Lipoprotein Metabolism, Dyslipidemia, and Lipid-Lowering Therapy in Women: A Comprehensive Review
Abstract
1. Introduction
2. Sex Differences in Lipoprotein Metabolism
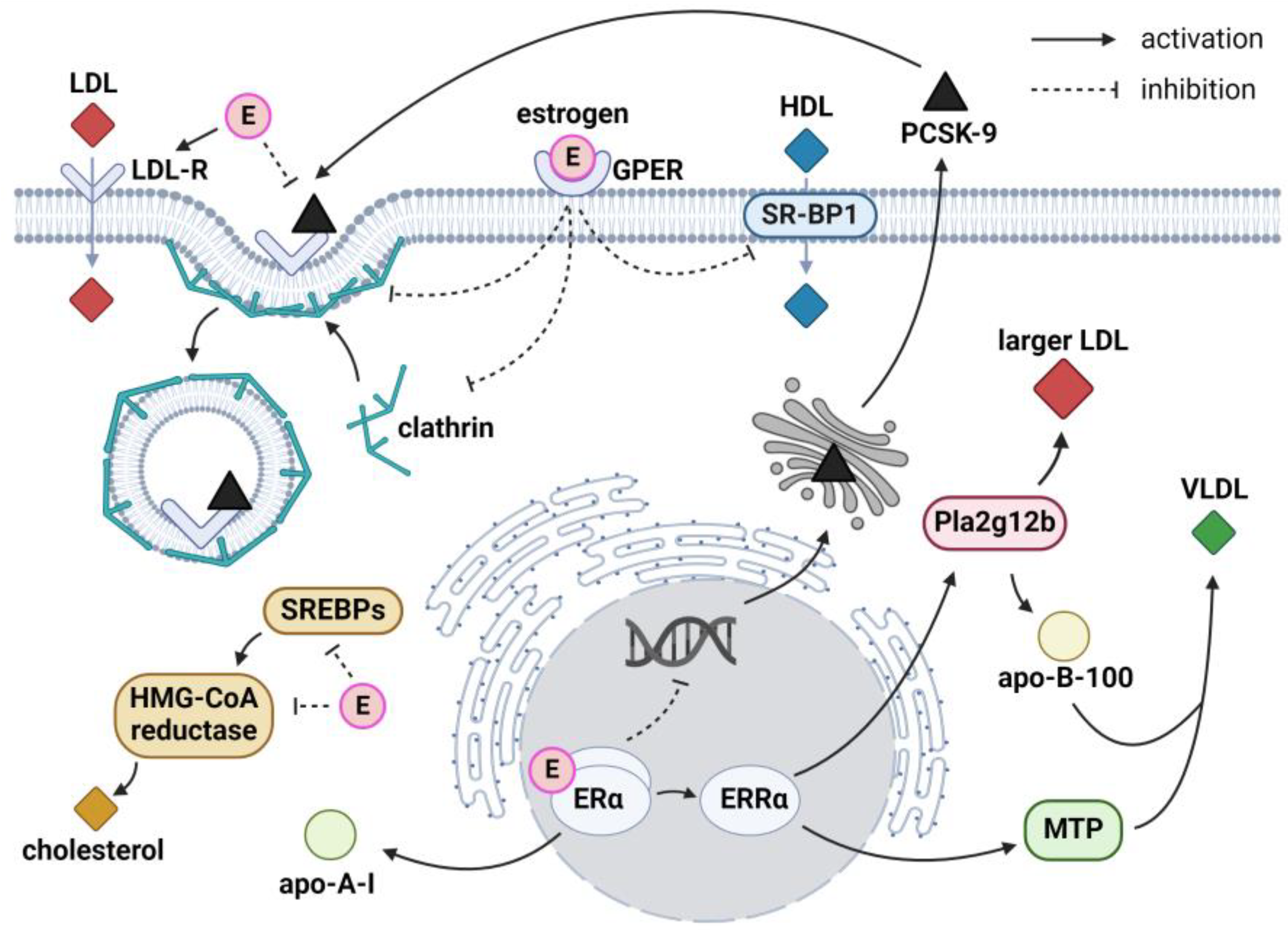
2.1. Role of Sex Hormones
2.1.1. Estrogens and Very-Low-Density Lipoproteins
2.1.2. Estrogens and Low-Density Lipoproteins
2.1.3. Estrogens and High-Density Lipoproteins
2.1.4. Estrogens and Cholesterol Metabolism
2.2. Role of Genetics
2.3. Key Messages
3. Sex Differences in Lipid Profiles
3.1. Lipid Profile throughout the Lifetime
3.2. Impact of Hormonal Status in Women
3.2.1. Menstrual Cycle
3.2.2. Pregnancy
3.2.3. Menopause
3.2.4. Female-Specific Comorbidities
3.3. Impact of Exogenous Estrogens
3.3.1. Contraception
3.3.2. Hormone Replacement Therapy
3.4. Key Messages
4. Women with Dyslipidemia in Clinical Practice
4.1. Patient Characteristics
4.1.1. Lipid Profile and Cardiovascular Risk in Women
4.1.2. Cardiovascular Risk Factors in Women
4.1.3. Atherosclerotic Cardiovascular Disease in Women
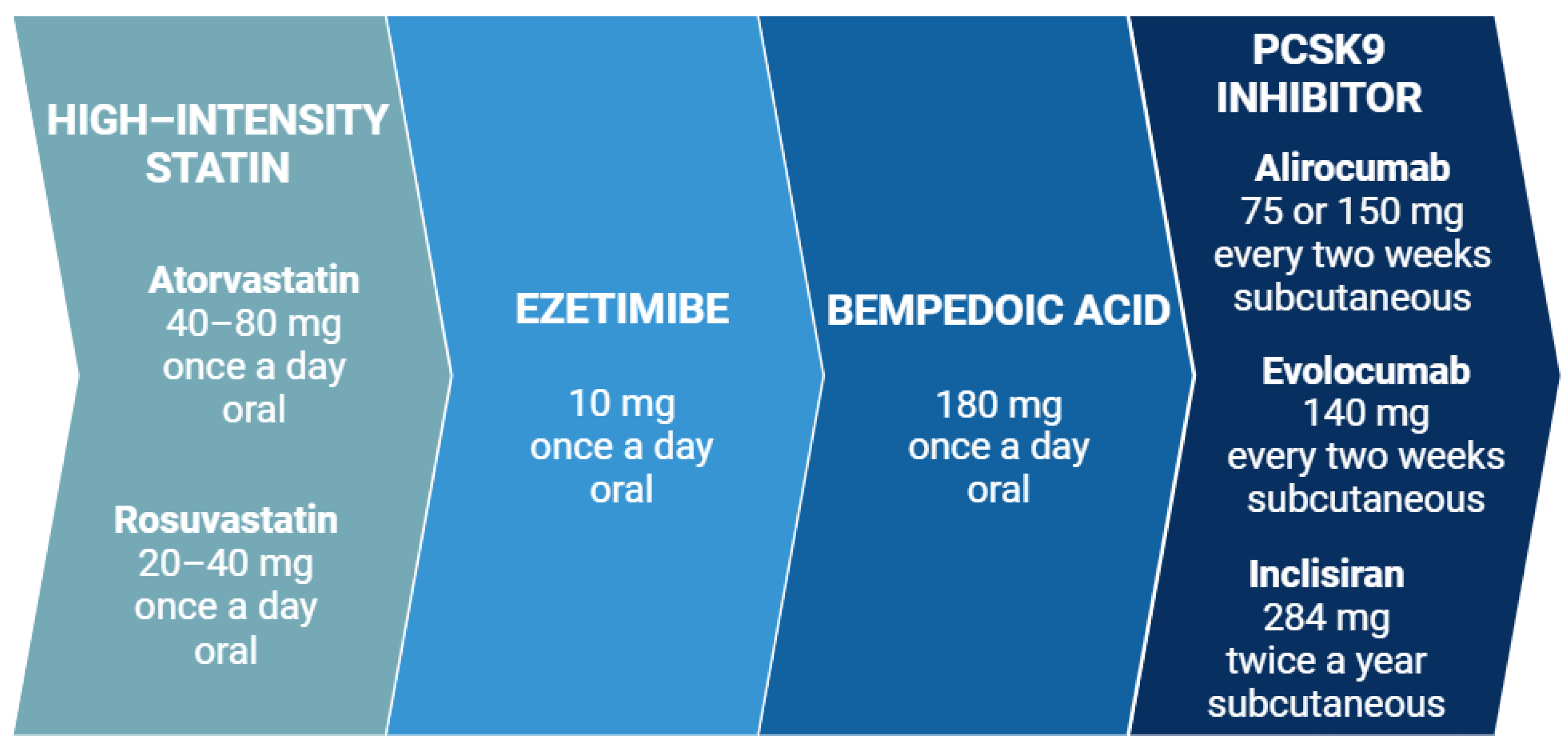
4.2. Lipid-Lowering Therapy
4.2.1. Effectiveness of Lipid-Lowering Agents in Women
4.2.2. Real-World Lipid-Lowering Therapy in Women
4.3. Key Messages
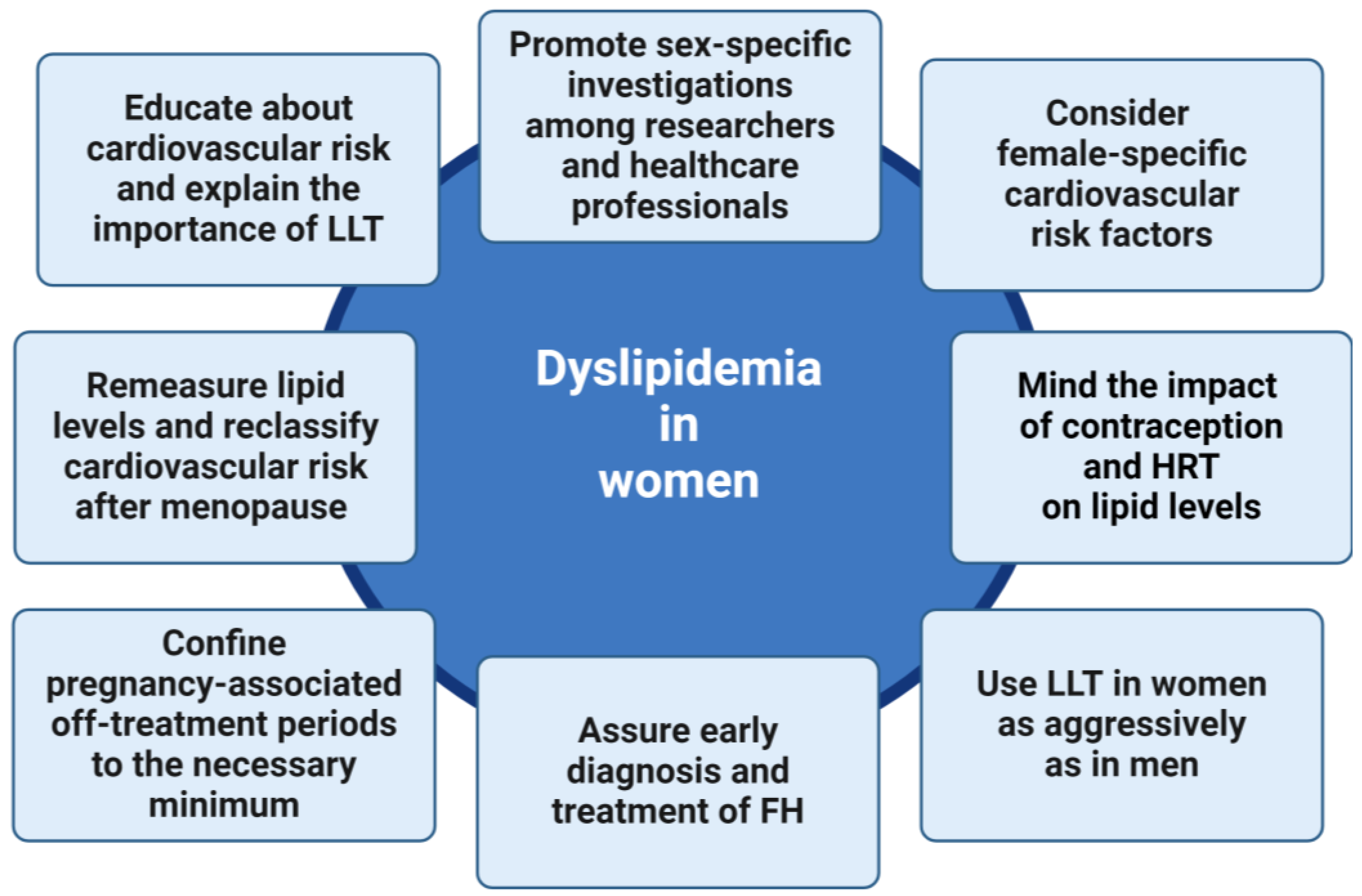
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ralapanawa, U.; Sivakanesan, R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: A Narrative Review. J. Epidemiol. Glob. Health 2021, 11, 169–177. [Google Scholar] [CrossRef]
- Libby, P. The Changing Landscape of Atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2022, 29, 5–115. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Banach, M.; Penson, P.E.; Farnier, M.; Fras, Z.; Latkovskis, G.; Laufs, U.; Paneni, F.; Parini, P.; Pirro, M.; Reiner, Ž. Bempedoic Acid in the Management of Lipid Disorders and Cardiovascular Risk. 2023 Position Paper of the International Lipid Expert Panel (ILEP). Prog. Cardiovasc. Dis. 2023, 79, 2–11. [Google Scholar] [CrossRef]
- Kronenberg, F.; Mora, S.; Stroes, E.S.G.; Ference, B.A.; Arsenault, B.J.; Berglund, L.; Dweck, M.R.; Koschinsky, M.; Lambert, G.; Mach, F.; et al. Lipoprotein(a) in Atherosclerotic Cardiovascular Disease and Aortic Stenosis: A European Atherosclerosis Society Consensus Statement. Eur. Heart J. 2022, 43, 3925–3946. [Google Scholar] [CrossRef] [PubMed]
- Gouni-Berthold, I.; Schwarz, J.; Berthold, H.K. PCSK9 Monoclonal Antibodies: New Developments and Their Relevance in a Nucleic Acid–Based Therapy Era. Curr. Atheroscler. Rep. 2022, 24, 779–790. [Google Scholar] [CrossRef]
- Katsiki, N.; Vrablik, M.; Banach, M.; Gouni-Berthold, I. Inclisiran, Low-Density Lipoprotein Cholesterol and Lipoprotein(a). Pharmaceuticals 2023, 16, 577. [Google Scholar] [CrossRef]
- Nanna, M.G.; Wang, T.Y.; Xiang, Q.; Goldberg, A.C.; Robinson, J.G.; Roger, V.L.; Virani, S.S.; Wilson, P.W.F.; Louie, M.J.; Koren, A. Sex Differences in the Use of Statins in Community Practice: The Patient and Provider Assessment of Lipid Management (PALM) Registry. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005562. [Google Scholar] [CrossRef]
- Peters, S.A.E.; Colantonio, L.D.; Zhao, H.; Bittner, V.; Dai, Y.; Farkouh, M.E.; Monda, K.L.; Safford, M.M.; Muntner, P.; Woodward, M. Sex Differences in High-Intensity Statin Use Following Myocardial Infarction in the United States. J. Am. Coll. Cardiol. 2018, 71, 1729–1737. [Google Scholar] [CrossRef]
- Vallejo-Vaz, A.J.; Stevens, C.A.T.; Lyons, A.R.M.; Dharmayat, K.I.; Freiberger, T.; Hovingh, G.K.; Mata, P.; Raal, F.J.; Santos, R.D.; Soran, H. Global Perspective of Familial Hypercholesterolaemia: A Cross-Sectional Study from the EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Lancet 2021, 398, 1713–1725. [Google Scholar] [CrossRef] [PubMed]
- März, W.; Schmidt, N.; an Haack, I.; Dressel, A.; Grammer, T.B.; Kleber, M.E.; Baessler, A.; Beil, F.U.; Gouni-Berthold, I.; Julius, U. The German CaRe High Registry for Familial Hypercholesterolemia–Sex Differences, Treatment Strategies, and Target Value Attainment. Atheroscler. Plus 2023, 53, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Conlon, D.M.; Welty, F.K.; Reyes-Soffer, G.; Amengual, J. Sex-Specific Differences in Lipoprotein Production and Clearance. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, B.T.; Zhu, L.; Stafford, J.M. Role of Estrogens in the Regulation of Liver Lipid Metabolism. Adv. Exp. Med. Biol. 2017, 1043, 227–256. [Google Scholar] [CrossRef] [PubMed]
- Holven, K.B.; Roeters van Lennep, J. Sex Differences in Lipids: A Life Course Approach. Atherosclerosis 2023, 384, 117270. [Google Scholar] [CrossRef] [PubMed]
- Klevmoen, M.; Mulder, J.W.C.M.; Roeters van Lennep, J.E.; Holven, K.B. Sex Differences in Familial Hypercholesterolemia. Curr. Atheroscler. Rep. 2023, 25, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Roeters Van Lennep, J.E.; Tokgözoǧlu, L.S.; Badimon, L.; Dumanski, S.M.; Gulati, M.; Hess, C.N.; Holven, K.B.; Kavousi, M.; Kaylkçloǧlu, M.; Lutgens, E. Women, Lipids, and Atherosclerotic Cardiovascular Disease: A Call to Action from the European Atherosclerosis Society. Eur. Heart J. 2023, 44, 4157–4173. [Google Scholar] [CrossRef]
- Vogel, B.; Acevedo, M.; Appelman, Y.; Bairey Merz, C.N.; Chieffo, A.; Figtree, G.A.; Guerrero, M.; Kunadian, V.; Lam, C.S.P.; Maas, A.H.E.M. The Lancet Women and Cardiovascular Disease Commission: Reducing the Global Burden by 2030. Lancet 2021, 397, 2385–2438. [Google Scholar] [CrossRef]
- Gavina, C.; Araújo, F.; Teixeira, C.; Ruivo, J.A.; Corte-Real, A.L.; Luz-Duarte, L.; Canelas-Pais, M.; Taveira-Gomes, T. Sex Differences in LDL-C Control in a Primary Care Population: The PORTRAIT-DYS Study. Atherosclerosis 2023, 384, 117148. [Google Scholar] [CrossRef]
- Rachamin, Y.; Grischott, T.; Rosemann, T.; Meyer, M.R. Inferior Control of Low-Density Lipoprotein Cholesterol in Women Is the Primary Sex Difference in Modifiable Cardiovascular Risk: A Large-Scale, Cross-Sectional Study in Primary Care. Atherosclerosis 2021, 324, 141–147. [Google Scholar] [CrossRef]
- Amrock, S.M.; Duell, P.B.; Knickelbine, T.; Martin, S.S.; O’Brien, E.C.; Watson, K.E.; Mitri, J.; Kindt, I.; Shrader, P.; Baum, S.J. Health Disparities among Adult Patients with a Phenotypic Diagnosis of Familial Hypercholesterolemia in the CASCADE-FHTM Patient Registry. Atherosclerosis 2017, 267, 19–26. [Google Scholar] [CrossRef]
