Unlocking the Green Gold: Exploring the Cancer Treatment and the Other Therapeutic Potential of Fucoxanthin Derivatives from Microalgae
Abstract
1. Introduction
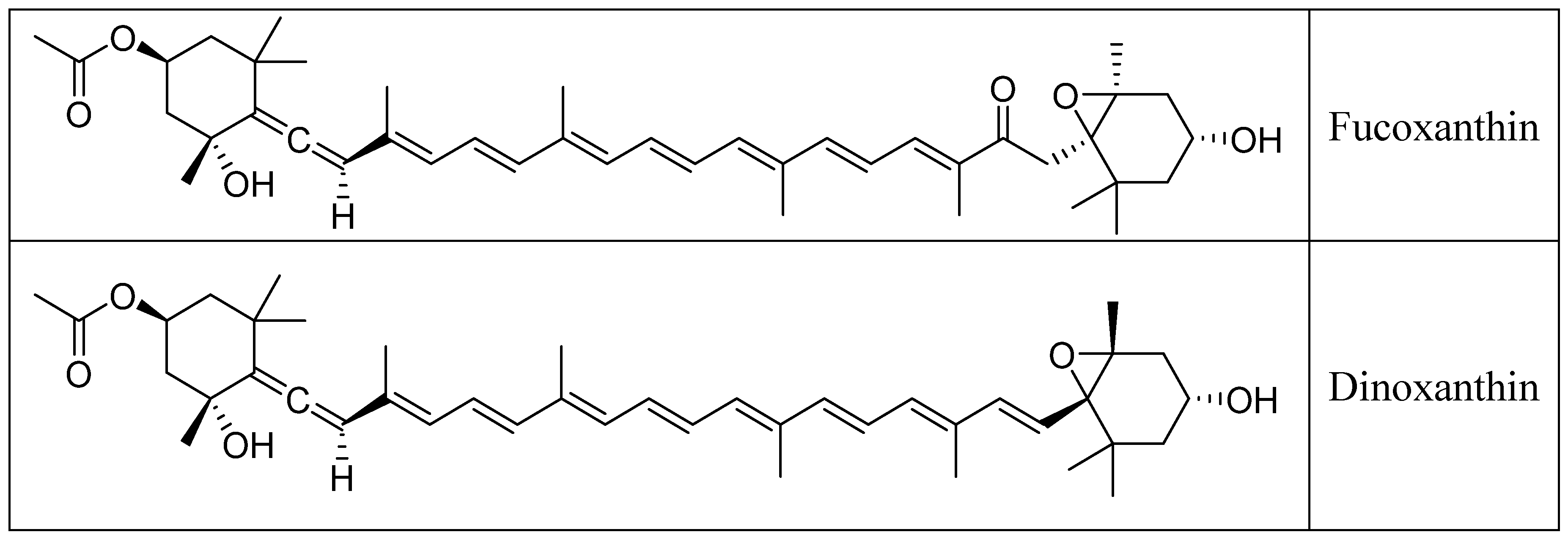
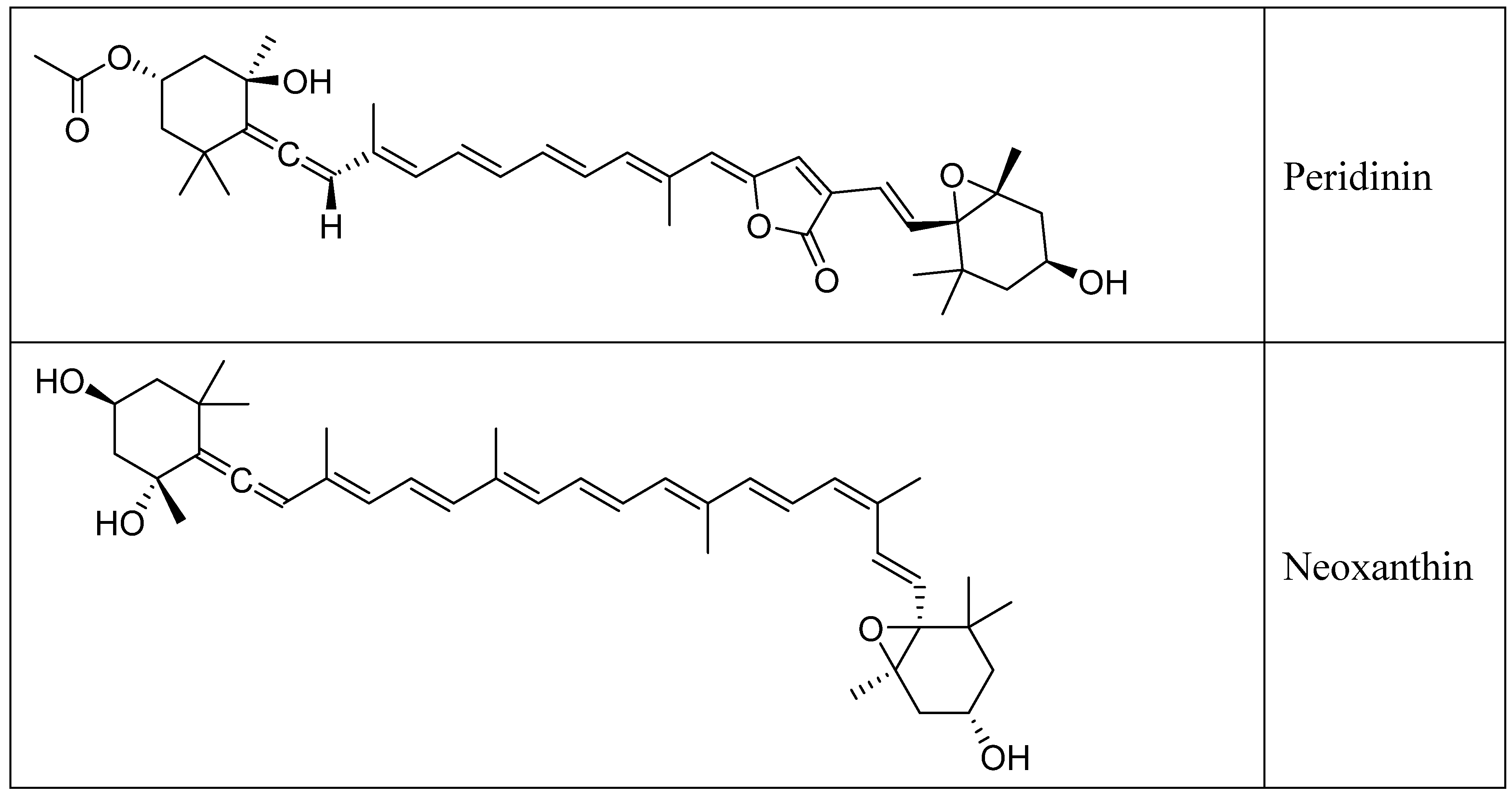
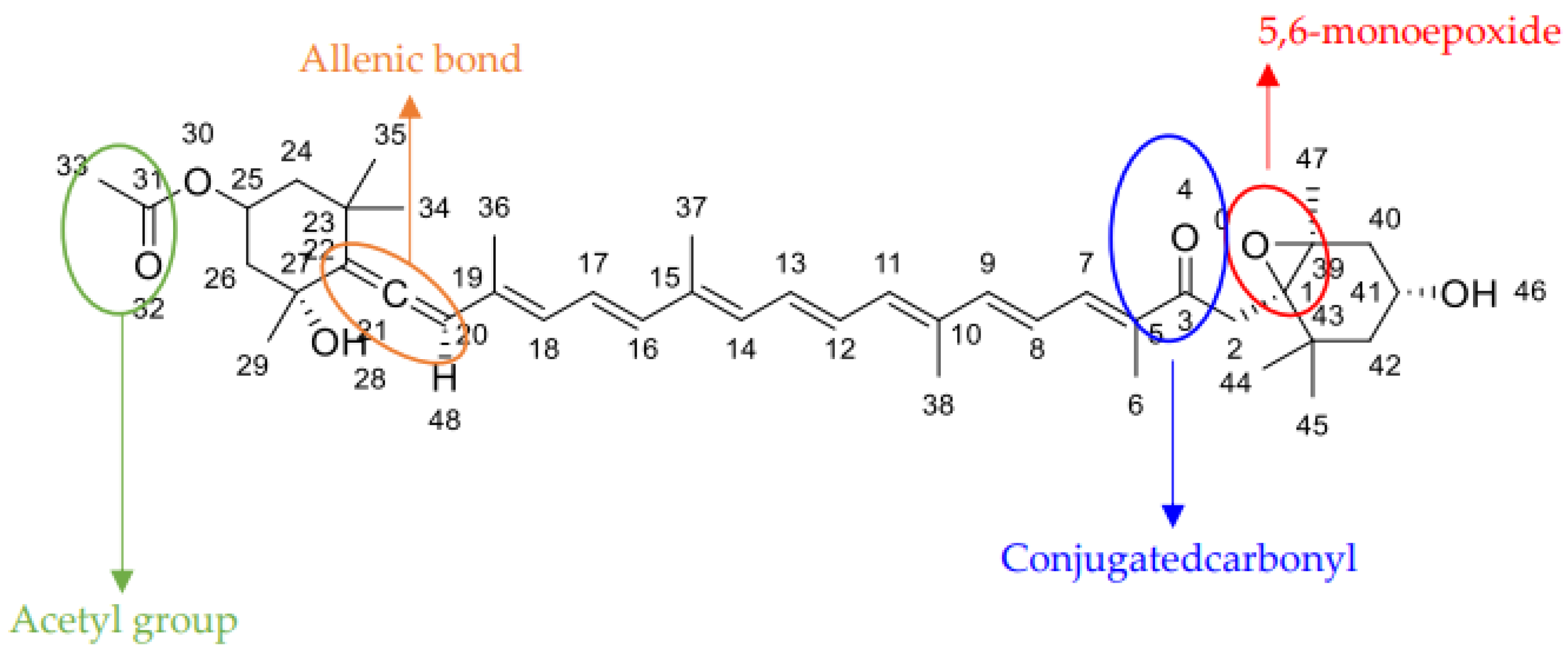
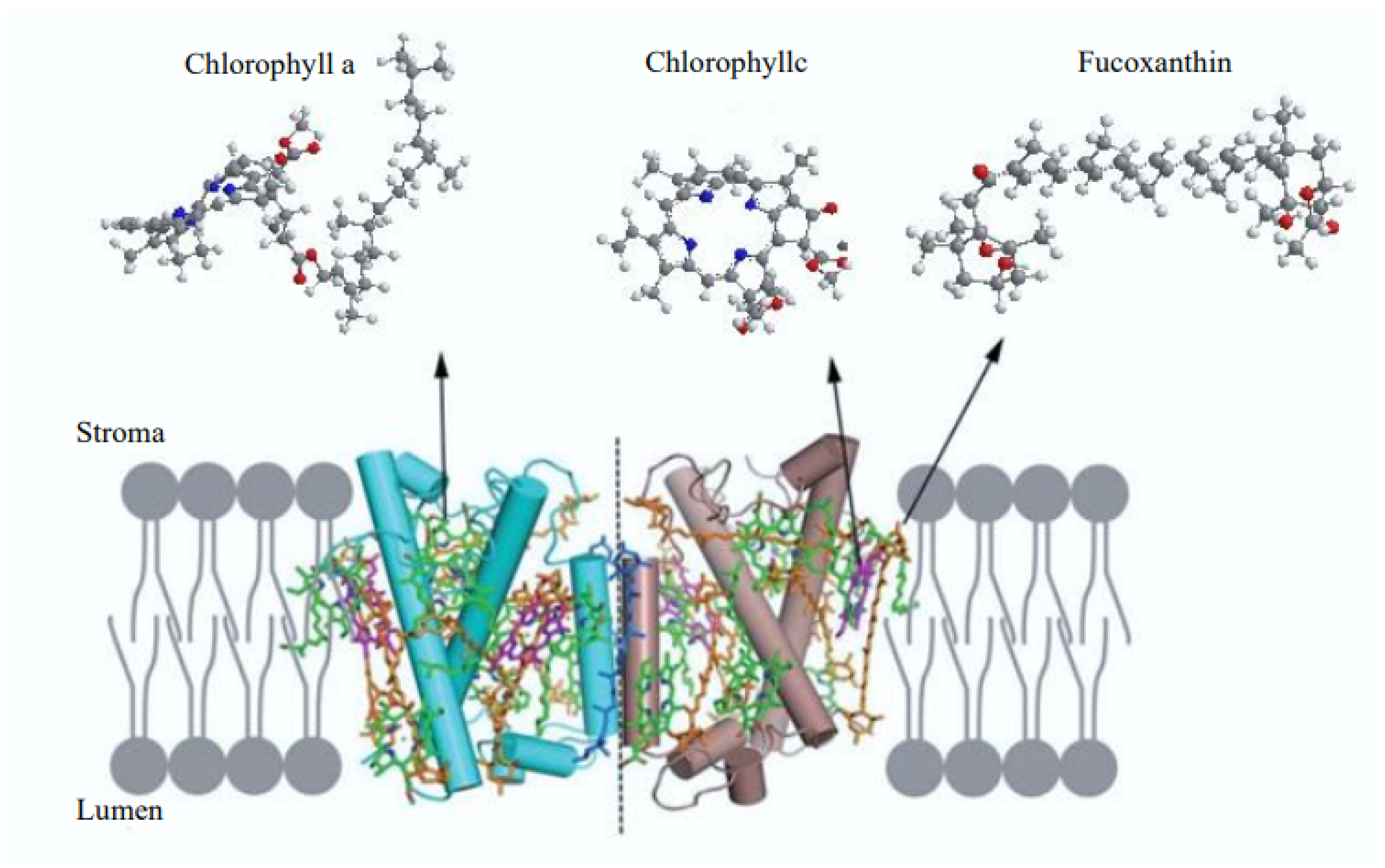
2. Chemistry of Fucoxanthin and Its Derived Compounds
3. Microalgae as a Source of Fucoxanthin
| Microalgae Species | Fucoxanthin (mg/g) | Reference |
|---|---|---|
| Amphora sp. | 1.2 | [29] |
| Chaetoceros muelleri | 2.9 | [29] |
| Phaeodactylum tricornutum | 5.5 | [34] |
| Cyclotella cryptica | 12.0 | [30] |
| Odontella aurita | 18.5 | [37] |
| Isochrysis zhangjiangensis | 23.3 | [36] |
| Cylindrotheca closterium | 25.5 | [32] |
