Where Are We Now with Oncolytic Viruses in Melanoma and Nonmelanoma Skin Malignancies?
Abstract
1. Introduction
2. Current State of OVs in Melanoma
2.1. Current State of T-VEC Usage in Melanoma
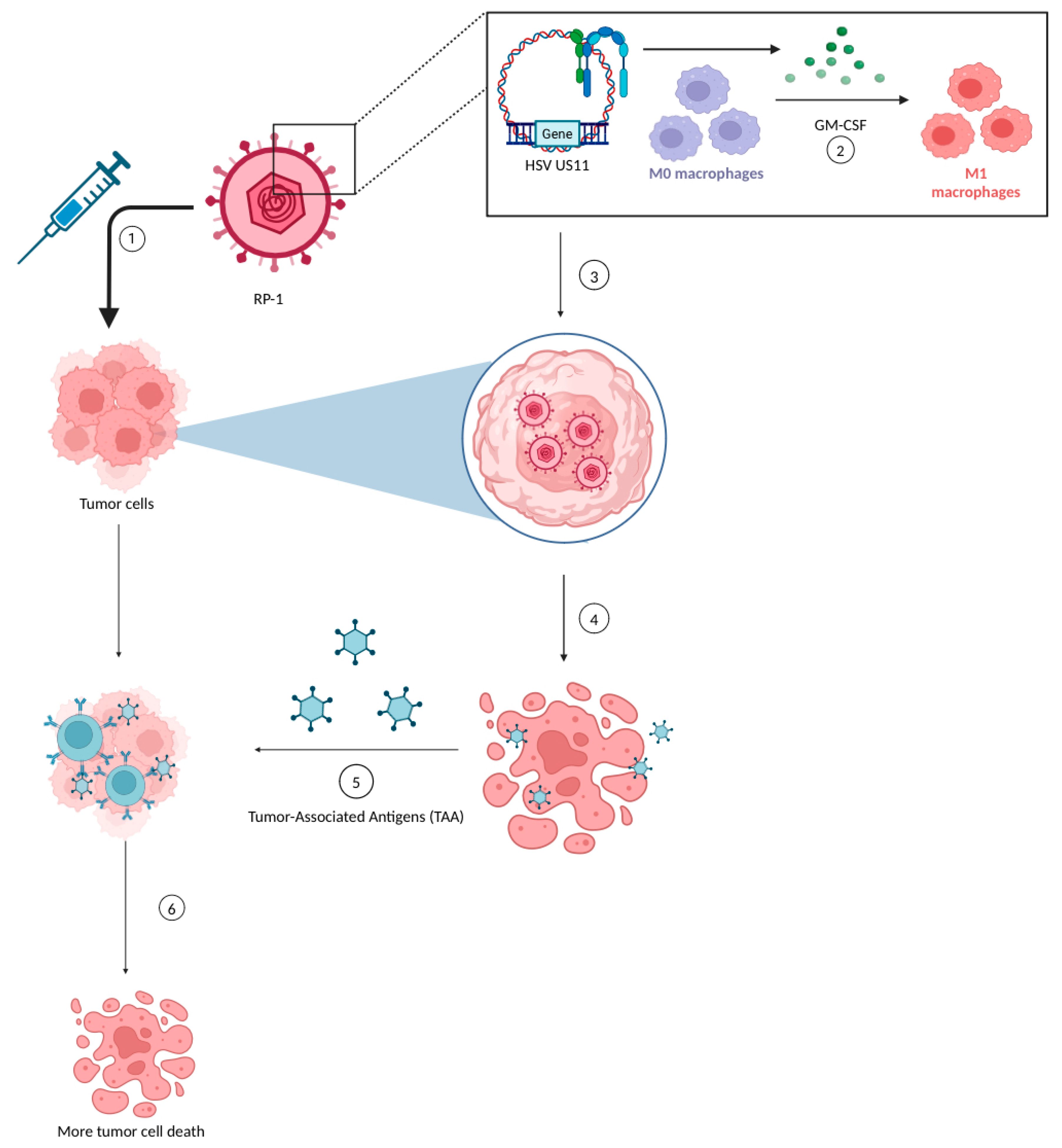
2.2. Current State of RP-1 Therapy
2.3. Advancements in Other OV Therapies
| Authors, Study Name, NCT # | Trial Stage | Therapeutic Approach | N | Disease/Stage | Outcomes | Common TRAE/Grade 3–4 |
|---|---|---|---|---|---|---|
| Ramelyte et al., 2023 (NCT03458117) [51] | Phase I (Completed) | T-VEC | 27 | Locally advanced SCC, BCC, MCC, and cutaneous T-cell lymphoma | 84% response in injected tumors, 40% non-injected response, 84% reduction in elevation, and 68% reduction in redness. | The most common TRAEs experienced were fever, flu-like symptoms, and the ulceration of the injected lesion. |
| (NCT04163952) | Phase I (Active, Not Recruiting) | T-VEC + Panitumumab | 5 | SCC | N/A | N/A |
| Haydon et al., 2022, CERPASS (NCT04050436) [52] | Phase II (Active, Not Recruiting) | RP-1 + Cemiplimab vs. Cemiplimab | 231 | SCC | There was a 48.1% CRR in RP1 + cemiplimab vs. 22.6% in cemiplimab alone. There were comparable ORRs between the combination (52.5%) and single-therapy groups (51.4%). | The RP1-receiving group experienced flu-like symptoms. The non-RP1-receiving group experienced fatigue, pyrexia, pruritis, nausea, and chills. |
| Curiel-Lewandrowski et al., 2024 (NCT03714828) [53] | Phase II (Completed) | T-VEC | 11 | SCC | ORR = 100%, mDoR = 209 days, Time to response = 35 days. In injected lesions, 95.8% CRR, and 4.2% partial response. | Most common TRAEs = fatigue, flu-like symptoms, and headache; all TRAEs were grade 1–2. |
| Migden et al., 2024, ARTACUS (NCT04349436) [54] | Phase Ιb (Recruiting) | RP-1 | 65 | SCC, BCC, MCC | Interim results: ORR = 35%, with a CRR of 22%. | Most common TRAEs were fatigue, chills, and pyrexia. |
| (NCT03921073) | Phase II (Terminated) | T-VEC | 5 | Angiosarcoma of the skin | N/A | Most common TRAEs were fever, flu-like symptoms, fatigue, and injection site pain. |
| Kelly, C. et al., 2023 (NCT03069378) [55] | Phase II (Active, Not Recruiting) | T-VEC + pembrolizumab | 21 | Locally advanced/metastatic sarcoma | The best ORR by 24 weeks was 11% for undifferentiated pleomorphic sarcoma and myxofibrosarcoma (UPS/MFS), 71% for cutaneous angiosarcoma (AS), and 0% for epithelioid sarcoma (ES). The mPFS was 14.9 for UPS/MFS and 54 for AS. | Only one participant in the AS expansion cohort experienced a grade 3 TRAE (immune-mediated hepatitis); no participants experienced grade 4 TRAEs. |
