Why Is Wnt/β-Catenin Not Yet Targeted in Routine Cancer Care?
Abstract
1. Introduction
2. Wnt/β-Catenin Inhibitors in Clinical Trials
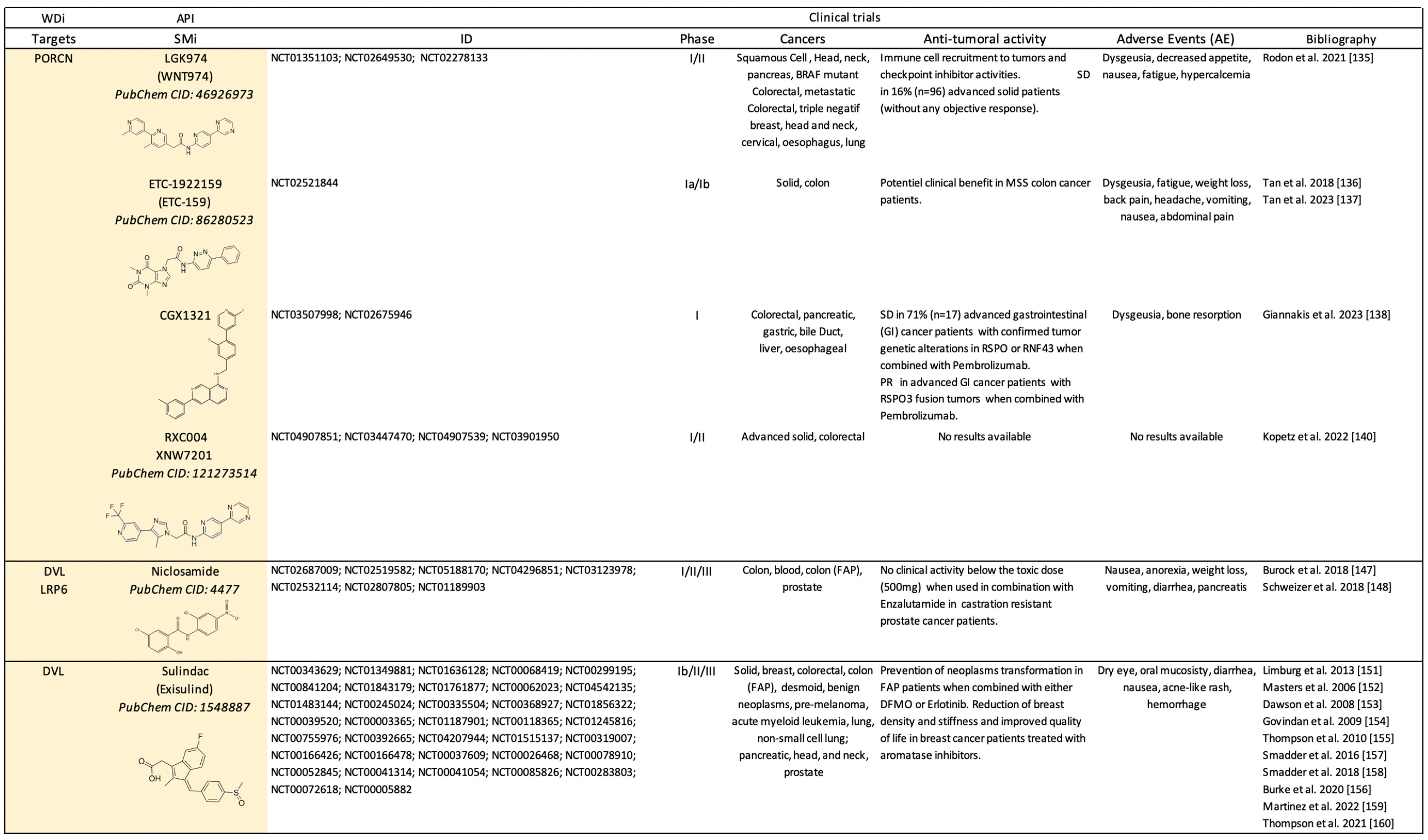
2.1. Clinical Trials Using Canonical Wnt-Dependent Inhibitors (WDi)
2.1.1. Antibody-Based Therapies
2.1.2. Small Molecule-Based Therapies
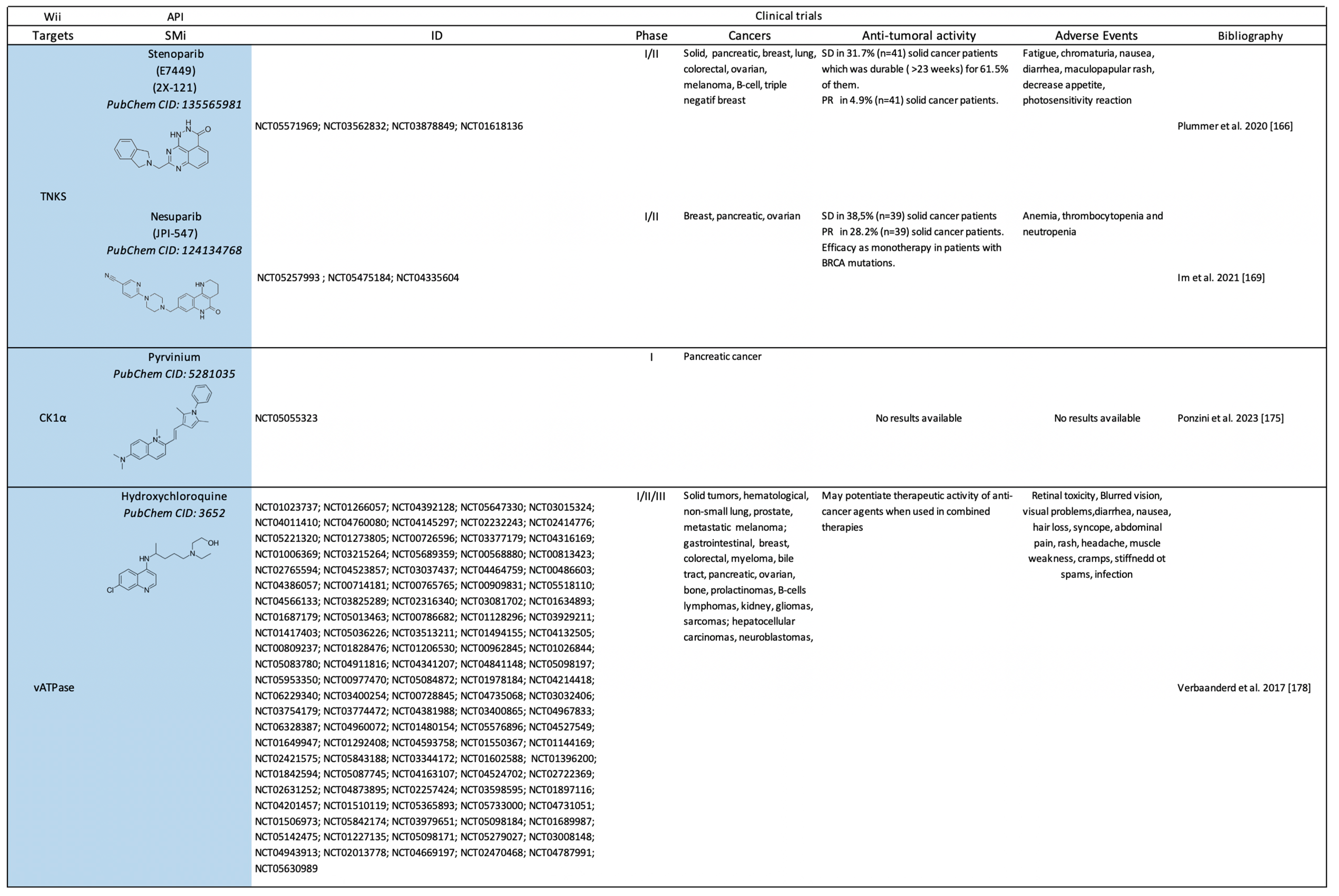
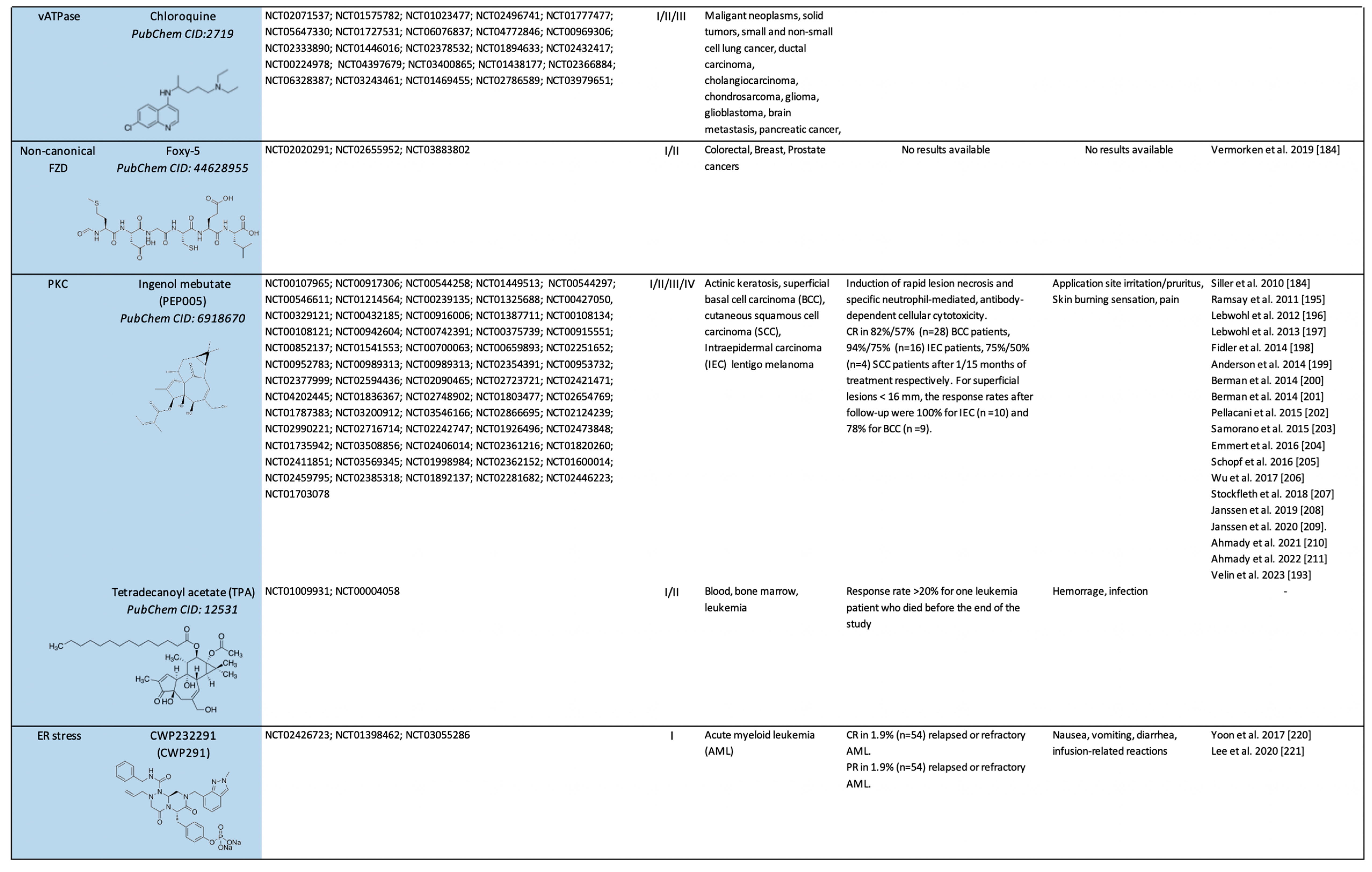
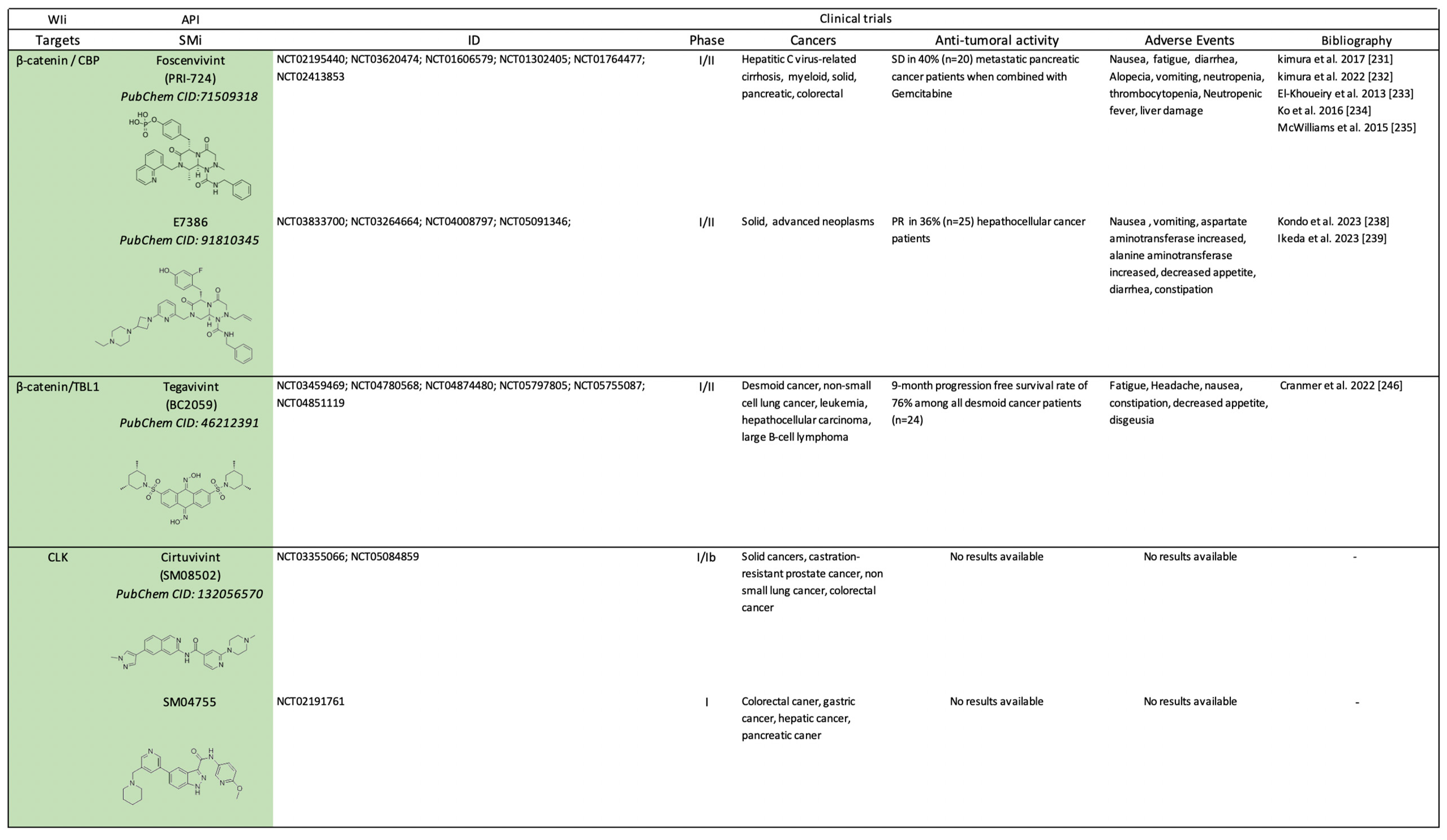
2.2. Clinical Trials on Canonical Wnt-Independent Inhibitors (WIi)
2.2.1. Small Molecule-Based Therapies to Prevent β-catenin Stabilization
2.2.2. Small Molecule-Based Therapies to Prevent β-catenin Co-Transcriptional Activity
3. Future Challenges
3.1. Drug Profiling
3.2. Drug Combinations
3.3. Drug Targeting
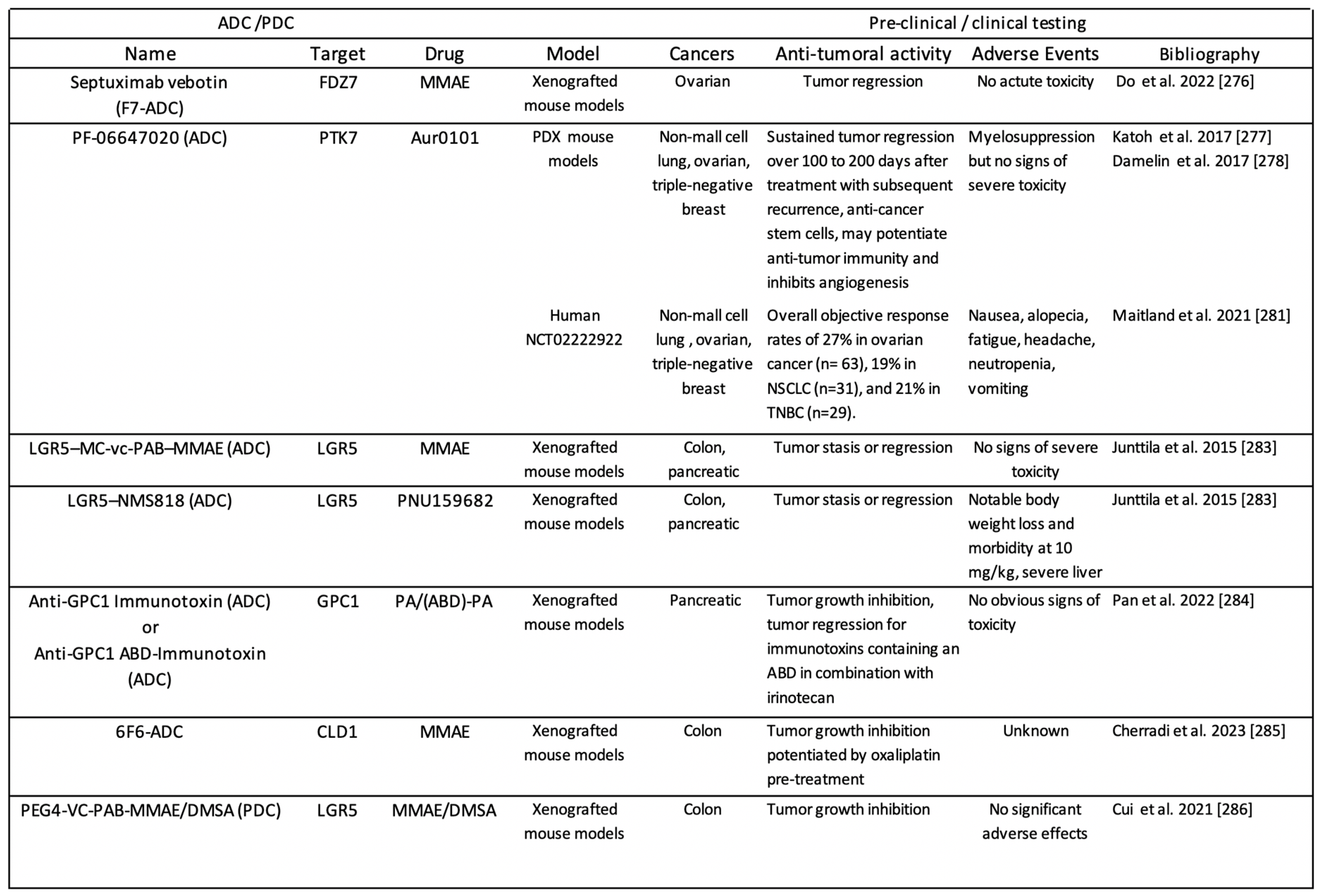
3.3.1. ADC-Based Approaches
3.3.2. Nanovectorization-Based Approaches
3.4. Patient Profiling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Van Ooyen, A.; Nusse, R. Structure and Nucleotide Sequence of the Putative Mammary Oncogene Int-1; Proviral Insertions Leave the Protein-Encoding Domain Intact. Cell 1984, 39, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Bittner, J.J. Some Possible Effects of Nursing on the Mammary Gland Tumor Incidence in Mice. Science 1936, 84, 162. [Google Scholar] [CrossRef] [PubMed]
- Lyons, M.J.; Moore, D.H. Purification of the Mouse Mammary Tumour Virus. Nature 1962, 194, 1141–1142. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Varmus, H.E. Many Tumors Induced by the Mouse Mammary Tumor Virus Contain a Provirus Integrated in the Same Region of the Host Genome. Cell 1982, 31, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Basham, K.J.; Rodriguez, S.; Turcu, A.F.; Lerario, A.M.; Logan, C.Y.; Rysztak, M.R.; Gomez-Sanchez, C.E.; Breault, D.T.; Koo, B.-K.; Clevers, H.; et al. A ZNRF3-Dependent Wnt/β-Catenin Signaling Gradient Is Required for Adrenal Homeostasis. Genes Dev. 2019, 33, 209–220. [Google Scholar] [CrossRef] [PubMed]
- van Ooyen, A.; Kwee, V.; Nusse, R. The Nucleotide Sequence of the Human Int-1 Mammary Oncogene; Evolutionary Conservation of Coding and Non-Coding Sequences. EMBO J. 1985, 4, 2905–2909. [Google Scholar] [CrossRef] [PubMed]
- Fung, Y.-K.T.; Shackleford, G.M.; Brown, A.M.C.; Sanders, G.S.; Varmus, H.E. Nucleotide Sequence and Expression In Vitro of cDNA Derived from mRNA of int-1, a Provirally Activated Mouse Mammary Oncogene. Mol. Cell. Biol. 1985, 5, 3337–3344. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, A.S.; Grosschedl, R.; Guzman, R.C.; Parslow, T.; Varmus, H.E. Expression of the Int-1 Gene in Transgenic Mice Is Associated with Mammary Gland Hyperplasia and Adenocarcinomas in Male and Female Mice. Cell 1988, 55, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.R.; Capecchi, M.R. Targeted Disruption of the Murine Int-1 Proto-Oncogene Resulting in Severe Abnormalities in Midbrain and Cerebellar Development. Nature 1990, 346, 847–850. [Google Scholar] [CrossRef]
- Gavin, B.J.; McMahon, J.A.; McMahon, A.P. Expression of Multiple Novel Wnt-1/Int-1-Related Genes during Fetal and Adult Mouse Development. Genes Dev. 1990, 4, 2319–2332. [Google Scholar] [CrossRef]
- Yost, C.; Torres, M.; Miller, J.R.; Huang, E.; Kimelman, D.; Moon, R.T. The Axis-Inducing Activity, Stability, and Subcellular Distribution of Beta-Catenin Is Regulated in Xenopus Embryos by Glycogen Synthase Kinase 3. Genes Dev. 1996, 10, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Brunner, E.; Peter, O.; Schweizer, L.; Basler, K. Pangolinencodes a Lef-1 Homologue That Acts Downstream of Armadillo to Transduce the Wingless Signal in Drosophila. Nature 1997, 385, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Van De Wetering, M. TCF/LEF Factors Earn Their Wings. Trends Genet. 1997, 13, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Hamada, F.; Tomoyasu, Y.; Takatsu, Y.; Nakamura, M.; Nagai, S.; Suzuki, A.; Fujita, F.; Shibuya, H.; Toyoshima, K.; Ueno, N.; et al. Negative Regulation of Wingless Signaling by D-Axin, a Drosophila Homolog of Axin. Science 1999, 283, 1739–1742. [Google Scholar] [CrossRef]
- Otero, L.; Lacunza, E.; Vasquez, V.; Arbelaez, V.; Cardier, F.; González, F. Variations in AXIN2 Predict Risk and Prognosis of Colorectal Cancer. BDJ Open 2019, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Groden, J.; Thliveris, A.; Samowitz, W.; Carlson, M.; Gelbert, L.; Albertsen, H.; Joslyn, G.; Stevens, J.; Spirio, L.; Robertson, M.; et al. Identification and Characterization of the Familial Adenomatous Polyposis Coli Gene. Cell 1991, 66, 589–600. [Google Scholar] [CrossRef]
- Kinzler, K.W.; Nilbert, M.C.; Su, L.-K.; Vogelstein, B.; Bryan, T.M.; Levy, D.B.; Smith, K.J.; Preisinger, A.C.; Hedge, P.; McKechnie, D.; et al. Identification of FAP Locus Genes from Chromosome 5q21. Science 1991, 253, 661–665. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Ando, H.; Nagase, H.; Nishisho, I.; Horii, A.; Miki, Y.; Mori, T.; Utsunomiya, J.; Baba, S.; Petersen, G. Germ-Line Mutations of the APC Gene in 53 Familial Adenomatous Polyposis Patients. Proc. Natl. Acad. Sci. USA 1992, 89, 4452–4456. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Iwao, K.; Nagasawa, Y.; Aihara, T.; Sasaki, Y.; Imaoka, S.; Murata, M.; Shimano, T.; Nakamura, Y. Activation of the Beta-Catenin Gene in Primary Hepatocellular Carcinomas by Somatic Alterations Involving Exon 3. Cancer Res. 1998, 58, 2524–2527. [Google Scholar] [PubMed]
- Kennerdell, J.R.; Carthew, R.W. Use of dsRNA-Mediated Genetic Interference to Demonstrate That Frizzled and Frizzled 2 Act in the Wingless Pathway. Cell 1998, 95, 1017–1026. [Google Scholar] [CrossRef]
- Liu, W.; Dong, X.; Mai, M.; Seelan, R.S.; Taniguchi, K.; Krishnadath, K.K.; Halling, K.C.; Cunningham, J.M.; Boardman, L.A.; Qian, C.; et al. Mutations in AXIN2 Cause Colorectal Cancer with Defective Mismatch Repair by Activating Beta-Catenin/TCF Signalling. Nat. Genet. 2000, 26, 146–147. [Google Scholar] [CrossRef] [PubMed]
- Lammi, L.; Arte, S.; Somer, M.; Järvinen, H.; Lahermo, P.; Thesleff, I.; Pirinen, S.; Nieminen, P. Mutations in AXIN2 Cause Familial Tooth Agenesis and Predispose to Colorectal Cancer. Am. J. Human. Genet. 2004, 74, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Park, W.S.; Nam, S.W.; Kim, S.Y.; Lee, S.H.; Yoo, N.J.; Lee, J.Y.; Park, C.K. Mutations of Beta-Catenin and AXIN I Genes Are a Late Event in Human Hepatocellular Carcinogenesis. Liver Int. 2005, 25, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Seshagiri, S.; Stawiski, E.W.; Durinck, S.; Modrusan, Z.; Storm, E.E.; Conboy, C.B.; Chaudhuri, S.; Guan, Y.; Janakiraman, V.; Jaiswal, B.S.; et al. Recurrent R-Spondin Fusions in Colon Cancer. Nature 2012, 488, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Hao, H.-X.; Growney, J.D.; Woolfenden, S.; Bottiglio, C.; Ng, N.; Lu, B.; Hsieh, M.H.; Bagdasarian, L.; Meyer, R.; et al. Inactivating Mutations of RNF43 Confer Wnt Dependency in Pancreatic Ductal Adenocarcinoma. Proc. Natl. Acad. Sci. USA 2013, 110, 12649–12654. [Google Scholar] [CrossRef] [PubMed]
- Sekine, S.; Yamashita, S.; Tanabe, T.; Hashimoto, T.; Yoshida, H.; Taniguchi, H.; Kojima, M.; Shinmura, K.; Saito, Y.; Hiraoka, N.; et al. Frequent PTPRK-RSPO3 Fusions and RNF43 Mutations in Colorectal Traditional Serrated Adenoma: RSPO3 Fusions and RNF43 Mutations in Colorectal TSA. J. Pathol. 2016, 239, 133–138. [Google Scholar] [CrossRef]
- Neumeyer, V.; Grandl, M.; Dietl, A.; Brutau-Abia, A.; Allgäuer, M.; Kalali, B.; Zhang, Y.; Pan, K.-F.; Steiger, K.; Vieth, M.; et al. Loss of Endogenous RNF43 Function Enhances Proliferation and Tumour Growth of Intestinal and Gastric Cells. Carcinogenesis 2019, 40, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Yu, M.; Fan, J.; Wang, H.; Zhao, P.; Zhao, G.; Zeng, W.; Chen, C.; Wang, Y.; Wang, A.; et al. Canonical and Noncanonical Wnt Signaling: Multilayered Mediators, Signaling Mechanisms and Major Signaling Crosstalk. Genes Dis. 2024, 11, 103–134. [Google Scholar] [CrossRef] [PubMed]