| Halamphora coffeaeformis | 38.0 | [33] |
| Phaeodactylum tricornutum | 59.2 | [35] |
| Tisochrysis lutea | 79.4 | [38] |
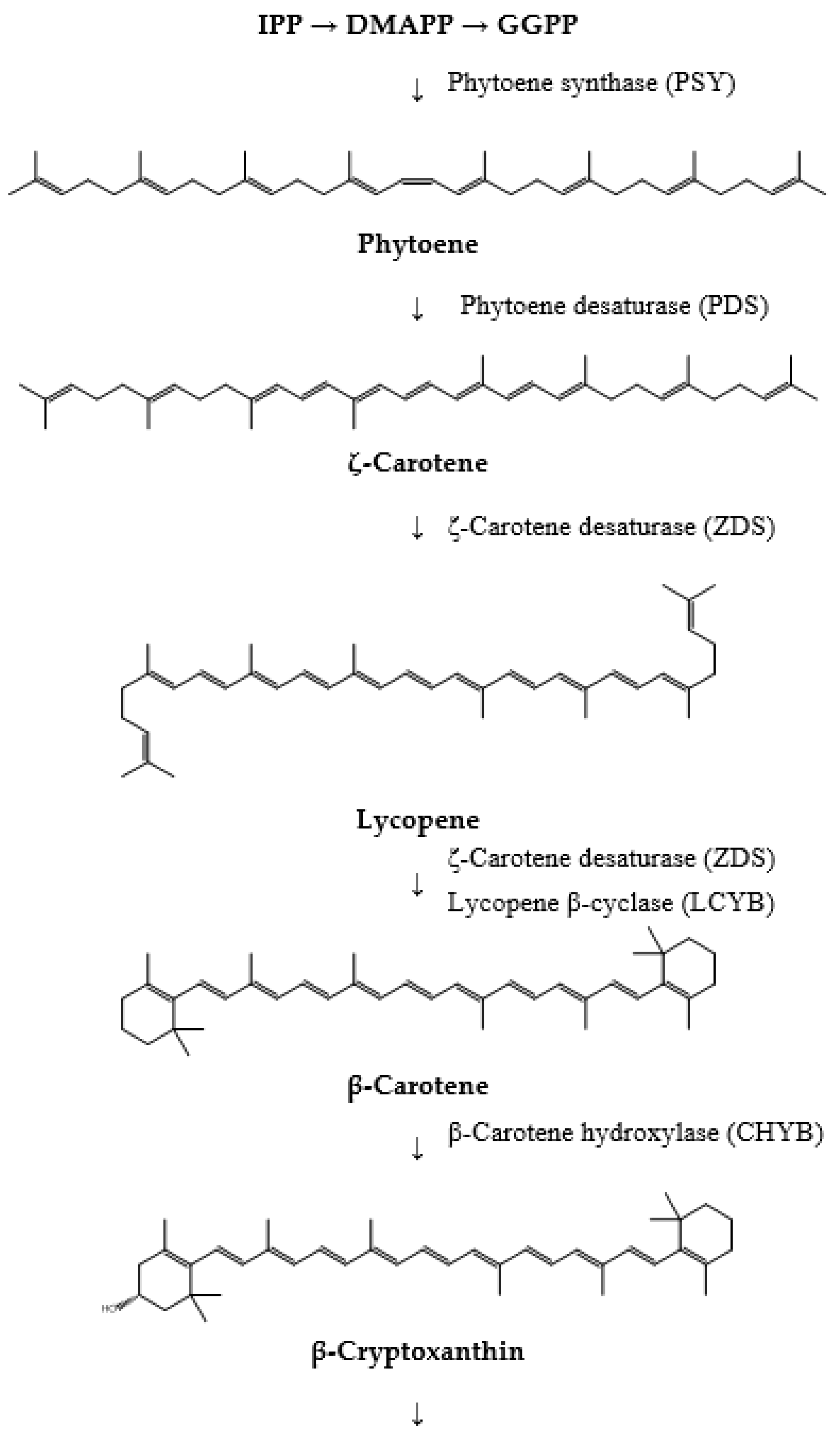
4. Biosynthesis of Fucoxanthin
- The initial step in fucoxanthin biosynthesis involves the production of the C5 building blocks, isopentenyl pyrophosphate (IPP) or its isomer, dimethylallyl diphosphate (DMAPP). These molecules can be synthesized from acetyl-CoA through the cytosolic mevalonic acid (MVA) pathway or from pyruvate and glyceraldehyde-3-phosphate (G3P) via the plastid methylerythritol 4-phosphate pathway (MEP). Although both pathways yield the same end product, carotenoid synthesis predominantly utilizes IPP or DMAPP derived from the MEP pathway.
