Abstract
Chronic lymphocytic leukemia (CLL) is a malignancy of mature B cells, and it is the most frequent form of leukemia diagnosed in Western countries. It is characterized by the proliferation and accumulation of neoplastic B lymphocytes in the blood, lymph nodes, bone marrow and spleen. We report the synthesis and antiproliferative effects of a series of novel ethanoanthracene compounds in CLL cell lines. Structural modifications were achieved via the Diels–Alder reaction of 9-(2-nitrovinyl)anthracene and 3-(anthracen-9-yl)-1-arylprop-2-en-1-ones (anthracene chalcones) with dienophiles, including maleic anhydride and N-substituted maleimides, to afford a series of 9-(E)-(2-nitrovinyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-diones, 9-(E)-3-oxo-3-phenylprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-diones and related compounds. Single-crystal X-ray analysis confirmed the structures of the novel ethanoanthracenes 23f, 23h, 24a, 24g, 25f and 27. The products were evaluated in HG-3 and PGA-1 CLL cell lines (representative of poor and good patient prognosis, respectively). The most potent compounds were identified as 20a, 20f, 23a and 25n with IC50 values in the ranges of 0.17–2.69 µM (HG-3) and 0.35–1.97 µM (PGA-1). The pro-apoptotic effects of the potent compounds 20a, 20f, 23a and 25n were demonstrated in CLL cell lines HG-3 (82–95%) and PGA-1 (87–97%) at 10 µM, with low toxicity (12–16%) observed in healthy-donor peripheral blood mononuclear cells (PBMCs) at concentrations representative of the compounds IC50 values for both the HG-3 and PGA-1 CLL cell lines. The antiproliferative effect of the selected compounds, 20a, 20f, 23a and 25n, was mediated through ROS flux with a marked increase in cell viability upon pretreatment with the antioxidant NAC. 25n also demonstrated sub-micromolar activity in the NCI 60 cancer cell line panel, with a mean GI50 value of 0.245 µM. This ethanoanthracene series of compounds offers potential for the further development of lead structures as novel chemotherapeutics to target CLL.
1. Introduction
Chronic lymphocytic leukemia (CLL) is a malignancy of mature B cells, and it is the most frequent form of leukemia diagnosed in Western countries. The incidence of CLL varies by race and geographical location [1,2], with Ireland having one of the highest incidence rates [3]. It is typically a slow-growing cancer, and it is characterized by the proliferation and accumulation of neoplastic B lymphocytes in the blood, lymph nodes, bone marrow and spleen. Traditional chemoimmunotherapy approaches have been the mainstay of CLL treatment. However, with an evolving understanding of the biology of CLL and the development of targeted therapies, the treatment of patients diagnosed with CLL has changed dramatically over the past 5–10 years [4,5,6,7].
The most commonly used chemotherapy drugs approved by the Food and Drug Administration (FDA) for the treatment of CLL include the alkylating agents bendamustine 1 [8] and cyclophosphamide, together with the nucleoside fludarabine phosphate 2, while the adenosine deaminase (ADA) inhibitor pentostatin 3 is also used in CLL patients who have relapsed (Figure 1). The development of small molecules targeting the B-cell receptor (BCR) signaling pathway has revolutionized the treatment of CLL [9]. Bruton’s tyrosine kinase (BTK) [10] and the phosphoinositide 3-kinase δ (PI3Kδ) isoform [11] are essential for BCR signaling [12]. The targeted orally active inhibitor ibrutinib 4 was the first kinase inhibitor to be approved for CLL treatment, and it is now used to treat patients with CLL regardless of their treatment history [13]. Ibrutinib 4 [14], acalabrutinib 5 [15], zanubrutinib 6 [16] and tirabrutinib 7 [17,18] interact with BTK to form a covalent bond with the cysteine residue Cys481, resulting in the inhibition of BTK activity. While these inhibitors have had a significant impact on the treatment of B-cell malignancies, acquired resistance frequently emerges in patients, leading to clinical relapse and disease progression [19]. Non-covalent inhibitors of BTK, such as pirtobrutinib 8 [20] and fenebrutinib 9 [21], are also effective in the treatment of CLL [22], and they have been evaluated in relapsed or refractory B-cell non-Hodgkin lymphoma (NHL) and CLL [23]. Many promising small-molecule, irreversible BTK inhibitors are under clinical evaluation in targeted cancer therapy development [24].
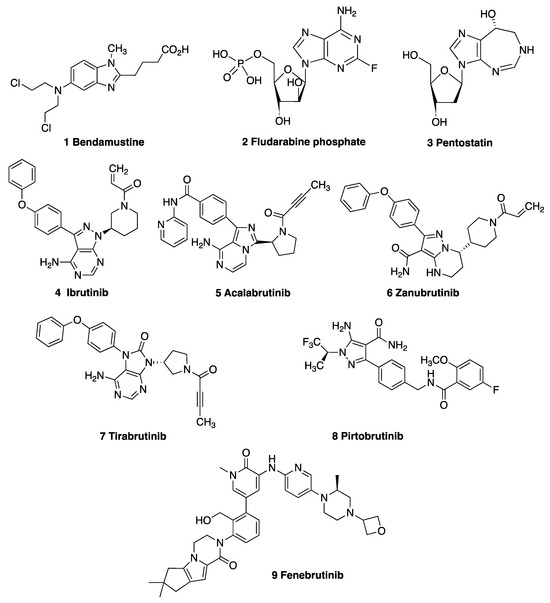
Figure 1.
Drugs used in the treatment of CLL: alkylating agents 1 bendamustine, 2 fludarabine phosphate and 3 pentostatin; covalent BTK inhibitors 4 ibrutinib, 5 acalabrutinib, 6 zanubrutinib and 7 tirabrutinib; and non-covalent BTK inhibitors 8 pirtobrutinib and 9 fenebrutinib.
The PI3Kδ inhibitor idelalisib 10 is an inhibitor of B-cell receptor signaling, and it is useful in patients with 17p deletion or TP53 mutation, who usually have a poor outcome, and in relapsed CLL [25,26]. Duvelisib 11 is an inhibitor of PI3Kδ and PI3Kγ, and it has been used in the treatment of high-risk CLL patients [27]. Venetoclax 12 is an orally bioavailable selective inhibitor of the anti-apoptotic B-cell lymphoma 2 (Bcl-2) protein, and it provides targeted therapy for the treatment of CLL patients, with a high overall response (80%) [28,29] (Figure 2); however, the development of clinical resistance is associated with its use [30]. Additionally, immunotherapies are now successfully used in the clinical treatment of CLL, e.g., the CD20 targeting monoclonal antibodies including rituximab, obinutuzumab and ofatumumab [27,31]. The programmed cell death protein 1 (PD-1) blocking antibodies pembrolizumab and nivolumab [27] can provide selective efficacy for CLL patients with Richter’s transformation (RT), an aggressive complication of CLL [28]. Many of the oral agents are more tolerable than the traditional combination of chemotherapy and immunotherapy programs, and they are suitable for the treatment of older and more frail patients [32].
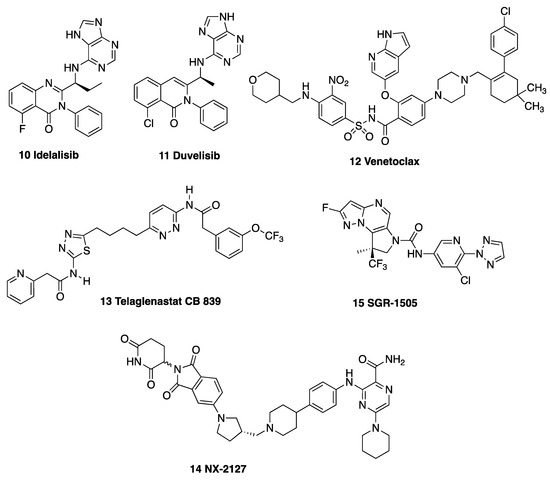
Figure 2.
Drugs targeting CLL: PI3Kδ inhibitor idelalasilib 10; PI3Kδ and PI3Kγ inhibitor duvelisib 11; Bcl-2 inhibitor venetoclax 12; glutaminase inhibitor telaglenastat CB 839 13; and dual BTK degrader NX-2127 14 and MALT-1 inhibitor SGR-1505 15.
Small molecules discovered in preclinical development studies for CLL include the glutaminase inhibitor CB-839 (telaglenastat 13) alone and in combinations with the widely used CLL drugs venetoclax, ibrutinib or MCL-1 inhibitor AZD-5991 [33]. The novel compound NX-2127 14, currently in Phase 1b clinical trials, combines the activity of a targeted BTK degrader with a second compound that degrades the Ikaros zinc finger transcription factors [34] (Figure 2). SGR-1505 15 is an investigational mucosa-associated lymphoid tissue–lymphoma translocation protein 1 (MALT1) inhibitor, and it is currently in Phase 1 clinical development for refractory B-cell neoplasms [35]. The pre-clinical modeling of novel therapeutics in CLL has resulted in the discovery of new agents with potential for targeted therapies [36]. Recently reported molecules that target the BCR-associated kinases BTK, PI3K and spleen tyrosine kinase (Syk), together with inhibitors of Bcl-2, are currently either approved or in additional clinical trials [22,37].
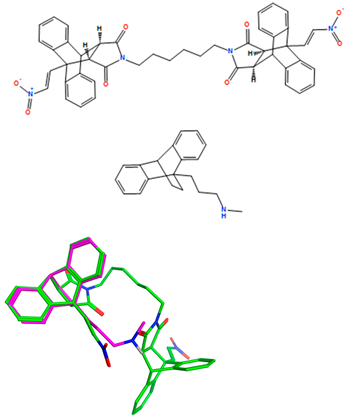
Previously, we identified a series of (E)-nitrostyrenes, e.g., 17a, 17b and (E)-9-(2-nitrovinyl)anthracenes 18a–e (Figure 3), which potently reduced cell viability in the Burkitt’s lymphoma (BL) cell lines MUTU-I and DG-75 [38]. In CLL cells associated with a poor patient prognosis, they demonstrated IC50 values of <10 µM [38,39,40] and were significantly more potent than fludarabine phosphate [39]. Anti-cancer and apoptotic effects were reported for nitrostyrenes and nitrovinyl compounds in oral and colon cancers, together with the modulation of tumorigenesis in colon and breast cancers via reactive oxygen species (ROS) effects [41,42]. We also demonstrated the potent antiproliferative and pro-apoptotic effects of (E)-9-(2-nitrovinyl)-9,10-dihydro-9,10-ethanoanthracenes in BL cells [43], indicating that the nitrovinylanthracene pharmacophore may have potential for design of CLL targeting compounds. The tetracyclic ethanoanthracene scaffold is similar to the antidepressant maprotiline 16 (Figure 3), which demonstrated antiproliferative effects in B-cell malignancies [44]. Maleimide-based ethanoanthracenes have demonstrated selective cannabinoid receptor CB2 [45], antimicrobial [46] and anti-inflammatory activities [47], as well as the inhibition of S100P/RAGE interaction in pancreatic cancer cells [48]. Glucocorticoid receptor modulation [49] and neuronal calcium channel and glycogen synthase kinase-3 beta (GSK-3 beta) modulation targeting Alzheimer’s disease [50] have also been reported for ethanoanthracene derivatives.
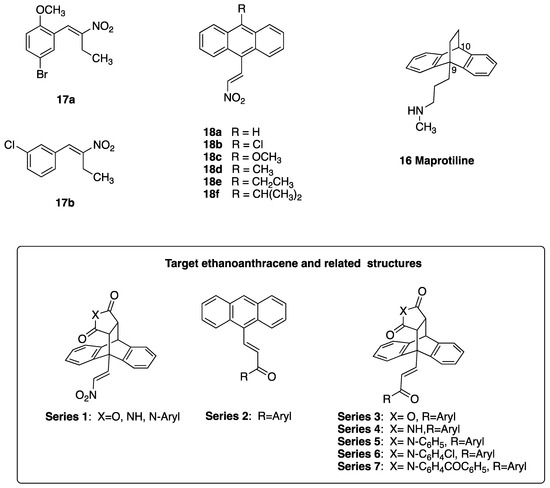
Figure 3.
Nitrostyrenes 17a, 17b, nitrovinylanthracenes 18a–e and maprotiline 16; target ethanoanthracene structures, Series 1–7.
In the present work, we report the design and synthesis of 110 novel compounds that are based on the 9,10-dihydro-9,10-ethanoanthracene and related scaffolds and that are grouped into seven series. Our objective was the identification of potent and selective compounds to target CLL with pro-apoptotic effects. The cycloaddition reaction of anthracene dienes with diverse dienophiles affords structurally varied ethanoanthracene products [43,45,46,47,48,49,50,51,52], while stereoselective Diels–Alder reactions of chiral C-9-substituted anthracenes with various maleimides have been reported [53]. Anthracenes suitably substituted with a nitrovinyl or α,β-unsaturated ketone group at C-9 were reacted with the dienophiles maleic anhydride, maleimide, N-phenylmaleimides, acrylonitrile and acetylenedicarboxylate to afford products containing a modified ethanoanthracene-bridgehead structure (Figure 3, target structures, Series 1–7). The ethanoanthracene compounds were biologically evaluated in the CLL cell lines HG-3 (containing unmutated immunoglobulin heavy-chain variable-region gene (IGHV), representing poor-prognosis CLL) and PGA-1 (containing mutated IGVH, representing good-prognosis disease).
2. Results and Discussion
2.1. Chemistry
2.1.1. Series 1: (E)-9-(2-Nitrovinyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-diones (20a–20h)
Based on our previous research with (E)-9-(2-nitrovinyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-diones in BL [43], a focused series of the most potent compounds was identified for further evaluation and testing in related B-cell malignancy CLL, (Scheme 1). (E)-9-(2-Nitrovinyl)anthracenes 18a and 18b were prepared via a piperidine-catalyzed Henry–Knoevenagel condensation reaction of 9-anthraldehyde with nitromethane [43]. The dienophiles acrylonitrile, maleimide, maleic anhydride, N-phenylmaleimides 19a–c, dimaleimide 19d and dimethyl acetylenedicarboxylate were chosen for Diels–Alder cycloaddition. The maleimide dienophiles 19b, 19c and 19d were prepared from 2,5-furandione via amic acid intermediates [43]. The cycloaddition of dienophiles 19a–c with (E)-9-(2-nitrovinyl)anthracene 18a afforded the adducts 20a–20c, whereas that with 18b provided compound 20g (30–51%), Scheme 1. Adducts 20d, 20e and 20h were obtained upon the reaction of maleimide, acrylonitrile and maleic anhydride, respectively, with diene 18a, while the dimer 20f was obtained upon the cycloaddition of anthracene 18a with the dimaleimide 19d, as previously reported [43] (Scheme 1). These lead compounds 20a–20h from BL studies bear a structural similarity to the tetracyclic antidepressant maprotiline 16, containing the three main characteristics of an electrophilic functional group chain at position 9 of the anthracene-based core, the polyaromatic core itself and the two carbon-aliphatic bridge structure-linking positions 9 and 10 of the center ring.
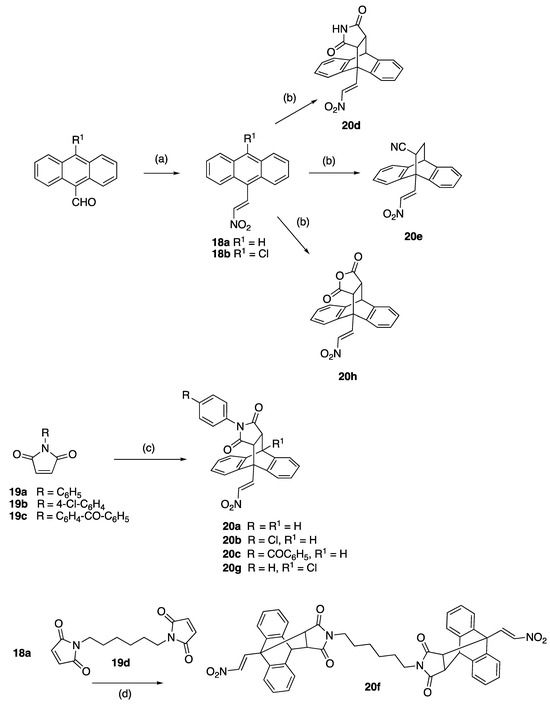
Scheme 1.
Synthesis of Series 1 ethanoanthracenes 20a–g reagents and conditions: (a) piperidine acetate, excess nitromethane (CH3NO2), 90 °C, N2, 1.5 h (71–99%); (b) dienophile (maleic anhydride) for 20h, maleimide for 20d, NCHC=CH2 for 20e, toluene, 90 °C, 48 h (30–80%); (c) dienophile 19a for N-arylmaleimides 20a and 20g, 19b for 20g, 19c for 20c, toluene, 90 °C, 48 h, (15–51%); and (d) toluene, 90 °C, 48 h (10%).
2.1.2. Series 2: (E)-3-(Anthracen-9-yl)-1-phenylprop-2-en-1-ones (21a–q)
The α,β-unsaturated carbonyl bioisosteric alternatives to the nitrovinyl group (located at C9 of the anthracene core) of our initial lead compounds (Series 1) were next investigated, principally due to potential nitrovinyl group genotoxicity and mutagenicity arising from biological metabolites of the nitro group [54]. Carcinogenicity, hepatotoxicity, mutagenicity and bone marrow suppression have been the major adverse toxicity issues associated with nitro-containing drugs [55]. The α,β-unsaturated ketone (chalcone) offers an alternative to the nitrovinyl group as an electrophilic system if required as a potential target for the enzymatic action of the compounds [56]. Covalent interactions of chalcones with biological targets can occur via the Michael-acceptor activity of the α,β-unsaturated carbonyl system, or they can also be mediated through radical-scavenging or reduction. [57] It is of interest that electrophilic compounds such as α,β-unsaturated ketones with CLL-selective cytotoxicity can interact with redox-sensitive proteins in primary CLL cells, resulting in the induction of nuclear factor erythroid 2–related factor 2 (Nrf2) signaling [58].
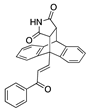
In the present work, a series of (E)-3-(anthracen-9-yl)-1-phenylprop-2-en-1-ones (Series 2) 21a–21q was prepared using a Claisen–Schmidt condensation reaction between 9-anthraldehyde and a panel of substituted aromatic and heterocyclic acetophenone derivatives in the presence of sodium hydroxide (Scheme 2, Table 1, Step a, yields 38–88%). The chalcones contained the diverse substitution of the aryl ring in both positions and atom types, including halogens, alkyl, methoxy groups and an unsubstituted benzoyl ring. The 2-naphthyl, 2 and 4-pyridyl rings, together with 2-furyl and 2-thienyl heterocycles, were also investigated (Scheme 2, Table 1). In the 1H NMR spectrum of 21a, H15 is identified as a doublet at δ 8.80 (J = 15.8 Hz), and it confirms the E alkene configuration [59]. The 13C NMR spectrum of compound 21a identified the alkene C-15 and C-16 signals at 142.5 and 130.4 ppm, respectively (see Supplementary Information). The E configuration of alkene 21k was confirmed via X-ray crystallography (Table 2) [60,61].
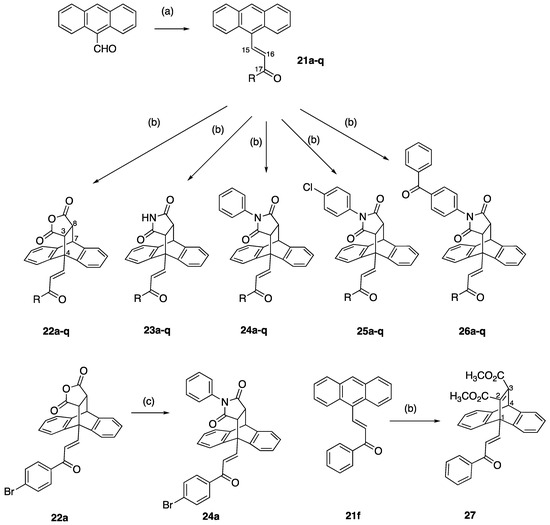
Scheme 2.
Synthesis of Series 2 anthracene chalcones 21a–q and Series 3–7 ethanoanthracenes 22a–q, 23a–q, 24a–q, 25a–q, 26a–q and 27 (see Table 1 for substituents and yields). Reagents and conditions: (a) Appropriate aryl methyl ketone, EtOH, NaOH, 20 °C, 24 h. (b) Appropriate anthracene chalcone 21a–q, dieneophile (maleic anhydride for 22a–q, maleimide for 23a–q, N-phenylmaleimide for 24a–q, N-(4-chlorophenyl)maleimide for 25a–q, N-(4-benzoylphenyl)maleimide for 26a–q, dimethyl acetylenedicarboxylate for 27), toluene, 90 °C, 48 h. (c) Aniline, acetic acid 120 °C, 2–3 h (72%).

Table 1.
Anthracene chalcones 21a–q and ethanoanthracenes 22a–q, 23a–q, 24a–q, 25a–q and 26a–q.

Table 2.
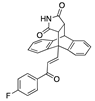
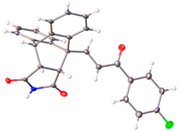
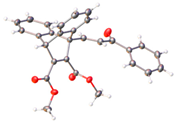
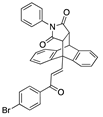
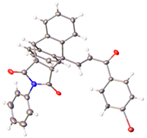
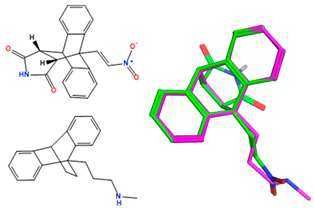
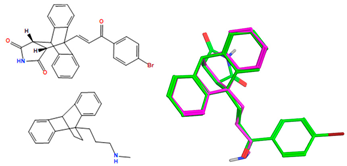
X-ray crystal structure of compounds 21k, 23f, 23h, 24a, 24g, 25f and 27 a,b.
2.1.3. Series 3: Maleic Anhydride-Substituted Ethanoanthracenes 22a–22q
The preparation of compounds 22a–22q was achieved using a Diels–Alder reaction, with the panel of substituted anthracene-chalcones 21a–21q acting as a diene system and maleic anhydride acting as the dienophile [51] in yields of 30–99% (Scheme 2, step (b), Table 1). The products contained three distinct structural features: a dihydrofuran-2,5-dione bridgehead, an anthracene-derived central core and an α,β-unsaturated ketone with a substituted aryl ring. The IR spectrum of 22e confirmed absorbances at 1669 cm−1 (α,β-unsaturated ketone) and 1774 cm−1 (maleic anhydride carbonyl). The 1H NMR spectrum of 22e confirmed the anthracene core protons at δ 3.63 (dd, J = 9.5, 3.1 Hz), δ 3.81 (d, J = 9.5 Hz) and δ 4.85 (d, J = 3.3 Hz) assigned to H8, H3 and H7, respectively. The alkene H22 is identified at δ 7.87 (d, J = 16.2 Hz). In the 13C NMR spectrum of 22e, C8, C3, C7 and quaternary C4 were identified at 54.1, 54.3, 49.4 and 56.33 ppm, respectively. The maleic anhydride C2, C9 and ketone C23 carbonyls were assigned at 175.4, 176.2 and 192.8 ppm. The alkenes C21 (136.8 ppm) and C22 (144.5 ppm) and ethanoanthracene bridges C3 (54.3, ppm), C7 (49.4 ppm) and C8 (54.1 ppm) were identified from the DEPT 90, HSQC and HMBC spectra (see Supplementary Information).
2.1.4. Series 4: Maleimide-Substituted Ethanoanthracenes (23a–23q)
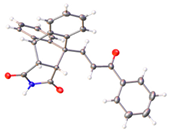
The ethanoanthracene compound panel 23a–23q was prepared using the Diels–Alder reaction of the substituted chalcones 21a–21q with maleimide (Scheme 2, Table 1). In the 1H NMR spectrum of 23a, the signals at δ 3.32 (m), 3.82 (d, J = 8.7 Hz) and 4.81 (J = 3.3 Hz) were assigned to the aliphatic H8, H3 and H7, respectively. The maleimide NH was identified at δ 10.87. The 13C NMR spectrum of 23a shows C8 of the ethanoanthracene bridge at 49.3 ppm, while the C3 was observed at 49.1 ppm. The signals at 45.0 and 51.7 ppm represent C7 and C4 of the anthracene core. The DEPT 90 spectrum was used to confirm the assignment of the alkene carbons C21 (143.1 ppm), C22 (131.5 ppm) and ethanoanthracene aliphatic bridge carbons C3 (49.1 ppm), C8 (49.3 ppm) and C7 (45 ppm) (Supplementary Information). The E configuration of the ethanoanthracene chalcone alkene of products 23f and 23h was confirmed via X-ray crystallography (Table 2) [61,62,63].
2.1.5. Series 5: N-Phenylmaleimide-Substituted Ethanoanthracenes (24a–24q)
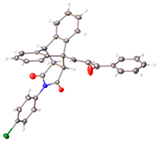
Compounds 24a–24q were obtained using the Diels–Alder reaction of the anthracene chalcones 21a–21q with N-phenylmaleimide (Table 1, Scheme 2). The N-phenylmaleimide adduct 24a was alternatively prepared by reacting the corresponding maleic anhydride chalcone adduct 22f with aniline in acid-catalyzed dehydration and the subsequent cyclization of the amic acid intermediate (Scheme 2, step c). This method resulted in a lower yield (72%) compared to the direct Diels–Alder cycloaddition of the chalcone 21f and N-phenylmaleimide (84%). In the 1H NMR spectrum of 24i, the characteristic aliphatic protons H8, H3 and H7 were identified at δ 3.55 (dd, J = 8.3, 3.3 Hz), 3.99 (d, J = 8.3 Hz) and 4.94 (d, J = 3.7 Hz), respectively, while the alkene protons H28 and H27 were observed at δ 7.81 and 7.89 (J = 16.3 Hz), confirming the E configuration. In the 13C NMR spectrum of 24i, the signals at 45.4, 48.2 and 48.3 ppm were assigned to C7, C3 and C8, respectively, of the aliphatic ethanoanthracene bridge using the HMBC and HSQC spectra, while C4 was identified at 52.04 ppm.
The stereo- and regioselectivity of Diels–Alder reaction and retention of the alkene E configuration was obtained from the X-ray crystallography of 24a and 24g (Table 2) [61,62,63]. Interestingly, the reaction of N-cyclohexylmaleimide and 9-hydroxymethylanthracene in the confined space inside carbon nanotubes resulted in the 1,4-exo Diels–Alder cycloaddition product instead of the 9,10-endo adduct obtained in conventional conditions [64].
2.1.6. Series 6: N-(4-Chlorophenyl)maleimide-Substituted Ethanoanthracenes (25a–25q)
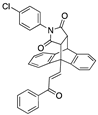
The ethanoanthracenes 25a–25q were prepared using the Diels–Alder reaction as above, with the substituted chalcones 21a–21q acting as the diene and N-(4-chlorophenyl)maleimide 19b as the dienophile (yields 3–95%) (Scheme 2, Table 1). The 1H NMR spectrum of 25k shows H-8, H-3 and H-7 at δ 3.53 (dd, J = 8.3, 3.3 Hz), δ 4.00 (d, J = 8.3 Hz) and δ 4.94 (J = 3.3 Hz), while the alkene H-26 and H-27 were identified as doublets at δ 7.80 and δ 7.88 (J = 16.2 Hz), respectively. In the 13C NMR spectrum of 25k, C-8, C-3, C-7 and C-4 of the ethanoanthracene bridge were identified at 45.4, 48.3, 48.6 and 52.11 ppm, respectively. (See Supplementary Information for HSQC, DEPT 90 and HMBC spectra.) The X-ray structure of 25f is displayed in Table 2.
2.1.7. Series 7: N-(4-Benzoylphenyl)maleimide-Substituted Ethanoanthracenes (26a–26q) and Adduct 27
The N-(4-benzoylphenyl)maleimide adducts 26a–26q were obtained using the Diels–Alder cycloaddition reaction of the anthracene chalcones 21a–21q with the N-(4-benzoylphenyl)maleimide 19c (Scheme 2, Step b, Table 1). In the 1H NMR spectrum of 26l, the ethanoanthracene carbon bridge protons H8, H3 and H7 were identified at δ 3.59 (J = 3.3, 8.3 Hz), 4.04 (d, J = 8.3 Hz) and δ 4.97 (d, J = 2.9 Hz). In the 13C NMR spectrum of 26l, the ethanoanthracene bridge carbons C3, C8 and C7 and alkenes C27 and C28 were identified at 48.5, 45.4, 52.2, 142.8 and 131.8 ppm, respectively (see Supplementary Information).
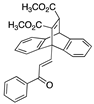
The Diels–Alder reaction of the chalcone 21f with dimethyl acetylenedicarboxylate afforded the adduct 27, containing an alkene on the central carbon bridge (Scheme 2, Step b) [65]. H-4 (center ring of the polycyclic core) was identified at δ 5.80 ppm, while H-10 and H-13 of the polycyclic core, together with alkene H-18, were identified at δ 7.55 as a multiplet; the doublet δ 7.73, J = 16.1 Hz, corresponded to alkene H-17. In the 13C NMR spectrum, the signals at 49.9, 52.9, 53.2 and 57.8 ppm were assigned to C1, C4, C2 and C3 (bridgehead alkene). The structure of the adduct 27 was confirmed via X-ray crystallography (Table 2) [61,62].
2.2. X-ray Crystallography Study of Ethanoanthracenes
The X-ray-crystallographic analysis of a series of the novel ethanoanthracene adducts was carried out, and it confirmed the regioisomer obtained from the Diels–Alder reactions (Table 2). Initially, the E configuration of the alkene 21k was observed via X-ray crystallography (Table 2). The C15–C16 bond length was consistent with an alkene (1.336 Å), and the C17–O18 bond length was consistent with a carbonyl group (1.228 Å) [60,61]. The trigonal–planar nature of the alkene bond was also evident in bond angles of 120.1° and 124.6° for C15-C16-C17 and C1-C15-C16, respectively (Supplementary Information, Table S1 and Figure S3 for crystal data and structure refinement for 21k).
The retention of the E configuration for the alkene double bond of the maleimide-substituted ethanoanthracenes products 23f and 23h was confirmed via X-ray crystallography with a C6–C7 alkene bond length 1.321 Å and a O3–C8 carbonyl bond length of 1.219 Å [61,62] (Table 2). Bond angles of 128.06° and 120.82° for C5–C6–C7 and C6–C7–C8, respectively, indicated the trigonal–planar configuration of the alkene. The core dihydroethanoanthracene moiety was rigid, with the flat succinimide ring fused to the ethano-bridge; the presence of the imide was supported by the carbonyl bond lengths of 1.205 Å for O1–C1 and O2–C2. In addition, the N1–C1 and N1–C2 bond lengths of 1.375 Å and 1.380 Å, respectively, confirm the C-N imide bond [63]. The X-ray crystal showed the presence of a racemic mixture, with the two stereocenters of the ethanoanthracene carbon bridge in the (R,R) and (S,S) stereochemical configuration (Supplementary Information, Table S1, and Figures S4, S7 and S8).
Additional evidence to confirm the stereo- and regioselectivity of the Diels–Alder reaction and retention of the alkene E configuration was obtained from the X-ray crystallography of the N-phenylmaleimide-substituted ethanoanthracenes 24a and 24g (Table 2). For compound 24a, the C10–C11 (1.335 Å) and O9–C8 (1.227 Å) bond lengths were consistent with alkene and carbonyl functional groups. Bond angles of 123.9° and 125.6° for C10–C11–C12 and C8–C10–C11, respectively supported the trigonal–planar configuration [61,62]. Bond lengths of 1.18 Å and 1.19 Å for O32–C31 and O29–C28 are typical of the maleimide carbonyl groups. Furthermore, the N30–C33 bond (1.36 Å) was within the expected range for conjugated C–N bonds [63]. (See Figure S5 and Table S1, Supplementary Information, for crystal data and structure refinement for 24a and 24g.)
The X-ray structure of the N-(4-chlorophenyl)maleimide-substituted ethanoanthracene 25f is displayed in Table 2, which confirms the structural assignment, showing the core dihydroethanoanthracene moiety to be rigid, with similar conformations observed in the various X-ray structures discussed above. (See Table 2 and Table S1, Supplementary Information, for crystal data and structure refinement for compound 25f.)
The structure of the adduct 27 obtained from the chalcone diene 21f with dienophile dimethyl acetylenedicarboxylate was also confirmed via X-ray crystallography (Table 2). The bond length of C15–C16 was consistent with an alkene (1.3269 Å), and the bond length of 1.2263 Å was consistent with a carbonyl (O28–C27) [61,62]. The bond angles for C14–C25–C26 and C27–C26–C25 were observed as 126.47° and 120.01°, respectively, indicating the trigonal–planar structure. The ester carbonyl bond lengths of 1.2073 Å and 1.2021 Å were observed for O18–C17 and O22–C21, while the O23–C24 and O19–C20 bond lengths of 1.45 Å and 1.4464 Å are typical of the ester C–O bond. The distinctive ethenoanthracene alkene bridgehead was observed with a bond length of 1.34 Å (see Figure S7 and Table S1, Supplementary Information, for crystal data and structure refinement for compound 27).
2.3. Stability Study for Chalcones 21a, 21i, Anhydride 22h, and Maleimides 23a, 23g, 23n, 24a, 24h, 26a and 26n
A preliminary stability study was carried out on selected chalcones, maleic anhydride and maleimide adducts at acidic, neutral and basic conditions (pH 4, 7.4 and 9) using HPLC to determine suitability for further preclinical investigations. Chalcones 21a and 21i, anhydride 22h, and maleimides 23a, 23g and 24a demonstrated poor stability over the pH range studied, with <50% recovery at 24 h (Figure 4 and Supplementary Information Table S2, Figure S1). At pH 4.0, the rate of degradation of 2-pyridyl-substituted ethanoanthracene 23n was minimal up to 8 h, with a half-life (t1/2) of 19 h, while at pH 7.4, degradation was slower; t1/2 >24 h. The compound remained stable at pH 9.0, with an 11% decrease in compound concentration over 24 h and t1/2> 24 h. Compound 23n was most stable at pH 9.0 (89% drug remaining at 24 h) in comparison with 70% and 39% remaining at pH 7.4 and 4.0 after 24 h. A possible degradation route for the maleimide 23n could be through the hydrolysis of the cyclic imide of the maleimide ring, leading to the resultant amic acid product [66,67]. Compound 23n was the most stable of the compounds evaluated at pH 9, and it would be suitable for further preclinical investigation. The N-phenylmaleimide adduct 24h demonstrated notable stability at pH 7.4 and pH 9, with 80% and 81%, respectively, remaining at 24 h, and t1/2 >24 h. At pH 4, it was less stable with 25% remaining (t1/2 = 14.8 h). The N-benzoylmaleimide 26a demonstrated good stability with 64%, 61% and 66% remaining after 24 h in pH conditions 4, 7.4 and 9, respectively, and t1/2> 24 h, while 26n was less stable with 12% remaining at pH 4 (24 h).
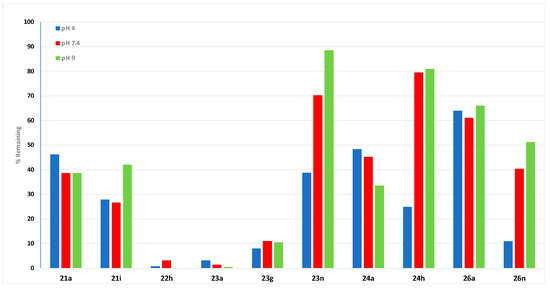
Figure 4.
Stability study for compounds 21a, 21i, 22h, 23a, 23g, 23n, 24a, 24h, 26a and 26n at pH 4.0, pH 7.5 and pH 9.0 over 24 h.
All samples were analyzed using acetonitrile–water (80:20%, 70:30%, 60:40% isocratic) as the mobile phase over 10 min and a flow rate of 1 mL/min. Stock solutions were prepared by dissolving 5 mg of compounds in a mobile phase ([(acetonitrile (80%): water (20%)], 10 mL). Anhydride 22h was not detected at its retention time of 5.2 min at pH 9.0. The data presented represent the results of single experiments.
2.4. Biological Results and Discussion
2.4.1. Evaluation of In Vitro Antiproliferative Activity of Chalcone and Nitrovinyl-Substituted Ethanoanthracenes in CLL Cell Lines HG-3 and PGA-1
The phenotypic cellular responses of the synthesized ethanoanthracenes were then investigated in the CLL cell lines HG-3 and PGA-1. The HG-3 cell line is representative of a poor patient prognosis; the cell line was established via EBV (Epstein–Barr virus) infection from an IGVH1–2 unmutated B1 lymphocyte-origin CLL patient clone [68]. The PGA-1 cell line, which is representative of a good patient prognosis, was established from leukemic B cells of a CLL patient with mutated IGVH1-2 [69]. The compounds were evaluated at 10-µM and 1-µM concentrations using an alamarBlue viability assay, and fludarabine was used as a clinically relevant comparative positive control [39]. Vehicle-treated cells [(DMSO (1% v/v)] were considered 100% viable, and the viabilities of each compound were calculated accordingly. The structure–activity relationships for the series were determined, and the most potent ethanoanthracene compounds were identified for further investigation. The present study was arranged according to structural type (Series 1–7).