- Karalis, D.G.; Wild, R.A.; Maki, K.C.; Gaskins, R.; Jacobson, T.A.; Sponseller, C.A.; Cohen, J.D. Gender Differences in Side Effects and Attitudes Regarding Statin Use in the Understanding Statin Use in America and Gaps in Patient Education (USAGE) Study. J. Clin. Lipidol. 2016, 10, 833–841. [Google Scholar] [CrossRef]
- Goldstein, K.M.; Zullig, L.L.; Bastian, L.A.; Bosworth, H.B. Statin Adherence: Does Gender Matter? Curr. Atheroscler. Rep. 2016, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Zamora, A.; Ramos, R.; Comas-Cufi, M.; García-Gil, M.; Martí-Lluch, R.; Plana, N.; Alves-Cabratosa, L.; Ponjoan, A.; Rodríguez-Borjabad, C.; Ibarretxe, D. Women with Familial Hypercholesterolemia Phenotype Are Undertreated and Poorly Controlled Compared to Men. Sci. Rep. 2023, 13, 1492. [Google Scholar] [CrossRef] [PubMed]
- Alshibani, B.; Iatan, I.; Guerin, A.; Ruel, I.; Cermakova, L.; Ramanakumar, A.V.; Pilote, L.; Coutinho, T.; Brunham, L.R.; Genest, J. Sex Differences in the Perception of Cardiovascular Risk in Familial Hypercholesterolemia. J. Clin. Lipidol. 2023, 18, e97–e104. [Google Scholar] [CrossRef]
- Maas, A.H.; Appelman, Y.E. Gender differences in coronary heart disease. Neth. Heart J. 2010, 18, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Ryczkowska, K.; Adach, W.; Janikowski, K.; Banach, M.; Bielecka-Dabrowa, A. Menopause and Women’s Cardiovascular Health: Is It Really an Obvious Relationship? Arch. Med. Sci. 2023, 19, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, Q.; Huang, T.; Tan, W.; Qu, L.; Chen, T.; Pan, H.; Chen, L.; Liu, J.; Wong, C.W. Dysfunction of Estrogen-Related Receptor Alpha-Dependent Hepatic VLDL Secretion Contributes to Sex Disparity in NAFLD/NASH Development. Theranostics 2020, 10, 10874–10891. [Google Scholar] [CrossRef]
- Palmisano, B.T.; Le, T.D.; Zhu, L.; Lee, Y.K.; Stafford, J.M. Cholesteryl Ester Transfer Protein Alters Liver and Plasma Triglyceride Metabolism through Two Liver Networks in Female Mice. J. Lipid Res. 2016, 57, 1541–1551. [Google Scholar] [CrossRef]
- Magkos, F.; Patterson, B.W.; Mohammed, B.S.; Klein, S.; Mittendorfer, B. Women Produce Fewer but Triglyceride-Richer Very Low-Density Lipoproteins than Men. J. Clin. Endocrinol. Metab. 2007, 92, 1311–1318. [Google Scholar] [CrossRef]
- Wang, X.; Magkos, F.; Mittendorfer, B. Sex Differences in Lipid and Lipoprotein Metabolism: It’s Not Just about Sex Hormones. J. Clin. Endocrinol. Metab. 2011, 96, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Matthan, N.R.; Jalbert, S.M.; Barrett, P.H.R.; Dolnikowski, G.G.; Schaefer, E.J.; Lichtenstein, A.H. Gender-Specific Differences in the Kinetics of Nonfasting TRL, IDL, and LDL Apolipoprotein B-100 in Men and Premenopausal Women. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1838–1843. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Reeds, D.N.; Okunade, A.L.; Patterson, B.W.; Mittendorfer, B. Systemic Delivery of Estradiol, but Not Testosterone or Progesterone, Alters Very Low Density Lipoprotein-Triglyceride Kinetics in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2014, 99, 1306–1310. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Patterson, B.W.; Mittendorfer, B. No Effect of Menstrual Cycle Phase on Basal Very-Low-Density Lipoprotein Triglyceride and Apolipoprotein B-100 Kinetics. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E1243–E1249. [Google Scholar] [CrossRef] [PubMed]
- Ndzie Noah, M.L.; Adzika, G.K.; Mprah, R.; Adekunle, A.O.; Adu-Amankwaah, J.; Sun, H. Sex–Gender Disparities in Cardiovascular Diseases: The Effects of Estrogen on ENOS, Lipid Profile, and NFATs During Catecholamine Stress. Front. Cardiovasc. Med. 2021, 8, 639946. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.T.S.; Yamamoto, T.; Goldstein, J.L.; Brown, M.S. Increased MRNA for Low Density Lipoprotein Receptor in Livers of Rabbits Treated with 17α-Ethinyl Estradiol. Proc. Natl. Acad. Sci. USA 1986, 83, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Owen, A.J.; Roach, P.D.; Abbey, M. Regulation of Low-Density Lipoprotein Receptor Activity by Estrogens and Phytoestrogens in a HepG2 Cell Model. Ann. Nutr. Metab. 2004, 48, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Gouni-Berthold, I.; Berthold, H.K. PCSK9 Antibodies for the Treatment of Hypercholesterolemia. Nutrients 2014, 6, 5517–5533. [Google Scholar] [CrossRef] [PubMed]
- Seidah, N.G.; Awan, Z.; Chrétien, M.; Mbikay, M. PCSK9: A Key Modulator of Cardiovascular Health. Circ. Res. 2014, 114, 1022–1036. [Google Scholar] [CrossRef]
- Ooi, T.C.; Raymond, A.; Cousins, M.; Favreau, C.; Taljaard, M.; Gavin, C.; Jolly, E.E.; Malone, S.; Eapen, L.; Chretien, M. Relationship between Testosterone, Estradiol and Circulating PCSK9: Cross-Sectional and Interventional Studies in Humans. Clin. Chim. Acta 2015, 446, 97–104. [Google Scholar] [CrossRef]
- Ghosh, M.; Gälman, C.; Rudling, M.; Angelin, B. Influence of Physiological Changes in Endogenous Estrogen on Circulating PCSK9 and LDL Cholesterol. J. Lipid Res. 2015, 56, 463–469. [Google Scholar] [CrossRef]
- Persson, L.; Henriksson, P.; Westerlund, E.; Hovatta, O.; Angelin, B.; Rudling, M. Endogenous Estrogens Lower Plasma PCSK9 and LDL Cholesterol but Not Lp(a) or Bile Acid Synthesis in Women. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Lakoski, S.G.; Lagace, T.A.; Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Genetic and Metabolic Determinants of Plasma PCSK9 Levels. J. Clin. Endocrinol. Metab. 2009, 94, 2537–2543. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Hu, T.; Lin, C.; Xiong, Q.; Liu, F.; Yuan, J.; Zhao, X.; Wang, R. Resveratrol Downregulates PCSK9 Expression and Attenuates Steatosis through Estrogen Receptor α-Mediated Pathway in L02 cells. Eur. J. Pharmacol. 2019, 855, 216–222. [Google Scholar] [CrossRef]
- Starr, A.E.; Lemieux, V.; Noad, J.; Moore, J.I.; Dewpura, T.; Raymond, A.; Chrétien, M.; Figeys, D.; Mayne, J. β-Estradiol Results in a Proprotein Convertase Subtilisin/Kexin Type 9-Dependent Increase in Low-Density Lipoprotein Receptor Levels in Human Hepatic HuH7 Cells. FEBS J. 2015, 282, 2682–2696. [Google Scholar] [CrossRef]
