Energy Transfer between AGuIX Nanoparticles and Photofrin under Light or X-ray Excitation for PDT Applications
Abstract
1. Introduction
2. Results and Discussion
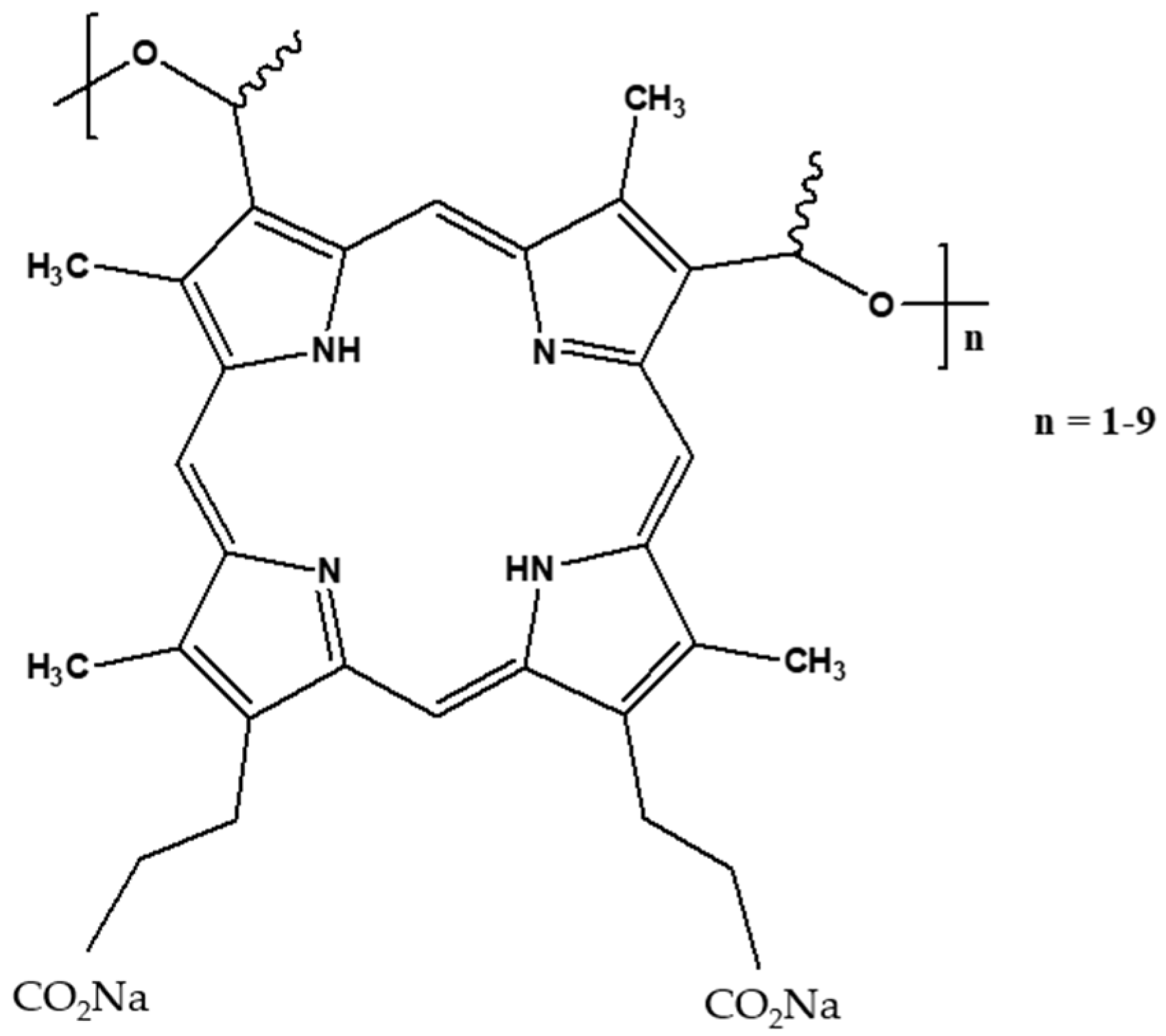
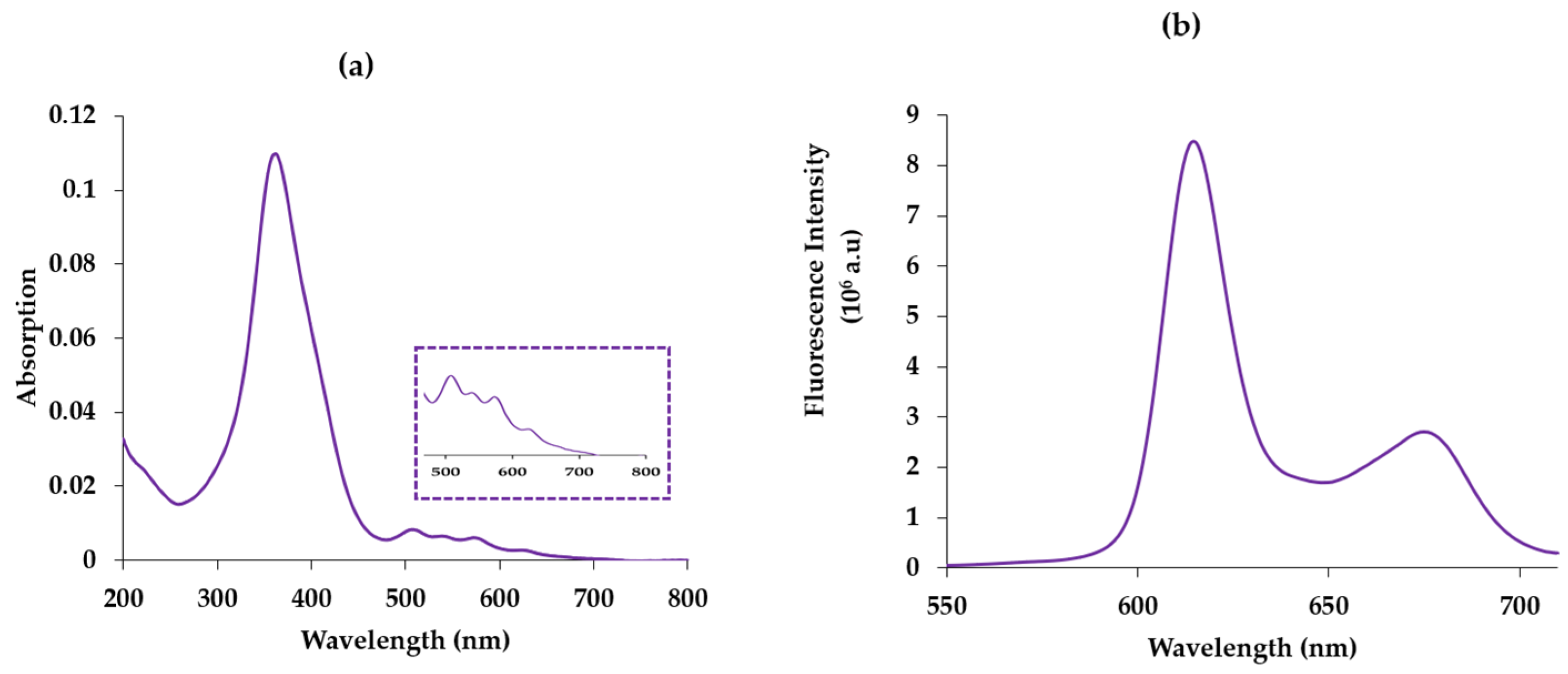
2.1. Photophysical Properties of Photofrin in Water
2.2. Study of the Energy Transfer between Free Lanthanides and Chelated Lanthanides in AGuIX in Solution
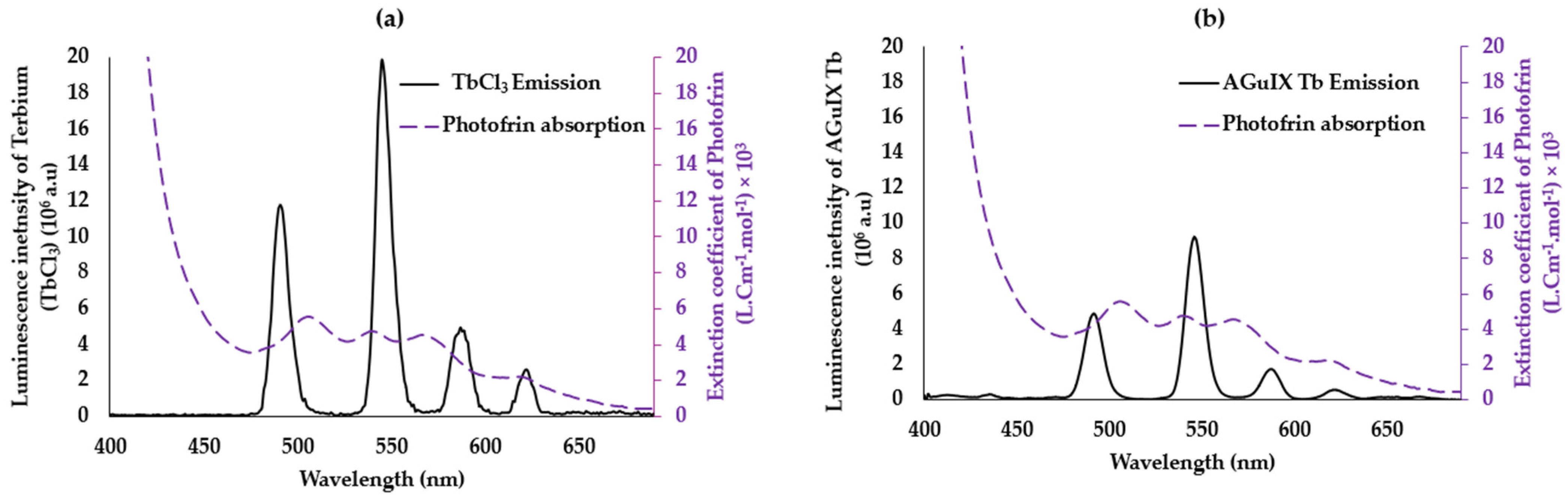
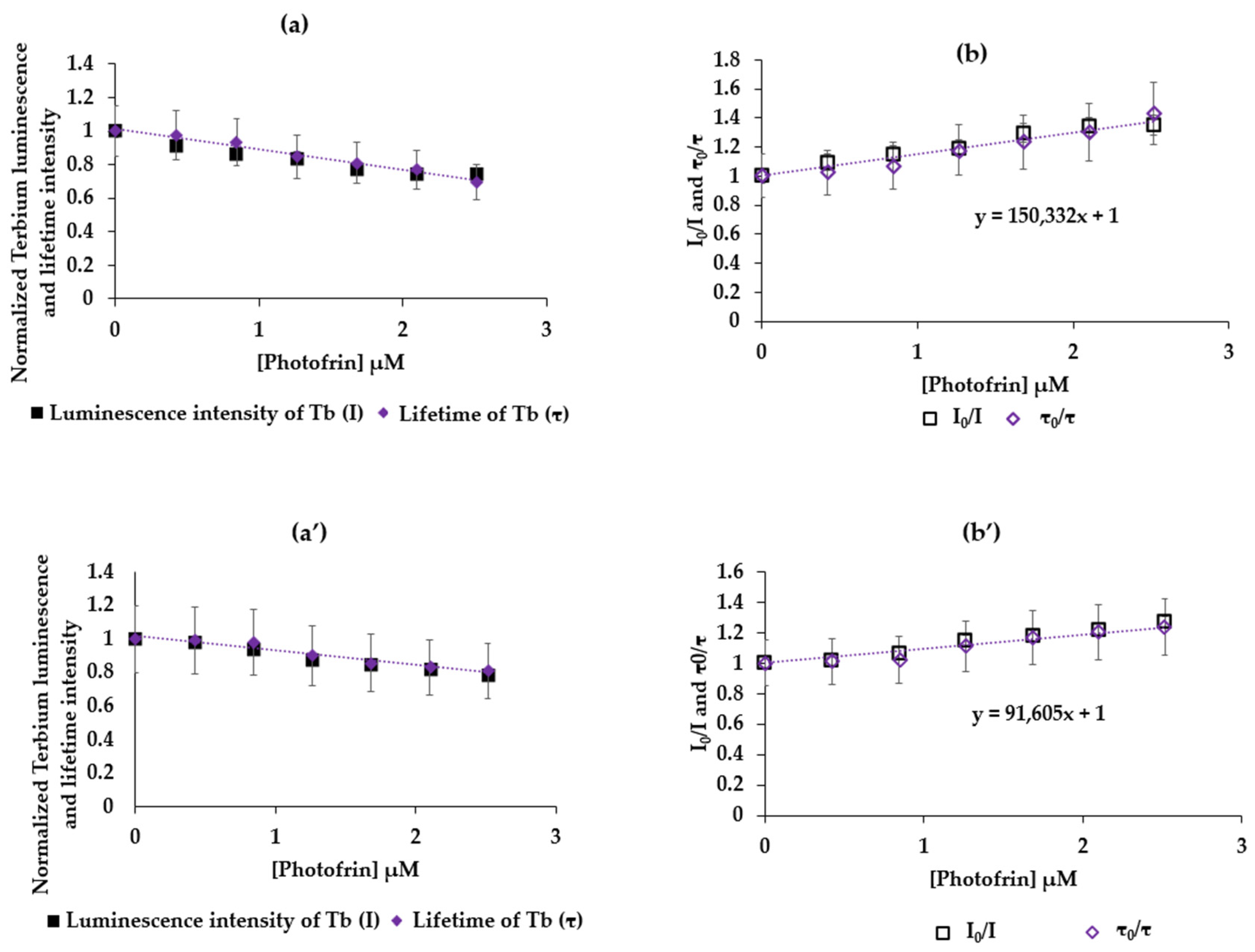
2.2.1. TbCl3/Photofrin and AGuIX Tb/Photofrin
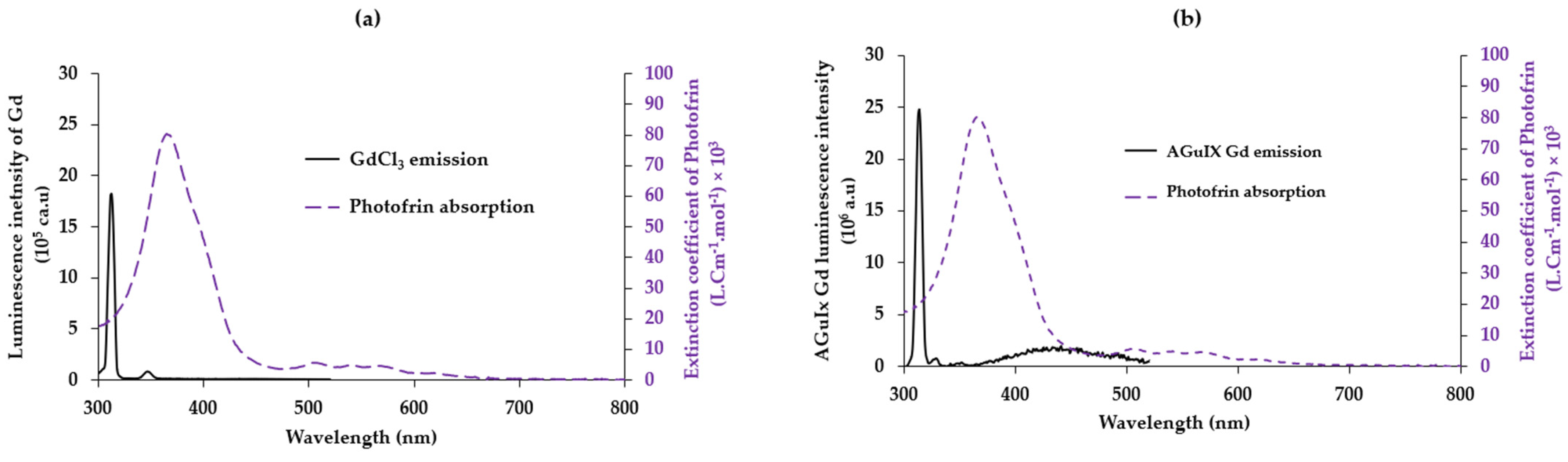
2.2.2. GdCl3/Photofrin and AGuIX Gd/Photofrin
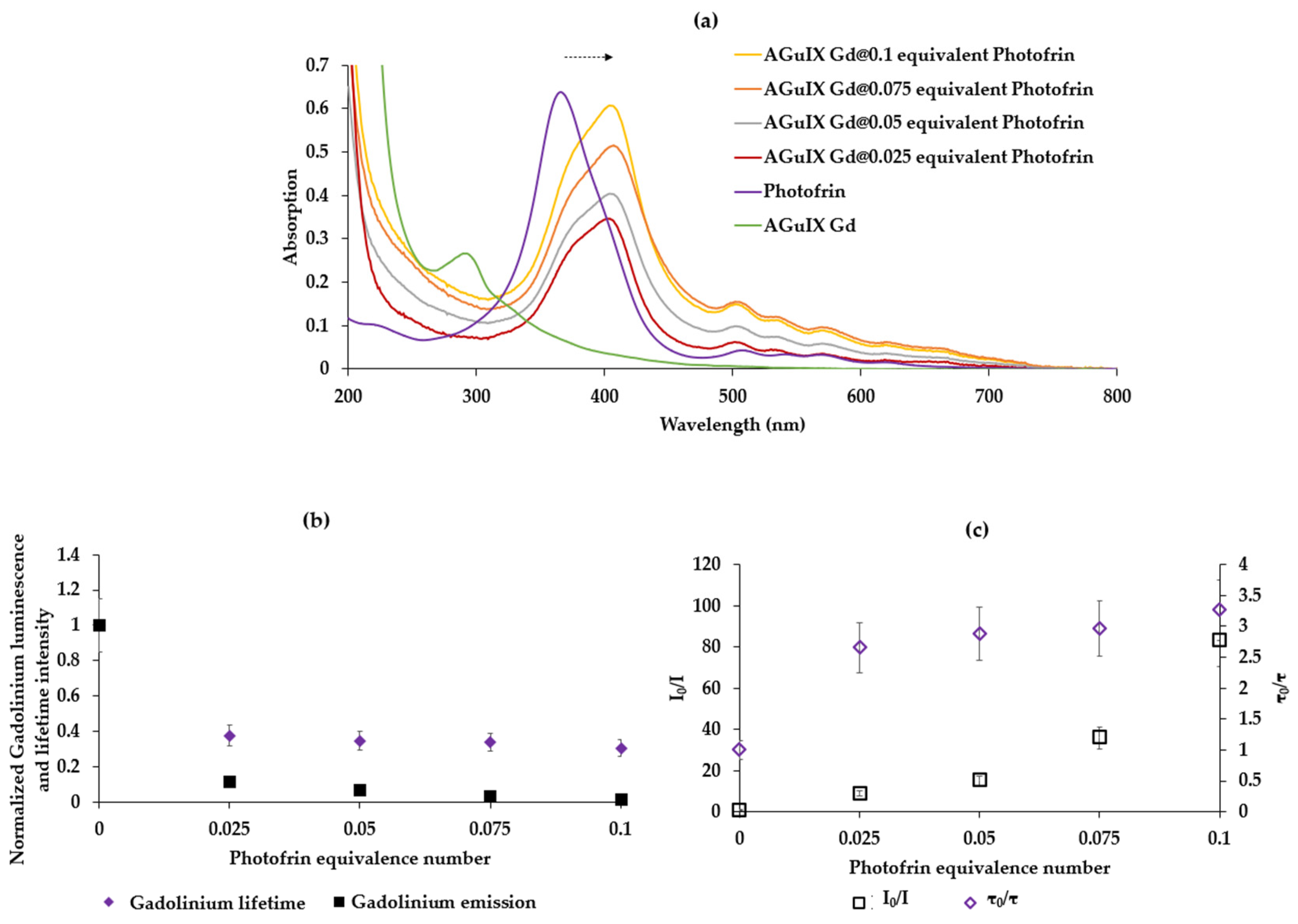
2.3. Study of the Energy Transfer between Photofrin Adsorbed on AGuIX Gd Nanoparticles
2.3.1. Energy Transfer between AGuIX Gd and Photofrin Adsorbed on AGuIX Gd
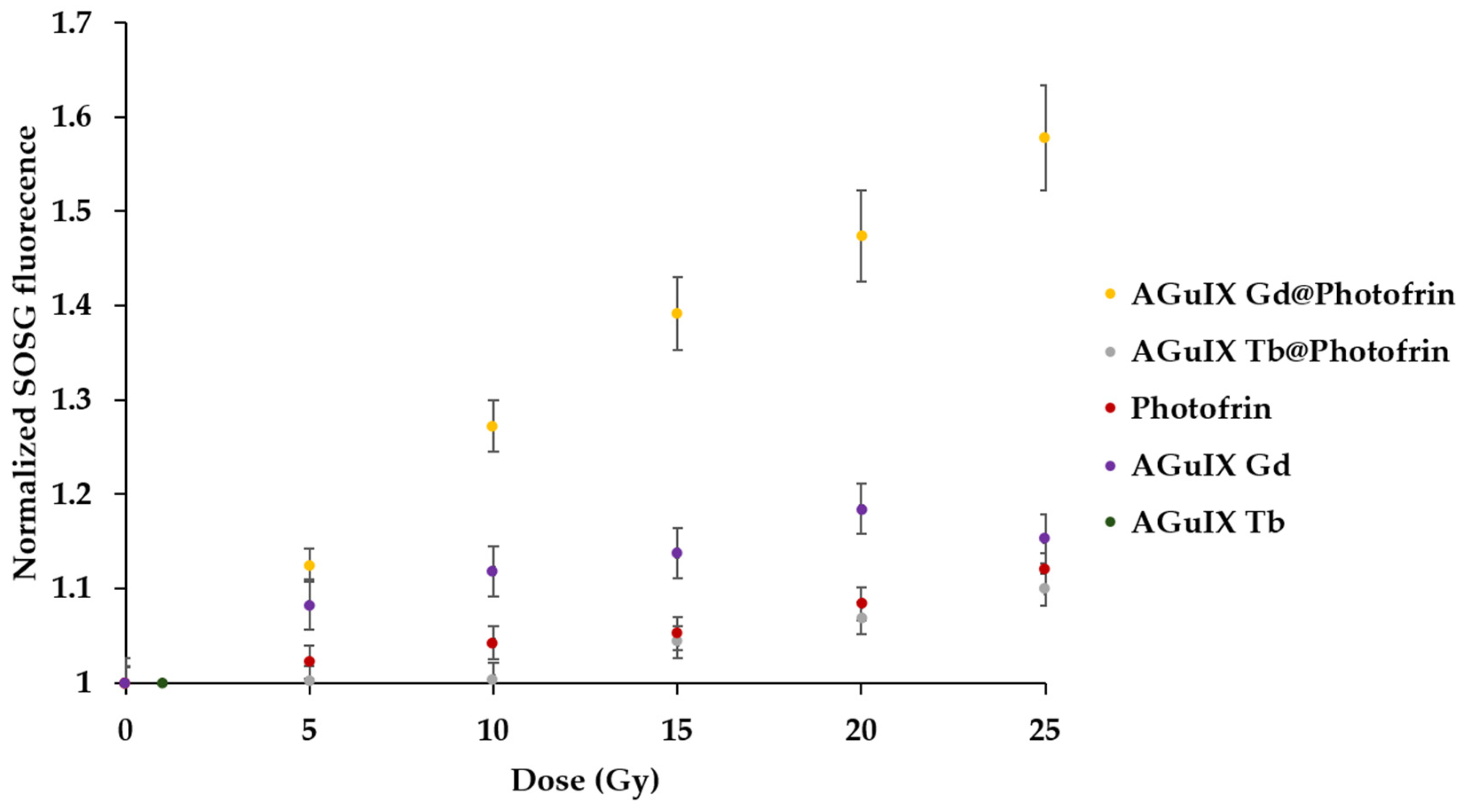
2.3.2. X-ray Excitation in Solution
3. Materials and Methods
3.1. Materials
3.2. Formatting of the Mathematical Components
- R0, the Forster radius, is the donor–acceptor distance for which the energy transfer efficiency is 50%.
- Quenching constant (kq).
- Stern–Volmer constant (KSV/slope of curve I0/I = f([Quencher]).
- τ0 is the luminescence lifetime of the donor without any quencher.
3.3. Adsorption of Photofrin on AGuIX Gd
