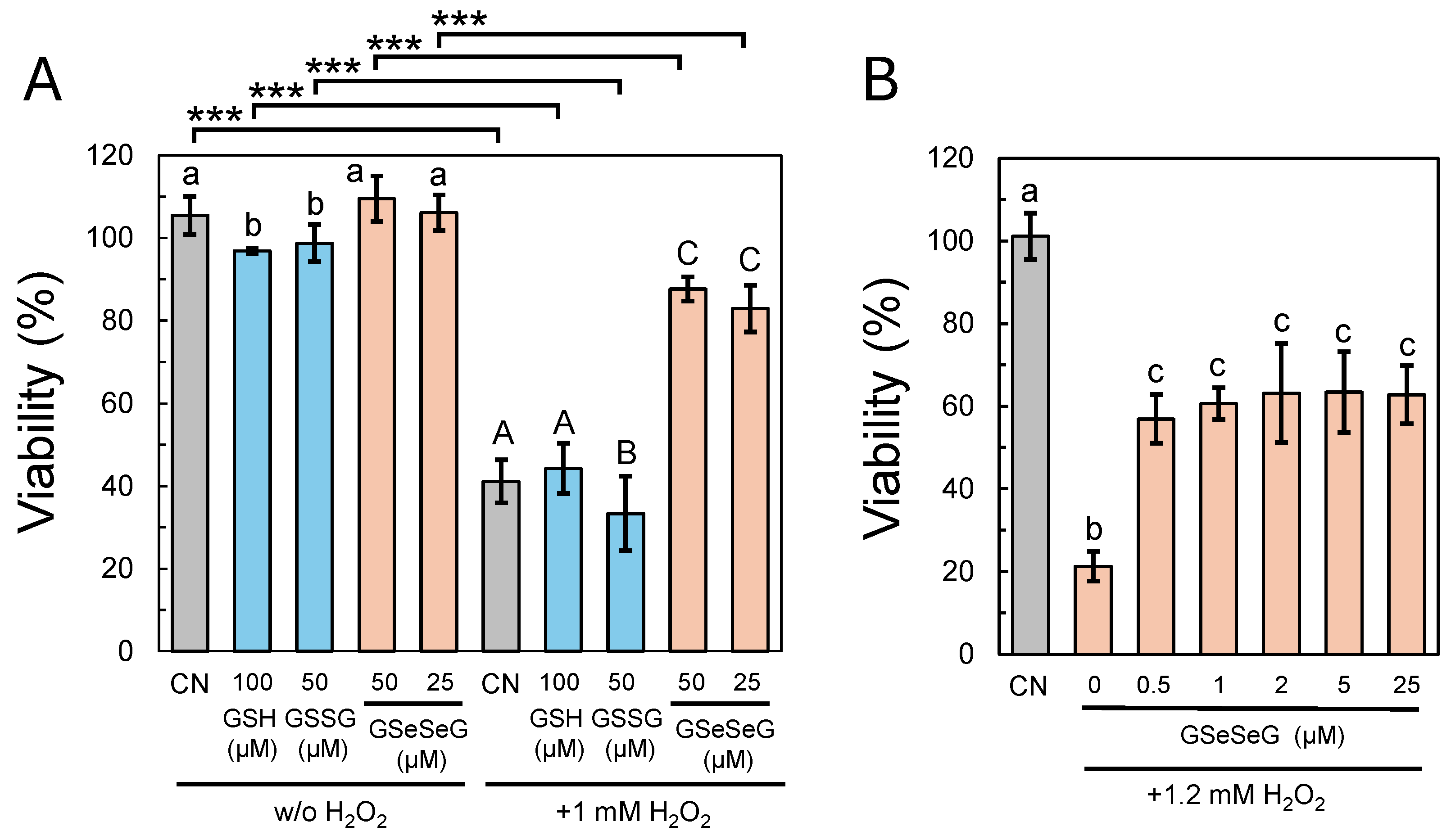
2.4. Effects of GSeSeG on Cell Viability against Oxidative Stress Caused by H2O2
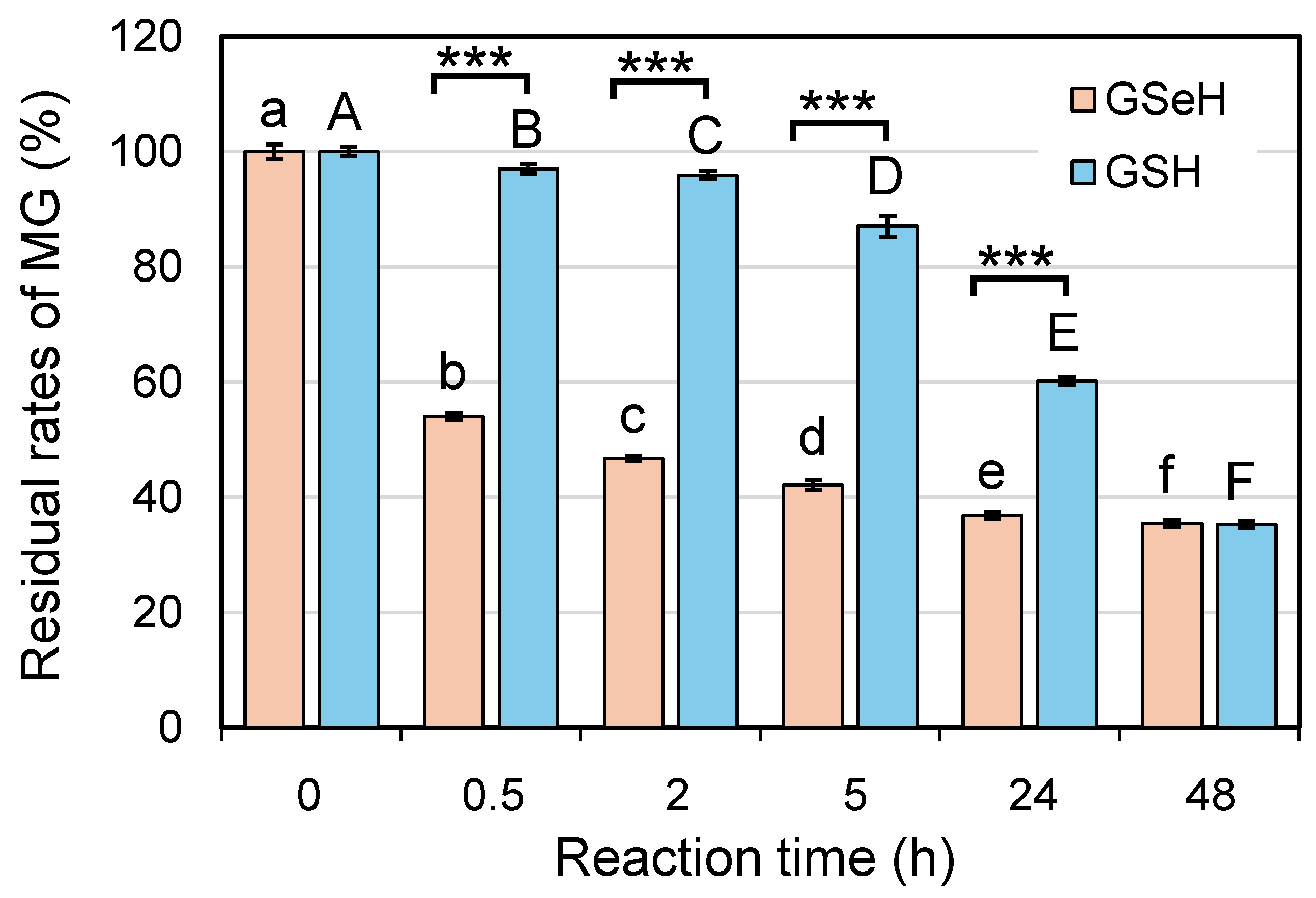
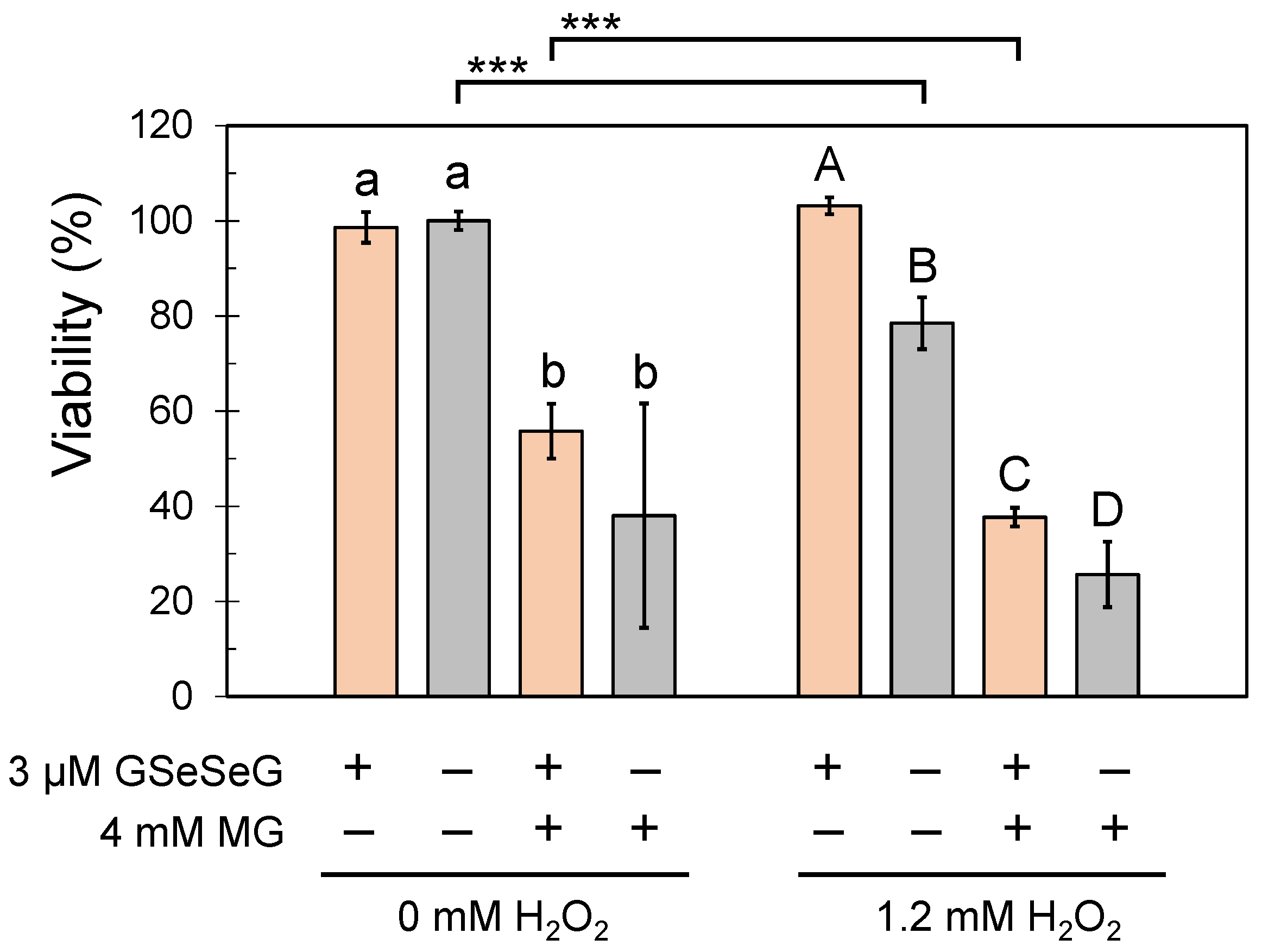
2.5. Effects of GSeSeG on Cell Viability against Glycative Stress Caused by MG and the Continuation of the Effects
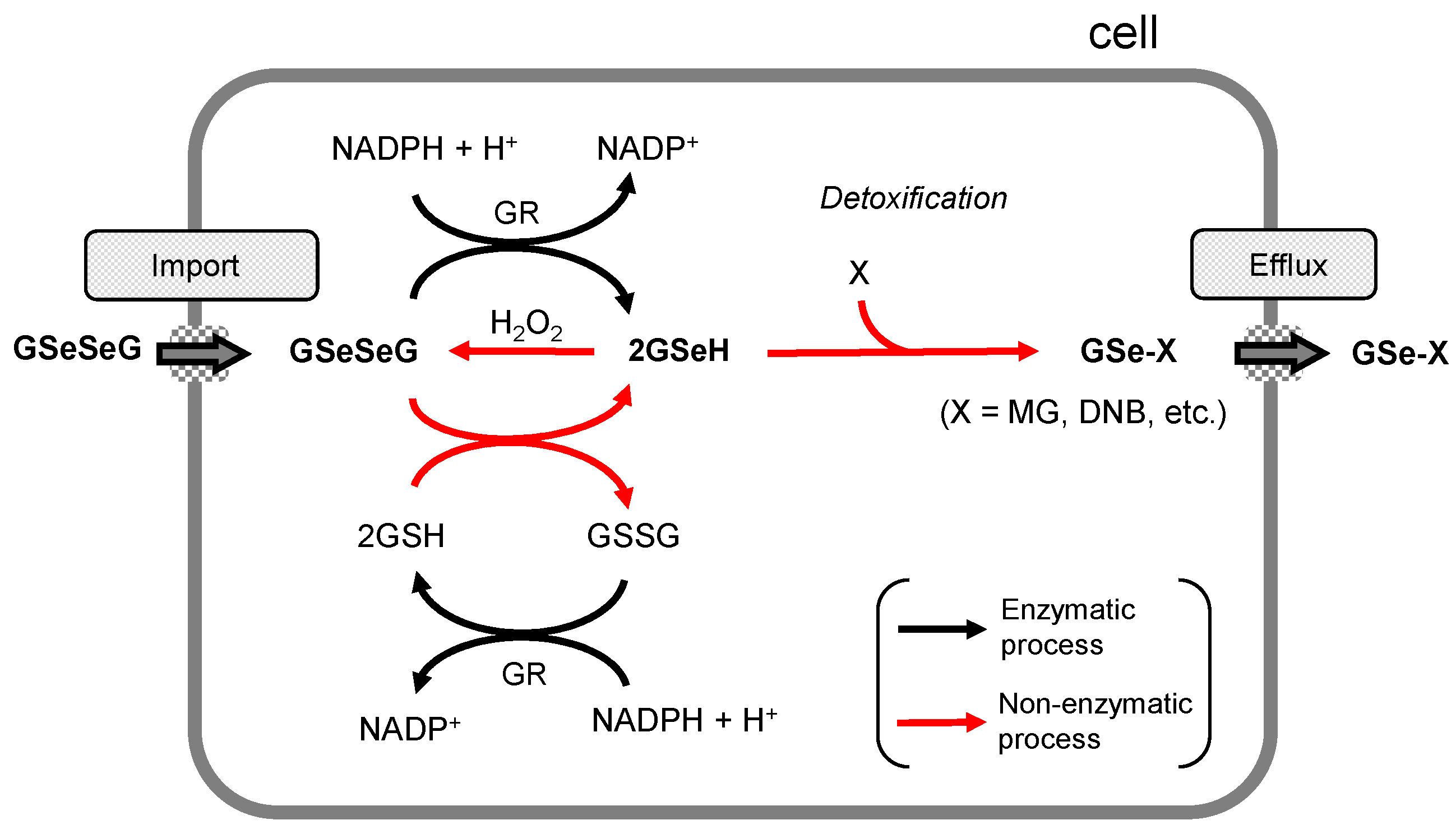
2.6. Detoxification Functions of GSeSeG in Cells
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of 1 mM GSeH in PBS from GSeSeG by Treatment with GR and NADPH
3.3. Determination of Glutathione Peroxidase (GPx)-like Antioxidant Activity
3.4. Determination of Glyoxalase 1 (GLO 1)-like Antiglycative Stress Activity
3.5. Determination of Glutathione S-Transferase (GST)-like Activity
3.6. Cell Cultures
3.7. Cell Viability Assays
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jo-Watanabe, A.; Ohse, T.; Nishimatsu, H.; Takahashi, M.; Ikeda, Y.; Wada, T.; Shirakawa, J.; Nagai, R.; Miyata, T.; Nagano, T.; et al. Glyoxalase I Reduces Glycative and Oxidative Stress and Prevents Age-Related Endothelial Dysfunction through Modulation of Endothelial Nitric Oxide Synthase Phosphorylation. Aging Cell 2014, 13, 519–528. [Google Scholar] [CrossRef]
- Vašková, J.; Kočan, L.; Vaško, L.; Perjési, P. Glutathione-Related Enzymes and Proteins: A Review. Molecules 2023, 28, 1447. [Google Scholar] [CrossRef] [PubMed]
- Georgiou-Siafis, S.K.; Tsiftsoglou, A.S. The Key Role of GSH in Keeping the Redox Balance in Mammalian Cells: Mechanisms and Significance of GSH in Detoxification via Formation of Conjugates. Antioxidants 2023, 12, 1953. [Google Scholar] [CrossRef] [PubMed]