- Roubtsova, A.; Chamberland, A.; Marcinkiewicz, J.; Essalmani, R.; Fazel, A.; Bergeron, J.J.; Seidah, N.G.; Prat, A. PCSK9 Deficiency Unmasks a Sex- and Tissue-Specific Subcellular Distribution of the LDL and VLDL Receptors in Mice. J. Lipid Res. 2015, 56, 2133–2142. [Google Scholar] [CrossRef]
- Fu, W.; Gao, X.P.; Zhang, S.; Dai, Y.P.; Zou, W.J.; Yue, L.M. 17β-Estradiol Inhibits PCSK9-Mediated LDLR Degradation through GPER/PLC Activation in HepG2 Cells. Front. Endocrinol. 2020, 10, 930. [Google Scholar] [CrossRef]
- Jia, F.; Fei, S.F.; Tong, D.B.; Xue, C.; Li, J.J. Sex Difference in Circulating PCSK9 and Its Clinical Implications. Front. Pharmacol. 2022, 13, 953845. [Google Scholar] [CrossRef]
- Xie, F.; Li, X.; Xu, Y.; Cheng, D.; Xia, X.; Lv, X.; Yuan, G.; Peng, C. Estrogen Mediates an Atherosclerotic-Protective Action via Estrogen Receptor Alpha/SREBP-1 Signaling. Front. Cardiovasc. Med. 2022, 9, 895916. [Google Scholar] [CrossRef]
- Thierer, J.H.; Ekker, S.C.; Farber, S.A. The LipoGlo Reporter System for Sensitive and Specific Monitoring of Atherogenic Lipoproteins. Nat. Commun. 2019, 10, 3426. [Google Scholar] [CrossRef]
- Demissie, S.; Cupples, L.A.; Shearman, A.M.; Gruenthal, K.M.; Peter, I.; Schmid, C.H.; Karas, R.H.; Housman, D.E.; Mendelsohn, M.E.; Ordovas, J.M. Estrogen Receptor-α Variants Are Associated with Lipoprotein Size Distribution and Particle Levels in Women: The Framingham Heart Study. Atherosclerosis 2006, 185, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Shearman, A.M.; Demissie, S.; Cupples, L.A.; Peter, I.; Schmid, C.H.; Ordovas, J.M.; Mendelsohn, M.E.; Housman, D.E. Tobacco Smoking, Estrogen Receptor α Gene Variation and Small Low Density Lipoprotein Level. Hum. Mol. Genet. 2005, 14, 2405–2413. [Google Scholar] [CrossRef] [PubMed]
- Shearman, A.M.; Cupples, L.A.; Demissie, S.; Peter, I.; Schmid, C.H.; Karas, R.H.; Mendelsohn, M.E.; Housman, D.E.; Levy, D. Association between Estrogen Receptor Gene Variation and Cardiovascular Disease. JAMA 2003, 290, 2263–2270. [Google Scholar] [CrossRef]
- Nordström, P.; Glader, C.A.; Dahlén, G.; Birgander, L.S.; Lorentzon, R.; Waldenström, A.; Lorentzon, M. Oestrogen Receptor α Gene Polymorphism Is Related to Aortic Valve Sclerosis in Postmenopausal Women. J. Intern. Med. 2003, 254, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Billon-Galés, A.; Fontaine, C.; Douin-Echinard, V.; Delpy, L.; Berges, H.; Calippe, B.; Lenfant, F.; Laurell, H.; Guéry, J.C.; Gourdy, P. Endothelial Estrogen Receptor-α Plays a Crucial Role in the Atheroprotective Action of 17β-Estradiol in Low-Density Lipoprotein Receptor-Deficient Mice. Circulation 2009, 120, 2567–2576. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.M.; Sugiyama, M.G.; Fung, K.Y.Y.; Gao, Y.; Wang, C.; Levy, A.S.; Azizi, P.; Roufaiel, M.; Zhu, S.N.; Neculai, D. A Novel Assay Uncovers an Unexpected Role for SR-BI in LDL Transcytosis. Cardiovasc. Res. 2015, 108, 268–277. [Google Scholar] [CrossRef]
- Ghaffari, S.; Nabi, F.N.; Sugiyama, M.G.; Lee, W.L. Estrogen Inhibits LDL (Low-Density Lipoprotein) Transcytosis by Human Coronary Artery Endothelial Cells via GPER (G-Protein-Coupled Estrogen Receptor) and SR-BI (Scavenger Receptor Class B Type 1). Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2283–2294. [Google Scholar] [CrossRef]
- Meyer, M.R.; Fredette, N.C.; Howard, T.A.; Hu, C.; Ramesh, C.; Daniel, C.; Amann, K.; Arterburn, J.B.; Barton, M.; Prossnitz, E.R. G Protein-Coupled Estrogen Receptor Protects from Atherosclerosis. Sci. Rep. 2014, 4, 7564. [Google Scholar] [CrossRef]
- Ruiz-Sanz, J.I.; Navarro, R.; Martínez, R.; Hernández, M.L.; Matorras, R.; Ruiz-Larrea, M.B. No Effect of Menstrual Cycle on LDL Oxidizability and Particle Size. Maturitas 2007, 57, 253–260. [Google Scholar] [CrossRef]
- Koh, K.K. Effects of Estrogen on the Vascular Wall: Vasomotor Function and Inflammation. Cardiovasc. Res. 2002, 55, 714–726. [Google Scholar] [CrossRef]
- Landschulz, K.T.; Pathak, R.K.; Rigotti, A.; Krieger, M.; Hobbs, H.H. Regulation of Scavenger Receptor, Class B, Type I, a High Density Lipoprotein Receptor, in Liver and Steroidogenic Tissues of the Rat. J. Clin. Investig. 1996, 98, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Stangl, H.; Graf, G.A.; Yu, L.; Cao, G.; Wyne, K. Effect of Estrogen on Scavenger Receptor BI Expression in the Rat. J. Endocrinol. 2002, 175, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Darabi, M.; Rabbani, M.; Ani, M.; Zarean, E.; Panjehpour, M.; Movahedian, A. Increased Leukocyte ABCA1 Gene Expression in Post-Menopausal Women on Hormone Replacement Therapy. Gynecol. Endocrinol. 2011, 27, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Zhu, L.; Wang, W.; Wan, Z.; Chen, F.; Wu, Y.; Zhou, J.; Yuan, Z. 17β-Estradiol Promotes Cholesterol Efflux from Vascular Smooth Muscle Cells through a Liver X Receptor α-Dependent Pathway. Int. J. Mol. Med. 2014, 33, 550–558. [Google Scholar] [CrossRef] [PubMed]
- El Khoudary, S.R.; Hutchins, P.M.; Matthews, K.A.; Brooks, M.M.; Orchard, T.J.; Ronsein, G.E.; Heinecke, J.W. Cholesterol Efflux Capacity and Subclasses of HDL Particles in Healthy Women Transitioning through Menopause. J. Clin. Endocrinol. Metab. 2016, 101, 3419–3428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhuang, Y.; Qiang, H.; Liu, X.; Xu, R.; Wu, Y. Relationship between Endogenous Estrogen Concentrations and Serum Cholesteryl Ester Transfer Protein Concentrations in Chinese Women. Clin. Chim. Acta 2001, 314, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Cazita, P.M.; Berti, J.A.; Aoki, C.; Gidlund, M.; Harada, L.M.; Nunes, V.S.; Quintão, E.C.R.; Oliveira, H.C.F. Cholesteryl Ester Transfer Protein Expression Attenuates Atherosclerosis in Ovariectomized Mice. J. Lipid Res. 2003, 44, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Ulioa, N.; Verdugo, C.; Rios, M.; Sep01veda, J.; Sepolveda, S.; Naveas, R.; Calvo, C. Increased Activity of Lecithin: Cholesterol Acyltransferase during Short-Term Oral Estrogen Progestin Replacement Therapy in a Group of Postmenopausal Women Metabolism. Metabolism 1998, 47, 297–300. [Google Scholar] [CrossRef]
- Brinton, E.A. Oral Estrogen Replacement Therapy in Postmenopausal Women Selectively Raises Levels and Production Rates of Lipoprotein A-I and Lowers Hepatic Lipase Activity without Lowering the Fractional Catabolic Rate. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 431–440. [Google Scholar] [CrossRef]