3.4. Singlet Oxygen Production during X-ray Irradiation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharman, W.M.; Allen, C.M.; Van Lier, J.E. Photodynamic therapeutics: Basic principles and clinical applications. Drug Discov. Today 1999, 4, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Josefsen, L.B.; Boyle, R.W. Photodynamic Therapy and the Development of Metal-Based Photosensitisers. Met. Based Drugs 2008, 2008, 276109. [Google Scholar] [CrossRef]
- Fisher, D.G.; Rypins, E.B.; Watson, L.R.; Nelson, J.S.; Berns, M.W. Colonic mucosectomy using laser photodynamic therapy. J. Surg. Res. 1989, 46, 579–583. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Derkay, C.S. Recurrent Respiratory Papillomatosis. Laryngoscope 2001, 111, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Myrzakhmetov, B.; Arnoux, P.; Mordon, S.; Acherar, S.; Tsoy, I.; Frochot, C. Photophysical Properties of Protoporphyrin IX, Pyropheophorbide-a and Photofrin® in Different Conditions. Pharmaceuticals 2021, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, F.; Helman, W.P.; Ross, A.B. Quantum Yields for the Photosensitized Formation of the Lowest Electronically Excited Singlet State of Molecular Oxygen in Solution. J. Phys. Chem. Ref. Data 1993, 22, 113–262. [Google Scholar] [CrossRef]