- Couto, N.; Wood, J.; Barber, J. The Role of Glutathione Reductase and Related Enzymes on Cellular Redox Homoeostasis Network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, D.A.; Forman, H.J. Cellular Glutathione and Thiols Metabolism. Biochem. Pharmacol. 2002, 64, 1019–1026. [Google Scholar] [CrossRef]
- He, Y.; Zhou, C.; Huang, M.; Tang, C.; Liu, X.; Yue, Y.; Diao, Q.; Zheng, Z.; Liu, D. Glyoxalase System: A Systematic Review of Its Biological Activity, Related-Diseases, Screening Methods and Small Molecule Regulators. Biomed. Pharmacother. 2020, 131, 110663. [Google Scholar] [CrossRef]
- Suravajjala, S.; Cohenford, M.; Frost, L.R.; Pampati, P.K.; Dain, J.A. Glycation of Human Erythrocyte Glutathione Peroxidase: Effect on the Physical and Kinetic Properties. Clin. Chim. Acta 2013, 421, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Allocati, N.; Masulli, M.; Di Ilio, C.; Federici, L. Glutathione Transferases: Substrates, Inihibitors and pro-Drugs in Cancer and Neurodegenerative Diseases. Oncogenesis 2018, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; McCooeye, M.; Windust, A.; Bramanti, E.; D’Ulivo, A.; Mester, Z. Mapping of Selenium Metabolic Pathway in Yeast by Liquid Chromatography-Orbitrap Mass Spectrometry. Anal. Chem. 2010, 82, 8121–8130. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Kumakura, F.; Komatsu, I.; Arai, K.; Onuma, Y.; Hojo, H.; Singh, B.G.; Priyadarsini, K.I.; Iwaoka, M. Antioxidative Glutathione Peroxidase Activity of Selenoglutathione. Angew. Chem. Int. Ed. 2011, 50, 2125–2128. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, D.; Nauser, T.; Beld, J.; Tanner, M.; Günther, D.; Bounds, P.L.; Koppenol, W.H. Kinetics of Tyrosyl Radical Reduction by Selenocysteine. Biochemistry 2008, 47, 9602–9607. [Google Scholar] [CrossRef]
- Carroll, L.; Gardiner, K.; Ignasiak, M.; Holmehave, J.; Shimodaira, S.; Breitenbach, T.; Iwaoka, M.; Ogilby, P.R.; Pattison, D.I.; Davies, M.J. Interaction Kinetics of Selenium-Containing Compounds with Oxidants. Free Radic. Biol. Med. 2020, 155, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Beld, J.; Woycechowsky, K.J.; Hilvert, D. Selenoglutathione: Efficient Oxidative Protein Folding by a Diselenide. Biochemistry 2007, 46, 5382–5390. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, D.; Nauser, T.; Koppenol, W.H. Selenium and Sulfur in Exchange Reactions: A Comparative Study. J. Org. Chem. 2010, 75, 6696–6699. [Google Scholar] [CrossRef]
- Beld, J.; Woycechowsky, K.J.; Hilvert, D. Catalysis of Oxidative Protein Folding by Small-Molecule Diselenides. Biochemistry 2008, 47, 6985–6987. [Google Scholar] [CrossRef] [PubMed]
- Beld, J.; Woycechowsky, K.J.; Hilvert, D. Diselenides as Universal Oxidative Folding Catalysts of Diverse Proteins. J. Biotechnol. 2010, 150, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, S.; Asano, Y.; Arai, K.; Iwaoka, M. Selenoglutathione Diselenide: Unique Redox Reactions in the GPx-Like Catalytic Cycle and Repairing of Disulfide Bonds in Scrambled Protein. Biochemistry 2017, 56, 5644–5653. [Google Scholar] [CrossRef]