2.4.2. Series 1: Nitrovinyl-Substituted Ethanoanthracenes (20a–20h)
The antiproliferative activities of the nitrovinyl-substituted ethanoanthracenes 20a–20h in HG-3 and PGA-1 cell lines are shown in Figure 5, with reference compounds maprotiline and fludarabine. These were among the most potent compounds initially identified in our previous work in the related B-cell malignancy BL cell lines (e.g., compound 20a IC50 = 0.21 µM in a chemosensitive MUTU-1 cell line and IC50 = 0.71 µM in a chemoresistant DG-75 cell line [43]). In HG-3 cells, at 10 µM treatment concentration, the most potent compounds were identified as the N-phenylmaleimide adducts 20g (2% cell viability), 20b (8%), and 20c (11%); the dimer 20f, N-phenylmaleimide 20a, maleimide 20d and acrylonitrile adduct 20e show reduced cell viability (14–43%). In PGA-1 cells, the most effective compounds were identified as 20g (1% viable cells), 20h (2%), 20b (3%), 20c (4%) and 20e (3%) at 10 µM concentration, while compounds 20a, 20d and 20f were less potent (11%, 17% and 17%, respectively). The compounds were more effective in PGA-1 compared to HG-3 cells with the p-chloro- and benzophenone-based N-phenylmaleimides 20b and 20c, as well as the acrylonitrile adduct 20e, initially identified as promising antiproliferative agents.
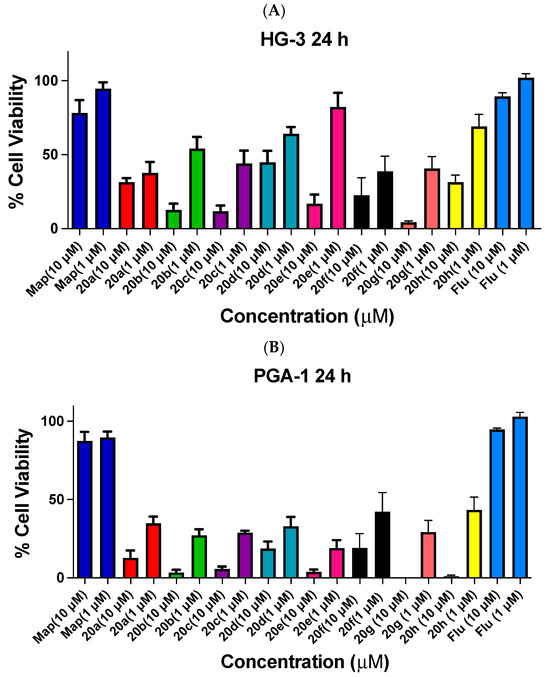
Figure 5.
Cell viability data for ethanoanthracenes 20a–e, 20g, 20h and 20f. Cell viability data for (E)-9-(2-Nitrovinyl)-9,10,11,15-tetrahydro-9,10-[3,4]epipyrroloanthracene-12,14-diones 20a–e, 20g and 20h and the related dimer 20f in CLL: (A) HG-3 cells (1 and 10 µM) and (B) PGA-1 cells (1 and 10 µM). The cell proliferation of HG-3 and PGA-1 cells was determined with an alamarBlue assay. Compound concentrations of either 1 µM or 10 µM for 24 h were used to treat the cells (in triplicate) with control wells containing vehicle DMSO (1% v/v). Map = maprotiline; Flu = fludarabine. The mean value for three experiments is shown.
2.4.3. Series 2: (E)-3-(Anthracen-9-yl)-1-phenylprop-2-en-1-ones (21a-21q)
The biochemical activity of the anthracene-based chalcones 21a–21q in the HG-3 and PGA-1 cell lines are shown in Figure 6A,B, with fludarabine and nitrovinylanthracene 18a acting as internal standards. These structures contain diverse functional group substitutions on the α,β-unsaturated ketone, ranging from ring-activating groups (4-nitro aryl, 21b) to ring-deactivating groups (3,4-dimethoxy aryl, 21e) and polyaromatic systems (2-naphthyl, 21p). From the initial HG-3 cell line screen, the most potent compounds observed were 21e (3,4-dimethoxy), 21n (2-pyridyl), 21k (3,4,5-trimethoxy), 21d (2,4-dichloro) and 21m (4-pyridyl) with % viability in the range of 37–54% at a 10 µM concentration and a 50–61% viability range at 1 µM (Figure 6A). In the PGA-1 cell line, the most potent compounds at a 10-µM treatment concentration were 21m (4-pyridyl, 43% cell viability), 21k (3,4,5-trimethoxy, 43%), 21n (2-pyridyl, 47%), 21i (4-methoxy, 49%), 21d (2,4-dichloro, 50%) and 21p (2-naphthyl, 50%). The halogenated compounds were weakly active at 10 µM with 21h (4-fluoro) and 21l (4-chloro) (70% viability) (Figure 6B). The anthracene chalcones from Series 2 were not evaluated further due to their poor activity, although the antiproliferative activity of chalcones was previously reported in various leukemia cell lines [70].
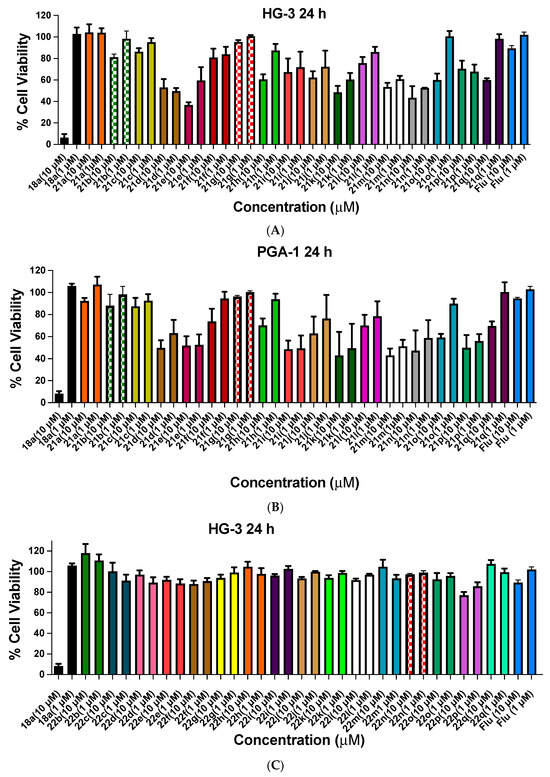
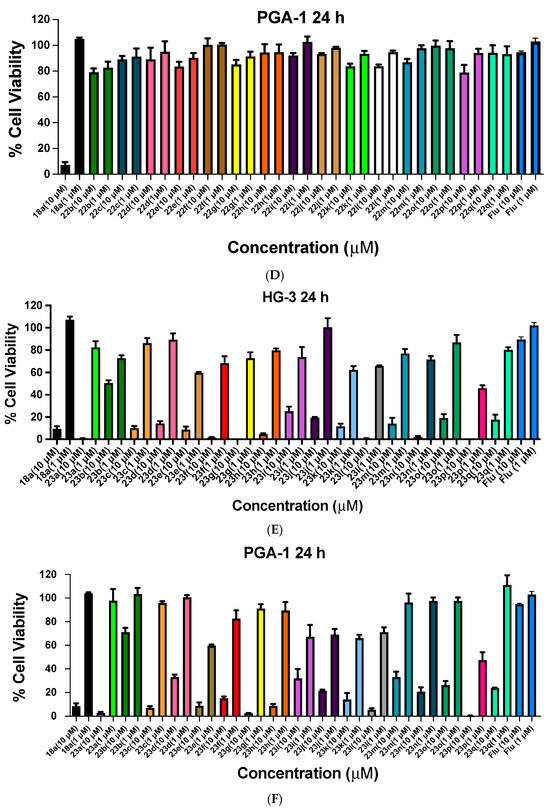
Figure 6.
Cell viability data for chalcones 21a–q and ethanoanthracenes 22a–22q and 23a–23q in CLL cell lines HG-3 and PGA-1. Cell viability data for chalcones 21a–q (A,B), maleic anhydride ethanoanthracene adducts 22a–22q (C,D) and maleimide ethanoanthracene adducts 23a–23q (E,F) in CLL cell lines: the cell proliferation of HG-3 and PGA-1 cells was determined with an alamarBlue assay. Compound concentrations of either 1 µM or 10 µM for 24 h were used to treat the cells (in triplicate) with control wells containing vehicle DMSO (1% v/v). Flu = fludarabine. The mean value for three independent experiments is shown.
2.4.4. Series 3: Maleic Anhydride-Substituted Ethanoanthracenes (22a–22q)
The cell viability of compounds 22a–22q in the HG-3 and PGA-1 cell lines is shown in Figure 6C,D. The majority of this compound series was weakly active or inactive across both treatment concentrations with the exception of 22p (2-naphthyl) with cell viability of 77% in HG-3 and 79% in PGA-1 cells. The poor activity displayed for these compounds in series 3 did not merit further investigation.
2.4.5. Series 4: Maleimide-Substituted Ethanoanthracenes (23a–23q)
The antiproliferative activities of the maleimide-substituted ethanoanthracenes compounds 23a–23q in the HG-3 cell line are shown in Figure 6E. In contrast to the maleic anhydride Series 3 compounds, the maleimide adducts demonstrated promising activity with the most potent of the panel identified as 23p (2-naphthyl, 0% viable cells), 23g (4-iodo 0%), 23a (4-bromo, 0.5%), 23l (4-chloro, 0.7%), 23f (phenyl, 2%) and 23n (2-pyridyl, 2%) when screened at a 10-µM concentration. Similar trends were observed at the 1-µM concentration, with the most potent compound identified as 23p (2-naphthyl derivative, 46%). The most potent of the panel screened in the PGA-1 cell line at 10 µM were identified as 23p (2-naphthyl, 0% viable cells), 23g (4-iodo, 2%), 23a (4-bromo, 3%), 23l (4-chloro, 5%), 23c (4-ethyl, 7%) and 23h (4-fluoro, 9%); see Figure 6F. With an increasing size of the halogen substituent and increasing electronegativity on the para position of the acetophenone-derived aryl ring, an increase in compound potency was observed (I > Br > Cl > F), as also noted for the HG-3 cells. Compared to maleic anhydride (Series 3), the maleimide-derived adducts elicited greater biological activity at both concentrations and in both cell lines tested. These diverse sets of aryl substituents (alkyl, halogens and nitro) for heterocycles eliciting a similar degree of antiproliferative activity in the CLL cells suggests a moderate tolerance for substitution on the acetophenone-derived portion of the molecules in relation to their anticancer effect. The Series 4 compounds are generally more effective in the aggressive HG-3 cell type than in PGA-1 cells; e.g., compounds 23n (2-pyridyl) and 23f (phenyl) were notably less effective at 10 µM in PGA-1 cells (19% higher viability for 23n and 13% higher viability for 23f compared to HG-3 cells).
2.4.6. Series 5: N-Phenylmaleimide Substituted Ethanoanthracenes (24a–24q)
The cell viability results for the N-phenylmaleimide compounds 24a–24q in the HG-3 cell line are shown in Figure 7A. All compounds were effective at a 10-µM concentration, with the most potent compounds identified in the screen as follows: 24o (2-furyl, 3% cell viability), 24n (2-pyridyl, 4%), 24b (4-nitro, 10%), 24a (4-bromo, 14%,), 24l (4-chloro, 24%,) and 24q (2-thienyl, 24%). The remaining compounds were also moderately effective, with cell viability in the range of 29–65%. At the lower treatment concentration (1 µM), 24n (2-pyridyl) showed the greatest activity (48% viable cells remaining). In the PGA-1 cell line (Figure 7B), a similar trend was observed with the most potent compounds identified as 24o (2-furyl, 9% viable cells), 24l (4-chloro, 17%), 24b (4-nitro, 22%), 24a (4-bromo, 25%) and 24f (phenyl, 26%).
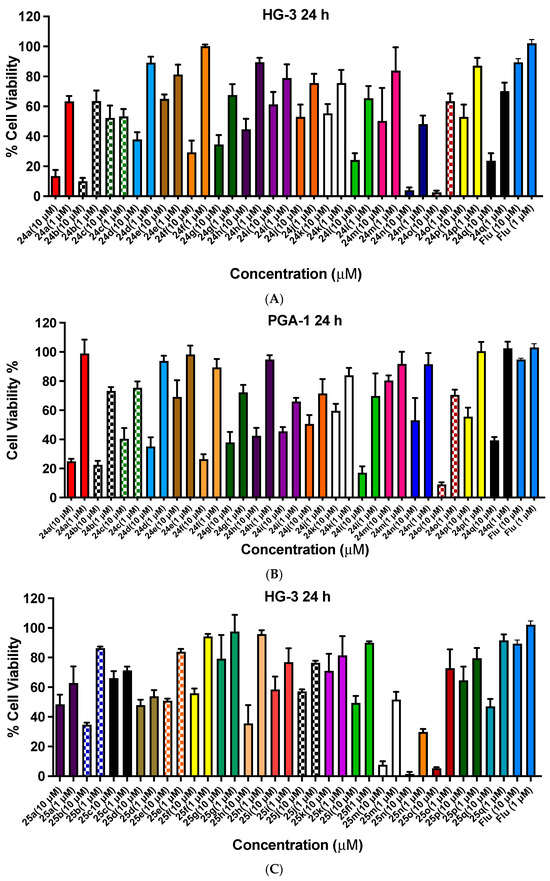
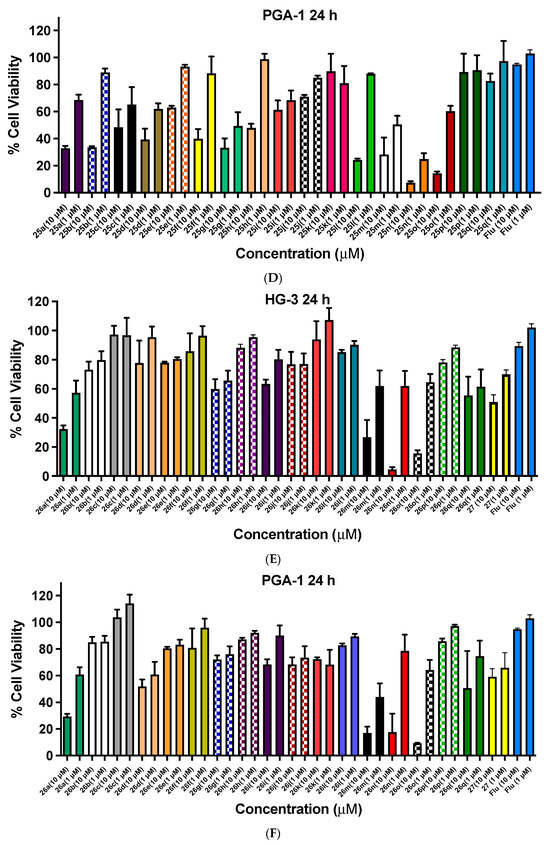
Figure 7.
Cell viability data for ethanoanthracenes 24a–q, 25a–q, 26a–q and 27 in CLL cell lines HG-3 and PGA-1. Cell viability data for N-phenylmaleimide-substituted ethanoanthracenes (24a–q, Panels A,B), N-(4-chlorophenyl)maleimide-substituted ethanoanthracenes (25a–q, Panels C and D) and N-(4-benzoylphenyl)maleimide-substituted ethanoanthracenes (26a–q, Panels E,F) and 27 (E,F) were determined in CLL cells HG-3 cells (1 and 10 µM) and PGA-1 cells (1 and 10 µM). The cell proliferation of HG-3 and PGA-1 cells was determined with an alamarBlue assay. Compound concentrations of either 1 µM or 10 µM for 24 h were used to treat the cells (in triplicate) with control wells containing vehicle DMSO (1% v/v). Flu = fludarabine. The mean value for three independent experiments is shown.
2.4.7. Series 6: N-(4-Chlorophenyl)maleimide-Substituted Ethanoanthracenes (25a–25q)
The antiproliferative activity of the N-(4-chlorophenyl)maleimide series 25a–25q in the HG-3 cell line is shown in Figure 7C. The most potent compounds of the series screened were identified as 25n (2-pyridyl, 2% viable cells), 25o (2-furyl, 5%,), 25m (4-pyridyl, 8%), 25h (4-fluoro, 35%,), 25b (4-nitro, 35%) and 25q (2-thienyl, 47%). 25n (2-pyridyl) was also identified as the most potent at 1 µM with 30% cell viability. The N-(4-chlorophenyl)maleimides identified with promising activity from the panel screened in the PGA-1 cell at a 10-µM concentration were 25n (2-pyridyl, 7% viable cells), 25o (2-furyl, 14%), 25l (24%, 4-chloro), 25m (4-pyridyl, 28%), 25a (4-bromo, 33%), 25g (4-iodo, 33%) and 25b (4-nitro, 34%). At the lower treatment concentration (1 µM), 25n was also observed to have the greatest activity (25% cell viability), (Figure 7D).
2.4.8. Series 7: N-(4-Benzoylphenyl)maleimide-Substituted Ethanoanthracenes (26a–26q)
The preliminary biochemical evaluation of the N-(4-benzoylphenyl)maleimide compound series 26a–26q in the HG-3 cell line is shown in Figure 7E, with the most potent compounds of the panel screened at 10 µM identified as 26n (2-pyridyl, 4% viable cells), 26o (2-furyl, 15%), 26m (4-pyridyl, 27%), 26q (2-thienyl, 56%) and 26a (4-bromo, 31%). At a 1-µM treatment concentration, 26a (4-bromo) was the most effective (55% cell viability). In the PGA-1 cell line (Figure 7F), 26o (2-furyl, 9% viable cells remaining), 26m (4-pyridyl, 17%), 26n (2-pyridyl, 18%) and 26a (4-bromo, 29%) were also identified as potent lead compounds. At a lower treatment concentration of 1 µM, 26m (4-pyridyl) demonstrated the greatest activity with 43% cell viability.
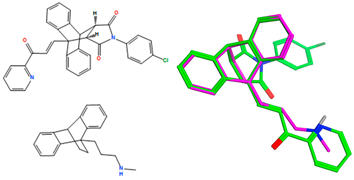
Following this preliminary evaluation of the panel of ethanoanthracene compounds described above, a number of key structural features were identified as critical for antiproliferative activity in CLL cells. The nitrovinyl and 1-phenyl-2-propen-1-one pharmacophores were demonstrated to be critical for cytotoxic effect. The maleimide-derived adducts (Series 4–7) were also found to produce a more potent effect than the corresponding furan-based products (Series 3). However, the nitrovinyl-based maleic anhydride adduct 20h (Series 1) demonstrated significantly greater efficacy in both cell lines (31% viability in HG-3; 2% in PGA-1 at 10 µM) when compared with the most potent maleic anhydride chalcone adduct 22p (>77% viability in both cell lines at 10 µM), thus confirming the requirement for the nitrovinyl pharmacophore for potency in the maleic anhydride-based series. For the maleimide-based compounds (Series 4–7), a comparison of the potent 2-pyridylchalcones compounds 23n (Series 4), 24n (Series 5), 25n (Series 6) and 26n (Series 7) confirmed the N-(4-benzoylphenyl)maleimide substitution as the most effective of the structural series evaluated.
In a more detailed analysis of the structure–activity relationships of the N-(4-benzoylphenyl)maleimide compounds (Series 7), it was observed that the replacement of the unsubstituted benzoyl aryl ring of 26f with the heterocycles in 26m (4-pyridyl), 26n (2-pyridyl) and 26q (2-thienyl) on the molecular scaffold resulted in an increase in potency for the compounds across both cell lines. The 4-pyridyl derivative 26m showed a notable decrease in cell viability of 64% (to 17% viable cells) at a 1-µM treatment concentration in comparison to the unsubstituted 26f in the PGA-1 cell line. In HG-3 cells, a 59% reduction in cell viability was observed at 10 µM for 26m and a 64% decrease at the 1-µM concentration compared to 26f. With 26n (2-pyridyl derivative), a significant reduction in cell viability was noted in PGA-1 cells, showing decreases of 63% and 20% at 10 µM and 1 µM, respectively. Similarly, in HG-3 cells, 26n showed substantial decreases in cell viability compared to 26f (82% greater decrease in cell viability at 10 µM and 34% greater decrease at 1 µM). In addition, the presence of bromine at C-4 of the benzoyl aryl ring of 26a resulted in a significant increase in biological activity when compared to 26f, leading to 20% and 70% reduction in cell viability at 10 µM and 1 µM in PGA-1 cells. In HG-3 cells, a similar decrease in cell viability of 29% and 64% at 10- and 1-µM treatment concentrations, respectively, was observed. The acetylene adduct 27 showed moderate activity (50% viability in HG-3 and 58% in PGA-1 cells) at 10 µM. This result indicated that the maleimide structure present in the maleimide series 4 adducts resulted in superior efficacy for corresponding compound 23f (0.5%, viability in HG-3, 13.9% viability in PGA-1 cells) when compared with the alkene-type bridge structure in compound 27.
The more potent compounds identified from Series 1 and Series 4–7 were then progressed to further biological investigations.
2.4.9. In vitro IC50 Determination of Selected Nitrovinyl-Based Ethanoanthracene and Chalcone-Based Ethanoanthracene Derivatives in CLL Cells
The IC50 values of the most promising anti-proliferative nitrovinyl-based ethanoanthracene and chalcone-based ethanoanthracene compounds identified from preliminary biological screening at 10-µM and 1-µM concentrations were obtained for both HG-3 and PGA-1 cell lines. Fludarabine was used as a clinically relevant positive control; IC50 = 28.1 µM (HG-3) and 32.0 µM (PGA-1).
2.4.10. (E)-9-(2-Nitrovinyl)-Substituted Ethanoanthracenes (Series 1)
Compounds 20a–20f were selected and tested across a concentration range of 5–0.01 µM and assessed for viability at 24 h using the alamarBlue assay. The compounds exerted a more potent effect than the fludarabine control (18–59-fold greater in HG-3 cells, 29–53-fold greater in PGA-1 cells) across both HG-3 and PGA-1 cell lines with IC50 ranges of 0.48–1.6 µM and 0.61–1.1 µM, respectively. In HG-3 cells, the most potent compounds identified were 20a (IC50 0.48 µM, N-phenylmaleimide derivative) and 20c (IC50 0.71 µM, N-(4-benzoylphenyl)maleimide; while in PGA-1 cells, the most potent compounds were 20a (IC50 0.61 µM) and 20b (IC50 0.66 µM, N-(4-chlorophenylmaleimide). Overall, the two most potent compounds across both cell lines were 20a (mean IC50: 0.55 µM) and 20b (mean IC50: 0.76 µM). In both cell lines, 20e (cyano-derivative) had the highest IC50 values (Table 3) with the remaining compounds having sub-micromolar activity. This suggests that the general maleimide structure plays an important role in the biological activity observed, as we previously reported in BL [71]. In addition, the presence of an unsubstituted aromatic ring on the maleimide-derived functional group 20a leads to better activity over the unsubstituted maleimide 20d, N-(4-chloromaleimide 20b and the N-(4-benzoylphenyl)maleimide 20c adducts. The novel dimeric compound 20f, which we identified with potent anti-proliferative activity in BL [43] [71], was also demonstrated to exert a more potent effect than the fludarabine control (165-fold greater in HG-3 cells, 91-fold greater in PGA-1 cells) across both HG-3 and PGA-1 cell lines with IC50 values of 0.17 µM and 0.35 µM, respectively, and a mean IC50 value of 0.53 µM.

Table 3.
IC50 values of selected (E)-9-(2-nitrovinyl)ethanoanthracenes 20a–20f in HG-3 and PGA-1 cell lines.
2.4.11. In Vitro IC50 Determination of the Most Potent Chalcone-Based Ethanoanthracene Derivatives in HG-3 and PGA-1 Cells (Series 4–7)
The most potent compounds from the preliminary screening of Series 2–7 (compounds 23a, 23c, 23f–23i, 23k, 23l, 23n, 23p, 24f, 24l, 25n and 26n) were chosen for IC50 determination in the PGA-1 and HG-3 cell lines. The compounds were tested over a concentration range of 50–0.01 µM and assessed at 24 h via an alamarBlue cell viability assay. The results are presented in Table 4. All compounds exerted a more potent effect than the fludarabine control (3–22-fold greater in HG-3 cells, 3–37-fold greater in PGA-1 cells) across both HG-3 and PGA-1 cell lines with IC50 ranges of 1.31–10.28 µM and 0.31–12.6 µM, respectively. In HG-3 cells, the most potent compounds identified were 25n (IC50 1.31 µM, 2-pyridyl, N-(4-chlorophenyl)maleimide derivative) and 23p (IC50 1.41 µM, 2-naphthyl, maleimide derivative), while in PGA-1 cells, the most potent compounds were 24l (IC50, 0.31 µM, 4-chloro, maleimide derivative) and 26n (IC50 0.39 µM, 2-pyridyl, N-(4-benzoylphenyl)maleimide derivative). Overall, the two most potent compounds across both cell lines were 25n (mean IC50 1.09 µM) and 26n (mean IC50 1.12 µM).

Table 4.
IC50 values of selected ethanoanthracene derivatives in HG-3 and PGA-1 CLL cell lines.
2.4.12. National Cancer Institute (NCI) 60-Cell-Line Panel Screening
A number of our most promising compounds identified through preliminary cell viability testing (23c, 23d, 23f, 23h, 23l, 23m, 23n, 23o, 24f, 24l, 25n and 26n) were selected for NCI drug screening in the 60-cell-line panel. The panel includes nine subcategories based on cancer tissue types, which include leukemia, ovarian, central nervous system, breast, lung, prostate, skin, renal and colon cancer. The compounds were initially assessed in a one-dose assay at a fixed treatment concentration of 10 µM. The compounds selected for further investigation were progressed to a five-dose assay across the concentration ranges of 0.01, 0.1, 1, 10 and 100 µM [72]. The endpoints assessed in the screening protocol were GI50, TGI and LC50 [72].
As the main focus of this work is the antiproliferative and anti-cancer effects of ethanoanthracenes in CLL, attention was focused primarily on leukemia cell line results within the wider 60-cell-line panel. The leukemia cell lines used for the assessment were as follows: CCRF-CEM (acute lymphoblastic leukemia), HL-60 (TB) (acute promyelocytic leukemia), K-562 (chronic myeloid leukemia), MOLT-4 (acute lymphoblastic leukemia), RPMI-8226 (plasmacytoma/myeloma) and SR (large cell immune blastic lymphoma). The resultant GI50 values obtained are presented in Table 5. All compounds demonstrated GI50 values in the low and sub-micromolar range across all leukemia cell lines, with compound 25n identified as the most potent with a mean GI50 of 0.29 µM, indicating the potential for the compound for further investigation. The mean GI50 values across the leukemia cell panel were determined in the range of 0.29–2.08 µM. Compounds 25n (0.29 µM), 24l (0.98 µM), 23h (1.00 µM) and 23n (1.32 µM) were the most promising overall anti-leukemic agents screened. These results are in good alignment with those determined in the HG-3 and PGA-1 CLL cell lines (Table 4), with compound 25n identified as the most potent compound in both CLL cell lines (HG-3, IC50 = 1.31 µM; PGA-1, IC50 = 0.87 µM and mean IC50 = 1.09 µM). They warrant further study as lead compounds for the development of more selective and potent anti-cancer agents.

Table 5.
Summary of NCI 60-cell-line screening results for selected ethanoanthracene compounds in the leukemia cell panel a.
In addition to the leukemia results above, promising anticancer activity was observed across the NCI 60-cell-line panel with particularly interesting activity in melanoma and breast cancer cell lines and notable GI50 value ranges of 25n (melanoma: 0.194–1.08 µM; breast: 0.295–0.627 µM), 23h (melanoma: 1.60–2.48 µM; breast: 0.885–1.88 µM), 23n (melanoma: 1.12–2.14 µM; breast: 0.295–0.627 µM) and 23l (melanoma: 1.40–1.81 µM; breast: 1.34–1.94 µM) (Table 6 and Table 7). These values suggest the potential exploration of these compounds in breast and skin cancers in the future.

Table 6.
Antitumor evaluation of compounds 23h, 23l and 25n in NCI 60-cell-line screen a.

Table 7.
Summary of NCI 60-cell lines’ mean screening results (GI50, TGI and LC50) for selected compounds.
The N-(4-chlorophenyl)maleimide ethanoanthracene adduct 25n was identified as the most potent compound evaluated in the NCI panel, with a mean GI50 value of 0.245 nM determined across the NCI-60 panel of cell lines screened. (See Table 6: NCI-60 cell line screen, Figure 8: Heatmap for the activity of compound 25n in the cell lines of the NCI-60 screen). Significantly, the GI50 values for 25n were in the sub-micromolar range for all but six of the cell lines investigated. 25n displayed significant potency in all breast cancer cell lines evaluated in the panel, with GI50 values in the range of 312–0.627 µM. GI50 values below 300 nM were obtained for compound 25n in 17 of the panel cell lines tested with activity against non-small-cell lung, colon, CNS, ovarian and prostate cell lines tested. Figure 8 displays a heatmap of the activity of compound 25n across the cell lines in the NCI-60 screen. In addition, compound 25n was particularly effective in the chemoresistant HT-29 human colorectal adenocarcinoma cell line with an IC50 value of 0.335 µM. The mean GI50 values for the panel of 60 cell lines for the most potent compounds evaluated were determined to be in the range of 0.245–2.511 µM (apart from compound 26n GI50 = 7.585 µM), and they are shown in Table 7.
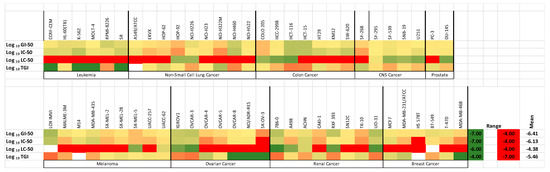
Figure 8.
Heatmap for compound 25n across cell lines in the NCI-60 screen.
Heatmap for the antiproliferative activity of compound 25n across cell lines in the NCI-60 screen using three different values (growth-inhibitory effect, GI50; drug concentration at which the response is reduced by half, IC50; cytostatic effect, TGI; cytotoxic effect, LC50; concentration in molar).
2.4.13. COMPARE Analysis of Compounds 23h, 23n, 24l and 25n
The COMPARE algorithm [74], allows for the comparison of compound activity profiles across the NCI 60-cell-line panel. Compounds that display similar activity profiles often result in cell growth inhibition through related mechanisms of action and display robust correlations in mean profiles across the screening assays. Representative potent compounds 23h, 23n, 24l and 25n were used as COMPARE seeds, with the NCI Standard Agents database selected for comparison. The COMPARE program was then used to probe the mechanism of action of the series of ethanoanthracene maleimide adducts [75]. The antiproliferative profiles (GI50, TGI and LC50 values) of potent compounds 23h, 23n, 24l and 25n were compared with compounds with a known mechanism of antiproliferative action in the NCI Standard Agents Database. The top five ranked compounds for each seed based on Pearson’s correlation coefficient were identified. Based on GI50 values, the chemotherapeutic agents that had fair to strong positive Pearson’s correlation coefficients (0.34–0.92) were identified (Supplementary Information, Tables S3–S6). Compound 23h demonstrated a good correlation with the antitumor purine analog pentostatin, effective in lymphoid neoplasms and a potent inhibitor of ADA (r = 0.918). The correlation of 23h with the anticancer ribonucleotide reductase inhibitor caracemide, (r = 0.663) was also observed. 24l correlates with the DNA-alkylating drugs fluorodopan (r = 0.631) and melphalan (r = 0.538), while 23n correlated with dichloroallyl lawsone (r = 0.505), an antineoplastic dihydroorotate dehydrogenase (DHOD) inhibitor (see Supplementary Information, Tables S3–S6). Many of the positively correlated chemotherapeutic agents identified in the COMPARE correlation analysis mediate cytotoxicity through DNA synthetic pathways and resultant DNA functionality, which may be relevant in determining the mechanism of action of the ethanoanthracene compounds screened.
2.4.14. Cheminformatics Analysis of Lead Ethanoanthracene Compounds
Estimation of ADME (absorption, distribution, metabolism and excretion) parameters early in the preclinical discovery phase can potentially reduce the number of pharmacokinetics-related drug failures in the clinical phases of drug development [76]. Based on the preliminary antiproliferative studies, a panel of novel compounds (20a–20e, 23a, 23c, 23d, 23f–23i, 23k–23p, 24f, 24l, 25n and 26n) was selected for further investigation of their ADME properties using the Swiss ADME cheminformatics webtool [77] (Supplementary Information Figure S2 and Tables S7–S9). The majority of the compounds satisfy the criteria for effective oral drug development, demonstrating logP values in the range of 1.86–4.98, HBD of 0–1, HBA of 3–5, rotatable bonds of 2–6 and TPSA of 54–91 Å2. The maleimide and N-phenylmaleimide series of compounds are not ionized at a physiological pH, (e.g., 20d, theoretical pKa value of 10.4, calculated from Chemicalise). High blood–brain barrier (BBB) absorption levels and high GI absorption were predicted for the compounds, indicating the potential of these compounds as lead compounds for further development. 20a was identified as the most potent analog evaluated in the nitrovinylanthracene series with an IC50 value of 0.48 µM (HG-3) and 0.61 µM (PGA-1) cell lines (MW = 422, HBD = 0, HBA = 4, Rot bonds = 3, cLogP 3.29, TPSA = 83.20 Å2). The lead compounds tested were not predicted to inhibit the metabolic activity of CYP2D6 or CYP3A4.
The aqueous solubility predicted for the compounds was in the range of 0.011–61.4 µg/mL, with the compounds 20d and 23m predicted to have the highest solubility in the series (61.4 µg/mL and 29.8 µg/mL, respectively). Potential correlations were assessed between the estimated physicochemical properties and biological activity. A negative correlation was determined for molar refractivity, log P and the skin permeation coefficient, while a positive correlation was observed for water solubility and may contribute to the identification of suitable lead compounds.
Pan Assay Interference Compounds (PAINS) contain functional groups or fragments that contribute to high reactivity. Lead compounds (20a–20e, 23a, 23c, 23d, 23f–23i, 23k–23p, 24f, 24l, 25n and 26n) were screened according to PAINS filters to reduce the possibility of such compounds being selected for progression and optimization. Despite the presence of the α,β-unsaturated ketone and nitrovinyl group (Michael acceptors) in the lead molecules considered, no PAINS alerts were flagged (Supplementary Information Table S9) [77]. The Brenk filter (used to identify fragments known to induce toxicity directly or post-metabolic activation for highly reactive and metabolically labile functional groups) identified two structural alerts in the series: the maleimide bridgehead structure and the α,β-unsaturated ketone (Michael acceptor) [77].
2.4.15. In vitro LDH Cytotoxicity Investigation of Selected Lead Compounds 20a, 20f, 23a and 25n
Further biochemical studies were then investigated for selected lead compounds 20a, 20f, 23a and 25n based on their potency in the antiproliferative assays in CLL cells. The lactate dehydrogenase (LDH) assay is used to assess the membrane integrity of cells as a function of the cytoplasmic LDH released. The loss of cell membrane integrity (due to cytotoxic insult) is detectable through LDH release into the cell growth medium. The assay is initiated via the reduction in cellular co-factor NAD+ to NADH catalyzed via the LDH enzyme. Newly formed NADH catalyzes the acidic reduction in iodonitrotetrazolium chloride (INT) to the highly colored formazan (red) (mediated via the diaphorase enzyme) [78,79]. The results for the LDH cytotoxicity assay of the HG-3 cell line for the selected compounds 20a, 20f, 23a and 25n (which were representative of the most potent ethanoanthracene compounds identified in preliminary biochemical screening) are shown in Figure 9A. At the 10-µM treatment concentration, mild to moderate cytotoxicity (19–47%) was observed. The nitrovinyl dimer 20f displayed the lowest LDH release at 19%, with 23a (chalcone maleimide, 25%), 20a (nitrovinyl N-phenylmaleimide 33%) and 25n (chalcone, N-(4-chlorophenyl)maleimide) having the highest cytotoxicity (47%). At the 1-µM treatment concentration, cytotoxicity remained mild to moderate with an observed range of 12–37% with 20f displaying the lowest % LDH release (12%). In the PGA-1 cell line (Figure 9B), 20a displayed the lowest LDH release (9%) at 10 µM, followed by 25n (15%), 20f (19%) and 23a (23%). Cytotoxicity remained low to mild at 1 µM (4–18%) with 23a displaying the lowest LDH release (4%). Interestingly, the assessed compounds were observed to cause a greater degree of LDH release from HG-3 cells compared to PGA-1, suggesting greater cytotoxicity against more aggressive variants of CLL. In addition, the mean TGI (total growth inhibition) value for the potent compound 25n (mean GI50: 0.245 µM) over the NCI 60-cancer-cell-line panel was 18.62 µM, indicating a wide therapeutic window for the compound (Table 7).