- The formation of phytoene marks the initial specific step in the carotenoid pathway. This process is catalyzed by phytoene synthase (PSY), which facilitates the head-to-head condensation of two geranylgeranyl diphosphate (GGPP) molecules to produce phytoene. Phytoene is pivotal as it serves as the first distinct intermediate in this biosynthetic pathway.
- The conversion of phytoene into lycopene follows next and involves several enzymes. Phytoene desaturase (PDS), ζ-carotene desaturase (ZDS), and carotene isomerase (CRTISO) sequentially desaturate phytoene to generate ζ-carotene, subsequently leading to the formation of lycopene. Lycopene represents the initial colored carotenoid produced in this biosynthesis pathway.
- Subsequently, lycopene undergoes cyclization to form β-carotene, a process catalyzed by lycopene β-cyclase (LCYB). β-carotene is an important precursor in fucoxanthin biosynthesis, marking a significant step in converting linear carotenoids into cyclic compounds.
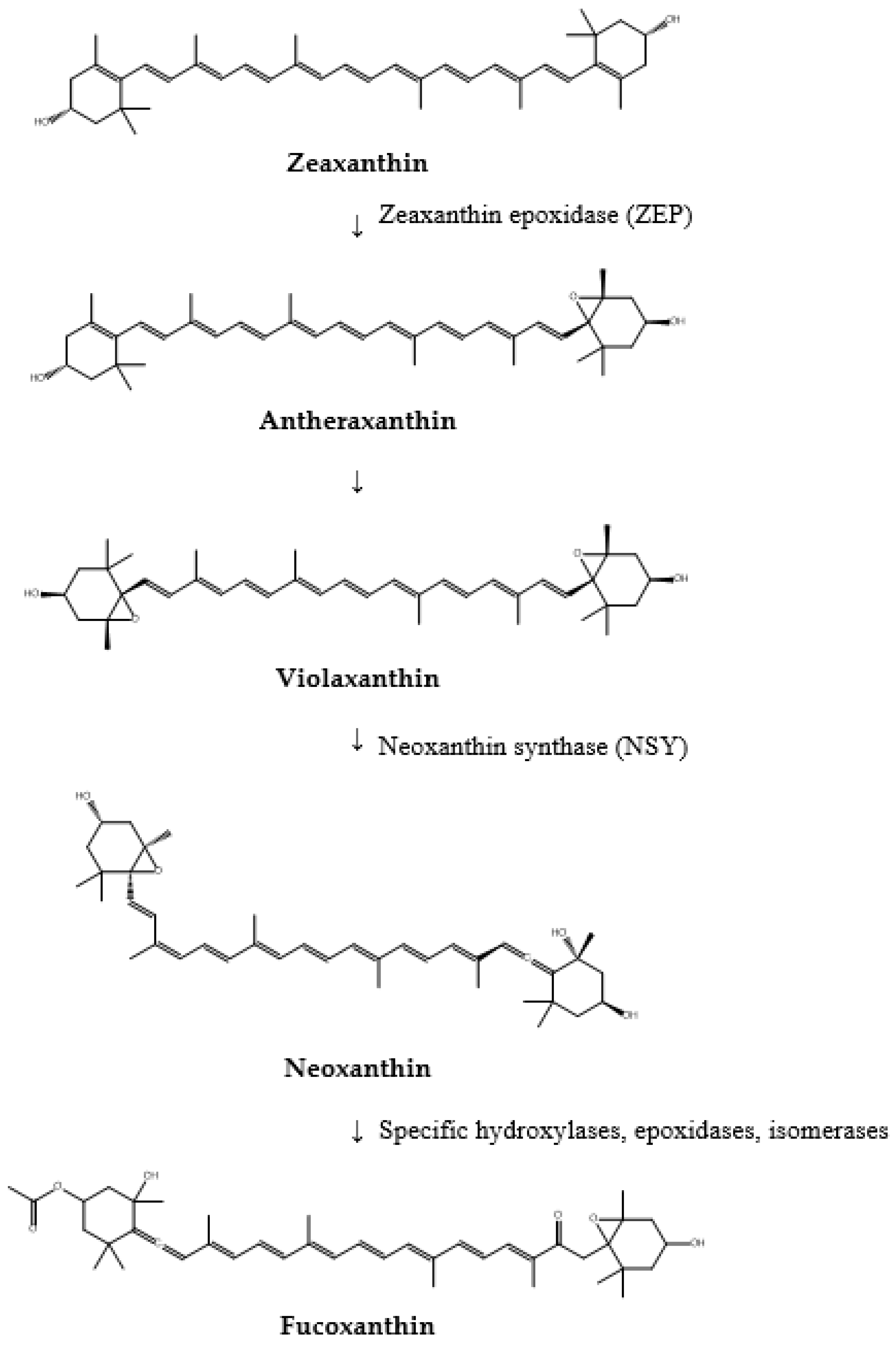
- β-carotene is then hydroxylated to produce zeaxanthin. This biosynthetic step involves β-carotene hydroxylase (CHYB), which introduces hydroxyl groups to β-carotene, forming β-cryptoxanthin and subsequently zeaxanthin.
- Zeaxanthin undergoes epoxidation to form antheraxanthin and then violaxanthin. Zeaxanthin epoxidase (ZEP) catalyzes this reaction by adding epoxy groups to zeaxanthin, thereby transforming the carotenoids into more complex and functional forms.
- Violaxanthin is further converted into neoxanthin by neoxanthin synthase (NSY). This transformation represents another critical step in the biosynthetic pathway, laying the groundwork for subsequent modifications that lead to the formation of fucoxanthin.
- Finally, neoxanthin undergoes a series of intricate enzymatic modifications to synthesize fucoxanthin. These reactions involve various enzymes, including hydroxylases, epoxidases, and specific isomerases, which introduce hydroxyl and epoxy groups and facilitate structural rearrangements necessary for forming fucoxanthin’s unique allene bond.
5. Anticancer Activities of Fucoxanthin
6. Medical Applications of Fucoxanthin
6.1. Antioxidative Activities
6.2. Antimicrobial Activities
6.3. Anti-Inflammatory Activities
6.4. Anti-Obesity Activity
6.5. Antidiabetic Activity
6.6. Other Medical Applications
7. Bioavailability, Stability and Toxicity
8. Challenges and Prospects of Microalgal Fucoxanthin
- Efficient Extraction: Extracting fucoxanthin from microalgae efficiently remains a significant hurdle. The carotenoid’s relatively low intracellular concentration necessitates sophisticated and economically viable extraction methods to achieve high yields. Moreover, purifying fucoxanthin to a degree suitable for medical applications requires advanced chromatography and filtration techniques.
- Stability Issues: Fucoxanthin is prone to physicochemical changes under light, heat, and oxygen, compromising its bioavailability and effectiveness in pharmaceutical and nutraceutical formulations. Therefore, developing effective stabilization methods is important to maintain its therapeutic potential.
- High Production Costs: The cost of producing fucoxanthin commercially is prohibitively high. Microalgae cultivation, harvesting, extraction, and purification processes are economically intensive. Reducing these costs while ensuring product quality is essential for broader adoption in medical and nutritional industries.
- Regulatory Standards: Establishing rigorous regulatory standards is essential to ensure fucoxanthin products’ safety, quality, and effectiveness. Harmonized regulations are needed to instill confidence among consumers and healthcare professionals in this promising natural substance.