- Beld, J.; Woycechowsky, K.J.; Hilvert, D. Small-Molecule Diselenides Catalyze Oxidative Protein Folding in Vivo. ACS Chem. Biol. 2010, 5, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research Progress of Glutathione Peroxidase Family (GPX) in Redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef]
- Bhabak, K.P.; Mugesh, G. Functional Mimics of Glutathione Peroxidase: Bioinspired Synthetic Antioxidants. Acc. Chem. Res. 2010, 43, 1408–1419. [Google Scholar] [CrossRef]
- Iwaoka, M. Antioxidant Organoselenium Molecules. In Organoselenium Chemistry Between Synthesis and Biochemistry; Bentham Science Publishers: Sharjah, United Arab Emirates, 2014; pp. 361–378. [Google Scholar]
- Wirth, T. Small Organoselenium Compounds: More than Just Glutathione Peroxidase Mimics. Angew. Chem. Int. Ed. 2015, 54, 10074–10076. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, N.V.; Nogueira, C.W.; Nogara, P.A.; De Bem, A.F.; Aschner, M.; Rocha, J.B.T. Organoselenium Compounds as Mimics of Selenoproteins and Thiol Modifier Agents. Metallomics 2017, 9, 1703–1734. [Google Scholar] [CrossRef] [PubMed]
- Capperucci, A.; Tanini, D. Tellurium-Containing Thiol-Peroxidase-like Antioxidants and Their Catalytic. Curr. Chem. Biol. 2022, 17, 13–25. [Google Scholar] [CrossRef]
