Phytochemical Profile, Antioxidant, Anti-Atopic, and Anti-Inflammatory Activities of Filipendula glaberrima Nakai at Different Growth Stages
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Polyphenol and Flavonoid Content (TPC and TFC)
2.2. Antioxidant Activities
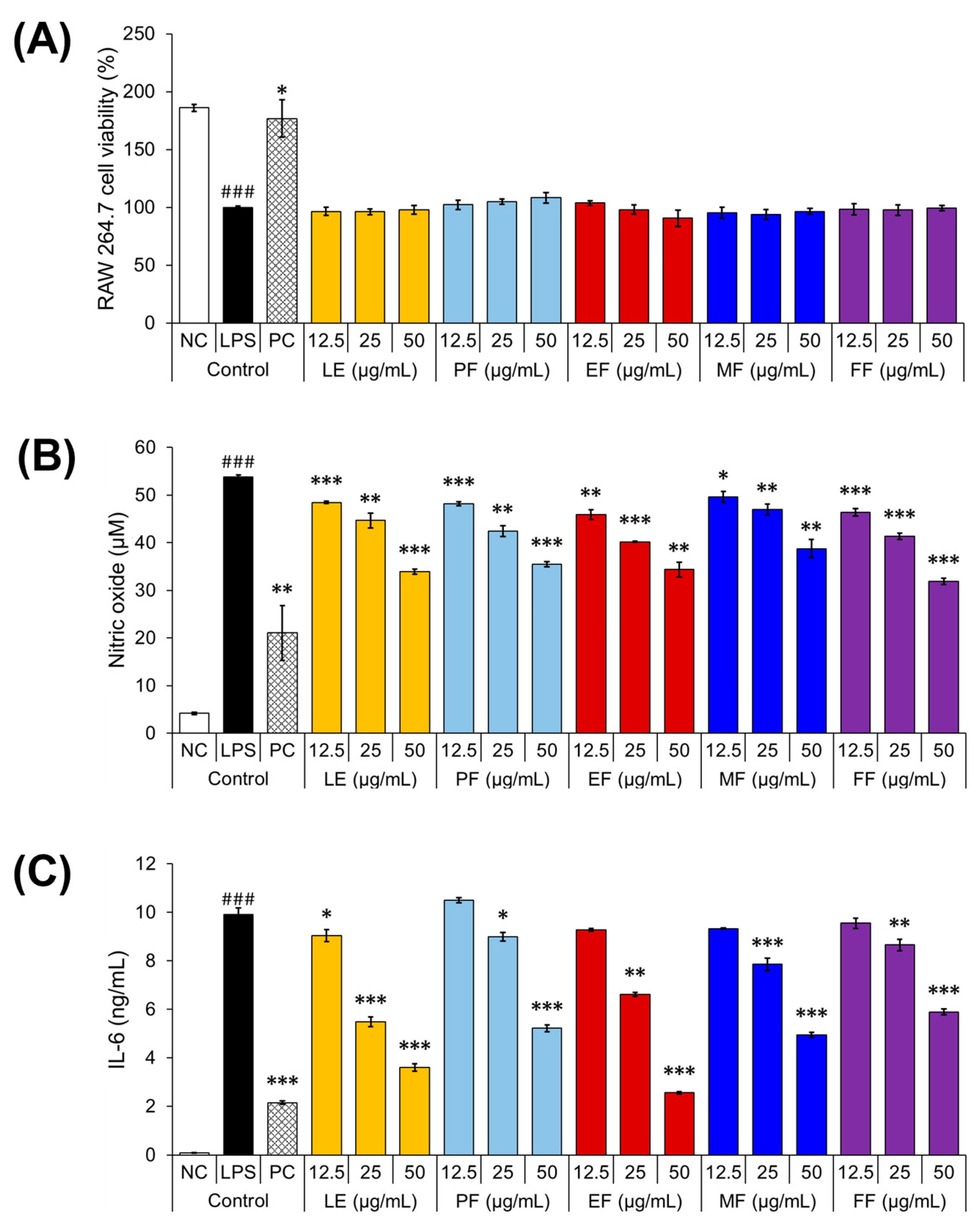
2.3. Anti-Inflammatory Activities
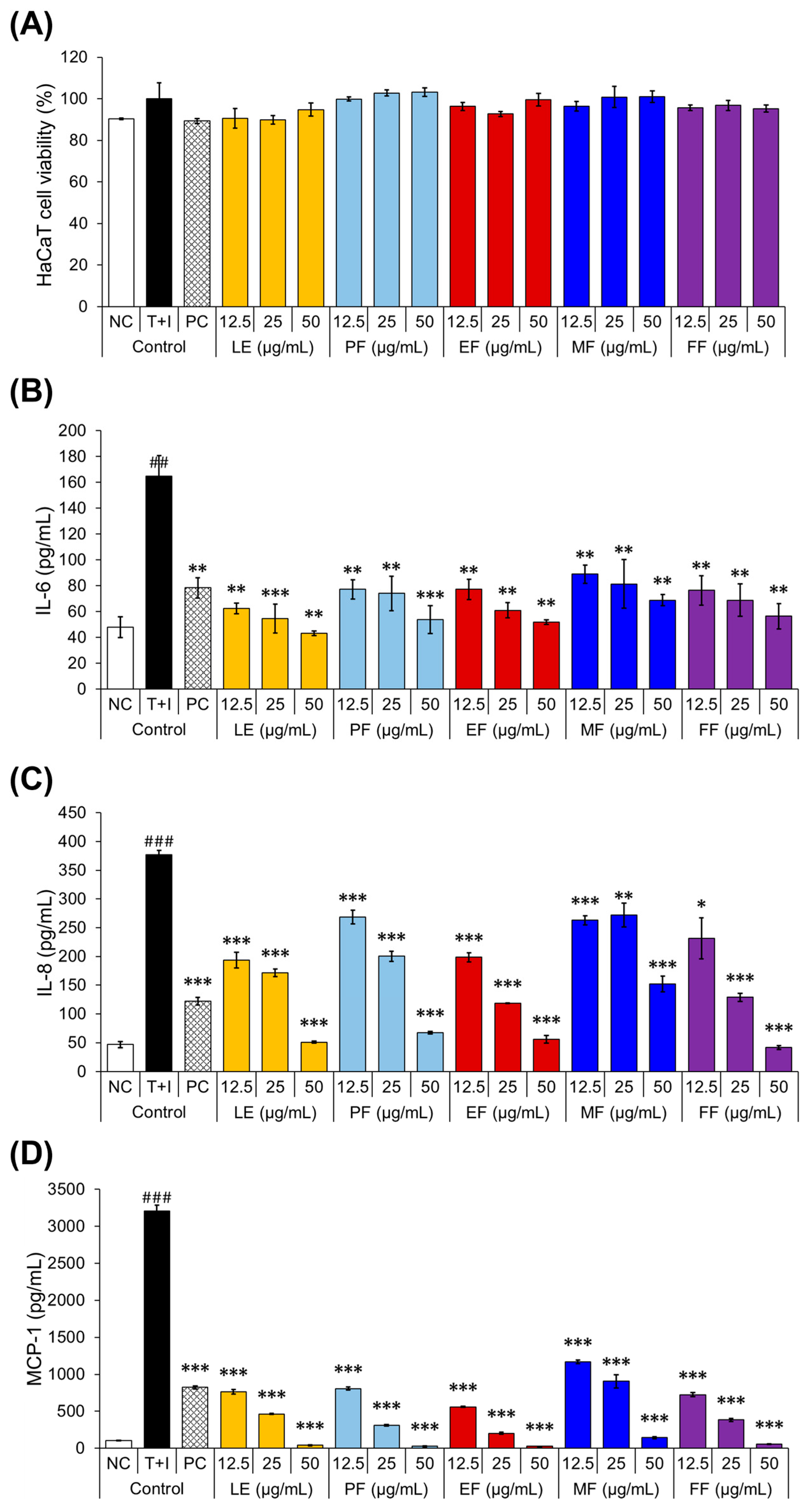
2.4. Anti-Atopic Activities
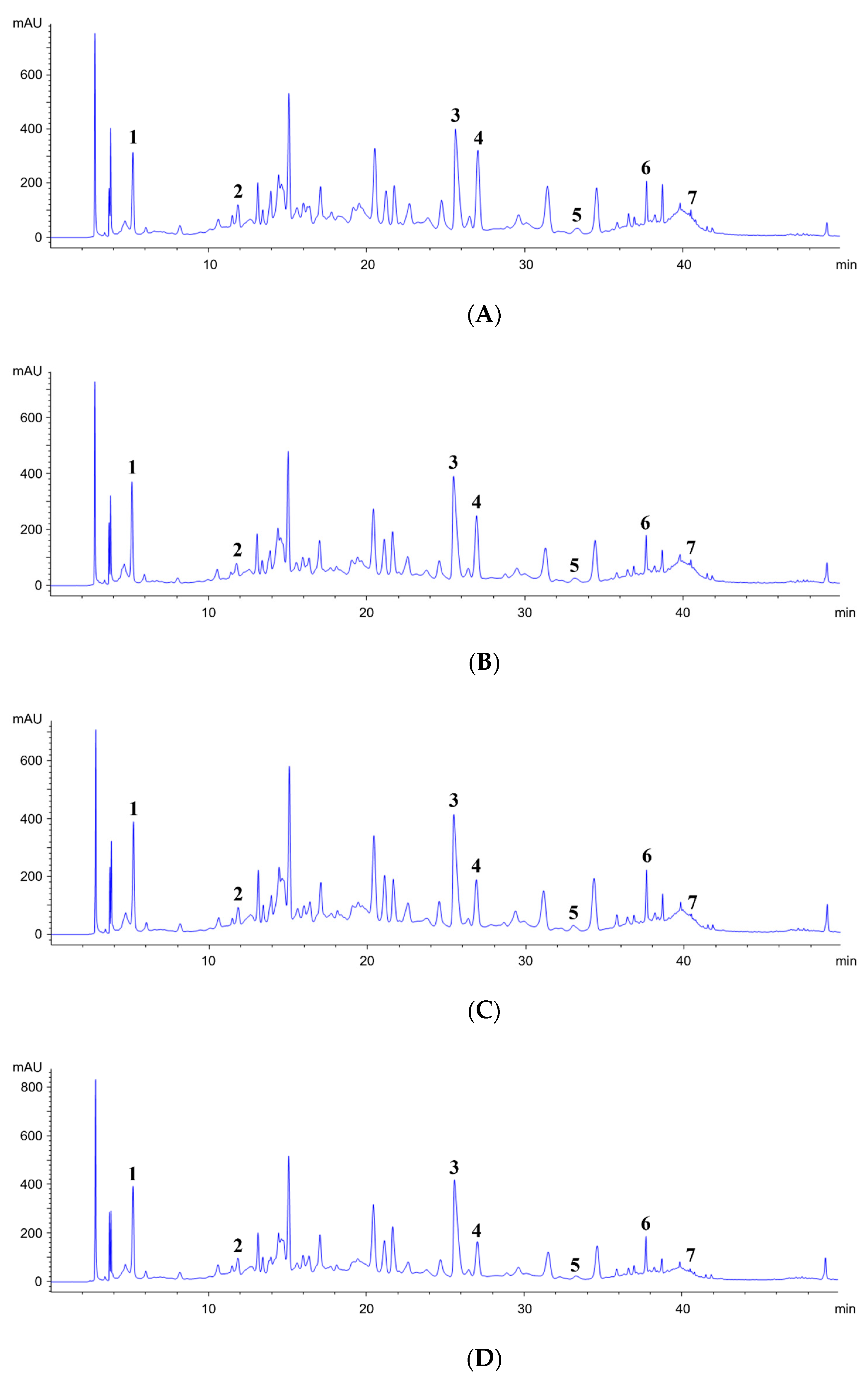
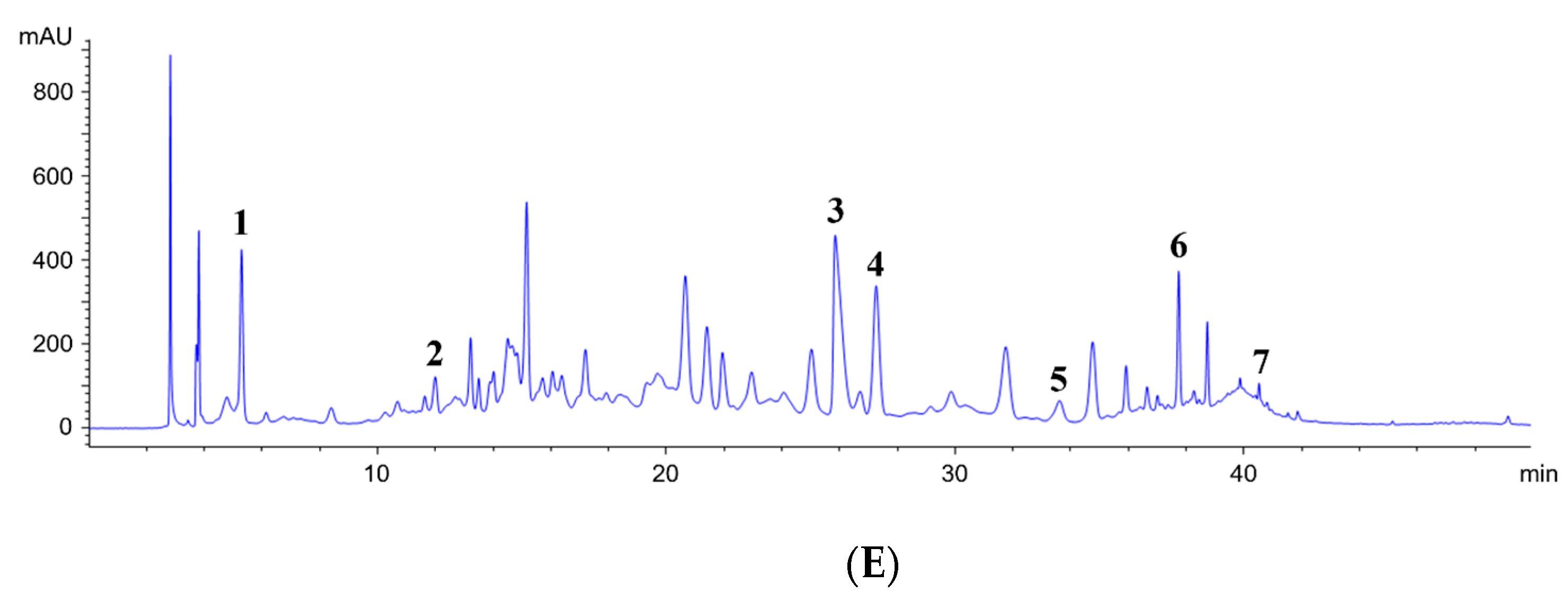
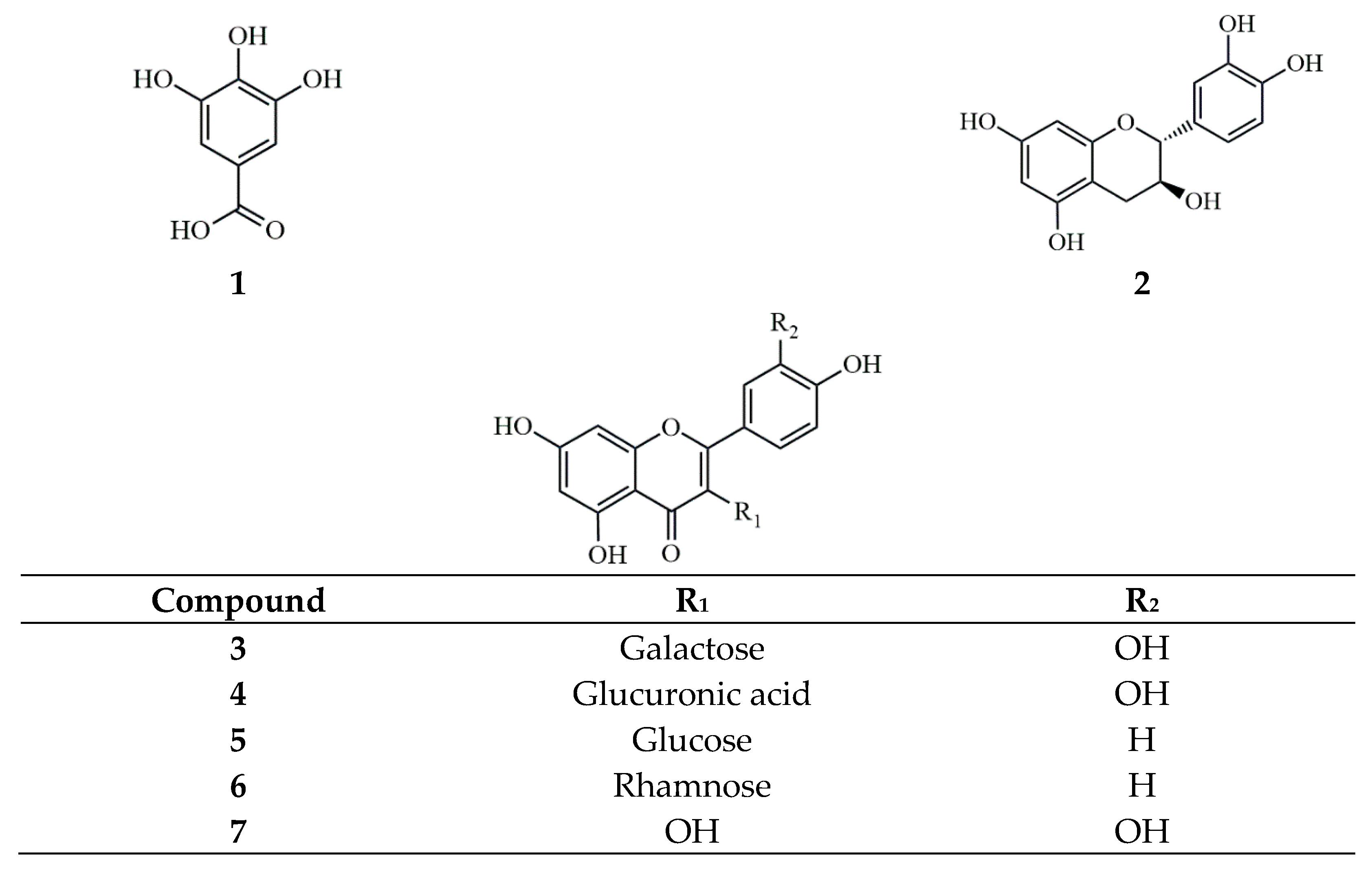
2.5. Characterization of Phytochemical Content by HPLC Analysis
3. Materials and Methods
3.1. Plant Materials
3.2. Instruments, Materials, and Reagents
3.3. Extraction and Isolation
3.4. Analysis of the TPC and TFC
3.5. DPPH Radical Scavenging Activity
3.6. ABTS Radical Scavenging Activity
3.7. Evaluation of Anti-Inflammatory Activity
3.8. Evaluation of Anti-Atopy Activity
3.9. Cell Viability, Griess Assay, and ELISA
3.10. Preparation of the Sample and Standard Solutions for HPLC
3.11. HPLC/UV Conditions
3.12. Calibration Curve
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, K.-J.; Yung, H.C. Monographic study of the endemic plants in Korea V. Taxonomy and interspecific relationships of the genus Filipendula. J. Plant Biol. 1986, 29, 19–40. [Google Scholar]
- Samardžić, S.; Arsenijević, J.; Božić, D.; Milenković, M.; Tešević, V.; Maksimović, Z. Antioxidant, anti-inflammatory and gastroprotective activity of Filipendula ulmaria (L.) Maxim. and Filipendula vulgaris Moench. J. Ethnopharmacol. 2018, 213, 132–137. [Google Scholar] [CrossRef]
- Farzaneh, A.; Hadjiakhoondi, A.; Khanavi, M.; Manayi, A.; Bahramsoltani, R.; Kalkhorani, M. Filipendula ulmaria (L.) Maxim. (Meadowsweet): A review of traditional uses, phytochemistry and pharmacology. Res. J. Pharmacogn. 2022, 9, 85–106. [Google Scholar] [CrossRef]