| Ji et al., 2023 (NCT05602792) [45] | Phase I/IIa (Recruiting) | T3011 | 233 | Advanced solid tumors | The confirmed ORR was 11%; the DCR was 49% in 55 pts, including patients with HNSCC, sarcoma, melanoma, breast cancer, etc., treated under RP2D who could be evaluated for their tumor response. The median PFS was 2.8 months in all 76 pts receiving T3011. | Both TRAEs of ≥G3 and treatment discontinuation in 2.2% of pts. The most frequently reported TRAEs were pyrexia (21.1%), influenza-like illness (8.9%), increased TSH (6.7%), proteinuria (6.7%), facial edema, white blood cell count decreased, AST increased, and anemia (5.6%). |
3. Current State of OVs in NMSCs
3.1. T-VEC Usage in NMSCs
3.2. RP-1 in NMSCs
3.3. Other OV Usage in NMSCs
4. Future Directions and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guy, G.P.; Thomas, C.C.; Thompson, T.; Watson, M.; Massetti, G.M.; Richardson, L.C. Centers for Disease Control and Prevention (CDC) Vital Signs: Melanoma Incidence and Mortality Trends and Projections—United States, 1982–2030. Morb. Mortal. Wkly. Rep. 2015, 64, 591–596. [Google Scholar]
- Guy, G.P.; Machlin, S.R.; Ekwueme, D.U.; Yabroff, K.R. Prevalence and Costs of Skin Cancer Treatment in the U.S., 2002–2006 and 2007–2011. Am. J. Prev. Med. 2015, 48, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Burden of Skin Disease. Available online: https://www.aad.org/member/clinical-quality/clinical-care/bsd (accessed on 8 January 2024).
- Skin Cancer. Available online: https://www.aad.org/media/stats-skin-cancer (accessed on 8 January 2024).
- Melanoma Skin Cancer Statistics. Available online: https://www.cancer.org/cancer/types/melanoma-skin-cancer/about/key-statistics.html (accessed on 22 January 2024).
- Cancer Facts & Figures 2023. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.html (accessed on 8 January 2024).
- Survival Statistics for Non-Melanoma Skin Cancer. Available online: https://cancer.ca/en/cancer-information/cancer-types/skin-non-melanoma/prognosis-and-survival/survival-statistics (accessed on 8 January 2024).
- Basal & Squamous Cell Skin Cancer Statistics. Available online: https://www.cancer.org/cancer/types/basal-and-squamous-cell-skin-cancer/about/key-statistics.html (accessed on 8 January 2024).