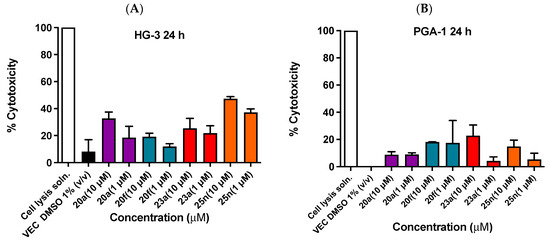
Figure 9.
LDH assay for cytotoxicity of compounds 20a, 20f, 23a and 25n in the HG-3 (Panel A) and PGA-1 (Panel B) cell lines.
Following the treatment of the HG-3 and PGA-1 cells at 10 µM for 24 hr, the amount of LDH released was determined using an LDH assay kit from Promega (G1780). Cell lysis solution = 100% cell lysis and LDH release). Data are presented as % cell death at a concentration of 10 µM. Cells were treated with ethanoanthracene compounds 20a, 20f, 23a and 25n (1 µM and 10 µM) for 24 h. Values are shown as the mean of three independent experiments.
2.4.16. Pro-Apoptotic Effects of Selected Compounds 20a, 20f, 23a and 25n in HG-3 Cells and PGA-1 Cells
The pro-apoptotic effects of selected nitrovinylethanoanthracene compounds 20a and 20f and anthracene chalcones 23a and 25n in HG-3 cells and PGA-1 cells was then investigated using FITC (fluorescein isothiocyanate), Annexin V/PI (propidium iodide) and FACS (fluorescence-activated cell sorting) analysis to characterize the mode of cellular death induced. Apoptosis was assessed as % total apoptosis (total of early (Q3) and late (Q2) apoptosis).
The results from the Annexin V/PI studies of representative potent lead ethanoanthracene compounds 20a, 20f, 23a and 25n are shown in Figure 10, together with our previously reported lead nitrovinylanthracene compound 18a [40]. In HG-3 cells, all compounds produced significant apoptosis that was largely concentration-dependent (Figure 10A,C). At the 10-µM concentration, the most active compounds with 95% total apoptosis were 23a (4-bromophenylchalcone, maleimide) and 20a (nitrostyrene, N-phenylmaleimide), followed by 25n (2-pyridylchalcone, N-(4-chlorophenyl)maleimide) and 20f (nitrostyrene dimer) with 93% and 82% apoptosis, respectively. At a 1-µM treatment concentration, the most potent pro-apoptotic effects were identified for the nitrostyrene derivatives 20a and 20f (both inducing 82% total apoptosis), while the chalcone ethanoanthracenes 25n (37%) and 23a (33% apoptosis) were less effective. The pro-apoptotic effect of nitrostyrene dimer 20f remained constant across all three concentrations, possibly associated with the potent antiproliferative effect in HG-3 cells (IC50 value of 0.17 µM). In PGA-1 cells, all compounds produced significant concentration-dependent apoptosis (Figure 10B,D). At the 10-µM treatment concentration, the nitrostyrene 20a induced the greatest apoptosis (97% total apoptosis), followed by 23a (89%), 20f (88%) and 25n (87%). At the 1-µM treatment concentration, the nitrovinyl compounds 20a and 20f produced the most potent effect with 83% and 76% apoptosis, respectively. These results from compounds 20a, 23a, 25n and 20f compare favorably with the effect of the clinically used drug fludarabine phosphate, which was found to induce a poorer increase in apoptosis (24.6%) for cancer cells isolated from CLL patients [39]. In summary, the results across both cell lines indicate that compounds 20a, 20f, 23a and 25n induce significant apoptosis in a largely concentration-dependent manner and support a pro-apoptotic mechanism of action for this class of ethanoanthracene compounds.
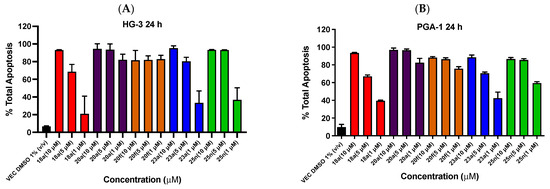
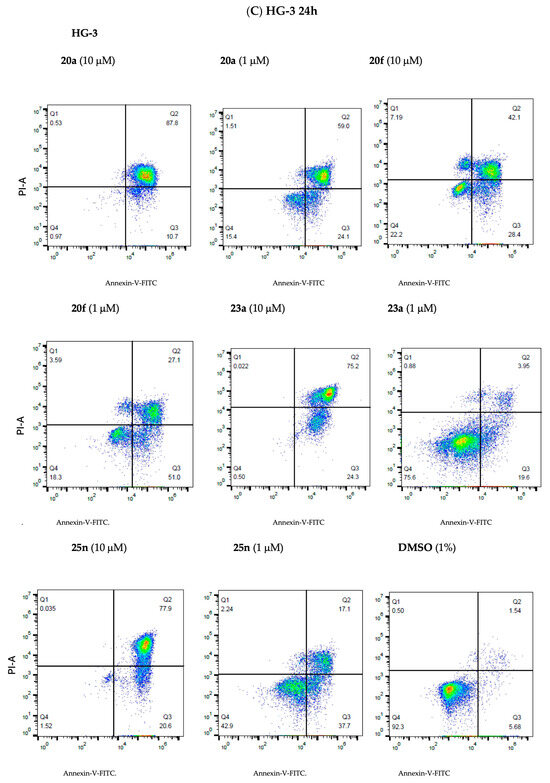
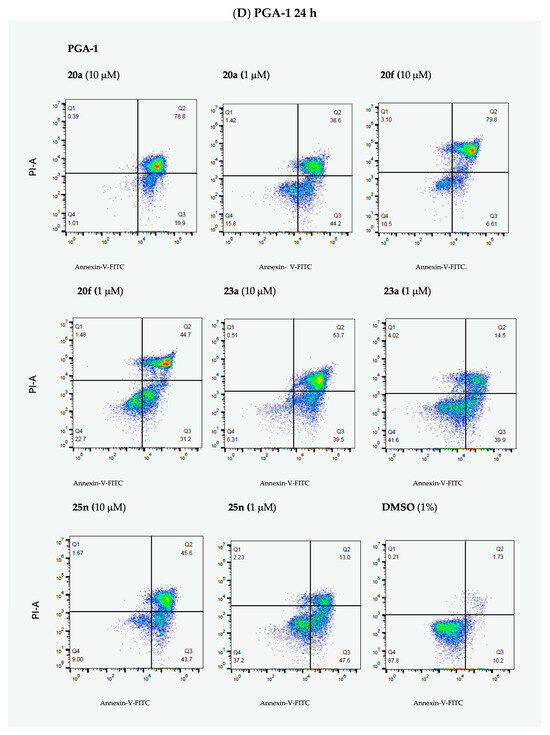
Figure 10.
Ethanoanthracene nitrostyrene compounds 18a, 20a, 20f, 23a and 25n induce apoptosis in HG-3 and PGA-1 CLL cells. Compounds 18a, 20a, 20f, 23a and 25n potently induce apoptosis in HG-3 and PGA-1 cell lines (Annexin V/PI FACS). HG-3 and PGA-1 leukemia cells were treated with 18a, 20a, 20f, 23a and 25n (10 µM, 5 µM and 1 µM) and a control vehicle [(1% DMSO (v/v))] at 48 h for panels A and B, respectively. The % of apoptotic cells was determined by staining with Annexin V-FITC and PI (Panels C and D show compounds 20a, 20f, 23a and 25n at 1- and 10-µM concentrations). The lower left quadrant cells are negative for both Annexin V-FITC and PI, and the upper left shows PI cells that are necrotic. The lower right quadrant shows Annexin-positive cells in the early apoptotic stage, and the upper right shows both Annexin- and PI-positive cells in the late apoptosis stage. The experiment was replicated on three independent days.
2.4.17. Ex Vivo Assessment of Selected Compounds 20a, 23a and 25n in Donors’ Peripheral Blood Mononuclear Cells (PBMCs)
Representative examples of the most potent ethanoanthracene compounds 20a, 23a and 25n were assessed in donor peripheral blood mononuclear cells (PBMCs). Whole blood samples from healthy donors (×5) were isolated, prepared and treated following the protocol previously described [40]. The percentage of apoptosis observed in the treatment of isolated donor PBMCs with nitrovinylethanoanthracene 20a is concentration-dependent (Figure 11A). 20a exhibited moderate toxicity (40%) towards the healthy-donor lymphocytes at the highest concentration tested of 1.25 µM. When compared to the IC50 values in both HG-3 and PGA-1 CLL cell lines of 0.48 µM and 0.61 µM, respectively, relatively low to moderate amounts of apoptotic death in healthy cells was noted (10–35% at concentrations of 0.34 and 0.68 µM). For ethanoanthracene 23a, the percentage of total apoptosis observed is illustrated in Figure 11B. 23a exhibited the lowest toxicity of the three compounds evaluated in healthy-donor PBMCs at its highest treatment concentration of 5 µM with observed total apoptosis of 28%. When these results were compared to the IC50 values of 23a in both HG-3 and PGA-1 CLL cell lines of 2.69 µM and 1.97 µM, respectively, very low amounts of lymphocyte cell death were detected (5–14% at 1.25–2.5 µM). The percentage of total apoptosis observed in the treatment of isolated donor PBMCs with the anthracene–chalcone 25n (normalized against vehicle 0.5% v/v DMSO) is illustrated in Figure 11C, and the dose-response behavior was also concentration-dependent, with compound 25n moderately toxic to healthy-donor PBMCs when treated at a 5.00 µM concentration (39%). Relatively low toxicity (12–16%) was observed at concentrations representative of the IC50 values in both HG-3 and PGA-1 CLL cell lines, which were 1.31 µM and 0.87 µM, respectively.
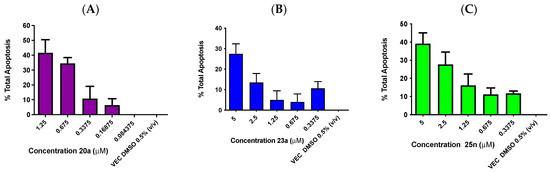
Figure 11.
Percentage of total apoptosis observed upon treatment of isolated donor PBMCs with compounds 20a, 23a and 25n. Ethanoanthracene compounds 20a, 23a and 25n induced apoptosis upon the treatment of isolated human-donor peripheral blood mononuclear cells (PBMCs). Isolated donor PBMCs were treated with compound 20a (Panel A) at 1.25 µM, 0.68 µM, 0.34 µM, 0.17 µM and 0.08 µM concentrations, compounds 23a (Panel B) and 25n (Panel C) at 5 µM, 2.5 µM, 1.25 µM, 0.68 µM and 0.34 µM concentrations, and all were normalized against a control vehicle (0.5% DMSO (v/v)) at 48 h. The % of apoptotic cells was determined via staining with Annexin V-FITC and PI. The experiment was performed individually and replicated on three independent days.
2.4.18. Effect of Pre-Treatment with Antioxidants on Cellular Viability of HG-3 and PGA-1 CLL Cells
Due to the pivotal role that reactive oxygen species (ROS) play in malignant cell transformation and progression, a wide variety of chemotherapeutic agents that rely on ROS modulation (either directly or indirectly) have been identified e.g., the quinone-based anticancer agents doxorubicin, mitomycin C and bleomycin used clinically for breast, small-cell lung and hematologic cancers [80,81,82].
Compounds 20a and 20f (potent nitrovinyl-based ethanoanthracenes), and 23a and 25n (potent chalcone-based ethanoanthracenes), were assessed at treatment concentrations of 10 µM and 1 µM in the absence and presence of the ROS inhibitor N-acetylcysteine (NAC) to assess the potential for the synthesized compounds to mediate their antiproliferative effect through ROS flux. In HG-3 cells, NAC addition impacts the ability of all compounds to reduce cell viability to varying degrees (Figure 12A). With the nitrovinyl 20a and chalcone compound 23a, a dramatic increase of 103% and 78%, respectively, in cell viability was noted at a 10-µM treatment concentration, following pre-treatment with NAC. Smaller relative increases in cell viability were observed for chalcone 25n and nitrovinyl dimer 20f (17% and 14%, respectively). A similar trend in viability was observed at the 1-µM treatment concentration. In PGA-1 cells, NAC addition also impacts on the ability of all compounds to reduce cell viability (Figure 12B). For 20a, viability was increased to 120% upon pre-treatment with NAC, while a moderate increase of 48% in cell viability was observed with chalcone 23a; 25n and 20f showed little change in cell viability. At the lower 1-µM treatment concentration, 20a was the most effective with a 92% increase in cell viability. These results strongly suggest a potential ROS-dependent mechanism for chalcone-based compound 23a and nitrovinyl-based compound 20a, and they support our previous reports of the ROS-dependent action of nitrovinyl-based ethanoantracenes in BL cell lines [43]; meanwhile, the antiproliferative action of chalcone 25n and nitrovinyl dimer 20f showed less dependence on ROS.
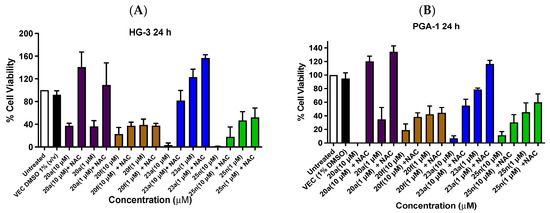
Figure 12.
Effect of antioxidant pre-treatment (N-acetylcysteine, NAC) on the viability of HG-3 and PGA-1 CLL cells treated with compounds 20a, 20f, 23a and 25n. The cell viability of HG-3 and PGA-1 cells was determined with an alamarBlue assay (seeding density: 2 × 105 cells/mL per well for 96-well plates). Compound concentrations of either 1 µM or 10 µM for 24 h were used to treat the HG-3 and PGA-1 CLL cells (in triplicate) with control wells containing vehicle DMSO (1% v/v). The cells were pre-treated with NAC (2 µL, 5 mM) for 1 h, (Panel A,B) and protected from light before then being treated with the compound. The mean value for three independent experiments is shown.
2.4.19. Caspase Inhibition Assay
Caspases (cysteine-aspartic proteases) are a family of aspartic acid residue-specific regulatory cysteine proteases that play an essential role in key cellular homeostatic processes such as inflammation and programmed cell death through the induction of apoptosis [83,84]. To assess whether the biological activity of both major ethanoanthracene classes (nitrostyrene and chalcone-based) is caspase-dependent, compounds 20a and 23a (representing Series 1 and Series 4, respectively) (Figure 13) were selected for evaluation at treatment concentrations of 10 µM and 1 µM across both CLL cell lines using a caspase assay protocol. In HG-3 cells, pre-treatment with pan-caspase inhibitor (CI) Z-VAD-FMK [85] (20 µM) resulted in an increase in the viable cells remaining of 24% for compound 23a (10 µM) and 21% at 1 µM (Figure 13A). In contrast to this, with compound 20a, a decrease of 10% in cell viability was noted at 10 µM and 2% at 1 µM. Little difference was found between cell viability responses (with or without the caspase inhibitor) at both 10-µM and 1-µM concentrations for compound 20a. In the PGA-1 cell lines, pre-treatment with Z-VAD-FMK (20 µM) resulted in a 68% increase in viable cells for compound 23a at 10 µM and an increase of 79% at 1 µM (Figure 13B). For compound 20a, an increase of 17% at 1 µM was observed, suggesting a correlation between antiproliferative activity and the induction of apoptotic cell death for nitrostyrene compound 20a. The involvement of caspases in the ability of compound 23a to reduces cell viability in HG-3 and PGA-1 cells was demonstrated, together with the pro-apoptotic effects of 20a in PGA-1 cells, suggesting the selective roles of the nitrovinyl and α,β-unsaturated ketone pharmacophores in the induction of apoptosis via caspases.
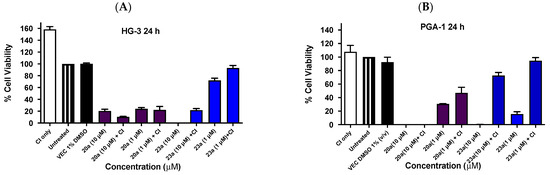
Figure 13.
Effect of pre-treatment with caspase inhibitor Z-VAD-FMK on HG-3 and PGA-1 cell viability for compounds 20a and 23a. Cell viability analysis (24 h) for inhibitor studies of compounds 20a and 23a in HG-3 (Panel A) and PGA-1 (Panel B) CLL cell lines: the HG-3 and PGA-1 CLL cells (2 × 105 cells/mL) were pre-treated at 37 °C with 40 µM of caspase inhibitor (CI) (Z-VAD-FMK) for 4 h prior to compound treatment at 1 µM and 10 µM for 24 h. The cell proliferation of HG-3 and PGA-1 cells was determined with an alamarBlue assay (CI = caspase inhibitor, n = 2).
2.5. Computational Study
2.5.1. Molecular Modeling Study
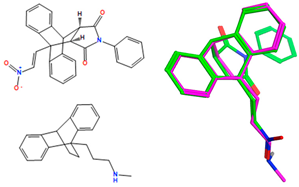
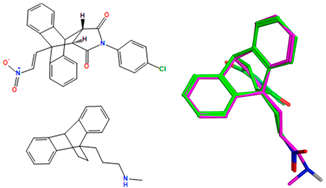
The synthesized ethanoanthracene compounds (series 1–7) are structurally related to the antidepressant maprotiline 16, which displays antiproliferative effects in B-cell malignancies and may have similar cellular antiproliferative effects. To examine the structural similarities, selected compounds (20a–20f, 23a, 23c, 23f–23i, 23k, 23l, 23n, 23p, 24f, 24l, 25n and 26n) were overlaid on maprotiline using MOE flexible alignment, as described in our previous work; this approach is based on similarity terms such as hydrogen bond donor/acceptor, aromaticity and partial charge [40]. Similar functional groups in each molecule are flexibly aligned and superimposed using a stochastic search procedure while the full conformational flexibility for each structure is samples. All databases and reference structures are provided in the Supplementary Information as .sdf or .mdb files.
An overlay of the most potent ethanoanthracenes 20a, 20b, 20d, 20f, 23a and 25n (displayed as green in their respective overlays with maprotiline pink) and reference nitrovinylanthracene compound 18b, together with the IC50 values in the HG-3 and PGA-1 CLL cell lines, is shown in Table 8. The overlay scores of the enthanoanthracene compounds were lower (better) than the lead nitrostyrene compound 18b. Common molecular features were clearly identified, e.g., the ethanoanthracene structure that overlays with the 9,10-dihydroanthracene core structure of maprotiline, whereas the nitrostyrene and α,β-unsaturated ketone mapped closely to the cyclic core of maprotiline. The MOE flexible alignment demonstrated a lack of correlation between the lowest-(best-) scored compounds and those with the most promising cellular data for the CLL cell lines (Table 8). The overlay results obtained for all the ethanoanthracene compounds in this study are inconclusive (Table S10, Supplementary Information), and they suggest that these compounds may have a different mechanism of action when compared with maprotiline.

Table 8.
Overlay of ethanoanthracenes (20a, 20b, 20d, 20f, 23a and 25n) and nitrostyrene 18b on maprotiline with their overlay scores a.
2.5.2. In Silico Target Prediction
Computational modeling was investigated to gain insight into the possible mechanisms of action of the ethanoanthracenes. Selected novel compounds (20a, 20b, 20d–20f, 23a, 23c, 23f–23i, 23k, 23l, 23n, 23p, 24f, 24l, 25n and 26n), together with lead anthracene 18b and maprotiline, were submitted to the SwissTargetPrediction service (STP) [86] for evaluation. The STP methodology is conceptually rooted in the SAR principle, which states that similar compounds tend to have similar biological activity. Thus, for a given query structure, STP finds structurally similar compounds with known activities and uses them to compile a list of potential targets for the given query structure.
STP generated a list of potential targets for each of the submitted compounds. Overall, all predictions were assigned relatively low internal confidence scores via STP, mostly at around 0.05–0.15. However, this was consistent with most predictions for maprotiline itself, except for a full 1.00 prediction confidence score for its several already experimentally confirmed targets. For dimeric compound 20f, STP was not able to provide predictions with any degree of confidence. The tested compounds share similar in vitro cytotoxic effects, and it could be assumed that these observed effects are caused by the compounds hitting the same unknown targets(s). Thus, targets predicted for the majority of the tested compounds became the focus of the STP-based analysis. To identify the most prevalent predicted targets among the 21 tested compounds, the STP target lists were aggregated by summing the individual prediction confidence scores for each compound. The resulting target list thus contains the cumulative prediction confidences for each given target, within the 21 tested compounds (Figure 14).
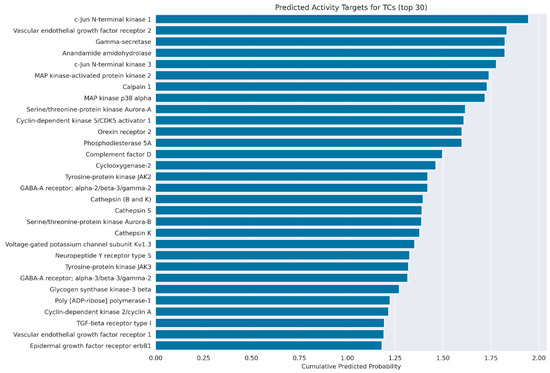
Figure 14.
Cumulative probability scores for compounds 20a, 20b, 20d–20f, 23a, 23c, 23f–23i, 23k, 23l, 23n, 23p, 24f, 24l, 25n and 26n for the 30 targets most strongly indicated via STP.
Since the score contributions of individual compounds are in the 0.1-score range, these targets were found to be a hit by STP for most of the 21 tested compounds. For a more general view of the target types predicted for the tested compounds, the cumulative probability scores for individual targets were further aggregated by summing based on their general protein family groups. To obtain a direct comparison between target groups predicted for the tested compounds and target groups predicted for maprotiline, both results were individually converted to standard z-scores (Figure 15).
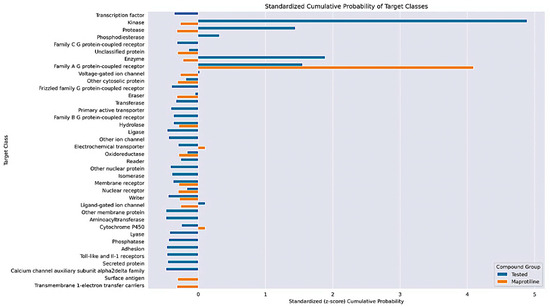
Figure 15.
Standardized cumulative probability scores of target groups indicated via STP for the tested compounds 20a, 20b, 20d–20f, 23a, 23c, 23f–23i, 23k, 23l, 23n, 23p, 24f, 24l, 25n and 26n compared to Maprotiline. Scores were standardized using z-scores (i.e., differences in standard deviations from their mean).
The aggregated STP outputs indicate that the tested compounds might, indeed, share some family A GPCR activity with maprotiline, which is a known antagonist of muscarinic acetylcholine receptors, having antiproliferative activity [44]. However, in contrast with maprotiline, there are stronger ligand-similarity hits against a variety of kinases, especially the c-Jun N-terminal (JNK), MAP and VEGFR kinases. These predicted kinase targets are reasonable, as each has a role in promoting cellular proliferation, so blocking them would promote the observed functional phenotype. It is interesting that the COMPARE analysis indicated that the compounds mediate cytotoxicity through DNA synthetic pathways. The in silico target evaluation remains inconclusive, but it offers insight into future investigations for these compounds, e.g., molecular docking with select family-A GPCRs, as well as JNK, MAP and VEGFR kinases.
3. Materials and Methods
3.1. Chemistry
All reagents were commercially available and were used without any further purification unless otherwise indicated. Melting points were measured on a Stuart Melting Point Apparatus, SMP20, and they are uncorrected. Infra-red (IR) spectra were recorded on a Perkin Elmer FT-IR Paragon 1000 spectrometer. 1H and 13C nuclear magnetic resonance spectra (NMR) were recorded at 25 °C on a Bruker DPX 400 spectrometer (400.13 MHz, 1H; 100.61 MHz, 13C), Bruker Avance III 400 or Avance II 600 (400.13 MHz/600.13 MHz, 1H; 100.61 MHz/150.61 MHz, 13C) in either CDCl3 (internal standard tetramethylsilane (TMS)) or DMSO-d6. For CDCl3, 1H-NMR spectra were assigned relative to the TMS peak at 0.00 ppm, and 13C-NMR spectra were assigned relative to the middle CDCl3 peak at 77.0 ppm. For CD3OD, 1H and 13C-NMR spectra were assigned relative to the center peaks of the CD3OD multiplets at 3.30 ppm and 49.00 ppm, respectively. Coupling constants are reported in Hertz. High-resolution mass spectrometry (HRMS) was carried out by Dr. Gary Hessman of the School of Chemistry, Trinity College Dublin, using atmospheric pressure chemical ionization (APCI) with the occasional use of electrospray ionization (ESI-MS) in both positive and negative modes. ESI mass spectra were acquired using a Bruker micrOTOF-Q III spectrometer interfaced to a Dionex UltiMate 3000 LC in positive and negative modes, as required. Masses were recorded over the range of 100–1400 m/z. APCI experiments were carried out on a Bruker micrOTOF-Q III spectrometer interfaced with a Dionex UltiMate 3000 LC or direct insertion probe. The instrument was operated in positive or negative mode, as required. Masses were recorded over a range of 100–1600 m/z. Mass measurement accuracies of <±5 ppm were obtained. TLC was carried out on silica gel on aluminum foils with fluorescent indicator F-254 nm. Flash-column chromatography was carried out on Merck Kieselgel 60 (particle size: 0.040–0.063 mm). Analytical high-performance liquid chromatography (HPLC) was performed using a Waters 2487 Dual Wavelength Absorbance detector, a Waters 1525 binary HPLC pump and a Waters 717plus Autosampler with a Thermo Scientific Hypersil GOLD C18 reverse-phase 5 µm 150 × 4.6 mm chromatography column. Samples were detected using wavelengths of 232 nm compounds. All samples were analyzed using acetonitrile (75%)–TFA (0.1%) in water (25%) over 15 min and a flow rate of 1 mL/min. Microwave experiments were carried out using the Discover CEM microwave synthesizer on standard power setting (300 watts) unless otherwise stated. Maleimides 19a–c and (E)-9-(2-nitrovinyl)-9,10,11,15- tetrahydro-9,10-[3,4]epipyrroloanthracene-12,14-diones 20a–h were prepared as we previously reported [43] (see Supplementary Information). (E)-3-(Anthracen-9-yl)-1- phenylprop-2-en-1-ones 21a–d, 21f–j and 21l–q were prepared as previously reported [87,88,89,90,91,92,93,94] (see Supplementary Information).
3.2. General Procedure for the Preparation of (E)-3-(anthracen-9-yl)-1-phenylprop-2-en-1-ones 21a–q
To a solution of NaOH (6 mmol, 0.24 g) in 50% aqueous EtOH (20 mL) was added the appropriate acetophenone (5.02 mmol). After the dissolution of the acetophenone, 9-anthracenecarboxaldehyde (5.02 mmol, 1.035 g) was added, resulting in a colored solution. This solution was stirred at room temperature for 24 h to achieve a colored suspension that was filtered, washed with minimal cold EtOH and dried. The crude product was then recrystallized from the minimal amount of EtOH or MeOH to afford a pure product.
(E)-3-(Anthracen-9-yl)-1-(3,4-dimethoxyphenyl)prop-2-en-1-one (21e) was synthesized from 3′,4′-dimethoxyacetophenone (5.02 mmol, 0.904 g) and 9-anthracenecarboxaldehyde (5.02 mmol, 1.035 g), according to the general procedure above; yellow powder (85%) Mp. 138–139 °C. 1H NMR (400 MHz, CDCl3) δ 3.97 (d, J = 11.61 Hz, 6 H, 2xCH3), 6.91 (d, J = 8.71 Hz, 1 H), 7.40–7.53 (m, 4 H, ArH), 7.54–7.58 (d, J = 15.76 Hz, 1 H,CH=C), 7.65–7.72 (m, 2 H, ArH), 8.01–8.05 (m, 2H, ArH), 8.29–8.32 (m, 2H, ArH), 8.47 (br.s, 1H, ArH), 8.77 (d, J = 15.76 Hz, 1H, ArH). 13C NMR (101 MHz, CDCl3) ppm 53.07, 53.11, 110.07, 110.85, 123.34, 125.39, 126.31, 128.20, 128.87, 129.61, 130.80, 131.03, 131.31, 141.00, 187.81 (C=O). IR ν max (KBr): 3081.44, 3049.16, 2968.80 (Ar C-H), 1656.98 (C=O), 1622.51 (trans C=C), 1591.24, 1580.66, 1515.04, 1442.23, 1416.45 (Ar C=C), 1266.73, 1014.80 (C-O) cm−1. HRMS (ESI) calculated for C25H20NaO3 [M++Na] 391.1305: found 391.1307.
(E)-3-(Anthracen-9-yl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (21k) was synthesized from 1-(3,4,5-trimethoxyphenyl)ethan-1-one (5.02 mmol, 0.65 mL) and 9-anthracenecarboxaldehyde (5.02 mmol, 1.035 g) according to the general procedure above; yellow crystals (86%), Mp. 160–164 °C. 1H NMR (400 MHz, CDCl3) δ 3.90–3.99 (m, 9H), 7.35 (s, 2 H), 7.49–7.56 (m, 5H), 8.03–8.08 (m, 2H), 8.28–8.35 (m, H), 8.50 (s, 1 H), 8.79 (d, J = 15.76 Hz, 1H). 13C NMR (101 MHz, CDCl3) ppm. 56.35, 61.00, 61.45, 62.52, 106.22, 125.38, 126.40, 128.37, 128.92, 129.62, 130.27, 130.70–131.49, 133.10, 141.70, 153.24, 188.33 (C=O). IR ν max (KBr): 3045.78 (Ar C-H), 1657.91 (C=O), 1592.99 (Ar C=C), 1268.62 (C-O) cm−1. HRMS (APCI) calculated for C26H22NaO4 [M++Na]: 421.1421; found: 421.1410.
3.3. General Preparation for (E)-9-(3-oxo-3-phenylprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-diones 22a-q
To a solution of the appropriate chalcone anthracene analog 21a-q (1.0 mmol) in toluene (2 mL) was added dienophile maleic anhydride (1.3 mmol). The mixture was stirred and heated at 90 °C for 48 h. The reaction mixture was then cooled to room temperature, and the resulting solid was isolated via filtration. The solid product was washed with toluene (2 mL) and diethyl ether (2 mL) and then recrystallized from toluene.
9-(E)-3-(4-Bromophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione (22a) was prepared from (E)-3-(anthracen-9-yl)-1-(4-bromophenyl)prop-2-en-1-one (1.0 mmol, 0.3873 g) and maleic anhydride (1.3 mmol, 0.13 g) according to the general procedure; light yellow powder (86%), Mp. 254–256 °C [51]. 1H NMR (400 MHz, CDCl3) δ 3.65 (dd, J = 9.12, 3.32 Hz, 1 H, CH), 3.81 (d, J = 9.12 Hz, 1 H, CH), 4.87 (d, J = 3.32 Hz, 1 H, CH), 7.16–7.36 (m, 6 H,5 x ArH, 1xC=CH), 7.39–7.44 (m, 2 H), 7.66–7.76 (m, 3 H, ArH), 7.95 (d, J = 16.17 Hz, 1 H, C=CH), 8.03 (d, J = 8.71 Hz, 2 H, ArH)13C NMR (101 MHz, CDCl3) ppm 45.46, 48.96, 49.28, 51.93, 123.52, 123.87, 124.18, 127.12, 127.63, 130.39, 130.92, 132.16, 136.24, 137.36, 138.85, 140.22, 141.51, 168.82, 169.74, 188.80 (C=O). IR ν max (KBr): 3328.89, 3074.11 (Ar C-H), 1773.04, 1665.51 (C=O), 1617.10, trans (C=C), 1581.33, 1458.65, 1397.18, 1076.35 (C-O) cm−1. LRMS (APCI) 485.04 [M++H].
9-(E)-3-(4-Nitrophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione (22b) was prepared from (E)-3-(anthracen-9-yl)-1-(4-nitrophenyl)prop-2-en-1-one (1.0 mmol, 0.3534 g) and maleic anhydride (1.3 mmol, 0.13 g) according to the general procedure above; cream powder (77%), Mp. >200 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.80 (dd, J = 9.12, 3.32 Hz, 1 H), 4.25 (d, J = 9.12 Hz, 1 H), 4.95 (d, J = 3.32 Hz, 1 H), 7.16–7.28 (m, 3 H), 7.31–7.36 (m, 1 H), 7.41 (dd, J = 5.60, 3.11 Hz, 1 H), 7.53–7.57 (m, 1 H), 7.55 (d, J = 7.05 Hz, 1 H), 7.86 (s, 1 H), 8.34–8.44 (m, 4 H). 13C NMR (101 MHz, DMSO-d6) 49.73, 49.78, 51.71, 56.89, 109.99, 123.47, 124.01, 124.46, 124.51, 125.00, 125.74, 125.77, 127.03, 127.53, 127.94, 130.55, 132.01, 138.92, 139.80, 141.19, 142.06, 142.92, 150.52, 170.66, 171.37, 189.02 (chalcone C=O) ppm. IR ν max (KBr): 2972.12, (C-H), 1770.72 (C=O), 1667.96, (Ar C=C), 1596.43, (NO2), 1516.78, 1346.74 (C-O) cm−1. HRMS (APCI) calculated for C27H16NO6 [M+−H]: 450.0983; found: 450.0982.
(E)-3-(4-Ethylphenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione (22c) was prepared from (E)-3-(anthracen-9-yl)-1-(4-ethylphenyl)prop-2-en-1-one (1.0 mmol, 0.336 g) and maleic anhydride (1.3 mmol, 0.13 g) according to the general procedure above; cream powder (70%), Mp. 195–200 °C. 1H NMR (400 MHz, DMSO-d6) δ 1.16–1.23 (m, 3H), 2.70 (q, J = 7.46 Hz, 7H), 3.79 (dd, J = 9.12, 3.32 Hz, 4H), 4.25 (d, J = 9.12 Hz, 4H), 4.94 (d, J = 3.32 Hz, 4H), 7.16–7.20 (m, 8H), 7.20–7.23 (m, H), 7.23–7.27 (m, 9H), 7.28–7.33 (m, 1H), 7.37–7.42 (m, 4H), 7.43 (d, J = 8.29 Hz, 2H), 7.54 (d, J = 6.63 Hz, 4H), 7.74–7.80 (m, 4H), 7.82–7.88 (m, 4H), 8.09 (d, J = 8.29 Hz, 7H). 13C NMR (101 MHz, DMSO-d6) ppm: 15.65, 28.70, 44.80, 49.37, 49.60, 51.58, 123.37, 123.91, 125.77, 127.03, 127.47, 127.79, 129.40, 132.06, 135.37, 138.95, 140.03, 140.65, 141.25, 142.31, 150.55, 170.57, 171.42, 189.00 (chalcone C=O) IR ν max (ATR): 3071.36, 2882.33 (Ar C-H), 1778.67, 1670.45 (C=O), 1619.17 (C=C), 1604.57, 1580.44 (Ar C=C), 1465.80 (CH), 1374.93 (CH), 1224.51 (C-O) cm−1. HRMS (APCI): calculated for C29H23O4: 435.1591 [M++H]; found: 435.1595.
9-(E)-3-(2,4-Dichlorophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione (22d) was prepared from (E)-3-(anthracen-9-yl)-1-(2,4-dichlorophenyl)prop-2-en-1-one (1.0 mmol, 0.377 g) and maleic anhydride (1.3 mmol, 0.13 g) according to the general procedure above; beige powder (76%), Mp. 232–236 °C. 1H NMR (400 MHz, CDCl3) δ 3.60 (dd, J = 9.12, 3.32 Hz, 1H), 3.72 (d, J = 9.54 Hz, 1H), 4.84 (d, J = 2.90 Hz, H), 7.20–7.28 (m, 5H, ArH), 7.28–7.34 (m, 2 H, ArH, 1, CH=C), 7.35–7.39 (m, 1H, ArH), 7.39–7.44 (m, 2H, ArH), 7.52 (d, J = 1.66 Hz, 1H, ArH), 7.68 (d, J = 8.29 Hz, 1H, ArH), 7.75 (d, J = 16.59 Hz, 1H, CH=C). 13C NMR (101 MHz, CDCl3) ppm 45.49, 49.12, 51.74, 123.44, 123.73, 124.30, 125.64, 127.11, 127.63, 127.92, 128.16, 130.46, 130.95, 134.60, 140.21, 141.14, 143.00, 168.53, 169.72, 191.11 (chalcone C=O) ppm. IR ν max (KBr): 3071.19 (Ar C-H), 1776.62, 1662.20 (C=O), 1619.95 (C=C), 1580.28, 1456.96 (Ar C=C), 1212.92 (C-O) cm−1. HRMS (APCI) calculated for C27H17Cl2O4: 475.0498 [M++H]; found 475.0491.