- Clinical Evaluation: While preclinical studies have demonstrated diverse therapeutic potential, rigorous clinical trials are essential to evaluate fucoxanthin’s efficacy in humans. These studies will determine optimal doses, administration routes, and potential adverse effects associated with medical use.
- Despite these challenges, promising prospects are emerging for fucoxanthin:
- Nutritional and Metabolic Health: Fucoxanthin may contribute to managing body weight and metabolic disorders such as hyperuricemia and hyperlipidemia, suggesting broader applications beyond traditional medicine.
- Formulation Innovations: Innovations in formulation technologies, such as encapsulation in nanoparticles and liposomes, aim to enhance the stability and bioavailability of fucoxanthin, thereby improving its absorption and effectiveness.
- Sustainability: Microalgae, as primary sources of fucoxanthin, offer a sustainable alternative to conventional sources of active compounds. Their controlled cultivation reduces environmental impact compared to agricultural or synthetic methods, supporting sustainable production practices.
- Education and Awareness: Educating consumers and healthcare professionals about the potential benefits and proper use of fucoxanthin is essential for widespread adoption. This includes raising awareness about quality challenges and regulatory considerations associated with products containing this carotenoid.
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yadav, K.; Vasistha, S.; Srivastava, A.; Rai, M.P. Microalgae as sustainable feedstock for biofuel production and value-added co-products. In Microalgal Biomass for Bioenergy Applications; Woodhead Publishing: Cambridge, UK, 2024; pp. 253–286. [Google Scholar]
- Occhipinti, P.S.; Russo, N.; Foti, P.; Zingale, I.M.; Pino, A.; Romeo, F.V.; Randazzo, C.L.; Caggia, C. Current challenges of microalgae applications: Exploiting the potential of non-conventional microalgae species. J. Sci. Food Agric. 2024, 104, 3823–3833. [Google Scholar] [CrossRef] [PubMed]
- Omoregie, I.P.; Ihotu, K.-E.I.; Osagie, A.D. Marine Green Microalgae Biomass Production and Application. In Marine Greens; CRC Press: Boca Raton, FL, USA, 2024; pp. 93–101. [Google Scholar]
- Bürck, M.; Ramos, S.D.P.; Braga, A.R.C. Enhancing the Biological Effects of Bioactive Compounds from Microalgae through Advanced Processing Techniques: Pioneering Ingredients for Next-Generation Food Production. Foods 2024, 13, 1811. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Xie, C.; Hill, D.R.A.; Barrow, C.J.; Dunshea, F.R.; Martin, G.J.O. Bioaccessibility, bioavailability and bioactivities of carotenoids in microalgae: A review. Food Rev. Int. 2024, 40, 230–259. [Google Scholar] [CrossRef]
- Yin, R.; Zhuang, G.; Lei, Y.; Han, J.; Li, Y.; Zhang, J.; Yan, X. Valorization of Nannochloropsis oceanica for integrated co-production of violaxanthin cycle carotenoids. Bioresour. Technol. 2024, 399, 130597. [Google Scholar] [CrossRef] [PubMed]
- Kneip, J.S.; Kniepkamp, N.; Jang, J.; Mortaro, M.G.; Jin, E.; Kruse, O.; Baier, T. CRISPR/Cas9-Mediated Knockout of the Lycopene ε-Cyclase for Efficient Astaxanthin Production in the Green Microalga Chlamydomonas reinhardtii. Plants 2024, 13, 1393. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Chang, Y.-F.; Prasetya, S.J.; Yu, F.-Y.; Lai, S.-Y.; Wang, M.-Y. An integrated process for enhanced production and purification of fucoxanthin and sulfated polysaccharides in diatom Hyalosynedra toxoneides cultures. J. Taiwan Inst. Chem. Eng. 2024, 155, 105308. [Google Scholar] [CrossRef]
- Razz, S.A. Comprehensive overview of microalgae-derived carotenoids and their applications in diverse industries. Algal Res. 2024, 78, 103422. [Google Scholar] [CrossRef]
- Huang, H.; Lang, Y.; Zhou, M. A comprehensive review on medical applications of microalgae. Algal Res. 2024, 80, 103504. [Google Scholar] [CrossRef]
- Gong, B.; Ma, S.; Yan, Y.; Wang, Z. Progress on the biological characteristics and physiological activities of fucoxanthin produced by marine microalgae. Front. Mar. Sci. 2024, 11, 1357425. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, Z.; Xue, C. Delivery systems for fucoxanthin: Research progress, applications and future prospects. Crit. Rev. Food Sci. Nutr. 2024, 64, 4643–4659. [Google Scholar] [CrossRef]
- Kumarasinghe, H.S.; Gunathilaka, M.D.T.L. A systematic review of fucoxanthin as a promising bioactive compound in drug development. Phytochem. Lett. 2024, 61, 52–65. [Google Scholar] [CrossRef]
- Pang, Y.; Song, W.; Fan, L. Progress in the Preparation and Application of Fucoxanthin from Algae. Int. J. Biol. Life Sci. 2024, 5, 46–51. [Google Scholar] [CrossRef]
- García-García, P.; Ospina, M.; Señoráns, F.J. Tisochrysis lutea as a source of omega-3 polar lipids and fucoxanthin: Extraction and characterization using green solvents and advanced techniques. J. Appl. Phycol. 2024, 2024, 1–12. [Google Scholar] [CrossRef]
- Phong, W.N.; Regmi, R.; Adhikari, K.C.; Bhattarai, R.R.; Show, P.L. Bioactive Compounds from Diatoms. In Diatoms Biotechnology; CRC Press: Boca Raton, FL, USA, 2023; pp. 20–37. [Google Scholar]
- Mc Gee, D.; Gillespie, E. The bioactivity and chemotaxonomy of microalgal carotenoids. Biodivers. Chemotaxon. 2019, 2019, 215–237. [Google Scholar]
- Gomez-Zavaglia, A.; Barros, L.; Prieto, M.A.; Cassani, L. Recent Progress in Understanding the Impact of Food Processing and Storage on the Structure–Activity Relationship of Fucoxanthin. Foods 2023, 12, 3167. [Google Scholar] [CrossRef]
- Bardakci, H.; Yücel, Ç.; Karatoprak, G.Ş.; Küpeli Akkol, E.; Hakan Barak, T.; Sobarzo-Sánchez, E. Oxidative stress and marine carotenoids: Application by using nanoformulations. Mar. Drugs 2020, 18, 423. [Google Scholar] [CrossRef]
- Wang, C.; Jin, M.; Sun, C.; Bai, Y.; Dong, X.; Qi, H. Rapeseed oil as the extraction solvent motivates fucoxanthin-loaded high internal phase emulsion preparation for food 3D printing. LWT 2023, 187, 115349. [Google Scholar] [CrossRef]
- Sun, H.; Yang, S.; Zhao, W.; Kong, Q.; Zhu, C.; Fu, X.; Zhang, F.; Liu, Z.; Zhan, Y.; Mou, H.; et al. Fucoxanthin from marine microalgae: A promising bioactive compound for industrial production and food application. Crit. Rev. Food Sci. Nutr. 2023, 63, 7996–8012. [Google Scholar] [CrossRef]
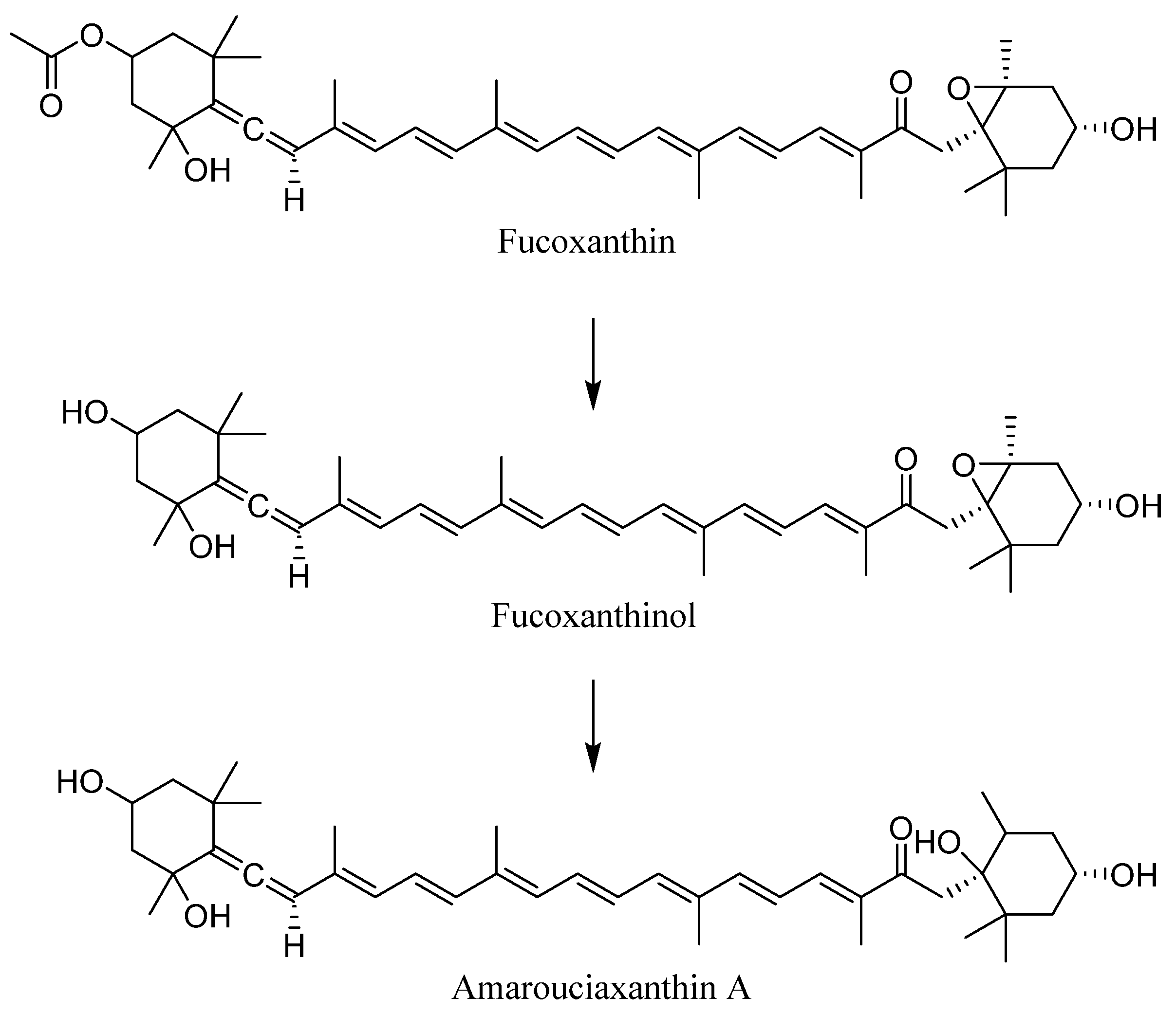
- Asai, A.; Sugawara, T.; Ono, H.; Nagao, A. Biotransformation of fucoxanthinol into amarouciaxanthin A in mice and HepG2 cells: Formation and cytotoxicity of fucoxanthin metabolites. Drug Metab. Dispos. 2004, 32, 205–211. [Google Scholar] [CrossRef]