- Hodi, F.S.; Chiarion -Sileni, V.; Lewis, K.D.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Survival in Advanced Melanoma for Patients Treated with Nivolumab plus Ipilimumab in CheckMate 067. J. Clin. Oncol. 2022, 40, 9522. [Google Scholar] [CrossRef]
- Jerby-Arnon, L.; Shah, P.; Cuoco, M.S.; Rodman, C.; Su, M.-J.; Melms, J.C.; Leeson, R.; Kanodia, A.; Mei, S.; Lin, J.-R.; et al. A Cancer Cell Program Promotes T Cell Exclusion and Resistance to Checkpoint Blockade. Cell 2018, 175, 984–997.e24. [Google Scholar] [CrossRef] [PubMed]
- Brastianos, P.K.; Kim, A.E.; Giobbie-Hurder, A.; Lee, E.Q.; Lin, N.U.; Overmoyer, B.; Wen, P.Y.; Nayak, L.; Cohen, J.V.; Dietrich, J.; et al. Pembrolizumab in Brain Metastases of Diverse Histologies: Phase 2 Trial Results. Nat. Med. 2023, 29, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Hussaini, S.; Chehade, R.; Boldt, R.G.; Raphael, J.; Blanchette, P.; Maleki Vareki, S.; Fernandes, R. Association between Immune-Related Side Effects and Efficacy and Benefit of Immune Checkpoint Inhibitors—A Systematic Review and Meta-Analysis. Cancer Treat. Rev. 2021, 92, 102134. [Google Scholar] [CrossRef] [PubMed]
- Pires da Silva, I.; Lo, S.; Quek, C.; Gonzalez, M.; Carlino, M.S.; Long, G.V.; Menzies, A.M. Site-Specific Response Patterns, Pseudoprogression, and Acquired Resistance in Patients with Melanoma Treated with Ipilimumab Combined with Anti-PD-1 Therapy. Cancer 2020, 126, 86–97. [Google Scholar] [CrossRef]
- Watanabe, D.; Goshima, F. Oncolytic Virotherapy by HSV. Adv. Exp. Med. Biol. 2018, 1045, 63–84. [Google Scholar] [CrossRef]
- Liu, B.L.; Robinson, M.; Han, Z.-Q.; Branston, R.H.; English, C.; Reay, P.; McGrath, Y.; Thomas, S.K.; Thornton, M.; Bullock, P.; et al. ICP34.5 Deleted Herpes Simplex Virus with Enhanced Oncolytic, Immune Stimulating, and Anti-Tumour Properties. Gene Ther. 2003, 10, 292–303. [Google Scholar] [CrossRef]
- Desjardins, A.; Vlahovic, G.; Friedman, H.S. Vaccine Therapy, Oncolytic Viruses, and Gliomas. Oncology 2016, 30, 211–218. [Google Scholar] [PubMed]
- Marelli, G.; Howells, A.; Lemoine, N.R.; Wang, Y. Oncolytic Viral Therapy and the Immune System: A Double-Edged Sword Against Cancer. Front. Immunol. 2018, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Marintcheva, B. Chapter 9—Virus-Based Therapeutic Approaches. In Harnessing the Power of Viruses; Marintcheva, B., Ed.; Academic Press: London, UK, 2018; pp. 243–276. ISBN 978-0-12-810514-6. [Google Scholar]
- Hwang, J.K.; Hong, J.; Yun, C.-O. Oncolytic Viruses and Immune Checkpoint Inhibitors: Preclinical Developments to Clinical Trials. Int. J. Mol. Sci. 2020, 21, 8627. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Shekarian, T.; Alcazer, V.; Valladeau-Guilemond, J.; Valsesia-Wittmann, S.; Amigorena, S.; Caux, C.; Depil, S. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front. Immunol. 2019, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Rosewell Shaw, A.; Suzuki, M. Oncolytic Viruses Partner With T-Cell Therapy for Solid Tumor Treatment. Front. Immunol. 2018, 9, 2103. [Google Scholar] [CrossRef]
- Ziogas, D.C.; Martinos, A.; Petsiou, D.-P.; Anastasopoulou, A.; Gogas, H. Beyond Immunotherapy: Seizing the Momentum of Oncolytic Viruses in the Ideal Platform of Skin Cancers. Cancers 2022, 14, 2873. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhang, X. Potent Systemic Antitumor Activity from an Oncolytic Herpes Simplex Virus of Syncytial Phenotype. Cancer Res. 2002, 62, 2306–2312. [Google Scholar] [PubMed]
- Haugh, A.M.; Daud, A.I. Current Role and Status for Intratumoral Injection Therapies in Metastatic Melanoma. Cancer J. 2024, 30, 108. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Kuncheria, L.; Roulstone, V.; Kyula, J.N.; Mansfield, D.; Bommareddy, P.K.; Smith, H.; Kaufman, H.L.; Harrington, K.J.; Coffin, R.S. Development of a New Fusion-Enhanced Oncolytic Immunotherapy Platform Based on Herpes Simplex Virus Type 1. J. Immunother. Cancer 2019, 7, 214. [Google Scholar] [CrossRef]
- Koch, M.S.; Lawler, S.E.; Chiocca, E.A. HSV-1 Oncolytic Viruses from Bench to Bedside: An Overview of Current Clinical Trials. Cancers 2020, 12, 3514. [Google Scholar] [CrossRef]
- Definition of Oncolytic Virus RP1—NCI Drug Dictionary—NCI. Available online: https://www.cancer.gov/publications/dictionaries/cancer-drug/def/vusolimogene-oderparepvec (accessed on 7 May 2024).