- Anghinoni, J.M.; Birmann, P.T.; da Rocha, M.J.; Gomes, C.S.; Davies, M.J.; Brüning, C.A.; Savegnago, L.; Lenardão, E.J. Recent Advances in the Synthesis and Antioxidant Activity of Low Molecular Mass Organoselenium Molecules. Molecules 2023, 28, 7349. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.K.; Priyadarsini, K.I. Selenium Compounds as Promising Antiviral Agents. New J. Chem. 2024, 48, 6534–6552. [Google Scholar] [CrossRef]
- Watanabe, N.; Forman, H.J. Autoxidation of Extracellular Hydroquinones Is a Causative Event for the Cytotoxicity of Menadione and DMNQ in A549-S Cells. Arch. Biochem. Biophys. 2003, 411, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L.; Gafvelin, G.; Arnér, E.S.J. Selenocysteine in Proteins—Properties and Biotechnological Use. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2005, 1726, 1–13. [Google Scholar] [CrossRef]
- Thornalley, P.J. Glyoxalase I—Structure, Function and a Critical Role in the Enzymatic Defence against Glycation. Biochem. Soc. Trans. 2003, 31, 1343–1348. [Google Scholar] [CrossRef]
- Distler, M.G.; Palmer, A.A. Role of Glyoxalase 1 (Glo1) and Methylglyoxal (MG) in Behavior: Recent Advances and Mechanistic Insights. Front. Genet. 2012, 3, 36946. [Google Scholar] [CrossRef]
- Ogasawara, Y.; Tanaka, R.; Koike, S.; Horiuchi, Y.; Miyashita, M.; Arai, M. Determination of Methylglyoxal in Human Blood Plasma Using Fluorescence High Performance Liquid Chromatography after Derivatization with 1,2-Diamino-4,5-Methylenedioxybenzene. J. Chromatogr. B 2016, 1029–1030, 102–105. [Google Scholar] [CrossRef]