- Melissari, Z.; Williams, R.M.; Senge, M.O. Porphyrinoids for Photodynamic Therapy. In Applications of Porphyrinoids as Functional Materials, 1st ed.; Lang, H., Rueffer, T., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2021; pp. 252–291. [Google Scholar] [CrossRef]
- Lang, H.; Rueffer, T. (Eds.) Front Matter. In Smart Materials Series; The Royal Society of Chemistry: Cambridge, UK, 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Hatz, S.; Lambert, J.D.C.; Ogilby, P.R. Measuring the lifetime of singlet oxygen in a single cell: Addressing the issue of cell viability. Photochem. Photobiol. Sci. 2007, 6, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Niedre, M.; Patterson, M.S.; Wilson, B.C. Direct Near-infrared Luminescence Detection of Singlet Oxygen Generated by Photodynamic Therapy in Cells In Vitro and Tissues In Vivo. Photochem. Photobiol. 2002, 75, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Vereecken, P.; Laporte, M.; Awada, A.; Velu, T.; Heenen, M. Thérapie photodynamique topique en cancérologie cutanée. Rev. Médicale Brux. 2004, 25, 512–520. [Google Scholar]
- Larue, L.; Mihoub, A.B.; Youssef, Z.; Colombeau, L.; Acherar, S.; André, J.C.; Arnoux, P.; Baros, F.; Vermandel, M.; Frochot, C. Using X-rays in photodynamic therapy: An overview. Photochem. Photobiol. Sci. 2018, 17, 1612–1650. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J. Using Nanoparticles to Enable Simultaneous Radiation and Photodynamic Therapies for Cancer Treatment. J. Nanosci. Nanotechnol. 2006, 6, 1159–1166. [Google Scholar] [CrossRef]