- Jung, J.; Hong, M.; Hwang, D. The water extract of Korean Filipendula glaberrima Nakai attenuates acute colitis by suppressing inflammation via the MAPK and NF-κB pathways. J. Funct. Foods 2023, 106, 105606. [Google Scholar] [CrossRef]
- Katanić, J.; Pferschy-Wenzig, E.M.; Mihailović, V.; Boroja, T.; Pan, S.P.; Nikles, S.; Kretschmer, N.; Rosić, G.; Selaković, D.; Joksimović, J.; et al. Phytochemical analysis and anti-inflammatory effects of Filipendula vulgaris Moench extracts. Food Chem. Toxicol. 2018, 122, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, E.; Krol, T.; Adamov, G.; Minyazeva, Y.; Baleev, D.; Sidelnikov, N. Total content and composition of phenolic compounds from Filipendula genus plants and their potential health-promoting properties. Molecules 2024, 29, 2013. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.D.; Lee, Y.; Kim, H.; Kim, H.; Park, C.G.; Lee, S. HPLC/UV quantification of (+)-catechin in Filipendula glaberrima from different regions and flowering stages. Korean J. Pharmacogn. 2020, 51, 291–296. [Google Scholar] [CrossRef]
- Pemp, E.; Reznicek, G.; Krenn, L. Fast quantification of flavonoids in Filipendulae ulmariae Flos by HPLC/ESI-MS using a nonporous stationary phase. J. Anal. Chem. 2014, 62, 669–673. [Google Scholar] [CrossRef]
- Yeo, H.S.; Kim, J.; Chung, B.S. Phytochemical studies on Filipendula glaberrima. Planta Med. 1990, 56, 539. [Google Scholar] [CrossRef]
- Cho, Y.B.; Lee, H.; Jeon, H.J.; Lee, J.Y.; Kim, H.J. Antioxidant and inhibitory activities of Filipendula glaberrima leaf constituents against HMG-CoA reductase and macrophage foam cell formation. Molecules 2024, 29, 354. [Google Scholar] [CrossRef]