- Vokařál, I.; Křížová, V.; Lamka, J.; Kubíček, V.; Szotáková, B.; Várady, M.; Nobilis, M.; Skálová, L. Effect of Flubendazole on Biotransformation Enzymes Activities in Haemonchus Contortus. Open Parasitol. J. 2010, 4, 24–28. [Google Scholar] [CrossRef][Green Version]
- Yamamoto, K.; Yamada, N. Identification of a Diazinon-Metabolizing Glutathione S-Transferase in the Silkworm, Bombyx Mori. Sci. Rep. 2016, 6, 30073. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.J.; Guiraud, P.; Didier, C.; Seve, M.; Flores, S.C.; Favier, A. Human Immunodeficiency Virus Type 1 Tat Protein Impairs Selenoglutathione Peroxidase Expression and Activity by a Mechanism Independent of Cellular Selenium Uptake: Consequences on Cellular Resistance to UV-A Radiation. Arch. Biochem. Biophys. 2001, 386, 213–220. [Google Scholar] [CrossRef]
- Allaman, I.; Bélanger, M.; Magistretti, P.J. Methylglyoxal, the Dark Side of Glycolysis. Front. Neurosci. 2015, 9, 123680. [Google Scholar] [CrossRef]
- Townsend, D.M.; Manevich, Y.; He, L.; Hutchens, S.; Pazoles, C.J.; Tew, K.D. Novel Role for Glutathione S-Transferase π: Regulator of Protein S-Glutathionylation Following Oxidative and Nitrosative Stress. J. Biol. Chem. 2009, 284, 436–445. [Google Scholar] [CrossRef]
- Luchese, C.; Nogueira, C.W. Diphenyl Diselenide in Its Selenol Form Has Dehydroascorbate Reductase and Glutathione S-Transferase-like Activity Dependent on the Glutathione Content. J. Pharm. Pharmacol. 2010, 62, 1146–1151. [Google Scholar] [CrossRef]