- Gadzhimagomedova, Z.; Zolotukhin, P.; Kit, O.; Kirsanova, D.; Soldatov, A. Nanocomposites for X-ray Photodynamic Therapy. Int. J. Mol. Sci. 2020, 21, 4004. [Google Scholar] [CrossRef]
- Cline, B.; Delahunty, I.; Xie, J. Nanoparticles to mediate X-ray-induced photodynamic therapy and Cherenkov radiation photodynamic therapy. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1541. [Google Scholar] [CrossRef]
- Yao, B.; Liu, X.; Zhang, W.; Lu, H. X-ray excited luminescent nanoparticles for deep photodynamic therapy. RSC Adv. 2023, 13, 30133–30150. [Google Scholar] [CrossRef]
- Souris, J.S.; Leoni, L.; Zhang, H.J.; Pan, A.; Tanios, E.; Tsai, H.-M.; Balyasnikova, I.V.; Bissonnette, M.; Chen, C.-T. X-ray Activated Nanoplatforms for Deep Tissue Photodynamic Therapy. Nanomaterials 2023, 13, 673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zheng, H.; Zhang, F.; Chu, C.; Liao, T.; Xie, L.; Liu, G.; Cai, W. Rare-earth scintillating nanoparticles for X-ray induced photodynamic therapy. J. Lumin. 2023, 261, 119862. [Google Scholar] [CrossRef]