- Doolaanea, A.A.; Alfatama, M.; Alkhatib, H.; Mawazi, S.M. Fucoxanthin: Structure, Sources, Properties and Benefits. In Handbook of Food Bioactive Ingredients: Properties and Applications; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–27. [Google Scholar]
- Mapelli-Brahm, P.; Gómez-Villegas, P.; Gonda, M.L.; León-Vaz, A.; León, R.; Mildenberger, J.; Rebours, C.; Saravia, V.; Vero, S.; Vila, E.; et al. Microalgae, seaweeds and aquatic bacteria, archaea, and yeasts: Sources of carotenoids with potential antioxidant and anti-inflammatory health-promoting actions in the sustainability era. Mar. Drugs 2023, 21, 340. [Google Scholar] [CrossRef]
- Li, D.; Chen, N.; Zhou, Z.; Zhong, C.; Fang, J.; Huang, L.; Sun, H.; Chi, C.; Zhou, Y.; He, Y. Isochrysis sp. cultivation in pilot-scale to concurrently produce sustainable triacylglycerols for human milk fat substitutes and fucoxanthin. Algal Res. 2023, 69, 102937. [Google Scholar] [CrossRef]
- Truong, T.Q.; Park, Y.J.; Koo, S.Y.; Choi, J.-H.; Enkhbayar, A.; Song, D.-G. Interdependence of fucoxanthin biosynthesis and fucoxanthin-chlorophyll a/c binding proteins in Phaeodactylum tricornutum under different light intensities. J. Appl. Phycol. 2023, 35, 25–42. [Google Scholar] [CrossRef]
- Zheng, M.; Zhou, C.; Wang, W.; Kuang, T.; Shen, J.; Tian, L. Origin of energy dissipation in the oligomeric fucoxanthin-chlorophyll a/c binding proteins. J. Phys. Chem. Lett. 2023, 14, 7967–7974. [Google Scholar] [CrossRef]
- Agostini, A.; Bína, D.; Carbonera, D.; Litvín, R. Conservation of triplet-triplet energy transfer photoprotective pathways in fucoxanthin chlorophyll-binding proteins across algal lineages. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2023, 1864, 148935. [Google Scholar] [CrossRef]
- Ishika, T.; Moheimani, N.R.; Bahri, P.A.; Laird, D.W.; Blair, S.; Parlevliet, D. Halo-adapted microalgae for fucoxanthin production: Effect of incremental increase in salinity. Algal Res. 2017, 28, 66–73. [Google Scholar] [CrossRef]
- Tokushima, H.; Inoue-Kashino, N.; Nakazato, Y.; Masuda, A.; Ifuku, K.; Kashino, Y. Advantageous characteristics of the diatom Chaetoceros gracilis as a sustainable biofuel producer. Biotechnol. Biofuels 2016, 9, 235. [Google Scholar] [CrossRef]
- Ryabushko, V.I.; Zheleznova, S.N.; Nekhoroshev, M.V. Effect of nitrogen on fucoxanthin accumulation in the diatom Cylindrotheca closterium (Ehrenb.) Reimann et Lewin. Int. J. Algae 2017, 19, 79–84. [Google Scholar] [CrossRef]
- Wang, S.; Verma, S.K.; Hakeem Said, I.; Thomsen, L.; Ullrich, M.S.; Kuhnert, N. Changes in the fucoxanthin production and protein profiles in Cylindrotheca closterium in response to blue light-emitting diode light. Microb. Cell Factories 2018, 17, 110. [Google Scholar] [CrossRef]
- Popovich, C.A.; Faraoni, M.B.; Sequeira, A.; Daglio, Y.; Martín, L.A.; Martínez, A.M.; Damiani, M.C.; Matulewicz, M.C.; Leonardi, P.I. Potential of the marine diatom Halamphora coffeaeformis to simultaneously produce omega-3 fatty acids, chrysolaminarin and fucoxanthin in a raceway pond. Algal Res. 2020, 51, 102030. [Google Scholar] [CrossRef]
- Wu, H.; Li, T.; Wang, G.; Dai, S.; He, H.; Xiang, W. A comparative analysis of fatty acid composition and fucoxanthin content in six Phaeodactylum tricornutum strains from diff erent origins. Chin. J. Oceanol. Limnol. 2016, 34, 391–398. [Google Scholar] [CrossRef]
- McClure, D.D.; Luiz, A.; Gerber, B.; Barton, G.W.; Kavanagh, J.M. An investigation into the effect of culture conditions on fucoxanthin production using the marine microalgae Phaeodactylum tricornutum. Algal Res. 2018, 29, 41–48. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Wu, T.; Fu, Y.; He, Y.; Mao, X.; Chen, F. Storage carbon metabolism of Isochrysis zhangjiangensis under different light intensities and its application for co-production of fucoxanthin and stearidonic acid. Bioresour. Technol. 2019, 282, 94–102. [Google Scholar] [CrossRef]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef]
- Mohamadnia, S.; Tavakoli, O.; Faramarzi, M.A.; Shamsollahi, Z. Production of fucoxanthin by the microalga Tisochrysis lutea: A review of recent developments. Aquaculture 2020, 516, 734637. [Google Scholar] [CrossRef]
- Seth, K.; Kumar, A.; Rastogi, R.P.; Meena, M.; Vinayak, V. Bioprospecting of fucoxanthin from diatoms—Challenges and perspectives. Algal Res. 2021, 60, 102475. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Vuong, T.T.; Choi, J.; Lee, T.S.; Um, J.-I.; Koo, S.Y.; Hwang, K.T. Fucoxanthin biosynthesis has a positive correlation with the specific growth rate in the culture of microalga Phaeodactylum tricornutum. J. Appl. Phycol. 2021, 33, 1473–1485. [Google Scholar] [CrossRef]
- Bai, Y.; Cao, T.; Dautermann, O.; Buschbeck, P.; Cantrell, M.B.; Chen, Y.; Lein, C.D.; Shi, X.; Ware, M.A.; Yan, F. Green diatom mutants reveal an intricate biosynthetic pathway of fucoxanthin. Proc. Natl. Acad. Sci. USA 2022, 119, e2203708119. [Google Scholar] [CrossRef]
- Shen, X.; Pan, K.; Zhang, L.; Zhu, B.; Li, Y.; Han, J. A DUF4281 domain-containing protein (homologue of ABA4) of Phaeodactylum tricornutum regulates the biosynthesis of fucoxanthin. Algal Res. 2022, 65, 102728. [Google Scholar] [CrossRef]
- Cao, T.; Bai, Y.; Buschbeck, P.; Tan, Q.; Cantrell, M.B.; Chen, Y.; Jiang, Y.; Liu, R.-Z.; Ries, N.K.; Shi, X.; et al. An unexpected hydratase synthesizes the green light-absorbing pigment fucoxanthin. Plant Cell 2023, 35, 3053–3072. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, J.; Li, Y.; Yang, S.; Di Chen, D.; Tu, Y.; Liu, J.; Sun, Z. Synthetic biology in microalgae towards fucoxanthin production for pharmacy and nutraceuticals. Biochem. Pharmacol. 2023, 115958. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhao, H.; Zhang, L.; Li, Z.; Han, J.; Zhou, C.; Xu, J.; Li, X.; Yan, X. The heat shock transcription factor PtHSF1 mediates triacylglycerol and fucoxanthin synthesis by regulating the expression of GPAT3 and DXS in Phaeodactylum tricornutum. Plant Cell Physiol. 2023, 64, 622–636. [Google Scholar] [CrossRef] [PubMed]
- Méresse, S.; Fodil, M.; Fleury, F.; Chénais, B. Fucoxanthin, a marine-derived carotenoid from brown seaweeds and microalgae: A promising bioactive compound for cancer therapy. Int. J. Mol. Sci. 2020, 21, 9273. [Google Scholar] [CrossRef] [PubMed]
- Salami, M.; Salami, R.; Aarabi, M.H.; Mafi, A.; Ghorbanhosseini, S.S.; Shafabakhsh, R.; Asemi, Z. Targeting glioma cells with nutraceuticals: Therapeutic effects based on molecular mechanisms, new evidence and perspectives. Mini Rev. Med. Chem. 2023, 23, 1167–1192. [Google Scholar] [CrossRef] [PubMed]