9-(E)-3-(3,4-Dimethoxyphenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione (22e) was prepared from (E)-3-(anthracen-9-yl)-1-(3,4-dimethoxyphenyl)prop-2-en-1-one (1.0 mmol, 0.3684 g) and maleic anhydride (1.3 mmol, 0.13 g) according to the general procedure above; cream/yellow powder (83%), Mp. 243–246 °C. 1H NMR (400 MHz, CDCl3) δ 3.63 (dd, J = 9.33, 3.11 Hz, 1H, CH), 3.81 (d, J = 9.54 Hz, 1H, CH), 3.87 (s, 3H, OCH3), 3.89 (s, 3H, OCH3), 4.85 (d, J = 3.32 Hz, 1H, CH), 6.96 (d, J = 8.29 Hz, 1H, ArH), 7.19–7.27 (m, 4H, ArH), 7.31–7.37 (m, 2H, ArH), 7.37–7.43 (m, 2H, ArH), 7.73–7.76 (m, 2H, ArH, CH=C), 7.78 (s, 1 H), 7.82 (dd, J = 8.29, 2.07 Hz, 1 H, ArH), 7.87 (d, J = 16.2 Hz, 1H, CH=C) ppm. 13C NMR (101 MHz, CDCl3) ppm 49.30, 54.1, 54.38, 56.31, 60.74, 61.03, 110.27, 111.02, 123.59, 123.70, 124.07, 125.53, 127.08, 127.54, 127.89, 128.04, 130.68, 131.47, 137.43, 139.09, 139.75, 140.26, 141.75, 149.30, 153.67, 175.36, 176.17, 192.77 (chalcone C=O). IR ν max (ATR): 3071.19 (Ar C-H), 1777.39, 1668.50 (C=O), 1619.46 (C=C), 1580.35, 1456.83 (Ar C=C), 1213.57, 1018.24 (C-O) cm−1. HRMS (ESI): calculated for C29H22NaO6; 489.1309 [M+−H] found: 489.1305.
(E)-9-(3-Oxo-3-phenylprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione (22f) was synthesized from (E)-3-(anthracene-9-yl)-1-phenylprop-2-en-1-one (1.0 mmol, 0.308 g) and maleic anhydride, (1.3 mmol, 0.13 g) according to the general procedure above; white powder (91%), Mp 214–218 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.81 (dd, J = 9.12, 3.32 Hz, 1H), 4.27 (d, J = 9.12 Hz, 1H), 4.96 (d, J = 3.32 Hz, 1H), 7.19–7.29 (m, 5H), 7.30–7.35 (m, 1H), 7.39–7.45 (m, 1H), 7.56 (d, J = 6.63 Hz, 1H), 7.58–7.65 (m, 2H), 7.68–7.74 (m, 1H), 7.77–7.92 (m, 2H), 8.14–8.21 (m, 2H). 13C NMR (101 MHz, DMSO-d6) 38.57–41.35, 44.84, 48.69–50.08, 51.64, 122.68–124.27,124.99, 125.48–125.98, 127.06, 127.40–128.13, 128.64, 128.91–129.60, 132.07, 134.01, 138.96,141.21, 142.29, 170.61, 171.43, 189.75 (chalcone C=O) ppm. IR ν max (ATR): 1774.31 (C=O), 1634.26 (C=C), 1221.80 (C-O) cm−1. HRMS (APCI): calculated for C27H18O4 [M++Na]:429.1097; found 429.1101.
9-(E)-3-(4-Iodophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione (22g) was prepared from (E)-3-(anthracen-9-yl)-1-(4-iodophenyl)prop-2-en-1-one (1.0 mmol, 0.434g) and maleic anhydride (1.3 mmol, 0.13 g) according to the general procedure above, light yellow powder (81%), Mp. 228–231 °C. 1H NMR (400 MHz, CDCl3) δ 3.63 (dd, J = 9.12, 2.90 Hz, 1H, CH), 3.79 (d, J = 9.12 Hz, 1H, CH), 4.86 (d, J = 3.32 Hz, 1H, CH), 7.15–7.34 (m, 6H, ArH), 7.36–7.44 (m, 2 H, ArH), 7.70 (d, J = 16.17 Hz, 1H, CH=C), 7.83–7.97 (m, 5 H, ArH, CH=C). 13C NMR (101 MHz, CDCl3) ppm 45.46, 48.97, 49.27, 51.93, 123.53, 123.88, 124.20, 125.59, 127.12, 127.63, 127.95, 128.13, 130.24, 130.88, 136.77, 137.38, 138.17, 138.86, 140.22, 141.53, 168.83, 189.12 (chalcone C=O). IR ν max (ATR): (C-H) 3016.49, 2876.80, (trans C=C) 1861.84, (C=O) 1771.38, (Ar C=C) 1619.73, 1579.29, 1479.43, 1456.94, (C-O) 1292.32, (C-I) 619.66 cm−1. LRMS (APCI): 531.20 [M+−H]. HRMS (APCI): calculated for C27H16IO4 [M+−H]: 531.0099; found 531.0102.
9-(E)-3-(4-Fluorophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione (22h) was prepared from (E)-3-(anthracen-9-yl)-1-(4-fluorophenyl)prop-2-en-1-one (1.0 mmol, 0.3264 g) and maleic anhydride (1.3 mmol, 0.13 g) according to the general procedure above; cream/yellow powder (79%), Mp. 242–247 °C. (HPLC: 97.8%, RT 6.17 min). 1H NMR (400 MHz, CDCl3) δ 3.64 (dd, J = 9.54, 3.32 Hz, 1H, CH), 3.81 (d, J = 9.12 Hz, 1H, CH), 4.87 (d, J = 3.32 Hz, 1H, CH), 7.18–7.28 (m, 6H, ArH), 7.29–7.36 (m, 2 H, ArH), 7.36–7.45 (m, 2H, ArH), 7.75 (d, J = 16.59 Hz, 1H, CH=C), 7.93 (d, J = 16.59 Hz, CH=C), 8.16–8.24 (m, 2H, ArH). 13C NMR (101 MHz, CDCl3) ppm 45.47, 48.96, 49.30, 51.91, 115.73–116.31, 123.54, 123.91, 124.17, 125.58, 127.12, 127.61, 128.03, 131.06, 131.59, 137.38, 140.23, 141.09, 141.58,168.86, 169.78, 188.26 (chalcone C=O). IR ν max (ATR): 3064.79, 2968.92 (Ar C-H), 1773.01 (C=O),1667.75 (C=C), 1625.39, 1585.13, 1481.57, 1458.78 (Ar C=C), 1292.88 (C-F), 1069.82 (C-O) cm−1. LRMS (APCI): 423.23 [M+−H]. HRMS (APCI): calculated for C27H16FO4 [M+−H]: 423.103811; found 423.103417.
(E)-9-(3-(4-Methoxyphenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione (22i) was synthesized from (E)-3-(anthracen-9-yl)-1-(4-methoxyphenyl)prop-2-en-1-one (1.0 mmol, 0.338 g) and maleic anhydride (1.3 mmol, 0.128 g) according to the general procedure above; (30%), Mp. 200–205 °C. 1H NMR (400 MHz, CDCl3) δ 3.81 (dd, J = 8.92, 3.11 Hz, 1H) 3.87 (s, 3H) 4.27 (d, J = 9.12 Hz, 1H) 4.96 (d, J = 3.32 Hz, 1H) 7.10–7.16 (m, 2H) 7.20 (d, J = 3.73 Hz, 2H) 7.21 -7.29 (m, 4H) 7.29–7.33 (m, 1H) 7.42 (dd, J = 4.98, 3.73 Hz, 1H) 7.56 (d, J = 6.63 Hz, 1H) 7.73–7.91 (m, 1H) 8.15–8.20 (m, 2H)13C NMR (101 MHz, CDCl3) ppm 40.15–40.69, 44.50, 49.19–50.26, 51.26, 51.57, 55.08, 57.04–58.17, 114.72, 122.69, 127.03, 130.49, 131.59, 138.97, 140.11, 141.27, 142.39, 170.58, 171.44, 175.90, 185.10, 187.87 (chalcone C=O). IR ν max (ATR): 1670.35 (C=O), 1600.48 (Ar C=C), 1229.19 (C-O) cm−1. HRMS (APCI): calculated for C28H19O5 [M+−H]: 435.1238; found: 435.1241.
(E)-9-(3-Oxo-3-(p-tolyl)prop-1-en-1-yl)-9,10-dihydro-9,10-[3,4] furanoanthracene-12,14-dione (22j) was synthesized from (E)-3-(anthracen-9-yl)-1-(p-tolyl)prop-2-en-1-one (1.0 mmol, 0.3384 g) and maleic anhydride (1.3 mmol, 0.13 g) according to the general procedure above; white powder (73%), Mp. 223–227 °C [51]. 1H NMR (400 MHz, CDCl3) δ 2.41 (s, 3H) 3.81 (dd, J = 8.92, 3.11 Hz, 1H) 4.27 (d, J = 9.12 Hz, 1H) 4.96 (d, J = 2.90 Hz, 1H) 7.20 (d, J = 3.73 Hz, 2H) 7.21–7.29 (m, 3H) 7.29–7.34 (m, H) 7.42 (d, J = 7.88 Hz, 3H) 7.56 (d, J = 6.63 Hz, 1H) 7.82 (q, J = 16.17 Hz, 2H) 8.08 (d, J = 7.88 Hz, 2H). 13C NMR (101 MHz, CDCl3) ppm 21.70, 44.81, 49.00–50.00, 123.38, 124.98, 127.04, 127.49, 127.85, 129.30, 129.99, 132.05, 135.15, 138.96, 140.06, 140.69, 142.33, 144.52, 170.59, 171.43, 189.16 (chalcone C=O). IR ν max (ATR): 1769.23 (C=O), 1603.35 (Ar C=C), 1458.64 (C-CH3), 1228.91 (C-O) cm−1. LRMS (APCI): 419.30 [M++H]. HRMS (APCI): calculated for C29H24NaO5 [M++Na]: 475.1516; found 475.1512.
(E)-9-(3-Oxo-3-(3,4,5-trimethoxyphenyl)-prop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione (22k) was synthesized from (E)-3-(anthracene-9-yl)-1-(4-chorophenyl)prop-2-en-1-one (1.0 mmol, 0.398 g) and maleic anhydride (1.3 mmol, 0.13 g) according to the general procedure above; white powder (78%), Mp. 262–266 °C. 1H NMR (400 MHz, CDCl3) δ 3.77 (s, 3H), 3.80 (dd, J = 9.12, 3.32 Hz, 1H), 3.87 (s, 6H) 4.27 (d, J = 9.12 Hz, 1H), 4.96 (d, J = 3.32 Hz, 1H) 7.20–7.23 (m, 2H) 7.23–7.28 (m, 3H) 7.32–7.35 (m, 1H) 7.40–7.43 (m, 1H) 7.48 (s, 2H) 7.56 (d, J = 6.22 Hz, 1H) 7.72–7.91 (m, 2 H). 13C NMR (101 MHz, CDCl3) ppm 43.55, 44.81, 49.23–49.97, 56.55, 60.70, 106.84, 123.39, 123.96, 125.00, 125.78, 127.50, 132.58, 132.95, 138.99, 139.83–140.53, 153.45, 171.40, 188.92 (chalcone C=O). IR ν max (ATR): 1662.19 (C=O), 1571.62 (Ar C=C), 1231.78 (C-O) cm−1. HRMS (APCI): calculated C30H25O7 [M++H]: 495.1449; found: 495.1449.
(E)-9-(3-(4-Chlorophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10[3,4]furanoanthracene-12,14-dione (22l) was synthesized from (E)-3-(anthracen-9-yl)-1-(4-chlorophenyl)prop-2-en-1-one (1.0 mmol, 0.3428 g) and maleic anhydride (1.3 mmol, 0.13 g) according to the general procedure above; white powder (89%), Mp. 248–252 °C. 1H NMR (400 MHz, CDCl3) δ 3.81 (dd, J = 8.71, 3.32 Hz, 1H), 4.27 (d, J = 9.12 Hz, 1H), 4.96 (d, J = 3.32 Hz, H), 7.18–7.29 (m, 5H), 7.30–7.35 (m, 1H), 7.42 (dd, J = 5.60, 3.11 Hz, 1H), 7.56 (d, J = 7.05 Hz, 1H), 7.67–7.72 (m, 2H), 7.79–7.90 (m, 2H), 8.16–8.21 (m, 2H). 13C NMR (101 MHz, CDCl3) ppm 39.13–40.72, 44.90, 125.51, 128.81, 129.41–129.54, 130.18, 131.84, 134.30, 135.66, 142.34, 143.69, 148.97, 149.27, 150.80, 171.10, 191.34 (chalcone C=O). IR ν max (ATR): 1773.83 (C=O), 1666.32 (C=C), 1618.07 (Ar C=C), 1221.94 (C-O), 712.12 (C-Cl) cm−1 HRMS (APCI): calculated for C27H17ClO4 [M++H]: 439.0743; found: 439.0739.
9-(E)-3-Oxo-3-(pyridin-4-yl)prop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione (22m) was prepared from (E)-3-(anthracen-9-yl)-1-(pyridin-4-yl)prop-2-en-1-one (1.0 mmol, 0.309 g) and maleic anhydride (1.3 mmol, 0.13 g) according to the general procedure above; light brown solid (99%), Mp. >200 °C. 1H NMR (400 MHz, CDCl3) δ 3.65 (dd, J = 9.12, 2.90 Hz, 1H, CH), 3.80 (d, J = 9.12 Hz, 1H, CH), 4.87 (d, J = 2.49 Hz, 1H, CH), 7.10–7.23 (m, 3H, ArH), 7.25–7.34 (m, 3H, ArH), 7.36–7.46 (m, 2H, ArH), 7.70 (d, J = 16.17 Hz, 1H, CH=C), 7.94 (d, J = 4.98 Hz, 2 H, ArH), 8.01 (d, J = 16.17 Hz, CH=C). 13C NMR (101 MHz, CDCl3) ppm 45.44, 48.99, 49.24, 51.99, 123.42, 123.71, 124.33, 125.70, 127.18, 127.77, 128.02, 128.28, 130.41, 140.20, 143.49, 150.40, 168.91, 169.60, 188.91 (chalcone C=O). IR ν max (KBr): 3064.92, 2969.14 (Ar C-H), 1773.15 (C=O), 1638.35, 1677.99 (C=C), 1625.35, 1585.28, 1458.89 (Ar C=C), 1069.92 (C-N) cm−1. HRMS (APCI) calculated for C26H18NO4 [M++H]: 408.1230 found: 408.1231.
9-(E)-3-Oxo-3-(pyridin-2-yl)prop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione (22n) prepared from (E)-3-(anthracen-9-yl)-1-(pyridin-2-yl)prop-2-en-1-one (1.0 mmol, 0.3094 g) and maleic anhydride (1.3 mmol, 0.13 g) according to the general procedure above; white powder (100%), Mp. 225–227 °C. 1H NMR (400 MHz, CDCl3) δ 3.62 (dd, J = 9.12, 3.32 Hz, 1H, CH), 3.93 (d, J = 9.54 Hz, 1H, CH), 4.85 (d, J = 3.32 Hz, 1H, CH), 7.13–7.24 (m, 3H, ArH), 7.25–7.31 (m, 3 H,ArH), 7.36–7.45 (m, 3 H ArH), 7.51 (ddd, J = 7.57, 4.67, 1.04 Hz, 1 ArH), 7.93 (td, J = 7.67, 1.66 Hz, 1H, ArH), 8.13 (d, J = 16.59 Hz, 1 H, CH=C), 8.29 (dt, J = 7.88, 1.04 Hz, 1H, ArH), 8.42 (d, J = 16.59 Hz, 1H, CH=C), 8.70–8.73 (m, 1H, ArH). IR ν max (ATR): 3087.05, 3064.46, 2968.93 (Ar C-H), 1770.84 (C=O), 1676.99 (C=C), 1625.37, 1584.71, 1479.76, 1456.99 (Ar C=C), 1325.60 (C-N), 1084.30 (C-O) cm−1. HRMS (APCI): calculated for C26H18NO4: 408.1230 [M++H]; found: 408.1237.
9-(E)-3-(Furan-2-yl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione (22o) was prepared from (E)-3-(anthracen-9-yl)-1-(furan-2-yl)prop-2-en-1-one (1.0 mmol, 0.298 g) and maleic anhydride (1.3 mmol, 0.13 g) according to the general procedure above; cream powder (81%), Mp. 222–225 °C. 1H NMR (400 MHz, CDCl3) δ 3.63 (dd, J = 9.54, 3.32 Hz, 1H, CH), 3.82 (d, J = 9.12 Hz, 1H, CH), 4.85 (d, J = 3.32 Hz, 1H, CH), 6.63 (dd, J = 3.73, 1.66 Hz, 1H, CH=C), 7.15–7.29, 5H, ArH, CH=C), 7.33–7.40 (m, 2H, ArH), 7.40–7.46 (m, 2H, ArH), 7.63 (d, J = 16.59 Hz, 1H, CH=C), 7.69 (d, J = 1.24 Hz, 1H, CH=C), 8.02 (d, J = 16.17 Hz, 1H, CH=C). 13C NMR (101 MHz, CDCl3) ppm 45.50, 49.08, 49.26, 51.82, 112.78, 118.91, 123.63, 123.95, 124.15, 125.53, 127.05, 127.56, 127.91, 128.04, 130.36, 137.38, 138.96, 140.29, 140.61, 141.55, 147.26, 153.19, 168.65, 169.93, 177.01 (chalcone C=O). IR ν max (ATR): 3071.48, 2882.79 (Ar C-H), 1776.90, 1668.01 (C=O), 1580.26, 1405.03 (Ar C=C), 1226.47, 1071.29 (C-O) cm−1. HRMS (APCI): calculated for C25H17O5: 397.1071 [M++H]; found: 397.1074.
9-(E)-3-(Naphthalen-2-yl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione (22p) was prepared from (E)-3-(anthracen-9-yl)-1-(naphthalen-2-yl)prop-2-en-1-one (1.0 mmol, 0.3584 g) and maleic anhydride (1.3 mmol, 0.13 g) according to the general procedure above; green crystals (86%), Mp. 226–231 °C. 1H NMR (400 MHz, CDCl3) δ 3.66 (dd, J = 9.12, 3.32 Hz, 1H), 3.86 (d, J = 9.54 Hz, 1 H), 4.88 (d, J = 3.32 Hz, 1H), 7.17–7.31 (m, 4H), 7.34–7.46 (m, 4H), 7.54–7.65 (m, 2H), 7.88–7.94 (m, 2H), 7.96–8.03 (m, 3H), 8.22 (dd, J = 8.71, 1.66 Hz, 1H), 8.71 (s, 1 H). 13C NMR (101 MHz, CDCl3) ppm 55.53–56.38, 60.88, 69.77–71.12, 106.07, 106.66, 107.75, 110.78–111.13, 114.44, 114.76, 123.43, 126.97, 127.76, 128.45. IR ν max (ATR): 3064.36, 2968.90 (Ar C-H), 1771.08 (C=O), 1676.62, 1625.39 (C=C), 1584.75, 1458.10 (Ar C=C),1 325.74 (C-O) cm−1. HRMS (APCI): calculated for C31H21O4 [M++H]: 457.1434; found: 457.1438.
9-(E)-3-Oxo-3-(thiophen-2-yl)prop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione (22q) was prepared from (E)-3-(anthracen-9-yl)-1-(thiophen-2-yl)prop-2-en-1-one (1.0 mmol, 0.3144 g) and maleic anhydride (1.3 mmol, 0.13 g) according to the general procedure above; cream powder (76%), Mp. 214–218 °C. 1H NMR (400 MHz, CDCl3) δ 3.64 (dd, J = 9.12, 2.90 Hz, 1H, CH), 3.81 (d, J = 9.12 Hz, 1H, CH), 4.86 (d, J = 2.90 Hz, 1H, CH), 7.11–7.28 (m, 5 H, ArH, CH=C), 7.28–7.46 (m, 4H, ArH), 7.66 (d, J = 16.17 Hz, 1H, CH=C), 7.75 (d, J = 4.98 Hz, 1H, CH=C), 7.91–8.01 (m, 2H, CH=C). 13C NMR (101 MHz, CDCl3) ppm 45.40, 48.99, 49.63, 52.01, 56.07, 110.20, 111.07, 123.48, 123.70, 123.93, 125.41, 126.70, 127.24, 127.41, 130.88, 131.48, 139.42, 140.66–140.97, 142.44, 149.24, 153.52, 174.93, 175.64, 188.72 (chalcone C=O). IR ν max (ATR): 3089.66, 2882.60 (Ar C-H), 1777.72, 1664.07 (C=O) 1616.88 (C=C), 1579.42, 1512.85, 1465.10, 1456.62, 1418.52 (Ar C=C), 1213.16 (C-O) cm-1. LRMS (APCI): 411.22 [M+−H]. HRMS (APCI): calculated for C25H16NaO4S [M++Na]: 435.0662; found: 435.0658.
3.4. General Preparation for (E)-9-(3-oxo-3-phenylprop-1-en-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-diones 23a-q
To a solution of the appropriate chalcone anthracene analog 21a–21q (1.0 mmol) in toluene (2 mL) was added dienophile maleimide (1.3 mmol). The mixture was heated and stirred at 90 °C for 48 h. The reaction mixture was then cooled to room temperature, and the resulting product was isolated via filtration and washed with toluene (2 mL) and diethyl ether (2 mL). The product was then recrystallized from toluene.
9-(E)-3-(4-Bromophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (23a) was prepared from (E)-3-(anthracen-9-yl)-1-(4-bromophenyl)prop-2-en-1-one (1.0 mmol, 0.387 g) and maleimide (1.3 mmol, 0.13g) according to the general procedure above; light yellow powder (91%), Mp. 295–297 °C. (HPLC: 87.8%, RT 5.02 min). 1H NMR (400 MHz, DMSO-d6) δ 3.32 (m, 1H, CH), 3.82 (d, J = 8.82 Hz, 1H, CH), 4.81 (d, J = 3.32 Hz, 1H, CH), 7.11–7.22 (m, 5 H, ArH, CH=C), 7.23–7.29 (m, 1H, ArH), 7.30–7.35 (m, 1H, ArH), 7.51 (d, J = 7.05 Hz, 1 H, ArH), 7.78–7.85 (m, 4H), 8.06–8.12 (m, 2H), 10.87 (s, 1H). 13C NMR (101 MHz, CDCl3) ppm 44.96, 49.09, 49.30, 51.70, 123.24, 123.66, 124.61, 126.65, 127.16, 127.28, 131.14, 131.47, 132.47, 136.76, 139.29, 140.24, 142.15, 143.10, 143.11, 177.48, 177.74, 189.08 (chalcone C=O). IR ν max (ATR): 3341 (N-H), 3096.65 (C-H), 1720 (C=O), 1629.57 (Ar C=C), 1603.21, 1522.86, 1458.09, (C-N) 1311, 1165, (C-Br) 1003.06 cm−1. HRMS (EI) calculated for C27H17BrNO3 [M+−H]: 482.0397; found 482.0391.
9-(E)-3-(4-Nitrophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (23b) was prepared from (E)-3-(anthracen-9-yl)-1-(4-nitrophenyl)prop-2-en-1-one (1.0 mmol, 0.353 g) and maleimide (1.3 mmol, 0.13 g) according to the general procedure above; beige powder (87%), Mp. >300 °C (dec). 1H NMR (400 MHz, DMSO-d6) δ 3.35 (d, J = 3.32 Hz, 1H, CH), 3.82 (d, J = 8.71 Hz, 1H, CH), 4.82 (d, J = 2.90 Hz, 1H, CH), 7.15–7.23 (m, 4H, ArH), 7.28–7.33 (m, 1 H, ArH), 7.34–7.39 (m, 1H, ArH), 7.52–7.57 (m, 1H, ArH), 7.82–7.89 (d, J = 15.8 Hz, 1 H, CH=C), 7.89–8.01 (d, J = 16.2 Hz, 1H, CH=C,), 8.37–8.47 (m, 4H, ArH), 10.88 (s, 1H). 13C NMR (101 MHz, DMSO-d6) 20.05, 24.86, 44.97, 49.24, 51.78, 63.44, 73.92, 78.91, 88.39, 109.61, 111.59, 118.64, 123.30, 123.71, 124.64, 125.65, 126.66, 127.25, 130.51, 131.69, 139.28, 140.12, 142.13, 142.52, 142.96, 144.40, 150.46,177.52, 177.70, 189.16 (chalcone C=O) ppm. IR ν max (ATR): 3343 (N-H), 3058.45 (C-H), 1719 (C=O), 1677.02, 1638.82 (C=C), 1599.84, 1458.22 (Ar C=C), 1517, 1340 (NO2), 1317, 1164 (C-N) cm-1. HRMS (EI): calculated for C27H17N2O3 [M+−H]: 449.1143; found: 449.1146.
9-((E)-3-(4-Ethylphenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (23c) was prepared from (E)-3-(anthracen-9-yl)-1-(4-ethylphenyl)prop-2-en-1-one (1.0 mmol, 0.336 g) and maleimide (1.3 mmol, 0.13 g) according to the general procedure above; white powder (82%), Mp. 265–269 °C. 1H NMR (400 MHz, DMSO-d6) δ 1.24 (t, J = 7.58 Hz, 3H), 2.73 (q, J = 7.83 Hz, 2H), 3.32 (d, J = 3.42 Hz, 1H), 3.82 (d, J = 8.31 Hz, 1H), 4.82 (d, J = 3.42 Hz, 1H), 7.11–7.26 (m, 5H), 7.27–7.32 (m, 6H), 7.34 (d, J = 6.85 Hz, 1H), 7.80–7.84 (m, 1H), 7.80–7.84 (m, 1H), 7.87 (d, J = 16.14 Hz, 1H), 8.13 (d, J = 8.31 Hz, 2H), 10.87 (br. s., 1H). IR ν max (ATR): (N-H) 3343 (C-H) 3088.25, (trans C=C), 1776.11, 1720 (C=O), 1626.04, 1603.36 (Ar C=C), 1522.96, 1457.88, (C-N) 1313, 1165 cm−1. HRMS (EI) calculated for C29H23NNaO3 [M++Na]: 456.1570; found: 456.1562.
9-(E)-3-(2,4-Dichlorophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (23d) was prepared from (E)-3-(anthracen-9-yl)-1-(2,4-dichlorophenyl)prop-2-en-1-one (1.0 mmol, 0.377 g) and maleimide (1.3 mmol, 0.13 g) according to the general procedure above; beige powder (82%), Mp. 265–268 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.24 (dd, J = 8.6, 3.2 Hz, 1H), 3.61 (d, J = 8.6 Hz, 1H), 4.76 (d, J = 3.2 Hz, 1H), 7.23–7.10 (m, 7H), 7.31 (dq, J = 4.7, 2.6 Hz, 1H), 7.49 (dd, J = 6.6, 1.8 Hz, 1H), 7.67–7.53 (m, 2H), 7.86–7.79 (m, 2H), 10.81 (s, 1H) 13C NMR (101 MHz, CDCl3) ppm 44.92, 49.24, 51.71, 123.17, 123.47, 124.73, 125.68, 126.63, 127.12, 127.26, 127.38, 128.23, 130.31, 131.41, 131.87, 135.33, 136.45, 137.33, 139.23, 139.82, 142.09, 142.59, 146.63, 177.28, 177.68, 192.42 (chalcone C=O) IR ν max (ATR): 3336 (N-H), 3062.21 (C-H), 1778.31, 1720 (C=O), 1601.84 (Ar C=C), 1578.57, 1521.96, 1457.78, (C-N) 1315, 1171 cm−1. HRMS (ESI): calculated for C27H16Cl2NO3 [M+−H]: 472.0513; found: 472.0519.
9-(E)-3-(3,4-Dimethoxyphenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (23e) was prepared from (E)-3-(anthracen-9-yl)-1-(3,4-dimethoxyphenyl)prop-2-en-1-one (1.0 mmol, 0.368 g) and maleimide (1.3 mmol, 0.13 g) according to the general procedure above, cream/yellow powder (83%), Mp. 269–271 °C. 1H NMR (400 MHz, CDCl3) δ 3.37 (dd, J = 8.5, 3.2 Hz, 1H), 3.56 (d, J = 8.6 Hz, 1H), 3.98 (d, J = 9.3 Hz, 6H), 4.81 (d, J = 3.2 Hz, 1H), 6.97 (d, J = 8.4 Hz, 1H), 7.14–7.24 (m, 4H), 7.35 (ddd, J = 10.9, 6.8, 2.5 Hz, 3H), 7.38–7.45 (m, 1H), 7.47 (s, 1H), 7.75–7.84 (m, 2H), 7.85 (dd, J = 8.4, 2.0 Hz, 1H), 7.91 (d, J = 16.2 Hz, 1H). 13C NMR (101 MHz, CDCl3) ppm 45.41, 48.99, 49.63, 52.00, 56.05, 56.09, 110.20, 111.07, 123.48, 123.70, 123.90, 123.95, 125.41, 126.70, 127.20, 127.27, 127.41, 130.88, 131.48, 137.96, 139.42, 140.87, 142.44, 149.24, 153.52, 174.95, 175.67, 188.72 (chalcone C=O). IR ν max (ATR): 3342 (N-H), 3061.45 (C-H), 1774.72, 1719 (C=O), 1626.19 (Ar C=C), 1598.13, 1517.42, 1458.15, (C-N) 1317, 1169, (C-O) 1268.92, 1017.26 cm−1. HRMS (ESI): calculated for C29H22NO5 [M+−H] 464.1504; found: 464.1504.
(E)-9-(3-oxo-3-phenylprop-1-en-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (23f) was synthesized from (E)-3-(anthracen-9-yl)-1-phenylprop-2-en-1-one (1.0 mmol, 0.308 g) and maleimide (1.3 mmol, 0.13 g) according to the general procedure above; (61%), Mp. 218–222 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.80 (d, J = 8.71 Hz, 1H) 4.79 (d, J = 3.32 Hz, 1H) 7.10–7.24 (m, 6H) 7.24–7.29 (m, 1H) 7.32–7.37 (m, 1H) 7.52 (d, J = 6.63 Hz, 1H) 7.57–7.65 (m, 2H) 7.67 -7.74 (m, 1H) 7.84 (dd, J = 16.60 Hz, 2H) 8.12–8.21 (m, 2H) 10.84 (s, 1H). 13C NMR (101 MHz, DMSO-d6) 123.23, 123.64, 124.62, 125.66, 125.76, 126.67 127.24, 128.64, 129.15, 129.30–129.46 131.65–131.81, 133.89, 137.78, 139.32, 140.34, 142.55, 143.20, 177.31–177.97, 189.92 (chalcone C=O) ppm. IR ν max (ATR): 3347.06 (N-H), 1720.18 (C=O), 1674.84, 1628.01 (C=C), 1000.09 (CN) cm−1. HRMS (APCI): calculated for C27H20NO3 [M++H]: 406.1438; found: 406.1450.
9-(E)-3-(4-Iodophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (23g) was prepared from (E)-3-(anthracene-9-yl)-1-(4-iodophenyl)prop-2-en-1-one (1.0 mmol, 0.449 g) and maleimide (1.3 mmol, 0.13 g) according to the general procedure above; yellow powder (93%), Mp. 288–293 °C. (HPLC: 98.22, RT 6.72 min). 1H NMR (400 MHz, DMSO-d6) δ 3.79 (d, J = 8.6 Hz, 1H), 3.31–3.29 (m,1H). 4.80 (d, J = 3.2 Hz, 1H), 7.29–7.24 (m,1H), 7.23–7.13 (m, 5H), 7.37–7.31 (m, 1H), 7.52 (dt, J = 7.0, 1.1 Hz, 1H), 7.82 (d, J = 6.7 Hz, 2H), 7.95–7.91 (m, 2H), 8.03–7.98 (m, 2H), 10.85 (s, 1H). 13C NMR (101 MHz, DMSO-d6) 44.98, 49.13, 49.32, 51.72, 102.63, 123.26, 123.67, 124.62, 125.65, 126.66, 127.17, 127.29, 130.84, 131.45, 137.06, 138.35, 139.30, 140.27, 142.17, 143.05, 143.12, 177.48, 177.74, 189.40. ppm IR ν max (ATR): 3339 (N-H), 2987.83 (C-H),1688.48 (C=O) 1633, 1621.45 (Ar C=C), 1557.57, 1485.56, 687.18 cm−1. HRMS (APCI): calculated C27H19INO3 [M++H]: 532.0404, found 532.0402.
9-(E)-3-(4-Fluorophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (23h) was prepared from (E)-3-(anthracen-9-yl)-1-(4-fluorophenyl)prop-2-en-1-one (1.0 mmol, 0.326 g) and maleimide (1.3 mmol, 0.13 g) according to the general procedure above; cream powder (100%), Mp. 260–268 °C. 1H NMR (400 MHz, CDCl3) δ 3.36 (dd, J = 8.5, 3.2 Hz, 1H), 3.54 (d, J = 8.5 Hz, 1H), 4.80 (d, J = 3.2 Hz, 1H), 7.12–7.26 (m, 6H), 7.32 (m, 3H), 7.37–7.44 (m, 1H), 7.49 (s, 1H), 7.76 (d, J = 16.2 Hz, 1H), 7.94 (d, J = 16.2 Hz, 1H), 8.16–8.26 (m, 2H). IR ν max (ATR): (N-H) 3340 (C-H) 2984.37, 1776.16, (C=O) 1718, 1630.99 (Ar C=C), 1595.20, 1457.80, (C-N) 1311, 1157, (C-F) 1224.84 cm−1. HRMS (APCI): calculated for C27H19FNO3 [M++H]: 412.1002; found: 412.1004.
(E)-9-(3-(4-Methoxyphenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (23i) was synthesized from (E)-3-(anthracen-9-yl)-1-(4-methoxyphenyl)prop-2-en-1-one (1.0 mmol, 0.338g) and maleimide (1.3 mmol, 0.13 g) according to the general procedure above; white solid (70%), Mp. 281–284 °C. 1H NMR (400 MHz, CDCl3) δ 3.80 (d, J = 8.29 Hz, 1H), 3.87 (s, 3H), 4.79 (d, J = 2.90 Hz, 1H), 7.10–7.19 (m, 5H), 7.19–7.23 (m, 3H), 7.25 (app t, J = 5.39 Hz, 2H), 7.32–7.36 (m, 1H), 7.52 (d, J = 6.63 Hz, 1H), 7.74–7.88 (m, 2H), 8.17 (d, J = 9.12 Hz, 2H). 13C NMR (101 MHz, CDCl3) ppm 39.15–40.72, 44.97, 49.37, 56.07, 123.21, 124.60, 125.65, 127.01–127.38, 130.62, 131.37–132.01, 140.43, 141.47, 143.30, 177.79, 188.12 (C=O chalcone). IR ν max (ATR): 3343.73 (N-H), 1670.36 (C=O), 1601.24 (Ar C=C), 1230.33 (C-O), 1019.60 (C-N) cm-1. LRMS (APCI): 434.30 [M+−H]. HRMS (APCI): calculated C28H20NO4 [M+−H]: 434.1398; found: 434.1392.
(E)-9-(3-Oxo-3-(p-tolyl)prop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (23j) was synthesized from (E)-3-(anthracen-9-yl)-1-(p-tolyl)prop-2-en-1-one (1.0 mmol, 0.338 g) and maleimide (1.3 mmol, 0.13 g) according to the general procedure above; white powder (46%), Mp. 129–134 °C. 1H NMR (400 MHz, CDCl3) δ 2.41 (s, 3H) 3.80 (d, J = 8.71 Hz, 1H) 4.79 (br. s., 1H) 7.10–7.30 (m, 7H) 7.30–7.45 (m, 3H) 7.52 (d, J = 6.63 Hz, 1H) 7.75–7.89 (m, 2H) 8.03–8.04 (m, 1H) 8.04–8.13 (m, 2H)13C NMR (101 MHz, CDCl3) ppm 21.69, 39.07–40.73, 44.99, 48.86–49.53, 55.35, 123.23, 123.64, 126.93–127.51, 129.04–129.58, 129.96, 135.30, 140.39, 141.79–142.33, 144.37, 177.47, 189.36 (chalcone C=O)IR ν max (ATR): 1710.75 (C=O), 1625.28 (Ar C=C), 1458.68 (C-CH3), 1231.30 (C-O), 1072.28 (C-N) cm−1. HRMS (APCI): calculated C28H22NO3 [M+−H]: 420.1594; found: 420.1606.