- Darlenski, R.; Kazandjieva, J.; Hristakieva, E.; Fluhr, J.W. Atopic dermatitis as a systemic disease. Clin. Dermatol. 2014, 32, 409–413. [Google Scholar] [CrossRef]
- Klonowska, J.; Gleń, J.; Nowicki, R.J.; Trzeciak, M. New cytokines in the pathogenesis of atopic dermatitis—New therapeutic targets. Int. J. Mol. Sci. 2018, 19, 3086. [Google Scholar] [CrossRef] [PubMed]
- Humeau, M.; Boniface, K.; Bodet, C. Cytokine-mediated crosstalk between keratinocytes and T cells in atopic dermatitis. Front. Immunol. 2022, 13, 801579. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, Y.; Nakashima, C.; Otsuka, A. Interplay of cytokines in the pathophysiology of atopic dermatitis: Insights from murin models and human. Front. Med. 2024, 11, 1342176. [Google Scholar] [CrossRef] [PubMed]
- Alsabbagh, M.; Ismaeel, A. The role of cytokines in atopic dermatitis: A breakthrough in immunopathogenesis and treatment. Acta Dermatovenerol. Alp. Pannonica Adriat. 2022, 31, 13–31. [Google Scholar] [CrossRef]
- Maina, S.; Ryu, D.H.; Bakari, G.; Misinzo, G.; Nho, C.W.; Kim, H.Y. Variation in phenolic compounds and antioxidant activity of various organs of African cabbage (Cleome gynandra L.) accessions at different growth stages. Antioxidants 2021, 10, 1952. [Google Scholar] [CrossRef]
- Makarova, K.; Sajkowska-Kozielewicz, J.J.; Zawada, K.; Olchowik-Grabarek, E.; Ciach, M.A.; Gogolewski, K.; Dobros, N.; Ciechowicz, P.; Freichels, H.; Gambin, A. Harvest time affects antioxidant capacity, total polyphenol and flavonoid content of Polish St John’s Wort’s (Hypericum perforatum L.) flowers. Sci. Rep. 2021, 11, 3989. [Google Scholar] [CrossRef]
- Cirak, C.; Radusiěnë, J.; Karabük, B.; Janulis, V.; Ivanauskas, L. Variation of bioactive compounds in Hypericum perforatum growing in Turkey during its phenological cycle. J. Integr. Plant Biol. 2007, 49, 615–620. [Google Scholar] [CrossRef]