- Jin, F.Y.; Kamanna, V.S.; Kashyap, M.L. Estradiol Stimulates Apolipoprotein A-I- but Not A-II-Containing Particle Synthesis and Secretion by Stimulating MRNA Transcription Rate in Hep G2 Cells. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 999–1006. [Google Scholar] [CrossRef][Green Version]
- Gardner, C.D.; Tribble, D.L.; Young, D.R.; Ahn, D.; Fortmann, S.P. Population Frequency Distributions of HDL, HDL2, and HDL3 Cholesterol and Apolipoproteins A-I and B in Healthy Men and Women and Associations with Age, Gender, Hormonal Status, and Sex Hormone Use: The Stanford Five City Project. Prev. Med. 2000, 31, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Badeau, R.M.; Metso, J.; Wähälä, K.; Tikkanen, M.J.; Jauhiainen, M. Human Macrophage Cholesterol Efflux Potential Is Enhanced by HDL-Associated 17β-Estradiol Fatty Acyl Esters. J. Steroid Biochem. Mol. Biol. 2009, 116, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Badeau, R.M.; Metso, J.; Kovanen, P.T.; Lee-Rueckert, M.; Tikkanen, M.J.; Jauhiainen, M. The Impact of Gender and Serum Estradiol Levels on HDL-Mediated Reverse Cholesterol Transport. Eur. J. Clin. Investig. 2013, 43, 3177–3323. [Google Scholar] [CrossRef] [PubMed]
- De Marinis, E.; Martini, C.; Trentalance, A.; Pallottini, V. Sex Differences in Hepatic Regulation of Cholesterol Homeostasis. J. Endocrinol. 2008, 198, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.P.; Wang, H.H.; Ohashi, A.; Wang, D.Q.H. Role of Intestinal Sterol Transporters Abcg5, Abcg8, and Npc111 in Cholesterol Absorption in Mice: Gender and Age Effects. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G269–G276. [Google Scholar] [CrossRef] [PubMed]
- Carrier, J.C.; Deblois, G.; Champigny, C.; Levy, E.; Giguère, V. Estrogen-Related Receptor α (ERRα) Is a Transcriptional Regulator of Apolipoprotein A-IV and Controls Lipid Handling in the Intestine. J. Biol. Chem. 2004, 279, 52052–52058. [Google Scholar] [CrossRef] [PubMed]
- Wiese, C.B.; Agle, Z.W.; Zhang, P.; Reue, K. Chromosomal and Gonadal Sex Drive Sex Differences in Lipids and Hepatic Gene Expression in Response to Hypercholesterolemia and Statin Treatment. Biol. Sex Differ. 2022, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- García-Calzón, S.; Perfilyev, A.; de Mello, V.D.; Pihlajamäki, J.; Ling, C. Sex Differences in the Methylome and Transcriptome of the Human Liver and Circulating HDL-Cholesterol Levels. J. Clin. Endocrinol. Metab. 2018, 103, 4395–4408. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Jeon, H.K.; Yoo, H.Y. Sex-Related Differences in Single Nucleotide Polymorphisms Associated with Dyslipidemia in a Korean Population. Lipids Health Dis. 2022, 21, 124. [Google Scholar] [CrossRef]
- Link, J.C.; Chen, X.; Prien, C.; Borja, M.S.; Hammerson, B.; Oda, M.N.; Arnold, A.P.; Reue, K. Increased High-Density Lipoprotein Cholesterol Levels in Mice with XX versus XY Sex Chromosomes. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1778–1786. [Google Scholar] [CrossRef]
- Van, P.L.; Bakalov, V.K.; Bondy, C.A. Monosomy for the X-Chromosome Is Associated with an Atherogenic Lipid Profile. J. Clin. Endocrinol. Metab. 2006, 91, 2867–2870. [Google Scholar] [CrossRef] [PubMed]
- AlSiraj, Y.; Chen, X.; Thatcher, S.E.; Temel, R.E.; Cai, L.; Blalock, E.; Katz, W.; Ali, H.M.; Petriello, M.; Deng, P. XX Sex Chromosome Complement Promotes Atherosclerosis in Mice. Nat. Commun. 2019, 10, 2631. [Google Scholar] [CrossRef] [PubMed]
- Gouni-Berthold, I.; Laufs, U. Special Aspects of Cholesterol Metabolism in Women. Dtsch. Arztebl. Int. 2024, 121, 401–406. [Google Scholar] [CrossRef] [PubMed]
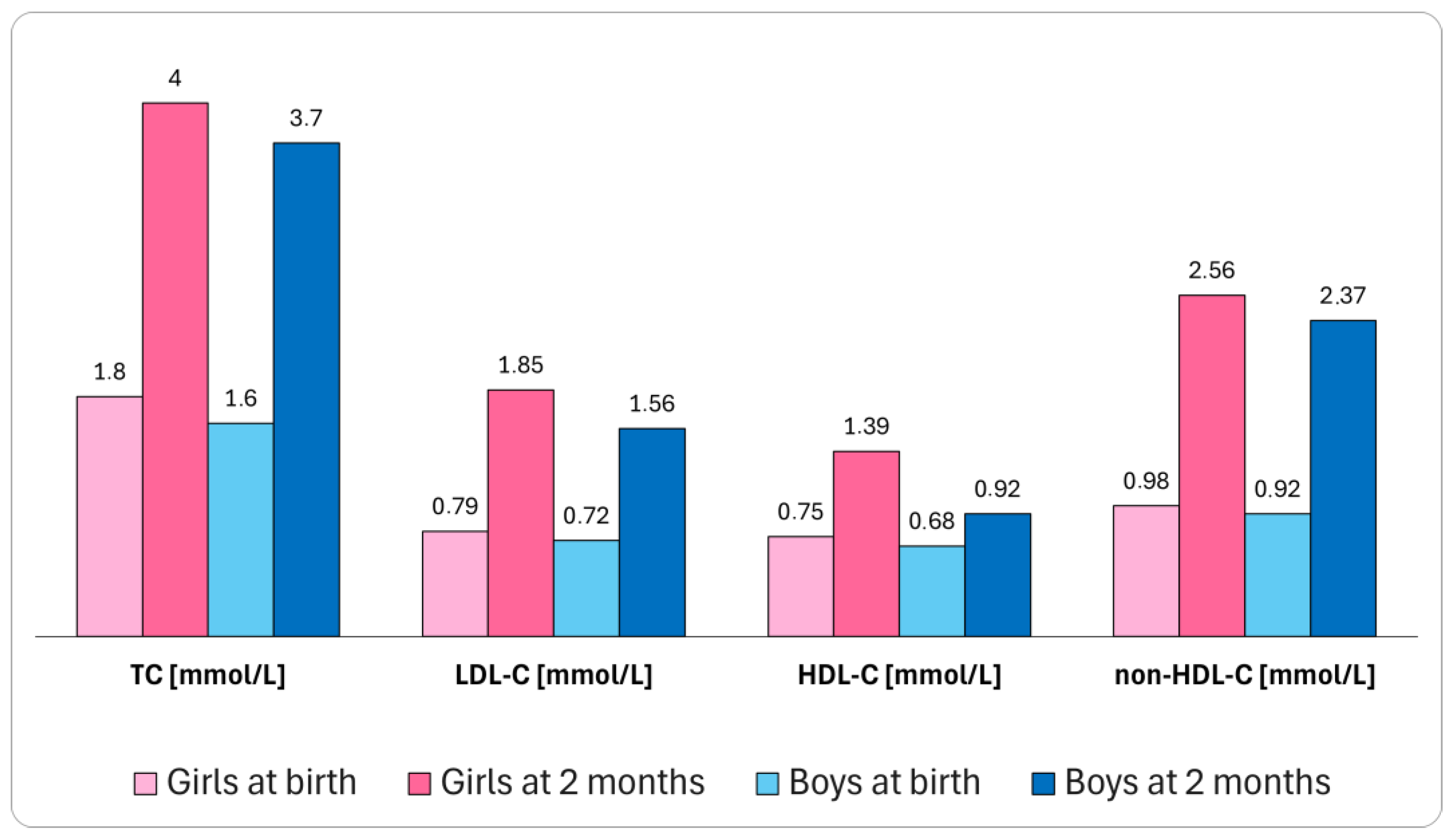
- Nielsen, S.T.; Lytsen, R.M.; Strandkjær, N.; Rasmussen, I.J.; Sillesen, A.S.; Vøgg, R.O.B.; Raja, A.A.; Nordestgaard, B.G.; Kamstrup, P.R.; Iversen, K. Significance of Lipids, Lipoproteins, and Apolipoproteins during the First 14–16 Months of Life. Eur. Heart J. 2023, 44, 178–183. [Google Scholar] [CrossRef]
- Felzer-Kim, I.T.; Visker, J.R.; Ferguson, D.P.; Hauck, J.L. Infant Blood Lipids: A Systematic Review of Predictive Value and Influential Factors. Expert. Rev. Cardiovasc. Ther. 2020, 18, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Enkhmaa, B.; Berglund, L. Non-genetic influences on lipoprotein(a) concentrations. Atherosclerosis 2022, 349, 53–62. [Google Scholar] [CrossRef]
- Frohlich, J.; Dobiášová, M.; Adler, L.; Francis, M. Gender Differences in Plasma Levels of Lipoprotein (a) in Patients with Angiographically Proven Coronary Artery Disease. Physiol. Res. 2004, 53, 481–486. [Google Scholar] [CrossRef]
- Nenseter, M.S.; Lindvig, H.W.; Ueland, T.; Langslet, G.; Ose, L.; Holven, K.B.; Retterstøl, K. Lipoprotein(a) Levels in Coronary Heart Disease-Susceptible and -Resistant Patients with Familial Hypercholesterolemia. Atherosclerosis 2011, 216, 426–432. [Google Scholar] [CrossRef]
- Øyri, L.K.L.; Bogsrud, M.P.; Christensen, J.J.; Ulven, S.M.; Brantsæter, A.L.; Retterstøl, K.; Brekke, H.K.; Michelsen, T.M.; Henriksen, T.; Roeters van Lennep, J.E. Novel Associations between Parental and Newborn Cord Blood Metabolic Profiles in the Norwegian Mother, Father and Child Cohort Study. BMC Med. 2021, 19, 91. [Google Scholar] [CrossRef]
- Holven, K.B.; Narverud, I.; van Lennep, J.R.; Versmissen, J.; Øyri, L.K.L.; Galema-Boers, A.; Langslet, G.; Ulven, S.M.; Veierød, M.B.; Retterstøl, K. Sex Differences in Cholesterol Levels from Birth to 19 Years of Age May Lead to Increased Cholesterol Burden in Females with FH. J. Clin. Lipidol. 2018, 12, 748–755. [Google Scholar] [CrossRef]
- Johansen, A.K.; Bogsrud, M.P.; Christensen, J.J.; Rundblad, A.; Narverud, I.; Ulven, S.; Langslet, G.; Retterstøl, K.; Holven, K.B. Young Women with Familial Hypercholesterolemia Have Higher LDL-Cholesterol Burden than Men: Novel Data Using Repeated Measurements during 12-Years Follow-Up. Atheroscler. Plus 2023, 51, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, M.M.; Hamkour, S.; Siegers, K.E.; Holven, K.B.; Johansen, A.K.; van de Ree, M.A.; Imholz, B.; Boersma, E.; Louters, L.; Bogsrud, M.P.; et al. LDL cholesterol targets rarely achieved in familial hypercholesterolemia patients: A sex and gender-specific analysis. Atherosclerosis 2023, 384, 117117. [Google Scholar] [CrossRef] [PubMed]