(E)-9-(3-Oxo-3-(3,4,5-trimethoxyphenyl)prop-1-en-1-yl)-9,10-dihydro-9,10-[3,4] epipyrroloanthracene-12,14-dione (23k) was synthesized from (E)-3-(anthracen-9-yl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (1.0 mmol, 0.398 g) and maleimide (1.3 mmol, 0.13 g) according to the general procedure above; white powder (53%), Mp. 257–260 °C. 1H NMR (400 MHz, CDCl3) δ 3.30 (d, J = 2.90 Hz, 1H), 3.76–3.79 (m, 3H), 3.81 (s, 1H), 3.87 (s, 6H), 4.79 (d, J = 3.32 Hz, 1H), 7.16–7.25 (m, 5H), 7.28 (d, J = 4.98 Hz, 1H), 7.32 -7.36 (m, 1H), 7.47–7.54 (m, 3H), 7.72–7.87 (m, 2H), 10.85 (s, 1H). 13C NMR (101 MHz, CDCl3) ppm 41.66, 43.00, 46.27, 49.08–49.42, 51.67, 56.51, 65.87, 106.82, 123.23, 123.67, 124.66, 125.66, 126.63,127.39, 132.39, 133.09, 139.33, 141.76, 142.56, 143.27,177.96, 189.32 (C=O chalcone). IR ν max (ATR): 1670.40 (C=O), 1600.25 (Ar C=C), 1229.98 (C-O), 1017.32 (C-N) cm−1. HRMS (APCI): calculated for C30H24NO6 [M+−H]: 494.1609; found: 494.1605.
(E)-9-(3-(4-Chlorophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (23l) was synthesized from (E)-3-(anthracen-9-yl)-1-(4-chlorophenyl)prop-2-en-1-one (1.0 mmol, 0.343 g) and maleimide (1.3 mmol, 0.13 g) according to the general procedure above; white solid (65%), Mp. 280–285 °C. (HPLC: 97.72%, RT 7.23 min). 1H NMR (400 MHz, CDCl3) δ 3.80 (d, J = 8.71 Hz, 1H) 4.80 (d, J = 2.90 Hz, 1H) 7.09–7.39 (m, 8H) 7.52 (d, J = 7.05 Hz, 1H) 7.68 (d, J = 8.29 Hz, H) 7.85 (s, 2 H) 8.19 (d, J = 8.29Hz, 2H) 10.85 (s, 1H). 13C NMR (101 MHz, CDCl3) ppm 44.98, 49.11, 49.32, 51.71, 123.26, 123.67,124.13, 124.62, 125.65, 125.75, 126.66, 127.18, 127.29, 128.64, 129.34, 129.54, 136.45, 140.28, 142.17, 143.11, 177.12–178.10, 198.62 (chalcone C=O). IR ν max (ATR): 1717.64 (C=O), 754.55 (C-Cl) cm−1. LRMS (APCI): 438.2284 [M+−H]. HRMS (APCI): calculated for C27H18ClNNaO3 [M++Na]: 462.0867; found 462.0864.
9-(E)-3-Oxo-3-(pyridin-4-yl)prop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (23m) was prepared from (E)-3-(anthracen-9-yl)-1-(pyridin-4-yl)prop-2-en-1-one (1.0 mmol, 0.3094 g) and maleimide (1.3 mmol, 0.13g) according to the general procedure above; powder (100%), Mp. 256–258 °C. 1H NMR (400 MHz, CDCl3) δ 3.36 (dd, J = 8.29, 3.32 Hz, 1H, CH), 3.54 (d, J = 8.29 Hz, 1H, CH), 4.81 (d, J = 3.32 Hz, 1H, CH), 7.12–7.25 (m, 6H, ArH), 7.26–7.31 (m, 1 H, ArH), 7.33–7.38 (m, 1H, ArH), 7.39–7.43 (m, 1H, ArH), 7.73 (d, J = 16.17 Hz, 1 H, CH=C), 7.95 (d, J = 5.39 Hz, 2H, ArH), 8.01 (d, J = 16.17 Hz, 1H, CH=C), 8.86 (br.s., 1 H, NH). 13C NMR (101 MHz, CDCl3) ppm 45.37, 48.96, 49.54, 52.07, 121.87, 123.29, 123.57, 124.14, 125.55, 126.76, 127.35, 127.60, 130.63, 1 (N-H), 3010.28 (C-H), 1776.21, 1721 (C=O), 1634.91 (Ar C=C), 1457.77, 1407.25, (C-N) 1317, 1166 cm−1. HRMS (ESI): calculated C26H17N2O3 [M+−H] 405.1245; found: 405.1247.
9-(E)-3-Oxo-3-(pyridin-2-yl)prop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (23n) was prepared from (E)-3-(anthracen-9-yl)-1-(pyridin-2-yl)prop-2-en-1-one (1.0 mmol, 0.309 g) and maleimide (1.3 mmol, 0.13 g) according to the general procedure above; brown powder (72%), Mp. > 200 °C. (HPLC: 92.20%, RT 3.80 min). 1H NMR (400 MHz, DMSO-d6) δ 3.33 (dd, J = 8.50, 3.11 Hz, 1H, CH), 3.64 (d, J = 8.29 Hz, 1H, CH), 4.78 (d, J = 3.32 Hz, 1H, CH), 7.10–7.22 (m, 4H, ArH), 7.29 (d, J = 7.46 Hz, 1H, ArH), 7.31–7.35 (m, 1H, ArH), 7.37–7.43 (m, 2H, ArH), 7.49 (ddd, J = 7.57, 4.66, 1.04 Hz, 2H, ArH), 7.90 (td, J = 7.67, 1.66 Hz, 1H, ArH), 8.11–8.19 (m, 1 H, ArH), 8.15 (d, J = 16.59 Hz, 1H, CH=C), 8.28 (d, J = 7.88 Hz, 1H, ArH), 8.40 (d, J = 16.59 Hz, 1H, CH=C), 8.67–8.72 (m, 1H, ArH). 13C NMR (101 MHz, DMSO-d6) 44.99, 49.37, 49.74–50.02, 51.79, 123.23, 123.29, 123.61, 124.67, 126.62, 127.12, 127.17, 127.30, 130.11, 135.68, 139.39, 140.2 (C-H), 1776.26, 1719 (C=O), 1623.69, 1603.44 (Ar C=C), 1523.46, 1458.07, (C-N) 1323, 1170 cm−1. HRMS (ESI): calculated for C26H19N2O3 [M+−H]: 407.1390; found: 407.1396.
9-(E)-3-(Furan-2-yl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (23o) was prepared from (E)-3-(anthracen-9-yl)-1-(furan-2-yl)prop-2-en-1-one (1.0 mmol, 0.293g) and maleimide (1.3 mmol, 0.13 g) according to the general procedure above; light yellow powder (81%), Mp. 268–270 °C. 1H NMR (400 MHz, CDCl3) δ 3.34 (dd, J = 8.6, 3.2 Hz, 1H), 3.54 (d, J = 8.6 Hz, 1H), 4.79 (d, J = 3.2 Hz, 1H), 6.61 (dd, J = 3.6, 1.7 Hz, 1H), 7.10–7.24 (m, 4H), 7.27 (dd, J = 6.9, 1.2 Hz, 1H), 7.34 (dd, J = 5.5, 3.2 Hz, 2H), 7.36–7.47 (m, 3H), 7.59–7.71 (m, 2H), 8.04 (d, J = 16.3 Hz, 1H). 13C NMR (101 MHz, CDCl3) ppm 45.42, 49.06, 49.55, 51.92, 67.18, 112.64, 118.71, 123.52, 123.82, 123.99, 125.41, 126.67, 127.22, 127.29, 127.43, 130.42, 137.89, 139.25, 140.95, 141.73, 142.21, 147.10, 153.32, 174.77, 175.62, 177.38. IR ν max (ATR): 3343 (N-H), 3033.42 (C-H), 1776.09, 1719 (C=O), 1626.86, 1602.84 (Ar C=C), 1522.49, 1458.42 (C-N), 1330, 1168, (C-O), 1287.87, 1010.65 cm−1 HRMS (ESI): calculated for C25H18NO4 [M++H]: 407.1390; found: 407.1396.
9-(E)-3-(Naphthalen-2-yl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (23p) was prepared from (E)-3-(anthracen-9-yl)-1-(naphthalen-2-yl)prop-2-en-1-one (1.0 mmol, 0.358 g) and maleimide (1.3 mmmol, 0.13 g) according to the general procedure above; cream/yellow powder (71%), Mp. 209–215 °C. 1H NMR (400 MHz, CDCl3) δ 3.42 (dd, J = 8.56, 3.18 Hz, 1H), 3.64 (d, J = 8.56 Hz, 1H), 4.86 (d, J = 3.18 Hz, 1H), 7.23–7.25 (m, 1H), 7.25–7.28 (m, 2H), 7.37–7.42 (m, 2H), 7.43–7.47 (m, 2H), 7.57–7.62 (m, 1H), 7.63–7.68 (m, 1H), 7.91–7.98 (m, 3H), 8.00–8.08 (m, 3H), 8.26 (dd, J = 8.56, 1.71 Hz, 3H), 8.76 (s, 3H). 13C NMR (101 MHz, CDCl3) ppm 45.5, 49.1, 49.6, 123.6, 123.9, 124.1, 124.6, 125.5, 126.8, 126.9, 127.3, 127.4, 127.5, 127.9, 128.6, 128.8, 129.8, 131.0, 131.6, 135.6, 142.1, 193.0. IR ν max (ATR): 3343 (N-H), 3062 (C-H), 1776.30 (C=O), 1719, (Ar C=C),1625.58, 1602.54 (C=C), 1522.97, 1457.95, (C-N) 1312, 1164 cm−1. HRMS (APCI): calculated for C31H22NO3 [M++H]: 456.1594; found: 456.1599.
9-(E)-3-Oxo-3-(thiophen-2-yl)prop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (23q) was prepared from (E)-3-(anthracen-9-yl)-1-(thiophen-2-yl)prop-2-en-1-one (1.0 mmol, 0.3144 g) and maleimide (1.3 mmol, 0.13 g) according to the general procedure above; beige/yellow powder (80%), Mp. 263–268 °C. 1H NMR (400 MHz, CDCl3) δ 3.35 (ddd, J = 8.5, 3.2, 0.6 Hz, 1H), 3.53 (d, J = 8.5 Hz, 1H), 4.80 (d, J = 3.1 Hz, 1H), 7.12–7.23 (m, 5H), 7.27–7.31 (m, 1H), 7.34 (ddd, J = 7.9, 4.1, 1.9 Hz, 2H), 7.38–7.44 (m, 1H), 7.63–7.78 (m, 2H), 7.94–8.03 (m, 2H). 13C NMR (101 MHz, CDCl3) ppm 45.40, 49.05, 49.60, 51.95, 123.51, 123.82, 124.00, 125.42, 126.71, 127.25, 127.31, 127.45, 128.42, 131.06, 132.95, 134.55, 137.91, 139.26, 140.93, 141.51, 142.25, 145.09, 174.85, 175.61, 181.86 (chalcone C=O). IR ν max (ATR): 3343 (N-H), 2963.67 (C-H), 1776.30, 1718, (C=O), 1623.56 (Ar C=C), 1582.14, 1524.12, 1457.91, 1324 (C-N), 1169, 720.73 cm−1. HRMS (APCI): calculated for C25H18NO3S [M++H]: 412.1002; found: 412.1004.
3.5. General Preparation for (E)-9-(3-oxo-3-phenylprop-1-en-yl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-diones 24a–q
To a solution of the appropriate chalcone anthracene 21a–q (1.0 mmol) in toluene (2 mL) was added dienophile N-phenylmaleimide (1.3 mmol). The mixture was heated and stirred at 90 °C for 48 h. The reaction was then cooled to room temperature, and the crude solid product was isolated via filtration, washed with toluene (2 mL) and diethyl ether (2 mL), and then recrystallized from toluene.
(E)-3-(4-Bromophenyl)-3-oxoprop-1-en-1-yl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (24a) was obtained from (E)-3-(anthracen-9-yl)-1-(4-bromophenyl)prop-2-en-1-one (1.0 mmol, 0.387 g) and N-phenylmaleimide (1.3 mmol, 0.23 g) according to the general procedure above; white powder (84%), Mp. 218 °C. (HPLC: 94.98%: RT 1.90 min). 1H NMR (400 MHz, CDCl3) δ 3.47 (dd, J = 8.29, 3.32 Hz, 2H), 3.64 (d, J = 8.29 Hz, 2H), 4.92 (d, J = 2.90 Hz, 2H), 6.46–6.53 (m, 3H), 7.19–7.23 (m, 2H), 7.25–7.30 (m, 8H), 7.30–7.35 (m, 3H), 7.35–7.43 (m, 4H), 7.45 (dd, J = 7.05, 1.24 Hz, 2H), 7.61–7.65 (m, 3H), 7.80 (d, J = 16.17 Hz, 1H), 7.97–8.05 (m, 3H). 13C NMR (101 MHz, CDCl3) ppm 45.94, 47.95, 48.48, 52.49, 123.51, 123.92, 124.17, 125.55, 126.05, 126.44, 126.82, 127.38, 127.55, 127.94, 128.36, 128.91, 129.11, 129.13, 130.49, 131.14, 131.25, 132.06, 134.17, 136.45, 138.17, 139.39, 140.80, 142.17, 142.8 (C=C), 1490, 1455 (Ar C=C), 1380.41 (C-N), 1180 (C-O), 691.05 cm−1. LRMS (APCI) 560.08 [M++H]. HRMS (ESI) calculated for C33H22BrNNaO3: 582.0675 [M++Na]; found: 582.0668. Alternative preparation of 24a: 9-(E)-3-(4-Bromophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione (22a) (0.575 mmol) was treated with aniline (0.07 g, 0.748 mmol) in acetic acid (5 mL) at 120 °C for 2–3 h. The reaction mixture was cooled in an ice bath, and then deionized water was added. The colorless, solid product was filtered and dried (72%). The product was identical when compared with the sample obtained above.
9-(E)-3-(4-Nitrophenyl)-3-oxoprop-1-en-1-yl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (24b) was prepared from (E)-3-(anthracen-9-yl)-1-(4-nitrophenyl)prop-2-en-1-one (1.0 mmol, 0.353 g) and N-phenylmaleimide (1.3 mmol, 0.23 g) according to the general procedure above; cream powder (86%), Mp. 242–246 °C. 1H NMR (400 MHz, CDCl3) δ 3.50 (dd, J = 8.4, 3.2 Hz, 1H), 3.67 (d, J = 8.5 Hz, 1H), 4.94 (d, J = 3.2 Hz, 1H), 6.54–6.45 (m, 2H), 7.40–7.17 (m, 9H), 7.46–7.37 (m, 1H), 7.51–7.43 (m, 1H), 7.85 (d, J = 16.2 Hz, 1H), 8.06 (d, J = 16.2 Hz, 1H), 8.38–8.26 (m, 4H). 13C NMR (101 MHz, CDCl3) ppm 45.92, 47.95, 48.43, 52.55, 123.39, 123.77, 123.95, 124.28, 125.65, 126.42, 126.87, 127.42, 127.51, 127.67, 128.99, 129.16, 129.96, 131.04, 131.17, 138.16, 139.18, 140.77, 141.95, 144.32, 174.72, 175.20 (chalcone C=O). IR ν max (ATR): 3335.77 (Ar C-H), 1771.68, 1700.63, 1671.17 (C=O), 1519, 1383 (NO2), 1179 (C-N) cm−1. LRMS (APCI): [M++H] 527.14. HRMS (ESI) calculated for C33H22N2NaO5, 549.1421 [M++Na]; found: 549.1424.
(E)-9-(3-(4-Ethylphenyl)-3-oxoprop-1-en-1-yl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (24c) was synthesized from (E)-3-(anthracen-9-yl)-1-(4-ethylphenyl)prop-2-en-1-one (1.0 mmol, 0.336 g) and N-phenylmaleimide (1.3 mmol, 0.23 g) according to the general procedure above; light green powder (90%), Mp. 207 °C. IR ν max (ATR): 2969.28, 2922.38, 1708.43, 1380.94, 1178.76, 715.69, 753.23, 689.68 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.57 (dd, J = 8.31, 3.42 Hz, 3H), 4.01 (d, J = 8.31 Hz, H), 4.97 (d, J = 2.93 Hz, 3H), 6.38–6.46 (m, 6H), 7.26–7.36 (m,2H), 7.36–7.39 (m, 3H), 7.40–7.43 (m, 1H), 7.59–7.65 (m, 1 H), 7.83 (d, J = 17.12 Hz, 1 H), 7.96 (d, J = 16.63 Hz, 1H), 8.01 (d, J = 5.87 Hz, 2 H), 8.89 (d, J = 5.87 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) 15.6, 25.9, 28.7, 45.4, 48.3, 52.1, 123.4, 123.9, 124.8, 125.7, 126.9, 127.0, 127.2, 127.3, 127.4, 127.5, 128.2, 128.6, 128.8, 129.0, 129.3, 129.4, 132.0, 132.2, 135.1, 135.5, 139.2, 140.2, 141.7, 141.9, 142.9, 150.4, 170.4, 175.3, 175.7, 189.4 (chalcone C=O) ppm. LRMS (APCI) 510.20 [M+H]. HRMS (ESI) calculated for C35H27KNO3, 548.1623 [M++K ]; found: 548.1627.
(E)-9-(3-(2,4-Dichlorophenyl)-3-oxoprop-1-en-1-yl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (24d) was synthesized from (E)-3-(anthracen-9-yl)-1-(2,4-dichlorophenyl)prop-2-en-1-one (1.0 mmol, 0.377 g) and N-phenylmaleimide (1.3 mmol, 0.23 g) according to the general procedure above; white powder (75%), Mp. 208–210 °C. 1H NMR (400 MHz, CDCl3) δ 3.44 (dd, J = 8.29, 3.32 Hz, 1H), 3.55 (d, J = 8.29 Hz, 1H), 4.91 (d, J = 3.32 Hz, 1H), 6.46 (m, 2H), 7.24 (m, 7H), 7.35 (m, 5H), 7.46 (m, 1H), 7.50 (d, J = 1.66 Hz, H), 7.66 (d, J = 8.29 Hz, 1 H), 7.83 (d, J = 17.00 Hz, 1H), 7.79 (d, J = 17.00 Hz, 1H). 13C NMR (101 MHz, CDCl3) ppm 45.97, 48.23, 48.34, 52.30, 123.43, 123.76, 124.28, 125.57, 126.38, 126.81, 127.33, 127.43, 127.45, 127.57, 128.88, 129.08, 130.87, 131.16, 132.66, 134.88, 136.80, 138.15, 139.07, 140.78, 141.69, 144.77, 174.43, 175.25, 191.55 (chalcone C=O). IR ν max (ATR): 3067.32 (C-H), 1776.64, 1710.54, 1630.86 (C=O), 1381.66 (C-N), 1183.14 (C-O) cm−1. HRMS (ESI) calculated for C33H20Cl2NO3 [M+−H]: 548.0826; found: 548.0825.
9-(E)-3-(3,4-Dimethoxyphenyl)-3-oxoprop-1-en-1-yl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (24e) was prepared from (E)-3-(anthracen-9-yl)-1-(3,4-dimethoxyphenyl)prop-2-en-1-one (1.0 mmol, 0.368 g) and N-phenylmaleimide (1.3 mmol, 0.23 g) according to the general procedure above; white powder (93%), Mp. 242–244 °C. 1H NMR (400 MHz, CDCl3) δ 3.47 (dd, J = 8.4, 3.3 Hz, 1H), 3.65 (d, J = 8.4 Hz, 1H), 3.94 (d, J = 9.4 Hz, 6H), 4.92 (d, J = 3.2 Hz, 1H), 6.45–6.54 (m, 2H), 6.91 (d, J = 8.4 Hz, 1H), 7.20 (td, J = 7.5, 1.6 Hz, 1H), 7.20–7.28 (m, 3H), 7.24–7.32 (m, 3H), 7.34–7.44 (m, 3H), 7.42–7.48 (m, 1H), 7.75 (d, J = 2.0 Hz, 1H), 7.80–7.89 (m, 2H), 7.95 (d, J = 16.1 Hz, 1H). 13C NMR (101 MHz, CDCl3) ppm 45.96, 47.99, 48.51, 52.48, 56.02, 56.08, 110.18, 110.99, 123.60, 123.84, 124.08, 125.49, 126.43, 126.79, 127.30, 127.33, 127.47, 128.82, 129.05, 130.89, 131.54, 138.21, 139.62, 140.83, 141.01, 142.42, 153.49, 188.72 (chalcone C=O). IR ν max (ATR): 3011.75 (Ar C-H), 1775.53, 1708.96, 1676.49 (C=O), 1379.80 (C-N), 1181.33 (C-O) cm−1. LRMS (APCI): 542.28 [M++H]. HRMS (ESI) calculated for C35H27NNaO5 [M++Na]: 564.1781; found 564.1783.
(E)-9-(3-Oxo-3-phenylprop-1-en-yl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (24f) was synthesized from (E)-3-(anthracen-9-yl)-1-phenylprop-2-en-1-one (1.0 mmol, 0.308 g) and N-phenylmaleimide (1.3 mmol, 0.23 g) according to the general procedure above; white powder (42%). Mp. 103–105 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.54 (dd, J = 8.29, 3.32 Hz, 1H) 4.00 (d, J = 8.29 Hz, 1H) 4.94 (d, J = 3.32 Hz, H) 6.39–6.43 (m, 2H) 7.19–7.27 (m, 3H) 7.27–7.34 (m, 6H) 7.36–7.41 (m, 1H) 7.57–7.62 (m, 3H) 7.66–7.71 (m, 1H) 7.88 (d, J = 1.66 Hz, 2H) 8.13–8.17 (m, 2H). 13C NMR (101 MHz, DMSO-d6) 45.41, 48.33, 52.15, 122.71, 123.42, 123.90, 124.86, 125.72, 126.88, 127.06, 127.44, 128.95, 129.20, 129.36, 132.01, 132.18, 133.88, 137.74, 139.24, 140.16, 141.70, 142.35, 142.82, 175.35, 175.76, 189.96 (chalcone C=O) ppm. IR ν max (ATR): 1707.50 (C=O), 1673.57 (C=C), 1636.62 (Ar C=C),1332.23 (C-N) cm−1. LRMS (APCI) 482.20 [M++H].
9-(E)-3-(4-Iodophenyl)-3-oxoprop-1-en-1-yl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (24g) was prepared from (E)-3-(anthracen-9-yl)-1-(4-iodophenyl)prop-2-en-1-one (1.0 mmol, 0.434 g) and N-phenylmaleimide (1.3 mmol, 0.23 g) according to the general procedure above; white crystals (20%), Mp. 214 °C. 1H NMR (400 MHz, DMSO-d6) δ ppm 3.51 (dd, J = 8.3, 3.2 Hz, 1H), 3.97 (d, J = 8.4 Hz, 1H), 4.92 (d, J = 3.2 Hz, 1H), 6.34–6.43 (m, 2H), 7.09–7.16 (m, 1H), 7.18–7.23 (m, 3H), 7.25–7.33 (m, 7H), 7.33–7.39 (m, 1H), 7.41–7.50 (m, 1H), 7.57 (dt, J = 7.1, 1.2 Hz, 1H), 7.78–7.85 (m, 2H), 7.86–7.91 (m, 2H), 7.94–8.01 (m, 2H). 13C NMR (101 MHz, DMSO-d6) 44.98, 49.13, 49.32, 51.72, 102.63, 123.26, 123.67, 124.62,125.65, 126.66, 127.17, 127.29, 130.84,131.45, 137.06, 138.35, 139.30, 140.27, 142.17, 143.05, 143.12, 177.48, 177.74, 189.40 (chalcone C=O) ppm. IR ν max (ATR): 3088.53 (C-H), 1772.98, 1705 (C=O), 1633.80 (Ar C=C), 1587.05, 1499.05, (C-N) 1467.03, 1381, 1180, 691.17 cm−1. LRMS (APCI) [M++H]: found: 608.03. HRMS (ESI) calculated for C33H21INO3, 606.0572 [M+−H]; found: 606.0567.
9-(E)-3-(4-Fluorophenyl)-3-oxoprop-1-en-1-yl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (24h) was prepared from (E)-3-(anthracen-9-yl)-1-(4-fluorophenyl)prop-2-en-1-one (1.0 mmol, 0.434 g) and N-phenylmaleimide (1.3 mmol, 0.23 g) according to the general procedure above; green solid (88%), Mp. 79 °C. (HPLC: 96.41%, RT 10.04 min). 1H NMR (400 MHz, CDCl3) δ 3.48 (dd, J = 8.4, 3.2 Hz, 1H), 3.66 (d, J = 8.4 Hz, 1H), 4.93 (d, J = 3.2 Hz, 1H), 6.44–6.54 (m, 2H), 7.13–7.21 (m, 2H), 7.22 (dd, J = 3.5, 1.6 Hz, 1H), 7.25–7.31 (m, 5H), 7.31–7.42 (m, 4H), 7.43–7.49 (m, 1H), 7.83 (d, J = 16.1 Hz, 1H), 8.00 (d, J = 16.1 Hz, 1H), 8.15–8.24 (m, 2H). 13C NMR (101 MHz, CDCl3) ppm 45.39, 48.93, 49.61, 52.01, 115.81, 116.03, 123.41, 123.79, 124.03, 125.47, 126.74, 127.28, 127.32, 127.49, 131.19, 131.58, 131.67, 134.09, 134.12, 137.94, 139.26, 140.92, 142.18, 142.28, 175.17, 175.73, 188.70 (chalcone C=O). LRMS (APCI) 500.20 [M++H]. HRMS (ESI) calculated for C33H22FNNaO3, 522.1476 [M++H]; found 522.1473.
(E)-9-(3-(4-Methoxyphenyl)-3-oxoprop-1-en-1-yl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (24i) was synthesized from (E)-3-(anthracen-9-yl)-1-(4-methoxyphenyl)prop-2-en-1-one (1.0 mmol, 0.338 g) and N-phenylmaleimide (1.3 mmol, 0.23 g) according to the general procedure above; white powder (56%), Mp. 207–212 °C. 1H NMR (400 MHz, CDCl3) δ 3.55 (dd, J = 8.29, 3.32 Hz, 1H), 3.85 (s, 3H), 3.99 (d, J = 8.29 Hz, 1H), 4.94 (d, 1H, J = 3.7 Hz, 1H), 7.09–7.18 (m, 5H), 7.20–7.34 (m, 11H), 7.81 (d, J = 16.3 Hz, 1H), 7.89 (d, J = 16.3 Hz, 1H), 8.16 (d, J = 9.12Hz, 2H). 13C NMR (101 MHz, CDCl3) ppm 45.42, 48.27, 48.34, 52.04, 56.03, 114.67, 122.22, 123.41, 123.90, 124.83, 125.71, 126.85, 127.07, 127.26–127.55, 128.64, 129.00, 129.32, 130.59, 131.53, 131.92, 132.19, 139.24, 140.28, 141.20, 141.72, 142.95, 163.88, 175.34, 175.76, 177.93, 188.14 (chalcone C=O). IR ν max (ATR): 3343.74 (N-H), 1670.84 (C=O), 1600.33 (Ar C=C), 1380.68, 1230.69 (C-O), 1024.50 (C-N) cm−1. LRMS (APCI) found: 512.19, [M++H]. HRMS (ESI) calculated for C34H24NO4 [M+−H]: 510.1705; found: 510.1708.
(E)-9-(3-Oxo-3-(p-tolyl)prop-1-en-1-yl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (24j) was synthesized from (E)-3-(anthracen-9-yl)-1-(p-tolyl)prop-2-en-1-one (1.0 mmol, 0.338 g) and N-phenylmaleimide (1.3 mmol, 0.23 g) according to the general procedure above; white powder (51%), Mp. 185–190 °C. 1H NMR (400 MHz, CDCl3) δ 2.39 (s, 3H), 3.53 (dd, J = 8.50, 3.11 Hz, 1H), 3.99 (d, J = 8.71 Hz, 1H), 4.94 (d, J = 2.90 Hz, 1H), 6.38–6.44 (m, 2H), 7.19–7.26 (m, 3H), 7.26–7.34 (m, 6H), 7.36–7.42 (m, 3H), 7.59 (d, J = 6.63 Hz, 1H), 7.86 (d, J = 3.32 Hz, 2H), 8.06 (d, J = 8.29 Hz, 2H). 13C NMR (101 MHz, CDCl3) ppm 21.69, 39.22–40.74, 48.34, 52.13, 123.89, 125.38 -125.98, 126.76–127.19, 127.26–127.61, 128.92–129.49, 129.95, 131.97, 135.25, 140.21, 141.91, 144.37, 175.34, 189.38 (chalcone C=O). IR ν max (ATR): 2917.28 (Ar C-H), 1772.13 (C=O), 1624.76 (Ar C=C), 1458.34 (C-CH3), 1230.53 (C-O), 1072.21 (C-N) cm−1. LRMS (APCI) 496.19 [M++H]. HRMS (ESI) calculated for C34H25KNO3 [M++K] 534.1466; found 534.1460.
(E)-9-(3-Oxo-3-(3,4,5-trimethoxyphenyl)prop-1-en-1-yl)-13-phenyl-9,10-dihydro-9,10-[3,4] epipyrroloanthracene-12,14-dione (24k) was synthesized from (E)-3-(anthracen-9-yl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (1.0 mmol, 0.398 g) and N-phenylmaleimide (1.3 mmol, 0.23 g) according to the general procedure above; white powder (62%), Mp. 242–246 °C. 1H NMR (400 MHz, CDCl3) δ 3.53 (dd, J = 8.50, 3.11 Hz, 1H), 3.76 (s, 3H), 3.68 (s, 6H), 4.00 (d, J = 8.29 Hz, 1H), 4.94 (d, J = 3.32 Hz, 1H), 6.42 (dd, J = 7.46, 2.07 Hz, 2H), 7.23–7.26 (m, 2H), 7.26–7.33 (m, 6H), 7.34 (s, 1H), 7.37–7.40 (m, H), 7.47 (s, 2H), 7.58–7.61 (m, 1H), 7.78–7.91 (m, 2H). 13C NMR (101 MHz, CDCl3) ppm 45.43, 48.1–48.62, 56.54, 60.67, 106.88, 123.42, 123.95, 124.88, 125.71, 126.75 -126.96, 127.29–127.48, 128.94, 129.26, 132.13, 132.50, 133.05, 140.18, 141.65, 153.41, 175.39, 175.73, 189.11 (chalcone C=O) IR ν max (ATR): 1702.71 (C=.O), 1123.37 (C-O) cm−1. LRMS (APCI) 572.19 [M++H]. HRMS (ESI) calculated for C36H28NO6 [M+−H]: 570.1922; found: 570.1920
(E)-9-(3-(4-Chlorophenyl)-3-oxoprop-1-en-1-yl)-13-phenyl-9,10-dihydro-9,10-[3,4] epipyrroloanthracene-12,14-dione (24l) was synthesized from (E)-3-(anthracen-9-yl)-1-(4-chlorophenyl) prop-2-en-1-one (1.0 mmol, 0.343 g) and N-phenylmaleimide (1.3 mmol, 0.23 g) according to the general procedure above; white powder (20%), Mp. 207–211 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.53 (dd, J = 8.29, 3.32 Hz, 1H), 3.99 (d, J = 8.29Hz, 1H), 4.94 (d, J = 3.32 Hz, 1H), 6.37–6.44 (m, 2H), 7.23 (d, J = 0.83 Hz, 3H), 7.27–7.35 (m, 6H), 7.35–7.40 (m, 1H), 7.59 (d, J = 6.63 Hz, 1H), 7.67 (d, J = 8.29 Hz, 2H), 7.87 (s, 2H), 8.16 (d, J = 8.29 Hz, 2H). 13C NMR (101 MHz, CDCl3) ppm: 39.12–40.77, 45.42, 52.17, 123.44, 123.92, 124.86, 125.72, 126.72 -127.66, 129.24–129.66, 131.03, 131.75, 132.16, 135.09, 138.84, 139.21, 141.68, 142.74, 142.92, 175.37, 175.76, 188.95 (chalcone C=O). IR ν max (ATR): 3058.71 (Ar C-H), 1708.71(C=O), 1674.04 (C=C), 1591.39 (Ar C=C), 1455.69 (C-C), 1015.46 (C-N), 743.22 (C-Cl) cm−1. LRMS (APCI) 516.13 [M++H]. HRMS (ESI) calculated for C33H23ClNO3 [M++H]: 516.1361; found: 516.1361.
9-(E)-3-Oxo-3-(pyridin-4-yl)prop-1-en-1-yl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (24m) was prepared from (E)-3-(anthracen-9-yl)-1-(pyridin-4-yl)prop-2-en-1-one (1.0 mmol, 0.309 g) and N-phenylmaleimide (1.3 mmol, 0.23 g) according to the general procedure above; cream crystals (82%), Mp. 203–206 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.57 (dd, J = 8.4, 3.2 Hz, 1H, H-8), 4.01 (d, J = 8.4 Hz, 1H, H-3), 4.97 (d, J = 3.2 Hz, 1H, H7), 6.42 (d, J = 15.1 Hz, 1H), 7.16–7.62 (m, 14H), 8.01 (m, 2H), 8.88 (m, 2H). 13C NMR (101 MHz, DMSO-d6) ppm 44.93 (C-7), 47.78 (C-8), 48.04 (C-3), 51.76 (C-4), 121.73, 123.03, 123.50, 124.43, 126.41, 126.58, 126.79, 126.93, 127.06, 128.55, 128.86, 131.30, 131.67, 134.67, 138.74, 139.46, 141.18, 142.09, 143.29, 143.96, 150.89, 174.93, 175.28, 189.58 (chalcone C=O). 15N (400 MHz, DMSO-d6) 194.8 maleimide), 329.7 (pyridine) ppm. HRMS (ESI) calculated for C32H22N2NaO3 [M++Na]: 505.1523; found 505.1519.
9-(E)-3-Oxo-3-(pyridin-2-yl)prop-1-en-1-yl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (24n) was prepared from (E)-3-(anthracen-9-yl)-1-(pyridin-2-yl)prop-2-en-1-one (1.0 mmol, 0.309 g) and N-phenylmaleimide (1.3 mmol, 0.23 g) according to the general procedure above; gray powder (91%), Mp. >256 °C (dec). 1H NMR (400 MHz, CDCl3) δ 3.47 (dd, J = 8.4, 3.2 Hz, 1H), 3.75 (d, J = 8.4 Hz, 1H), 4.92 (d, J = 3.2 Hz, 1H), 6.44–6.53 (m, 2H), 7.14–7.30 (m, 7H), 7.31–7.42 (m, 2H), 7.46 (ddt, J = 7.2, 6.1, 1.1 Hz, 3H), 7.88 (td, J = 7.7, 1.8 Hz, 1H), 8.21 (d, J = 16.6 Hz, 1H), 8.26 (dt, J = 7.9, 1.1 Hz, 1H), 8.49 (d, J = 16.5 Hz, 1H), 8.69 (ddd, J = 4.8, 1.8, 1.0 Hz, 1H). 13C NMR (101 MHz, CDCl3) ppm 46.06, 48.18, 48.39, 52.51, 123.15, 123.74, 124.07, 124.15, 125.42, 126.41, 126.70, 127.00, 127.21, 127.31, 127.38, 128.69, 128.94, 130.07, 131.31, 137.00, 138.22, 139.70, 140.93, 142.30, 142.60, 149.08, 174.40, 175.50, 188.99 (chalcone C=O). IR ν max (ATR): 3054.46 (Ar C-H), 1779.59, 1713.79, 1674.55 (C=O), 1201.95 (C-N), 1181.33 (C-O) cm−1. LRMS (APCI) 483.23 [M++H].