- Cooke, K.; Rottman, J.; Zhan, J.; Mitchell, P.; Ikotun, O.; Yerby, B.; Chong, A.; Glaus, C.; Moesta, A.K.; Pedro, B. Oncovex MGM-CSF –Mediated Regression of Contralateral (Non-Injected) Tumors in the A20 Murine Lymphoma Model Does Not Involve Direct Viral Oncolysis. J. Immunother. Cancer 2015, 3, P336. [Google Scholar] [CrossRef]
- Piasecki, J.; Tiep, L.; Zhou, J.; Beers, C. Talilmogene Iaherparepvec Generates Systemic T-Cell-Mediated Anti-Tumor Immunity. J. Immunother. Cancer 2013, 1, P198. [Google Scholar] [CrossRef]
- Moesta, A.K.; Cooke, K.; Piasecki, J.; Mitchell, P.; Rottman, J.B.; Fitzgerald, K.; Zhan, J.; Yang, B.; Le, T.; Belmontes, B.; et al. Local Delivery of OncoVEXmGM-CSF Generates Systemic Antitumor Immune Responses Enhanced by Cytotoxic T-Lymphocyte-Associated Protein Blockade. Clin. Cancer Res. 2017, 23, 6190–6202. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.; Zhan, J.; Mitchell, P.; Werner, J.; Beltran, P.J.; DeVoss, J.; Qing, J.; Cooke, K.S. OncoVEXmGM-CSFexpands Tumor Antigen-Specific CD8+ T-Cell Response in Preclinical Models. J. Immunother. Cancer 2023, 11, e006374. [Google Scholar] [CrossRef]
- Andtbacka, R.H.I.; Collichio, F.; Harrington, K.J.; Middleton, M.R.; Downey, G.; Ӧhrling, K.; Kaufman, H.L. Final Analyses of OPTiM: A Randomized Phase III Trial of Talimogene Laherparepvec versus Granulocyte-Macrophage Colony-Stimulating Factor in Unresectable Stage III–IV Melanoma. J. Immunother. Cancer 2019, 7, 145. [Google Scholar] [CrossRef] [PubMed]
- Gogas, H.; Samoylenko, I.; Schadendorf, D.; Gutzmer, R.; Grob, J.J.; Sacco, J.J.; Gorski, K.; Anderson, A.; Liu, C.; Malvehy, J. Talimogene Laherparepvec (T-VEC) Treatment Increases Intratumoral Effector T-Cell and Natural Killer (NK) Cell Density in Noninjected Tumors in Patients (Pts) with Stage IIIB–IVM1c Melanoma: Evidence for Systemic Effects in a Phase II, Single-Arm Study. Ann. Oncol. 2018, 29, viii443. [Google Scholar] [CrossRef]
- Ribas, A.; Chesney, J.; Long, G.V.; Kirkwood, J.M.; Dummer, R.; Puzanov, I.; Hoeller, C.; Gajewski, T.F.; Gutzmer, R.; Rutkowski, P.; et al. 1037O MASTERKEY-265: A Phase III, Randomized, Placebo (Pbo)-Controlled Study of Talimogene Laherparepvec (T) plus Pembrolizumab (P) for Unresectable Stage IIIB–IVM1c Melanoma (MEL). Ann. Oncol. 2021, 32, S868–S869. [Google Scholar] [CrossRef]
- Chesney, J.A.; Ribas, A.; Long, G.V.; Kirkwood, J.M.; Dummer, R.; Puzanov, I.; Hoeller, C.; Gajewski, T.F.; Gutzmer, R.; Rutkowski, P.; et al. Randomized, Double-Blind, Placebo-Controlled, Global Phase III Trial of Talimogene Laherparepvec Combined With Pembrolizumab for Advanced Melanoma. J. Clin. Oncol. 2023, 41, 528–540. [Google Scholar] [CrossRef]
- Gastman, B.; Robert, C.; Gogas, H.; Rutkowski, P.; Long, G.V.; Chaney, M.F.; Joshi, H.; Lin, Y.-L.; Snyder, W.; Chesney, J.A. Primary Analysis of a Phase 2, Open-Label, Multicenter Trial of Talimogene Laherparepvec (T-VEC) plus Pembrolizumab (Pembro) for the Treatment (Tx) of Patients (Pts) with Advanced Melanoma (MEL) Who Progressed on Prior Anti–PD-1 Therapy: MASTERKEY-115. J. Clin. Oncol. 2022, 40, 9518. [Google Scholar] [CrossRef]
- Zijlker, L.P.; van Houdt, W.J.; Stahlie, E.H.A.; Franke, V.; Rohaan, M.W.; Delatzakis, A.; Zuur, C.; Klop, W.M.C.; van de Wiel, B.A.; Kuijpers, A.; et al. Neoadjuvant T-VEC + Nivolumab Combination Therapy for Resectable Early Metastatic (Stage IIIB/C/D-IV M1a) Melanoma with Injectable Disease: NIVEC Trial. J. Clin. Oncol. 2023, 41, 9546. [Google Scholar] [CrossRef]
- Schwarze, J.K.; Tijtgat, J.; Awada, G.; Cras, L.; Vasaturo, A.; Bagnall, C.; Forsyth, R.; Dufait, I.; Tuyaerts, S.; Van Riet, I.; et al. Intratumoral Administration of CD1c (BDCA-1)+ and CD141 (BDCA-3)+ Myeloid Dendritic Cells in Combination with Talimogene Laherparepvec in Immune Checkpoint Blockade Refractory Advanced Melanoma Patients: A Phase I Clinical Trial. J. Immunother. Cancer 2022, 10, e005141. [Google Scholar] [CrossRef]