- Ren, X.-D.; Hao, X.-Y.; Li, H.-C.; Ke, M.-R.; Zheng, B.-Y.; Huang, J.-D. Progress in the development of nanosensitizers for X-ray-induced photodynamic therapy. Drug Discov. Today 2018, 23, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhou, Z.; Pratx, G.; Chen, X.; Chen, H. Nanoscintillator-Mediated X-ray Induced Photodynamic Therapy for Deep-Seated Tumors: From Concept to Biomedical Applications. Theranostics 2020, 10, 1296–1318. [Google Scholar] [CrossRef] [PubMed]
- Le Duc, G.; Roux, S.; Paruta-Tuarez, A.; Dufort, S.; Brauer, E.; Marais, A.; Truillet, C.; Sancey, L.; Perriat, P.; Lux, F.; et al. Advantages of gadolinium based ultrasmall nanoparticles vs molecular gadolinium chelates for radiotherapy guided by MRI for glioma treatment. Cancer Nanotechnol. 2014, 5, 4. [Google Scholar] [CrossRef]
- Mignot, A.; Truillet, C.; Lux, F.; Sancey, L.; Louis, C.; Denat, F.; Boschetti, F.; Bocher, L.; Gloter, A.; Stéphan, O.; et al. A Top-Down Synthesis Route to Ultrasmall Multifunctional Gd-Based Silica Nanoparticles for Theranostic Applications. Chem. Eur. J. 2013, 19, 6122–6136. [Google Scholar] [CrossRef]
- Lux, F.; Mignot, A.; Mowat, P.; Louis, C.; Dufort, S.; Bernhard, C.; Denat, F.; Boschetti, F.; Brunet, C.; Antoine, R.; et al. Ultrasmall Rigid Particles as Multimodal Probes for Medical Applications. Angew. Chem. 2011, 50, 12299–12303. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yu, X.; Li, W. Recent Progress and Trends in X-ray-Induced Photodynamic Therapy with Low Radiation Doses. ACS Nano 2022, 16, 19691–19721. [Google Scholar] [CrossRef] [PubMed]