- Lau, T.Y.; Kwan, H.Y. Fucoxanthin is a potential therapeutic agent for the treatment of breast cancer. Mar. Drugs 2022, 20, 370. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Mendonca, P.; Elhag, R.; Soliman, K.F. Anticancer effects of fucoxanthin through cell cycle arrest, apoptosis induction, angiogenesis inhibition, and autophagy modulation. Int. J. Mol. Sci. 2022, 23, 16091. [Google Scholar] [CrossRef] [PubMed]
- Moga, M.A.; Dima, L.; Balan, A.; Blidaru, A.; Dimienescu, O.G.; Podasca, C.; Toma, S. Are bioactive molecules from seaweeds a novel and challenging option for the prevention of HPV infection and cervical cancer therapy?—A review. Int. J. Mol. Sci. 2021, 22, 629. [Google Scholar] [CrossRef] [PubMed]
- Manochkumar, J.; Doss CG, P.; Efferth, T.; Ramamoorthy, S. Tumor preventive properties of selected marine pigments against colon and breast cancer. Algal Res. 2022, 61, 102594. [Google Scholar] [CrossRef]
- Sangavi, P.; Langeswaran, K.; KumaR, S.G.O. Anticarcinogenic Efficacy of Fucoxanthin on HepG2 Cell Lines. J. Clin. Diagn. Res. 2022, 16, XC05–XC09. [Google Scholar] [CrossRef]
- Almeida, T.P.; Ramos, A.A.; Ferreira, J.; Azqueta, A.; Rocha, E. Bioactive compounds from seaweed with anti-leukemic activity: A mini-review on carotenoids and phlorotannins. Mini Rev. Med. Chem. 2020, 20, 39–53. [Google Scholar] [CrossRef]
- Wang, J.; Dong, X.; Li, D.; Fang, Z.; Wan, X.; Liu, J. Fucoxanthin inhibits gastric cancer lymphangiogenesis and metastasis by regulating Ran expression. Phytomedicine 2023, 118, 154926. [Google Scholar] [CrossRef]
- Januar, H.I.; Dewi, A.S.; Marraskuranto, E.; Wikanta, T. In silico study of fucoxanthin as a tumor cytotoxic agent. J. Pharm. Bioallied Sci. 2012, 4, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Roy, A.; Choi, J.S. In vitro monoamine oxidase A and B inhibitory activity and molecular docking simulations of fucoxanthin. Fish. Sci. 2017, 83, 123–132. [Google Scholar] [CrossRef]
- Garg, S.; Afzal, S.; Elwakeel, A.; Sharma, D.; Radhakrishnan, N.; Dhanjal, J.K.; Sundar, D.; Kaul, S.C.; Wadhwa, R. Marine carotenoid fucoxanthin possesses anti-metastasis activity: Molecular evidence. Mar. Drugs 2019, 17, 338. [Google Scholar] [CrossRef] [PubMed]
- Dibha, A.F.; Wahyuningsih, S.; Ansori AN, M.; Kharisma, V.D.; Widyananda, M.H.; Parikesit, A.A.; Sibero, M.T.; Probojati, R.T.; Murtadlo, A.A.; Trinugroho, J.P.; et al. Utilization of secondary metabolites in algae Kappaphycus alvarezii as a breast cancer drug with a computational method. Pharmacogn. J. 2022, 14, 536–543. [Google Scholar] [CrossRef]
- Padmi, H.; Fitriani, F.; Saraswati PB, A.; Nurhidayati, S.Z.; Ilhami BT, K.; Sunarwidhi, A.L.; Widyastuti, S.; Prasedya, E.S. Anti-melanoma potential of fucoxanthin by inhibition MC1R receptor: In Silico study. In AIP Conference Proceedings; AIP Publishing: New York, NY, USA, 2023; Volume 2956. [Google Scholar]
- Miyashita, K.; Beppu, F.; Hosokawa, M.; Liu, X.; Wang, S. Bioactive significance of fucoxanthin and its effective extraction. Biocatal. Agric. Biotechnol. 2020, 26, 101639. [Google Scholar] [CrossRef]
- Mumu, M.; Das, A.; Emran, T.B.; Mitra, S.; Islam, F.; Roy, A.; Dasc, M.K.R.; Park, M.N.; Chandran, D.; Sharma,, R. Fucoxanthin: A promising phytochemical on diverse pharmacological targets. Front. Pharmacol. 2022, 13, 929442. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, Y.; Ruan, J.; Yang, Z.; Wang, C.; Hong, Z.; Zuo, Z. Protective effects of fucoxanthin and fucoxanthinol against tributyltin-induced oxidative stress in HepG2 cells. Environ. Sci. Pollut. Res. 2018, 25, 5582–5589. [Google Scholar] [CrossRef] [PubMed]
- Neumann, U.; Derwenskus, F.; Flaiz Flister, V.; Schmid-Staiger, U.; Hirth, T.; Bischoff, S.C. Fucoxanthin, a carotenoid derived from Phaeodactylum tricornutum exerts antiproliferative and antioxidant activities in vitro. Antioxidants 2019, 8, 183. [Google Scholar] [CrossRef]
- Younas, U.; Tehseen, S.; Khan, F.; Niaz, K. Brown Algae (Fucoxanthin) Against Cancer. In Nutraceuticals and Cancer Signaling: Clinical Aspects and Mode of Action; Springer: Cham, Switzerland, 2021; pp. 99–127. [Google Scholar]
- Shen, Y.; Zhang, H.; Cheng, L.; Wang, L.; Qian, H.; Qi, X. In vitro and in vivo antioxidant activity of polyphenols extracted from black highland barley. Food Chem. 2016, 194, 1003–1012. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, Z.; Zhang, S.; Li, K.; Bu, Y.; Zheng, N.; Zhao, S.; Wang, J. Enrichment of milk antioxidant activity by dietary supplementation of red clover isoflavone in cows and its improvement on mice intestinal health. Food Chem. 2024, 446, 138764. [Google Scholar] [CrossRef]
- Chiang, Y.F.; Chen, H.Y.; Chang, Y.J.; Shih, Y.H.; Shieh, T.M.; Wang, K.L.; Hsia, S.M. Protective effects of fucoxanthin on high glucose-and 4-hydroxynonenal (4-HNE)-induced injury in human retinal pigment epithelial cells. Antioxidants 2020, 9, 1176. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, M.; Zhang, X.; Chen, Q.; Chen, H.; Sun, L.; Liu, G. Protective effect of fucoxanthin isolated from Laminaria japonica against visible light-induced retinal damage both in vitro and in vivo. J. Agric. Food Chem. 2016, 64, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Adamczak, A. Fucoxanthin—An antibacterial carotenoid. Antioxidants 2019, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Ożarowski, M.; Alam, R.; Łochyńska, M.; Stasiewicz, M. What do we know about antimicrobial activity of astaxanthin and fucoxanthin? Mar. Drugs 2021, 20, 36. [Google Scholar] [CrossRef] [PubMed]
- Abdoul-Latif, F.M.; Ainane, A.; Houmed Aboubaker, I.; Mohamed, J.; Ainane, T. An Overview of Cancer in Djibouti: Current Status, Therapeutic Approaches, and Promising Endeavors in Local Essential Oil Treatment. Pharmaceuticals 2023, 16, 1617. [Google Scholar] [CrossRef] [PubMed]
- Šudomová, M.; Shariati, M.A.; Echeverría, J.; Berindan-Neagoe, I.; Nabavi, S.M.; Hassan, S.T. A microbiological, toxicological, and biochemical study of the effects of fucoxanthin, a marine carotenoid, on Mycobacterium tuberculosis and the enzymes implicated in its cell wall: A link between mycobacterial infection and autoimmune diseases. Mar. Drugs 2019, 17, 641. [Google Scholar] [CrossRef] [PubMed]
- Rubiño, S.; Aymerich, T.; Peteiro, C.; Bover-Cid, S.; Hortós, M. Antimicrobial potential of Ericaria selaginoides extracts against Listeria monocytogenes in “mató”, a Catalan fresh cheese. J. Appl. Phycol. 2023, 35, 949–959. [Google Scholar] [CrossRef]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Maadane, A.; Merghoub, N.; El Mernissi, N.; Ainane, T.; Amzazi, S.; Wahby, I.; Bakri, Y. Antimicrobial activity of marine microalgae isolated from Moroccan coastlines. J. Microbiol. Biotechnol. Food Sci. 2017, 6, 1257. [Google Scholar] [CrossRef]