- Savina, T.; Lisun, V.; Feduraev, P.; Skrypnik, L. Variation in phenolic compounds, antioxidant and antibacterial activities of extracts from different plant organs of meadowsweet (Filipendula ulmaria (L.) Maxim.). Molecules 2023, 28, 3512. [Google Scholar] [CrossRef]
- Han, M.; Zhao, Y.; Meng, J.; Yin, J.; Li, H. Analysis of physicochemical and antioxidant properties of Malus spp. petals reveals factors involved in flower color change and market value. Sci. Hortic. 2023, 310, 111688. [Google Scholar] [CrossRef]
- Ayele, D.T.; Akele, M.L.; Melese, A.T. Analysis of total phenolic contents, flavonoids, antioxidant and antibacterial activities of Croton macrostachyus root extracts. BMC Chem. 2022, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Dibacto, R.E.K.; Tchuente, B.R.T.; Nguedjo, M.W.; Tientcheu, Y.M.T.; Nyobe, E.C.; Edoun, F.L.E.; Kamini, M.F.G.; Dibanda, R.F.; Medoua, G.N. Total polyphenol and flavonoid content and antioxidant capacity of some varieties of Persea americana peels consumed in Cameroon. Sci. World J. 2021, 2021, 8882594. [Google Scholar] [CrossRef] [PubMed]
- Quyen, N.T.C.; Quyen, N.T.N.; Quy, N.N.; Quan, P.M. Evaluation of total polyphenol content, total flavonoid content, and antioxidant activity of Centella asiatica. IOP Conf. Ser. Mater. Sci. Eng. 2020, 991, 012020. [Google Scholar] [CrossRef]
- Önder, D. Variation in antioxidant capacity, antioxidant activity and mineral composition during flower development of oil-bearing rose (Rosa damascena Mill.). Sci. Rep. 2023, 13, 17255. [Google Scholar] [CrossRef] [PubMed]