- Chmielowski, B.; Milhem, M.M.; Sacco, J.J.; Bowles, T.L.; Tsai, K.K.; In, G.K.; Muñoz-Couselo, E.; Vanderwalde, A.M.; Chesney, J.A.; Michels, J.; et al. Initial Efficacy and Safety of RP1 + Nivolumab in Patients with Anti–PD-1–Failed Melanoma from the Ongoing Phase 1/2 IGNYTE Study. J. Clin. Oncol. 2023, 41, 9509. [Google Scholar] [CrossRef]
- Barker, C.A.; D’Angelo, S.P.; Steckler, A.M.; Lian, M.; Wasilewski, G.; Lacouture, M.E.; Chapman, P.B.; Shoushtari, A.N.; Ariyan, C.E. A Phase II Randomized Trial of Talimogene Laherparepvec (T-VEC) Oncolytic Immunotherapy with or without Radiotherapy for Patients with Cutaneous Metastases from Solid Tumors. J. Clin. Oncol. 2023, 41, 2639. [Google Scholar] [CrossRef]
- Yamazaki, N.; Isei, T.; Kiyohara, Y.; Koga, H.; Kojima, T.; Takenouchi, T.; Yokota, K.; Namikawa, K.; Yi, M.; Keegan, A.; et al. A Phase I Study of the Safety and Efficacy of Talimogene Laherparepvec in Japanese Patients with Advanced Melanoma. Cancer Sci. 2022, 113, 2798–2806. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Gyorki, D.E.; Hyngstrom, J.R.; Ning, M.; Lawrence, T.; Ross, M.I. Final 5-Year Follow-Up Results Evaluating Neoadjuvant Talimogene Laherparepvec Plus Surgery in Advanced Melanoma: A Randomized Clinical Trial. JAMA Oncol. 2023, 9, 1457–1459. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, X.; Bai, X.; Li, C.; Mao, L.; Chi, Z.; Lian, B.; Bixia, T.; Kong, Y.; Dai, J.; et al. 795P A Phase Ib Trial of Neoadjuvant Oncolytic Virus OrienX010 (Ori) and Anti-PD-1 Toripalimab (Tori) Combo in Patients (Pts) with Resectable Stage IIIb-IV (M1a) Acral Melanoma. Ann. Oncol. 2022, 33, S907. [Google Scholar] [CrossRef]
- Ji, D.; Weitao, Y.; Tong, X.; Zhang, C.; Wang, F.; Chen, Z.; Zhou, Y.; Li, Z.; Deng, Y.; Huang, G.; et al. A Phase 1/2a Study of T3011, an Oncolytic HSV Expressing IL-12 and PD-1 Antibody, Administered via Intratumoral (IT) Injection as Monotherapy in Advanced Solid Tumors. J. Clin. Oncol. 2023, 41, 2520. [Google Scholar] [CrossRef]
- Santos, J.; Svane, I.; Peltola, K.; Alanko, T.; Korpisaari, R.; Juteau, S.; Jaakkola, M.; Sormunen, J.; Kononen, J.; Ellebaek, E.; et al. 749 Early Phase Oncology Experience on the Use of an Oncolytic Adenovirus Encoding for TNFa and IL-2 for the Treatment of Solid Tumors—Interim Results. J. Immunother. Cancer 2023, 11, 845. [Google Scholar] [CrossRef]
- Monberg, T.J.; Pakola, S.; Dreno, B.; Cervera-Carrascon, V.; Ellebaek, E.; Donia, M.; Khammari, A.; Kistler, C.; Santos, J.; Clubb, J.; et al. 48O Safety and Efficacy of Combined Treatment with Tumor Infiltrating Lymphocytes (TILs) and Oncolytic Adenovirus TILT-123 for Patients with Metastatic Melanoma: Results from a Phase I Trial. Immuno-Oncol. Technol. 2023, 20, 100521. [Google Scholar] [CrossRef]
- Namikawa, K.; Yamazaki, N. Targeted Therapy and Immunotherapy for Melanoma in Japan. Curr. Treat. Options Oncol. 2019, 20, 7. [Google Scholar] [CrossRef]
- Dummer, R.; Gyorki, D.E.; Hyngstrom, J.; Berger, A.C.; Conry, R.; Demidov, L.; Sharma, A.; Treichel, S.A.; Radcliffe, H.; Gorski, K.S.; et al. Neoadjuvant Talimogene Laherparepvec plus Surgery versus Surgery Alone for Resectable Stage IIIB–IVM1a Melanoma: A Randomized, Open-Label, Phase 2 Trial. Nat. Med. 2021, 27, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Havunen, R.; Kalliokoski, R.; Siurala, M.; Sorsa, S.; Santos, J.M.; Cervera-Carrascon, V.; Anttila, M.; Hemminki, A. Cytokine-Coding Oncolytic Adenovirus TILT-123 Is Safe, Selective, and Effective as a Single Agent and in Combination with Immune Checkpoint Inhibitor Anti-PD-1. Cells 2021, 10, 246. [Google Scholar] [CrossRef] [PubMed]