- Mulder, J.W.C.M.; Tromp, T.R.; Al-Khnifsawi, M.; Blom, D.J.; Chlebus, K.; Cuchel, M.; D’Erasmo, L.; Gallo, A.; Hovingh, G.K.; Kim, N.T.; et al. Homozygous Familial Hypercholesterolemia International Clinical Collaborators. Sex Differences in Diagnosis, Treatment, and Cardiovascular Outcomes in Homozygous Familial Hypercholesterolemia. JAMA Cardiol. 2024, 9, 313–322. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, K.A.; Ho, F.K.; Celis-Morales, C.A.; Pell, J.P.; Gallagher, I.J.; Moran, C.N. Association between Menstrual Cycle Phase and Metabolites in Healthy, Regularly Menstruating Women in UK Biobank, and Effect Modification by Inflammatory Markers and Risk Factors for Metabolic Disease. BMC Med. 2023, 21, 488. [Google Scholar] [CrossRef]
- Mumford, S.L.; Schisterman, E.F.; Siega-Riz, A.M.; Browne, R.W.; Gaskins, A.J.; Trevisan, M.; Steiner, A.Z.; Daniels, J.L.; Zhang, C.; Perkins, N.J. A Longitudinal Study of Serum Lipoproteins in Relation to Endogenous Reproductive Hormones during the Menstrual Cycle: Findings from the BioCycle Study. J. Clin. Endocrinol. Metab. 2010, 95, E80–E85. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.B.; Woods, M.N.; Lamon-Fava, S.; Schaefer, E.J.; McNamara, J.R.; Spiegelman, D.; Hertzmark, E.; Goldin, B.; Longcope, C.; Gorbach, S.L. Plasma Lipid and Lipoprotein Levels during the Follicular and Luteal Phases of the Menstrual Cycle. J. Clin. Endocrinol. Metab. 2004, 89, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Vashishta, S.; Gahlot, S.; Goyal, R. Effect of Menstrual Cycle Phases on Plasma Lipid and Lipoprotein Levels in Regularly Menstruating Women. J. Clin. Diagn. Res. 2017, 11, CC05–CC07. [Google Scholar] [CrossRef] [PubMed]
- Bartels, Ä.; O’Donoghue, K. Cholesterol in Pregnancy: A Review of Knowns and Unknowns. Obstet. Med. 2011, 4, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Lewek, J.; Banach, M. Dyslipidemia Management in Pregnancy: Why Is It Not Covered in the Guidelines? Curr. Atheroscler. Rep. 2022, 24, 547–556. [Google Scholar] [CrossRef]
- Bartels, Ä.; Egan, N.; Broadhurst, D.I.; Khashan, A.S.; Joyce, C.; Stapleton, M.; O’Mullane, J.; O’Donoghue, K. Maternal Serum Cholesterol Levels Are Elevated from the 1st Trimester of Pregnancy: A Cross-Sectional Study. J. Obstet. Gynaecol. 2012, 32, 747–752. [Google Scholar] [CrossRef]
- Belo, L.; Caslake, M.; Santos-Silva, A.; Castro, E.M.B.; Pereira-Leite, L.; Quintanilha, A.; Rebelo, I. LDL Size, Total Antioxidant Status and Oxidised LDL in Normal Human Pregnancy: A Longitudinal Study. Atherosclerosis 2004, 177, 391–399. [Google Scholar] [CrossRef]
- Stuebe, A.M.; Rich-Edwards, J.W. The Reset Hypothesis: Lactation and Maternal Metabolism. Am. J. Perinatol. 2009, 26, 81–88. [Google Scholar] [CrossRef]
- Gunderson, E.P.; Lewis, C.E.; Wei, G.S.; Whitmer, R.A.; Quesenberry, C.P.; Sidney, S. Lactation and Changes in Maternal Metabolic Risk Factors. Obstet. Gynecol. 2007, 109, 729–738. [Google Scholar] [CrossRef]
- Kallio, M.J.T.; Siimes, M.A.; Perheentupa, J.; Salmenperä, L.; Miettinen, T.A. Serum Cholesterol and Lipoprotein Concentrations in Mothers during and after Prolonged Exclusive Lactation. Metabolism 1992, 41, 1327–1330. [Google Scholar] [CrossRef]
- Matthews, K.A.; Crawford, S.L.; Chae, C.U.; Everson-Rose, S.A.; Sowers, M.F.; Sternfeld, B.; Sutton-Tyrrell, K. Are Changes in Cardiovascular Disease Risk Factors in Midlife Women Due to Chronological Aging or to the Menopausal Transition? J. Am. Coll. Cardiol. 2009, 54, 2366–2372. [Google Scholar] [CrossRef]
- Ambikairajah, A.; Walsh, E.; Cherbuin, N. Lipid Profile Differences during Menopause: A Review with Meta-Analysis. Menopause 2019, 26, 1327–1333. [Google Scholar] [CrossRef]
- Song, D.K.; Hong, Y.S.; Sung, Y.A.; Lee, H. The Effect of Menopause on Cardiovascular Risk Factors According to Body Mass Index in Middle-Aged Korean Women. PLoS ONE 2023, 18, e0283393. [Google Scholar] [CrossRef]
- Lou, Z.; Huang, Y.; Lan, Y.; Li, C.; Chu, K.; Chen, P.; Xu, W.; Ma, L.; Zhou, J. Relationship between Years since Menopause and Lipid Variation in Postmenopausal Women: A Cross-Sectional Study. Medicine 2023, 102, E32684. [Google Scholar] [CrossRef]
- El Khoudary, S.R. HDL and the Menopause. Curr. Opin. Lipidol. 2017, 28, 328–336. [Google Scholar] [CrossRef]
- Simony, S.B.; Mortensen, M.B.; Langsted, A.; Afzal, S.; Kamstrup, P.R.; Nordestgaard, B.G. Sex Differences of Lipoprotein(a) Levels and Associated Risk of Morbidity and Mortality by Age: The Copenhagen General Population Study. Atherosclerosis 2022, 355, 76–82. [Google Scholar] [CrossRef]
- Roeters Van Lennep, J.E.; Heida, K.Y.; Bots, M.L.; Hoek, A. Cardiovascular Disease Risk in Women with Premature Ovarian Insufficiency: A Systematic Review and Meta-Analysis. Eur. J. Prev. Cardiol. 2016, 23, 178–186. [Google Scholar] [CrossRef]
- Knauff, E.A.H.; Westerveld, H.E.; Goverde, A.J.; Eijkemans, M.J.; Valkenburg, O.; Van Santbrink, E.J.P.; Fauser, B.C.J.M.; Van Der Schouw, Y.T. Lipid Profile of Women with Premature Ovarian Failure. Menopause 2008, 15, 919–923. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, L.; Wu, Z.; Li, Y.; Jia, Q.; Cheng, J.C.; Sun, Y.P. A Meta-Analysis of Serum Lipid Profiles in Premature Ovarian Insufficiency. Reprod. Biomed. Online 2022, 44, 539–547. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, B.; Hu, Y.; Na, Z.; Li, D. Effects of Steroid Hormones on Lipid Metabolism in Sexual Dimorphism: A Mendelian Randomization Study. Front Endocrinol 2023, 13, 1119154. [Google Scholar] [CrossRef]
- Wekker, V.; Van Dammen, L.; Koning, A.; Heida, K.Y.; Painter, R.C.; Limpens, J.; Laven, J.S.E.; Van Lennep, J.E.R.; Roseboom, T.J.; Hoek, A. Long-Term Cardiometabolic Disease Risk in Women with PCOS: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2020, 26, 942–960. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xie, N.; Qu, F.; Zhou, J.; Wang, F. The Association Between Polycystic Ovary Syndrome and Metabolic Syndrome in Adolescents: A Systematic Review and Meta-Analysis. Reprod. Sci. 2023, 30, 28–40. [Google Scholar] [CrossRef]