- Pandur, P.; Maurus, D.; Kühl, M. Increasingly Complex: New Players Enter the Wnt Signaling Network. BioEssays 2002, 24, 881–884. [Google Scholar] [CrossRef]
- Park, W.-J.; Liu, J.; Adler, P.N. The Frizzled Gene of Drosophila Encodes a Membrane Protein with an Odd Number of Transmembrane Domains. Mech. Dev. 1994, 45, 127–137. [Google Scholar] [CrossRef]
- Vinson, C.R.; Conover, S.; Adler, P.N. A Drosophila Tissue Polarity Locus Encodes a Protein Containing Seven Potential Transmembrane Domains. Nature 1989, 338, 263–264. [Google Scholar] [CrossRef]
- Dijksterhuis, J.P.; Baljinnyam, B.; Stanger, K.; Sercan, H.O.; Ji, Y.; Andres, O.; Rubin, J.S.; Hannoush, R.N.; Schulte, G. Systematic Mapping of WNT-FZD Protein Interactions Reveals Functional Selectivity by Distinct WNT-FZD Pairs. J. Biol. Chem. 2015, 290, 6789–6798. [Google Scholar] [CrossRef]
- Rulifson, E.J.; Wu, C.H.; Nusse, R. Pathway Specificity by the Bifunctional Receptor Frizzled Is Determined by Affinity for Wingless. Mol. Cell 2000, 6, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Janda, C.Y.; Waghray, D.; Levin, A.M.; Thomas, C.; Garcia, K.C. Structural Basis of Wnt Recognition by Frizzled. Science 2012, 337, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, N.; Hwang, S.; Waghray, D.; Hansen, S.; Jude, K.M.; Wang, N.; Miao, Y.; Glassman, C.R.; Caveney, N.A.; Janda, C.Y.; et al. Structure of the Wnt-Frizzled-LRP6 Initiation Complex Reveals the Basis for Coreceptor Discrimination. Proc. Natl. Acad. Sci. USA 2023, 120, e2218238120. [Google Scholar] [CrossRef]
- Van Neerven, S.M.; De Groot, N.E.; Nijman, L.E.; Scicluna, B.P.; Van Driel, M.S.; Lecca, M.C.; Warmerdam, D.O.; Kakkar, V.; Moreno, L.F.; Vieira Braga, F.A.; et al. Apc-Mutant Cells Act as Supercompetitors in Intestinal Tumour Initiation. Nature 2021, 594, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Verkaar, F.; Zaman, G.J.R. A Model for Signaling Specificity of Wnt/Frizzled Combinations through Co-receptor Recruitment. FEBS Lett. 2010, 584, 3850–3854. [Google Scholar] [CrossRef]
- Kikuchi, A.; Yamamoto, H.; Kishida, S. Multiplicity of the Interactions of Wnt Proteins and Their Receptors. Cell. Signal. 2007, 19, 659–671. [Google Scholar] [CrossRef]
- Mao, J.; Wang, J.; Liu, B.; Pan, W.; Farr, G.H.; Flynn, C.; Yuan, H.; Takada, S.; Kimelman, D.; Li, L.; et al. Low-Density Lipoprotein Receptor-Related Protein-5 Binds to Axin and Regulates the Canonical Wnt Signaling Pathway. Mol. Cell 2001, 7, 801–809. [Google Scholar] [CrossRef]
- Cadigan, K.M.; Liu, Y.I. Wnt Signaling: Complexity at the Surface. J. Cell Sci. 2006, 119, 395–402. [Google Scholar] [CrossRef]
- Billiard, J.; Way, D.S.; Seestaller-Wehr, L.M.; Moran, R.A.; Mangine, A.; Bodine, P.V.N. The Orphan Receptor Tyrosine Kinase Ror2 Modulates Canonical Wnt Signaling in Osteoblastic Cells. Mol. Endocrinol. 2005, 19, 90–101. [Google Scholar] [CrossRef]
- Patthy, L. The WIF Module. Trends Biochem. Sci. 2000, 25, 12–13. [Google Scholar] [CrossRef]
- Menck, K.; Heinrichs, S.; Baden, C.; Bleckmann, A. The WNT/ROR Pathway in Cancer: From Signaling to Therapeutic Intervention. Cells 2021, 10, 142. [Google Scholar] [CrossRef]
- Shi, F.; Mendrola, J.M.; Sheetz, J.B.; Wu, N.; Sommer, A.; Speer, K.F.; Noordermeer, J.N.; Kan, Z.-Y.; Perry, K.; Englander, S.W.; et al. ROR and RYK Extracellular Region Structures Suggest That Receptor Tyrosine Kinases Have Distinct WNT-Recognition Modes. Cell Rep. 2021, 37, 109834. [Google Scholar] [CrossRef]
- Ren, Q.; Chen, J.; Liu, Y. LRP5 and LRP6 in Wnt Signaling: Similarity and Divergence. Front. Cell Dev. Biol. 2021, 9, 670960. [Google Scholar] [CrossRef]
- Green, J.; Nusse, R.; Van Amerongen, R. The Role of Ryk and Ror Receptor Tyrosine Kinases in Wnt Signal Transduction. Cold Spring Harb. Perspect. Biol. 2014, 6, a009175. [Google Scholar] [CrossRef] [PubMed]
- Bafico, A.; Liu, G.; Yaniv, A.; Gazit, A.; Aaronson, S.A. Novel Mechanism of Wnt Signalling Inhibition Mediated by Dickkopf-1 Interaction with LRP6/Arrow. Nat. Cell Biol. 2001, 3, 683–686. [Google Scholar] [CrossRef]
- Mao, B.; Wu, W.; Li, Y.; Hoppe, D.; Stannek, P.; Glinka, A.; Niehrs, C. LDL-Receptor-Related Protein 6 Is a Receptor for Dickkopf Proteins. Nature 2001, 411, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Semënov, M.V.; Tamai, K.; Brott, B.K.; Kühl, M.; Sokol, S.; He, X. Head Inducer Dickkopf-1 Is a Ligand for Wnt Coreceptor LRP6. Curr. Biol. 2001, 11, 951–961. [Google Scholar] [CrossRef]
- Oishi, I.; Suzuki, H.; Onishi, N.; Takada, R.; Kani, S.; Ohkawara, B.; Koshida, I.; Suzuki, K.; Yamada, G.; Schwabe, G.C.; et al. The Receptor Tyrosine Kinase Ror2 Is Involved in Non-Canonical Wnt5a/JNK Signalling Pathway. Genes Cells 2003, 8, 645–654. [Google Scholar] [CrossRef]
- Yoda, A.; Oishi, I.; Minami, Y. Expression and Function of the Ror-Family Receptor Tyrosine Kinases during Development: Lessons from Genetic Analyses of Nematodes, Mice, and Humans. J. Recept. Signal Transduct. Res. 2003, 23, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, S.; McKinnon, R.D.; Kokel, M.; Thomas, J.B. Wnt-Mediated Axon Guidance via the Drosophila Derailed Receptor. Nature 2003, 422, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Tamai, K.; Doble, B.; Li, S.; Huang, H.; Habas, R.; Okamura, H.; Woodgett, J.; He, X. A Dual-Kinase Mechanism for Wnt Co-Receptor Phosphorylation and Activation. Nature 2005, 438, 873–877. [Google Scholar] [CrossRef]
- Li, V.S.W.; Ng, S.S.; Boersema, P.J.; Low, T.Y.; Karthaus, W.R.; Gerlach, J.P.; Mohammed, S.; Heck, A.J.R.; Maurice, M.M.; Mahmoudi, T.; et al. Wnt Signaling through Inhibition of β-Catenin Degradation in an Intact Axin1 Complex. Cell 2012, 149, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Aberle, H.; Bauer, A.; Stappert, J.; Kispert, A.; Kemler, R. β-Catenin Is a Target for the Ubiquitin–Proteasome Pathway. EMBO J. 1997, 16, 3797–3804. [Google Scholar] [CrossRef] [PubMed]
- Orford, K.; Crockett, C.; Jensen, J.P.; Weissman, A.M.; Byers, S.W. Serine Phosphorylation-Regulated Ubiquitination and Degradation of β-Catenin. J. Biol. Chem. 1997, 272, 24735–24738. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, K.N.; Bonello, T.T.; Zhang, S.; Williams, C.E.; Roberts, D.M.; McKay, D.J.; Peifer, M. Supramolecular Assembly of the Beta-Catenin Destruction Complex and the Effect of Wnt Signaling on Its Localization, Molecular Size, and Activity in Vivo. PLoS Genet. 2018, 14, e1007339. [Google Scholar] [CrossRef]
- Munemitsu, S.; Albert, I.; Souza, B.; Rubinfeld, B.; Polakis, P. Regulation of Intracellular Beta-Catenin Levels by the Adenomatous Polyposis Coli (APC) Tumor-Suppressor Protein. Proc. Natl. Acad. Sci. USA 1995, 92, 3046–3050. [Google Scholar] [CrossRef] [PubMed]
- Behrens, J.; Jerchow, B.-A.; Würtele, M.; Grimm, J.; Asbrand, C.; Wirtz, R.; Kühl, M.; Wedlich, D.; Birchmeier, W. Functional Interaction of an Axin Homolog, Conductin, with β-Catenin, APC, and GSK3β. Science 1998, 280, 596–599. [Google Scholar] [CrossRef]
- Hart, M.J.; De Los Santos, R.; Albert, I.N.; Rubinfeld, B.; Polakis, P. Downregulation of β-Catenin by Human Axin and Its Association with the APC Tumor Suppressor, β-Catenin and GSK3β. Curr. Biol. 1998, 8, 573–581. [Google Scholar] [CrossRef]
- Kishida, S.; Yamamoto, H.; Ikeda, S.; Kishida, M.; Sakamoto, I.; Koyama, S.; Kikuchi, A. Axin, a Negative Regulator of the Wnt Signaling Pathway, Directly Interacts with Adenomatous Polyposis Coli and Regulates the Stabilization of β-Catenin. J. Biol. Chem. 1998, 273, 10823–10826. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Hamada, F.; Ishidate, T.; Anai, K.; Kawahara, K.; Toyoshima, K.; Akiyama, T. Axin, an Inhibitor of the Wnt Signalling Pathway, Interacts with Β-catenin, GSK-3β and APC and Reduces the Β-catenin Level. Genes Cells 1998, 3, 395–403. [Google Scholar] [CrossRef]