- Ramelyte, E.; Pawlik, L.; Turko, P.; Sella, F.; Roshardt Prieto, N.M.; Stäger, R.; Maul, J.-T.; Dummer, R. Intralesional Oncolytic Virotherapy with Talimogene Laherparepvec in Patients with Cutaneous Lymphomas and Non-Melanoma Skin Cancers. J. Clin. Oncol. 2023, 41, 9581. [Google Scholar] [CrossRef]
- Haydon, A.M.; Khushalani, N.I.; Robert, C.; Brungs, D.; Collichio, F.A.; Colevas, A.D.; Lim, A.M.L.; Kudchadkar, R.R.; Chai-Ho, W.; Daniels, G.A.; et al. A Randomized, Controlled, Open-Label, Phase 2 Study of Cemiplimab ± RP1 in Patients with Advanced Cutaneous Squamous Cell Carcinoma (CERPASS). J. Clin. Oncol. 2022, 40, TPS9593. [Google Scholar] [CrossRef]
- Curiel-Lewandrowski, C.; Stratton, D.B.; Adams, A.C.; Cui, H.; Roe, D.; Sundararajan, S. Abstract CT004: A Single Arm Phase 2 Study of TVEC in Patients with Invasive Cutaneous SCC: A Novel Therapeutic Approach for Low Risk Tumors. Cancer Res. 2024, 84, CT004. [Google Scholar] [CrossRef]
- Migden, M.R.; Chai-Ho, W.; Daniels, G.A.; Medina, T.; Wise-Draper, T.M.; Kheterpal, M.; Tang, J.C.; Ibrahim, S.F.; Bolotin, D.; Verschraegen, C.; et al. Abstract CT003: Initial Results from an Open-Label Phase 1b/2 Study of RP1 Oncolytic Immunotherapy in Solid Organ Transplant Recipients with Advanced Cutaneous Malignancies (ARTACUS). Cancer Res. 2024, 84, CT003. [Google Scholar] [CrossRef]
- Kelly, C.M.; Avutu, V.; Chi, P.; Dickson, M.A.; Gounder, M.M.; Keohan, M.L.; Movva, S.; Rosenbaum, E.; Cordover, E.; Ariyan, C.E.; et al. A Phase II Study of Talimogene Laherparepvec (T-VEC) and Pembrolizumab in Patients with Advanced Sarcoma: Results of Expansion Cohorts. J. Clin. Oncol. 2023, 41, 11570. [Google Scholar] [CrossRef]
- Salloum, A.; Koblinski, J.; Bazzi, N.; Zeitouni, N.C. Talimogene Laherparepvec in Non-Melanoma Cancers. J. Clin. Aesthet. Dermatol. 2021, 14, 18–25. [Google Scholar] [PubMed]
- Wu, S.-E.; Chen, Y.-H.; Hung, C.-T.; Yang, B.-H. Therapeutic Cancer Vaccines for Nonmelanoma Skin Cancer. Curr. Treat. Options Oncol. 2023, 24, 496–514. [Google Scholar] [CrossRef]
- Shalhout, S.Z.; Kaufman, H.L.; Emerick, K.S.; Miller, D.M. Immunotherapy for Nonmelanoma Skin Cancer: Facts and Hopes. Clin. Cancer Res. 2022, 28, 2211–2220. [Google Scholar] [CrossRef]
- Curiel, C.N.; Stratton, D.; Cui, H.; Roe, D.; Arif Tiwari, H.; Sundararajan, S. A Single Arm Phase 2 Study of Talimogene Laherparepvec in Patients with Low-Risk Invasive Cutaneous Squamous Cell Cancer. Interim Analysis. J. Clin. Oncol. 2022, 40, e21583. [Google Scholar] [CrossRef]
- Hall, E.T.; Fernandez-Lopez, E.; Silk, A.W.; Dummer, R.; Bhatia, S. Immunologic Characteristics of Nonmelanoma Skin Cancers: Implications for Immunotherapy. In American Society of Clinical Oncology Educational Book; American Society of Clinical Oncology: Alexandria, VA, USA, 2020; Volume 40, pp. 1–10. [Google Scholar] [CrossRef]
- Harrington, K.J.; Kong, A.; Mach, N.; Chesney, J.A.; Castelo, B.; Rischin, D.; Cohen, E.E.W.; Radcliffe, H.-S.; Gumuscu, B.; Snyder, W.; et al. Final Analysis of a Phase 1b, Randomized, Multicenter Study of Talimogene Laherparepvec (T-VEC) plus Pembrolizumab (Pembro) Combination for the Treatment (Tx) of Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck (R/M HNSCC): MASTERKEY-232. J. Clin. Oncol. 2021, 39, 6036. [Google Scholar] [CrossRef]
- Niu, J.; Milhem, M.; Vanderwalde, A.M.; Chmielowski, B.; Beasley, G.; Samson, A.; Sacco, J.J.; Bowles, T.; Jew, T.; He, S.; et al. Safety and Efficacy of RP1 + Nivolumab in Patients With Non-Melanoma Skin Cancer of the Head and Neck: Results From IGNYTE Phase 1/2 Multi-Cohort Clinical Trial. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, e8–e9. [Google Scholar] [CrossRef]
- FDA Grants Fast Track Designation to MVR-T3011 in HNSCC. Available online: https://www.targetedonc.com/view/fda-grants-fast-track-designation-to-mvr-t3011-in-hnscc (accessed on 26 April 2024).