9-(E)-3-(Furan-2-yl)-3-oxoprop-1-en-1-yl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (24o) was prepared from (E)-3-(anthracen-9-yl)-1-(furan-2-yl)prop-2-en-1-one (1.0 mmol, 0.298 g) and N-phenylmaleimide according to the general procedure above; cream powder (87%), Mp. >200 °C. 1H NMR (400 MHz, CDCl3) δ 3.47 (dd, J = 8.4, 3.2 Hz, 1H), 3.65 (d, J = 8.4 Hz, 1H), 4.92 (d, J = 3.2 Hz, 1H), 6.43–6.53 (m, 2H), 6.58 (dd, J = 3.6, 1.7 Hz, 1H), 7.13–7.29 (m, 5H), 7.25–7.31 (m, 2H), 7.33 (dd, J = 7.4, 1.5 Hz, 1H), 7.36–7.44 (m, 2H), 7.41–7.49 (m, 2H), 7.66 (dd, J = 1.7, 0.8 Hz, 1H), 7.71 (d, J = 16.3 Hz, 1H), 8.09 (d, J = 16.3 Hz, 1H). 13C NMR (101 MHz, CDCl3) ppm 45.96, 48.07, 48.46, 52.41, 112.61, 118.83, 123.63, 123.99, 124.13, 125.49, 126.40, 126.78, 127.34, 127.50, 128.81, 129.04, 130.61, 131.25, 138.15, 139.45, 140.83, 141.76, 142.19, 147.13, 175.35. IR ν max (ATR): 1738.78, 1695.65, 1665.48, 1646.52, 1616.16, 1562.62 cm−1. LRMS (APCI) 472.19 [M++H]. HRMS (ESI) calculated for C31H20NO4 [M+−H]: 470.1398; found: 470.1394
9-(E)-3-(Naphthalen-2-yl)-3-oxoprop-1-en-1-yl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (24p) was prepared from (E)-3-(anthracen-9-yl)-1-(naphthalen-2-yl)prop-2-en-1-one (1.0 mmol, 0.358 g) and N-phenylmaleimide (1.3 mmol, 0.23g) according to the general procedure above; light green powder (65%), Mp. 202–206 °C. 1H NMR (400 MHz, CDCl3) δ 3.52 (dd, J = 8.4, 3.2 Hz, 1H), 3.69 (d, J = 8.5 Hz, 1H), 4.94 (d, J = 3.2 Hz, 1H), 6.64–6.71 (m, 2H), 7.10–7.22 (m, 2H), 7.19–7.25 (m, 1H), 7.22–7.31 (m, 3H), 7.33–7.42 (m, 2H), 7.38–7.48 (m, 3H), 7.44–7.64 (m, 2H), 7.67–7.75 (m, 4H), 7.77 (s, 1H), 7.97 (dd, J = 3.8, 1.1 Hz, 1H), 8.05 (d, J = 16.1 Hz, 1H). IR ν max (ATR): 3068.21 (Ar C-H), 1773.41, 1710.67, 1675.13 (C=O), 1380.71 (C-N), 1195.89 (C-O) cm−1. LRMS (APCI) 530.31 [M+−H]. HRMS (ESI) calculated for C37H24NO3 [M+−H]: 530.1762; found: 530.1751.
9-(E)-3-Oxo-3-(thiophen-2-yl)prop-1-en-1-yl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (24q) was prepared from (E)-3-(anthracen-9-yl)-1-(thiophen-2-yl)prop-2-en-1-one (1.0 mmol, 0.314 g) and N-phenylmaleimide (1.3 mmol, 0.23 g) according to the general procedure above; cream powder (81%), Mp. 233–236 °C. 1H NMR (400 MHz, CDCl3) δ 3.48 (dd, J = 8.4, 3.2 Hz, 1H), 3.65 (d, J = 8.4 Hz, 1H), 4.93 (d, J = 3.2 Hz, 1H), 6.45–6.54 (m, 2H), 7.14–7.19 (m, 1H), 7.19–7.24 (m, 1H), 7.24–7.31 (m, 6H), 7.38 (ddd, J = 13.1, 6.4, 2.5 Hz, 3H), 7.44–7.49 (m, 1H), 7.70 (dd, J = 4.9, 1.1 Hz, 1H), 7.77 (d, J = 16.1 Hz, 1H), 7.98 (dd, J = 3.8, 1.1 Hz, 1H), 8.04 (d, J = 16.1 Hz, 1H). 13C NMR (101 MHz, CDCl3) ppm 45.96, 48.06, 48.51, 52.44, 123.63, 124.01, 124.14, 125.51, 126.43, 126.82, 127.36, 127.52, 128.43, 128.85, 129.08, 131.20, 133.10, 134.54, 138.17, 139.46, 140.82, 141.60, 142.24, 174.57. IR ν max (ATR): 1729.12, 1660.73, 1393.28, 1327.06, 1204.75 cm−1. LRMS (APCI) [M++Na]: 510.30. HRMS (ESI) calculated for C31H21NNaO3S [M++Na]: 510.1134; found: 510.1132.
3.6. General Preparation for (E)-13-(4-Chlorophenyl)-9-(3-oxo-3-phenylprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-diones 25a–q
To a solution of the appropriate chalcone anthracene 21a–q (1.0 mmol) in toluene (2 mL) was added dienophile 1-(4-chlorophenyl)-1H-pyrrole-2,5-dione (1.3 mmol). The mixture was stirred and heated at 90 °C for 48 h. The reaction was then cooled to room temperature, and the resulting solid was isolated via filtration. The crude product was washed with toluene (2 mL) and diethyl ether (2 mL) and then recrystallized from toluene.
(E)-9-(3-(4-Bromophenyl)-3-oxoprop-1-en-1-yl)-13-(4-chlorophenyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (25a) was prepared from (E)-3-(anthracen-9-yl)-1-(4-bromophenyl)prop-2-en-1-one (1.0 mmol, 0.387 g) and 1-(4-chlorophenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.27 g) according to the general procedure above; white powder (95%) Mp. 253–254 °C. 1H NMR (400 MHz, CDCl3) δ 3.52 (dd, J = 8.32, 3.15 Hz, 1H), 4.01 (d, J = 8.33 Hz, 1H), 4.94 (d, J = 3.22 Hz, 1H), 6.47 (d, J = 8.28 Hz, 2H), 7.14–7.32 (m, 9H), 7.45–7.56 (m, 1H), 7.60 (d, J = 6.3 Hz, 1H), 7.82 (m, 2H), 7.85 (d, J = 16.12 Hz, 1 H), 7.97 (d, J = 8.4 Hz, 2H). 13C NMR (101 MHz, CDCl3) 45.96, 48.00, 48.47, 52.52, 123.53, 123.98, 124.22, 125.56, 126.92, 127.43, 127.48, 127.62, 127.71, 128.46, 129.38, 129.65, 130.50, 131.21, 132.12, 134.86, 136.44, 138.13,139.37, 140.66, 142.05, 142.68, 174.42, 175.10, 189.26 ppm. IR ν max (ATR): 1708.82, 1673.68, 1624.31, 1585.32, 1492.81, 1386.87, 1315.79, 1189.52, 1067.58, 1005.54, 850.69, 830.15 cm−1. HRMS (ESI) calculated for C33H21BrClNNaO3 [M++Na]: 616.0286; found: 616.0287.
(E)-13-(4-Chlorophenyl)-9-(3-(4-nitrophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (25b) was synthesized from (E)-3-(anthracen-9-yl)-1-(4-nitrophenyl)prop-2-en-1-one (1.0 mmol, 0.353 g) and 1-(4-chlorophenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.27 g) according to the general procedure above; white powder (82%), 1H NMR (400 MHz, CDCl3) δ 3.51 (dd, J = 8.50, 3.11 Hz, 4 H), 3.67 (d, J = 8.29 Hz, 1 H), 4.94 (d, J = 3.32 Hz, 1 H), 6.47 (d, J = 9.12 Hz, 2 H), 7.20–7.31 (m, 6 H), 7.31–7.38 (m, 2 H), 7.39–7.45 (m, 1 H), 7.46–7.51 (m, 1 H), 7.84 (d, J = 16.17 Hz, 1 H), 8.06 (d, J = 16.17 Hz, 1 H), 8.32 (d, J = 8.71 Hz, 2 H), 8.37 (d, J = 8.71 Hz, 2 H. IR ν max (ATR): 1739.76, 1665.28, 1646.57, 1562.09, 1494.06 cm−1. 13C NMR (101 MHz, CDCl3) 121.93, 123.44, 123.80, 125.15, 125.30, 125.58, 125.62, 126.00, 126.39, 126.51, 127.27, 127.35, 127.52, 127.72, 127.77, 128.06, 128.56, 128.73, 128.80, 129.03, 130.79, 131.16, 133.12, 134.17, 136.03, 138.44. HRMS (ESI) calculated for C33H21Cl2N2O5 [M++Cl]: 595.0833; found: 595.0820.
(E)-13-(4-Chlorophenyl)-9-(3-(4-ethylphenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (25c) was synthesized from (E)-3-(anthracen-9-yl)-1-(4-ethylphenyl)prop-2-en-1-one (1 mmol, 0.336 g) and 1-(4-chlorophenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.27 g) according to the general procedure above; white powder (3%), Mp. >220 (dec). 1H NMR (400 MHz, CDCl3) δ 1.19–1.25 (m, 3H), 2.37 (m, 2H), 3.54 (dd, J = 8.27, 3.39 Hz, 1H), 3.98 (d, J = 8.27 Hz, 1 H), 4.97 (d, J = 2.91 Hz, 1 H), 6.39–6.47 (m, 2H), 7.23–7.32 (m, 5H), 7.34–7.36 (m, 1 H), 7.37–7.43 (m, 3H), 7.47–7.59 (m, 2 H), 7.78 (d, J = 16.2 Hz, 1 H), 7.83 (d, J = 5.87 Hz, 2 H), 7.98 (d, J = 5.87 Hz, 2 H). IR ν max (ATR): 1733.88, 1695.56, 1665.62, 1646.02, 1616.24, 1562.03, 1520.74 cm−1. 13C NMR (101 MHz, CDCl3) 15.22, 29.04, 46.00, 48.05, 48.49, 52.51, 123.66, 124.12, 124.14, 125.49, 126.89, 127.39, 127.53, 127.72, 128.31, 129.22, 129.34, 129.36, 129.71, 131.71, 134.78, 135.42, 138.15, 139.56, 140.70, 141.53, 142.24, 150.28, 174.32, 175.15, 189.78 ppm. HRMS (ESI) calculated for C35H26ClNNaO3 [M++Na]: 566.1493; found: 556.1500.
(E)-13-(4-Chlorophenyl)-9-(3-(2,4-dichlorophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (25d) was synthesized from (E)-3-(anthracen-9-yl)-1-(2,4-dichlorophenyl)prop-2-en-1-one (1.0 mmol, 0.309 g) and 1-(4-chlorophenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.27 g) according to the general procedure above; white powder (87%), Mp. 251 (dec) °C. 1H NMR (400 MHz, CDCl3) δ 3.45 (dd, J = 8.50, 3.11 Hz, 3H), 3.56 (d, J = 8.29 Hz, 2H), 4.91 (d, J = 3.32 Hz, 2H), 6.43 (d, J = 9.12 Hz, 2H), 7.19–7.23 (m, 1H), 7.25 -7.30 (m, 4H), 7.30–7.37 (m, 3H), 7.41 (d, J = 2.07 Hz, 1H), 7.43 -7.48 (m, 1H), 7.52 (d, J = 1.66 Hz, 1H), 7.67 (d, J = 8.29 Hz, 1H), 7.81 (d, J = 16.59 Hz, 1H). IR ν max (ATR): 1738.28, 1695.68, 1648.38, 1616.26, 1562.04, 1520.75, 1451.55 cm−1. 13C NMR (101 MHz, CDCl3) 46.00, 48.23, 48.37, 52.33, 123.46, 123.83, 124.34, 125.59, 126.92, 127.40, 127.53, 27.64, 127.67, 129.36, 129.59, 130.45, 130.91, 132.71, 134.84, 134.94, 136.81, 137.53, 138.13, 139.06, 140.66, 141.59, 144.60, 174.21, 175.04, 191.56.ppm. HRMS (ESI) calculated for C32H20Cl4NO3 [M++Cl]: 618.0203; found: 618.0193.
(E)-13-(4-Chlorophenyl)-9-(3-(3,4-dimethoxyphenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (25e) was synthesized from (E)-3-(anthracen-9-yl)-1-(3,4-dimethoxyphenyl)prop-2-en-1-one (1.0 mmol, 0.368 g) and 1-(4-chlorophenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.27 g) according to the general procedure above; white powder (4%) Mp. 167–169 °C. 1H NMR (400 MHz, CDCl3) δ 2.83 (d, J = 4.98 Hz, 1H), 2.88 (d, J = 4.98 Hz, 1H), 3.49 (dd, J = 8.09, 3.11 Hz, 1H), 3.71 (d, J = 9.12 Hz, 1H), 4.93 (d, J = 3.73 Hz, 1H), 6.48 (d, J = 9.12 Hz, 1H), 7.50–7.67 (m, 5H), 7.87–7.91 (m, 1H), 7.93–7.99 (m, 1H), 8.06 (d, J = 15.76 Hz, 1H), 8.21 (d, J = 9.54 Hz, 1H). IR ν max (ATR): 1713.42, 1604.96, 1542.00, 1493.58, 1397.57, 1247.61, 1184.72 1091.31 cm−1. 13C NMR (101 MHz, CDCl3) 35.72, 67.62, 123.46, 123.83, 124.34, 125.59, 126.92, 127.40, 127.53, 127.64, 127.67, 129.36, 129.59, 130.45, 130.91, 132.71, 134.84, 134.94, 136.81, 137.53, 138.13, 139.06, 140.66, 141.60, 144.40, 169.94, 171.92, 172.24 ppm; LRMS (APCI) 514.20. HRMS 621.1041, found 621.1008.
(E)-13-(4-Chlorophenyl)-9-(3-oxo-3-phenylprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (25f) was synthesized from (E)-3-(anthracen-9-yl)-1-phenylprop-2-en-1-one (1.0 mmol, 0.308g) and 1-(4-chlorophenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.27 g) according to the general procedure above; white powder (48%), Mp. 205–210 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.53 (dd, J = 8.50, 3.11 Hz, 1H) 4.00 (d, J = 8.71 Hz, 1H) 4.94 (d, J = 3.32 Hz, 1H) 6.43–6.49 (m, 2H) 7.20–7.29 (m, 5H) 7.29–7.35 (m, 1H) 7.36–7.44 (m, 3H) 7.55–7.62 (m, 3H) 7.68 (d, J = 7.46 Hz, 1H) 7.86 (d, J = 1.24 Hz, 2H) 8.13–8.18 (m, 2H). 13C NMR (101 MHz, DMSO-d6) 39.13–40.81, 48.39, 52.14, 123.39, 123.92, 124.88, 125.69, 127.46, 129.11, 129.44, 130.91, 132.03, 133.89, 137.73, 139.18, 140.10, 141.60, 142.26, 142.73, 175.14, 175.56, 189.95 (chalcone C=O) ppm. IR ν max (ATR): 3059.03 (Ar C-H), 1707.71 (C=O), 1673.57 (C=C), 1637.61 (Ar C=C), 1327.46 (C-N), 767.80 (C-Cl) cm−1. LRMS (APCI) 514.21 [M+−H]. HRMS (ESI) calculated for C33H21ClNO3 [M+−H]: 514.1209; found: 514.1191.
(E)-13-(4-Chlorophenyl)-9-(3-(4-iodophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (25g) was synthesized (E)-3-(anthracen-9-yl)-1-(4-iodophenyl)prop-2-en-1-one (1.0 mmol, 0.4493 g) and 1-(4-chlorophenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.27 g) according to the general procedure above; white powder (92%), Mp. 225–228 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.52 (dd, J = 8.29, 3.32 Hz, H), 3.98 (d, J = 8.29 Hz, 2H), 4.93 (d, J = 3.32 Hz, 2H), 6.45 (d, J = 8.29 Hz, 2H), 7.16–7.23 (m, 2H), 7.23–7.28 (m, 2H), 7.29–7.34 (m, 1H), 7.36 (d, J = 3.73 Hz, H), 7.37–7.41 (m, H), 7.54–7.59 (m, 1H), 7.81 (d, J = 16.59 Hz, 1H), 7.84–7.92 (m, H), 7.97 (d, J = 8.29 Hz, 2H). IR ν max (ATR): 1716.83, 1661.60, 1493.51, 1392.83, 1325.76 cm−1. LRMS (APCI) [M++H]: 642.04. HRMS (ESI) calculated for C33H21ClINNaO3 [M+−H]: 664.0147; found: 664.0133.
(E)-13-(4-Chlorophenyl)-9-(3-(4-fluorophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10[3,4]epipyrroloanthracene-12,14-dione (25h) was synthesized from (E)-3-(anthracen-9-yl)-1-(4-fluorophenyl)prop-2-en-1-one (1.0 mmol, 0.326 g) and 1-(4-chlorophenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.27 g) according to the general procedure above; white powder (88%) Mp. >250 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.49 (dd, J = 8.50, 3.11 Hz, 4 H), 3.67 (d, J = 8.29 Hz, 5 H), 4.93 (d, J = 3.32 Hz, 1 H), 6.47 (d, J = 8.71 Hz, 2 H), 7.16–7.23 (m, 3 H), 7.23–7.31 (m, 6 H), 7.34–7.43 (m, 4 H), 7.47 (d, J = 6.63 Hz, 1 H), 7.83 (d, J = 15.76 Hz, 1 H), 8.00 (d, J = 15.76 Hz, 1 H), 8.21 (dd, J = 8.71, 5.39 Hz, 5 H). 13C NMR (101 MHz, DMSO-d6) 45.4, 48.4, 48.4, 52.1, 116.4, 116.6, 123.4, 123.9, 124.9, 125.7, 126.9, 127.4, 127.4, 127.5, 128.7, 129.5, 130.9, 131.9, 132.1, 132.2, 133.6, 134.4, 134.5, 139.2, 140.1, 141.6, 142.4, 142.7, 175.2, 175.5, 188.5 ppm. IR ν max (ATR): 1709.12, 1681.34, 1638.55, 1596.79, 1494.89, 1480.52, 1390.28, 1324.82 cm−1. HRMS (ESI) calculated for C33H21ClFNNaO3 [M++Na]: 556.1086; found: 556.1081.
(E)-13-(4-Chlorophenyl)-9-(3-(4-methoxyphenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (25i) was synthesized from (E)-3-(anthracen-9-yl)-1-(4-methoxyphenyl)prop-2-en-1-one (1.0 mmol, 0.338 g) and 1-(4-chlorophenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.27 g) according to the general procedure above; white powder (63%), Mp. 220–225 °C. 1H NMR (400 MHz, CDCl3) δ 3.53 (dd, J = 8.50, 3.11 Hz, 1H), 3.85 (s, 3H), 3.99 (d, J = 8.29 Hz, 1H), 4.94 (d, J = 3.32 Hz, 1H), 6.44–6.48 (m, 2H), 7.11 (d, J = 8.71 Hz, 2H), 7.19–7.34 (m, 6H), 7.35–7.40 (m, 1H), 7.39–7.43 (m, 2H), 7.59 (d, J = 7.05 Hz, 1H), 7.77–7.91 (m, 2H), 8.15 (d, J = 9.12 Hz, 2H). 13C NMR (101 MHz, CDCl3) ppm 39.21–40.74, 45.39, 48.40, 56.07, 123.39, 123.92, 124.86, 125.68, 126.88, 127.23–127.57, 128.72, 129.46, 131.53, 131.94, 140.21, 141.11, 142.86, 175.13, 175.56, 188.11 (chalcone C=O). IR ν max (ATR): 3344.59 (N-H), 1669.34 (C=O), 1599.27 (Ar C=C), 1230.74 (C-O), 1021.65 (C-N), 766.69 (C-Cl) cm−1. LRMS (APCI) 544.24 [M+−H]. HRMS (ESI) calculated for C34H24ClNNaO4 [M++Na]: 568.1286; found 568.1283.
(E)-13-(4-Chlorophenyl)-9-(3-oxo-3-(p-tolyl)prop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (25j) was synthesized from (E)-3-(anthracen-9-yl)-1-(p-tolyl)prop-2-en-1-one (1.0 mmol, 0.338 g) and 1-(4-chlorophenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.27 g) according to the general procedure above; white powder (60%), Mp. 215–220 °C. 1H NMR (400 MHz, CDCl3) δ 2.39 (s, 3H), 3.53 (dd, J = 8.29, 3.32 Hz, 1H), 3.99 (d, J = 8.29 Hz, 1H), 4.94 (d, J = 2.90 Hz, 1H), 6.43–6.49 (m, 2H, 7.20–7.29 (m, 5H), 7.29–7.34 (m, 1H), 7.35–7.43 (m, 5H), 7.55–7.61 (m, 1H), 7.85 (d, J = 2.49 Hz, 2H), 8.06 (d, J = 7.88 Hz, 2H). 13C NMR (101 MHz, CDCl3) ppm 21.69, 39.15–40.74, 48.40, 52.13, 123.39, 123.92, 124.87, 125.72, 126.88, 127.18–127.58, 128.44–128.84, 129.05 -129.59, 129.95, 132.00, 135.24, 140.15, 141.53–141.91, 142.78, 175.13, 189.36 (chalcone C=O) IR ν max (ATR): 3340.19 (N-H), 2970.43 (C-H), 1709.33 (C=O), 1623.92 (Ar C=C), 1464.76 (C-C), 1224.81 (C-O), 1014.05 (C-N), 728.59 (C-Cl) cm−1. LRMS (APCI) 528.33 [M+−H]. HRMS (ESI) calculated for C34H24ClNNaO3 [M++Na]: 552.1337; found 552.1337.
(E)-13-(4-Chlorophenyl)-9-(3-oxo-3-(3,4,5-trimethoxyphenyl)-prop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (25k) was synthesized from (E)-3-(anthracen-9-yl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (1.0 mmol, 0.398 g) and 1-(4-chlorophenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.27 g) according to the general procedure above; white powder (35%), Mp. 160–163 °C. 1H NMR (400 MHz, CDCl3) δ 3.53 (dd, J = 8.29, 3.32 Hz, 1H), 3.76 (s, 3H), 3.85 (s, 6H), 4.00 (d, J = 8.29 Hz, 1H), 4.94 (d, J = 3.32 Hz, 1H), 6.45–6.49 (m, 2H), 7.20–7.31 (m, 5H), 7.31–7.43 (m, 4H), 7.47 (s, 2H), 7.57–7.61 (m, 1H), 7.80 (d, J = 16.0 Hz, 1H), 7.88 (d, J = 16.0 Hz, 1H). 13C NMR (101 MHz, CDCl3) ppm 45.41, 48.3, 48.6, 52.11, 56.54, 60.67, 106.87, 124.90, 125.68, 126.85, 127.24–127.60, 128.55, 129.30–129.66, 132.54, 141.50, 142.60, 142.78, 153.42, 175.19, 189.10 (chalcone C=O). IR ν max (ATR): 2940.25 (C-H), 1707.34 (C=O), 1579.08 (Ar C=C), 1234.05 (C-O), 1090.23 (C-N), 766.71 cm−1. LRMS (APCI): 606.21 [M++H].
(E)-13-(4-Chlorophenyl)-9-(3-(4-chlorophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (25l) was synthesized from (E)-3-(anthracen-9-yl)-1-(4-chlorophenyl)prop-2-en-1-one (1.0 mmol, 0.343 g) and 1-(4-chlorophenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.27 g) according to the general procedure above; white powder (39%), Mp. 268–271 °C. 1H NMR (400 MHz, CDCl3) δ 3.53 (dd, J = 8.29, 3.32 Hz, 1H), 4.00 (d, J = 8.29 Hz, 1H), 4.94 (d, J = 3.32 Hz, 1H), 6.42–6.48 (m, 2H), 7.20–7.31 (m, 6H), 7.32 (s, H), 7.36–7.43 (m, 3H), 7.59 (d, J = 7.05 Hz, 1H), 7.65–7.70 (m, 2H), 7.87 (s, 2H), 8.14–8.19 (m, 2H). 13C NMR (101 MHz, CDCl3) ppm 39.07–40.76, 45.39, 48.39, 52.16, 123.42, 123.94, 124.89, 125.72, 127.46, 128.68, 129.32–129.73, 130.69–131.19, 131.78, 133.58, 138.84, 139.17, 140.04, 141.59, 142.74, 175.16, 175.55, 188.92 (chalcone C=O). IR ν max (ATR): 1707.42 (C=O), 1673.82 (C=C), 1589.01 (Ar C=C), 1015.71 (C-N), 746.57 cm−1. LRMS (APCI) 548.09 [M+−H]. HRMS Found 645.4544 [M++H3Cl2Na}; C33H24Cl4NO3Na requires 645.0408.
(E)-13-(4-Chlorophenyl)-9-(3-oxo-3-(pyridin-4-yl)prop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (25m) was synthesized from (E)-3-(anthracen-9-yl)-1-(pyridin-2-yl)prop-2-en-1-one (1.0 mmol, 0.309 g) and 1-(4-chlorophenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.27 g) according to the general procedure (4), (92%), powder, Mp. 197–198 °C. IR ν max (ATR): 1703.19, 1493.90, 777.18 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.52 (dd, J = 8.29, 3.32 Hz, 1H), 3.97 (d, J = 8.71 Hz, 1H), 4.93 (d, J = 2.90 Hz, 1H), 6.44 (d, J = 8.71 Hz, 2H), 7.24–7.29 (m, 4H), 7.30–7.35 (m, 1H), 7.35–7.42 (m, 3H), 7.54–7.60 (m, 2H), 7.78 (d, J = 16.17 Hz, 1H), 7.91 (d, J = 15.76 Hz, 1H), 7.97 (d, J = 6.63 Hz, 2H), 8.85 (d, J = 6.22 Hz, 2H). 13C NMR (101 MHz, CDCl3) 44.89. 47.79, 48.23, 51.82, 123.07, 123.53, 123.59, 124.47, 125.23, 126.40, 126.89, 126.97, 127.11, 128.22, 129.00, 130.39, 131.39, 133.12, 138.68, 139.29, 141.07, 141.88, 145.03, 147.47 ppm. HRMS (ESI) calculated for C32H22Cl2NO3 [M++H]: 551.0935; found: 551.0924.
(E)-13-(4-Chlorophenyl)-9-(3-oxo-3-(pyridin-2-yl)prop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (25n) was synthesized from (E)-3-(anthracen-9-yl)-1-(pyridin-2-yl)prop-2-en-1-one (1.0 mmol, 0.309 g) and 1-(4-chlorophenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.27 g), according to the general procedure above; cream powder, 57%. IR ν max (ATR): 1710.53, 1677.50, 1624.02, 1490.52, 1388.35, 1324.42, 1457.44, 1190.22, 1085.27, 1015.50, 996.38 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.50 (dd, J = 8.29, 3.32 Hz, 3H), 3.84 (d, J = 8.71 Hz, H), 4.94 (d, J = 2.90 Hz, 3 H), 6.45 (d, J = 8.29 Hz, H), 7.11–7.22 (m, 1 H), 7.22–7.30 (m, 4H), 7.30–7.35 (m, 1H), 7.36–7.42 (m, 3H), 7.59 (d, J = 7.05 Hz, 1H), 7.70 (dd, J = 7.46, 4.98 Hz, 1H), 8.04 (d, J = 17.00 Hz, 1H), 8.07–8.12 (m, 1H), 8.21 (d, J = 7.88 Hz, 1H), 8.27 (d, J = 16.59 Hz, 1H), 8.76 (d, J = 4.98 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) 45.41, 48.40, 48.96, 52.25, 127.37, 127.41, 127.54, 128.31, 128.64, 129.4, 130.28, 130.89, 133.51, 138.34, 139.29, 140.07, 141.66, 142.60, 143.09, 149.75, 153.68, 174.99, 175.58, 188.85 ppm. HRMS (ESI) calculated for C23H22ClN2O3 [M++H]: 517.1313; found: 517.1032.
(E)-13-(4-Chlorophenyl)-9-(3-(furan-2-yl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (25o) was prepared from (E)-3-(anthracen-9-yl)-1-(furan-2-yl)prop-2-en-1-one (1.0 mmol, 0.298 g) and 1-(4-chlorophenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.27 g) according to the general procedure above; white powder (46%) Mp. 256 °C (dec). 1H NMR (400 MHz, CDCl3) δ 3.54 (dd, J = 8.33, 3.18 Hz, 1H), 4.03 (d, J = 8.21 Hz, 1H), 4.94 (d, J = 3.3 Hz, 1H), 6.61 (m, 2H), 7.12–7.32 (m, 6H), 7.35–7.43 (m, 2H), 7.45–7.49 (m, 2H), 7.55–7.63 (m, 2H), 7.66 (m, 1H), 7.75 (d, J = 16.18 Hz, 1H), 8.13 (d, J = 16.18 Hz, 1H). 13C NMR (101 MHz, CDCl3) ppm 45.4, 48.3, 48.8, 52.0, 113.4, 120.6, 123.4, 123.9, 124.9, 125.7, 125.7, 126.9, 127.4, 127.5, 128.6, 129.3, 129.4, 130.9, 131.4, 133.5, 139.2, 140.0, 141.5, 141.6, 142.6, 149.2, 153.1, 167.3, 175.1, 175.5, 176.8. IR ν max (ATR): 1709.72, 1675.02, 1626.02, 1586.77, 1494.51, 1480.32, 1388.67 cm−1. LRMS (APCI): 506.14 [M++H].
(E)-13-(4-Chlorophenyl)-9-(3-(naphthalen-2-yl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (25p) was prepared from (E)-3-(anthracen-9-yl)-1-(naphthalen-2-yl)prop-2-en-1-one (1.0 mmol, 0.358 g) and 1-(4-chlorophenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.27 g) according to the general procedure above; gray powder (88%), Mp. 243–244 °C. 1H NMR (400 MHz, CDCl3) δ 3.51 (dd, J = 8.50, 3.11 Hz, 3H), 3.72 (d, J = 8.29 Hz, 3H), 4.95 (d, J = 3.32 Hz, 1H), 6.51 (d, J = 8.71 Hz, 2H), 7.24–7.32 (m, 6H), 7.38–7.43 (m, 2H), 7.43–7.50 (m, 2H), 7.52–7.58 (m, 1H), 7.58–7.64 (m, 1H), 7.91 (d, J = 7.88 Hz, 1H), 7.94–8.02 (m, H), 8.05–8.11 (m, 1H), 8.23 (dd, J = 8.50, 1.45 Hz, 1H), 8.71 (s, 1H). 13C NMR (101 MHz, CDCl3) ppm 46.00, 48.1, 48.47, 52.5, 123.63, 124.08, 124.16, 124.57, 125.50, 126.78, 126.89, 127.40, 127.54, 127.70, 127.82, 128.57, 128.71, 129.31, 129.48, 129.71, 129.74, 130.81, 131.62, 132.59, 134.74, 135.05, 135.68, 138.16, 140.69, 142.05, 142.19, 174.34, 175.15, 189.99. IR ν max (ATR): 1709.81, 1668.28, 1645.28, 1584.47, 1459.45, 1420.04, 1389.07, 1493.53, 1442.07 cm−1. LRMS (APCI): [M++Cl] 602.31. HRMS calculated for C37H24Cl2NO3 [M+Cl-2H] 598.0977; found 598.1371.
(E)-13-(4-Chlorophenyl)-9-(3-oxo-3-(thiophen-2-yl)prop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (25q) was prepared from (E)-3-(anthracen-9-yl)-1-(thiophen-2-yl)prop-2-en-1-one (1.0 mmol, 0.314 g) and 1-(4-chlorophenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.27 g) according to the general procedure above; white powder (70%), Mp. 180–181 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.56 (dd, J = 8.29, 3.32 Hz, 1H), 3.99 (d, J = 8.29 Hz, 1H), 4.95 (d, J = 2.90 Hz, 1H), 6.48 (d, J = 8.71 Hz, 2H), 7.09–7.17 (m, 2H), 7.18–7.32 (m, H), 7.32–7.36 (m, 1 H), 7.36–7.43 (m, 3H), 7.59 (d, J = 6.63 Hz, 1H), 7.84 (d, J = 15.76 Hz, H), 7.93 (d, J = 16.59 Hz, 1H), 8.10 (d, J = 4.56 Hz, 1H), 8.21 (d, J = 3.32 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) 45.45, 48.35, 48.68, 52.08, 123.42, 123.9, 124.88, 125.7, 125.74, 126.88, 127.3, 127.5, 129.33, 129.44, 129.4, 130.90, 131.70, 133.58, 134.6, 136.50, 137.79, 139.18, 140.05, 141.60, 142.67, 145.27 ppm. IR ν max (ATR): 1733.84, 1708.84, 1699.66, 1662.25, 1616.18, 1582.06, 1546.74, 1562.06 cm−1. LRMS (APCI): 520.30 [M+−H]. HRMS (APCI) calculated for C31H20ClNNaO3S [M++Na]: 544.0745; found: 544.0735.
3.7. General Preparation for (E)-13-(4-Benzoylphenyl)-9-(3-oxo-3-phenylprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4] Epipyrroloanthracene-12,14-diones 26a–q
To a solution of the appropriate chalcone anthracene 21a–q (1.0 mmol) in toluene (2 mL) was added dienophile 1-(4-benzoylphenyl)-1H-pyrrole-2,5-dione (1.3 mmol). The mixture was heated with stirring at 90 °C for 48 h. The reaction mixture was then cooled to room temperature, and the resulting crude solid product was isolated via filtration. The product was washed with toluene (2 mL) and diethyl ether (2 mL) and then recrystallized from toluene.
(E)-13-(4-Benzoylphenyl)-9-(3-(4-bromophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (26a) was synthesized from (E)-3-(anthracen-9-yl)-1-(4-bromophenyl)prop-2-en-1-one (1.0 mmol, 0.387 g) and 1-(4-benzoylphenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.361 g) according to the general procedure above; white powder (71%), Mp. 209–210 °C. (HPLC: 93.25%, RT 2.57 min). 1H NMR (400 MHz, DMSO-d6) δ 3.57 (dd, J = 8.29, 2.90 Hz, 3H), 4.03 (d, J = 8.71 Hz, 3H), 4.96 (d, J = 2.90 Hz, 3H), 6.65 (d, J = 8.29 Hz, 6H), 7.20–7.30 (m, 15H), 7.32–7.36 (m, 3H), 7.36–7.41 (m, 3H), 7.48–7.54 (m, 6H), 7.56–7.61 (m, 3H), 7.61–7.69 (m, 15H), 7.80 (d, J = 8.71 Hz, 6H), 7.83–7.87 (m, 2H), 7.88–7.93 (m, 1H), 8.08 (d, J = 8.71 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) 45.4, 48.4, 48.5, 52.2, 123.4, 124.0, 124.9, 125.7, 126.9, 127.0, 127.4, 127.5, 127.6, 128.0, 129.1, 130.1, 130.6, 131.1, 131.8, 132.5, 133.4, 135.5, 136.7, 137.0, 137.4, 139.2, 140.0, 141.6, 142.7, 142.8, 175.1, 175.5, 189.1, 195.3 ppm. LRMS (APCI) [M++H]: 664.16.
(E)-13-(4-Benzoylphenyl)-9-(3-(4-nitrophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (26b) was prepared from (E)-3-(anthracen-9-yl)-1-(4-nitrophenyl)prop-2-en-1-one (1.0 mmol, 0.353 g) and 1-(4-benzoylphenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.361 g) according to the general procedure above; white powder (75%); Mp. 175–177 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.59 (dd, J = 8.50, 3.11 Hz, 2H), 4.04 (d, J = 8.71 Hz, 2H), 4.98 (d, J = 3.32 Hz, 2H), 6.66 (d, J = 8.29 Hz, 3H), 7.09–7.17 (m, 4H), 7.19–7.26 (m, 5H), 7.26–7.32 (m, 6H), 7.34–7.42 (m, 4H), 7.50–7.55 (m, 4H), 7.58–7.64 (m, 2H), 7.65–7.70 (m, 8H), 7.92 (dd, J = 16.60 Hz, 2H), 8.34–8.43 (m, 4H). 13C NMR (101 MHz, DMSO-d6) 45.45, 48.43, 48.66, 52.30, 123.51, 124.02, 124.49, 124.94, 125.75, 126.91, 127.01, 127.48, 127.63, 128.6, 129.07, 129.34, 130.08, 130.50, 130.61, 132.03, 133.36, 135.54, 136.98, 137.40, 137.79, 139.17, 139.91, 141.60, 142.52, 142.54, 144.14, 150.47, 175.15, 175.51, 189.23 (chalcone C=O), 195.28 (benzophenone C=O) ppm. IR ν max (ATR): 1714.57, 1687.42, 1639.11, 1653.38, 1604.55, 1557.12, 1506.50, 1449.13, 1409.43, 1377.97, 1275.37, 1173.59 cm−1. LRMS (APCI): 631.19 [M++H]. HRMS (APCI) calculated for C40H26KN2O6 [M++K]: 669.1423; found: 669.1418.
(E)-13-(4-Benzoylphenyl)-9-(3-(4-ethylphenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (26c) was prepared from (E)-3-(anthracen-9-yl)-1-(4-ethylphenyl)prop-2-en-1-one (1.0 mmol, 0.336 g) and 1-(4-benzoylphenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.361 g) according to the general procedure above; white powder (76%), Mp. 246–247 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.58 (dd, J = 8.50, 3.11 Hz, 1H), 3.84 (s, 2H), 3.85 (s, 3H), 4.04 (d, J = 8.71 Hz, 1H), 4.97 (d, J = 3.32 Hz, 1H), 6.67 (d, J = 8.29 Hz, 2H), 7.13 (d, J = 8.29 Hz, H), 7.19–7.32 (m, 5), 7.32–7.36 (m, 1H), 7.37–7.42 (m, 1H), 7.49–7.56 (m, 2H), 7.60 (d, J = 6.63 Hz, 1H), 7.63–7.72 (m, 5H), 7.81 (d, J = 16.17 Hz, 1H), 7.78–7.84 (m, 1H), 7.87 (dd, J = 8.71, 2.07 Hz, 1H), 7.91 (d, J = 16.17 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) 45.43,48.47, 48.55, 52.14, 56.01, 56.29, 111.31, 111.51,123.39, 123.93, 124.08, 124.89, 125.71, 126.88, 126.97, 127.37, 127.45, 127.55, 129.08, 130.08, 130.60, 132.11, 133.36, 135.56, 136.97, 137.35, 139.20, 140.21, 141.65, 149.34, 175.10, 175.52, 188.25 (chalcone C=O), 195.30 (benzophenone C=O) ppm. IR ν max (ATR): 1716.45, 1660.71, 1391.17, 1325.22 cm−1. LRMS (APCI) 614.14 [M++H].