- Facchin, B.M.; dos Reis, G.O.; Vieira, G.N.; Mohr, E.T.B.; da Rosa, J.S.; Kretzer, I.F.; Demarchi, I.G.; Dalmarco, E.M. Inflammatory biomarkers on an LPS-induced RAW 264.7 cell model: A systematic review and meta-analysis. Inflamm. Res. 2022, 71, 741–758. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Giampieri, F.; Afrin, S.; Alvarez-Suarez, J.M.; Mazzoni, L.; Mezzetti, B.; Quiles, J.L.; Battino, M. Anti-inflammatory effect of strawberry extract against LPS-induced stress in RAW 264.7 macrophages. Food Chem. Toxicol. 2017, 102, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Sun, L.; Liu, B.; Deng, Y.S.; Zhan, D.; Chen, Y.L.; He, Y.; Liu, J.; Zhang, Z.J.; Sun, J.; et al. Resveratrol inhibits LPS-induced MAPKs activation via activation of the phosphatidylinositol 3-kinase pathway in murine RAW 264.7 macrophage cells. PLoS ONE 2012, 7, e44107. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Dhandapani, S.; Wang, R.; cheol Hwang, K.; Kim, H.; Kim, Y.J. Exploring the potential anti-inflammatory effect of biosynthesized gold nanoparticles using Isodon excisus leaf tissue in human keratinocytes. Arab. J. Chem. 2023, 16, 105113. [Google Scholar] [CrossRef]
- Vaughan, M.M.; Block, A.; Christensen, S.A.; Allen, L.H.; Schmelz, E.A. The effects of climate change associated abiotic stresses on maize phytochemical defenses. Phytochem. Rev. 2018, 17, 37–49. [Google Scholar] [CrossRef]
- Zhang, B.; Murtaza, A.; Iqbal, A.; Zhang, J.; Bai, T.; Ma, W.; Xu, X.; Pan, S.; Hu, W. Comparative study on nutrient composition and antioxidant capacity of potato based on geographical and climatic factors. Food Biosci. 2022, 46, 101536. [Google Scholar] [CrossRef]
- Karlová, K. Accumulation of flavonoid compounds in flowering shoots of Achillea collina Becker ex. Rchb. alba during flower development. Hortic. Sci. 2006, 33, 158–162. [Google Scholar] [CrossRef]