- Malvehy, J.; Samoylenko, I.; Schadendorf, D.; Gutzmer, R.; Grob, J.-J.; Sacco, J.J.; Gorski, K.S.; Anderson, A.; Pickett, C.A.; Liu, K.; et al. Original Research: Talimogene Laherparepvec Upregulates Immune-Cell Populations in Non-Injected Lesions: Findings from a Phase II, Multicenter, Open-Label Study in Patients with Stage IIIB–IVM1c Melanoma. J. Immunother. Cancer 2021, 9, e001621. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-T.; Ramsay, L.; Ahanfeshar-Adams, M.; Lajoie, M.; Schadendorf, D.; Alain, T.; Watson, I.R. Mutations in the IFNγ-JAK-STAT Pathway Causing Resistance to Immune Checkpoint Inhibitors in Melanoma Increase Sensitivity to Oncolytic Virus Treatment. Clin. Cancer Res. 2021, 27, 3432–3442. [Google Scholar] [CrossRef]
- Khaddour, K.; Dowling, J.; Huang, J.; Council, M.; Chen, D.; Cornelius, L.; Johanns, T.; Dahiya, S.; Ansstas, G. Successful Administration of Sequential TVEC and Pembrolizumab Followed by Temozolomide in Immunotherapy Refractory Intracranial Metastatic Melanoma with Acquired B2M Mutation. Oncotarget 2020, 11, 4836–4844. [Google Scholar] [CrossRef]
- Bommareddy, P.K.; Aspromonte, S.; Zloza, A.; Rabkin, S.D.; Kaufman, H.L. MEK Inhibition Enhances Oncolytic Virus Immunotherapy through Increased Tumor Cell Killing and T Cell Activation. Sci. Transl. Med. 2018, 10, eaau0417. [Google Scholar] [CrossRef]
| Authors, Study Name, NCT # | Trial Stage | Therapeutic Approach | N | Disease/Stage | Outcomes | Common TRAEs/Grade 3–4 |
|---|---|---|---|---|---|---|
| Zijlker L. et al., 2023 NIVEC (NCT04330430) [38] | Phase II (Active, Not Recruiting) | Neoadjuvant T-VEC + Nivolumab for 8 weeks | 24 | Resectable stage IIIB-IVA melanoma | pCR in 65% of pts and a partial response in 9%. | Grade 3 TRAEs = 8%. No grade 4 TRAEs. |
| (NCT03842943) | Phase II (Recruiting) | T-VEC + pembrolizumab | 28 | Stage III | N/A | N/A |
| (NCT02965716) | Phase II (Active, Not Recruiting) | T-VEC + pembrolizumab | 42 | Stage III-IV | N/A | N/A |
| Gastman B. et al., 2022 MASTERKEY-115 (NCT04068181) [37] | Phase II (Completed) | T-VEC + pembrolizumab | 72 | Stage IIIB-IV | ORR ranged from 0 to 46.7% among different cohorts. | Most common TRAEs: pyrexia, fatigue, flu-like illness. Grade ≥ 3: 12.7%. |
| Schwarze J. et al., 2022 myDCTV (NCT03747744) [39] | Phase I (Status Unknown) | CD1c (BDCA-1) + myDC + T-VEC | 18 | Advanced/metastatic melanoma | 15% developed a durable pCR, 8% partial unconfirmed response, and 15% mixed response. | Most common TRAEs: fatigue, fever, chills/flu-like symptoms. |
| Chmielowski B. et al., 2023 IGNYTE (NCT03767348) [40] | Phase II (Recruiting) | RP-1 vs. RP-1 + nivolumab | 340 | Advanced and/or refractory solid tumors, including melanoma | ORR was 37.4%, and CR was 18.7%. | Most common TRAEs: fatigue, chills, pyrexia, and nausea. |
| Barker C. et al., 2023 (NCT02819843) [41] | Phase II (Active, Not Recruiting) | T-VEC + Hypofractionated Radiotherapy vs. T-VEC alone | 19 | Solid tumors, including melanoma | Composite response rate in T-VEC = 7% and T-VEC+RT = 22%. (Trial was closed early due to slow accrual) | Most common TRAEs was fever. |
| Yamazaki N. et al., 2022 (NCT03064763) [42] | Phase I (Completed) | T-VEC | 18 | Stage IIIB-IV | 11.1% had a durable partial response ≥ 6 months. | Most common TRAEs were pyrexia, malaise, chills, decreased appetite, pruritus, and skin ulcers. |
| Dummer R. et al., 2023 (NCT02211131) [43] | Phase II (Completed) | Neoadjuvant T-VEC for 6 doses + surgical resection vs. immediate surgical resection | 150 | Resectable stage IIIB- IVM1a | For group 1 (T-VEC then surgery) and group 2 (immediate surgery), respectively, 5-year Kaplan–Meier plots showed 22.3% and 15.2% for RFS; 43.7% and 27.4% for EFS; and 77.3% and 62.7% for OS. HRs for local RFS, regional RFS, and DMFS were 0.82 0.81, and 0.73, respectively. | No new safety signals were reported. |