- Bulin, A.-L.; Truillet, C.; Chouikrat, R.; Lux, F.; Frochot, C.; Amans, D.; Ledoux, G.; Tillement, O.; Perriat, P.; Barberi-Heyob, M. X-ray-Induced Singlet Oxygen Activation with Nanoscintillator-Coupled Porphyrins. J. Phys. Chem. C 2013, 117, 21583–21589. [Google Scholar] [CrossRef]
- Daouk, J.; Iltis, M.; Dhaini, B.; Béchet, D.; Arnoux, P.; Rocchi, P.; Delconte, A.; Habermeyer, B.; Lux, F.; Frochot, C.; et al. Terbium-Based AGuIX-Design Nanoparticle to Mediate X-ray-Induced Photodynamic Therapy. Pharmaceuticals 2021, 14, 396. [Google Scholar] [CrossRef] [PubMed]
- Chouikrat, R.; Baros, F.; André, J.-C.; Vanderesse, R.; Viana, B.; Bulin, A.-L.; Dujardin, C.; Arnoux, P.; Verelst, M.; Frochot, C. A Photosensitizer Lanthanide Nanoparticle Formulation that Induces Singlet Oxygen with Direct Light Excitation, but Not By Photon or X-ray Energy Transfer. Photochem. Photobiol. 2017, 93, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Abliz, E.; Collins, J.E.; Friedberg, J.S.; Kumar, A.; Bell, H.; Waynant, R.W.; Tata, D.B. Novel applications of diagnostic X-rays in activating photo-agents through X-ray induced visible luminescence from rare-earth particles: An in vitro study. In BiOS; Chen, W.R., Ed.; SPIE: San Francisco, CA, USA, 2010. [Google Scholar] [CrossRef]