- So, J.; Lee, H.D.; Kim, J.H.; Lee, S.; Lim, J.H. Antioxidant, antimicrobial, and skin-whitening effects and quantitative analysis of phenolic compounds in Korean wild Chrysanthemum flowers via HPLC/UV. Hortic. Environ. Biotechnol. 2024, 65, 215–227. [Google Scholar] [CrossRef]
- Kim, J.; Uy, N.P.; Kim, D.; Lee, S. Analysis of phenolic acid content and antioxidant activity of chestnut honey from different regions of Korea. Nat. Prod. Sci. 2023, 29, 127–131. [Google Scholar] [CrossRef]
- Gupta, N.; Sharma, S.K.; Rana, J.C.; Chauhan, R.S. Expression of flavonoid biosynthesis genes vis-à-vis rutin content variation in different growth stages of Fagopyrum species. J. Plant Physiol. 2011, 168, 2117–2123. [Google Scholar] [CrossRef] [PubMed]
- Vvedenskaya, I.O.; Vorsa, N. Flavonoid composition over fruit development and maturation in American cranberry, Vaccinium macrocarpon Ait. Plant Sci. 2004, 167, 1043–1054. [Google Scholar] [CrossRef]
- Khatiwora, E.; Adsul, V.B.; Kulkarni, M.M.; Deshpande, N.R.; Kashalkar, R.V. Spectroscopic determination of total phenol and flavonoid contents of Ipomoea carnea. Int. J. ChemTech Res. 2010, 2, 1698–1701. [Google Scholar]
- Baraldi, R.; Isacchi, B.; Predieri, S.; Marconi, G.; Vincieri, F.F.; Bilia, A.R. Distribution of artemisinin and bioactive flavonoids from Artemisia annua L. during plant growth. Biochem. Syst. Ecol. 2008, 36, 340–348. [Google Scholar] [CrossRef]
- Choi, J.; An, J.; Lee, H.D.; Kim, W.J.; Lee, S.; Lee, S. Comprehensive analysis of phenolic compounds, carotenoids, and antioxidant activities in Lactuca sativa var. longifolia cultivated in a smart farm system. Processes 2023, 11, 2993. [Google Scholar] [CrossRef]
- Doan, T.T.M.; Tran, G.H.; Nguyen, T.K.; Lim, J.H.; Lee, S. Antioxidant activity of different cultivars of Chrysanthemum morifolium and quantitative analysis of phenolic compounds by HPLC/UV. Appl. Biol. Chem. 2024, 67, 17. [Google Scholar] [CrossRef]