- Melo, A.S.; Rosa-E-Silva, J.C.; De Sá Rosa-E-Silva, A.C.J.; Poli-Neto, O.B.; Ferriani, R.A.; Vieira, C.S. Unfavorable Lipid Profile in Women with Endometriosis. Fertil. Steril. 2010, 93, 2433–2436. [Google Scholar] [CrossRef] [PubMed]
- Dragoman, M.; Curtis, K.M.; Gaffield, M.E. Combined Hormonal Contraceptive Use among Women with Known Dyslipidemias: A Systematic Review of Critical Safety Outcomes. Contraception 2016, 94, 280–287. [Google Scholar] [CrossRef]
- Palacios, S.; Colli, E.; Regidor, P.A. Metabolic and Laboratory Effects of a Progestin-Only Pill Containing Drospirenone 4 Mg in Comparison to Desogestrel 75 Μg: A Double-Blind, Double-Dummy, Prospective, Randomised Study. Eur. J. Contracept. Reprod. Health Care 2021, 26, 454–461. [Google Scholar] [CrossRef]
- Lobo, R.A.; Skinner, J.B.; Lippman, J.S.; Cirillo, S.J. Plasma Lipids and Desogestrel and Ethinyl Estradiol: A Meta-Analysis. Fertil. Steril. 1996, 65, 1100–1109. [Google Scholar] [CrossRef]
- Klipping, C.; Duijkers, I.; Mawet, M.; Maillard, C.; Bastidas, A.; Jost, M.; Foidart, J.M. Endocrine and Metabolic Effects of an Oral Contraceptive Containing Estetrol and Drospirenone. Contraception 2021, 103, 213–221. [Google Scholar] [CrossRef]
- Mawet, M.; Maillard, C.; Klipping, C.; Zimmerman, Y.; Foidart, J.M.; Bennink, H.J.T.C. Unique Effects on Hepatic Function, Lipid Metabolism, Bone and Growth Endocrine Parameters of Estetrol in Combined Oral Contraceptives. Eur. J. Contracept. Reprod. Health Care 2015, 20, 463–475. [Google Scholar] [CrossRef]
- Guazzelli, C.A.F.; Barreiros, F.A.; Barbosa, R.; Torloni, M.R.; Barbieri, M. Extended Regimens of the Contraceptive Vaginal Ring versus Hormonal Oral Contraceptives: Effects on Lipid Metabolism. Contraception 2012, 85, 389–393. [Google Scholar] [CrossRef]
- Creasy, G.W.; Fisher, A.C.; Hall, N.; Shangold, G.A. Transdermal Contraceptive Patch Delivering Norelgestromin and Ethinyl Estradiol: Effects on the Lipid Profile. J. Reprod. Med. 2003, 48, 179–186. [Google Scholar]
- Porkka, K.V.K.; Erkkola, R.; Taimela, S.; Raitakari, O.T.; Dahlen, G.H.; Viikari, J.S.A. Influence of Oral Contraceptive Use on Lipoprotein(a) and Other Coronary Heart Disease Risk Factors. Ann. Med. 1995, 27, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Barkfeldt, J.; Virkkunen, A.; Dieben, T. The Effects of Two Progestogen-Only Pills Containing Either Desogestrel (75 Μg/Day) or Levonorgestrel (30 Μg/Day) on Lipid Metabolism. Contraception 2001, 64, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.W.; Liang, S.; Singh, K. Effects of Mirena (Levonorgestrel-Releasing Intrauterine System) and Ortho Gynae T380 Intrauterine Copper Device on Lipid Metabolism-a Randomized Comparative Study. Contraception 2009, 79, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Nie, G.; Yang, X.; Wang, Y.; Liang, W.; Li, X.; Luo, Q.; Yang, H.; Liu, J.; Wang, J.; Guo, Q.; et al. The Effects of Menopause Hormone Therapy on Lipid Profile in Postmenopausal Women: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 850815. [Google Scholar] [CrossRef]
- Gregersen, I.; Høibraaten, E.; Holven, K.B.; Løvdahl, L.; Ueland, T.; Mowinckel, M.C.; Dahl, T.B.; Aukrust, P.; Halvorsen, B.; Sandset, P.M. Effect of Hormone Replacement Therapy on Atherogenic Lipid Profile in Postmenopausal Women. Thromb. Res. 2019, 184, 1–7. [Google Scholar] [CrossRef]
- Anagnostis, P.; Galanis, P.; Chatzistergiou, V.; Stevenson, J.C.; Godsland, I.F.; Lambrinoudaki, I.; Theodorou, M.; Goulis, D.G. The Effect of Hormone Replacement Therapy and Tibolone on Lipoprotein(a) Concentrations in Postmenopausal Women: A Systematic Review and Meta-Analysis. Maturitas 2017, 99, 27–36. [Google Scholar] [CrossRef]
- Rezende, G.P.; Dassie, T.; Gomes, D.A.Y.; Benetti-Pinto, C.L. Cardiovascular Risk Factors in Premature Ovarian Insufficiency Using Hormonal Therapy. Rev. Bras. De Ginecol. E Obstet. 2022, 45, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Goldštajn, M.Š.; Mikuš, M.; Ferrari, F.A.; Bosco, M.; Uccella, S.; Noventa, M.; Török, P.; Terzic, S.; Laganà, A.S.; Garzon, S. Effects of Transdermal versus Oral Hormone Replacement Therapy in Postmenopause: A Systematic Review. Arch. Gynecol. Obstet. 2023, 307, 1727–1745. [Google Scholar] [CrossRef] [PubMed]
- Allard, M.D.; Saeedi, R.; Yousefi, M.; Frohlich, J. Risk Stratification of Patients with Familial Hypercholesterolemia in a Multi-Ethnic Cohort. Lipids Health Dis. 2014, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Markus, M.R.P.; Ittermann, T.; Schipf, S.; Bahls, M.; Nauck, M.; Völzke, H.; Santos, R.D.; Peters, A.; Zeller, T.; Felix, S.B. Association of Sex-Specific Differences in Lipoprotein(a) Concentrations with Cardiovascular Mortality in Individuals with Type 2 Diabetes Mellitus. Cardiovasc. Diabetol. 2021, 20, 168. [Google Scholar] [CrossRef]
- Pino, B.D.; Gorini, F.; Gaggini, M.; Landi, P.; Pingitore, A.; Vassalle, C. Lipoprotein(a), Cardiovascular Events and Sex Differences: A Single Cardiological Unit Experience. J. Clin. Med. 2023, 12, 764. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.R.; Mora, S.; Ridker, P.M. Lipoprotein(a) and Cardiovascular Risk Prediction among Women. J. Am. Coll. Cardiol. 2018, 72, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Benn, M.; Schnohr, P.; Tybjærg-Hansen, A. Nonfasting Triglycerides and Risk of Myocardial Infarction, Ischemic Heart Disease, and Death in Men and Women. JAMA 2007, 298, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Wadström, B.N.; Wulff, A.B.; Pedersen, K.M.; Jensen, G.B.; Nordestgaard, B.G. Elevated Remnant Cholesterol Increases the Risk of Peripheral Artery Disease, Myocardial Infarction, and Ischaemic Stroke: A Cohort-Based Study. Eur. Heart J. 2022, 43, 3258–3269. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.H.E.M.; Rosano, G.; Cifkova, R.; Chieffo, A.; van Dijken, D.; Hamoda, H.; Kunadian, V.; Laan, E.; Lambrinoudaki, I.; Maclaran, K.; et al. Cardiovascular health after menopause transition, pregnancy disorders, and other gynaecologic conditions: A consensus document from European cardiologists, gynaecologists, and endocrinologists. Eur. Heart J. 2021, 42, 967–984. [Google Scholar] [CrossRef]
- Mehta, P.K.; Levit, R.D.; Wood, M.J.; Aggarwal, N.; O’Donoghue, M.L.; Lim, S.S.; Lindley, K.; Gaignard, S.; Quesada, O.; Vatsa, N. Chronic Rheumatologic Disorders and Cardiovascular Disease Risk in Women. Am. Heart J. Plus 2023, 27, 100267. [Google Scholar] [CrossRef]