- Spink, K.E.; Polakis, P.; Weis, W.I. Structural Basis of the Axin–Adenomatous Polyposis Coli Interaction. EMBO J. 2000, 19, 2270–2279. [Google Scholar] [CrossRef] [PubMed]
- Amit, S.; Hatzubai, A.; Birman, Y.; Andersen, J.S.; Ben-Shushan, E.; Mann, M.; Ben-Neriah, Y.; Alkalay, I. Axin-Mediated CKI Phosphorylation of β-Catenin at Ser 45: A Molecular Switch for the Wnt Pathway. Genes Dev. 2002, 16, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Semenov, M.; Han, C.; Baeg, G.H.; Tan, Y.; Zhang, Z.; Lin, X.; He, X. Control of Beta-Catenin Phosphorylation/Degradation by a Dual-Kinase Mechanism. Cell 2002, 108, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Salic, A.; Krüger, R.; Heinrich, R.; Kirschner, M.W. The Roles of APC and Axin Derived from Experimental and Theoretical Analysis of the Wnt Pathway. PLoS Biol. 2003, 1, E10. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.M.; Pronobis, M.I.; Poulton, J.S.; Waldmann, J.D.; Stephenson, E.M.; Hanna, S.; Peifer, M. Deconstructing the SScatenin Destruction Complex: Mechanistic Roles for the Tumor Suppressor APC in Regulating Wnt Signaling. Mol. Biol. Cell 2011, 22, 1845–1863. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Fu, C.; Ishikawa, S.; Stella, A.; Kojima, M.; Shitoh, K.; Schreiber, E.M.; Day, B.W.; Liu, B. APC Is Essential for Targeting Phosphorylated β-Catenin to the SCFβ-TrCP Ubiquitin Ligase. Mol. Cell 2008, 32, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.-C.; Tonozuka, T.; Stamos, J.L.; Choi, H.-J.; Weis, W.I. Mechanism of Phosphorylation-Dependent Binding of APC to β-Catenin and Its Role in β-Catenin Degradation. Mol. Cell 2004, 15, 511–521. [Google Scholar] [CrossRef]
- Tauriello, D.V.F.; Jordens, I.; Kirchner, K.; Slootstra, J.W.; Kruitwagen, T.; Bouwman, B.A.M.; Noutsou, M.; Rüdiger, S.G.D.; Schwamborn, K.; Schambony, A.; et al. Wnt/β-Catenin Signaling Requires Interaction of the Dishevelled DEP Domain and C Terminus with a Discontinuous Motif in Frizzled. Proc. Natl. Acad. Sci. USA 2012, 109, E812–E820. [Google Scholar] [CrossRef]
- Finch, P.W.; He, X.; Kelley, M.J.; Uren, A.; Schaudies, R.P.; Popescu, N.C.; Rudikoff, S.; Aaronson, S.A.; Varmus, H.E.; Rubin, J.S. Purification and Molecular Cloning of a Secreted, Frizzled-Related Antagonist of Wnt Action. Proc. Natl. Acad. Sci. USA 1997, 94, 6770–6775. [Google Scholar] [CrossRef] [PubMed]
- Leyns, L.; Bouwmeester, T.; Kim, S.H.; Piccolo, S.; De Robertis, E.M. Frzb-1 Is a Secreted Antagonist of Wnt Signaling Expressed in the Spemann Organizer. Cell 1997, 88, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Rattner, A.; Hsieh, J.C.; Smallwood, P.M.; Gilbert, D.J.; Copeland, N.G.; Jenkins, N.A.; Nathans, J. A Family of Secreted Proteins Contains Homology to the Cysteine-Rich Ligand-Binding Domain of Frizzled Receptors. Proc. Natl. Acad. Sci. USA 1997, 94, 2859–2863. [Google Scholar] [CrossRef]
- Cliffe, A.; Hamada, F.; Bienz, M. A Role of Dishevelled in Relocating Axin to the Plasma Membrane during Wingless Signaling. Curr. Biol. 2003, 13, 960–966. [Google Scholar] [CrossRef]
- Schwarz-Romond, T.; Metcalfe, C.; Bienz, M. Dynamic Recruitment of Axin by Dishevelled Protein Assemblies. J. Cell Sci. 2007, 120, 2402–2412. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/Beta-Catenin Signaling: Components, Mechanisms, and Diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- The Wnt Home Page. Available online: https://web.stanford.edu/group/nusselab/cgi-bin/wnt/target_genes (accessed on 1 April 2024).
- Van Tienen, L.M.; Mieszczanek, J.; Fiedler, M.; Rutherford, T.J.; Bienz, M. Constitutive Scaffolding of Multiple Wnt Enhanceosome Components by Legless/BCL9. eLife 2017, 6, e20882. [Google Scholar] [CrossRef] [PubMed]
- Barker, N. The Chromatin Remodelling Factor Brg-1 Interacts with Beta-Catenin to Promote Target Gene Activation. EMBO J. 2001, 20, 4935–4943. [Google Scholar] [CrossRef]
- Townsley, F.M.; Thompson, B.; Bienz, M. Pygopus Residues Required for Its Binding to Legless Are Critical for Transcription and Development. J. Biol. Chem. 2004, 279, 5177–5183. [Google Scholar] [CrossRef]
- Hecht, A.; Vleminckx, K.; Stemmler, M.P.; van Roy, F.; Kemler, R. The P300/CBP Acetyltransferases Function as Transcriptional Coactivators of Beta-Catenin in Vertebrates. EMBO J. 2000, 19, 1839–1850. [Google Scholar] [CrossRef]
- Sansom, O.J.; Meniel, V.S.; Muncan, V.; Phesse, T.J.; Wilkins, J.A.; Reed, K.R.; Vass, J.K.; Athineos, D.; Clevers, H.; Clarke, A.R. Myc Deletion Rescues Apc Deficiency in the Small Intestine. Nature 2007, 446, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, A.-B.; Cadigan, K.M. Wnt Target Genes and Where to Find Them. F1000Research 2017, 6, 746. [Google Scholar] [CrossRef] [PubMed]
- He, T.C.; Sparks, A.B.; Rago, C.; Hermeking, H.; Zawel, L.; da Costa, L.T.; Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Identification of C-MYC as a Target of the APC Pathway. Science 1998, 281, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Jackstadt, R.; Hodder, M.C.; Sansom, O.J. WNT and β-Catenin in Cancer: Genes and Therapy. Annu. Rev. Cancer Biol. 2020, 4, 177–196. [Google Scholar] [CrossRef]
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/β-Catenin Signaling in Cancers and Targeted Therapies. Sig. Transduct. Target. Ther. 2021, 6, 307. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Kurzrock, R. Targeting the Wnt/Beta-Catenin Pathway in Cancer: Update on Effectors and Inhibitors. Cancer Treat. Rev. 2018, 62, 50–60. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-Catenin Signaling Pathway in Cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Wang, Z.; Moscoso-Castro, M.; D’Souza, P.; Lei, C.; Xu, J.; Gu, J. Biology Drives the Discovery of Bispecific Antibodies as Innovative Therapeutics. Antib. Ther. 2020, 3, 18–62. [Google Scholar] [CrossRef]
- Jimeno, A.; Gordon, M.; Chugh, R.; Messersmith, W.; Mendelson, D.; Dupont, J.; Stagg, R.; Kapoun, A.M.; Xu, L.; Uttamsingh, S.; et al. A First-in-Human Phase I Study of the Anticancer Stem Cell Agent Ipafricept (OMP-54F28), a Decoy Receptor for Wnt Ligands, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2017, 23, 7490–7497. [Google Scholar] [CrossRef]
- Dotan, E.; Cardin, D.B.; Lenz, H.-J.; Messersmith, W.; O’Neil, B.; Cohen, S.J.; Denlinger, C.S.; Shahda, S.; Astsaturov, I.; Kapoun, A.M.; et al. Phase Ib Study of Wnt Inhibitor Ipafricept with Gemcitabine and Nab-Paclitaxel in Patients with Previously Untreated Stage IV Pancreatic Cancer. Clin. Cancer Res. 2020, 26, 5348–5357. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Gunderson, C.C.; Sabbatini, P.; McMeekin, D.S.; Mantia-Smaldone, G.; Burger, R.A.; Morgan, M.A.; Kapoun, A.M.; Brachmann, R.K.; Stagg, R.; et al. A Phase 1b Dose Escalation Study of Ipafricept (OMP 54F28) in Combination with Paclitaxel and Carboplatin in Patients with Recurrent Platinum-Sensitive Ovarian Cancer. Gynecol. Oncol. 2019, 154, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Vlashi, R.; Zhang, X.; Wu, M.; Chen, G. Wnt Signaling: Essential Roles in Osteoblast Differentiation, Bone Metabolism and Therapeutic Implications for Bone and Skeletal Disorders. Genes Dis. 2023, 10, 1291–1317. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.M.; Cancilla, B.; Yeung, V.P.; Cattaruzza, F.; Chartier, C.; Murriel, C.L.; Cain, J.; Tam, R.; Cheng, C.-Y.; Evans, J.W.; et al. WNT Antagonists Exhibit Unique Combinatorial Antitumor Activity with Taxanes by Potentiating Mitotic Cell Death. Sci. Adv. 2017, 3, e1700090. [Google Scholar] [CrossRef]
- OncoMed, P.I. OncoMed Pre-Announces 2014 Year-End Cash Balance and Provides 2015 Guidance. Available online: https://www.globenewswire.com/news-release/2015/01/12/696685/10115034/en/oncomed-pre-announces-2014-year-end-cash-balance-and-provides-2015-guidance.html (accessed on 15 April 2024).