(E)-13-(4-Benzoylphenyl)-9-(3-(2,4-dichlorophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (26d) was prepared from (E)-3-(anthracen-9-yl)-1-(2,4-dichlorophenyl)prop-2-en-1-one (1.0 mmol, 0.377 g) and 1-(4-benzoylphenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.361 g) according to the general procedure above; white powder (63%). 1H NMR (400 MHz, DMSO-d6) δ 3.58 (dd, J = 8.29, 3.32 Hz, 1H), 4.04 (d, J = 8.29 Hz, 1H), 4.97 (d, J = 2.90 Hz, 1H), 6.67 (d, J = 8.29 Hz, 2H), 7.09–7.16 (m, 1H), 7.19–7.32 (m, 5H), 7.32–7.36 (m, 1H), 7.37–7.43 (m, 1H), 7.50–7.56 (m, 2H), 7.60 (d, J = 6.22 Hz, 1H), 7.63–7.71 (m, 6H), 7.81 (d, J = 16.17 Hz, 1H), 7.86 (d, J = 1.66 Hz, 1H), 7.91 (d, J = 16.17 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) 48.5, 52.1, 56.0, 56.3, 111.3, 111.5, 123.9, 124.1, 124.9, 126.9, 127.0, 127.5, 129.1, 130.1, 130.6, 132.1, 133.4, 135.6, 137.0, 137.4, 139.2, 140.2, 140.9, 141.7, 142.9, 144.5, 145.7, 149.3, 153.9, 175.1, 175.5, 188.2 (chalcone C=O), 195.3 (benzophenone C=O) ppm. LRMS (APCI): 614.15 [M+-C3H3].
(E)-13-(4-Benzoylphenyl)-9-(3-(3,4-dimethoxyphenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (26e) was prepared from (E)-3-(anthracen-9-yl)-1-(3,4-dimethoxyphenyl)prop-2-en-1-one (1.0 mmol, 0.368 g) and 1-(4-benzoylphenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.361 g) according to the general procedure above; white powder (72%), Mp. 161–164 °C. IR ν max (ATR): 2969, 1714.39, 1651.24, 1603.13, 1507.90, 1458.19, 1378.67, 1275.61, 1177.36, 929.79, 925.10 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 1.20 (t, J = 7.46 Hz, 2H), 2.28 (s, 2H), 3.58 (dd, J = 8.50, 3.11 Hz, 1H), 4.03 (d, J = 8.29 Hz, 1H), 4.97 (d, J = 3.32 Hz, 1H), 6.67 (d, J = 8.29 Hz, 1H), 7.09–7.18 (m, 2H), 7.19–7.25 (m, 3H), 7.26–7.32 (m, 2H), 7.32–7.36 (m, H), 7.38–7.45 (m, 2H), 7.50–7.55 (m, 2H), 7.58–7.63 (m, 1H), 7.63–7.71 (m, 4H), 7.82–7.93 (m, 2H), 7.87 (dd, J = 16.20 Hz, 2H), 8.10 (d, J = 7.88 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) 21.49, 28.70, 45.4, 48.47, 48.52, 52.18, 123.41, 123.93, 124.89, 126.90, 127.02, 127.38, 127.48, 127.57, 128.64, 128.80, 129.07, 129.34, 129.39, 130.08, 130.60, 132.09, 133.36, 135.50, 135.56, 136.97, 137.38, 137.78, 139.18, 140.14, 141.63, 141.76, 142.80, 150.41, 175.09, 175.51, 189.38 (chalcone C=O), 195.29 (benzophenone C=O) ppm. LRMS (APCI) 614.19 [M+-OCH3].
(E)-13-(4-Benzoylphenyl)-9-(3-oxo-3-phenylprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (26f) was synthesized from (E)-3-(anthracen-9-yl)-1-phenylprop-2-en-1-one (1.0 mmol, 0.308 g) and 1-(4-benzoylphenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.361 g) according to the general procedure above; white powder (49%), Mp. 240–241 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.58 (dd, J = 8.50, 3.11 Hz, 1H), 4.04 (d, J = 8.29 Hz, 1H), 4.97 (d, J = 2.90 Hz, 1H), 6.67 (d, J = 8.29 Hz, 2H), 7.19–7.32 (m, 6H), 7.34 (s, 1H), 7.37–7.42 (m, 1H), 7.49–7.56 (m, 2H), 7.57–7.64 (m, 4H), 7.64–7.72 (m, 6H), 7.89 (s, 2H), 8.16 (d, J = 7.05 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) 39.21–40.63, 48.48, 52.20, 55.34, 125.75, 127.03, 127.48, 128.94–129.60, 130.08, 130.61, 131.99, 136.97, 137.39, 139.17, 142.75, 174.93–175.59, 176.01, 189.96 (chalcone C=O), 195.31 (benzophenone C=O) ppm. IR ν max (ATR): 1708.57 (C=O), 1670.41 (C=C), 1603.92 (Ar C=C), 1308.22 (C-N) cm−1. HRMS (APCI) calculated for C40H27NNaO4 [M++Na]: 608.1832; found: 608.1830.
(E)-13-(4-Benzoylphenyl)-9-(3-(4-iodophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (26g) was prepared from (E)-3-(anthracen-9-yl)-1-(4-iodophenyl)prop-2-en-1-one (1.0 mmol, 0.338 g) and 1-(4-benzoylphenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.361 g) according to the general procedure above; white powder (85%), Mp. 186–188 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.59 (dd, J = 8.29, 2.90 Hz, 1H), 4.04 (d, J = 8.71 Hz, 1H), 4.98 (d, J = 3.32 Hz, 1H), 6.67 (d, J = 8.29 Hz, 2H), 7.10–7.17 (m, 2H), 7.19–7.32 (m, 6H), 7.33–7.37 (m, 1H), 7.38–7.43 (m, 1H), 7.50–7.56 (m, 2H), 7.61 (d, J = 7.05 Hz, 1H), 7.63–7.71 (m, 5H), 7.86 (d, J = 16.59 Hz, 1H), 7.89–7.95 (m, 3H), 7.96–8.03 (m, 2H). 13C NMR (101 MHz, DMSO-d6) 45.45, 48.4, 48.55, 52.23, 102.63, 123.45, 123.97, 124.90, 125.72, 125.7, 126.91, 127.03, 127.40, 127.48, 127.59, 128.64, 129.07, 129.34, 130.09, 130.61, 130.82, 131.76, 133.35, 135.56, 136.98, 137.04, 137.39, 137.79, 138.36, 139.17, 140.04, 141.61, 142.69, 142.77, 175.11, 175.50, 189.44, 195.28 ppm. IR ν max (ATR): 1715.20, 1687.34, 1638.37, 1653.19, 1604.04, 1448.80, 1379.55, 1275.10, 1173.70, 1190.33 cm−1. LRMS (APCI) 710.13 [M+−H]. HRMS (ESI) calculated for C40H25INO4 [M+−H]: 710.0834; found: 710.0823.
(E)-13-(4-Benzoylphenyl)-9-(3-(4-fluorophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (26h) was synthesized from (E)-3-(anthracen-9-yl)-1-(4-fluorophenyl)prop-2-en-1-one (1.0 mmol, 0.387 g) and 1-(4-benzoylphenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.361 g) according to the general procedure above, white powder (77%). 1H NMR (400 MHz, DMSO-d6) δ 3.51 (dd, J = 8.22, 3.17 Hz, 1H), 4.02 (d, J = 8.4 Hz, 1H), 4.98 (d, J = 3.28 Hz, 1H), 6.49–6.54 (m, 2H), 7.15–7.32 (m, 5H), 7.35 (m, 1H), 7.36–7.40 (m, 4H), 7.41–7.46 (m, 4H), 7.47–7.49 (m, 1H), 7.83 (m, 3H), 8.02 (d, J = 16.18 Hz, 1H), 8.13–8.23 (m, 2H).
(E)-13-(4-Benzoylphenyl)-9-(3-(4-methoxyphenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (26i) was prepared from (E)-3-(anthracen-9-yl)-1-(4-methoxyphenyl)prop-2-en-1-one (1.0 mmol, 0.338 g) and 1-(4-benzoylphenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.361 g) according to the general procedure above; white powder (50%), Mp. 224–228 °C. 1H NMR (400 MHz, CDCl3) δ 3.58 (dd, J = 8.29, 3.32 Hz, 1H), 3.86 (s, 3H), 4.04 (d, J = 8.29 Hz, 1H), 4.97 (d, J = 3.32 Hz, H), 6.66 (d, J = 8.29 Hz, 2H), 7.12 (d, J = 8.71 Hz, 2H), 7.20–7.31 (m, 5H), 7.31–7.35 (m, 1H), 7.38–7.41 (m, 1H), 7.51–7.56 (m, 2H), 7.60 (d, J = 6.63 Hz, 1H), 7.64–7.71 (m, 5H), 7.79–7.93 (m, 2H), 8.16 (d, J = 8.71 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) 39.26, 40.72, 48.48, 52.15, 56.07, 114.67, 123.42, 123.93, 124.87, 125.71, 126.76, 127.69, 130.08, 131.53, 133.36, 136.97, 139.18, 141.10, 141.64, 142.88, 163.89, 175.09, 175.52, 188.12 (chalcone C=O), 195.29 (benzophenone C=O) ppm. IR ν max (ATR): 3344.36 (N-H), 1659.32 (C=O), 1599.79 (Ar C=C), 1231.15 (C-O), 1020.42 (C-N) cm−1. HRMS (ESI) calculated for C41H29NNaO5 [M++Na]: 638.1938; found: 638.1943.
(E)-13-(4-Benzoylphenyl)-9-(3-oxo-3-(p-tolyl)prop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (26j) was prepared from (E)-3-(anthracen-9-yl)-1-(p-tolyl)prop-2-en-1-one (1.0 mmol, 0.3384 g) and 1-(4-benzoylphenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.361g) according to the general procedure above; white powder (59%), Mp. 256–260 °C. 1H NMR (400 MHz, CDCl3) δ 2.40 (s, 3H), 3.57 (dd, J = 8.29, 2.90 Hz, 1H), 4.03 (d, J = 8.29 Hz, 1H), 4.96 (d, J = 2.90 Hz, 1H), 6.66 (d, J = 8.29 Hz, 2H), 7.20–7.31 (m, 5H), 7.31–7.36 (m, 1H), 7.40 (d, J = 7.88 Hz, 3H), 7.50–7.56 (m, 2H), 7.56–7.71 (m, 6H), 7.86 (d, J = 3.32 Hz, 2H), 8.06 (d, J = 7.88 Hz, 2H). 13C NMR (101 MHz, CDCl3) 21.69, 39.21–40.78, 48.49, 55.35, 123.41, 127.03, 128.83–129.51, 130.02, 136.97, 137.38, 139.18, 142.80, 144.38, 175.51 (chalcone C=O), 189.37 (benzophenone C=O) ppm. IR ν max (ATR): 2965 (O-H), 1712.89 (C=O), 1626.70 (Ar C=C), 1458.41 (C-C), 1231.54 (C-O), 1128.77 (C-N) cm−1. HRMS (ESI) calculated for C41H29NNaO4 [M++Na]: 622.1989; found: 622.1982.
(E)-13-(4-Benzoylphenyl)-9-(3-oxo-3-(3,4,5-trimethoxyphenyl)-prop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (26k) was prepared from (E)-3-(anthracen-9-yl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (1 mmol, 0.398 g) and 1-(4-benzoylphenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.361 g) according to the general procedure above; white powder (44%), Mp. 210–216 °C. 1H NMR (400 MHz, CDCl3) δ 3.58 (dd, J = 8.50, 3.11 Hz, 1H), 3.76 (s, 3H), 3.85 (s, 6 H), 4.04 (d, J = 8.71 Hz, 1H), 4.97 (d, J = 3.32 Hz, 1H), 6.65–6.70 (m, 2H), 7.21–7.31 (m, 5H), 7.35–7.38 (m, 1H), 7.38–7.42 (m, 1H), 7.48 (s, 2H), 7.51–7.56 (m, 2H), 7.59–7.62 (m, 1H), 7.63–7.70 (m, 5H), 7.80–7.93 (m, 2H). 13C NMR (101 MHz, CDCl3) 39.24, 40.78, 48.40, 52.16, 56.56, 60.68, 106.89, 125.53, 125.91, 126.85, 127.40, 128.64, 129.07, 129.33, 130.07, 130.56, 132.54, 133.05, 133.34, 137.00, 140.12, 141.56, 153.43, 175.03, 175.61, 189.07 (chalcone C=O), 195.29 (benzophenone C=O) ppm. IR ν max (ATR): 2941.10 (O-H), 1708.03 (C=O), 1579.54 (Ar C=C), 1234.15 (C-O), 1091.54 (C-N) cm−1. HRMS (ESI) calculated for C43H33NNaO7 [M++Na]: 698.2149; found: 698.2141.
(E)-13-(4-Benzoylphenyl)-9-(3-(4-chlorophenyl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (26l) was synthesized from (E)-3-(anthracen-9-yl)-1-(4-chorophenyl)prop-2-en-1-one (1.0 mmol, 0.343 g) and 1-(4-benzoylphenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.361 g) according to the general procedure above; white powder (30%), Mp. 217–220 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.59 (dd, J = 3.32, 8.29 Hz, 1H), 4.04 (d, J = 8.29 Hz, 1H), 4.97 (d, J = 2.90 Hz, 1H), 6.66 (d, J = 8.71 Hz, 2H), 7.20–7.31 (m, 6H), 7.34 (m, 1H), 7.39 (m, 1H), 7.50–7.56 (m, 2H), 7.60 (d, J = 6.63 Hz, 1H), 7.66 (m, 6H), 7.89 (m, 2H), 8.18 (d, J = 8.0 Hz, 2H) 13C NMR (101 MHz, DMSO-d6) 42.31, 45.42, 48.48, 52.20, 123.44, 123.95, 124.90, 125.64, 125.78, 126.85, 127.14, 127.35, 127.66, 128.64, 129.08, 129.55, 130.08, 130.61, 131.03, 131.81, 136.41, 138.85, 139.16, 142.68, 142.81, 175.12, 175.51, 195.31 (chalcone C=O), 197.42 (benzophenone C=O) ppm. IR ν max (ATR): 3024.65 (Ar C-H), 1712.90 (C=O), 1672.35 (C=C), 1603.46 (Ar C=C), 1013.52 (C-N), 769.64 cm−1. HRMS (ESI) calculated for C40H25ClNO4 [M+−H]: 618.1478; found: 618.1487.
(E)-13-(4-Benzoylphenyl)-9-(3-oxo-3-(pyridin-4-yl)prop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (26m) was synthesized from (E)-3-(anthracen-9-yl)-1-(pyridin-4-yl)prop-2-en-1-one (1.0 mmol, 0.309 g) and 1-(4-benzoylphenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.361 g) according to the general procedure above; white powder (62%), Mp. 230–231 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.58 (dd, J = 8.29, 3.32 Hz, 2H), 4.02 (d, J = 8.29 Hz, 2H), 4.97 (d, J = 3.32 Hz, 2H), 6.66 (d, J = 8.71 Hz, 4H), 7.19–7.32 (m, 10H), 7.33–7.38 (m, 2 H), 7.38–7.42 (m, 2 H), 7.49–7.56 (m, 4H), 7.59–7.64 (m, 3H), 7.64–7.69 (m, 9H), 7.78–7.85 (m, 2H), 7.81 (d, J = 16.17 Hz, 1H), 7.91–7.97 (m, 2H), 7.94 (d, J = 16.17 Hz, 3H), 7.99 (d, J = 5.81 Hz, 4H). 13C NMR (101 MHz, DMSO-d6) 45.41, 48.40, 48.67, 52.27, 122.20, 123.48, 123.99, 124.94, 125.73, 126.93, 127.02, 127.48, 127.63, 129.08, 130.08, 130.61, 131.80, 133.38, 135.51, 136.96, 137.40, 139.14, 139.85, 141.57, 142.48, 143.77, 144.35, 151.35, 175.14, 175.51, 190.04 (chalcone C=O), 195.32 (benzophenone C=O) ppm. IR ν max (ATR): 1714.95, 1687.33, 1654.19, 1604.43, 1449.30, 1377.44, 1275.15, 1173.54, 1190.08 cm−1. LRMS (APCI) 587.37 [M++H]. HRMS (ESI) calculated for C39H25N2O4 [M+−H]: 585.1820; found: 585.1818.
(E)-13-(4-Benzoylphenyl)-9-(3-oxo-3-(pyridin-2-yl)prop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (26n) was prepared from (E)-3-(anthracen-9-yl)-1-(pyridin-2-yl)prop-2-en-1-one (1.0 mmol, 0.309 g) and 1-(4-benzoylphenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.361 g) according to the general procedure above; gray powder (75%), Mp. > 240 °C (dec). HPLC: 96.29%, RT 8.88 min). 1H NMR (400 MHz, DMSO-d6) δ 3.54 (dd, J = 4.98, 3.32 Hz, 1H), 3.86–3.91 (m, 1H), 4.96 (d, J = 3.32 Hz, 1H), 6.66 (d, J = 8.71 Hz, 2H), 7.19–7.31 (m, 6H), 7.32–7.38 (m, 1H), 7.38–7.42 (m, 1H), 7.52 (s, 2H), 7.58–7.64 (m, 2H), 7.64–7.73 (m, H), 8.08 (d, J = 16.59 Hz, 1H), 8.09–8.12 (m, H), 8.20–8.23 (m, 1H), 8.27 (d, J = 16.59 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) 45.44, 48.50, 49.04, 52.29, 123.30, 123.42, 123.89, 124.96, 125.75, 126.89, 126.95, 127.44, 128.31, 128.64, 129.07, 129.33, 130.06, 130.32, 130.56, 133.36, 135.53, 136.97, 137.33, 137.79, 138.34, 139.23, 140.06, 141.67, 142.62, 143.08, 149.7, 153.68, 174.9, 175.54, 188.8 (chalcone C=O), 195.32 (benzophenone C=O) ppm. LRMS (APCI) 587.49 [M++H]. IR ν max (ATR): 1710.44, 1678.62, 1651.53, 1622.75, 1372.38, 1278.33, 1323.21, 1022.35, 999.00, 926.58 cm−1. HRMS (ESI) calculated for C39H26N2NaO4 [M++Na]: 609.1785; found: 609.1786.
(E)-13-(4-Benzoylphenyl)-9-(3-(furan-2-yl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (26o) was prepared from (E)-3-(anthracen-9-yl)-1-(furan-2-yl)prop-2-en-1-one (1.0 mmol, 0.298 g) and 1-(4-benzoylphenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.361 g) according to the general procedure above; white powder (60%), Mp. >230 °C (dec). IR ν max (ATR) 2961.18, 1709.80, 1667.15, 1661.00, 1652.47, 1620.31, 1604.15, 1577.76, 1461.92, 1373.31, 1276.89, 1156.72, 940.89, 724.18 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.60 (dd, J = 8.56, 3.18 Hz, 1H), 3.98 (d, J = 8.31 Hz, 1H), 4.99 (d, J = 3.42 Hz, 3H), 6.68 (d, J = 8.31 Hz, 2H), 7.21–7.28 (m, 2H), 7.28–7.33 (m, 2 H), 7.33–7.38 (m, 1H), 7.39–7.44 (m, 1H), 7.52–7.60 (m, 2H), 7.60–7.64 (m, 1H), 7.65–7.74 (m, 6H), 7.75–7.80 (m, 1H), 7.95 (d, J = 16.63 Hz, 1H), 8.14 (d, J = 1.47 Hz, 1H). LRMS (APCI) 576.11 [M++H].
(E)-13-(4-Benzoylphenyl)-9-(3-(naphthalen-2-yl)-3-oxoprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (26p) was synthesized from (E)-3-(anthracen-9-yl)-1-(naphthalen-2-yl)prop-2-en-1-one (1.0 mmol, 0.358 g) and 1-(4-benzoylphenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.361 g) according to the general procedure above; gray powder (88%). 1H NMR (400 MHz, DMSO-d6) δ 3.58 (dd, J = 8.31, 3.42 Hz, 1H), 3.91 (d, J = 8.80 Hz, 1H), 5.00 (d, J = 3.42 Hz, 1H), 6.69 (d, J = 8.31 Hz, 2H), 7.19–7.34 (m, 5H), 7.38 (d, J = 4.89 Hz, 1H), 7.41–7.45 (m, H), 7.51–7.58 (m, 2 H), 7.60–7.67 (m, 1H), 7.67–7.77 (m, 5H), 8.11 (d, J = 16.14 Hz, H), 8.24 (d, J = 7.83 Hz, 1H), 8.32 (d, J = 16.63 Hz, 1H), 8.79 (d, J = 4.40 Hz, 1H). LRMS (APCI): 636.24 [M++H].
(E)-13-(4-Benzoylphenyl)-9-(3-oxo-3-(thiophen-2-yl)prop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (26q) was synthesized from (E)-3-(anthracen-9-yl)-1-(thiophen-2-yl)prop-2-en-1-one (1.0 mmol, 0.3144 g) and 1-(4-benzoylphenyl)-1H-pyrrole-2,5-dione (1.3 mmol, 0.361 g) according to the general procedure above; white solid (51%). Mp. 255–257 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.59 (dd, J = 5.39, 3.73 Hz, 1H), 4.02 (d, J = 8.71 Hz, 1H), 4.97 (d, J = 3.32 Hz, 1H), 6.66 (d, J = 8.29 Hz, 2H), 7.19–7.32 (m, 6H), 7.34 (s, 1H), 7.38–7.42 (m, 1H), 7.54 (d, J = 7.46 Hz, 2H), 7.60 (s, 1H), 7.65–7.71 (m, 5H), 7.85 (d, J = 15.76 Hz, 1H), 7.89 (d, J = 16.59 Hz, 1H), 8.12 (dd, J = 4.98, 0.83 Hz, 1H), 8.21 (dd, J = 3.73, 0.83 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) 45.4, 48.4, 48.8, 52.1, 123.4, 124.0, 124.9, 125.7, 127.0, 127.4, 127.5, 127.6, 128.6, 129.1, 129.3, 129.5, 130.1, 130.6, 131.7, 133.3, 134.6, 135.5, 136.5, 137.0, 137.4, 139.2, 140.0, 141.5, 141.6, 142.7, 145.2, 175.1, 175.5, 182.0, 195.3 ppm. IR ν max (ATR): 1734.00, 1699.51, 1665.19, 1646.71, 1618.07, 1520.87, 1493.38, 1460.27 cm−1. LRMS (APCI) [M+−H]: 590.54. HRMS (ESI) calculated for C38H24NO4S [M+−H] 590.1431; Found 590.1430.
Dimethyl (E)-9-(3-oxo-3-phenylprop-1-en-1-yl)-9,10-dihydro-9,10-ethenoanthracene-11,12-dicarboxylate (27) was synthesized from (E)-3-(anthracen-9-yl)-1-phenylprop-2-en-1-one (1 mmol, 0.3084 g) and dimethyl acetylenedicarboxylate (1.3 mmol, 0.16 mL) according to the general procedure above; orange/yellow crystalline solid (62%), [65], Mp. >250 °C. IR ν max (ATR): 1726.80, 1714.47, 1679.84, 1644.84, 1601.46, 1448.65, 1325.56, 1294.56, 1276.25, 947.76 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.64 (s, 3H), 3.72 (s, 3H), 5.80 (s, 1H), 7.07–7.15 (m, 4H), 7.45–7.50 (m, 2H), 7.52–7.58 (m, 3H), 7.61 (m, 2H), 7.73 (d, J = 16.12 Hz, 2H), 8.08 (d, J = 7.77 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) 49.98, 52.95, 53.23, 57.83, 122.94, 124.84, 125.77, 126.31, 129.13, 129.56, 133.42, 134.17, 137.31, 137.80, 142.62, 143.97, 145.09, 154.91, 163.57, 167.01, 189.21 ppm. HRMS (ESI) calculated for C29H22NaO5 [M++Na]: 473.1360; found: 473.1351.
3.8. Stability Study of Compounds 21a, 21i, 22h, 23a, 23g, 23n, 24a, 24h, 26a and 26n
The HPLC stability studies for compounds 21a, 21i, 22h, 23a, 23g, 23n, 24a, 24h, 26a and 26n were performed using a Symmetry® column (C18, 5 µm, 4.6 × 150 mm), a Waters 2487 Dual Wavelength Absorbance detector, and an HPLC pump: Waters 1525 binary and Autosampler; Waters 717 plus (Waters Corporation, Milford, MA, USA), mobile phase [(acetonitrile (80%) and water (20%)] with a typical run time of 10 min and a flow rate of 1 mL/min with detection at 254 nm. The selected compounds 21a, 21i, 22h, 23a, 23g, 23n, 24a, 24h, 26a and 26n (5 mg) in the mobile phase (10 mL) provided the stock solution for the analysis. The phosphate buffers were prepared at pH values of 4, 7.4 and 9, as described in the British Pharmacopoeia monograph 2019. The stock solution (30 µL) was added to the appropriate buffer (1 mL) and retained at 37 °C. Samples were then analyzed over the following 24-h period at selected time intervals (t = 0 min, 5 min, 30 min, 60 min, 90 min, 120 min and 24 h).
3.9. X-ray Crystallography Analysis
Samples were mounted on a MiTeGen micromount with NVH immersion oil. Data for 21k, 23f, 23h, 24a and 24g were collected from a shock-cooled single crystal at 100(2) K on a Bruker D8 Quest ECO three-circle diffractometer with a standard sealed X-ray tube using a graphite monochromator and a CMOS area detector. The diffractometer was equipped with an Oxford Cryostream 800 low-temperature device, and it used MoKα radiation (λ = 0.71073 Å). Data for 25f and 27 were collected from a shock-cooled single crystal at 100(2) K on a Bruker APEX2 Kappa Duo Kappa diffractometer with a microfocus sealed X-ray tube using mirror optics as a monochromator and an APEX2 detector. The diffractometer was equipped with an Oxford Cobra low-temperature device, and it used Cu Kα radiation (λ = 1.54178 Å). All data were integrated with SAINT, and a multi-scan absorption correction using SADABS was applied [95,96]. Structures were solved with dual methods using XT and refined with full-matrix least-squares methods against F2 by XL using Olex2 [97,98,99]. All non-hydrogen atoms were refined with anisotropic displacement parameters. All hydrogen atoms were refined in an isotropic manner on calculated positions using a riding model with their Uiso values constrained to 1.5 times the Ueq of their pivot atoms for terminal sp3 carbon atoms and 1.2 times for all other carbon atoms. Disordered moieties were refined using bond lengths’ restraints and displacement parameter restraints.
In 23f, the N-H hydrogen was located on the difference map and refined with restraints (DFIX). The molecule has a chiral center at C3 and C4; however, it is a racemate, and the R configuration is shown (centrosymmetric space group). In 23h, the pendant fluorophenyl group was modeled as disordered over two positions (C24–F1) with 50% occupancy. Restraints (SADI, DFIX and RIGU) and constraints (EADP) were used to model the disorder.
In 24a, the majority of the molecule was disordered over two positions with approx. 61:39% occupancy. Restraints (SIMU and ISOR) and some constraints (EADP) were used in the model for L.S. convergence. 25f was a poorly diffracting small crystal with weak high-angle data and incomplete Friedel pair collection. The model has chirality at C25 and C31, S (Polar Space Group).
The crystallographic data for the structures reported here were deposited with the Cambridge Crystallographic Data Centre [100] (CCDC) for 21k (Deposition Number 2341799), 23f (Deposition Number 2341800), 23h (Deposition Number 2341801), 24a (Deposition Number 2341802), 24g (Deposition Number 2341803), 25f (Deposition Number 2341804) and 27 (Deposition Number 2341805), and they contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures.
3.10. In Silico Computational Study
Two-dimensional structures of the 21 novel anthracene compounds and maprotiline were exported from MOE post-standardization in the SMILES format [101]. The exported structures were then individually submitted as queries to the SwissTargetPrediction (STP) web service [86]. For each of the 22 submitted structures, STP automatically generated its structural fingerprints and estimated its 3D shape. Based on these characteristics, STP then searched its internal database of known active compounds (based mainly on ChEMBL data) [102] for ones that are similar in structure and/or shape [103]. All compounds discovered through the similarity search were then tracked via STP to their specific biological targets. All targets hit by these similar compounds were then compiled into a prioritized list. This potential target list was generated and downloaded in a tabular format for each of the 21 + 1 query structures. The resulting target lists were aggregated and further processed in a standard Python/Pandas data analysis environment [104].
3.11. Molecular Modeling: Computational Overlay Study
All the compounds were opened in a database viewer. The compounds were washed with default values, and explicit hydrogens were added. For each compound, MMFF94x partial charges were calculated, and each was minimized to a gradient of 0.001 kcal/mol/Å. The compounds were then overlaid individually on a 3D structure of maprotiline using flexible alignment on MOE with default values [105].
3.12. Biochemistry
3.12.1. Materials
The EBV-transformed CLL PGA-1 (M-IGVH, good prognosis) and HG-3 (UM-IGVH, poor prognosis) cell lines were provided by Professor Anders Rosén (Linköping University, Linköping, Sweden) [106]. alamarBlue was obtained from BioSource, Nivelles, Belgium, and fetal bovine serum (FBS) was sourced from Invitrogen, U.K. RPMI 1640 medium, HEPES, gentamycin (G418) and glutamine were sourced from Thermo Fisher Scientific, Dun Laoghaire, Ireland. Cell culture consumables were obtained from Greiner Bio-One Ltd., Stonehouse, U.K., while all other reagents used were obtained from Sigma-Aldrich (now Merck), Arklow, Ireland.
3.12.2. Cell Culture
The CLL PGA-1 and HG-3 leukemia cell lines were grown in RPMI-1640 (Glutamax; Thermo Fisher Scientific, Inc., Dun Laoghaire, Dublin, Ireland) medium that was supplemented with 10% (v/v) FBS, 50 µg/mL of streptomycin and 50 U/mL of penicillin. The cells were seeded at a density of 2 × 105 cells/mL. The cell cultures were maintained at 37 ◦C in a humidified atmosphere of 5% CO2/95% O2, and the cells were passaged at least twice weekly, according to their confluency.
3.12.3. AlamarBlue Cell Viability Assay
CLL cells (HG-3 and PGA-1) were seeded at a density of 2 × 105 cells/well (200 µL per well) in 96-well plates. The cells were treated with the appropriate concentrations of test compounds or control drugs (maprotiline and fludarabine), and the samples were incubated for 24 h. alamarBlue (20 µL) was then added to each well, and the samples were further incubated at 37 ◦C in the dark for 4 h. Wells containing only the reagent and cell culture medium (without cells) were used as blank controls, and the vehicle used was DMSO (1% v/v) in all experiments. The 96-well plates were analyzed on a fluorescence plate reader (SpectraMax Gemini, Molecular Devices) with emission and excitement wavelengths of 590 nm and 544 nm, respectively, and fluorescence was measured. The decrease in cell viability was obtained with reference to the vehicle (100% viability). An RPMI medium containing alamarBlue was used as a blank. The viabilities of the compounds were calculated with reference to the vehicle-treated cells (100% viable). A non-linear, sigmoidal dose–response curve was obtained from transformed data (Final Concentration = Log (Final Concentration); IC50 values (concentration of drug required for 50% reduction in cell survival) were obtained using Prism (Prism 10 for mac OS 10.0.2 GraphPad Software, Inc., La Jolla, CA, USA). The biochemical assays were performed in triplicate on at least three independent occasions, and the mean values were determined.
3.12.4. Annexin V/PI Apoptosis Assay
CLL cells PGA-1 and HG-3 (1 × 106 cells/mL) were treated at 37 °C with either the vehicle (1% (v/v) DMSO) or the ethanoanthracene compounds 20a, 20f, 23a and 25n (10 µM, 5 µM and 1 µM) for 48 h, following the protocol previously described [40]. The cells were then harvested via centrifugation at 400× g in a temperature-controlled Sorvall centrifuge. The cells were rinsed with Ca2+ Annexin-V-binding buffer (0.1 M HEPES, pH 7.4; 0.14 M NaCl; 25 mM CaCl2, 0.5 mL). The samples were resuspended in FITC Annexin V (diluted 1:33 in Ca2+ Annexin V-binding buffer, 50 µL) and incubated for 10 min (on ice and protected from light). The samples were washed with Annexin-V-binding buffer and resuspended in a PI solution (0.5 µg/mL, 500 µL) and analyzed within 1 h using a BD Accuri C6 flow cytometer counting 10,000 cells using the FlowJo software Version 10 (FlowJo LLC, Ashland, OR, USA).
3.12.5. Inhibitor Studies: Reactive Oxygen Species (ROS)
The ROS cell viability assay included the pre-treatment of CLL cells PGA-1 and HG-3 with N-acetyl cysteine. N-acetyl cysteine (NAC) (Sigma, Berrinba, Queensland, Australia) was dissolved in sterile water and subsequently diluted to obtain a 5-mM stock solution concentration. Fresh solutions were prepared for each experiment. CLL cells PGA-1 and HG-3 were seeded at a density of 2 × 105 cells/mL in a 96-well plate. The cells were protected from light and pre-treated with NAC (2 µL) for 1 h before then being treated with compounds 20a, 20f, 23a and 25n at concentrations 1 µM or 10 µM for 24 h. The remainder of the assay was carried out as described previously for the alamarBlue cell viability assay.
3.12.6. Caspase Inhibitor Study
Compounds 20a and 23a (representing Series 1 and Series 4, respectively) were selected for evaluation at two treatment concentrations, 10 µM and 1 µM, across both HG-3 and PGA-1 CLL cells. The HG-3 and PGA-1 CLL cells were seeded at a density of 2 × 105 cells/mL and were subsequently pre-treated at 37 ◦C with 40 µM of pan-caspase inhibitor Z-VAD-FMK (G-Biosciences, Geno Technology Inc., St. Louis, MO, USA) per well for 4 h prior to compound treatment for 24 h. The caspase assay protocol was adapted from Bright et al. [85], and the remainder of the assay was carried out as described above for the alamarBlue cell viability assay and completed in triplicate on two independent days. The output was statistically analyzed using a one-way ANOVA with a Bonferroni multiple-comparison test. Statistical significance was defined as any comparison with a generated p-value of <0.05.
3.12.7. Cytotoxicity Assay
The cytotoxicity of the selected compounds 20a, 20f, 23a and 25n was determined using the CytoTox 96 non-radioactive cytotoxicity assay (Promega Corporation; 2800 Woods Hollow Road, Madison, WI, USA). CLL cells HG-3 and PGA-1 were incubated for 24 h and then treated with the selected compounds 20a, 20f, 23a and 25n at 10-µM and 1-µM concentrations, following the protocol outlined above in the cell viability assay. After 24 h, the lysis solution (10×) (20 µL) was added, and the cells were incubated for a further 1 h to ensure complete cell death. Supernatant (50 µL) was removed from each well and transferred to a clean 96-well plate. “Substrate mix” (50 µL) was added, and the plate was stored in the dark at 20 °C for 30 min. “Stop solution” (50 µL) was added to each well, and the absorbance at 490 nm was recorded using a Dynatech MR5000 plate reader; the % cell death was determined relative to the control cell lysis solution.
3.12.8. Evaluation of Selected Compounds 20a, 23a and 25n in Donor Peripheral Blood Mononuclear Cells (PBMCs)
The human peripheral blood mononuclear cells (PBMCs) were generated as follows: peripheral blood was obtained from healthy donors (n = 2) after informed consent was received. The blood was then placed into a 50-mL falcon tube and diluted with an equal volume of phosphate-buffered saline (PBS). PBMCs were isolated using density-gradient centrifugation with LymphoPrep, as described previously [107]. The cells were resuspended in RPMI media containing 10% FBS and 1% penicillin/streptomycin (P/S). The cells were counted using a haemocytometer and seeded into 96 well plates at a density of 1 × 105 cells/well (200 µL), and they were incubated at 37 °C for 1 h prior to compound treatment. The Annexin V/PI assay was used as a preliminary method of assessing the selective toxicity of the above compounds when compared with a previous alamarBlue cell viability assay in CLL cell lines. The assay was carried out as described above for the Annexin V/PI assay previously described above, and it was completed in triplicate on two independent days. Approval for this study was obtained from the School of Pharmacy and Pharmaceutical Sciences Trinity College Dublin Research Ethics Committee (2020-06-01-MS).
4. Conclusions
Three new classes of targeted agents have been approved for the treatment of CLL in the last decade: BTK inhibitors (ibrutinib, acalabrutinib and zanubrutinib), the BCL2 inhibitor (venetoclax) and PI3K inhibitors (idelalisib and duvelisib). However, the development of resistance or intolerance to these multiple classes of drugs is now observed, and new therapeutic options for this growing population of patients is identified as an unmet medical need [7,30,34,36,108,109,110,111].