| LUMINOS-102 (NCT04577807) | Phase II (Active, Not Recruiting) | PVSRIPO vs. PVSRIPO + anti-PD-1 | 56 | Advanced melanoma refractory to anti-PD-1 | N/A | N/A |
| Wei X. et al., 2022 (NCT04197882) [44] | Phase IB (Active, Not Recruiting) | Neoadjuvant OrienX010 + toripalimab for 8–12 weeks and Adjuvant toripalimab for a year | 30 | Resectable stage IIIB-IV (M1a) acral melanoma. | Radiological ORR = 36.7% and pathological ORR = 77.8%. CRR = 3.3%. 1-year RFS = 80%. | Grade 3 TRAEs= 17% (soft tissue infections, transaminitis, peripheral neuropathy, and neutropenia). |
| (NCT06216938) | Phase I (Recruiting) | RP-1 | 25 | Primary melanoma | N/A | N/A |
| (NCT05961111) | Phase I (Recruiting) | R130 OV | 20 | Advanced solid tumors, including melanoma | N/A | N/A |
| (NCT05868707) | Phase III (Recruiting) | OH2 vs. Salvage chemotherapy or best supportive care | 340 | Unresectable or metastatic melanoma | N/A | N/A |
| Ji, D. et al., 2023 (NCT05602792) [45] | Phase I/IIa (Recruiting) | T3011 | 233 | Advanced solid tumors, including melanoma | The confirmed ORR was 11%, the DCR was 49% in 55 pts including HNSCC, sarcoma, melanoma, breast cancer, etc., treated under RP2D evaluable for tumor response. The median PFS was 2.8 months in all 76 pts receiving T3011. | Both TRAEs of ≥G3 and treatment discontinuation in 2.2% of pts. The most frequently reported TRAEs were pyrexia (21.1%), influenza-like illness (8.9%), increased TSH (6.7%), proteinuria (6.7%), facial edema, white blood cell count decreased, AST increased, and anemia (5.6%). |
| (NCT04725331) | Phase I/II (Recruiting) | BT-001 alone or in combination with pembrolizumab | 48 | Advanced solid tumors, including melanoma | N/A | N/A |
| (NCT05076760) | Phase I (Recruiting) | MEM-288 alone or in combination with nivolumab | 61 | Advanced solid tumors, including melanoma | N/A | N/A |
| (NCT06265025) | Phase I/II (Recruiting) | GM103 alone and in combination with pembrolizumab | 125 | Locally advanced, unresectable, refractory, and/or metastatic solid tumors, including melanoma | N/A | N/A |
| Santos, J. et al., 2023 AVENTIL (NCT05222932) [46] | Phase I (Recruiting) | TILT-123 and avelumab | 15 | Solid tumors refractory to or progressing after anti-PD-1, including melanoma | N/A | N/A |
| Monberg T. J. et al., 2023 TUNINTIL (NCT04217473) [47] | Phase I (Active, Not Recruiting) | TILT-123 with tumor-infiltrating lymphocytes | 17 | Advanced melanoma | Disease control rate of 38.0%. | Most common AEs from TILT-123 were fever and pain at injection site. |
| (NCT06171178) | Phase I (Recruiting) | ASP1012 | 229 | Advanced/metastatic solid tumors, including melanoma | N/A | N/A |
| (NCT04616443) | Phase I/II (Recruiting) | OH2 in combination with HX008 | 60 | Advanced melanoma | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nassief, G.; Anaeme, A.; Moussa, K.; Chen, D.; Ansstas, G. Where Are We Now with Oncolytic Viruses in Melanoma and Nonmelanoma Skin Malignancies? Pharmaceuticals 2024, 17, 916. https://doi.org/10.3390/ph17070916
Nassief G, Anaeme A, Moussa K, Chen D, Ansstas G. Where Are We Now with Oncolytic Viruses in Melanoma and Nonmelanoma Skin Malignancies? Pharmaceuticals. 2024; 17(7):916. https://doi.org/10.3390/ph17070916
Chicago/Turabian StyleNassief, George, Angela Anaeme, Karen Moussa, David Chen, and George Ansstas. 2024. "Where Are We Now with Oncolytic Viruses in Melanoma and Nonmelanoma Skin Malignancies?" Pharmaceuticals 17, no. 7: 916. https://doi.org/10.3390/ph17070916
APA StyleNassief, G., Anaeme, A., Moussa, K., Chen, D., & Ansstas, G. (2024). Where Are We Now with Oncolytic Viruses in Melanoma and Nonmelanoma Skin Malignancies? Pharmaceuticals, 17(7), 916. https://doi.org/10.3390/ph17070916