- Smith, D.C.; Rosen, L.S.; Chugh, R.; Goldman, J.W.; Xu, L.; Kapoun, A.; Brachmann, R.K.; Dupont, J.; Stagg, R.J.; Tolcher, A.W.; et al. First-in-Human Evaluation of the Human Monoclonal Antibody Vantictumab (OMP-18R5; Anti-Frizzled) Targeting the WNT Pathway in a Phase I Study for Patients with Advanced Solid Tumors. J. Clin. Oncol. 2013, 31, 2540. [Google Scholar] [CrossRef]
- Diamond, J.R.; Becerra, C.; Richards, D.; Mita, A.; Osborne, C.; O’Shaughnessy, J.; Zhang, C.; Henner, R.; Kapoun, A.M.; Xu, L.; et al. Phase Ib Clinical Trial of the Anti-Frizzled Antibody Vantictumab (OMP-18R5) plus Paclitaxel in Patients with Locally Advanced or Metastatic HER2-Negative Breast Cancer. Breast Cancer Res. Treat. 2020, 184, 53–62. [Google Scholar] [CrossRef]
- Davis, S.L.; Cardin, D.B.; Shahda, S.; Lenz, H.-J.; Dotan, E.; O’Neil, B.H.; Kapoun, A.M.; Stagg, R.J.; Berlin, J.; Messersmith, W.A.; et al. A Phase 1b Dose Escalation Study of Wnt Pathway Inhibitor Vantictumab in Combination with Nab-Paclitaxel and Gemcitabine in Patients with Previously Untreated Metastatic Pancreatic Cancer. Investig. New Drugs 2020, 38, 821–830. [Google Scholar] [CrossRef]
- Giraudet, A.-L.; Cassier, P.A.; Iwao-Fukukawa, C.; Garin, G.; Badel, J.-N.; Kryza, D.; Chabaud, S.; Gilles-Afchain, L.; Clapisson, G.; Desuzinges, C.; et al. A First-in-Human Study Investigating Biodistribution, Safety and Recommended Dose of a New Radiolabeled MAb Targeting FZD10 in Metastatic Synovial Sarcoma Patients. BMC Cancer 2018, 18, 646. [Google Scholar] [CrossRef]
- Inglis, D.J.; Licari, J.; Georgiou, K.R.; Wittwer, N.L.; Hamilton, R.W.; Beaumont, D.M.; Scherer, M.A.; Lavranos, T.C. Abstract 3910: Characterization of BNC101 a Human Specific Monoclonal Antibody Targeting the GPCR LGR5: First-in-Human Evidence of Target Engagement. Cancer Res. 2018, 78, 3910. [Google Scholar] [CrossRef]
- Carmon, K.S.; Lin, Q.; Gong, X.; Thomas, A.; Liu, Q. LGR5 Interacts and Cointernalizes with Wnt Receptors To Modulate Wnt/β-Catenin Signaling. Mol. Cell. Biol. 2012, 32, 2054–2064. [Google Scholar] [CrossRef]
- Xu, L.; Lin, W.; Wen, L.; Li, G. Lgr5 in Cancer Biology: Functional Identification of Lgr5 in Cancer Progression and Potential Opportunities for Novel Therapy. Stem Cell Res. Ther. 2019, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Élez, E.; Lenz, H.-J.; De Jonge, M.; Yaeger, R.; Doi, T.; Pronk, L.; Teufel, M.; Marzin, K.; Tabernero, J. Abstract CT514: A Phase I, Open-Label, Dose-Escalation Study Investigating a Low-Density Lipoprotein Receptor-Related Protein (LRP) 5/6 Inhibitor, BI 905677, in Patients with Advanced Solid Tumors. Cancer Res. 2022, 82, CT514. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Z.; Yu, Y.; Chu, H.Y.; Yu, S.; Yao, S.; Zhang, G.; Zhang, B.-T. Drug Discovery of DKK1 Inhibitors. Front. Pharmacol. 2022, 13, 847387. [Google Scholar] [CrossRef] [PubMed]
- Ahn, V.E.; Chu, M.L.-H.; Choi, H.-J.; Tran, D.; Abo, A.; Weis, W.I. Structural Basis of Wnt Signaling Inhibition by Dickkopf Binding to LRP5/6. Dev. Cell 2011, 21, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Wall, J.A.; Klempner, S.J.; Arend, R.C. The Anti-DKK1 Antibody DKN-01 as an Immunomodulatory Combination Partner for the Treatment of Cancer. Expert. Opin. Investig. Drugs 2020, 29, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Goyal, L.; Sirard, C.; Schrag, M.; Kagey, M.H.; Eads, J.R.; Stein, S.; El-Khoueiry, A.B.; Manji, G.A.; Abrams, T.A.; Khorana, A.A.; et al. Phase I and Biomarker Study of the Wnt Pathway Modulator DKN-01 in Combination with Gemcitabine/Cisplatin in Advanced Biliary Tract Cancer. Clin. Cancer Res. 2020, 26, 6158–6167. [Google Scholar] [CrossRef]
- Turkes, F.S.; Crux, R.; Tran, A.; Cartwright, E.; Rana, I.; Johnston, E.; Dunlop, A.; Thomas, J.; Smith, A.; Smyth, E.; et al. 1253P Safety and Efficacy of Wnt Inhibition with a DKK1 Inhibitor, DKN-01, in Combination with Atezolizumab in Patients with Advanced Oesophagogastric Adenocarcinoma: Phase IIa Results of the WAKING Trial. Ann. Oncol. 2022, 33, S1120–S1121. [Google Scholar] [CrossRef]
- Heath, D.J.; Chantry, A.D.; Buckle, C.H.; Coulton, L.; Shaughnessy, J.D.; Evans, H.R.; Snowden, J.A.; Stover, D.R.; Vanderkerken, K.; Croucher, P.I. Inhibiting Dickkopf-1 (Dkk1) Removes Suppression of Bone Formation and Prevents the Development of Osteolytic Bone Disease in Multiple Myeloma. J. Bone Miner. Res. 2009, 24, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Fulciniti, M.; Tassone, P.; Hideshima, T.; Vallet, S.; Nanjappa, P.; Ettenberg, S.A.; Shen, Z.; Patel, N.; Tai, Y.; Chauhan, D.; et al. Anti-DKK1 mAb (BHQ880) as a Potential Therapeutic Agent for Multiple Myeloma. Blood 2009, 114, 371–379. [Google Scholar] [CrossRef]
- Iyer, S.P.; Beck, J.T.; Stewart, A.K.; Shah, J.; Kelly, K.R.; Isaacs, R.; Bilic, S.; Sen, S.; Munshi, N.C. A Phase IB Multicentre Dose-determination Study of BHQ 880 in Combination with Anti-myeloma Therapy and Zoledronic Acid in Patients with Relapsed or Refractory Multiple Myeloma and Prior Skeletal-related Events. Br. J. Haematol. 2014, 167, 366–375. [Google Scholar] [CrossRef]
- Fischer, M.M.; Yeung, V.P.; Cattaruzza, F.; Hussein, R.; Yen, W.-C.; Murriel, C.; Evans, J.W.; O’Young, G.; Brunner, A.L.; Wang, M.; et al. RSPO3 Antagonism Inhibits Growth and Tumorigenicity in Colorectal Tumors Harboring Common Wnt Pathway Mutations. Sci. Rep. 2017, 7, 15270. [Google Scholar] [CrossRef]
- Flanagan, D.J.; Pentinmikko, N.; Luopajärvi, K.; Willis, N.J.; Gilroy, K.; Raven, A.P.; Mcgarry, L.; Englund, J.I.; Webb, A.T.; Scharaw, S.; et al. NOTUM from Apc-Mutant Cells Biases Clonal Competition to Initiate Cancer. Nature 2021, 594, 430–435. [Google Scholar] [CrossRef]
- Hao, H.-X.; Jiang, X.; Cong, F. Control of Wnt Receptor Turnover by R-Spondin-ZNRF3/RNF43 Signaling Module and Its Dysregulation in Cancer. Cancers 2016, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-B.; Kim, J.-W.; Baek, K.-H. Regulation of Wnt Signaling through Ubiquitination and Deubiquitination in Cancers. Int. J. Mol. Sci. 2020, 21, 3904. [Google Scholar] [CrossRef]
- Yan, K.S.; Janda, C.Y.; Chang, J.; Zheng, G.X.Y.; Larkin, K.A.; Luca, V.C.; Chia, L.A.; Mah, A.T.; Han, A.; Terry, J.M.; et al. Non-Equivalence of Wnt and R-Spondin Ligands during Lgr5+ Intestinal Stem-Cell Self-Renewal. Nature 2017, 545, 238–242. [Google Scholar] [CrossRef]
- Gong, X.; Yi, J.; Carmon, K.S.; Crumbley, C.A.; Xiong, W.; Thomas, A.; Fan, X.; Guo, S.; An, Z.; Chang, J.T.; et al. Aberrant RSPO3-LGR4 Signaling in Keap1-Deficient Lung Adenocarcinomas Promotes Tumor Aggressiveness. Oncogene 2015, 34, 4692–4701. [Google Scholar] [CrossRef]
- Bendell, J.; Eckhardt, G.S.; Hochster, H.S.; Morris, V.K.; Strickler, J.; Kapoun, A.M.; Wang, M.; Xu, L.; McGuire, K.; Dupont, J.; et al. Initial Results from a Phase 1a/b Study of OMP-131R10, a First-in-Class Anti-RSPO3 Antibody, in Advanced Solid Tumors and Previously Treated Metastatic Colorectal Cancer (CRC). Eur. J. Cancer 2016, 69, S29–S30. [Google Scholar] [CrossRef]
- Zhang, M.; Haughey, M.; Wang, N.-Y.; Blease, K.; Kapoun, A.M.; Couto, S.; Belka, I.; Hoey, T.; Groza, M.; Hartke, J.; et al. Targeting the Wnt Signaling Pathway through R-Spondin 3 Identifies an Anti-Fibrosis Treatment Strategy for Multiple Organs. PLoS ONE 2020, 15, e0229445. [Google Scholar] [CrossRef]
- Herzog, B.H.; Baer, J.M.; Borcherding, N.; Kingston, N.L.; Belle, J.I.; Knolhoff, B.L.; Hogg, G.D.; Ahmad, F.; Kang, L.-I.; Petrone, J.; et al. Tumor-Associated Fibrosis Impairs Immune Surveillance and Response to Immune Checkpoint Blockade in Non–Small Cell Lung Cancer. Sci. Transl. Med. 2023, 15, eadh8005. [Google Scholar] [CrossRef] [PubMed]
- Piersma, B.; Hayward, M.-K.; Weaver, V.M. Fibrosis and Cancer: A Strained Relationship. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2020, 1873, 188356. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Sodji, Q.H.; Oyelere, A.K. Inflammation, Fibrosis and Cancer: Mechanisms, Therapeutic Options and Challenges. Cancers 2022, 14, 552. [Google Scholar] [CrossRef] [PubMed]
- Bayle, E.D.; Svensson, F.; Atkinson, B.N.; Steadman, D.; Willis, N.J.; Woodward, H.L.; Whiting, P.; Vincent, J.-P.; Fish, P.V. Carboxylesterase Notum Is a Druggable Target to Modulate Wnt Signaling. J. Med. Chem. 2021, 64, 4289–4311. [Google Scholar] [CrossRef] [PubMed]
- Kakugawa, S.; Langton, P.F.; Zebisch, M.; Howell, S.A.; Chang, T.-H.; Liu, Y.; Feizi, T.; Bineva, G.; O’Reilly, N.; Snijders, A.P.; et al. Notum Deacylates Wnt Proteins to Suppress Signalling Activity. Nature 2015, 519, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Panchal, S.; Patel, B. Porcupine Inhibitors: Novel and Emerging Anti-Cancer Therapeutics Targeting the Wnt Signaling Pathway. Pharmacol. Res. 2021, 167, 105532. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Wilder, E.; Klingensmith, J.; Zachary, K.; Perrimon, N. The Segment Polarity Gene Porcupine Encodes a Putative Multitransmembrane Protein Involved in Wingless Processing. Genes Dev. 1996, 10, 3116–3128. [Google Scholar] [CrossRef] [PubMed]
- Van Den Heuvel, M.; Harryman-Samos, C.; Klingensmith, J.; Perrimon, N.; Nusse, R. Mutations in the Segment Polarity Genes Wingless and Porcupine Impair Secretion of the Wingless Protein. EMBO J. 1993, 12, 5293–5302. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cao, J.; Zhang, N.; Tu, M.; Xu, F.; Wei, S.; Chen, X.; Xu, Y. Identification of RSPO2 Fusion Mutations and Target Therapy Using a Porcupine Inhibitor. Sci. Rep. 2018, 8, 14244. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pan, S.; Hsieh, M.H.; Ng, N.; Sun, F.; Wang, T.; Kasibhatla, S.; Schuller, A.G.; Li, A.G.; Cheng, D.; et al. Targeting Wnt-Driven Cancer through the Inhibition of Porcupine by LGK974. Proc. Natl. Acad. Sci. USA 2013, 110, 20224–20229. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.-K.; van Es, J.H.; van den Born, M.; Clevers, H. Porcupine Inhibitor Suppresses Paracrine Wnt-Driven Growth of Rnf43;Znrf3-Mutant Neoplasia. Proc. Natl. Acad. Sci. USA 2015, 112, 7548–7550. [Google Scholar] [CrossRef]
- Madan, B.; Ke, Z.; Harmston, N.; Ho, S.Y.; Frois, A.O.; Alam, J.; Jeyaraj, D.A.; Pendharkar, V.; Ghosh, K.; Virshup, I.H.; et al. Wnt Addiction of Genetically Defined Cancers Reversed by PORCN Inhibition. Oncogene 2016, 35, 2197–2207. [Google Scholar] [CrossRef]
- Bhamra, I.; Adams, N.; Armer, R.; Bingham, M.; McKeever, H.; Phillips, C.; Thompson, B.; Woodcock, S. Novel Porcupine (PORCN) Inhibitor RXC004: Evaluation in Models of RNF43 Loss of Function Cancers. J. Clin. Oncol. 2017, 35, e14094. [Google Scholar] [CrossRef]
- Picco, G.; Petti, C.; Centonze, A.; Torchiaro, E.; Crisafulli, G.; Novara, L.; Acquaviva, A.; Bardelli, A.; Medico, E. Loss of AXIN1 Drives Acquired Resistance to WNT Pathway Blockade in Colorectal Cancer Cells Carrying RSPO 3 Fusions. EMBO Mol. Med. 2017, 9, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Rodon, J.; Argilés, G.; Connolly, R.M.; Vaishampayan, U.; De Jonge, M.; Garralda, E.; Giannakis, M.; Smith, D.C.; Dobson, J.R.; McLaughlin, M.E.; et al. Phase 1 Study of Single-Agent WNT974, a First-in-Class Porcupine Inhibitor, in Patients with Advanced Solid Tumours. Br. J. Cancer 2021, 125, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Ng, M.; Subbiah, V.; Messersmith, W.; Teneggi, V.; Diermayr, V.; Ethirajulu, K.; Yeo, P.; Gan, B.H.; Lee, L.H.; et al. Phase I Extension Study of ETC-159 an Oral PORCN Inhibitor Administered with Bone Protective Treatment, in Patients with Advanced Solid Tumours. Ann. Oncol. 2018, 29, ix23–ix24. [Google Scholar] [CrossRef]
- Tan, D.S.P.; Ng, M.C.H.; Subbiah, V.; Messersmith, W.A.; Strickler, J.H.; Diermayr, V.; Cometa, J.; Blanchard, S.; Nellore, R.; Pendharkar, V.; et al. A Phase 1B Dose Escalation Study of ETC-159 in Combination with Pembrolizumab in Advanced or Metastatic Solid Tumours. J. Clin. Oncol. 2023, 41, 2601. [Google Scholar] [CrossRef]
- Giannakis, M.; Le, D.T.; Pishvaian, M.J.; Weinberg, B.A.; Papadopoulos, K.P.; Shen, L.; Gong, J.; Li, J.; Strickler, J.H.; Zhou, A.; et al. Phase 1 Study of WNT Pathway Porcupine Inhibitor CGX1321 and Phase 1b Study of CGX1321 + Pembrolizumab (Pembro) in Patients (Pts) with Advanced Gastrointestinal (GI) Tumors. J. Clin. Oncol. 2023, 41, 3514. [Google Scholar] [CrossRef]
- Pharmaceutical Technology CGX-1321 by Curegenix for Pancreatic Cancer: Likelihood of Approval. Available online: https://www.pharmaceutical-technology.com/data-insights/cgx-1321-curegenix-pancreatic-cancer-likelihood-of-approval/ (accessed on 15 April 2024).