- Lillig, C.H.; Lönn, M.E.; Enoksson, M.; Fernandes, A.P.; Holmgren, A. Short Interfering RNA-Mediated Silencing of Glutaredoxin 2 Increases the Sensitivity of HeLa Cells toward Doxorubicin and Phenylarsine Oxide. Proc. Natl. Acad. Sci. USA 2004, 101, 13227–13232. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanamori, A.; Egawa, N.; Yamasaki, S.; Ikeda, T.; da Rocha, M.J.; Bortolatto, C.F.; Savegnago, L.; Brüning, C.A.; Iwaoka, M. Antioxidative and Antiglycative Stress Activities of Selenoglutathione Diselenide. Pharmaceuticals 2024, 17, 1049. https://doi.org/10.3390/ph17081049
Kanamori A, Egawa N, Yamasaki S, Ikeda T, da Rocha MJ, Bortolatto CF, Savegnago L, Brüning CA, Iwaoka M. Antioxidative and Antiglycative Stress Activities of Selenoglutathione Diselenide. Pharmaceuticals. 2024; 17(8):1049. https://doi.org/10.3390/ph17081049
Chicago/Turabian StyleKanamori, Akiko, Nana Egawa, Suyako Yamasaki, Takehito Ikeda, Marcia Juciele da Rocha, Cristiani Folharini Bortolatto, Lucielli Savegnago, César Augusto Brüning, and Michio Iwaoka. 2024. "Antioxidative and Antiglycative Stress Activities of Selenoglutathione Diselenide" Pharmaceuticals 17, no. 8: 1049. https://doi.org/10.3390/ph17081049
APA StyleKanamori, A., Egawa, N., Yamasaki, S., Ikeda, T., da Rocha, M. J., Bortolatto, C. F., Savegnago, L., Brüning, C. A., & Iwaoka, M. (2024). Antioxidative and Antiglycative Stress Activities of Selenoglutathione Diselenide. Pharmaceuticals, 17(8), 1049. https://doi.org/10.3390/ph17081049