- Choi, J.; Lee, H.D.; Cho, H.; Lee, C.D.; Tran, G.H.; Kim, H.; Moon, S.K.; Lee, S. Antioxidative phenolic compounds from the aerial parts of Cyperus exaltatus var. iwasakii and their HPLC analysis. Appl. Biol. Chem. 2023, 66, 61. [Google Scholar] [CrossRef]
| Samples | TPC (mg GAE/mL) | TFC (mg QE/mL) | IC50 (mg/mL) | |
|---|---|---|---|---|
| ABTS | DPPH | |||
| LE | 12.75 ± 1.91 ab | 7.49 ± 0.59 a | 0.23 ± 0.01 c | 0.69 ± 0.03 a |
| PF | 9.06 ± 1.50 bc | 5.31 ± 0.29 b | 0.27 ± 0.01 ab | 0.67 ± 0.06 ab |
| EF | 7.01 ± 0.91 c | 5.97 ± 0.13 ab | 0.25 ± 0.00 bc | 0.65 ± 0.06 ab |
| MF | 5.53 ± 0.90 c | 5.37 ± 0.23 b | 0.27 ± 0.00 a | 0.72 ± 0.07 a |
| FF | 14.75 ± 2.69 a | 7.75 ± 1.31 a | 0.16 ± 0.01 d | 0.55 ± 0.02 b |
| Ascorbic acid | 0.10 ± 0.00 | 0.08 ± 0.00 | ||
| Compound | tR | Linear Range (µg/mL) | LOD (µg/mL) | LOQ (µg/mL) | Calibration Equation | Correlation Factor, r2 |
|---|---|---|---|---|---|---|
| Gallic acid (1) | 5.01 | 0–500 | 0.33 | 0.98 | Y = 32.134X + 169.17 | 1.0000 |
| (+)-Catechin (2) | 11.9 | 0–500 | 0.48 | 1.45 | Y = 3.3541X + 31.146 | 0.9996 |
| Hyperin (3) | 25.9 | 0–500 | 0.39 | 1.19 | Y = 15.666X + 79.491 | 0.9999 |
| Miquelianin (4) | 27.1 | 0–500 | 0.23 | 0.69 | Y = 19.653X + 81.667 | 1.0000 |
| Astragalin (5) | 33.2 | 0–500 | 0.31 | 0.95 | Y = 16.769X + 65.244 | 0.9998 |
| Afzelin (6) | 37.9 | 0–500 | 0.81 | 2.41 | Y = 14.891X + 137.51 | 0.9995 |
| Quercetin (7) | 40.3 | 0–50 | 0.54 | 1.65 | Y = 23.209X + 19.113 | 0.9995 |
| Compound | Content (mg/g ext.) | ||||
|---|---|---|---|---|---|
| LE | PF | EF | MF | FF | |
| Gallic acid (1) | 1.98 ± 0.02 e | 2.47 ± 0.02 d | 2.73 ± 0.01 b | 2.59 ± 0.01 c | 2.84 ± 0.02 a |
| (+)-Catechin (2) | 9.90 ± 0.02 b | 7.91 ± 0.17 c | 7.57 ± 0.05 d | 5.12 ± 0.05 e | 10.30 ± 0.07 a |