- Kopetz, S.; Morris, V.K.; O’Neil, B.; Bridgewater, J.A.; Graham, J.; Parkes, E.E.; Saunders, M.P.; Asken, E.; Goodwin, L.; Phillips, C.; et al. A Multi-Arm, Phase 2, Open-Label Study to Assess the Efficacy of RXC004 as Monotherapy and in Combination with Nivolumab in Patients with Ring Finger Protein 43 (RNF43) or R-Spondin (RSPO) Aberrated, Metastatic, Microsatellite Stable Colorectal Cancer Following Standard Treatments. J. Clin. Oncol. 2022, 40, TPS3637. [Google Scholar] [CrossRef]
- Yang, Q.; Qin, T.; An, T.; Wu, H.; Xu, G.; Xiang, J.; Lei, K.; Zhang, S.; Xia, J.; Su, G.; et al. Novel PORCN Inhibitor WHN-88 Targets Wnt/β-Catenin Pathway and Prevents the Growth of Wnt-Driven Cancers. Eur. J. Pharmacol. 2023, 945, 175628. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, M.; Barak, L.S. Development of Small Molecules Targeting the Wnt Pathway for the Treatment of Colon Cancer: A High-Throughput Screening Approach. Am. J. Physiol. -Gastrointest. Liver Physiol. 2010, 299, G293–G300. [Google Scholar] [CrossRef]
- Chen, M.; Wang, J.; Lu, J.; Bond, M.C.; Ren, X.-R.; Lyerly, H.K.; Barak, L.S.; Chen, W. The Anti-Helminthic Niclosamide Inhibits Wnt/Frizzled1 Signaling. Biochemistry 2009, 48, 10267–10274. [Google Scholar] [CrossRef]
- Osada, T.; Chen, M.; Yang, X.Y.; Spasojevic, I.; Vandeusen, J.B.; Hsu, D.; Clary, B.M.; Clay, T.M.; Chen, W.; Morse, M.A.; et al. Antihelminth Compound Niclosamide Downregulates Wnt Signaling and Elicits Antitumor Responses in Tumors with Activating APC Mutations. Cancer Res. 2011, 71, 4172–4182. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, J.; Tian, R.; Wang, J.; Xie, C.; Gao, H.; Shan, Y.; Hong, J.; Zhang, Z.; Xu, M.; et al. Down-regulation of Dishevelled-2 Inhibits Cell Proliferation and Invasion in Hepatoblastoma. Pediatr. Blood Cancer 2018, 65, e27032. [Google Scholar] [CrossRef] [PubMed]
- Zeyada, M.S.; Abdel-Rahman, N.; El-Karef, A.; Yahia, S.; El-Sherbiny, I.M.; Eissa, L.A. Niclosamide-Loaded Polymeric Micelles Ameliorate Hepatocellular Carcinoma in Vivo through Targeting Wnt and Notch Pathways. Life Sci. 2020, 261, 118458. [Google Scholar] [CrossRef] [PubMed]
- Burock, S.; Daum, S.; Keilholz, U.; Neumann, K.; Walther, W.; Stein, U. Phase II Trial to Investigate the Safety and Efficacy of Orally Applied Niclosamide in Patients with Metachronous or Sychronous Metastases of a Colorectal Cancer Progressing after Therapy: The NIKOLO Trial. BMC Cancer 2018, 18, 297. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.T.; Haugk, K.; McKiernan, J.S.; Gulati, R.; Cheng, H.H.; Maes, J.L.; Dumpit, R.F.; Nelson, P.S.; Montgomery, B.; McCune, J.S.; et al. A Phase I Study of Niclosamide in Combination with Enzalutamide in Men with Castration-Resistant Prostate Cancer. PLoS ONE 2018, 13, e0198389. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Wang, N.X.; Shi, D.; Zheng, J.J. Sulindac Inhibits Canonical Wnt Signaling by Blocking the PDZ Domain of the Protein Dishevelled. Angew. Chem. Int. Ed. 2009, 48, 6448–6452. [Google Scholar] [CrossRef] [PubMed]
- Tutter, A.V.; Fryer, C.J.; Jones, K.A. Chromatin-Specific Regulation of LEF-1–β-Catenin Transcription Activation and Inhibition in Vitro. Genes Dev. 2001, 15, 3342–3354. [Google Scholar] [CrossRef]
- Limburg, P.J.; Mandrekar, S.J.; Aubry, M.C.; Ziegler, K.L.A.; Zhang, J.; Yi, J.E.; Henry, M.; Tazelaar, H.D.; Lam, S.; McWilliams, A.; et al. Randomized Phase II Trial of Sulindac for Lung Cancer Chemoprevention. Lung Cancer 2013, 79, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Masters, G.A.; Li, S.; Dowlati, A.; Madajewicz, S.; Langer, C.; Schiller, J.; Johnson, D. A Phase II Trial of Carboplatin and Gemcitabine with Exisulind (IND #65,056) in Patients with Advanced Non-Small Cell Lung Cancer: An Eastern Cooperative Oncology Group Study (E1501). J. Thorac. Oncol. 2006, 1, 673–678. [Google Scholar]
- Dawson, N.A.; Halabi, S.; Ou, S.-S.; Biggs, D.D.; Kessinger, A.; Vogelzang, N.; Clamon, G.H.; Nanus, D.M.; Kelly, W.K.; Small, E.J. A Phase II Study of Estramustine, Docetaxel, and Exisulind in Patients with Hormone-Refractory Prostate Cancer: Results of Cancer and Leukemia Group B Trial 90004. Clin. Genitourin. Cancer 2008, 6, 110–116. [Google Scholar] [CrossRef]
- Govindan, R.; Wang, X.; Baggstrom, M.Q.; Burdette-Radoux, S.; Hodgson, L.; Vokes, E.E.; Green, M.R. A Phase II Study of Carboplatin, Etoposide, and Exisulind in Patients with Extensive Small Cell Lung Cancer: CALGB 30104. J. Thorac. Oncol. 2009, 4, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.A.; Wertheim, B.C.; Zell, J.A.; Chen, W.-P.; McLaren, C.E.; LaFleur, B.J.; Meyskens, F.L.; Gerner, E.W. Levels of Rectal Mucosal Polyamines and Prostaglandin E2 Predict Ability of DFMO and Sulindac to Prevent Colorectal Adenoma. Gastroenterology 2010, 139, 797–805.e1. [Google Scholar] [CrossRef]
- Burke, C.A.; Dekker, E.; Lynch, P.; Samadder, N.J.; Balaguer, F.; Hüneburg, R.; Burn, J.; Castells, A.; Gallinger, S.; Lim, R.; et al. Eflornithine plus Sulindac for Prevention of Progression in Familial Adenomatous Polyposis. N. Engl. J. Med. 2020, 383, 1028–1039. [Google Scholar] [CrossRef] [PubMed]
- Samadder, N.J.; Kuwada, S.K.; Boucher, K.M.; Byrne, K.; Kanth, P.; Samowitz, W.; Jones, D.; Tavtigian, S.V.; Westover, M.; Berry, T.; et al. Association of Sulindac and Erlotinib vs Placebo With Colorectal Neoplasia in Familial Adenomatous Polyposis: Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018, 4, 671. [Google Scholar] [CrossRef]
- Samadder, N.J.; Neklason, D.W.; Boucher, K.M.; Byrne, K.R.; Kanth, P.; Samowitz, W.; Jones, D.; Tavtigian, S.V.; Done, M.W.; Berry, T.; et al. Effect of Sulindac and Erlotinib vs Placebo on Duodenal Neoplasia in Familial Adenomatous Polyposis: A Randomized Clinical Trial. J. Am. Med. Assoc. 2016, 315, 1266. [Google Scholar] [CrossRef]
- Martinez, J.A.; Wertheim, B.C.; Roe, D.J.; Chalasani, P.; Cohen, J.; Baer, L.; Chow, H.-H.S.; Stopeck, A.T.; Thompson, P.A. Sulindac Improves Stiffness and Quality of Life in Women Taking Aromatase Inhibitors for Breast Cancer. Breast Cancer Res. Treat. 2022, 192, 113–122. [Google Scholar] [CrossRef]
- Thompson, P.A.; Huang, C.; Yang, J.; Wertheim, B.C.; Roe, D.; Zhang, X.; Ding, J.; Chalasani, P.; Preece, C.; Martinez, J.; et al. Sulindac, a Nonselective NSAID, Reduces Breast Density in Postmenopausal Women with Breast Cancer Treated with Aromatase Inhibitors. Clin. Cancer Res. 2021, 27, 5660–5668. [Google Scholar] [CrossRef] [PubMed]
- Croy, H.E.; Fuller, C.N.; Giannotti, J.; Robinson, P.; Foley, A.V.A.; Yamulla, R.J.; Cosgriff, S.; Greaves, B.D.; Von Kleeck, R.A.; An, H.H.; et al. The Poly(ADP-Ribose) Polymerase Enzyme Tankyrase Antagonizes Activity of the β-Catenin Destruction Complex through ADP-Ribosylation of Axin and APC2. J. Biol. Chem. 2016, 291, 12747–12760. [Google Scholar] [CrossRef]
- McGonigle, S.; Chen, Z.; Wu, J.; Chang, P.; Kolber-Simonds, D.; Ackermann, K.; Twine, N.C.; Shie, J.-L.; Miu, J.T.; Huang, K.-C.; et al. E7449: A Dual Inhibitor of PARP1/2 and Tankyrase1/2 Inhibits Growth of DNA Repair Deficient Tumors and Antagonizes Wnt Signaling. Oncotarget 2015, 6, 41307–41323. [Google Scholar] [CrossRef]
- Huang, S.-M.A.; Mishina, Y.M.; Liu, S.; Cheung, A.; Stegmeier, F.; Michaud, G.A.; Charlat, O.; Wiellette, E.; Zhang, Y.; Wiessner, S.; et al. Tankyrase Inhibition Stabilizes Axin and Antagonizes Wnt Signalling. Nature 2009, 461, 614–620. [Google Scholar] [CrossRef]
- Mariotti, L.; Templeton, C.M.; Ranes, M.; Paracuellos, P.; Cronin, N.; Beuron, F.; Morris, E.; Guettler, S. Tankyrase Requires SAM Domain-Dependent Polymerization to Support Wnt-β-Catenin Signaling. Mol. Cell 2016, 63, 498–513. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Mashima, T.; Mizutani, A.; Sato, A.; Aoyama, A.; Gong, B.; Yoshida, H.; Muramatsu, Y.; Nakata, K.; Matsuura, M.; et al. APC Mutations as a Potential Biomarker for Sensitivity to Tankyrase Inhibitors in Colorectal Cancer. Mol. Cancer Ther. 2017, 16, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Plummer, R.; Dua, D.; Cresti, N.; Drew, Y.; Stephens, P.; Foegh, M.; Knudsen, S.; Sachdev, P.; Mistry, B.M.; Dixit, V.; et al. First-in-Human Study of the PARP/Tankyrase Inhibitor E7449 in Patients with Advanced Solid Tumours and Evaluation of a Novel Drug-Response Predictor. Br. J. Cancer 2020, 123, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Katuwal, N.; Ghosh, M.; Jeong, Y.K.; Deok Hong, S.; Park, S.M.; Kim, J.; Cha, H.; Cheon, B.; Kim, S.-G.; et al. Abstract 4485: JPI-547, a Novel Dual Inhibitor of PARP 1/2 and Tankyrase 1/2 Overcomes Olaparib Resistance in BRCA 1/2 Mutant Ovary and Breast Cancer Preclinical Model. Cancer Res. 2023, 83, 4485. [Google Scholar] [CrossRef]
- Oh, K.-S.; Nam, A.-R.; Bang, J.-H.; Jeong, Y.; Choo, S.Y.; Kim, H.J.; Lee, S.I.; Kim, J.-M.; Yoon, J.; Kim, T.-Y.; et al. Abstract 4496: JPI-547, a Dual Inhibitor of PARP/Tankyrase, Shows Promising Antitumor Activity against Pancreatic Cancers with Homologous Recombination Repair Deficiency or Wnt-Addiction. Cancer Res. 2023, 83, 4496. [Google Scholar] [CrossRef]
- Im, S.-A.; Lee, S.; Lee, K.W.; Lee, Y.; Sohn, J.; Kim, J.H.; Im, Y.-H.; Park, K.H.; Oh, D.-Y.; Kim, M.H.; et al. A Phase I Dose-Escalation and Expansion Study of JPI-547, a Dual Inhibitor of PARP/Tankyrase in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2021, 39, 3113. [Google Scholar] [CrossRef]
- Pharmaceutical Technology Nesuparib by Onconic Therapeutics for Epithelial Ovarian Cancer: Likelihood of Approval. Available online: https://www.pharmaceutical-technology.com/data-insights/nesuparib-onconic-therapeutics-epithelial-ovarian-cancer-likelihood-of-approval/ (accessed on 14 February 2024).
- Shen, C.; Li, B.; Astudillo, L.; Deutscher, M.P.; Cobb, M.H.; Capobianco, A.J.; Lee, E.; Robbins, D.J. The CK1α Activator Pyrvinium Enhances the Catalytic Efficiency (kcat/Km) of CK1α. Biochemistry 2019, 58, 5102–5106. [Google Scholar] [CrossRef] [PubMed]
- Saraswati, S.; Alfaro, M.P.; Thorne, C.A.; Atkinson, J.; Lee, E.; Young, P.P. Pyrvinium, a Potent Small Molecule Wnt Inhibitor, Promotes Wound Repair and Post-MI Cardiac Remodeling. PLoS ONE 2010, 5, e15521. [Google Scholar] [CrossRef]
- Thorne, C.A.; Hanson, A.J.; Schneider, J.; Tahinci, E.; Orton, D.; Cselenyi, C.S.; Jernigan, K.K.; Meyers, K.C.; Hang, B.I.; Waterson, A.G.; et al. Small-Molecule Inhibition of Wnt Signaling through Activation of Casein Kinase 1α. Nat. Chem. Biol. 2010, 6, 829–836. [Google Scholar] [CrossRef]
- Schultz, C.W.; Nevler, A. Pyrvinium Pamoate: Past, Present, and Future as an Anti-Cancer Drug. Biomedicines 2022, 10, 3249. [Google Scholar] [CrossRef]
- Ponzini, F.M.; Schultz, C.W.; Leiby, B.E.; Cannaday, S.; Yeo, T.; Posey, J.; Bowne, W.B.; Yeo, C.; Brody, J.R.; Lavu, H.; et al. Repurposing the FDA-Approved Anthelmintic Pyrvinium Pamoate for Pancreatic Cancer Treatment: Study Protocol for a Phase I Clinical Trial in Early-Stage Pancreatic Ductal Adenocarcinoma. BMJ Open 2023, 13, e073839. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Stratton, S.A.; Lee, S.H.; Kim, M.; Jun, S.; Zhang, J.; Zheng, B.; Cervantes, C.L.; Cha, J.; Barton, M.C.; et al. TMEM9-v-ATPase Activates Wnt/β-Catenin Signaling Via APC Lysosomal Degradation for Liver Regeneration and Tumorigenesis. Hepatology 2021, 73, 776–794. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Choudhury, D.; Das, A.; Mukherjee, D.D.; Dasgupta, M.; Bandopadhyay, S.; Chakrabarti, G. Autophagy Inhibition with Chloroquine Reverts Paclitaxel Resistance and Attenuates Metastatic Potential in Human Nonsmall Lung Adenocarcinoma A549 Cells via ROS Mediated Modulation of β-Catenin Pathway. Apoptosis 2019, 24, 414–433. [Google Scholar] [CrossRef]
- Verbaanderd, C.; Maes, H.; Schaaf, M.B.; Sukhatme, V.P.; Pantziarka, P.; Sukhatme, V.; Agostinis, P.; Bouche, G. Repurposing Drugs in Oncology (ReDO)—Chloroquine and Hydroxychloroquine as Anti-Cancer Agents. Ecancermedicalscience 2017, 11, 781. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Kim, I.S.; Kim, H.; Lee, J.S.; Kim, K.; Yim, H.Y.; Jeong, J.; Kim, J.H.; Kim, J.-Y.; Lee, H.; et al. RORα Attenuates Wnt/β-Catenin Signaling by PKCα-Dependent Phosphorylation in Colon Cancer. Mol. Cell 2010, 37, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Osman, J.; Bellamkonda, K.; Liu, Q.; Andersson, T.; Sjölander, A. The WNT5A Agonist Foxy5 Reduces the Number of Colonic Cancer Stem Cells in a Xenograft Mouse Model of Human Colonic Cancer. Anticancer. Res. 2019, 39, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C.P.; Manchanda, M.; Mohapatra, P.; Andersson, T. WNT5A as a Therapeutic Target in Breast Cancer. Cancer Metastasis Rev. 2018, 37, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Canesin, G.; Evans-Axelsson, S.; Hellsten, R.; Krzyzanowska, A.; Prasad, C.P.; Bjartell, A.; Andersson, T. Treatment with the WNT5A-Mimicking Peptide Foxy-5 Effectively Reduces the Metastatic Spread of WNT5A-Low Prostate Cancer Cells in an Orthotopic Mouse Model. PLoS ONE 2017, 12, e0184418. [Google Scholar] [CrossRef] [PubMed]
- Säfholm, A.; Tuomela, J.; Rosenkvist, J.; Dejmek, J.; Härkönen, P.; Andersson, T. The Wnt-5a–Derived Hexapeptide Foxy-5 Inhibits Breast Cancer Metastasis In Vivo by Targeting Cell Motility. Clin. Cancer Res. 2008, 14, 6556–6563. [Google Scholar] [CrossRef]
- Vermorken, J.; Cervantes, A.; Morsing, P.; Johansson, K.; Andersson, T.; Roest, N.L.; Gullbo, J.; Salazar, R. A Randomized, Multicenter, Open-Label Controlled Phase 2 Trial of Foxy-5 as Neoadjuvant Therapy in Patients with WNT5A Negative Colon Cancer. Ann. Oncol. 2019, 30, iv36. [Google Scholar] [CrossRef]