- Yang, W.; Read, P.W.; Mi, J.; Baisden, J.M.; Reardon, K.A.; Larner, J.M.; Helmke, B.P.; Sheng, K. Semiconductor nanoparticles as energy mediators for photosensitizer-enhanced radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 633–635. [Google Scholar] [CrossRef]
- Wang, L.; Yang, W.; Read, P.; Larner, J.; Sheng, K. Tumor cell apoptosis induced by nanoparticle conjugate in combination with radiation therapy. Nanotechnology 2010, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Kulka, U.; Schaffer, M.; Siefert, A.; Schaffer, P.M.; Ölsner, A.; Kasseb, K.; Hofstetter, A.; Dühmke, E.; Jori, G. Photofrin as a radiosensitizer in an in vitro cell survival assay. Biochem. Biophys. Res. Commun. 2003, 311, 98–103. [Google Scholar] [CrossRef]
- Schaffer, M.; Schaffer, P.M.; Jori, G.; Corti, L.; Sotti, G.; Hofstetter, A.; Dühmke, E. Radiation therapy combined with photofrin or 5-ALA: Effect on Lewis sarcoma tumor lines implanted in mice. Preliminary results. Tumori J. 2002, 88, 407–410. [Google Scholar] [CrossRef]
- Chin, K.K.; Trevithick-Sutton, C.C.; McCallum, J.; Jockusch, S.; Turro, N.J.; Scaiano, J.C.; Foote, C.S.; Garcia-Garibay, M.A. Quantitative Determination of Singlet Oxygen Generated by Excited State Aromatic Amino Acids, Proteins, and Immunoglobulins. J. Am. Chem. Soc. 2008, 130, 6912–6913. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhaini, B.; Arnoux, P.; Daouk, J.; Lux, F.; Tillement, O.; Hagège, A.; Hamieh, T.; Shafirstein, G.; Frochot, C. Energy Transfer between AGuIX Nanoparticles and Photofrin under Light or X-ray Excitation for PDT Applications. Pharmaceuticals 2024, 17, 1033. https://doi.org/10.3390/ph17081033
Dhaini B, Arnoux P, Daouk J, Lux F, Tillement O, Hagège A, Hamieh T, Shafirstein G, Frochot C. Energy Transfer between AGuIX Nanoparticles and Photofrin under Light or X-ray Excitation for PDT Applications. Pharmaceuticals. 2024; 17(8):1033. https://doi.org/10.3390/ph17081033
Chicago/Turabian StyleDhaini, Batoul, Philippe Arnoux, Joël Daouk, François Lux, Olivier Tillement, Agnès Hagège, Tayssir Hamieh, Gal Shafirstein, and Céline Frochot. 2024. "Energy Transfer between AGuIX Nanoparticles and Photofrin under Light or X-ray Excitation for PDT Applications" Pharmaceuticals 17, no. 8: 1033. https://doi.org/10.3390/ph17081033
APA StyleDhaini, B., Arnoux, P., Daouk, J., Lux, F., Tillement, O., Hagège, A., Hamieh, T., Shafirstein, G., & Frochot, C. (2024). Energy Transfer between AGuIX Nanoparticles and Photofrin under Light or X-ray Excitation for PDT Applications. Pharmaceuticals, 17(8), 1033. https://doi.org/10.3390/ph17081033