- Peraman, M.; Nachimuthu, S. Identification and quantification of fucoxanthin in selected carotenoid-producing marine microalgae and evaluation for their chemotherapeutic potential. Pharmacogn. Mag. 2019, 15, 243–249. [Google Scholar] [CrossRef]
- Tsushima, Y.; Moka, T.; Katsuyama, M.; Kozuka, M.; Matsuno, T.; Tokuda, H.; Nishino, S.; Iwashima, A. Inhibitory effect of natural carotenoids on Epstein-Barr virus activation activity of a tumor promoter in Raji cells. A screening study for anti-tumor promoters. Biol. Pharm. Bull. 1995, 18, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, C.; Manabe, Y.; Tomonaga, N.; Wada, T.; Maoka, T.; Sugawara, T. Evaluation of intestinal absorption of dietary halocynthiaxanthin, a carotenoid from the sea squirt Halocynthia roretzi. Mar. Drugs 2020, 18, 588. [Google Scholar] [CrossRef] [PubMed]
- Tamama, K. Potential benefits of dietary seaweeds as protection against COVID-19. Nutr. Rev. 2021, 79, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Heo, S.Y.; Kim, E.A.; Cha, S.H.; Ryu, B.; Heo, S.J. Antiviral effect of fucoxanthin obtained from Sargassum siliquastrum (Fucales, Phaeophyceae) against severe acute respiratory syndrome coronavirus 2. Algae 2023, 38, 295–306. [Google Scholar] [CrossRef]
- Kang, N.; Kim, E.A.; Park, A.; Heo, S.Y.; Heo, J.H.; Heo, S.J. Antiviral Potential of Fucoxanthin, an Edible Carotenoid Purified from Sargassum siliquastrum, against Zika Virus. Mar. Drugs 2024, 22, 247. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Bishnoi, M.; Kondepudi, K.K.; Shukla, G. Isolation, characterization and anti-inflammatory mechanism of probiotics in lipopolysaccharide-stimulated RAW 264.7 macrophages. World J. Microbiol. Biotechnol. 2020, 36, 74. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, M.-B.; Baek, S.; Lee, J.; Lee, H.; Cao, B.; Kim, Y.; Cao, L.; Lee, S. Suppression of Pro-Inflammatory M1 Polarization of LPS-Stimulated RAW 264.7 Macrophage Cells by Fucoxanthin-Rich Sargassum hemiphyllum. Mar. Drugs 2023, 21, 533. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe AM, K.; Kirindage KG, I.S.; Fernando IP, S.; Kim, K.N.; Oh, J.Y.; Ahn, G. The anti-inflammatory effect of low molecular weight fucoidan from sargassum siliquastrum in lipopolysaccharide-stimulated RAW 264.7 macrophages via inhibiting NF-κB/MAPK signaling pathways. Mar. Drugs 2023, 21, 347. [Google Scholar] [CrossRef]
- Kim, K.N.; Heo, S.J.; Yoon, W.J.; Kang, S.M.; Ahn, G.; Yi, T.H.; Jeon, Y.J. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-κB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur. J. Pharmacol. 2010, 649, 369–375. [Google Scholar]
- Lee, A.H.; Shin, H.Y.; Park, J.H.; Koo, S.Y.; Kim, S.M.; Yang, S.H. Fucoxanthin from microalgae Phaeodactylum tricornutum inhibits pro-inflammatory cytokines by regulating both NF-κB and NLRP3 inflammasome activation. Sci. Rep. 2021, 11, 543. [Google Scholar] [CrossRef]
- Vostálová, J.; Zdařilová, A.; Svobodová, A. Prunella vulgaris extract and rosmarinic acid prevent UVB-induced DNA damage and oxidative stress in HaCaT keratinocytes. Arch. Dermatol. Res. 2010, 302, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Mohibbullah, M.; Haque, M.N.; Sohag, A.A.M.; Hossain, M.T.; Zahan, M.S.; Uddin, M.J.; Hannan, M.A.; Moon, I.S.; Choi, J.-S. A systematic review on marine algae-derived fucoxanthin: An update of pharmacological insights. Mar. Drugs 2022, 20, 279. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Abdoul-Latif, F.; Ainane, A.; Houmed Aboubaker, I.; Mohamed Ahmed, N.; Ainane, T. Effectiveness of a diet for type 2 diabetics based on vegetables and fruits of the Cucurbitaceae family. J. Anal. Sci. Appl. Biotechnol. 2021, 3, 107–113. [Google Scholar]
- Hitoe, S.; Shimoda, H. Seaweed fucoxanthin supplementation improves obesity parameters in mild obese Japanese subjects. Funct. Foods Health Dis. 2017, 7, 246–262. [Google Scholar] [CrossRef]
- Woo, M.N.; Jeon, S.M.; Shin, Y.C.; Lee, M.K.; Kang, M.A.; Choi, M.S. Anti-obese property of fucoxanthin is partly mediated by altering lipid-regulating enzymes and uncoupling proteins of visceral adipose tissue in mice. Mol. Nutr. Food Res. 2009, 53, 1603–1611. [Google Scholar] [CrossRef]
- Sharma, P.P.; Baskaran, V. Polysaccharide (laminaran and fucoidan), fucoxanthin and lipids as functional components from brown algae (Padina tetrastromatica) modulates adipogenesis and thermogenesis in diet-induced obesity in C57BL6 mice. Algal Res. 2021, 54, 102187. [Google Scholar] [CrossRef]
- Gopal, S.S.; Maradgi, T.; Ponesakki, G. Antiobese properties of carotenoids: An overview of underlying molecular mechanisms. Carotenoids: Prop. Process. Appl. 2020, 2020, 75–105. [Google Scholar]
- Ferreira, Y.A.M.; Jamar, G.; Estadella, D.; Pisani, L.P. Role of carotenoids in adipose tissue through the AMPK-mediated pathway. Food Funct. 2023, 14, 3454–3462. [Google Scholar] [CrossRef]
- Vidyashree, J.S.; Shetti, P.P.; Ghagane, S.C. Seaweeds as a potential resource in diabetes management: A review. Future J. Pharm. Sci. 2024, 10, 12. [Google Scholar] [CrossRef]
- Oliyaei, N.; Moosavi-Nasab, M.; Tamaddon, A.M.; Tanideh, N. Antidiabetic effect of fucoxanthin extracted from Sargassum angustifolium on streptozotocin-nicotinamide-induced type 2 diabetic mice. Food Sci. Nutr. 2021, 9, 3521–3529. [Google Scholar] [CrossRef]
- Bae, M.; Kim, M.B.; Park, Y.K.; Lee, J.Y. Health benefits of fucoxanthin in the prevention of chronic diseases. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158618. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Singh, V.; Chauhan, K. Antidiabetic potential of seaweed and their bioactive compounds: A review of developments in last decade. Crit. Rev. Food Sci. Nutr. 2023, 63, 5739–5770. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Kawakami, F.; Chiba, M.; Kanzaki, M.; Maruyama, H. Undaria pinnatifida (Wakame) Intake Ameliorates High-Fat Diet-Induced Glucose Intolerance via Promoting GLUT4 Expression and Membrane Translocation in Muscle. J. Nutr. Metab. 2023, 2023, 9774157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, W.; Huang, X.; Zhao, Y.; Ren, Q.; Hong, Z.; Huang, M.; Xing, X. Fucoxanthin ameliorates hyperglycemia, hyperlipidemia and insulin resistance in diabetic mice partially through IRS-1/PI3K/Akt and AMPK pathways. J. Funct. Foods 2018, 48, 515–524. [Google Scholar] [CrossRef]
- Pruccoli, L.; Balducci, M.; Pagliarani, B.; Tarozzi, A. Antioxidant and Neuroprotective Effects of Fucoxanthin and Its Metabolite Fucoxanthinol: A Comparative In Vitro Study. Curr. Issues Mol. Biol. 2024, 46, 5984–5998. [Google Scholar] [CrossRef] [PubMed]