- Yadav, V.; Islam, R.; Tuli, H.S. Patent Landscape Highlighting Double-Edged Scaffold of a WNT5A-Agonizing Peptide, Foxy5. Pharm. Pat. Anal. 2023, 12, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Inokuchi, J.; Eto, M.; Murata, M.; Kang, J.-H. Activators and Inhibitors of Protein Kinase C (PKC): Their Applications in Clinical Trials. Pharmaceutics 2021, 13, 1748. [Google Scholar] [CrossRef]
- Antal, C.E.; Hudson, A.M.; Kang, E.; Zanca, C.; Wirth, C.; Stephenson, N.L.; Trotter, E.W.; Gallegos, L.L.; Miller, C.J.; Furnari, F.B.; et al. Cancer-Associated Protein Kinase C Mutations Reveal Kinase’s Role as Tumor Suppressor. Cell 2015, 160, 489–502. [Google Scholar] [CrossRef]
- Shah, K.; Kazi, J.U. Phosphorylation-Dependent Regulation of WNT/Beta-Catenin Signaling. Front. Oncol. 2022, 12, 858782. [Google Scholar] [CrossRef]
- Dupasquier, S.; Blache, P.; Picque Lasorsa, L.; Zhao, H.; Abraham, J.-D.; Haigh, J.J.; Ychou, M.; Prévostel, C. Modulating PKCα Activity to Target Wnt/β-Catenin Signaling in Colon Cancer. Cancers 2019, 11, 693. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Maqueda, J.G.; Luna-Ulloa, L.B.; Santoyo-Ramos, P.; Castañeda-Patlán, M.C.; Robles-Flores, M. Protein Kinase C Delta Negatively Modulates Canonical Wnt Pathway and Cell Proliferation in Colon Tumor Cell Lines. PLoS ONE 2013, 8, e58540. [Google Scholar] [CrossRef] [PubMed]
- Gwak, J.; Cho, M.; Gong, S.-J.; Won, J.; Kim, D.-E.; Kim, E.-Y.; Lee, S.S.; Kim, M.; Kim, T.K.; Shin, J.-G.; et al. Protein-Kinase-C-Mediated β-Catenin Phosphorylation Negatively Regulates the Wnt/β-Catenin Pathway. J. Cell Sci. 2006, 119, 4702–4709. [Google Scholar] [CrossRef] [PubMed]
- Llado, V.; Nakanishi, Y.; Duran, A.; Reina-Campos, M.; Shelton, P.M.; Linares, J.F.; Yajima, T.; Campos, A.; Aza-Blanc, P.; Leitges, M.; et al. Repression of Intestinal Stem Cell Function and Tumorigenesis through Direct Phosphorylation of β-Catenin and Yap by PKCζ. Cell Rep. 2015, 10, 740–754. [Google Scholar] [CrossRef]
- Velin, M.; Cardot-Leccia, N.; Cathelineau, A.; Duteil, L.; Queille-Roussel, C.; Passeron, T.; Bahadoran, P. Efficacy and Safety of 0.05% Ingenol Mebutate in the Treatment of Basal Cell Carcinoma: A Prospective Study. Skin. Health Dis. 2023, 3, e150. [Google Scholar] [CrossRef]
- Siller, G.; Rosen, R.; Freeman, M.; Welburn, P.; Katsamas, J.; Ogbourne, S.M. PEP005 (Ingenol Mebutate) Gel for the Topical Treatment of Superficial Basal Cell Carcinoma: Results of a Randomized Phase IIa Trial. Aust. J. Dermatol. 2010, 51, 99–105. [Google Scholar] [CrossRef]
- Ramsay, J.R.; Suhrbier, A.; Aylward, J.H.; Ogbourne, S.; Cozzi, S.-J.; Poulsen, M.G.; Baumann, K.C.; Welburn, P.; Redlich, G.L.; Parsons, P.G. The Sap from Euphorbia Peplus Is Effective against Human Nonmelanoma Skin Cancers: Euphorbia Peplus Sap Is Effective against Skin Cancers. Br. J. Dermatol. 2011, 164, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, M.; Swanson, N.; Anderson, L.L.; Melgaard, A.; Xu, Z.; Berman, B. Ingenol Mebutate Gel for Actinic Keratosis. N. Engl. J. Med. 2012, 366, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, M.; Shumack, S.; Gold, L.S.; Melgaard, A.; Larsson, T.; Tyring, S.K. Long-Term Follow-up Study of Ingenol Mebutate Gel for the Treatment of Actinic Keratoses. JAMA Dermatol. 2013, 149, 666. [Google Scholar] [CrossRef] [PubMed]
- Fidler, B.; Goldberg, T. Ingenol Mebutate Gel (Picato): A Novel Agent for the Treatment of Actinic Keratoses. Pharm. Ther. 2014, 39, 40–46. [Google Scholar]
- Anderson, L.; Jarratt, M.; Schmieder, G.; Shumack, S.; Katsamas, J.; Welburn, P. Tolerability and Pharmacokinetics of Ingenol Mebutate 0.05% Gel Applied to Treatment Areas up to 100cm(2) on the Forearm(s) of Patients with Actinic Keratosis. J. Clin. Aesthet. Dermatol. 2014, 7, 19–29. [Google Scholar] [PubMed]
- Berman, B.; Goldenberg, G.; Hanke, C.W.; Tyring, S.K.; Werschler, W.P.; Knudsen, K.M.; Goncalves, J.; Larsson, T.; Skov, T.; Swanson, N. Efficacy and Safety of Ingenol Mebutate 0.015% Gel 3 Weeks after Cryosurgery of Actinic Keratosis: 11-Week Results. J. Drugs Dermatol. 2014, 13, 154–160. [Google Scholar] [PubMed]
- Berman, B.; Goldenberg, G.; Hanke, C.W.; Tyring, S.K.; Werschler, W.P.; Knudsen, K.M.; Larsson, T.; Swanson, N. Efficacy and Safety of Ingenol Mebutate 0.015% Gel after Cryosurgery of Actinic Keratosis: 12-Month Results. J. Drugs Dermatol. 2014, 13, 741–747. [Google Scholar]
- Pellacani, G.; Peris, K.; Guillen, C.; Clonier, F.; Larsson, T.; Venkata, R.; Puig, S. A Randomized Trial Comparing Simultaneous vs. Sequential Field Treatment of Actinic Keratosis with Ingenol Mebutate on Two Separate Areas of the Head and Body. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2192–2198. [Google Scholar] [CrossRef]
- Samorano, L.P.; Torezan, L.A.; Sanches, J.A. Evaluation of the Tolerability and Safety of a 0.015% Ingenol Mebutate Gel Compared to 5% 5-fluorouracil Cream for the Treatment of Facial Actinic Keratosis: A Prospective Randomized Trial. Acad. Dermatol. Venereol. 2015, 29, 1822–1827. [Google Scholar] [CrossRef]
- Emmert, S.; Haenssle, H.A.; Zibert, J.R.; Schön, M.; Hald, A.; Hansen, M.H.; Litman, T.; Schön, M.P. Tumor-Preferential Induction of Immune Responses and Epidermal Cell Death in Actinic Keratoses by Ingenol Mebutate. PLoS ONE 2016, 11, e0160096. [Google Scholar] [CrossRef]
- Schopf, R.E. Ingenol Mebutate Gel Is Effective against Anogenital Warts—A Case Series in 17 Patients. Acad. Dermatol. Venereol. 2016, 30, 1041–1043. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.C.; Guiha, I.; Goldman, M.P. A Prospective Pilot Clinical Trial to Evaluate the Efficacy and Safety of Topical Therapy with Ingenol Mebutate Gel 0.015% for Actinic Keratosis on an Expanded Area of the Chest. J. Clin. Aesthet. Dermatol. 2017, 10, 31–36. [Google Scholar] [PubMed]
- Stockfleth, E.; Harwood, C.A.; Serra-Guillén, C.; Larsson, T.; Østerdal, M.L.; Skov, T. Phase IV Head-to-Head Randomized Controlled Trial Comparing Ingenol Mebutate 0·015% Gel with Diclofenac Sodium 3% Gel for the Treatment of Actinic Keratosis on the Face or Scalp. Br. J. Dermatol. 2018, 178, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.H.E.; Kessels, J.P.H.M.; Nelemans, P.J.; Kouloubis, N.; Arits, A.H.M.M.; Van Pelt, H.P.A.; Quaedvlieg, P.J.F.; Essers, B.A.B.; Steijlen, P.M.; Kelleners-Smeets, N.W.J.; et al. Randomized Trial of Four Treatment Approaches for Actinic Keratosis. N. Engl. J. Med. 2019, 380, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.H.E.; Kessels, J.P.H.M.; Merks, I.; Nelemans, P.J.; Kelleners-Smeets, N.W.J.; Mosterd, K.; Essers, B.A.B. A Trial-based Cost-effectiveness Analysis of Topical 5-fluorouracil vs. Imiquimod vs. Ingenol Mebutate vs. Methyl Aminolaevulinate Conventional Photodynamic Therapy for the Treatment of Actinic Keratosis in the Head and Neck Area Performed in the Netherlands. Br. J. Dermatol. 2020, 183, 738–744. [Google Scholar] [CrossRef]
- Ahmady, S.; Jansen, M.H.E.; Nelemans, P.J.; Essers, B.A.B.; Kessels, J.P.H.M.; Kelleners-Smeets, N.W.J.; Mosterd, K. The Effect of Four Approaches to Treat Actinic Keratosis on the Health-Related QOL, as Assessed by the Skindex-29 and Actinic Keratosis QOL. J. Investig. Dermatol. 2021, 141, 1830–1832. [Google Scholar] [CrossRef]
- Ahmady, S.; Jansen, M.H.E.; Nelemans, P.J.; Kessels, J.P.H.M.; Arits, A.H.M.M.; De Rooij, M.J.M.; Essers, B.A.B.; Quaedvlieg, P.J.F.; Kelleners-Smeets, N.W.J.; Mosterd, K. Risk of Invasive Cutaneous Squamous Cell Carcinoma After Different Treatments for Actinic Keratosis: A Secondary Analysis of a Randomized Clinical Trial. JAMA Dermatol. 2022, 158, 634. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.H.; Gupta, A.K.; Tyring, S.K. Dual Mechanism of Action of Ingenol Mebutate Gel for Topical Treatment of Actinic Keratoses: Rapid Lesion Necrosis Followed by Lesion-Specific Immune Response. J. Am. Acad. Dermatol. 2012, 66, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.T.; Zhu, X.X.; Yang, R.Y.; Sun, J.Z.; Tian, G.F.; Liu, X.J.; Cao, G.S.; Newmark, H.L.; Conney, A.H.; Chang, R.L. Effect of Intravenous Infusions of 12-O-Tetradecanoylphorbol-13-Acetate (TPA) in Patients with Myelocytic Leukemia: Preliminary Studies on Therapeutic Efficacy and Toxicity. Proc. Natl. Acad. Sci. USA 1998, 95, 5357–5361. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Song, Y.; Han, Z.; Wei, X.; Lin, Q.; Zhu, X.; Yang, R.; Sun, J.; Tian, G.; Liu, X.; et al. Synergistic Interactions between 12-0-Tetradecanoylphorbol-13-Acetate (TPA) and Imatinib in Patients with Chronic Myeloid Leukemia in Blastic Phase That Is Resistant to Standard-Dose Imatinib. Leuk. Res. 2007, 31, 1441–1444. [Google Scholar] [CrossRef]
- Fürstenberger, G.; Berry, D.L.; Sorg, B.; Marks, F. Skin Tumor Promotion by Phorbol Esters Is a Two-Stage Process. Proc. Natl. Acad. Sci. USA 1981, 78, 7722–7726. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cho, U.; Yoo, A.; Jung, C.-L.; Kim, B.; Kim, H.; Lee, J.; Jo, H.; Han, Y.; Song, M.-H.; et al. Wnt/β-Catenin Inhibition by CWP232291 as a Novel Therapeutic Strategy in Ovarian Cancer. Front. Oncol. 2022, 12, 852260. [Google Scholar] [CrossRef] [PubMed]
- Pak, S.; Park, S.; Kim, Y.; Park, J.-H.; Park, C.-H.; Lee, K.-J.; Kim, C.; Ahn, H. The Small Molecule WNT/β-Catenin Inhibitor CWP232291 Blocks the Growth of Castration-Resistant Prostate Cancer by Activating the Endoplasmic Reticulum Stress Pathway. J. Exp. Clin. Cancer Res. 2019, 38, 342. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Um, H.; Yang, H.; Cha, J.Y.; Lee, K.-J.; Kim, H.K. CWP232291, a Wnt/β-Catenin Inhibitor, to Suppress the Growth and Development of Gastrointestinal Cancers. J. Clin. Oncol. 2017, 35, e15534. [Google Scholar] [CrossRef]
- Cha, J.Y.; Jung, J.-E.; Lee, K.-H.; Briaud, I.; Tenzin, F.; Jung, H.K.; Pyon, Y.; Lee, D.; Chung, J.U.; Lee, J.H.; et al. Anti-Tumor Activity of Novel Small Molecule Wnt Signaling Inhibitor, CWP232291, In Multiple Myeloma. Blood 2010, 116, 3038. [Google Scholar] [CrossRef]
- Yoon, S.-S.; Manasanch, E.E.; Min, C.K.; Kim, J.S.; Hauptschein, R.S.; Choi, J.; Chun, J.K. Novel Phase 1a/1b Dose-Finding Study Design of CWP232291 (CWP291) in Relapsed or Refractory Myeloma (MM). J. Clin. Oncol. 2017, 35, TPS8058. [Google Scholar] [CrossRef]
- Lee, J.-H.; Faderl, S.; Pagel, J.M.; Jung, C.W.; Yoon, S.-S.; Pardanani, A.D.; Becker, P.S.; Lee, H.; Choi, J.; Lee, K.; et al. Phase 1 Study of CWP232291 in Patients with Relapsed or Refractory Acute Myeloid Leukemia and Myelodysplastic Syndrome. Blood Advances 2020, 4, 2032–2043. [Google Scholar] [CrossRef] [PubMed]
- Teo, J.-L.; Ma, H.; Nguyen, C.; Lam, C.; Kahn, M. Specific Inhibition of CBP/Beta-Catenin Interaction Rescues Defects in Neuronal Differentiation Caused by a Presenilin-1 Mutation. Proc. Natl. Acad. Sci. USA 2005, 102, 12171–12176. [Google Scholar] [CrossRef] [PubMed]
- McMillan, M.; Kahn, M. Investigating Wnt Signaling: A Chemogenomic Safari. Drug Discov. Today 2005, 10, 1467–1474. [Google Scholar] [CrossRef]
- Eguchi, M.; Nguyen, C.; Lee, S.C.; Kahn, M. ICG-001, a Novel Small Molecule Regulator of TCF/Beta-Catenin Transcription. Med. Chem. 2005, 1, 467–472. [Google Scholar] [CrossRef]
- Ma, H.; Nguyen, C.; Lee, K.-S.; Kahn, M. Differential Roles for the Coactivators CBP and P300 on TCF/Beta-Catenin-Mediated Survivin Gene Expression. Oncogene 2005, 24, 3619–3631. [Google Scholar] [CrossRef] [PubMed]
- Gutova, M.; Hibbard, J.C.; Ma, E.; Natri, H.M.; Adhikarla, V.; Chimge, N.-O.; Qiu, R.; Nguyen, C.; Melendez, E.; Aguilar, B.; et al. Targeting Wnt Signaling for Improved Glioma Immunotherapy. Front. Immunol. 2024, 15, 1342625. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Parish, M.; Brennan, J.T.; Winer, B.L.; Segars, J.H. Targeting Fibrotic Signaling Pathways by EGCG as a Therapeutic Strategy for Uterine Fibroids. Sci. Rep. 2023, 13, 8492. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, H.; Sato, S.; Koyama, K.; Morizumi, S.; Abe, S.; Azuma, M.; Chen, Y.; Goto, H.; Aono, Y.; Ogawa, H.; et al. The Novel Inhibitor PRI-724 for Wnt/β-Catenin/CBP Signaling Ameliorates Bleomycin-Induced Pulmonary Fibrosis in Mice. Exp. Lung Res. 2019, 45, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-S.; Ryu, G.; Kim, J.H.; Kim, E.H.; Rhee, Y.H.; Chung, Y.-J.; Kim, D.W.; Lim, S.; Chung, P.-S.; Shin, H.-W.; et al. Effects of Wnt Signaling on Epithelial to Mesenchymal Transition in Chronic Rhinosinusitis with Nasal Polyp. Thorax 2020, 75, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Osawa, Y.; Kimura, K. Wnt/β-Catenin Signaling as a Potential Target for the Treatment of Liver Cirrhosis Using Antifibrotic Drugs. Int. J. Mol. Sci. 2018, 19, 3103. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Ikoma, A.; Shibakawa, M.; Shimoda, S.; Harada, K.; Saio, M.; Imamura, J.; Osawa, Y.; Kimura, M.; Nishikawa, K.; et al. Safety, Tolerability, and Preliminary Efficacy of the Anti-Fibrotic Small Molecule PRI-724, a CBP/β-Catenin Inhibitor, in Patients with Hepatitis C Virus-Related Cirrhosis: A Single-Center, Open-Label, Dose Escalation Phase 1 Trial. EBioMedicine 2017, 23, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Kanto, T.; Shimoda, S.; Harada, K.; Kimura, M.; Nishikawa, K.; Imamura, J.; Ogawa, E.; Saio, M.; Ikura, Y.; et al. Safety, Tolerability, and Anti-Fibrotic Efficacy of the CBP/β-Catenin Inhibitor PRI-724 in Patients with Hepatitis C and B Virus-Induced Liver Cirrhosis: An Investigator-Initiated, Open-Label, Non-Randomised, Multicentre, Phase 1/2a Study. EBioMedicine 2022, 80, 104069. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Ning, Y.; Yang, D.; Cole, S.; Kahn, M.; Zoghbi, M.; Berg, J.; Fujimori, M.; Inada, T.; Kouji, H.; et al. A Phase I First-in-Human Study of PRI-724 in Patients (Pts) with Advanced Solid Tumors. J. Clin. Oncol. 2013, 31, 2501. [Google Scholar] [CrossRef]