In our work, we report the synthesis of compounds that demonstrate a significant antiproliferative action in CLL cell lines and also promote apoptosis. However, we have not yet identified the molecular target(s) for these compounds. The further investigation of the mechanism of action of the compounds reported in our research (particularly against the targets identified in our in silico study) may offer an alternative approach to the development of effective drugs for the treatment of CLL patients who have development resistance to the targeted therapy drugs.
In the present work, we have identified a series of potent lead compounds that have nitrostyrene-ethanoanthracene and chalcone-ethanoanthracene scaffold structures. The structures of the novel ethanoanthracene compounds were confirmed via X-ray crystallography. According to preliminary biochemical screening in CLL cell lines HG-3 and PGA-1, the compounds displayed highly effective antiproliferative activity in the CLL cell lines. The lead compounds were identified as 20a, 20f, 23a and 25n, with IC50 values in the ranges of 0.17–2.69 µM (HG-3) and 0.35–1.97 µM (PGA-1, with a more potent effect than the fludarabine control drug). 25n was the most potent compound in both CLL cell lines (HG-3, IC50 = 1.31 µM; PGA-1, IC50 = 0.87 µM). The lead compounds were subsequently found to induce cell death via a pro-apoptotic mechanism. The compounds also demonstrated potent antiproliferative activity in a panel of leukemia cell lines, with the mean GI50 values for compound 25n across the leukemia cell panel determined as 0.29 µM. Evidence for ROS involvement in their antiproliferative activity suggested a structure-dependent factor linked to the potential degree of ROS involvement. Caspase dependence was indicated for the representative anthracene–chalcone lead compound 23a. The compounds were demonstrated to be relatively non-toxic in LDH assays and also in PBMCs. While the biological macromolecular target(s) for the antiproliferative action of these compounds has yet to be identified, they may be effective through the disruption of signaling pathways or tumor-microenvironmental signaling. The antiproliferative nitroalkenes and chalcones identified in this study may act as electrophilic substrates for Michael-addition reactions of nucleophiles occurring in CLL cells and biological systems. The in silico target prediction remains inconclusive, as no singular target responsible for the activity has been identified. However, the target prediction indicated some potential targets for future investigations of molecular docking with selected family-A GPCRs, together with JNK, MAP and VEGFR kinases. This docking campaign, followed by molecular dynamics confirmation of the simulated protein–ligand interactions, could indicate which of these targets, if any, should be selected for in vitro experiments.
Overall, the identified lead compounds 20a, 23a, 25n and 20f represent promising potential scaffolds for CLL drug discovery, and they warrant further preclinical study as lead compounds for the development of more selective and potent antileukemic agents.
Supplementary Materials
The following supplementary information is available online at https://www.mdpi.com/1424-8247/17/8/1034/s1: Experimental details for the preparation of nitrovinylethanoanthracenes 20a–h and (E)-3-(anthracen-9-yl)-1-phenylprop-2-en-1-ones 21a-q; Figure S1: Stability analysis for 23n at pH 4.0, pH 7.5 and pH 9.0; Figure S2: Bioavailability analysis for compound 23a; Figures S3-S8; X-ray analysis for 21k, 23f, 24a, 23h and 27; Figures S9–49: 1H NMR, 13C NMR, DEPT-90, HSQC and HMBC for ethanoanthracenes; Table S1: X-ray data for compounds 21k, 23f, 23h, 24a, 24g, 25f and 27; Table S2: Stability data for ethanoanthracene 23n at pH 4.0, pH 7.5 and pH 9.0; Tables S3–S6; COMPARE analysis for compounds 23n, 23h, 25n and 24l; Tables S7–S9: Tier-1 profiling screen, physicochemical descriptors, Lipinski properties pharmacokinetic, ADMET and drug-likeness predictions for selected ethanoanthracenes; and Table S10: Overlay of ethanoanthracene compounds series with maprotiline with their overlay scores.
Author Contributions
Conceptualization, M.J.M., D.C.W., S.A.B. and A.M.M. formal analysis, A.J.B., J.P.M., B.T., D.F., S.K., I.Č., D.C.W., S.A.B. and M.J.M.; funding acquisition, M.J.M.; investigation, A.J.B., J.P.M., S.A.B., C.E.C., S.K., I.Č., D.F., B.T. and A.J.B.; methodology, A.J.B., S.A.B., J.P.M., D.F., I.Č. and S.K.; supervision, D.C.W., S.A.B., A.M.M., N.M.O., M.J.M. and D.F.; writing—original draft, M.J.M. and J.P.M.; writing—review and editing, M.J.M., J.P.M., A.J.B., N.M.O., S.A.B., B.T. and D.F. All authors have read and agreed to the published version of the manuscript.
Funding
Part of the research conducted in this publication was funded by the Irish Research Council under grant numbers GOIPG/2021/954 and GOIPD/2023/1294 and Trinity College Dublin Postgraduate research scholarships (AJB, JPMcK).
Institutional Review Board Statement
Approval for this study was obtained from the School of Pharmacy and Pharmaceutical Sciences Trinity College Dublin Research Ethics Committee (2020-06-01-MS).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Acknowledgments
Postgraduate research scholarships from Trinity College Dublin (AJB, JPMcK) and Irish Research Council Postgraduate Fellowship (GOIPG/2021/954 and GOIPD/2023/1294; SK and IČ) are gratefully acknowledged. We thank John O’Brien and Manuel Ruether for NMR spectroscopy, Gary Hessman for high-resolution mass spectrometry, Brian Talbot for HPLC and Barry Moran for flow cytometry. We also thank Thomas Fraisse, Christopher Teodorescu and Zaid Alobaidi for HPLC analysis, Adam Bergin and Keith Ferris for biochemical analysis and Peadar Grant for manuscript preparation. The Trinity Biomedical Sciences Institute (TBSI) is supported by a capital infrastructure investment from Cycle 5 of the Irish Higher Education Authority’s Programme for Research in Third Level Institutions (PRTLI). This study was also co-funded under the European Regional Development Fund. D.F., I.C. and S.K. thank the software vendors for their continuing support of academic research efforts, in particular the contributions of the Chemical Computing Group (CCG) and OpenEye, Cadence Molecular Sciences. The support and provisions of Dell Ireland, the Trinity Centre for High Performance Computing (TCHPC) and the Irish Centre for High-End Computing (ICHEC) are also gratefully acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the correspondence contact information. This change does not affect the scientific content of the article.
Abbreviations
| ADA | Adenosine deaminase |
| ADME | Absorption, distribution, metabolism and excretion |
| BBB | Blood–brain barrier |
| Bcl-2 | B-cell lymphoma-2 protein |
| BCR | B-cell receptor |
| BTK | Bruton’s tyrosine kinase |
| BL | Burkitt’s lymphoma |
| CB2 | Cannabinoid receptor |
| CLL | Chronic lymphocytic leukemia |
| CYP450 | Cytochrome P450 |
| CYP24A1 | 25-Hydroxyvitamin D-24-hydroxylase |
| DHOD | Dihydroorotate dehydrogenase |
| DEPT | Distortionless enhancement via polarization transfer |
| DMSO | Dimethylsulfoxide |
| EBV | Epstein–Barr virus |
| FACS | Fluorescence-activated cell sorting |
| FBS | Fetal bovine serum |
| FDA | The United States Food and Drug Administration |
| FITC | Fluorescein isothiocyanate |
| GSK-3 beta | Glycogen synthase kinase-3 beta |
| HBD | Hydrogen bond donors |
| HBA | Hydrogen bond acceptors |
| HEPES | N-2-Hydroxyethylpiperazine-N′-2-ethanesulfonic acid |
| HMBC | Heteronuclear multiple-bond correlation |
| HSQC | Heteronuclear single quantum coherence |
| HRMS | High-resolution mass spectrometry |
| IC50 | Half-maximal inhibitory concentration |
| IGVH | Immunoglobulin heavy-chain gene |
| INT | Iodonitrotetrazolium chloride |
| IR | Infrared |
| LDH | Lactate dehydrogenase |
| MALT1 | Mucosa-associated lymphoid tissue–lymphoma translocation protein 1 |
| MEM | Eagle’s minimum essential medium |
| NAC | N-acetylcysteine |
| NCI | National Cancer Institute |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| NHL | Non-Hodgkin lymphoma |
| NMR | Nuclear magnetic resonance |
| PAINS | Pan-assay interference compounds |
| PBMCs | Peripheral blood mononuclear cells |
| PI3Kδ | Phosphoinositide 3-kinase δ isoform |
| PI | Propidium iodide |
| PD-1 | Programmed cell death protein 1 |
| ROS | Reactive oxygen species |
| RPMI 1640 | Roswell Park Memorial Institute 1640 medium |
| RT | Richter’s transformation |
| Syk | Spleen tyrosine kinase |
| TPSA | Topological polar surface area |
References
- Cancer Stat Facts: Leukemia—Chronic Lymphocytic Leukemia (CLL). National Cancer Institute. 2022. Available online: https://seer.cancer.gov/statfacts/html/clyl.html (accessed on 26 April 2024).
- Cronin, K.A.; Scott, S.; Firth, A.U.; Sung, H.; Henley, S.J.; Sherman, R.L.; Siegel, R.L.; Anderson, R.N.; Kohler, B.A.; Benard, V.B.; et al. Annual report to the nation on the status of cancer, part 1: National cancer statistics. Cancer 2022, 128, 4251–4284. [Google Scholar] [CrossRef] [PubMed]
- Waldron, C.; O’Brien, D.; Brophy, S.; Perera, K.; Crotty, G.M.; Dunlea, E.; Walsh, A.; Connolly, M.; Clifford, R.; O’Leary, H.; et al. Epidemiology of chronic lymphocytic leukaemia in an Irish subpopulation with total case ascertainment: An additional tool for health economic planning. Br. J. Haematol. 2022, 196, e47–e49. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M. Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment. Am. J. Hematol. 2019, 94, 1266–1287. [Google Scholar] [CrossRef] [PubMed]
- Shadman, M. Diagnosis and treatment of chronic lymphocytic leukemia: A review. JAMA 2023, 329, 918–932. [Google Scholar] [CrossRef] [PubMed]
- Hampel, P.J.; Parikh, S.A. Chronic lymphocytic leukemia treatment algorithm 2022. Blood Cancer J. 2022, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.; Seymour, J.F. Update on the management of relapsed/refractory chronic lymphocytic leukemia. Blood Cancer J. 2024, 14, 33. [Google Scholar] [CrossRef]
- Lalic, H.; Aurer, I.; Batinic, D.; Visnjic, D.; Smoljo, T.; Babic, A. Bendamustine: A review of pharmacology, clinical use and immunological effects (review). Oncol. Rep. 2022, 47, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, N.; O’Brien, S. Novel agents in chronic lymphocytic leukemia. Hematol. Am. Soc. Hematol. Educ. Program. 2016, 2016, 137–145. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.; Xanthopoulos, C.; Kostareli, E. The role of Bruton’s tyrosine kinase in the immune system and disease. Immunology 2021, 164, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Hus, I.; Pula, B.; Robak, T. PI3K inhibitors for the treatment of chronic lymphocytic leukemia: Current status and future perspectives. Cancers 2022, 14, 1571. [Google Scholar] [CrossRef]
- Wiestner, A. BCR pathway inhibition as therapy for chronic lymphocytic leukemia and lymphoplasmacytic lymphoma. Hematol. Am. Soc. Hematol. Educ. Program. 2014, 2014, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hu, P.; Yan, X.; Zhang, Y.; Shi, W. Ibrutinib in chronic lymphocytic leukemia: Clinical applications, drug resistance, and prospects. Onco Targets Ther. 2020, 13, 4877–4892. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.S.; Brown, J.R. Ibrutinib: A first in class covalent inhibitor of Bruton’s tyrosine kinase. Future Oncol. 2014, 10, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.A.; Wierda, W.G. Acalabrutinib: A selective Bruton tyrosine kinase inhibitor for the treatment of B-cell malignancies. Front. Oncol. 2021, 11, 668162. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Munoz, J.L.; Seymour, J.F.; Opat, S. Zanubrutinib: Past, present, and future. Blood Cancer J. 2023, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Munakata, W.; Ando, K.; Yokoyama, M.; Fukuhara, N.; Yamamoto, K.; Fukuhara, S.; Ohmachi, K.; Mishima, Y.; Ichikawa, S.; Ogiya, D.; et al. Long-term safety profile of tirabrutinib: Final results of a Japanese phase I study in patients with relapsed or refractory B-cell malignancies. Int. J. Hematol. 2023, 117, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Walter, H.S.; Rule, S.A.; Dyer, M.J.; Karlin, L.; Jones, C.; Cazin, B.; Quittet, P.; Shah, N.; Hutchinson, C.V.; Honda, H.; et al. A phase 1 clinical trial of the selective BTK inhibitor ONO/GS-4059 in relapsed and refractory mature B-cell malignancies. Blood 2016, 127, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Woyach, J.A.; Ruppert, A.S.; Guinn, D.; Lehman, A.; Blachly, J.S.; Lozanski, A.; Heerema, N.A.; Zhao, W.; Coleman, J.; Jones, D.; et al. BTK(C481S)-mediated resistance to ibrutinib in chronic lymphocytic leukemia. J. Clin. Oncol. 2017, 35, 1437–1443. [Google Scholar] [CrossRef]
- Mato, A.R.; Shah, N.N.; Jurczak, W.; Cheah, C.Y.; Pagel, J.M.; Woyach, J.A.; Fakhri, B.; Eyre, T.A.; Lamanna, N.; Patel, M.R.; et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): A phase 1/2 study. Lancet 2021, 397, 892–901. [Google Scholar] [CrossRef]
- Crawford, J.J.; Johnson, A.R.; Misner, D.L.; Belmont, L.D.; Castanedo, G.; Choy, R.; Coraggio, M.; Dong, L.; Eigenbrot, C.; Erickson, R.; et al. Discovery of GDC-0853: A potent, selective, and noncovalent Bruton’s tyrosine kinase inhibitor in early clinical development. J. Med. Chem. 2018, 61, 2227–2245. [Google Scholar] [CrossRef]
- Zhang, Q.; Wen, C.; Zhao, L.; Wang, Y. A comprehensive review of small-molecule inhibitors targeting Bruton tyrosine kinase: Synthetic approaches and clinical applications. Molecules 2023, 28, 8037. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Smith, S.; Wagner-Johnston, N.; Sharman, J.; Chen, A.I.; Advani, R.; Augustson, B.; Marlton, P.; Renee Commerford, S.; Okrah, K.; et al. First-in-human phase 1 study of the BTK inhibitor GDC-0853 in relapsed or refractory B-cell NHL and CLL. Oncotarget 2018, 9, 13023–13035. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- von Tresckow, J.; Heyl, N.; Robrecht, S.; Giza, A.; Aldaoud, A.; Schlag, R.; Klausmann, M.; Linde, H.; Stein, W.; Schwarzer, A.; et al. Treatment with idelalisib in patients with chronic lymphocytic leukemia—Real world data from the registry of the German CLL study group. Ann. Hematol. 2023, 102, 3083–3090. [Google Scholar] [CrossRef] [PubMed]
- Sharman, J.P.; Coutre, S.E.; Furman, R.R.; Cheson, B.D.; Pagel, J.M.; Hillmen, P.; Barrientos, J.C.; Zelenetz, A.D.; Kipps, T.J.; Flinn, I.W.; et al. Final results of a randomized, phase III study of rituximab with or without idelalisib followed by open-label idelalisib in patients with relapsed chronic lymphocytic leukemia. J. Clin. Oncol. 2019, 37, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.; Wang, L. Emerging therapies in CLL in the era of precision medicine. Cancers 2023, 15, 1583. [Google Scholar] [CrossRef] [PubMed]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective Bcl-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Birkinshaw, R.W.; Gong, J.N.; Luo, C.S.; Lio, D.; White, C.A.; Anderson, M.A.; Blombery, P.; Lessene, G.; Majewski, I.J.; Thijssen, R.; et al. Structures of Bcl-2 in complex with venetoclax reveal the molecular basis of resistance mutations. Nat. Commun. 2019, 10, 2385. [Google Scholar] [CrossRef] [PubMed]
- Furstenau, M.; Eichhorst, B. Novel agents in chronic lymphocytic leukemia: New combination therapies and strategies to overcome resistance. Cancers 2021, 13, 1336. [Google Scholar] [CrossRef]
- Hagenbeek, A.; Gadeberg, O.; Johnson, P.; Pedersen, L.M.; Walewski, J.; Hellmann, A.; Link, B.K.; Robak, T.; Wojtukiewicz, M.; Pfreundschuh, M.; et al. First clinical use of ofatumumab, a novel fully human anti-CD20 monoclonal antibody in relapsed or refractory follicular lymphoma: Results of a phase 1/2 trial. Blood 2008, 111, 5486–5495. [Google Scholar] [CrossRef]
- O’Reilly, A.; Murphy, J.; Rawe, S.; Garvey, M. Chronic lymphocytic leukemia: A review of front-line treatment options, with a focus on elderly CLL patients. Clin. Lymphoma Myeloma Leuk. 2018, 18, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, N.; Ayres, M.L.; Baran, N.; Santiago-O’Farrill, J.M.; Bildik, G.; Lu, Z.; Konopleva, M.; Gandhi, V. Preclinical investigations of the efficacy of the glutaminase inhibitor CB-839 alone and in combinations in chronic lymphocytic leukemia. Front. Oncol. 2023, 13, 1161254. [Google Scholar] [CrossRef] [PubMed]
- Robbins, D.W.; Noviski, M.A.; Tan, Y.S.; Konst, Z.A.; Kelly, A.; Auger, P.; Brathaban, N.; Cass, R.; Chan, M.L.; Cherala, G.; et al. Discovery and preclinical pharmacology of NX-2127, an orally bioavailable degrader of Bruton’s tyrosine kinase with immunomodulatory activity for the treatment of patients with B cell malignancies. J. Med. Chem. 2024, 67, 2321–2336. [Google Scholar] [CrossRef] [PubMed]
- Study of SGR-1505 in Mature B-Cell Neoplasms. Schrödinger, Inc. 2023. Available online: https://classic.Clinicaltrials.Gov/ct2/show/nct05544019 (accessed on 27 March 2024).
- Herman, S.E.; Wiestner, A. Preclinical modeling of novel therapeutics in chronic lymphocytic leukemia: The tools of the trade. Semin. Oncol. 2016, 43, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, N. New oral small molecules in the treatment of chronic lymphocytic leukemia. Cancer 2015, 121, 1917–1926. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.J.; Bright, S.A.; Fayne, D.; McKeown, J.P.; McCabe, T.; Twamley, B.; Williams, C.; Meegan, M.J. Synthesis, antiproliferative and pro-apoptotic effects of nitrostyrenes and related compounds in Burkitt’s lymphoma. Med. Chem. 2018, 14, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Bright, S.A.; Byrne, A.J.; Vandenberghe, E.; Browne, P.V.; McElligott, A.M.; Meegan, M.J.; Williams, D.C. Selected nitrostyrene compounds demonstrate potent activity in chronic lymphocytic leukaemia cells, including those with poor prognostic markers. Oncol. Rep. 2019, 41, 3127–3136. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.J.; Bright, S.A.; McKeown, J.P.; Bergin, A.; Twamley, B.; McElligott, A.M.; Noorani, S.; Kandwal, S.; Fayne, D.; O’Boyle, N.M.; et al. Synthesis and pro-apoptotic effects of nitrovinylanthracenes and related compounds in chronic lymphocytic leukaemia (CLL) and Burkitt’s lymphoma (BL). Molecules 2023, 28, 8095. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.C.; Tsai, C.H.; Hou, M.F.; Chang, W.L.; Wang, C.H.; Lee, Y.C.; Ko, A.; Hu, S.C.; Chang, F.R.; Hsieh, P.W.; et al. The synthetic beta-nitrostyrene derivative CYT-Rx20 induces breast cancer cell death and autophagy via ROS-mediated MEK/ERK pathway. Cancer Lett. 2016, 371, 251–261. [Google Scholar] [CrossRef]
- Tsai, C.H.; Hung, A.C.; Chen, Y.Y.; Chiu, Y.W.; Hsieh, P.W.; Lee, Y.C.; Su, Y.H.; Chang, P.C.; Hu, S.C.; Yuan, S.F. 3′-hydroxy-4′-methoxy-beta-methyl-beta-nitrostyrene inhibits tumorigenesis in colorectal cancer cells through ROS-mediated DNA damage and mitochondrial dysfunction. Oncotarget 2017, 8, 18106–18117. [Google Scholar] [CrossRef]
- Byrne, A.J.; Bright, S.A.; McKeown, J.P.; O’Brien, J.E.; Twamley, B.; Fayne, D.; Williams, D.C.; Meegan, M.J. Design, synthesis and biochemical evaluation of novel ethanoanthracenes and related compounds to target Burkitt’s lymphoma. Pharmaceuticals 2020, 13, 16. [Google Scholar] [CrossRef]
- Cloonan, S.M.; Williams, D.C. The antidepressants maprotiline and fluoxetine induce type II autophagic cell death in drug-resistant Burkitt’s lymphoma. Int. J. Cancer 2011, 128, 1712–1723. [Google Scholar] [CrossRef]
- Bisi, A.; Mokhtar Mahmoud, A.; Allara, M.; Naldi, M.; Belluti, F.; Gobbi, S.; Ligresti, A.; Rampa, A. Polycyclic maleimide-based scaffold as new privileged structure for navigating the cannabinoid system opportunities. ACS Med. Chem. Lett. 2019, 10, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Bonvicini, F.; Manet, I.; Belluti, F.; Gobbi, S.; Rampa, A.; Gentilomi, G.A.; Bisi, A. Targeting the bacterial membrane with a new polycyclic privileged structure: A powerful tool to face Staphylococcus aureus infections. ACS Infect. Dis. 2019, 5, 1524–1535. [Google Scholar] [CrossRef]
- Arya, S.K.; Kumar, S.; Rani, R.; Kumar, N.; Roy, P.; Sondhi, S.M. Synthesis, anti-inflammatory, and cytotoxicity evaluation of 9,10-dihydroanthracene-9,10-α,β-succinimide and bis-succinimide derivatives. Med. Chem. Res. 2013, 22, 4278–4285. [Google Scholar] [CrossRef]
- Camara, R.; Ogbeni, D.; Gerstmann, L.; Ostovar, M.; Hurer, E.; Scott, M.; Mahmoud, N.G.; Radon, T.; Crnogorac-Jurcevic, T.; Patel, P.; et al. Discovery of novel small molecule inhibitors of S100P with in vitro anti-metastatic effects on pancreatic cancer cells. Eur. J. Med. Chem. 2020, 203, 112621. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.V.; Vaccaro, W.; Doweyko, A.M.; Doweyko, L.M.; Huynh, T.; Tortolani, D.; Nadler, S.G.; McKay, L.; Somerville, J.; Holloway, D.A.; et al. Discovery of novel dihydro-9,10-ethano-anthracene carboxamides as glucocorticoid receptor modulators. Bioorganic Med. Chem. Lett. 2009, 19, 2139–2143. [Google Scholar] [CrossRef] [PubMed]
- Bisi, A.; Arribas, R.L.; Micucci, M.; Budriesi, R.; Feoli, A.; Castellano, S.; Belluti, F.; Gobbi, S.; de Los Rios, C.; Rampa, A. Polycyclic maleimide-based derivatives as first dual modulators of neuronal calcium channels and GSK-3beta for Alzheimer’s disease treatment. Eur. J. Med. Chem. 2019, 163, 394–402. [Google Scholar] [CrossRef]
- Sadeq, H.; Thiemann, T.; Graham, J.P.; al Jasem, Y.; Bugenhagen, B.; Al-Rawashdeh, N.; al Sulaibi, M. Preparation of 3-(9-anthryl)acrylates and 9-aroylethenylanthracenes as Pi-extended anthracenes and their Diels–Alder type adducts with electron-poor dienophiles. Polycycl. Aromat. Compd. 2017, 37, 148–160. [Google Scholar] [CrossRef]
- Zhang, Q.; Lv, J.; Luo, S. Enantioselective Diels-Alder reaction of anthracene by chiral tritylium catalysis. Beilstein J. Org. Chem. 2019, 15, 1304–1312. [Google Scholar] [CrossRef]
- Sanyal, A.S.; Snyder, J.K. Stereoselective Diels−Alder reactions of chiral anthracenes. Org. Lett. 2000, 2, 2527–2530. [Google Scholar] [CrossRef] [PubMed]
- Bandowe, B.A.M.; Meusel, H. Nitrated polycyclic aromatic hydrocarbons (nitro-pahs) in the environment—A review. Sci. Total Environ. 2017, 581, 237–257. [Google Scholar] [CrossRef] [PubMed]
- Nepali, K.; Lee, H.Y.; Liou, J.P. Nitro-group-containing drugs. J. Med. Chem. 2019, 62, 2851–2893. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A privileged structure in medicinal chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Kenari, F.; Molnar, S.; Perjesi, P. Reaction of chalcones with cellular thiols. The effect of the 4-substitution of chalcones and protonation state of the thiols on the addition process. Diastereoselective thiol addition. Molecules 2021, 26, 4332. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.P.; Hayashi, T.; Cottam, H.B.; Jin, G.; Yao, S.; Wu, C.C.; Rosenbach, M.D.; Corr, M.; Schwab, R.B.; Carson, D.A. Nrf2 responses and the therapeutic selectivity of electrophilic compounds in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2010, 107, 7479–7484. [Google Scholar] [CrossRef]
- Wijayanti, L.W.; Swasono, R.T.; Lee, W.; Jumina, J. Synthesis and evaluation of chalcone derivatives as novel sunscreen agent. Molecules 2021, 26, 2698. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Vuelvas, O.F.; Enriquez-Figueroa, R.A.; Garcia-Ortega, H.; Flores-Alamo, M.; Pineda-Contreras, A. Crystal structure of the chalcone (E)-3-(furan-2-yl)-1-phenylprop-2-en-1-one. Acta Crystallogr. Sect. E Crystallogr. Commun. 2015, 71, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Khan, A.; Usman, R.; Wang, M. Understanding relationship between stacking modes and optical properties of organic charge transfer cocrystals involving anthracyl chalcones and TCNB. J. Photochem. Photobiol. A Chem. 2019, 371, 315–326. [Google Scholar] [CrossRef]
- Feng, Q.; Wang, J.; Huan, W.; Shen, C.; Guo, F.; Lu, J.; Diao, G. Polymorphism and configurational isomerism in 3-(9-anthryl)-1-(3-hydroxyphenyl)prop-2-en-1-one. J. Mol. Struct. 2019, 1195, 355–363. [Google Scholar]
- Kemnitz, C.R.; Loewen, M.J. “Amide resonance” correlates with a breadth of C−N rotation barriers. J. Am. Chem. Soc. 2007, 129, 2521–2528. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.M.; Iyer, K.S.; Corry, B. The confined space inside carbon nanotubes can dictate the stereo- and regioselectivity of Diels-Alder reactions. Phys. Chem. Chem. Phys. 2014, 16, 6986–6989. [Google Scholar] [CrossRef] [PubMed]
- Aswathia, C.S.; Unnikrishnana, P.A.; Saumya, T.S. Synthesis, characterization and DFT studies of a few enone appended dibenzobarrelenes. Chem. Data Collect. 2020, 25, 100340. [Google Scholar] [CrossRef]
- Sato, Y.; Kojima, T.; Goto, T.; Oomikawa, R.; Watanabe, H.; Wakabayashi, K. Hydrolysis and phytotoxic activity of cyclic imides. Agric. Biol. Chem. 1991, 55, 2677–2681. [Google Scholar][Green Version]
- Shriner, R.; Kurosawa, T. Chalcones. II. Decomposition by alkali. J. Am. Chem. Soc. 1930, 52, 2538–2540. [Google Scholar] [CrossRef]
- Rosen, A.; Bergh, A.C.; Gogok, P.; Evaldsson, C.; Myhrinder, A.L.; Hellqvist, E.; Rasul, A.; Bjorkholm, M.; Jansson, M.; Mansouri, L.; et al. Lymphoblastoid cell line with B1 cell characteristics established from a chronic lymphocytic leukemia clone by in vitro EBV infection. Oncoimmunology 2012, 1, 18–27. [Google Scholar] [CrossRef]
- Lewin, N.; Aman, P.; Mellstedt, H.; Zech, L.; Klein, G. Direct outgrowth of in vivo Epstein-Barr virus (EBV)-infected chronic lymphocytic leukemia (CLL) cells into permanent lines. Int. J. Cancer 1988, 41, 892–895. [Google Scholar] [CrossRef]
- Singh, P.; Anand, A.; Kumar, V. Recent developments in biological activities of chalcones: A mini review. Eur. J. Med. Chem. 2014, 85, 758–777. [Google Scholar] [CrossRef]
- McNamara, Y.M.; Bright, S.A.; Byrne, A.J.; Cloonan, S.M.; McCabe, T.; Williams, D.C.; Meegan, M.J. Synthesis and antiproliferative action of a novel series of maprotiline analogues. Eur. J. Med. Chem. 2014, 71, 333–353. [Google Scholar] [CrossRef]
- National Cancer Institute (NCI), NCI-60 screening methodology. Available online: https://dtp.cancer.gov/discovery_development/nci-60/methodology.htm (accessed on 26 April 2024).
- Biological Testing Branch, National Cancer Institute, Bethesda, MD. 2018. Available online: https://dtp.Nci.Nih.Gov/branches/btb/hfa.Html (accessed on 26 April 2024).
- Cleaveland, E.S.; Monks, A.; Vaigro-Wolff, A.; Zaharevitz, D.W.; Paull, K.; Ardalan, K.; Cooney, D.A.; Ford, H., Jr. Site of action of two novel pyrimidine biosynthesis inhibitors accurately predicted by the compare program. Biochem. Pharmacol. 1995, 49, 947–954. [Google Scholar] [CrossRef]
- Compare Analysis. Available online: https://dtp.Cancer.Gov/databases_tools/compare.Htm (accessed on 26 April 2024).
- Dahlin, J.L.; Inglese, J.; Walters, M.A. Mitigating risk in academic preclinical drug discovery. Nat. Rev. Drug Discov. 2015, 14, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Decker, T.; Lohmann-Matthes, M.L. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J. Immunol. Methods 1988, 115, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Nachlas, M.M.; Margulies, S.I.; Goldberg, J.D.; Seligman, A.M. The determination of lactic dehydrogenase with a tetrazolium salt. Anal. Biochem. 1960, 1, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Ahmed, K.R.; Haque, F.; Park, M.N.; Kim, B. Recent advances in cellular signaling interplay between redox metabolism and autophagy modulation in cancer: An overview of molecular mechanisms and therapeutic interventions. Antioxidants 2023, 12, 428. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Hounsell, C.; Fan, Y. The duality of caspases in cancer, as told through the fly. Int. J. Mol. Sci. 2021, 22, 8927. [Google Scholar] [CrossRef] [PubMed]
- Boice, A.; Bouchier-Hayes, L. Targeting apoptotic caspases in cancer. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118688. [Google Scholar] [CrossRef]
- Li, X.; Yao, X.; Zhu, Y.; Zhang, H.; Wang, H.; Ma, Q.; Yan, F.; Yang, Y.; Zhang, J.; Shi, H.; et al. The caspase inhibitor Z-VAD-FMK alleviates endotoxic shock via inducing macrophages necroptosis and promoting MDSCS-mediated inhibition of macrophages activation. Front. Immunol. 2019, 10, 1824. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwisstTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- James, J.P.; Ishwar, B.K.; More, U.A.; Joshi, S.D. Design, synthesis, molecular modeling, and ADMET studies of some pyrazoline derivatives as shikimate kinase inhibitors. Med. Chem. Res. 2018, 27, 546–559. [Google Scholar] [CrossRef]
- Becker, H.D.; Beckers, H.C.; Sandros, K.; Andersson, K. Photochemistry of the anthracene chromophore: Novel isomerization of 1-(9-anthryl)-2-benzoylethylenes. Tetrahedron Lett. 1985, 26, 1589–1592. [Google Scholar] [CrossRef]
- Velagapudi, N.; Sharma, N.; Bandla, R. Synthesis, screening and QSAR analysis of chalcone derivatives as potential anti bacterial agents. Int. J. Pharm. Chem. Biol. Sci. 2017, 7, 211–218. [Google Scholar]
- Gonzalez, J.J.; Ortega, E.; Rothemund, M.; Gold, M.; Vicente, C.; de Haro, C.; Bautista, D.; Schobert, R.; Ruiz, J. Luminescent gold(I) complexes of 1-pyridyl-3-anthracenylchalcone inducing apoptosis in colon carcinoma cells and antivascular effects. Inorg. Chem. 2019, 58, 12954–12963. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Sasmal, D. Development of selective and reversible pyrazoline based MAO-B inhibitors: Virtual screening, synthesis and biological evaluation. Bioorganic Med. Chem. Lett. 2011, 21, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, C.; Alam, M.S.; Hamid, H.; Javed, K.; Dhulap, A.; Bano, S.; Ali, Y. Antidiabetic effect of novel benzenesulfonylureas as PPAR-γ agonists and their anticancer effect. Bioorganic Med. Chem. Lett. 2015, 25, 4601–4605. [Google Scholar] [CrossRef] [PubMed]
- Levai, A.; Jeko, J. Synthesis of 1-substituted 3,5-diaryl-2-pyrazolines by the reaction of α,β-unsaturated ketones with hydrazines. J. Heterocycl. Chem. 2006, 43, 111–115. [Google Scholar] [CrossRef]
- Buu-Hoi, N.P.; Hoan, N. Some anthracene derivatives of potential biological interest. J. Org. Chem. 1951, 16, 874–881. [Google Scholar] [CrossRef]
- Bruker AXS Inc. SAINT, v8.40A; Bruker AXS Inc.: Madison, WI, USA, 2019. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus x-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Chemical Computing Group Inc. Molecular Operating Environment (MOE) Version 2022.02, Chemical Computing Group Inc.: Montreal, QC, Canada, 2022.
- Zdrazil, B.; Felix, E.; Hunter, F.; Manners, E.J.; Blackshaw, J.; Corbett, S.; de Veij, M.; Ioannidis, H.; Lopez, D.M.; Mosquera, J.F.; et al. The ChEMBL database in 2023: A drug discovery platform spanning multiple bioactivity data types and time periods. Nucleic Acids Res. 2024, 52, D1180–D1192. [Google Scholar] [CrossRef] [PubMed]
- Gfeller, D.; Michielin, O.; Zoete, V. Shaping the interaction landscape of bioactive molecules. Bioinformatics 2013, 29, 3073–3079. [Google Scholar] [CrossRef] [PubMed]
- The Pandas Development Team. Pandas-dev/pandas: Pandas. V2.2.2. 2024. Available online: https://doi.Org/10.5281/zenodo.3509134 (accessed on 23 April 2024).
- Omega 4.2.2.0. OpenEye, Cadence Molecular Sciences, Santa Fe, NM, USA. Available online: http://www.eyesopen.com (accessed on 10 April 2024).
- Lanemo Myhrinder, A.; Hellqvist, E.; Sidorova, E.; Soderberg, A.; Baxendale, H.; Dahle, C.; Willander, K.; Tobin, G.; Backman, E.; Soderberg, O.; et al. A new perspective: Molecular motifs on oxidized LDL, apoptotic cells, and bacteria are targets for chronic lymphocytic leukemia antibodies. Blood 2008, 111, 3838–3848. [Google Scholar] [CrossRef] [PubMed]
- Brophy, S.; Amet, R.; Foy-Stones, H.; Gardiner, N.; McElligott, A.M. Isolation and cryopreservation of mononuclear cells from peripheral blood and bone marrow of blood cancer patients. Methods Mol. Biol. 2023, 2645, 179–187. [Google Scholar]
- Timofeeva, N.; Gandhi, V. Ibrutinib combinations in CLL therapy: Scientific rationale and clinical results. Blood Cancer J. 2021, 11, 79. [Google Scholar] [CrossRef]
- Zygmunciak, P.; Robak, T.; Pula, B. Treatment of double-refractory chronic lymphocytic leukemia-an unmet clinical need. Int. J. Mol. Sci. 2024, 25, 1589. [Google Scholar] [CrossRef]
- Liang, E.C.; Albittar, A.; Huang, J.J.; Hirayama, A.V.; Kimble, E.L.; Portuguese, A.J.; Chapuis, A.; Shadman, M.; Till, B.G.; Cassaday, R.D.; et al. Factors associated with long-term outcomes of CD19 CAR T-cell therapy for relapsed/refractory CLL. Blood Adv. 2023, 7, 6990–7005. [Google Scholar] [CrossRef] [PubMed]
- Borogovac, A.; Siddiqi, T. Transforming CLL management with immunotherapy: Investigating the potential of CAR T-cells and bispecific antibodies. Semin. Hematol. 2024, 61, 119–130. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).