| Hyperin (3) | 12.76 ± 0.03 e | 13.12 ± 0.07 d | 13.77 ± 0.03 c | 14.45 ± 0.02 b | 16.21 ± 0.03 a |
| Miquelianin (4) | 7.11 ± 0.03 b | 5.42 ± 0.03 c | 3.95 ± 0.06 d | 3.41 ± 0.01 e | 7.62 ± 0.01 a |
| Astragalin (5) | 0.94 ± 0.03 b | 0.71 ± 0.02 d | 0.84 ± 0.01 c | 0.60 ± 0.01 e | 2.12 ± 0.02 a |
| Afzelin (6) | 1.92 ± 0.01 c | 1.65 ± 0.02 e | 2.27 ± 0.02 b | 1.77 ± 0.01 d | 4.06 ± 0.02 a |
| Quercetin (7) | 0.11 ± 0.00 b | 0.10 ± 0.00 c | tr | tr | 0.18 ± 0.00 a |
| Total content | 34.72 | 31.38 | 31.13 | 24.53 | 43.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-D.; Tonog, G.; Uy, N.P.; Lee, Y.; Kim, K.-Y.; Kim, H.; Lee, S. Phytochemical Profile, Antioxidant, Anti-Atopic, and Anti-Inflammatory Activities of Filipendula glaberrima Nakai at Different Growth Stages. Pharmaceuticals 2024, 17, 928. https://doi.org/10.3390/ph17070928
Lee H-D, Tonog G, Uy NP, Lee Y, Kim K-Y, Kim H, Lee S. Phytochemical Profile, Antioxidant, Anti-Atopic, and Anti-Inflammatory Activities of Filipendula glaberrima Nakai at Different Growth Stages. Pharmaceuticals. 2024; 17(7):928. https://doi.org/10.3390/ph17070928
Chicago/Turabian StyleLee, Hak-Dong, Genevieve Tonog, Neil Patrick Uy, Yunji Lee, Ki-Young Kim, Hangeun Kim, and Sanghyun Lee. 2024. "Phytochemical Profile, Antioxidant, Anti-Atopic, and Anti-Inflammatory Activities of Filipendula glaberrima Nakai at Different Growth Stages" Pharmaceuticals 17, no. 7: 928. https://doi.org/10.3390/ph17070928
APA StyleLee, H.-D., Tonog, G., Uy, N. P., Lee, Y., Kim, K.-Y., Kim, H., & Lee, S. (2024). Phytochemical Profile, Antioxidant, Anti-Atopic, and Anti-Inflammatory Activities of Filipendula glaberrima Nakai at Different Growth Stages. Pharmaceuticals, 17(7), 928. https://doi.org/10.3390/ph17070928