- Lu, Y.; Li, S.X.; Liu, Y.; Rodriguez, F.; Watson, K.E.; Dreyer, R.P.; Khera, R.; Murugiah, K.; D’Onofrio, G.; Spatz, E.S. Sex-Specific Risk Factors Associated with First Acute Myocardial Infarction in Young Adults. JAMA Netw. Open 2022, 5, E229953. [Google Scholar] [CrossRef]
- Okoth, K.; Chandan, J.S.; Marshall, T.; Thangaratinam, S.; Thomas, G.N.; Nirantharakumar, K.; Adderley, N.J. Association between the Reproductive Health of Young Women and Cardiovascular Disease in Later Life: Umbrella Review. BMJ 2020, 371, m3502. [Google Scholar] [CrossRef]
- Fabunmi, O.A.; Dludla, P.V.; Nkambule, B.B. Investigating Cardiovascular Risk in Premenopausal Women on Oral Contraceptives: Systematic Review with Meta-Analysis. Front. Cardiovasc. Med. 2023, 10, 1127104. [Google Scholar] [CrossRef]
- Yoon, C.W.; Bushnell, C.D. Stroke in Women: A Review Focused on Epidemiology, Risk Factors, and Outcomes. J. Stroke 2023, 25, 2–15. [Google Scholar] [CrossRef]
- Hodis, H.N.; Mack, W.J. Menopausal Hormone Replacement Therapy and Reduction of All-Cause Mortality and Cardiovascular Disease: It Is about Time and Timing. Cancer J. 2022, 28, 208–223. [Google Scholar] [CrossRef]
- Greenlee, H.; Iribarren, C.; Rana, J.S.; Cheng, R.; Nguyen-Huynh, M.; Rillamas-Sun, E.; Shi, Z.; Laurent, C.A.; Lee, V.S.; Roh, J.M. Risk of Cardiovascular Disease in Women with and without Breast Cancer: The Pathways Heart Study. J. Clin. Oncol. 2022, 40, 1647–1658. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Gebhard, C. Gender Medicine: Effects of Sex and Gender on Cardiovascular Disease Manifestation and Outcomes. Nat. Rev. Cardiol. 2023, 20, 236–247. [Google Scholar] [CrossRef]
- Van Dam-Nolen, D.H.K.; Van Egmond, N.C.M.; Koudstaal, P.J.; Van Der Lugt, A.; Bos, D. Sex Differences in Carotid Atherosclerosis: A Systematic Review and Meta-Analysis. Stroke 2023, 54, 315–326. [Google Scholar] [CrossRef]
- ten Haaf, M.; Rijndertse, M.; Cheng, J.; de Boer, S.; Garcia-Garcia, H.; van Geuns, R.-J.; Regar, E.; Lenzen, M.; Appelman, Y.; Boersma, E. Sex Differences in Plaque Characteristics by Intravascular Imaging in Patients with Coronary Artery Disease. EuroIntervention 2017, 13, 320–328. [Google Scholar] [CrossRef]
- Kerkhof, P.L.M.; Tona, F. Sex Differences in Diagnostic Modalities of Atherosclerosis in the Macrocirculation. Atherosclerosis 2023, 384, 117275. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Shaw, L.J.; Min, J.K.; Spertus, J.A.; Chaitman, B.R.; Berman, D.S.; Picard, M.H.; Kwong, R.Y.; Bairey-Merz, C.N.; Cyr, D.D. Association of Sex with Severity of Coronary Artery Disease, Ischemia, and Symptom Burden in Patients with Moderate or Severe Ischemia: Secondary Analysis of the ISCHEMIA Randomized Clinical Trial. JAMA Cardiol. 2020, 5, 773–778. [Google Scholar] [CrossRef]
- Gasecka, A.; Zimodro, J.M.; Appelman, Y. Sex Differences in Antiplatelet Therapy: State-of-the Art. Platelets 2023, 34, 2176173. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Y.; Dong, X.; Zhang, X.; Ma, J.; Li, N.; Shi, H.; Yin, Z.; Xue, Y.; Hu, Y. Sex Disparities in Management and Outcomes among Patients with Acute Coronary Syndrome. JAMA Netw. Open 2023, 6, E2338707. [Google Scholar] [CrossRef]
- Kavurma, M.M.; Boccanfuso, L.; Cutmore, C.; Passam, F.; Patel, S.; Hennessy, A.; Loa, J.; Figtree, G.A.; Golledge, J.; Robinson, D.A. A Hidden Problem: Peripheral Artery Disease in Women. Eur. Heart J. Qual. Care Clin. Outcomes 2023, 9, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, G.; Bosisio, R.; Calabresi, L.; Magni, P.; Pavanello, C.; Pazzucconi, F.; Sirtori, C.R. Gender-Related Lipid and/or Lipoprotein Responses to Statins in Subjects in Primary and Secondary Prevention. J. Clin. Lipidol. 2015, 9, 226–233. [Google Scholar] [CrossRef]
- Fulcher, J.; O’Connell, R.; Voysey, M.; Emberson, J.; Blackwell, L.; Mihaylova, B.; Simes, J.; Collins, R.; Kirby, A.; Colhoun, H. Efficacy and Safety of LDL-Lowering Therapy among Men and Women: Meta-Analysis of Individual Data from 174 000 Participants in 27 Randomised Trials. Lancet 2015, 385, 1397–1405. [Google Scholar] [CrossRef]
- Hunt, N.B.; Emmens, J.E.; Irawati, S.; de Vos, S.; Bos, J.H.J.; Wilffert, B.; Hak, E.; de Boer, R.A. Sex Disparities in the Effect of Statins on Lipid Parameters The PharmLines Initiative. Medicine 2022, 101, e28394. [Google Scholar] [CrossRef]
- Kato, E.T.; Cannon, C.P.; Blazing, M.A.; Bohula, E.; Guneri, S.; White, J.A.; Murphy, S.A.; Park, J.G.; Braunwald, E.; Giugliano, R.P. Efficacy and Safety of Adding Ezetimibe to Statin Therapy among Women and Men: Insight from IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). J. Am. Heart Assoc. 2017, 6, e006901. [Google Scholar] [CrossRef]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Abramson, B.L.; Benlian, P.; Hanson, M.E.; Lin, J.; Shah, A.; Tershakovec, A.M. Response by Sex to Statin plus Ezetimibe or Statin Monotherapy: A Pooled Analysis of 22,231 Hyperlipidemic Patients. Lipids Health Dis. 2011, 10, 146. [Google Scholar] [CrossRef]
- Kim, B.G.; Lee, S.J.; Lee, Y.J.; You, S.C.; Hong, S.J.; Yun, K.H.; Hong, B.K.; Heo, J.H.; Rha, S.W.; Hong, S.J. Effect of Moderate-Intensity Statin with Ezetimibe Combination vs. High-Intensity Statin Therapy According to Sex in Patients with Atherosclerosis. Sci. Rep. 2023, 13, 972–998. [Google Scholar] [CrossRef]
- Goldberg, A.C.; Banach, M.; Catapano, A.L.; Duell, P.B.; Leiter, L.A.; Hanselman, J.C.; Lei, L.; Mancini, G.B.J. Evaluation of the Efficacy and Safety of Bempedoic Acid in Women and Men: Pooled Analyses from Phase 3 Trials. Atherosclerosis 2023, 384, 117192. [Google Scholar] [CrossRef]
- Cho, L.; Plutzky, J.; Brennan, D.; Louie, M.J.; Lei, L.; Robinson, P.; Powell, H.A.; Nicholls, S.J.; Lincoff, A.M.; Nissen, S.E. Impact of Bempedoic Acid on Cardiovascular Outcomes by Sex. Circulation 2024, 149, 1775–1777. [Google Scholar] [CrossRef]
- Sever, P.; Gouni-Berthold, I.; Keech, A.; Giugliano, R.; Pedersen, T.R.; Im, K.A.; Wang, H.; Knusel, B.; Sabatine, M.S.; O’donoghue, M.L. LDL-Cholesterol Lowering with Evolocumab, and Outcomes According to Age and Sex in Patients in the FOURIER Trial. Eur. J. Prev. Cardiol. 2021, 28, 805–812. [Google Scholar] [CrossRef]