- Ko, A.H.; Chiorean, E.G.; Kwak, E.L.; Lenz, H.-J.; Nadler, P.I.; Wood, D.L.; Fujimori, M.; Inada, T.; Kouji, H.; McWilliams, R.R. Final Results of a Phase Ib Dose-Escalation Study of PRI-724, a CBP/Beta-Catenin Modulator, plus Gemcitabine (GEM) in Patients with Advanced Pancreatic Adenocarcinoma (APC) as Second-Line Therapy after FOLFIRINOX or FOLFOX. J. Clin. Oncol. 2016, 34, e15721. [Google Scholar] [CrossRef]
- McWilliams, R.R.; Ko, A.H.; Chiorean, E.G.; Kwak, E.L.; Lenz, H.-J.; Nadler, P.I.; Wood, D.L.; Fujimori, M.; Morita, K.; Inada, T.; et al. A Phase Ib Dose-Escalation Study of PRI-724, a CBP/Beta-Catenin Modulator, plus Gemcitabine (GEM) in Patients with Advanced Pancreatic Adenocarcinoma (APC) as Second-Line Therapy after FOLFIRINOX or FOLFOX. J. Clin. Oncol. 2015, 33, e15270. [Google Scholar] [CrossRef]
- Yamada, K.; Hori, Y.; Inoue, S.; Yamamoto, Y.; Iso, K.; Kamiyama, H.; Yamaguchi, A.; Kimura, T.; Uesugi, M.; Ito, J.; et al. E7386, a Selective Inhibitor of the Interaction between β-Catenin and CBP, Exerts Antitumor Activity in Tumor Models with Activated Canonical Wnt Signaling. Cancer Res. 2021, 81, 1052–1062. [Google Scholar] [CrossRef]
- Higuchi, Y.; Nguyen, C.; Chimge, N.-O.; Ouyang, C.; Teo, J.-L.; Kahn, M. E7386 Is Not a Specific CBP/β-Catenin Antagonist. Curr. Mol. Pharmacol. 2023, 17, e290523217409. [Google Scholar] [CrossRef]
- Kondo, S.; Kawazoe, A.; Iwasa, S.; Yamamoto, N.; Ueda, Y.; Nagao, S.; Kimura, T.; Suzuki, I.; Hayata, N.; Tamai, T.; et al. A Phase 1 Study of E7386, a CREB-Binding Protein (CBP)/β-Catenin Interaction Inhibitor, in Patients (Pts) with Advanced Solid Tumors Including Colorectal Cancer: Updated Dose-Escalation Part. J. Clin. Oncol. 2023, 41, 106. [Google Scholar] [CrossRef]
- Ikeda, M.; Kato, N.; Kondo, S.; Inaba, Y.; Ueshima, K.; Sasaki, M.; Kanzaki, H.; Ida, H.; Imaoka, H.; Minami, Y.; et al. A Phase 1b Study of E7386, a CREB-Binding Protein (CBP)/β-Catenin Interaction Inhibitor, in Combination with Lenvatinib in Patients with Advanced Hepatocellular Carcinoma. J. Clin. Oncol. 2023, 41, 4075. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.-Y. TBL1–TBLR1 and β-Catenin Recruit Each Other to Wnt Target-Gene Promoter for Transcription Activation and Oncogenesis. Nat. Cell Biol. 2008, 10, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Braggio, D.A.; de Faria, F.C.C.; Koller, D.; Jin, F.; Zewdu, A.; Lopez, G.; Batte, K.; Casadei, L.; Welliver, M.; Horrigan, S.K.; et al. Preclinical Efficacy of the Wnt/β-Catenin Pathway Inhibitor BC2059 for the Treatment of Desmoid Tumors. PLoS ONE 2022, 17, e0276047. [Google Scholar] [CrossRef] [PubMed]
- Perissi, V.; Aggarwal, A.; Glass, C.K.; Rose, D.W.; Rosenfeld, M.G. A Corepressor/Coactivator Exchange Complex Required for Transcriptional Activation by Nuclear Receptors and Other Regulated Transcription Factors. Cell 2004, 116, 511–526. [Google Scholar] [CrossRef]
- Nomura, M.; Rainusso, N.C.; Han, R.; Larson, J.; Shuck, R.L.; Kurenbekova, L.; Yustein, J.T. Abstract 3186: Tegavivint Suppresses Progression and Metastasis of Osteosarcoma via Blockade of Wnt Signaling/ALDH1 Axis: Preclinical Study of a Novel Wnt/β-Catenin Pathway Inhibitor. Cancer Res. 2018, 78, 3186. [Google Scholar] [CrossRef]
- Dimitrova, Y.N.; Li, J.; Lee, Y.-T.; Rios-Esteves, J.; Friedman, D.B.; Choi, H.-J.; Weis, W.I.; Wang, C.-Y.; Chazin, W.J. Direct Ubiquitination of β-Catenin by Siah-1 and Regulation by the Exchange Factor TBL1. J. Biol. Chem. 2010, 285, 13507–13516. [Google Scholar] [CrossRef]
- Liu, J.; Stevens, J.; Rote, C.A.; Yost, H.J.; Hu, Y.; Neufeld, K.L.; White, R.L.; Matsunami, N. Siah-1 Mediates a Novel β-Catenin Degradation Pathway Linking P53 to the Adenomatous Polyposis Coli Protein. Mol. Cell 2001, 7, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Cranmer, L.D.; Abdul Razak, A.R.; Ratan, R.; Choy, E.; George, S.; Liebner, D.A.; Stenehjem, D.D.; Gounder, M.M. Results of a Phase I Dose Escalation and Expansion Study of Tegavivint (BC2059), a First-in-Class TBL1 Inhibitor for Patients with Progressive, Unresectable Desmoid Tumor. J. Clin. Oncol. 2022, 40, 11523. [Google Scholar] [CrossRef]
- Tam, B.Y.; Chiu, K.; Chung, H.; Bossard, C.; Nguyen, J.D.; Creger, E.; Eastman, B.W.; Mak, C.C.; Ibanez, M.; Ghias, A.; et al. The CLK Inhibitor SM08502 Induces Anti-Tumor Activity and Reduces Wnt Pathway Gene Expression in Gastrointestinal Cancer Models. Cancer Lett. 2020, 473, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Corr, B.R.; Moroney, M.R.; Woodruff, E.; Watson, Z.L.; Jordan, K.R.; Danhorn, T.; Bailey, C.; Wolsky, R.J.; Bitler, B.G. Combination CDC-like Kinase Inhibition (CLK)/Dual-Specificity Tyrosine-Regulated Kinase (DYRK) and Taxane Therapy in CTNNB1 -Mutated Endometrial Cancer. bioRxiv 2023. preprint. [Google Scholar]
- Deshmukh, V.; O’Green, A.L.; Bossard, C.; Seo, T.; Lamangan, L.; Ibanez, M.; Ghias, A.; Lai, C.; Do, L.; Cho, S.; et al. Modulation of the Wnt Pathway through Inhibition of CLK2 and DYRK1A by Lorecivivint as a Novel, Potentially Disease-Modifying Approach for Knee Osteoarthritis Treatment. Osteoarthr. Cartil. 2019, 27, 1347–1360. [Google Scholar] [CrossRef]
- Bossard, C.; Chiu, K.; Chung, H.; Nguyen, J.D.; Creger, E.; Eastman, B.; Mak, C.C.; Do, L.; Cho, S.; Kc, S.; et al. Effects of SM08502, a Novel, Oral Small-Molecule Inhibitor of Wnt Pathway Signaling, on Gene Expression and Antitumor Activity in Colorectal Cancer (CRC) Models. J. Clin. Oncol. 2019, 37, e15185. [Google Scholar] [CrossRef]
- Deshmukh, V.; Seo, T.; O’Green, A.L.; Ibanez, M.; Hofilena, B.; Kc, S.; Stewart, J.; Dellamary, L.; Chiu, K.; Ghias, A.; et al. SM04755, a Small-molecule Inhibitor of the Wnt Pathway, as a Potential Topical Treatment for Tendinopathy. J. Orthop. Res. 2021, 39, 2048–2061. [Google Scholar] [CrossRef]
- Jantan, I.; Haque, M.A.; Arshad, L.; Harikrishnan, H.; Septama, A.W.; Mohamed-Hussein, Z.-A. Dietary Polyphenols Suppress Chronic Inflammation by Modulation of Multiple Inflammation-Associated Cell Signaling Pathways. J. Nutr. Biochem. 2021, 93, 108634. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.-S.; Ninfali, P. Phytochemicals as Innovative Therapeutic Tools against Cancer Stem Cells. IJMS 2015, 16, 15727–15742. [Google Scholar] [CrossRef]
- Naujokat, C.; McKee, D.L. The “Big Five” Phytochemicals Targeting Cancer Stem Cells: Curcumin, EGCG, Sulforaphane, Resveratrol and Genistein. Curr. Med. Chem. 2021, 28, 4321–4342. [Google Scholar] [CrossRef]
- Liao, W.; Zhang, L.; Chen, X.; Xiang, J.; Zheng, Q.; Chen, N.; Zhao, M.; Zhang, G.; Xiao, X.; Zhou, G.; et al. Targeting Cancer Stem Cells and Signalling Pathways through Phytochemicals: A Promising Approach against Colorectal Cancer. Phytomedicine 2023, 108, 154524. [Google Scholar] [CrossRef] [PubMed]
- Tafrihi, M.; Nakhaei Sistani, R. E-Cadherin/β-Catenin Complex: A Target for Anticancer and Antimetastasis Plants/Plant-Derived Compounds. Nutr. Cancer 2017, 69, 702–722. [Google Scholar] [CrossRef] [PubMed]
- Avila-Carrasco, L.; Majano, P.; Sánchez-Toméro, J.A.; Selgas, R.; López-Cabrera, M.; Aguilera, A.; González Mateo, G. Natural Plants Compounds as Modulators of Epithelial-to-Mesenchymal Transition. Front. Pharmacol. 2019, 10, 715. [Google Scholar] [CrossRef]
- Motallebi, M.; Bhia, M.; Rajani, H.F.; Bhia, I.; Tabarraei, H.; Mohammadkhani, N.; Pereira-Silva, M.; Kasaii, M.S.; Nouri-Majd, S.; Mueller, A.-L.; et al. Naringenin: A Potential Flavonoid Phytochemical for Cancer Therapy. Life Sci. 2022, 305, 120752. [Google Scholar] [CrossRef] [PubMed]
- Morin, P.J.; Sparks, A.B.; Korinek, V.; Barker, N.; Clevers, H.; Vogelstein, B.; Kinzler, K.W. Activation of Beta-Catenin-Tcf Signaling in Colon Cancer by Mutations in Beta-Catenin or APC. Science 1997, 275, 1787–1790. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.-K.; Spit, M.; Jordens, I.; Low, T.Y.; Stange, D.E.; van de Wetering, M.; van Es, J.H.; Mohammed, S.; Heck, A.J.R.; Maurice, M.M.; et al. Tumour Suppressor RNF43 Is a Stem-Cell E3 Ligase That Induces Endocytosis of Wnt Receptors. Nature 2012, 488, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Preisler, L.; Ben-Yosef, D.; Mayshar, Y. Adenomatous Polyposis Coli as a Major Regulator of Human Embryonic Stem Cells Self-Renewal. Stem Cells 2019, 37, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S. The Importance of Clinical Trials in Drug Development. Avicenna J. Med. Biotechnol. 2016, 8, 151. [Google Scholar]
- Li, B.; Liang, J.; Lu, F.; Zeng, G.; Zhang, J.; Ma, Y.; Liu, P.; Wang, Q.; Zhou, Q.; Chen, L. Discovery of Novel Inhibitor for WNT/β-Catenin Pathway by Tankyrase 1/2 Structure-Based Virtual Screening. Molecules 2020, 25, 1680. [Google Scholar] [CrossRef]
- Low, J.-L.; Du, W.; Gocha, T.; Oguz, G.; Zhang, X.; Chen, M.W.; Masirevic, S.; Yim, D.G.R.; Tan, I.B.H.; Ramasamy, A.; et al. Molecular Docking-Aided Identification of Small Molecule Inhibitors Targeting β-Catenin-TCF4 Interaction. iScience 2021, 24, 102544. [Google Scholar] [CrossRef]
- Yan, M.; Li, G.; An, J. Discovery of Small Molecule Inhibitors of the Wnt/β-Catenin Signaling Pathway by Targeting β-Catenin/Tcf4 Interactions. Exp. Biol. Med. 2017, 242, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, Y.; Wang, H.; Zhang, X.; Wang, M.; He, J.; Li, S.; Zhang, L.; Li, K.; Cao, L. Advances of Artificial Intelligence in Anti-Cancer Drug Design: A Review of the Past Decade. Pharmaceuticals 2023, 16, 253. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wang, B.; Wu, Q.; Wang, G. Combination of Niclosamide and Current Therapies to Overcome Resistance for Cancer: New Frontiers for an Old Drug. Biomed. Pharmacother. 2022, 155, 113789. [Google Scholar] [CrossRef] [PubMed]
- Lehár, J.; Krueger, A.S.; Avery, W.; Heilbut, A.M.; Johansen, L.M.; Price, E.R.; Rickles, R.J.; Short Iii, G.F.; Staunton, J.E.; Jin, X.; et al. Synergistic Drug Combinations Tend to Improve Therapeutically Relevant Selectivity. Nat. Biotechnol. 2009, 27, 659–666. [Google Scholar] [CrossRef]
- Borisy, A.A.; Elliott, P.J.; Hurst, N.W.; Lee, M.S.; Lehár, J.; Price, E.R.; Serbedzija, G.; Zimmermann, G.R.; Foley, M.A.; Stockwell, B.R.; et al. Systematic Discovery of Multicomponent Therapeutics. Proc. Natl. Acad. Sci. USA 2003, 100, 7977–7982. [Google Scholar] [CrossRef]
- Duarte, D.; Vale, N. Evaluation of Synergism in Drug Combinations and Reference Models for Future Orientations in Oncology. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100110. [Google Scholar] [CrossRef] [PubMed]
- Tallarida, R.J. Quantitative Methods for Assessing Drug Synergism. Genes Cancer 2011, 2, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P. Immune Checkpoint Therapy and the Search for Predictive Biomarkers. Cancer J. 2016, 22, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Spranger, S.; Gajewski, T.F. A New Paradigm for Tumor Immune Escape: β-Catenin-Driven Immune Exclusion. J. Immunother. Cancer 2015, 3, 43. [Google Scholar] [CrossRef]
- Biopahrma PEG FDA Approved Antibody-Drug Conjugates (ADCs) by 2024. Available online: https://www.biochempeg.com/article/74.html (accessed on 15 April 2024).
- Do, M.; Wu, C.C.N.; Sonavane, P.R.; Juarez, E.F.; Adams, S.R.; Ross, J.; Rodriguez Y Baena, A.; Patel, C.; Mesirov, J.P.; Carson, D.A.; et al. A FZD7-Specific Antibody–Drug Conjugate Induces Ovarian Tumor Regression in Preclinical Models. Mol. Cancer Ther. 2022, 21, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M. Antibody-Drug Conjugate Targeting Protein Tyrosine Kinase 7, a Receptor Tyrosine Kinase-like Molecule Involved in WNT and Vascular Endothelial Growth Factor Signaling: Effects on Cancer Stem Cells, Tumor Microenvironment and Whole-Body Homeostasis. Ann. Transl. Med. 2017, 5, 462. [Google Scholar] [CrossRef] [PubMed]
- Damelin, M.; Bankovich, A.; Bernstein, J.; Lucas, J.; Chen, L.; Williams, S.; Park, A.; Aguilar, J.; Ernstoff, E.; Charati, M.; et al. A PTK7-Targeted Antibody-Drug Conjugate Reduces Tumor-Initiating Cells and Induces Sustained Tumor Regressions. Sci. Transl. Med. 2017, 9, eaag2611. [Google Scholar] [CrossRef] [PubMed]
- Berger, H.; Breuer, M.; Peradziryi, H.; Podleschny, M.; Jacob, R.; Borchers, A. PTK7 Localization and Protein Stability Is Affected by Canonical Wnt Ligands. J. Cell Sci. 2017, 130, 1890–1903. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.; Scerbo, P.; Giordano, M.; Daulat, A.M.; Lhoumeau, A.-C.; Thomé, V.; Kodjabachian, L.; Borg, J.-P. The PTK7 and ROR2 Protein Receptors Interact in the Vertebrate WNT/Planar Cell Polarity (PCP) Pathway. J. Biol. Chem. 2015, 290, 30562–30572. [Google Scholar] [CrossRef] [PubMed]
- Maitland, M.L.; Sachdev, J.C.; Sharma, M.R.; Moreno, V.; Boni, V.; Kummar, S.; Stringer-Reasor, E.; Lakhani, N.; Moreau, A.R.; Xuan, D.; et al. First-in-Human Study of PF-06647020 (Cofetuzumab Pelidotin), an Antibody–Drug Conjugate Targeting Protein Tyrosine Kinase 7, in Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 4511–4520. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, D.; Ried, T. Targeting Colorectal Cancer (Stem-like) Cells Using LGR5 Directed Antibody Drug Conjugates. Ann. Transl. Med. 2016, 4, 508. [Google Scholar] [CrossRef]