- Paudel, P.; Seong, S.H.; Jung, H.A.; Choi, J.S. Characterizing fucoxanthin as a selective dopamine D3/D4 receptor agonist: Relevance to Parkinson’s disease. Chem.-Biol. Interact. 2019, 310, 108757. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Cheng, C.Y.; Liu, C.T.; Sue, Y.M.; Chen, T.H.; Hsu, Y.H.; Huang, N.-J.; Chen, C.H. Combined protective effects of oligo-fucoidan, fucoxanthin, and L-carnitine on the kidneys of chronic kidney disease mice. Eur. J. Pharmacol. 2021, 892, 173708. [Google Scholar] [CrossRef]
- Tsukui, T.; Baba, N.; Hosokawa, M.; Sashima, T.; Miyashita, K. Enhancement of hepatic docosahexaenoic acid and arachidonic acid contents in C57BL/6J mice by dietary fucoxanthin. Fish. Sci. 2009, 75, 261–263. [Google Scholar] [CrossRef]
- Guo, L.; Dang, M.; Song, Q.; Zhang, W.; Li, B. Protective effect of fucoxanthin on ovariectomy-induced osteoporosis in rats. Pharmacogn. Mag. 2020, 16, 242–249. [Google Scholar]
- Ma, S.Y.; Park, W.S.; Lee, D.S.; Choi, G.; Yim, M.J.; Lee, J.M.; Jung, W.-K.; Park, S.G.; Seo, S.-K.; Park, S.J. Fucoxanthin inhibits profibrotic protein expression in vitro and attenuates bleomycin-induced lung fibrosis in vivo. Eur. J. Pharmacol. 2017, 811, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.T.; Chen, H.Y.; Lin, P.H.; Hsia, S.M. Preventive effects of fucoidan and fucoxanthin on hyperuricemic rats induced by potassium oxonate. Mar. Drugs 2019, 17, 343. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ozaki, Y.; Mizuno, M.; Yoshida, M.; Nishitani, Y.; Azuma, T.; Komoto, A.; Maoka, T.; Tanino, Y.; Kanazawa, K. Pharmacokinetics of fucoxanthinol in human plasma after the oral administration of kombu extract. Br. J. Nutr. 2012, 107, 1566–1569. [Google Scholar] [CrossRef] [PubMed]
- Beppu, F.; Niwano, Y.; Tsukui, T.; Hosokawa, M.; Miyashita, K. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. J. Toxicol. Sci. 2009, 34, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Mok, I.K.; Lee, J.K.; Kim, J.H.; Pan, C.H.; Kim, S.M. Fucoxanthin bioavailability from fucoxanthin-fortified milk: In vivo and in vitro study. Food Chem. 2018, 258, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Bharathiraja, K.; Babu, L.H.; Vijayaprakash, S.; Tamilselvan, P.; Balasubramanian, M.P. Fucoxanthin, a marine carotenoid protects cadmium-induced oxidative renal dysfunction in rats. Biomed. Prev. Nutr. 2013, 3, 201–207. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Garcia-Oliveira, P.; Carpena, M.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Scientific approaches on extraction, purification and stability for the commercialization of fucoxanthin recovered from brown algae. Foods 2020, 9, 1113. [Google Scholar] [CrossRef] [PubMed]
- Lykov, A.; Rachkovsky, E.; Gevorgiz, R.; Zheleznova, S.; Kotlyarova, A. Toxicity of fucoxanthin on Balb/c mice splenocytes and thymocytes. In Proceedings of the 2020 Cognitive Sciences, Genomics and Bioinformatics (CSGB), Novosibirsk, Russia, 6–10 July 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 277–280. [Google Scholar]
- Ming, J.X.; Wang, Z.C.; Huang, Y.; Ohishi, H.; Wu, R.J.; Shao, Y.; Wang, H.; Qin, M.Y.; Wu, Z.L.; Li, Y.Y.; et al. Fucoxanthin extracted from Laminaria Japonica inhibits metastasis and enhances the sensitivity of lung cancer to Gefitinib. J. Ethnopharmacol. 2021, 265, 113302. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ozaki, Y.; Taminato, M.; Das, S.K.; Mizuno, M.; Yoshimura, K.; Kanazawa, K. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br. J. Nutr. 2009, 102, 242–248. [Google Scholar] [CrossRef]

| Organs | Mechanisms of Action |
|---|---|
| Brain |
|
| Breasts |
|
| Lungs |
|
| Cervix |
|
| Colon |
|
| Liver |
|
| Blood |
|
| Stomach |
|
| Bacterial Strain | ZOI * (mm) | MIC ** (µg/mL) |
|---|---|---|
| Streptococcus agalactiae | 12.2 ± 0.7 | 62.5 |
| Staphylococcus epidermidis | 11.2 ± 0.7 | 125 |
| Staphylococcus aureus | 11.0 ± 0.6 | 125 |
| Escherichia coli | 10.2 ± 0.7 | 125 |
| Streptococcus pyogenes | 10.0 ± 0.6 | 125 |
| Klebsiella oxytoca | 9.2 ± 0.7 | 125–250 |
| Enterococcus faecalis | 9.0 ± 0.8 | 125–250 |
| Streptococcus pneumoniae | 9.7 ± 0.5 | 125 |
| Staphylococcus aureus | 11.0 ± 0.6 | 125 |
| Klebsiella pneumoniae | 8.8 ± 0.7 | 250 |
| Acinetobacter lwoffii | 8.2 ± 0.4 | 250 |
| Pseudomonas aeruginosa | 7.5 ± 0.5 | 250–500 |
| Serratia marcescens | 7.3 ± 0.5 | 500 |
| Proteus mirabilis | 7.2 ± 0.4 | 500 |
| Cutibacterium acnes | 6.0 ± 0.1 | >1000 |
| Veillonella parvula | 6.0 ± 0.1 | >1000 |
| Porphyromonas gingivalis | 6.0 ± 0.1 | >1000 |
| Mechanism of Action Enzymatic | Description | Reactions Involved |
|---|---|---|
| Regulation of UCP1 | Stimulates the expression of Uncoupling Protein 1 (UCP1) in brown adipose tissue (BAT). | Unspecified |
| Browning of white adipose tissue (WAT) | Transforms some white adipose tissue depots into brown adipose tissue via induction of UCP1. | Unspecified |
| Inhibition of adipogenesis | Inhibits adipocyte differentiation by negatively regulating adipogenic genes like PPARγ. | PPARγ inhibition Pancreatic lipase inhibition |
| Reduced fatty acid synthesis | Increases phosphorylation of AMP-activated protein kinase (AMPK). | Reduced acetyl-CoA carboxylase activity |
| Inhibition of digestive enzymes | Inhibits the enzymes α-amylase and α-glucosidase, reducing glucose absorption. | Inhibition of α-amylase Inhibition of α-glucosidase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed Abdoul-Latif, F.; Ainane, A.; Houmed Aboubaker, I.; Merito Ali, A.; Mohamed, H.; Jutur, P.P.; Ainane, T. Unlocking the Green Gold: Exploring the Cancer Treatment and the Other Therapeutic Potential of Fucoxanthin Derivatives from Microalgae. Pharmaceuticals 2024, 17, 960. https://doi.org/10.3390/ph17070960
Mohamed Abdoul-Latif F, Ainane A, Houmed Aboubaker I, Merito Ali A, Mohamed H, Jutur PP, Ainane T. Unlocking the Green Gold: Exploring the Cancer Treatment and the Other Therapeutic Potential of Fucoxanthin Derivatives from Microalgae. Pharmaceuticals. 2024; 17(7):960. https://doi.org/10.3390/ph17070960
Chicago/Turabian StyleMohamed Abdoul-Latif, Fatouma, Ayoub Ainane, Ibrahim Houmed Aboubaker, Ali Merito Ali, Houda Mohamed, Pannaga Pavan Jutur, and Tarik Ainane. 2024. "Unlocking the Green Gold: Exploring the Cancer Treatment and the Other Therapeutic Potential of Fucoxanthin Derivatives from Microalgae" Pharmaceuticals 17, no. 7: 960. https://doi.org/10.3390/ph17070960
APA StyleMohamed Abdoul-Latif, F., Ainane, A., Houmed Aboubaker, I., Merito Ali, A., Mohamed, H., Jutur, P. P., & Ainane, T. (2024). Unlocking the Green Gold: Exploring the Cancer Treatment and the Other Therapeutic Potential of Fucoxanthin Derivatives from Microalgae. Pharmaceuticals, 17(7), 960. https://doi.org/10.3390/ph17070960