- Fogacci, F.; Yerlitaş, S.İ.; Giovannini, M.; Zararsız, G.; Lido, P.; Borghi, C.; Cicero, A.F.G. Sex X Time Interactions in Lp(a) and LDL-C Response to Evolocumab. Biomedicines 2023, 11, 3271. [Google Scholar] [CrossRef]
- Vallejo-Vaz, A.J.; Ginsberg, H.N.; Davidson, M.H.; Eckel, R.H.; Cannon, C.P.; Lee, L.V.; Bessac, L.; Pordy, R.; Letierce, A.; Ray, K.K. Lower On-Treatment Low-Density Lipoprotein Cholesterol and Major Adverse Cardiovascular Events in Women and Men: Pooled Analysis of 10 ODYSSEY Phase 3 Alirocumab Trials. J. Am. Heart Assoc. 2018, 7, e009221. [Google Scholar] [CrossRef]
- Myasoedova, V.A.; Rimbert, A.; Camera, M.; Le May, C.; Capoulade, R.; Cariou, B.; Poggio, P. LDL Lowering Effect of PCSK9 Inhibition Is Reduced in Women. Eur. Heart J. Cardiovasc. Pharmacother. 2023, 9, 337–342. [Google Scholar] [CrossRef]
- Rivera, F.B.; Cha, S.W.; Aparece, J.P.; Rocimo, A.; Ong, B.A.; Golbin, J.M.; Alfonso, P.G.; Enkhmaa, B.; Khan, S.U.; Cainzos-Achirica, M. Sex Differences in Cardiovascular Outcomes and Cholesterol-Lowering Efficacy of PCSK9 Inhibitors: Systematic Review and Meta-Analysis. JACC Adv. 2023, 2, 100669. [Google Scholar] [CrossRef]
- Galema-Boers, A.M.H.; Mulder, J.W.C.M.; Steward, K.; Roeters van Lennep, J.E. Sex Differences in Efficacy and Safety of PCSK9 Monoclonal Antibodies: A Real-World Registry. Atherosclerosis 2023, 384, 117108. [Google Scholar] [CrossRef]
- Paquette, M.; Faubert, S.; Saint-Pierre, N.; Baass, A.; Bernard, S. Sex Differences in LDL-C Response to PCSK9 Inhibitors: A Real World Experience. J. Clin. Lipidol. 2023, 17, 142–149. [Google Scholar] [CrossRef]
- Zhang, H.; Plutzky, J.; Shubina, M.; Turchin, A. Drivers of the Sex Disparity in Statin Therapy in Patients with Coronary Artery Disease: A Cohort Study. PLoS ONE 2016, 11, e0155228. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Molemans, B.; Marieke Schoonen, W.; Giovas, P.; Bray, S.; Kiru, G.; Murphy, J.; Banach, M.; De Servi, S.; Gaita, D. EU-Wide Cross-Sectional Observational Study of Lipid-Modifying Therapy Use in Secondary and Primary Care: The DAVINCI Study. Eur. J. Prev. Cardiol. 2021, 28, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Vrablik, M.; Seifert, B.; Parkhomenko, A.; Banach, M.; Jóźwiak, J.J.; Kiss, R.G.; Gaita, D.; Rašlová, K.; Zachlederova, M.; Bray, S. Lipid-Lowering Therapy Use in Primary and Secondary Care in Central and Eastern Europe: DA VINCI Observational Study. Atherosclerosis 2021, 334, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Gouni-Berthold, I.; Schaper, F.; Schatz, U.; Tabbert-Zitzler, A.; Fraass, U.; Sauer, S.; Ray, K.K. Low-Density Lipoprotein Cholesterol Goal Attainment in Germany: Results from the DA VINCI Study: LDL-C Goal Attainment in Germany. Atheroscler. Plus 2022, 50, 10–16. [Google Scholar] [CrossRef]
- Cannon, C.P.; De Lemos, J.A.; Rosenson, R.S.; Ballantyne, C.M.; Liu, Y.; Gao, Q.; Palagashvilli, T.; Alam, S.; Mues, K.E.; Bhatt, D.L. Use of Lipid-Lowering Therapies over 2 Years in GOULD, a Registry of Patients with Atherosclerotic Cardiovascular Disease in the US. JAMA Cardiol. 2021, 6, 1060–1068. [Google Scholar] [CrossRef]
- Agarwala, A.; Deych, E.; Jones, L.K.; Sturm, A.C.; Aspry, K.; Ahmad, Z.; Ballantyne, C.M.; Goldberg, A.C. Sex-Related Differences in Premature Cardiovascular Disease in Familial Hypercholesterolemia. J. Clin. Lipidol. 2023, 17, 150–156. [Google Scholar] [CrossRef]
- Iyen, B.; Qureshi, N.; Weng, S.; Roderick, P.; Kai, J.; Capps, N.; Durrington, P.N.; McDowell, I.F.; Soran, H.; Neil, A. Sex Differences in Cardiovascular Morbidity Associated with Familial Hypercholesterolaemia: A Retrospective Cohort Study of the UK Simon Broome Register Linked to National Hospital Records. Atherosclerosis 2020, 315, 131–137. [Google Scholar] [CrossRef]
- Klevmoen, M.; Bogsrud, M.P.; Retterstøl, K.; Svilaas, T.; Vesterbekkmo, E.K.; Hovland, A.; Berge, C.; Roeters van Lennep, J.; Holven, K.B. Loss of statin treatment years during pregnancy and breastfeeding periods in women with familial hypercholesterolemia. Atherosclerosis 2021, 335, 8–15. [Google Scholar] [CrossRef]
- Christensen, J.J.; Retterstøl, K.; Godang, K.; Roland, M.C.; Qvigstad, E.; Bollerslev, J.; Ueland, T.; Henriksen, T.; Holven, K.B. LDL cholesterol in early pregnancy and offspring cardiovascular disease risk factors. J. Clin. Lipidol. 2016, 10, 1369–1378.e7. [Google Scholar] [CrossRef]
- Ray, K.K.; Raal, F.J.; Kallend, D.G.; Jaros, M.J.; Koenig, W.; Leiter, L.A.; Landmesser, U.; Schwartz, G.G.; Lawrence, D.; Friedman, A. Inclisiran and Cardiovascular Events: A Patient-Level Analysis of Phase III Trials. Eur. Heart J. 2023, 44, 129–138. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; López, J.A.G.; Knusel, B.; Gencer, B.; Wang, H.; Wu, Y.; Kassahun, H.; Sabatine, M.S. Study Design and Rationale for the Olpasiran Trials of Cardiovascular Events And LipoproteiN(a) Reduction-DOSE Finding Study (OCEAN(a)-DOSE). Am. Heart J. 2022, 251, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Van Diemen, J.; Verdonk, P.; Chieffo, A.; Regar, E.; Mauri, F.; Kunadian, V.; Sharma, G.; Mehran, R.; Appelman, Y. The Importance of Achieving Sex- And Gender-Based Equity in Clinical Trials: A Call to Action. Eur. Heart J. 2021, 42, 2990–2994. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimodro, J.M.; Mucha, M.; Berthold, H.K.; Gouni-Berthold, I. Lipoprotein Metabolism, Dyslipidemia, and Lipid-Lowering Therapy in Women: A Comprehensive Review. Pharmaceuticals 2024, 17, 913. https://doi.org/10.3390/ph17070913
Zimodro JM, Mucha M, Berthold HK, Gouni-Berthold I. Lipoprotein Metabolism, Dyslipidemia, and Lipid-Lowering Therapy in Women: A Comprehensive Review. Pharmaceuticals. 2024; 17(7):913. https://doi.org/10.3390/ph17070913
Chicago/Turabian StyleZimodro, Jakub Michal, Magda Mucha, Heiner K. Berthold, and Ioanna Gouni-Berthold. 2024. "Lipoprotein Metabolism, Dyslipidemia, and Lipid-Lowering Therapy in Women: A Comprehensive Review" Pharmaceuticals 17, no. 7: 913. https://doi.org/10.3390/ph17070913
APA StyleZimodro, J. M., Mucha, M., Berthold, H. K., & Gouni-Berthold, I. (2024). Lipoprotein Metabolism, Dyslipidemia, and Lipid-Lowering Therapy in Women: A Comprehensive Review. Pharmaceuticals, 17(7), 913. https://doi.org/10.3390/ph17070913