- Junttila, M.R.; Mao, W.; Wang, X.; Wang, B.-E.; Pham, T.; Flygare, J.; Yu, S.-F.; Yee, S.; Goldenberg, D.; Fields, C.; et al. Targeting LGR5 + Cells with an Antibody-Drug Conjugate for the Treatment of Colon Cancer. Sci. Transl. Med. 2015, 7, 314ra186. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Li, N.; Renn, A.; Zhu, H.; Chen, L.; Shen, M.; Hall, M.D.; Qian, M.; Pastan, I.; Ho, M. GPC1-Targeted Immunotoxins Inhibit Pancreatic Tumor Growth in Mice via Depletion of Short-Lived GPC1 and Downregulation of Wnt Signaling. Mol. Cancer Ther. 2022, 21, 960–973. [Google Scholar] [CrossRef]
- Cherradi, S.; Garambois, V.; Marines, J.; Andrade, A.F.; Fauvre, A.; Morand, O.; Fargal, M.; Mancouri, F.; Ayrolles-Torro, A.; Vezzo-Vié, N.; et al. Improving the Response to Oxaliplatin by Targeting Chemotherapy-Induced CLDN1 in Resistant Metastatic Colorectal Cancer Cells. Cell Biosci. 2023, 13, 72. [Google Scholar] [CrossRef]
- Cui, J.; Park, S.; Yu, W.; Carmon, K.; Liu, Q.J. Drug Conjugates of Antagonistic RSPO4 Mutant For Simultaneous Targeting of LGR4/5/6 for Cancer Treatment. J. Med. Chem. 2021, 64, 12572. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, A.; Nejati, M.; Homayoonfal, M.; Arj, A.; Razavi, Z.S.; Ostadian, A.; Mohammadzadeh, B.; Vosough, M.; Karimi, M.; Rahimian, N.; et al. Doxorubicin-Loaded Zymosan Nanoparticles: Synergistic Cytotoxicity and Modulation of Apoptosis and Wnt/β-Catenin Signaling Pathway in C26 Colorectal Cancer Cells. Int. J. Biol. Macromol. 2024, 260, 128949. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.A.; Kwon, S.-J.; Bale, S.S.; Banerjee, A.; Dordick, J.S.; Kane, R.S. Regulation of Stem Cell Signaling by Nanoparticle-Mediated Intracellular Protein Delivery. Biomaterials 2011, 32, 3210–3219. [Google Scholar] [CrossRef] [PubMed]
- Hong, I.-S.; Jang, G.-B.; Lee, H.-Y.; Nam, J.-S.; An, S.S. Targeting Cancer Stem Cells by Using the Nanoparticles. Int. J. Nanomed. 2015, 10, 251–260. [Google Scholar] [CrossRef]
- Yi, Y.; Tang, H.; Pi, P.; Zhang, H.; Du, S.; Ge, W.; Dai, Q.; Zhao, Z.; Li, J.; Sun, Z. Melatonin in Cancer Biology: Pathways, Derivatives, and the Promise of Targeted Delivery. Drug Metab. Rev. 2024, 56, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Acharya, A.; Ray, R.S.; Agrawal, R.; Raghuwanshi, R.; Jain, P. Cellular and Molecular Mechanisms of Curcumin in Prevention and Treatment of Disease. Crit. Rev. Food Sci. Nutr. 2020, 60, 887–939. [Google Scholar] [CrossRef] [PubMed]
- Shelash Al-Hawary, S.I.; Abdalkareem Jasim, S.; Kadhim, M.M.; Jaafar Saadoon, S.; Ahmad, I.; Romero Parra, R.M.; Hasan Hammoodi, S.; Abulkassim, R.; Hameed, N.M.; Alkhafaje, W.K.; et al. Curcumin in the Treatment of Liver Cancer: From Mechanisms of Action to Nanoformulations. Phytother. Res. 2023, 37, 1624–1639. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.H.; Alhumaydhi, F.A.; Khan, M.A.; Younus, H. Therapeutic Potential of Polyphenols and Their Nanoformulations in the Treatment of Colorectal Cancer. Anti-Cancer Agents Med. Chem. 2021, 21, 2117–2129. [Google Scholar] [CrossRef] [PubMed]
- Najafiyan, B.; Bokaii Hosseini, Z.; Esmaelian, S.; Firuzpour, F.; Rahimipour Anaraki, S.; Kalantari, L.; Hheidari, A.; Mesgari, H.; Nabi-Afjadi, M. Unveiling the Potential Effects of Resveratrol in Lung Cancer Treatment: Mechanisms and Nanoparticle-Based Drug Delivery Strategies. Biomed. Pharmacother. 2024, 172, 116207. [Google Scholar] [CrossRef]
- Mohapatra, P.; Madhulika, S.; Behera, S.; Singh, P.; Sa, P.; Prasad, P.; Swain, R.K.; Sahoo, S.K. Nimbolide-Based Nanomedicine Inhibits Breast Cancer Stem-like Cells by Epigenetic Reprogramming of DNMTs-SFRP1-Wnt/β-Catenin Signaling Axis. Mol. Ther.—Nucleic Acids 2023, 34, 102031. [Google Scholar] [CrossRef]
- Liu, Y.; Guerrero, D.; Lechuga-Ballesteros, D.; Tan, M.; Ahmad, F.; Aleiwi, B.; Ellsworth, E.; Chen, B.; Chua, M.-S.; So, S. Lipid-Based Self-Microemulsion of Niclosamide Achieved Enhanced Oral Delivery and Anti-Tumor Efficacy in Orthotopic Patient-Derived Xenograft of Hepatocellular Carcinoma in Mice. Int. J. Nanomed. 2024, 19, 2639–2653. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, M.; Luo, W.; Zhang, Y.; Ji, H. Discovery of 2-(3-(3-Carbamoylpiperidin-1-Yl)Phenoxy)Acetic Acid Derivatives as Novel Small-Molecule Inhibitors of the β-Catenin/B-Cell Lymphoma 9 Protein–Protein Interaction. J. Med. Chem. 2021, 64, 5886–5904. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Ma, F.; Zhang, Z.; Yan, J. Turning a Targeting β-Catenin/Bcl9 Peptide Inhibitor into a GdOF@Au Core/Shell Nanoflower for Enhancing Immune Response to Cancer Therapy in Combination with Immune Checkpoint Inhibitors. Pharmaceutics 2022, 14, 1306. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, J.; You, W.; Zhao, X.; Hou, P.; He, W.; Yan, J.; Guo, H. Targeted Disruption of the BCL9/β-Catenin Interaction by Endosomal-Escapable Nanoparticles Functionalized with an E-Cadherin-Derived Peptide. Nanotechnology 2020, 31, 115102. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, D.; Sharda, N.; Banerjee, A.; Denisenko, K.; Basalious, E.B.; Shukla, H.; Waddell, J.; Hamdy, N.M.; Banerjee, A. Differential Signaling Pathways in Medulloblastoma: Nano-Biomedicine TargetingNon-Coding Epigenetics to Improve Current and Future Therapeutics. Curr. Pharm. Des. 2024, 30, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Yousefnia, S.; Seyed Forootan, F.; Seyed Forootan, S.; Nasr Esfahani, M.H.; Gure, A.O.; Ghaedi, K. Mechanistic Pathways of Malignancy in Breast Cancer Stem Cells. Front. Oncol. 2020, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-Y.; Gong, T.; Huang, Y.-K.; Kang, L.; Warner, C.A.; Xie, H.; Chen, L.-M.; Duan, X.-Q. Role of Noncoding RNAs in Liver Fibrosis. World J. Gastroenterol. 2023, 29, 1446–1459. [Google Scholar] [CrossRef] [PubMed]
- Chandramohan, K.; Balan, D.J.; Devi, K.P.; Nabavi, S.F.; Reshadat, S.; Khayatkashani, M.; Mahmoodifar, S.; Filosa, R.; Amirkhalili, N.; Pishvaei, S.; et al. Short Interfering RNA in Colorectal Cancer: Is It Wise to Shoot the Messenger? Eur. J. Pharmacol. 2023, 949, 175699. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Saxena, S.; Sharma, H.; Paudel, K.R.; Chakraborty, A.; MacLoughlin, R.; Oliver, B.G.; Gupta, G.; Negi, P.; Singh, S.K.; et al. Emerging Role of Tumor Suppressing microRNAs as Therapeutics in Managing Non-Small Cell Lung Cancer. Pathology—Res. Pract. 2024, 256, 155222. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Papadopoulos, K.P.; Patnaik, A.; Rasco, D.W.; Martinez, D.; Wood, D.L.; Fielman, B.; Sharma, M.; Janisch, L.A.; Brown, B.D.; et al. Safety and Activity of DCR-MYC, a First-in-Class Dicer-Substrate Small Interfering RNA (DsiRNA) Targeting MYC, in a Phase I Study in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2015, 33, 11006. [Google Scholar] [CrossRef]
- Rodriguez-Rivera, I.I.; Wu, T.-H.; Ciotti, R.; Senapedis, W.; Sullivan, K.; Gao, J.Z.; Palakurthi, S.; McCauley, T.; Moore, Y. A Phase 1/2 Open-Label Study to Evaluate the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Preliminary Antitumor Activity of OTX-2002 as a Single Agent and in Combination with Standard of Care in Patients with Hepatocellular Carcinoma and Other Solid Tumor Types Known for Association with the MYC Oncogene (MYCHELANGELO I). J. Clin. Oncol. 2023, 41, TPS627. [Google Scholar] [CrossRef]
- CancerNetwork OTX-2002 Shows Encouraging Safety in Small Hepatocellular Carcinoma Cohort. Available online: https://www.cancernetwork.com/view/otx-2002-shows-encouraging-safety-in-small-hepatocellular-carcinoma-cohort (accessed on 28 February 2024).
- Zhang, L.-M.; Li, M.; Tian, C.-C.; Wang, T.-T.; Mi, S.-F. CCAAT Enhancer Binding Protein α Suppresses Proliferation, Metastasis, and Epithelial-Mesenchymal Transition of Ovarian Cancer Cells via Suppressing the Wnt/β-Catenin Signaling. Neoplasma 2021, 68, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Setten, R.L.; Lightfoot, H.L.; Habib, N.A.; Rossi, J.J. Development of MTL-CEBPA: Small Activating RNA Drug for Hepatocellular Carcinoma. Curr. Pharm. Biotechnol. 2018, 19, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Sarker, D.; Plummer, R.; Meyer, T.; Sodergren, M.H.; Basu, B.; Chee, C.E.; Huang, K.-W.; Palmer, D.H.; Ma, Y.T.; Evans, T.R.J.; et al. MTL-CEBPA, a Small Activating RNA Therapeutic Upregulating C/EBP-α, in Patients with Advanced Liver Cancer: A First-in-Human, Multicenter, Open-Label, Phase I Trial. Clin. Cancer Res. 2020, 26, 3936–3946. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Moradi, S.Z.; Faraji, F.; Farhadi, T.; Hesami, O.; Iranpanah, A.; Webber, K.; Bishayee, A. Current Advances in Nanoformulations of Therapeutic Agents Targeting Tumor Microenvironment to Overcome Drug Resistance. Cancer Metastasis Rev. 2023, 42, 959–1020. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Thiruvengadam, M.; Venkidasamy, B.; Alomary, M.N.; Salawi, A.; Chung, I.-M.; Shariati, M.A.; Rebezov, M. Exosome-Based Nanomedicine for Cancer Treatment by Targeting Inflammatory Pathways: Current Status and Future Perspectives. Semin. Cancer Biol. 2022, 86, 678–696. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.-B.; Cai, L. Smart Nanoparticles for Cancer Therapy. Sig Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, C.; Franken, P.; Molenaar, L.; Breukel, C.; Van Der Valk, M.; Smits, R.; Fodde, R. A Targeted Constitutive Mutation in the Apc Tumor Suppressor Gene Underlies Mammary But Not Intestinal Tumorigenesis. PLoS Genet. 2009, 5, e1000547. [Google Scholar] [CrossRef]
- Letai, A.; Bhola, P.; Welm, A.L. Functional Precision Oncology: Testing Tumors with Drugs to Identify Vulnerabilities and Novel Combinations. Cancer Cell 2022, 40, 26–35. [Google Scholar] [CrossRef]
- Rodríguez, N.; Viñal, D.; Rodríguez-Cobos, J.; De Castro, J.; Domínguez, G. Genomic Profiling in Oncology Clinical Practice. Clin. Transl. Oncol. 2020, 22, 1430–1439. [Google Scholar] [CrossRef]
- Cook, D.P.; Vanderhyden, B.C. Ovarian Cancer and the Evolution of Subtype Classifications Using Transcriptional Profiling†. Biol. Reprod. 2019, 101, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, K.; Peymani, M.; Rashidi, M.; Hushmandi, K.; Ghaedi, K.; Taheriazam, A.; Hashemi, M. Colon Cancer Transcriptome. Prog. Biophys. Mol. Biol. 2023, 180–181, 49–82. [Google Scholar] [CrossRef]
- Huang, P.; Gao, W.; Fu, C.; Tian, R. Functional and Clinical Proteomic Exploration of Pancreatic Cancer. Mol. Cell. Proteom. 2023, 22, 100575. [Google Scholar] [CrossRef]
- Wong, G.Y.M.; Diakos, C.; Hugh, T.J.; Molloy, M.P. Proteomic Profiling and Biomarker Discovery in Colorectal Liver Metastases. Int. J. Mol. Sci. 2022, 23, 6091. [Google Scholar] [CrossRef] [PubMed]
- Madama, D.; Martins, R.; Pires, A.S.; Botelho, M.F.; Alves, M.G.; Abrantes, A.M.; Cordeiro, C.R. Metabolomic Profiling in Lung Cancer: A Systematic Review. Metabolites 2021, 11, 630. [Google Scholar] [CrossRef] [PubMed]
- Aurilio, G.; Santoni, M.; Massari, F.; Cimadamore, A.; Rizzo, A.; Mollica, V.; Verri, E.; Battelli, N.; Montironi, R. Metabolomic Profiling in Renal Cell Carcinoma Patients: News and Views. Cancers 2021, 13, 5229. [Google Scholar] [CrossRef] [PubMed]
- Wojas-Krawczyk, K.; Paśnik, I.; Kucharczyk, T.; Wieleba, I.; Krzyżanowska, N.; Gil, M.; Krawczyk, P.; Milanowski, J. Immunoprofiling: An Encouraging Method for Predictive Factors Examination in Lung Cancer Patients Treated with Immunotherapy. Int. J. Mol. Sci. 2021, 22, 9133. [Google Scholar] [CrossRef] [PubMed]
- Koelzer, V.H.; Sirinukunwattana, K.; Rittscher, J.; Mertz, K.D. Precision Immunoprofiling by Image Analysis and Artificial Intelligence. Virchows Arch. 2019, 474, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, D.; Solit, D.B. Clinical Cancer Genomic Profiling. Nat. Rev. Genet. 2021, 22, 483–501. [Google Scholar] [CrossRef]
- Borhani, A.A.; Catania, R.; Velichko, Y.S.; Hectors, S.; Taouli, B.; Lewis, S. Radiomics of Hepatocellular Carcinoma: Promising Roles in Patient Selection, Prediction, and Assessment of Treatment Response. Abdom. Radiol. 2021, 46, 3674–3685. [Google Scholar] [CrossRef]
- Babu, M.; Snyder, M. Multi-Omics Profiling for Health. Mol. Cell. Proteom. 2023, 22, 100561. [Google Scholar] [CrossRef] [PubMed]
- Fountzilas, E.; Tsimberidou, A.M.; Vo, H.H.; Kurzrock, R. Clinical Trial Design in the Era of Precision Medicine. Genome Med. 2022, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A. Biomarkers and Precision Medicine in Oncology Practice and Clinical Trials. In Advancing the Science of Cancer in Latinos; Ramirez, A.G., Trapido, E.J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 113–123. ISBN 978-3-030-29285-0. [Google Scholar]
- Mortezaee, K. WNT/β-Catenin Regulatory Roles on PD-(L)1 and Immunotherapy Responses. Clin. Exp. Med. 2024, 24, 15. [Google Scholar] [CrossRef] [PubMed]
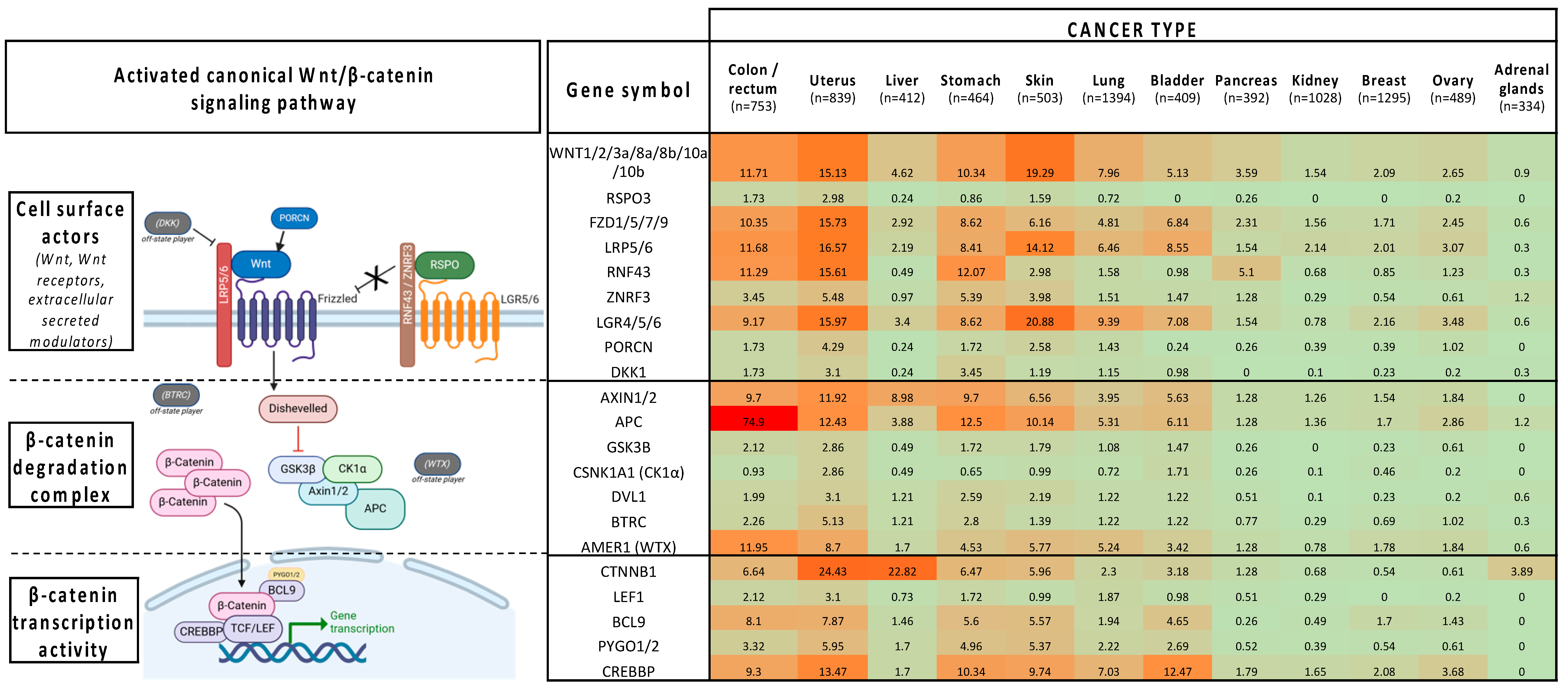
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Pellegars-Malhortie, A.; Picque Lasorsa, L.; Mazard, T.; Granier, F.; Prévostel, C. Why Is Wnt/β-Catenin Not Yet Targeted in Routine Cancer Care? Pharmaceuticals 2024, 17, 949. https://doi.org/10.3390/ph17070949
de Pellegars-Malhortie A, Picque Lasorsa L, Mazard T, Granier F, Prévostel C. Why Is Wnt/β-Catenin Not Yet Targeted in Routine Cancer Care? Pharmaceuticals. 2024; 17(7):949. https://doi.org/10.3390/ph17070949
Chicago/Turabian Stylede Pellegars-Malhortie, Auriane, Laurence Picque Lasorsa, Thibault Mazard, Fabien Granier, and Corinne Prévostel. 2024. "Why Is Wnt/β-Catenin Not Yet Targeted in Routine Cancer Care?" Pharmaceuticals 17, no. 7: 949. https://doi.org/10.3390/ph17070949
APA Stylede Pellegars-Malhortie, A., Picque Lasorsa, L., Mazard, T., Granier, F., & Prévostel, C. (2024). Why Is Wnt/β-Catenin Not Yet Targeted in Routine Cancer Care? Pharmaceuticals, 17(7), 949. https://doi.org/10.3390/ph17070949







